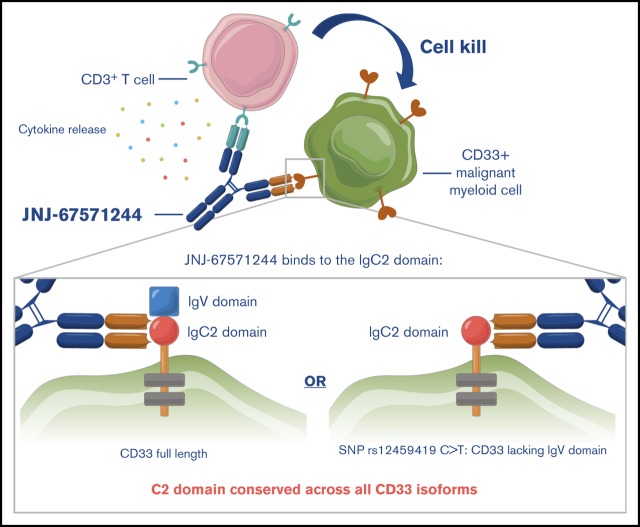
Key Points
JNJ-67571244 binds the C2 domain of CD33 and mediates killing of AML cells independently of the genotype status of SNP rs12459419.
JNJ-67571244 is currently in phase 1 clinical trials in patients with relapsed/refractory AML and high-risk myelodysplastic syndrome.
Abstract
CD33 is expressed in 90% of patients with acute myeloid leukemia (AML), and its extracellular portion consists of a V domain and a C2 domain. A recent study showed that a single nucleotide polymorphism (SNP), rs12459419 (C > T), results in the reduced expression of V domain–containing CD33 and limited efficacy of V domain–binding anti-CD33 antibodies. We developed JNJ-67571244, a novel human bispecific antibody capable of binding to the C2 domain of CD33 and to CD3, to induce T-cell recruitment and CD33+ tumor cell cytotoxicity independently of their SNP genotype status. JNJ-67571244 specifically binds to CD33-expressing target cells and induces cytotoxicity of CD33+ AML cell lines in vitro along with T-cell activation and cytokine release. JNJ-67571244 also exhibited statistically significant antitumor activity in vivo in established disseminated and subcutaneous mouse models of human AML. Furthermore, this antibody depletes CD33+ blasts in AML patient blood samples with concurrent T-cell activation. JNJ-67571244 also cross-reacts with cynomolgus monkey CD33 and CD3, and dosing of JNJ-67571244 in cynomolgus monkeys resulted in T-cell activation, transient cytokine release, and sustained reduction in CD33+ leukocyte populations. JNJ-67571244 was well tolerated in cynomolgus monkeys up to 30 mg/kg. Lastly, JNJ-67571244 mediated efficient cytotoxicity of cell lines and primary samples regardless of their SNP genotype status, suggesting a potential therapeutic benefit over other V-binding antibodies. JNJ-67571244 is currently in phase 1 clinical trials in patients with relapsed/refractory AML and high-risk myelodysplastic syndrome.
Visual Abstract
Introduction
Acute myeloid leukemia (AML) is a genetically heterogeneous disease characterized by clonal expansion of leukemic cells. Despite an increased understanding of the underlying disease biology in AML, the standard treatment with cytotoxic chemotherapy has remained largely unchanged over the last decades, and the overall 5-year survival remains poor (<30%).1,2 Thus, there is a pressing need for novel therapies with increased efficacy and decreased toxicity.
CD33 is a 67 kD single-pass transmembrane glycoprotein and is a member of the sialic acid–binding immunoglobulin-like lectins family. Expression of CD33 is restricted to the hematopoietic lineage,3,4 with low levels present in myeloid progenitors, neutrophils, and macrophages and high levels detected in circulating monocytes and dendritic cells. Importantly, CD33 is absent on normal hematopoietic stem cells5-7 but is expressed on blasts and leukemic stem cells of 85% to 90% of patients presenting with AML.7,8 These findings suggest that CD33 is a suitable target for an antibody-based therapy in AML.
The structure of CD33 consists of an amino-terminal V-set Ig-like domain (coded by exon 2 of CD33) and a C2-set Ig-like domain (coded by exons 3 and 4) in its extracellular portion.9 Alternative splicing of CD33 RNA can lead to a shorter isoform that is expressed on the cell surface, which lacks the V-set but retains the C2-set Ig-like domain.8,9 The biological relevance of this splicing process was largely unknown until recent studies showed that a single nucleotide polymorphism (SNP), rs12459419 (C > T; Ala14Val), was present in ∼50% of the North and South American and European AML population and leads to skipping of exon 2 of CD33, which results in the deletion of the V domain of CD33.10 Interestingly, several CD33-antibody–based therapies, including gemtuzumab ozogamicin (GO), the only approved antibody drug conjugate (ADC) for AML, bind and recognize the V domain of CD33. In fact, recent studies have shown that limited clinical activity was observed for GO in AML patients with the CT or TT genotype for SNP rs12459419 (∼50% of the study entrants).10,11 Given these data with GO, it is reasonable to hypothesize that the efficacy of other V-binding anti-CD33 antibodies will also be limited to a pool of patients with AML, specifically the ones with homozygous genotype of CC in SNP rs12459419.
The current article describes the development of JNJ-67571244, a human bispecific antibody capable of binding to the C2 domain of CD33 and to CD3, to induce T-cell recruitment and tumor cell cytotoxicity. We present in vitro, in vivo, and ex vivo evidence showing the potent cytotoxicity and T-cell activation mediated by JNJ-67571244 that results in tumor cell depletion and clearance. The safety profile of the molecule is also demonstrated in an exploratory IV toxicokinetics and tolerability study in cynomolgus monkeys with clinical tolerability achieved in the range of 0.01 to 30 mg/kg per week along with evidence of lymphocyte margination and reduction in CD33+ myeloid cells. Moreover, we present ex vivo assays with healthy and diseased primary samples and show that JNJ-67571244 mediated cytotoxicity of primary human CD33+ cells regardless of their SNP genotype status. These data indicate that JNJ-67571244 has the potential to be broadly active in most AML patient samples. JNJ-67571244 represents a novel anti-CD33 therapeutic agent for the treatment of AML; it is currently in phase 1 clinical trials to treat patients with relapsed/refractory AML and high-risk myelodysplastic syndrome (#NCT03915379).
Methods
Information on the detailed experimental procedures is provided in the supplemental Methods.
Production of DuoBody antibodies
JNJ-67571244 DuoBody antibody was generated by controlled antigen binding arm exchange from an anti-CD33 monoclonal antibody (mAb) and an anti-CD3ε mAb. After isolation and manipulation, the resulting genes expressing human mAbs against CD33 and CD3 were transfected into separate Chinese hamster ovary cell lines for production, purification, and formulation.
CD33 antigen density quantification
Quantum Simply Cellular microspheres were used according to manufacturer’s instructions to correlate geometric mean fluorescence intensity with CD33 antigen expression as molecules per cell on AML cell lines and patient samples. CD33 positivity was established as >0 receptors per cell postcorrection of nonspecific binding.
In vitro and ex vivo cytotoxicity assays
For the in vitro assays, carboxyfluorescein succinimidyl ester–labeled tumor cell lines or thawed frozen monocytes (2 × 104 cells) were plated with thawed purified frozen T cells (1 × 105) and bispecific antibodies and incubated at 37°C with 5% carbon dioxide for 48 hours. The supernatant was collected for cytokine analysis, and the cells were stained for analysis on a FACSCanto II (BD Biosciences). Similarly, for the ex vivo assays, primary AML whole blood or healthy whole blood (in heparin tubes) was treated with bispecific antibodies for 48 hours. Samples were stained with various markers before acquisition and analysis on a FACSCanto II.
In vivo studies
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, and the Office of Laboratory Animal Welfare. They were approved by the Institutional Animal Care and Use Committee of Janssen R&D.
Rodent studies
For all studies, 5- to 8-week-old female NSG mice (The Jackson Laboratory) were used. To generate KG-1 xenografts, tumor cells were injected subcutaneously into the right flank before humanization of mice with T cells. Mice were randomized into groups of 10 according to tumor volume, and treatment was initiated with intraperitoneal dosing twice a week of JNJ-67571244 (0.1, 0.5, or 1 mg/kg) or nullxCD3 control (1 mg/kg) for a total of 12 doses. To generate the systemic model, 1 × 105 luciferase-tagged MOLM-13 (MOLM-13-luc) cells were IV injected into the tail vein before randomization. Mice were treated intraperitoneally twice a week with JNJ-67571244 at 0.005, 0.05, or 0.5 mg/kg or nullxCD3 control (0.5 mg/kg) for a total of 13 doses. To assess T-cell infiltration, flushed bone marrow was harvested for flow analysis, and whole femurs were collected for immunohistochemistry (IHC) staining.
Cynomolgus studies
The pharmacokinetic (PK) and pharmacodynamic (PD) properties of JNJ-67571244 were evaluated in naive cynomolgus monkeys at Charles River Laboratories. Cynomolgus monkeys were treated with a single IV dose of control (vehicle) or 0.05, 0.2, or 1 mg/kg of JNJ-67571244. Blood samples were collected by venipuncture via the femoral vein prestudy and at selected time points up to 28 days after dosing for analysis of hematology, PK, and PD end points, including T lymphocyte activation and cytokines.
Results
Development of JNJ-67571244 antibody that targets CD33, an antigen abundantly expressed by leukemic blasts
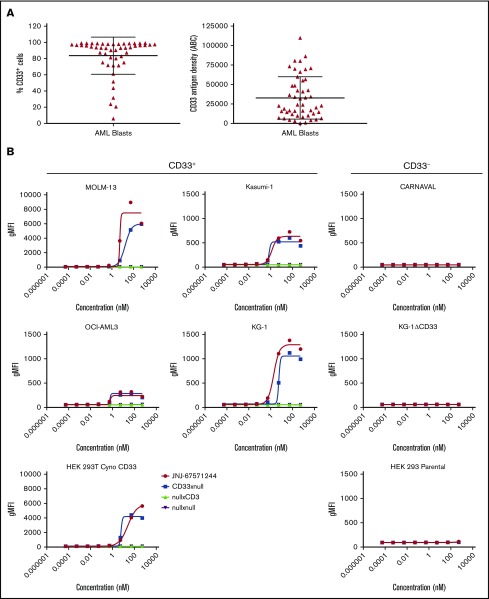
CD33 has been identified as a potential target for the destruction of malignant cells in patients with AML because of its expression in a large proportion (∼90%) of these patients.7,12,13 Indeed, we confirmed from a panel of 49 primary AML samples that an average of ∼84% of blasts per sample expressed CD33 (range of 21%-99% CD33+ blasts). The antigen density levels ranged from ∼400 to ∼110 000 molecules per cell with a mean of 32 800 molecules per cell (Figure 1A). However, CD33 is also reportedly expressed on healthy hematopoietic subsets, including monocytes and granulocytes.8 We confirmed the expression of CD33 and found that the mean surface density of CD33 is higher on AML blasts (∼32 800) than on healthy human neutrophils (∼3800) and is similar to the mean expression on healthy human monocytes (∼44 000) (supplemental Figure 1A).
Figure 1.
JNJ-67571244 binds to CD33, an antigen abundantly expressed by leukemic blasts. (A) Expression and antigen density of CD33 was evaluated in 49 AML samples. Mean ± standard deviation are graphed. (B) CD33+ and CD33– cell lines were stained for 4 hours with various concentrations of JNJ-67571244 to characterize the surface-binding profiles of the bispecific antibody. Binding of the bispecific antibody was detected by staining with mouse anti-human IgG4 (details provided in the supplemental Methods).
We developed JNJ-67571244, a fully human immunoglobulin G (IgG)4–PAA bispecific DuoBody antibody targeting CD33 on myeloid cells and the CD3 receptor complex on T cells. JNJ-67571244 bound specifically to CD33-expressing AML cells lines KG-1, MOLM-13, Kasumi-1, and OCI-AML3 (Figure 1B). A similar binding pattern was seen with the negative control CD33xnull antibody, as expected, because it contains a single anti-CD33 Fab arm and a nontargeting (null) arm. Negative control bispecific antibodies nullxCD3 as well as nullxnull showed no significant binding on these cells. None of the bispecific antibodies tested bound to the CD33-negative cell lines, CARNAVAL and KG-1ΔCD33 (ie, KG-1 cells with genetic deletion of CD33) using CRISPR. In addition, in contrast to the parental HEK-293T cells, JNJ-67571244 bound to HEK-293T cells expressing cynomolgus monkey CD33, demonstrating their cross-reactivity.
JNJ-67571244 kills CD33+ AML cell lines and activates T cells in vitro
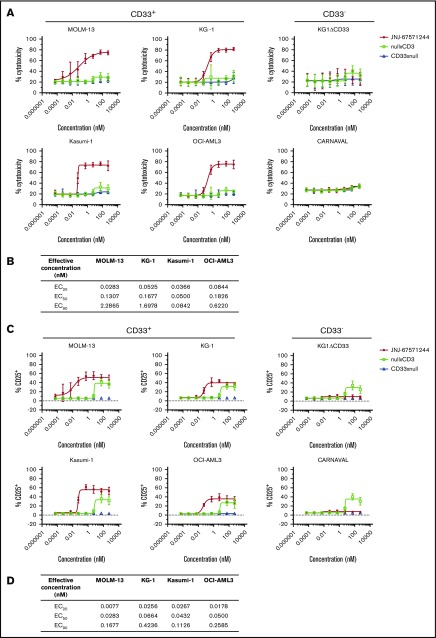
We next evaluated the activity of JNJ-67571244 in vitro. JNJ-67571244 demonstrated T cell–mediated cytotoxicity of CD33+ AML cell lines (representing a range of CD33 antigen densities shown in supplemental Figure 1B) after 48 hours (Figure 2A). The median half-maximal effective concentration (EC50) and the concentration producing 20% of the maximum possible effect (EC20) are shown in Figure 2B. No cytotoxicity was observed with the CD33– cell lines or with control bispecific antibodies. We confirmed that KG1ΔCD33 cells could indeed be targeted by T cells, by performing cytotoxicity assays with a CD123xCD3 bispecific antibody (supplemental Figure 2A).
Figure 2.
JNJ-67571244 kills CD33+AML cell lines and activates T cells in vitro. (A) T cells from 6 healthy donors were tested in T-cell redirection assays with the indicated cell lines, and percentage of cytotoxicity was determined by flow cytometry. Mean ± standard deviation is graphed. (B) Median values showing EC20, EC50, and concentration producing 90% of the maximum possible effect (EC90) for the cytotoxicity readout from 6 healthy donors. (C) Similar to panel A but here T-cell activation (CD25 upregulation on CD3+ T cells) was measured. (D) Similar to panel B but here median EC20, EC50, and EC90 values are shown for the T-cell activation readout from 6 healthy donors.
Concomitantly, JNJ-67571244 induced T-cell activation (measured by CD25 upregulation) in response to CD33+ tumor cell lines while minimal or no T-cell activation was observed with CD33– cells (Figure 2C; median EC50 and EC20 values in Figure 2D). The nullxCD3 control antibody induced T-cell activation at the highest concentrations of 53 and 533 nM in the presence of CD33+ and CD33– cell lines but failed to mediate activation at any other dose. Importantly, JNJ-67571244 showed specific induction of T-cell activation, only in the presence of CD33+ cell lines and not in the presence of CD33– cell lines or when T cells were incubated in the absence of target cells (supplemental Figure 2B).
Consistent with the cytotoxicity and T-cell activation data, JNJ-67571244 also led to the secretion of several cytokines, including interferon γ, tumor necrosis factor α, interleukin (IL)-2, and IL-8 (supplemental Figure 3). A summary of median EC50 and EC20 values for the cytokine responses is reported in Table 1.
Table 1.
Effective concentration values for JNJ-67571244–mediated cytokine release in a T-cell redirection assay with purified T cells and Kasumi-1 target cells
| Cytokine | n | Median EC20, nM | Median EC50, nM | Median EC90, nM |
|---|---|---|---|---|
| IFN-γ | 6 | 0.048 | 0.16 | 1.409 |
| IL-1beta | 6 | 0.013 | 0.045 | 0.226 |
| IL-2 | 4 | 0.090 | 0.346 | 5.282 |
| IL-4 | 4 | 0.054 | 0.078 | 0.153 |
| IL-8 | 6 | 0.015 | 0.042 | 0.246 |
| IL-10 | 6 | 0.052 | 0.123 | 1.271 |
| IL-13 | 5 | 0.008 | 0.034 | 0.103 |
| TNF-α | 6 | 0.049 | 0.208 | 3.530 |
"n" represents the number of donors out of 6 for which EC values could be determined.
IFN-γ, interferon γ; TNF-α, tumor necrosis factor α.
JNJ-67571244 shows effective antitumor activity in vivo
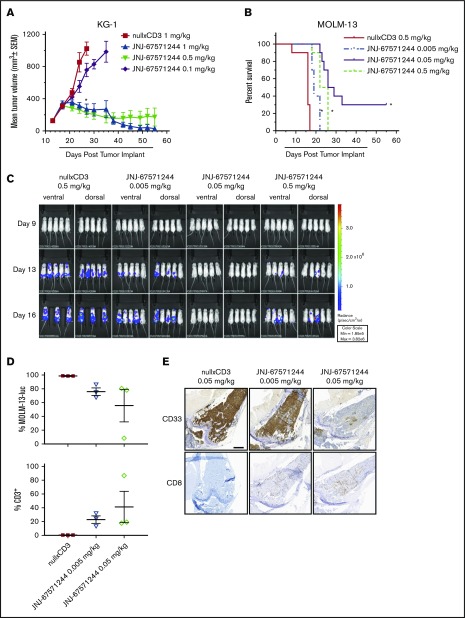
We next evaluated the function of JNJ-67571244 in 2 established xenograft tumor models in NSG mice engrafted with human T cells. In established subcutaneous KG-1-tumor–bearing mice, treatment with JNJ-67571244 at 0.1, 0.5, and 1 mg/kg elicited tumor growth inhibition of 41%, 92%, and 87%, respectively, compared with nullxCD3 treated control animals (P < .0001 for 0.5 and 1 mg/kg) (Figure 3A). JNJ-67571244 at 0.5 and 1 mg/kg also resulted in 6 and 7 complete responses at day 55, respectively.
Figure 3.
JNJ-67571244 mediates potent tumor activity in vivo in 2 established murine AML models. (A) T cell–engrafted NSG mice bearing established KG-1 tumors were intraperitoneally dosed with JNJ-67571244 at 0.1, 0.5, and 1 mg/kg (denoted by bar below x-axis). Tumor volume was measured twice weekly and the results presented as the mean tumor volume ± standard error of the mean for each group (statistical significance denoted by asterisk). (B) T cell–engrafted NSG mice bearing disseminated MOLM-13-luc cells were intraperitoneally dosed with JNJ-67571244 at 0.005, 0.05, and 0.5 mg/kg (denoted by bar below x-axis). Survival was determined by using Kaplan-Meier survival analysis (statistical significance denoted by asterisks). (C) Same as panel B but here bioluminescence was measured twice weekly, and representative images of live animal imaging of bioluminescence (ventral and dorsal views of n = 3-5 animals) on days 9, 13, and 16 are shown (n = 3 in control group on day 16 due to mortality). (D) Similar to panel B but here mice were dosed with JNJ-67571244 at 0.005 and 0.05 mg/kg for 3 doses. T-cell infiltration in the bone marrow was measured by flow cytometric analysis, and results are presented as percent tumor cells (top panel) or percent CD3+ T cells (bottom panel). (E) Same as panel D but here T-cell infiltration in bone marrow was measured by IHC staining, and results are presented as CD33+ tumor cells (top panel) or CD8+ T cells (bottom panel). Scale bar, 500 μm.
In the established disseminated MOLM-13-luc model, JNJ-67571244 treatment was initiated after homing of AML cells to bone marrow was confirmed following IV injection. JNJ-67571244 at 0.005, 0.05, and 0.5 mg/kg significantly inhibited tumor growth as assessed by bioluminescence (76%, 100%, and 82%, respectively) compared with nullxCD3 control-treated mice (supplemental Figure 4). JNJ-67571244 at 0.005, 0.05, and 0.5 mg/kg resulted in a statistically significant increased life span of 19%, 72%, and 50% (P < .0001) (Figure 3B), correlating with reduced tumor burden in the bone marrow, spine, and hind limb as observed by bioluminescence (Figure 3C). At the end of the study on day 55, three animals treated with JNJ-67571244 at 0.05 mg/kg exhibited complete response as assessed by bioluminescence.
Furthermore, MOLM-13-luc tumor-bearing mice treated with JNJ-67571244 at 0.05 mg/kg, and to a lesser extent at 0.005 mg/kg, showed decreased tumor cells and increased CD3+ T-cell infiltration in the bone marrow as measured by using flow cytometry (Figure 3D) and increased CD8+ T-cell infiltration according to IHC (Figure 3E) on day 11. These data are relevant because the bone marrow is often a site of resistance for leukemic stem cells in AML and persistence of minimal residual disease. Together, these data show that JNJ-67571244 inhibits tumor growth of two AML tumor models by recruiting T cells to the tumor site.
JNJ-67571244 mediates cytotoxicity of AML blasts from primary patient samples
Healthy and patient samples could contain soluble CD33 (sCD33) because the extracellular domain of CD33 can be shed from cells. One study found that there is ∼0.6 to 5.8 ng/mL and 0.4 to 29.6 ng/mL of sCD33 detected in healthy human serum and the plasma of patients with AML, respectively.14 We determined the physiological C2 domain sCD33 levels in healthy and AML donors and found that normal and AML serum samples exhibited similar mean sCD33 levels of 6.12 ng/mL (0.23 nM) and 3.94 ng/mL (0.15 nM) (Table 2).
Table 2.
Assessment of C2-domain sCD33 levels in healthy and AML patient samples
| Variable | Sample | Concentration, nM | Concentration, ng/mL |
|---|---|---|---|
| NHS | Individual 1 | 0.23 | 6.15 |
| Individual 2 | NR | NR | |
| Individual 3 | NR | NR | |
| Individual 4 | 0.21 | 5.59 | |
| Individual 5 | NR | NR | |
| Individual 6 | 0.25 | 6.73 | |
| Individual 7 | 0.24 | 6.39 | |
| Individual 8 | NR | NR | |
| Individual 9 | 0.23 | 6.14 | |
| Individual 10 | 0.21 | 5.69 | |
| Mean NHS (reportable) | 0.23 | 6.12 | |
| AML donor serum (AML) | AML Donor 1 | 0.14 | 3.76 |
| AML Donor 2 | 0.14 | 3.71 | |
| AML Donor 3 | NR | NR | |
| AML Donor 4 | 0.15 | 3.92 | |
| AML Donor 5 | 0.16 | 4.32 | |
| AML Donor 6 | 0.13 | 3.61 | |
| AML Donor 7 | NR | NR | |
| AML Donor 8 | 0.17 | 4.44 | |
| AML Donor 9 | 0.14 | 3.81 | |
| AML Donor 10 | NR | NR | |
| Mean AML (reportable) | 0.15 | 3.94 |
Healthy human and AML patient serum samples (n = 10 per each) were analyzed for sCD33 levels using mass spectroscopy. The concentration of C2-domain sCD33 in these samples was <3 ng/mL (0.11 nM).
NHS, normal human serum; NR, not reportable.
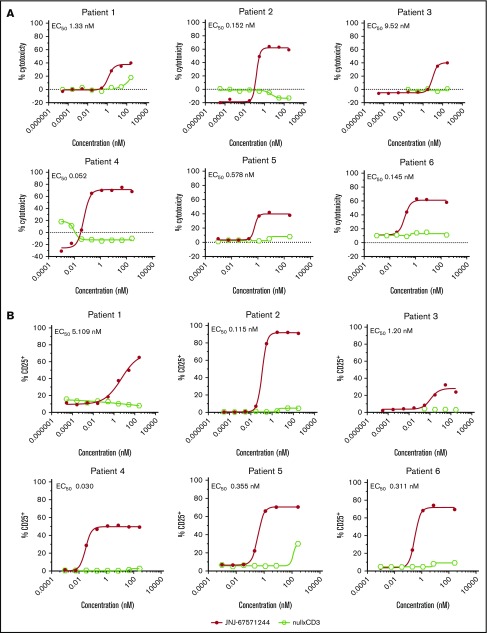
The ability of JNJ-67571244 to induce cytotoxicity in a more clinically relevant context was next assessed in an ex vivo cytotoxicity assay using whole blood from AML donors (see Table 3 for AML sample information). This system relies on the presence of autologous T cells in the patient’s own blood to kill AML cells. Unlike the null armxCD3 control, JNJ-67571244 induced a concentration-dependent cytotoxicity of CD33+ blasts (EC50, 0.052-9.52 nM) (Figure 4A) that also correlated with increased T-cell activation (EC50, 0.03 to 5.109 nM) (Figure 4B) in all 6 patient samples. These data indicate that JNJ-67571244 was effective in killing CD33+ AML cells in a more physiological ex vivo setting and in the presence of baseline sCD33 levels.
Table 3.
Characteristics of patient samples used in ex vivo cytotoxicity experiments
| Patient | Sex | Age, y | Diagnosis | Subtype | Disease phase | % blasts in the blood | Cytogenetics |
|---|---|---|---|---|---|---|---|
| 1 | M | 57 | AML | M5 | Relapsed | 64.2 | Normal |
| 2 | F | 77 | AML | M5 | De novo | 25.5 | 46,XX,del(7)(q22q32)[19]/46,XX[1] |
| 3 | M | 80 | AML | M1 | De novo | 96 | Not done |
| 4 | M | 61 | AML | Not documented | De novo | 53 | Not documented |
| 5 | M | 65 | AML | Not documented | De novo | 80 | Not documented |
| 6 | M | 79 | AML | Not documented | De novo | 49 | Not documented |
| 7 | M | 49 | AML | Not documented | De novo | 78 | inv(16)(p13.1q22) or t(16;6)(p13.1;q22); CBEB-MYH11 FLT3-ITD+ |
| 8 | M | 66 | AML | Not documented | De novo | 68.8 | Not documented |
| 9 | F | 65 | AML | M0 | De novo | 75.8 | Normal |
| 10 | M | 33 | AML | M5 | De novo | 59.4 | 46,XY,t(9;11)(p21;q23)[5] |
Figure 4.
JNJ-67571244 mediates cytotoxicity of AML blasts from primary patient samples. (A) Ex vivo assessment of JNJ-67571244–mediated cytotoxicity of CD33+ blasts in fresh AML patient whole blood after 48 hours. Individual EC50 values are shown for each patient sample. (B) Same as panel A but here T-cell activation (CD25 upregulation on CD3+ T cells) was measured.
Assessment of cynomolgus monkey cross-reactivity for JNJ-67571244
To assess if the cynomolgus monkey was an appropriate model to evaluate the activity of JNJ-67571244, we first investigated by flow cytometry the CD33 expression on leukocytes obtained from 6 healthy cynomolgus monkeys. T and B cells in cynomolgus monkey peripheral blood were found to have low to zero levels of CD33 expression (supplemental Figure 5). Cynomolgus monkey neutrophils (∼75%–96%) expressed CD33 at a mean antigen density of 7545 molecules per cell (supplemental Figure 6). Conversely, the percentage of CD33+ cyno monocytes was variable among the 6 donors, ranging from 0% to 84% with a mean antigen density of 2146 molecules per cell.
We next demonstrated the cytotoxicity potential of JNJ-67571244 at eliminating normal cynomolgus monkey monocytes and neutrophils by performing ex vivo cytotoxicity assays using healthy cynomolgus monkey whole blood. Indeed, JNJ-67571244 mediated killing of CD33+ normal cynomolgus monkey monocytes (EC50, 0.625-5.636 nM) and normal cynomolgus monkey neutrophils (EC50, 0.013-0.714 nM) as well as activation of cynomolgus T cells (EC50, 0.004-0.039 nM) after 48 hours of ex vivo incubation (supplemental Figure 6). When the same assay was performed with healthy human blood, we observed similar results and noted depletion of human CD33+ monocytes (EC50, 0.1-2.6 nM) and CD33+ neutrophils (EC50, 0.03-1.3 nM), as well as activation of T cells (EC50, 0.02-0.20 nM) (supplemental Figure 7). Together, these data show functional cynomolgus monkey cross-reactivity of JNJ-67571244 and establish CD33+ cynomolgus monkey monocytes and neutrophils as potential PD markers in nonhuman primate studies. These results also show comparable activity of JNJ-67571244 in human and cynomolgus monkey blood and lend support for using the cynomolgus monkey as an appropriate model to evaluate safety and activity of JNJ-67571244 and to potentially be predictive of effects in normal human tissues.
JNJ-67571244 mediates reduction of CD33+ leukocytes in cynomolgus monkeys
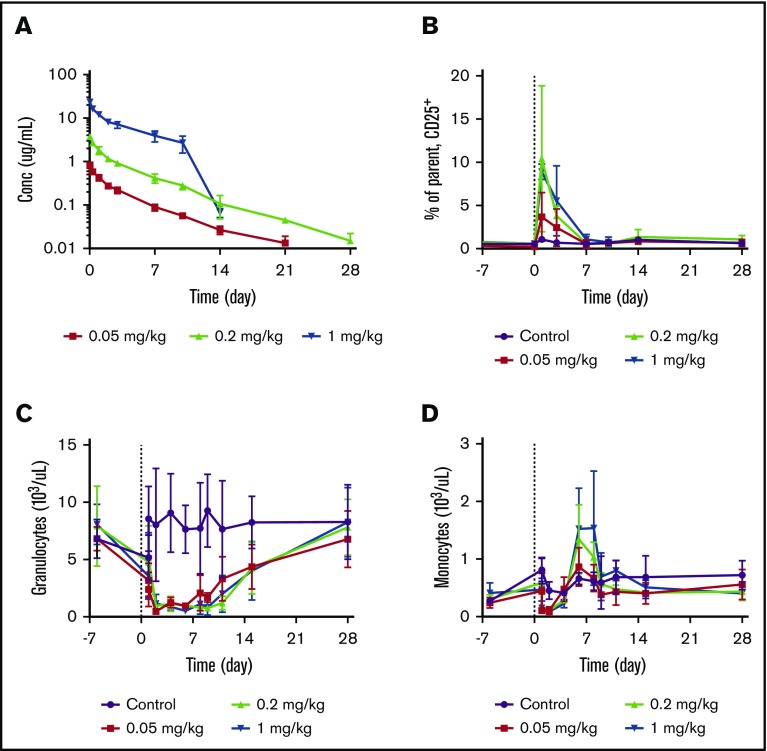
To assess the PK and PD properties of JNJ-67571244 in vivo, JNJ-67571244 was administered as a single IV dose to cynomolgus monkeys. JNJ-67571244 exhibited PK characteristics of a typical mAb with approximately linear PK properties over the 0.05 to 1 mg/kg dose range (Figure 5A). The estimated mean total clearance of JNJ-67571244 was 13.03 to 21.39 mL/d per kilogram, volume of distribution was 89.14 to 154.91 mL/kg, and terminal half-life was 4.20 to 5.06 days. An accelerated elimination of JNJ-67571244 after day 10 was observed in animals in the 1 mg/kg dose group. This finding is most likely related to the development of antidrug antibodies, although these antibodies were not tested in this study.
Figure 5.
JNJ-67571244 mediates reduction of CD33+leukocytes in cynomolgus monkeys. Cynomolgus monkeys were treated with a single IV dose of control (vehicle), 0.05, 0.2, or 1 mg/kg of JNJ-67571244. (A) JNJ-67571244 concentration over time profiles. (B) T-cell activation (% CD25+ on CD8+ T cells) in peripheral blood. (C) The effect of JNJ-67571244 on granulocytes (neutrophils). (D) The effect of JNJ-67571244 on monocytes.
Consistent with the anticipated mechanism of action, dose-dependent increases in T-cell activation (% CD25+) were observed after a single IV dose of JNJ-67571244, with peak CD25 upregulation on CD8+ T cells observed at 24 hours postdose (Figure 5B). Similar results were observed with CD4+ T cells (data not shown). Administration of JNJ-67571244 also resulted in a dose-dependent increase in plasma concentrations of the cytokines being analyzed (interferon γ, IL-10, IL-2, IL-6, monocyte chemoattractant protein-1, and tumor necrosis factor-α) at 2 hours postdose (supplemental Figure 8). With the exception of IL-10 and monocyte chemoattractant protein-1, the cytokines returned to below the lower limit of quantification levels by 24 hours postdose.
Dosing of JNJ-67571244 led to a sustained reduction in CD33+ granulocytes (neutrophils) (Figure 5C). Consistent with the lower CD33 expression levels on monocytes, a more transient reduction in CD33+ monocytes was also observed (Figure 5D). Although the initial rapid disappearance of granulocytes and monocytes from peripheral blood could be related to the transient leukocyte margination associated with T-cell activation,15 the reduction of CD33+ granulocytes was much more sustained and continued to be at a near-maximum level through day 8, and gradually recovered after that. The rebound of monocytes, however, happened earlier and was more prominent.
JNJ-67571244 was also studied in 2 other cynomolgus monkey studies after multiple IV administrations at dose levels ranging from 0.01 to 30 mg/kg. The PK and PD effects described earlier were also observed in the additional studies, and JNJ-67571244 was well tolerated at the dose levels tested (data not shown). Together, these data provide evidence of JNJ-67571244–mediating activity while maintaining tolerability in cynomolgus monkeys.
JNJ-67571244–mediated cytotoxicity of CD33+ cell lines and patient samples regardless of genotypes of rs12459419 SNP
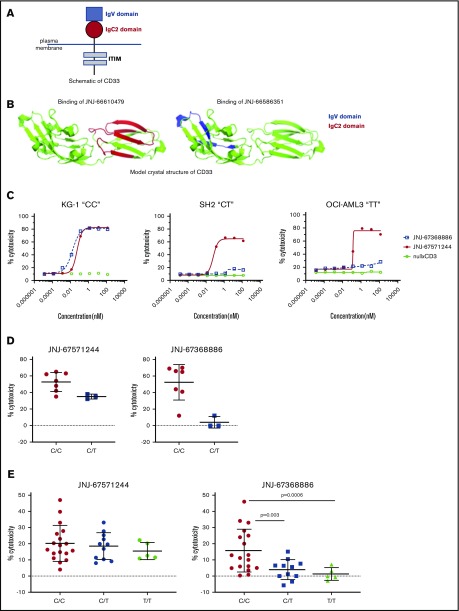
The extracellular domain of CD33 consists of an amino-terminal V domain and a membrane-proximal C2 domain (schematic shown in Figure 6A). SNP rs12459419 (C>T; Ala14Val in exon 2) is associated with preferential expression of an alternatively spliced CD33 isoform lacking exon2, resulting in the deletion of the CD33 V domain. Recent data showed that unlike patients with CT or TT genotypes, only patients with the SNP rs12459419 CC genotype (∼50% of the study participants) receiving GO had a significantly lower risk of relapse and increased event-free and disease-free survival.10 Given the earlier data with GO, we assessed the impact of SNP-rs12459419 genotypes on the activity of JNJ-67571244 and compared it vs the V domain–binding CD33xCD3 bispecific antibody JNJ-67368886. We first confirmed via hydrogen deuterium exchange mapping that JNJ-66610479 (CD33 parental mAb of JNJ-67571244) binds to distinct regions in the C2 domain of CD33 and has no binding in the V region (Figure 6B). In contrast, JNJ-66586351 (CD33 parental mAb of JNJ-67368886) binds to the V domain and has no binding in the C2 region of CD33. We next used in vitro T cell–mediated cytotoxicity assays to compare responses mediated by JNJ-67368886 to JNJ-67571244. Unlike the nullxCD3 control, both V- and C2-binding CD33xCD3 antibodies induced T cell–redirected cell cytotoxicity of the CD33+ KG-1 “CC” cell line at 48 h (Figure 6C). In contrast, unlike V-binder JNJ-67368886, only the C2-binding JNJ-67571244 mediated cytotoxicity of SH2 “CT” cell line and OCI-AML3 “TT” cell lines.
Figure 6.
JNJ-67571244 binds to the C2 domain and mediates cytotoxicity of primary samples regardless of their SNP 12459419 genotype status. (A) Schematic showing the structure of CD33. (B) Hydrogen deuterium exchange mapping and subsequent illustration of epitope regions in IgC2 and IgV domain of CD33 extracellular domain protein for V- and C2-binder mAbs. The V epitope region is colored in blue, and the C2 epitope region is colored in red. (C) T cell–mediated cytotoxicity assays using JNJ-67368886 (CD33 V-binder) and JNJ-67571244 (CD33 C2-binder) in various CD33+ SNP rs12459419 CC, CT, or TT cell lines. The percent cytotoxicity was determined by flow cytometry. (D) Similar to panel B but here ex vivo assessment of cytotoxicity of CD33+ blasts in fresh AML patient whole blood was performed at 27 nM of bispecific antibody concentration. Mean ± standard deviation is graphed. (E) Similar to panel B but here JNJ-67571244–mediated cytotoxicity of frozen purified monocytes from 34 normal donors was assessed at 0.27 nM of bispecific antibody concentration. Mean ± standard deviation is graphed. P values were calculated by GraphPad Prism using Welch’s unequal variance t test.
We then performed ex vivo cytotoxicity assays using AML patient whole blood to extend and confirm our earlier observations. V-binding and C2-binding CD33xCD3 bispecific antibodies indeed induced comparable cytotoxicity of AML samples that were identified as CC genotype; in contrast, C2-binding JNJ-67571244 showed enhanced cytotoxicity of AML samples that were heterozygous (CT) for the SNP rs12459419 mutation, compared with the V-binding JNJ-67368886 (Figure 6D). We were unable to obtain primary AML samples that were homozygous TT for the CD33 SNP rs12459419 due to its low frequency.
Given the difficulty in obtaining sufficient numbers of primary AML samples of each genotype and the fact that SNP rs12459419 is a germline mutation, we next conducted cytotoxicity assays with purified primary monocytes and matched autologous T cells from 34 healthy donors. Consistent with the fact that JNJ-67571244 binds to the C2 domain of CD33, JNJ-67571244 mediated cytotoxicity of primary human monocytes regardless of their SNP genotype status (Figure 6E). In contrast, while V-binding JNJ-67368886 mediated appreciable cytotoxic responses against samples that were CC for the SNP rs12459419, it mediated limited to no cytotoxicity when samples were CT or TT. These differences in the cytotoxic activity of JNJ-67368886 were also statistically significant (P < .005). Together, these 3 lines of evidence suggest that JNJ-67571244 could demonstrate efficacy in a broader group of patients with AML by targeting the conserved C2 epitope.
Discussion
The myeloid malignancies, and AML specifically, remain areas of high unmet medical need. Poor overall survival rates for AML result in ∼11 000 deaths annually in the United States.16 After decades of limited progress, recently there has been a shift toward more targeted therapies with 4 new therapies approved: (1) anti–FMS-like tyrosine kinase 3 inhibitors, including midostaurin, gilteritinib, and quizartinib (in Japan); (2) an isocitrate dehydrogenase 2 inhibitor (enasidenib); (3) CPX-351 (liposomal cytarabine and daunorubicin); and (4) GO.17 However, the improvements are incremental, with clinical activity limited to responses of short duration and quick emergence of resistant clones, leaving room for the development of better therapeutic agents. As such, T-cell redirection approaches that target suitable antigens are poised to become a prime focus in the field and to have a clinical impact in patients with AML.
In this study, we developed JNJ-67571244, a bispecific antibody that binds to the C2 domain of CD33 and CD3 on T cells. We showed that JNJ-67571244 binds specifically to CD33-expressing AML cell lines and primary AML patient samples and mediates specific in vitro T cell–dependent cytotoxicity in AML cell lines and patient samples. JNJ-67571244 also cross-reacts with cynomolgus monkey CD33 and CD3 and mediated decrease of cynomolgus monkey CD33+ leukocytes in ex vivo assays and in vivo. Moreover, JNJ-67571244 induced potent in vivo antitumor activity in 2 established murine models of AML. Consistent with binding to the C2 domain of CD33, JNJ-67571244 also mediated cytotoxic responses in primary samples regardless of their genotypes of the SNP rs12459419.
Although our studies, along with others,10,18 demonstrate a case for SNP rs12459419 to predict the activity of anti-CD33 therapeutics, some recent studies found no difference in the response to GO or SGN-33A (anti-CD33 ADC targeting the same epitope as GO) in the genotype groups.19,20 It is speculated that the age difference between the patient populations (0-29 years in Lamba et al10 vs 13-69 years in Gale et al19 and 27-83 years in Stanchina et al20) may play a role in the reduced efficacy of anti-CD33 ADCs in the older population due to resistance facilitated by P-glycoprotein–mediated drug efflux,21 which is higher in the older patients.22 Preclinically, the rs12459419 SNP genotype was not found to affect the cytotoxic abilities of V-binding GO or AMG-330.23 However, GO or AMG-330 failed to show any efficacy in cells that were engineered to have a deletion of exon 2 in CD33.24 In light of these studies, it is possible that there is heterogeneous expression of the V domain epitope in the CT genotype, and there still may be limited expression of the V domain in the TT genotype, which may be sufficient to drive responses by V-binding anti-CD33 therapeutics in preclinical assays. However, it is reasonable to speculate that in the clinic, limited expression of the CD33 V domain may affect the efficacy of anti-CD33 therapeutic agents and could lead to potential resistance.
In addition to rs12459419, there are several SNPs present on the CD33 gene that are associated with expression levels of CD33 isoform lacking exon 2 in AML patient samples and correlated with CD33 density on leukemic blasts.18,25 It will be interesting to assess if and to what extent all the different SNPs can predict the activity of V domain vs C2 domain binding CD33 antibodies in various preclinical studies in AML cell lines and patient samples.
In addition, targeting epitopes closer to the membrane reportedly results in the exclusion of the negative regulatory protein CD45 in the synapse between T cells and target cells,26 translating into increased potency of the T-cell response and killing.27 As such, binding the membrane proximal CD33 C2 epitope for a CD3 redirection bispecific antibody may offer an additional benefit over the membrane distal V domain epitope.
Our in vitro and in vivo results indicate that JNJ-67571244 specifically binds to CD33-expressing cells, induces T-cell activation, and effectively redirects T cells to induce cytotoxicity of CD33-expressing cells. In addition, JNJ-67571244 binds to the C2 domain of CD33 and is expected to have broader activity regardless of the genotypes of the SNP rs12459419. JNJ-67571244 is currently in a phase 1 clinical trial in patients with relapsed/refractory AML and high-risk myelodysplastic syndrome (#NCT03915379).
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful to all members of the Gaudet group for technical assistance and helpful discussions. They also thank Anna Hughes and Lorraine Angelillo for the IHC staining, as well as Emily Garrigan and Katherine Kraft for assistance with the SNP genotyping experiments. Additionally, they thank Gwenn-ael H. Danet-Desnoyers from the University of Pennsylvania for providing some of the primary AML patient samples used in the manuscript.
Footnotes
For original data, please contact the corresponding author (François Gaudet; e-mail: fgaudet@its.jnj.com).
Authorship
Contribution: P.N.-G. conceived, designed and performed experiments, analyzed data, wrote the manuscript and co-led antibody discovery for JNJ-67571244; M.D. conceived, led antibody generation, and co-led antibody discovery for JNJ-67571244; R.S. and W.W. designed and led the cynomolgus monkey studies; K.S. and B.M. designed and performed the in vivo rodent experiments; D.R., B.H., M.M., J.J., J.S., B.F., G.C., D.F., Q.J., and S.-J.W. performed experiments and contributed to data; K.P. oversaw design and execution of in vivo experiments; R.A. and Y.E. oversaw the project; F.G. conceived, contributed to project design, and oversaw the project; and all authors reviewed and revised the manuscript, approved the final version, and agreed to submit the manuscript for publication.
Conflict-of-interest disclosure: All authors are employees of Janssen Research and Development, LLC.
Correspondence: François Gaudet, Janssen Research & Development LLC, 1400 McKean Rd, Spring House, PA 19477; e-mail: fgaudet@its.jnj.com.
References
- 1.Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487-494. [DOI] [PubMed] [Google Scholar]
- 2.Ley TJ, Miller C, Ding L, et al. ; Cancer Genome Atlas Research Network . Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul SP, Taylor LS, Stansbury EK, McVicar DW. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood. 2000;96(2):483-490. [PubMed] [Google Scholar]
- 4.Ulyanova T, Blasioli J, Woodford-Thomas TA, Thomas ML. The sialoadhesin CD33 is a myeloid-specific inhibitory receptor. Eur J Immunol. 1999;29(11):3440-3449. [DOI] [PubMed] [Google Scholar]
- 5.Andrews RG, Torok-Storb B, Bernstein ID. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983;62(1):124-132. [PubMed] [Google Scholar]
- 6.Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8(4):521-534. [DOI] [PubMed] [Google Scholar]
- 7.Jilani I, Estey E, Huh Y, et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol. 2002;118(4):560-566. [DOI] [PubMed] [Google Scholar]
- 8.Laszlo GS, Estey EH, Walter RB. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014;28(4):143-153. [DOI] [PubMed] [Google Scholar]
- 9.Laszlo GS, Harrington KH, Gudgeon CJ, et al. Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia. Oncotarget. 2016;7(28):43281-43294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamba JK, Chauhan L, Shin M, et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: report from randomized Phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol. 2017;35(23):2674-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamba JK, Pounds S, Cao X, et al. Coding polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML: a pilot study. Leukemia. 2009;23(2):402-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krupka C, Kufer P, Kischel R, et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood. 2014;123(3):356-365. [DOI] [PubMed] [Google Scholar]
- 13.Pollard JA, Alonzo TA, Loken M, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119(16):3705-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biedermann B, Gil D, Bowen DT, Crocker PR. Analysis of the CD33-related siglec family reveals that Siglec-9 is an endocytic receptor expressed on subsets of acute myeloid leukemia cells and absent from normal hematopoietic progenitors. Leuk Res. 2007;31(2):211-220. [DOI] [PubMed] [Google Scholar]
- 15.Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226-6233. [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. [DOI] [PubMed] [Google Scholar]
- 17.Bewersdorf JP, Stahl M, Zeidan AM. Are we witnessing the start of a therapeutic revolution in acute myeloid leukemia? Leuk Lymphoma. 2019;60(6):1354-1369. [DOI] [PubMed] [Google Scholar]
- 18.Mortland L, Alonzo TA, Walter RB, et al. Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clin Cancer Res. 2013;19(6):1620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale RE, Popa T, Wright M, et al. No evidence that CD33 splicing SNP impacts the response to GO in younger adults with AML treated on UK MRC/NCRI trials. Blood. 2018;131(4):468-471. [DOI] [PubMed] [Google Scholar]
- 20.Stanchina M, Pastore A, Devlin S, Famulare C, Stein E, Taylor J. CD33 splice site genotype was not associated with outcomes of patients receiving the anti-CD33 drug conjugate SGN-CD33A. J Hematol Oncol. 2019;12(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter RB, Gooley TA, van der Velden VH, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109(10):4168-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood. 1999;94(3):1086-1099. [PubMed] [Google Scholar]
- 23.Laszlo GS, Beddoe ME, Godwin CD, et al. Relationship between CD33 expression, splicing polymorphism, and in vitro cytotoxicity of gemtuzumab ozogamicin and the CD33/CD3 BiTE® AMG 330. Haematologica. 2019;104(2):e59-e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humbert O, Laszlo GS, Sichel S, et al. Engineering resistance to CD33-targeted immunotherapy in normal hematopoiesis by CRISPR/Cas9-deletion of CD33 exon 2. Leukemia. 2019;33(3):762-808. [DOI] [PubMed] [Google Scholar]
- 25.Raj T, Ryan KJ, Replogle JM, et al. CD33: increased inclusion of exon 2 implicates the Ig V-set domain in Alzheimer’s disease susceptibility. Hum Mol Genet. 2014;23(10):2729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Stagg NJ, Johnston J, et al. Membrane-proximal epitope facilitates efficient T cell synapse formation by anti-FcRH5/CD3 and is a requirement for myeloma cell killing. Cancer Cell. 2017;31(3):383-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strohl WR, Naso M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies (Basel). 2019;8(3):E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.