Abstract
Generation of amyloid-β peptides (Aβs) by proteolytic cleavage of the amyloid-β protein precursor (AβPP), especially increased production of Aβ42/Aβ43 over Aβ40, and their aggregation as oligomers and plaques, represent a characteristic feature of Alzheimer’s disease (AD). In familial AD (FAD), altered Aβ production originates from specific mutations of AβPP or presenilins 1/2 (PS1/PS2), the catalytic subunits of γ-secretase. In sporadic AD, the origin of altered production of Aβs remains unknown. We hypothesize that the ‘human chemical exposome’ contains products able to favor the production of Aβ42/Aβ43 over and shorter Aβs. To detect such products, we screened a library of 3500 + compounds in a cell-based assay for enhanced Aβ42/Aβ43 production. Nine pyrazole insecticides were found to induce a β- and γ-secretase-dependent, 3–10-fold increase in the production of extracellular Aβ42 in various cell lines and neurons differentiated from induced pluripotent stem cells derived from healthy and FAD patients. Immunoprecipitation/mass spectrometry analyses showed increased production of Aβs cleaved at positions 42/43, and reduced production of peptides cleaved at positions 38 and shorter. Strongly supporting a direct effect on γ-secretase activity, pyrazoles shifted the cleavage pattern of another γ-secretase substrate, alcadeinα, and shifted the cleavage of AβPP by highly purified γ-secretase toward Aβ42/Aβ43. Focusing on fipronil, we showed that some of its metabolites, in particular the persistent fipronil sulfone, also favor the production of Aβ42/Aβ43 in both cell-based and cell-free systems. Fipronil administered orally to mice and rats is known to be metabolized rapidly, mostly to fipronil sulfone, which stably accumulates in adipose tissue and brain. In conclusion, several widely used pyrazole insecticides enhance the production of toxic, aggregation prone Aβ42/Aβ43 peptides, suggesting the possible existence of environmental “Alzheimerogens” which may contribute to the initiation and propagation of the amyloidogenic process in sporadic AD.
Keywords: Aβ38, Aβ40, Aβ42, Aβ43, Aβ42/Aβ40 ratio, aftins, Alzheimer’s disease, alzheimerogen, amyloid-β, amyloid-β protein precursor, fipronil, γ-secretase, human chemical exposome, pesticides, phenylpyrazoles, prevention, pyrazoles, triazines
INTRODUCTION
Alzheimer’s disease (AD) is a major disease in countries with aging populations. Despite unresolved questions on the initial causes of AD and numerous clinical trial failures in recent years, the lack of satisfactory treatments and the extremely high prevalence of AD calls for fundamental research to determine its underlying molecular and cellular causes and mechanisms and for applied research to identify options for prevention, therapeutic targets and disease-modifying drug candidates (reviews in [1–4]).
Although the aggregation of specific forms of amyloid-β peptides (Aβs) into soluble oligomers is undoubtedly associated with the onset of AD, the upstream etiological events underlying this pathological process remain unclear, including whether there is an absolute, or relative, increase in the production of longer, aggregation-prone Aβ species. Aβs are derived from the successive action of two proteases, β-secretase and γ-secretase, on one of their numerous substrates, the transmembrane amyloid-β protein precursor (AβPP). According to its cleavage site, γ-secretase liberates Aβs of different sizes. While Aβ40 is considered as the main physiological product, the appearance of Aβ42 and Aβ43, or increase of the Aβ42-Aβ43/Aβ40 ratio is clearly associated with the onset of AD [5–7]. These longer Aβs show an increased propensity to form oligomers, which can assemble into large extracellular deposits, the amyloid plaques, a characteristic feature of AD. Aβ42/Aβ43 oligomers are now considered as the toxic, AD initiating elements rather than the more prominent plaques initially discovered by Aloïs Alzheimer. This ‘amyloid cascade theory’ is further supported by the identification of the genetic causes of early onset familial AD (FAD). These rare forms of AD (less than 1% of all AD cases) are indeed all associated with specific mutations of either AβPP or PS1 or PS2 (Presenilins) genes (the latter encode the catalytic subunits of the γ-secretase complex) (review in [8, 9]). In addition, a specific, protective mutation of AβPP was recently found to be associated with reduced risk for developing AD [10]. Furthermore, animal models that express mutated forms of AβPP and/or PS1, or human Aβ42/Aβ43, develop molecular, cellular and cognitive deficits reminiscent of AD ([6], review in [11, 12]). Yet, the level of the different forms of Aβs is not well correlated with cognitive deficits in mice, suggesting that Aβs may represent markers rather than inducers of AD in these models [13]. An altered processing of γ-secretase substrates other than AβPP might also be important in the pathogenesis of AD. Although FAD is clearly a consequence of genetic mutations of genes encoding the proteins responsible for the production of Aβs, the initial trigger(s) of late-onset, sporadic AD (>99% of AD cases) remain unknown, despite extensive genome-wide association studies (GWAS) and the identification of various risk factors (review in [14]).
Environmental neurotoxic agents including pesticides, organic solvents, metals and some natural toxins (cyanobacteria) are likely sources of AD-inducing factors (reviews in [15–25]). According to the US Environmental Protection Agency (EPA) Toxic Substances Control Act, over 84,000 chemicals are manufactured or imported at levels >10 tons per year, not including pesticides, cosmetics, food stuffs, and food additives which are covered by other legislations (http://www.epa.gov). It is estimated that we are exposed to over 85,000 substances which, along with all natural substances to which we are exposed from conception to death, constitute the ‘human chemical exposome’ (HCE) [26–34]. In search for potential “Alzheimerogens” (named by analogy with carcinogens), and encouraged by the discovery of Aftins (Amyloid β Forty-Two Inducers) [35–37], a class of synthetic compounds which specifically induce the production of Aβ42, we have started to assemble and screen a library of compounds, belonging to the HCE, for Aβ42-inducing products. Our first study identified some triazine herbicides as products stimulating the production of Aβ42/Aβ43 in numerous cell lines [38].
In this new study, we show that several members of the pyrazole class of insecticides, exemplified by fipronil, also increase the γ-secretase-dependent production of Aβ42/Aβ43 peptides in various cell lines and in a cell-free system with highly purified γ-secretase. Interestingly fipronil is metabolized to fipronil sulfone, a very persistent product that accumulates in adipose tissue and brain. These results show that the HCE contains various brain permeable products able to induce the production of pathogenic Aβs. If long exposures occur in real life and if accumulation of Aβ42/Aβ43 is sufficient to trigger neurodegeneration/neuroinflammation, such products may contribute to the initiation, development and acceleration of sporadic AD and might thus be collectively qualified as potential “Alzheimerogens”. Some of them might also be used to develop useful chemically-induced animal models of AD.
MATERIAL AND METHODS
Material and methods are described in full in the Supplementary Material They include: 1) Pyrazoles and other reagents; 2) Cell cultures: cell lines, iPSCs-derived neurons and HEK293-alcadeinα cells; 3) Purified β-secretase preparation; 4) Aβ cell-based and cell-free assays; 5) Cell viability assay; 6) Pharmacokinetics studies; 7) Proteomics study.
RESULTS
Screening HCE compounds reveals pyrazole insecticides as Aβ42 inducers
We first screened a library of over 3,500 low molecular weight compounds representative of the HCE for their ability to induce the production and secretion of Aβ42 by N2a cells stably expressing AβPP695 (N2a-AβPP695). An MTS-based cell viability assay was run in parallel to assess cell survival. Although the vast majority of compounds were unable to induce Aβ42 production, a few active products including several triazine herbicides [38] and pyrazole insecticides (this article) were identified. We next assembled a small library of 18 pyrazoles (1–18, Supplementary Tables 1 and 2) that we further tested, along with aftin-5 (19) as a positive control, for their ability to trigger Aβ42 production at 5, 10, 25, or 50 μM, in both N2a and CHO cells stably expressing AβPP695 and AβPP751, respectively (Supplementary Table 2 and Fig. 1). Nine pyrazoles were found to induce more than a 3-fold change in Aβ42 levels, showing that Aβ42 induction is an intrinsic property of some, but not all, pyrazoles: fenpyroximate (2), tebufenpyrad (3), bixafen (4), fluxapyroxad (5), isopyrazam (6), penthiopyrad (8), chlorantraniliprole (14), tolfenpyrad (16) and fipronil (18) (Figs. 1 and 2). We next focused on fipronil (18)2, a widely produced insecticide used to treat crops and pet parasites (reviews in [39–44]), and we assembled a small library of fipronil analogues and metabolites (Fig. 3) which were tested for their Aβ42 production ability in N2a-AβPP695 cells (Supplementary Table 3). The two fipronil enantiomers (18a, 18b) were separated by SFC chiral chromatography (Regis Whelk O1 SS column) and found to be roughly equipotent. Interestingly the main metabolites of fipronil (18) [45], fipronil sulfone (20), fipronil desulfinyl (21) and fipronil sulfide (22) displayed Aβ42 inducing activity equivalent or close to that of the parent compound. In contrast, a few other environmental metabolites such as fipronil chloramine (24) [46] and hydroxy-fipronil (25) [47] were found to be inactive (Supplementary Table 3).
Fig. 1.
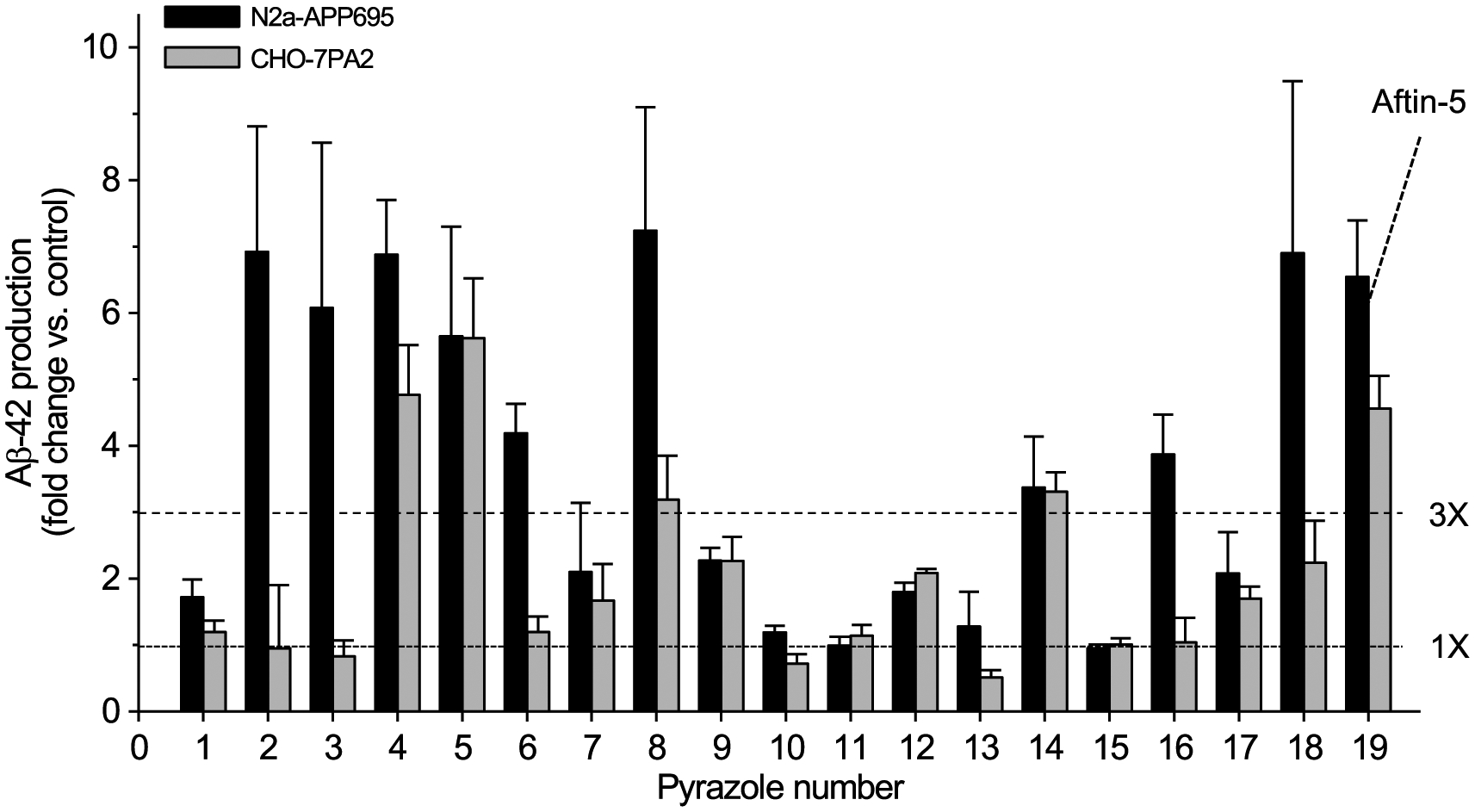
Some pyrazoles trigger the production of extracellular amyloid Aβ42. Effect of 18 pyrazoles on extracellular amyloid Aβ42 production by N2a-APP695 and CHO-7PA2-APPsw cells. Cells were treated with 100 μM of each compound for 18 h and cell supernatants were collected for extracellular Aβ42 levels measurement by an ELISA assay. Aftin-5 was used as a positive control and the corresponding volume of vehicle (DMSO) was used as a negative control. Levels are expressed as fold change, ± SE, of Aβ42 levels over the Aβ42 level of control, vehicle-treated cells. Average of five experiments performed in triplicate. Horizontal dotted lines indicate levels for 1- and 3- fold increases in Aβ42 concentration.
Fig. 2.
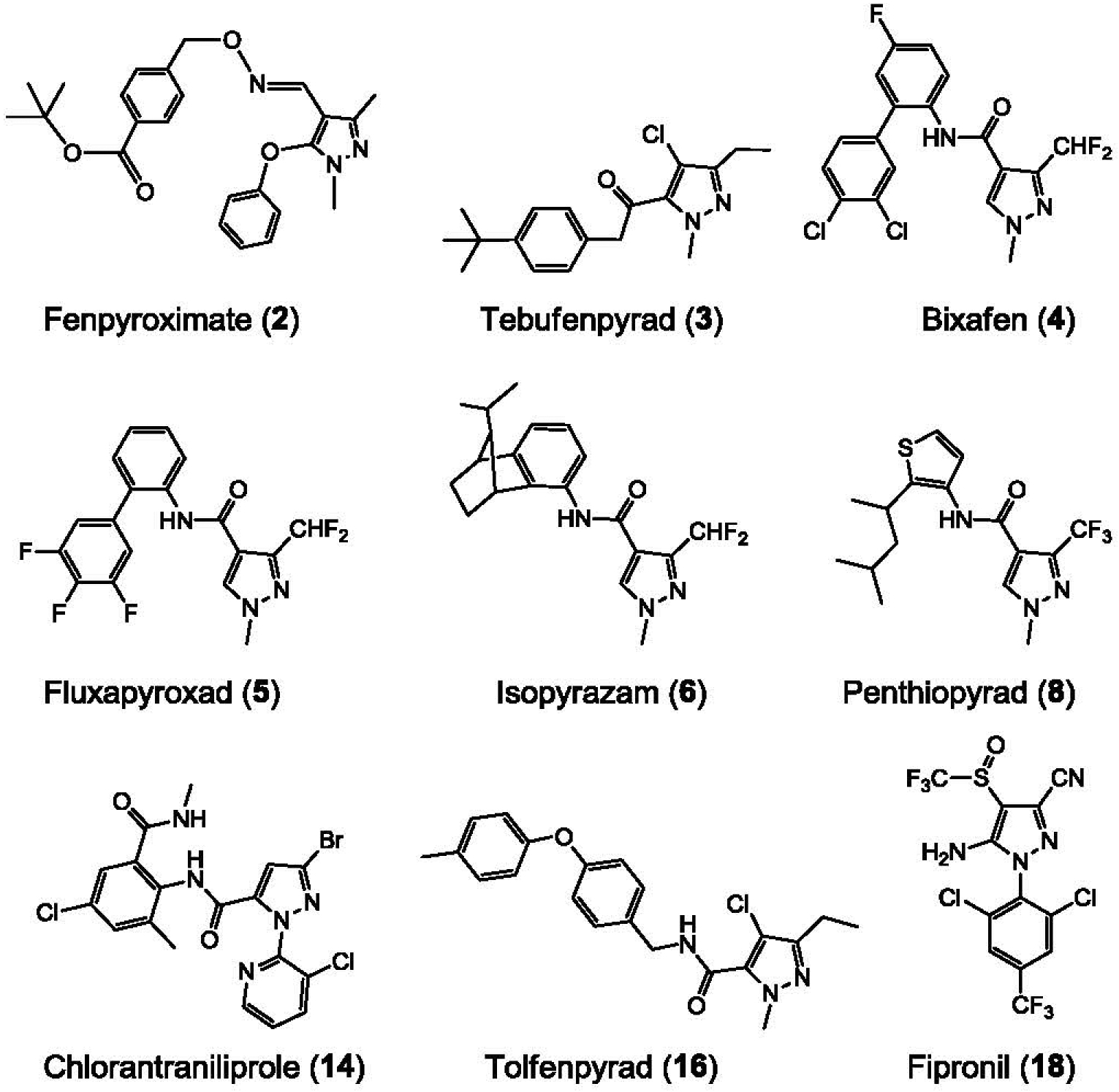
Molecular structure of the nine active pyrazoles.
Fig. 3.
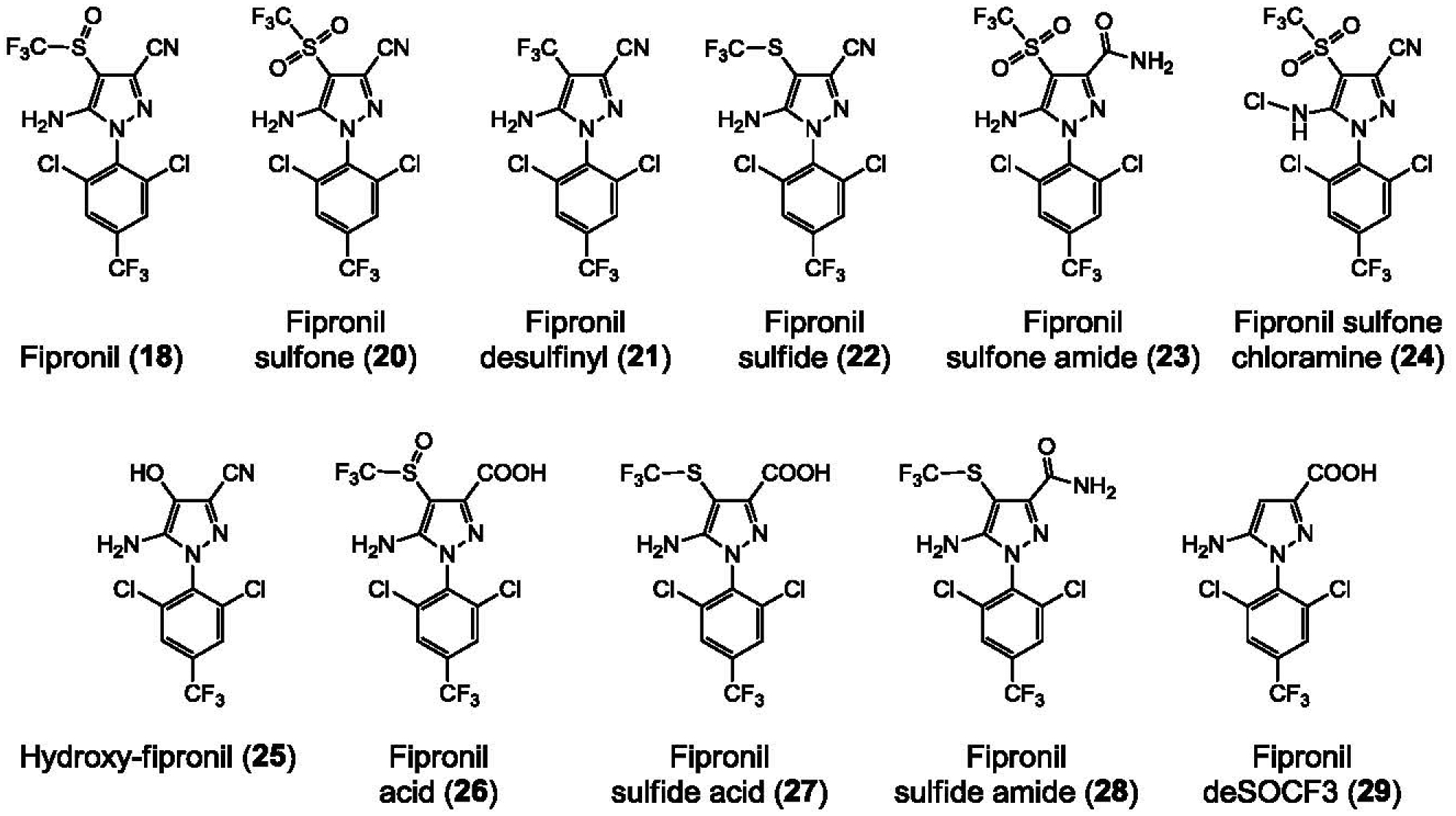
Molecular structure of fipronil and some of its metabolites and derivatives.
Aβ42 production triggered by pyrazoles in N2a-APP695 cells requires β- and γ-secretase activity as demonstrated by the fact that it was strongly inhibited by β- (inhibitor IV) and γ-secretases (BMS 299897, DAPT) inhibitors and by a γ-secretase modulator (‘Torrey Pines’ compound, inducing an increase in Aβ38 and a decrease in Aβ42 and Aβ40) [48,49] (Fig. 4). Aβ38 production was also strongly reduced following pyrazole treatment, while Aβ40 levels were only modestly affected, as measured by ELISA (data not shown) and by mass spectrometry (Fig. 5). Increased Aβ42 production triggered by pyrazoles was farther confirmed in HEK293 cells stably expressing AβPPsw (data not shown) and neurons derived from human iPSCs (see below).
Fig. 4.
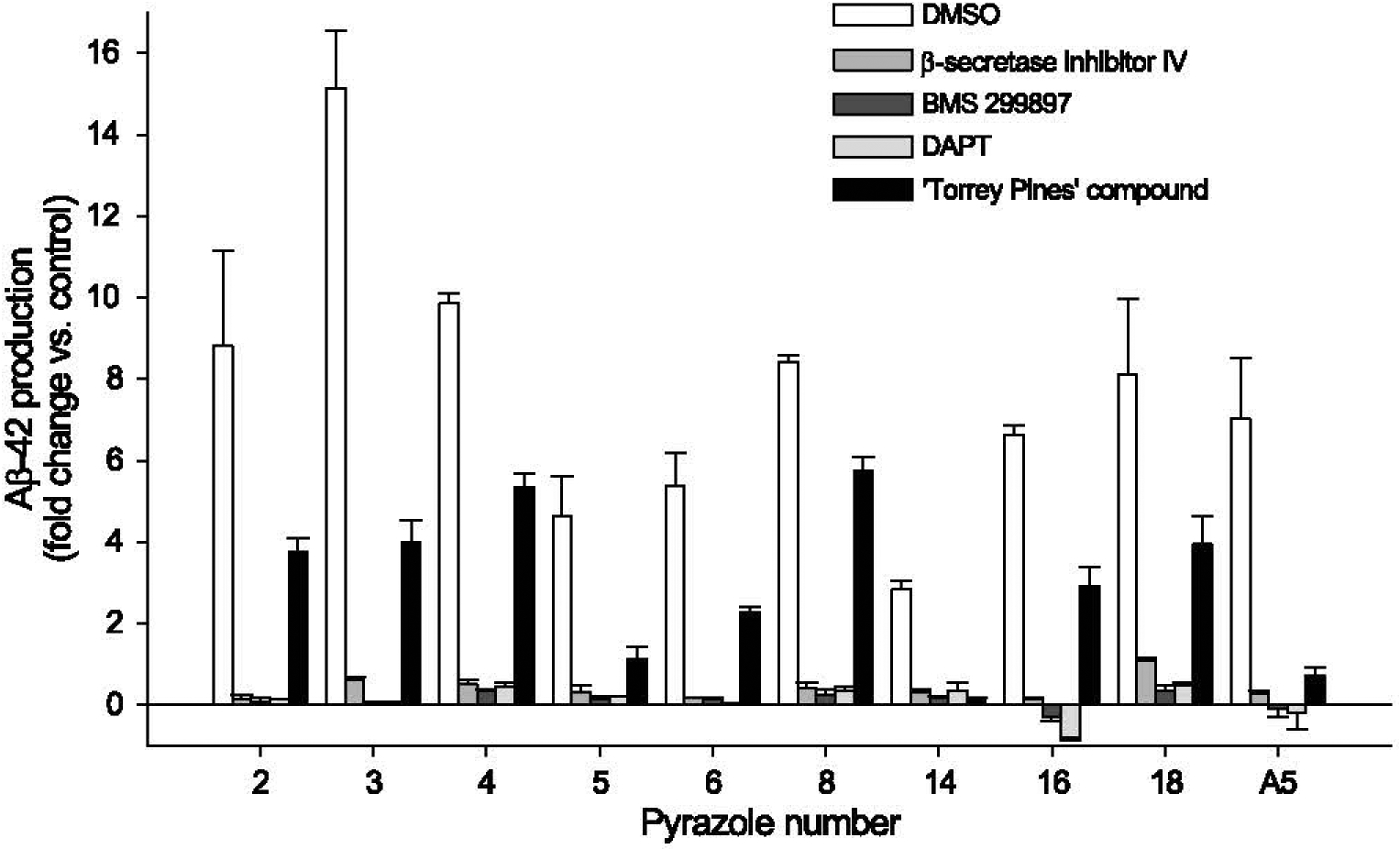
Extracellular Aβ42 production induced by pyrazoles is inhibited by β-secretase inhibitor IV, γ-secretase inhibitors DAPT and BMS 299897, and γ-secretase modulator ‘Torrey Pines’. N2a-APP695 cells were exposed to 10 μM β-secretase inhibitor IV, 2 μM BMS 299897, 2 μM DAPT or 10 μM ‘Torrey Pines’ compound. 1.5 h later cells were exposed to 50 μM (2,5,8,14,16,18) or 25 μM (3, 4, 6) pyrazoles or 50 μM aftin-5. Extracellular Aβ42 levels were measured after 18 h and are expressed as fold change, ± SE, of Aβ42 level in pyrazole-treated cells over the Aβ42 level of control, vehicle-treated cells. Representative of two independent experiments performed in triplicates. Errors bars represent SE of all six values.
Fig. 5.
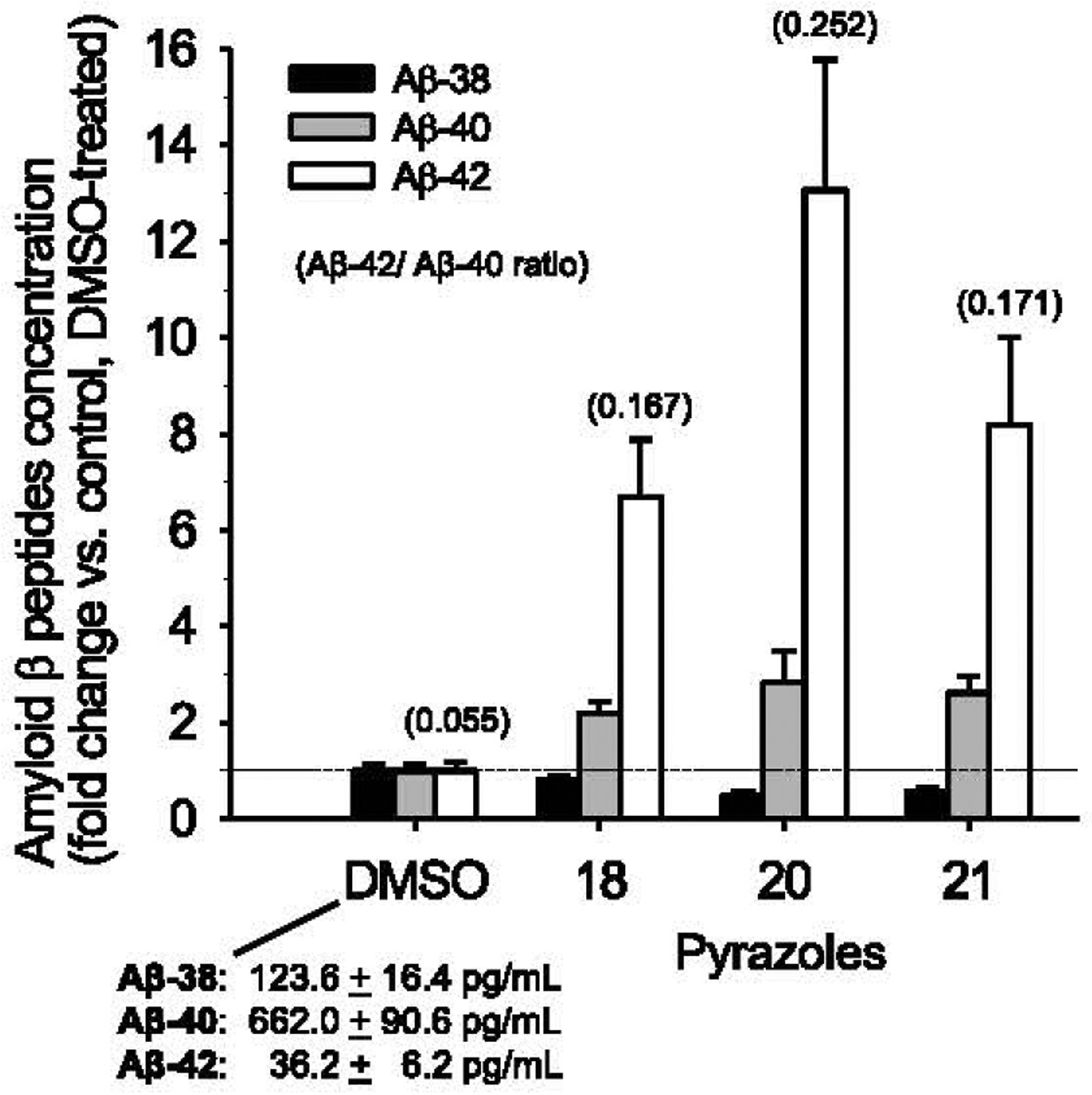
Absolute quantification of Aβ38, Aβ40, and Aβ42 using LC-MS/MS. Levels of the three Aβs were determined by mass spectrometry in supernatants of N2a-APP695 cells following 18 h treatment with DMSO, 100 μM of pyrazoles 18,20, or 21. Amyloid levels are expressed as percentage of levels in vehicle-treated cells (average ± SE of quadriplicate values; absolute values in control cell supernatants are indicated) and Aβ42/Aβ40 ratios are indicated in parentheses. Horizontal dotted line indicates basal Aβ levels versus the values in DMSO-treated cells.
Mass spectrometric quantification and profile analysis of pyrazole-induced Aβs
Aβ38, Aβ40, and Aβ42 were quantified in N2a-AβPP695 cell culture supernatants using LC-MS/MS [50,51]. Like aftins [35, 36] and triazines [38], pyrazoles induced a reduction in Aβ38 levels, a slight increase in Aβ40 levels and a strong increase in Aβ42 levels (Fig. 5).
We next analyzed, by IP-MS [52], the profile of Afis produced by N2a-AβPP695 exposed to fipronil (18), fipronil sulfone (20) and fipronil desulfinyl (21) (Fig. 6). Cell supernatants were collected and Aβs were immunoprecipitated and analyzed using MALDI-TOF/TOF [52] (Fig. 6). Exposure to pyrazoles decreased the production of Aβ1–18, Aβ1–19, Aβ1–33, Aβ1–37, and Aβ1–38. Aβ1–40 levels showed a modest increase. In contrast, the two highly neurotoxic [6, 7, 53–55] amyloid peptides Aβ1–42 and Aβ1–43, which were undetectable in supernatants of control cells, were strongly induced in cells treated with fipronil (18) and its two metabolites.
Fig. 6.
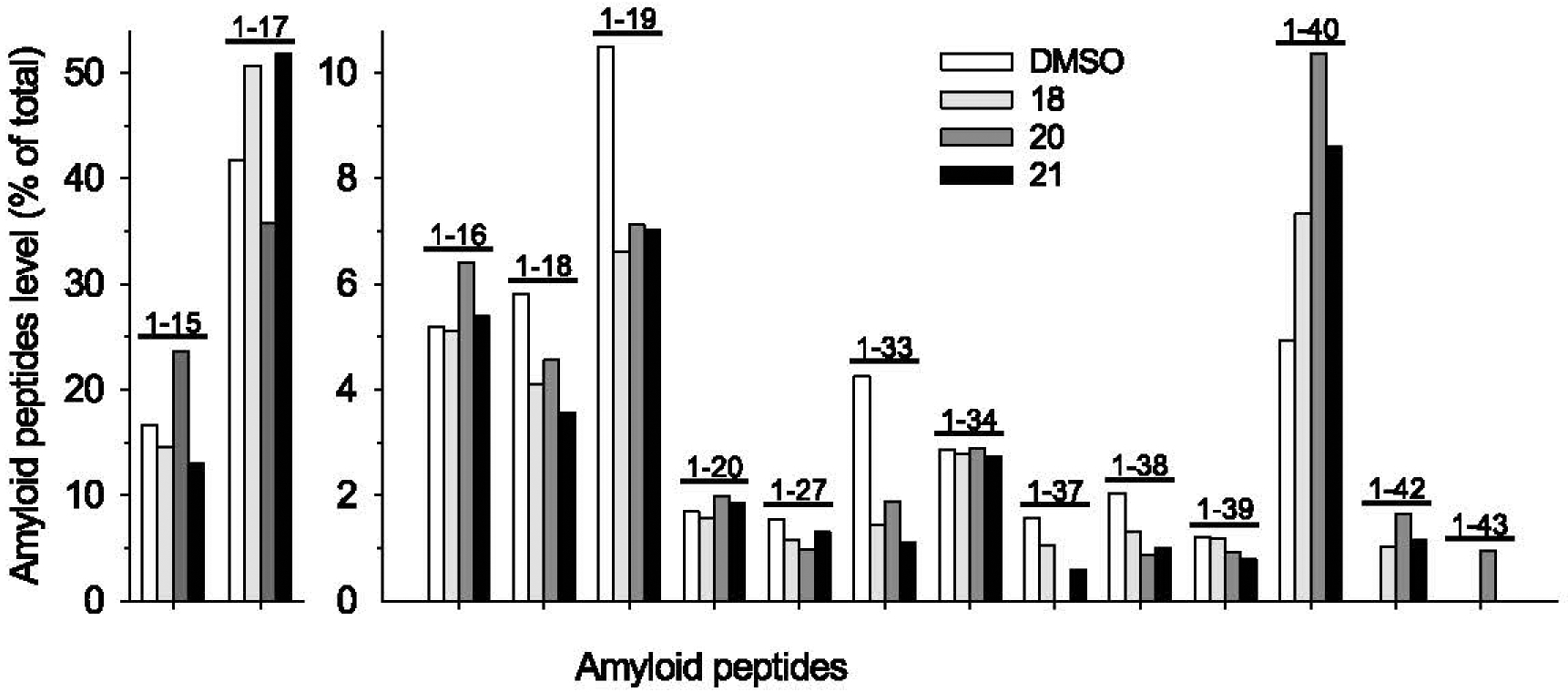
Pattern of Aβs produced by N2a-APP695 cells exposed to pyrazoles 18, 20, or 21. Cells were treated for 18 h with DMSO or 20 μM of each pyrazole. Cell supernatants were collected and analyzed as described. Quantification of all Aβs in N2a-APP695 cells supernatants are presented as percentage of total amyloids. Note the decrease in peptides Aβ1–19, Aβ1–37, Aβ1–38, the increase in Aβ1–40 and the appearance of Aβ1–42 and Aβ1–43 in the supernatants of pyrazole-treated cells (these two peptides are undetectable in the supernatant of DMSO-treated cells).
Neurons differentiated from human iPSCs of AD patient and healthy control
We next tested the effects of the active pyrazoles and aftin-5 on neurons differentiated for 4 weeks from human iPSCs derived from a healthy individual (AβPP WT, wild-type) or from a patient with familial AD (AβPP K724N mutation) [56–58] (Fig. 7). AβPP K724N neurons produced more Aβ42 versus Aβ40 compared to AβPP WT neurons. As observed with triazines [38], addition of any of the active pyrazoles or aftin-5 resulted in further increase in Aβ42 production, in both AβPP WT and AβPP K724N neurons (Fig. 7).
Fig. 7.
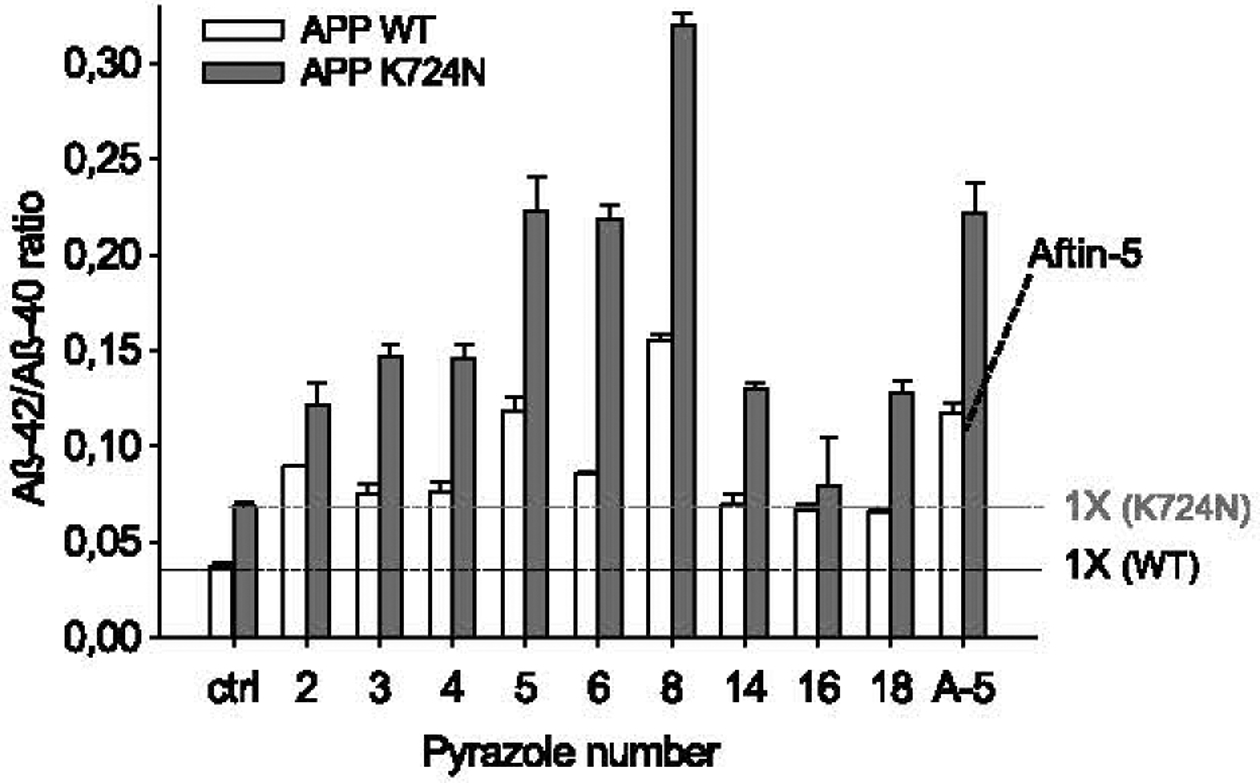
Pyrazoles trigger enhanced production of Aβ42 versus Aβ40 in neurons differentiated from human induced pluripotent stem cells. Neurons were derived from iPSCs obtained from healthy donor (APP WT, white bars) or from an AD patient with APP K724N mutation (grey bars). They were exposed for 24 h to DMSO (control), 100 μM aftin-5 or the nine pyrazoles. Cell supernatants were collected for extracellular Aβ40 and Aβ42 levels measurement by an ELISA assay. Levels are expressed as Aβ42/Aβ40 ratios ± SE of triplicate values. Horizontal dotted line indicates level for basal Aβ42/Aβ40 ratios.
Pyrazoles affect the specificity of APP-C99 cleavage and Aβ production in a cell-free γ-secretase activity assay
In order to assess whether the pyrazole-based compounds modulate Aβ production through a direct effect on the specific processing of APP-C99 by the γ-secretase complex, we tested fipronil (18), fipronil sulfone (20), and fipronil desulfinyl (21) in a previously described cell-free activity assay performed with highly purified γ-secretase protease and APP-C99 substrate (a recombinant APP C-terminal fragment expressed in Escherichia coli as a fusion protein consisting of a N-terminal Met for translation initiation, amino acids 597–695 of the 695-amino acid human isoform of APP, and a C-terminal Flag tag sequence) [59,60]. More specifically, Aβs generated by purified γ-secretase in the presence of 100 μM of the pyrazole compounds were analyzed by immunoprecipitation and MALDI-TOF (IP/MS), as previously described [60]. We found that all three pyrazole compounds increased the Aβ42/Aβ40 ratio when compared to the DMSO control (Fig. 8). Importantly, our analysis further revealed that fipronil sulfone (20), but not fipronil (18) or fipronil desulfinyl (21), drastically increased the Aβ43/Aβ40 ratio by ~2-fold when compared to the DMSO control (Fig. 8). These results strongly support the increased Aβ43 production seen exclusively with fipronil sulfone (20) in N2a-AβPP695 cells (Fig. 6). Altogether, we found that pyrazoles favor the production of the toxic Aβ42 and/or Aβ43 peptides, both of which contribute to the formation of amyloid plaques and the development of AD.
Fig. 8.
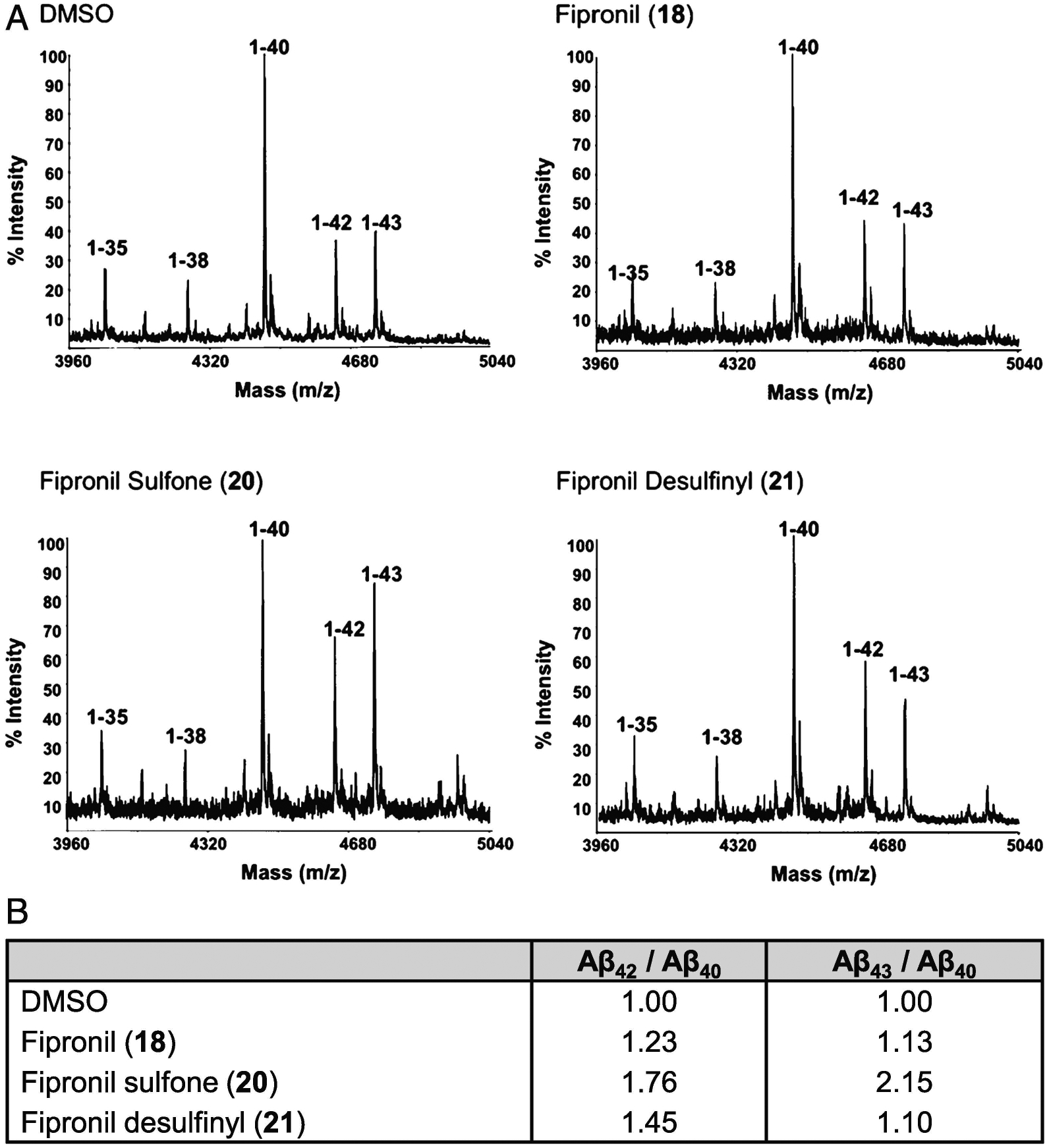
Mass spectrometric analysis of the Aβs generated in cell-free γ-secretase assays, in the presence of DMSO or fipronils 18, 20, or 21. A) The Aβs generated by highly purified γ-secretase in the presence of 100 μM of fipronil (18), fipronil sulfone (20) or fipronil desulfinyl (21) or DMSO (vehicle) were pooled from triplicates of the activity assays, immunoprecipitated overnight with the anti-Aβ antibody 4G8 and protein G and analyzed by MALDI-TOF in a reflectron mode. The Aβs generated from the recombinant APP-C99 contain an N-terminal methionine, which results in a mass shift of +149m/z when compared to endogenous Aβ peptides. B) Note the increased Aβ42/Aβ40 ratio for fipronils 18, 20, and 21, and the increased Aβ43/Aβ40 ratio for fipronil sulfone (20).
Pyrazoles shift the cleavage pattern of another γ-secretase substrate, alcadeinα
Like AβPP, alcadeins (Ales) are sequentially cleaved by secretases, first by α-secretase, leading to N- and C-terminal fragments, the latter being then cleaved by γ-secretase to an intracellular domain and the p3-Alcs peptide, in a way similar to AβPP [61, 62] (see Fig. 6A in [38]). HEK293 cells stably expressing full length alcadeinα were used to investigate the effects of pyrazoles on alcadein cleavage. Alcadeinα is first cleaved on the N-terminal side (two possible sites) followed by cleavage by γ-secretase leading to p3-Alcα35 and p3-Alcα 2N+35, the latter representing the major peptide in cultured cells. HEK293-alcadeinα cells were grown till 70% confluence and treated with 100 μM pyrazoles for 48 h. The secreted p3-Alcα peptides were recovered by immunoprecipitation and analyzed by MALDITOF/MS (Fig. 9A). Quantification of p3-Alcα peptides showed that, compared to the p3-Alcα peptide profile in vehicle treated cells, concentrations of the main alcadeinα peptide (p3-Alcα2N+35) and p3-Alcα2N+37 peptide remained stable. In contrast, both p3-Alcα2N+34 and p3-Alcα2N+36 concentrations dropped significantly while the level of p p3-Alcα2N+38 peptide concentration strongly increased (Fig. 9B). These results show that, like for AβPP, pyrazoles induce a shift in the cleavage pattern of Alcadeinα, another γ-secretase substrate. Similar effects were seen previously with aftin-5 and triazines [38]. These observations further suggest that pyrazoles are more likely to interact with γ-secretase than with their substrates.
Fig. 9.
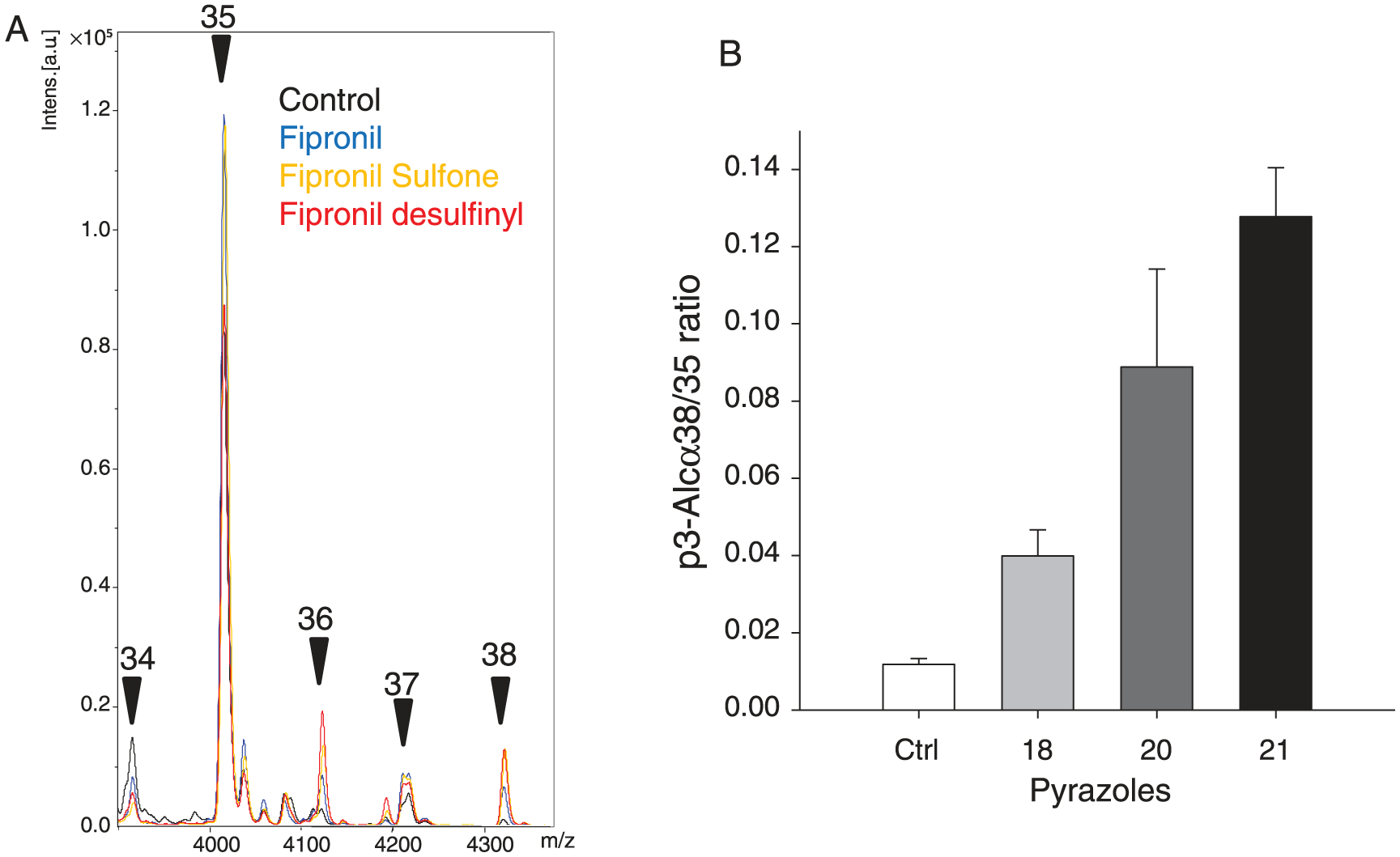
Pyrazoles alter the cleavage pattern of Alcadeinα, leading to increased p3-Alcα38 production. A) Immunoprecipitation/mass spectrometry spectra of p3-Alcα peptides produced by HEK cells expressing full length Alcadeinα exposed to various pyrazoles or DMSO (control). Cells were treated for 48 h with 100 μM of each reagent and p3-Alc peptides were analyzed by MALDI-TOF/MS. A) Representative profile for each product showing the the p3-Alcα2N+34, p3-Alcα2N+35, p3-Alcα2N+36, p3-Alcα2N+37 and p3-Alcα2N+38 peaks. B) Relative quantification of p3-Alcα peptides produced by cells exposed to DMSO (control), fipronil (18), fipronil sulfone (20) or fipronil desulfinyl (21). Levels of each peptide are presented as ratios of p3-Alcα2N+38 versus p3-Alcα2N+35 (average ± SE of triplicate values).
Proteomics study offipmnil’s effects
In order to understand the molecular mechanisms of action of pyrazoles, we decided to analyze protein expression of cells exposed to fipronil sulfone (20) and dipropetryn, two structurally unrelated products which, despite their unidentified targets, share the property of inducing Aβ42 production. N2a-AβPP cells were exposed for 18 h to 20 μM fipronil sulfone (20), 100 μM dipropetryn or 0.1% DMSO (control) and were processed for proteomics analysis. Cell supernatant Aβ42 levels were increased as expected (data not shown). A total of 8,027 proteins were identified in the multiplex experiment (data not shown). The expression of 1,634 and 1,638 proteins was modified, respectively, in fipronil sulfone (20)- and dipropetryn-treated cells compared to control, DMSO-treated cells (Fig. 10A). Among these, 261 proteins were shared by cells treated with both products, out of which 178 were upregulated or downregulated in the same direction for both products (Supplementary Table 4). DAVID analysis of these 178 proteins showed that they gather in mitochondrial pathways, cell cycle control, AD (Fig. 10B).
Fig. 10.
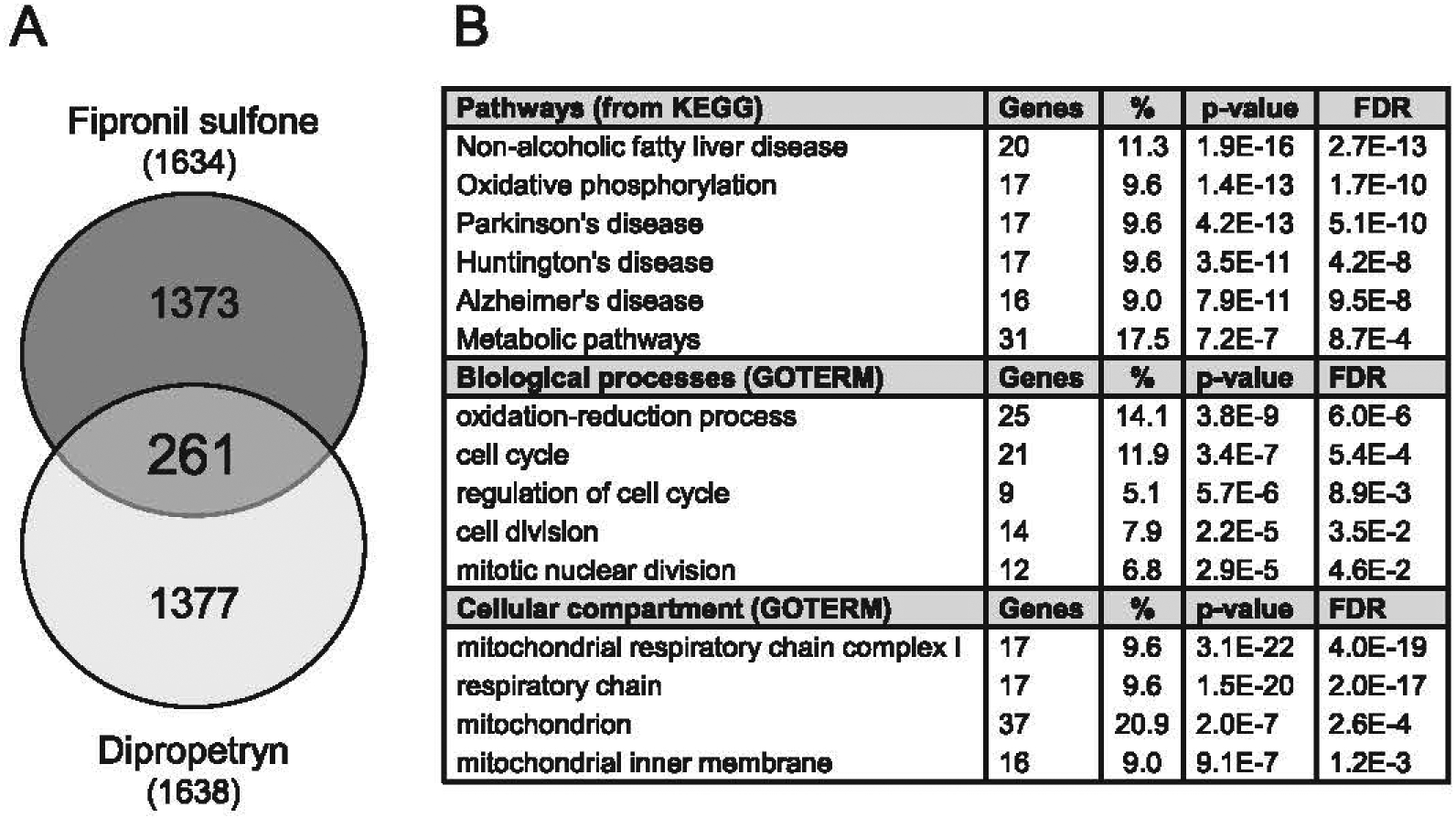
Proteomics and phosphoproteomics analysis of N2a-APP695 cells exposed to fipronil sulfone (20) and dipropetryn. A) N2a-APP695 cells were exposed for 18 h to 20 μM fipronil sulfone (20), 100 μM dipropetryn or DMSO. This led to increased extracellular Aβ42 expression (3.58 ± 0.05 and 7.87 ± 0.81 fold change for fipronil sulfone (20) and dipropetryn, respectively, compared to DMSO-treated cells). Up/downregulated proteins in fipronil sulfone- (1634) and dipropetryn- (1638) treated cells versus DMSO-treated cells were identified and compared. Among the 261 common proteins, 178 proteins (listed in Supplementary Table 1) were either upregulated by both treatments or downregulated by both treatments. B) DAVID analysis of the 178 proteins common to fipronil sulfone (20) and dipropetryn treatments. Data are selected with p-value < 0.01 and FDR < 0.05. FDR, False Discovery Rate.
Fipronil is metabolized to fipronil sulfone, a stable metabolite which accumulates in adipose tissue and brain
We next investigated the pharmacokinetics and biodistribution of fipronil (18) and its metabolites in mice (Fig. 11) and rats (Supplementary Figure 2). Mice first received a single oral dose of fipronil (18) and blood, brain and adipose tissue were collected at various times during 72 h (Fig. 11). Plasma pharmacokinetics showed rapid elimination of fipronil (18) and parallel appearance of fipronil sulfone (20) which remained stable during the 72 h time-course (Supplementary Figure 1). No fipronil desulfinyl (21) was detected. Similarly, fipronil (18) transiently appeared in brain and adipose tissues, while fipronil sulfone (20) accumulated to a stable level in both tissues (Fig. 11 A, B). Fipronil sulfone (20) reached a 5–6-fold higher concentration in adipose tissue compared to brain. Given the metabolic stability of fipronil sulfone (20), we next ran a two months duration study following a single oral administration of fipronil (18) (Fig. 12A). Fipronil sulfone (20) levels were determined at various times up to 56 days after the single administration. The half-life of fipronil sulfone (20) was found to be 14 ± 3 days, 17 ± 2 days, and 26 ± 3 days in plasma, brain, and adipose tissue, respectively (Fig. 11 A). We next administered fipronil (18) daily, 5 days/week, for 3 weeks and measured fipronil sulfone (20) in plasma, brain and adipose tissue (Fig. 12B). This repeated oral dosing allowed the maintenance of a stable level of fipronil sulfone (20) in plasma, brain and adipose tissue (Fig. 12B). Pharmacokinetics and biodistribution of fipronil (18) and fipronil sulfone (20) were next studied in rats during a 14 days period, following a single oral administration. Results confirmed rapid metabolism of fipronil (18) to fipronil sulfone (20) (Supplementary Figure 2A). Fipronil sulfone (20) was much more stable and showed extensive accumulation in adipose tissue (peak concentration 48 h after oral administration of fipronil (18)) followed by slow release and/or metabolism (Supplementary Figure 2B).
Fig. 11.
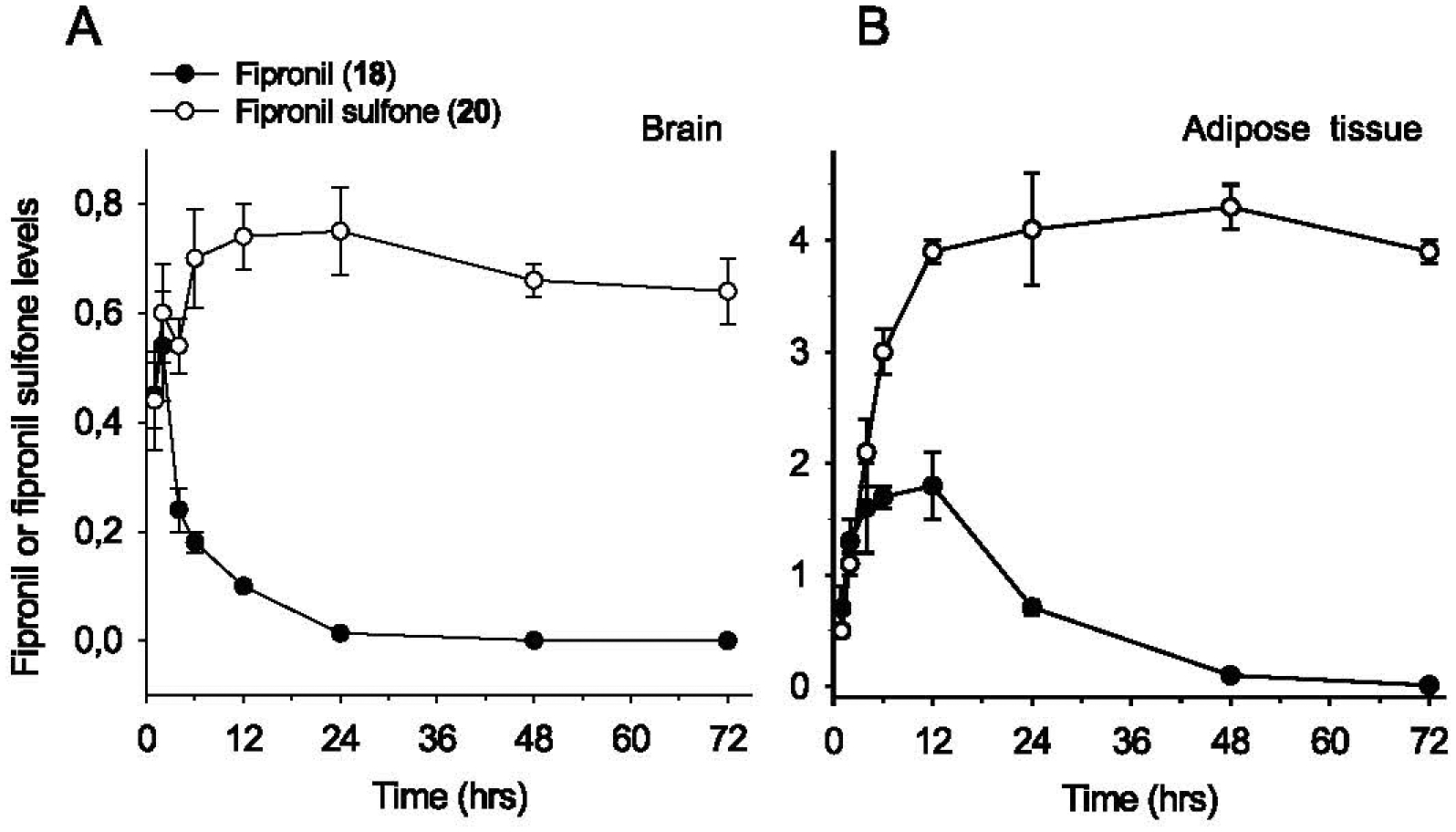
Short-term (72 hrs) time-course of fipronil (18) and fipronil sulfone (20) bioaccumulation in brain (A) and epididymal adipose tissue (B) following a single oral administration of fipronil (18) to mice. Fipronil (18) (10 mg/kg) was administrated by oral gavage at time 0. Animals were sacrificed at various times and plasma, epididymal adipose tissue and brain were collected. Fipronil (18) and fipronil sulfone (20) levels were quantified by LC-MS/MS. Concentrations are expressed as μg/brain or (μg/epididymal adipose tissue.
Fig. 12.
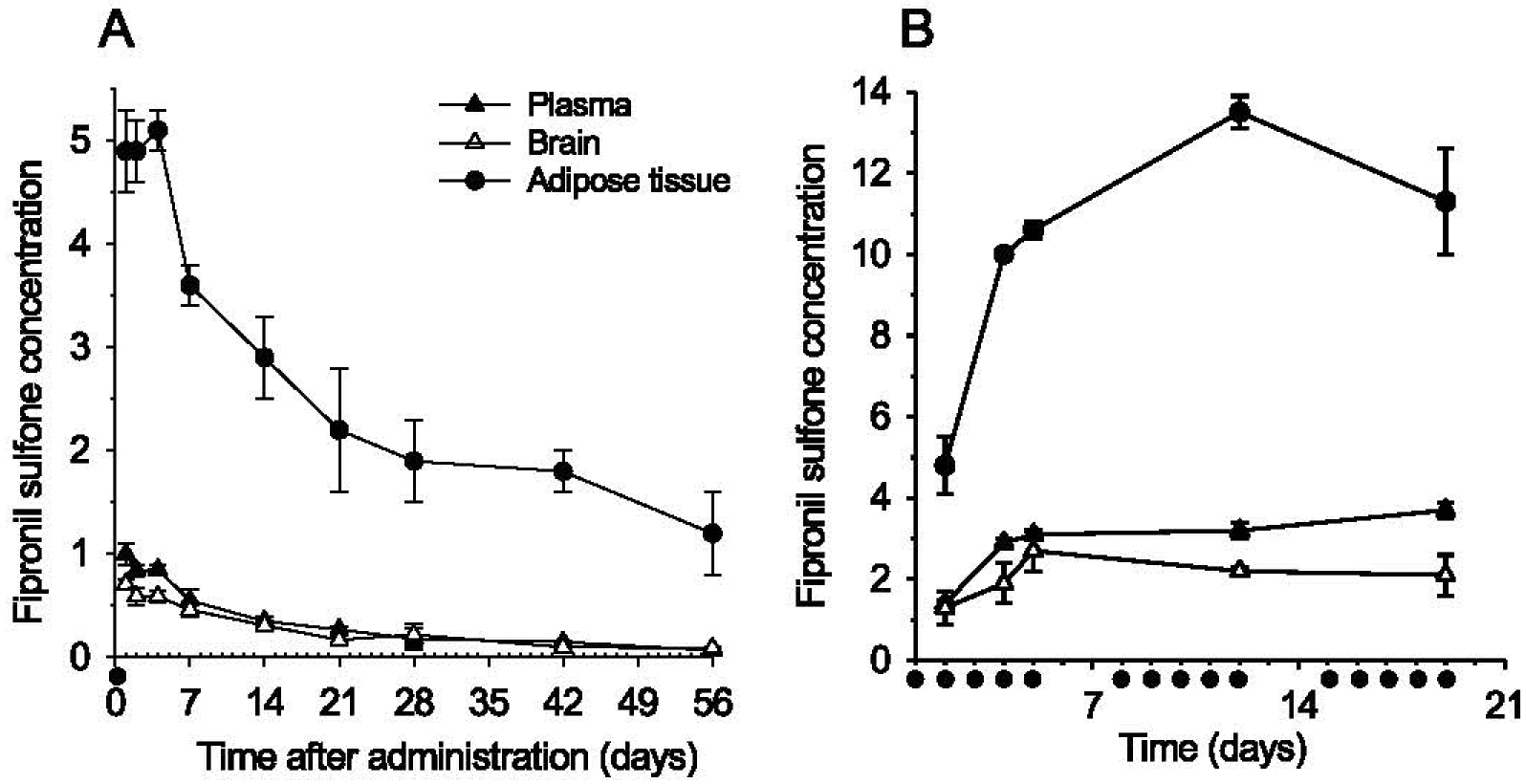
Long-term (56 days) time-course of fipronil sulfone (20) production and accumulation following single (A) or repeated (B) oral administrations of fipronil (18) to mice. A) Fipronil (18) (10 mg/kg) was administrated by oral gavage on day 0. Animals were sacrificed at various times and plasma, epididymal adipose tissue and brain were collected. Fipronil sulfone (20) levels were quantified by LC-MS/MS. B) Fipronil (18) (10 mg/kg) was administrated by oral gavage 5 days/week for 3 weeks (times of administration are indicated by black dots). Animals were sacrificed at various times and plasma, epididymal adipose tissue and brain were collected. Fipronil sulfone (20) levels were quantified by LC-MS/MS. Concentrations are expressed as μg/brain, μg/epididymal adipose tissue or μg/mL plasma.
DISCUSSION
This study reports on the induction of Aβ42/Aβ43 production, and the increased ratio of long versus short Aβs, in various cell cultures and by highly purified γ-secretase following exposure to several pyrazole insecticides. These results support previous results obtained with various drugs (fenofibrate, celecoxib, indomethacin, isoprenoids) [63], DAPT under certain conditions [64, 65], steroids [66], ceramide analogs [67], the peroxynitrite donor SIN-1 [68], Zinc [60], aftins [35–37] and several triazine herbicides [38]. Altogether these results have two main implications:
They support the idea that the HCE contains several compounds able to shift the cleavage of AβPP toward the production of long, toxic, aggregation-prone Aβs, such as Aβ42/Aβ43, those which are classically associated with AD in humans and numerous animal models of AD. These compounds, if proven to modify this balance in animals (as suggested by the first preliminary results obtained with aftin-4 [28] and celecoxib or FT-1 [63]) and in human, may contribute to the onset, development and/or acceleration of AD. We suggest that they be collectively named “Alzheimerogens”, by analogy with cancer-inducing carcinogens. Identification of such compounds in our environment appears to be a priority to develop a prevention strategy.
Some of these compounds, particularly the metabolically stable, brain-permeable molecules, constitute new pharmacological research tools to investigate the role of γ-secretase, its substrates and products, in the onset of AD, in cell lines and animal models. In this context, one of our objectives is, by analogy with MPTP (1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine)-induced Parkinsonism, to develop a pesticide-induced animal model of AD, which would allow the study of AD onset in wild-type (WT) animals. This is exemplified by the orally available fipronil (18) which is rapidly metabolized to fipronil sulfone (20), a very stable compound which accumulates in adipose tissue, crosses the blood-brain barrier and reaches stable levels in the brain (Figs. 11 and 12; Supplementary Figure 2). We are currently running long-term exposure experiments with fipronil (18) administered orally in WT mice and Tg2576 mice (overexpressing isoform 695 of human AβPP with the Swedish mutation (KM670/671NL)).
Mechanism of action of fipronil and other pyrazoles
As also observed with aftins [36] andtriazines [38], pyrazoles insecticides, including fipronil derivatives, show a structure/activity relationship (Supplementary Table 1) in their effects on Aβ production - some are active, others are inactive-, showing that pyrazole insecticides devoid of Aβ42 induction properties can be developed. The limited number of pyrazoles and their wide structural diversity precludes a clear identification of key structural features interacting with the unknown target leading to Aβ42 production. Unspecific, detergent, hydrophobic, membrane, or protein structure disrupting actions are unlikely to account for the effects of pyrazoles. Several arguments support an effect on γ-secretase and/or its micro-environment: 1) induction of Aβ production by pyrazoles is inhibited by γ-secretase inhibitors or modulators (Fig. 4); 2) pyrazoles also affect the cleavage of another substrate of γ-secretase, alcadeins (Fig. 9); and 3) pyrazoles modify the cleavage pattern of AβPP by highly purified γ-secretase.
Our cell-free γ-secretase activity assays performed with highly purified enzyme and substrate (Fig. 8) show that fipronil (18), Fipronil sulfone (20), and fipronil desulfinyl (21) clearly increased the Aβ42/Aβ40 ratio. Remarkably, Fipronil sulfone (20) additionally increased by ~2-fold the Aβ43/Aβ40 ratio. At the molecular level, the γ-secretase complex cleaves the APP-C99 substrate within the transmembrane domain (TMD), first at the e-site located at the intracellular interface, to generate two different C-terminal APP intracellular domains (AICDs) of 50 or 51 aa length [69]. To generate Aβs of different lengths, γ-secretase next cleaves the N-terminus of the TMD every α-helical turn (corresponding to 3–4 amino acids), starting from e-sites, following two production lines: the AICD5I/Aβ48 pathway (Aβ48⇒Aβ45⇒Aβ42⇒Aβ38) and the AICD50/Aβ49 pathway (Aβ49=>Aβ46⇒Aβ43⇒Aβ40) [69]. Consistent with a direct effect of pyrazoles on APP-C99 processing, our results support an altered Aβ48 pathway leading to an increased Aβ42 production. Surprisingly, our study further revealed that fipronil sulfone (20) unambiguously and drastically increases the Aβ43/Aβ40 ratio (Fig. 8). This observation is important since Aβ43 is known to be highly amyloidogenic and a main species involved in the formation of senile plaques [6, 7, 70]. As of today, Aβ43 is known to be a direct precursor of Aβ40 [69]. Therefore, the increased Aβ43 production in the cell-free assay suggests that fipronil sulfone (20) blocks the processing of APP-C99 at position 43 in the Aβ49 pathway, an event which is likely associated with reduced Aβ40 production.
Further quantitative experiments are required to explain the increased Aβ42/Aβ40 or Aβ43/Aβ40 ratios, caused either by increased Aβ42 or Aβ43 production, a reduced Aβ40 production or both. In any case, an altered Aft profile in the presence of pyrazole insecticides strongly suggests that the compounds directly affect the positioning of the AβPP substrate in the lipid-bilayer, as previously observed for compounds known as inverse γ-secretase modulators (iGSM) [64], Similarly to what we observed with fipronil (18) and fipronil desulfinyl (21), the iGSM nonsteroidal anti-inflammatory drugs (NSAIDs) Fenofibrate and Celecoxib have indeed been reported to be Aβ42-raising and Aβ38-lowering compounds [63]. Interestingly, Celecoxib is a pyrazole. The pyrazole scaffold has in fact been used extensively in therapeutic drug development (review in [71]). In contrast to iGSMs, some NSAID-based γ-secretase modulators (GSM), including sulindacsulfide, lower Aβ42 and increase Aβ38 productions [63]. GSMs are of great pharmaceutical interest and are currently under clinical evaluation for their ability to reduce the production of the amyloidogenic Aβ42 and Aβ43 peptides. At the same time the potential risk from Aβ42 and Aβ43 producing iGSMs needs careful evaluation since some of these compounds are FDA-approved and widely used as NSAIDs. At the molecular level, it has further been shown that residues 29–36 (GAHGLMV) of the Aβ motif in APP-C99 located at the TMD N-terminus represent the binding site of both Aβ42-lowering GSMs and Aβ42-raising iGSMs [63]. This raises the question whether the same binding site could be involved in the pyrazole-induced Aβ42/Aβ40 and Aβ43/Aβ40 ratio increase. Interestingly, we recently found that zinc can drastically raise the Aβ43/Aβ40 ratio by influencing the positioning of the APP-C99 TMD through binding to Lysine 28, which is located at the surface of the membrane and is involved in anchoring APP-C99 in the lipid bilayer [69]. This observation further supports the possibility that Lys28 and more globally residues 29–36 in APP-C99 are involved in the pyrazole-induced Aβ43/Aβ40 increase. Finally, novel GSMs have recently been reported that directly bind on PS1 [72], Binding of these compounds to PS 1 induced a conformational change in the enzyme-substrate interaction that modulated the protease activity and consequently the Aβ profile. Thus, one cannot exclude that fipronil (18), fipronil sulfone (20), or fipronil desulfinyl (21) also modulate AβPP-processing by directly binding to the γ-secretase complex. Further experiments are needed to decipher the mode of action of these compounds (see Supplementary Figure 3 for working model).
Fipronil exposure and consequences
Fipronil has been developed a selective inhibitor of insect GABA-gated chloride channels (review in [44]). It is one of the most widely used pyrazole insecticides in agriculture (seed, culture, and crop treatment), in urban pest control (coackroaches, termites, wasps, flies, and ants management in and around buildings), and in veterinary applications (fleas and ticks control for pets and cattle). Fipronil and its metabolites are therefore widely found [73] in urban soil, dust, wastewater, and residential runoff [74], in rural soil, rivers, and atmosphere [46, 73, 75, 76], but also in various tissues of pet, farmland, and wild-life animals [75, 77,78] (only a very small number of references are cited). Although fipronil (18) is relatively resistant to degradation, it is mostly oxidized to fipronil sulfone (20), reduced to fipronil sulfide (22), photodegradated to fipronil desulfinyl (21), hydrolyzed to fipronil amide (23, 28). Many other metabolites have been described ([45–47, 73], review in [39]). Although fipronil shows low affinity for mammalian GABA-gated chloride channels compared to those of insects, toxicity studies carried out with fipronil and its metabolites in various vertebrates [39,40,43,44,77] as well as studies performed with mammalian cells studies [79–83] cast some doubts about the safety of fipronil to humans (see Supplementary Table 5). This concern is amplified by 1) the existence of numerous metabolites which are poorly characterized in terms of toxicity, 2) their long persistence in the environment (soil, water, sediment, and atmosphere), 3) their accumulation in tissues, especially adipose tissue (Figs. 11 and 12), 4) the potency of fipronil sulfone on mammalian GABA-receptor [84], and 5) the fact that fipronil and its metabolites essentially meet all draggability criteria of Lipinski’s rule of Five (Supplementary Table 6). In addition, fipronil (along with neonicotinoids) is a major worldwide concern for the survival of pollinators [85–89]. In mammals, fipronil shows oxidative stress, neurotoxic, thyroid, endocrine, lung inflammation, blood pressure, and reproductive effects (review in [44]) at high doses. In humans, fipronil triggers mild nervous troubles as seen in acute intoxication cases [90, 91] and apparently impacted thyroid functions of workers following occupational exposure [92].
As shown in Figs. 11 and 12, fipronil (18) is rapidly metabolized to a very stable product, fipronil sulfone (20), which readily accumulates in fat tissue. Table 1 reviews plasma and tissue levels of fipronil sulfone (20) reached experimentally in mice and rat or accidentally in humans (our data + literature data). The highest plasma levels reached experimentally during these relatively short exposures (3.7 μ/mL, i.e., 8.2 (μM) are not very far from those which induce Aβ42 and Aβ43 production. Adipose tissue and brain levels reached experimentally are probably higher. 72 h after a single oral administration of 14C-fipronil to rats, about 50% and 2% of the radioactivity (essentially fipronil sulfone) were found in adipose tissue and brain, respectively [93]. Fipronil sulfone is also the primary metabolite produced in human via hepatocyte cytochrome P-450 oxydation [90, 92–95]. Fipronil sulfone was detected in about 25% of 96 human serum samples (0.1–39ng/mL which corresponds to 0.22–86 nM) [45]. These samples were obtained from individuals with no known fipronil exposure. However, fipronil applied to the fur of pets is easily and rapidly transferred to humans [96, 97], Fipronil and fipronil sulfone levels were measured in urine and blood samples of 159 workers in a factory manufacturing fipronil-containing veterinary products [92]: 33 and 155 workers had detectable serum fipronil and fipronil sulfone (0.37–42.45 ng/mL, i.e., 0.82–9.32 nM), respectively. Incidentally, fipronil has recently been shown to promote adipogenesis [98] and adiposity/obesity has been suggested as a risk factor for AD [99, 100]. Adipose tissue may thus act as a trap and storage tissue for lipophilic, Aβ42/Aβ43 inducers like fipronil, other pyrazoles, other pesticides. The persistence of fipronil sulfone, its accumulation in adipose tissue, and its ability to enter the brain raises concerns on its possible long-term effects on the central nervous system, in particular on its possible effects on the production of toxic, aggregation-prone amyloidogenic Aβ42 and Aβ43; as shown in this article.
Table 1.
Concentrations of fipronil sulfone reached in mammals following fipronil administration (oral, ip, iv, general exposure). Fipronil sulfone (20) levels are expressed either in μg/mL or in μg/g. A 4.53 μg/mL concentration is equivalent to a concentration of 10 μM. bw, body weight
| Species/tissue | Exposure conditions | Fipronil sulfone (20) levels reached | Refs. |
|---|---|---|---|
| Mouse | |||
| plasma | 10 mg/kg bw, oral, single administration | 1–1.1 μg/mL | Fig. 11, 12A |
| plasma | 10 mg/kg bw, oral, 5 days/week/3 weeks | 3.7 μg/mL | Fig. 12B |
| plasma | 2.5 or 10 mg/kg bw, oral, 1 day/week/2 weeks | 0.029 or 0.210 μg/mL | SK, unpubl. |
| plasma | 2.5 or 10 mg/kg bw, oral, 3 days/week/2 weeks | 0.211 or 0.662 μg/mL | SK, unpubl. |
| adipose tissue | 2 mg/kg bw, ip, daily/6 days | 22–24 ppm | [102] |
| adipose tissue | 10 mg/kg bw, oral, single administration | 4.3–4.9 μg/epididymal adipose pad | Fig. 11, 12A |
| adipose tissue | 10 mg/kg bw, oral, 5 days/week/3 weeks | 13.5 μg/epididymal adipose pad | Fig. 12B |
| adipose tissue | 2.5 or 10 mg/kg bw, oral, 1 day/week/2 weeks | 0.730 or 5.276 μg/g | SK, unpubl. |
| adipose tissue | 2.5 or 10 mg/kg bw, oral, 3 days/week/2 weeks | 6.098 or 12.48 μg/g | SK, unpubl. |
| brain | 40 mg/kg bw, ip, 6 or 20 min, single administration | 19 or 32 ppm (6 and 20 min, respectively) | [103] |
| brain | 10 mg/kg bw, oral, single administration | 0.71–0.75 μg/brain | Fig. 11, 12A |
| brain | 10 mg/kg bw, oral, 5 days/week/3 weeks | 2.7 μg/brain | Fig. 12B |
| brain | 2.5 or 10 mg/kg bw, oral, 1 day/week/2 weeks | 0.049 or 0.405 μg/g | SK, unpubl. |
| brain | 2.5 or 10 mg/kg bw, oral, 3 days/week/2 weeks | 0.585 or 1.746 μg/g | SK, unpubl. |
| Rat | |||
| plasma | 10 mg/kg bw, oral, single administration | 1.4 μg/mL | Suppl. Fig. 1 |
| plasma | 5 or 10 mg/kg bw, oral, daily/2 weeks | 2.4 or 3.6 μg/mL | [104] |
| plasma | 30 mg Regent ®/kg bw, oral, daily/15 days | 0.46 μg/mL | [105] |
| plasma | 4 μg/kg bw, oral, 3X/week/90 days | 0.008–0.39 ng/mL | [106] |
| plasma | 3.4 (po) or 6.9 (iv) μmole/kg bw, single administration | 0.2 (po) or 0.25 (iv) μg/mL | [107] |
| plasma | 3.4 μmole/kg bw, oral, daily/14 days | 1.5 μg/mL | [107] |
| plasma | 3 mg/kg bw, oral, daily/13 days | 0.043 – 2.8 μg/mL | [108] |
| adipose tissue | 10 mg/kg bw, oral, single administration | 152.3 μg/g | Suppl. Fig. 3 |
| adipose tissue | 10 mg/kg bw, oral, 72 h, administration | 47.87 μg fipronil equivalent (>90% fipronil sulfone)/g wet weight | [93] |
| brain | 10 mg/kg bw, oral, single dose | 3.74 μg/g | Suppl. Fig. 2 |
| urine | 5 or 10 mg/kg bw, oral, daily/2 weeks or single dose | 0.023–0.026 μg/mL | [47] |
| urine | 5 or 10 mg/kg bw, oral, daily/2 weeks | 0.024 or 0.032 μg/mL | [104] |
| urine | 4 μg/kg bw, oral, 3X/week/90 days | 0.009–4.27 ng/mL | [106] |
| hair | 4 μg/kg bw, oral, 3X/week/90 days | 4.58–306 pg/mg | [106] |
| Human | |||
| plasma | no known fipronil exposure (96 plasma samples) | 0.1–3.9 ng/mL; detected in 25% of the samples. | [104] |
| plasma | conducted on 159 workers from a factory manufacturing fipronil-containing veterinary products in France | mean: 7.79 ng/mL (range: 0.37–42.45 ng/mL) detected in 155 out of 159 workers. | [92] |
| plasma | 6 cases of self-poisoning with Regent ® | maximum fipronil + fipronil sulfone level measured was 3.74 (μg/mL | [109] |
| urine | no known fipronil exposure (84 urine samples) | undetectable | [104] |
In conclusion, we have added some pyrazole insecticides, especially the main metabolite of fipronil, in the growing list of HCE products which are able to alter the specificity of Aβ production. Our hypothesis is that these products may, on a long-term, cumulative and additive basis, possibly in parallel with microorganisms [101] and microorganisms-derived products, alter the Aβ production pattern sufficiently to lead to AD pathogenesis, and thus qualify as potential “Alzheimerogens”. Gradual accumulation in and chronic release from adipose tissue and transfer to the brain may contribute to the onset, development, and acceleration of sporadic AD.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Laetitia Bailly (Laboratoire Cobra, University of Rouen) for purifying the two fipronil enantiomers. We are grateful to Jasna Padovan and Zeljko Javorscak (Fidelta) for the rat PK studies, to Rebecca L. McMahen, Mark J. Strynar for providing fipronil sulfone chloramine. This work was supported by ‘Fonds Unique Interministériel’ PHARMASEA/TRIAD projects, « Agence nationale de sécurité sanitaire, de 1’alimentation, de l’environnement et du travail » (ANSES), Fondation “Jérôme Lejeune” and CRITT-Santé Bretagne/FEDER (LM). This research was also partly supported by an FP7-KBBE-2012 grant (BlueGenics) to LM. MC is CIFRE/ManRos Therapeutics PhD fellowship recipient. KB is a Torsten Soderberg Professor. HZ is a Wallenberg Academy Fellow. This work was supported in part by Grant-in-aid for Scientific Research 26293010 (TS) and 24790062 (SH) from the Ministry of Education, Culture, Sports, Science and Technology in Japan. HG, JF, and PCF were supported by the Fondation Eclosion. Partial support was provided by NIEH S/ROIES002710, NffiH S Superfund Research Program P42 ES004699 and NIH/454 NS079202.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-0875rl).
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170875.
Although fipronil is not the most potent Aβ42 inducer, it was selected for more detailed studies, among other pyrazoles, for several reasons: 1) its very wide use all over the world (despite its ban in Europe in America for food plant cultures), 2) its wide use against fleas and ticks in domestic animals, 3) its wide presence in urban and rural environment, 4) its chemical stability and environmental persistence (especially that of its metabolite, fipronil sulfone), 5) the biological activity of the main metabolite, 6) the fact fipronil sulfone accumulates in adipose tissue, crosses the blood-brain barrier and accumulates in the brain, and 8) its availability in large quantities for very cheap prices (in preparation of our current long term exposure experiments the supply issue needed to be solved). Incidentally, fipronil was selected before the public health scandal of summer 2017 in Europe (contamination of eggs by fipronil, presumably even more by fipronil sulfone)!
REFERENCES
- [1].Alzheimer’s Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimers Dement 13, 325–373. [Google Scholar]
- [2].Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148,1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vinters HV (2015) Emerging concepts in Alzheimer’s disease. Annu Rev Pathol 10, 291–319. [DOI] [PubMed] [Google Scholar]
- [4].Canter RG, Penney J, Tsai LH (2016) The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539,187–196. [DOI] [PubMed] [Google Scholar]
- [5].Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D’Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B (2010) Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J 29, 3408–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saito T, Suemoto T, Brouwers N, Sleegers K, Funamoto S, Mihira N, Matsuba Y, Yamada K, Nilsson P, Takano J, Nishimura M, Iwata N, Van Broeckhoven C, Ihara Y, Saido TC (2011) Potent amyloidogenicity and pathogenicity of Aβ43. Nat Neurosci 14, 1023–1032. [DOI] [PubMed] [Google Scholar]
- [7].Sandebring A, Welander H, Winblad B, Graff C, Tjemberg LO (2013) The pathogenic Aβ43 is enriched in familial and sporadic Alzheimer disease. PLoS One 8, e55847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C (2011) Autosomal-dominant Alzheimer’s disease a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gaiteri C, Mostafavi S, Honey CJ, De Jager PL, Bennett DA (2016) Genetic variants in Alzheimer disease - molecular and brain network approaches. Nat Rev Neurol 12, 413–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Benilova I, Gallardo R, Ungureanu AA, Castillo Cano V, Snellinx A, Ramakers M, Bartic C, Rousseau F, Schymkowitz J, De Strooper B (2014) The Alzheimer disease protective mutation A2T modulates kinetic and thermodynamic properties of amyloid-β (Aβ) aggregation. J Biol Chem 289, 30977–30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Puzzo D, Gulisano W, Palmeri A, Arancio O (2015) Rodent models for Alzheimer’s disease drug discovery. Expert Opin Drug Discov 10, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Van Dam D, De Deyn PP (2017) Non human primate models for Alzheimer’s disease-related research and drug discovery. Expert Opin Drug Discov 12, 187–200. [DOI] [PubMed] [Google Scholar]
- [13].Foley AM, Ammar ZM, Lee RH, Mitchell CS (2015) Systematic review of the relationship between amyloid-β levels and measures of transgenic mouse cognitive deficit in Alzheimer’s disease. J Alzheimers Dis 44,787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cuyvers E, Sleegers K (2016) Genetic variations underlying Alzheimer’s disease: Evidence from genome-wide association studies and beyond. Lancet Neurol 15, 857–868. [DOI] [PubMed] [Google Scholar]
- [15].Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals. Lancet 368, 2167–2178. [DOI] [PubMed] [Google Scholar]
- [16].Grandjean P, Landrigan PJ (2014) Neurobehavioural effects of developmental toxicity. Lancet Neurol 13, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cannon JR, Greenamyre JT (2011) The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 124, 225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grandjean P (2013) Only one chance. How environmental pollution impairs brain development - and how to protect the brains of the next generation Oxford University Press, pp. 212. [Google Scholar]
- [19].Demeneix B (2014) Losing our minds. How environmental pollution impairs human intelligence and mental health Oxford University Press, pp. 284. [Google Scholar]
- [20].Chin-Chan M, Navarro-Yepes J, QuintianiUa-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yegambaram M, Manivannan B, Beach TG, Halden RU (2015) Role of environmental contaminants in the etiology of Alzheimer’s disease: A review. Curr Alzheimer Res 12, 116–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nicolia V, Lucarelli M, Fuso A (2015) Environment, epigenetics and neurodegeneration: Focus on nutrition in Alzheimer’s disease. Exp Gerontol 68, 8–12. [DOI] [PubMed] [Google Scholar]
- [23].Killin LO, Starr JM, Shiue U, Russ TC (2016) Environmental risk factors for dementia: A systematic review. BMC Geriatr 16, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ (2016) Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun 7, 11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pearson BL, Ehninger D (2017) Environmental chemicals and aging. Curr Environ Health Rep 4, 38–43. [DOI] [PubMed] [Google Scholar]
- [26].Wild CP (2012) The exposome from concept to utility. Int J Epidemiol 41, 24–32. [DOI] [PubMed] [Google Scholar]
- [27].Juarez PD, Matthews-Juarez P, Hood DB, Im W, Levine RS, Kilboume BJ, Langston MA, Al-Hamdan MZ, Crosson WL, Estes MG, Estes SM, Agboto VK, Robinson P, Wilson S, Lichtveld MY (2014) The public health exposome a population-based, exposure science approach to health disparities research. Int J Environ Res Public Health 11, 12866–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den Hazel P, Thomsen C, Wright J, Athersuch TJ, Avellana N, Basagana X, Brochot C, Bucchini L, Bustamante M, Carracedo A, Casas M, Estivill X, Fairley L, van Gent D, Gonzalez JR, Granum B, Gražulevičiene R, Gutzkow KB, Julvez J, Keun HC, Kogevinas M, McEachan RR, Meltzer HM, Sabidó E, Schwarze PE, Siroux V, Sunyer J, Want EJ, Zeman F, Nieuwenhuij senMJ(2014)Thehumanearly-life exposome (HELIX), project rationale and design. Environ Health Perspect 122, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wishart D, Arndt D, Pon A, Sajed T, Guo AC, Djoumbou Y, Knox C, Wilson M, Liang Y, Grant J, Liu Y, Goldansaz SA, Rappaport SM (2015) T3DB the toxic exposome database. Nucleic Acids Res 43, D928–D934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Goldsmith MR, Grulke CM, Brooks RD, Transue TR, Tan YM, Frame A, Egeghy PP, Edwards R, Chang DT, Tomero-Velez R, Isaacs K, Wang A, Johnson J, Holm K, Reich M, Mitchell J, Vallero DA, Phillips L, Phillips M, Wambaugh JF, Judson RS, Buckley TJ, Dary CC (2014) Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem Toxicol 65, 269–279. [DOI] [PubMed] [Google Scholar]
- [31].Siroux V, Agier L, Slama R (2016) The exposome concept: A challenge and a potential driver for environmental health research. Eur Respir Rev 25, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Turner MC, Nieuwenhuij sen M, Anderson K, Balshaw D, Cui Y, Dunton G, Hoppin JA, Koutrakis P, Jerrett M (2017) Assessing the exposome with external measures: Commentary on the state of the science and research recommendations. Annu Rev Public Health 38,215–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Buck Louis GM, Smarr MM, Patel CJ (2017) The exposome research paradigm: An opportunity to understand the environmental basis for human health and disease. Curr Environ Health Rep 4, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Escher BI, Hackermiiller J, Polte T, Scholz S, Aigner A, Altenburger R, Bohme A, Bopp SK, Brack W, Busch W, Chadeau-Hyam M, Covaci A, Eisenträger A, Galligan JJ, Garcia-Reyero N, Hartung T, Hein M, Herberth G, Jahnke A, Kleinjans J, Klüver N, Krauss M, Lamoree M, Lehmann I, Luckenbach T, Miller GW, Muller A, Phillips DH, Reemtsma T, Rolle-Kampczyk U, Schüürmann G, Schwikowski B, Tan YM, Trump S, Walter-Rohde S, Wambaugh JF (2017) From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int 99,97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bettayeb K, Oumata N, Zhang Y, Luo W, Bustos V, Galons H, Greengard P, Meijer L, Flajolet M (2012) Small molecule inducers of Aβ42 peptide production share a common mechanism of action. FASEBJ 26, 5115–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hochard A, Oumata N, Bettayeb K, Gloulou O, Fant X, Durieu E, Buron N, Porceddu M, Borgne-Sanchez A, Galons H, Flajolet M, Meijer L (2013) Aftins increase amyloid-β42, lower amyloid-β38 and do not alter amyloid-β40 in vitro production towards a chemical model of Alzheimer’s disease? J Alzheimers Dis 35, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Meunier J, Boijini N, Gillis C, Villard V, Maurice T (2015) Brain toxicity and inflammation induced in vivo in mice by the amyloid-β forty-two inducer aftin-4, a roscovitine derivative. J Alzheimers Dis 44, 507–524. [DOI] [PubMed] [Google Scholar]
- [38].Portelius E, Durieu E, Bodin M, Cam M, Pannee J, Leuxe C, Mabondzo A, Oumata N, Galons H, Lee J, Chang YT, Stüber K, Koch P, Fontaine G, Potier MC, Manousopoulou A, Garbis S, Covaci A, Van Dam D, De Deyn P, Karg F, Flajolet M, Omori C, Hata S, Suzuki T, Blennow K, Zetterberg H, Meijer L (2016) Specific triazine herbicides induce amyloid β 42 production. J Alzheimers Dis 54, 1593–1605. [DOI] [PubMed] [Google Scholar]
- [39].Tingle CC, Rother JA, Dewhurst CF, Lauer S, King WJ (2003) Fipronil: Environmental fate, ecotoxicology, and human health concerns. Rev Environ Contam Toxicol 176, 1–66. [DOI] [PubMed] [Google Scholar]
- [40].Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EA, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehom PR, Wiemers M (2015) Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int 22, 5–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Giorio C, Safer A, Sánchez-Bayo F, Tapparo A, Lentola A, Girolami V, van Lexmond MB, Bonmatin JM (2017) An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 1: New molecules, metabolism, fate, and transport. Environ Sci Pollut Res Int, doi: 10.1007/s11356-017-0394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, Van der Sluijs JP, Van Dyck H, Wiemers M (2015) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res Int 22, 68–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van der Sluijs JP, Amaral-Rogers V, Belzunces LP, Bijleveld van Lexmond MF, Bonmatin JM, Chagnon M, Downs CA, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, McField M, Mineau P, Mitchell EA, Morrissey CA, Noome DA, Pisa L, Settele J, Simon-Delso N, Stark JD, Tapparo A, Van Dyck H, van Praagh J, Whitehom PR, Wiemers M (2015) Conclusions of the Worldwide Integrated Assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environ Sci Pollut Res Int 22, 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang X, Martínez MA, Wu Q, Ares I, Martínez-Larrañaga MR, Anadón A, Yuan Z (2016) Fipronil insecticide toxicology: Oxidative stress and metabolism. CritRev Toxicol 46, 876–899. [DOI] [PubMed] [Google Scholar]
- [45].McMahen RL, Strynar MJ, Dagnino S, Herr DW, Moser VC, Garantziotis S, Andersen EM, Freeborn DL, McMillan L, Lindstrom AB (2015) Identification of fipronil metabolites by time-of-flight mass spectrometry for application in a human exposure study. Environ Int 78, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McMahen RL, Strynar MJ, McMillan L, DeRose E, Lindstrom AB (2016) Comparison of fipronil sources in North Carolina surface water and identification of a novel fipronil transformation product in recycled wastewater. Sci Total Environ 569–570, 880–887. [DOI] [PubMed] [Google Scholar]
- [47].Vasylieva N, Bamych B, Wan D, El-Sheikh EA, Nguyen HM, Wulff H, McMahen R, Strynar M, Gee SJ, Hammock BD (2017) Hydroxy-fipronil is a new urinary biomarker of exposure to fipronil. Environ Int 103,91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL (2010) Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron 67,769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kretner B, Fukumori A, Gutsmiedl A, Page RM, Luebbers T, Galley G, Baumann K, Haass C, Steiner H (2011) Attenuated Abeta42 responses to low potency gamma-secretase modulators can be overcome for many pathogenic presenilin mutants by second-generation compounds. J Biol Chem 286, 15240–15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leinenbach A, Pannee J, Dülffer T, Huber A, Bittner T, Andreasson U, Gobom J, Zetterberg H, Kobold U, Portelius E, Blennow K, Scientific Division Working Group on IFCC, CSF, proteins (2014) Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-β in cerebrospinal fluid. Clin Chem 60, 987–994. [DOI] [PubMed] [Google Scholar]
- [51].Pannee J, Portelius E, Oppermann M, Atkins A, Homshaw M, Zegers I, Hojrup P, Minthon L, Hansson O, Zetterberg H, Blennow K, Gobom J (2013) A selected reaction monitoring (SRM)-based method for absolute quantification of Aβ−38, Aβ−40, and Aβ42 in cerebrospinal fluid of Alzheimer’s disease patients and healthy controls. j Alzheimers Dis 33, 1021–1032. [DOI] [PubMed] [Google Scholar]
- [52].Portelius E, Olsson M, Brinkmalm G, Rüetschi U, Mattsson N, Andreasson U, Gobom J, Brinkmalm A, Hölttä M, Blennow K, Zetterberg H (2013) Mass spectrometric characterization of amyloid-β species in the 7PA2 cell model of Alzheimer’s disease. J Alzheimers Dis 33, 85–93. [DOI] [PubMed] [Google Scholar]
- [53].Welander H, Frånberg J, Graff C, Sundstrom E, Winblad B, Tjemberg LO (2009) Abeta43 is more frequent than Abeta40 in amyloid plaque cores from Alzheimer disease brains. J Neurochem 110, 697–706. [DOI] [PubMed] [Google Scholar]
- [54].Conicella AE, Fawzi NL (2014) The C-terminal threo-nine of Aβ43 nucleates toxic aggregation via structural and dynamical changes in monomers and protofibrils. Biochemistry 53, 3095–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Almdahl IS, Lauridsen C, Seines P, Kalheim LF, Coello C, Gajdzik B, Møller I, Wettergreen M, Grambaite R, Bjømerud A, Bråthen G, Sando SB, White LR, Fladby T (2017) Cerebrospinal fluid levels of amyloid beta 1–43 mirror 1–42 in relation to imaging biomarkers of Alzheimer’s disease. Front Aging Neurosci 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mertens J, Stüber K, Wunderlich P, Ladewig J, Kesavan JC, Vandenberghe R, Vandenbulcke M, van Damme P, Walter J, Brüstle O, Koch P (2013) APP processing in human pluripotent stem cell-derived neurons is resistant to NSAID-based γ-secretase modulation. Stem Cell Reports 1,491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Koch P, Tamboli IY, Mertens J, Wunderlich P, Ladewig J, Stüber K, Esselmann H, Wiltfang J, Brüstle O, Walter J (2012) Presenilin-1 L166P mutant human pluripotent stem cell-derived neurons exhibit partial loss of γ-secretase activity in endogenous amyloid-β generation. Am J Pathol 180,2404–2416. [DOI] [PubMed] [Google Scholar]
- [58].Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O (2009) A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U SA 106,3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fraering PC, Ye W, Strub J-M, Dolios G, LaVoie MJ, Ostaszewski BL, van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS (2004) Purification and characterization of the human gamma-secretase complex. Biochemistry 43,9774–9789. [DOI] [PubMed] [Google Scholar]
- [60].Gerber H, Wu F, Dimitrov M, Garcia Osuna GM, Fraering PC (2017) Zinc and copper differentially modulate amyloid precursor protein processing by γ-secretase and amyloid-β peptide production. J Biol Chem 292, 3751–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hata S, Fujishige S, Araki Y, Kato N, Araseki M, Nishimura M, Hartmann D, Saftig P, Fahrenholz F, Taniguchi M, Urakami K, Akatsu H, Martins RN, Yamamoto K, Maeda M, Yamamoto T, Nakaya T, Gandy S, Suzuki T (2009) Alcadein cleavages by amyloid beta-precursor protein (APP) alpha- and gamma-secretases generate small peptides, p3-Alcs, indicating Alzheimer disease-related gamma-secretase dysfunction. J Biol Chem 284, 36024–36033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Piao Y, Kimura A, Urano S, Saito Y, Taru H, Yamamoto T, Hata S, Suzuki T (2013) Mechanism of intramembrane cleavage of alcadeins by γ-secretase. PLoS One 8, e62431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols W, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE (2005) Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med 11, 545–550. [DOI] [PubMed] [Google Scholar]
- [64].Svedružić ŽM, Popović K, Šendula-Jengić V (2013) Modulators of γ-secretase activity can facilitate the toxic side-effects and pathogenesis of Alzheimer’s disease. PLoS One 8, e50759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Barnwell E, Padmaraju V, Baranello R, Pacheco-Quinto J, Crosson C, Ablonczy Z, Eckman E, Eckman CB, Ramakrishnan V, Greig NH, Pappolla MA, Sambamurti K (2014) Evidence of a novel mechanism for partial γ-secretase inhibition induced paradoxical increase in secreted amyloid β protein. PLoS One 9, e91531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jung JI, Ladd TB, Kukar T, Price AR, Moore BD, Koo EH, Golde TE, Felsenstein KM (2013) Steroids as γ-secretase modulators. FASEB J 27, 3775–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Takasugi N, Sasaki T, Shinohara M, Iwatsubo T, Tomita T (2015) Synthetic ceramide analogues increase amyloid-β42 production by modulating γ-secretase activity. Biochem Biophys Res Commun 457, 194–199. [DOI] [PubMed] [Google Scholar]
- [68].Guix FX, Wahle T, Vennekens K, Snellinx A, Chávez Gutiérrez L, Ill-Raga G, Ramos-Femandez E, Guardia-Laguarta C, Lleó A, Arimon M, Berezovska O, Muñoz FJ, Dotti CG, De Strooper B (2012) Modification of γ-secretase by nitrosative stress links neuronal ageing to sporadic Alzheimer’s disease. EMBO Mol Med 4, 660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y (2009) gamma-Secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 29, 13042–13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bumouf S, Gorsky MK, Dols J, Grönke S, Partridge L (2015) Aβ34 is neurotoxic and primes aggregation of Ap40 in vivo. Acta Neuropathol 130, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Khan MF, Alam MM, Verma G, Akhtar W, Akhter M, Shaquiquzzaman M (2016) The therapeutic voyage of pyrazole and its analogs: A review. Eur J Med Chem 120, 170–201. [DOI] [PubMed] [Google Scholar]
- [72].Ebke A, Luebbers T, Fukumori A, Shirotani K, Haass C, Baumann K, Steiner H (2011) Novel γ-secretase enzyme modulators directly target presenilin protein. J Biol Chem 286, 37181–37186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].deToffoli AL, da Mata K, Bisinoti MC, Moreira AB (2015) Development, validation, and application of a method for the GC-MS analysis of fipronil and three of its degradation products in samples of water, soil, and sediment. J Environ Sci Health B 50,753–759. [DOI] [PubMed] [Google Scholar]
- [74].Gan J, Bondarenko S, Oki L, Haver D, Li JX (2012) Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ Sci Technol 46, 1489–1495. [DOI] [PubMed] [Google Scholar]
- [75].Michel N, Freese M, Brinkmann M, Pohlmann JD, Hollert H, Kammann U, Haarich M, Theobald N, Gerwinski W, Rotard W, Hanel R (2016) Fipronil and two of its transformation products in water and European eel from the river Elbe. Sci Total Environ 568,171–179. [DOI] [PubMed] [Google Scholar]
- [76].Socorro J, Durand A, Temime-Roussel B, Gligorovski S, Wortham H, Quivet E (2016) The persistence of pesticides in atmospheric particulate phase: An emerging air quality issue. Sci Rep 6, 33456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lopez-Antia A, Ortiz-Santaliestra ME, Camarero PR, Mougeot F, Mateo R (2015) Assessing the risk of fipronil-treated seed ingestion and associated adverse effects in the red-legged partridge. Environ Sci Technol 49, 13649–13657. [DOI] [PubMed] [Google Scholar]
- [78].Gibbons D, Morrissey C, Mineau P (2015) A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res Int 22, 103–118. Erratum in: Environ Sci Pollut Res Int 23, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Romero A, Ramos E, Ares I, Castellano V, Martínez M, Martínez-Larrañaga MR, Anadón A, Martínez MA (2016) Fipronil sulfone induced higher cytotoxicity than fipronil inSH-SY5Y cells: Protection by antioxidants. Toxicol Lett 252, 42–49. [DOI] [PubMed] [Google Scholar]
- [80].Sidiropoulou E, Sachana M, Flaskos J, Harris W, Hargreaves AJ, Woldehiwet Z (2011) Fipronil interferes with the differentiation of mouse N2a neuroblastoma cells. Toxicol Lett 201, 86–91. [DOI] [PubMed] [Google Scholar]
- [81].Lee JE, Kang JS, Ki YW, Lee SH, Lee SJ, Lee KS, Koh HC (2011) Akt/GSK3β signaling is involved in fipronil-induced apoptotic cell death of human neuroblastoma SH-SY5Y cells. Toxicol Lett 202, 133–141. [DOI] [PubMed] [Google Scholar]
- [82].Park JH, Park YS, Lee JB, Park KH, Paik MK, Jeong M, Koh HC (2016) Meloxicam inhibits fipronil-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. J Appl Toxicol 36,10–23. [DOI] [PubMed] [Google Scholar]
- [83].Ruangjaroon T, Chokchaichamnankit D, Srisomsap C, Svasti J, Paricharttanakul NM (2017) Involvement of vimentin in neurite outgrowth damage induced by fipronil in SH-SY5Y cells. Biochem Biophys Res Commun 486, 652–658. [DOI] [PubMed] [Google Scholar]
- [84].Zhao X, Yeh JZ, Salgado VL, Narahashi T (2005) Sulfone metabolite of fipronil blocks gamma-aminobutyric acid- and glutamate-activated chloride channels in mammalian and insect neurons. J Pharmacol Exp Ther 314, 363–373. [DOI] [PubMed] [Google Scholar]
- [85].Kairo G, Poquet Y, Haji H, Tchamitchian S, Cousin M, Bonnet M, Pelissier M, Kretzschmar A, Belzunces LP, Brunet JL (2017) Assessment of the toxic effect of pesticides on honey bee drone fertility using laboratory and semifield approaches: A case study of fipronil. Environ Toxicol Chem 36, 2345–2351. [DOI] [PubMed] [Google Scholar]
- [86].Roat TC, Carvalho SM, Palma MS, Malaspina O (2017) Biochemical response of the Africanized honeybee exposed to fipronil. Environ Toxicol Chem 36,1652–1660. [DOI] [PubMed] [Google Scholar]
- [87].Kairo G, Provost B, Tchamitchian S, Ben Abdelkader F, Bonnet M, Cousin M, Sénéchal J, Benet P, Kretzschmar A, Belzunces LP, Brunet JL (2016) Drone exposure to the systemic insecticide Fipronil indirectly impairs queen reproductive potential. Sci Rep 6, 31904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Erickson BE (2013) Europe to ban fipronil pesticide to protect bees. Chem Eng News 91, 21. [Google Scholar]
- [89].Kairo G, Biron DG, Ben Abdelkader F, Bonnet M, Tchamitchian S, Cousin M, Dussaubat C, Benoit B, Kretzschmar A, Belzunces LP, Brunet JL (2017) Nosema ceranae, Fipronil and their combination compromise honey bee reproduction via changes in male physiology. Sci Rep 7, 8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, Azher S, Hittarage A, Dissanayake W, Sheriff MH, Davies W, Buckley NA, Eddleston M (2004) Acute human self-poisoning with the N-phenylpyrazole insecticide fipronil - a GABAA-gated chloride channel blocker. J Toxicol Clin Toxicol 42, 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lee SJ, Mulay P, Diebolt-Brown B, Lackovic MJ, Mehler LN, Beckman J, Waltz J, Prado JB, Mitchell YA, Higgins SA, Schwartz A, Calvert GM (2010) Acute illnesses associated with exposure to fipronil-surveillance data from 11 states in the United States, 2001–2007. Clin Toxicol (Phila) 48,737–744. [DOI] [PubMed] [Google Scholar]
- [92].Herin F, Boutet-Robinet E, Levant A, Dulaurent S, Manika M, Galatry-Bouju F, Caron P, Soulat JM (2011) Thyroid function tests in persons with occupational exposure to fipronil. Thyroid 21, 701–706. [DOI] [PubMed] [Google Scholar]
- [93].Cravedi JP, Delous G, Zalko D, Viguié C, Debrauwer L (2013) Disposition of fipronil in rats. Chemosphere 93, 2276–2283. [DOI] [PubMed] [Google Scholar]
- [94].Roques BB, Lacroix MZ, Puel S, Gayrard V, Picard-Hagen N, Jouanin I, Perdu E, Martin PG, Viguié C (2012) CYP450-dependent biotransformation of the insecticide fipronil into fipronil sulfone can mediate fipronil-induced thyroid disruption in rats. Toxicol Sci 127,29–41. Erratum in: Toxicol Sci 130,444–445. [DOI] [PubMed] [Google Scholar]
- [95].Tang JA, Usmani K, Hodgson E, Rose RL (2003) In vitro metabolims of fipronil by human and rat cytochrome P450 and its interactions with testosterone and diazepam. Chem Biol Interact 147, 319–329. [DOI] [PubMed] [Google Scholar]
- [96].Cochran RC, Yu L, Krieger RI, Ross JH (2015) Post application fipronil exposure following use on pets. J Toxicol Environ Health A 78, 1217–1226. [DOI] [PubMed] [Google Scholar]
- [97].Bigelow Dyk M, Liu Y, Chen Z, Vega H, Krieger RI (2012) Fate and distribution of fipronil on companion animals and in their indoor residences following spot-on flea treatments. J Environ Sci Health B 47, 913–924. [DOI] [PubMed] [Google Scholar]
- [98].Sun Q, Qi W, Yang JJ, Yoon KS, Clark JM, Park Y (2016) Fipronil promotes adipogenesis via AMPKα-mediated pathway in 3T3-L1 adipocytes. Food Chem Toxicol 92, 217–223. [DOI] [PubMed] [Google Scholar]
- [99].Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Griffin WS, Haas J, Hudson AP, Kamer AR, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strand berg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA (2016) Microbes and Alzheimer’s disease. J Alzheimers Dis 51, 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Pedditizi E, Peters R, Beckett N (2016) The risk of over-weight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 45, 14–21. Erratum in: Age Ageing 45, 740. [DOI] [PubMed] [Google Scholar]
- [101].Mazon JN, de Mello AH, Ferreira GK, Rezin GT (2017) The impact of obesity on neurodegenerative diseases. Life Sci 182,22–28. [DOI] [PubMed] [Google Scholar]
- [102].Hainzl D, Casida JE (1996) Fipronil insecticide: Novel photochemical desulfinylation with retention of neurotoxicity. Proc Natl Acad Sci USA 93, 12764–12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hainzl D, Cole LM, Casida JE (1998) Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol 11, 1529–1535. [DOI] [PubMed] [Google Scholar]
- [104].McMahen RL, Strynar MJ, Dagnino S, Herr DW, Moser VC, Garantziotis S, Andersen EM, Freeborn DL, McMillan L, Lindstrom AB (2015) Identification of fipronil metabolites by time-of-fiight mass spectrometry for application in a human exposure study. Environ Int 78, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chaguri JL, Godinho AF, Horta DF, Gonçalves-Rizzi VH, Possomato-Vieira JS, Nascimento RA, Dias-Junior CA (2016) Exposure to fipronil elevates systolic blood pressure and disturbs related biomarkers in plasma of rats. Environ Toxicol Pharmacol 42, 63–68. [DOI] [PubMed] [Google Scholar]
- [106].Appenzeller BMR, Hardy EM, Grova N, Chata C, Faÿs F, Briand O, Schroeder H, Duca RC (2017) Hair analysis for the biomonitoring of pesticide exposure: Comparison with blood and urine in a rat model. Arch Toxicol 91, 2813–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Roques BB, Lacroix MZ, Puel S, Gayrard V, Picard-Hagen N, Jouanin I, Perdu E, Martin PG, Viguié C (2012) CYP450-dependent biotransformation of the insecticide fipronil into fipronil sulfone can mediate fipronil-induced thyroid disruption in rats. Toxicol Sci 127, 29–41. [DOI] [PubMed] [Google Scholar]
- [108].Lacroix MZ, Puel S, Toutain PL, Viguié C (2010) Quantification of fipronil and its metabolite fipronil sulfone in rat plasma over a wide range of concentrations by LC/UV/MS. J Chromatogr B Analyt Technol Biomed Life Sci 878,1934–1938. [DOI] [PubMed] [Google Scholar]
- [109].Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, Azher S, Hittarage A, Dissanayake W, Sheriff MH, Davies W, Buckley NA, Eddleston M (2004) Acute human self-poisoning with the N-phenylpyrazole insecticide fipronil—a GABAA-gated chloride channel blocker. J Toxicol Clin Toxicol 42, 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


