Abstract
Despite major efforts to reduce atherosclerotic cardiovascular disease (ASCVD) burden with conventional risk factor control, significant residual risk remains. Recent evidence on non-traditional determinants of cardiometabolic health has advanced our understanding of lifestyle–disease interactions. Chronic exposure to environmental stressors like poor diet quality, sedentarism, ambient air pollution and noise, sleep deprivation and psychosocial stress affect numerous traditional and non-traditional intermediary pathways related to ASCVD. These include body composition, cardiorespiratory fitness, muscle strength and functionality and the intestinal microbiome, which are increasingly recognized as major determinants of cardiovascular health. Evidence points to partially overlapping mechanisms, including effects on inflammatory and nutrient sensing pathways, endocrine signalling, autonomic function and autophagy. Of particular relevance is the potential of low-risk lifestyle factors to impact on plaque vulnerability through altered adipose tissue and skeletal muscle phenotype and secretome. Collectively, low-risk lifestyle factors cause a set of phenotypic adaptations shifting tissue cross-talk from a proinflammatory milieu conducive for high-risk atherosclerosis to an anti-atherogenic milieu. The ketone body ß-hydroxybutyrate, through inhibition of the NLRP-3 inflammasome, is likely to be an intermediary for many of these observed benefits. Adhering to low-risk lifestyle factors adds to the prognostic value of optimal risk factor management, and benefit occurs even when the impact on conventional risk markers is discouragingly minimal or not present. The aims of this review are (a) to discuss novel lifestyle risk factors and their underlying biochemical principles and (b) to provide new perspectives on potentially more feasible recommendations to improve long-term adherence to low-risk lifestyle factors.
Keywords: Atherosclerotic cardiovascular disease, plaque phenotype, novel lifestyle risk factors, adipose tissue phenotype, ketone body ß-hydroxybutyrate, n-3 fatty acids
Introduction
The global burden of obesity, type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS) is continuously increasing and may be the largest yet-known non-infectious pandemic, subsequently leading to an enormous increase of atherosclerotic cardiovascular diseases (ASCVDs).1 ASCVD heavily depends on modifiable factors,2 but despite major efforts to manage conventional risk factors, significant residual risk, which varies across studies and methods of statistical analysis, remains.3
To date, lifestyle guidance has failed to substantially impact the burden from the twin epidemics of metabolic disease and ASCVD. Rising healthcare costs highlight the need for new strategies that have the potential to improve long-term adherence.
The aims of this review are (a) to discuss new lifestyle risk factors, their underlying biochemical principles, and public heath interventions that have the potential to reduce the burden from ASCVD and (b) to provide novel perspectives on potentially more feasible recommendations to improve long-term adherence to low-risk lifestyle risk factors.
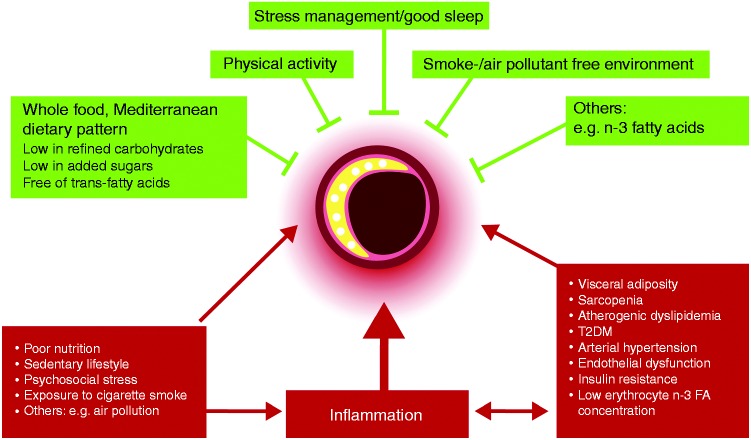
Of particular relevance in cardiovascular prevention is the effect of lifestyle risk factors on plaque phenotype and vulnerability, as depicted in Figure 1 and as discussed below.
Figure 1.
Lifestyle and high-risk atherosclerosis. Lifestyle risk factors influence plaque composition by modulating traditional and novel pathways associated with cardiovascular risk. Red colour indicates initiation of high-risk atherosclerosis, green colour indicates inhibition of high-risk atherosclerosis.
FA: fatty acids; T2DM: type 2 diabetes mellitus.
Recommendations regarding lifestyle risk factors
Diet
Malnutrition, in terms of quantity and quality, has become the leading risk factor for disability and death worldwide.2 During the past decades, dietary guidance has focused on calorie- and nutrient-based approaches, with one of the major targets being restriction of dietary (in particular, saturated) fat.4 However, there is no evidence that this has substantially reduced the cardiometabolic disease burden. A major limitation of the calorie- and nutrient-centric model is that it disregards the complex and weight-independent effects of food matrix and dietary patterns on metabolic and hormonal responses related to satiety and other intermediary pathways relevant to cardiovascular and overall health, such as the intestinal microbiome.4–7 Another layer of complexity is added by concerns about the use of error-prone methodology in nutritional epidemiology to inform dietary advice. Memory-based assessment methods such as food frequency questionnaires can produce physiologically implausible data (i.e. incompatible with survival).8,9 Therefore, this review focuses on evidence from randomized controlled trials (RCTs) on surrogate markers for cardiometabolic health and clinical endpoints. The resulting conceptual frameworks might help inform dietary recommendations in clinical practice.
Food- versus nutrient-based recommendations
First and foremost, judging a food’s health effects by its individual nutrients without considering food matrix does not confer meaningful information.2 Evidence from RCTs on clinical endpoints provides robust support for the superiority of food-based recommendations without specific guidance regarding calories, as opposed to nutrient- and calorie-based recommendations.7 Outcome evidence supporting this concept came from the two largest dietary intervention trials with cardiovascular endpoints. The PREDIMED trial demonstrated that adhering to a calorie-unrestricted Mediterranean-style dietary pattern supplemented with four tablespoons of extra virgin olive oil or 30 g mixed nuts per day over 4.9 years in high-risk patients reduced inflammation10 and the incidence of major cardiovascular events by 31% and 28%, respectively.11 Of note, these effects were independent of body weight and lipid-lowering.11 This stands in stark contrast to the Women’s Health Initiative (WHI), the largest dietary RCT ever performed. In the WHI, a fat-restricted dietary pattern involving specific guidance on caloric intake failed to significantly reduce cardiovascular endpoints after eight years despite a substantial achieved reduction in total fat intake (37.8% to 28.8%).12
Body composition versus body weight
Second, the effects of diet on health seem to be more closely related to body composition than to body weight and total fat mass per se, suggesting that body mass index (BMI) can be an inadequate measure for assessing the effects of diet on health.13 In the CENTRAL-MRI trial, a Mediterranean low carbohydrate dietary pattern was superior to a low fat diet in mobilizing atherogenic and diabetogenic fat depots in liver, pancreas and pericardium; changes in lipid traits related to MetS (triglycerides and triglyceride/high-density lipoprotein cholesterol ratio) correlated with reductions in visceral/hepatic fat, but not with BMI.14 This supports the notion that visceral, intra-organ and subcutaneous adipose tissue depots have distinct associations with cardiometabolic health,15,16 and selective mobilization of visceral/ectopic fat is a fundamental mechanism underlying improved metabolic function. Overall, the combination of anthropometric and laboratory markers indicative of visceral adiposity (e.g. waist circumference and triglycerides) appears more indicative of adipose tissue distribution and phenotype than BMI.17 Hypertriglyceridemic waist, a visceral adiposity marker combining elevated waist circumference (≥90 cm) and elevated fasting plasma triglycerides (≥2 mmol/L), is indicative of the high-risk metabolic phenotype18 and/or high risk atherosclerosis.15 Waist-to-height-ratio is another inexpensive metric to better inform cardiovascular risk stratification than BMI and/or waist circumference alone; the suggested general cutoff being 0.5.16,19
Carbohydrate restriction/nutritional ketosis
Third, insulin resistance alters metabolic responses to dietary cues. Although of immense and yet to be fully elucidated complexity on a cellular and molecular level, insulin resistance clinically manifests itself as an intolerance to dietary carbohydrate; glycogen synthesis is impaired and dietary carbohydrate is diverted at increasing rates into hepatic de novo lipogenesis.20 Conceptually, this is of clinical relevance for dietary recommendations in MetS and T2DM and provides a reasonable explanation for evidence supporting use of dietary carbohydrate restriction and (intermittent) caloric restriction in the dyslipidaemic, insulin resistant phenotype, where favourable metabolic changes are observed in the absence of weight loss.21–23
It is worth noting that carbohydrate restriction is not a new concept but was successfully applied to treat diabetes mellitus prior to the discovery of insulin.24 With the discovery of insulin in 1923, which allowed for acute symptom control in diabetes even with high carbohydrate diets, this potent therapy has been largely forgotten.
With the delineation of the cellular and metabolic responses to carbohydrate restriction, these dietary regimens have recently regained scientific interest. In the insulin resistant phenotype, very low carbohydrate dietary patterns resulting in nutritional ketosis reduce broad, systemic inflammation and most biomarkers of ASCVD risk.25 Mechanistically, reduced inflammation has been linked to the ketone body ß-hydroxybutyrate (BHB),26,27 which is elevated in the state of nutritional ketosis and, in addition to its role in metabolism, acts as a signalling molecule.28 BHB blocks an innate immune sensor, the NLRP3 inflammasome, with downstream effects resulting in reduced levels of interleukin 1 beta (IL-1b).27 IL-1β and the NLRP3 inflammasome are crucial mediators conferring cardiovascular risk,29,30 and inhibition of IL-1β effectively reduced cardiovascular events in the CANTOS trial.31
Furthermore, BHB is an endogenous inhibitor of class 1 histone deacetylases (HDACs) and thus affects gene expression via chromatin modifications.32 HDACs regulate numerous pathways implicated in longevity and cardiometabolic disease, including autophagy and insulin-like growth factor (IGF) signalling. HDAC1 inhibition by BHB provides an example of the close link between metabolic status/diet and epigenetic gene regulation.28,32 Other effects of BHB relevant to cardiovascular aging include attenuated vascular aging33 and the promotion of a healthy microbiome.34
(Intermittent) caloric restriction
Caloric restriction is an umbrella term summarizing a set of dietary interventions involving either chronically or periodically reduced energy intake without malnutrition.35 Caloric restriction has consistently been found to extend healthy life span across a variety of species, yet the major limitation in humans is long-term sustainability for the vast majority. It is thus encouraging that intermittent fasting regimens evoke similar cellular and metabolic adaptations to chronic caloric restriction. Time-restricted food intake has been linked to cardiometabolic health in animal models36 and humans.22
Intermittent states of negative energy balance activate a set of adaptations where metabolism switches from lipid synthesis and fat storage to mobilization of fat as free fatty acids and fatty acid-derived ketones. This has been linked to improvement in surrogate markers for cardiometabolic health (e.g. weight loss) and beneficial effects on body composition such as mobilization of visceral fat and retention of lean mass.22,37 Further adaptations include increased circulating ketone and decreased inflammatory cytokine-, fatty acid-, amino acid-, glucose- and insulin concentrations, which translates to ameliorated insulin sensitivity and lipoprotein metabolism.22,37 In people with insulin resistance, metabolic outcomes appear more favourable upon consumption of the daily dietary allotment in the first half of the day rather than the same caloric intake divided into six meals throughout the day.38 Furthermore, in a strictly controlled feeding trial in men with prediabetes, those who consumed their meals in a 6-h time window in the morning experienced a greater amelioration of metabolic markers than those on a control schedule (12-h time window) within five weeks, independent of body weight.39 Another study comparing two isocaloric weight-loss interventions showed greater improvement of metabolic markers in the group consuming a bigger breakfast and a smaller dinner than vice versa.40 Overall, the evidence supports the notion that both the amount of time spent eating during each day35 and the time at which food is consumed relative to the circadian rhythm (chrono-nutrition)41 modulate the effects of diet on cardiometabolic health. Importantly, no safety signals such as electrolyte imbalance, nausea and vomiting, hyperuricemia and others have been reported in intermittent fasting regimens.37
Dietary patterns
Collectively, for maintaining cardiovascular and overall health, strong evidence and broad consensus speak to minimizing consumption of added sugars and refined grains and avoiding industrial trans-fatty acids, while replacing them with plant- and animal-based whole foods.2
In the insulin resistant phenotype, an overwhelming body of scientific literature has documented the superiority of low carbohydrate dietary patterns for glycaemic and weight control in T2DM.21,42–44 It is therefore not surprising that the recently published Consensus Report of the American Diabetes Association and the European Association for the Study of Diabetes endorsed low carbohydrate diets (<26% of total energy) as one strategy to manage hyperglycemia and hyperinsulinaemia in T2DM.45
The so called ‘Mediterranean dietary pattern’ supplemented by extra virgin olive oil and nuts is the most established dietary pattern with regard to reducing cardiovascular endpoints11 and lowering apolipoprotein B.46 However, it should be mentioned that (a) the Mediterranean diet is highly heterogeneous, (b) it has mostly been tested in a Mediterranean country, and (c) it might not be a feasible option for large parts of the population globally.
Emerging evidence from animal and human studies suggests the utility of time restricted feeding regimens in cardiometabolic disease. Interestingly, ketogenic diets seem to phenocopy some of the biochemical characteristics of fasting, including several pathways that are associated with longevity and cardiovascular health. Nutritional ketosis has been linked to an inhibition of major signalling pathways associated with growth (insulin, IGF-1 and mTOR), activation of AMP-activated protein kinase (AMPK), and an induction of antioxidant genes. BHB is likely to be an intermediary for many of the benefits observed with nutritional ketosis and caloric restriction.26,27
Marine n-3 fatty acids
Mechanisms of action
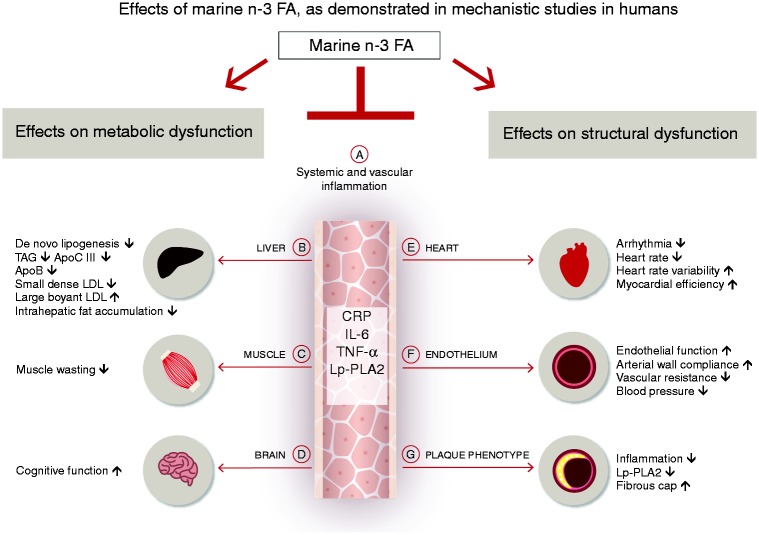
The marine n-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are essential to cell membrane health in all tissues, modulating a variety of humoral (Figure 2(a)), metabolic (Figure 2(b) to (d)) and structural parameters (Figure 2(e) to (g)) related to cardiometabolic risk – in part due to their anti-inflammatory properties.47,48 Of particular relevance to ASCVD is the beneficial effect of EPA on oxidative stress/inflammation, endothelial function and plaque phenotype, and lipid metabolism, which may delay the onset of atherosclerosis and the clinical sequelae associated with acute plaque rupture (reviewed in Ganda et al.49). Relevant mechanisms include inhibition of inflammation-related pathways and reduced leukocyte-endothelial adhesive interactions including reduced circulating plasma levels of IL-6, tumour necrosis factor α,50 monocyte chemoattractant protein-1 and asymmetric dimethylarginine.51 Furthermore, n-3 fatty acids are precursors of specialized pro-resolving mediators, such as resolvins, protectins and maresins,47 which support resolution of inflammation and plaque stability.52
Figure 2.
Marine n-3 fatty acids and mechanisms related to cardiovascular risk. Mechanistic studies in humans have demonstrated that the marine n-3 fatty acids eicosapentaenoic acid (EPA) and/or docosahexaenoic acid (DHA) reduce serum triglycerides, shift low-density lipoprotein subfractions and reduce ApoB (b), favourably change body composition (b, c), have direct effects on cardiac electrophysiology and autonomic function, and mitigate adverse remodelling of the left ventricle after myocardial infarction (e), improve endothelial function and lower systolic and diastolic blood pressure (f), and increase plaque stability (g) – in part due to their inflammatory resolving properties (a). Furthermore, EPA/DHA improve cognitive function across different age groups, including in the elderly and in children (d).
FA: fatty acids; TAG: triglycerides; Apo: apolipoprotein; LDL: low-density lipoprotein; CRP: C-reactive protein; IL-6: interleukin 6; TNF-α: tumour necrosis factor α; Lp-PLA2: lipoprotein-associated phospholipase A2.
The most consistent cardiovascular benefit of DHA and EPA is protection from sudden cardiac death.48,53 This has been linked, in part, to their membrane stabilizing and antiarrhythmic properties in the setting of ischaemia-induced ventricular fibrillation.54
The incorporation of EPA/DHA into cellular membranes associated with arterial plaque has been shown to have local inflammatory resolving effects, including reductions in IL-6, matrix metalloproteinases and lipoprotein-associated phospholipase A2 (Lp-PLA2).49 This has been linked to increased plaque stability and protection from the clinical sequelae associated with acute plaque destabilization, occlusion of arterial blood flow and tissue infarction.49,54,55
With regard to hepatic lipid metabolism (Figure 2(b)), n-3 fatty acids have been shown to downregulate hepatic genes involved in hepatic de novo lipogenesis, thus depleting the hepatic pool of triglycerides.48 High intakes of DHA/EPA ameliorate atherogenic dyslipidaemia and particle distribution48,56 and have furthermore been linked to reductions in ApoB46 and ApoCIII.56
Trial evidence
Short-term supplementation trials with high doses of both EPA57 and DHA58 affected surrogate markers relevant to ASCVD. EPA at 4, but not 2, g/day reduced oxidized-low density lipoprotein, Lp-PLA2 and high-sensitivity C-reactive protein (hsCRP) levels compared with placebo.57 DHA at 3 g/day has been shown to reduce levels of IL-6, hsCRP and granulocyte monocyte-colony stimulating factor and to increase the anti-inflammatory matrix-metalloproteinase-2 in hypertriglyceridaemic men after three months.58
However, despite biological plausibility and favourable short-term effects on surrogate markers of cardiometabolic health, results of large trials assessing EPA and/or DHA supplementation with cardiovascular endpoints have been mixed. In ASCEND, 1 g n-3 fatty acids/day did not reduce adverse vascular events in individuals with T2DM.59 Conversely, in REDUCE-IT, treatment of patients at elevated cardiovascular risk (elevated triglyceride levels and previous cardiovascular event or T2DM) with 4 g of EPA/day resulted in a 25% relative risk reduction (number needed to treat, 21) of the primary composite end point of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization or unstable angina compared with the placebo group.60 It is noteworthy that these results were achieved on top of statin therapy, suggesting additive value of EPA.60
One explanation for these diverging results might be the large variabilities in baseline levels and in bioavailability of EPA and/or DHA, which combine to produce substantial overlaps of n-3 levels in active treatment and placebo groups during supplementation trials.61 Group discrimination can be augmented by using a high dose, as in REDUCE-IT or JELIS,62 or by recruiting participants with low baseline levels, as in GISSI-HF,63 with the consequence of positive trial results. Additionally, it may be prudent to monitor erythrocyte levels of EPA and/or DHA during supplementation trials and in clinical practice, because rather than with group assignment or intake, clinical events correlate with tissue levels of EPA and/or DHA.61
Collectively, cumulative evidence from controlled studies on risk factors and recent clinical trials with endpoints, combined with the low risk profile of n-3 polyunsaturated fatty acids, justifies supplementation as a reasonable option to lower risk of ASCVD in individuals who do not frequently consume fish or are at high risk of ASCVD.7
Physical activity
Physical inactivity and low cardiorespiratory fitness (CRF) are under-recognized cardiometabolic risk factors and are strong independent predictors of outcomes in primary and secondary prevention of ASCVD across different BMI groups.64–67 Encouragingly, high levels of physical activity and CRF largely neutralize the adverse effects of adiposity and other traditional cardiovascular disease risk factors, including the metabolic syndrome.64 Furthermore, there is clinically relevant and plausible (although not conclusive) evidence that a sedentary lifestyle independent of physical activity and loss of muscle mass, strength and functionality might independently contribute to ASCVD risk.68–70 Importantly, these metrics can be improved, including in the elderly population.71,72 Even if started late in life, physical activity improves functional independence and reduces mortality, maintaining a strong effect even after controlling for potential confounders such as smoking, hypertension, obesity and diabetes.70 This implies therapeutic potential and underscores the importance of addressing CRF, muscle strength and functionality, and sedentary behaviour reduction as independent therapeutic targets in cardiovascular disease prevention and management.
Mechanisms of action
Physical activity evokes a complex systemic network of endo- and paracrine responses linked to cardiometabolic health. This has been attributed, in large part, to exercise-induced alterations in white adipose tissue and skeletal muscle phenotype and secretome. Muscle and white adipose tissue impact on inter-organ cross-talk, an effect mediated by adipokines, myokines, and gaseous messengers such as nitric oxide. Physical activity, particularly resistance training, affects body composition in a way that promotes an overall anti-inflammatory and anti-atherogenic milieu.15,29,71 Of particular current interest is skeletal muscle maintenance as a way of attenuating immunosenescence and inflammation, a hallmark of chronic, age-related diseases.73
The effect of physical activity on brown adipose tissue is less clear and studies have yielded conflicting results.74 One plausible explanation for improved body composition in overweight/obese individuals with physical exercise75–77 is the potential for physical activity to induce adipose tissue browning, an effect that has been linked to the PGC-1α-dependent myokine irisin secreted by muscle fibres upon exercise. Browning of adipose tissue encompasses a phenotypic set of adaptations that results in increased basal metabolic rate and total energy expenditure.
Further mechanisms of action relevant to cardiovascular health include improved endothelial function,78 preservation of the stable phenotype of pre-existing atherosclerotic plaque,71 increased autonomic balance, and downregulation of pathways related to nutrient sensing, growth signalling and inflammation – with compounding effects.14,71,79,80 The mild antidepressant effect of physical activity may constitute another intermediary pathway that reduces cardiovascular risk.
Recommendations
Guidelines recommend 150–300 min of moderate-intensity or 75–150 min of vigorous-intensity activity weekly for adults, supplemented by two sessions of muscle-building exercise. Furthermore, the updated Physical Activity Guidelines for Americans have included recommendations on sedentary time reduction.81
Moderate-to-vigorous physical activity
The recommended amount of 150 min per week of physical activity is associated with a 15% decreased risk of coronary heart disease and a 4.5 year increase in life expectancy.82 Given the problems associated with long-term adherence to moderate-to-vigorous exercise it is encouraging to note that even 15 min per day of moderate-intensity exercise delivers significant health benefits and has been associated with a 14% reduction in all-cause mortality and increased life expectancy of 3.5 years.83
Sedentary behaviour ‘sitting time’
Sedentary time is ubiquitous and accumulates while commuting, at school, in the workplace, at home and in leisure contexts.84 Objective measurements from accelerometers indicate US adults are exposed to an average of 6–8 h of prolonged sitting time, and adults > 60 years old to an average of 8.5–9.6 h sedentary time per day – the metabolic consequences of which are deleterious.68
Epidemiological evidence imply that greater time spent in sedentary behaviour is associated with all-cause and cardiovascular morbidity and mortality, suggesting prolonged sitting time as a population-wide, ubiquitous health risk. While definitive evidence from randomized clinical trials in humans is lacking, evidence from preclinical and short-term mechanistic studies in humans has identified potential underlying biological mechanisms,68,81 including decreased insulin sensitivity and impaired vascular endothelial-dependent dilation as key antecedents of age-related ASCVD risk. Encouragingly, these can be reversed by introducing several short bouts of exercise to uninterrupted sitting,68,81,85 including in the elderly.86
This highlights the importance of working on population-wide initiatives to reduce sedentary behaviour.84 This can be implemented by workplace-based interventions that offer activity-permissive workstations by enabling office workers to stand, walk or pedal while doing desk-based tasks and the use of smartphone applications to interrupt sedentary time.68,81
Overall, exercise training can be an effective and safe strategy for primary and secondary prevention of ASCVD across all weight groups.66,71 Combination of all modalities, endurance/resistance training and sedentary time reduction is likely to convey the greatest cumulative benefit with regard to improved body composition, glycaemic control and reduced inflammation, given their activation of different physiological signals and adaptations.78 Although the evidence on the adverse cardiometabolic effects of time spent in sedentary behaviour is less robust, it seems appropriate to promote ‘Sit less, move more’ as an important public health recommendation.68,81
Passive exposure to tobacco smoke
While smoking is an acknowledged risk factor for ASCVD, the detrimental health effects of exposure to passive smoke are frequently overlooked. Evidence from studies in humans shows that even brief exposure to secondhand smoke acutely increases the risk of acute myocardial infarction (AMI) by 30%.87 Mechanistically, this has been linked to increased platelet activity, impaired endothelial function and increased inflammation/oxidative stress.87 Similarly, epidemiological data shows an overall reduction in hospital admissions for AMI by 17% following smoking bans in public areas, with the greatest effect in non-smokers.88 Noteworthy, Helena, Montana experienced a temporary decline in hospital admissions for AMI of 40% within six months after smoke-free legislation – with admission rates readily returning to baseline (+46%) after the ban was suspended.88 On the basis of available evidence, smoke-free legislation appears to be a prudent public health action in countries that have not yet endorsed this.
Alternative modes of nicotine delivery
Electronic nicotine delivery systems (ENDSs) such as e-cigarettes are increasingly used as potentially less harmful, alternative modes of nicotine delivery.89 Although results have been mixed, there is evidence to suggest that smokers switching to ENDS improve lung function90 and disease symptoms attributable to asthma and chronic obstructive pulmonary disease.90,91 However, considering the use of ENDS relative to no use (absolute harm), the best advisory is likely to be complete abstinence from smoking.
Recreational use of shisha smoking has gained popularity worldwide. This is of concern, given that reported potential short-term effects on markers of cardiovascular risk are similar to that of cigarette smoking, including increased oxidative stress, platelet dysfunction, increased blood pressure and heart rate.92,93 Even more concerning is that one session of waterpipe tobacco smoking has been shown to elicit larger acute effects compared with smoking one cigarette.94
Novel lifestyle risk factors
Chronic exposure to (a) environmental stressors like ambient air pollution and nocturnal and diurnal noise,95,96 (b) quantitative and qualitative sleep deprivation97–99,100 and (c) psychosocial stress such as loneliness and depression101 are increasingly recognized as independent risk factors for ASCVD. Evidence points to partially overlapping disease mechanisms that are closely related to sympathoadrenergic activation, chronic upregulation of the hypothalamic–pituitary–adrenal (HPA) axis and cerebral and systemic chronic low grade inflammation with downstream effects on endocrine signalling and vascular health.95,96,98,101
Exposure to ambient air pollution
Epidemiological and toxicological studies have established ambient air pollution as an independent risk factor for ASCVD.95,96 Like exposure to tobacco smoke, ambient particulate matter (PM), a principal component of air pollutants, has acute and chronic effects on the cardiovascular system. The acute toxic effects (seconds–hours) of exposure to ambient PM are mediated by oxidative stress, sympathoadrenergic activation, endothelial dysfunction and effects on blood coagulability.95 Of note, highest risk for acute toxicity occurs within the framework of chronic exposure in susceptible patients characterized by pre-existing ‘vulnerable plaque’, ‘vulnerable, proarrhythmic myocardium’ and ‘vulnerable, prothrombotic circulation’.95 These associations seem to be strongest for fine particulate air pollutants (PM < 2.5 µm in diameter (PM2.5)), which are a principal component of the combustion-derived nanoparticulate in diesel exhaust.95 Public health interventions targeting reduced burden from ambient PM from air pollution, particularly PM2.5, have the potential to reduce morbidity and mortality from ASCVD on a global scale.102
Exposure to noise
Interestingly, exposure to noise mediates its detrimental effects on cardiovascular health largely through similar mechanisms as exposure to ambient air pollution.95,96 For example, exposure to nocturnal aircraft noise dose-dependently impaired endothelial function and caused sympathoadrenergic activation in a cohort of healthy adults free from ASCVD.103 Mechanistic studies in mice have established increased oxidative stress as a causal mechanism leading to noise-induced endothelial dysfunction.104 Chronic non-resolving inflammation thus constitutes a biologically plausible explanation for the observed association between chronic noise exposure and ASCVD, rendering it an important public health target.103,104
Sleep deprivation
Chronically disrupted circadian rhythms, through adipose tissue dysfunction and associated high-risk metabolic traits, create a milieu conducive for ASCVD.105 A scientific statement from the American Heart Association argues in favour of a sleep duration of ≥7 h per night for adults.98 This concurs with epidemiological evidence that total sleep duration of 6–8 h per day was associated with the lowest risk of deaths and major cardiovascular events in a large cohort of 116,632 people from 21 countries.99 One common cause for sleep fragmentation in adults is obstructive sleep apnoea syndrome (OSAS), which speaks for a broader screening for OSAS in cardiovascular prevention.98
Psychosocial stress
One of the principal mechanisms translating chronic stress into adverse cardiometabolic outcomes is upregulation of the HPA axis. Chronic elevation of the stress hormone cortisol enhances a set of phenotypic adaptations which promote an overall pro-inflammatory and pro-atherogenic milieu. This includes visceral fat accumulation,105 increased peripheral protein/muscle catabolism, and insulin resistance.101,106 In line with this, evidence from preclinical studies in rabbit models of chronic stress shows that exposure to physical and social stress for eight weeks results in markedly increased plaque instability, which is manifested by thinner fibrous caps, larger lipid cores and more inflammation, but fewer smooth muscle cells and elastic fibres.107 This provides a biologically plausible concept for the observation of increased risk for ASCVD with chronic stress in humans and might justify the implementation of stress management programmes such as mind–body medicine, meditation or physical activity to prevent and manage ASCVD.101 This is of particular importance given that psychosocial distress serves as a barrier to behavioural changes/adoption of a healthy lifestyle on the one hand and, on the other hand, stress-induced behavioural compensations (e.g. smoking, increased alcohol consumption or unhealthy food choices exacerbate novel and traditional risk factors.108
Intestinal microbiome: intermediary pathway for lifestyle mediated effects on cardiometabolic health?
In recent years, microbiome–health interactions have been found to play a fundamental role in cardiovascular health.109 One major link between the intestinal microbiome and high-risk atherosclerosis is gut dysbiosis-related inflammation.110 Gut dysbiosis has been linked to increased mucosal barrier permeability, which allows the penetration of bacteria and their products – including pathogen-associated molecular patterns, damage-associated molecular patterns and microbial-associated molecular patterns – into the circulatory system.29 This elicits a systemic, chronic, pro-inflammatory condition and provides a mechanistic basis for the observed association of ASCVD and a wide array of non-communicable diseases with gut dysbiosis.29 Additional mechanisms linking gut microbiome and dysbiosis to cardiometabolic risk include gut microbiota-derived metabolites such as short chain fatty acids and trimethylamine N-oxide (TMAO), which are observed in lower and higher concentrations, respectively, in non-communicable diseases (reviewed in Tang et al.109). However, it is less clear whether dietary sources of TMAO (e.g. l-carnitine in red meat and lecithin) are the mediating risk factor in TMAO’s relationship with cardiometabolic risk; the richest dietary source of preformed TMAO is deep sea fish, yet fish consumption is associated with favourable vascular outcomes in a dose-dependent manner.111
Adhering to low-risk lifestyle factors has been linked to favourable changes in the intestinal microbiome; 112 exercise training,71 (intermittent) caloric restriction29,112 and certain foods, such as nuts,113 rich in soluble fibre, are associated with increased bacterial gut diversity, a shift towards health-promoting and butyrate-producing bacterial species and a protection of the intestinal barrier. Of note, the ketone body BHB, which is elevated in the state of nutritional ketosis, chemically differs from butyrate by only one hydroxyl group. It is thus tempting to speculate that the observed benefits of ketogenic diets on inflammatory diseases may in part be attributable to increased intestinal barrier function and gut health.34
Future directions
Shifting the focus (a) from BMI to body composition and cardiorespiratory fitness,114 (b) from nutrient- and calorie-based approaches to food-based, calorie-unrestricted dietary recommendations115 and (c) addressing exposure to novel lifestyle risk factors through public health interventions102 might result in more feasible lifestyle guidance, improve long-term adherence and ameliorate prognosis115 with combined approaches achieving cumulative benefit.116
Of emerging interest is the potential health benefit of ‘metabolic switching’ (i.e. intermittent metabolic states of low cellular energy levels). This can be achieved by intermittent caloric restriction and physical exercise. Interestingly, carbohydrate restricted diets resulting in nutritional ketosis phenocopy the effects of caloric restriction and/or physical exercise by activating nutrient sensitive pathways linked to low energy metabolic states (AMPK).117 States of low energy result in reduced anabolic processes (synthesis, growth and reproduction) and enhanced maintenance systems, including increased stress resistance, tissue repair and recycling of damaged molecules (autophagy).35 Autophagy, also referred to as the body’s innate recycling programme, has been linked to attenuation of cardiovascular aging through numerous mechanisms, including reduced inflammation/oxidative stress, suppression of mTOR signalling, increased mitochondrial biogenesis and enhanced endothelial function, among others.22,118 Collectively, evidence points to similar cellular, molecular and tissue/organ adaptations observed with exercise, (intermittent) caloric restriction and carbohydrate restriction27 – the ketone body BHB through NLRP3 inflammasome- and HDAC inhibition likely being an intermediary for many of the observed benefits.22,27,28 It is thus tempting to speculate that BHB, the main circulating ketone body, underlies many of the long-recognized (cardiovascular) health benefits of caloric restriction, carbohydrate restriction and exercise.26,27
Several approaches have been proposed to encourage lifestyle modification; (a) peer group-based interventions have proven effective for global cardiovascular risk factor modification and tobacco cessation in particular,119 (b) e-health devices,120 (c) rehabilitation programmes,121 (d) motivational interviewing and (e) the use of positive messages rather than negative ones.115
Concluding remarks
In conclusion, we posit that cardiovascular disease risk factors such as visceral adiposity, diabetes, hypertension and dyslipidaemia might in part be seen as symptoms and consequences of high-risk lifestyle risk factors such as poor diet quality, sedentary behaviour, exposure to ambient air pollution/noise, sleep deprivation and psychosocial stress. Adhering to low-risk lifestyle factors reduces cardiovascular disease risk in a multifactorial manner, thus significantly adding to the prognostic benefit of conventional risk factor control. Collectively, low-risk lifestyle factors cause a set of phenotypic adaptations that shift tissue cross-talk from a proinflammatory milieu conducive for high-risk atherosclerosis, to an anti-atherogenic milieu. This is a powerful public health message for clinicians and patients alike.
Acknowledgements
The authors are indebted to ALM for language and grammar editing, and to Nicola Bernhart for graphical design.
Author contribution
All authors contributed to the review. KL did the literature search, and drafted the manuscript. CvS, ALM, NW, UN, BL, NK, MH, RMK and JS critically revised and edited the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KL, NW, UN, BL, NK, MH and JS declare that they have no conflict of interest to disclose with respect to this manuscript. ALM is employed by Virta Health and has been offered stock options. CvS operates Omegametrix, a laboratory for fatty acid analyses. He consults for BASF/Pronova, and Huntsworth Medical, and received speaker’s honoraria from Abbott, DSM and Norsan. RMK is on the Scientific Advisory Board of Virta Health and Day Two, and has a licensed patent for lipoprotein particle analysis by ion mobility.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.WHO. WHO/Fact sheets/Detail/Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (2017, 14 March 2019).
- 2.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016; 133: 187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaasenbrood L, Boekholdt SM, van der Graaf Y, et al. Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation 2016; 134: 1419–1429. [DOI] [PubMed] [Google Scholar]
- 4.Astrup A, Bertram HC, Bonjour JP, et al. WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach? BMJ 2019; 366: l4137–l4137. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: A review of emerging biologic pathways. Circ Res 2018; 122: 369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanhope KL, Goran MI, Bosy-Westphal A, et al. Pathways and mechanisms linking dietary components to cardiometabolic disease: Thinking beyond calories. Obes Rev 2018; 19: 1205–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. Epub ahead of print 16 May 2019. DOI: 10.1038/s41569-019-0206-1. [DOI] [PubMed] [Google Scholar]
- 8.Archer E, Marlow ML, Lavie CJ. Controversy and debate: Memory-Based Methods Paper 1: The fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J Clin Epidemiol 2018; 104: 113–124. [DOI] [PubMed] [Google Scholar]
- 9.Archer E, Pavela G, Lavie CJ. The inadmissibility of what we eat in America and NHANES dietary data in nutrition and obesity research and the scientific formulation of national dietary guidelines. Mayo Clin Proc 2015; 90: 911–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estruch R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc NutR Soc 2010; 69: 333–340. [DOI] [PubMed] [Google Scholar]
- 11.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018; 378: e34–e34. . DOI: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: The Women’s Health Initiative randomized controlled dietary modification trial. JAMA 2006; 295: 655–666. [DOI] [PubMed] [Google Scholar]
- 13.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012; 126: 1301–1313. [DOI] [PubMed] [Google Scholar]
- 14.Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: The CENTRAL MRI randomized controlled trial. Circulation 2018; 137: 1143–1157. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc S, Coulombe F, Bertrand OF, et al. Hypertriglyceridemic waist: A simple marker of high-risk atherosclerosis features associated with excess visceral adiposity/ectopic fat. J Am Heart Assoc 2018; 7: e008139–e008139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashwell M, Gibson S. Waist-to-height ratio as an indicator of ‘early health risk’: Simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open 2016; 6: e010159–e010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 2019; 16: 83–99. [DOI] [PubMed] [Google Scholar]
- 18.Von Bibra H, Saha S, Hapfelmeier A, et al. Impact of the triglyceride/high-density lipoprotein cholesterol ratio and the hypertriglyceremic-waist phenotype to predict the metabolic syndrome and insulin resistance. Horm Metab Res 2017; 49: 542–549. [DOI] [PubMed] [Google Scholar]
- 19.Schneider HJ, Friedrich N, Klotsche J, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab 2010; 95: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig DS, Willett WC, Volek JS, et al. Dietary fat: From foe to friend? Science 2018; 362: 764–770. [DOI] [PubMed] [Google Scholar]
- 21.Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015; 31: 1–13. [DOI] [PubMed] [Google Scholar]
- 22.Anton SD, Moehl K, Donahoo WT, et al. Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity (Silver Spring) 2018; 26: 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyde PN, Sapper TN, Crabtree CD, et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019; 4: 128308–128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joslin EP. The treatment of diabetes mellitus. Can Med Assoc J 1916; 6: 673–684. [PMC free article] [PubMed] [Google Scholar]
- 25.Bhanpuri NH, Hallberg SJ, Williams PT, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc Diabetol 2018; 17: 56–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Park MH, Ha S, et al. Anti-inflammatory action of beta-hydroxybutyrate via modulation of PGC-1alpha and FoxO1, mimicking calorie restriction. Aging 2019; 11: 1283–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015; 21: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014; 25: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018; 15: 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ Res 2016; 118: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 32.Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han YM, Bedarida T, Ding Y, et al. Beta-hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated Upregulation of Oct4. Mol Cell 2018; 71: 1064–1078.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavaleri F, Bashar E. Potential synergies of β-hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J Nutr Metab 2018; 2018: 7195760–7195760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Francesco A, Di Germanio C, Bernier M, et al. A time to fast. Science 2018; 362: 770–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Lopez N, Tarabra E, Toledo M, et al. System-wide benefits of intermeal fasting by autophagy. Cell Metab 2017; 26: 856–871.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J, Sowers JR, Zhang Y. Metabolic stress, autophagy, and cardiovascular aging: From pathophysiology to therapeutics. Trends Endocrinol Metab 2018; 29: 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahleova H, Belinova L, Malinska H, et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: A randomised crossover study. Diabetologia 2014; 57: 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton EF, Beyl R, Early KS, et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 2018; 27: 1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakubowicz D, Barnea M, Wainstein J, et al. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013; 21: 2504–2512. [DOI] [PubMed] [Google Scholar]
- 41.Asher G, Sassone-Corsi P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015; 161: 84–92. [DOI] [PubMed] [Google Scholar]
- 42.Schwingshackl L, Chaimani A, Hoffmann G, et al. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol 2018; 33: 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008; 359: 229–241. [DOI] [PubMed] [Google Scholar]
- 44.Taylor R, Barnes AC. Translating aetiological insight into sustainable management of type 2 diabetes. Diabetologia 2018; 61: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamantia V, Sniderman A, Faraj M. Nutritional management of hyperapoB. Nutr Res Rev 2016; 29: 202–233. [DOI] [PubMed] [Google Scholar]
- 47.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015; 1851: 469–484. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011; 58: 2047–2067. [DOI] [PubMed] [Google Scholar]
- 49.Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol 2018; 72: 330–343. [DOI] [PubMed] [Google Scholar]
- 50.Moertl D, Hammer A, Steiner S, et al. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: A double-blind, placebo-controlled, 3-arm study. Am Heart J 2011; 161: 915.e1–9. [DOI] [PubMed] [Google Scholar]
- 51.Kohashi K, Nakagomi A, Saiki Y, et al. Effects of eicosapentaenoic acid on the levels of inflammatory markers, cardiac function and long-term prognosis in chronic heart failure patients with dyslipidemia. J Atheroscler Thromb 2014; 21: 712–729. [DOI] [PubMed] [Google Scholar]
- 52.Kasikara C, Doran AC, Cai B, et al. The role of non-resolving inflammation in atherosclerosis. J Clin Invest 2018; 128: 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rimm EB, Appel LJ, Chiuve SE, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American Heart Association. Circulation 2018; 138: e35–e47. . DOI: 10.1161/CIR.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003; 361: 477–485. [DOI] [PubMed] [Google Scholar]
- 55.Kasikara C, Doran C, Cai A, et al. The role of non-resolving inflammation in atherosclerosis. J Clin Invest 2018; 128: 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunbar RL, Nicholls SJ, Maki KC, et al. Effects of omega-3 carboxylic acids on lipoprotein particles and other cardiovascular risk markers in high-risk statin-treated patients with residual hypertriglyceridemia: A randomized, controlled, double-blind trial. Lipids Health Dis 2015; 14: 98–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bays HE, Ballantyne CM, Braeckman RA, et al. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: Effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs 2013; 13: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelley DS, Siegel D, Fedor DM, et al. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr 2009; 139: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Effects of n−3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018; 379: 1540–1550. [DOI] [PubMed]
- 60.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2018; 379: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 61.Heydari B, Abdullah S, Pottala JV, et al. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: The OMEGA-REMODEL randomized clinical trial. Circulation 2016; 134: 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007; 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 63.Harris WS, Masson S, Barlera S, et al. Red blood cell oleic acid levels reflect olive oil intake while omega-3 levels reflect fish intake and the use of omega-3 acid ethyl esters: The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico-Heart Failure trial. Nutr Res 2016; 36: 989–994. [DOI] [PubMed] [Google Scholar]
- 64.Oktay AA, Lavie CJ, Kokkinos PF, et al. The interaction of cardiorespiratory fitness with obesity and the obesity paradox in cardiovascular disease. Prog Cardiovasc Dis 2017; 60: 30–44. [DOI] [PubMed] [Google Scholar]
- 65.Moholdt T, Lavie CJ, Nauman J. Sustained physical activity, not weight loss, associated with improved survival in coronary heart disease. J Am Coll Cardiol 2018; 71: 1094–1101. [DOI] [PubMed] [Google Scholar]
- 66.Lavie CJ, Arena R, Swift DL, et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ Res 2015; 117: 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steell L, Ho FK, Sillars A, et al. Dose–response associations of cardiorespiratory fitness with all-cause mortality and incidence and mortality of cancer and cardiovascular and respiratory diseases: The UK Biobank cohort study. Br J Sports Med. Epub ahead of print 22 Feb 2019. DOI: 10.1136/bjsports-2018-099093. [DOI] [PubMed] [Google Scholar]
- 68.Young DR, Hivert MF, Alhassan S, et al. Sedentary behavior and cardiovascular morbidity and mortality: A science advisory from the American Heart Association. Circulation 2016; 134: e262–e279. [DOI] [PubMed] [Google Scholar]
- 69.Matsubara Y, Matsumoto T, Inoue K, et al. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J Vasc Surg 2017; 65: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 70.Marzetti E, Calvani R, Tosato M, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res 2017; 29: 35–42. [DOI] [PubMed] [Google Scholar]
- 71.Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat Rev Cardiol 2018; 15: 731–743. [DOI] [PubMed] [Google Scholar]
- 72.Melov S, Tarnopolsky MA, Beckman K, et al. Resistance exercise reverses aging in human skeletal muscle. PLoS One 2007; 2: e465–e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duggal NA, Niemiro G, Harridge SDR, et al. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. Epub ahead of print 7 June 2019. DOI: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 74.Lehnig AC, Stanford KI. Exercise-induced adaptations to white and brown adipose tissue. J Exper Biol 2018; 221(Pt Suppl. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leal LG, Lopes MA, Batista ML. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front Physiol 2018; 9: 1307–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8: 457–465. [DOI] [PubMed] [Google Scholar]
- 77.Seale P, Lazar MA. Brown fat in humans: Turning up the heat on obesity. Diabetes 2009; 58: 1482–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krankel N, Bahls M, van Craenenbroeck EM, et al. Exercise training to reduce cardiovascular risk in patients with metabolic syndrome and type 2 diabetes mellitus: How does it work? Eur J Prev Cardiol 2019; 26: 701–708. [DOI] [PubMed] [Google Scholar]
- 79.Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest 2017; 47: 600–611. [DOI] [PubMed] [Google Scholar]
- 80.Bernardo BC, Ooi JYY, Weeks KL, et al. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: Current knowledge and emerging concepts. Physiol Rev 2018; 98: 419–475. [DOI] [PubMed] [Google Scholar]
- 81.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018; 320: 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation 2011; 124: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen CP, Wai JPM, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011; 378: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 84.Owen N, Salmon J, Koohsari MJ, et al. Sedentary behaviour and health: Mapping environmental and social contexts to underpin chronic disease prevention. Br J Sports Med 2014; 48: 174–174. [DOI] [PubMed] [Google Scholar]
- 85.Cartee GD. Once is enough for acute exercise benefits on insulin sensitivity. J Physiol 2019; 597: 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reidy PT, McKenzie AI, Mahmassani Z, et al. Skeletal muscle ceramides and relationship with insulin sensitivity after 2 weeks of simulated sedentary behaviour and recovery in healthy older adults. J Physiol 2018; 596: 5217–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 2005; 111: 2684–2698. [DOI] [PubMed] [Google Scholar]
- 88.Meyers DG, Neuberger JS, He J. Cardiovascular effect of bans on smoking in public places: A systematic review and meta-analysis. J Am Coll Cardiol 2009; 54: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 89.Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: A systematic review. Am J Prev Med 2017; 52: e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Polosa R, Morjaria JB, Caponnetto P, et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med 2016; 21: 99–108. [PubMed] [Google Scholar]
- 91.Farsalinos KE, Romagna G, Tsiapras D, et al. Characteristics, perceived side effects and benefits of electronic cigarette use: A worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health 2014; 11: 4356–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kadhum M, Jaffery A, Haq A, et al. Measuring the acute cardiovascular effects of shisha smoking: A cross-sectional study. JRSM Open 2014; 5: 2054270414531127–2054270414531127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolfram RM, Chehne F, Oguogho A, et al. Narghile (water pipe) smoking influences platelet function and (iso-)eicosanoids. Life Sci 2003; 74: 47–53. [DOI] [PubMed] [Google Scholar]
- 94.Primack BA, Carroll MV, Weiss PM, et al. Systematic review and meta-analysis of inhaled toxicants from waterpipe and cigarette smoking. Public Health Rep 2016; 131: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Münzel T, Sørensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: Part II –Mechanistic insights. Eur Heart J 2017; 38: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munzel T, Sorensen M, Gori T. Set al. Environmental stressors and cardio-metabolic disease: part I –Epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017; 38: 550–556. [DOI] [PubMed] [Google Scholar]
- 97.Koren D, Taveras EM. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism 2018; 84: 67–75. [DOI] [PubMed] [Google Scholar]
- 98.St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation 2016; 134: e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang C, Bangdiwala SI, Rangarajan S, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: A study of 116 632 people from 21 countries. Eur Heart J 2019; 40: 1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 2010; 95: 2963–2968. [DOI] [PubMed]
- 101.Vaccarino V, Badimon L, Bremner JD, et al. Depression and coronary heart disease: 2018 ESC position paper of the working group of coronary pathophysiology and microcirculation developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J. Epub ahead of print 28 January 2019. DOI: 10.1093/eurheartj/ehy913.
- 102.Lelieveld J, Klingmüller K, Pozzer A, et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 2019; 40: 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schmidt FP, Basner M, Kroger G, et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J 2013; 34: 3508–3514a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munzel T, Daiber A, Steven S, et al. Effects of noise on vascular function, oxidative stress, and inflammation: Mechanistic insight from studies in mice. Eur Heart J 2017; 38: 2838–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pagano ES, Spinedi E, Gagliardino JJ. White adipose tissue and circadian rhythm dysfunctions in obesity: Pathogenesis and available therapies. Neuroendocrinology 2017; 104: 347–363. [DOI] [PubMed] [Google Scholar]
- 106.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress – a modifiable risk factor. Nat Rev Endocrinol 2017; 13: 547–560. [DOI] [PubMed] [Google Scholar]
- 107.Yu ZM, Deng XT, Qi RM, et al. Mechanism of chronic stress-induced reduced atherosclerotic medial area and increased plaque instability in rabbit models of chronic stress. Chin Med J (Engl) 2018; 131: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pedersen SS, von Kanel R, Tully PJ, et al. Psychosocial perspectives in cardiovascular disease. Eur J Prev Cardiol 2017; 24(3_Suppl): 108–115. [DOI] [PubMed] [Google Scholar]
- 109.Tang WHW, Bäckhed F, Landmesser U, et al. Intestinal microbiota in cardiovascular health and disease. J Am Coll Cardiol 2019; 73: 2089–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma J, Li H. The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol 2018; 9: 1082–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DiNicolantonio JJ, McCarty M, OKeefe J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019; 6: e000890–e000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science 2018; 362: 776–776. [DOI] [PubMed] [Google Scholar]
- 113.Coates AM, Hill AM, Tan SY. Nuts and cardiovascular disease prevention. Curr Atheroscler Rep 2018; 20: 48–48. [DOI] [PubMed] [Google Scholar]
- 114.Lavie CJ, Laddu D, Arena R, et al. Reprint of: Healthy weight and obesity prevention: JACC Health Promotion Series. J Am Coll Cardiol 2018; 72: 3027–3052. [DOI] [PubMed] [Google Scholar]
- 115.Masana L, Ros E, Sudano I, et al. Is there a role for lifestyle changes in cardiovascular prevention? What, when and how? Atheroscler Suppl 2017; 26: 2–15. [DOI] [PubMed] [Google Scholar]
- 116.Alvarez-Alvarez I, de Rojas JP, Fernandez-Montero A, et al. Strong inverse associations of Mediterranean diet, physical activity and their combination with cardiovascular disease: The Seguimiento Universidad de Navarra (SUN) cohort. Eur J Prev Cardiol 2018; 25: 1186–1197. [DOI] [PubMed] [Google Scholar]
- 117.Kroemer G, Lopez-Otin C, Madeo F, et al. Carbotoxicity – noxious effects of carbohydrates. Cell 2018; 175: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abdellatif M, Sedej S, Carmona-Gutierrez D, et al. Autophagy in cardiovascular aging. Circ Res 2018; 123: 803–824. [DOI] [PubMed] [Google Scholar]
- 119.Gomez-Pardo E, Fernandez-Alvira JM, Vilanova M, et al. A comprehensive lifestyle peer group-based intervention on cardiovascular risk factors: The randomized controlled Fifty-Fifty Program. J Am Coll Cardiol 2016; 67: 476–485. [DOI] [PubMed] [Google Scholar]
- 120.Coorey GM, Neubeck L, Mulley J, et al. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: Systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol 2018; 25: 505–521. [DOI] [PubMed] [Google Scholar]
- 121.Kotseva K, Wood D, De Bacquer D. Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROASPIRE IV survey. Eur J Prev Cardiol 2018; 25: 1242–1251. [DOI] [PubMed] [Google Scholar]