Abstract
The study assessed the feasibility of using a Turkish-version of the Modified Checklist for Autism in Toddlers, Revised (M-CHAT-R/F) as a screening tool for an urban low risk population of young children. M-CHAT-R/F was completed for 6,712 children between ages 16 to 36 months living in Istanbul, Turkey. Autism Diagnostic Observation Schedule-2 was served as the main measure for diagnosis. M-CHAT-R/F screen was positive for 9.8% of children. At follow up interview, 39.7% of initial screen-positive children met criteria for ASD. The study identified 57 (1 in 117) children with ASD (0.8%; 95% CI: 0.063%−1.05%). M-CHAT-R/F performed comparably in Turkey as in United States. Implications of the study for future universal screening for autism in Turkey is also discussed.
Keywords: Autism, MCHAT-R/F, urban population, screening, diagnosis, Turkey
Introduction
Autism Spectrum Disorder (ASD) is a lifelong serious neurodevelopmental disorder characterized by early onset of impairments in socio-communicative skills and restricted interests and/or repetitive stereotypical behaviors. It is now widely accepted that the estimates of prevalence of ASD have increased over time across the globe (Elsabbagh et al. 2012). Recently, a Center for Disease Control and Prevention (CDC) report for 8-year old children across sureveillance sites in the United States found an estimate of 1 in 59 children with ASD, a further increase from 1 in 68 two years earlier (CDC 2018). The CDC has also been directing resources for development of Early Autism and Developmental Disabilities Monitoring (Early ADDM) Network (Christensen et al. 2019).
The parents of children with ASD have been noted to have their first concerns on their child’s development as early as 11-months of age, and usually, when the child is around 17 to 18-months of age (Kleinman et al. 2008). Nevertheless, the median age of diagnosis of ASD can be as late as 50-months (Christensen et al. 2016) underscoring an alarming delay in early diagnosis in practice, especially in low resource regions, and in low-and-middle-income country contexts, given that ASD is currently shown to be reliably diagnosable within the first 36-months of life (Zwaigenbaum et al. 2013).
The American Academy of Pediatrics (AAP) has recommended that all children should systematically be screened at 18 and 24-months of age (American Academy of Pediatrics 2006; Zwaigenbaum et al. 2015). Early identification of young children with ASD has therefore ought to be a public health priority worldwide. Such a public policy is particularly salient since it is also increasingly recognized that early intensive behavioral interventions can lead to better symptom improvement as well as improved overall long-term outcomes (Thompson 2013; Howlin et al. 2009; Dawson et al. 2010).
Current best practice recommendations for early ASD detection among young children include: ongoing developmental surveillance; broad developmental screening at age 9-, 18-, and 24/30-month during well-child check-ups; and ASD-specific screening for all children at the 18- and 24-month check-ups (Gupta et al. 2007; Johnson and Myers 2007). There remain several barriers for achievement of public health goals for universal and successful ASD screening worldwide. For general practitioners, the most common barriers include: lack of time, lack of training, and lack of funding to support screening programs (Fenikile et al. 2015; Khowaja et al. 2014).
Successful universal screening also depends on the availability of standardized and reliable screening tools that are culturally adaptable and acceptable as an initial critical step (Al Qabandi et al. 2011). While there are several ASD screening instruments, only few have been validated in low-risk samples of children younger than 3-years of age (for a review, see Johnson and Myers 2007) and overall psychometric performance needs further improvement (National Institute for Health and Clinical Excellence [NICE] 2011; U.S. Preventive Services Task Force 2016). The Modified Checklist for Autism in Toddlers (M-CHAT; Robins et al. 2001), and its revision, the Modified Checklist for Autism in Toddlers, Revised, with Follow-Up (M-CHAT-R/F) (Robins et al. 2009; Robins et al. 2014) are the most widely used screening instruments worldwide. The M-CHAT and M-CHAT-R/F have been used in several countries (e.g. Baduel et al. 2017; Brennan et al. 2016, Kamio et al. 2015; Stenberg et al. 2014; Garcia-Primo et al. 2014; Tsai et al. 2019; Guo et al. 2019) and have been shown to perform as valid and reliable measures. The M-CHAT and M-CHAT-R have also been shown to identify children in different culturally contexts (e.g. Brennan et al. 2016; Kamio et al. 2015; Seung et al. 2015).
The main aim of the present study was to assess the feasibility of using a Turkish version of the M-CHAT-R/F as a screening tool among an urban low risk population of young children living in Istanbul, Turkey. The Istanbul Metropolitan Municipality (Istanbul Province) has a total population of over 18 million residents. According to the Turkish Statistical Institute (TUIK), there were more than 723,300 children between 18 to 48-months of age at the time of the study. Therefore, screening of young children for ASD remains a huge task which needs to be planned thoroughly. Additional aims included training of participating practitioners in the collaborating Family Healthcare Centers (FHCs) in the Istanbul Metropolitan Municipality for the use of the MCHAT-R/F, as well as the evaluation of parental factors associated with barriers to ASD screening. The Autism Diagnostic Observation Schedule-2 (ADOS-2) was used to inform the diagnosis of ASD and final diagnosis was made by clinical evaluations based on DSM-5 Autism Spectrum Disorder criteria. An overarching aim of the study was to assess the feasibility of a future universal screening program for ASD in Turkey.
Methods
The study was supported by the Istanbul Development Agency and conducted collaboratively under the auspices of the TOHUM Autism Foundation (the leading and largest national autism non-governmental organization in Turkey), the Istanbul Metropolitan Municipality, the Istanbul Directorate of Public Health, and the Istanbul Directorate of Education. Institutional Review Board of the Istanbul Directorate of Public Health approved the study and informed consent was obtained from all participating parents.
Sample:
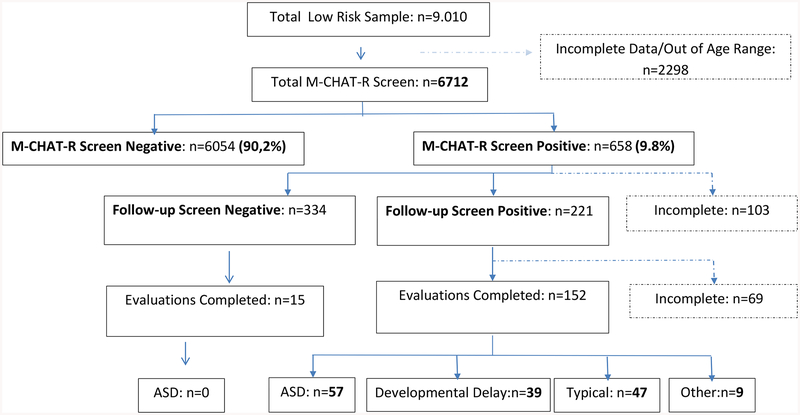
Study flowchart is summarized in Figure 1. The total low-risk sample included 9,010 children that came from 75 FHCs; volunteer family practitioners in the FHCs were invited to participate in the study. The FHCs in Turkey are responsible for free immunization of all children registered in their designated catchment area, besides other duties such as pregnancy and post-natal follow-up. The FHCs were revitalized as part of a national network under the primary healthcare reforms that began implemention in 2003 under the Health Transformation Program. The goals of the reforms were to improve access as well as provision of better targeted medical services for families and children across the 81 provinces. A single FHCs may be responsible for up to 3000 families. The M-CHAT-R/F was obtained from 6,712 children between ages of 16 to 36-months (mean age: 26.75 months; SD: 5.76 months); 48.5% of the sample were females.
Figure 1:
Flowchart of screening results. ASD: Autism Spectrum Disorder.
Measures:
The M-CHAT-R/F is a two-stage ASD screener (see www.mchatscreen.com). It is freely available for clinical, research and educational use and is comprised of 20 yes/no questions. Translation and back-translation of the M-CHAT-R/F version (Robins et al. 2014) was conducted and checked for comprehensibility. It usually takes about 10 minutes or less to fill the form. M-CHAT-R/F was read aloud to parents by participating practitioners in the FHCs. Among consenting parents completing the form, if the child screened positive (i.e., ≥3–20 endorsed items), a structured follow-up interview was conducted by psychologists who were familiar with the instrument; each follow-up interview with the parents took 20 minutes or less to complete.
Autism Diagnostic Observation Schedule-2 (ADOS-2, Lord et al. 2012a, 2012b) was used to inform ASD diagnosis. Previous edition of the ADOS was translated by our team with the permission of the publisher, Western Psychological Services (WPS), for use in research Turkey. All examinations were conducted by the first author, research certified for use of the instrument.
Denver Developmental Screening-II (Frankenburg and Dodds, 1990; Anlar et al. 2009) was used to evaluate developmental level of the children during clinical evaluation. Denver II is a widely used developmental screening test in Turkey. It has been shown to be valid and reliable in Turkish children, and it is one of the very few tests with available population norms.
Procedures:
M-CHAT-R/F was translated by the first author to Turkish and back translated by two different translators. Back translation was sent to the original authors for control. Comprehensibility of the translation was checked, and pretests were carried out in order to make the translation more understandable and remove any ambiguous phrases. The comments of the respondents as well as those of the researchers in the pretest measurements were incorporated into the translated version whenever appropriate.
After the translation, the participating family practitioners were trained for use of M-CHAT-R/F in the corresponding FHCs in Istanbul Province. The family practitioners were responsible for free immunization of all children registered in the FHC designated catchment area, besides other duties such as pregnancy and postnatal follow-up of mothers and children. The family practitioners in the correponding FHCs therefore were in a unique position with close and trusted relationships with the parents. Participation by the practitioners as well as the families were voluntary. There were 75 FHCs sites comprising 148 practitioners who volunteered to participate in the study. The contributions of the paractitioners in terms of the number of children they screened varied widely (between 1 to 346). Accordingly, we did not compare screening results from individual practitioners and FHCs.
Initial screening were administered by the practitioners at the FHCs who were trained in the use of the MCHAT-R/F. All the second stage follow-up interviews were conducted by trained psychologists. Since we aimed to see whether follow-up interview results were similar between children who scored higher or lower than 7, follow-up interviews were conducted for all children who scored higher than 3. Research staff contacted parents of children screened positive at follow-up interviews for further clinical evaluation. Over 30 children who were positive at the first screen stage, but not at the follow up interview, were also additionally randomly invited for further clinical evaluations, with 15 such children completing evaluations. Clinical evaluations were conducted by the first author trained in the use of the ADOS. The final diagnosis of ASD integrated all available information and used the best estimate clinical judgment to evaluate the presence of DSM-5 based criteria (APA, 2013). For children who did not meet ASD criteria, attribution of language-based learning disorder, global developmental delay (without ASD), and other disorders were made as clinically appropriate. Written reports of all evaluations were shared with the parents and the family practitioners. For those with diagnosis of ASD, the two relevant Istanbul Directorates of Public Health, and of National Education, further helped parents to obtain treatment as usual.
Results
There were a total of 298 participating practitioners in the FHCs who received training in the use of M-CHAT-R/F. Majority of the children were M-CHAT-R/F screen negative (90.2%, n=6054) (total score 0–2, low risk). The rate of screen-positive children was 9.8% (95% CI: 9.1%−10.5%); 89.7% (n=590) of screen-positive children had 3–7 total score (moderate-risk), and 10.3% had 8–20 total score (n=68, high-risk). A total of 555 follow up interviews were conducted. Of the initial screen-positive children, 334 (60.2%: 95% CI: 56.2%−64.4%) were not noted to be positive after the follow-up interview assessments. Of the 555 children, 221 (39.8%) were positive after follow-up interview. Among children positive after follow-up interview, 173 (78.7%) had 3–7 total score (moderate-risk), and the remaining 48 children had 8–20 total score (high-risk).
The outcome of follow up interview was significantly different between children who had medium-risk or high-risk (x2(df:2) 262,4; p<.001). The follow-up interview was positive in 33.2% of children with medium-risk (165/497), and 96.6% (56/58) of children with high-risk. The outcome of examination was also different between children who were at medium (n=173) or high risk (n=48) at follow up interview (x2(df:1) 22,1; p<.001). The rate of children diagnosed with ASD among those who completed clinical evaluation was 22.1%, and among those with medium-risk after follow-up interview was 18.5% (32/173). The rate of ASD diagnosis (25/48) was significantly higher among children who were in the high-risk after follow up interview 52.1% (25/48), compared to 61.5% among children who completed clinical evaluation.
The Cronbach’s α coefficient was used as a measure of internal consistency of the M-CHAT-R/F at the global level evaluating its unidimensionality. Nonetheless, M-CHAT-R/F is not a unitary ASD scale as it includes non-ASD foil questions, e.g., motor items “does your child like climbing on things?” (item 4), “does your child walk?” (item 13). The Cronbach α for all the M-CHAT-R/F items was 0.67; this is consistent with the subthreshold value of 0.63 reported by Robins et al (2014). We accompanied the Cronbach α measure with the McDonald omega (ω) coefficient (McDonald 1999), also known as Jöreskog Rho (Stone, Janssens, Vermulst, Van Der Maten, Engels & Otten, 2015), as an alternative means for calculating reliability (Jöreskog, 1971. McDonald ω has the advantage of taking into account the strength of association between item and construct and item-specific measurement error. The McDonald ω for the M-CHAT-R/F was 0.73. When the 2-stage follow up screen was examined, the overall internal consistency values for M-CHAT-R/F were higher (Cronbach’s α = 0.82 and McDonald’s ω = 0.83). This is again consistent with the above-threshold Cronbach α (0.79) reported by Robins et al (2014) for the 2-stage screen.
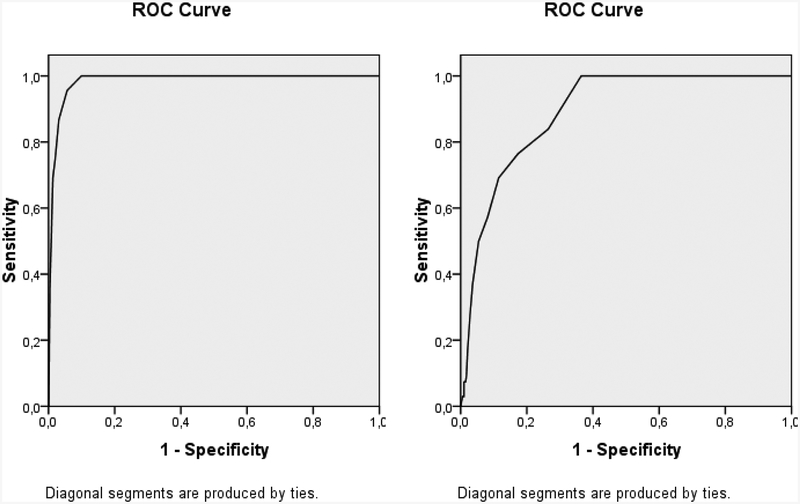
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for M-CHAT-R/F were 1.0, 0.91, 0.086, and 1.0, respectively (Table 1). The area under the curve in terms of the Receiver Operant Characteristic (ROC) was 0.985. The ROC analysis indicated that optimal cut-off score was 3. Sensitivity, specificity, positive predictive value, and negative predictive value for follow-up interview questions were 1.0, 0.67, 0.26, and 1.0, respectively (Table 1, Figure 2). Nevertheless, it must be noted that true negativity data included only 15 children, and therefore these results must be interpreted with caution.
Table 1:
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of M-CHAT-R and Follow-up Interview in the present sample. *Based on 15 subjects.
| Screen Positive | Screen Negative | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| M-CHAT-R | ||||||
| ASD+ | 57 | 0* | 57/57=1 | 6054/6655=0.91 | 57/658=0.086 | 6054/6054=1 |
| ASD − | 601 | 6054 | ||||
| Follow-up Interview | ||||||
| ASD+ | 57 | 0* | 57/57=1 | 334/498=0.67 | 57/221=0.26 | 334/334=1 |
| ASD − | 164 | 334 |
Figure 2:
ROC curves for M-CHAT-R (right) and follow-up interviews (left). Areas under the curve are.985 and .892, respectively. ROC: receiver operational characteristic.
After all clinical evaluations, a total of 57 (1 in 117) children were diagnosed with ASD (0.8%; 95% CI: 0.063%−1.05%).
Barriers for Screening:
Effects of maternal education and household income on screening performance and clinical examination were evaluated (Tables 2 and 3). Positive first screen-positive rates were higher among children of parents with lower education and lower socioeconomic status. The results of positive rates after follow-up interview, and subsequent lack of attendance to follow-up interview and clinical evaluation, were not associated with maternal education and family income.
Table 2:
Maternal education, screening results and attendance at follow-up. a: x2: 48.9 (df:10): p<.001. b: x2: 2.6 (df:10): p>.90. c: x2: 3.4 (df:5): p>.64. d: x2: 5.2 (df:5): p>.35.
| Maternal Education | ||||||
|---|---|---|---|---|---|---|
| None (n:346, 5.2%) |
Primary (n:1907, 28,4%) |
Secondary (n:1588, 23,7%) |
High-School (n:1724, 25.7%) |
College (n:1059, 15.8%) |
Higher (n:87, 1,3%) |
|
| MCHAT R Screen Resulta | ||||||
| Low Risk | 82,9% | 88,4% | 90,3% | 91,9% | 92,4% | 96,6% |
| Moderate Risk | 15,9% | 10,3% | 8,9% | 6,9% | 6,9% | 3,4% |
| High Risk | 1,2% | 1,3% | ,8% | 1,2% | ,7% | ,0% |
| MCHAT R Follow up Screen Resultb | ||||||
| Low Risk | 66,0% | 58,7% | 60,7% | 58,5% | 62,5% | 66,7% |
| Moderate Risk | 30,0% | 31,5% | 31,1% | 32,2% | 29,7% | 33,3% |
| High Risk | 4,0% | 9,8% | 8,1% | 9,3% | 7,8% | ,0% |
| Attendance to Follow-up Screen and Clinical Evaluation | ||||||
| Not attending follow-up screenc | 15,3% | 17,1% | 12,3% | 15,1% | 20,0% | |
| Not attending clinical evaluationd | 29,4% | 35,5% | 39,6% | 24,0% | 20,8% | |
Table 3:
Economic status, screening results and attendance at follow-up a: x2: 5.1 (df:2): p:0.08. b: x2: 2.1 (df:2): p>.35. c: x2: 32.3 (df:4): p<.001. d: x2: 5.1 (df:4): p>.27.
| Economic Status | ||||
|---|---|---|---|---|
| Low | Middle | High | ||
| Attendance | Not attending follow-up screena | 18,9% | 12,8% | 20,8% |
| Not attending clinical evaluationb | 37,7% | 27,9% | 35,3% | |
| First Screenc | Low Risk | 87,0% | 91,2% | 92,2% |
Risk groups in the first and follow-up interview was also examined. The parents of children in the medium-risk group in the first screen were as likely to attend the follow up interview when compared with the high-risk group (15.8 % vs. 14.7%; x2: 0.5, Fisher’s exact test: NS). However, parents of children in the medium-risk group in the follow up interview were more likely not to attend the clinical evaluation (35.3% vs. 17.0%; x2: 5.7, Fisher’s exact test: 0.02).
Discussion
In this study we evaluated the feasibility of M-CHAT-R/F in an urban low-risk sample of young children living in the Istanbul Metropolitan Municipality. Our results showed that almost 1 in 10 (9.8%) of low-risk children, 16 to 36 months of age, were positive at the initial screen. The follow-up interviews were positive for 3.3% of the total sample, and for 39.7% of the children who were positive at the initial screen. Although the ASD prevalence for this community-based sample of children attending participating FHCs is not an epidemiologically representative figure, the proportion of children with ASD, i.e., 1 in 117 (0.8%), nevertheless, the figure reflects a significant finding in a low and middle income country low risk community setting. Although the sensitivity and specificity were high, the PPV was low, particularly for the initial screening. Therefore, the follow-up interview represents a methodologically, as well as ethically, essential step to decrease the false positive screening outcomes.
The results of the present study are similar to those reported by other international groups that evaluated M-CHAT as a valid screening tool (Baduel et al. 2017; Brennan et al. 2016; Kamio et al. 2015; Seung et al. 2015). The results of the present study are also highly similar to those reported by Robins and associates (2014), that underscore that M-CHAT-R/F screening procedures, performed similarly, among cross-national settings lead to comparable findings, as between the US and Turkish samples. It is of interest that the positive first-screen rates have varied widely between studies, e.g., from 26.6% (Seung et al. 2015) to 1.4% (Srisinghasongkram et al. 2016). The positive follow-up rates were also usually lower in studies with higher positive initial screen rates.
Children who scored higher than 7 (8–20) on the M-CHAT-R/F initial screen were 96.6% positive in the follow-up interview. This suggests that for these high-risk children, especially in low resource settings, the follow-up interview may not be as informative, and these children may be referred directly to clinical evaluation. This finding is also consistent with that of Robins and colleagues (2014).
Our results showed that parents with lower education and lower economic status had inflated positive first screen rates. This was consistent with the results of Khowaja and associates (2015). On the other hand, positive first-screen rates at the follow-up interview, and among those not attending to follow-up interview, as well as clinical evaluation, were not associated with maternal education and family income, suggesting that inflated first-screen positivity might be due to poor comprehension of M-CHAT-R items or due to reduced knowledge of child development (Reich, 2005). This result, as suggested before by Khowaja and colleagues (2015), indicated that follow-up interview was essential to decrease false positive screen rate. A more detailed approach at follow-up interview might be better understood by parents. The parents of children in the medium-risk group in the follow-up interview, were more likely not to attend the clinical evaluation. The results of the first screen are also consistent with the Rosenthal effect whereby target expectations by researchers may affect the parents’ screen performance based on their level of education and economic status (Rosenthal et al. 1992).
Using the values obtained from the present study, we can provide a feasibility analysis of a future ASD screening using the M-CHAT-R/F in Istanbul. Given the TUIK data of about 289,000 children for each of the 12- to 48-month age group, the expected yield will be about 28,000 children screened positive on M-CHAT-R/F who will need follow up interview, and 11,000 children who will need clinical evaluation. There will be about 2300 children in each year group who will be diagnosed with ASD. Of further import will be identification of children with other neurodevelopmental and co-ouccring mental health problems that will require attention and referral for appropriate services (Munir 2016). The present study therefore provides an important opportunity to estimate the scope of the public health problem and points of allocation of requisite resources both in terms of early treatment efforts and early intensive educational interventions. There is clealry need for coordination and leveraging of services between health, education and social service sectors (Munir et al. 2016).
Limitations
The results of this study needs to be interpreted with caution given a number of limitations. It must be kept in mind that in our study false negative rate, sensitivity and NPV values were dependent on a very small sample, and the real values at the wider population level will be different. Any judgment about prevalence estimate of ASD must also be evaluated with caution, since this study did not aim to detect prevalence of ASD in a representative sample, but to evaluate M-CHAT-R/F as a screening tool in Turkey.
Conclusions
As reported by the US Preventive Services Task Force (Silverstein and Radesky 2016), both the heterogeneity of ASD, as well as the disparities in screening and service outreach, make the process of ASD screening highly challenging. The Task Force therefore suggested that, as in the case of population screening for major depression, screening of a disorder may not be advisable when systems for accurate diagnosis, effective treatment, and follow-up are limited or absent. On the other hand, this does not necessarily mean that these services are not urgently wanting. Indeed, research related to ASD, even in many low and middle income country settings is leading to important advances in addressing childhood neurodevelopmental disorders across the board. In this regard, universal screening of ASD in low and middle income countries is an important advance for clinical as well as research capacity development, enhancement of family health centers, combined with efforts to increase parental education and engagement about early child development, and implementation of steps necessary to integrate early childhood development and educational services (Munir et al. 2016)
Supplementary Material
Acknowledgements:
Drs. Ozgur Oner and Kerim Munir were supported, in part, by Fogarty International Center/NIMH Grant (D43TW009680) at Boston Children’s Hospital
Funding: This study was funded by İstanbul Kalkinma Ajansi (ISTKA).
Footnotes
Conflict of Interest: Ozgur Oner declares that he has no conflict of interest. Kerim Munir declares that he has no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Ozgur Oner, Bahcesehir University School of Medicine, Department of Child Psychiatry, Boston Children’s Hospital, MA.
Kerim M. Munir, Harvard Medical School, Boston Children’s Hospital, MA.
References
- Al-Qabandi M, Gorter JW, & Rosenbaum P (2011). Early Autism Detection: Are We Ready for Routine Screening? Pediatrics, 128(1), e211–7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Association. [Google Scholar]
- Anlar B, Bayoglu BU, & Yalaz K (2009). Denver II Screening Test: Standardization and Adaptation for Turkish Children.
- Baduel S, Guillon Q, Afzali MH, Foudon N, Kruck J, & Rogé B (2017). The French Version of the Modified-Checklist for Autism in Toddlers (M-CHAT): A Validation Study on a French Sample of 24 Month-Old Children. Journal of Autism and Developmental Disorders, 47(2), 297–304. [DOI] [PubMed] [Google Scholar]
- Brennan L, Fein D, Como A, Rathwell IC, & Chen CM (2016). Use of the Modified Checklist for Autism, Revised with Follow Up-Albanian to Screen for ASD in Albania. Journal of Autism and Developmental Disorders, 46(11), 3392–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Braun KVN, Baio J, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee LC, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, & Yeargin-Allsopp M (2012). Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, MMWR Surveillance Summaries (2018), 65(13), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Bilder DA, & Zahorodny W, et al. (2016). Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the Autism and Developmental Disabilities Monitoring Network. Journal of Developmental and Behavioral Pediatrics, 37, 1–8. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Maenner MJ, Bilder DA, & Constantino JN, et al. (2019). Prevalence and characteristics of autism spectrum disorder among children aged 4-years- Early Autism and Developmental Disabilities Monitoring Network, Seven Sites, United States, 2010, 2012 and 2014. MMWR Surveillance Summaries, 68, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council on Children with Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. (2006). Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics, 118(1), 405–20. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, & Varley J (2010). Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics, 125(1), e17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, & Fombonne E (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5(3), 160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenikilé TS, Ellerbeck K, Filippi MK, & Daley CM (2015). Barriers to autism screening in family medicine practice: a qualitative study. Primary Health Care Research and Development, 16(4), 356–66. [DOI] [PubMed] [Google Scholar]
- Frankenburg WK, & Dodds JB (1990). Denver II Screening Manual. Denver Developmental Materials, Inc; Denver. [Google Scholar]
- García-Primo P, Hellendoorn A, Charman T, Roeyers H, Dereu M, Roge B, Baduel S, Muratori F, Narzisi A, Van Daalen E, Moilanen I, de la Paz MP, & Canal-Bedia R (2014). Screening for autism spectrum disorders: state of the art in Europe. European Child and Adolescent Psychiatry, 23(11), 1005–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Luo M, Wang X, Huang S, Meng Z, Shao J, Zhang X, Shao Z, Wu J, Robins DL, & Jing J (2019). Reliability and Validity of the Chinese Version of Modified Checklist for Autism in Toddlers, Revised, with Follow-Up (M-CHAT-R/F). Journal of Autism and Developmental Disorders, 49(1),185–196. [DOI] [PubMed] [Google Scholar]
- Gupta VB, Hyman SL, Johnson CP, Bryant J, Byers B, Kallen R, Levy SE, Myers SM, Rosenblatt AI, & Yeargin-Allsopp M (2007). Identifying Children With Autism Early? Pediatrics, 119(1), 152–3. [DOI] [PubMed] [Google Scholar]
- Howlin P, Magiati I, & Charman T (2009). Systematic review of early intensive behavioral interventions for children with autism. American Journal on Intellectual and Developmental Disabilities, 114(1), 23–41. [DOI] [PubMed] [Google Scholar]
- Johnson CP, & Myers SM (2007). American Academy of Pediatrics Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics, 120, 1183–215. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG (1971). Statistical analysis of sets of congeneric tests. Psychometrika, 36, 109–133. [Google Scholar]
- Kamio Y, Haraguchi H, Stickley A, Ogino K, Ishitobi M, & Takahashi H (2015). Brief Report: Best Discriminators for Identifying Children with Autism Spectrum Disorder at an 18-Month Health Check-Up in Japan. Journal of Autism and Developmental Disorders, 45(12), 4147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khowaja MK, Hazzard AP, & Robins DL (2015). Sociodemographic Barriers to Early Detection of Autism: Screening and Evaluation Using the M-CHAT, M-CHAT-R, and Follow-Up. Journal of Autism and Developmental Disorders, 45(6), 1797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JM, Ventola PE, Pandey J, Verbalis AD, Barton M, Hodgson S, Green J, Dumont-Mathieu T, Robins DL, & Fein D (2008). Diagnostic stability in very young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 38(4), 606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (Part I): Modules 1–4 Torrance, CA; Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (Part II): Toddler Module Torrance, CA; Western Psychological Services. [Google Scholar]
- McDonald RP (1999). Test theory: A unified treatment. Mahwah, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Munir KM, Lavelle TA, Helm DT, Thompson D, Presstt J, Azeem MW (2016). Autism: Global Framework for Action. Doha, Qatar: World Innovation Summit in Health. [Google Scholar]
- Munir K (2016). Co-occurrence of mental disorders in children and adolescents with intellectual disability/intellectual developmental disorder. Current Opinion in Psychiatry. 29(2), 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Women’s and Children’s Health (UK). (2011). Autism: Recognition, Referral and Diagnosis of Children and Young People on the Autism Spectrum. London: RCOG Press. [PubMed] [Google Scholar]
- Reich S (2005). What do mothers know? Maternal knowledge of child development. Infant Mental Health Journal, 26(2), 143–156. [DOI] [PubMed] [Google Scholar]
- Robins DL, Casagrande K, Barton ML, Chen C, Dumont- Mathieu T, & Fein D (2014). Validation of the Modified Checklist for Autism in Toddlers-Revised with Follow-Up (MCHAT- R/F), Pediatrics, 133(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton ML, & Green JA (2001). The Modified Checklist for Autism in Toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. Journal of Autism and Developmental Disorders, 31(2), 131–44. [DOI] [PubMed] [Google Scholar]
- Robins DL, Fein D, & Barton M (2009). The Modified Checklist for Autism in Toddlers, Revised with Follow-Up (M-CHAT-R/F). [DOI] [PMC free article] [PubMed]
- Rosenthal Robert, Jacobson Lenore (1992). Pygmalion in the classroom: Teacher expectation and pupils’ intellectual development. Bancyfelin, Carmarthen, Wales: Crown House Pub; ISBN 978–1904424062]. [Google Scholar]
- Seung H, Ji J, Kim SJ, Sung I, Youn YA, Hong G, Lee H, Lee YH, Lee H, & Youm HK (2015). Examination of the Korean Modified Checklist of Autism in Toddlers: Item Response Theory. Journal of Autism and Developmental Disorders, 45(9), 2744–57. [DOI] [PubMed] [Google Scholar]
- Silverstein M, & Radesky J (2016). Embrace the Complexity: The US Preventive Services Task Force Recommendation on Screening for Autism Spectrum Disorder. JAMA, 315(7), 661–2. [DOI] [PubMed] [Google Scholar]
- Srisinghasongkram P, Pruksananonda C, & Chonchaiya W (2016). Two-Step Screening of the Modified Checklist for Autism in Toddlers in Thai Children with Language Delay and Typically Developing Children. Journal of Autism and Developmental Disorders, 46(10), 3317–29. [DOI] [PubMed] [Google Scholar]
- Stenberg N, Bresnahan M, Gunnes N, Hirtz D, Hornig M, Lie KK, Lipkin WI, Lord C, Magnus P, Reichborn-Kjennerud T, Schjølberg S, Surén P, Susser E, Svendsen BK, Von Tetzchner S, Oyen AS, & Stoltenberg C (2014). Identifying children with autism spectrum disorder at 18 months in a general population sample. Paediatric and Perinatal Epidemiology, 28(3), 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Janssens JMAM, Vermulst AA, Van Der Maten M, Engels RCME, Otten R (2015). The Strengths and Difficulties Questionnaire: psychometric properties of the parents and tacher version in children aged 4–7. BMC Psychol, 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Chawarska K, Dawson G, Georgiades S, Landa R, Lord C, Messinger DS, Thurm A, & Halladay A (2016). Prospective Longitudinal Studies of Infant Siblings of Children with Autism: Lessons Learned and Future Directions. Journal of the American Academy of Child and Adolescent Psychiatry, 55(3), 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T (2013). Autism research and services for young children: History, progress and challenges. Journal of Applied Research in Intellectual Disabilities, 26(2), 81–107. [DOI] [PubMed] [Google Scholar]
- Tsai JM, Lu L, Jeng SF, Cheong PL, Gau SS, Huang YH, & Wu YT (2019). Validation of the modified checklist for autism in toddlers, revised with follow-up in Taiwanese toddlers. Research in Developmental Disabilities, 85, 205–216. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Fein D, Pierce K, Buie T, Davis PA, Newschaffer C, Robins DL, Wetherby A, Choueiri R, Kasari C, Stone WL, Yirmiya N, Estes A, Hansen RL, McPartland JC, Natowicz MR, Carter A, Granpeesheh D, Mailloux Z, Smith Roley S, & Wagner S (2015). Early Screening of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics, 136 Suppl 1, 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, & Garon N (2013). Early identification of autism spectrum disorders. Behavioural Brain Research, 251, 133–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.