Abstract
Background:
Examination of IgE cross-reactivity among nuts has been limited to in vitro experiments. Cross-reactivity studies of nuts at the T cell level are difficult to interpret because of the inability to determine which cellular responses are from a true sensitization and which are due to cross-reactivity. Using a mouse model in which the sensitizing nuts are controlled may provide novel methods to investigate in vivo and T cell cross-reactivity.
Methods:
C3H/HeJ mice were sensitized by intraperitoneal injection of cashew alone (monosensitized mice), or cashew plus walnut, utilizing alum as an adjuvant. Both groups underwent challenges to cashew, walnut and peanut, with subsequent monitoring of anaphylactic reactions. Anaphylactic antibodies were quantified by ELISA, and protein allergens were identified by Western blotting. Cellular responses were studied via splenocyte proliferation assay and measurement of secreted cytokines.
Results:
The monosensitized mice reacted to cashew and walnut during challenges, with significantly weaker reactions induced on challenge with peanut. Cross-reactive IgE to walnut and peanut were detected by ELISA, and the cross-reactive allergens were identified as vicilin proteins. In cellular assays, splenocytes from the monosensitized mice proliferated and produced IL-4 and IL-5 in response to cashew, walnut and peanut. The cashew- plus walnut-sensitized mice experienced stronger clinical reactions to walnut, recognized additional walnut allergens and secreted significantly more IL-4 and IL-5 in walnut-stimulated splenocyte assays compared to the monosensitized mice.
Conclusions:
Cross-reactivity in vivo was found between cashew and walnut, while cross-reactivity among cashew, walnut and peanut was demonstrated at the T cell level.
Key Words: Cross-reactivity, Tree nut, Food allergy, Walnut, Cashew, Peanut, Vicilin
Introduction
In predisposed individuals, virtually any food can cause an IgE-mediated reaction; however, there are 8 foods responsible for the vast majority of type I allergic reactions: milk, egg, peanuts, fish, shellfish, soy, tree nuts and wheat [1]. While many individuals outgrow food allergies, allergies to peanuts and tree nuts are concerning because there is only an approximately 20 and 10% chance, respectively, of outgrowing these allergies [2, 3]. This fact is troubling for the approximately 1.2% of children in the USA with peanut and/or tree nut allergies [4], because peanuts and tree nuts are implicated as the major culprits of fatal food-induced anaphylactic reactions [5, 6].
Tree nuts, such as walnut, cashew, hazelnut, almond, pecan, Brazil nut, pine nut, macadamia, pistachio and coconut, are all capable of inducing allergic reactions in a sensitized patient [7, 8]. Walnut and cashew allergies have been identified as the most frequent tree nut allergies in the USA [2, 4, 8]. One study found that 86% of children allergic to nuts (peanuts and/or tree nuts) are sensitized to multiple nuts and 47% are multiallergic [9], while reports of allergic reactions due to tree nut cross-reactivity have also appeared in the literature [10, 11]. These findings often lead to nut-allergic patients being instructed to avoid all tree nuts and peanuts once a diagnosis of allergy to any one nut is made [8, 9].
Taxonomic classification of walnut, cashew and peanut shows that all three belong to the Magnoliopsida class (dicotyledons), while peanut (Arachis hypogaea) and cashew (Anacardium occidentale) belong to the same subclass, Rosidae, and walnut (Juglans regia) is a member of the Hamamelidae subclass [12]. However, phylogenetic trees based on amino acid sequence alignments of vicilins, legumins and 2S albumins from these nuts indicate that cashew and walnut are evolutionarily more closely related to each other than either is to peanut [13]. Cross-reactive studies using human IgE have shown relatively low cross-reactivity between cashew and peanut, moderate cross-reactivity between walnut and cashew and little or no cross-reactivity between walnut and peanut [14,15,16,17,18]. T cell cross-reactivity of nuts has rarely been studied, likely because of the implicit problems in determining patients' true sensitizations, with the only available report finding no cross-reactivity of peanut-specific T cell clones stimulated with hazelnut or Brazil nut extracts [19].
Studying cross-reactivity in vivo in a mouse model may help determine which in vitro allergen cross-reactivity is clinically relevant. Determining the T cell cross-reactivity among nuts in a mouse model in which the sensitizing antigen is known can provide pertinent information with regard to the development of multi-nut allergy in humans and potential immunotherapy approaches. We used C3H/HeJ mice as a model for studying the cross-reactivity of cashew, walnut and peanut in vivo and compared cross-reactive B and T cell responses in monosensitized, cashew-allergic mice to those in truly multisensitized, cashew- plus walnut-allergic mice.
Materials and Methods
Cashew, Walnut, Peanut, Macadamia and Egg Extracts
Cashew, walnut (English), peanut and macadamia extracts were prepared from raw seeds. Ground seeds were mixed in a 1:5 (w:v) ratio in 2× PBS and homogenized for 30 min while maintaining an alkaline pH (approx. 8.5). Centrifugation at 30,000 g for 30 min at 4°C was carried out to clarify the supernatant by removal of insoluble material and the fat layer. Ammonium sulfate precipitation at 90% saturation was used to concentrate the proteins, with final resuspension in PBS. The protein solutions were dialyzed against water to remove excess salt, and then exchanged into PBS using dialysis. After sterile filtration of the extracts, the protein concentration was determined via bicinchoninic acid assay (Pierce, Rockford, Ill., USA), and all extracts were subjected to polyacrylamide gel electrophoresis under denaturing and reducing conditions utilizing Coomassie blue staining to visualize protein bands. Egg extract was prepared from dried egg whites using a procedure similar to the nut extractions.
Sensitization of Mice
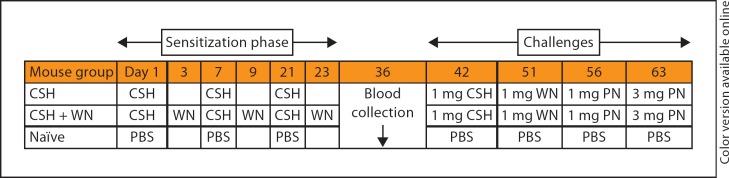
Five-week-old female C3H/HeJ mice purchased from the Jackson Laboratory (Bar Harbor, Me., USA) were maintained on tree-nut- and peanut-free food (PMI Nutrition International, St. Louis, Mo., USA) under pathogen-free conditions following standard guidelines for care and use [20]. The sensitization schedule was based on our previous study using intraperitoneal sensitization to peanut [21]. Mice were divided into 3 groups consisting of 5 mice each: monosensitization to cashew (CSH mice), double sensitization to both cashew and walnut (CSH + WN mice) and sham control (naïve mice). Injections consisted of 0.5 mg of the appropriate nut extract or PBS alone, with 2.0 mg of alum given intraperitoneally. The schedule is summarized in figure 1.
Fig. 1.
Schedule of sensitization, blood collection and challenges for the CSH mice, CSH + WN mice and naïve mice. During the sensitization phase, mice were injected intraperitoneally with the nut extract (cashew or walnut) or PBS, plus alum as an adjuvant on the days shown. Blood was collected 2 weeks following the last immunization for studies on the immunoglobulin responses. Mice were then challenged intraperitoneally with cashew, walnut and peanut (PN) on the days shown.
Challenges and Assessment of Reactions
The sensitized animals were first challenged 19 days after the last sensitization doses. Mice were challenged intraperitoneally with cashew (1 mg), walnut (1 mg) and peanut (1 and 3 mg), spaced approximately 1 week apart (fig. 1). Anaphylactic reactions were assessed 30 min after challenge and scored on a scale from 0 to 5 using a pre-established scoring method from our previous studies [21]. Core body temperature was measured before challenge and immediately following the score assessment using a rectal probe.
IgE and IgG1 Measurements
Submandibular facial sampling was used to collect sera from individual mice 2 weeks following administration of the last sensitizing dose (fig. 1). Cashew-, walnut- and peanut-specific IgE and IgG1 were measured by ELISA using a reference curve, as described previously for peanut [21]. Plates were coated with appropriate nut extracts (20 µg/ml) in carbonate-bicarbonate buffer at pH 9.6. For IgE measurements, sera were pretreated with protein G beads (Pierce) to remove IgG, otherwise serum dilutions were optimized and used without further treatment. Detection of IgE was performed with sheep anti-mouse IgE (0.5 µg/ml; Binding Site, Birmingham, UK), followed by biotinylated donkey anti-sheep IgG (0.5 µg/ml; Accurate Chemical, Westbury, N.Y., USA) and neutravidin-horseradish peroxidase (HRP; 0.2 µg/ml; Pierce). IgG1 was detected by HRP-conjugated goat anti-mouse IgG1 (Southern Biotech, Birmingham, Ala., USA) used at 1:40,000. The HRP activity was measured with color development of 3,3′,5,5′-tetramethylbenzidine substrate (KPL, Gaithersburg, Md., USA).
Western Blotting for IgG1-Reactive Proteins
Protein extracts were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, Calif., USA) and then transferred onto Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, Mass., USA). Blots were blocked using 2% BSA in PBS containing 0.05% Tween-20 (PBST-BSA). Sera from each group of mice were pooled and then diluted in PBST-BSA before incubation with the blots. IgG1 was detected with HRP-conjugated goat anti-mouse IgG1 (Southern Biotech). The blots were developed with a chemiluminescence detection kit (Super Signal West Pico Chemiluminescent Substrate, Pierce). For inhibition studies to verify cross-reactivity, sera were incubated with 50 µg of walnut or peanut extracts for 6 h before overnight incubation with the blots.
Identification of IgG1-Binding Proteins
Walnut and peanut bands were excised after separation on NuPAGE 4–12% Bis-Tris gels (Invitrogen) and processed by the Duke Proteomics Core Facility. Proteins underwent in-gel trypsin digestion and were identified using liquid chromatograpy-tandem mass spectrometry (LC-MS/MS) on a Q-ToF Premier mass spectrometer (Waters, Milford, Mass., USA) coupled to a Nano Acquity liquid chromatograph (Waters). MS/MS data were processed using Mascot Distiller (Matrix Sciences Ltd., Boston, Mass., USA) and searched with Mascot v2.2 (Matrix Sciences) against either the Swissprot 51.6 database or the Trembl 35.5 database with Viridiplantae taxonomy.
Proliferation Assay and Measurement of Secreted Cytokines
Splenocytes were isolated from individual mice at least 2 weeks following completion of challenges. Cells were plated at a density of 2.5 × 106/ml in complete media (RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM glutamine). Antigens, also in complete media, were used at the following concentrations: 100 µg/ml for cashew, walnut, peanut and macadamia, and 200 µg/ml for egg. All cultures were carried out in a humidified incubator at 37°C in 5% CO2. Proliferation assays were carried out in triplicate in 96-well round-bottom plates, with cells being pulsed with 0.5 µCi of [3H]thymidine/well at 78 h, harvested 18 h later and counted for β-radioactivity (Wallac, Turku, Finland). Cultures for cytokine assays were performed in 24-well plates with supernatant collection at 96 h. Cytokines secreted into the culture media were quantified by ELISA, using antibodies from Biolegend (San Diego, Calif., USA; clones used were: IL-4: 11B11 for capture, BVD6-24G2 for detection; IL-5: TRFK5 for capture, TRFK4 for detection) following the manufacturer's instructions.
Statistics
Statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, Ill., USA). Differences in the symptom scores were assessed with the nonparametric Mann-Whitney U test. All other statistical comparisons were made with the independent sample t test, not assuming equal variances. Differences were considered statistically significant with a p value of less than 0.05.
Results
Assessment of in vivo Cross-Reactivity between Cashew, Walnut and Peanut
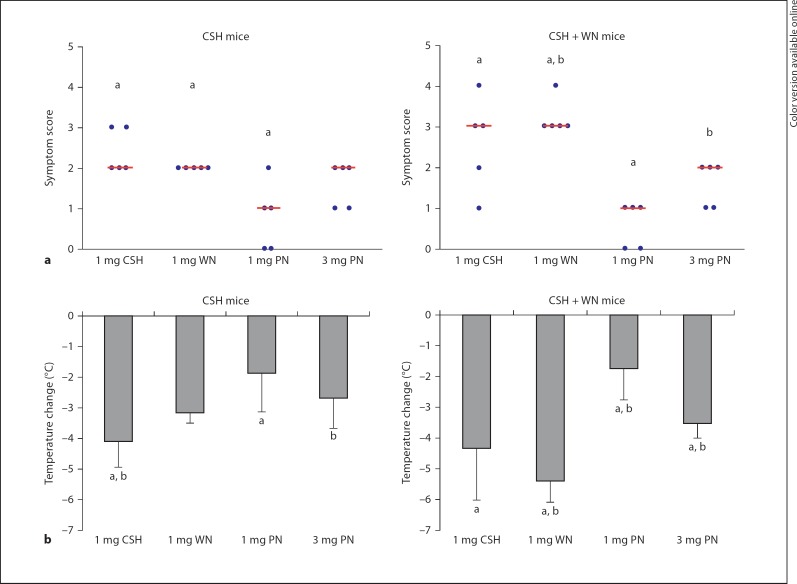
Following sensitization, mice were challenged intraperitoneally with cashew, walnut and peanut. The CSH mice reacted to both cashew and walnut with median symptom scores of 2, demonstrating in vivocross-reactivity between these nuts (fig. 2a). The symptom scores correlated with the measured decrease in body temperature, with the cashew challenge producing a slightly larger drop in temperature, i.e. 4.1°C, than the 3.1°C decrease following the walnut challenge (fig. 2b). Significantly milder reactions were observed on challenge with the same dose (1 mg) of peanut (p < 0.05), resulting in a median symptom score of 1 and a modest 1.9°C temperature decrease. Interestingly, the mice could be pushed to have stronger reactions to peanut with an increased challenge dose of 3 mg (fig. 2).
Fig. 2.
Anaphylactic symptom scores and change in body temperature following challenges. Groups of mice were challenged intraperitoneally with the quantities of nuts shown (PN = peanut). Anaphylactic reactions were assessed by monitoring clinical symptoms and measuring changes in body temperature. a Symptom scores for the two groups. Circles represent individual mice. Bars represent the median outcome for each challenge. b Temperature changes for the two groups. The average is shown with the standard deviation. a p < 0.05: Statistically significant difference compared to 1 mg of peanut; b p < 0.05: statistically significant difference compared to 3 mg of peanut.
The CSH + WN mice had stronger anaphylactic scores than the CSH group in response to both the cashew and walnut challenges, although only the differences in the walnut challenges reached statistical significance between the two groups of mice (p < 0.01). Cashew challenge produced a median score of 3 and a temperature drop of 4.3°C, while walnut reactions produced a median symptom score of 3 and a temperature decrease of 5.4°C. The 1-mg peanut challenge produced significantly weaker anaphylactic reactions than either the cashew or walnut challenges (p < 0.05), giving a median symptom score of 1, which could again be exacerbated by administering a larger dose of 3 mg (fig. 2).
In vitro Characterization of Antibody Cross-Reactivity between Cashew, Walnut and Peanut
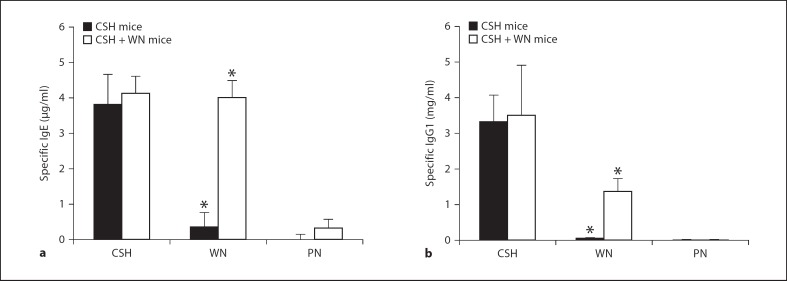
To investigate the extent of cross-reacting antibodies responsible for the in vivo allergic reactions, specific IgE and IgG1 for cashew, walnut and peanut were measured by ELISA (fig. 3). The highest IgE levels for the CSH mice were found with cashew, while lower levels were measured in response to walnut and peanut (fig. 3a). Walnut-specific IgE in the CSH mice appeared to be above a threshold capable of inducing strong anaphylactic reactions, whereas peanut-specific IgE was below this critical threshold and therefore triggered weak reactions at the same challenge dose. The CSH + WN mice developed strong IgE responses to both cashew and walnut, while lower concentrations were found for peanut. Approximately 11-fold more walnut-specific IgE was found in the CSH + WN mice compared to the CSH mice, demonstrating a highly significant difference (p < 0.01). Specific IgG1 was also quantified with similar patterns as for IgE (fig. 3b), and a highly significant difference in walnut-specific IgG1 between the two groups was found (p < 0.01).
Fig. 3.
Nut-specific antibodies in the serum of mice following sensitization. Values shown are averages with standard deviation. a IgE in response to cashew, walnut and peanut in the sensitized mice. IgE levels in naïve mice for each nut were subtracted from those in the sensitized mice and are thus set to zero (not shown). b IgG1 in response to cashew, walnut and peanut in the sensitized mice. IgG1 levels in naïve mice were less than 0.3 µg/ml for each nut and are not shown. * p < 0.01: Statistically significant differences between the two groups of mice for the walnut-specific immunoglobulins. PN = peanut.
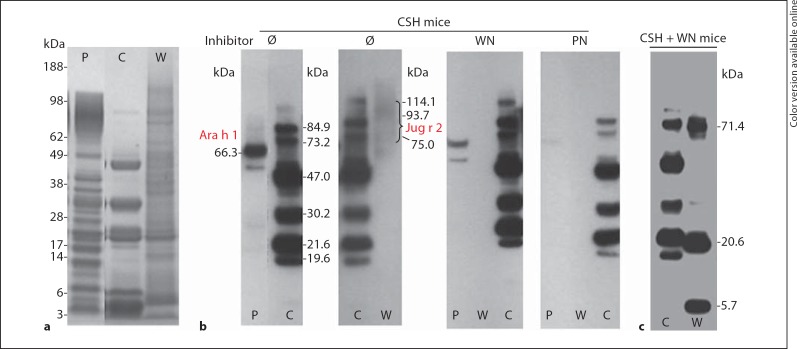
Western blotting was used to identify the allergenic proteins in each nut extract. The CSH mouse IgG1 recognized 6 bands in the cashew extract ranging from 19.6 to 84.9 kDa. The proteins at 47.0 and 30.2 kDa in cashew (fig. 4b) presumably correspond to Ana o 1 and Ana o 2, the 7S and 11S globulins, respectively, which were identified in a previous report [22]. The overall banding pattern was nearly identical to that found in a previous report using human IgE [22], except that the 2S albumin was absent from the mouse immunoblot, a phenomenon also seen with walnut allergens in the atopic dog model of Teuber et al. [23]. Cross-reactive proteins were detected in walnut at 75.0 kDa and a smeared band with a molecular weight range of 93.7–114.1 kDa, and in peanut at 66.3 kDa, and were identified as Jug r 2 and Ara h 1 by tryptic digestion LC-MS/MS (fig. 4b). Interestingly, the inhibition studies revealed cross-reactivity between walnut and peanut, as the walnut binding antibodies are inhibited after preincubation with peanut. Cross-reactivity between walnut and peanut vicilins has previously been observed in a walnut- plus peanut-allergic subject [11].
Fig. 4.
Western blot analysis showing IgG1-binding proteins in the CSH mice and CSH + WN mice. a Coomassie blue-stained SDS-PAGE gel showing the protein profile of peanut (P), cashew (C) and walnut (W) extracts. b Proteins recognized by IgG1 in pooled CSH mice sera for peanut (P), cashew (C) and walnut (W). Inhibition studies were performed with no inhibitor (Ø), walnut or peanut (PN), and demonstrated that the cross-reacting proteins can be inhibited with the appropriate nut extract. The cross-reactive peanut protein was identified as Ara h 1, and the cross-reactive walnut protein was found to be Jug r 2. c Western blot showing the cashew (C) and walnut (W) allergens in the CSH + WN mice. Notice that additional bands are recognized in walnut compared to the CSH mice.
The CSH + WN mice displayed the same banding pattern in response to the cashew extract as the CSH mice, but recognized additional bands at 71.4, 20.6 and 5.7 kDa in the walnut extract (fig. 4c). These additional bands, which we identified by LC-MS/MS, corresponded to proteins previously shown to bind human IgE [24, 25]. The 5.7-kDa allergen was identified as the large subunit of Jug r 1, the walnut 2S albumin; the 20.6-kDa band contained the basic subunit of Jug r 4, the legumin in walnut, and a fragment of Jug r 2, the walnut vicilin, and the 71.4-kDa band was identified as Jug r 2.
Investigation of T Cell Cross-Reactivity of Cashew, Walnut and Peanut
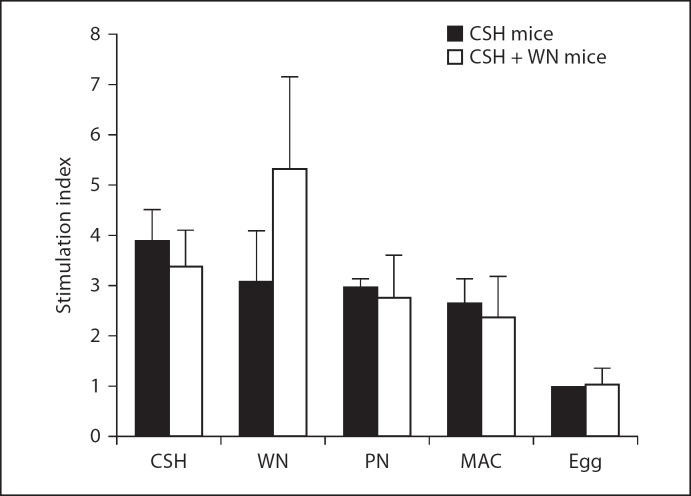
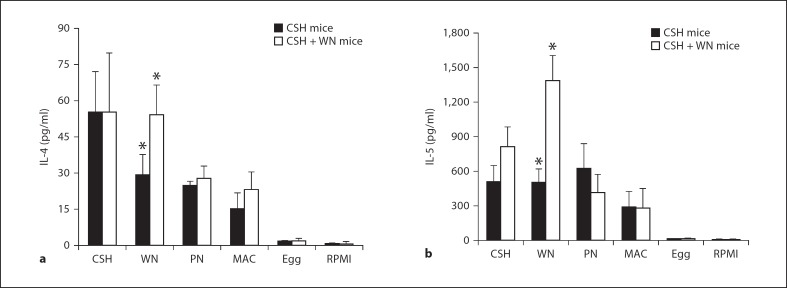
A proliferation assay and quantification of secreted cytokines were used to assess the cellular responses to cashew, walnut and peanut, along with macadamia, a tree nut that the mice never received, and the unrelated allergenic food, egg. The CSH mice showed the strongest proliferation response to cashew, with slightly weaker responses to walnut, peanut and macadamia, and no appreciable proliferation in response to egg (fig. 5). The highest IL-4 secretion for these mice was in response to cashew stimulation, with lower levels produced by walnut, peanut and macadamia stimulations (fig. 6a). IL-5 was produced at equal levels by cashew, walnut and peanut stimulation of CSH mouse splenocytes, with a lower level produced by macadamia (fig. 6b). All antigens except macadamia induced significantly different levels of IL-4 and IL-5 secretion in the CSH mice compared to those produced in response to egg (p < 0.05).
Fig. 5.
Splenocyte proliferation assay for CSH mice and CSH + WN mice in response to various food antigens. Triplicate cultures of splenocytes were used to calculate the stimulation indices, using RPMI stimulation as the reference, for an individual mouse in response to cashew, walnut, peanut (PN), macadamia (MAC) or egg. Naïve mice were always normalized to a stimulation index of 1.0, and the other groups were adjusted accordingly. Values shown are averages of at least 3 individual mouse stimulation indices, with the exception of egg stimulation, which was only carried out on 2 mice per group.
Fig. 6.
Secreted IL-4 and IL-5 levels from tree-nut-sensitized mice. Splenocytes were stimulated with cashew, walnut, peanut (PN), macadamia (MAC), egg or medium alone (RPMI) and cultured for 96 h. ELISA was used to quantify IL-4 (a) and IL-5 (b). Values shown are averages with standard deviations and represent at least 3 mice per condition, except for egg, which represents only 2 mice. * p < 0.05: Statistically significant difference between the two groups of mice for walnut-stimulated IL-4 and IL-5 secretion.
The CSH + WN mice showed strong proliferation responses to both cashew and walnut, with milder responses to peanut and macadamia (fig. 5). These mice secreted high levels of IL-4 and IL-5 when stimulated with either cashew or walnut, with lower levels in response to peanut and macadamia, and virtually no response to egg (fig. 6). Importantly, there was a significant difference between the CSH mice and CSH + WN mice with regard to IL-4 and IL-5 secretion following walnut stimulation (p < 0.05), which correlates well with the differences observed in walnut-specific IgE and IgG1 between these groups of mice.
Discussion
Tree nut allergy develops during childhood and usually persists throughout the life of the patient [2]. Similar to peanut allergy, tree nut allergy results from IgE antibodies aimed at 3 classes of seed storage proteins: vicilins (7S globulins), legumins (11S globulins) and 2S albumins [7]. The homology among these tree nut and peanut proteins has been studied in vitro using human IgE [14,15,16,17]. In this report, we studied cross-reactivity among cashew, walnut and peanut using an in vivo approach. Our findings show a high degree of in vivo cross-reactivity between cashew and walnut, with a weaker cross-reactivity between cashew and peanut (fig. 2).
The in vivo cross-reactivity between cashew and walnut observed in our study seems to mimic what has been reported in the clinic with tree nut allergy, namely that if a patient has an allergic reaction to one nut, they will likely react to others and are therefore advised to avoid all nuts [2, 9]. Mice sensitized to only cashew reacted almost as strongly to walnut as they did to the cashew extract, with the vicilin Jug r 2 being implicated as the cross-reactive allergen (fig. 4b). Interestingly, we saw only mild symptoms with our standard challenge dose of peanut in these mice, but could induce more significant reactions to peanut when the dose was increased, indicating that there is a threshold for peanut-induced reactions in a CSH mouse (fig. 2). The concept of studying cross-reactive food-induced anaphylactic reactions in mice is not new, although previous work by Lifrani et al. [26] on peanut and lupine (legumes) failed to demonstrate in vivo cross-reactivity despite the presence of cross-reactive IgE and IgG1. It is uncertain why this study was unsuccessful in showing cross-allergenicity, but it could be that the lupine challenge dose was too low, as the researchers administered 1 mg of lupine intraperitoneally, the same dose used to induce strong peanut reactions in that study. The C3H/HeJ strain appears to be an acceptable model to study in vivo cross-reactivity of food allergens and may be used in conjunction with other studies using human cells, such as recent work using a basophil histamine release assay to assess cross-reactivity between the walnut and sesame 11S globulins [27], to further characterize clinically relevant cross-reactive allergens.
Our study was designed so that we could compare allergic reactions from cross-reactivity to reactions involving true multisensitization. The CSH + WN mice experienced significantly severer anaphylactic reactions to walnut than the CSH mice, demonstrating that allergic reactions resulting from cross-reactive proteins are weaker than allergic reactions from proteins to which sensitization has occurred. The IgG1-binding proteins in the two allergic groups of mice were the same for cashew proteins, but different bands in walnut were recognized by the CSH + WN mice. This agrees with our finding of increased walnut-specific IgE and IgG1 in the CSH + WN mice compared to the monosensitized CSH mice (fig. 3). Interestingly, this phenomenon was recently reported on by Peeters et al. [28] in their study of cross-reactivity of legumes versus true sensitization. The key finding was that different proteins in lupine are recognized by lupine-sensitized individuals when compared to peanut-sensitized patients who have cross-reactive antibodies to lupine [28].
From a therapeutic standpoint, cross-reactive T cell epitopes are even more important than cross-reactive antibodies. Immunotherapy has been shown to work by modulation of the cellular response, inducing a shift from Th1 to Th2 and/or generating Treg cells, both of which downregulate the allergic state [29]. Cross-reactivity at the T cell level should allow for treatment of multiple allergies by administering only one relevant allergen, an effect which has indeed been shown clinically, albeit with some conflicting reports, for oral allergy syndrome patients undergoing pollen immunotherapy [30,31,32]. This presents an obvious advantage for the treatment of life-threatening tree nut or shellfish allergies in which the known allergens are similar among tree nuts (i.e. vicilin, legumin and 2S albumin proteins) and within shellfish (i.e. tropomyosins).
Surprisingly, very little work has been reported on cross-reactivity at the T cell level among tree nuts and peanut [19]. Since studies on human cells would be difficult to interpret because of inherent problems in determining which tree nuts represent true sensitizations and which are only cross-reactive, turning to a mouse model in which we control the sensitizing tree nut is an attractive option. Here, we used monosensitized CSH mice to study cellular cross-reactivity. Cross-reactivity was shown between cashew and walnut via proliferation assay and Th2 cytokine production. Cashew and walnut stimulation both gave a stimulation index of greater than 3.0, while walnut stimulation led to lower IL-4 secretion, but equal IL-5 production, thus indicating that walnut can activate cashew-specific T cells. Importantly, peanut had a nearly identical effect to that of walnut on the cashew-specific T cells, while macadamia was slightly less effective and egg showed no cross-reactivity. Similar T cell responses to both peanut and walnut in the CSH mice are especially interesting from an immunotherapy perspective because these two foods are distantly related both evolutionarily and taxonomically [12, 13, 21]. This may mean that there will be enough T cell cross-reactivity among diverse nuts, and possibly even other allergenic seeds, such as sesame, that treatment for allergy to one nut may downregulate allergy to many distinct nuts, legumes or seeds. This work also sheds light on a possible explanation as to why many patients are sensitized to both peanuts and tree nuts [8]. This phenomenon may be caused by the high cross-reactivity between T cells, such that once a sensitization is established, future peanut or tree nut consumption will activate the already established Th2 clones, leading to a cytokine milieu dominated by IL-4, and thus the generation of specific IgE to this latter ingested nut.
In conclusion, we have demonstrated in vivo cross-reactivity between cashew and walnut, with a weaker response to peanut, and showed evidence of cross-reactivity between cashew, walnut and peanut at the T cell level. The T cell cross-reactivity among these phylogenetically diverse nuts implies two important consequences: T cell cross-reactivity may account for the high prevalence of multi-nut allergy in nut-allergic populations, and this cross-reactivity may be exploited in immunotherapy aimed at treating multi-nut allergy.
Acknowledgement
Michael Kulis is holder of the ‘American Academy of Allergy, Asthma and Immunology's Strategic Training in Allergy Research (ST*AR) Award’.
References
- 1.Lee LA, Burks AW. Food allergies: prevalence, molecular characterization, and treatment/prevention strategies. Annu Rev Nutr. 2006;26:539–565. doi: 10.1146/annurev.nutr.26.061505.111211. [DOI] [PubMed] [Google Scholar]
- 2.Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005;116:1087–1093. doi: 10.1016/j.jaci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W., Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107:367–374. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Munoz-Furlong A., Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 5.Bock SA, Munoz-Furlong A., Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 6.Bock SA, Munoz-Furlong A., Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 7.Roux KH, Teuber SS, Sathe SK. Tree nut allergens. Int Arch Allergy Immunol. 2003;131:234–244. doi: 10.1159/000072135. [DOI] [PubMed] [Google Scholar]
- 8.Sicherer SH, Furlong TJ, Munoz-Furlong A., Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5,149 registrants. J Allergy Clin Immunol. 2001;108:128–132. doi: 10.1067/mai.2001.115755. [DOI] [PubMed] [Google Scholar]
- 9.Clark AT, Ewan PW. The development and progression of allergy to multiple nuts at different ages. Pediatr Allergy Immunol. 2005;16:507–511. doi: 10.1111/j.1399-3038.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 10.Senna G., Bonadonna P., Crivellaro M., Schiappoli M., Passalacqua G. Anaphylaxis due to Brazil nut skin testing in a walnut-allergic subject. J Investig Allergol Clin Immunol. 2005;15:225–227. [PubMed] [Google Scholar]
- 11.Teuber SS, Peterson WR. Systemic allergic reaction to coconut (Cocos nucifera) in 2 subjects with hypersensitivity to tree nut and demonstration of cross-reactivity to legumin-like seed storage proteins: new coconut and walnut food allergens. J Allergy Clin Immunol. 1999;103:1180–1185. doi: 10.1016/s0091-6749(99)70196-x. [DOI] [PubMed] [Google Scholar]
- 12.Integrated Taxonomic Information System Database ITISo-l. 2007 http://www.itis.gov. [Google Scholar]
- 13.Radauer C., Breiteneder H. Evolutionary biology of plant food allergens. J Allergy Clin Immunol. 2007;120:518–525. doi: 10.1016/j.jaci.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 14.de Leon MP, Drew AC, Glaspole IN, Suphioglu C., O'Hehir RE, Rolland JM. IgE cross-reactivity between the major peanut allergen Ara h 2 and tree nut allergens. Mol Immunol. 2007;44:463–471. doi: 10.1016/j.molimm.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 15.de Leon MP, Drew AC, Glaspole IN, Suphioglu C., Rolland JM, O'Hehir RE. Functional analysis of cross-reactive immunoglobulin E antibodies: peanut-specific immunoglobulin E sensitizes basophils to tree nut allergens. Clin Exp Allergy. 2005;35:1056–1064. doi: 10.1111/j.1365-2222.2005.02310.x. [DOI] [PubMed] [Google Scholar]
- 16.de Leon MP, Glaspole IN, Drew AC, Rolland JM, O'Hehir RE, Suphioglu C. Immunological analysis of allergenic cross-reactivity between peanut and tree nuts. Clin Exp Allergy. 2003;33:1273–1280. doi: 10.1046/j.1365-2222.2003.01761.x. [DOI] [PubMed] [Google Scholar]
- 17.Goetz DW, Whisman BA, Goetz AD. Cross-reactivity among edible nuts: double immunodiffusion, crossed immunoelectrophoresis, and human specific IgE serologic surveys. Ann Allergy Asthma Immunol. 2005;95:45–52. doi: 10.1016/S1081-1206(10)61187-8. [DOI] [PubMed] [Google Scholar]
- 18.Teuber SS, Jarvis KC, Dandekar AM, Peterson WR, Ansari AA. Identification and cloning of a complementary DNA encoding a vicilin-like proprotein, Jug r 2, from English walnut kernel (Juglans regia), a major food allergen. J Allergy Clin Immunol. 1999;104:1311–1320. doi: 10.1016/s0091-6749(99)70029-1. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JA, Lamb JR, Lake RA, O'Hehir RE. Polyclonal and clonal analysis of human CD4+ T-lymphocyte responses to nut extracts. Immunology. 1995;84:91–97. [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Laboratory Animal Resources Commission of Life Sciences NRC Guide for the Care and Use of Laboratory Animals. Washington, National Academy Press. 1996 [Google Scholar]
- 21.Pons L., Ponnappan U., Hall RA, Simpson P., Cockrell G., West CM, Sampson HA, Helm RM, Burks AW. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004;114:915–921. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Teuber SS, Sathe SK, Peterson WR, Roux KH. Characterization of the soluble allergenic proteins of cashew nut (Anacardium occidentale L.) J Agric Food Chem. 2002;50:6543–6549. doi: 10.1021/jf025757j. [DOI] [PubMed] [Google Scholar]
- 23.Teuber SS, Del Val G., Morigasaki S., Jung HR, Eisele PH, Frick OL, Buchanan BB. The atopic dog as a model of peanut and tree nut food allergy. J Allergy Clin Immunol. 2002;110:921–927. doi: 10.1067/mai.2002.130056. [DOI] [PubMed] [Google Scholar]
- 24.Wallowitz M, Peterson WR, Uratsu S, Comstock SS, Dandekar AM, Teuber SS. Jug r 4, a legumin group food allergen from walnut (Juglans regia cv. Chandler) J Agric Food Chem. 2006;54:8369–8375. doi: 10.1021/jf061329s. [DOI] [PubMed] [Google Scholar]
- 25.Comstock SS, McGranahan G, Peterson WR, Teuber SS. Extensive in vitro cross-reactivity to seed storage proteins is present among walnut (Juglans) cultivars and species. Clin Exp Allergy. 2004;34:1583–1590. doi: 10.1111/j.1365-2222.2004.02049.x. [DOI] [PubMed] [Google Scholar]
- 26.Lifrani A, Dubarry M, Rautureau M, Aattouri N, Boyaka PN, Tome D. Peanut-lupine antibody cross-reactivity is not associated to cross-allergenicity in peanut-sensitized mouse strains. Int Immunopharmacol. 2005;5:1427–1435. doi: 10.1016/j.intimp.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Wallowitz ML, Chen RJ, Tzen JT, Teuber SS. Ses i 6, the sesame 11s globulin, can activate basophils and shows cross-reactivity with walnut in vitro. Clin Exp Allergy. 2007;37:929–938. doi: 10.1111/j.1365-2222.2007.02725.x. [DOI] [PubMed] [Google Scholar]
- 28.Peeters KA, Nordlee JA, Penninks AH, Chen L, Goodman RE, Bruijnzeel-Koomen CA, Hefle SL, Taylor SL, Knulst AC. Lupine allergy: not simply cross-reactivity with peanut or soy. J Allergy Clin Immunol. 2007;120:647–653. doi: 10.1016/j.jaci.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 29.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–791. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwolfer B, Schreiber C, Francis JN, Ebner C, Bohle B. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119:937–943. doi: 10.1016/j.jaci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Bolhaar ST, Tiemessen MM, Zuidmeer L, van Leeuwen A, Hoffmann-Sommergruber K, Bruijnzeel-Koomen CA, Taams LS, Knol EF, van Hoffen E, van Ree R, Knulst AC. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004;34:761–769. doi: 10.1111/j.1365-2222.2004.1939.x. [DOI] [PubMed] [Google Scholar]
- 32.Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59:1272–1276. doi: 10.1111/j.1398-9995.2004.00626.x. [DOI] [PubMed] [Google Scholar]