Abstract
Background:
Mast cells are involved in allergy and inflammation by secreting multiple mediators including histamine, cytokines and platelet-activating factor. Certain histamine 1 receptor antagonists have been reported to inhibit histamine secretion, but the effect on cytokine release from human mast cells triggered by allergic and other stimuli is not well known. We investigated the ability of rupatadine, a potent histamine 1 receptor antagonist that also blocks platelet-activating factor actions, to also inhibit mast cell mediator release.
Methods:
Rupatadine (1–50 μM) was used before stimulation by: (1) interleukin (IL)-1 to induce IL-6 from human leukemic mast cells (HMC-1 cells), (2) substance P for histamine, IL-8 and vascular endothelial growth factor release from LAD2 cells, and (3) IgE/anti-IgE for cytokine release from human cord blood-derived cultured mast cells. Mediators were measured in the supernatant fluid by ELISA or by Milliplex microbead arrays.
Results:
Rupatadine (10–50 μM) inhibited IL-6 release (80% at 50 μM) from HMC-1 cells, whether added 10 min or 24 h prior to stimulation. Rupatadine (10–50 μM for 10 min) inhibited IL-8 (80%), vascular endothelial growth factor (73%) and histamine (88%) release from LAD2 cells, as well as IL-6, IL-8, IL-10, IL-13 and tumor necrosis factor release from human cord blood-derived cultured mast cells.
Conclusion:
Rupatadine can inhibit histamine and cytokine secretion from human mast cells in response to allergic, immune and neuropeptide triggers. These actions endow rupatadine with unique properties in treating allergic inflammation, especially perennial rhinitis and idiopathic urticaria.
Key Words: Cytokines, Inflammation, Mast cells, Rupatadine, Urticaria
Introduction
Mast cells are important effector cells in IgE-mediated allergic reactions, but also in immunity [1, 2] and inflammatory processes [3] due to their ability to secrete numerous vasoactive molecules and cytokines in response to a variety of stimuli. For instance, mast cells can secrete histamine, interleukin (IL)-6, IL-8, platelet-activating factor (PAF), tumor necrosis factor (TNF), tryptase and vascular endothelial growth factor (VEGF) [4]. IL-6 participates in inflammation and acute-phase reactions [5], while IL-8 is a potent neutrophil chemotactic molecule [6], and VEGF also has vasodilatory actions [7]. In view of such findings, mast cells are now recognized to have a more critical role in diseases that may also involve inflammation, such as in asthma [8], but also in skin diseases [9].
Certain histamine 1 (H1) receptor antagonists have been reported to have variable antiallergic effects [10, 11]. Rupatadine is a newer H1 receptor antagonist [12, 13] with the ability to also inhibit actions of PAF [14, 15]. Rupatadine appears to be more potent than other antihistamines in the treatment of allergic rhinitis [16, 17] and chronic idiopathic urticaria [18,19,20]. We hypothesized that this apparent superior action might be due to, in addition to blocking PAF actions, the ability of rupatadine to inhibit the release of proinflammatory cytokines from mast cells.
Therefore, we examined the effect of rupatadine on human mast cell activation by 3 different triggers: (1) IL-1, which stimulates the release of IL-6 from human leukemic mast cells (HMC-1), (2) substance P (SP), which stimulates LAD2 human mast cells, to release histamine and cytokines, and (3) IgE/anti-IgE, which stimulates human umbilical cultured cord blood-derived mast cells (hCBMCs) to release histamine, IL-6, IL-8, IL-10, IL-13, TNF and VEGF. The present results indicate that rupatadine inhibits human mast cell activation by allergic and nonallergic triggers, effects that may explain its potent actions in rhinitis and chronic idiopathic urticaria.
Materials and Methods
Rupatadine was provided by Uriach, Inc., Barcelona, Spain. Rupatadine concentrations (1, 5, 10, 25 and 50 µM) were prepared from stock (10–3M) made as follows: 5.3 mg of rupatadine was diluted with 0.1 N HCl (5 drops) and 8 ml of distilled water; pH was adjusted to 5.0–5.5 with NaOH and the volume was adjusted to 10 ml. This stock solution was aliquoted and stored at −80°C. Working dilutions were prepared in distilled water immediately before use.
Mast Cell Culture
HMC-1 [21] and LAD2 [22] cells were cultured for 5 and 14 days, respectively, as previously reported. Human umbilical cord blood was collected at Tufts Medical Center, Boston, Mass., USA, as approved by the Medical Center Human Investigation Review Board. Cord blood was diluted with Dulbecco's phosphate-buffered saline (DPBS) from Gibco BRL (Life Technologies, Grand Island, N.Y., USA) containing 2 mM ethylenediaminetetraacetic acid (Sigma). Mononuclear cells were separated using Lymphocyte Separation Medium from Organon Teknika Corp., Durham, N.C., USA. CD34+ cells were isolated by positive selection of CD34+/AC133+ cells by magnetic associated cell sorting using an AC133+ cell isolation kit (Milltenyi Biotec, Auburn, Calif., USA), as reported previously [23]. CD34+ cells were cultured in Iscove's Modified Dulbecco's Medium (Gibco BRL) containing 100 ng/ml recombinant human stem cell factor, 50 ng/ml IL-6 (Chemicon International, Inc., Temecula, Calif., USA), 5% fetal bovine serum (Biowhittaker, Walkesville, Md., USA), 5 × 10–5M 2-mercaptoethanol, and 1% penicillin-streptomycin (Gibco BRL) for 4 weeks except during the last week for the Milliplex microbead assays; they were then cultured another 4 weeks in the same medium to which IL-4 (20 ng/ml; Chemicon International, Inc.) was added to increase maturation. During this culture period, the cells were washed with DPBS every week and resuspended using fresh complete culture medium. The purity of hCBMCs was evaluated by immunocytochemical staining for tryptase as previously described [23], and mast cell viability was determined by trypan blue (0.3%) exclusion; hCBMCs cultured for over 8 weeks were used in these experiments.
HMC-1 and LAD2 Cell Stimulation
HMC-1 cells (105 cells/condition) were preincubated with rupatadine for 10 min at the concentrations indicated, before stimulation with IL-1 (100 ng/ml) for 6 h at 37°C in 95%/5% CO2 atmosphere, as most cytokines are synthesized de novo[24]. For the time course experiments, rupatadine was added first at the doses and times indicated, and the cells were incubated until they were stimulated by the specific trigger. LAD2 cells (105 cells/condition for IL-8 assay, 5 × 104 cells/condition for VEGF assay, 3 × 104 cells/condition for histamine assay) were preincubated with rupatadine for 10 min at the concentrations indicated, before stimulation with SP (1 µM) for 24 h for IL-8 and VEGF release, and for 20 min for histamine release in human Tyrode's buffer at 37°C in a shaking water bath. Control cells were treated with the same volume of culture medium/Tyrode's buffer. IL-6, IL-8 and VEGF were measured in the supernatant fluid by ELISA (Quantikine, R&D Systems, Minneapolis, Minn., USA). Histamine levels in the supernatant fluid and pellet were measured fluorometrically using an LS-50B Luminescence Spectrometer (Perkin Elmer, Norwalk, Conn., USA) as reported previously [25]. Histamine release was expressed as percentage of the total cellular histamine content = [(histamine in supernatant)/(histamine in supernatant + histamine in pellet)] × 100.
Sensitization and Stimulation of hCBMCs
hCBMCs were washed with DPBS and plain culture medium once in each and resuspended in serum-free complete culture medium. Cells (106 cells/ml) were then incubated with human myeloma-IgE (2 µg/ml; Chemicon International, Inc.) at 37°C for 48 h in 24-well Falcon cell culture plates (Becton Dickinson, Franklin Lakes, N.J., USA). These sensitized hCBMCs (1.8 × 105 cells/condition) were incubated with rupatadine for 10 min at the concentrations indicated, before stimulation with anti-human IgE (10 µg/ml) for 24 h; supernatant fluid was then collected and analyzed for several mediators using Milliplex microbead arrays (Millipore, Billerica, Mass., USA).
HMC-1 and LAD2 cells do not respond to stimulation by IgE/anti-IgE while LAD2 cells do not produce IL-6; hence, IL-1 stimulation was used only with HMC-1 cells.
Statistics
Cytokine results are presented as actual amounts released since most cytokines are de novo synthesized and are not stored in the granules; in contrast, histamine results are presented as percent net release, since histamine is stored in the granules and can be measured in the cell pellet. Results from all samples testing inhibition were individually compared with the corresponding stimulated samples using the Student's t test, as indicated in the legends, except for human results that were compared using the nonparametric Mann-Whitney U test. Data are represented as the mean ± SD. The level of statistical significance was set at p < 0.05.
Results
Effect of Rupatadine on IL-1 Stimulation of HMC-1 Cells
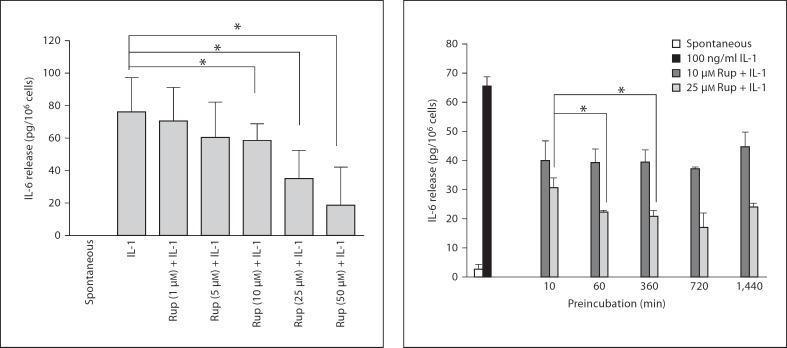
We had previously shown that IL-1 can stimulate HMC-1 cells to release only IL-6. Therefore, we tested the effect of rupatadine on IL-1 stimulation of HMC-1 cells. Rupatadine at 1, 5, 10, 25 and 50 µM added 10 min prior to IL-1 reduced IL-6 release from HMC-1 cells by 8, 20, 22, 54 and 77%, respectively (fig. 1). A preincubation time course showed that there was no difference whether rupatadine (10 µM) was added 10 min or 24 h prior to stimulation, but there was better inhibition when 25 µM was used for 60 min prior to stimulation (fig. 2). Spontaneous release and loss of cell viability were negligible at these rupatadine concentrations.
Fig. 1.
Dose response of the inhibitory effect of rupatadine on IL-6 release from HMC-1 cells. HMC-1 cells were preincubated with rupatadine (Rup) for 10 min at 1, 5, 10, 25 and 50 µM and stimulated with IL-1 (100 ng/ml, for 6 h). Control mast cells were treated with culture medium only for 6 h. After the incubation period, the supernatant solution was collected and IL-6 was assayed by ELISA. Results were analyzed using the unpaired equal, 2-tailed, Student's t test (n = 11). Spontaneous release was below the level of detection. * p < 0.05, significance of comparisons between conditions and samples treated with the trigger alone, as shown by the corresponding brackets.
Fig. 2.
Time course of the inhibitory effect of rupatadine on IL-6 release from HMC-1 cells. HMC-1 cells were preincubated with rupatadine (RUP) at 10 and 25 µM for 10 min, 1, 6, 12 and 24 h and stimulated with IL-1 (at 100 ng/ml, for 6 h). Control mast cells were treated with culture medium only for 6 h. After the incubation period, the supernatant solution was collected and IL-6 was assayed by ELISA. Results were analyzed using the paired equal, 2-tailed, Student's t test (n = 3). * p < 0.05, significance of comparisons between the results of the same concentration at different times of preincubation and 10 min of preincubation, as shown by the corresponding brackets.
Effect of Rupatadine on SP Stimulation of LAD2 Cells
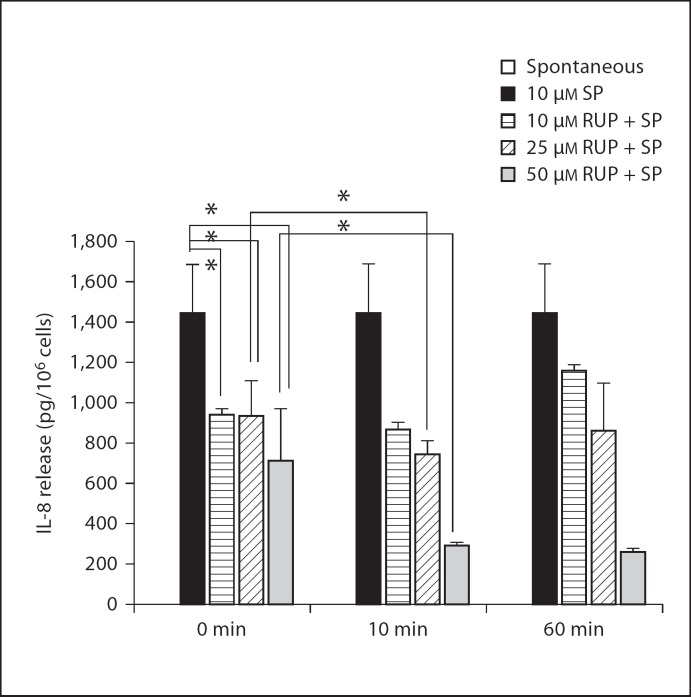
We then investigated the effect of rupatadine on LAD2 cells stimulated by SP. Rupatadine at 10, 25 and 50 µM added 10 min prior to SP (10 µM) inhibited IL-8 release from LAD2 cells by 40, 48 and 80%, respectively (fig. 3). We also compared the effect of rupatadine added together with or 10 and 60 min prior to SP. Rupatadine inhibited IL-8 release by 50% at 50 µM even when added together with SP (fig. 4). Although adding rupatadine 10 min prior to the trigger further reduced IL-8 release at all concentrations tested (fig. 4), there was no difference between 10- or 60-min preincubation.
Fig. 3.
Dose response of the inhibitory effect of rupatadine on IL-8 release from LAD2 cells. LAD2 cells were preincubated with rupatadine (Rup) at 10 (n = 4), 25 (n = 15) and 50 µM (n = 9) for 10 min and were subsequently stimulated with SP (10 µM) for 24 h. Spontaneous release was below the level of detection. Results were analyzed using the unpaired equal, 2-tailed, Student's t test (for the comparison between SP and 10 or 25 µM rupatadine + SP, for each time of preincubation) and the unpaired unequal, 2-tailed, Student's t test for the rest. * p < 0.05, significance of comparisons between conditions and samples treated with the trigger alone, as shown by the corresponding brackets.
Fig. 4.
Time course of the inhibitory effect of rupatadine on IL-8 release from LAD2 cells. LAD2 cells were preincubated with rupatadine (Rup) at 10, 25 and 50 µM for 0 min, 10 min and 1 h and were subsequently stimulated with SP (1 µM, for 24 h) 10 (n = 4), 25 (n = 15) and 50 µM (n = 9). IL-8 was measured in the supernatant fluid by ELISA. Results were analyzed using the paired equal, 2-tailed, Student's t test. Spontaneous release was below the level of detection. * p < 0.05, significance of comparisons between the results of the same concentration at different times of preincubation and 10 min of preincubation, as shown by the corresponding brackets.
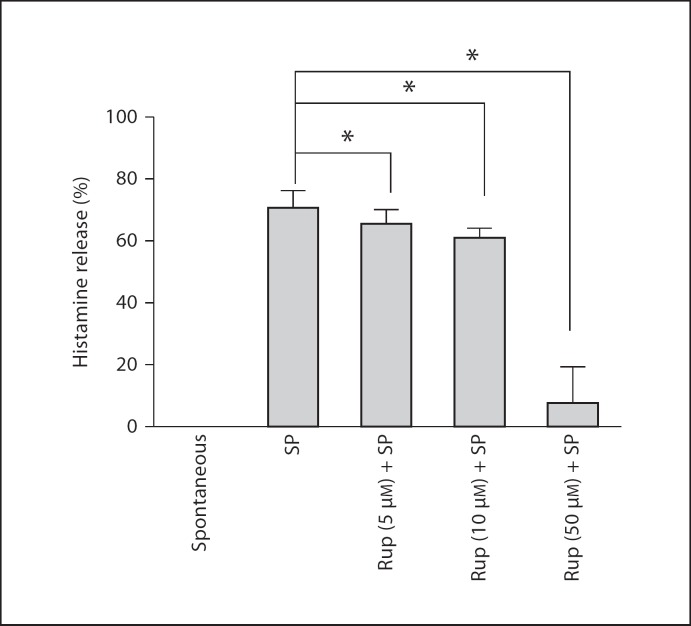
Rupatadine at 5, 10 and 50 µM for 10 min prior to SP inhibited VEGF release by 18, 32 and 73%, respectively (fig. 5); histamine release was inhibited by 7, 13 and 88%, respectively (fig. 6).
Fig. 5.
Dose response of the inhibitory effect of rupatadine on VEGF release from LAD2 cells. LAD2 cells were preincubated with rupatadine (Rup) at 5, 10 and 50 µM for 10 min and stimulated with SP (at 10 µM, n = 6) for 24 h. VEGF was measured in the supernatant fluid by ELISA. Results were analyzed by the unpaired equal, 2-tailed, Student's t test. * p < 0.05, significance of comparisons between conditions and samples treated with the trigger alone, as shown by the corresponding brackets.
Fig. 6.
Dose response of the inhibitory effect of rupatadine on histamine release from LAD2 cells. LAD2 cells were preincubated with rupatadine (Rup) at 5, 10 and 50 µM for 10 min and stimulated with SP (at 10 µM, n = 9) for 20 min. Histamine in the supernatant and pellet was measured fluorometrically. Spontaneous release was below the level of detection. Results are presented as percent net release, and data were analyzed by the unpaired equal, 2-tailed, Student's t test. * p < 0.05, significance of comparisons between conditions and samples treated with the trigger alone, as shown by the corresponding brackets.
Effect of Rupatadine on IgE/Anti-IgE Stimulation of hCBMCs
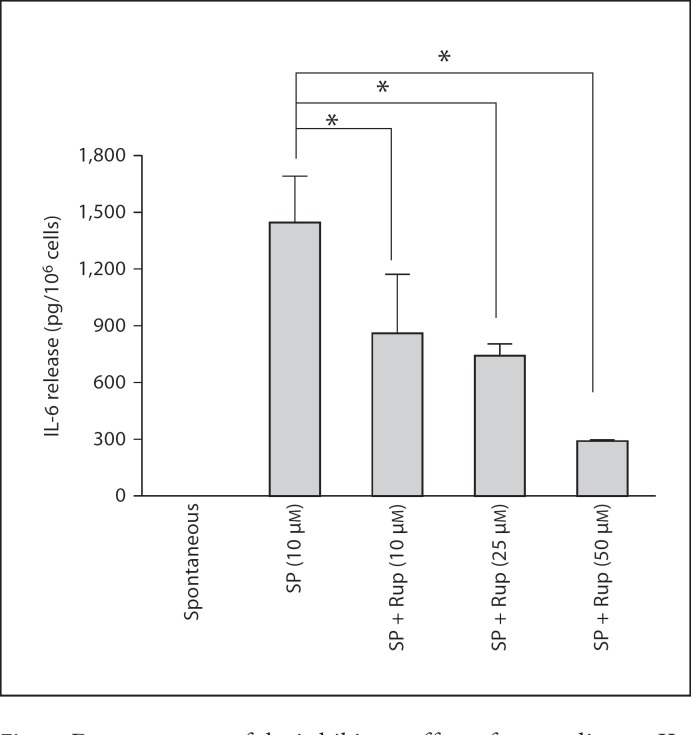
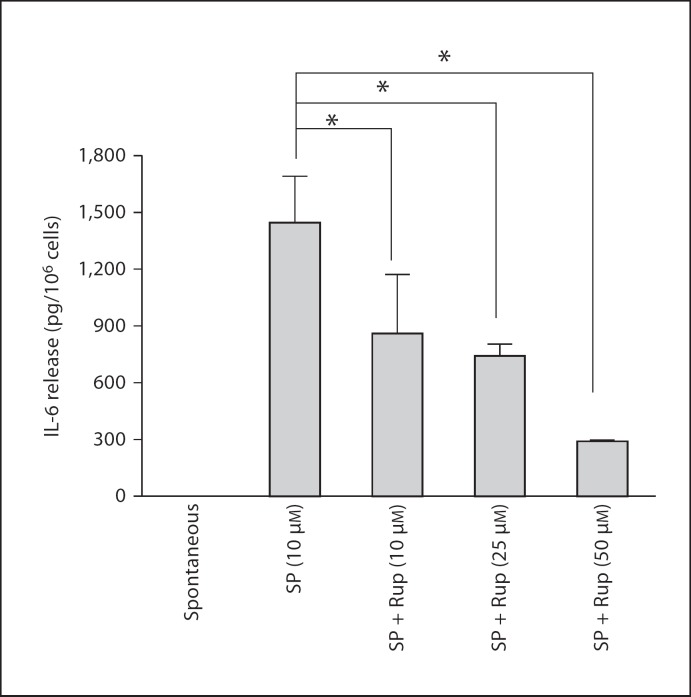
We then investigated the effect of rupatadine on stimulation of hCBMCs by IgE/anti-IgE. Rupatadine at 10 and 25 µM was added 10 min prior to stimulation with anti-IgE (10 µg/ml). Supernatant fluid was collected and analyzed for histamine fluorometrically, as well as for 37 molecules [IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IL-18, IL-23, VEGF, TNF-α, TNF-β, stem cell factor, interferon-γ, monocyte chemoattractant protein 1, macrophage inflammatory protein (MIP)-1, MIP-1α, MIP-2β, matrix metalloproteinase (MMP)-2, MMP-3, MMP-9, brain-derived neurotrophic factor, eotaxin, factor VII, granulocyte macrophage colony-stimulating factor, intercellular adhesion molecule 1, RANTES, interferon-γ and epidermal growth factor] using Milliplex microbead arrays.
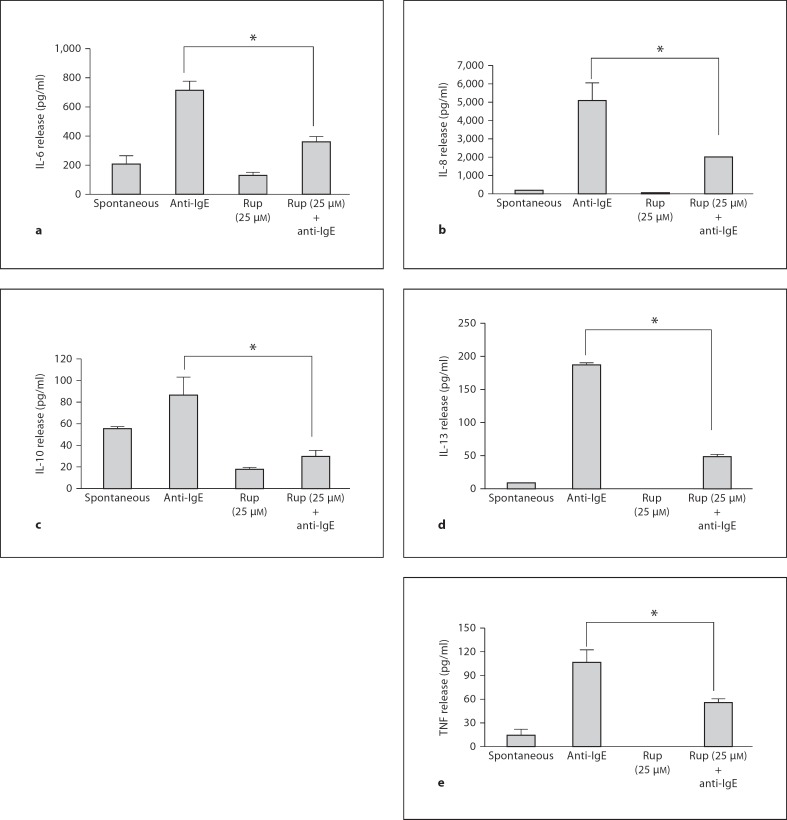
IgE/anti-IgE stimulation significantly increased only IL-6 (fig. 7a), IL-8 (fig. 7b), IL-10 (fig. 7c), IL-13 (fig. 7d) and TNF release (fig. 7e); VEGF results were too variable. Rupatadine at 25 µM reduced IL-6 release from 715 ± 65.8 pg/ml after anti-IgE to 364 ± 36.4 pg/ml (69% inhibition, p = 0.0012), IL-8 release from 5,120 ± 934.9 to 2,012 ± 29.4 pg/ml (63% inhibition, p = 0.0045), IL-10 release from 86 ± 16.8 to 30 ± 4.9 pg/ml (34% inhibition, p = 0.005), IL-13 release from 188 ± 3.3 to 48 ± 3.4 pg/ml (79% inhibition, p = 0.00000001), and TNF release from 107 ± 15.3 to 56 ± 4.5 pg/ml (55% inhibition, p = 0.0055), as shown in figure 7.
Fig. 7.
Effect of rupatadine (25 µM for 10 min) on IL-6 (a), IL-8 (b), IL-10 (c), IL-13 (d) and TNF release (e) release from hCBMCs measured by Milliplex microbead array (n = 3). Data were analyzed by the unpaired equal, 2-tailed, Student's t test. * p < 0.05, significance of comparisons between conditions and samples treated with the trigger alone, as shown by the corresponding brackets.
Discussion
In the present paper, we investigated stimulation of human mast cells by different triggers. HMC-1 mast cells were stimulated by IL-1, which we had previously shown to release only IL-6 [24]; HMC-1 cells were also used because they grow rapidly in culture allowing us to carry out both dose-response and time-course experiments. We did not use IL-1 stimulation with LAD2 cells because these cells do not secrete IL-6. Instead, we stimulated LAD2 cells with the neuropeptide SP [3], to which HMC-1 cells do not respond, and measured the most abundant mediators produced, histamine, IL-8 and VEGF. Finally, we stimulated primary cultured human mast cells (hCBMCs), which grow slowly and are not as plentiful, with IgE/anti-IgE and measured a number of mediators. It is obvious from the above that the same stimuli could not be compared in all these types of human mast cells. The present results indicate that rupatadine can inhibit the secretion of histamine and the proinflammatory mediators IL-6, IL-8, IL-10, IL-13, TNF and VEGF from human mast cells, stimulated by allergic and other triggers.
The inhibitory action of rupatadine did not exhibit a classic dose-response curve, as it was limited to within 1 log (5–50 µM). The IC50 for the inhibitory effect of rupatadine on human mast cell secretion was about 25 µM, within the range previously reported for rupatadine's inhibition of histamine and TNF release from canine skin mast cells [26]. This concentration is 250–1,000 times higher than the concentration required for H1 receptor antagonism depending on the assays used [12]. Even though rupatadine is marketed as 10 mg/day, it is increasingly used by clinicians at 20 mg/day for chronic idiopathic urticaria [18]. The rupatadine plasma concentration achieved with a single 20 mg/day in humans is Cmax = 4.3 µg/l [27]. Assuming this Cmax and the molecular weight of rupatadine (fumarate salt) to be 415.96, the plasma concentration is approximately 0.01 µM. Primary mast cells in the nose or skin may be more sensitive to the action of rupatadine than cultured mast cells, to be tested in the future with ex vivo treatment of nasal polyp or skin biopsies from patients. In addition, much higher rupatadine local concentrations could be achieved after instillation into the nasal mucosa of a topical rupatadine solution. For instance, topical application into a nostril of 150 µl of 1 mg/ml rupatadine solution (feasible) means 150 µg rupatadine that could produce a local concentration closer to the values used in this study. Other H1 receptor antagonists had previously been shown to inhibit mast cell granule-stored mediators in similar concentrations to what we report here for rupatadine [10, 11]. For instance, azelastine resulted in a maximal inhibition of tryptase (55%) and histamine (41%) release [28], as well as of IL-6 (83%) release from hCBMCs at 24 µM[25]. Azelastine also inhibited IL-6, IL-8 and TNF release from hCBMCs stimulated by IgE/anti-IgE [29]. More recently, the H1 receptor antagonists [30] epinastine (40 µM) and azelastine (200 µM) reduced IL-8 release by almost 30%, epinastine and olopatadine reduced IL-10 release by 30–50%, while epinastine and olopatadine reduced TNF release by about 30% [30]. However, as we also observed with rupatadine, there was no clear dose-response effect [30], and the extent of inhibition also varied considerably, with epinastine showing the strongest inhibition of IL-8 and IL-10, while azelastine was the most potent inhibitor for TNF. In contrast, rupatadine inhibited the release of all these cytokines (IL-8, IL-10, TNF) at 25–50 µM. However, no comparative studies with other antihistamines were performed.
Mast cell activation by IgE has traditionally relied on the measurement of preformed mast cell mediators, such as histamine, which is stored in the secretory granules [31,32,33]. It is now recognized that human mast cells produce a number of cytokines, most of which are newly synthesized upon activation [34]. However, there is considerable heterogeneity on the cytokine content of various human cell types [34,35,36,37], making it necessary to measure different mediators from different types of mast cells in different pathophysiological settings. Moreover, mast cells can secrete cytokines selectively, without degranulation [38]. In particular, we had shown that IL-1 can stimulate selective release of IL-6 [24], while corticotropin-releasing hormone can release VEGF selectively without degranulation [39].
The mechanism of the inhibitory action of rupatadine is not known at present. The H1 receptor is not likely to be involved as rupatadine can effectively antagonize this receptor at <0.1 nM, while >10 µM are required for mast cell inhibition. Moreover, pretreatment of mast cells with diphenhydramine (50 µM), which totally blocks the H1 receptor, could not prevent the ability of rupatadine to inhibit mast cells (results not shown). Rupatadine may inhibit intracellular calcium ion elevations and/or nuclear factor-ĸB activation, as previously shown for azelastine [29]. The ability of rupatadine to antagonize PAF may also somehow contribute to mast cell inhibition, especially if PAF is secreted from mast cells and has an autocrine stimulatory action.
Conclusion
Rupatadine is a potent H1 receptor antagonist with additional anti-PAF and mast cell secretion blocking effects. These properties could be particularly useful in the treatment of allergic inflammation present in a variety of airway and skin diseases, such as perennial rhinitis and chronic idiopathic urticaria, both of which are fairly resistant to common H1 receptor antagonists. However, the present studies did not use primary mast cells from the respective tissues and, therefore, cannot be directly extrapolated to the clinical settings.
Disclosure
US patents No. 6624148, 6689748, 6984667, 7115278, 10/811825 and EPO 136577 (awarded to T.C.T.) cover the use of methods and compositions of mast cell blockers, including the combination of H1 receptor antagonists (and rupatadine specifically) together with flavonoids and proteoglycans in allergic and inflammatory diseases.
Acknowledgments
We thank Uriach, Inc., Barcelona, Spain, for providing rupatadine and for partially funding this study, as well as Amgen, Inc., Thousand Oaks, Calif., USA, for their generous gift of recombinant human stem cell factor. We also thank Millipore Inc., Billerica, Mass., USA, for performing the Luminex microbead array analysis, as well as Dr. Butterfield (Mayo Clinic, Rochester, Minn., USA) for providing the HMC-1 cells, and Drs. D. Metcalfe and A.S. Kirshenbaum (NIH, Bethesda, Md., USA) for providing the LAD2 mast cells. We also thank the personnel of the Maternal-Fetal Division of Tufts Medical Center for collecting the umbilical cord blood used in these studies.
References
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 3.Theoharides TC, Kalogeromitros D. The critical role of mast cell in allergy and inflammation. Ann NY Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 4.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcε receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papanicolaou D, Wilder RL, Manolagas SC, Chrousos G. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Salamon P, Shoham NG, Gavrieli R, Wolach B, Mekori YA. Human mast cells release interleukin-8 and induce neutrophil chemotaxis on contact with activated T cells. Allergy. 2005;60:1316–1319. doi: 10.1111/j.1398-9995.2005.00886.x. [DOI] [PubMed] [Google Scholar]
- 7.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A. Synthesis, storage and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay AB. Allergy and allergic diseases: first of two parts. N Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 9.Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Estelle F, Simons R. The antiallergic effects of antihistamines (H1-receptor antagonists) J Allergy Clin Immunol. 1992;90:705–715. doi: 10.1016/0091-6749(92)90156-v. [DOI] [PubMed] [Google Scholar]
- 11.Keam SJ, Plosker GL. Rupatadine: a review of its use in the management of allergic disorders. Drugs. 2007;67:457–474. doi: 10.2165/00003495-200767030-00008. [DOI] [PubMed] [Google Scholar]
- 12.Merlos M, Giral M, Balsa D, Ferrando R, Queralt M, Puigdemont A, Garcia-Rafanell J, Forn J. Rupatadine, a new potent, orally active dual antagonist of histamine and platelet-activating factor (PAF) J Pharmacol Exp Ther. 1997;280:114–121. [PubMed] [Google Scholar]
- 13.Katiyar S, Prakash S. Pharmacological profile, efficacy and safety of rupatadine in allergic rhinitis. Prim Care Respir J. 2008:8–12. doi: 10.3132/pcrj.2008.00043. DOI: 10.3132/pcrj.2008.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picado C. Rupatadine: pharmacological profile and its use in the treatment of allergic disorders. Expert Opin Pharmacother. 2006;7:1989–2001. doi: 10.1517/14656566.7.14.1989. [DOI] [PubMed] [Google Scholar]
- 15.Izquierdo I, Merlos M, Garcia-Rafanell J. Rupatadine: a new selective histamine H1 receptor and platelet-activating factor (PAF) antagonist. A review of pharmacological profile and clinical management of allergic rhinitis. Drugs Today (Barc) 2003;39:451–468. doi: 10.1358/dot.2003.39.6.799450. [DOI] [PubMed] [Google Scholar]
- 16.Stuebner P, Horak F, Zieglmayer R, Arnaiz E, Leuratti C, Perez I, Izquierdo I. Effects of rupatadine vs placebo on allergen-induced symptoms in patients exposed to aeroallergens in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol. 2006;96:37–44. doi: 10.1016/S1081-1206(10)61038-1. [DOI] [PubMed] [Google Scholar]
- 17.Fantin S, Maspero J, Bisbal C, Agache I, Donado E, Borja J, Mola O, Izquierdo I. A 12-week placebo-controlled study of rupatadine 10 mg once daily compared with cetirizine 10 mg once daily, in the treatment of persistent allergic rhinitis. Allergy. 2008;63:924–931. doi: 10.1111/j.1398-9995.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- 18.Dubertret L, Zalupca L, Cristodoulo T, Benea V, Medina I, Fantin S, Lahfa M, Perez I, Izquierdo I, Arnaiz E. Once-daily rupatadine improves the symptoms of chronic idiopathic urticaria: a randomised, double-blind, placebo-controlled study. Eur J Dermatol. 2007;17:223–228. doi: 10.1684/ejd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Cocera C, De Molina M, Marti-Guadano E, Pola J, Conde J, Borja J, Perez I, Arnaiz E, Izquierdo I. Rupatadine 10 mg and cetirizine 10 mg in seasonal allergic rhinitis: a randomised, double-blind parallel study. J Investig Allergol Clin Immunol. 2005;15:22–29. [PubMed] [Google Scholar]
- 20.Mullol J, Bousquet J, Bachert C, Canonica WG, Gimenez-Arnau A, Kowalski ML, Marti-Guadano E, Maurer M, Picado C, Scadding G, Van Cauwenberge P. Rupatadine in allergic rhinitis and chronic urticaria. Allergy. 2008;63((suppl 87)):5–28. doi: 10.1111/j.1398-9995.2008.01640.x. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 22.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 23.Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, Tomikawa M, Tachimoto H, Ebisawa M, Akasawa A, Miyagi T, Kimura H, Nakajima T, Tsuji K, Nakahata T. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- 24.Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, Athanassiou A, Theoharides TC. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 25.Kempuraj D, Huang M, Kandere K, Boucher W, Leutourneau R, Jeudy S, Fitzgerald K, Spear K, Athanasiou A, Theoharides TC. Azelastine is more potent than olopatadine in inhibiting interleukin-6 and tryptase release from human umbilical cord blood-derived cultured mast cells. Ann Allergy Asthma Immunol. 2002;88:501–506. doi: 10.1016/s1081-1206(10)62389-7. [DOI] [PubMed] [Google Scholar]
- 26.Queralt M, Brazis P, Merlos M, de Mora F, Puigdemont A. In vitro inhibitory effect of rupatadine on histamine and TNF-alpha release from dispersed canine skin mast cells and the human mast cell line HMC-1. Inflamm Res. 2000;49:355–360. doi: 10.1007/PL00000216. [DOI] [PubMed] [Google Scholar]
- 27.Solans A, Carbo ML, Pena J, Nadl T, Izquierdo I, Merlos M. Influence of food on the bioavailability of rupatadine tablets in healthy volunteers; a single-dose, randomized, open-label, two-way crossover study. Clin Ther. 2007;29:900–908. doi: 10.1016/j.clinthera.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Lytinas M, Kempuraj D, Huang M, Kandere K, Boucher W, Letourneau R, Jeudy S, Fitzgerald K, Spear K, Athanasiou A, Theoharides TC. Azelastine's inhibition of histamine and tryptase release from human umbilical cord blood-derived cultured mast cells, as well as rat skin mast cell-induced vascular permeability: comparison with olopatadine. Allergy Asthma Proc. 2002;23:45–51. [PubMed] [Google Scholar]
- 29.Kempuraj D, Huang M, Kandere-Grzybowska K, Basu S, Boucher W, Letourneau R, Athanasiou A, Theoharides TC. Azelastine inhibits secretion of IL-6, TNF-α and IL-8 as well as NF-κB activation and intracellular calcium ion levels in normal human mast cells. Int Arch Allergy Immunol. 2003;132:231–239. doi: 10.1159/000074304. [DOI] [PubMed] [Google Scholar]
- 30.Galatowicz G, Ajayi Y, Stern ME, Calder VL. Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. Clin Exp Allergy. 2007;37:1648–1656. doi: 10.1111/j.1365-2222.2007.02782.x. [DOI] [PubMed] [Google Scholar]
- 31.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Kraft S, Rana S, Jouvin MH, Kinet JP. The role of the FcepsilonRI beta-chain in allergic diseases. Int Arch Allergy Immunol. 2004;135:62–72. doi: 10.1159/000080231. [DOI] [PubMed] [Google Scholar]
- 34.Grabbe J, Welker P, Möller A, Dippel E, Ashman LK, Czarnetzki BM. Comparative cytokine release from human monocytes, monocyte-derived immature mast cells and a human mast cell line (HMC-1) J Invest Dermatol. 1994;103:504–508. doi: 10.1111/1523-1747.ep12395649. [DOI] [PubMed] [Google Scholar]
- 35.Bradding P, Okayama Y, Howarth PH, Church MK, Holgate ST. Heterogeneity of human mast cells based on cytokine content. J Immunol. 1995;155:297–307. [PubMed] [Google Scholar]
- 36.Krüger-Krasagakes S, Möller AM, Kolde G, Lippert U, Weber M, Henz BM. Production of inteuleukin-6 by human mast cells and basophilic cells. J Invest Dermatol. 1996;106:75–79. doi: 10.1111/1523-1747.ep12327815. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cell rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- 38.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 39.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]