Abstract
Many metals have biological functions and play important roles in human health. Copper (Cu) is an essential metal that supports normal cellular physiology. Significant research efforts have focused on identifying the molecules and pathways involved in dietary Cu uptake in the digestive tract. The lack of an adequate in vitro model for assessing Cu transport processes in the gut has led to contradictory data and gaps in our understanding of the mechanisms involved in dietary Cu acquisition. The recent development of organoid technology has provided a tractable model system for assessing the detailed mechanistic processes involved in Cu utilization and transport in the context of nutrition. Enteroid (intestinal epithelial organoid)-based studies have identified new links between intestinal Cu metabolism and dietary fat processing. Evidence for a metabolic coupling between the dietary uptake of Cu and uptake of fat (which were previously thought to be independent) is a new and exciting finding that highlights the utility of these three-dimensional primary culture systems. This review has three goals: (a) to critically discuss the roles of key Cu transport enzymes in dietary Cu uptake; (b) to assess the use, utility, and limitations of organoid technology in research into nutritional Cu transport and Cu-based diseases; and (c) to highlight emerging connections between nutritional Cu homeostasis and fat metabolism.
Keywords: copper, organoid, enteroid, nutrition, intestine, fat, ATP7B, Wilson disease
1. INTRODUCTION
1.1. Copper in Human Biology
Copper (Cu) is an essential micronutrient for living organisms. Common Cu-rich foods include shellfish, meats, seeds, nuts, lentils, leafy greens, and cocoa. The Recommended Dietary Allowance (RDA) for adults is 0.9 mg/day (99), and the average daily consumption of Cu in North America is estimated to be between 1.0 and 1.6 mg/day, which is well above the RDA. However, whether the average adult in North America has an adequate Cu status is debatable. A recent National Health and Nutrition Examination Survey demonstrated that as much as 40% of the North American population is in a state of functional Cu deficit (70). Insufficient dietary Cu intake is cited as a probable cause for the low serum Cu values identified in such a significant portion of the population. These findings are supported by additional studies suggesting that the optimal dietary Cu intake for adults should be adjusted to 2.6 mg/day (11).
In humans, Cu plays a role in several fundamental processes that are critical for optimal health. Respiration, the formation of connective tissues, wound repair, macronutrient energy metabolism, synthesis of catecholamines, and iron flux all depend on Cu. Although Cu is critical to normal physiological functions, it becomes toxic at high levels. Therefore, tight regulation of Cu levels within cells and tissues is essential. Both Cu overload and deficiency lead to severe, often fatal, pathologies (64, 105). Menkes disease is a fatal genetic disorder of systemic Cu deficit caused by an inability to absorb dietary Cu (46, 63, 64, 68, 85). Cu deficiency, whether caused by genetic dysfunction or nutritional deficit, has been linked to cardiac dysfunction, abnormal blood vessels, severe neurological abnormalities, and premature death. Wilson disease (WD) is a disorder of systemic Cu overload caused by hepatic inability to remove excess Cu from circulation. In this disease, an excess of Cu leads to neurological abnormalities, acute liver failure, and premature death (57, 105).
The delicate balance of Cu in the body is maintained by two membrane-bound Cu-transporting adenosine triphosphatases (ATPases), ATP7A and ATP7B. Menkes disease and WD result from inactivating mutations in ATP7A and ATP7B, respectively. ATP7A and ATP7B have essential and complementary roles in Cu transport and distribution within cells and between tissues (reviewed in 57). Both transporters play important roles in the digestive tract. ATP7A facilitates efflux of Cu from absorptive cells (enterocytes) for delivery of nutritional Cu into circulation (68, 85). Enteric ATP7B facilitates storage of Cu in intracellular vesicles to maintain Cu balance, which is required for normal lipid homeostasis in the absorptive epithelium (84). The final destination of Cu stored in ATP7B vesicles remains unknown; however, it is possible that Cu may be released from vesicles as cellular or systemic need dictates. The roles of ATP7A and ATP7B in enteric absorptive epithelium are illustrated in Figure 1.
Figure 1.
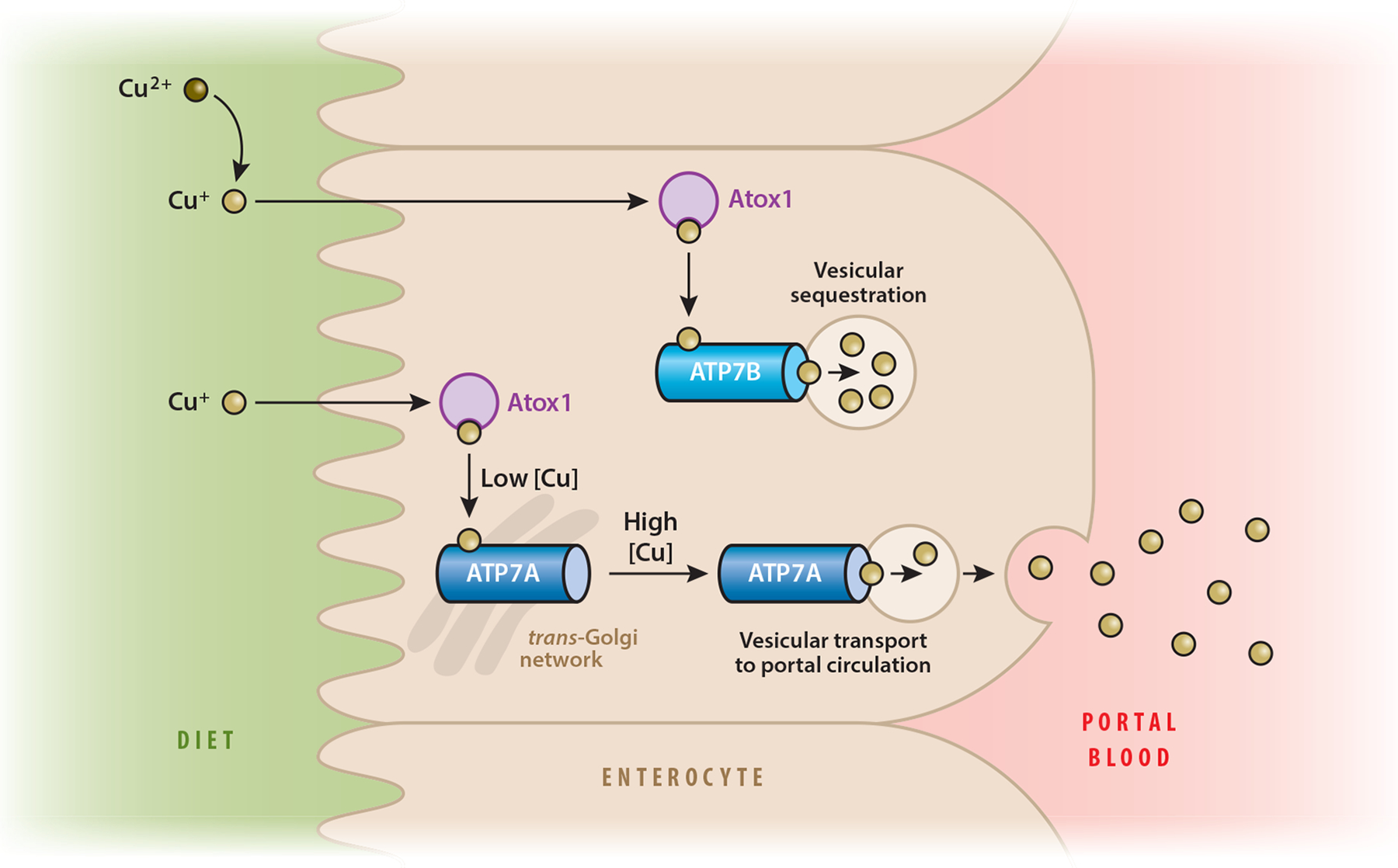
Model of dietary copper (Cu) acquisition and storage in human absorptive epithelium. Dietary Cu is in the Cu2+ oxidation state. Enzymes at the brush border reduce Cu2+ to Cu+ prior to importation into the cell (80). Reduced Cu is bound by Cu chaperones. Atox1 is a Cu chaperone that delivers Cu to ATP7A and ATP7B (30). Under low-Cu conditions, ATP7A resides in the trans-Golgi network (78). When intracellular Cu loads are high, ATP7A traffics to the basolateral membrane and transports Cu+ into portal circulation. ATP7B sequesters Cu in vesicles, presumably to buffer cytosolic Cu levels or deliver Cu to yet-to-be-identified Cu-dependent enzymes located in the vesicles.
1.2. What Are Organoids and How Do They Open Avenues for Discovery?
Organoids are three-dimensional (3D) self-organized multicellular cultures that originate from, and model properties of, primary tissues. Currently, methods are available to generate organoids from mammary gland (18), trachea (5), esophagus (41, 90), pancreas (27), prostate (34, 40), stomach (6), intestine (61, 91), colon (90), brain (50, 59), and liver (35, 73), as well as uterine endometrium (9). Evidence is accumulating to demonstrate that these 3D tissue culture systems faithfully recapitulate the core properties of the tissues from which they are derived (89–91). Regardless of the tissue of origin, organoid culture systems provide an excellent platform for biological and biochemical studies in physiologically relevant contexts.
Organoids may be derived from primary tissues through adult organ-restricted stem cells or induced pluripotent stem cells (iPSCs). These 3D primary culture systems mitigate the concerns related to altered physiological responses in cancer-based or otherwise immortalized cell lines. Organoids also provide stable heterogeneous cultures, as more than one cell type can be simultaneously grown, allowing for physical and functional interactions between different cell types (89). Such interactions provide a more physiologically relevant context, as well as the opportunity to investigate the roles of different cell types in integrated tissue responses. Studies on protein trafficking, signal transduction, and metabolic regulation in live animals, although highly desirable, are still extremely challenging, particularly if studies involve endogenous untagged proteins. Organoids bridge this gap and facilitate live-cell imaging in primary culture (32, 81). Animals may not be easily amenable to the manipulations and treatments required to address certain scientific questions (such as the use of genetically encoded molecular sensors and measurements of nutrient fluxes across different membranes), and organoids provide more tractable systems for these types of investigations. Methods are emerging to make organoid cultures compatible with transfection and genetic manipulations, opening even more avenues for mechanistic queries (47, 51, 60). Finally, organoids can be derived from patients’ biopsies as well as excised tissues, allowing for personalized medicine approaches and basic research on the mechanisms driving disease (23).
The recent development of 3D primary intestinal epithelial culture models (91) (enteroids) has generated significant excitement in the fields of gastroenterology and nutrition and opened new avenues for studies of Cu physiology in the gut. Prior to the development of enteroids, the lack of a workable experimental system limited mechanistic investigation into Cu homeostasis and dietary Cu acquisition. As a result, the understanding of Cu handling in the intestine is incomplete and even controversial (see specific examples in Section 2). Advances in enteroid technologies are facilitating complex experiments aimed at gaining a better understanding of micronutrient absorption and transport. Enteroids are rapidly becoming the gold standard for cell-based studies of digestive epithelial biology.
2. DIETARY COPPER UPTAKE IN THE INTESTINE AND THE UNRESOLVED ISSUES
2.1. Unique Characteristics of Intestinal Copper Transport
The importance of Cu homeostasis and balanced Cu nutrition for human health is not yet fully appreciated by the general public because genetic disorders of Cu homeostasis are relatively rare, and the consequences of dietary Cu misbalance take a long time to manifest. Nevertheless, the discovery of important Cu-dependent enzymes, the high mortality rate of patients with Menkes disease, and the significant burden of ill health affecting patients with WD have motivated the research community to better understand how nutritional Cu enters the human body and is utilized. Initial attempts to dissect the processes underlying dietary Cu uptake were made between 1940 and 1960. These early studies, using radioactive Cu isotopes either in vivo or in everted intestinal sacs, determined the primary role of the small intestine in dietary Cu uptake. These studies also established that the transfer of Cu from the digestive lumen to the bloodstream involved distinct transport mechanisms. While the uptake of Cu through the apical (gut luminal) membrane of enterocytes can be significantly increased by increasing Cu concentrations in the gut lumen, the transport out of intestinal cells through the basolateral membrane has a limited capacity and can be saturated at relatively low concentrations of Cu (15).
It has also been shown that dietary Cu uptake is regulated (56). Varying concentrations of Cu in the diet affects not only the overall Cu flux through the intestine but also the retention of Cu by the intestinal epithelial cells. Depleting dietary Cu prior to measuring Cu flux increases both the fraction of Cu that is absorbed from the diet upon refeeding and Cu export from enterocytes. In other words, following Cu depletion, the Cu flux through the intestine is high and the retention of Cu in the intestinal tissue is low. In contrast, chronic dietary Cu excess increases Cu accumulation in the intestine but significantly decreases the overall Cu flux out of the gut. Similar effects of cellular Cu status on Cu fluxes were observed in polarized intestinal Caco2-cells grown as a monolayer on Transwells (Corning Life Sciences) (4, 108). It was also noted that high Cu2+ decreases the permeability of chloride (100). Reciprocally, the decrease of mucosal sodium chloride (NaCl) levels inhibits Cu transport, whereas increasing NaCl concentrations stimulates Cu uptake by intestinal cells from the apical membrane (71). Lastly, it was found that while Cu uptake into enterocytes did not require ATP, Cu efflux depended on cellular metabolic state and energy availability, indicative of distinct active transport mechanisms. During the past two decades, we have gained a better understanding of the molecular basis of processes that regulate Cu flux through the intestine, but many details are missing and important questions remain to be answered.
2.2. Copper-Transporting ATPase ATP7A Provides the Primary Route of Copper Efflux from Enterocytes
The discovery and characterization of the ATP-driven Cu transporter ATP7A (defective in patients with Menkes disease) identified the mechanism of Cu efflux from enterocytes. Humans and mice with genetically inactivated ATP7A accumulate Cu in the intestine and fail to export Cu into the bloodstream (44, 67). Several other lines of evidence point to a primary role for ATP7A in the transfer of dietary Cu to the body. ATP7A is strongly expressed in the small intestine and is more abundant in the villi compared with the crypts (Figure 2a). Basolateral localization of ATP7A in enterocytes has been firmly established (78, 84). The data also suggest that in the intestine, this localization is regulated by Cu availability. Studies on intestine perfused with Cu chelating reagents or with excess Cu have shown that Cu depletion causes perinuclear clustering of ATP7A in enterocytes, whereas Cu excess is associated with the distribution of ATP7A to vesicles and trafficking toward the plasma membrane. Little ATP7A was found on the plasma membrane per se. These results suggest that intestinal ATP7A transports Cu into vesicles in the immediate vicinity of the basolateral membrane and that the Cu export step involves the fusion of vesicles with the membrane. This conclusion found support in studies using surface biotinylation of polarized intestinal cells (78). These latter experiments revealed that only 8–10% of total ATP7A is found at the plasma membrane even when Cu is elevated.
Figure 2.
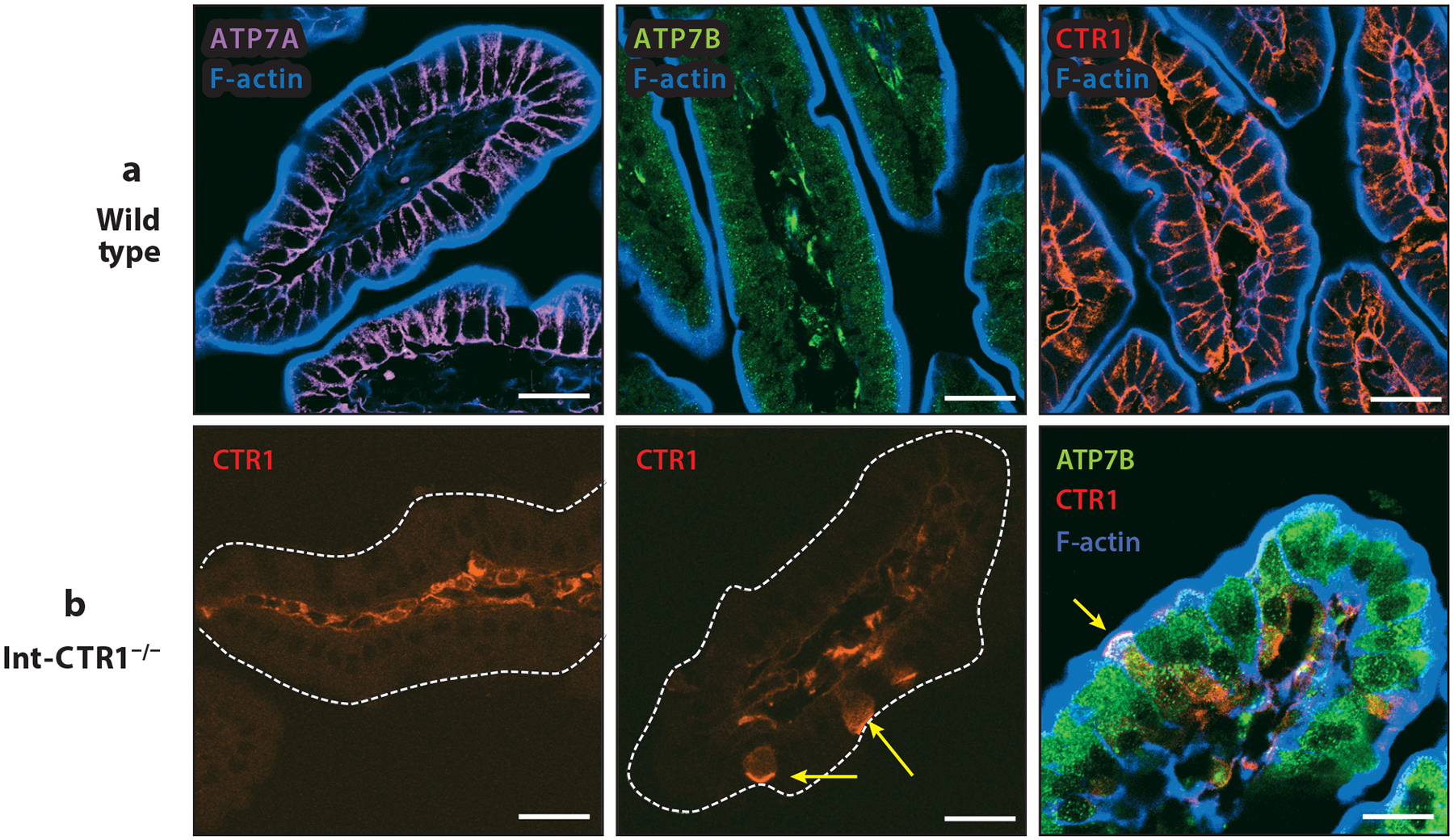
Expression and distribution of ATP7A, ATP7B, and CTR1 in murine small intestine. Duodenal intestinal tissues from 2-week-old sex-matched mice were used to assess the expression and intracellular distribution of the key players in intestinal copper (Cu) homeostasis. (a) In wild-type tissues, ATP7A (probed with anti-ATP7A sc376467, Santa Cruz Biotechnology) showed strong basolateral staining consistent with previous reports. ATP7B (probed with anti-ATP7b ab124973, Abcam) had a strong vesicular pattern, and CTR1 (probed with 8G11-F10, hybridoma monoclonal antibody; H. Pierson, H. Yang & S. Lutsenko, unpublished data) was detected specifically at the basolateral membrane. F-actin (blue) marks the apical cell border. (b) Tissues with intestine-specific deletion of CTR1 in enterocytes (Int-CTR1−/−) specifically lack CTR1 expression in the intestinal epithelium and show no basolateral staining of CTR1 in the epithelial layer. However, sparse epithelial cells did show CTR1 expression in the apical membrane in the context of the Int-CTR1−/− tissues (yellow arrows). All scale bars: 25 μm.
The abundance of ATP7A in enterocytes is also regulated by Cu, although more data are needed to fully understand the species- and age-dependent effects of Cu on ATP7A abundance. Specifically, suckling rat pups (but not adult rats) upregulate ATP7A in response to increased Cu in the diet (7). In mice, high Cu does not seem to have a significant effect on ATP7A abundance; instead, a systemic Cu deficiency is the main causative factor of upregulation of intestinal ATP7A (13). It has been proposed that the regulation of ATP7A abundance in the intestine could be a key homeostatic control for Cu export into the circulation. The direct proof of the critical role of ATP7A in Cu efflux from intestinal cells was generated in experiments in mice that had a targeted deletion of ATP7A in enterocytes. These studies found that mice lacking enteric ATP7A had high Cu in the intestine but showed marked growth impairment, neurological deterioration, and early mortality caused by Cu deficiency in other organs (101).
2.3. Enteric ATP7B Is Responsible for Vesicular Copper Storage and Uneven Copper Distribution Along the Crypt–Villus Axis
ATP7B is also highly expressed in the intestine, especially in the duodenum and jejunum (103). The distribution of ATP7B along the crypt–villus axis is opposite to that of ATP7A. ATP7B is easily detected in enterocytes by immunostaining, but the highest levels of ATP7B are in the crypts (84). Both Paneth cells and stem cells appear to express ATP7B, although more formal evidence is needed. ATP7B in enterocytes is vesicular, regardless of the Cu load. This observation led to the suggestion that the role of intestinal ATP7B is not in facilitating Cu transport out of the cell (as it would be in hepatocytes), but rather in sequestering Cu for storage. Studies in enteroids and Cu-overloaded intestinal tissue found that ATP7B levels positively correlate with Cu load, and ATP7B vesicles expand in size by 35–40% in response to high Cu, in agreement with ATP7B’s role in Cu storage (84). Furthermore, the loss of ATP7B in the mouse intestine is associated with a lower Cu content in the tissue (presumably caused by the elimination of intracellular Cu stores) and the disappearance of a Cu gradient along the crypt–villus axis. Intestinal ATP7A is not significantly affected by ATP7B inactivation. This suggests that in the intestine, the two transporters, while competing for Cu, may otherwise operate independently and be required for different physiological processes. Studies of the metabolic consequences of ATP7B inactivation in the animal model of WD support this notion (discussed in Section 4).
2.4. The Role of the High-Affinity Copper Transporter CTR1 in Dietary Copper Uptake
While ATP7A has been firmly established as the major route of Cu exit from the intestine, the identities of the pathways regulating Cu entry into enterocytes from the diet remain controversial. It is generally agreed that the cells of most organs acquire their Cu from circulation primarily via the high-affinity Cu transporter CTR1, which is located at the basolateral membrane (52). The intestine expresses high levels of CTR1; however, the localization of CTR1 in intestinal cells has proven to be controversial. Immunostaining of fixed intestinal tissue suggested that CTR1 might be located at the apical membrane (76, 77). In contrast, surface biotinylation and functional studies of polarized intestinal cells provided evidence for active CTR1 being localized primarily at the basolateral membrane (110). Furthermore, the inactivation of CTR1 in enterocytes does not block Cu acquisition from the lumen of the gut via the apical membrane (76), but it renders Cu unavailable for further export by ATP7A, further complicating the phenotype.
Our recent studies revealed that the loss of CTR1 in enterocytes is also associated with the overexpression of ATP7B, which is likely responsible for sequestration of the excess Cu and, subsequently, the loss of Cu availability (84). In addition, we observed changes to the intracellular distribution of ATP7B in response to CTR1 knockout, indicative of pleiotropic effects of CTR1 inactivation. These results prompted us to independently examine the localization of CTR1 in the intestine using three distinct monoclonal antibodies. These antibodies consistently showed a basolateral pattern of CTR1 in the villi of the small intestine of 2-week-old healthy mouse pups (Figure 2b). Intestinal tissue from age-matched mice with selective deletion of CTR1 in enterocytes (Int-CTR1−/−) served as a control for staining specificity and showed little to no labeling in the bulk of the epithelial cell layer. The 2-week time point was selected because survival of the Int-CTR1−/− mice sharply declines after 2 weeks of age. Surprisingly, these experiments also revealed the presence of sparse CTR1-positive cells in the Int-CTR1−/− intestinal tissue, with a clear apical localization of the transporter. This result may explain the previously reported finding of CTR1 at the apical membrane (77). The Int-CTR1−/− mice were generated using the villin promoter; therefore, the epithelial cells retaining CTR1 expression at the apical membrane are likely villin negative, that is, not likely to be differentiated absorptive cells. The identity of these cells remains to be established. ATP7B expression is upregulated significantly in Int-CTR1−/− tissues, and the ATP7B vesicles are enlarged. Cells showing apical CTR1 staining also show large ATP7B-positive vesicles, similar to neighboring absorptive cells. Taken together, the data strongly support the important role of CTR1 in the proper development and function of the small intestine. However, the specific role of this important protein in enterocytes needs further study.
The interesting phenotype of Int-CTR1−/− mice (with accumulation of Cu in the intestine in an apparently sequestered form) suggests that Cu, which enters the enterocyte from the lumen of the gut, either is sequestered by upregulated ATP7B in vesicles or is present in the cytosol in a form that cannot be recognized by the Cu efflux transporter ATP7A. Currently, a cohesive explanation that accounts for all of the data is lacking, and many questions remain. If CTR1 transports Cu only from the bloodstream into enterocytes (as it does in other cell types), then which transporter moves dietary Cu into enterocytes from the lumen? In the absence of CTR1 why is Cu, which enters via the apical plasma membrane, not available for further processing by ATP7A? If CTR1 contributes to the entry of Cu via the apical membrane, how does the loss of CTR1 cause intestinal Cu overload? Our recent screen of HeLa cells for proteins affecting intracellular Cu levels (58) suggests that members of other transporter families contribute to the Cu balance in cells, but further experiments are needed to explore the role of these transporters in Cu uptake by the intestine. Studies in fish provide evidence for coregulation of DMT1, with several other proteins involved in regulating Cu balance in fish intestine (12). However, recent reports argue against the direct involvement of DMT1 in apical Cu uptake (72, 109) and suggest that anion transporters could be involved in the uptake of Cu from the lumen of the gut. The uptake of Cu through the apical membrane was unaffected by the removal of Na ions from the media, eliminating any significant involvement of Na-dependent uptake pathways. In contrast, apical Cu uptake is greatly inhibited following the replacement of chloride ions by sulfate or gluconate. Furthermore, inhibitors of the anion exchange transporters reduce Cu uptake (109). Clearly, more work needs to be done to delineate the Cu entry pathways in the small intestine.
3. ENTERIC ORGANOIDS: ENTEROIDS AND MINIGUTS
3.1. Introduction to 3D Intestinal Culture Systems
Studies aimed at understanding dietary nutrient absorption have generally relied on animal models and intestinal epithelial cell lines derived from colorectal tumors. Experiments based on animal models often depend on ex vivo approaches and are limited in their temporal and spatial resolution. There are also technical challenges. For example, analyzing protein distribution in the intestine can often be complicated by the presence of different tissue layers and mucus that cause nonspecific antibody staining and skew quantitation. Cancer-derived cell lines, such as Caco-2, are amenable to transfection and can be used for live-cell imaging and substrate transport assays. However, these cells express markers from both the colon and small bowel, and they often exhibit a mixed phenotype, which limits their biological relevance.
Mechanistic investigation into intestinal Cu homeostasis using animal experiments and Caco-2 models has produced interesting and novel data, but also lasting controversies (76, 110). The recently developed enteroid system (Figure 3) shows promise in resolving these ongoing debates (91). Specifically, enteroids represent not only a primary differentiated system but also fully polarized epithelial cultures that can be used in 3D or 2D formats. Enteroids are established by harvesting adult intestinal stem cells and cultivating them in a cocktail of carefully balanced growth factors (91). Enteroids have been established from tissue explants as well as small biopsy samples obtained during routine clinical procedures. This recently developed system has caused significant excitement due to its faithful expression of segment-specific intestinal markers, polarization, and longevity in culture (22, 55, 90). Enteroids closely mimic the native intestinal epithelium, and in the past 2–3 years, their utilization has grown exponentially. Because enteroids are maintained in culture, they allow precise control of Cu exposure, which is difficult in the animals or human participants from which they are established. Other studies that cannot be conducted in animals are also possible, including studies of epithelial development and single-cell RNA sequencing (29). Another exciting feature of enteroids is that they can be readily used in live-cell imaging experiments to assess processes in the gut in real time.
Figure 3.
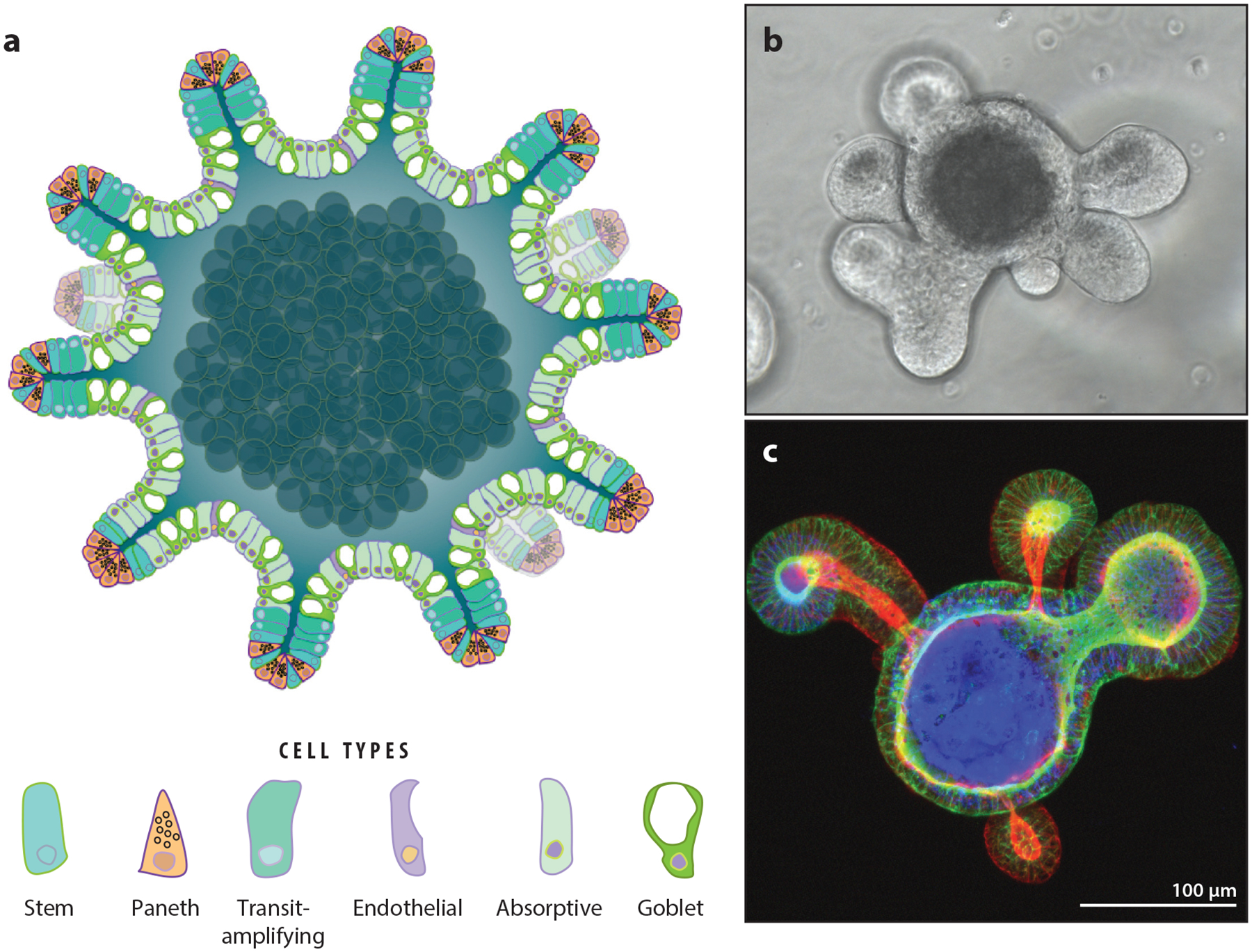
Three-dimensional (3D) enteroids: sophisticated model systems for investigating intestinal epithelium. (a) Schematic of an enteroid. Small crypt-like projections decorate the surface of the enteroid. The apical cell surface faces the lumen, analogous to the morphology of the digestive tract. Panel a adapted with permission from Reference 84. (b) Bright-field image of a single, live differentiated mouse enteroid in culture. (c) 3D projection of a single differentiated mouse enteroid with the apical membranes stained for F-actin demonstrates apical–inward polarity.
Enteroids can be cultured in two states: proliferative and differentiated. The proliferative state is obtained when the growth factors Wnt-3a and R-spondin are maintained at high levels in actively growing cultures. Terminal differentiation of the culture into mature intestinal epithelial cell types is achieved through extended culture in the absence of Wnt-3a and R-spondin. Differentiated enteroid cultures contain the major cell types found in mature epithelium (absorptive, goblet, endocrine, tuft, Paneth) in appropriate ratios (91). Initial studies indicate that proliferative cultures are rapidly dividing and provide a suitable model for the crypt base epithelium, whereas terminally differentiated cultures provide an excellent model system for studying processes occurring in the villi.
3.2. Advantages and Limitations of 3D Enteroids for Transport Studies
3D intestinal organoids and enteroids both provide several advantages over animal models and cancer cell lines. Intestinal organoids are derived from iPSCs and contain microtissue layers, including the epithelium, fibroblasts, and smooth muscle (96, 102). Enteroids are derived from adult intestinal stem cells that contain only epithelial cell types (91). There are benefits and drawbacks to each system. The intestinal organoids provide a model system that is much more organ-like, and they are arguably more akin to native tissue. However, the presence of multiple tissue layers imparts complications and challenges similar to those in animal or whole-tissue experiments. Additionally, because organoids are derived from iPSCs, they may harbor a mixed phenotype, expressing markers of multiple intestinal segments concurrently. Enteroids provide tissue culture only for the intestinal epithelium. However, these cultures faithfully recapitulate the expression and distribution of key markers of the specific parental tissue segment. They are also more amenable to transport measurements, especially in the 2D format (see below for details).
The multicellular nature of enteroid cell cultures provides many advantages. The presence of various cell types in one culture better replicates the properties of the native epithelial layer and allows the cultures to produce and respond to native enteroendocrine signals. These properties not only yield more accurate physiological responses to treatments but also provide avenues for studying cell–cell interactions between different cellular lineages. Epithelial enteroids are excellent for confocal microscopy–based studies. They can be imaged as live cultures using 3D imaging techniques similar to methods frequently used with classical tissue culture models, and they can also be treated like tissues and cryosectioned for fixed imaging approaches (84). Small-scale enteroid cultures are amenable to Western blot or quantitative polymerase chain reaction analyses. However, enteroid cultures provide limited material for biochemical applications and studies. Bulk culture can overcome some of these limitations, but large-scale cultures may be prohibitively expensive.
3D enteroids can be an excellent experimental system for transport assays involving basolateralto-apical membrane movement because the basolateral membrane faces the outside environment, or growth medium. Transport assay applications based on 3D enteroids can also be employed in clinical settings for testing drug toxicity and therapeutic potential. Epithelial enteroids derived from colorectal biopsies have been implemented in efficacy testing of therapeutic reagents for cystic fibrosis (CF) through the development of a swelling assay (16, 92). CF is caused by inactivating mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. Decreased activity of CFTR or the inability to reach the plasma membrane, or both, impairs water transport. Enteroids derived from CF patients with different CFTR mutations have been used as a litmus test for determining the therapeutic efficacy of specific drugs applied basolaterally. Therapeutic compounds that successfully activated the CFTR channel resulted in basolateral-to-apical transport of water (through CFTR), causing the enteroids to swell. The change in luminal volume of individual enteroids can be recorded over time, providing insights into the best therapeutic combination for a given CF patient: Higher levels of swelling in the enteroids translated to higher-efficacy drugs.
However, the architecture of 3D enteroids (with the apical surface oriented inward) effectively makes the apical side of the epithelium inaccessible to test reagents. This lack of access presents significant challenges to the utility of 3D enteroids. Three approaches have been developed to work around this challenge: (a) microinjection, (b) mechanical rupture, and (c) development of polarized monolayer enteroid cultures. Microinjection of reagents into the lumen of individual enteroids has been implemented (19); however, the approach may have limited utility. It is difficult to calculate the internal lumen volume to allow for accurate and consistent dosing of individual enteroids. Additionally, in many cases, a large set of individual enteroids would need to be injected to obtain statistically significant and reproducible results. High-throughput microinjection technologies are emerging but are not yet widely available (104). Microinjection could be a highly successful approach in imaging experiments in which smaller numbers of enteroids were analyzed individually.
Studies aimed at investigating the transport of fats in enteroid cultures circumvented the apical–inward problem by using a mechanical rupture technique in which enteroids were sheared with a pipette to generate fragments (39). The enteroid fragments were bathed in treatment solution, allowing access to both the basolateral and apical sides simultaneously. While the rupture approach does provide apical access and certainly has utility, it does not isolate the apical and basolateral sides for treatment and the stress effects caused by mechanical fracture are unknown, both of which may complicate data interpretation.
Finally, efforts have been made to develop and characterize fully differentiated and polarized monolayer enteroid cultures. Such cultures obviate many of the challenges involved in conducting apical-to-basolateral transport assays in the 3D culture system. To obtain 2D enteroids, the enteroids are first grown and maintained in 3D culture, then dispersed and seeded on Transwells (37, 38, 69, 75). The monolayer format and permeable support allow equal access to apical and basolateral cell surfaces for physiologically relevant treatments (similar to studies previously conducted in Caco-2 cells), while simultaneously imparting the benefits of primary tissue culture. Presently, monolayer technology is available for both human- and murine-derived cultures (48), yet careful evaluation of the differentiation status of murine monolayer enteroids and their full functionality remains to be done.
4. ENTEROIDS AS A MODEL FOR STUDIES OF NUTRITIONAL COPPER METABOLISM IN HEALTH AND DISEASE
4.1. Enterocyte-Specific Copper Acquisition and Utilization
Enterocytes have a unique dual role in Cu-dependent metabolic processes. They are responsible for acquiring Cu from dietary sources and exporting it to the circulation, that is, they act as a Cu conduit for the entire organism. At the same time, enterocytes require Cu for their own normal cellular functions, such as mitochondrial respiration and the detoxification of reactive oxygen species. It is not known whether Cu, which is required for normal cellular functions, is obtained via the basolateral membrane from circulation (as suggested by CTR1 localization; see Figure 2 and 110) or from dietary sources. Given that peritoneal Cu injections rescue the phenotype of Int-CTR1−/− mice, it is reasonable to speculate that Cu entering cells from circulation may provide enterocytes with Cu for housekeeping tasks. Further study in polarized enteroid models has the potential to directly address this gap in our understanding.
In addition to Cu requirements in the fundamental processes shared by many cells (such as mitochondrial respiration), enterocytes have specialized Cu-dependent processes. Hephaestin (HP) is a Cu-dependent ferroxidase specifically expressed in gut epithelium. HP facilitates iron efflux from the intestine into the bloodstream and requires Cu for this activity. Dietary Cu deficiency and, interestingly, inactivation of ATP7B are associated with iron accumulation in enterocytes (84). The specific role of ATP7B (and the vesicular Cu pool) in activating HP remains to be explored.
4.2. New Insights Into the Pathogenesis of Wilson Disease Yielded by Studies of the Intestine and Enteroids
Hepatic ATP7B is well characterized for its role as a central player in maintaining systemic Cu balance. In the liver, ATP7B functions to deliver Cu to the secretory pathway (to activate ceruloplasmin, which is a structural and functional homolog of HP) and to shuttle excess Cu from the liver into the bile for removal from circulation. WD is a disorder of systemic Cu misbalance that is caused by inactivating mutations of ATP7B. The clinical presentation of WD is highly variable, but it can be grouped into two major categories: neurological WD and hepatic WD. Within each subgroup there are a spectrum of presentations. The factors that tip the progression of this disease toward either hepatic or neurological manifestation are beginning to emerge (62).
Classically, WD is viewed as a hepatic or neurological disorder, which is reasonable given the clinical presentations. Since the liver is primarily thought to be responsible for maintaining systemic Cu balance, the trend in the field is to attribute most symptoms of WD to hepatic dysfunction. Recent studies suggest that the contribution of enteric ATP7B and the gastrointestinal tract to the pathobiology of WD might have been underappreciated. Our recent study on healthy control enteroids and enteroids from Atp7b−/− mice (WD animal model) have highlighted the important roles of ATP7B in the gastrointestinal tract (84). Analyses of ATP7B’s response to changing Cu levels, and Cu depletion in response to ATP7B inactivation, demonstrated that intestinal ATP7B acts in a different fashion than the well-characterized hepatic ATP7B. Importantly, the study defined some of the functional consequences of ATP7B inactivation in intestinal epithelium and enteroids. The loss of ATP7B activity has a direct impact on the processing of dietary fats and the synthesis of intestine-specific lipoproteins (84). This observation is significant because it identifies a previously unappreciated link between Cu homeostasis and the processing of dietary fat. Furthermore, one of the common features of WD is liver steatosis. The abnormal lipid homeostasis seen in WD patients has historically been attributed to Cu misbalance in the liver alone. The abnormal processing of dietary fat in the intestine of Atp7b−/− mice suggests that there is an intestinal contribution to the dysregulation of lipid balance seen in WD.
5. NEW CONNECTIONS BETWEEN COPPER AND FAT IN CELL BIOLOGY AND NUTRITION
Dietary Cu load appears to influence the lipid content of tissues, and both low and high Cu have been linked to changes in lipid metabolism and systemic fat deposition (10). Increased systemic fat accumulation has been observed in the context of Cu deficiency (70, 106). Conversely, dietary Cu overload appears to result in fat mobilization and the reduction of fat stores in tissues. The precise mechanistic link between Cu homeostasis and fat metabolism remains unclear, but mounting evidence points to a strong and important connection between these two distinct transport and metabolic networks. Using enteroids as a model system has the potential to change how we assess lipid processing in the digestive tract. At present, investigations involving enteroid models and lipid metabolic processes are limited. However, the characterization of dietary fat absorption along with the synthesis and export dynamics of lipoproteins in enteroid cultures (39) provide a platform for further investigations.
Feeding animals with physiologically relevant concentrations of Cu, which are above RDAs, alters lipid metabolism in several animal species. Dietary Cu supplementation in rabbits increases their body mass but decreases adipose tissue mass (53). Similarly, a Cu-supplemented diet (20 mg Cu/kg dry matter) fed to steers diminishes subcutaneous fat (20). Dietary Cu supplementation in sheep was shown to increase lipolysis in adipose tissue (93). The relationship between Cu and lipolysis was further investigated in 3T3-L1 cells, the in vitro model for white adipocytes. This study showed that Cu treatment decreased isoproterenol-stimulated lipolysis at the level of the second messenger, cyclic adenosine monophosphate (cAMP) (49). The Cu-dependent inhibition of cAMP-degrading phosphodiesterase PDE3B was proposed as a mechanism for reduced lipolysis in the context of Cu overload.
It is important to appreciate that the short-term effects of Cu misbalance seen in enteroids may not precisely replicate the consequences of long-term dietary Cu misbalance. In enteroids, Cu deficiency results in fat retention in enterocytes and impaired formation of chylomicrons. In rats, Cu deficiency also causes the accumulation of long-chain fatty acids in triglycerides in multiple tissues (1) and increased body weight and fat deposition (94). At the same time, serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels are significantly higher in the context of Cu deficiency (42). A Cu-deficient diet in combination with high-fat feeding increased lipid droplet volume and compromised the contractile function of the heart (45). Restricting dietary Cu in rats induces hepatic steatosis, inflammation, and fibrosis, leading to nonalcoholic fatty liver disease (NAFLD)-like symptoms (2, 94, 95, 97). Taken together, these findings suggest that insufficient Cu results in the improper allocation of fats to different tissues or the misuse of fats by those tissues, or both. Studies in enteroids may help to distinguish between the initial events associated with dietary fat processing and absorption and the subsequent metabolic events in the liver, and adipose and other tissues.
Recent data generated in animal studies also suggest that Cu deficiency may shift the utilization of metabolic fuels (33). In rats, Cu limitation decreases carbohydrate utilization in favor of fat, resulting in an unaltered total intake of metabolizable energy. In combination with a high-fructose diet, the physiological manifestations of Cu deficiency are more severe (8, 66) and may involve the inhibition of glycolysis. The role of Cu in lipogenesis and glycolysis is not fully understood. Recently, Cu deficiency was shown to be associated with enlarged cell size and elevated lipid content in 3T3-L1 adipocytes. Yang et al. (106) linked this phenomenon to the diminished activity of the Cu-dependent enzyme semicarbazide-sensitive amine oxidase (SSAO), which alters the uptake and utilization of glucose and fatty acids. Systemic Cu deficiency decreases SSAO activity (106), which may partly explain the metabolic misbalances seen in animals fed a Cu-deficient diet.
The incidence of metabolic diseases such as NAFLD, obesity, and diabetes has dramatically increased during the past decade. These disorders often manifest with systemic Cu misbalance (86). Low hepatic Cu was reported in NAFLD patients, and the extent of hepatic steatosis in these patients was inversely correlated with hepatic Cu loads (2). Ceruloplasmin is a Cu-dependent ferroxidase enzyme mainly secreted by the liver, as well as being the major Cu-binding protein in serum. Consistent with the hepatic Cu levels found in NAFLD, serum Cu levels and ceruloplasmin levels are also lower in these patients (3, 74). Mouse models of diet-induced NAFLD (or ob/ob mice) also show hepatic Cu deficiency (14, 31). In this NAFLD model, high fat increased the expression of the Cu-transporting ATPases, ATP7A and ATP7B (31). These results once again demonstrate the link between Cu and fat metabolism and suggest that enhanced Cu export from the liver may represent a response to fat overload. How dietary fat affects Cu uptake and transport in the intestine remains to be investigated.
A recent study demonstrated that in the context of obesity, Cu levels positively correlate with body mass index (21). Unlike in NAFLD, serum Cu levels are elevated in obese patients (83, 107). However, in ob/ob mice, liver Cu levels are lower, while serum Cu levels are elevated (54). As discussed above, Cu deficiency can induce fat accumulation in hepatocytes and adipocytes. Conversely, fat deposition and expansion appear to result in elevated serum Cu levels. Along with serum Cu levels, the main Cu-binding enzymes in the blood, ceruloplasmin and SSAO, are also increased in obese individuals (87). Finally, patients who have undergone bariatric surgical procedures have an increased risk of developing Cu deficiency (28, 79, 82) due to the marked decrease of dietary Cu absorption. Organoids derived from various tissues not only help us to gain a better mechanistic understanding of Cu absorption and processing, but may also highlight both the common and the tissue-specific links between Cu and fat metabolism.
6. OTHER ORGANOID MODELS EMERGING AS TOOLS IN COPPER BIOLOGY
The hepatic and neurological concerns involved in WD and other disorders of Cu homeostasis have made brain- and liver-derived organoid technology highly desirable. First efforts to establish these model systems have been published (35, 36). Hepatic organoids are not as well characterized, nor as widely utilized, as enteric organoids and enteroids. Methods are available to generate hepatic organoids from both tissue explants and surgical biopsy samples. The resulting organoids are stable in long-term culture conditions and adopt a spheroid morphology (35, 36). Established proliferative cultures are composed of bile duct cholangiocytes, but specific additives are required to prevent a drift in cell identity over time in culture (36, 73). The differentiation protocols available for hepatic organoid cultures result in a hybrid phenotype. Differentiation appears to tip cell identity closer toward hepatocytes, but it should be noted that differentiation appears to be incomplete; the culture retains markers of both cholangiocytes and mature hepatocytes (73). Nevertheless, the potential of hepatic organoid technology to address liver disease has been investigated in a canine model of pathogenic Cu overload (73). Dogs with inactivating mutations in the copper metabolism domain containing 1 (COMMD1) gene accumulate Cu in the liver and display many of the hallmarks of WD. Liver organoids grown from COMMD1−/− animals have been transfected with functional recombinant COMMD1 using lentivirus, resulting in the rescue of the culture’s ability to survive in high concentrations of Cu. The authors posit that this work constitutes the first step in correcting genetic errors in patients’ livers, with the hope that the cultivation of large-volume organoid culture may permit the transplantation of the genetically edited biomass and, therefore, facilitate correction of the disease (73).
The contribution of Cu to neurological development and function is well established. However, there is little information about how Cu functions mechanistically in central nervous system–specific processes. Culture methods are available to produce cerebral organoids (also known as brain balls) that contain cell types mimicking multiple brain regions (50). To the best of our knowledge, the neurobiology of classical Cu-based disorders has not yet been explored in brain-derived organoids. Cerebral organoids have been assessed for Cu content using X-ray fluorescence microscopy techniques, which revealed that Cu was low in the regions examined (88). Further characterization and implementation of cerebral organoids may yield a promising experimental platform for understanding neuronal degeneration caused by Cu misbalance.
Cu transport in the mammary gland is a critical component of nutrition for neonates. Breast milk supplies the infant with the nutritional Cu required for rapid cellular division, growth, and neurological development. The rate of Cu absorption in lactating mammary tissue (17) as well as the levels of Cu in breast milk are tightly regulated (26, 43). In mammary tissue, ATP7B functions to secrete Cu into the breast milk (65). In WD, breast milk Cu concentrations are reduced drastically by the systemic Cu misbalance and mammary-specific deactivation of ATP7B. Murine models of WD are characterized by a failure to deliver Cu into milk, causing a toxic-milk phenotype and pup death (98). Mammary organoid models have been developed and are a well-established model for milk-producing tissue (18). One study has linked posttranslational regulation (detailed mechanism unknown) of CTR1 levels in mammary organoids to basolateral Cu uptake (24). Enhanced levels of CTR1 in the basolateral membrane of mammary cells led to Cu accumulation, presumably to supply Cu for export into milk. Further investigation in mammary organoids also revealed that the intracellular Cu concentration regulates the secretion of ceruloplasmin into breast milk (25).
7. CONCLUSIONS
The rapid development of organoid culture systems is providing better and better models for physiologically relevant studies on a wide array of tissues. In the short span of 10 years since their discovery and creation, organoids have already become a highly useful technology. Enteroid cultures provide an exciting addition to the nutritional biology tool kit. From a basic research perspective, 3D enteroids have a strong potential for use in imaging-based assessments of protein distribution and trafficking, biochemical investigations into genetic mutations affecting digestive health, and tracking physiological processes in real time. The recent development of 2D monolayer enteroid technology shows exciting promise in the arena of transport assays. Monolayer enteroid cultures offer isolated access to both the apical and basolateral side of primary mixed-population intestinal epithelium. Monolayers provide a useful platform for studies on the transport of individual nutrients and compounds across absorptive epithelium. The opportunity to assess the co-transport processes between dietary Cu and nutritional fats will help to identify the mechanistic link between these disparate pathways. The utility of organoids in diagnostic and personalized medicine is also changing the way we approach drug screening both in the clinic and in early-phase pharmaceutical trials. With the continued fast-paced development of organoid technologies, the present limitations will certainly be overcome.
ACKNOWLEDGMENTS
This work was supported by a US National Institute of Health grant to S.L. (R01DK071865) and a fellowship to H.P. from the Hopkins Conte Digestive Diseases Basic and Translational Research Core Center (2T32DK007632-26).
Glossary
- Cu
copper
- RDA
Recommended Dietary Allowance
- WD
Wilson disease
- iPSCs
induced pluripotent stem cells
- Int-CTR1−/−
enterocyte-specific deletion of CTR1
- DMT1
divalent metal transporter 1
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- cAMP
cyclic adenosine monophosphate
- NAFLD
nonalcoholic fatty liver disease
- SSAO
semicarbazide-sensitive amine oxidase
- COMMD1
copper metabolism domain containing 1
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abdel-Mageed AB, Oehme FW. 1990. A review of the biochemical roles, toxicity and interactions of zinc, copper and iron: III. Iron. Vet. Hum. Toxicol 32:324–28 [PubMed] [Google Scholar]
- 2.Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, et al. 2010. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am. J. Gastroenterol 105:1978–85 [DOI] [PubMed] [Google Scholar]
- 3.Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, et al. 2008. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology 135:680–88 [DOI] [PubMed] [Google Scholar]
- 4.Arredondo M, Uauy R, Gonzalez M. 2000. Regulation of copper uptake and transport in intestinal cell monolayers by acute and chronic copper exposure. Biochim. Biophys. Acta Gen. Subj 1474:169–76 [DOI] [PubMed] [Google Scholar]
- 5.Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BL. 2017. Lung organoids: current uses and future promise. Development 144:986–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, et al. 2010. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6:25–36 [DOI] [PubMed] [Google Scholar]
- 7.Bauerly KA, Kelleher SL, Lönnerdal B. 2005. Effects of copper supplementation on copper absorption, tissue distribution, and copper transporter expression in an infant rat model. Am. J. Physiol. Gastrointest. Liver Physiol 288:G1007–14 [DOI] [PubMed] [Google Scholar]
- 8.Bellomo G, Comstock JP, Wen D, Hazelwood RL. 1987. Prolonged fructose feeding and aldose reductase inhibition: effect on the polyol pathway in kidneys of normal rats. Proc. Soc. Exp. Biol. Med 186:348–54 [DOI] [PubMed] [Google Scholar]
- 9.Bertinato J, Iskandar M, L’Abbe MR. 2003. Copper deficiency induces the upregulation of the copper chaperone for Cu/Zn superoxide dismutase in weanling male rats. J. Nutr 133:28–31 [DOI] [PubMed] [Google Scholar]
- 10.Burkhead JL, Lutsenko S. 2013. The role of copper as a modifier of lipid metabolism In Lipid Metabolism, ed. RV Baez, pp. 39–60. London: InTech Open [Google Scholar]
- 11.Chambers A, Krewski D, Birkett N, Plunkett L, Hertzberg R, et al. 2010. An exposure–response curve for copper excess and deficiency. J. Toxicol. Environ. Health B Crit. Rev 13:546–78 [DOI] [PubMed] [Google Scholar]
- 12.Cheng J, Luo Z, Chen GH, Wei CC, Zhuo MQ. 2017. Identification of eight copper (Cu) uptake related genes from yellow catfish Pelteobagrus fulvidraco, and their tissue expression and transcriptional responses to dietborne Cu exposure. J. Trace Elem. Med. Biol 44:256–65 [DOI] [PubMed] [Google Scholar]
- 13.Chun H, Catterton T, Kim H, Lee J, Kim BE. 2017. Organ-specific regulation of ATP7A abundance is coordinated with systemic copper homeostasis. Sci. Rep 7:12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church SJ, Begley P, Kureishy N, McHarg S, Bishop PN, et al. 2015. Deficient copper concentrations in dried-defatted hepatic tissue from ob/ob mice: a potential model for study of defective copper regulation in metabolic liver disease. Biochem. Biophys. Res. Commun 460:549–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crampton RF, Matthews DM, Poisner R. 1965. Observations on the mechanism of absorption of copper by the small intestine. J. Physiol 178:111–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, et al. 2016. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med 8:344ra384. [DOI] [PubMed] [Google Scholar]
- 17.Donley SA, Ilagan BJ, Rim H, Linder MC. 2002. Copper transport to mammary gland and milk during lactation in rats. Am. J. Physiol. Endocrinol. Metab 283:E667–675 [DOI] [PubMed] [Google Scholar]
- 18.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, et al. 2003. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17:1253–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta D, Heo I, Clevers H. 2017. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med 23:393–410 [DOI] [PubMed] [Google Scholar]
- 20.Engle TE, Spears JW. 2000. Dietary copper effects on lipid metabolism, performance, and ruminal fermentation in finishing steers. J. Anim. Sci 78:2452–58 [DOI] [PubMed] [Google Scholar]
- 21.Fan Y, Zhang C, Bu J. 2017. Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S. Nutrients 9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Ettayebi K, et al. 2014. Human enteroids as an ex-vivo model of host–pathogen interactions in the gastrointestinal tract. Exp. Biol. Med 239:1124–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, et al. 2016. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150:638–49.e638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freestone D, Cater MA, Ackland ML, Paterson D, Howard DL, et al. 2014. Copper and lactational hormones influence the CTR1 copper transporter in PMC42-LA mammary epithelial cell culture models. J. Nutr. Biochem 25:377–87 [DOI] [PubMed] [Google Scholar]
- 25.Freestone D, Denoyer D, Jakab M, Leigh Ackland M, Cater MA, Michalczyk A. 2016. Ceruloplasmin is regulated by copper and lactational hormones in PMC42-LA mammary epithelial cell culture models. Metallomics 8:941–50 [DOI] [PubMed] [Google Scholar]
- 26.Friel JK, Andrews WL, Jackson SE, Longerich HP, Mercer C, et al. 1999. Elemental composition of human milk from mothers of premature and full-term infants during the first 3 months of lactation. Biol. Trace Elem. Res 67:225–47 [DOI] [PubMed] [Google Scholar]
- 27.Greggio C, De Franceschi F, Figueiredo-Larsen M, Grapin-Botton A. 2014. In vitro pancreas organo-genesis from dispersed mouse embryonic progenitors. J. Vis. Exp 89:51725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith DP, Liff DA, Ziegler TR, Esper GJ, Winton EF. 2009. Acquired copper deficiency: a potentially serious and preventable complication following gastric bypass surgery. Obesity 17:827–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grun D, Lyubimova A, Kester L, Wiebrands K, Basak O, et al. 2015. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525:251–55 [DOI] [PubMed] [Google Scholar]
- 30.Hamza I, Prohaska J, Gitlin JD. 2003. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. PNAS 100:1215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffern MC, Park HM, Au-Yeung HY, Van de Bittner GC, Ackerman CM, et al. 2016. In vivo bioluminescence imaging reveals copper deficiency in a murine model of nonalcoholic fatty liver disease. PNAS 113:14219–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Held M, Santeramo I, Wilm B, Murray P, Levy R. 2018. Ex vivo live cell tracking in kidney organoids using light sheet fluorescence microscopy. PLOS ONE 13:e0199918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoogeveen RC, Reaves SK, Reid PM, Reid BL, Lei KY. 1994. Copper deficiency shifts energy substrate utilization from carbohydrate to fat and reduces fat mass in rats. J. Nutr 124:1660–66 [DOI] [PubMed] [Google Scholar]
- 34.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, et al. 2013. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32:2708–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, et al. 2013. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494:247–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, et al. 2015. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160:299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.In J, Foulke-Abel J, Zachos NC, Hansen AM, Kaper JB, et al. 2016. Enterohemorrhagic Escherichia coli reduces mucus and intermicrovillar bridges in human stem cell–derived colonoids. Cell. Mol. Gastroenterol. Hepatol 2:48–62.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, et al. 2014. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLOS ONE 9:e107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jattan J, Rodia C, Li D, Diakhate A, Dong H, et al. 2017. Using primary murine intestinal enteroids to study dietary TAG absorption, lipoprotein synthesis, and the role of apoC-III in the intestine. J. Lipid Res 58:853–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, et al. 2014. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159:163–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasagi Y, Chandramouleeswaran PM, Whelan KA, Tanaka K, Giroux V, et al. 2018. The esophageal organoid system reveals functional interplay between Notch and cytokines in reactive epithelial changes. Cell. Mol. Gastroenterol. Hepatol 5:333–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaya A, Altiner A, Ozpinar A. 2006. Effect of copper deficiency on blood lipid profile and haematological parameters in broilers. J. Vet. Med. A Physiol. Pathol. Clin. Med 53:399–404 [DOI] [PubMed] [Google Scholar]
- 43.Kelleher SL, Lönnerdal B. 2006. Mammary gland copper transport is stimulated by prolactin through alterations in Ctr1 and Atp7A localization. Am. J. Physiol. Regul. Integr. Comp. Physiol 291:R1181–91 [DOI] [PubMed] [Google Scholar]
- 44.Kelly EJ, Palmiter RD. 1996. A murine model of Menkes disease reveals a physiological function of metallothionein. Nat. Genet 13:219–22 [DOI] [PubMed] [Google Scholar]
- 45.Klevay LM. 2000. Cardiovascular disease from copper deficiency—a history. J. Nutr 130:489S–92S [DOI] [PubMed] [Google Scholar]
- 46.Kodama H, Murata Y, Kobayashi M. 1999. Clinical manifestations and treatment of Menkes disease and its variants. Pediatr. Int 41:423–29 [DOI] [PubMed] [Google Scholar]
- 47.Koo BK, Sasselli V, Clevers H. 2013. Retroviral gene expression control in primary organoid cultures. Curr. Protoc. Stem Cell Biol 27:5A.6.1–5A.6.8 [DOI] [PubMed] [Google Scholar]
- 48.Kozuka K, He Y, Koo-McCoy S, Kumaraswamy P, Nie B, et al. 2017. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep. 9:1976–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnamoorthy L, Cotruvo JA Jr., Chan J, Kaluarachchi H, Muchenditsi A, et al. 2016. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol 12:586–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, et al. 2013. Cerebral organoids model human brain development and microcephaly. Nature 501:373–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laperrousaz B, Porte S, Gerbaud S, Harma V, Kermarrec F, et al. 2018. Direct transfection of clonal organoids in Matrigel microbeads: a promising approach toward organoid-based genetic screens. Nucleic Acids Res. 46:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Pena MM, Nose Y, Thiele DJ. 2002. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem 277:4380–87 [DOI] [PubMed] [Google Scholar]
- 53.Lei L, Xiaoyi S, Fuchang L. 2017. Effect of dietary copper addition on lipid metabolism in rabbits. Food Nutr. Res 61:1348866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin WH, Chen MD, Wang CC, Lin PY. 1995. Dietary copper supplementation increases the catecholamine levels in genetically obese (ob/ob) mice. Biol. Trace Elem. Res 50:243–47 [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Walker NM, Cook MT, Ootani A, Clarke LL. 2012. Functional Cftr in crypt epithelium of organ-otypic enteroid cultures from murine small intestine. Am. J. Physiol. Cell Physiol 302:C1492–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lönnerdal B. 2008. Intestinal regulation of copper homeostasis: a developmental perspective. Am. J. Clin. Nutr 88:846S–50S [DOI] [PubMed] [Google Scholar]
- 57.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. 2007. Function and regulation of human copper-transporting ATPases. Physiol. Rev 87:1011–46 [DOI] [PubMed] [Google Scholar]
- 58.Malinouski M, Hasan NM, Zhang Y, Seravalli J, Lin J, et al. 2014. Genome-wide RNAi ionomics screen reveals new genes and regulation of human trace element metabolism. Nat. Commun 5:3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, et al. 2012. Modeling human cortical development in vitro using induced pluripotent stem cells. PNAS 109:12770–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matano M, Date S, Shimokawa M, Takano A, Fujii M, et al. 2015. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat. Med 21:256–62 [DOI] [PubMed] [Google Scholar]
- 61.McCracken KW, Howell JC, Wells JM, Spence JR. 2011. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc 6:1920–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medici V, Weiss KH. 2017. Genetic and environmental modifiers of Wilson disease. Handb. Clin. Neurol 142:35–41 [DOI] [PubMed] [Google Scholar]
- 63.Menkes JH. 1972. Kinky hair disease. Pediatrics 50:181–83 [PubMed] [Google Scholar]
- 64.Menkes JH, Alter M, Steigleder GK, Weakley DR, Sung JH. 1962. A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics 29:764–79 [PubMed] [Google Scholar]
- 65.Michalczyk A, Bastow E, Greenough M, Camakaris J, Freestone D, et al. 2008. ATP7B expression in human breast epithelial cells is mediated by lactational hormones. J. Histochem. Cytochem 56:389–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millo H, Werman MJ. 2000. Hepatic fructose-metabolizing enzymes and related metabolites: role of dietary copper and gender. J. Nutr. Biochem 11:374–81 [DOI] [PubMed] [Google Scholar]
- 67.Miyayama T, Ogra Y, Osima Y, Suzuki KT. 2008. Narrow-bore HPLC–ICP–MS for speciation of copper in mutant mouse neonates bearing a defect in Cu metabolism. Anal. Bioanal. Chem 390:1799–803 [DOI] [PubMed] [Google Scholar]
- 68.Monty JF, Llanos RM, Mercer JF, Kramer DR. 2005. Copper exposure induces trafficking of the Menkes protein in intestinal epithelium of ATP7A transgenic mice. J. Nutr 135:2762–66 [DOI] [PubMed] [Google Scholar]
- 69.Moon C, VanDussen KL, Miyoshi H, Stappenbeck TS. 2014. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 7:818–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morrell A, Tallino S, Yu L, Burkhead JL. 2017. The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life 69:263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nadella SR, Grosell M, Wood CM. 2007. Mechanisms of dietary Cu uptake in freshwater rainbow trout: evidence for Na-assisted Cu transport and a specific metal carrier in the intestine. J. Comp. Physiol. B 177:433–46 [DOI] [PubMed] [Google Scholar]
- 72.Nadella SR, Hung CC, Wood CM. 2011. Mechanistic characterization of gastric copper transport in rainbow trout. J. Comp. Physiol. B 181:27–41 [DOI] [PubMed] [Google Scholar]
- 73.Nantasanti S, Spee B, Kruitwagen HS, Chen C, Geijsen N, et al. 2015. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Rep. 5:895–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nobili V, Siotto M, Bedogni G, Rava L, Pietrobattista A, et al. 2013. Levels of serum ceruloplasmin associate with pediatric nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr 56:370–75 [DOI] [PubMed] [Google Scholar]
- 75.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, et al. 2017. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host–pathogen interactions. Sci. Rep 7:45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nose Y, Kim BE, Thiele DJ. 2006. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 4:235–44 [DOI] [PubMed] [Google Scholar]
- 77.Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, et al. 2010. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Biol. Chem 285:32385–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. 2007. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am. J. Physiol. Gastrointest. Liver Physiol 292:G1181–94 [DOI] [PubMed] [Google Scholar]
- 79.O’Donnell KB, Simmons M. 2011. Early-onset copper deficiency following Roux-en-Y gastric bypass. Nutr. Clin. Pract 26:66–69 [DOI] [PubMed] [Google Scholar]
- 80.Ohgami RS, Campagna DR, McDonald A, Fleming MD. 2006. The Steap proteins are metalloreductases. Blood 108:1388–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okkelman IA, Foley T, Papkovsky DB, Dmitriev RI. 2017. Live cell imaging of mouse intestinal organoids reveals heterogeneity in their oxygenation. Biomaterials 146:86–96 [DOI] [PubMed] [Google Scholar]
- 82.Papamargaritis D, Aasheim ET, Sampson B, le Roux CW. 2015. Copper, selenium and zinc levels after bariatric surgery in patients recommended to take multivitamin–mineral supplementation. J. Trace Elem. Med. Biol 31:167–72 [DOI] [PubMed] [Google Scholar]
- 83.Perrone L, Gialanella G, Moro R, Feng SL, Boccia E, et al. 1998. Zinc, copper, and iron in obese children and adolescents. Nutr. Res 18:183–89 [Google Scholar]
- 84.Pierson H, Muchenditsi A, Kim BE, Ralle M, Zachos N, et al. 2018. The function of ATPase copper transporter ATP7B in intestine. Gastroenterology 154:168–80.e165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ravia JJ, Stephen RM, Ghishan FK, Collins JF. 2005. Menkes copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J. Biol. Chem 280:36221–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruhl CE, Everhart JE. 2015. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther 41:65–76 [DOI] [PubMed] [Google Scholar]
- 87.Safavi SM, Ziaei R, Maracy MR. 2012. Association of serum ceruloplasmin level with obesity: some components of metabolic syndrome and high-sensitive C-reactive protein in Iran. J. Obes 2012:951093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sartore RC, Cardoso SC, Lages YV, Paraguassu JM, Stelling MP, et al. 2017. Trace elements during primordial plexiform network formation in human cerebral organoids. PeerJ 5:e2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sato T, Clevers H. 2013. Primary mouse small intestinal epithelial cell cultures. Methods Mol. Biol 945:319–28 [DOI] [PubMed] [Google Scholar]
- 90.Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, et al. 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–72 [DOI] [PubMed] [Google Scholar]
- 91.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, et al. 2009. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459:262–65 [DOI] [PubMed] [Google Scholar]
- 92.Sinha G. 2017. The organoid architect. Science 357:746–49 [DOI] [PubMed] [Google Scholar]
- 93.Sinnett-Smith PA, Woolliams JA. 1987. Antilipogenic but not lipolytic effects of recombinant DNA-derived bovine somatotropin treatment on ovine adipose tissue: variation with genetic type. Int. J. Biochem 21:535–40 [DOI] [PubMed] [Google Scholar]
- 94.Song M, Schuschke DA, Zhou Z, Chen T, Pierce WM Jr., et al. 2012. High fructose feeding induces copper deficiency in Sprague-Dawley rats: a novel mechanism for obesity related fatty liver. J. Hepatol 56:433–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song M, Schuschke DA, Zhou Z, Chen T, Shi X, et al. 2013. Modest fructose beverage intake causes liver injury and fat accumulation in marginal copper deficient rats. Obesity 21:1669–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, et al. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tallino S, Duffy M, Ralle M, Cortes MP, Latorre M, Burkhead JL. 2015. Nutrigenomics analysis reveals that copper deficiency and dietary sucrose up-regulate inflammation, fibrosis and lipogenic pathways in a mature rat model of nonalcoholic fatty liver disease. J. Nutr. Biochem 26:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Theophilos MB, Cox DW, Mercer JF. 1996. The toxic milk mouse is a murine model of Wilson disease. Hum. Mol. Genet 5:1619–24 [DOI] [PubMed] [Google Scholar]
- 99.Trumbo P, Yates AA, Schlicker S, Poos M. 2001. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc 101:294–301 [DOI] [PubMed] [Google Scholar]
- 100.Ussing HH, Zerahn K. 1951. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand 23:110–27 [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Zhu S, Hodgkinson V, Prohaska JR, Weisman GA, et al. 2012. Maternofetal and neonatal copper requirements revealed by enterocyte-specific deletion of the Menkes disease protein. Am. J. Physiol. Gastrointest. Liver Physiol 303:G1236–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, et al. 2014. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med 20:1310–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weiss KH, Wurz J, Gotthardt D, Merle U, Stremmel W, Fullekrug J. 2008. Localization of the Wilson disease protein in murine intestine. J. Anat 213:232–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williamson IA, Arnold JW, Samsa LA, Gaynor L, DiSalvo M, et al. 2018. A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell. Mol. Gastroenterol. Hepatol 6:301–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson SAK. 1912. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain 34:295–507 [DOI] [PubMed] [Google Scholar]
- 106.Yang H, Ralle M, Wolfgang MJ, Dhawan N, Burkhead JL, et al. 2018. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLOS Biol. 16:e2006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yerlikaya FH, Toker A, Aribas A. 2013. Serum trace elements in obese women with or without diabetes. Indian J. Med. Res 137:339–45 [PMC free article] [PubMed] [Google Scholar]
- 108.Zerounian NR, Redekosky C, Malpe R, Linder MC. 2003. Regulation of copper absorption by copper availability in the Caco-2 cell intestinal model. Am. J. Physiol. Gastrointest. Liver Physiol 284:G739–47 [DOI] [PubMed] [Google Scholar]
- 109.Zimnicka AM, Ivy K, Kaplan JH. 2011. Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am. J. Physiol. Cell Physiol 300:C588–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zimnicka AM, Maryon EB, Kaplan JH. 2007. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J. Biol. Chem 282:26471–80 [DOI] [PubMed] [Google Scholar]


