Abstract
Values and preferences relate to the importance that patients place on health outcomes (eg, bleeding, having a deep venous thrombosis) and are essential when weighing benefits and harms in guideline recommendations. To inform the American Society of Hematology guidelines for management of venous thromboembolism (VTE) disease, we conducted a systematic review of patients’ values and preferences related to VTE. We searched Medline, Embase, Cochrane Central Register of Controlled Trials, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature from inception to April of 2018 (PROSPERO-CRD42018094003). We included quantitative and qualitative studies. We followed Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidance for rating the certainty and presenting findings for quantitative research about the relative importance of health outcomes and a grounded theory approach for qualitative thematic synthesis. We identified 14 quantitative studies (2465 participants) describing the relative importance of VTE-related health states in a widely diverse population of patients, showing overall small to important impact on patients’ lives (certainty of the evidence from low to moderate). Additionally, evidence from 34 quantitative studies (6424 participants) and 15 qualitative studies (570 participants) revealed that patients put higher value on VTE risk reduction than on the potential harms of the treatment (certainty of evidence from low to moderate). Studies also suggested a clear preference for oral medication over subcutaneous medication (moderate certainty). The observed variability in health state values may be a result of differences in the approaches used to elicit them and the diversity of included populations rather than true variability in values. This finding highlights the necessity to explore the variability induced by different approaches to ascertain values.
Introduction
The American Society of Hematology (ASH), together with the MacGRADE Centre at McMaster University, developed clinical practice guidelines for management of venous thromboembolism disease (VTE) (see the 10 topics in supplemental Material).1-7 To develop these guidelines, we followed an evidence synthesis and guideline development approach based on the GIN-McMaster Guideline Development Checklist8 and the Handbook in Grading of Recommendations Assessment, Development and Evaluation's (GRADE) official application GRADEpro (www.gradepro.org). We used the GRADE Evidence to Decision frameworks,9-11 which integrate, among other criteria, patients’ values and preferences in balancing desirable and undesirable consequences of investigated options to arrive at evidence-based recommendations.
The GRADE approach defines patients’ values and preferences as the relative importance that people place on health outcomes (eg, bleeding, having a deep venous thrombosis [DVT]).12 Considering this importance is essential when weighing benefits and harms in guideline recommendations. Incorporating values and preferences in the guideline processes also promotes the development of recommendations with greater acceptability and adherence by those intended to benefit from them.12 Such recommendations are also useful to inform individual patient decision making.12-15
To inform the judgments by the guideline panels of the ASH VTE guidelines, we conducted a systematic review focused on patients’ values and preferences for the health outcomes prioritized by the panels.
Methods
Overview
We developed a protocol for this systematic review (PROSPERO 2018 CRD42018094003). The review was part of the overall project coordinated by the McMaster GRADE Center to support the development of the ASH VTE guidelines.1-7 We adopted an inclusive systematic review methodology to reach breadth and depth of understanding of the topic under review.16 To do so, we systematically reviewed findings from quantitative studies and aggregated results from qualitative primary studies.6-8 Information was shared with the systematic review team and the panel members using an interactive approach during which the experts were asked for additional suggestions about the research evidence and synthesis of the findings prior to the in-person panel meetings. For the purpose of this article, 2 reviewers with qualitative research experience (F.B. and I.D.F.). independently extracted, assessed, analyzed, and interpreted qualitative data and regularly met with other authors to discuss and integrate the analytical findings.
Search strategy
We developed sensitive electronic search strategies based on previously published work and a search filter for values and preferences,17 which incorporates terms to capture content areas (utilities, direct choice, structured scales or questionnaires, and qualitative studies) in combination with terms related to VTE disease, the interventions prioritized across the different guidelines, and terms defining prioritized health outcomes rated as critical by the panels.
We first searched Medline, Embase, the Cochrane Central Register of Controlled Trials, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature from inception to October 2016 (see “Search strategies” in supplemental Material) and we set up and monitored search alerts until April 2018 to identify and incorporate newly published research.
Eligibility criteria
We included original studies assessing the values and preferences of individuals at risk or with a VTE-related condition that was covered as a topic in the guidelines. Supplemental Table 1 defines the selection criteria.
Selection of studies
First, we screened titles and abstracts of a set of references following a well-defined selection framework (supplemental Figure 1). We conducted a piloting exercise to ensure consistency among 22 screeners, with different levels of expertise in values and preferences research (Yuan Zhang, I.E.-I., H.B., C.A.C., Y. Roldan, R. Chen, C.D., R.L.M., J.J.R., Yuqing Zhang, R. Charide, A.A., S.B., G.P.M., J.J.Y.-N., Y. Rehman, I.N., N.S., T.B., C.B., and M.F.R.), who screened sets of references independently and in duplicate. A third reviewer resolved disagreements when needed. The screening results, together with the content of the search strategy, the selection criteria, and the framework, were used to develop and calibrate a machine-learning screening model in the Collaboratron platform. The screening model allowed us to predict the probability of a citation being relevant for full-text screening. We chose a ≤1% probability of relevance, specified by the machine-learning model, as the threshold to exclude irrelevant references.
Second, the model was used in the remaining set of references, and the titles and abstracts of relevant records (those with >1% probability of relevance, determined by machine-learning model) were screened. Finally, the full texts of included records were independently assessed in duplicate, and disagreements were resolved by consensus or by consulting a third reviewer.
Data abstraction and data analysis
Seven reviewers (Yuan Zhang, I.E.-I., H.B., C.A.C., Y. Roldan, R. Chen, and C.D.) independently, and in duplicate, extracted data from studies using quantitative methods, and 2 other reviewers (F.B. and I.D.F.) extracted qualitative research study data. Using structured abstraction forms, we extracted 2 types of information for qualitative data: descriptive characteristics of studies (identification data, objectives, participant characteristics, study design and methodology, and country) and study findings, including original data excerpts (participant quotes, stories, or incidents). Disagreement was resolved through discussion and consensus. If necessary, a third party was consulted. QSR NVivo v.11 and Microsoft Excel software were used to extract and analyze the qualitative data.
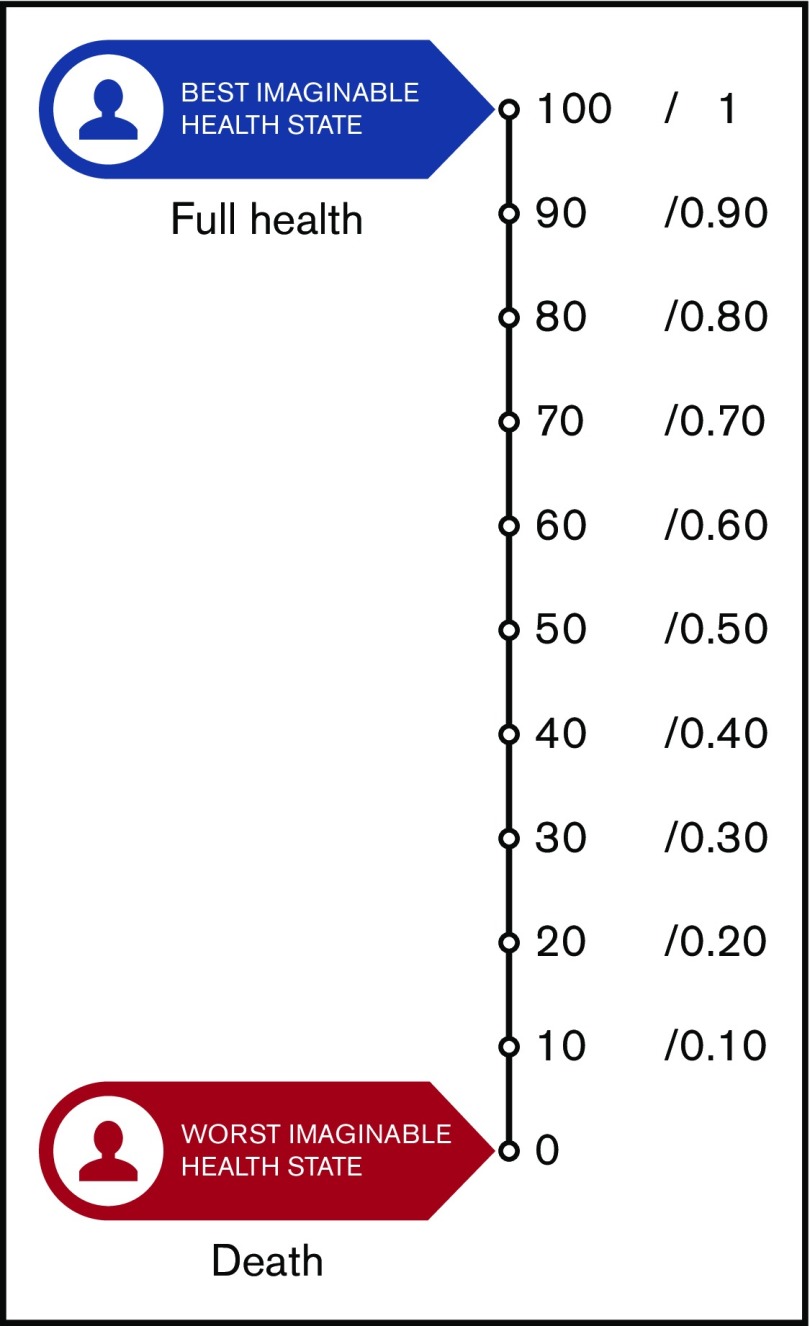
For the synthesis of findings from quantitative-method studies, we present the raw data, ranges, and narrative descriptions, because, as expected, there was high heterogeneity in methods and measurement of outcomes that limited the ability to conduct any meta-analysis. We categorized the results into relative importance of outcomes (RIOs) or utilities and nonutility results. Utilities represent the strength of an individual’s preferences for different health outcomes. They are measured on an interval scale, with 0 reflecting states of health equivalent to being dead and 1 reflecting perfect health. The nonutility results were summarized in predetermined subgroups of patients who were the target population of the guidelines, such as medical patients, surgical patients, patients with cancer, or pregnant women.
For studies using qualitative methods, we followed an analytical approach adapted from grounded theory to extract and analyze qualitative data.18,19 This inductive analytical technique, based on a constant comparison of data, suits the aims of aggregating qualitative evidence and providing new conceptual interpretations that integrate findings across studies.19 We did not apply an overall certainty of evidence framework like GRADE-CERQual,20 because our search strategy was not sensitive enough to ensure a comprehensive identification of all of the qualitative evidence, and only the qualitative studies identified with our search strategy were included.
Risk of bias and certainty of the body of evidence
To assess the risk of bias (RoB) for individual quantitative studies, we used a priori defined items, including selection of study population, completeness of data, selection of measurement instruments, administration of measurement instruments, presentation of outcomes, understanding of participants, and data analysis.21 To assess the certainty of the body of evidence from quantitative studies, we applied the GRADE approach to assess the certainty of evidence for RIOs or values and preferences.12,21,22 The approach includes the following domains: RoB, inconsistency, indirectness, imprecision, and publication bias. For qualitative research studies, we used the CASP Qualitative Checklist tool23 to appraise the RoB of individual studies.
Results
Searches
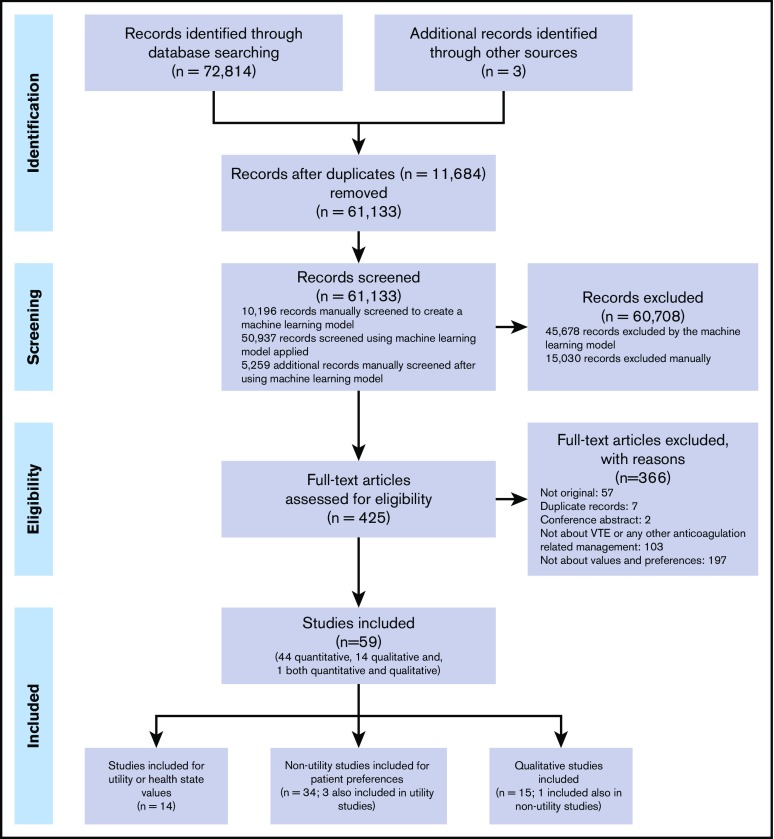
Our search strategy identified 72 814 records from the electronic database searches (61 130 nonduplicate records). After applying the machine learning (which required manual prescreening of a set of 10 196 records and its performance on the remaining 50 937 references), we selected and manually screened the title and abstract of 5259 records classified as having >1% probability of being relevant; 422 publications were retrieved for full-text screening. A set of 56 records identified from electronic database searches was selected for inclusion,24-79 and 3 additional studies80-82 were identified by checking references included in a previously published guideline on the same topic.83 Overall, we included 44 quantitative studies,24-53,55-65,80-82 14 qualitative studies,66-79 and 1 study with both types of evidence54 (Figure 1). Fourteen of the included studies reported patients’ values or utilities,24-37 34 nonutility studies used quantitative methods,24,32,35,38-65,80-82 and another 15 studies used qualitative methods.54,66-79 Supplemental Table 2 lists the studies that did not fulfill the inclusion criteria with the reasons for exclusion at full-text screening.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Quantitative research evidence.
Supplemental Table 3 summarizes the individual study characteristics, results, and the overall RoB assessment. Supplemental Table 4 summarizes the individual RoB criteria for utility and nonutility of the 45 quantitative studies.
RIOs, utility, or health state values
We identified 14 studies,24-37 including 2465 participants, reporting utility or health state values of DVT, pulmonary embolism (PE), postthrombotic syndrome (PTS) (mild and severe), and bleeding events, as well as utilities of using treatments such as vitamin K antagonists (VKAs) and low molecular weight heparin (LMWH) (Figure 2; Tables 1 and 2).
Figure 2.
Utilities represent the strength of an individual’s preferences for different health outcomes. They are measured on an interval scale, with 0 reflecting states of health equivalent to death and 1 reflecting perfect health.
Table 1.
Summary of findings for overall population: utility, RIO, or health state value information
| Health state/outcome (categories of values and preferences) | Estimates of utilities | Certainty in evidence | Interpretation of findings |
|---|---|---|---|
| No. of participants/studies | |||
| DVT* (Hogg et al,27,28 Lloyd et al,31 Locadia et al,32 Marvig et al,34 Utne et al37) | Range across studies: 0.61-0.99 Standard gamble: 0.81-0.99 Time trade-off: 0.84 VAS: 0.65-0.72 EQ-5D utility: 0.61-0.79 SF-6D: 0.64 |
⊕⊕⊕○ Moderate certainty due to inconsistency† |
People may probably find DVT having a moderate or a trivial impact on their lives. There is likely an important variability for this assessment. |
| 1702 participants from 6 studies‡
Standard gamble: 260 participants from 2 studies Time trade-off: 124 participants from 1 study VAS: 485 participants from 3 studies EQ-5D utility: 1318 participants from 4 studies SF-6D: 44 participants from 1 study | |||
| PE* (Hogg et al,27,28 Lloyd et al,31 Locadia et al,32 Marvig et al,34 Tavoly et al,36 Utne et al37) | Range across studies: 0.63-0.93 Standard gamble: 0.75-0.93 Time trade-off: 0.63 VAS: 0.67-0.70 Rating scale modeled: EQ-5D: 0.621-0.80 SF-6D: 0.68 |
⊕⊕⊕○ Moderate certainty due to inconsistency† |
People may probably find PE having a moderate or a small impact on their lives. There is likely an important variability for this assessment. |
| 1474 participants from 5 studies§
Standard gamble: 260 participants from 2 studies Time trade-off: 124 participants from 1 study Rating scale modeled: EQ-5D: 877 participants from 1 study VAS: 257 participants from 2 studies EQ-5D: 213 participants from 1 study SF-6D: 44 participants from 1 study | |||
| PTS (Locadia et al32) | 0.82 (IQR, 0.66-0.94) | ⊕⊕⊕○ Moderate certainty due to indirectness¶ |
People may probably find PTS having a small impact on their lives. There is likely an important variability for this assessment. |
| 124 participants from 1 study based on time trade-off | |||
| Mild PTS (Lenert and Soetikno,30 O’Meara et al35) | Range across studies: 0.99-1.00 | ⊕⊕○○ Low certainty due to indirectness and imprecision||,# |
People might possibly find mild PTS having a trivial impact on their lives. There is likely no important variability for this assessment. |
| 66 participants from 2 studies based on standard gamble | |||
| Severe PTS (Lenert and Soetikno,30 O’Meara et al35) | Range across studies: 0.93-0.98 | ⊕⊕○○ Low certainty due to indirectness and imprecision||,# |
People might possibly find severe PTS having a trivial or a small impact on their lives. There is likely no important variability for this assessment. |
| 66 participants from 2 studies based on standard gamble | |||
| Gastrointestinal tract bleeding event (Hogg et al,27 Lloyd et al,31 Locadia et al32) | Range across studies: 0.59-0.65 Standard gamble: 0.65 (IQR, 0.15-0.86) Time trade-off: 0.65 (IQR, 0.49-0.86) Rating scale modeled: EQ-5D 0.59 (95% CI, 0.46-0.69) |
⊕⊕⊕○ Moderate certainty due¶ to indirectness** |
People may probably find gastrointestinal tract bleeding having a moderate impact on their lives. There is likely an important variability for this assessment. |
| 1217 participants Standard gamble: 216 participants from 1 study Time trade-off: 124 participants from 1 study Rating scale modeled: EQ-5D: 877 patients from 1 study | |||
| Muscular bleeding event (Locadia et al32) | 0.76 (IQR, 0.59-0.95) | ⊕⊕⊕○ Moderate certainty due to indirectness¶ |
People may probably find a muscular bleeding event having a moderate impact on their lives. There is likely an important variability for this assessment. |
| 124 participants from 1 study based on time trade-off | |||
| Central nervous system bleeding (including intracranial bleeding) (Lenert and Soetikno,30 O’Meara et al35) | Range across studies: 0.29-0.60 | ⊕○○ Very low certainty due to inconsistency, indirectness, and imprecision||,#,†† |
People appear to find central nervous system bleeding having an important or a moderate impact on their lives. There is likely an important variability for this assessment. |
| 66 participants from 2 studies based on standard gamble | |||
| Major intracranial bleeding event (Hogg et al27) | Standard gamble: 0.15 (IQR, 0.00-0.65) | ⊕⊕⊕⊕ High certainty |
People find major intracranial bleeding having a large impact on their lives. There is likely an important variability for this assessment. |
| 216 participants from 1 study based on standard gamble | |||
| Minor intracranial bleeding event (Hogg et al27) | Standard gamble: 0.75 (0.55-0.92) | ⊕⊕⊕⊕ High certainty |
People find minor intracranial bleeding having a moderate impact on their lives. There is likely an important variability for this assessment. |
| 216 participants from 1 study based on standard gamble | |||
| Treatment with VKAs (Dranitsaris et al,25 Keita et al,29 Locadia et al,32 Marchetti et al33) | Time trade-off: range 0.38 to 0.99, EQ-5D VAS: mean 0.61 | ⊕⊕⊕○ Moderate certainty due to inconsistency and indirectness‡‡ |
People may probably find treatment with VKAs having an important and a trivial impact on their lives. There is likely an important variability for this assessment. |
| 296 participants in 4 studies (3 for time trade-off, 1 in EQ-5D VAS) | |||
| Treatment with LMWH (Dranitsaris et al,25 Marchetti et al33) | Range: 0.66-0.99 | ⊕⊕○○ Low certainty due to inconsistency/indirectness and imprecision‡‡,a |
People might possibly find treatment with LMWH having a moderate or trivial impact on their lives. There is likely an important variability for this assessment. |
| 72 participants in 2 studies based on time trade-off | |||
| Treatment with standard systemic thrombolysis (Enden et al26) | 0.84 (95% CI, 0.81-0.87) | ⊕⊕⊕○ Moderate certainty due to imprecisiona |
People may probably find treatment with thrombolysis having a small impact on their lives. After 24 mo, there were probably no differences in treatment burden between the standard treatment arms and the additional CDT. There is likely no important variability for this assessment. |
| 99 participants from standard treatment group (n = 189), 1 study based on EQ-5D utility | |||
| Treatment with CDT (Enden et al26) | 0.80 (95% CI, 0.75-0.85) | ⊕⊕⊕○ Moderate certainty due to imprecisiona |
|
| 90 participants from CDT treatment group (n = 189), 1 study based on EQ-5D utility |
High certainty in evidence: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
CDT, catheter-directed thrombolysis; CI, confidence interval; IQR, interquartile range, VAS, visual analog scale.
Utilities measured with VAS, time trade-off, and standard gamble, as well as EQ-5D and SF-6D utility.
Of the 6 studies included, we can observe heterogeneity around the estimates across different studies. Study participants or measurement methodology alone could not explain the differences.
Hogg et al28 reported standard gamble, VAS, and SF-6D utility information; Marvig et al34 and Utne et al37 reported EQ-5D VAS and EQ-5D utility; and Lloyd et al31 reported VAS and EQ-5D utility.
Hogg et al28 reported standard gamble, VAS, and SF-6D utility information; Lloyd et al31 reported VAS and EQ-5D utility.
Locadia et al32 is a cross-sectional study interviewing participants with decision analysis. This study included 3 groups of patients, which may compromise the applicability of the overall results: (1) newly diagnosed patients with a first or second episode of VTE for whom treatment with VKAs had been started, (2) patients who had experienced an episode of major bleeding during treatment with VKAs in the previous year, and (3) patients with a PTS, diagnosed ≥1 year after an episode of DVT, who had been treated with VKAs for ≥3 months. The latter 2 patient groups were patients who have had the experience and, therefore, were not the optimal population who would be at risk for decision making.
The included studies have different populations than the patients facing the choice: 30 community volunteers (Lenert and Soetikno30), 36 patients with DVT (16/36 participants) and without DVT (O'Meara et al35).
In total, the sample size was too small; there were only 66 participants (excluding 30 physicians) from 2 studies.
Downgraded due to indirectness. Locadia et al32 included different group of population of patients who had experienced VTE, major bleeding and post–thrombotic syndrome, and Lloyd et al31 reported on major bleeding, rather than specific gastrointestinal bleeding.
The estimates from the 2 studies were very different, although both studies used standard gamble and measured the same outcome.
Downgraded considering both quality criteria, because the observed variability (people finding taking VKAs as having both important or small impact on their lives) might be explained by the variability in the included populations across studies (people from the general population [Dranitsaris et al25]); patients attending the local anticoagulation clinic (Marchetti et al33); and patients with newly diagnosed first or second episode of VTE, patients with an episode of major bleeding during treatment the previous year, and patients with a PTS after an episode of DVT treated for ≥3 months (Locadia et al32), as well as VTE outpatients receiving anticoagulation therapy by VKA or DOAC (Keita et al29).
Small sample size of included study or subgroup of patients.
Table 2.
Summary of findings for pregnancy subgroup: utility, RIO, or health state value information
| Health state/outcome (categories of values and preferences) | Estimates of utilities | Certainty in evidence | Interpretation of findings |
|---|---|---|---|
| No. of participants/studies | |||
| Pregnancy-related DVT (Bates et al24) | VAS: 46 (IQR, 30-65) | ⊕⊕○○ Low certainty due to inconsistency and imprecision*,† |
Women might possibly find pregnancy-related DVT having an important impact on their lives. There is likely an important variability for this assessment. |
| 123 participants from 1 study based on VAS | |||
| Pregnancy-related PE (Bates et al24) | VAS: 30 (IQR, 15-50) | ⊕⊕○○ Low certainty due to inconsistency and imprecision*,† |
Most women might possibly find pregnancy-related PE having an important impact on their lives. There is likely an important variability for this assessment. |
| 123 participants from 1 study based on VAS | |||
| Obstetric bleeding (Bates et al24) | VAS: 30 (IQR, 15-50) | ⊕⊕○○ Low certainty due to inconsistency and imprecision*,† |
Most women might possibly find obstetric bleeding having an important impact on their lives. There is likely an important variability. |
| 123 participants from 1 study based on VAS | |||
| Treatment with LMWH (Bates et al24) | Visual analog scale: 83 (IQR, 70-90) | ⊕⊕⊕○ Moderate certainty due to imprecision† |
Women may probably find treatment with LMWH having a small impact on their lives. There is some variability for this assessment. |
| 123 participants from 1 study based on VAS |
Moderate certainty in evidence: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty in evidence: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
IQR, interquartile range; VAS, visual analog scale.
Inconsistency due to important variability.
Imprecision due to small sample size.
A total of 1702 participants from 6 studies,27,28,31,32,34,37 including the overall population, provided their estimates of RIOs for DVT, with a range from 0.61 to 0.99 across studies (Table 1). Five studies with 1474 participants27,28,31,32,36 reported an RIO for PE in a range from 0.63 to 0.93. Across different techniques to elicit utilities, standard gamble appeared to yield higher utilities (or lower disutilities) for DVT (0.81-0.99) and PE (0.75-0.93). Meanwhile, EuroQol-5 Dimension (EQ-5D) (EQ-5D) and Short-Form Six-Dimension (SF-6D) utilities were lower. The EQ-5D utility ranged from 0.61 to 0.79 for DVT and from 0.62 to 0.80 for PE. The SF-6D utilities are 0.64 for DVT and 0.68 for PE. In general, people may probably find DVT and PE as having a small to moderate impact on their lives, although there is likely important variability for this assessment. Because of the inconsistency and the potential variability observed, we downgraded the certainty of evidence to moderate for the DVT and PE outcomes.
For PTS, 1 study32 based on 124 participants using time trade-off techniques, reported a utility of 0.82 (moderate certainty of evidence), suggesting a probable small impact on people’s lives. Two other studies elicited the utility for mild and severe PTS30,35 with the standard gamble technique (66 participants from 2 studies) and reported utilities from 0.99 to 1.00 for mild PTS and from 0.93 to 0.98 for severe PTS (Table 1). This suggests that people might possibly find PTS having a trivial to small impact on their lives; however, most of the participants were healthy volunteers, and studies included small sample sizes. Therefore, we downgraded the certainty of evidence to low for the importance of mild and severe PTS as a result of indirectness and imprecision.
The included studies reported a variety of utilities for bleeding events. The utilities ranged from 0.59 to 0.65 for gastrointestinal tract bleeding (1217 participants from 3 studies),27,31,32 0.76 for muscular bleeding (124 participants from 1 study),32 from 0.29 to 0.60 for central nervous system bleeding (66 participants from 2 studies),30,35 0.75 for minor intracranial bleeding, and 0.15 for major intracranial bleeding (216 participants from 1 study).27 The certainty was graded as moderate for gastrointestinal and muscular bleeding due to indirectness, as very low for central nervous system bleeding, and as high for minor and major intracranial bleeding (Table 1).
One of the studies24 assessing thromboprophylaxis during pregnancy using a feeling thermometer (anchored at death [0] and full health [100]) reported a utility value of 46 (interquartile range [IQR], 30-65) for pregnancy-related DVT and 30 (IQR, 15-50) for pregnancy-related PE and obstetrical bleeding. The certainty was graded as low for the DVT, PE, and bleeding utilities due to inconsistency and imprecision (Table 2).
The utilities participants place on different treatment options are summarized in Table 1 (overall population) and Table 2 (pregnant women). People may probably find the inconvenience of medication treatment ranging from having a trivial to an important impact on their lives. Utility values ranged from 0.38 to 0.99 for VKA treatment (296 participants from 4 studies).25,29,32,33 For LMWH treatment, a range of 0.66 to 0.99 was reported in the general population (72 participants from 2 studies)25,33 and 83 (IQR, 70-90) was reported in pregnant women (123 participants from 1 study).24 Overall, results showed considerable variability, and certainty was judged as moderate or low. The observed variability is explained, in part, by the different approaches used to determine the RIO and, in part, by the diversity of the respondents, including the general population, patients who recently started treatment, and patients with long-term treatment experience.
Nonutility results
We identified 34 quantitative studies,24,32,35,38-65,80-82 including 6424 participants, reporting patients’ preferences or experiences with VTE management options. Tables 3 through 6 summarize the evidence and the certainty of RIOs for VTE management consequences (Table 3), burden of treatment (Table 4), different treatment alternatives (Table 5), and VTE diagnosis management (Table 6) classified by different subgroups of patients, such as medical or surgical patients receiving VTE prophylaxis, those receiving treatment of VTE, patients with cancer, or pregnant women. No evidence was identified for the population with heparin-induced thrombocytopenia or the pediatric population.
Table 3.
Summary of findings: nonutility results for VTE management
| Health state/outcome (categories of values and preferences) | Estimates | Certainty in evidence |
|---|---|---|
| No. of participants/studies | ||
| Prophylaxis | ||
| VTE risk reduction (Haac et al,49 Quante et al,56 Westrich et al38) | Patients highly value the benefits of VTE risk reduction of VTE prophylaxis. Preferences changed in favor of subcutaneous injections with an absolute risk reduction of only 1.27% in VTE, also with the assumption of a generally better effectivity (47.4% of patients). VAS for the importance of VTE prevention with mechanical devices: 7.09 (scale, 1-9). | ⊕⊕⊕⊕ High certainty due to serious RoB |
| 510 participants from 1 RCT and 2 cross-sectional studies | ||
| Treatment | ||
| VTE risk reduction (Lutsey et al52) | Patients held the greatest concern for recurrent VTE (33% of them being extremely concerned) and mortality (29%), regardless of which treatment they were prescribed | ⊕⊕⊕○ Moderate certainty due to RoB* |
| 519 participants from 1 cross-sectional study | ||
| Adverse events (Barcellona et al,42 Keita et al,29 Lutsey et al,52 O’Meara et al35) | Patients considered adverse events burdensome, and the majority would like to avoid them. Some patients are fearful of hemorrhagic events (21%-25%), although most patients (87%) who have had a negative episode are “not afraid of” negative consequences. | ⊕⊕⊕○ Moderate certainty due to RoB† |
| 1019 participants from 4 cross-sectional studies | ||
| Cancer | ||
| VTE risk reduction and adverse events (Cajfinger et al,46 Noble et al54) | Patients place more importance on decreased risks for new or recurring blood clot than on decreased risks for minor and major bleeding | ⊕⊕⊕○ Moderate certainty due to RoB‡ |
| 509 participants from 2 cross-sectional studies |
High certainty in evidence: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty in evidence: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
RCT, randomized controlled trial; VAS, visual analog scale.
Lutsey et al52 directly asked which administration type the participants preferred.
Only 1 study (O’Meara et al35) judged as an overall low RoB. Two studies (Barcellona et al,42 O’Meara et al35) showed unclear response rates, and another study (Lutsey et al52) used unclear sampling methods. Two studies (Barcellona et al,42 Lutsey et al52) directly asked which administration route the participants preferred, without describing the possible consequence of the treatment. Therefore, the measurement instrument was judged to be at serious RoB for both studies, in addition to health state presentation for 1 of them (Barcellona et al42).
Cajfinger et al46 directly asked which administration route the participants would prefer, without describing the possible consequence of the treatment; the response rate was unknown in both studies.
Table 6.
Summary of findings: nonutility results for diagnostic management
| Health state/outcome (categories of values and preferences) | Estimates | Certainty in evidence |
|---|---|---|
| No. of participants/studies | ||
| Prophylaxis | ||
| Thrombophilia testing preferences (van Korlaar et al61) | Participants, members of families with heritable protein C deficiency who had not been tested before, were quite interested in getting a genetic test for protein C deficiency (mean, 4.6; standard deviation, 2.4 (on a scale from 1 to 7), with that decision being primarily a matter of concern for the family. | ⊕⊕○○ Low certainty due to serious RoB* and imprecision† |
| 168 participants from 1 cross-sectional study | ||
| Treatment | ||
| Diagnostic management preferences: false-negative results (Geyer et al48) | The majority (63%) of patients favored undergoing CTPA in a low pretest probability scenario. The most common cited factors for declining the test were risk of radiation-associated malignancy, contrast-induced nephropathy, or allergy. Others deferred CTPA testing because they believed it was unnecessary; however, patients with a previous PE diagnosis were less likely to defer CTPA. Most patients (85%) who accepted CTPA testing had concerns about missing a PE. | ⊕⊕⊕⊕ High certainty |
| 203 participants from 1 cross-sectional study |
High certainty in evidence: We are very confident that the true effect lies close to that of the estimate of the effect. Low certainty in evidence: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
CTPA, computed tomography pulmonary angiography.
van Korlaar et al61 was a study with unclear sampling methods, risk of measurement of instrument, and health state presentations.
Estimation referring to a subgroup of 76 participants who had not been previously tested.
Table 4.
Summary of findings: nonutility results for burden of treatments
| Health state/outcome (categories of values and preferences) | Estimates | Certainty in evidence |
|---|---|---|
| No. of participants/studies | ||
| Prophylaxis | ||
| Treatment burden of mechanical methods (Anand and Asumu,39 Brady et al,44 Chan et al80) | More patients wearing thigh-length SCDs and TEDs complained of discomfort compared with patients wearing knee-length SCDs and TEDs. Many patients using foot pumps reported sleep disturbance (range, 28%-57%), “heat intolerance” (43%), and found them soothing (54%). | ⊕⊕○○ Low certainty due to very serious RoB* and indirectness† |
| 210 participants from 3 cross-sectional studies | ||
| Treatment | ||
| Treatment burden of DOACs (Zolfaghari et al65) | Some patients (4/33; 12%) using DOACs may switch to VKAs due to complications, such as hair loss, after 9 mo of treatment or general discomfort, due to fear of side effects on DOACs, or because of no reimbursement from the health insurer. | ⊕⊕○○ Low certainty due to RoB‡ and imprecision§ |
| 19 participants (subgroup of 1001 participants study sample) from 1 cross-sectional study | ||
| Treatment burden of VKAs (Attaya et al,40 Brekelman et al,45 Elewa et al,47 Lutsey et al,52 Zolfaghari et al65) | Although routine monitoring does not represent a limitation for patients accepting the VKA, many patients would like to switch to an equally effective anticoagulant, primarily due to the burden associated with monitoring and dietary change (58%-64% of patients; mean willingness to change, 3.3 [on a scale of 1-5]). | ⊕⊕○○ Low certainty due to serious RoB¶ and inconsistency|| |
| 2070 participants from 5 cross-sectional studies | ||
| Treatment burden of ECSs (Bouman et al43) | Significant determinants of preference were PTS risk reduction, putting on ECSs, duration of ECS therapy, reduction in current complaints, comfort of wearing ECSs, and ease of washing ECSs. Cost and appearance of ECSs did not significantly influence preference. | ⊕⊕⊕○ Moderate due to serious RoB# |
| 300 participants from 1 RCT | ||
| Trade-off between treatment burden and benefits with VKAs (Locadia et al32) | Men were willing to take greater risks regarding recurrent VTE during cessation of treatment than women. Patients with a low educational level were more willing to opt for continuation of treatment, regardless of the risk for VTE, compared with patients with a medium or high educational level. | ⊕⊕⊕○ Moderate certainty due to serious imprecision** |
| 124 participants from 1 case-control study | ||
| Cancer | ||
| Treatment burden of injection (Lemke et al,51 Maxwell et al,53 Noble et al,54 Sousou and Khorana60) | Many patients (range across studies, 46%-55.7%) had a positive attitude toward receiving shots, and very few (4%) reported swelling, pain, or anxiety related to the shot. | ⊕⊕⊕○ Moderate certainty due to RoB†† |
| 601 participants from 4 cross-sectional studies | ||
| Treatment burden of EPCs (Maxwell et al53) | The majority of patients were satisfied with treatment with EPC, although 26% of patients experienced discomfort, inconvenience, problems, and/or side effects related to EPC. | ⊕⊕○○ Low certainty due to RoB† and imprecision‡‡ |
| 211 participants from 1 cross-sectional study | ||
| Trade-off between treatment burden and benefits with LMWH (Cajfinger et al,46 Noble et al54) | Cancer patients place highest value on “the interference with cancer treatment,” followed by “efficacy of the VTE treatment” and “risk for major bleeding.” They place low value on monitoring through blood tests, frequency of administration, mistakes, and costs. | ⊕⊕⊕○ Moderate certainty due to RoBa |
| 509 participants from 2 cross-sectional studies | ||
| Pregnancy | ||
| Willingness to be treated with LMWH (Bates et al24) | The majority of women with a previous VTE (76%) were willing to take LMWH prophylaxis throughout the antepartum period: 86% of women at high risk and 60% at low risk for recurrent VTE. The threshold reduction in VTE risk at which they would accept the use of LMWH was 2% higher for women with <2 wk of previous experience with LMWH during pregnancy (vs with >2 wk of LMWH experience) and 1.6% higher for those who were pregnant or planning pregnancy (vs neither pregnant nor planning a pregnancy). | ⊕⊕⊕○ Moderate certainty due to imprecision** |
| 123 participants from 1 cross-sectional study |
Moderate certainty in evidence: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty in evidence: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
DOAC, direct oral anticoagulant; ECS, elastic compression stocking; EPC, external pneumatic compression; RCT, randomized controlled trial; SCD, sequential compression device; TED, thromboembolic deterrent stocking.
Brady et al44 assessed SCDs and stockings, whereas the other 2 studies assessed foot pump devices.
The studies directly asked about the administration route the participants would prefer, without describing the possible consequence of the treatment. High RoB for health state presentation and measurement instrument.
Patients undergoing anticoagulation treatment, very likely in long-term treatment.
Although the overall sample size is large, this information comes from a subgroup of 19 patients who changed from DOACs to VKAs, not included in the final analysis.
Two studies directly asked which administration route the participants would prefer, without describing the possible consequence of the treatment, with high RoB for measurement instrument and unknown sampling methods and high risk in health state presentation.
Studies’ findings showing variability in the value of the monitoring burden.
Bouman et al43 discrete choice analysis of results with RoB bias due to unknown sampling methods and high risk in health state presentation.
Imprecision due to small sample size.
Sousou and Khorana60 with high RoB for health state presentation and, together with Maxwell et al53 and Lemke et al,51 measurement instrument; the sampling methods are unclear in 2 studies, and the response rate is unknown in 2 studies.
Maxwell et al53 used forced choice method to elicit values and preferences, and the response rate was not reported.
Cajfinger et al46 directly asked which administration route the participants would prefer, without describing the possible consequence of the treatment; the response rate is unknown in both studies.
Table 5.
Summary of findings: nonutility results for different treatment alternatives
| Health state/outcome (categories of values and preferences) | Estimates | Certainty in evidence |
|---|---|---|
| No. of participants/studies | ||
| Prophylaxis | ||
| Treatment method preferences: injections vs oral (Haac et al,49 Popoola et al,55 Quante et al,56 Sousou and Khorana,60 Wilke et al,62 Wong et al64) | The majority of patients prefer oral pills to subcutaneous injection (range, 60%-86%); the stated reasons include: easier to take and to integrate into the daily routine, less painful, and cost. For patients preferring the injection route, the reasons included faster onset of action, pill burden, and ease of use. | ⊕⊕⊕○ Moderate certainty due to serious RoB* |
| 1645 participants 1 RCT 3 cross-sectional studies 2 mixed-methods surveys | ||
| Treatment method preferences: LMWH vs EPC (Maxwell et al53) | The majority of patients were satisfied with the prophylactic method that they received to the extent that they would prefer the treatment they had received; a similar proportion of participants chose to receive the options they had received again after surgery (78% of those receiving LMWH and 74% of those receiving EPC). | ⊕⊕⊕○ Moderate certainty due to RoB† |
| 211 participants 1 cross-sectional study | ||
| Treatment method preferences: SCDs vs foot pumps (Roberston et al57) | Of a subgroup of 35 patients who had used both devices, 24 preferred the foot pump (69%), 7 preferred SCDs (20%), and 4 had no preference (11%). | ⊕○○○ Very low certainty due to serious RoB‡ and very serious imprecision§ |
| Subgroup of 35 participants who used both interventions from 1 cross-sectional study | ||
| Treatment management preferences: self-administration vs professional vs family/friend administration (Baba et al,41 Spahn82) | Variability in results, 1 study reporting a majority preference for professional or family administration of injections, whereas the other reported a majority preferring self-management. | ⊕⊕○○ Low certainty due to serious RoB¶ and inconsistency|| |
| 425 participants from 2 cross-sectional studies | ||
| Treatment | ||
| Treatment method preferences: warfarin vs DOACs (Lutsey et al52) | The majority of patients strongly prefer anticoagulants that are reversible (53%), and many of them (30%) prefer anticoagulants for which blood drug levels can be monitored. | ⊕⊕⊕○ Moderate due to serious RoB# |
| 519 participants from 1 cross-sectional study | ||
| Treatment method preferences: GCS type preferences (Lattimer et al,50 Williams et al63) | The majority (52%) of participants wanted to change their first assigned compression stocking, changes (in length, class, or size) may be required in determining a suitable GCS. Important variability is seen across studies: in 1 study, an above-knee thigh-length stocking was preferred by many patients (38%), whereas in the other study, all women preferred to wear below-knee stockings. | ⊕○○○ Very low certainty due to serious RoB** and very serious imprecision†† |
| 81 participants; 1 cohort study and 1 cross-sectional study | ||
| Management-type preferences/doctor-patient relationship (Barcellona et al42) | The doctor-patient relationship was considered very important by almost all patients (96%). They (93%) considered it important to be assessed by the doctor at the anticoagulation clinic and believed that doctors should always present the results personally (83%). | ⊕⊕⊕○ Moderate certainty due to RoB‡‡ |
| 264 participants from 1 cross-sectional study | ||
| Treatment management preferences: home based vs hospital based (Rymes et al59) | The majority of respondents preferred treatment at home (79%), and some expressed no preference (9%). Of all of these respondents, patients who had suffered a previous DVT and were treated in hospital stated that they preferred home treatment. | ⊕⊕○○ Low certainty due to very serious RoBa |
| 344 participants from 1 cross-sectional study | ||
| Cancer | ||
| Treatment method preferences: injections vs oral (Noble et al,54 Sousou and Khorana60) | Preference for oral administration over injection had moderate importance, although more patients were willing to use daily oral anticoagulants (86%) than to administer daily injections of anticoagulants (46%). | ⊕⊕⊕○ Moderate certainty due to RoBb |
| 290 participants from 2 cross-sectional studies | ||
| Treatment method preferences: LMWH vs EPC (Maxwell et al53) | The majority of patients were satisfied with the prophylactic method that they received to the extent that they would prefer the treatment they had received; a similar proportion of participants chose to receive the options they had received again after surgery (78% of those receiving LMWH and 74% of those receiving EPC). | ⊕⊕⊕○ Moderate certainty due to RoB† |
| 211 participants from 1 cross-sectional study | ||
| Treatment management preferences: self-administration vs professional/family administration (Baba et al41) | The majority of patients prefer a nurse to give the injection (40%), followed by family/friend (33.6%), and self-injection (25.6%). | ⊕⊕○○ Low certainty due to serious RoBc and imprecisiond |
| 160 participants from 1 cross-sectional study |
Moderate certainty in evidence: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty in evidence: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty in evidence: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
DOAC, direct oral anticoagulant; EPC, external pneumatic compression; GCS, graduated compression stockings; RCT, randomized controlled trial; SCD, sequential compression devices.
Popoola et al,55 Quante et al,56 Sousou and Khorana,60 and Wong et al64 directly asked which administration route the participants would prefer, without describing the possible consequence of the treatment.
Maxwell et al53 used forced choice method to elicit values and preferences, and the response rate was not reported.
Study directly asked which intervention the participants would prefer; potential bias due to measurement instrument, with only a very small subset of participants who had used both interventions.
Imprecision due to small sample size.
Studies directly asking which administration type the participants would prefer, with high RoB for measurement instrument, unclear sampling or response rates, and data analysis.
Variability in estimates: in 1 study, 38% preferred self-management, whereas in the other study, 92% preferred self-management.
Lutsey et al49 directly asked which administration type the participants would prefer.
The studies directly asked which administration route the participants would prefer, and the measurement instruments used are not validated.
Imprecision due to very small sample size.
Barcellona et al42 directly asked which administration route the participants would prefer, without describing the possible consequence of the treatment. High risk in health state presentation and instrument measurement.
The study directly asked which management option the participants would prefer, without describing the possible consequence of the treatment. High risk in health state presentation and instrument measurement.
Sousou and Khorana60 with high RoB for health state presentation and measurement instrument; the sampling methods are unclear in 2 studies, and the response rate is unknown in both studies.
Baba et al41 directly asked which type of care the participants would prefer, with high RoB for measurement instrument and unclear sampling and response rates.
Imprecision due to small sample size.
Studies assessing the RIOs that medical and surgical patients place on VTE prophylaxis benefits and adverse events showed that VTE risk reduction is a more important factor influencing their preferences than the potential harms of the treatment (510 participants from 3 studies; high certainty)38,49,56 (Table 3). The same was true for patients treated for VTE, who held greater concerns for recurrent VTE risk (519 participants from 1 study; moderate certainty).52 Studies also suggested that, although the majority of patients treated for VTE would like to avoid adverse events, only 21% to 25% are afraid of hemorrhagic events. Moreover, 87% of patients who had experienced a negative episode reported being “not afraid of” negative consequences (1019 participants from 4 studies; moderate certainty)29,35,42,52 (Table 3). Similarly, studies including cancer patients and patients with cancer-related thrombosis suggest “the interference with cancer treatment” is the most important attribute influencing their preferences for the medication, followed by “efficacy of the VTE treatment” and “the risk of major bleeding” (509 participants from 2 conjoint analyses; moderate certainty)46,54 (Table 3).
Sixteen studies evaluated patient RIOs for the burden associated with treatments.24,32,39,40,43-47,51-54,60,65,80 One study, including patients taking long-term anticoagulation, reported that only some (12%) patients using direct oral anticoagulants (DOACs) may switch to VKAs because of complications and fear of adverse effects, as well as for not being reimbursed (subgroup of 19 participants from 1 study including 1001 total participants; low certainty)65 (Table 4). With regard to the use of oral VKAs in general, although routine monitoring does not seem to represent a limitation for patients accepting it, many (58-64%) would switch to another anticoagulant if it were equally effective and required less monitoring or dietary restrictions (2070 participants from 5 studies; low certainty)40,45,47,52,65 (Table 4). Two studies in cancer patients receiving LMWH treatment also showed lower RIOs for attributes such as monitoring through blood tests, frequency of administration, mistakes, and costs (509 participants from 2 studies; moderate certainty)46,54 (Table 4). One study evaluating elastic compression stockings showed that participants would accept an increase in the risk of complications if they could put them on by themselves (300 participants from 1 study; moderate certainty)43 (Table 4, treatment subgroup). Another study using a direct-choice exercise showed that, a majority of women with a previous VTE, who were at low or high risk for recurrent VTE (86% and 60%, respectively), and who were pregnant or planning a pregnancy, were willing to take LMWH (123 participants from 1 study; moderate certainty)24 (Table 4).
Sixteen studies, including 3452 participants, assessed RIOs for different treatment alternatives (Table 5).41,42,49,50,52-57,59,60,62-64,82 Six of those studies evaluated the preference for injections or oral administration routes in 1645 medical or surgical patients receiving prophylaxis.49,55,56,60,62,64 There was a clear preference for oral medication (range, 60-86% of patients) over subcutaneous injection, because it is easier to take and to integrate into the daily routine, and it is also perceived as less painful and less expensive. For participants preferring the injection route, the stated reasons for the preferences included faster onset of action, avoidance of pill burden, and ease of use (1645 participants from 6 studies; moderate certainty).
Lastly, we found 1 study addressing patient preferences related to different diagnostic management options. A survey assessed preferences for the use of computed tomography pulmonary angiography (CTPA) for PE diagnosis in a hypothetical scenario of low pretest probability compared with not using it when results of D-dimer testing left patients with uncertainty. The majority of participants (63%) favored undergoing CTPA, regardless of its adverse effects. Most patients (85%) who accepted CTPA testing had concerns about missing a PE (203 participants from 1 study; high certainty)48 (Table 6).
Qualitative research evidence.
The 15 included qualitative studies involved individuals (570 participants) who had, or were at risk for having, VTE.54,66-79 Supplemental Table 5 summarizes the individual study characteristics, and supplemental Table 6 provides a descriptive summary of the study designs, qualitative methodologies, and study locations. Supplemental Table 7 reports a narrative assessment of the major strengths and weaknesses of included studies, based on the CASP Qualitative Checklist tool.23
Table 7 outlines the 4 analytic themes that summarize patients’ experiences and perspectives when thinking about VTE disease management. For each theme, we describe how patients understand and experience the intervention and its outcomes. In the following sections, we summarize the 2 analytic themes related to the importance of outcomes.
Table 7.
Qualitative findings: main analytic themes and quotes
| Themes | Quotes |
|---|---|
| Disease and treatment benefits and burden | I hope it’s positive because I want to do the [LWMH] … you know, 'cause I’m scared to have a miscarriage.71 |
| …operation, colostomy, chemotherapy, and radio (LMWH) is a walk in the park compared with what I've been through76 | |
| Because I do it as soon as I get up and then if I’ve got to go anywhere it’s all done. Done and dusted.54 | |
| I can't beat the tumor but I can fight its effects…the heparin helps me face the future. I choose to inject myself because I feel I can face the future on my own terms…the DVT was terrible, I couldn't face the day.75 | |
| The timing is very pressuring, he told me that an hour [before or after] it doesn’t make a difference, but it is a big pressure for me to remember every single day at the same time…and what happens if I forget 1 day…the pressure that I shouldn’t forget, this is the most hard thing.71 | |
| And every time I had them [panic attacks], I kept thinking it was a PE so I was having a panic attack. All the time! Every day! I was…it was just ridiculous. And I just felt like it was a big dark cloud [starts to cry].68 | |
| Healthcare provider communication and relationship | I took them because my doctor advised it. And I had a lot of confidence in him based on my primary care physician, her very high recommendation.74 |
| It’s sort of reassuring knowing that people are still doing something for me. People keep talking to me about controlling my symptoms. It seems just as important to me to prevent anything that may cause bad symptoms.76 | |
| I’m sure they were giving me something, they were giving me various pills, but again, they didn’t necessarily tell me, as I recall, what it was for.66 | |
| I’ve got the option to ring if I need to speak to somebody. If you were to remove that option, then I think…although I don’t think about it on a day-today basis, I think…a piece of me would be quite worried.70 | |
| Awareness and perception of risk | Well, if it keeps me alive it’s as simple as that. I’d take poison to stay alive. It’s not nice, you’re tired every morning, pants down and injection but there you go – that’s life, isn’t it, and as I say, it’s keeping me alive, so that’s the important bit.78 |
| Not having a stroke or a heart attack or a PE! Or having a clot so big that it blocks your leg. Then you have to have it taken care of.74 | |
| I know generally if I’m high because my gums might start bleeding a bit, so I’ll probably have a test then. All the little tell-tale.70 | |
| I do know that they can make you bleed too much. That’s one of the biggest. Sometimes you don’t know because it can be internal you know and you can bleed into your gut.74 | |
| Day-to-day routine | I do ‘em, you know, it’s to suit me. So, I do ‘em in the morning. And the day’s my own then like, do you know what I mean?54 |
| I usually take them between 8 and half past 8. And then I know it’s done, and I don’t forget for the day, then, because someone I was talking to, he was saying “You don’t do it in the night, do you?” and I said, “No, I get up, have my cup of tea then 8, half past 8 do it.”78 |
Disease and treatment benefits and burden
Diagnosis of VTE and treatment benefits brought feelings of relief, empowerment, and control54,70,71,74,75,78 that increased patient motivation and self-discipline to initiate and pursue the treatment while acknowledging its inconveniences. VTE treatment provided a solution to live without fear and restrictions68,70; patients described treatment as going “back to normal”54,67,76-78 by learning to self-manage their condition and reprioritize their life goals and habits.70,71,74,77 Patients expressed preferences for heparin injections, because these relieved them from the burdens of constant international normalized ratio monitoring.54,75,76 For pregnant women, the diagnosis also provided reasons for past miscarriages and the treatment hopes for a healthy current pregnancy.71
In contrast, patients revealed feelings of distress, emotional discomfort, and hopelessness due to the unpredictability of the disease and recurrence of VTE episodes.66,70,71,78 Most patients described the fear of the uncertainty of bleeding events, sudden death,66,74,76 thrombophilia diagnosis, and the distressful feelings caused by misdiagnosis.54,68,71 The burden of daily self-injections, the discomfort of stocking use, and the antithrombotic treatments’ side effects, such as the bleeding risk and bruising events, were concerns related to the treatment raised across the included studies.67,68,74,79
Awareness and perceptions of risk
Personal risk assessment of VTE events influences patients’ treatment preferences and decisions for undergoing prophylaxis and treatment. When patients lack clarity in understanding the risks of the disease,72,76 the need for treatment,54,66,67,71,72,74,76 and the treatment’s side effects,66,74,76 initiating treatment in the absence of symptoms might be confusing.54,66,67,74,76 Overall, understanding the rationale and benefits for using anticoagulation treatment made patients more aware and accepting of the need for the intervention.66,70,72,74,76,78
Discussion
We systematically reviewed quantitative and qualitative evidence of patients’ values and preferences related to VTE. The review directly informed ASH guidelines for management of VTE, aiming to inform guideline panel members’ decision making about the balance between benefits and harms in prophylaxis, treatment, and diagnosis of VTE.
Strengths and weaknesses
This systematic review has a number of strengths. First, we used a comprehensive literature search strategy and set no time or language limit on the search, ensuring the comprehensiveness and directness of summarized evidence specific to VTE. We developed a machine-learning algorithm to balance efficiency and accuracy of screening a broad area of the literature. The training and calibration exercise, together with the 10 196 records initially screened for the algorithm development, gave us high confidence in the accuracy of the process. Second, we used the GRADE concept of outcome importance to determine the eligibility criteria, including studies reporting the importance that patients place on health outcomes, as well as studies about preferences for or against a treatment, because they implicitly provide us the RIOs.12 We also summarized the results within the GRADE Evidence-to-Decision framework for guideline decision making.10,14
Moreover, this review includes quantitative and qualitative research evidence on the topic, which enabled us to reach a wide breadth and depth of understanding and substantive knowledge of patients’ values and preferences. Finally, for the quantitative studies, we assessed the RoB of the included individual studies, as well as the certainty of the overall body of evidence. For qualitative research, we used the CASP Qualitative Checklist tool to appraise the quality of each included study to help readers assess the trustworthiness of our synthesis.23
Our study also has limitations. Because of time constraints, we used the machine-learning model to rule out irrelevant records, which was only validated in a set of the complete reference list. However, to overcome the risk of losing relevant information, we consulted our guideline panels and compared our included studies list with other systematic reviews on similar topics84-87 to identify potentially missed relevant studies. Additionally, although we included quantitative and qualitative studies, the search strategy may not have been sensitive enough to ensure the identification of the full body of qualitative evidence, and the inclusion of these types of studies could be considered unsystematic. Nevertheless, our aim of aggregating identified qualitative studies’ findings was to complement quantitative findings rather than synthesizing the entire body of evidence. We consider our approach appropriate to summarize patient values and preferences related to VTE management, although we also acknowledge the opportunity for further improvement in the synthesis of the qualitative body of evidence.
Principal findings
Findings from quantitative research studies show high variability in the utility values for DVT, PE, and PTS, with a certainty of evidence varying from low to moderate, primarily due to the diverse methods used to obtain the RIOs and the diversity of the patient populations included in the studies. Overall, the studies suggest VTE-related health states have small to important impacts on patients’ lives. Qualitative findings also corroborate quantitative results regarding patients’ preferences for oral medication over subcutaneous medication. However, because all of the included qualitative studies focused on VKAs, which still require constant international normalized ratio monitoring, rather than on DOACs, as well as on subcutaneous injections, overall, they report similar preferences for treatment modalities that do not require monitoring or dietary changes. Finally, quantitative and qualitative findings reveal patients’ positive perceptions of treatment and diagnosis when informed and when included in the treatment decision-making process.
Context in relation to the body of literature
In this systematic review, we only included studies on VTE, excluding other diseases for which anticoagulation treatment might also be indicated. Results regarding RIOs were similar to another systematic review88 that included, among other diseases, 3 studies on VTE disease, illustrating the significant variability in patients’ values and preferences regarding prophylaxis and treatment. In our systematic review, the highly sensitive search strategy allowed us to identify and include more studies, and the new machine-learning approach positively impacted the efficiency of the screening process.
In conclusion, the results of our review show high variability in how patients value the impact of VTE-related health outcomes, which is partially explained by the different approaches used to determine the RIOs and the diversity of the included participants. These findings highlight the necessity to explore methods-induced variability, which might be facilitated by the use of health outcome descriptors, patient scenarios, or hypothetical health states,89,90 to differentiate true variability from methods-induced variability.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Rachel Couban (McMaster University) for reviewing the search strategy and translating it into PsycINFO and Cumulative Index to Nursing and Allied Health Literature database languages and Daniel Perez Rada (Epistemonikos Foundation) and Aldo Canepa (Epistemonikos Foundation) for setting up and running the Collaboratron platform.
This work was supported by ASH to inform guideline recommendations in the ASH Guidelines for VTE Management.
Footnotes
Data sharing requests should be sent to Itziar Etxeandia-Ikobaltzeta (e-mail: itzi.etxe@gmail.com).
Authorship
Contribution: Yuan Zhang, I.E.-I., and H.J.S. designed the study; I.E.-I., Yuan Zhang, W.W., R.N., F.B., I.D.F., H.B., C.A.C., Y. Roldan, R. Chen, C.D., R.L.M., J.J.R., Yuqing Zhang, R. Charide, A.A., S.B., G.P.M., J.J.Y.-N., Y. Rehman, I.N., N.S., T.B., C.B., and M.F.R. screened the literature and/or abstracted the data; I.E.-I., Yuan Zhang, F.B., I.D.F., and H.J.S. drafted the manuscript; H.J.S., W.W., and R.N. coordinated the overall project; H.J.S. is cochair of the GRADE working group and was the principal investigator for this project; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Itziar Etxeandia-Ikobaltzeta, McMaster University, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; e-mail: itzi.etxe@gmail.com.
References
- 1.Bates SM, Rajasekhar A, Middeldorp S, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuker A, Arepally GM, Chong BH, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim W, Le Gal G, Bates SM, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2(22):3226-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monagle P, Cuello CA, Augustine C, et al. . American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schünemann HJ, Cushman M, Burnett AE, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witt DM, Nieuwlaat R, Clark NP, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DR, Morgano GP, Bennett C, et al. . American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3(23):3898-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schunemann HJ, Wiercioch W, Etxeandia I, et al. . Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186(3):E123-E142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso-Coello P, Schünemann HJ, Moberg J, et al. ; GRADE Working Group . GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group . GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. [DOI] [PubMed] [Google Scholar]
- 11.Schünemann Brożek H J, Guyatt G, Oxman A, eds. GRADE Handbook: Introduction to GRADE Handbook. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. Accessed 2 October 2016.
- 12.Zhang Y, Coello PA, Brożek J, et al. . Using patient values and preferences to inform the importance of health outcomes in practice guideline development following the GRADE approach. Health Qual Life Outcomes. 2017;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Weijden T, Légaré F, Boivin A, et al. . How to integrate individual patient values and preferences in clinical practice guidelines? A research protocol. Implement Sci. 2010;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews JC, Schünemann HJ, Oxman AD, et al. . GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726-735. [DOI] [PubMed] [Google Scholar]
- 15.Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310(23):2503-2504. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence M, Kinn S. Defining and measuring patient-centred care: an example from a mixed-methods systematic review of the stroke literature. Health Expect. 2012;15(3):295-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selva A, Solà I, Zhang Y, et al. . Development and use of a content search strategy for retrieving studies on patients’ views and preferences. Health Qual Life Outcomes. 2017;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charmaz K. Constructing Grounded Theory. London: United Kingdom: SAGE Publications Ltd.; 2014. [Google Scholar]
- 19.Sandelowski M, Barroso J. Toward a metasynthesis of qualitative findings on motherhood in HIV-positive women. Res Nurs Health. 2003;26(2):153-170. [DOI] [PubMed] [Google Scholar]
- 20.Lewin S, Bohren M, Rashidian A, et al. . Applying GRADE-CERQual to qualitative evidence synthesis findings-paper 2: how to make an overall CERQual assessment of confidence and create a Summary of Qualitative Findings table. Implement Sci. 2018;13(suppl 1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Alonso Coello P, Guyatt G, et al. . GRADE Guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferences–risk of bias and indirectness. J Clin Epidemiol. 2019;111:94-104. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Alonso Coello P, Guyatt G, et al. . GRADE Guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences; inconsistency, imprecision, and other domains. J Clin Epidemiol. 2019;111:83-93. [DOI] [PubMed] [Google Scholar]
- 23.Clinical Appraisal Skills Programme CASP Qualitative Checklist. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist.pdf. Accessed 12 October 2018.
- 24.Bates SM, Alonso-Coello P, Tikkinen KA, et al. . Women’s values and preferences and health state valuations for thromboprophylaxis during pregnancy: a cross-sectional interview. Thromb Res. 2016;140:22-29. [DOI] [PubMed] [Google Scholar]
- 25.Dranitsaris G, Shane L, Burgers L, Woodruff S. Economic analysis comparing dalteparin to vitamin K antagonists to prevent recurrent venous thromboembolism in patients with cancer having renal impairment. Clin Appl Thrombosis/Hemost. 2016;22(7):617-626. [DOI] [PubMed] [Google Scholar]
- 26.Enden T, Wik HS, Kvam AK, Haig Y, Kløw NE, Sandset PM. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study. BMJ Open. 2013;3(8):e002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg K, Kimpton M, Carrier M, Coyle D, Forgie M, Wells P. Estimating quality of life in acute venous thrombosis. JAMA Intern Med. 2013;173(12):1067-1072. [DOI] [PubMed] [Google Scholar]
- 28.Hogg K, Shaw J, Coyle D, Fallah P, Carrier M, Wells P. Validity of standard gamble estimated quality of life in acute venous thrombosis. Thromb Res. 2014;134(4):819-825. [DOI] [PubMed] [Google Scholar]
- 29.Keita I, Aubin-Auger I, Lalanne C, et al. . Assessment of quality of life, satisfaction with anticoagulation therapy, and adherence to treatment in patients receiving long-course vitamin K antagonists or direct oral anticoagulants for venous thromboembolism. Patient Prefer Adherence. 2017;11:1625-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc. 1997;4(1):49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd AJ, Dewilde S, Noble S, Reimer E, Lee AYY. What impact does venous thromboembolism and bleeding have on cancer patients’ quality of life? Value Health. 2018;21(4):449-455. [DOI] [PubMed] [Google Scholar]
- 32.Locadia M, Bossuyt PM, Stalmeier PF, et al. . Treatment of venous thromboembolism with vitamin K antagonists: patients’ health state valuations and treatment preferences. Thromb Haemost. 2004;92(6):1336-1341. [DOI] [PubMed] [Google Scholar]
- 33.Marchetti M, Pistorio A, Barone M, Serafini S, Barosi G. Low-molecular-weight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: a cost-effectiveness analysis. Am J Med. 2001;111(2):130-139. [DOI] [PubMed] [Google Scholar]
- 34.Marvig CL, Verhoef TI, de Boer A, et al. ; EU-PACT consortium . Quality of life in patients with venous thromboembolism and atrial fibrillation treated with coumarin anticoagulants. Thromb Res. 2015;136(1):69-75. [DOI] [PubMed] [Google Scholar]
- 35.O’Meara JJ III, McNutt RA, Evans AT, Moore SW, Downs SM. A decision analysis of streptokinase plus heparin as compared with heparin alone for deep-vein thrombosis. N Engl J Med. 1994;330(26):1864-1869. [DOI] [PubMed] [Google Scholar]
- 36.Tavoly M, Utne KK, Jelsness-Jørgensen LP, et al. . Health-related quality of life after pulmonary embolism: a cross-sectional study. BMJ Open. 2016;6(11):e013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utne KK, Tavoly M, Wik HS, et al. . Health-related quality of life after deep vein thrombosis. Springerplus. 2016;5(1):1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westrich GH, Jhon PH, Sánchez PM. Compliance in using a pneumatic compression device after total knee arthroplasty. Am J Orthop. 2003;32(3):135-140. [PubMed] [Google Scholar]
- 39.Anand S, Asumu T. Patient acceptance of a foot pump device used for thromboprophylaxis. Acta Orthop Belg. 2007;73(3):386-389. [PubMed] [Google Scholar]
- 40.Attaya S, Bornstein T, Ronquillo N, et al. . Study of warfarin patients investigating attitudes toward therapy change (SWITCH Survey). Am J Ther. 2012;19(6):432-435. [DOI] [PubMed] [Google Scholar]
- 41.Baba M, Al-Masri M, Salhab M, El-Ghanem M. Patient’s compliance on the use of extended low molecular weight heparin post major pelvic surgeries in cancer patients at King Hussein Cancer Center. Gulf J Oncolog. 2015;1(17):73-81. [PubMed] [Google Scholar]
- 42.Barcellona D, Contu P, Sorano GG, Pengo V, Marongiu F. The management of oral anticoagulant therapy: the patient’s point of view. Thromb Haemost. 2000;83(1):49-53. [PubMed] [Google Scholar]
- 43.Bouman AC, Ten Cate-Hoek AJ, Dirksen CD, Joore MA. Eliciting patients’ preferences for elastic compression stocking therapy after deep vein thrombosis: potential for improving compliance. J Thromb Haemost. 2016;14(3):510-517. [DOI] [PubMed] [Google Scholar]
- 44.Brady D, Raingruber B, Peterson J, et al. . The use of knee-length versus thigh-length compression stockings and sequential compression devices. Crit Care Nurs Q. 2007;30(3):255-262. [DOI] [PubMed] [Google Scholar]
- 45.Brekelmans MP, Kappelhof M, Nieuwkerk PT, Nierman M, Buller HR, Coppens M. Preference for direct oral anticoagulants in patients treated with vitamin K antagonists for venous thromboembolism. Neth J Med. 2017;75(2):50-55. [PubMed] [Google Scholar]
- 46.Cajfinger F, Debourdeau P, Lamblin A, et al. ; TROPIQUE investigators . Low-molecular-weight heparins for cancer-associated thrombosis: adherence to clinical practice guidelines and patient perception in TROPIQUE, a 409-patient prospective observational study. Thromb Res. 2016;144:85-92. [DOI] [PubMed] [Google Scholar]
- 47.Elewa HF, DeRemer CE, Keller K, Gujral J, Joshua TV. Patients satisfaction with warfarin and willingness to switch to dabigatran: a patient survey. J Thromb Thrombolysis. 2014;38(1):115-120. [DOI] [PubMed] [Google Scholar]
- 48.Geyer BC, Xu M, Kabrhel C. Patient preferences for testing for pulmonary embolism in the ED using a shared decision-making model. Am J Emerg Med. 2014;32(3):233-236. [DOI] [PubMed] [Google Scholar]
- 49.Haac BE, O’Hara NN, Mullins CD, et al. . Patient preferences for venous thromboembolism prophylaxis after injury: a discrete choice experiment [published correction appears in BMJ Open. 2017;7(12):e016676corr1]. BMJ Open. 2017;7(8):e016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lattimer CR, Azzam M, Kalodiki E, Makris GC, Geroulakos G. Compression stockings significantly improve hemodynamic performance in post-thrombotic syndrome irrespective of class or length. J Vasc Surg. 2013;58(1):158-165. [DOI] [PubMed] [Google Scholar]
- 51.Lemke M, Beyfuss K, Hallet J, Coburn NG, Law CH, Karanicolas PJ. Patient adherence and experience with extended use of prophylactic low-molecular-weight heparin following pancreas and liver resection. J Gastrointestinal Surg. 2016;20(12):1986-1996. [DOI] [PubMed] [Google Scholar]
- 52.Lutsey PL, Horvath KJ, Fullam L, et al. . Anticoagulant preferences and concerns among venous thromboembolism patients. Thromb Haemost. 2018;118(3):553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. [DOI] [PubMed] [Google Scholar]
- 54.Noble S, Matzdorff A, Maraveyas A, Holm MV, Pisa G. Assessing patients’ anticoagulation preferences for the treatment of cancer-associated thrombosis using conjoint methodology. Haematologica. 2015;100(11):1486-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popoola VO, Lau BD, Shihab HM, et al. . Patient preferences for receiving education on venous thromboembolism prevention - a survey of stakeholder organizations. PLoS One. 2016;11(3):e0152084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quante M, Thate-Waschke I, Schofer M. [What are the reasons for patient preference? A comparison between oral and subcutaneous administration]. Z Orthop Unfall. 2012;150(4):397-403. [DOI] [PubMed] [Google Scholar]
- 57.Robertson KA, Bertot AJ, Wolfe MW, Barrack RL. Patient compliance and satisfaction with mechanical devices for preventing deep venous thrombosis after joint replacement. J South Orthop Assoc. 2000;9(3):182-186. [PubMed] [Google Scholar]
- 58.Robinson AM, McLean KA, Greaves M, Channer KS. Subcutaneous versus intravenous administration of heparin in the treatment of deep vein thrombosis; which do patients prefer? A randomized cross-over study. Postgrad Med J. 1993;69(808):115-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rymes NL, Lester W, Connor C, Chakrabarti S, Fegan CD. Outpatient management of DVT using low molecular weight heparin and a hospital outreach service. Clin Lab Haematol. 2002;24(3):165-170. [DOI] [PubMed] [Google Scholar]
- 60.Sousou T, Khorana AA. Cancer patients and awareness of venous thromboembolism. Cancer Invest. 2010;28(1):44-45. [DOI] [PubMed] [Google Scholar]
- 61.van Korlaar IM, Vossen CY, Rosendaal FR, et al. . Attitudes toward genetic testing for thrombophilia in asymptomatic members of a large family with heritable protein C deficiency. J Thromb Haemost. 2005;3(11):2437-2444. [DOI] [PubMed] [Google Scholar]
- 62.Wilke T, Tesch S, Scholz A, Kohlmann T, Greinacher A. The costs of heparin-induced thrombocytopenia: a patient-based cost of illness analysis. J Thromb Haemost. 2009;7(5):766-773. [DOI] [PubMed] [Google Scholar]
- 63.Williams LA, Owen TD. Above-knee versus below-knee stockings in total knee arthroplasty. Ann R Coll Surg Engl. 2006;88(3):302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong A, Kraus PS, Lau BD, et al. . Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015;10(2):108-111. [DOI] [PubMed] [Google Scholar]
- 65.Zolfaghari S, Harenberg J, Frölich L, et al. . Development of recommendations to continue anticoagulation with one of the two types of oral anticoagulants based on the identification of patients’ preference. Semin Thromb Hemost. 2015;41(2):166-177. [DOI] [PubMed] [Google Scholar]
- 66.Apenteng PN, Fitzmaurice D, Litchfield I, et al. . Patients’ perceptions and experiences of the prevention of hospital-acquired thrombosis: a qualitative study. BMJ Open. 2016;6(12):e013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haxaire C, Tromeur C, Couturaud F, Leroyer C. A qualitative study to appraise patients and family members perceptions, knowledge, and attitudes towards venous thromboembolism risk. PLoS One. 2015;10(11):e0142070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunter R, Lewis S, Noble S, Rance J, Bennett PD. “Post-thrombotic panic syndrome”: a thematic analysis of the experience of venous thromboembolism. Br J Health Psychol. 2017;22(1):8-25. [DOI] [PubMed] [Google Scholar]
- 69.Kline JA, Runyon MS, Webb WB, Jones AE, Mitchell AM. Prospective study of the diagnostic accuracy of the simplify D-dimer assay for pulmonary embolism in emergency department patients. Chest. 2006;129(6):1417-1423. [DOI] [PubMed] [Google Scholar]
- 70.Kuljis J, Money AG, Perry M, Barnett J, Young T. Technology-assisted self-testing and management of oral anticoagulation therapy: a qualitative patient-focused study. Scand J Caring Sci. 2017;31(3):603-617. [DOI] [PubMed] [Google Scholar]
- 71.Martens TZ, Emed JD. The experiences and challenges of pregnant women coping with thrombophilia. J Obstet Gynecol Neonatal Nurs. 2007;36(1):55-62. [DOI] [PubMed] [Google Scholar]
- 72.May V, Clarke T, Coulling S, et al. . What information patients require on graduated compression stockings. Br J Nurs. 2006;15(5):263-270. [DOI] [PubMed] [Google Scholar]
- 73.Mockler A, O’Brien B, Emed J, Ciccotosto G. The experience of patients with cancer who develop venous thromboembolism: an exploratory study. Oncol Nurs Forum. 2012;39(3):E233-E240. [DOI] [PubMed] [Google Scholar]
- 74.Najafzadeh M, Kim SC, Patterson C, et al. . Patients’ perception about risks and benefits of antithrombotic treatment for the prevention of venous thromboembolism (VTE) after orthopedic surgery: a qualitative study. BMC Musculoskelet Disord. 2015;16(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noble SI, Finlay IG. Is long-term low-molecular-weight heparin acceptable to palliative care patients in the treatment of cancer related venous thromboembolism? A qualitative study. Palliat Med. 2005;19(3):197-201. [DOI] [PubMed] [Google Scholar]
- 76.Noble SIR, Nelson A, Turner C, Finlay IG. Acceptability of low molecular weight heparin thromboprophylaxis for inpatients receiving palliative care: qualitative study. BMJ. 2006;332(7541):577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saukko PM, Ellard S, Richards SH, Shepherd MH, Campbell JL. Patients’ understanding of genetic susceptibility testing in mainstream medicine: qualitative study on thrombophilia. BMC Health Serv Res. 2007;7(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seaman S, Nelson A, Noble S. Cancer-associated thrombosis, low-molecular-weight heparin, and the patient experience: a qualitative study. Patient Prefer Adherence. 2014;8:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wild D, Murray M, Donatti C. Patient perspectives on taking vitamin K antagonists: a qualitative study in the UK, USA and Spain. Expert Rev Pharmacoecon Outcomes Res. 2009;9(5):467-474. [DOI] [PubMed] [Google Scholar]
- 80.Chan JC, Roche SJ, Lenehan B, O’sullivan M, Kaar K. Compliance and satisfaction with foot compression devices: an orthopaedic perspective. Arch Orthop Trauma Surg. 2007;127(7):567-571. [DOI] [PubMed] [Google Scholar]
- 81.Chiou-Tan FY, Garza H, Chan KT, et al. . Comparison of dalteparin and enoxaparin for deep venous thrombosis prophylaxis in patients with spinal cord injury. Am J Phys Med Rehabil. 2003;82(9):678-685. [DOI] [PubMed] [Google Scholar]
- 82.Spahn G. Compliance with self-administration of heparin injections in outpatients. Eur J Trauma. 2002;28(2):104-109. [Google Scholar]
- 83.National Clinical Guideline Centre Venous Thromboembolism: Reducing the Risk of Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Hospital. https://www.ncbi.nlm.nih.gov/books/NBK116518/pdf/Bookshelf_NBK116518.pdf. Accessed 2 October 2016.
- 84.Barnes GD, Izzo B, Conte ML, Chopra V, Holbrook A, Fagerlin A. Use of decision aids for shared decision making in venous thromboembolism: a systematic review. Thromb Res. 2016;143:71-75. [DOI] [PubMed] [Google Scholar]
- 85.McLean S, Ryan K, O’Donnell JS. Primary thromboprophylaxis in the palliative care setting: a qualitative systematic review. Palliat Med. 2010;24(4):386-395. [DOI] [PubMed] [Google Scholar]
- 86.Mott DJ, Najafzadeh M. Whose preferences should be elicited for use in health-care decision-making? A case study using anticoagulant therapy. Expert Rev Pharmacoecon Outcomes Res. 2016;16(1):33-39. [DOI] [PubMed] [Google Scholar]
- 87.Wade R, Sideris E, Paton F, et al. . Graduated compression stockings for the prevention of deep-vein thrombosis in postoperative surgical patients: a systematic review and economic model with a value of information analysis. Health Technol Assess. 2015;19(98):1-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacLean S, Mulla S, Akl EA, et al. . Patient values and preferences in decision making for antithrombotic therapy: a systematic review: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest. 2012e1S-e23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bremner KE, Tomlinson G, Krahn MD. Marker states and a health state prompt provide modest improvements in the reliability and validity of the standard gamble and rating scale in prostate cancer patients. Qual Life Res. 2007;16(10):1665-1675. [DOI] [PubMed] [Google Scholar]
- 90.Schünemann HJ, Goldstein R, Mador MJ, et al. . Do clinical marker states improve responsiveness and construct validity of the standard gamble and feeling thermometer: a randomized multi-center trial in patients with chronic respiratory disease. Qual Life Res. 2006;15(1):1-14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.