Abstract
Multiple myeloma represents 2% of all new cancer diagnoses in the United Kingdom and accounts for 2% of all cancer deaths. In the past few decades, there have been huge improvements in life expectancy which have been driven by novel therapeutic agents, autologous stem cell transplants and intensified supportive care. This review will discuss the pathogenesis of multiple myeloma, current management approaches and the direction of future treatments. In addition, this review will highlight the high burden of symptoms that patients experience and therefore the great benefits that can be gained from specialist palliative care input.
Keywords: management, multiple myeloma, palliative care, pathogenesis, prognosis, treatment
Introduction
Multiple myeloma (MM) is a malignancy of plasma cells which represents about 2% of all new cancer cases in the United Kingdom.1 The disease can cause failure of the bone marrow leading to anaemia, immune paresis with resultant infection, bone pain and fractures, high calcium levels and renal failure. In recent years, the therapeutic options to treat MM have rapidly expanded leading to increased length of survival. However, despite these improvements, MM remains an incurable condition for the majority of patients; most patients will die of their disease as it becomes refractory to treatment. This review aims to outline the haematological management of MM for a palliative care audience and highlight the need for a multidisciplinary team approach, combining active treatment and symptomatic support.
An overview of the pathology of MM
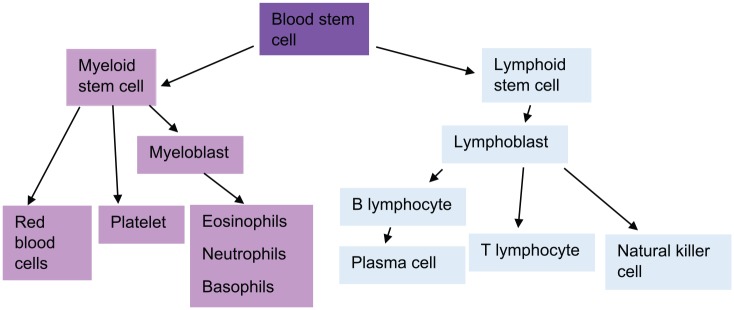
MM is a B-cell malignancy involving a subset of cells known as long-lived plasma cells. These are terminally differentiated, non-dividing cells that survive for months to years in the bone marrow and produce antigen-specific immunoglobulin, thus forming an integral part of the immune defence system (Figure 1).2 Malignant plasma cell clones make an excess of a specific immunoglobulin (which comprises two heavy chains and two light chains) and also an excess of additional light chains. These proteins can be detected in the blood and are useful in both the diagnosis and monitoring of MM.
Figure 1.
Haematopoietic stem cell differentiation pathways.
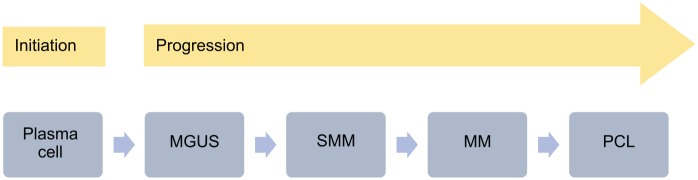
MM develops from a pre-malignant plasma cell dyscrasia, namely monoclonal gammopathy of unknown significance (MGUS). Over time, MGUS has the potential to evolve into smouldering (asymptomatic) multiple myeloma (SMM), which then progresses into MM. Rarely, MM can evolve into plasma cell leukaemia (PCL), a condition in which malignant plasma cells no longer rely on the bone marrow niche and circulate in the peripheral blood (Figure 2).2,3 Together, these conditions form a spectrum of clonal disorders involving long-lived plasma cells.2 However, progression can be very slow and therefore the vast majority of patients do not progress through the full spectrum of disease.
Figure 2.
The spectrum of plasma cell dyscrasias.2
MGUS, monoclonal gammopathy of unknown significance; MM, multiple myeloma; PCL, plasma cell leukaemia; SMM, smouldering multiple myeloma.
Sequencing of the immunoglobulin heavy chain (IGH) variable region of MM cells has shown that the first oncogenic event occurs when the plasma cells are developing in the germinal centres of lymph nodes.2 Patients with the pre-malignant state of MGUS also carry these initial mutations, and the acquisition of multiple further oncogenic events is required in the founder cell in order to drive the progression from pre-malignancy (MGUS) to malignancy (SMM, MM, PCL). MGUS is relatively common, the incidence increasing with age to approximately 5% in the over-80s. However, most patients with MGUS do not progress to myeloma, the risk being approximately 1% per year.2 MGUS is not currently screened for, but is often detected as an incidental finding and, if detected, should be monitored due to the small ongoing risk of development into MM.
Epidemiology and prognosis
MM is the second most common haematological malignancy, representing 10–15% of all new diagnoses.4 Around 5700 new cases of MM are diagnosed in the United Kingdom per year and there are about 3000 deaths per year.1 MM incidence is strongly related to age, and almost half of new cases are in people aged 75 years and above.1 The condition is more common in men than women; in the United Kingdom, 58% of MM cases are in males and 42% are in females.1 There are also significant racial differences in disease occurrence with MM occurring around twice as frequently in Black people compared with White or Asian people.1 Median survival is currently 4–8 years, partly depending on age at presentation.1 MM survival has greatly improved in the past 40 years; in the 1970s, roughly 5% of newly diagnosed people survived with their disease beyond 10 years, and by 2011, this had improved to a third.1
Diagnosis and presenting features
MM often presents with a high symptomatic burden, most commonly with fatigue and bone pain (Table 1).5 In the United Kingdom, the most frequent route to diagnosis, accounting for roughly one-third of cases, is emergency presentation to hospital, either via self-referral to accident and emergency departments or referral by a general practitioner.6
Table 1.
Presenting features of multiple myeloma.5
| Presenting features of multiple myeloma |
|---|
| Fatigue |
| Bone pain and fractures |
| Recurrent infections |
| Easy bruising and bleeding |
| Loss of weight |
As the bone marrow becomes filled with malignant plasma cells, the ability of haematopoietic stem cells to produce new blood cells is diminished and this in turn leads to anaemia and, less commonly, neutropenia and thrombocytopenia.7 Severe destructive bone disease is a hallmark of MM and a frequent cause of presenting symptoms. Lytic skeletal lesions are detected in approximately 80% of patients.5 The spine and ribs are frequently affected and around 3% of patients present with spinal cord compression caused by vertebral compression fractures or spinal soft tissue tumours.8 The primary cause of bone disease is increased osteoclastic activity, which occurs in close proximity to active myeloma cells.9 Hypercalcaemia is caused by bone lysis and can lead to abdominal pain, constipation and confusion.5 Other common findings at presentation include impairment of renal function, which is contributed to by high light chain load, hypercalcaemia and use of non-steroidal anti-inflammatory drugs (NSAIDs) for pain.5 A further common complication of MM is infection, particularly pneumonia. This is because patients have immune paresis, defined by suppression of the production of normal immunoglobulins.
As discussed above, MM follows on from pre-malignant plasma cell dyscrasias such as MGUS and SMM. In 2014, the International Myeloma Working Group (IMWG) updated the diagnostic criteria for these conditions and added three highly specific biomarkers to the existing criteria for MM (see bold text in Table 2).5 The addition of these criteria allows earlier diagnosis of MM with the aim of initiating treatment before organ damage occurs.5
Table 2.
Diagnostic criteria for plasma cell disorders.
| Plasma cell disorder | Definition |
|---|---|
| MGUS | The following three criteria must be met: • Serum monoclonal protein of <3 g/dL • Clonal bone marrow plasma cells of <10% • Absence of end organ damage using the CRAB criteria (hypercalcaemia, renal impairment, anaemia and bone lesions) |
| SMM | The following two criteria must be met: • Serum monoclonal protein (IgG or IgA) of ⩾3 g/dL, or urinary monoclonal protein of ⩾500 mg/24 h and clonal bone marrow plasma cells of 10–60% • Absence of myeloma-defining events or amyloidosis |
| MM | The following two criteria must be met: • Clonal bone marrow plasma cells of ⩾10% or biopsy-proven bony or extramedullary plasmacytoma • Any one or more of the following myeloma-defining events: • Evidence of end organ damage using the CRAB criteria • Hypercalcaemia: serum calcium of >0.25 mmol/L higher than the upper limit of normal or >2.75 mmol/L • Renal insufficiency: creatinine clearance of <40 mL/min or serum creatinine of >177 µmol/L • Anaemia: Hb of >2 g/dL below the lower limit of normal or a Hb value of <10 g/dL • Bone lesions: one or more osteolytic lesions on skeletal XR, CT or PET-CT • Clonal bone marrow plasma cells of ⩾60% • Involved: uninvolved serum free light chain ratio of ⩾100 (involved free light chain level must be ⩾100 mg/L) • More than one focal lesion on MRI studies (at least 5 mm in size) |
CT, computed tomography; MGUS, monoclonal gammopathy of unknown significance; MM, multiple myeloma; MRI, magnetic resonance imaging; PET-CT, positron emission tomography/computed tomography; SMM, smouldering multiple myeloma; XR, X-ray.
The new criteria, added in 2014, are indicated in bold.5
The treatment of MM
The impressive improvements in survival seen in the past few decades have largely been driven by better treatments, including novel drugs, autologous stem cell transplantation and enhanced supportive care.
Active drug classes in MM
Corticosteroids
Steroids remain one of the fundamental drug treatments in MM, with dexamethasone or prednisolone initially forming part of the vast majority of treatment regimes. Glucocorticoids as a single agent have action against myeloma cells and work by the promotion of a broad range of anti-inflammatory and immunosuppressive activities.10 However, the side effect profile of steroids, which includes mood symptoms, insomnia, muscle weakness and increased risk of infection, can be problematic, especially in more elderly patients. The doses of steroids used to treat MM are often much higher than in other fields, with 40 mg of dexamethasone once weekly being a standard dose.
Standard chemotherapy
Alkylating agents (such as cyclophosphamide, melphalan and bendamustine) and anthracyclines (such as doxorubicin and idarubicin) are types of chemotherapy that act, albeit through different mechanisms, by damaging deoxyribonucleic acid (DNA) and are used to treat a broad spectrum of haematological and solid organ cancers.11 DNA damage tends to affect malignant cells to a greater extent than healthy cells because malignant cells proliferate faster and have reduced DNA error-correcting capabilities. High-dose melphalan remains a standard of care as part of the induction for stem cell transplantation.
Proteasome inhibitors
The first-in-class proteasome inhibitor (PI), bortezomib, was approved for use by the US Food and Drug Administration (FDA) in 2003 and has been followed by newer agents such as carfilzomib and ixazomib.12 Proteasomes are large protein complexes within the cell which degrade proteins that the cell no longer requires.12 PIs enhance apoptosis by disrupting the proteasomal degradation of proteins involved in several critical cellular pathways.12 One key mechanism in MM is the inhibition of inhibitory kappa B (IκB) breakdown which leads to stabilisation of the nuclear factor kappa B (NFκB) complex. This prevents NFκB translocation to the nucleus and leads to inactivation of multiple downstream pathways known to be important in myeloma cell signalling.12
Immunomodulatory drugs
Thalidomide was initially used in Europe as a sedative anti-emetic in hyperemesis gravidarum and was withdrawn from the market in 1961 due to a proven association with congenital birth defects.13 It was later shown to have anti-angiogenic properties and broad immunomodulatory and anti-inflammatory effects.13 It was first found to be useful in MM treatment in the late 1990s, and analogues such as lenalidomide and pomalidomide were developed and introduced into clinical practice.14 The mechanism of action of immunomodulatory drugs (IMiDs) has only recently begun to be understood; IMiDs bind to the E3 ligase cereblon and this affects targeting of proteins to the proteasome system.15
Histone deacetylase inhibitors
Histone deacetylase inhibitors (HDACis), such as panobinostat, represent another new class of drug treatment for use in MM.16 Epigenetic changes, which are changes in the configuration of DNA rather than the nucleotide sequence, are increasingly being recognised as important to the transcription of tumour suppressor genes and oncogenes. The genetic information in cells is stored as chromatin: DNA wrapped around protein components called histones. HDACis cause histone hyperacetylation, leading to alterations in the structure of chromatin and thereby gene expression.16
Monoclonal antibodies
Daratumumab was the first monoclonal antibody approved by the FDA for the treatment of MM and is licensed for use in the relapsed setting.17 It is a human monoclonal antibody that targets the cell surface protein CD38 which is universally expressed on malignant plasma cells. Daratumumab induces cell death through a number of mechanisms including complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis and apoptosis.17 It has significant activity as a monotherapy and has been shown to dramatically improve response rates and remission times when combined with a number of other agents.18–20 As such, it probably represents the most important new therapy of the past decade. Other anti-CD38 monoclonal antibodies are now in clinical trials.
Elotuzumab is also approved for treatment of MM and it targets SLAMF7, a cell surface protein which is highly expressed on normal plasma cells, malignant plasma cells and natural killer (NK) cells.21 Elotuzumab again has multiple mechanisms of action including the mediation of antibody cell-mediated cytotoxicity, enhancing the cytotoxicity of NK cells and inhibition of myeloma cell interaction with bone marrow stromal cells.22
Treatment approach
When a patient is first diagnosed with myeloma, it is important to accurately classify and stage their disease. Patients with asymptomatic myeloma are usually placed under close surveillance but are not initially treated as only 50% of these patients will eventually require active therapy.8 The exceptions are patients who have either ⩾60% bone marrow infiltration, >1 focal lesions on magnetic resonance imaging (MRI) scanning or a serum-free light chain ratio of ⩾100; these patients are deemed to have a very high chance of developing symptoms within a short time frame and therefore treatment is expedited.5 Therefore, management of asymptomatic patients includes monitoring in clinic every few months with close attention to blood parameters including renal function, calcium level, paraprotein (the monoclonal protein produced by plasma cells) and light chains. The goal in this patient cohort is to commence treatment before bone disease, kidney damage or other effects of myeloma occur.
In patients who already have evidence of end organ damage caused by myeloma such as bone damage, renal impairment, hypercalcaemia or bone marrow failure, or those with asymptomatic disease but high-risk features, treatment is initiated immediately. The initial aim of treatment is to reduce the burden of disease to a low level so that further damage cannot occur.
Patients who require treatment (MM) are initially divided into two broad categories: those fit enough for stem cell transplantation and those who would not tolerate this intensive procedure (Figure 3).23 Factors to consider when assessing fitness include medical comorbidities and performance status. Older age in itself is a factor but many haematology units will consider performing autologous transplants in fit patients up to the age of 75 years, although rarely above this age.
Figure 3.
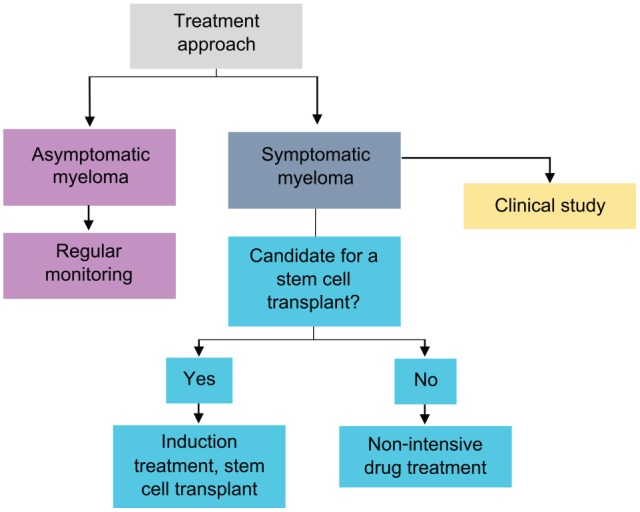
Treatment paradigm in MM.23
Initial treatment for stem cell–eligible patients
The treatment of MM for stem cell–eligible patients can be divided into different phases, namely, induction therapy, stem cell transplantation and consolidation or maintenance therapy.5 The aim of induction therapy is to reduce the burden of disease before consolidating this response with transplantation. Induction therapy usually comprises a combination of three drugs from different classes, for example, bortezomib, thalidomide and dexamethasone.23 Bortezomib is given by weekly subcutaneous injection, thalidomide is a daily capsule and dexamethasone comes as tablets that are taken weekly. One cycle lasts for 4 weeks, is delivered entirely as an outpatient, and most patients will receive four to six cycles.
Following induction therapy, autologous stem cell transplant is performed. An autologous transplant is a mechanism to provide high-dose chemotherapy to destroy malignant cells. This aggressive treatment also destroys the normal function of the bone marrow and leaves the patient pancytopenic and thus at high risk of opportunistic infection. In autologous transplantation, patient’s stem cells are harvested before the administration of chemotherapy and then infused back into the body, allowing ‘stem cell rescue’ and early re-constitution of bone marrow function. Autologous transplantation improves complete response rates and prolongs remission times in MM by an average of 12–18 months.5
In autologous transplantation, the first step is to collect the patient’s stem cells. Haemopoietic stem cells usually circulate in the peripheral blood in low numbers and therefore must be mobilised from the bone marrow into the blood stream for collection.24 This is usually done by injection of cytokines, such as granulocyte-colony-stimulating factor (G-CSF), alone or in combination with chemotherapy, such as cyclophosphamide.24 The patient undergoes apheresis and the collected cells are then frozen. High-dose chemotherapy is given to the patient, usually melphalan, and the stem cells are de-frosted and transfused into the patient intravenously. The stem cell transplant involves a period as an inpatient, usually up to 3 weeks, although many centres now run much of this procedure as an outpatient under very close supervision.
Post-transplant some patients may receive medication to prolong response. The Myeloma XI study showed a significant improvement in progression-free survival from 28 to 50 months with the addition of maintenance lenalidomide which translates into improved overall survival.25 Although now considered a standard of care in some other countries, it is not currently available in the National Health Service (NHS), United Kingdom, and there are some additional side effects compared with nonmaintenance, including an increased risk of other malignancies and venous thromboembolism.26,27
Initial treatment for non-transplant eligible patients
Patients who are not deemed to be fit for stem cell transplantation are initially treated with a combination of two or three drugs from different classes, with steroids usually forming one of the components. The doses used, and the dose intensity, is often lower than would be used for fitter patients, although the combination of drugs given is similar. Examples of drug combinations used as initial therapy for older, frailer patients are shown in Table 3.23 As there is no consolidation with transplantation, therapy is often extended for longer than the four to six cycles used for younger, fitter patients.
Table 3.
Commonly used initial treatment combinations for MM in patients not fit for stem cell transplantation.23
| Thalidomide based | Velcade based | Lenalidomide based |
|---|---|---|
| • CTD Cyclophosphamide Thalidomide Dexamethasone • MPT Melphalan Prednisolone Thalidomide |
• VMP Velcade Melphalan Prednisolone • CVD Cyclophosphamide Velcade Dexamethasone |
• Lenalidomide and dexamethasone |
MM, multiple myeloma.
Treatment at relapse
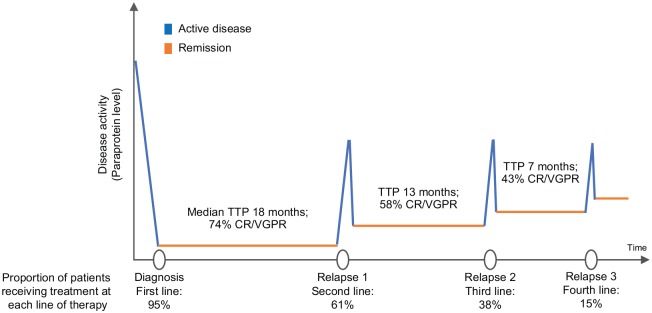
Although the majority of patients respond well to initial therapy and disease levels are greatly reduced, treatment is generally not curative and most patients will relapse at some point. The average remission times are approximately 4–5 years after transplant if maintenance lenalidomide is used, 2–3 years after transplant if no maintenance is used and 1–2 years if patients are not transplanted, although there is great variation in these outcomes. Most patients can be successfully retreated at relapse; however, each remission is usually associated with diminishing duration and depth of response over time (Figure 4).28–31 If possible, different combinations of drugs are used compared with initial therapy. In addition, a second autologous transplant can be performed if the patient received a significant benefit from the first transplant, commonly considered as a minimum of 18–24 months of remission.32 In this way, many patients control their disease with sequential lines of treatment for many years; 47% of people diagnosed with MM in England and Wales survive their disease for 5 years or more and 33% survive their disease for 10 years or more.1
Figure 4.
The average response rates and remission times to first-, second-, third- and fourth-line therapies. Data from retrospective analysis of 4997 patient charts in Belgium, France, Germany, Italy, Spain, Switzerland and the United Kingdom. The chart review was performed in 2014. Note that only 38% of patients received third-line therapy which reduces to 15% at fourth-line therapy. CR, complete response; TTP, time to progression; VGPR, very good partial response.
Source: Data taken from Yong and colleagues.27
Supportive care
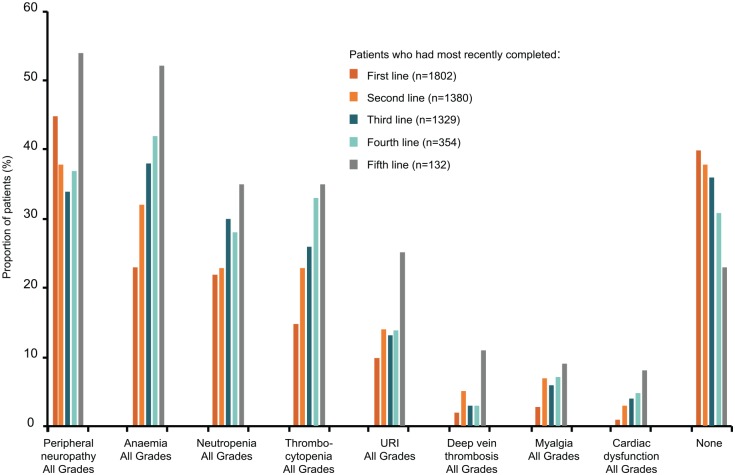
Alongside active MM treatment, supportive care for patients is critical. The most common comorbidities and toxicities at first-line therapy include peripheral neuropathy, anaemia, neutropenia and thrombocytopenia, and the proportion of patients with one or more toxicity or comorbidity at the end of treatment increases with lines of therapy (Figure 5).29
Figure 5.
Frequency of adverse events experienced by patients at different stages of treatment. Data from 4997 patient charts in Belgium, France, Germany, Italy, Spain, Switzerland and the United Kingdom. Only patients who had progressed at the time of inclusion in the study were included in this analysis. Events generally increased with number of lines of therapy. Less than 25% of patients have no events by fifth line of treatment. URI, upper respiratory infection.
Source: Adapted from Yong and colleagues and reproduced with permission from John Wiley & Sons.27
Bone disease, including osteolytic lesions and osteoporosis, is a significant concern in MM as it can lead to severe pain, bone fractures and spinal cord compression.33 To improve bone health, patients should routinely be treated with bisphosphonates such as zoledronic acid.5 Bisphosphonates bind to hydroxyapatite crystals in the bone where they are absorbed by osteoclasts and inhibit their catabolic effects.33 In addition, they also display anti-tumour and immunomodulatory activity and these combined actions have been shown to increase patient survival.5,33,34 Denosumab, a RANK (receptor activator of NFκB) ligand inhibitor, is an alternative to bisphosphonates. All patients with significant spinal disease should be referred to a specialist spinal surgery team for assessment as they may benefit from bracing, vertebroplasty or kyphoplasty.5
Patients with MM have a weakened immune system and infection is a common cause of presentation and of death, especially in the initial months following diagnosis while the disease is still very active. Therefore, minimising the risk of contracting infections is important. Patients are advised to receive pneumococcal and influenza vaccinations and those with hypogammaglobulinaemia and recurrent infections can be considered for regular infusions of intravenous immunoglobulins, although the efficacy of this approach is unproven.23 Prophylaxis medications are commonly prescribed alongside chemotherapy, including aciclovir to prevent shingles. The use of additional prophylactic antibiotics remains controversial. Many patients with MM die before they have time to respond to anti-myeloma therapy, in particular from pneumonia and other infections.35 The Tackling Early Morbidity and Mortality in Myeloma (TEAMM) trial randomised patients to levofloxacin and placebo during the first 3 months of treatment. Prophylactic levofloxacin did not greatly increase the risk of acquisition of carriage of resistant bacteria, such as Clostridium difficile, methicillin-resistant Staphylococcus aureus (MRSA) and faecal extended spectrum beta-lactamase (ESBL)-positive gram-negative bacteria, but did reduce the incidence of febrile episodes and deaths.36
Patients also commonly experience neuropathies which can greatly affect activities of daily living.29,37 Neuronal injury can be caused by malignant infiltration, immune-mediated antibody deposition or local compression of nerve roots.37 Furthermore, many of the drugs used to treat MM, particularly bortezomib, cause peripheral neuropathy.29 Neuropathy can be managed by stopping or reducing the dose of any causative agents and commencing pain medications such as gabapentin. In addition, use of IMiDs significantly increases the risk of venous thromboembolism and patients need to be assessed for use of prophylactic low molecular weight heparin or aspirin to prevent clots.26 Furthermore, if treatable causes of anaemia have been excluded, erythropoietin analogues to maintain a haemoglobin of 110–120 g/L may help to improve fatigue in patients with symptomatic anaemia.23
Direction of future treatments
Treatment in MM is rapidly evolving as new research is having proven clinical impact. Three areas that may become increasingly important include risk-stratified treatment, targeted therapies and immunotherapy including chimeric antigen receptor T (CAR-T) cells.
Risk stratification in MM
The aim of stratified therapy is to define patients with adverse prognostic features at the outset and alter therapy in order to try and improve their outcomes. MM is considered to be a single disease but is actually a collection of several cytogenetically distinct plasma cell malignancies.5 The recurrent genetic lesions that give rise to myeloma are reasonably well defined. Broadly, in approximately half of patients with myeloma, the initiating genetic event is hyperdiploidy, characterised by trisomies of chromosomes. The remaining 50% of patients usually have a translocation of genetic material between one of five partner chromosomes and the IGH gene on 14q32.38 These initial events are followed by secondary genetic events which include gain of genetic material (e.g. chromosome 1q) and deletion of parts of chromosomes (e.g. 1p, 13q, 17p).38 These genetic lesions can be tested for, usually by a method called flow in situ hybridisation (FISH), and several have been associated with shorter remission times and impaired survival, namely, t(4;14), t(14;16), t(14;20), +1q21 and del(17p13).38 Prognosis can be stratified based on an assessment of these genetic lesions of the plasma cell clone (Figure 6).38
Figure 6.
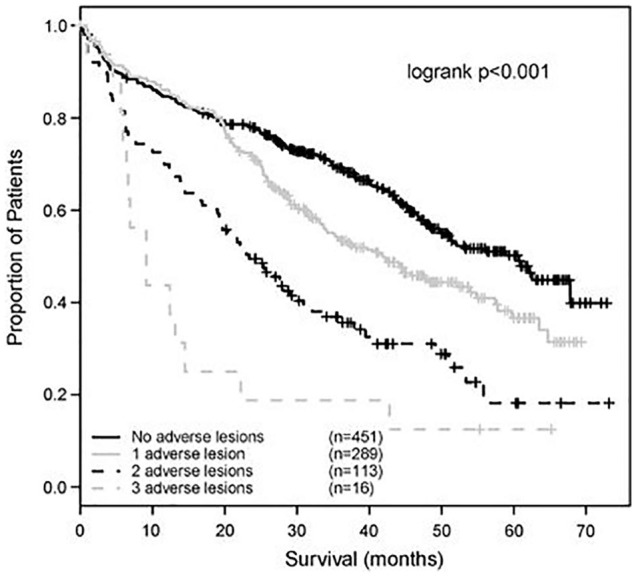
The impact of adverse cytogenetic lesions on patient survival.
Source: Reproduced with permission from Springer Nature.38
Gene expression profiling is an alternative method of assessing genetic risk, with several high-risk gene expression signatures being defined including the SKY92 and UAMS profiles.39 Several groups have looked to improve patient outcomes by tailoring initial treatment based on these genetic tests, although the best combination of tests to use and the best tailored treatment is not proven. The MUK9 trial in the United Kingdom is seeking to address these questions (trial ongoing, clinicaltrials.gov, NCT03188172).
Targeted therapies in MM
As it becomes quicker and cheaper to determine the mutations driving MM, we may be able to perform a mutation analysis for each patient and then implement targeted therapies. For example, mutations of the RAS/mitogen-activated protein kinase (MAPK) pathway are present in up to 50% of newly diagnosed MM cases and this could be an important drug target for this group of patients.40 Indeed, trametinib, an oral, allosteric inhibitor of MEK1/2, has shown early promise as a myeloma therapeutic.40 Another good example of targeted therapy is the use of venetoclax. Venetoclax inhibits the anti-apoptotic B-cell lymphoma-2 (BCL2) protein and is approved for use as a monotherapy in patients with relapsed or refractory chronic lymphocytic leukaemia (CLL) with 17p deletion.41 By inhibiting BCL2, the drug induces apoptosis in CLL cells. Myeloma cells, particularly the 15% of myeloma cases with a t(11;14) translocation, express high levels of BCL2, and venetoclax also induces cell death in this group of patients.42
Immunotherapy
CAR-T cells are a rapidly emerging form of immunotherapy, currently being trialled in a range of haematological malignancies. These engineered T cells not only have potent anti-tumour activity but also have a novel profile of toxic side effects.43 T cells are collected from a patient’s peripheral blood using an apheresis machine. These cells are then genetically modified in a laboratory to express an artificial chimeric antigen receptor (CAR). CARs chiefly consist of three components, an extracellular domain derived from a monoclonal antibody which binds to an antigen on the malignant cell, a transmembrane domain which anchors the CAR to the T cells and an intracellular T-cell activation domain, CD3ζ with or without a costimulatory domain, that confers the T cell with sustained anti-tumour activity (Figure 7).43 Once the CAR-T cells have been made, they are allowed to replicate in vitro, and millions of copies are produced. Patients then receive immunosuppressive chemotherapy before the CAR-T cells are infused intravenously.
Figure 7.
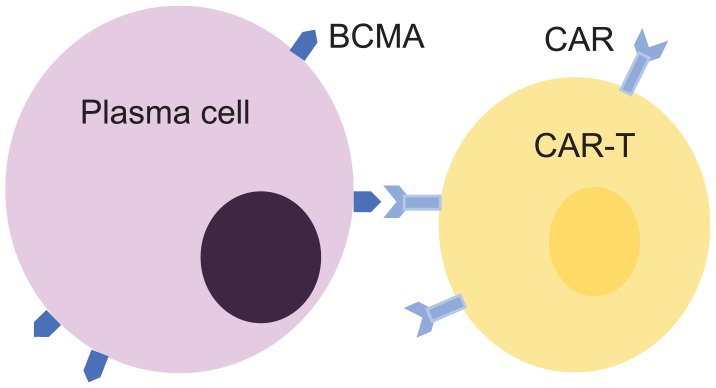
CAR-T cell’ interaction with myeloma cell.43
CAR-T cells targeting CD19 (a transmembrane protein expressed by all B lineage cells except plasma cells) are now licensed and National Institute for Health and Care Excellence (NICE) approved for use in children and young people with B-cell acute lymphoblastic leukaemia (B-ALL) and adults with diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma.44 The use of CAR-T cells in MM is currently in the trial phase. For example, the Bluebird group is using CAR-T cells which target the B-cell maturation antigen (BCMA) found on malignant plasma cells. In 22 heavily pre-treated patients who received the highest cell dose used in the study, the overall response rate was 95.5%, with 50% complete responses and a median response duration of 10.8 months.45 Cytokine release syndrome was the predominant side effect.45 The reasonably short remission duration has tempered some of the early enthusiasm for this treatment, but it is still at a very early stage of development in myeloma and the response rates are unparalleled in this patient population. Therefore, it remains a very promising line of exploration.
A summary of palliative care considerations
Patients with MM often present with a significant burden of symptoms, including pain, fatigue and dyspnoea.46 Bone pain is a particular concern in this disease. Although many cancers can metastasise to bone and cause symptoms, MM has a specific propensity to cause osteolysis and the majority of patients have some evidence of bone damage on computed tomography (CT) or MRI scans. Vertebral fractures and back pain are very common and symptoms will often continue for the rest of a patient’s life, even if the MM is brought under control. Patients in remission with no detectable disease may often have an ongoing high burden of symptoms, which is unusual in other cancers. Many patients with chronic pain will require a holistic approach to their pain management and involvement with pain specialists and palliative care can be very helpful.23 In later stages of the disease, when active anti-myeloma drug therapy has been deemed inappropriate, palliative radiotherapy can often be useful for bone pain and has minimal toxicity.23
Renal impairment is very common in patients with MM, usually caused by the toxic effects of a high light chain load.47 Even if renal function is not impacted by early disease, it can often deteriorate in later stages when the MM is less well controlled.47 This can have implications for drug dosing, especially of opiates, and more frequent testing of renal function to guide these decisions may be justified compared with other patient groups in the palliative setting.
It is also important to consider the significant psychological impact of an incurable malignant diagnosis and a large number of patients suffer from anxiety and depression.46 As discussed above, patients may go through multiple lines of treatment as the disease progresses. The chance of remission at each line decreases and the side effect profile frequently worsens.29 There are more treatment options compared with many other cancers with up to six different lines of treatment currently approved and funded on the NHS in England. It is therefore often difficult to decide when to take a purely palliative approach and stop active MM therapy; although this is not unique to myeloma, the range of therapeutic options means that patients will often undergo more lines of treatment than in other malignancies.
As a general comment, the palliative care needs of patients with advanced haematological malignancies, including MM, have been widely acknowledged and early referral to palliative care teams has been shown to improve end-of-life care as measured by fewer emergency department attendances, fewer admissions and fewer hospital deaths in the last 30 days of life.48,49 However, individuals with haematological malignancies are less frequently referred to palliative care services and often receive more aggressive treatments near the end of life than those with solid tumours.49,50 The reasons for this have been explored by many groups and a common theme is great prognostic uncertainty in this group of cancers.51 Other potential factors include unrealistic expectations from both doctors and patients, long-term patient–doctor relationships resulting in difficulty conducting discussions regarding the end of life and organisational issues.51,52
Patients with haematological malignancies are also more likely to die in hospital compared with those with other forms of cancer, despite the fact that many patients express a preference to die at home.53 Again, this may be related to a range of factors including difficulties predicting prognosis and identifying if and when to withdraw treatment.53,54 Advanced planning, with improved communication between hospital-based and community-based teams, may help to improve end-of-life care in this patient cohort.54 It is important to note that some end-of-life quality measures developed for solid tumours may be less applicable to patients with blood cancers given their unique needs, for example, palliative blood product transfusions.47 Therefore, there is a great opportunity for research into improved models for when to refer to palliative care and also updated quality measures for research purposes.
Conclusion
Despite improvements in treatment and outcomes, most patients diagnosed with MM die as a result of their malignancy. Patients can be treated with multiples lines of therapy but the depth and length of remission usually decrease with each relapse and the disease eventually becomes refractory. End-stage disease can cause a range of issues, including bone pain, renal failure, marrow failure and infection. Indeed, infection is frequently the final cause of death in MM patients. Providing tolerable active treatment and supportive medications, such as infection prophylaxis and analgesia, is the mainstay of management. This remains an exciting time for new MM therapies and the introduction of targeted therapies and CAR-T cells is likely to change the future of this condition. Due to the high burden of symptoms and complexity of MM patients, a holistic approach needs to be employed and specialist palliative input can be hugely beneficial for patients at all stages of their disease.
Footnotes
Author Contribution: SAB and KB wrote this manuscript and approved the final version.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Contributor Information
Sarah Anne Bird, Royal Marsden Hospital, Downs Road, Sutton SM2 5PT, UK.
Kevin Boyd, Royal Marsden Hospital, Sutton, UK.
References
- 1. Cancer Research UK. Myeloma statistics, www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma (accessed 4 June 2019).
- 2. Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood 2015; 125: 3049–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jelinek T, Kryukov F, Rihova L, et al. Plasma cell leukemia: from biology to treatment. Eur J Haematol 2015; 95: 16–26. [DOI] [PubMed] [Google Scholar]
- 4. Smith D, Yong K. Multiple myeloma. BMJ 2013; 346: 3863. [DOI] [PubMed] [Google Scholar]
- 5. Rajkumar VS, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc 2016; 91: 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howell D, Smith A, Appleton S, et al. Multiple myeloma: routes to diagnosis, clinical characteristics and survival: findings from a UK population-based study. Br J Haematol 2017; 177: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol 2011; 6: 249–274. [DOI] [PubMed] [Google Scholar]
- 8. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78: 21–33. [DOI] [PubMed] [Google Scholar]
- 9. Kristinsson SY, Minter AR, Korde N, et al. Bone disease in multiple myeloma and precursor disease: novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn 2011; 11: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burwick N, Sharma S. Glucocorticoids in multiple myeloma: past, present, and future. Ann Hematol 2019; 98: 19–28. [DOI] [PubMed] [Google Scholar]
- 11. Neal AJ, Hoskin PJ. Clinical oncology. 4th ed. London: CRC Press, 2012. [Google Scholar]
- 12. Field-Smith A, Morgan GJ, Davies FE. Bortezomib (Velcade™) in the treatment of multiple myeloma. Ther Clin Risk Manag 2006; 2: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palumbo A, Facon T, Sonneveld P, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood 2008; 111: 3968–3977. [DOI] [PubMed] [Google Scholar]
- 14. Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–1571. [DOI] [PubMed] [Google Scholar]
- 15. Collins I, Wang H, Caldwell JJ, et al. Chemical approaches to targeted protein degradation through modulation of the ubiquitin-proteasome pathway. Biochem J 2017; 474: 1127–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deleu S, Menu E, Van Valckenborgh E, et al. Histone deacetylase inhibitors in multiple myeloma. Hematol Rev 2009; 1: e9. [DOI] [PubMed] [Google Scholar]
- 17. Sanchez L, Wang Y, Siegel DS, et al. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol 2016; 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016; 128: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 20. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754–766. [DOI] [PubMed] [Google Scholar]
- 21. Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Sanchez L, Siegel DS, et al. Elotuzumab for the treatment of multiple myeloma. J Hematol Oncol 2016; 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institute for Health and Care Excellence. Myeloma: diagnosis and management (NICE guidelines [NG35]), 2016, https://www.nice.org.uk/guidance/ng35
- 24. Gertz MA, Dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood 2014; 124: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide is a highly effective maintenance therapy in myeloma patients of all ages: results of the phase III Myeloma XI study. Blood 2016; 128: 1143. [Google Scholar]
- 26. Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008; 22: 414–423. [DOI] [PubMed] [Google Scholar]
- 27. Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol 2017; 28: 228–245. [DOI] [PubMed] [Google Scholar]
- 28. Durie BGM. Concise review of the disease and treatment options, https://www.myeloma.org/sites/default/files/resource/ConciseReview.pdf (accessed 22 July 2019).
- 29. Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol 2016; 175: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc 2004; 79: 867–874. [DOI] [PubMed] [Google Scholar]
- 31. Moreau P, Touzeau C. Multiple myeloma: from front-line to relapsed therapies. Am Soc Clin Oncol Educ Book 2015; 35: e504–e511. [DOI] [PubMed] [Google Scholar]
- 32. Mina R, Lonial S. Is there still a role for stem cell transplantation in multiple myeloma? Cancer 2019; 125: 2534–2543. [DOI] [PubMed] [Google Scholar]
- 33. Vallet S, Filzmoser JM, Pecherstorfer M, et al. Myeloma bone disease: update on pathogenesis and novel treatment strategies. Pharmaceutics 2018; 10: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morgan GJ, Davies FE, Gregory WM, et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council Myeloma IX Trial. Blood 2012; 119: 5374–5383. [DOI] [PubMed] [Google Scholar]
- 35. Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002: Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005; 23: 9219–9226. [DOI] [PubMed] [Google Scholar]
- 36. Drayson MT, Bowcock S, Planche T, et al. Tackling early morbidity and mortality in myeloma (TEAMM): assessing the benefit of antibiotic prophylaxis and its effect on healthcare associated infections in 977 patients. Blood 2017; 130: 903.28637661 [Google Scholar]
- 37. Rosenbaum E, Marks D, Raza S. Diagnosis and management of neuropathies associated with plasma cell dyscrasias. Hematol Oncol 2018; 36: 3–14. [DOI] [PubMed] [Google Scholar]
- 38. Boyd KD, Ross FM, Chiecchio L, et al. A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia 2012; 26: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Beers EH, van Vliet MH, Kuiper R, et al. Prognostic validation of SKY92 and its combination with ISS in an independent cohort of patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 2017; 17: 555–562. [DOI] [PubMed] [Google Scholar]
- 40. Heuck CJ, Jethava Y, Khan R, et al. Inhibiting MEK in MAPK pathway-activated myeloma. Leukemia 2016; 30: 976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mihalyova J, Jelinek T, Growkova K, et al. Venetoclax: a new wave in hematooncology. Exp Hematol 2018; 61: 10–25. [DOI] [PubMed] [Google Scholar]
- 42. Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017; 130: 2401–2409. [DOI] [PubMed] [Google Scholar]
- 43. Graham C, Hewitson R, Pagliuca A, et al. Cancer immunotherapy with CAR-T cells: behold the future. Clin Med (Lond) 2018; 18: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. NHS England. https://www.england.nhs.uk/cancer/cdf/car-t-therapy/ (accessed 4 June 2019).
- 45. Raje NS, Berdeja JG, Lin Y, et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: updated results from a multicenter phase I study. J Clin Oncol 2018; 36: 8007. [Google Scholar]
- 46. Kiely F, Cran A, Finnerty D, et al. Self-reported quality of life and symptom burden in ambulatory patients with multiple myeloma on disease-modifying treatment. Am J Hosp Palliat Care 2017; 34: 671–676. [DOI] [PubMed] [Google Scholar]
- 47. Dimopoulos MA, Kastritis E, Rosinol L, et al. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 2008; 22: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 48. Hui D, Kim SH, Roquemore J, et al. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer 2014; 120: 1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beaussant Y, Daguindau E, Chauchet A, et al. Hospital end-of-life care in haematological malignancies. BMJ Support Palliat Care 2018; 8: 314–324. [DOI] [PubMed] [Google Scholar]
- 50. LeBlanc TW, Roeland EJ, El-Jawahri A. Early palliative care for patients with hematologic malignancies: is it really so difficult to achieve? Curr Hematol Malig Rep 2017; 12: 300–308. [DOI] [PubMed] [Google Scholar]
- 51. Odejide OO, Salas Coronado DY, Watts CD, et al. End-of-life care for blood cancers: a series of focus groups with hematologic oncologists. J Oncol Pract 2014; 10: e396–e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCaughan D, Roman E, Smith AG, et al. Palliative care specialists’ perceptions concerning referral of haematology patients to their services: findings from a qualitative study. BMC Palliat Care 2018; 17: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCaughan D, Roman E, Smith AG, et al. Perspectives of bereaved relatives of patients with haematological malignancies concerning preferred place of care and death: a qualitative study. Palliat Med 2019; 33: 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCaughan D, Roman E, Smith AG, et al. Determinants of hospital death in haematological cancers: findings from a qualitative study. BMJ Support Palliat Care 2018; 8: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]