Abstract
Atmospheric particulate matter (PM) is a complex component of air pollution and the leading environmental risk factor for death worldwide. PM is formed from a combination of primary sources that emit PM directly into the atmosphere and secondary sources that emit gaseous precursors which oxidize in the atmosphere to form PM and composed of both inorganic and organic components. Currently, all PM is regulated by total mass. This regulatory strategy does not consider individual chemical constituents and assumes all PM is equally toxic. The chemically-extracted organic fraction (OF) of PM retains most organic constituents such as polycyclic aromatic hydrocarbons (PAHs) and excludes inorganics. PAHs are ubiquitous environmental toxicants and known aryl hydrocarbon receptor (AHR) ligands. This study addressed the role of individual components, specifically PAHs, of PM and how differences in chemical composition of real-world samples drive differential responses. This study tested the hypothesis that real-world extracts containing different combinations and concentrations of PAHs activate the AHR and enhance T helper (Th)17 differentiation to different degrees based on specific PAHs present in each sample, and not simply the mass of PM. The ability of the real-world OF, with different PAH compositions, to enhance Th17 differentiation and activate the AHR was tested in vitro. Cumulatively, the results identified PAHs as possible candidate components of PM contributing to increased inflammation and demonstrated that the sum concentration of PAHs does not determine the extent to which each PM activates the AHR and enhances Th17 differentiation suggesting individual components of each PM, and interactions of those components with others in the mixture, contribute to the inflammatory response. This demonstrates that not all PM are the same and suggests that when regulating PM based on its ability to cause human pathology, a strategy based on PM mass may not reduce pathologic potential of exposures.
Keywords: Atmospheric particulate matter, Chemically-Extracted organic fraction, Th17 differentiation, Polycyclic aromatic hydrocarbons, Aryl hydrocarbon receptor
1. Introduction
Air pollution has emerged as a leading risk factor for disease and deaths worldwide. Currently, atmospheric PM1 poses the greatest environmental risk for death worldwide, ranking as the 6th leading cause of mortality (Institute, 2018). According to the World Health Organization’s Air Quality Guideline for PM, over 95% of the world’s population breathes unhealthy air (Institute, 2018). PM is a formed from a combination of primary sources that emit PM directly into the atmosphere and secondary sources that emit gaseous precursors which oxidize in the atmosphere (Bell, 2012; Council, N.R., 2010; Kelly and Fussell, 2012). Each individual component of PM can be generated from multiple sources and each of these sources has the capacity to generate multiple different components all of which contributes to the complexity of PM (Kelly and Fussell, 2012). These diverse primary emission sources in conjunction with secondary atmospheric chemical reactions lead to the complex mixtures of PM components that include inorganics such as metals, nitrates, sulfates, and organic compounds, such as PAHs2 (Cheung et al., 2011; Vincent et al., 1997). Given the complexity of these mixtures, identifying specific exposures, routes of exposure, and mechanisms leading to death and disease has proven particularly difficult.
All atmospheric PM is currently regulated by total mass of PM and does not consider individual chemical constituents. This regulatory strategy assumes that all PM is equally toxic, despite its complexity and heterogeneity. Efforts have been made to identify the active component (s) of PM responsible for increased morbidity by testing the effects of the intact PM or the chemically-extracted OF3 adsorbed to it (Ma and Ma, 2002). The OF of PM retains the organic constituents, including toxicants such as PAHs, and excludes inorganics. Among the PAHs that are present, 15 are classified by the U.S. EPA as priority pollutants and are disease and cancer-causing agents (Agency 2002; Program 2016). Additionally, PAHs are known ligands of the AHR4 (DeGroot et al., 2011). Not only are classical U.S. EPA priority PAHs potent inducers of AHR, additional PAHs as well as their polar derivatives have also been shown to be important contributors to AHR-mediated activities in animal and human cells (Andrysik et al., 2011; Libalova et al., 2014; Vondracek et al., 2017).
The AHR is a ligand-activated transcription factor that responds to xenobiotic toxicants, such as PAHs, as well as endogenous ligands (Carver and Bradfield, 1997). Once activated, the AHR upregulates transcription of CYP5 metabolizing enzymes among other targets (Carver and Bradfield, 1997). The AHR is expressed in several T cell subsets, but most highly expressed in Th617 cells and is minimally expressed in naïve Th0 cells (Mascanfroni et al., 2015; Veldhoen et al., 2008). Likewise, Th17 cells have increased expression of interleukin (IL)-17, a pro-inflammatory cytokine, while naïve Th0 cells do not express any IL-17 (Veldhoen et al., 2008). The AHR is critical in the balance of Treg7 and Th17 cells (Quintana et al., 2008; Veldhoen et al., 2008). The extent and duration of AHR activation shifts the balance between these effector and regulatory responses (Ehrlich et al., 2018). Th17 cells are characterized by the production of IL-17 and are generally pro-inflammatory T cells (Tesmer et al., 2008), which may play role in pathology derived from PM exposure. Our recent work has found that an ambient urban dust particle, SRM1649b, and it’s chemically-extracted OF, both of which contain PAHs, enhanced Th17 differentiation in an AHR-dependent manner in vitro (van Voorhis et al., 2013). Moreover, a diesel exhaust extract and cigarette smoke extract, both which contain PAHs, enhanced Th17 differentiation and an individual PAH, benzo[k]fluoranthene enhanced Th17 differentiation in in an AHR-dependent manner in vitro (van Voorhis et al., 2013). We also recently reported that synthetic PAH mixtures containing 15 PAHs, most of which are from the U.S.EPA priority pollutant list, that replicate the PAH milieu present in standardized PM samples enhanced Th17 differentiation in an AHR-dependent manner (O’Driscoll et al., 2018). Finally, multiple studies have demonstrated that PAHs are a major contributor of AHR-mediated responses in vitro (Andrysik et al., 2011; Libalova et al., 2014). Taken together, these results demonstrate that PAHs enhance Th17 differentiation through the AHR and have been shown to be a major contributor of AHR-mediated responses, suggesting that PAHs present in PM and derivative OF may activate the AHR and enhance Th17 differentiation.
The current study addresses the overarching question of whether all PM samples of the same size elicit the same biological effects and thus should be regulated the same. More specifically, it addresses the role of individual components of PM and how the differences in chemical composition drive differential responses. The diversity of PAHs found in different PM samples leads to differences in the extent and duration of AHR activation, which can impact T cell status, by shifting the balance from regulatory to effector responses. Given this, we hypothesized that real-world samples containing different mixtures and concentrations of PAHs would activate the AHR and enhance Th17 differentiation to different degrees based on individual components of each sample, and not simply the mass of PM. We tested the ability of the real-world OF, with different PAH compositions, to enhance Th17 differentiation and activate the AHR in vitro. Motor oil, which does not contain PAHs, had no effect on Th17 differentiation or AHR activation. Barley straw smoke, the sample with the highest PAH concentrations, was the most potent sample measured by the dose sufficient to enhance Th17 differentiation and activate AHR. Interestingly, Riverside, an ambient urban dust particle collected in California, was more potent than Pine wood smoke, despite having fewer PAHs and a lower total sum of PAH concentrations. These results identify PAHs as possible candidate components of PM contributing to increased morbidity. Moreover, the specific PAHs present and in each PM, rather than the sum concentration of PAHs, may determine the extent to which each PM activates the AHR and enhances Th17 differentiation leading to the observed differences. Together these results strongly demonstrate that not all PM have the same potential for causing inflammation. The implications of these results suggest that PAHs present in PM elicit different biological effects and specific individual PAHs, as well as the mixtures they makeup, alter these responses. More effort should be put forth to identify the specific PAHs and other active components that may lead to increased biological responses that could cause human disease, as not all PM responds the same and even those samples that have more PAHs do not have the strongest response.
2. Methods
2.1. Mice
Wild-type (WT), C57BL/6 J mice were obtained from Jackson Laboratories (stock# 000,664) or bred in house in a Specific Pathogen Free facility. All mice were maintained under specific, pathogen free conditions. All animal experiments were performed in accordance with protocols approved by the School of Medicine and Public Health Institutional Animal Care and Use Committee at the University of Wisconsin-Madison. All mice were anesthetized using isoflurane anesthesia, and all efforts were made to minimize suffering.
2.2. Sample collection
Riverside, Barley straw smoke, and Pine wood smoke samples were collected as PM and extracted using a methylene chloride (DCM8) dimethyl sulfoxide (DMSO9) solvent extraction process to get the OF. The chemically-extracted OF excludes the metals and inorganics and retain most of the organic constituents.
Motor oil is a sample of Valvoline Premium Conventional 5W-20 motor oil. Motor oil is composed of virtually all organic constituents and because of this Motor oil was dissolved in the same solvents, DCM followed by DMSO, as all other samples, but did not undergo an extraction.
Riverside is an ambient urban dust particle collected at the University of Southern California in Riverside, representing a downwind site of the LA basin, every 6 days from May 2009 to April 2010 (Heo et al., 2013). A detailed description of the sampling method is described elsewhere (Heo et al., 2013). The Riverside sample used in the current study is an annual composite of the PM collected. Briefly, the PM2.5 samples were collected on quartz-fiber filters by a URG-3000B medium volume sampler (URG, Chapel Hill, NC).
Barley straw smoke is a smoke sample collected from an experiment conducted at the National Institute for Agro-Environmental Sciences (Tsukuba, Japan) in which barley straw was burned and the smoke PM emissions were collected (Fushimi et al., 2017). An in-depth description of the burning experiment and emission collections are reported elsewhere (Fushimi et al., 2017). Briefly, a stainless-steel combustion hood was used to simulate open burning of barley straw. The hood was placed on the soil surface on a field and the barley straw was placed inside the hood and ignited. The PM emissions were collected from the exhaust duct of the hood. A portion of the exhaust was continuously sampled from ignition until the end of combustion.
Pine wood smoke is a smoke sample collected from burning kiln dried red pine. The combustion emissions of kiln dried red pine were collected in a controlled experiment similar to that described in Olson et al. (Olson et al., 2015). Pine logs were combusted in a cast iron stove and emissions were diluted with high efficiency particle air (HEPA) filtered, activated carbon scrubbed primary dilution air collected through a sample port located approximately one foot below the stack opening. The split-stream of the diluted emission was collected on 47 mm pre-baked quartz filters. The PM was size segregated using a PM2.5 size selective cyclone at a flow rate of approximately 13 liters per minute. The pine logs were burned for approximately 1 h prior to sampling and were ignited using an electric coil starter. Similar source emissions are described in more detail by Schauer et al. (Schauer et al., 2001).
2.3. OF extraction
The Pine wood smoke was prepared by extracting the samples with DCM using a Soxhlet extractor. The DCM extraction was conducted for 24 h with nominally 5 solvent cycles per hour. The extracts were then reduced in volume by evaporating the solvent by directing a high-purity, gaseous nitrogen stream into the sample, which was held at 50 °C in a water bath. The solvent evaporation continued until the extract volume reached 0.1 mL. 1.0 mL of hexane was then added to the concentrated extract and the volume was reduced by the nitrogen blow down to 0.1 mL. This process was repeated twice to transfer the extract into hexane and remove the DCM. After the three hexane transfers, 0.2 mL of DMSO was added the residual hexane was evaporated with nitrogen under the same conditions for 30 min. Additional DMSO was then added to obtain the final extract concentration.
The OF of the Riverside and Barley straw smoke samples were isolated from filters with 2 mg of OC10 in 250 mL glass jars containing 30 mL of DCM. The glass jars were sonicated for 3 times for 20 min for a total of 60 min, with 30 mL s of DCM added each time resulting in 90 mL s. Using a rotovapor, the volume of the solution was decreased from 90 mL to 2.5 mL at 40 °C. The remaining 2.5 mL of concentrate was transferred to a 10 mL test tube and blown down with nitrogen to 2 mL at 50 °C. The extract was filtered and transferred to a new 10 mL test tube and dried down under nitrogen to 0.5 mL. Then, the extract was transferred to a 2 mL amber vial and dried down under nitrogen to 0.1 mL. To remove any remaining DCM, 0.5 mL of n-hexane was added to the 0.1 mL and dried down again to 0.1 mL, this was repeated 3 times. Lastly, 0.25 mL of DMSO was added and placed under nitrogen for about 1 h. After 1 h, all the n-hexane was completely evaporated.
For all samples, PAH levels were measured in the extracts using a GC/MS (6980 GC and a 5973 Inert MS; Agilent) and a HP-5MS 30-meter-long, 0.25 mm inner diameter, 0.25 μm film thickness column (Agilent).
2.4. Isolation of naïve T cells and T-cell differentiation
Naïve CD4+ T cells were isolated by negative selection and purified from male or female adult C57BL/6 J WT using CD4+ Isolation Kit (Miltenyi) in conjunction with QuadroMACS separator (Miltenyi). Media used for cultures was RPMI 1640 (Cell Gro) supplemented with Hepes buffer (Cell Gro), non-essential amino acids (Cell Gro), sodium pyruvate (Cell Gro), penicillin/streptomycin/glutamine (Cell Gro), 2-Mercaptoethanol (Life Technologies) and 5% FBS (Hyclone).
Purified naïve CD4+ T cells were plated in 96-well plates at 150,000 cells per well in 100 μL and stimulated with plate-coated anti-CD3 (1 μg/ml; R&D Systems) at 4 °C for 24 h and by soluble anti-CD28 (1 μg/mL, BD) added at time 0. Cells were differentiated under Th17 conditions (human TGF-β (5 ng/mL; R&D Systems), murine IL11-6 (50 ng/mL; R&D Systems), for 72 h (3 days) at 37 °C and 5% CO2. All cultures included one positive control, FICZ12 (200 nM; Enzo Life Sciences), which is a tryptophan photoproduct and endogenous high affinity AHR ligand. The positive control was added to the culture at a final concentration of 200 nM and used to determine whether the differentiation cultures were prepared appropriately, and naïve cells responded and differentiated (Supplementary Fig. 1). All treatments were done in duplicate or triplicate on each 96-well plate and cultured for 3 days. On day 3, cells were harvested for flow cytometry and mRNA analysis and cell supernatants were collected for ELISA.
2.5. OF treatments
Cells were exposed to a dose response of Riverside, Barley straw smoke, Motor oil, or Pine wood smoke, SRM131649b, SRM1650b, or SRM2975 OFs (Dr. James Schauer, WSLH, Madison, WI) and Solvent control (Dr. James Schauer, WSLH, Madison, WI) for 72 h added to the culture at time 0. The treatments were diluted into media for a final volume of 100 μL, making the total volume in each well of the 96-well plate 200 μL. The treatment doses chosen were based on OC content because it is extractable and all OF treatments can be normalized to it. Motor oil was normalized based on volume of solvent because the sample was dissolved in solvent and not extracted. The highest dose was 10 μg/mL OC and the lowest dose was 0.00001 μg/mL OC.
2.6. Intracellular cytokine staining for T cells
Intracellular cytokine staining for T cells was conducted on day 3, after the T cells were cultured for 72 h. The cultured cells were stimulated with Cell Stimulation Cocktail (eBioscience) for 5 h. Brefeldin A 1000X (eBioscience) was added for the final 4.5 h. Cells were then fixed and permeabilized with Intracellular Fixation & Permeabilization Buffer (eBioscience) or Foxp3/Transcription Factor Staining Buffer Set (eBioscience) and intracellular cytokines overnight at 4 °C T cells were stained with LIVE/DEAD Fixable Blue Dead Cell Stain Kit for UV Excitation (Invitrogen) or Ghost 780 (Tonbo) prior to fixation. Cells were stained with CD4 (BUV395; BD or PE, PeCy5, FITC; eBioscience) and TCRB (PeCy7; eBioscience) for extracellular markers. Cells were stained with IL-17 A (FITC or PE; eBioscience), and/or KI67 (PE; eBioscience). Stained cells were analyzed on Fortessa (BD), Attune NxT (Invitrogen), or Accuri C6 (BD). Data was analyzed using FlowJo software (TreeStar). Flow plots showing percent live cells are showing the percent live cells of total lymphocytes. Flow plots show IL-17 producing cells as percent cytokine producing cells of CD4+TCRβ+ Live cells or TCRβ+ Live cells.
2.7. ELISAs
Supernatant was collected from in vitro Th17 cultures on day 3 and analyzed for mouse IL-17 by ELISA according to manufacturer’s instructions (R&D Systems).
2.8. Quantitative real time PCR (qRT-PCR)
Total RNA was extracted using a RNeasy Mini Kit (Qiagen). cDNA synthesis was completed using SuperScript IV VILO Master Mix (Invitrogen). The qRT-PCR was performed on the Applied Biosystems 7900 H T Fast Real-Time PCR system using Taqman Gene Expression Assays and Taqman Universal PCR Mastermix (Life Technologies). Taqman Gene Expression Assays used include: Actin b, Mm02619580_g1; IL-17 A, mm00439619_m1; Cyp1 A1, Mm00487218_m1. Data were analyzed using the ΔΔCt method with actin serving as the endogenous reference.
2.9. Statistics
Statistics were analyzed using GraphPad Prism version 8. For all in vitro analyses the question asked was: Is there an interaction between treatment and dose for the different samples? The two variables tested were treatment and dose. A test of normality was conducted to determine whether the statistics would be parametric or nonparametric. A 2-way repeated measures analysis of variance (ANOVA) was performed with p value < 0.05. The treatments were Riverside, Barley straw smoke, Motor oil, Pine wood smoke or solvent control and 8 doses were tested with the highest dose being 10 μg/mL OC and the lowest dose 0.00001 μg/mL OC. For the comparison of different endpoints across samples, a 2-way repeated measures ANOVA was used if each sample had the same number of data points for a given measure. In cases, where each sample did not have the same number of data points for a given measure, a Mixed-effects model was conducted.
3. Results
3.1. Riverside OF enhances Th17 differentiation and increases CYP1 A1 mRNA at high concentrations in vitro
Given that our lab previously showed that the chemically-extracted OF of an ambient urban dust PM, SRM1649b, enhanced Th17 differentiation to the same extent as the PM and that individual PAHs (van Voorhis et al., 2013) and synthetic PAH mixtures based on PAHs present in PM enhanced Th17 differentiation in vitro (O’Driscoll et al., 2018; van Voorhis et al., 2013), we tested real-world sample OFs and hypothesized that those samples containing the highest levels of PAHs would activate AHR and enhance Th17 differentiation to a greater degree than other samples and samples containing no PAHs would have no effect on AHR activation or Th17 differentiation in vitro. To test this hypothesis, Riverside, an ambient pollution sample collected Riverside, CA between 2009–2010, was fractionated and its derivative OF tested. Naïve CD4 positive T cells were isolated from spleens of C57BL/6 J WT mice and exposed to a dose-response of Riverside OF or solvent control on day 0, cultured under Th17 conditions for 3 days. Supernatant was collected for IL-17 ELISA, mRNA was collected, and flow cytometry was performed to measure the percent live cells, percent IL-17 expressing cells, and percent of proliferating cells using KI67 stain.
There was no significant difference in percent live cells at any concentration for Riverside except at 10 μg/mL OC, in which Riverside had significantly more live cells than solvent control (Fig. 1A and B). Moreover, Riverside significantly enhanced Th17 differentiation, measured by percent of IL-17 positive cells, at 10 and 5 μg/mL OC (Fig. 1C and D). Additionally, an IL-17 ELISA was performed on supernatants collected on day 3 of culture and a significant increase of IL-17 protein was measured at 5 μg/mL OC Riverside compared to solvent control (Fig. 1E). There was no difference in IL-17 mRNA expression (Fig. 1F). To test whether Riverside activated the AHR, CYP1 A1 mRNA levels were measured and Riverside exposure increased CYP1 A1 mRNA levels at 10 and 5 μg/mL OC (Fig. 1G). Next, we measured the percent of KI67 high cells, a marker for proliferating cells, to determine if the enhanced Th17 differentiation measured after Riverside exposure was due to enhanced proliferation. There was a significant difference in the percent of KI67 high cells of the total CD4+TCRβ cells at 10 μg/mL OC, but no difference in the percent of KI67 high cells of IL-17 positive cells (Fig. 1H).
Fig. 1. Riverside enhanced Th17 differentiation and activated the AHR in vitro.
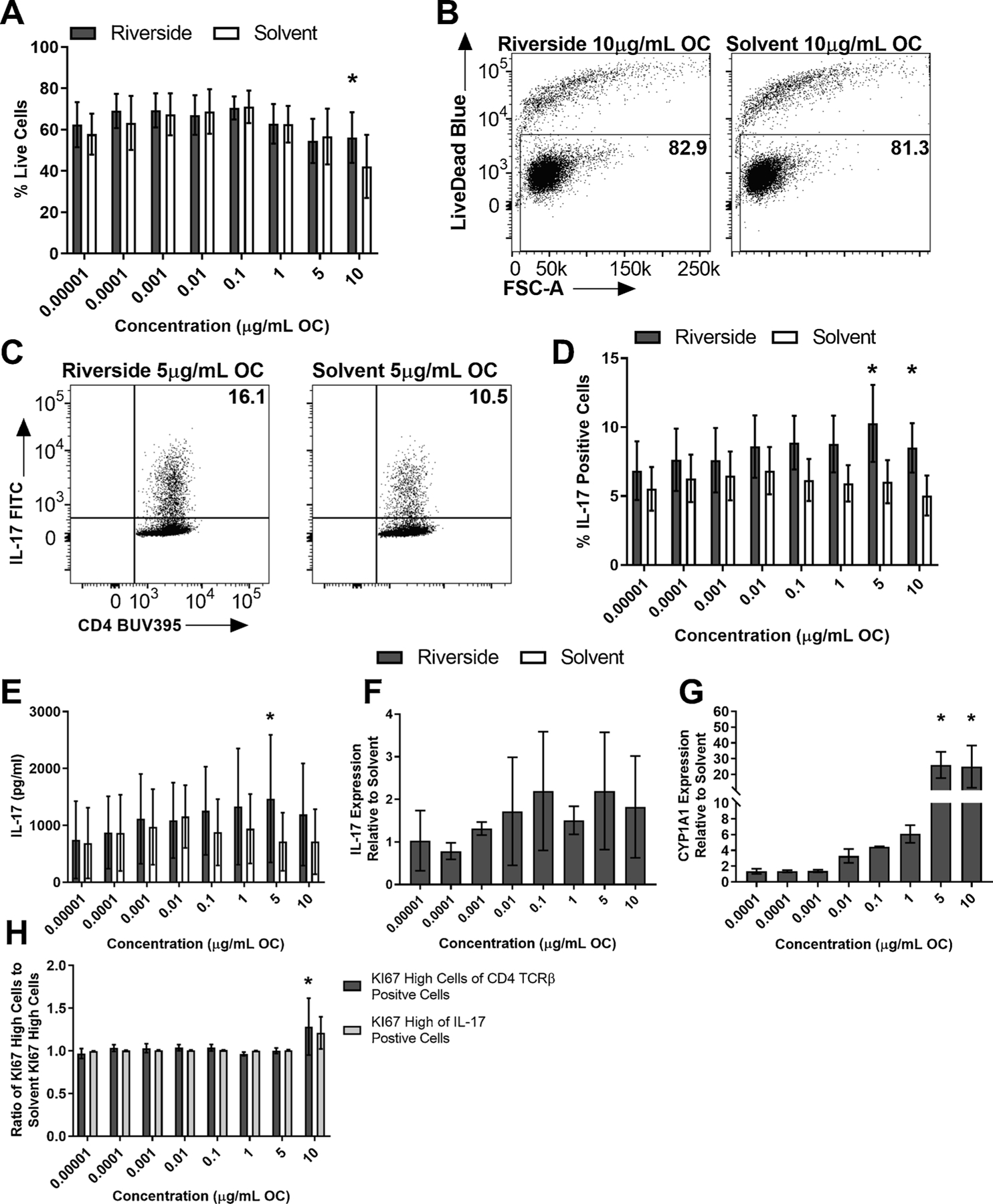
Naïve CD4+ T cells were isolated from WT (C57BL/6 J) mice and cultured under Th17 conditions. At time 0, cells were exposed to a dose-response (8 doses) of Riverside or DCM/DMSO solvent control and cultured for 3 days. (A) Riverside exposure did not cause significant cell death, however at the 10 μg/mL OC concentration there was a significant reduction in percent live cells for solvent (n = 4). (B) Representative flow plots of WT percent of live cells at 10.0 μg/mL OF, measured as percent live cells of total CD4 cells. (C) Representative flow plots of WT Th17 differentiation measured by the percent of IL-17 positive cells at dose 5 μg/mL OC. (D) Riverside enhanced Th17 differentiation at the two highest doses tested, measured by the percent of IL-17 positive cells (n = 4). (E) Riverside increased the total production of IL-17 after the 3-day culture at 5 μg/mL OC (n = 3). (F) There was no significant difference in IL-17 mRNA between Riverside treatment and solvent control (n = 2). (G) Riverside significantly upregulated CYP1 A1 mRNA at 10 and 5 μg/mL OC (n = 3). (H) There was a significant difference in the percent of CD4 + TCRβ+ cells that proliferated, measured by KI67 expression, between Riverside and solvent control. Results are mean ± SEM. Significant differences among groups (p < 0.05) are indicated by an asterisk. Abbreviations: DCM/DMSO, methylene chloride/dimethyl sulfoxide; OC, organic carbon; OF, organic fraction.
3.2. Barley straw smoke enhances Th17 differentiation and increases CYP1 A1 mRNA at high concentrations in vitro
Next, a real-world source sample was tested. Barley straw smoke, a smoke sample collected from barley straw burning in Japan, was tested for its ability to enhance Th17 differentiation and activate the AHR. Naïve CD4 positive T cells were isolated from spleens of C57BL/6 J WT mice and exposed to a dose-response of Barley straw smoke OF or solvent control on day 0, cultured under Th17 conditions for 3 days, and supernatant was collected for IL-17 ELISA, mRNA was collected, and flow cytometry was performed. Barley straw smoke exposure caused significant increase in cell death, measured by percent live cells, at 10 and 5 μg/mL OC (Fig. 2A and B). For this reason, those concentrations were excluded from the rest of the analysis. Barley straw smoke enhanced Th17 differentiation, measured by increased percent of IL-17 positive cells at 1, 0.1, and 0.001 μg/mL OC (Fig. 2C and D). Barley straw smoke exposure increased IL-17 protein, measured by ELISA, and 1 and 0.1 μg/mL (Fig. 2E) and no difference was observed for IL-17 mRNA at any concentration measured (Fig. 2F). AHR activation was measured using CYP1 A1 mRNA as a marker and Barley straw smoke increased CYP1 A1 mRNA compared to solvent control at 1 μg/mL (Fig. 2G). To determine whether proliferation explained the enhanced Th17 differentiation and increased AHR activation, KI67 stain was performed. There was no significant difference between Barley straw and solvent at any concentrations tested for KI67 (Fig. 2H).
Fig. 2. Barley straw smoke enhanced Th17 differentiation and activated the AHR in vitro.
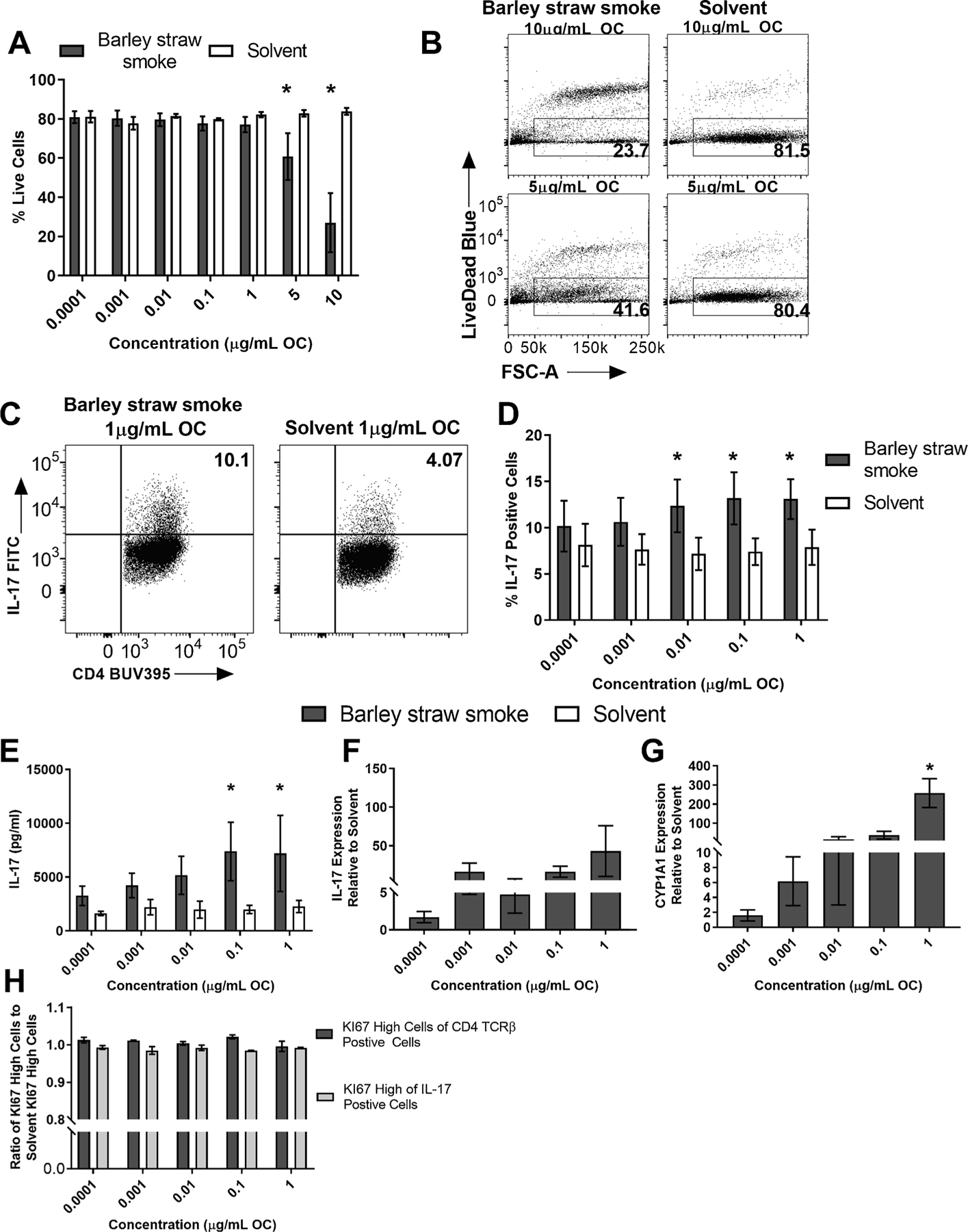
Naïve CD4+ T cells were isolated from WT (C57BL/6 J) mice and cultured under Th17 conditions. At time 0, cells were exposed to a dose-response (8 doses) of Barley straw smoke or DCM/DMSO solvent control and cultured for 3 days. (A) Barley straw exposure caused significant cell death at 10 and 5 μg/mL OC measured by the percent live cells of total CD4 cells (n = 3). These doses were subsequently excluded from further analysis (B) Representative flow plots of WT percent of live cells at 10.0 μg/mL OF (top) and 5 μg/mL OC (bottom) (C) Representative flow plots of WT Th17 differentiation measured by the percent of IL-17 positive cells at dose 1 μg/mL OC. (D) Barley straw smoke enhanced Th17 differentiation at 1, 0.1, and 0.01 μg/mL OC, measured by the percent of IL-17 positive cells (n = 3). (E) Barley straw smoke increased the total production of IL-17 after the 3-day culture at 1 and 0.1 μg/mL OC (n = 3). (F) There was no significant difference in IL-17 mRNA between Barley straw smoke treatment and solvent control (n = 3). (G) Barley straw significantly upregulated CYP1 A1 mRNA at 1 μg/mL OC (n = 3). (H) There was no significant difference in percent of proliferating cells, measured by KI67 expression, between Barley straw smoke and solvent control. Results are mean ± SEM. Significant differences among groups (p < 0.05) are indicated by an asterisk. Abbreviations: DCM/DMSO, methylene chloride/dimethyl sulfoxide ;OC, organic carbon; OF, organic fraction.
3.3. Motor oil has no effect on Th17 differentiation or CYP1 A1 mRNA levels in vitro
Given that all of the real-world samples tested contain PAHs and enhanced Th17 differentiation and activated the AHR, we wanted to test a real-world sample that does not contain PAHs to test whether PAHs could be the component driving the enhanced responses. Motor oil was prepared by the WSLH and contains no measurable PAHs. Naïve CD4 positive T cells were isolated from spleens of C57BL/6 J WT mice and exposed to a dose-response of Motor oil or solvent control on day 0, cultured under Th17 conditions for 3 days, and supernatant was collected for IL-17 ELISA, mRNA was collected, and flow cytometry was performed. There was no significant difference in percent live cells at any concentration for Motor oil except at 10 μg/mL OC, in which Motor oil had significantly more live cells than solvent control (Fig. 3A and B). Motor oil had no effect on Th17 differentiation (Fig. 3C and D), IL-17 protein levels (Fig. 3E), IL-17 mRNA (Fig. 3F), or CYP1 A1 mRNA (Fig. 3G). There was a significant difference in the percent of KI67 high cells of the total CD4+TCRβ cells at 10 μg/mL OC, and in the percent of KI67 high cells of IL-17 positive cells at 10 μg/mL OC (Fig. 3H).
Fig. 3. Motor oil had no effect on Th17 differentiation or AHR activation in vitro.
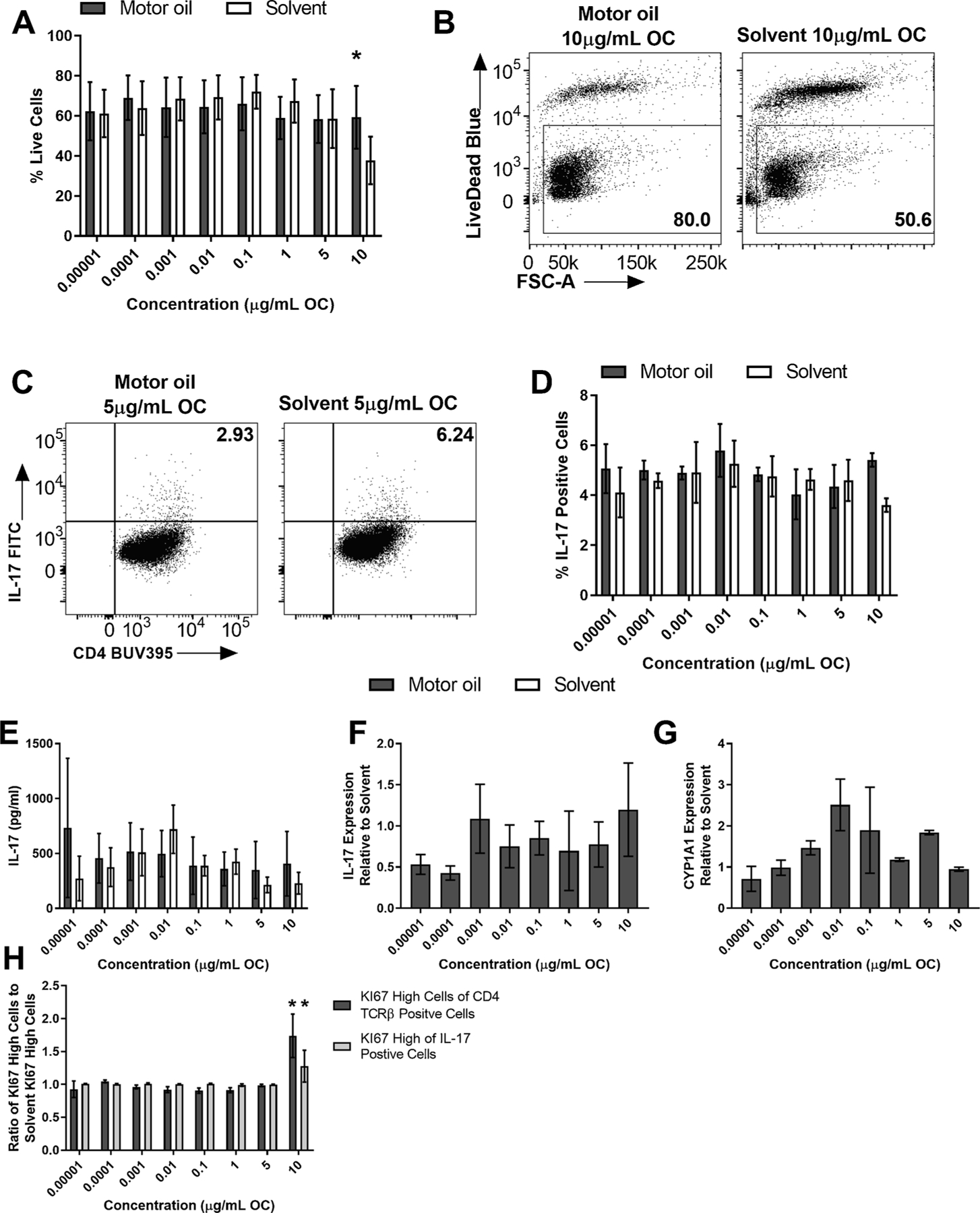
Naïve CD4+ T cells were isolated from WT (C57BL/6 J) mice and cultured under Th17 conditions. At time 0, cells were exposed to a dose-response (8 doses) of Motor oil or DCM/DMSO solvent control and cultured for 3 days. (A) Motor oil exposure did not cause significant cell death, however at the 10 μg/mL OC concentration there was a significant reduction in percent live cells for solvent (n = 4). (B) Representative flow plots of WT percent of live cells at 10.0 μg/mL OF, measured as percent live cells of total CD4 cells. (C) Representative flow plots of WT Th17 differentiation measured by the percent of IL-17 positive cells at dose 5 μg/mL OC. (D) Motor oil did not significantly enhance Th17 differentiation at any dose tested compared to solvent control (n = 3). (E) Motor oil did not significantly increase the total production of IL-17 after the 3-day culture (n = 3). (F) There was no significant difference in IL-17 mRNA between Motor oil treatment and solvent control (n = 2). (G) There was no significant difference in CYP1 A1 mRNA expression between Motor oil treatment and solvent control at any dose tested (n = 2). (H) There was a significant difference in the percent of CD4 + TCRβ+ cells that proliferated and IL-17 positive cells that proliferated, measured by KI67 expression in those populations, between solvent and control. Results are mean ± SEM. Significant differences among groups (p < 0.05) are indicated by an asterisk. Abbreviations: DCM/DMSO, methylene chloride/dimethyl sulfoxide; OC, organic carbon; OF, organic fraction.
3.4. Pine wood smoke enhances Th17 differentiation at the highest concentration in vitro
Barley straw smoke was the most potent sample in its ability to enhance Th17 differentiation because it enhanced differentiation at lower concentrations than Riverside or Motor oil. Given this, we tested another real-world source sample, Pine wood smoke, a smoke sample collected from burning pine wood to determine if all samples from similar sources enhanced Th17 differentiation to the same degree. Pine wood smoke is similar to Barley straw smoke in composition as both are from burning biomass. Naïve CD4 positive T cells were isolated from spleens of C57BL/6 J WT mice and exposed to a dose-response of Pine wood smoke OF or solvent control on day 0, cultured under Th17 conditions for 3 days. There was no significant difference in percent live cells between Pine wood smoke exposure and solvent, however there was a reduction in the overall percent cells alive at 10 and 5 μg/mL OC (Fig. 4A and B). Pine wood smoke significantly enhanced Th17 differentiation at the highest concentration tested 10 μg/mL OC (Fig. 4C and D). The enhanced Th17 differentiation observed at 10 μg/mL OC was associated with a significant increase in cell proliferation, measured by KI67 high cells of IL-17 positive cells at 10 μg/mL OC. Although there was no significant difference in percent live cells between Pine wood smoke and solvent control, there was a reduction in the overall percent live cells at 10 μg/mL OC which could explain the significant increase in percent IL-17 positive cells suggesting it is artificially high due to cell death. Alternatively, the significant increase in the percent of KI67 high IL-17 positive cells could suggest that the IL-17 positive cells are selected for and more viable in the culture.
Fig. 4. Pine wood smoke enhanced Th17 differentiation in vitro.
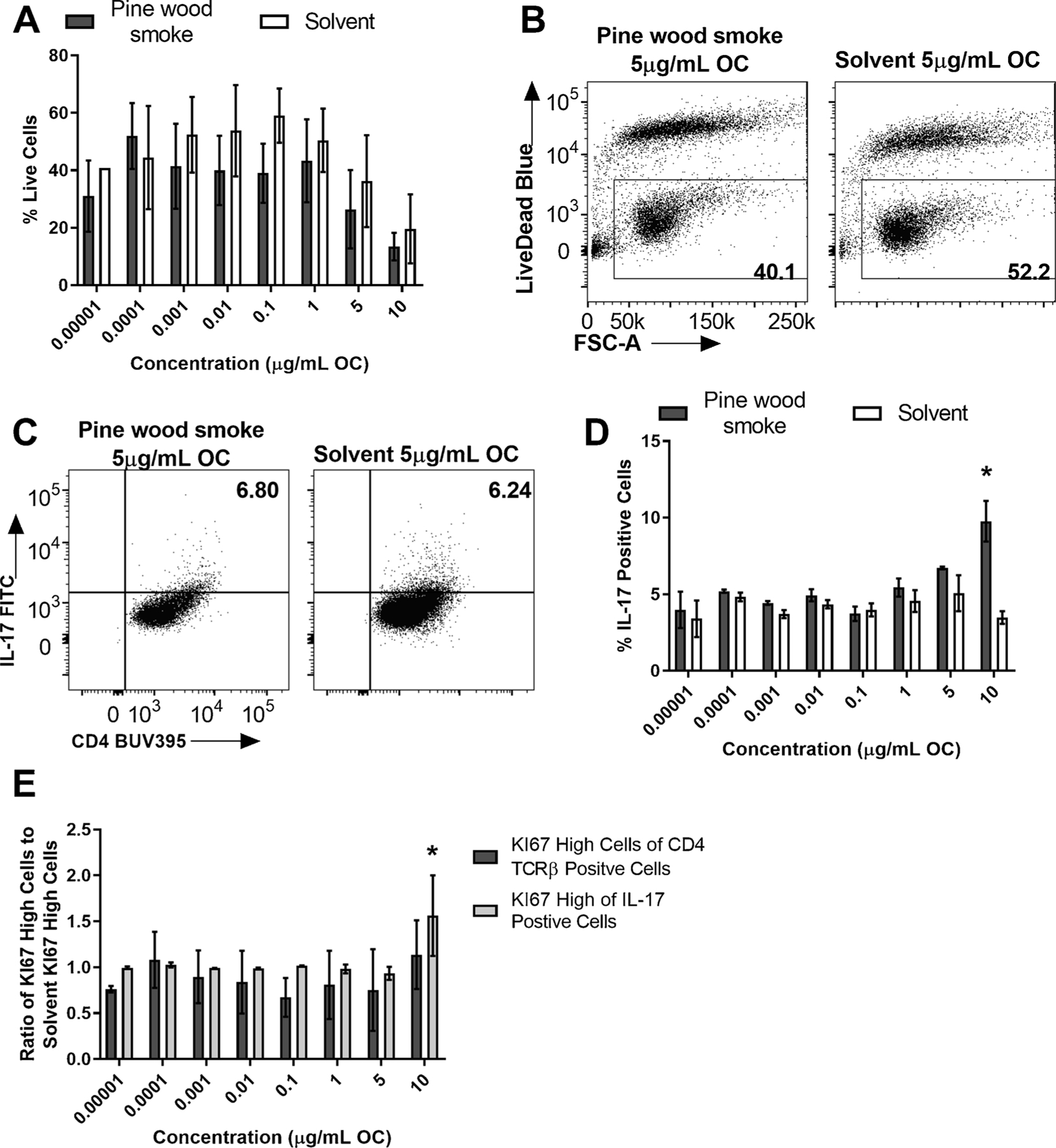
Naïve CD4+ T cells were isolated from WT (C57BL/6 J) mice and cultured under Th17 conditions. At time 0, cells were exposed to a dose-response (8 doses) of Pine smoke or DCM/DMSO solvent control and cultured for 3 days. (A) There was no significant difference in percent live cells between Pine wood smoke treated and solvent treated cells (n = 2). (B) Representative flow plots of WT percent of live cells at 5 μg/mL OF, measured as percent live cells of total CD4 cells. (C) Representative flow plots of WT Th17 differentiation measured by the percent of IL-17 positive cells at the 10 μg/mL OC concentration. (D) Pine wood smoke enhanced Th17 at 10 μg/mL OC, measured by the percent of IL-17 positive cells (n = 2). (E) There was a significant difference in the percent of IL-17 positive cells that proliferated, measured by KI67 expression, between Pine wood smoke and control. Results are mean ± SEM. Significant differences among groups (p < 0.05) are indicated by an asterisk. Abbreviations: DCM/DMSO, methylene chloride/dimethyl sulfoxide ;OC, organic carbon; OF, organic fraction.
3.5. Extracts of standard and real-world samples enhance Th17 differentiation to different degrees and contain different PAHs at different levels
In addition to real-world samples, we also tested standard reference materials (SRMs) 1649b, 1650b, and 2975. SRM1649b is an ambient urban dust PM, SRM1650b is a 4-cylinder diesel exhaust PM, and SRM2975 is a 2-cylinder diesel exhaust PM. All three SRM OFs enhanced Th17 differentiation and increased CYP1 A1 mRNA, albeit to different degrees (data not shown). In order to compare the real-world samples to each other, percent IL-17 positive cells, IL-17 protein levels, IL-17 mRNA levels, and CYP1 A1 mRNA levels were normalized to their solvent controls and compared to each other (Fig. 5A–D). There was a significant difference in the percent of IL-17 positive cells between Barley straw smoke and motor oil at 1 μg/mL OC (Fig. 5A). When comparing IL-17 protein levels there was significant difference in IL-17 protein levels at 10 μg/mL OC between Riverside and Barley straw smoke (Fig. 5B). It is important to note that Barley straw smoke exhibited significant cell death at 10 and 5 μg/mL but was included for comparison. Although Barley straw smoke had higher levels of IL-17 and CYP1 A1 mRNA compared to Riverside or Motor oil (Fig. 5C and D, respectively) there was no significant difference between samples. Our hypothesis for this study was that different OFs of real-world samples would enhance Th17 to different degrees based on the number of PAHs and concentrations. To address this, a non-linear regression was conducted, and a dose-response curve was generated for the real-world samples as well as the SRMs (Fig. 5E). As expected, Motor oil was the least potent sample (Fig. 5E) and contains no PAHs (Fig. 6F). Barley straw has the highest concentration of PAHs (Fig. 6C) and was the most potent sample at enhancing Th17 differentiation with a logEC50 of −2.12 (Fig. 5E). SRM1649b and Riverside are almost equally potent with logEC50 values around −0.9 (Fig. 5E). SRM1650b and SRM2975 are less potent than SRM1649b and Riverside (Fig. 5E). Riverside has multiple PAHs but at low levels (Fig. 6E) compared to SRM1649b (Fig. 6A) and SRM1650b (Fig. 6B). SRM2975 has the fewest number of PAHs (Fig. 6DE) but is not the least potent sample at enhancing Th17 differentiation (Fig. 5E). Pine wood smoke has many PAHs at high levels compared to other samples (Fig. 6G) but Pine wood smoke is least potent along with Motor oil (Fig. 5E).
Fig. 5. Comparing extent of Th17 differentiation and AHR activation by real-world PM samples.
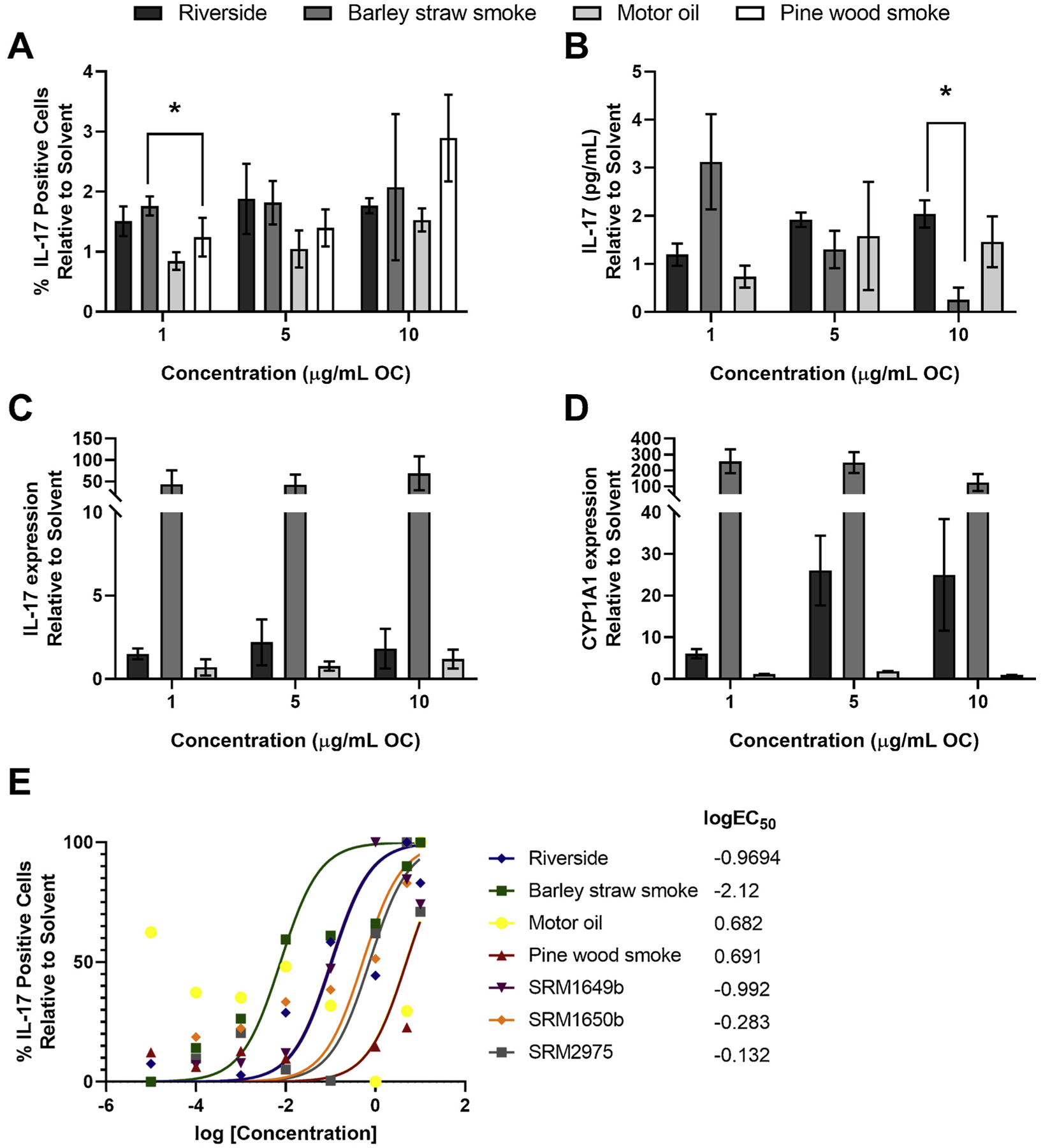
Naïve CD4+ T cells were isolated from WT (C57BL/6 J) mice and cultured under Th17 conditions. At time 0, cells were exposed to a dose-response (8 doses) of Riverside, Barley straw smoke, Motor oil, Pine wood smoke, or DCM/DMSO solvent control and cultured for 3 days. For panels A–D treatments with real-world samples were normalized to DCM/DMSO solvent controls. (A) A significant difference in the % IL-17 positive cells was observed between Barley straw smoke and Motor oil at 1.0 μg/mL. (B) There was a significant difference in the level of IL-17 protein between Riverside and Barley straw smoke at 10 μg/mL, however Barley straw smoke exhibited significant cell death at the 10 μg/mL concentration. (C) There was no significant difference between IL-17mRNA levels of real-world samples (D) or CYP1 A1 mRNA levels. However, Barley straw smoke trended to have higher levels compared to Riverside or Motor oil. (E) A non-linear regression was fit to the raw flow cytometry data. In addition to the real-world samples tested, three standard reference materials (SRMs) 1649b, 1650b, and 2975 were used tested in the Th17 differentiation assay and included in the dose response. The percent IL-17 positive cell data was normalized to set the largest mean in each data set as 100%. Abbreviations: DCM/DMSO, methylene chloride/dimethyl sulfoxide ;SRM, standard reference material; IL, interleukin; CYP, cytochrome P450; PAHs, polycyclic aromatic hydrocarbons.
Fig. 6. PAH profiles based of PAHs present in PM samples.
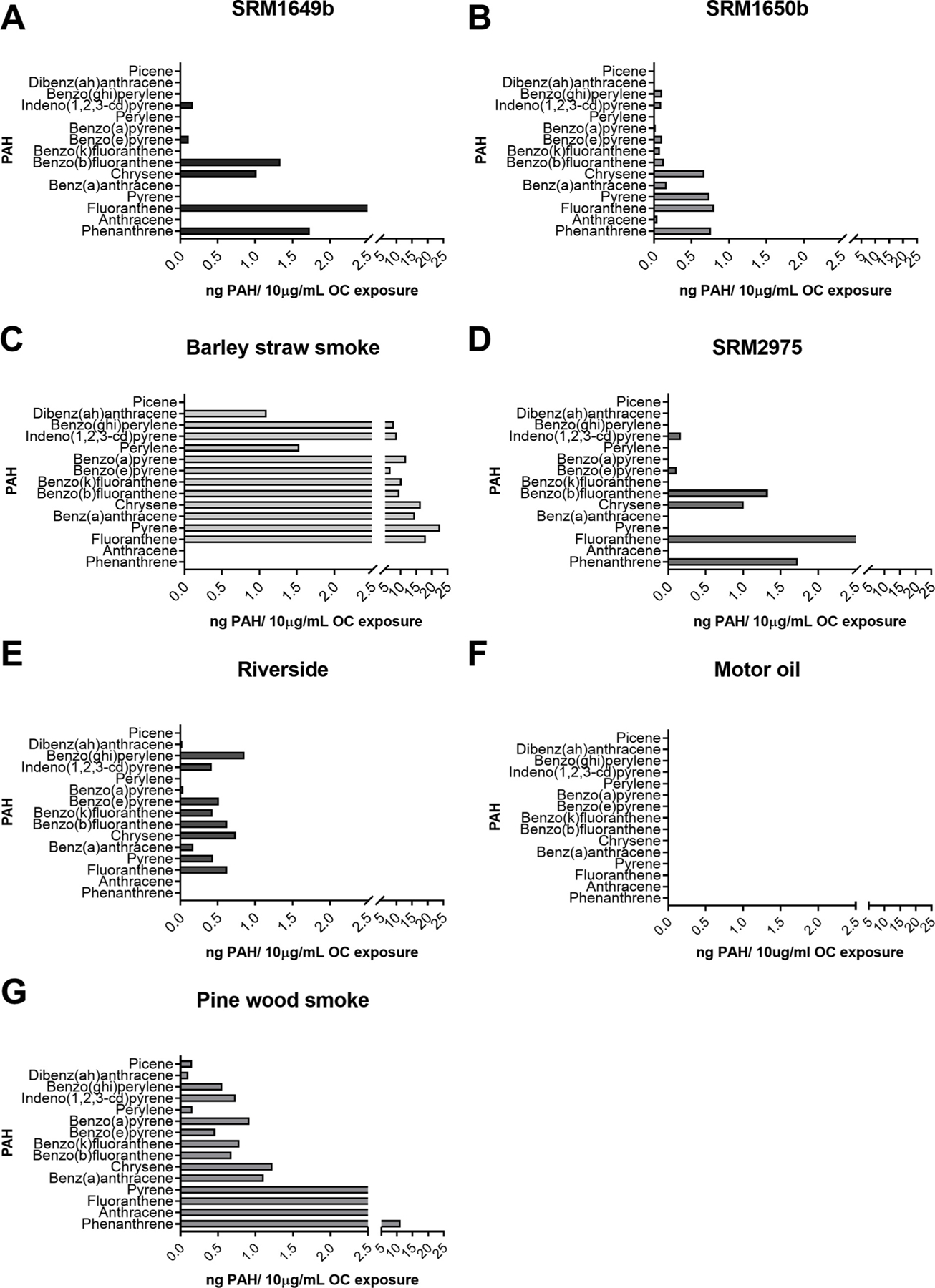
The PAH concentrations are represented are PAH concentrations present in the 10 μg/mL OC concentration used. The PAHs were measured in each sample using GC/MS. (A–G) PAH profiles of the nanograms of 15 PAHs, most of which are U.S. EPA priority PAHs, present in each sample at the 10 μg/mL OC concentration tested. Abbreviations: EPA, Environmental Protection Agency; PAH, polycyclic aromatic hydrocarbon; OC, organic carbon.
4. Discussion
The current study aimed to demonstrate that all PM of the same mass do not elicit the same biological effects and to identify PAHs as candidate components present in PM that contribute to pro-inflammatory responses. It is important to note that real-world PM samples contain high affinity AHR ligands other than PAHs, such as polychlorinated biphenyls and polychlorinated dibenzodioxins. Recently, the polar components present in diesel exhaust particles, which contains the polar components present in the PM mixture as well as polar PAH derivatives formed as a result of metabolism, were shown to be more active than PAHs in human cells (Vondracek et al., 2017). However, PAHs have been considered the main contributors to genotoxicity, dioxin-like toxicity, and carcinogenicity of complex PM mixtures (Libalova et al., 2014). It was shown that in an organic extract of a standard ambient urban dust the AHR-mediated activity of the neutral fraction was linked to PAHs and their polar derivatives, which are generated after metabolism by CYP enzymes, and polychlorinated di-benzo-p-dioxins, dibenzofurans and biphenyls were only minor contributors to the overall AHR-mediated activities (Andrysik et al., 2011). Additionally, it was shown that induction of AHR target gene expression by extractable organic matter was not antagonized by other components of extractable organic matter of PM as the induction of AHR target genes reached maximum levels induced by PAHs suggesting that the induction of AHR target genes is induced by PAHs present in the PM mixtures (Libalova et al., 2014). Based on these studies, we originally hypothesized that the samples with the largest sum concentration of PAHs would enhance Th17 differentiation and activate the AHR to the greatest extent. This hypothesis was based on previous data from our lab demonstrating that synthetic PAH mixtures composed of 15 PAHs, most of which are from the EPA priority pollutant list, that replicate the PAH milieu present in PM samples enhanced Th17 differentiation in an AHR-dependent manner (O’Driscoll et al., 2018). We demonstrated that the chemically-extracted OF of real-world PM samples, that contain PAHs, enhanced the generation of pro-inflammatory Th17 cells and activated the AHR, but samples lacking PAHs did not. The real-world sample with the lowest observable adverse effect level (LOAEL) was Barley straw smoke, followed by Riverside, Pine wood smoke, and Motor oil (Table 1). Motor oil does not contain any PAHs and did not enhance Th17 differentiation or activate the AHR at any concentration tested (Table 1).
Table 1.
Lowest observable adverse effect level of real-world samples. The table shows three endpoints, Th17 differentiation measured by the percent of IL-17 positive cells, IL-17 production measured by IL-17 cytokine levels, and AHR activation measured by CYP1 A1 mRNA expression. Each row is real-world sample and the values are the LOAEL or lowest concentration for each sample that a significant effect was observed.
| Th17 differentiation % IL-17 Positive cells |
IL-17 production IL-17 (pg/mL) |
AHR Activation CYP1 A1 mRNA Expression Relative to Solvent |
|
|---|---|---|---|
| Lowest observable adverse effect level (LOAEL) (μg/mL OC) | |||
| Riverside | 5 | 5 | 5 |
| Barley straw smoke | 0.01 | 0.1 | 1.0 |
| Motor oil | None | None | None |
| Pine wood smoke | 10 | N/A | N/A |
Barley straw smoke, smoke collected from barley straw burning in Japan, with high concentrations of PAHs (Table 2), resulted in significant cell death at high doses, but at lower doses enhanced Th17 differentiation and activated the AHR (Table 1). Interestingly, Riverside, an ambient PM sample collected outside of Los Angeles in Riverside, CA with low concentrations of PAHs (Table 2), enhanced Th17 differentiation and activated the AHR (Table 1) more strongly than samples containing higher levels and more PAHs (Table 2). On the other hand, Pine wood smoke, smoke collected from burning pine wood, exhibited increased Th17 differentiation only at the highest dose tested (Table 1), despite having the second highest sum total PAH concentration of the samples tested (Table 2). These data suggest that the sum concentration of PAHs present in PM alone does not predict the extent of Th17 differentiation and AHR activation. The OFs containing PAHs can activate the AHR and enhance Th17 differentiation, however certain individual PAHs or the specific PAH mixtures present may play a more important role than the sum concentration of PAHs.
Table 2.
Concentrations of PAHs present in real-world samples. The table lists 15 PAHs, most of which are U.S. EPA priority PAHs, and gives the PAH concentration in nanogram per milliliter concentration. This table also calculates the nanogram PAH concentration at the highest dose tested 10 μg/mL OC. The last two rows of the table represent the average PAH concentration and the total sum of PAH concentration. Motor oil is not included in this table because it does not contain any PAHs.
| PAHs | Riverside | Barley straw smoke | Pine wood smoke | |||
|---|---|---|---|---|---|---|
| ng PAH/mL extracted | ng PAH/ 10 μg/mL dose exposure | ng PAH/mL extracted | ng PAH/ 10 μg/mL dose exposure | ng PAH/mL extracted | ng PAH/ 10 μg/mL dose exposure | |
| Phenanthrene | 0 | 0 | 0 | 0 | 1087.98 | 11.27 |
| Anthracene | 0 | 0 | 0 | 0 | 269.45 | 2.79 |
| Fluoranthene | 64 | 0.63 | 1859 | 18.05 | 406.3 | 4.21 |
| Pyrene | 45 | 0.44 | 2330 | 22.62 | 336.7 | 3.49 |
| Benz[a]anthracene | 18 | 0.18 | 1500 | 14.56 | 107.03 | 1.11 |
| Chrysene | 76 | 0.74 | 1694 | 16.45 | 118.46 | 1.23 |
| Benzo[b]fluoranthene | 64 | 0.63 | 993 | 9.64 | 65.52 | 0.68 |
| Benzo[k]fluoranthene | 44 | 0.43 | 1069 | 10.38 | 76.03 | 0.79 |
| Benzo[e] pyrene | 53 | 0.52 | 705 | 6.84 | 44.86 | 0.46 |
| Benzo[a]pyrene | 4 | 0.04 | 1218 | 11.83 | 89.07 | 0.92 |
| Perylene | 0 | 0 | 158 | 1.53 | 15.55 | 0.16 |
| Indeno[l,2,3-cd]pyrene | 43 | 0.42 | 914 | 8.87 | 71.12 | 0.74 |
| Benzo[ghi]perylene | 88 | 0.86 | 812 | 7.88 | 53.85 | 0.56 |
| Dibenz[ah]anthracene | 3 | 0.03 | 113 | 1.10 | 10.08 | 0.10 |
| Picene | 1 | 0.01 | 0 | 0 | 14.9 | 0.15 |
| Average PAH concentration | 0.33 | 8.65 | 1.91 | |||
| Sum of PAH concentration | 4.91 | 129.76 | 28.65 | |||
The level of AHR activation by real-world PM samples was a primary focus of this study due to the ability of PAHs present in the OF of PM to activate the receptor and its modulatory role in the immune system. More specifically, AHR has been shown to play a role in the balance between Tregs and Th17 cells with different ligands promoting the differentiation of either T cell subset (Quintana et al., 2008; Veldhoen et al., 2008). The AHR plays a critical role in balancing effector and regulatory immune responses (Quintana et al., 2008; Veldhoen et al., 2008) and the extent and duration of AHR activation by AHR ligands, such as PAHs, can lead to enhanced regulation or enhanced inflammation (Ehrlich et al., 2018). In the current study, the OF of real-world PM samples enhanced Th17 differentiation and activated the AHR to different degrees. The enhanced Th17 differentiation observed after exposure to real-world PM samples containing PAHs was concentration-dependent in that lower doses exhibited no observable effect and higher doses significantly enhanced Th17 differentiation. This concentration dependence was true for AHR activation as well. The loss of enhanced Th17 differentiation and AHR activation at low doses in the real-world samples could be due to reduced level of PAHs present in the samples so the AHR is not being activated and Th17 differentiation is not enhanced. Additionally, antagonistic or synergistic effects of other organic constituents in the real-world samples could be inhibiting the PAHs that are present from activating the AHR (Libalova et al., 2014). PAHs were identified as candidate components of PM that drive enhanced Th17 differentiation because they are present in the OF of real-world PM samples at high levels compared to other AHR ligands and they exhibit high affinity for the AHR. Differences in the specific PAHs present and the combination of PAHs present led to different potencies of enhanced Th17 differentiation as well as AHR activation. One possibility is that only a subset of the PAHs measured are required to activate the AHR sufficiently to shift the balance from a regulatory T cell response to an effector Th17 response, leading to enhanced Th17 differentiation. Another possibility is that the specific PAH mixtures, with certain combinations of individual PAHs can have synergistic effects and shift the balance towards Th17 differentiation. It has been shown that the genotoxic effects of individual PAHs can be inhibited by other organic compounds adhered to PM suggesting other components present in the PM may have synergistic or antagonistic effects on the responses elicited by specific components (Libalova et al., 2014). We have previously published that benzo[k]fluoranthene enhanced Th17 differentiation in and AHR-dependent manner (van Voorhis et al., 2013) and others have shown that priority PAHs, including benzo[k] fluoranthene, were the principal contributors to AHR-mediated activity (Andrysik et al., 2011). The mixtures may also alter the ability of cytochrome p450 enzymes, downstream targets of AHR, to metabolize PAHs present in the PM sample, which could affect the immune response of that mixture as well as other PAHs.
5. Conclusions
A major goal of the current study was to address the longstanding gap in knowledge as to whether all PM behaves the same and should be regulated by mass or if individual chemical constituents should be considered. Currently, in the United States and worldwide, PM is regulated by total mass as a function of aerodynamic size, ignoring individual particle characteristics and components (Organization, W.H., 2006). The World Health Organization regulates PM based on total mass instead of fractions or constituents because scientific evidence that any one property or constituent of PM is responsible for adverse health effects has not been found (Organization, W.H., 2006). The current study demonstrates that not all PM is the same and individual components such as PAHs likely contribute to the observed biologic effect by activating the AHR and enhancing pro-inflammatory Th17 cells. Further studies need to be conducted looking at the levels of AHR activation and pro-inflammatory effects of PAHs in human cells to assess which specific PAHs are most toxic to humans considering differences in AHR activation, since humans harbor a lower affinity AHR compared to C57BL/6 J mice and CYP metabolism to account for metabolic differences in humans. Certain exposures may be more pathologic than others, depending on the specific composition of each sample and the biologic endpoint. The specific exposures and components that increase the risk of inflammation may be different than those that increase the risk of cancer and other human pathologies. This suggests that further efforts to reduce disease derived from PM should pursue more targeted strategies focused on reducing specific sources that emit certain constituents, rather than focusing on total mass. Additionally, efforts to reduce PM in the context of protecting human health should explicitly include efforts to reduce specific PAHs that exacerbate human disease.
Supplementary Material
Acknowledgements
Thank you to the University of Wisconsin-Carbone Cancer Center Flow Cytometry Laboratory [University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520 and Special BD LSR Fortessa Project Grant 1S100OD018202-01]. Thank you to Michael Olson for providing the Pine wood smoke sample and to Akihiro Fushimi and Ana Maria Villalobos Igor for providing the Barley straw smoke and Riverside samples. Also, thank you to Brandon Shelton at the Wisconsin State Lab of Hygiene for extraction and analysis of the extracts as well as preparation of the motor oil sample.
Funding
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) [RO1 ES023842 to J.D.M, R21 ES025304 to J.D.M], and [T32 ES007015 (C.A.O)]. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tox.2019.01.002.
Particulate matter
Polycyclic aromatic hydrocarbons
Organic fraction
Aryl hydrocarbon receptor
Cytochrome p450
T helper
Regulatory T cells
Methylene chloride
Dimethyl sulfoxide
Organic carbon
interleukin
6-formylindolo[3,2-b] carbazole
Standard reference material
References
- Andrysik Z, Vondracek J, Marvanova S, Ciganek M, Neca J, Pencikova K, Mahadevan B, Topinka J, Baird WM, Kozubik A, Machala M, 2011. Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: the role of polycyclic aromatic hydrocarbons. Mutat. Res 714, 53–62. [DOI] [PubMed] [Google Scholar]
- Bell ML, 2012. Assessment of the Health Impacts of Particulate Matter Characteristics. Health Effects Institute, Boston, Massachusetts. [PubMed] [Google Scholar]
- Carver LA, Bradfield CA, 1997. Ligand-dependent Interaction of the Aryl Hydrocarbon Receptor With a Novel Immunophilin Homolog in Vivo. [DOI] [PubMed]
- Cheung K, Daher N, Kam W, Shafer MM, Ning Z, Schauer JJ, Sioutas C, 2011. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10–2.5) in the Los Angeles area. Atmos. Environ 45, 2651–2662. [Google Scholar]
- Council, N.R, 2010. In: Press, T.N.A. (Ed.), Global Sources of Local Pollution: An Assessment of Long-Range Transport of Key Air Pollutants to and from the United States. Council, N.R., Washington, DC. [Google Scholar]
- DeGroot D, He G, Fraccalvieri D, Bonati L, Pandini A, Denison M, 2011. AhR ligands: promiscuity in binding and diversity in response In: Pohjanvirta R (Ed.), The AH Receptor in Biology and Toxicology. Wiley, Hoboken, NJ, pp. 63–79. [Google Scholar]
- Ehrlich AK, Pennington JM, Bisson WH, Kolluri SK, Kerkvliet NI, 2018. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol. Sci 161, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi A, Saitoh K, Hayashi K, Ono K, Fujitani Y, Villalobos AM, Shelton BR, Takami A, Tanabe K, Schauer JJ, 2017. Chemical characterization and oxidative potential of particles emitted from open burning of cereal straws and rice husk under flaming and smoldering conditions. Atmos. Environ 163, 118–127. [Google Scholar]
- Heo J, Dulger M, Olson MR, McGinnis JE, Shelton BR, Matsunaga A, Sioutas C, Schauer JJ, 2013. Source apportionments of PM2.5 organic carbon using molecular marker Positive Matrix Factorization and comparison of results from different receptor models. Atmos. Environ 73, 51–61. [Google Scholar]
- Institute HE, 2018. State of Global Air 2018. Health Effects Institute, Boston, MA. [Google Scholar]
- Kelly FJ, Fussell JC, 2012. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ 60, 504–526. [Google Scholar]
- Libalova H, Krckova S, Uhlirova K, Milcova A, Schmuczerova J, Ciganek M, Klema J, Machala M, Sram RJ, Topinka J, 2014. Genotoxicity but not the AhR-mediated activity of PAHs is inhibited by other components of complex mixtures of ambient air pollutants. Toxicol. Lett 225, 350–357. [DOI] [PubMed] [Google Scholar]
- Ma JY, Ma JK, 2002. The dual effect of the particulate and organic components of diesel exhaust particles on the alteration of pulmonary immune/inflammatory responses and metabolic enzymes. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev 20, 117–147. [DOI] [PubMed] [Google Scholar]
- Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, Pan F, Priel A, Clish CB, Robson SC, Quintana FJ, 2015. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat. Med 21, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll CA, Owens LA, Gallo ME, Hoffmann EJ, Afrazi A, Han M, Fechner JH, Schauer JJ, Bradfield CA, Mezrich JD, 2018. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part. Fibre Toxicol 15, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Garcia Mercedes Victoria, Robinson Michael A., Van Rooy Paul., Dietenberger Mark A., Bergin Michael, Schauer James Jay, 2015. Investigation of black and brown carbon multiple-wavelength-dependent light absorption from biomass and fossil fuel combustion source emissions - Olson - 2015 - Journal of Geophysical Research: Atmospheres - Wiley Online Library. J. Geophys. Res.: Atmos 120, 6682–6697. [Google Scholar]
- Organization, W.H, 2006. In: W.H. Organization (Ed.), WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and sulfur Dioxide Global Update 2005 Summary of Risk Assessment. WHO Press, Geneva, Switzerland. [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL, 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BR, 2001. Measurement of emissions from air pollution sources. 3. C1-C29 organic compounds from fireplace combustion of wood. Environ. Sci. Technol 35, 1716–1728. [DOI] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA, 2008. Th17 cells in human disease. Immunol. Rev 223, 87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, Mezrich JD, 2013. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One 8, e82545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B, 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109. [DOI] [PubMed] [Google Scholar]
- Vincent R, Bjarnason SG, Adamson IY, Hedgecock C, Kumarathasan P, Guénette J, Potvin M, Goegan P, Bouthillier L, 1997. Acute pulmonary toxicity of urban particulate matter and ozone. Am. J. Pathol 151, 1563–1570. [PMC free article] [PubMed] [Google Scholar]
- Vondracek J, Pencikova K, Neca J, Ciganek M, Grycova A, Dvorak Z, Machala M, 2017. Assessment of the aryl hydrocarbon receptor-mediated activities of polycyclic aromatic hydrocarbons in a human cell-based reporter gene assay. Environ. Pollut 220, 307–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


