Abstract
Enthesitis is a common clinical feature of PsA, which is characterized by inflammation at the site of insertion of tendons, ligaments and joint capsule fibres into bone. Enthesitis is relatively unique to the spondyloarthritides, setting this group of diseases apart from other rheumatological conditions. The pathophysiological underpinnings of this clinical domain, and the imaging assessment of it, are described in accompanying articles in this supplement. The focus of this article is on the assessment of enthesitis by physical examination, the impact of enthesitis on function and quality of life, the impact of concomitant FM on clinical assessment, and the evidence for therapy of enthesitis garnered in trials of biologic and targeted synthetic DMARDs. Several physical examination measures of enthesitis have been developed and have proved reliable in assessment of enthesitis. Enthesitis has a significant deleterious impact on function and quality of life. The presence of concomitant FM in ≤20% of patients may result in artefactual worsening of assessment of disease severity and hinder achievement of the goal of low disease activity or remission. Several targeted therapies, which, for example, target the TNF, IL-17, IL-23, phosphodiesterase 4 or Janus kinase pathways, have shown significant efficacy in the treatment of enthesitis, resulting in improvement of function and quality of life for patients with PsA.
Keywords: psoriatic arthritis, enthesitis, enthesitis clinical assessment, enthesitis impact, treatment of enthesitis
Rheumatology key messages
Enthesitis is a hallmark of PsA with significant impact on function and quality of life.
Enthesitis is now assessed routinely in clinical trials to determine efficacy of treatment.
Current and emerging therapies for PsA demonstrate significant efficacy in the treatment of enthesitis.
Introduction
This article reviews the physical examination measures that have been developed to assess enthesial tenderness, the impact of enthesitis as clinically measured in long-term observational studies and registries, and the effect of current and emerging therapies on enthesitis as assessed in clinical trials.
An important caveat is that the physical examination measures used in studies and in practice assess patient-reported tenderness, which may represent true inflammation at the enthesial site (i.e. enthesitis), but, as will be discussed, may instead, or at least in part, reflect the phenomenon of centralized pain, also known by terms or phrases such as FM, chronic widespread pain (CWP) or central sensitization syndrome. The fact that an -itis may not always be present in a tender area is borne out, in some studies, by lack of correlation with US or MRI evidence of inflammation. Despite this potential imprecision of assessment, the clinical indices have performed fairly well in their ability to discriminate between active treatment and placebo in clinical trials, as will be demonstrated.
Several current and emerging therapies are proving to be highly effective in the treatment of enthesitis. However, it is important to note that not all treatments have across-the-board effectiveness in the different clinical domains of PsA (arthritis/synovitis, enthesitis, dactylitis, spondylitis, psoriasis skin and nail disease) and, within a single patient, responsiveness of the different domains may differ in both magnitude and time of response. Thus, it is important to assess each domain individually, both initially and in follow-up visits, to determine quality of treatment. I liken this to a symphony orchestra concert, in which different instruments and sections may be playing together in concert or as individual solos at different times, thus emphasizing the need to listen to the patient, not only about what they are reporting, but what you as a clinician are picking up on examination and through imaging and laboratory evaluation. Given that enthesitis can cause such significant pain, physical dysfunction and reduced quality of life, it is important to work towards a treatment regimen that can achieve low disease activity or remission in this domain. Even if, for example, arthritis and skin disease are well controlled with a given treatment, if significant enthesitis, documented to be caused by persistent inflammation, is stubbornly resistant to that medication, and physical therapy, injection and surgical approaches are not helpful or not indicated, then trial of an alternative medication may need to be pursued.
Enthesitis physical examination indices
The first physical examination approach to enthesitis, using a standardized index, was the Mander index [1]. This was developed by Mander, a physiatrist in Newcastle, UK. The Mander index required palpation of 66 enthesial insertion sites. Although comprehensive in its scope, it proved impractical for use in clinical trials or practice because of the large number of sites to be assessed, paucity of routine use by investigators and other clinicians, and the consequent potential difficulty of achieving good intra- and inter-rater reliability. However, it did provide a map for subsequent development of simpler clinical indices now used in clinical trials.
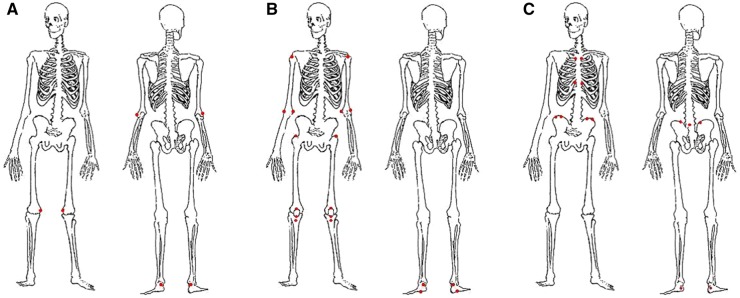
Three enthesitis physical examination indices have been developed and used in PsA clinical trials and in trials in axial spondyloarthritis (AxSpA), including both radiographic AxSpA (r-AxSpA), also known as AS, and non-radiographic AxSpA (nr-AxSpA) (Fig. 1). With each of the indices, it is recommended that the examiner use a standardized assessment method for each entheseal site (e.g. use of the thumb or index finger, applying enough pressure to blanch the finger about a fifth of the way from the tip of the fingernail).
Fig. 1.
Three enthesitis indices used in clinical trials
(A) The Leeds enthesitis index (LEI): six sites. (B) The Spondyloarthritis Research Consortium of Canada (SPARCC) index: 18 sites, with a score of 16. (C) The Maastricht ankylosing spondylitis enthesitis score (MASES): 13 sites.
The Leeds enthesitis index (LEI) was developed by Philip Helliwell and others in patients with PsA [1, 2]. The LEI consists of six sites: bilateral lateral epicondyles, medial femoral condyles, and Achilles tendon insertion sites. The LEI is simple to use, because only six sites are assessed, but in some studies it has lacked discriminative power because of the small number of sites assessed.
The Spondyloarthritis Research Consortium of Canada (SPARCC) index, developed in Canada by Walter Maksymowych and others, was developed in patients with spondyloarthritis and includes assessment of 18 sites for tenderness [1, 3]. The total score is 16, because the distal patella and tibial tuberosity, in close proximity, are considered as one site. Although only more recently used in PsA trials, this index has demonstrated consistent ability to discriminate between treatment and placebo. Recently, studies have used SPARCC plus one (i.e. having the investigator assess each of the SPARCC entheseal sites in addition to the medial condyle of the femur), which then allows calculation of both the SPARCC and Leeds indices, because the other four sites of the LEI are included in the SPARCC. This allows for greater comparability between studies.
The Maastricht ankylosing spondylitis enthesitis score (MASES), developed in patients with AS, has been used in AS and AxSpA consistently, with good performance [1, 4]. It has also been used in some PsA studies and has demonstrated the ability to discriminate treatment from placebo in most studies, despite its relative lack of peripheral entheseal sites, but not in all studies; therefore, it is no longer being used in PsA studies. Its performance in studies enriched for patients with PsA spondylitis has not yet been assessed.
Effect of central sensitization on assessment of enthesitis
Physical examination detects tenderness. Physical examination does not discriminate between the true presence of inflammation (i.e. enthesitis) vs tenderness attributable to other factors, including what we label FM/CWP/central sensitization, which are conditions with overlapping definitions and features. These are relatively common chronic central pain syndromes, which arise in individuals with genetic, biological and psychosocial predisposing factors and are characterized by CWP, often accompanied by fatigue, sleep disturbance and other symptoms. These conditions occur more commonly in patients with chronic pain and inflammatory conditions. We might use the term enthesalgia to describe this phenomenon when it influences tenderness at entheseal insertion points. It is possible that in some individuals, tenderness is attributable only to -itis, in others only to -algia and in others, a combination of the two.
The phenomenon of coexistent central pain syndromes accompanying chronic rheumatic diseases has become an item of research and clinical importance because of its influence on disease severity measures and determination of treatment response in clinical trials and in practice. Numerous studies of cohorts of patients with various rheumatological conditions, including RA, SLE, SS, OA, PsA and AS, have demonstrated that 15–20% of these cohorts, on average, will have a concomitant diagnosis of FM based on various classification criteria [5]. Brikman et al. [6] noted, in a Tel Aviv cohort of PsA patients, that concomitant FM was present in 18% and that all of the disease severity measures that included a subjective element reported by the patient, such as pain or patient global, such as Disease Activity in PsA (DAPSA), minimal disease activity (MDA), HAQ and LEI, were twice as severe as the same measures in patients without concomitant FM. Højsgaard et al. [7] studied 69 PsA patients initiating treatment with physical and US examination of joints and entheses and also performed measures for FM/CWP, such as the widespread pain index (WPI) and PainDetect questionnaire. Responses consistent with FM/CWP on the WPI were seen in 35%. These patients were not able to achieve a state of MDA, and there was little correlation between examination of joints and entheses and US findings. These findings emphasize the importance of evaluating patients for concomitant FM/CWP in order to contextualize our assessment of disease severity and treatment response better in individual patients.
Enthesitis in clinical registries
With the above caveats in mind, data on the prevalence, impact and response to treatment in clinical registries and clinical trials is reviewed below. Using a modification of the SPARCC index, enthesitis is assessed routinely in the University of Toronto long-term PsA registry directed by Dafna Gladman. The prevalence of enthesitis in this registry is 35% [8]. Investigators in the Corrona registry in the USA use the SPARCC and LEI indices, finding the prevalence of enthesitis in PsA in this cohort to be 27% [9]. In an analysis of data from the Corrona registry of 1567 patients with PsA, Mease observed that patients with enthesitis had significantly greater disease activity than those without enthesitis, exemplified by worse severity of arthritis as measured by the 68 tender and 66 swollen joint counts, higher DAS28CRP and CDAI scores. Subjects with enthesitis were less likely to achieve MDA status with PsA treatments. Patients with enthesitis reported higher levels of pain and fatigue, poorer function and quality of life and greater impairment at work [9]. Polachek et al. [10] analysed a group of 223 PsA subjects and noted that enthesitis, as measured by US, showed a correlation with more peripheral and axial damage, as measured by X-ray. Baskan et al. [11] noted a correlation between the presence of enthesitis and worse quality of life in 52 PsA patients. In 41 PsA patients, a correlation was noted between poor sleep quality and the presence of enthesitis, compared with healthy controls [12].
Enthesitis in clinical trials: biologic treatment
TNF inhibition
The first measurement of enthesitis in PsA therapeutic trials occurred with infliximab. In IMPACT 2, using a four-point technique of assessing tenderness of the Achilles tendon and plantar fascia insertions, a statistically significant reduction in the presence of enthesitis was noted at week 12 compared with placebo. In that study, 30% of patients treated with infliximab demonstrated Achilles tenderness at baseline compared with 14% at week 12 [13].
Using the same assessment in the adalimumab pivotal PsA trial, ADalimumab Effectiveness in Psoriatic arthritis Trial, 38% of patients had evidence of enthesitis at baseline. Although the mean change in enthesitis score was greater with adalimumab than with placebo, there was not a statistically significant separation [14]. Given subsequent studies, which show adalimumab to have clear efficacy in treating enthesitis, this result reflects the fact that the study was not powered to assess enthesitis, in addition to the limitation of using the four-point technique.
In the golimumab phase 3 trial, GO-REVEAL, using a modified MASES index (adding plantar fascia insertion sites), 75% of patients had evidence of enthesitis at baseline, and there was a median change of 50% in the approved dose (50 mg) treatment group, statistically superior to placebo at 14 and 24 weeks [15].
In the RAPID-PsA trial, at 24 weeks certolizumab demonstrated significant improvement of enthesitis, noted in 64% of subjects at baseline, using the mean change of LEI [16].
The etanercept phase 3 study, which was the first of the TNF inhibitor trials in PsA, did not include an enthesitis measure. The efficacy of etanercept for enthesitis has been assessed subsequently, most recently in the SEAM trial, wherein patients with early PsA (median disease duration of 6 months) were randomized to etanercept or MTX monotherapy or a combination of these medications [17]. Using the SPARCC index, of 67–69% with enthesitis at baseline, 53% of the etanercept monotherapy group achieved complete resolution of enthesitis at week 24 and 66% at week 48. In the MTX monotherapy arm, these percentages were 43 and 51%, respectively. These two arms were statistically separated at week 48 but not at week 24. A similar degree of improvement was seen between the etanercept monotherapy and combination arms, consistent with other musculoskeletal outcomes in this study, suggesting that there was no additional benefit of using this biologic agent combined with MTX. In this study, the LEI was also used and showed similar results to the SPARCC. Although not placebo controlled, the results with MTX are suggestive that this medication can yield benefit in the treatment of enthesitis.
Each of these five TNF inhibitors has also been approved for the treatment of AS (r-AxSpA), and several have been approved in various countries for nr-AxSpA [18]. Efficacy of these agents for the treatment of enthesitis in AxSpA has been demonstrated.
IL-12/23 inhibition
The first non-TNF inhibitor to be approved for PsA was ustekinumab, a p40 IL-12/IL-23 inhibitor. In two pivotal trials in PsA, PSUMMIT 1 and 2, ∼70% of subjects had enthesitis at baseline according to the PsA-modified MASES (addition of plantar fascia assessment) [19, 20]. The median improvement at 24 weeks in the usual dose arm (45 mg) was 40% in PSUMMIT 1 and slightly less in PSUMMIT 2, both significantly separated from placebo. A small, open label trial studied the effect of ustekinumab in 23 PsA subjects vs infliximab or adalimumab in 24, all of whom had evidence of enthesitis [21]. Using the SPARCC and MASES indices, ustekinumab showed statistically superior efficacy compared with the TNF inhibitors. Significant separation could not be demonstrated with the LEI, partly because of the low number of affected sites at baseline using that index. In a phase 3 trial programme, ustekinumab did not show efficacy in the treatment of AxSpA [22]. This result came as a surprise, because an earlier open label trial had suggested potential efficacy [23]. Whether ustekinumab could be effective in peripheral enthesitis in AxSpA is unclear. These results suggest that there might be differential effectiveness between peripheral enthesitis, as seen in the PsA trial results, and the enthesial component of axial disease [24].
IL-17 inhibition
Two IL-17A inhibitors have been approved for the treatment of PsA, secukinumab and ixekizumab, and one IL-17A and F inhibitor, bimekizumab, is in development for psoriasis and PsA.
Using a four-point scoring technique, 61% of patients in FUTURE 1, in which an i.v. loading dose of secukinumab was used, followed by monthly s.c. injections, had evidence of enthesitis at baseline [25]. At week 24, 46% of these patients demonstrated complete resolution of enthesitis. In FUTURE 2, in which an s.c. loading dose (the currently approved regimen) was followed by monthly s.c. dosing, of the ∼60% at baseline demonstrating enthesitis, 48 and 42% treated with 300 and 150 mg of secukinumab, respectively, demonstrated complete resolution of enthesitis [26]. As patients were tracked out to 104 weeks, the percentage demonstrating complete resolution increased to ∼70% in the various arms of the study. In FUTURE 5, the largest study to date in PsA (996 patients), of the 60% with enthesitis at baseline per LEI assessment, 65 and 53% in the 300 and 150 mg arms, respectively, achieved complete resolution of enthesitis at week 24 [27]. In FUTURE 5, the SPARCC index was also performed and displayed performance characteristics similar to the LEI. Owing to the robustness of these results, there are several studies underway to investigate the effect of secukinumab in studies focused on enthesitis, using not only clinical but also US and/or MRI assessment.
In the SPIRIT-P1 study of biologic-naïve PsA patients, enthesitis was present at baseline in 58% of subjects [28]. The mean change of LEI score showed numerical but not statistically significant improvement in the ixekizumab-treated patients compared with placebo at 24 weeks. Statistical significance compared with placebo was demonstrated in a post hoc analysis of complete resolution of enthesitis assessed by LEI in ∼40% of the patients in the study arms. In a TNF inhibitor-exposed PsA population in SPIRIT-P2, 56% had enthesitis at baseline and, of these, 35% displayed complete resolution using LEI. This did not achieve statistical significance vs placebo at week 24, the primary end point, but it did at earlier time points.
In the first trial comparing two biologics head to head in the treatment of PsA, SPIRIT H2H, ixekizumab was compared with adalimumab in an open-label, assessor-blinded approach [29]. Enthesitis was present in slightly >60% of patients as assessed by the SPARCC index. At week 24, 57% of patients treated with ixekizumab demonstrated resolution of enthesitis, whilst 45% treated with adalimumab did so, a statistically significant separation. Similar improvement was demonstrated using the LEI, although not statistically separated.
In the phase 2B BE-ACTIVE trial of bimekizumab, an IL-17A and F inhibitor, ∼50% of the patients demonstrated enthesitis according to LEI at baseline. At 12 weeks, two 160 mg dose arms, with and without a loading dose, demonstrated 59% complete resolution of enthesitis vs 29% in the placebo group, a statistically significant separation.
Secukinumab is approved for the treatment of AS/r-AxSpA. Ixekizumab has shown efficacy in a phase 3 group of studies in this indication, and bimekizumab is also being developed for this indication, for which enthesitis is an important clinical domain.
IL-23 inhibition
Three p19 IL-23 inhibitors are in development for PsA: guselkumab, risankizumab and tildrakizumab.
Guselkumab enthesitis data were reported in a phase 2 trial, in which 57% of patients with enthesitis demonstrated LEI complete resolution compared with 29% in the placebo group, a statistically significant difference [30].
In a phase 2 trial of risankizumab, 65% of subjects had enthesitis according to the SPARCC index at baseline; statistically significant improvement in two of five risankizumab dose arms was noted at week 24 [31].
Partial data from a phase 2 study of tildrakizumab has been reported [32]. Approximately 50% of subjects had evidence of enthesitis at baseline according to LEI assessment; all dose arms showed numerical mean change differentiation from placebo at week 24 but achieved statistical differentiation only in the highest dose group of 200 mg every 4 weeks.
A clinical trial of risankizumab in AxSpA failed to show efficacy [33].
T-cell modulation
Abatacept, a T-cell modulator that inhibits T-cell activation through a co-stimulatory blockade mechanism, is approved for PsA [34]. In a phase 3 study, 65% of patients had enthesitis at baseline. Of these, 33% achieved LEI resolution compared with 21% in the placebo group at week 24, a numerical but not statistically significant difference. Abatacept is not effective for the treatment of AxSpA.
IL-6 inhibitor
Although IL-6 inhibition is not a mechanism that is in current development for PsA, an exploratory phase 2 study with a direct IL-6 inhibitor, clazakizumab, was performed in PsA. In that study, >75% of subjects had evidence of enthesitis at baseline, using both SPARCC and LEI assessments [35]. The SPARCC index demonstrated numerical improvement in all clazakizumab treatment arms compared with placebo at week 24. Of note, the LEI was not able to discriminate between the treatment arms and placebo. IL-6 inhibition is not effective for the treatment of AxSpA.
Enthesitis in clinical trials: targeted synthetic disease modification
There are two oral medications that have been approved for the treatment of PsA, apremilast and tofacitinib, and numerous others in the development pipeline [36]. These are known as targeted synthetic DMARDs (tsDMARDs), because their mechanism of action targets specific immunological mechanisms more precisely than older oral medications, such as MTX, SSZ and LEF, which are known as conventional synthetic DMARDs (csDMARDs). Some patients may prefer oral over parenteral administration of medication, partly related to the lack of need for refrigeration while travelling. Also, there is no immunogenicity with an oral medication, which can sometimes lead to loss of efficacy with the parenterally administered biologic agents.
Phosphodiesterase 4 inhibition
Apremilast is a phosphodiesterase 4 (PDE4) inhibitor, which, by inhibiting PDE4, modulates intracellular signal transduction, decreases conversion of cyclic adenosine monophosphate to adenosine monophosphate and, by this mechanism in immunologically active cells, results in less pro-inflammatory cytokine production. In PALACE-1, a phase 3 trial in PsA, enthesitis was noted at baseline in >60% of patients using the MASES [36]. The mean change of the MASES score was statistically higher in apremilast-treated patients than placebo at week 16, and at week 24 statistically more apremilast-treated patients achieved resolution of MASES. Apremilast has not shown efficacy in AxSpA.
Janus kinase inhibition
Tofacitinib, a Janus kinase (JAK) inhibitor, is approved for treatment of PsA. Enthesitis was assessed in two pivotal phase 3 studies, OPAL Broaden and OPAL Beyond, the former with biologic-naïve patients and the latter with patients who had had lack of efficacy or intolerance of at least one TNF inhibitor [37, 38]. In OPAL Broaden, at month 3, the mean change in LEI in the 61–71% who had enthesitis at baseline was significantly greater than placebo for the 10 mg twice daily dose but not for the approved 5 mg twice daily dose. However, in OPAL Beyond, both doses of tofacitinib demonstrated statistically significant improvement compared with placebo at month 3 in the 63–75% of patients who had enthesitis at baseline.
Several other JAK inhibitors are actively in development for PsA, including filgotinib and upadacitinib, which are both relatively specific for JAK1 inhibition. In the EQUATOR trial, 59 and 74% in the filgotinib and placebo arms, respectively, had enthesitis at baseline according to SPARCC and LEI assessment [39]. At week 16, of those with enthesitis, 35% achieved SPARCC resolution in the treatment arm and 23% in placebo, and the similar percentages for LEI resolution were 52 and 26%, respectively, which were statistically significant separations. Data from the upadacitinib development programme are expected soon. Several other JAK inhibitors, customized for differing JAK selectivities, are anticipated to be studied in PsA in the near future. These agents are also demonstrating efficacy in AxSpA clinical development.
Enthesitis in OMERACT core domain and core assessment sets
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)-OMERACT has identified enthesitis as part of the core domain and assessment set of PsA [40]. At present, the GRAPPA group considers each of the indices for assessment of enthesitis as valid and feasible, although in more recent trials, the SPARCC and LEI are the most commonly used indices to assess enthesitis. The SPARCC has reliably shown ability to discriminate between treatment and placebo and response to treatment. The LEI has generally performed well, but in a few trials it has shown less discriminatory capability, partly owing to the smaller number of sites assessed, although for this same reason, it may be easier to use in clinical settings. A formal analysis of these performance characteristics will be forthcoming as part of the OMERACT core set of outcome measures for PsA.
Enthesitis in treatment recommendations
Treatment guidelines developed by prominent international and dermatology associations highlight the importance of assessing and effectively treating enthesitis as one of the core clinical domains of PsA (GRAPPA [41], EULAR [42], ACR/National Psoriasis Foundation [43] and American Academy of Dermatology [44]). The approach of the GRAPPA group is to evaluate the evidence for therapy of each major clinical domain (peripheral arthritis, enthesitis, dactylitis, axial disease, skin and nail disease) and recommend treatments that have proven efficacy in each domain. For enthesitis, for example, based on the paucity of evidence for effectiveness of csDMARDs, the recommendation is to go from NSAID therapy directly to biologic or tsDMARD therapy. The fact that enthesitis is singled out for assessment and treatment in each of these guidelines emphasizes the need for the clinician to do so in day-to-day practice.
Future directions
Recognition of the unique clinical presentation and significant patient impact of enthesitis in PsA has led to increased research on the pathophysiology of this domain, which will continue to burgeon. More research is needed regarding the accurate assessment of inflammation in the enthesium (i.e. distinguishing -itis from -algia), including detailed studies to correlate clinical evaluation with advanced imaging assessment (e.g. US, MRI and other modalities). Additionally, it is hoped that serum biomarkers can be identified that will help with the assessment of enthesitis. As new treatments emerge, assessment of their impact on enthesitis will be a core part of clinical trials.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript. This paper was published as part of a supplement funded by an educational grant from Novartis.
Disclosure statement: P.M. has received consultancy fees from AbbVie, Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Sun, UCB; research grants from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer, Sun, UCB; and speakers fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Genentech, Janssen, Lilly, Novartis, Pfizer, UCB.
References
- 1. Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res 2011;63(Suppl 11):S64–85. [DOI] [PubMed] [Google Scholar]
- 2. Healy PJ, Helliwell PS.. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 3. Maksymowych WP, Mallon C, Morrow S. et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index. Ann Rheum Dis 2009;68:948–53. [DOI] [PubMed] [Google Scholar]
- 4. Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A. et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol 2017;29:304–10. [DOI] [PubMed] [Google Scholar]
- 6. Brikman S, Furer V, Wollman J. et al. The effect of the presence of fibromyalgia on common clinical disease activity indices in patients with psoriatic arthritis: a cross-sectional study. J Rheumatol 2016;43:1749–54. [DOI] [PubMed] [Google Scholar]
- 7. Højgaard P, Ellegaard K, Nielsen SM. et al. Pain mechanisms and ultrasonic inflammatory activity as prognostic factors in patients with psoriatic arthritis: a prospective cohort study. Arthritis Care Res 2018;71:798–810. [DOI] [PubMed] [Google Scholar]
- 8. Polachek A, Li S, Chandran V. et al. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res 2017;69:1685–91. [DOI] [PubMed] [Google Scholar]
- 9. Mease PJ, Karki C, Palmer JB. et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res 2017;69:1692–9. [DOI] [PubMed] [Google Scholar]
- 10. Polachek A, Cook R, Chandran V. et al. The association between sonographic enthesitis and radiographic damage in psoriatic arthritis. Arthritis Res Ther 2017;19:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baskan B, Oten E, Sivas F. et al. The relationship between vitamin D, vertebral deformity and quality of life in psoriatic arthritis. Acta Reumatol Port 2016;41:350–8. [PubMed] [Google Scholar]
- 12. Gezer O, Batmaz I, Sariyildiz MA. et al. Sleep quality in patients with psoriatic arthritis. Int J Rheum Dis 2017;20:1212–8. [DOI] [PubMed] [Google Scholar]
- 13. Antoni C, Krueger GG, de Vlam K. et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mease P, Gladman D, Ritchlin C.. Adalimumab in the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. [DOI] [PubMed] [Google Scholar]
- 15. Kavanaugh A, McInnes I, Mease P. et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 16. Mease PJ, Fleischmann R, Deodhar AA. et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mease PJ, Gladman DD, Collier DH. et al. etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized controlled phase III trial. Arthritis Rheumatol 2019. ;71:1112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mease P. Emerging immunomodulatory therapies and new treatment paradigms for axial spondyloarthritis. Curr Rheumatol Rep 2019;21:35. [DOI] [PubMed] [Google Scholar]
- 19. McInnes IB, Kavanaugh A, Gottlieb AB. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. [DOI] [PubMed] [Google Scholar]
- 20. Ritchlin C, Rahman P, Kavanaugh A. et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Araujo EG, Englbrecht M, Hoepken S. et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum 2019;48:632–7. [DOI] [PubMed] [Google Scholar]
- 22. Deodhar A, Gensler LS, Sieper J. et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019;71:258–70. [DOI] [PubMed] [Google Scholar]
- 23. Poddubnyy D, Hermann KG, Callhoff J. et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 2014;73:817–23. [DOI] [PubMed] [Google Scholar]
- 24. Mease P. Ustekinumab fails to show efficacy in a phase III axial spondyloarthritis program: the importance of negative results. Arthritis Rheum 2019;71:179–81. [DOI] [PubMed] [Google Scholar]
- 25. Mease PJ, McInnes IB, Kirkham B. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 26. McInnes IB, Mease PJ, Kirkham B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 27. Mease P, van der Heijde D, Landewé R. et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 2018;77:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mease PJ, van der Heijde D, Ritchlin CT. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mease PJ, Smolen JS, Behrens F. et al. Multicentre, randomized, open-label, assessor-blinded, parallel-group head-to-head comparison of the efficacy of ixekizumab versus adalimumab in patients with psoriatic arthritis naive to biologic disease-modifying anti-rheumatic drugs: 24-week results. Ann Rheum Dis 2020;79:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deodhar A, Gottlieb AB, Boehncke WH. et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2018;391:2213–24. [DOI] [PubMed] [Google Scholar]
- 31. Mease PJ, Kellner H, Morita A. et al. Efficacy and safety of risankizumab, a selective il-23p19 inhibitor, in patients with active psoriatic arthritis over 24 weeks: results from a phase 2 trial. Arthritis Rheum 2017;69:12–6. [Google Scholar]
- 32. Mease PJ, Chohan S, Fructuoso FJG. et al. Randomized, double-blind, placebo-controlled, multiple-dose, Phase 2B study to demonstrate the safety and efficacy of tildrakizumab, a high-affinity anti-interleukin-23P19 monoclonal antibody in patients with active psoriatic arthritis . Ann Rheum Dis 2019;78:78–9. [Google Scholar]
- 33. Baeten D, Østergaard M, Wei JC. et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis 2018;77:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mease PJ, Gottlieb AB, van der Heijde D. et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017;76:1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mease PJ, Gottlieb AB, Berman A. et al. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol 2016;68:2163–73. [DOI] [PubMed] [Google Scholar]
- 36. Kavanaugh A, Mease PJ, Gomez-Reino JJ. et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014;73:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mease P, Hall S, FitzGerald O. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 38. Gladman D, Rigby W, Azevedo VF. et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- 39. Mease P, Coates LC, Helliwell PS. et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2367–77. [DOI] [PubMed] [Google Scholar]
- 40. Orbai AM, de Wit M, Mease PJ. et al. Updating the psoriatic arthritis (PsA) core domain set: a report from the PsA workshop at OMERACT 2016. J Rheumatol 2017;44:1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coates LC, Kavanaugh A, Mease PJ. et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 42. Gossec L, Smolen JS, Ramiro S. et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 43. Singh JA, Guyatt G, Ogdie A. et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elmets CA, Leonardi CL, Davis DMR. et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol 2019;80:1073–113. [DOI] [PubMed] [Google Scholar]