Abstract
Although screening is effective in reducing incidence, mortality, and costs of treating colorectal cancer (CRC), it remains underutilized, in part due to limited insurance access. We used microsimulation to estimate the health and financial effects of insurance expansion and reduction scenarios in North Carolina (NC). We simulated the full lifetime of a simulated population of 3,298,265 residents age-eligible for CRC screening (ages 50–75) during a 5-year period starting January 1, 2018, including polyp incidence and progression and CRC screening, diagnosis, treatment, and mortality. Insurance scenarios included: status quo, which in NC includes access to the Health Insurance Exchange (HIE) under the Affordable Care Act (ACA); no ACA; NC Medicaid expansion, and Medicare-for-all. The insurance expansion scenarios would increase percent up-to-date with screening by 0.3 and 7.1 percentage points for Medicaid expansion and Medicare-for-all, respectively, while insurance reduction would reduce percent up-to-date by 1.1 percentage points, compared to the status quo (51.7% up-to-date), at the end of the 5-year period. Throughout these individuals' lifetimes, this change in CRC screening/testing results in an estimated 498 CRC cases averted with Medicaid expansion and 6031 averted with Medicare-for-all, and an additional 1782 cases if health insurance gains associated with ACA are lost. Estimated cost savings – balancing increased CRC screening/testing costs against decreased cancer treatment costs – are approximately $30M and $970M for Medicaid expansion and Medicare-for-all scenarios, respectively, compared to status quo. Insurance expansion is likely to improve CRC screening both overall and in underserved populations while saving money, with the largest savings realized by Medicare.
1. Introduction
Colorectal cancer (CRC) has the fourth highest incidence and is the second leading cause of cancer-related death in the United States (American Cancer Society, 2017). Given the prevalence of this disease, the economic burden associated with CRC is high and anticipated to increase (Mariotto et at, 2011). Fortunately, routine CRC screening – for which multiple modalities are recommended – is effective (U.S. Preventive Services Task Force et at, 2016). Two modalities are widely used. Of these, colonoscopy is more accurate (higher sensitivity and specificity) in detecting CRC and allows for immediate removal of any pre-cancerous polyps found, but includes the potential risks and costs associated with an invasive exam. Fecal testing, such as fecal immunochemical testing (FIT), can also detect CRC and can be completed at home or in a clinical office setting. However, it must be followed by a colonoscopy in the event of an abnormal result to confirm CRC and remove any polyps found. Screening is recommended for average-risk individuals 50–75 years of age – either a colonoscopy every ten years or fecal testing annually (U.S. Preventive Services Task Force et al., 2016).
Despite the ability to prevent most CRC, screening rates nationally remain relatively low, with approximately two-thirds (67.3%) of age-eligible individuals self-reporting being up-to-date with recommendations in 2016 (Joseph et al., 2018). This rate falls below the Healthy People 2020 goal of 70.5% (Office of Disease Prevention and Health Promotion, 2019), and well below the National Colorectal Cancer Roundtable's target of 80% by 2018 (National Colorectal Cancer Roundtable, 2019). The gap between target and actual rates is likely even greater, however, as self-reported screening has been shown to overestimate up-to-datedness (Pierannunzi et al., 2013) – by 12 to 13 percentage points in North Carolina (NC) (Hassmiller Lich et al., 2017).
There have been many efforts to improve CRC screening rates through implementation of single and multi-pronged evidence-based interventions (EBIs) (Sabatino et al., 2012; Dougherty et al., 2018; Davis et al., 2018). Substantial state-level financial investments have been made to improve screening rates in diverse subpopulations, including research-supported interventions and technical assistance projects funded by the Centers for Disease Control and Prevention (CDC) (Coughlin et al., 2006; Joseph et al., 2011). In a prior analysis, we found that investing $1–4 million on top of the cost of care in NC to target CRC screening non-compliance using current EBIs – without addressing access to care more broadly – would have limited effects on improving CRC screening at the population level (Hassmiller Lich et al., 2017).
Access to health insurance is an important barrier to CRC screening, as evidenced by the substantial gap in the probability of getting screened between those with and without insurance (National Center for Health Statistics, 2015). Among adults ages 50 to 64 years, those with private insurance were 2.5 times more likely to be screened for CRC compared to the uninsured in 2015 (National Center for Health Statistics, 2015). Low-income Medicaid enrollees, who are known to have relatively low screening rates, also have higher rates than the uninsured (Medicaid and CHIP Payment and Access Commission, 2016; Davis et al., 2017). Improved access to insurance will likely reduce current barriers and disincentives to getting preventive care, but insurance expansion remains actively under debate at the state and national levels.
In this paper, we build on prior efforts to estimate the population-level impact of EBIs to estimate the impact of insurance expansion or reduction scenarios on CRC screening, incidence, mortality, and related costs (e.g., CRC screening and treatment) among NC residents age-eligible for screening. As before (Hassmiller Lich et al., 2017), we use microsimulation – a type of modeling that simulates individuals with diverse characteristics as they age and change over time, tracking both individual and population-level outcomes (Wheeler et al., 2018). Specifically, we use microsimulation to compare CRC outcomes and cost implications under five health insurance scenarios: status quo, which, in NC, reflects some increase in insurance when the Health Insurance Exchange (HIE) was implemented under the Affordable Care Act (ACA); reversal of the ACA; expansion of the state's Medicaid program; and two Medicare-for-all scenarios (with more and less conservative screening uptake). The results provide insight into how changing access to insurance coverage may help to narrow (or widen) the gap between established targets and current levels of screening, both overall and among subpopulations experiencing disparities.
2. Methods
We used an individual-based microsimulation model that integrates best available data to simulate lifetime CRC outcomes under each of the five insurance scenarios for the full population of 3,298,265 NC residents age-eligible for CRC screening over a five-year study period (January 1, 2018-December 31, 2022). Key model parameter values are reported in Table 1. This study was approved by the University of North Carolina Institutional Review Board.
Table 1.
Model parameter values.
Both existing literature and expert opinion were used to inform the selection of parameter values.
2.1. Population simulated
We used a synthetic population, which is a realistic but not real population of simulated individuals whose characteristics are based on those of the state population at a single point in time. The simulated population was created using the Topologically Integrated Geographic Encoding and Referencing (TIGER), Summary File 3 (SF3), and American Community Survey Public Use Microdata Sample (PUMS) datasets from the U.S. Census Bureau from 2007 to 2011, to represent the NC population in 2009 (Wheaton et al., 2009; RTI International, 2019; The University of North Carolina at Chapel Hill, 2019). In this analysis, we restrict focus to the simulated individuals in the state who, if they did not die before January 1, 2018, would be age-eligible for screening at some time during the five-year study period.
Time-invariant characteristics of simulated individuals are based on the synthetic population and include race, gender, and county of residence (we assume no migration for simplicity). Time-variant characteristics are changed within the simulation model and include age, income, insurance status, preferred routine screening modality, and polyp/cancer status. These characteristics may affect an individual's screening and/or cancer risk. Aging, non-CRC mortality, initial insurance (i.e., in 2009), and status-quo screening are based on our previous work and described elsewhere (Hassmiller Lich et al., 2017).
For this analysis, we updated how we simulate individuals' income, insurance, and polyp/CRC status over time. To account for income change, each individual's income is updated from the 2009 simulated population value using multipliers based on U.S. Census Bureau data on per-capita income, stratified by race (white, black, other), sex (male, female), and age category (35–44, 45–54, 55–64, 65–74, 75+) (United States Census Bureau, 2019). From these data, we obtained multipliers and used them to convert each individual's income value in 2009 to his or her expected income value in each year thereafter. Because 2017 was the most recent year in which mean per-capita income was available at the time of this study, the annual rates of change beyond 2017 were obtained through extrapolation. Insurance, initially based on Census data, changes over simulated individuals' life course based on simple rules (Hassmiller Lich et al., 2017), which have been updated to reflect insurance scenarios and described in more detail below.
2.2. Polyp incidence and progression
Simulated individuals have a chance of developing one or more polyps during their lifetime. Polyps can grow from birth, with incidence rates changing across the life course (Lansdorp-Vogelaar et al., 2009), and are characterized as small, medium, or large. All polyps start out small (<6 mm), but can transition to medium (6–9 mm) or large (≥10mm) over time (Subramanian et al., 2009). Although possible to detect, small polyps do not pose immediate risk of cancer. Medium and large polyps can transition into and across pre-clinical CRC stages, or become clinically relevant when diagnosed based on symptoms or through screening/testing. Each time-to-transition is modeled according to an exponential distribution, with the average number of years for a small polyp to transition to a medium polyp and for a medium polyp to transition to a large polyp set to 15 years and 5 years, respectively, based on prior research (Subramanian et al., 2009).
2.3. Status quo CRC screening and diagnostic testing
In the status quo scenario, all simulated individuals may receive routine CRC screening when they become age-eligible at 50 years of age, with some colonoscopies happening as early as 45 years of age; this matches observed variation in age at first screen in claims data and accounts for differences in screening guidelines, such as an earlier recommended starting age for African Americans compared to other groups (Williams et al., 2016; Rex et al., 2017). Receipt and modality of screening/testing are simulated with randomness using predicted probabilities estimated from multi-variable statistical models that are a function of both individual characteristics (insurance, sex, race/ethnicity, county of residence, and distance between zip code centroid and nearest endoscopy facility) and characteristics of individuals' county of residence (population-adjusted number of medical generalists, percentage of residents below federal poverty level) (Hassmiller Lich et al., 2017). Individuals are considered up-to-date and not offered screening if they completed a colonoscopy within the past 10 years or a fecal test in the past 12 months. We do not simulate any other CRC screening tests, as they are observed infrequently, comprising <4% of all CRC tests in our underlying claims analysis (Wheeler et al., 2017). See Table 1 for assumptions about screen/test accuracy.
We simulate CRC screening for all individuals as soon as they become age-eligible, regardless of whether this occurred prior to or during the study period, to fully capture all screening, polyp identification/removal, and cancer outcomes in their full lifetimes. Predicted probabilities of screening estimated with the statistical models are adjusted over time to capture underlying temporal trends in clinical practice, norms, and other relevant patient-level factors (Hassmiller Lich et al., 2017). When polyp(s) are found during colonoscopy, we assume they are removed and analyzed, and assign costs for polypectomy and pathology. For those who screen by fecal test and have a positive (abnormal) result, they are offered a diagnostic colonoscopy, consistent with national guidelines (U.S. Preventive Services Task Force et al., 2016). Assuming imperfect follow-up, 85% of these individuals will receive diagnostic colonoscopies (Bogie & Sanduleanu, 2016). Individuals undergoing a colonoscopy have a small chance of bleeding or perforation, both of which are assumed to be treated (Lin et al., 2016).
2.4. CRC detection, treatment, and mortality
For individuals with pre-clinical CRC whose diagnostic test is inaccurately negative or those who do not have a diagnostic test, the cancerous polyp remains clinically undetected and continues to progress until it is diagnosed at some future point or the individual dies of causes other than CRC. We assume that all CRC is diagnosed before patients die from cancer. Patients who are diagnosed with CRC are treated, and associated costs (adjusted by stage and age) and survival (adjusted by stage, age, race, and sex) are tracked. CRC stage is based on the extent of malignancy, and modeled according to the definitions established by the American Joint Committee on Cancer (AJCC) (Edge & Compton, 2010). Mortality is determined as the minimum of their natural life expectancy (Arias, 2004) and cancer mortality risk (National Cancer Institute, 2019). Rates of transition to and between pre-clinical CRC stages are calibrated using CRC incidence data from the NC Central Cancer Registry for the years 2008 to 2014 among individuals 37–92 years of age (NC State Center for Health Statistics, 2019).
2.5. CRC screening, surveillance, and treatment costs
We estimated the costs of CRC-related preventive care and treatment for each insurance scenario from the perspective of individual payers. The total cost of screening/testing for each scenario includes the costs of routine screening by colonoscopy or fecal test, diagnostic colonoscopies in the event of abnormal fecal test results or polyps found on routine colonoscopy, surveillance examinations, the removal of polyps during colonoscopy, pathology, treatment for complications during colonoscopy, and cancer treatment. We identified the Current Procedural Terminology (CPT) codes for these procedures using the 2018 NC Medicare Part B Fee Schedule (Centers for Medicare & Medicaid Services, 2018). We assigned the associated costs directly to the Medicare payer perspective, and then used these costs to estimate costs for other payers based on assumptions informed by expert opinion. Specifically, we assumed the costs for privately insured and Medicaid enrollees are, on average, three times the Medicare costs and 95% of Medicare costs, respectively. We assumed the costs for patients dually enrolled in Medicare/Medicaid are the same as for Medicaid alone. For the uninsured, we accounted for charity care cost estimates, set to 40% of Medicare reimbursement, which would be borne by the facility/provider that performs the screening or provides cancer treatment while the patient is uninsured. Costs assigned to cancer care, including treatment and surveillance, are based on prior research (Yabroff et al., 2008; Zauber et al., 2007). We converted all costs to 2018 U.S. dollars, with future dollars discounted at a rate of 3% per year.
2.6. Model calibration
We calibrated model parameters to match two primary data points: 1) existing survey-based estimates of the percentage of age-eligible individuals up-to-date on CRC screening adjusted to correct for self-report bias (Joseph et al., 2018; Pierannunzi et al., 2013; Hassmiller Lich et al., 2017), and 2) the number of incident CRC cases, including the distribution of stage at diagnosis, from the NC Central Cancer Registry (NC State Center for Health Statistics, 2019). See model documentation for more detail (The University of North Carolina at Chapel Hill, 2019).
2.7. Insurance scenarios
We estimate and compare the impact of five health insurance change scenarios, described in Table 2, on CRC screening and outcomes. Briefly, these scenarios are: status quo (ACA), no ACA, Medicaid expansion, conservative Medicare-for-all, and enhanced Medicare-for-all. For the status quo scenario, we update 2009 insurance based on NCspecific insurance data between 2013 and 2016 to capture insurance acquisition associated with the ACA. Specifically, we estimated the likelihood that each uninsured simulated individual would gain insurance in 2014 and 2015, with insurance gains based on individuals' household income, age, gender, marital status, and race. Insurance gains level out between 2015 and 2016, so no further increases in insurance are simulated thereafter due to ACA. In the conservative Medicare-for-all scenario, we assume people will screen at rates consistent with current screening among others like themselves (i.e., this is based on income/Medicaid eligibility). In the more optimistic Medicare-for-all scenario, we assume screening rates for all individuals level out at current screening rates among individuals with higher incomes.
Table 2.
Insurance change scenarios that are simulated and compared, starting January 1, 2018 in North Carolina.
| Scenario name | Scenario description |
|---|---|
| ACA (status quo) | This scenario represents NC's actual level of increase in insurance coverage, since NC did not expand eligibility for Medicaid. The ACA/status quo scenario includes the option/incentives for individuals and households without insurance to begin purchasing health insurance plans through the HIE on HealthCare.gov as of January 1, 2014, or to enroll in Medicaid if they were previously eligible. |
| No ACA | This scenario undoes insurance gains through ACA, retaining insurance levels from pre-ACA (in 2013). |
| Medicaid expansion | This scenario expands the state's Medicaid program. The income eligibility limit for Medicaid increases to 138% of the federal poverty level (FPL) for all individuals, regardless of their age, employment status, number of dependents, or disability status – consistent with expanded eligibility among states that have opted to expand Medicaid (The Henry J. Kaiser Family Foundation, 2018). In NC, Medicaid expansion involves an increase in the income eligibility limit for adults with dependents from 43% to 138% of the FPL, and the ability for childless adults who previously could not qualify for Medicaid coverage to be eligible if they meet income criteria (The Henry J. Kaiser Family Foundation, 2018). |
| Conservative Medicare-for-all | This scenario implements a Medicare-for-all program of universal health coverage. Under this type of single-payer system, all individuals would have access to health coverage through the federal government. By simulating this fully inclusive scenario, we estimate the uptake in CRC screening if insurance access were removed as a barrier to preventive healthcare. In this more conservative scenario (compared to the enhanced version below), we assume that screening patterns depend on an individual's income level. Individuals with an income at or below 138% of the FPL screen like those with Medicaid currently, whereas individuals with incomes above the 138% FPL threshold screen at higher rates like those observed among current private insurance enrollees. |
| Enhanced Medicare-for-all | This scenario is a more optimistic version of the Medicare-for-all scenario above. We assume that all individuals screen according to current higher levels observed among those with private insurance – driven, for example, by additional efforts to increase the accessibility and affordability of care for all. |
ACA, Affordable Care Act, CRC, Colorectal Cancer, HIE, Health Insurance Exchange, FPL, Federal Poverty Level, NC, North Carolina.
2.8. Simulation outcomes and analyses
The simulation model was programmed in AnyLogic (version 7.3.6). To ensure an individual's life course is otherwise identical across insurance scenarios, an application of common random numbers is used (Cornejo et al., 2014). We track percent up-to-date overall and within subgroups after the five-year study period (i.e., on December 31, 2022). We also track years up-to-date, CRC incidence (overall and by AJCC stage), CRC mortality, life-years, and CRC costs overall and by payer over the full lifetime of the simulated population. We present results for the status quo scenario, and for other insurance change scenarios compared to this scenario. Five replications were run for this analysis, and average outcomes are presented along with uncertainty intervals, defined here as the replication minimum and maximum, for primary simulation outcomes (percent up-to-date after five years and total CRC cases and total life-years within simulated individuals' lifetimes in each scenario). To assess robustness in estimated relative differences across scenarios, we rank order scenarios based on each primary outcome, within each replication, and average scenario rankings across replications.
Given the large simulated population size, we needed few replications to obtain precise simulation estimates. The use of common random numbers also contributes to the consistent results across replications and the quick stabilization of cross-replication statistics (Cornejo et al., 2014). Adding the fifth replication was sufficient to reach our stopping condition – when adding another replication changed the cross-replication average for each primary simulated outcome by <1%. All statistical analyses were conducted using R Statistical Software (version 3.3.3).
3. Results
Table 3 presents the demographic and insurance mix in the simulated NC population age-eligible for CRC screening (50–75) on December 31, 2013 (just before ACA was implemented), on December 31, 2014 (after one year of ACA) and on December 31, 2017 (just before our study period). As expected, the most noteworthy differences in these snapshots include a decrease in the percent uninsured as a result of insurance reform and aging of the population, occurring in NC much like many other states. We project that a total of 3,298,265 individuals who would have been alive on December 31, 2017 will be between 50 and 75 years of age at some point during the five-year study period.
Table 3.
Demographic characteristics and insurance among North Carolina synthetic population aged 50–75 on December 31st of each year in the ACA scenario.
| Characteristic | 2013 |
2014 |
2017 |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 2,358,433 | 100.0% | 2,473,341 | 100.0% | 2,760,775 | 100.0% |
| Sex | ||||||
| Male | 1,106,271 | 46.9% | 1,159,278 | 46.9% | 1,287,101 | 46.6% |
| Female | 1,252,162 | 53.1% | 1,314,062 | 53.1% | 1,473,674 | 53.4% |
| Race | ||||||
| White | 1,809,927 | 76.7% | 1,894,483 | 76.6% | 2,109,327 | 76.4% |
| Black | 440,935 | 18.7% | 462,598 | 18.7% | 511,133 | 18.5% |
| Other | 107,570 | 4.6% | 116,260 | 4.7% | 140,316 | 5.1% |
| Ethnicity | ||||||
| Hispanic | 70,353 | 3.0% | 77,409 | 3.1% | 101,029 | 3.7% |
| Age | ||||||
| 50–54 | 668,682 | 28.4% | 673,294 | 27.2% | 655,440 | 23.7% |
| 55–59 | 612,039 | 26.0% | 621,640 | 25.1% | 643,098 | 23.3% |
| 60–64 | 556,021 | 23.6% | 556,182 | 22.5% | 577,292 | 20.9% |
| 65+ | 521,690 | 22.1% | 622,225 | 25.2% | 884,947 | 32.1% |
| Type of insurance | ||||||
| Private | 1,337,046 | 56.7% | 1,392,202 | 56.3% | 1,445,415 | 52.4% |
| Medicare | 558,147 | 23.7% | 642,825 | 26.0% | 857,945 | 31.1% |
| Medicaid | 84,383 | 3.6% | 89,823 | 3.6% | 97,515 | 3.5% |
| Dual eligible | 103,823 | 4.4% | 111,800 | 4.5% | 126,958 | 4.6% |
| Uninsured | 275,032 | 11.7% | 236,692 | 9.6% | 232,942 | 8.4% |
Table 4 presents the difference in the percent of age-eligible individuals up-to-date with CRC screening/testing in each insurance scenario as compared to ACA at the end of the study period – both overall and by sociodemographic characteristics. On December 31, 2022, we estimate that 51.7% of the population would be up-to-date with screening under ACA. With no ACA, the percent up-to-date would be 1.1 percentage points lower. In contrast, the insurance expansion scenarios increase the screened population compared to ACA: 0.3 percentage points with Medicaid expansion, 7.1 percentage points for conservative Medicare-for-all, and 8.6 percentage points for enhanced Medicare-for-all. The magnitude of these differences varies by demographic subgroups. For example, among Hispanics, there is an expected increase in up-to-datedness of 1.3 percentage points for Medicaid expansion, 11.6 percentage points for conservative Medicare-for-all, and 14.5 percentage points for enhanced Medicare-for-all, relative to ACA (44.7% up-to-date).
Table 4.
Percent of simulated age-eligible North Carolina population up-to-date with CRC screening/testing on January 1, 2023 under the ACA scenario, and percentage point changes under alternate insurance scenarios.
| Variable | ACA (status quo) | Percentage point change compared with the status quo |
|||
|---|---|---|---|---|---|
| No ACA | Medicaid expansion | Conservative Medicare-for all | Enhanced Medicare-for-all | ||
| Overall | 51.7% | −1.1 | +0.3 | +7.1 | +8.6 |
| (Min,Max)b | (51.7%,51.8%) | (−1.1,−1.0) | (+0.3,+0.3) | (+7.0,+7.1) | (+8.5,+8.6) |
| By sex | |||||
| Male | 50.2% | −0.9 | +0.2 | +6.3 | +8.1 |
| Female | 53.1% | −1.3 | +0.4 | +7.7 | +8.9 |
| By race | |||||
| White | 52.4% | −0.9 | +0.2 | +6.5 | +7.7 |
| Black | 50.3% | −1.5 | +0.5 | +8.3 | +10.9 |
| Other | 48.0% | −2.4 | +0.9 | +11.1 | +13.5 |
| By ethnicity | |||||
| Hispanic | 44.7% | −2.3 | +1.3 | +11.6 | +14.5 |
| By insurance | |||||
| Private | 54.3% | −0.1 | a | NA | NA |
| Medicare | 56.7% | a | a | +2.1 | +3.6 |
| Medicaid | 42.0% | −0.1 | +1.4 | NA | NA |
| Dual | 47.9% | a | +0.4 | NA | NA |
| Uninsured | 18.3% | a | a | NA | NA |
Represents differences that are between −0.1 and 0.1.
Minimum and maximum results (100% uncertainty interval) presented for overall results only.
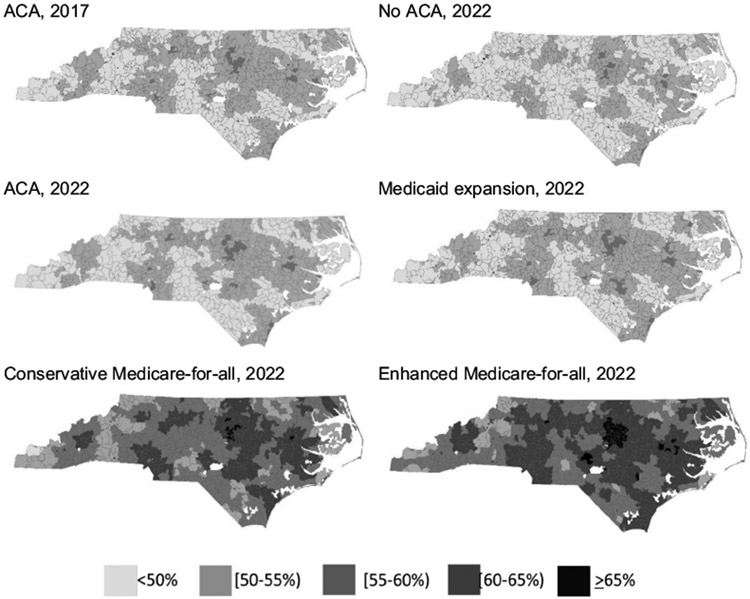
Fig. 1 is comprised of a panel of maps indicating the percent of age-eligible residents in each zip code up-to-date with CRC screening/testing at two time points - December 31, 2017 (ACA) and December 31, 2022 (under each insurance change scenario). No zip codes would reach 70.5% up-to-date targets under any scenario; the zip code with the highest percent up-to-date under enhanced Medicare-for-all is 67.7%.
Fig. 1.
Maps of percent up-to-date with CRC screening/testing by zip code in North Carolina under the ACA (December 31, 2017 before the study period and December 31, 2022 at the end of the study period) and other insurance change scenarios (December 31, 2022 at the end of the study period).
Table 5 presents the difference in lifetime CRC diagnoses and deaths for each scenario compared to ACA between January 1, 2018 when insurance change scenarios were implemented and 2072 when the full simulated population is deceased. During this period, a total of 153,806 incident CRC cases will be diagnosed, of which 33,754 will be Stage 1, 32,603 will be Stage 2, 45,201 will be Stage 3, and 42,248 will be Stage 4. Compared to ACA, the number of CRC cases averted by insurance expansion would be 498 for Medicaid expansion, 6031 for conservative Medicare-for-all, and 7602 for enhanced Medicare-for-all. The no ACA scenario, however, would result in 1782 additional CRC cases relative to ACA. CRC-attributable deaths follow a similar pattern, as shown in Table 5. In addition, insurance expansion is expected to increase both total years up-to-date with CRC screening and total years of life among the simulated population, while insurance reduction would have the opposite effect, compared to ACA (see Supplemental Table 1). For example, the population will gain 5431 life-years with Medicaid expansion, 56,248 life-years with conservative Medicare-for all, and 68,399 life-years with enhanced Medicare-for-all, but lose 14,531 life-years if the ACA is reversed, across the individuals' cumulative lifespans, relative to the ACA. In terms of the total number of years up-to-date with CRC screening compared to the ACA, Medicaid expansion will result in 92,887 more years, conservative Medicare-for-all will result in 1,452,876 more years, and enhanced Medicare-for-all will result in 2,178,523 more years, while the no ACA scenario is expected to have 384,842 fewer years up-to-date.
Table 5.
CRC cases and deaths among the synthetic cohort age-eligible for CRC screening during the study period and throughout their remaining lifetimes under the ACA scenario, and changes under alternate insurance scenarios.a
| ACA (status quo) | Incremental change in CRC cases and deaths, compared with ACA |
||||
|---|---|---|---|---|---|
| No ACA | Medicaid expansion | Conservative Medicare-for-all | Enhanced Medicare-for-all | ||
| Total CRC cases | 153,806 | +1782 | −498 | −6031 | −7602 |
| (Minimum, Maximum)b | (149,266, 162,796) | (−11,538, 12,605) | (−5413, 4229) | (−19,547, 5821) | (−20,138, 4108) |
| CRC cases by stage at diagnosis | |||||
| Stage 1 | 33,754 | +254 | −170 | −1358 | −1428 |
| Stage 2 | 32,603 | +386 | −24 | −1253 | −1449 |
| Stage 3 | 45,201 | +551 | −159 | −1831 | −2358 |
| Stage 4 | 42,248 | +591 | −146 | −1589 | −2366 |
| CRC deaths | 76,588 | +791 | −226 | −2813 | −3732 |
Cumulative CRC cases and deaths are reported through 2072, when the last synthetic individual dies.
Minimum and maximum results (100% uncertainty interval) presented for overall results only.
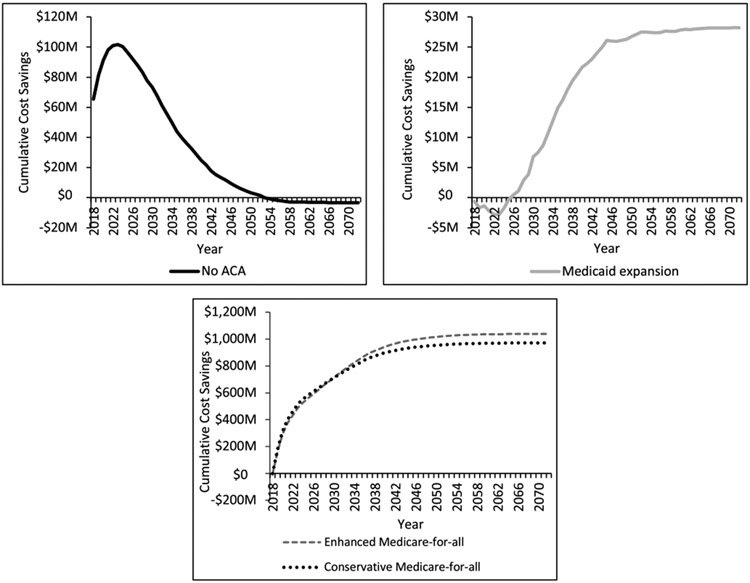
Fig. 2 presents cumulative CRC cost savings for each insurance change scenario compared to ACA in 2018 U.S. dollars, discounted at 3% per year. Compared to ACA, the no ACA scenario is cost saving through 2054, when marginal costs associated with worse CRC outcomes offset earlier gains from reduced screening/testing. This scenario is associated with a cumulative discounted cost increase of $3.2 million. In contrast, cumulative discounted costs under the Medicaid expansion scenario are initially higher than the ACA scenario, breaking even in 2025 and ultimately saving an additional $28.2 million. Both Medicare-for-all scenarios are immediately cost saving, with cumulative discounted cost savings reaching $970.9 and $1037.7 million, respectively, for the conservative and enhanced scenarios compared to ACA.
Fig. 2.
Cumulative cost savings across payers discounted at 3% per year, comparing each insurance change scenario to ACA.
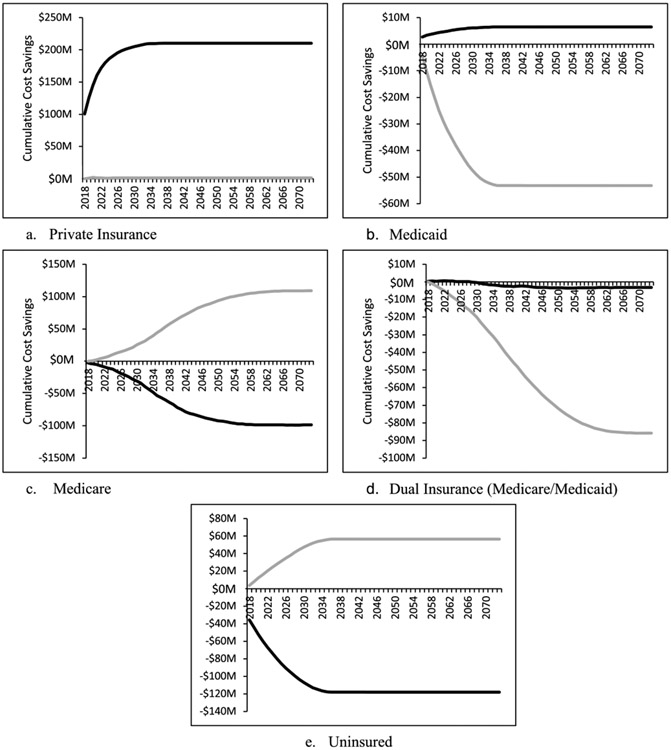
Fig. 3 presents cumulative CRC cost savings for each payer under the no ACA and Medicaid expansion scenarios compared to the ACA scenario in 2018 U.S. dollars, discounted at 3% per year. We do not show payer-specific costs for the Medicare-for-all scenarios, as all costs will be shifted to Medicare under these scenarios. From the perspective of private insurers, the Medicaid expansion scenario would be slightly cost saving (compared to the ACA), whereas the removal of ACA would save approximately $200 million by 2030. From the Medicaid perspective, removing the ACA would save about $8 million in CRC-related costs and expanding Medicaid would cost an additional $50 million by 2030, compared to ACA. In the case of dual Medicaid/Medicare enrollees, both removal of ACA and Medicaid expansion would cost more, compared to ACA. The removal of ACA would be less expensive, increasing cumulative discounted costs by approximately $5 million while Medicaid expansion would increase costs by approximately $85 million (though with a delay). Medicare would quickly reap cost savings from Medicaid expansion, increasing to about $100 million in 2055, while removal of ACA would result in an approximate $100 million increase in cumulative costs by the same time, both compared to ACA. Medicaid expansion would save nearly $60 million in uncompensated care by 2035, while removal of ACA would increase cumulative costs by nearly $120 million by 2034, compared to the ACA.
Fig. 3.
Cumulative cost savings discounted at 3% per year, by payer (insurance type) for the no ACA (heavy black line) and Medicaid expansion (heavy gray line) scenarios compared to the ACA scenario.
We present uncertainty intervals (the minimum and maximum values across replications) for primary simulation outcomes in Tables 4 and 5 and Supplemental Table 1. The uncertainty intervals for percent up-to-date (Table 4) are fairly narrow, though the corresponding intervals for CRC diagnoses and life-years are wider. While these intervals overlap across scenarios, analysis of scenario rankings within each replication indicate our conclusions about scenario dominance are robust to uncertainty. This is particularly true for scenario rankings based on percent up-to-date; scenario rankings across every replication were the same as the rankings based on average cross-replication results presented in Table 4. While there was some heterogeneity in the relative impact of enhanced insurance access (and thus screening) on the number of CRC cases, the rank orderings in terms of life-years gained were more robust across scenarios. This indicates that increased insurance (and thus screening) can result in more CRC diagnoses, but typically at earlier stages and producing a net increase in life-years.
4. Discussion
Our findings suggest that, at the population-level, insurance expansion will improve CRC screening, reduce the number of incident CRC cases, shift the burden of CRC cases from later to earlier stages at diagnosis, reduce CRC-attributable mortality, and add years to people's lives – at a cost savings across payers. If NC expands its Medicaid program, which would add short-term costs associated with screening, annual costs would be expected to decline within seven years, saving more than $28 million for this population of all NC residents age-eligible for CRC screening over the five-year study period. Nearly 500 fewer people in this population will develop CRC, saving more than 200 lives and adding more than 5000 years of life. These numbers increase by more than a factor of 12 for the conservative scenario and 15–16 for the enhanced scenario if insurance expansion via Medicare is extended to all. Cost savings increase even more – by a factor of 34–36, depending on whether the more conservative or enhanced Medicare-for-all scenario is considered. That said, the expected impact varies across payers, with some benefitting more than others. For example, if Medicaid is expanded (on top of ACA), the costs to Medicaid will rise in the short term whereas Medicare costs and the cost of uncompensated care will decrease. Some of these cost savings could be shifted to offset the increased burden on the state to expand its Medicaid program, increasing the likelihood that insurance access will be expanded to more low-income individuals. Medicare-for-all scenarios demonstrate the potentially sizeable cost savings attributable to both enhanced prevention and the impact of lower rates associated with volume-based purchasing.
As much as insurance expansion improves health and saves money, this analysis also highlights the reductions in insurance access that would stem from the reversal of ACA and the corresponding negative impact on health and cost outcomes. Among this population simulated, reversal of ACA would lead to 1782 additional CRC diagnoses, 791 more deaths, and 14,531 fewer years of life, compared to the status quo. If we consider that there were an estimated 3,298,265 individuals exposed to CRC screening in the study period, this implies increased CRC incidence and mortality rates of 54 and 24 per 100,000 population, respectively.
Despite the sizable improvements in health and cost outcomes associated with the insurance expansion scenarios for most payers, none of these scenarios are capable of increasing screening to the 70.5% (Office of Disease Prevention and Health Promotion, 2019) or 80% (National Colorectal Cancer Roundtable, 2019) targets by 2022. Indeed, these targets are not reached in a single zip code across NC. Furthermore, insurance expansion by itself is not able to eliminate CRC screening disparities by gender, race/ethnicity, or geography. Projections in Table 4 and Fig. 1 could inform more attainable state and national screening targets. Considering both these results and our previous analysis of EBIs in NC (Hassmiller Lich et al., 2017), it is clear that a combination of insurance expansion and multiple EBIs will be needed to reach established targets.
While it is difficult to reach current national screening targets of 70.5% and 80%, research has shown that we are getting closer to these targets, especially among subpopulations or within individual clinics or facilities. For instance, our microsimulation in Oregon found that a mailed FIT plus patient navigation intervention has the potential to increase the percent of Oregon residents enrolled in Medicaid coordinated care organizations by 20.2 percentage points (to 70.3%) after five years (Davis et al., 2019). The difficulty lies in reaching these targets at the population level, either state-wide or country-wide. While it is important to note, though, that screening programs and national targets differ greatly from country to country (Young et al., 2019; Navarro et al., 2017), international comparisons indicate that the 80% target has not been reached at the country level, although substantial progress has – and thus can – be made toward the 70.5% target when EBIs and broad access to insurance are combined (Young et al., 2019; Navarro et al., 2017).
Like all microsimulation initiatives, this study has limitations. While such models can be used to integrate fragmented datasets, they require structural and numeric (parameter value) assumptions. We attempted to make all modeling assumptions transparent here and in the model documentation. However, as the context of CRC care evolves, these assumptions will need to be updated and conclusions might change. For example, if the cost of CRC treatment decreases (Mennini et al., 2019) while the cost of screening remains fixed, cost savings associated with insurance expansion may shift. This is not likely to be the case, though, as cancer treatment costs have risen dramatically in the recent past. While both polyp size and histology affect the progression to CRC and CRC detection, our natural history model currently only distinguishes between different sized polyps (though it is well-calibrated to historical screening and CRC cancer registry data). As another limitation, we focused on outcomes among a five-year population of age-eligible individuals. Until more up-to-date simulated populations are available, we feel this decision offers a balance between projecting outcomes of interest (e.g., impact on percent up-to-date, ability to meet established targets in the future) with concerns about the generalizability of the simulated population far beyond 2009. Lastly, we make a simplifying assumption of no migration. Given that the rates of movement into and out of the state are approximately balanced (Tippett, 2018), and with the older adult population (the focus of this study) less transient in this state (Rosenthal, 2017), we believe the impact on our results to be minimal. Future work to update the synthetic population, to account for migration patterns, to further develop the natural history model to account for polyp histology, to simulate combined insurance expansion and EBI scenarios, and to consider the impact of parameter uncertainty on conclusions is needed.
5. Conclusions
Our state-level microsimulation results suggest that insurance expansion is a powerful approach to increasing CRC screening and improving CRC-related health outcomes while reducing costs of care. In NC, the ACA has increased the percent of the population up-to-date with CRC screening/testing by 1.1 percentage points – more than the multimillion dollar investments designed to improve screening simulated in prior work (Hassmiller Lich et al., 2017). Medicaid expansion would increase this by another 0.3 percentage points (more on par with these EBIs). Microsimulation models can be used as illustrated here to support decision makers in choosing between approaches, or in better understanding what it will take to reach established targets, efficiently.
Supplementary Material
Acknowledgments
This study was supported, in part, by Cooperative Agreement Numbers U48-DP005017 (University of North Carolina at Chapel Hill) and U48-DP005006 (Oregon Health & Science University) from the Centers for Disease Control and Prevention (CDC) Prevention Research Centers (PRC) Program and the National Cancer Institute (NCI), as part of the Cancer Prevention and Control Research Network (CPCRN). Melinda Davis was supported by an Agency for Healthcare Research & Quality patient-centered outcomes research (PCOR) K12 award (Award # K12 HS022981 01, PI: Jeanne-Marie Guise) and an NCI K07 award (1K07CA211971-01A1, PI: Davis). The content provided is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Publication of this supplement was supported by the Cancer Prevention and Control Network (CPCRN), University of North Carolina at Chapel Hill and the following co-funders: Case Western Reserve University, Oregon Health & Science University, University of South Carolina, University of Iowa, University of Kentucky, University of Pennsylvania and University of Washington.
Abbreviations:
- ACA
Affordable Care Act
- AJCC
American Joint Committee on Cancer
- CDC
Centers for Disease Control and Prevention
- CPT
Current Procedural Terminology
- CRC
colorectal cancer
- EBI
evidence-based intervention
- FIT
fecal immunochemical test
- FPL
federal poverty level
- FOBT
fecal occult blood test
- HIE
Health Insurance Exchange
- NC
North Carolina
Footnotes
Declaration of competing interest
Stephanie Wheeler receives unrelated grant funding to her institution from Pfizer. All other authors declare no conflicts of interest.
References
- Allison JE, Sakoda LC, Levin TR, et al. , 2007. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J. Natl. Cancer Inst 99 (19), 1462–1470. [DOI] [PubMed] [Google Scholar]
- American Cancer Society, 2017. Colorectal Cancer Facts & Figures 2017–2019. American Cancer Society, Atlanta. [Google Scholar]
- Arias E, 2004. United States life tables, 2002 In: National Vital Statistics Reports, vol. 53, No. 6 National Center for Health Statistics, Hyattsville (MD). [PubMed] [Google Scholar]
- Bogie R, Sanduleanu S, 2016. Optimizing post-polypectomy surveillance: a practical guide for the endoscopist. Dig. Endosc 28, 348–359. 10.1111/den.12510. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services, 2017. Clinical Diagnostic Laboratory Fee Schedule. [Google Scholar]
- Centers for Medicare & Medicaid Services, 2018. North Carolina Medicare Physician Fee Schedule. [Google Scholar]
- Cornejo D, Mayorga ME, Lich KH, 2014. Creating common patients and evaluating individual results: issues in individual simulation for health policy analysis. In: Proceedings of the 2014 Winter Simulation Conference. [Google Scholar]
- Coughlin SS, Costanza ME, Fernandez ME, Glanz K, Lee JW, Smith SA, 2006. CDC-funded intervention research aimed at promoting colorectal cancer screening in communities. Cancer 107, 1196–1204. 10.1002/cncr.22017. [DOI] [PubMed] [Google Scholar]
- Davis MM, Renfro S, Pham R, et al. , 2017. Geographic and population-level disparities in colorectal cancer testing: a multilevel analysis of Medicaid and commercial claims data. Prev. Med 101 (8), 44–52. 10.1016/j.ypmed.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Freeman M, Shannon J, et al. , 2018. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States - how, what and when? BMC Cancer 18 (1), 40 10.1186/s12885-017-3813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Nambiar S, Mayorga ME, et al. , 2019. Mailed FIT (Fecal Immunochemical Test), Navigation or Patient Reminders? Using Microsimulation to Inform Selection of Interventions to Increase Colorectal Cancer Screening in Medicaid Enrollees. 10.1016/j.ypmed.2019105836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty MK, Brenner AT, Crockett SD, et al. , 2018. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern. Med 178 (12), 1645–1658. 10.1001/jamainternmed.2018.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge SB, Compton CC, 2010. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM Ann. Surg. Oncol. 17 (6), 1471–1474. 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Hassmiller Lich K, Cornejo DA, Mayorga ME, et al. , 2017. Cost-effectiveness analysis of four simulated colorectal cancer screening interventions, North Carolina. Prev. Chronic Dis 14, 160158 10.5888/pcd14.160158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph DA, DeGroff AS, Hayes NS, Wong FI, Plescia M, 2011. The Colorectal Cancer Control Program: partnering to increase population level screening. Gastrointest. Endosc 73 (3), 429–434. 10.1016/j.gie.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Joseph DA, King JB, Richards TB, Thomas CC, Richardson LC, 2018. Use of colorectal cancer screening tests by state. Prev. Chronic Dis 15, 170535 10.5888/pcd15.170535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp-Vogelaar I, van Bellegooijen M, Zauber AG, et al. , 2009. Individualizing colonoscopy screening by gender and race. Gastrointest. Endosc 70 (1), 96–10924. 10.1016/j.gie.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin TR, Zhao W, Conell C, et al. , 2006. Complications of colonoscopy in an integrated health care delivery system. Ann. Intern. Med 145 (12), 880–886. [DOI] [PubMed] [Google Scholar]
- Lin JS, Piper M, Perdue LA, et al. , 2016. Screening for colorectal cancer: A systematic review for the US Preventive Services Task Force: Evidence synthesis no. 135 In: AHRQ Publication 14–05203-EF-1. Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML, 2011. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 103, 117–128. 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicaid and CHIP Payment and Access Commission. Access in brief: use of cervical, breast, and colon cancer tests among adult Medicaid enrollees. November 2016. Available at: https://www.macpac.gov/wp-content/uploads/2016/11/Use-of-Cervical-Breast-and-Colon-Cancer-Tests-among-Adult-Medicaid-Enrollees.pdf Last accessed July 14, 2019. [Google Scholar]
- Mennini FS, Marcellusi A, Fabiano G, Rimassa L, Santoro A, Personeni N, 2019. Budget impact of bimonthly use of cetuximab in patients diagnosed with metastatic colorectal cancer. Future Oncol. 10.2217/fon-2018-0904. epub. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute, 2019. Surveillance, Epidemiology, and End Results Program. SEER*Explorer. Retrieved from: http://seer.cancer.gov/explorer/ Last accessed July 14, 2019.. [Google Scholar]
- National Colorectal Cancer Roundtable. Shared goal: reaching 80% screened for color-ectal cancer by 2018. Available at: https://nccrt.org/what-we-do/80-percent-by-2018/ Last accessed July 14, 2019. [Google Scholar]
- National Center for Health Statistics, 2015. Persons receiving a recommended colorectal cancer screening by insurance status. National Health Interview Survey. Reported in Office of Disease Prevention and Health Promotion. Healthy People 2020: colorectal cancer screening (C-16) Available at https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Clinical-Preventive-Services/data#c16 (Last updated July 12, 2019). [Google Scholar]
- Navarro M, Nicolas A, Ferrandez A, Lanas A, 2017. Colorectal cancer population screening programs worldwide in 2016: an update. World J. Gastroenterol. 23 (20), 3632–3642. 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North Carolina State Center for Health Statistics, 2018. Cancer Incidence and Mortality in North Carolina. Central Cancer Registry. North Carolina Division of Public Health. [Google Scholar]
- Office of Disease Prevention and Health Promotion 2019. Healthy People 2020: colorectal cancer screening (C-16). Available at: https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Clinical-Preventive-Services/data#c16Last updated 7/12/19. [Google Scholar]
- Pierannunzi C, Ss Hu, Balluz L, 2013. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Med. Res. Methodol 13, 49 10.1186/1471-2288-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DK, Cutler CS, Lemmel GT, et al. , 1997. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 112 (1), 24–28. 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- Rex DK, Boland CR, Dominitz JA, et al. , 2017. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest. Endosc 86 (1), 18–33. 10.1016/j.gie.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Rosenthal J Staying home: declining interstate migration and its impact on North Carolina. North Carolina Department of Commerce. Available at: https://www.nccommerce.com/blog/2017/05/22/staying-home-declining-interstate-migration-and-its-impact-north-carolina Published on May 22, 2017. Last accessed August 19, 2019. [Google Scholar]
- RTI International. RTI U.S. synthetic household population. Available at: https://www.rti.org/impact/rti-us-synthetic-household-population™ Last accessed July 14, 2019. [Google Scholar]
- Sabatino SA, Lawrence B, Elder R, et al. , 2012. Effectiveness of interventions to in-crease screening for breast, cervical, and colorectal cancers: Nine updated systematic reviews for the Guide to Community Preventive Services. Am. J. Prev. Med 43 (1), 97–118. 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Smith A, Young GP, Cole SR, Bampton P, 2006. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 107 (9), 2152–2159. 10.1002/cncr.22230. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Bobashev G, Morris RJ, 2009. Modeling the cost-effectiveness of colorectal cancer screening: Policy guidance based on patient preferences and compliance. Cancer Epidemiol. Biomark. Prev 18 (7), 1971–1978. 10.1158/1055-9965.EPI-09-0083. [DOI] [PubMed] [Google Scholar]
- The Henry J Kaiser Family Foundation, 2018. Where are states today? Medicaid and CHIP eligibility levels for children, pregnant women, and adults. Available at. https://www.kff.org/medicaid/fact-sheet/where-are-states-today-medicaid-and-chip/ (Last updated March 31, 2019). [Google Scholar]
- The University of North Carolina at Chapel Hill; Population simulation model: simulating colorectal cancer screening and outcomes Model documentation. Available at: http://crcsim.web.unc.edu Last accessed July 14, 2019. [Google Scholar]
- Tippett R 5 facts to know about migration between NC and other states. Carolina Population Center. Available at: https://www.ncdemography.org/2018/01/09/5-facts-to-know-about-migration-between-nc-and-other-states/ Published on January 9, 2018. Last accessed August 19, 2019. [Google Scholar]
- U.S. Preventive Services Task Force, U.S., Bibbins-Domingo K, Grossman DC, et al. , 2016. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 315 (23), 2564–2575. 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau Historical income tables: people. Available at. https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-income-people.html (Last updated August 27, 2019). [Google Scholar]
- Van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, Van Deventer SJ, Dekker E, 2006. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am. J. Gastroenterol 101 (2), 343–350. 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- Wheaton WD, Cajka JC, Chasteen BM, et al. , 2009. Synthesized population databases: a US geospatial database for agent-based models. Methods Rep 10, 905 10.3768/rtipress.2009.mr.0010.0905. RTI Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SB, Kuo T, Meyer AM, et al. , 2017. Multilevel predictors of colorectal cancer testing modality among publicly and privately insured people turning 50. Prev. Med. Rep 6, 9–16. 10.1016/j.pmedr.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SB, Leeman J, Hassmiller Lich K, Tangka FKL, Davis MM, Richardson LC, 2018. Data-powered participatory decision making: leveraging systems thinking and simulation to guide selection and implementation of evidence-based colorectal cancer screening interventions. Cancer J. 24 (3), 132–139. 10.1097/PPO.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, White P, Nieto J, Vieira D, Francois F, Hamilton F, 2016. Colorectal cancer in African Americans: an update. Clin. Transl. Gastroenterol 7, e185 10.1038/ctg.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabroff KR, Lamont KB, Mariotto A, et al. , 2008. Cost of care for elderly cancer patients in the United States. JNCI 100 (9), 630–641. 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- Young GP, Rabeneck L, Winawer SJ, 2019. The global paradigm shift in screening for colorectal cancer. Gastroenterology 156 (4), 843–851.e2. 10.1053/j.gastro.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Zauber A, Lansdorp-Vogelaar I, Wilschut J, Knudsen A, Van Ballegooijen M, Kuntz K. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer: Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; 2007. December 20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.