Abstract
Background
An acute burn wound is a complex and evolving injury. Extensive burns produce systemic consequences, in addition to local tissue damage. Treatment of partial thickness burn wounds is directed towards promoting healing and a wide variety of dressings are currently available. Improvements in technology and advances in understanding of wound healing have driven the development of new dressings. Dressing selection should be based on their effects on healing, but ease of application and removal, dressing change requirements, cost and patient comfort should also be considered.
Objectives
To assess the effects of burn wound dressings on superficial and partial thickness burns.
Search methods
For this first update we searched The Cochrane Wounds Group Specialised Register (searched 8 November 2012); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 10); Ovid MEDLINE (2008 to October Week 4 2012); Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, November 07, 2012); Ovid EMBASE (2008 to 2012 Week 44); AND EBSCO CINAHL (1982 to 2 November 2012).
Selection criteria
All randomised controlled trials (RCTs) that evaluated the effects of burn wound dressings on the healing of superficial and partial thickness burns.
Data collection and analysis
Two authors extracted the data independently using standardised forms. We assessed each trial for internal validity and resolved differences by discussion.
Main results
A total of 30 RCTs are included in this review. Overall both the quality of trial reporting and trial conduct were generally poor and meta analysis was largely precluded due to study heterogeneity or poor data reporting. In the context of this poor quality evidence, silver sulphadiazine (SSD) was consistently associated with poorer healing outcomes than biosynthetic (skin substitute) dressings, silver‐containing dressings and silicon‐coated dressings. Burns treated with hydrogel dressings appear to heal more quickly than those treated with usual care.
Authors' conclusions
There is a paucity of high‐quality evidence regarding the effect of different dressings on the healing of superficial and partial thickness burn injuries. The studies summarised in this review evaluated a variety of interventions, comparators and clinical endpoints and all were at risk of bias. It is impossible to draw firm and confident conclusions about the effectiveness of specific dressings, however silver sulphadiazine was consistently associated with poorer healing outcomes than biosynthetic, silicon‐coated and silver dressings whilst hydrogel‐treated burns had better healing outcomes than those treated with usual care.
Keywords: Humans; Wound Healing; Bandages; Bandages/classification; Bandages/standards; Bandages, Hydrocolloid; Burns; Burns/physiopathology; Burns/therapy; Randomized Controlled Trials as Topic; Silicon Compounds; Silicon Compounds/therapeutic use; Silver Sulfadiazine; Silver Sulfadiazine/therapeutic use; Skin, Artificial
Plain language summary
Dressings for treating superficial and partial thickness burns
Superficial burns are those which involve the epidermal skin layer and partial thickness burns involve deeper damage to structures such as blood vessels and nerves. There are many dressing materials available to treat these burns but none has strong evidence to support their use. Evidence from poor quality, small trials, suggests that superficial and partial thickness burns heal more quickly with silicon‐coated nylon, silver containing dressings and biosynthetic dressings than with silver sulphadiazine cream. Burns treated with hydrogel dressings healed more quickly than those treated with usual care.
Background
Description of the condition
Burn injury occurs in all age groups, from many causes, and may range from the very minor, when no or self treatment is sufficient, through to the most severe which require the highest levels of intensive care and surgery. Thus, patients suffering a burn injury present with a wide spectrum of injury severity depending on the depth of the wound and the surface area of the body affected. This variability of injury makes it difficult to describe the number of people who suffer burn injuries each year accurately; only the most serious are admitted to hospital and these are the least common of burn injuries (Burd 2005).
Full thickness burns involve all layers of the skin and may involve the structures beneath such as muscle and bone. A superficial burn involves just the epidermal layer of the skin, while partial thickness burns involve damage to deeper structures within the skin such as blood vessels, nerves and hair follicles. Whilst causing considerable pain and distress, these types of burns can heal without the need for surgical intervention and, if only involving relatively small areas, can be managed safely in an outpatient environment. It is these types of burn wounds that are the focus of this review.
Accurate assessment of burn depth is important in making the right decision about treatment. Most extensive burns are a mixture of different depths and burn depth can change and deepen following initial injury (Hettiaratachy 2004). The management of burn wounds can have a considerable influence on the time taken for the wound to heal. Ensuring that the wound is managed in a way that promotes healing will influence the long term quality and appearance of the scar, and also minimise the risk of burn wound infection. Superficial and partial thickness wounds can progress to a deeper burn if the wound dries out or becomes infected.
Description of the intervention
Numerous dressing materials are available for treating partial thickness burns, the most common being a combination of paraffin‐impregnated gauze and an absorbent cotton wool layer (Hudspith 2004). Silver sulphadiazine (SSD) cream has also been commonly used in burn wound management since 1968 to minimise the risk of wound infection. However, these conventional dressings tend to adhere to the wound surface (Thomas 1995) and their need for frequent changes traumatises newly epithelialised surfaces and delays healing. Silver sulphadiazine cream itself is also thought to delay wound healing due to a toxic effect on regenerating keratinocytes (Wasiak 2005).
The limitations of conventional dressings, improvements in technology and advances in our understanding of wound healing have led to an enormous expansion in the range of dressing options that can be used on minor burns. Burn wounds may lose large amounts of fluid through evaporation and exudation, so dressings must absorb fluid, but also maintain a high humidity at the wound site to encourage granulation and assist epithelialisation. The burn dressing should provide a bacterial barrier to prevent infection entering the wound or being transmitted from the wound. Burn dressings should also possess mechanical characteristics to accommodate movement (Quinn 1985)
The range of dressings now available can be sub‐categorised into different types based upon the materials used in their manufacture (Queen 1987). These sub‐categories can include: films, foams, composites, sprays and gels. Also available as an alternative to traditional gauze dressings are the biological skin replacements and the bioengineered skin substitutes, including autologous cultured and non‐cultured products, and the newer biosynthetic skin dressings that are available to produce physiological wound closure until the epidermal layer has repaired. Further details of these dressing categories are as follows:
1. Hydrocolloid dressings
Hydrocolloid dressings contain a variety of constituents including gelatin, pectin and sodium carboxymethylcellulose in an adhesive polymer matrix. These dressings form a gel when their inner layer comes into contact with exudate which in turn facilitates autolytic debridement of the wound. Examples of a hydrocolloid dressing include Comfeel (Coloplast) and DuoDerm (ConvaTec) (Lawrence 1997).
2. Polyurethane film dressings
Polyurethane films are transparent, adhesive‐coated sheets that are applied directly to the wound. They are permeable to water vapour, oxygen and carbon dioxide but not to liquid water or bacteria. Depending on the amount of wound exudate, the dressings can be left in place for several days. Film dressings are suitable for lightly exuding wounds (Lawrence 1997). Two examples of a polyurethane film include OpSite (Smith & Nephew) or Tegaderm (3M Company).
3. Hydrogel dressings
Hydrogel dressings are high water content gels containing insoluble polymers. Their constituents include modified carboxymethylcellulose, hemicellulose, agar, glycerol and pectin. Unlike the film dressings, they have more capacity to absorb fluid, and can therefore cope with higher levels of wound exudate. Their fluid donating properties may also aid wound debridement and assist in maintaining a moist wound environment. Hydrogels are available in amorphous form (a loose gel) and in a sheet form where the gel is presented with a fixed three‐dimensional macro structure. Amorphous hydrogels include products such as IntraSite (Smith & Nephew) and Solugel, while sheet hydrogels include Aqua clear and Nu‐gel (Johnson & Johnson).
4. Silicon‐coated nylon dressings
This group of dressings consist of a flexible polyamide net coated with soft silicone containing no biological compounds. They act as a direct wound contact layer and their mesh structure allows drainage of exudate from the burned surface. They function primarily as a non‐adherent dressing layer and therefore to reduce potential damage during dressing changes. An example includes Mepitel (Mölnlycke)(Walmsley 2002).
5. Biosynthetic skin substitute dressings
Biosynthetic skin substitute dressings are a family of materials which have been developed to mimic a function of skin by replacing the epidermis or dermis, or both. Generally speaking, manufactured epidermal substitutes will allow for re‐epithelialisation to occur while permitting a gas and fluid exchange which in turn provides both protection from bacterial influx and mechanical coverage (Demling 2013). Examples include Biobrane (Dow Hickam/Bertek Pharmaceuticals) and TransCyte (Advanced Tissue Sciences) (Walmsley 2002).
6. Antimicrobial (silver and iodine‐containing) dressings
Antimicrobial dressings claim to manage the bio burden of the wound: they are thought to reduce the risk of invasive infection by minimising the bacterial colonisation of wounds. Specialist products containing antimicrobials include the Acticoat range (delivering nano‐crystalline silver) and the Iodosorb range (cadexomer iodine). Several types of product have now been produced with added silver, including: Contreet (hydrocolloid with silver), Avance (foam with silver) and Aquacel Ag (fibre dressing with silver, ConvaTec).
7. Fibre dressings
Fibre dressings such as the calcium alginate dressings are absorbent, biodegradable and derived from seaweed. Alginate dressings may help to maintain a moist microenvironment conducive to healing, whilst limiting wound secretions and minimising bacterial contamination. They are useful for moderate to heavily exudating wounds. Alginates can be rinsed away with saline irrigation, which minimises interference with the healing process and may reduce pain experienced by patients. Some examples of alginate dressings are: Algosteril (Johnson & Johnson), Comfeel Alginate Dressing (Coloplast), Carrasorb H (Carrington Laboratories), Kaltostat (ConvaTec) (Walmsley 2002).
8. Wound dressing pads
This group of dressings include simple non‐adherent dressings, knitted viscose dressings (e.g. N/A Dressing ‐ Johnson & Johnson), tulle and gauze dressings. They are usually in the form of woven cotton pads that are applied directly to the wound surface. They can be either non‐medicated (e.g. paraffin gauze dressing) or medicated (e.g. containing povidone iodine or chlorhexidine).
Why it is important to do this review
Despite the increase in the types of dressings available, traditional dressings of paraffin‐impregnated gauze and absorbent cotton wool or gauze are still commonly used (Hudspith 2004). The purpose of this review is to establish which type of dressing from the many now available is more effective in promoting healing and minimising discomfort and infection for patients with superficial and partial thickness burns.
Objectives
The objective of this review was to assess the effects of burn wound dressings for treating superficial and partial thickness burns.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that evaluated the effects of burn wound dressings used in the treatment of superficial and partial thickness burns.
Types of participants
We focused on people of any age with a superficial or partial thickness burn determined by either clinical evaluation or objective assessment, or both, which required treatment in any health care setting. We did not include trials that recruited people with full thickness burns.
Types of interventions
We included any wound dressing used singly and in combination to treat superficial and partial thickness burns. The groups of products considered included:
hydrocolloid dressings;
polyurethane film dressings;
hydrogel dressings;
silicon‐coated nylon dressings;
biosynthetic skin substitute dressings;
antimicrobial (silver and iodine containing) dressings;
fibre dressings;
wound dressing pads.
We excluded topical skin agents, biological skin replacements and autologous cultured and non‐cultured skin engineering products, as these products tend to be used on people with deep dermal and full thickness burns, both of which were not within the remit of this review. We excluded trials which considered the treatment of hand burns. This decision was taken post hoc as it was felt that the treatment regime for these particular types of burns was different due to the anatomical site.
Types of outcome measures
Studies were eligible for inclusion if they reported any of the following outcome measures:
Primary outcomes
Time to complete wound healing/proportion of burns completely healed in a specified time period.
Change in wound surface area over time/proportion of wounds partly healed in a specified time period.
Secondary outcomes
Number of dressing changes.
Cost of the dressings.
Level of pain associated with the application and removal, or both, of the wound dressing.
Patient perception, level of satisfaction with the application and removal of dressing.
Quality of life.
Hospital length of stay (LOS).
Need for surgery.
Incidence of infection.
Adverse events.
Search methods for identification of studies
Electronic searches
For this first update we conducted searches of the following databases:
The Cochrane Wounds Group Specialised Register (searched 8 November 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 10);
Ovid MEDLINE (2008 to October Week 4 2012);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, November 07, 2012);
Ovid EMBASE (2008 to 2012 Week 44);
EBSCO CINAHL (1982 to 2 November 2012).
We used the following search strategy in the Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Bandages, Hydrocolloid explode all trees #2 hydrocolloid* or askina or biofilm or combiderm or comfeel or cutinova or duoderm or duoderm or (hydroactive NEXT gel*) or granuflex or hydrocoll or replicare or tegasorb or sureskin or hydrofibre or hydrofiber or aquacel #3 MeSH descriptor Alginates explode all trees #4 alginate NEXT dressing* #5 alginate* or calcium or algosteril or kaltostat or melgisorb or seasorb or sorbalgon or sorbsan or tegagen or “algisite M” #6 foam NEXT dressing* #7 allevyn or avance or biatain or cavi‐care or flexipore or lyofoam or spyrosorb or tielle or mepilex #8 MeSH descriptor Hydrogels explode all trees #9 hydrogel* or aquaform or debrisan or geliperm or granugel or hydrosorb or novogel or nu‐gel or "nu gel" or purilon or sterigel #10 film or films or arglaes or omiderm or polyurethane or tegaderm or opsite #11 MeSH descriptor Occlusive Dressings explode all trees #12 paraffin NEAR gauze #13 paranet or paratulle or unitulle or jelonet or bactigras or cuticerin or adaptic or atrauman #14 "retention tape" or hypafix or mefix or fixamul #15 biosynthetic NEAR substitute* #16 (biosynthetic NEAR dressing*) #17 transcyte or biobrane #18 (antimicrobial NEXT dressing*) or acticoat #19 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We combined the EMBASE and CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2010). We applied no date or language restrictions.
Searching other resources
We handsearched the references of all identified studies and contacted authors for information about other published and unpublished studies. We contacted all dressing manufacturers to request information on trials evaluating dressings.
Data collection and analysis
Selection of studies
FC and JW scanned records retrieved by the initial search to exclude obviously irrelevant studies, and then three review authors (FC, HC and JW) screened titles and abstracts identified by the search against the inclusion criteria for the additional updates. Two review authors (FC and JW) retrieved and reviewed full‐text articles independently for the purpose of applying the inclusion criteria. In all instances, we resolved differences of opinion by discussion among the review authors.
Data extraction and management
Two review authors (FC and JW) extracted data from the studies independently, using standardised forms. The standardised forms allowed for the extraction of specific data such as type of care setting, key baseline variables of each group, e.g. depth of burn wound, size of burn wound, burn type, age, sex, description of the intervention and the control or co‐intervention including: secondary dressings used, frequency of dressings changes and length of treatment. We resolved all differences by discussion among the review authors.
Assessment of risk of bias in included studies
Risk of bias assessment was based on the method outlined in The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). Two review authors (FC and JW) extracted data for risk of bias assessment and they are presented in a descriptive manner. We assessed the following characteristics: sequence generation; allocation concealment; blinding (of participants, personnel and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias. We categorised these judgements as low risk of bias, high risk of bias or 'unclear'. We resolved differences of opinion by discussion among the review authors.
Data synthesis
For proportions (dichotomous outcomes, e.g. percentage of burns healed), we used risk ratios (RR). We calculated the mean difference (MD) for continuous data and pooled data in a meta‐analysis.
We made all analyses on an intention‐to‐treat basis, where possible, and where not possible this was clearly stated. Time to wound healing was to be analysed as survival (time‐to‐event) outcomes if possible, using the appropriate analytical method (as per the Cochrane Handbook for Systematic Reviews of Interventions version 5.0)(Deeks 2011).
We gave consideration to the appropriateness of pooling and meta‐analysis. Two review authors extracted and summarised data from all eligible studies independently using a standard data extraction tool.
Subgroup analysis and investigation of heterogeneity
We used a fixed‐effect model where there was no evidence of significant heterogeneity between studies (I2 statistic less than 40%), and employed a random‐effects model when heterogeneity was likely (I2 statistic more than 40%) (DerSimonian 1986; Higgins 2003).
We gave consideration to the appropriateness of subgroup analyses based on the type of burn injury, i.e. superficial or deep partial thickness burn, but many of the studies did not report on the extent of burn depth. If they did, subgroup analysis was to be done by calculation of RR or mean difference (MD) in each subgroup with examination of the 95% confidence intervals (95% CI). We would take non‐overlap in intervals to indicate a statistically significant difference between subgroups.
Results
Description of studies
From independent scrutiny of the titles and abstracts from all the searches conducted to date, a total of 30 studies met the inclusion criteria (see Characteristics of included studies). Twenty‐one trials did not meet the inclusion criteria and we excluded them from the review; the reasons for exclusion are detailed in the Characteristics of excluded studies. Seven studies are classified as 'awaiting assessment' (Mabrouk 2012; Mostaque 2011; Ostlie 2012; Piatkowski 2011; Silverstein 2011; Verbelen 2011; Zhou 2011).
1. Hydrocolloid dressings
Five studies with 442 participants compared hydrocolloid dressings with other conventional burn wound dressings (Afilalo 1992; Phipps 1988; Thomas 1995; Wright 1993; Wyatt 1990). The studies were published between 1984 and 1995 and carried out in Canada (Afilalo 1992), the United Kingdom (Phipps 1988; Thomas 1995; Wright 1993) and the United States (Wyatt 1990). Studies took place in emergency departments, outpatient clinics or tertiary burn care centres. The type of burn injury was generally limited to partial thickness burns. The definition of superficial or partial thickness burns was described in only one study (Afilalo 1992). The inclusion and exclusion criteria did not differ considerably between the studies. Within the studies, patients were generally well matched for sex, age, location and size of burn injury.
The traditional treatments which acted as controls included chlorhexidine‐impregnated tulle‐gras in three of the trials (Phipps 1988; Thomas 1995; Wright 1993) and silver sulphadiazine (SSD) in two trials (Afilalo 1992; Wyatt 1990). The time for changing the comparator dressings differed in the trials, ranging from twice daily to every three to five days to when required. The number of dressing changes or ease of dressing change was reported in four studies (Afilalo 1992; Thomas 1995; Wright 1993; Wyatt 1990). The hydrocolloid dressings were changed every five days or when required in trials where this was reported.
2. Polyurethane film dressings
Two trials with 106 patients compared polyurethane film dressings with conventional burn wound therapy (Neal 1981; Poulsen 1991). The studies were carried out in an outpatient clinic of an accident and emergency department. The type of burn injury examined was limited to partial thickness burns although its definition was described in only one study (Poulsen 1991). The mechanism of burn injury was described in both studies (Neal 1981; Poulsen 1991). The inclusion and exclusion criteria did not differ considerably amongst the two studies. Within the studies, patients were generally well matched for sex, age, location and size of burn injury.
The conventional (control) dressing varied slightly between the studies and included chlorhexidine‐impregnated gauze (Neal 1981) and paraffin‐impregnated gauze (Poulsen 1991). The polyurethane film dressing was changed only if leakage, infection or an adverse skin reaction occurred (Poulsen 1991). The control dressings were changed on day six post‐burn in the Poulsen 1991 study. The control or conventional dressings in the Neal 1981 study were not changed until the third or fifth day post‐burn.
3. Hydrogel dressings
Three studies with 235 patients compared hydrogel dressings with SSD or paraffin gauze with or without topical antibiotics for a partial thickness burn injury (Grippaudo 2010; Guilbaud 1992; Guilbaud 1993). In Guilbaud 1992 and Guilbaud 1993, each patient acted as his or her own control; a total of 310 wound sites with similar depth and surface area, contiguous or anatomically separated, were evaluated with the following measures: healing time expressed in days, assessment of pain, quality of healing, sensitivity of the scar and frequency of dressing changes. The endpoint of healing was defined as the complete epithelialisation of the wound. Examinations were performed on days 0, 2, 4 and 8 and on the day of complete healing in both studies. The studies were undertaken in burn centres in Europe (Guilbaud 1992; Guilbaud 1993). Grippaudo 2010 evaluated time to wound healing at 6, 9, 12, 15, 18 and 21 days, wound infection, pain and adverse effects.
4. Silicon‐coated nylon dressings
Two studies (Bugmann 1998; Gotschall 1998) compared the effectiveness of silicon‐coated nylon dressings with SSD in 142 children presenting within 24 hours of injury with a partial thickness burn. A secondary dressing was applied over the silicon‐coated nylon dressing which consisted of a gauze dressing soaked with chlorhexidine in one study (Bugmann 1998) and wet and dry cotton gauze in the second study (Gotschall 1998).
Outcome measures assessed by Bugmann 1998 included depth of the burn, the number of cumulative dressings, presence or absence of complete epithelial cover, and number of reported cases of infection and bleeding. The criterion used to define the complete epithelial cover time was the time when a full surface shining layer of epithelial cells was observed. Evaluation of the burn was made between day three and six after injury. In Gotschall 1998, trained burn specialist nurses assessed the following outcome measures: wound healing, eschar formation, pain at dressing with the use of an objective pain scale tool and the time required for dressing changes.
5. Biosynthetic skin substitute dressings
Ten studies compared the effectiveness of biosynthetic dressings with twice‐daily application of SSD or other comparators in 434 patients. Five studies used Biobrane (Smith & Nephew) (Barret 2000; Cassidy 2005; Gerding 1988; Gerding 1990; Lal 1999), three used Hydron (Abbott Laboratories) (Curreri 1980; Fang 1987; Husain 1983) and one study used TransCyte (Smith & Nephew) (Noordenbos 1999). An additional study by Kumar 2004 had a three arm design in which patients were randomised to receive either Biobrane or TransCyte or SSD. A total of four studies (Fang 1987; Gerding 1988; Gerding 1990; Husain 1983) had patients serve as their own controls and similar areas of burns were randomised to receive either the intervention or control dressing.
The inclusion and exclusion criteria did not differ considerably between the 10 studies. Within the studies, patients were generally well matched for sex, age and location, although size of burn injury could vary. Outcome measures were similar across the studies with emphasis placed on healing times, infection rates, cost of dressings, levels of pain and length of stay in hospital.
6. Antimicrobial (silver and iodine‐containing) dressings
Five studies compared the efficacy of silver‐impregnated dressing (Acticoat, Smith and Nephew) with SSD on pain levels during dressing changes in 331 patients with partial thickness burns (Gong 2009; Huang 2004; Muangman 2006; Opasanon 2010; Varas 2005). The study by Huang 2004 reported on 166 wound sites rather than number of patients. The studies were carried out in tertiary burn centres with patients serving as their own controls (Varas 2005) or randomised to SSD (Gong 2009; Muangman 2006; Opasanon 2010) or SSD powder (Huang 2004). The outcome of interest (pain scores as assessed and reported using the visual analogue pain scale score) were collected during the initial application of the dressing (Muangman 2006) and once during the dressing change for Opasanon 2010 and Varas 2005. Other outcomes of interest for Huang 2004 and Opasanon 2010 included healing time expressed in number of days, and for Gong 2009 number of people healed.
7. Fibre dressings
Three studies evaluated the efficacy and safety of fibre‐type dressings. In the first study by Costagliola 2002, calcium alginate was compared with SSD in the treatment of 59 patients with 73 partial thickness burns. In Caruso 2006 and Muangman 2010 hydrofibre dressings were compared with SSD in 154 patients. With burn characteristics similar in all groups, all patients in the Caruso 2006 study were observed for a maximum of three weeks until the wound had completely healed. Outcomes measures of interest included length of time to onset of healing, pain, amount of care and treatment safety required and evaluated on a weekly basis. Cost outcomes were also measured by Muangman 2010, including total dressing cost, total hospital cost and transport cost.
Patient baseline characteristics
Most studies enrolled patients with a partial thickness burn. The definition of a partial thickness burn injury was absent in most studies with only Afilalo 1992, Gerding 1988, Gerding 1990 and Poulsen 1991 providing the reader with a definition of a burn wound. Huang 2004 defined burn depth according to an unusual nomenclature, i.e. three levels, four categories method and the size of the burn according to the nine categories method. Kumar 2004 matched burn depth estimates with laser Doppler specific criteria. The percentage of total burn surface area (%TBSA) and specific inclusion and exclusion criteria was reported in all studies except for Wright 1993. All trials had clear inclusion and exclusion criteria and there was some consistency between studies. Within the studies, patients were generally well matched for sex, age and size of burn injury.
Risk of bias in included studies
Details of the quality assessment based on the method outlined in Higgins 2011 are given in the table 'Characteristics of included studies', Figure 1 and Figure 2. Additionally, a brief descriptive analyses of the studies is provided below. In general, we assessed study quality as poor to very poor. The trials included had serious methodological or reporting shortcomings or both.
1.
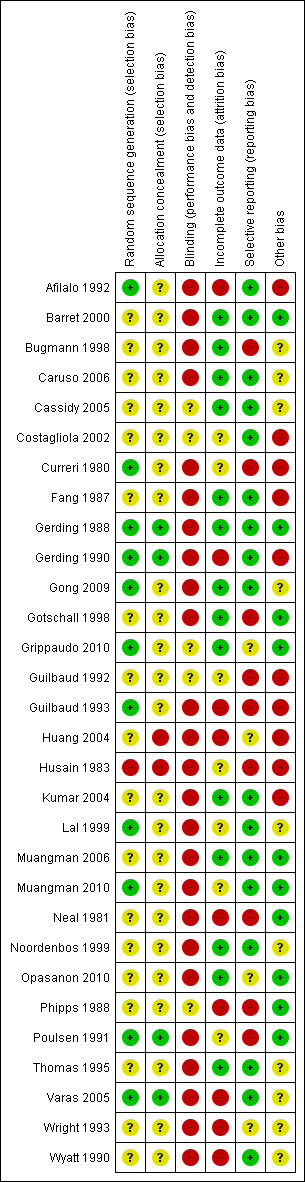
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomisation and adequacy of allocation concealment
The method of randomisation was adequate in only 11 of the 30 studies (Afilalo 1992; Curreri 1980; Gerding 1988; Gerding 1990; Gong 2009; Grippaudo 2010; Guilbaud 1993; Lal 1999; Muangman 2010; Poulsen 1991; Varas 2005). Six trials used matched controls by randomising paired wounds to treatment by opposite modalities (Fang 1987; Gerding 1988; Gerding 1990; Guilbaud 1992; Guilbaud 1993; Varas 2005). Husain 1983 and Varas 2005 had patients serve as their own controls. Allocation concealment was adequately documented and described in only four studies (Gerding 1988; Gerding 1990; Poulsen 1991; Varas 2005).
Blinding
Only two trials used blinded outcome assessors to measure an overall impression of healing (Wyatt 1990) and wound evaluation (Fang 1987).
Incomplete outcome data
Seventeen studies detail patients lost to follow‐up (Bugmann 1998; Caruso 2006; Cassidy 2005; Fang 1987; Gerding 1988; Gong 2009; Gotschall 1998; Grippaudo 2010; Husain 1983; Kumar 2004; Muangman 2006; Neal 1981; Noordenbos 1999; Opasanon 2010; Phipps 1988; Poulsen 1991; Thomas 1995). None of the studies were analysed by intention‐to‐treat.
Selective reporting
Seventeen studies were free from obvious selective reporting (Afilalo 1992; Barret 2000; Caruso 2006; Cassidy 2005; Costagliola 2002; Fang 1987; Gerding 1988; Gerding 1990; Gong 2009; Kumar 2004; Lal 1999; Muangman 2006; Muangman 2010; Noordenbos 1999; Thomas 1995; Varas 2005; Wyatt 1990).
Other potential sources of bias
Eleven studies were free from other obvious sources of potential bias (Barret 2000; Curreri 1980; Gerding 1988; Gotschall 1998; Grippaudo 2010; Muangman 2006; Muangman 2010; Neal 1981; Opasanon 2010; Phipps 1988; Poulsen 1991).
Effects of interventions
Results are presented for each dressing comparison and primary and secondary outcomes are presented when reported. Although the trials included a number of similar outcomes, sometimes the heterogeneous nature of the studies (i.e. use of different comparators), the absence of data, poor reporting or variations in reporting precluded formal statistical analysis. In most instances, we synthesised the results in a narrative review. Where studies analysed time‐to‐event data using methods for continuous outcomes (e.g. time to healing as "mean healing time") we present the results narratively and did not pool studies. The most appropriate way of summarising time‐to‐event data such as time to healing is by survival analysis with the hazard ratio as the measure of effect. It is not appropriate to analyse time to healing as continuous data since the relevant times are only known for the subset of participants who experienced the (healing) event. Censored participants cannot be included in such analyses, which almost certainly will introduce bias (Higgins 2011).
1. Hydrocolloid dressings
A total of five trials comparing hydrocolloid dressings with other dressing types or with different hydrocolloid dressings were included in this review.
a. Hydrocolloid dressings compared with chlorhexidine‐impregnated paraffin gauze dressing (three trials, 344 people)
Time to complete wound healing
We found three randomised controlled trials (RCTs) which compared hydrocolloid dressings with chlorhexidine‐impregnated paraffin gauze dressings (Phipps 1988; Thomas 1995; Wright 1993). None of the trials found a significant difference in healing rates. The trials could not be pooled as no variance data were reported.
Wright 1993 found no significant difference in time to wound healing (median wound healing time: 12 days in each group; P = 0.89).
Thomas 1995 had three study arms; hydrocolloid dressing, hydrocolloid dressing plus silver sulphadiazine (SSD) and chlorhexidine‐impregnated paraffin gauze dressing. There was no significant difference in mean time to wound healing between hydrocolloid dressing and chlorhexidine‐impregnated paraffin gauze dressing (10.6 days with hydrocolloid versus 11.1 days with chlorhexidine‐impregnated paraffin gauze; P value reported as not significant). No variance data were reported in the study.
Phipps 1988 reported that there was no statistically significant difference between the total mean time to wound healing between hydrocolloid dressing and chlorhexidine‐impregnated paraffin gauze dressing (14.18 days with hydrocolloid versus 11.83 days with chlorhexidine‐impregnated paraffin gauze; P value reported as not significant). No variance data were reported in the study.
Patient perception/level of satisfaction
In the study by Wright 1993, investigators and participants rated the hydrocolloid dressing more highly than the chlorhexidine‐impregnated paraffin gauze (10‐item visual analogue scale (VAS), with 0 = useless and 10 = excellent: participants' rating: 9.04 with hydrocolloid versus 6.86 with chlorhexidine‐impregnated paraffin gauze; P < 0.02; investigators' rating: 9.31 with hydrocolloid versus 6.9 with chlorhexidine‐impregnated paraffin gauze; P = 0.005); the study does not report if these ratings were mean values. The study does not report that the raters were blinded therefore bias cannot be ruled out.
Level of pain
Wright 1993 found no significant difference between treatments in background pain, pain associated with dressing changes (pain rated using a visual analogue scale), or ease of dressing removal (background pain: mean scores not reported; P = 0.28; pain on dressing change: mean scores not reported; P = 0.96; ease of dressing removal: mean scores not reported; P = 0.49).
Similarly, Thomas 1995 recorded pain using a visual analogue score of zero to 10 (zero = no pain and 10 = severe pain) by the clinician and by the patient (where possible). No significant difference between the pain scores of patients was reported (mean scores not reported; P = 0.82). During the clinical assessment, however, patients receiving the chlorhexidine‐impregnated paraffin gauze dressing sometimes complained that the dressing would stick to the wound surface, causing pain. Patients in the hydrocolloid group complained of pain when the adhesive border was removed from surrounding unshaved area (numerical or graphical results not presented).
Pain as an outcome measures was not reported by Phipps 1988.
Number of dressing changes
Only two of the three trials reported the frequency of dressing changes. In the Wright 1993 study, dressings were changed more often because of leakage in the hydrocolloid group compared with the chlorhexidine‐impregnated paraffin gauze group (15/94 (15%) with hydrocolloid versus 3/89 (3%) with chlorhexidine‐impregnated paraffin gauze dressing; P < 0.02). This difference was statistically significant.
In contrast Thomas 1995 reported significantly fewer dressing changes per patient during treatment with hydrocolloid dressing compared with chlorhexidine‐impregnated paraffin gauze dressing (2.3 with hydrocolloid dressing versus 4.1 with chlorhexidine‐impregnated paraffin gauze dressing; P < 0.0001; reasons for dressing changes were not reported and no variance data were reported in the study). Dressing changes were not reported by Phipps 1988.
Adverse events
In the study by Wright 1993, pain was reported in one person and rash in two people with hydrocolloid dressing.
Incidence of infection
Wright 1993 reported that one person in the hydrocolloid group withdrew from the study because of infection, but did not report any cases of infection in those who remained in the study (Analysis 1.1) and this was not significant. Thomas 1995 reported no significant difference in increase in pathogenic bacterial isolates between the hydrocolloid dressing and the chlorhexidine‐impregnated paraffin gauze dressing (P = 0.12). In Phipps 1988 no significant difference in pathogenic bacterial isolates between hydrocolloid dressing and the chlorhexidine‐impregnated paraffin gauze dressing was noted (P = 0.02), although the organism most commonly acquired in both groups was Staphylococcus aureus.
1.1. Analysis.

Comparison 1 Hydrocolloid dressing vs chlorhexidine‐impregnated gauze dressing, Outcome 1 Withdrawal due to wound infection.
Other outcome measures such as change in wound surface area, cost of the dressings, quality of life, length of stay and need for surgery were not addressed by any of these studies.
Summary
Overall there is no evidence of a difference between hydrocolloid dressings and chlorhexidine‐impregnated paraffin gauze, although the evidence is of poor quality.
b. Hydrocolloid dressings compared with chlorhexidine‐impregnated paraffin gauze dressing plus silver sulphadiazine (SSD) cream (one trial, 48 people)
Time to complete wound healing
One study by Afilalo 1992 compared hydrocolloid dressings with chlorhexidine‐impregnated paraffin gauze plus SSD after initial burn in 48 adults with partial thickness burns. They found no statistically significant difference between treatments for the time to wound healing (time to wound healing in the hydrocolloid group: 10.7 days (4.8), paraffin gauze group:11.2 days (4.2) P = 0.76), however 18 out of 48 participants were lost to follow‐up (nine from each group) and this may have introduced bias.
Number of dressing changes
Dressings were changed less frequently with hydrocolloid dressing compared with chlorhexidine‐impregnated paraffin gauze plus SSD (mean number of dressing changes: three with hydrocolloid dressing, eight with chlorhexidine‐impregnated paraffin gauze plus SSD; P < 0.02). Although reasons for dressing changes were not given, this result was to be expected, as chlorhexidine‐impregnated paraffin gauze plus SSD dressings were changed routinely, whereas hydrocolloid dressings were only changed when there was an indication of leakage or suspected infection.
Level of pain
There was no significant difference between treatment groups for pain. Afilalo 1992 reported median pain score baseline: 3/10 in the hydrocolloid group, 2/10 in the chlorhexidine‐impregnated paraffin gauze plus SSD group; this was reported as non‐significant; the variance data and the P value were not reported. The median pain score at second visit: 0/10 with hydrocolloid compared with 1/10 with chlorhexidine‐impregnated paraffin gauze plus SSD; reported as non‐significant; the variance data and P value were not reported.
Patient perception, level of satisfaction with the application and removal of dressing
Afilalo 1992 reported that application and removal were more frequently rated as "easy" with hydrocolloid dressing compared with chlorhexidine‐impregnated paraffin gauze plus silver sulphadiazine.
Adverse events
Afilalo 1992 did not report any wound infections. However, three people who developed cellulitis during treatment were excluded from the RCT.
Other outcome measures such as change in wound surface area, cost of the dressings, quality of life, length of hospital stay and need for surgery were not addressed by this study.
Summary
Overall we found no evidence of a difference between hydrocolloid dressing and chlorhexidine‐impregnated paraffin gauze dressing plus SSD cream, although there is only poor quality evidence.
c. Hydrocolloid dressing compared with silver sulphadiazine cream (one trial, 50 people)
Time to complete wound healing
We found one study (Wyatt 1990) which compared hydrocolloid dressings with sterile gauze plus SSD after initial burn cleaning. Hydrocolloid dressing significantly reduced mean healing time when compared with SSD. Hydrocolloid dressing (10.23 days +/‐ 3.19) versus SSD (15.59 days +/‐ 8.32) (P < 0.01). Wyatt also found that after complete wound healing, wound appearance, re‐pigmentation and overall investigator/participant satisfaction were significantly better with the hydrocolloid dressing compared with SSD (wound appearance: P < 0.01; re‐pigmentation: P < 0.01; investigator/participant satisfaction: P < 0.001). An assessor, blind to treatment allocation, rated overall wound healing and reported that 64% of wounds in the hydrocolloid group appeared healthy and well hydrated compared with 35% of wounds in the SSD group.
Number of dressing changes
There were significantly fewer dressing changes with the hydrocolloid dressing compared with SSD (mean number of dressing changes: 3.55 with hydrocolloid dressing versus 22.2 with SSD; mean difference (MD) ‐18.65 95% confidence interval (CI) ‐22.54 to ‐14.76; P < 0.00001) (Analysis 2.1). The number of minutes taken to change the dressing was 4.82 minutes with hydrocolloid versus 9.05 minutes with SSD; P < 001). However, this result was to be expected, as SSD dressings were changed routinely, whereas there was no indication to change the hydrocolloid dressings without leakage or suspected infection.
2.1. Analysis.
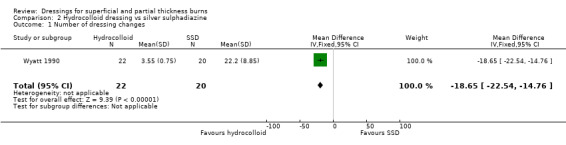
Comparison 2 Hydrocolloid dressing vs silver sulphadiazine, Outcome 1 Number of dressing changes.
Level of pain
Patients graded pain on a scale of 0 to 10 (0 = no pain, 10 = maximum pain). Pain was significantly more severe in the those treated with SSD than those treated with a hydrocolloid dressing (mean pain score 2.28 for those in the SSD group versus 1.09 for those treated with a hydrocolloid dressing; MD ‐1.19 95%CI ‐1.82 to ‐0.56; P < 0.00002) (Analysis 2.2).
2.2. Analysis.
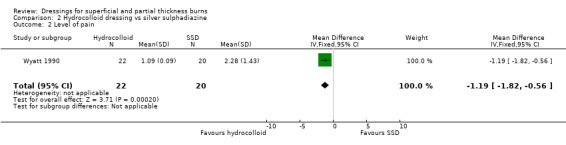
Comparison 2 Hydrocolloid dressing vs silver sulphadiazine, Outcome 2 Level of pain.
Patient perception, level of satisfaction with the application and removal of dressing
Dressing application and removal were rated as easier, and dressing comfort as better with hydrocolloid dressing compared with SSD (P < 0.01).
Other outcome measures such as change in wound surface area, cost of the dressings, quality of life, length of hospital stay, adverse events and need for surgery were not addressed by this study.
Summary
Overall we found that hydrocolloid dressings may heal burns more quickly than SSD cream, although this evidence is low quality.
2. Polyurethane film dressing
A total of two trials compared polyurethane film dressings with alternatives.
a. Polyurethane film dressing compared with paraffin gauze dressing (one trial, 55 people)
We found one study by Poulsen 1991, which compared polyurethane film with paraffin gauze dressing.
Time to complete wound healing
The study reported that no significant difference was found between polyurethane film and the paraffin‐impregnated gauze in time to wound healing (median days to wound healing: seven days (range six to 30 days) with paraffin gauze compared with 10 days (range five to 24 days) with polyurethane film; P > 0.05).
Patient perception, level of satisfaction with the application and removal of dressing
The same study reported no significant difference between groups in participant satisfaction (satisfaction ratings were self assessed or, in the case of children, assessed by their parents; proportion of people "satisfied": 27/29 (96%) with polyurethane film versus 20/25 (80%) with paraffin gauze; reported as not significant; P value not reported).
Level of pain
Patients were assessed for pain on a four‐item scale for degrees of no pain, mild, moderate and severe pain. A total of 3/30 (10%) patients with polyurethane film compared with 4/24 (16%) with paraffin gauze reported moderate to severe pain; differences reported as not significant; P value not reported.
Incidence of infection
There was no difference in rates of wound infection, 3/30 (10%) people in the polyurethane group and 2/25 (8%) people in the paraffin gauze group (risk ratio (RR) 1.25, 95% CI 0.23 to 6.90; P = 0.80) (Analysis 3.1). No infection required antibiotic treatment.
3.1. Analysis.

Comparison 3 Polyurethane film dressing vs paraffin gauze dressing, Outcome 1 Wound infection.
Adverse events
Poulsen 1991 reported skin reactions such as follicular exanthema and itching in 2/30 (7%) people with polyurethane film (data for control group not reported).
Other outcome measures such as change in wound surface area, cost of the dressings, number of dressing changes, quality of life, length of hospital stay, adverse events and need for surgery were not addressed by this study.
Summary
Overall we found no evidence of a difference between polyurethane film and paraffin gauze dressing, although there is only poor quality evidence.
b. Polyurethane film dressing compared with chlorhexidine‐impregnated paraffin gauze dressing (one trial, 51 people)
Time to complete wound healing
We found one study by Neal 1981 which compared polyurethane film with chlorhexidine‐impregnated paraffin gauze dressing. The author found polyurethane film significantly reduced healing time compared with chlorhexidine‐impregnated paraffin gauze (mean healing time: 10.0 days (standard deviation (SD) 5.00) with polyurethane film, 14.1 days (SD 7.00) with chlorhexidine‐impregnated paraffin gauze; P = 0.02). The RCT found that at 10 days after injury, polyurethane film significantly increased healing compared with chlorhexidine‐impregnated paraffin gauze (results presented graphically; P < 0.05). However, more than 10 days after injury, there was no significant difference in wound healing between the two treatment groups (results presented graphically; P value not given but study author reported as not significant).
Level of pain
Less pain (by comparative ranking on a "pain" perception diagram assessing intensity and duration) was experienced with polyurethane film compared with chlorhexidine‐impregnated paraffin gauze (P < 0.01; results presented graphically).
Incidence of infection
There was no significant difference in rates of wound infection between the two groups (1/26 (4%) with polyurethane film versus 2/25 (8%) with chlorhexidine‐impregnated paraffin gauze; RR 0.48, 95% CI 0.05 to 4.98; P = 0.54) (Analysis 4.1).
4.1. Analysis.
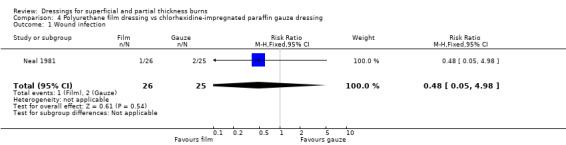
Comparison 4 Polyurethane film dressing vs chlorhexidine‐impregnated paraffin gauze dressing, Outcome 1 Wound infection.
Other outcome measures such as change in wound surface area, cost of the dressings, number of dressing changes, quality of life, length of hospital stay and need for surgery were not addressed by this study.
Summary
Overall, there was some evidence that polyurethane film dressings may be more effective in healing partial thickness burns than chlorhexidine‐impregnated paraffin gauze dressings, although there is only poor quality evidence.
3. Hydrogel dressings
a. Hydrogel dressing compared with usual care (three trials, 235 people)
We found three RCTs which compared hydrogel dressings with usual care (either SSD, paraffin gauze or paraffin gauze with antibiotics) (Grippaudo 2010; Guilbaud 1992; Guilbaud 1993).
Time to complete wound healing
Guilbaud 1992 found healing times to be shorter in the group allocated to the hydrogel dressing (mean wound healing times: 11.92 days (SD 5.91) with hydrogel dressing (n = 51) versus 13.55 days (SD 6.70) with usual care (n = 51); P < 0.02). Guilbaud 1993 showed no statistical difference although a trend in favour of the hydrogel was noted (mean healing time: 13.6 days (SD 9.6) with hydrogel dressing versus 15.1 days (SD 6.45) with usual care; P = 0.07).
Number of people healed
Grippaudo 2010 found significantly more people in the hydrogel treatment group had healed at nine days (RR 2.00, 95% CI 1.08 to 3.72; P = 0.03) (Analysis 5.2) and 12 days (RR 1.68, 95% CI 1.17 to 2.42; P = 0.005) (Analysis 5.4). No significant differences were found between the treatment groups in the number of people healed at six days, 15 days, 18 days and 21 days.
5.2. Analysis.

Comparison 5 Hydrogel dressing vs usual care, Outcome 2 Wound healing: number of people healed at 9 days.
5.4. Analysis.
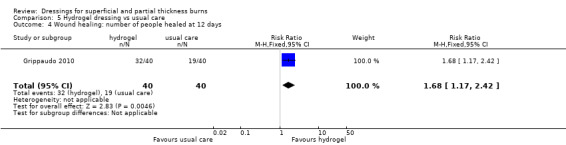
Comparison 5 Hydrogel dressing vs usual care, Outcome 4 Wound healing: number of people healed at 12 days.
Level of pain
Two studies report on pain at dressing application and removal. The tool used to describe pain assessment in the study by Guilbaud 1993 was not described and data not reported, although it was reported narratively that pain following dressing application was reduced at days two, four and eight; P < 0.0001. Guilbaud 1992 reported pain assessments at baseline, 30 minutes after treatment, at days two, four and eight and an overall assessment at the end of the study. There was no significant difference between the groups at baseline but there was significant less pain in the hydrogel group at the end of the study (MD ‐1.31, 95% CI ‐2.37 to ‐0.25) (Analysis 5.7; Analysis 5.9).
5.7. Analysis.

Comparison 5 Hydrogel dressing vs usual care, Outcome 7 Assessment of pain at baseline.
5.9. Analysis.
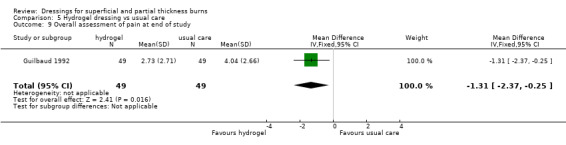
Comparison 5 Hydrogel dressing vs usual care, Outcome 9 Overall assessment of pain at end of study.
Number of dressing changes
Guilbaud 1993 found fewer dressing changes with the hydrogel dressings compared with the control (mean number of dressings reported graphically). Guilbaud 1992 also found the rate of renewal (the ratio between healing time and number of dressings) was 8.2 days for the hydrogel dressing with 3.5 days for control sites. Twenty‐seven (51.9%) treated with a hydrogel dressing had one application whereas two treated (3.8%) in the control group had one application.
Adverse events
Guilbaud 1992 noted the incidence of local events, especially exudate and suppuration and was similar with both groups, but only noticed 6/52 (11.5%) patients revealing positive bacteriological cultures. In the six patients (12 sites), three specimens were positive in the hydrogel sites versus six in the control sites.
Infection with Pseudomonas aeruginosa requiring antibiotic therapy
Grippaudo 2010 found that there was no difference in the number of patients becoming infected with Pseudomonas aeruginosa that required antibiotic therapy (RR 0.33, 95% CI 0.01 to 7.95; P = 0.50)(Analysis 5.10).
5.10. Analysis.
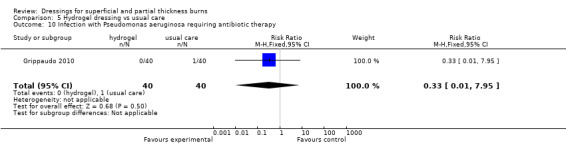
Comparison 5 Hydrogel dressing vs usual care, Outcome 10 Infection with Pseudomonas aeruginosa requiring antibiotic therapy.
Other outcome measures such as change in wound surface area, cost of the dressings, quality of life, length of hospital stay and need for surgery were not addressed by these studies.
Summary
Overall hydrogel dressings may heal partial thickness burns more quickly than usual care, although the evidence is of low quality.
4. Silicon‐coated nylon dressings
a. Silicon‐coated nylon dressings compared with silver sulphadiazine (two trials, 142 people)
Time to complete wound healing
We found two RCTs which compared silicon‐coated nylon dressings with SSD (Bugmann 1998; Gotschall 1998). Bugmann 1998 found the mean time to full epithelialisation to be significantly shorter with silicon‐coated nylon dressings (mean healing time: 7.58 days (+/‐ 3.12) with silicone‐coated nylon versus 11.26 days (+/‐ 6.02) with silver sulphadiazine; P < 0.01). Gotschall 1998 reported the median time to full epithelialisation to be shorter with silicon‐coated nylon dressings (median time to full re‐epithelialisation of the wound: 10.5 days with silicone mesh dressing compared with 27.6 days with SSD; P = 0.0002). No variance data were reported for this outcome.
Level of pain
Gotschall 1998 found that the silicon‐coated mesh nylon dressing reduced pain (measured on the Objective Pain Scale (OPS), where 0 = no pain and 10 = severe pain) in the first five days after injury compared with SSD (mean pain score over first five days on pain scale: 4.0 with silicone mesh dressing versus 4.9 with SSD; P < 0.025). No variance data were reported for this outcome. They also found that mean pain score at dressing change (measured on the OPS) was significantly lower with silicone mesh dressing compared with SSD in the first five days after burn injury. Bugmann 1998 did not report on pain.
Number of dressing changes
Bugmann 1998 noted that there were significantly fewer dressing changes with silicone‐coated nylon net dressing than with SSD (3.64 with silicone‐coated nylon net dressing versus 5.13 with SSD; MD ‐1.49, 95% CI ‐2.64 to ‐0.34; P < 0.01) (Analysis 6.1). As the dressings were changed every two to three days until complete healing was obtained, this result was not surprising but simply a result of the longer healing period with SSD. The RCT found no fluid collection, haematoma or secondary displacement in either group.
6.1. Analysis.
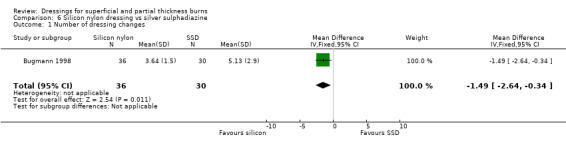
Comparison 6 Silicon nylon dressing vs silver sulphadiazine, Outcome 1 Number of dressing changes.
Cost of the dressing
Gotschall 1998 reported on resource use and noted that children treated with silicone‐coated nylon net dressing incurred lower total charges for dressing changes from USD 739 per hospitalisation versus USD 413 for those treated with SSD (P<0.05).
Adverse events
Gotschall 1998 noted that SSD significantly increased the risk of moderate to severe eschar formation compared with silicone mesh dressing (42% with SSD versus 6% with silicone mesh dressing; P < 0.0001). Gotschall 1998 also noted that none of the wounds in either treatment arm exhibited signs of infection during the dressing changes. However, it was reported that wound cultures for children treated with silicone mesh dressing did yield both a wider variety of bacterial flora and larger amounts of bacterial growth. Three children in the silicone mesh dressing group developed fevers of unknown origin followed by a diffuse maculopapular rash. They were excluded from the RCT on a precautionary basis, although their wounds healed without complication. Treatment regimens for these three children were not reported. Bugmann 1998 reported one case of infection and two cases of bleeding in the SSD group, with one case of bleeding reported in the silicon dressing group.
Other outcome measures such as change in wound surface area, quality of life, patient perception and level of satisfaction with application and removal of dressing, length of hospital stay and need for surgery were not addressed by these studies.
Summary
Overall the evidence suggests that silicon‐coated nylon dressings may heal partial thickness burns more quickly than SSD, although there is only poor quality evidence.
5. Biosynthetic skin substitute dressings
A total of 10 trials (11 comparisons) compared various biosynthetic dressings with a range of alternatives.
a. Biosynthetic dressings compared with silver sulphadiazine (six trials, 267 people)
Time to complete wound healing
We found six studies that compared biosynthetic dressings with SSD. Five studies compared one type of biosynthetic dressing with SSD (Barret 2000; Gerding 1988; Gerding 1990; Lal 1999; Noordenbos 1999) and one study compared two different types of biosynthetic dressings with SSD in a three‐arm design. All six studies individually reported a significantly shorter wound healing time with the use of biosynthetic dressing compared with SSD. Barret 2000 noted mean healing times to be 9.7 days (+/‐ 0.7) with biosynthetic dressing (Biobrane) compared with 16.1 days (+/‐ 0.6) with SSD (P < 0.001). Gerding 1988 found healing times to be 13.7 days (+/‐ 6.75) with biosynthetic dressing (Biobrane) compared with 21.3 days (+/‐ 11.03) with SSD (P < 0.01). Gerding 1990 noted that, in their sample, the greatest difference in healing time was observed in grease/tar burns (mean healing time in biosynthetic dressing (Biobrane) group: 8.4 days (+/‐ 1.0) compared with 18.5 days (+/‐ 5.0) for those in the SSD group (P < 0.02)). Lal 1999 reported on time to heal per percent total body surface area burned (in participants < 3 years old: 1.52 days in biosynthetic dressing (Biobrane) compared with 2.35 days with SSD (P 0.025); in participants aged 3 to 17 years: 1.00 days in biosynthetic dressing compared with 2.40 days with SSD (P 0.026) (information presented graphically)). Noordenbos 1999 included 14 people and identified paired wound sites which were randomised to treatment with a biosynthetic dressing (TransCyte) or SSD. The author reported on days until 90% healed and found that the biosynthetic dressing significantly reduced healing time compared with SSD (days until 90% healed: 11.14 days (SD 4.37) with biosynthetic dressing versus 18.14 days (SD 6.05) with SSD (paired t test P = 0.002). Kumar 2004 randomised 33 people with 58 wound sites to three different burn dressings (TransCyte, Biobrane and SSD) Wound healing, measured as mean time to re‐epithelialisation, was 7.5 days for TransCyte; 9.5 days for Biobrane and 11.2 days for SSD (P < 0.001). No variance data were reported. Healing progression was estimated visually by two independent observers but it was not reported whether or not they were blind to treatment allocation and this could be a source of bias. It was not appropriate to pool these studies due to heterogeneity, missing variance data, different types of burns and unit of analysis errors.
Level of pain
Pain was assessed by Barret 2000; Gerding 1988 and Gerding 1990 using scales of different magnitudes. Barret 2000 using a visual analogue scale plus face scale noted a difference in pre‐treatment pain baseline scores (3.3 for those randomised to biosynthetic dressing versus 3.8 for those assigned to SSD; P value not significant). Relief following dressing application was reduced to 2.4 with biosynthetic dressing and 3.7 with those assigned SSD at day 1; P <0.001; and 2.6 with biosynthetic dressing and 3.8 with those assigned SSD at day 2; P < 0.001. Gerding 1988 and Gerding 1990 noted a difference in pain scores (measured on a visual analogue scale using a five‐point scale with 1 = no pain and 5 = severe pain) at first follow‐up visit. Pooling these two trials using a random effects model (I2 = 75.5%) demonstrated a statistically significant difference (MD ‐1.63, 95% CI ‐2.20 to ‐1.06) (Analysis 7.1). Although Kumar 2004 did not report pain scores using any form of validated pain scale, patients treated with biosynthetic dressings required significantly fewer pain medications than those treated with SSD (P = 0.0001; type, route and dose of analgesia not reported).Pain was not reported by Lal 1999.
7.1. Analysis.
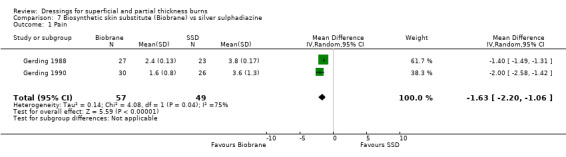
Comparison 7 Biosynthetic skin substitute (Biobrane) vs silver sulphadiazine, Outcome 1 Pain.
Out of interest, Gerding 1988 also found that patients in the biosynthetic dressing treatment arm used fewer pain relieving tablets than those receiving SSD (1.4 tablets in biosynthetic dressing versus 3.5 tablets in SSD; P < 0.01, dosage and type of analgesic not reported). In the Gerding 1990 study, on average, fewer doses of narcotics were also given to those receiving biosynthetic dressing (12 doses in the biosynthetic dressing group versus 16.9 doses in the SSD group; P value not significant, dosage and type of analgesic not reported). Barret 2000 found similar results: (0.5 doses/person/day in biosynthetic dressing versus 1.9 doses/person/day with SSD; P <0.002, dosage and type of analgesia not reported).
Need for surgery
Gerding 1988 also noted that five participants (22%) in the SSD arm obtained split‐thickness skin graft to close the granulation defects compared with four patients (15%) who were treated with biosynthetic dressing (RR 0.68; 95% CI 0.21 to 2.24;P = 0.53) (Analysis 7.2). Kumar 2004 found that there were five wounds in the SSD group that required auto‐grafting, three in the Biobrane and one in the TransCyte group. Patients treated with biosynthetic dressings underwent auto‐grafting due to infection and loss of product. Patients treated with SSD underwent grafting due to delay to re‐epithelialisation.
7.2. Analysis.

Comparison 7 Biosynthetic skin substitute (Biobrane) vs silver sulphadiazine, Outcome 2 Need for surgery.
Length of hospital stay
Lal 1999 noted that hospital length of stay was shorter in those receiving biosynthetic dressing compared with SSD in both toddlers and infants (age 0 to 3 years; P = 0.002) and older children (age > 3 years; P = 0.0026).
Incidence of infection
Wound infection and other systemic complications were reported in three studies (Gerding 1988; Gerding 1990; Noordenbos 1999). The remaining studies reported no infection (Barret 2000) or only suspected, but not confirmed (Lal 1999). Infection was poorly defined by many of the studies. Gerding 1988 reported the development of bacterial growth in four wounds in each group with two of the infected in each group requiring surgical excision and grafting. Gerding 1990 noted that there were three infections in those patients assigned to biological dressings and two infections in those assigned to SSD. One patient in each group required skin grafting. Noordenbos 1999 noted that six patients developed mild cellulitis in the SSD arm of the trial, and all incidents responded to intravenous antibiotics. No wounds became infected during treatment with the biosynthetic dressing.
Number of dressing changes
Kumar 2004 found fewer dressing changes with either TransCyte or Biobrane compared with SSD. The number of dressing changes: 1.5 with TransCyte and 2.4 with Biobrane compared with 9.2 with Silvazene cream (P < 0.0001).
Other outcome measures such as change in wound surface area, cost of the dressing and quality of life were not addressed by these studies.
Summary
Overall there is consistent evidence that biosynthetic dressings are more effective than SSD, although there is only poor quality evidence.
b. Biosynthetic dressing (Biobrane) compared with hydrocolloid dressing (one trial, 72 people)
Time to complete wound healing
We found one study by Cassidy 2005 which compared a biosynthetic dressing with a hydrocolloid dressing. No significant difference was found between the biosynthetic dressing and the hydrocolloid dressing in mean time to wound healing: 12.24 days with the biosynthetic dressing compared with 11.21 days for the hydrocolloid dressing (MD 1.03, 95% CI ‐1.66 to 3.72; P = 0.45).
Level of pain
Pain assessment was performed using the Oucher Scale in 34 participants and the VAS utilised for the remaining 37 patients. The study authors do not make it clear if the use of these two scales was balanced across both groups. Cassidy 2005 noted no statistically significant difference in mean aggregate scores (2.36 for those randomised to biosynthetic dressing versus 2.37 with hydrocolloid dressing; P = 0.99).
Cost of the dressing
Cassidy 2005 reported that cost of each treatment was higher in the biosynthetic dressing group, regardless of the size or thickness of the dressing (P < 0.0001). This cost was obvious and not unexpected given the nature of biosynthetic dressing technology compared with older, simpler dressings such as a hydrocolloid.
Other outcome measures such as change in wound surface area, number of dressing changes, adverse events, quality of life, need for surgery and patient perception and level of satisfaction with application and removal of dressing were not addressed by this study.
Summary
Overall there was no evidence of a difference in burn healing between biosynthetic dressings and hydrocolloid dressings, although the single trial was poorly reported and may be at risk of bias.
c. Antimicrobial‐releasing biosynthetic dressings (Hydron) compared with silver sulphadiazine or other agents (three trials, 95 people)
Time to complete wound healing
We found three RCTs which compared antimicrobial‐releasing biosynthetic dressings with SSD (Curreri 1980; Fang 1987; Husain 1983). Husain 1983 reported average healing times to be significantly shorter with antimicrobial releasing biosynthetic dressing (6.8 days) compared with 11.7 days with SSD; P value and variance data not reported. Curreri 1980 reported time to complete wound healing to be more rapid in wounds covered with antimicrobial releasing biosynthetic dressing rather than SSD (numerical or graphical data not provided; P value not reported).
Number of dressing changes
Fang 1987 noted that on average there were 93 dressing applications making it an average of more than three dressings per patient. In most patients (number of patients not reported), the antimicrobial‐releasing biosynthetic dressing remained in place for almost four days, in the same time period the control site required four dressing changes. Although the number of dressing changes was not stated by Husain 1983, the authors reported on their response to the dressing. Treating nurses and participants rated the antimicrobial releasing biosynthetic dressing more highly than the control using the classification favourable, unfavourable or no difference. Favourable ratings were self‐assessed and proportion of treating nurses in favour was: 41/50 (82%) treating nurses versus 34/50 (68%) patients; unfavourable: 9/50 (18%) treating nurses versus 11/50 (22%) patients and no difference was recorded in 5/50 (10%) of patients.
Patient perception, level of satisfaction with the application and removal of dressing
In contrast, Curreri 1980 reported that 81% of the sample population found that the antimicrobial‐releasing biosynthetic dressing was more difficult and time‐consuming to apply than SSD (definition and description of difficulty not provided; number of minutes defining time‐consuming not reported).
Need for surgery
Fang 1987 noted that eight patients in the antimicrobial releasing biosynthetic dressing required grafting and seven in the SSD group.
Incidence of infection
Husain 1983 noted that wound infection developed in 15/50 (30%) sites treated with antimicrobial‐releasing biosynthetic dressing and 8/50 (16%) sites for those treated with SSD; this difference was not statistically significant (RR 1.88 95%CI 0.87 to 4.02; P = 0.11) (Analysis 8.1). Fang 1987 reported on bacterial colonisation rather than infection.
8.1. Analysis.
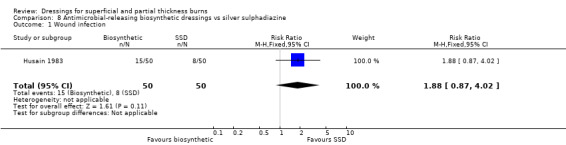
Comparison 8 Antimicrobial‐releasing biosynthetic dressings vs silver sulphadiazine, Outcome 1 Wound infection.
Other outcome measures such as change in wound surface area, cost of the dressings, quality of life and length of hospital stay were not addressed by these studies.
Summary
Overall we found some evidence that antimicrobial‐releasing biosynthetic dressings may heal burns more quickly than SSD or other agents, although the evidence is generally of poor quality
6. Antimicrobial (silver‐containing) dressings
a. Silver‐impregnated dressings compared with silver sulphadiazine (five trials, 331 patients)
We found five RCTs which compared silver‐impregnated dressing (Acticoat, Smith and Nephew USA) with SSD (Gong 2009; Huang 2004; Muangman 2006; Opasanon 2010; Varas 2005).
Time to complete wound healing
Gong 2009 and Opasanon 2010 found mean healing time significantly shorter in those patients treated with silver dressings compared with SSD. We have pooled these studies as they appear to report on all wounds until complete healing (MD ‐4.22, 95% CI ‐5.92 to ‐2.52; P < 0.00001) (Analysis 9.1). Huang 2004 also found mean healing time to be significantly shorter in the intervention group, but we have not included this study in the meta‐analysis because of censored data. Muangman 2006 and Varas 2005 did not report on time to complete wound healing.
9.1. Analysis.
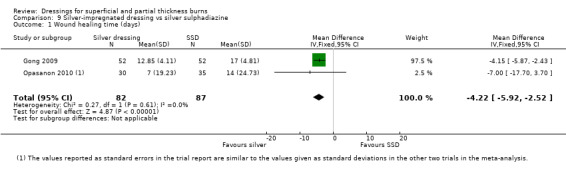
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 1 Wound healing time (days).
Number of people healed
Gong 2009 found that the number of people healed at seven days, 10 days and 17 days was not significantly different for silver dressings compared with SSD (Analysis 9.2; Analysis 9.3; Analysis 9.5). However, at 15 days, Gong 2009 and Huang 2007 found that the number of people healed was significantly more for silver dressings than SSD (RR 1.17, 95% CI 1.02 to 1.35; P = 0.03) (Analysis 9.4) and at 21 days Gong 2009 also found a significant effect in favour of silver dressings (RR 1.21, 95% CI 1.06 to 1.37; P = 0.004) (Analysis 9.6).
9.2. Analysis.
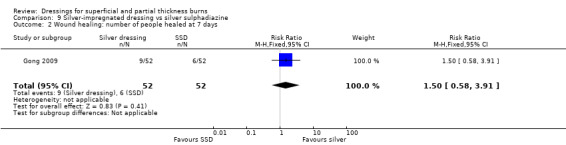
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 2 Wound healing: number of people healed at 7 days.
9.3. Analysis.
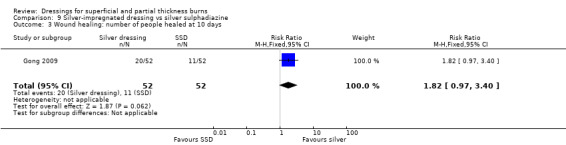
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 3 Wound healing: number of people healed at 10 days.
9.5. Analysis.
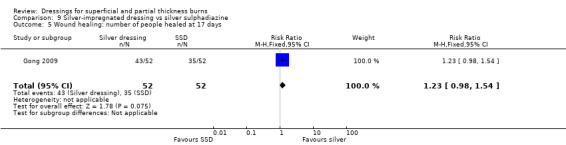
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 5 Wound healing: number of people healed at 17 days.
9.4. Analysis.
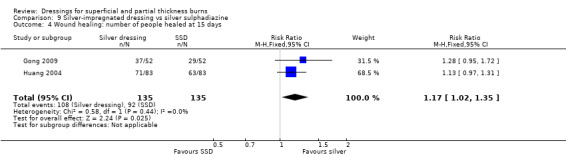
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 4 Wound healing: number of people healed at 15 days.
9.6. Analysis.
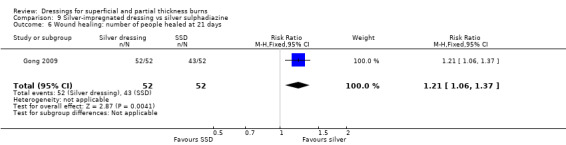
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 6 Wound healing: number of people healed at 21 days.
Healing rate
Huang 2004 found that there was no difference in the rate of healing between silver dressings and SSD (MD 2.21, 95% CI ‐2.37 to 6.79; P = 0.34) (Analysis 9.7).
9.7. Analysis.
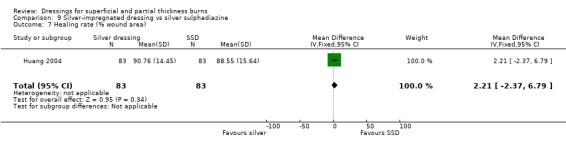
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 7 Healing rate (% wound area).
Level of pain
The studies by Muangman 2006, Opasanon 2010 and Varas 2005 found that silver‐impregnated dressings reduced pain (measured on a visual analogue scale (VAS ‐ scale 1 to 10) compared with SSD. These trials were pooled using a random‐effects model due to the high level of heterogeneity (I2 = 81%) and the difference was not statistically significant (MD ‐2.84; 95% CI ‐5.89 to 0.21) (Analysis 9.8).
9.8. Analysis.
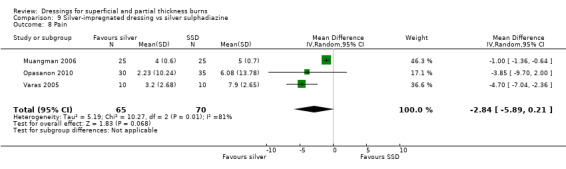
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 8 Pain.
Need for surgery
Muangman 2006 noted that six participants (24%) in the SSD arm obtained split‐thickness skin graft to close the granulation defects compared with four patients (16%) who were treated with silver dressing (RR 0.67; 95% CI 0.21 to 2.08 P = 0.48) (Analysis 9.9).
9.9. Analysis.
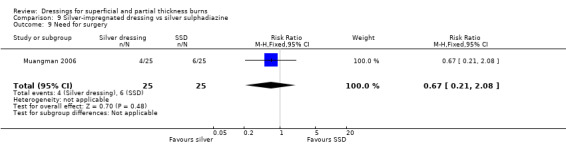
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 9 Need for surgery.
Hospital length of stay
Muangman 2006 reported no difference in hospital length of stay between the two groups.
Incidence of infection
Gong 2009, Huang 2004, Muangman 2006 and Varas 2005 found that there was no significant difference between silver dressings and SSD in the number of infections (RR 1.04, 95% CI 0.64 to 1.67) (Analysis 9.10).
9.10. Analysis.
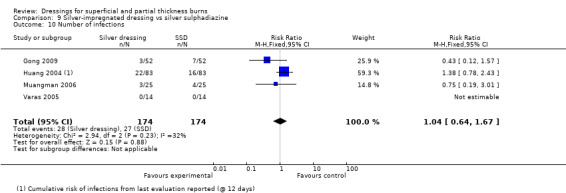
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 10 Number of infections.
Huang 2004 found a total of 56 bacterial strains in 166 wounds which cleared on the 6th and 12th day post antibiotic treatment.
Number of wound dressings
Opasanon 2010 found that there were significantly fewer wound dressings used for silver dressings than for SSD (MD ‐11.07, 95% CI ‐19.58 to ‐2.56; P = 0.01) (Analysis 9.11).
9.11. Analysis.
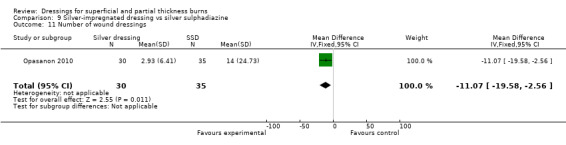
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 11 Number of wound dressings.
Nursing time
Opasanon 2010 found that there was no difference in nursing time between the silver dressing group and the SSD group (MD ‐4.82, 95% CI ‐19.42 to 9.78; P = 0.52) (Analysis 9.12).
9.12. Analysis.
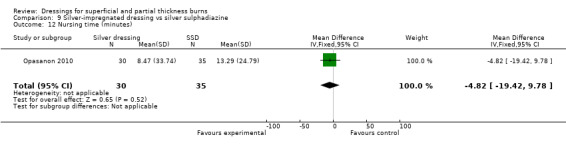
Comparison 9 Silver‐impregnated dressing vs silver sulphadiazine, Outcome 12 Nursing time (minutes).
Other outcome measures such as change in wound surface area, cost of the dressings, quality of life, patient perception and level of satisfaction with application and removal of dressing were not addressed by these studies.
Summary
Overall there was evidence that silver‐impregnated dressings heal burns more quickly than SSD, although the evidence is of poor quality.
7. Fibre dressings
a. Calcium alginate compared with silver sulphadiazine (one trial, 59 people)
Time to complete wound healing
We found one RCT by Costagliola 2002 (59 people with 73 partial thickness burns) which compared calcium alginate with SSD. It found no significant difference between calcium alginate and SSD in time to healing (12.1 days with calcium alginate versus 11.7 days with SSD; P value and variance data not reported).
The author states that he found no significant difference between groups in terms of pain and the amount of care required (however pain scale assessment and scores not provided; definition of amount of care are not described).
Other outcome measures such as change in wound surface area, cost of the dressings, quality of life, patient perception and level of satisfaction with application and removal of dressing, and length of hospital stay were not addressed by this study.
Summary
Overall there was no evidence that calcium alginate dressings are more effective than SSD, although the evidence is of poor quality.
b. Hydrofibre dressing compared with silver sulphadiazine (two trials, 154 people)
Time to complete wound healing
Muangman 2010 found a significantly shorter healing time for hydrogel fibre dressing when compared with SSD (MD ‐3.70, 95% CI ‐5.44 to ‐1.96; P < 0.0001) (Analysis 10.1). Caruso 2006 also compared a hydrogel fibre dressing with SSD. No significant difference was found between the hydrogel dressing and SSD in time to wound healing: median wound healing time 16 days with hydrogel fibre versus 17 days with SSD; P = 0.517. However, these data were not pooled as no variance data were reported.
10.1. Analysis.
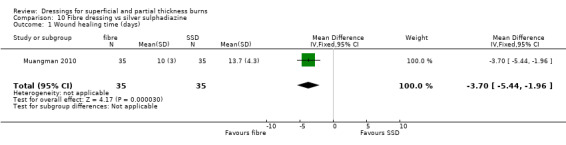
Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 1 Wound healing time (days).
Level of pain
Caruso 2006 evaluated pain using the Johns Hopkins visual analogue scale for those aged four years and older and investigator‐reported pain scores for the pre‐verbal population. Hydrogel fibre dressing reduced pain during dressing changes (mean pain score: 3.63 with hydrogel fibre dressing versus 4.77 with SSD; P = 0.003). There was no difference in the investigator‐reported pain scores between the hydrogel fibre and SSD groups (mean pain scores 3.52 with hydrogel fibre dressing versus 3.32 with SSD; P = 0.991). Fewer types of procedural medications (2.4 doses versus 3.4 doses; P = 0.18; and procedural opiates (1.5 doses versus 2.1 doses; P = 0.022) were administered in the hydrogel fibre dressing group compared with the SSD group. Drug names, routes and dosages were not reported.
Muangman 2010 evaluated level of pain on a 10‐point Likert scale and found that hydrofibre was superior to SSD in the level of pain at day one (MD ‐2.00, 95% CI ‐3.03 to ‐0.97; P = 0.0001) (Analysis 10.2), day three (MD ‐3.10, 95% CI ‐4.02 to ‐2.18; P < 0.00001) (Analysis 10.3) and day seven (MD ‐2.40, 95% CI ‐3.18 to ‐1.62; P < 0.00001) (Analysis 10.4).
10.2. Analysis.

Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 2 Pain at day 1.
10.3. Analysis.

Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 3 Pain at day 3.
10.4. Analysis.

Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 4 Pain at day 7.
Number of dressing changes
Caruso 2006 found fewer dressing changes with hydrogel fibre dressing compared with SSD (mean number of dressing changes: 7.7 with hydrogel fibre dressing versus 19.1 with SSD (MD ‐11.40, 95% CI ‐15.66 to ‐7.14; P <0.0001) (Analysis 10.5). However, this result was to be expected, as SSD dressings were changed routinely, whereas there was no indication to change hydrogel fibre dressings other than every second day. Dressing application, comfort of dressing and patient comfort were not significantly different between treatment groups.
10.5. Analysis.
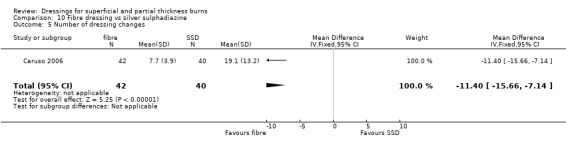
Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 5 Number of dressing changes.
Cost of dressings
Mean total cost of primary dressings during the study was significantly greater for the hydrogel fibre dressing than for SSD (mean cost of primary dressing: USD 684 with hydrogel fibre dressings versus USD 398 in SSD group; P = 0.007). As expected, the mean cost for the secondary dressing (i.e. gauze dressing application over primary dressing) was lower for the hydrogel fibre group than the SSD group (mean secondary cost USD 68.10 with hydrogel fibre dressings versus USD138.00 in SSD group; P = 0.004). When total treatment costs were compared, costs were comparable amongst the two groups (mean total dressing cost: USD 848.50 with hydrogel fibre dressing versus USD 759.60 with SSD group).
Incidence of infection
Caruso 2006 noted similar rates of wound infection in the two treatment groups (8/42 with hydrogel fibre dressing compared with 6/40 with SSD; RR 1.27, 95% CI 0.48 to 3.34) (Analysis 10.6). Patients who developed infections were treated with antibiotics.
10.6. Analysis.
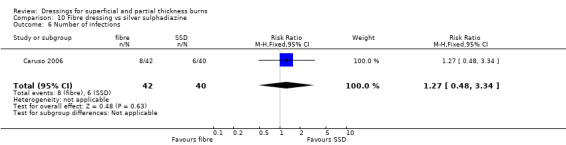
Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 6 Number of infections.
Need for surgery
The need for skin grafting due to re‐classification of a partial thickness burn as a full thickness burn or because of infection was required in both groups (5.04/42 (12%) with hydrogel fibre dressing versus 7.2/40 (18%) SSD (RR 0.68, 95% CI 0.24 to 1.97; P = 0.48) (Analysis 10.7).
10.7. Analysis.
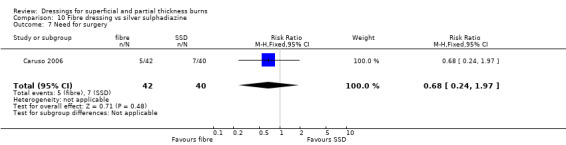
Comparison 10 Fibre dressing vs silver sulphadiazine, Outcome 7 Need for surgery.
Other outcome measures such as change in wound surface area, quality of life and length of hospital stay were not addressed by this study.
Summary
Overall there was no clear difference between hydrofibre dressings and SSD, although the trials were of poor quality.
Discussion
This systematic review summarises the best available evidence relating to the effects of dressings used to treat superficial or partial thickness burns. A total of 30 randomised controlled trials met the inclusion criteria for the review. Overall the quality of the evidence for dressings for burns is low, comprising small studies which are poorly reported and at risk of bias.
Trial results suggest that burn wounds dressed with hydrogel dressings healed more rapidly than those dressed with a variety of usual care regimens. We also found evidence that burn wounds dressed with silicon‐coated dressings, biosynthetic dressings and silver‐impregnated dressings heal more rapidly than those dressed with SSD dressings.There is inadequate evidence to determine the effects of hydrocolloids and polyurethane dressings; five studies out of the seven included (Afilalo 1992; Phipps 1988; Poulsen 1991; Thomas 1995; Wright 1993) found no statistically significant difference between the intervention and control groups. One study suggested that fibre dressings improve rates of healing when hydrofibre dressing is compared with SSD (Muangman 2010), although Caruso 2006 did not find a difference in the rates of healing; there was no evidence that calcium alginate fibre dressing had reduced healing times compared to SSD. There was no evidence of a difference in healing time between biosynthetic dressings and hydrocolloids.
There was some evidence that the pain experienced by patients (where reported) appeared to be reduced with the use of the intervention dressing against comparator dressings. This finding was not statistically significant in all studies but was consistent for all intervention dressings, except antimicrobial dressings where the difference was not significant. There was no significant difference in pain levels between biosynthetic dressings and hydrocolloids when compared directly.
The evidence for the effectiveness of the different dressings for protecting from wound infection is limited by the inconsistent measurement and reporting of this outcome. Where infection rates are reported there does not appear to be a significant difference between intervention dressings and comparison groups.
The number of dressing changes required appeared to favour several of the intervention dressings. This difference was however also a reflection of different protocol regimens with SSD gauze dressings requiring daily changes and intervention dressings changed as required.
These results, however, must be interpreted with caution. The included studies were generally of very poor quality and poorly reported. In many cases the number of people included in the trials was small and the time to wound healing data and subsequent statistical analysis were often not reported in a way that allowed the results to be reproduced by the review authors. Time to burn healing is invariably treated as a continuous outcome rather than a time to event outcome with censoring; however it is often unclear whether (a) all participants were fully followed up and (b) all healed. If either or both of these conditions are not met then it is incorrect to treat the time to event as continuous in nature.
The quality of the evidence provided by these studies was low and limited in the following ways:
a. Poor clinical definition of a superficial or partial thickness burn injury in many studies. b. Unreported burn depth estimates or no formal or direct assessment of burn wound depth. This may have erroneously led to the inclusion of a number of studies that were a mixture of various burn depths. c. Failure to report on randomisation techniques and allocation concealment, small sample sizes, subjective outcome assessment, lack of blinding at outcome assessment and poor reporting of withdrawals and adverse events data. d. Poor measurement of outcomes that are important, such as levels of pain, patient satisfaction, wound infection and scar appearance. The limited use of objective outcome measures and insufficient reporting of results makes the analysis and usefulness of these results doubtful.
In conclusion, a number of dressings may have some benefit over other products in the management of superficial and partial thickness burns. This advantage relates to time to wound healing, the number of dressing changes and pain experienced. However our confidence in these conclusions is reduced by the low quality of the evidence and small sample sizes of these trials.
Authors' conclusions
Implications for practice.
A number of dressings may have some benefits over alternatives for the management of superficial and partial thickness burns. There is some, albeit poor quality, research evidence to suggest that silver‐based dressings, silicon‐coated nylon and biosynthetic dressings are associated with better healing outcomes than SSD. Hydrogel dressings were associated with better healing outcomes than usual care.
Implications for research.
There is a need for large, well‐designed trials for dressing interventions. There is a need to clearly estimate burn depth in order to use properly defined dressing interventions. Trials should address key methodological criteria (allocation concealment, blinding of participants and outcome assessors, adequate follow‐up and appropriate statistical analysis) and should follow CONSORT guidelines on reporting. The review did not conduct an economic analysis and that would usefully inform decisions about the most effective and cost‐effective treatments for patients with burn wounds.
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2012 | New citation required but conclusions have not changed | New search, four new studies were added to the review (Gong 2009; Grippaudo 2010; Muangman 2010; Opasanon 2010) but the conclusions remain unchanged. |
| 9 November 2012 | New search has been performed | First update with a risk of bias assessment included. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | Amended | Corrections made to data for two trials (Wyatt 1990 and Caruso 2006). |
| 28 May 2008 | Amended | Converted to new review format. |
| 6 April 2007 | Amended | New protocol published 2007, Issue 3. Title change. |
Acknowledgements
Acknowledgements to Kate Seers for previous work that was the foundation of the current review and to Greg Duncan for his assistance in reading the first draft review. The authors would like to acknowledge the peer referees, Wounds Group Editors (Andrew Jull, Dirk Ubbink, Gill Worthy) and Catriona McDaid, Mary Mondozzi and Janet Yarrow. Acknowledgements to the Cochrane Editorial Unit (Toby Lasserson, John Hilton and Rachel Marshall), Karla‐Soares Weiser, for search appraisal, data extraction and updating the text, and Anne Lethaby for extracting risk of bias data in the most recent review update. This work was funded by the NIHR.
Appendices
Appendix 1. Search methods from the original version ‐ 2008
We conducted searches of the following databases:
The Cochrane Wounds Group Specialised Register (searched 29 May 2008)
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2008, Issue 2
Ovid MEDLINE ‐ 1950 to May Week 3 2008
Ovid EMBASE ‐ 1980 to 2008 Week 21
Ovid CINAHL ‐ 1982 to May Week 4 2008
The following search strategy was used in CENTRAL and modified as appropriate for other databases:
#1 MeSH descriptor Bandages, Hydrocolloid explode all trees #2 hydrocolloid* or askina or biofilm or combiderm or comfeel or cutinova or duoderm or duoderm or (hydroactive NEXT gel*) or granuflex or hydrocoll or replicare or tegasorb or sureskin or hydrofibre or hydrofiber or aquacel # 3MeSH descriptor Alginates explode all trees #4 alginate NEXT dressing* #5 alginate* or calcium or algosteril or kaltostat or melgisorb or seasorb or sorbalgon or sorbsan or tegagen or “algisite M” #6 foam NEXT dressing* #7 allevyn or avance or biatain or cavi‐care or flexipore or lyofoam or spyrosorb or tielle or mepilex #8 MeSH descriptor Hydrogels explode all trees #9 hydrogel* or aquaform or debrisan or geliperm or granugel or hydrosorb or novogel or nu‐gel or "nu gel" or purilon or sterigel #10 film or films or arglaes or omiderm or polyurethane or tegaderm or opsite #11 MeSH descriptor Occlusive Dressings explode all trees #12 paraffin NEAR gauze #13 paranet or paratulle or unitulle or jelonet or bactigras or cuticerin or adaptic or atrauman #14 "retention tape" or hypafix or mefix or fixamul #15 biosynthetic NEAR substitute* #16 (biosynthetic NEAR dressing*) #17 transcyte or biobrane #18 (antimicrobial NEXT dressing*) or acticoat #19 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format. The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network. No date or language restrictions were applied.
We handsearched the references of all identified studies and contacted authors for information about other published and unpublished studies. All dressing manufacturers were contacted to request information on trials evaluating dressings.
Appendix 2. Ovid MEDLINE search strategy
1 exp Bandages, Hydrocolloid/ 2 (hydrocolloid$ or askina or biofilm or combiderm or comfeel or cutinova or duoderm or duoderm or hydroactive gel$ or granuflex or hydrocoll or replicare or tegasorb or sureskin or hydrofibre or hydrofiber or aquacel).tw. 3 exp Alginates/ 4 alginate dressing$.tw. 5 (alginate$ or calcium or algosteril or kaltostat or melgisorb or seasorb or sorbalgon or sorbsan or tegagen or algisite M).tw. 6 foam dressing$.tw. 7 (allevyn or avance or biatain or cavi‐care or flexipore or lyofoam or spyrosorb or tielle or mepilex).tw. 8 exp Hydrogels/ 9 (hydrogel$ or aquaform or debrisan or geliperm or granugel or hydrosorb or novogel or nu‐gel or purilon or sterigel).tw. 10 (film or films or arglaes or omiderm or polyurethane or tegaderm or opsite).tw. 11 exp Occlusive Dressings/ 12 (paraffin adj10 gauze).tw. 13 (paranet or paratulle or unitulle or jelonet or bactigras or cuticerin or adaptic or atrauman).tw. 14 (retention tape or hypafix or mefix or fixamul).tw. 15 (biosynthetic adj10 substitute$).tw. 16 (biosynthetic adj10 dressing$).tw. 17 (biobrane or transcyte).tw. 18 (antimicrobial dressing$ or acticoat).tw. 19 or/1‐18 20 exp Burns/ 21 (burn or burns or burned).tw. 22 or/20‐21 23 19 and 22
Appendix 3. Ovid EMBASE search strategy
1 exp Hydrocolloid Dressing/ 2 (hydrocolloid$ or askina or biofilm or combiderm or comfeel or cutinova or duoderm or duoderm or hydroactive gel$ or granuflex or hydrocoll or replicare or tegasorb or sureskin or hydrofibre or hydrofiber or aquacel).tw. 3 exp Calcium Alginate/ 4 alginate dressing$.tw. 5 (alginate$ or calcium or algosteril or kaltostat or melgisorb or seasorb or sorbalgon or sorbsan or tegagen or algisite M).tw. 6 foam dressing$.tw. 7 (allevyn or avance or biatain or cavi‐care or flexipore or lyofoam or spyrosorb or tielle or mepilex).tw. 8 exp Hydrogel/ 9 (hydrogel$ or aquaform or debrisan or geliperm or granugel or hydrosorb or novogel or nu‐gel or purilon or sterigel).tw. 10 (film or films or arglaes or omiderm or polyurethane or tegaderm or opsite).tw. 11 occlusive dressing$.tw. 12 (paraffin adj10 gauze).tw. 13 (paranet or paratulle or unitulle or jelonet or bactigras or cuticerin or adaptic or atrauman).tw. 14 (retention tape or hypafix or mefix or fixamul).tw. 15 exp Biobrane/ 16 (biosynthetic adj10 substitute$).tw. 17 (biosynthetic adj10 dressing$).tw. 18 (biobrane or transcyte).tw. 19 (antimicrobial dressing$ or acticoat).tw. 20 or/1‐19 21 exp Burn/ 22 (burn or burns or burned).tw. 23 or/21‐22 24 20 and 23
Appendix 4. EBSCO CINAHL search strategy
S25 S21 and S24 S24 S22 or S23 S23 TI ( burn or burns or burned ) or AB ( burn or burns or burned ) S22 (MH "Burns+") S21 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 S20 TI ( antimicrobial dressing* or acticoat ) or AB ( antimicrobial dressing* or acticoat ) S19 (MH "Antimicrobial Dressings") S18 (MH "Antimicrobial Dressings") S17 TI ( biobrane or transcyte ) or AB ( biobrane or transcyte ) S16 TI biosynthetic N10 dressing* or AB biosynthetic N10 dressing* S15 TI biosynthetic N10 substitute* or AB biosynthetic N10 substitute* S14 TI ( retention tape or hypafix or mefix or fixamul ) or AB ( retention tape or hypafix or mefix or fixamul ) S13 TI ( paranet or paratulle or unitulle or jelonet or bactigras or cuticerin or adaptic or atrauman ) or AB ( paranet or paratulle or unitulle or jelonet or bactigras or cuticerin or adaptic or atrauman ) S12 TI paraffin N10 gauze or AB paraffin N10 gauze S11 (MH "Gauze Dressings") S10 (MH "Occlusive Dressings") S9 TI ( film or films or arglaes or omiderm or polyurethane or tegaderm or opsite ) or AB ( film or films or arglaes or omiderm or polyurethane or tegaderm or opsite ) S8 TI ( hydrogel* or aquaform or debrisan or geliperm or granugel or hydrosorb or novogel or nu‐gel or purilon or sterigel ) or AB ( hydrogel* or aquaform or debrisan or geliperm or granugel or hydrosorb or novogel or nu‐gel or purilon or sterigel ) S7 (MH "Hydrogel Dressings") S6 TI ( allevyn or avance or biatain or cavi‐care or flexipore or lyofoam or spyrosorb or tielle or mepilex ) or AB ( allevyn or avance or biatain or cavi‐care or flexipore or lyofoam or spyrosorb or tielle or mepilex ) S5 (MH "Foam Dressings") S4 TI ( alginate* or calcium or algosteril or kaltostat or melgisorb or seasorb or sorbalgon or sorbsan or tegagen or algisite M ) or AB ( alginate* or calcium or algosteril or kaltostat or melgisorb or seasorb or sorbalgon or sorbsan or tegagen or algisite M ) S3 (MH "Alginates") S2 TI ( hydrocolloid* or askina or biofilm or combiderm or comfeel or cutinova or duoderm or duoderm or hydroactive gel* or granuflex or hydrocoll or replicare or tegasorb or sureskin or hydrofibre or hydrofiber or aquacel ) or AB ( hydrocolloid* or askina or biofilm or combiderm or comfeel or cutinova or duoderm or duoderm or hydroactive gel* or granuflex or hydrocoll or replicare or tegasorb or sureskin or hydrofibre or hydrofiber or aquacel ) S1 (MH "Hydrocolloid Dressings")
Data and analyses
Comparison 1. Hydrocolloid dressing vs chlorhexidine‐impregnated gauze dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to wound infection | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.11, 59.90] |
Comparison 2. Hydrocolloid dressing vs silver sulphadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of dressing changes | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐18.65 [‐22.54, ‐14.76] |
| 2 Level of pain | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.82, ‐0.56] |
Comparison 3. Polyurethane film dressing vs paraffin gauze dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.23, 6.90] |
Comparison 4. Polyurethane film dressing vs chlorhexidine‐impregnated paraffin gauze dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 4.98] |
Comparison 5. Hydrogel dressing vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing: number of people healed at 6 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.46, 4.91] |
| 2 Wound healing: number of people healed at 9 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.08, 3.72] |
| 3 Wound healing: number of people healed at 21 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.95, 1.05] |
| 4 Wound healing: number of people healed at 12 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.17, 2.42] |
| 5 Wound healing: number of people healed at 15 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.95, 1.41] |
| 6 Wound healing: number of people healed at 18 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| 7 Assessment of pain at baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Pain 30 minutes after treatment | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.64, 0.06] |
| 9 Overall assessment of pain at end of study | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐2.37, ‐0.25] |
| 10 Infection with Pseudomonas aeruginosa requiring antibiotic therapy | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
5.1. Analysis.
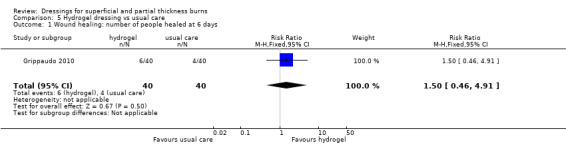
Comparison 5 Hydrogel dressing vs usual care, Outcome 1 Wound healing: number of people healed at 6 days.
5.3. Analysis.
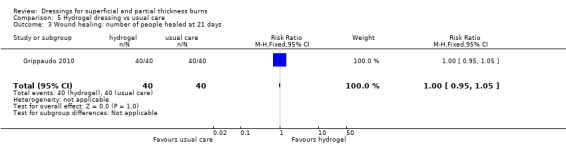
Comparison 5 Hydrogel dressing vs usual care, Outcome 3 Wound healing: number of people healed at 21 days.
5.5. Analysis.
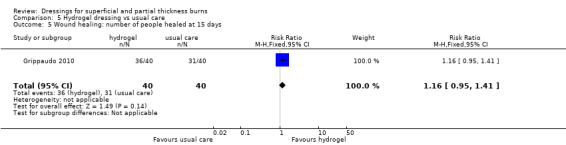
Comparison 5 Hydrogel dressing vs usual care, Outcome 5 Wound healing: number of people healed at 15 days.
5.6. Analysis.
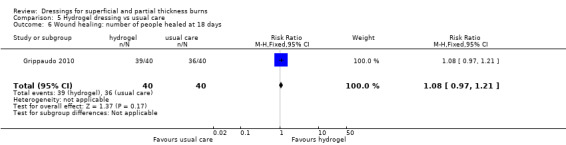
Comparison 5 Hydrogel dressing vs usual care, Outcome 6 Wound healing: number of people healed at 18 days.
5.8. Analysis.
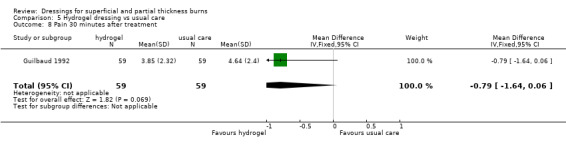
Comparison 5 Hydrogel dressing vs usual care, Outcome 8 Pain 30 minutes after treatment.
Comparison 6. Silicon nylon dressing vs silver sulphadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of dressing changes | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐2.64, ‐0.34] |
Comparison 7. Biosynthetic skin substitute (Biobrane) vs silver sulphadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 2 | 106 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.20, ‐1.06] |
| 2 Need for surgery | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.21, 2.24] |
Comparison 8. Antimicrobial‐releasing biosynthetic dressings vs silver sulphadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.87, 4.02] |
Comparison 9. Silver‐impregnated dressing vs silver sulphadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing time (days) | 2 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐4.22 [‐5.92, ‐2.52] |
| 2 Wound healing: number of people healed at 7 days | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.58, 3.91] |
| 3 Wound healing: number of people healed at 10 days | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.97, 3.40] |
| 4 Wound healing: number of people healed at 15 days | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 5 Wound healing: number of people healed at 17 days | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.98, 1.54] |
| 6 Wound healing: number of people healed at 21 days | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.06, 1.37] |
| 7 Healing rate (% wound area) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 2.21 [‐2.37, 6.79] |
| 8 Pain | 3 | 135 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐5.89, 0.21] |
| 9 Need for surgery | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.08] |
| 10 Number of infections | 4 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.67] |
| 11 Number of wound dressings | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐11.07 [‐19.58, ‐2.56] |
| 12 Nursing time (minutes) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐4.82 [‐19.42, 9.78] |
Comparison 10. Fibre dressing vs silver sulphadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound healing time (days) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐5.44, ‐1.96] |
| 2 Pain at day 1 | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.03, ‐0.97] |
| 3 Pain at day 3 | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.1 [‐4.02, ‐2.18] |
| 4 Pain at day 7 | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐3.18, ‐1.62] |
| 5 Number of dressing changes | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐11.40 [‐15.66, ‐7.14] |
| 6 Number of infections | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.48, 3.34] |
| 7 Need for surgery | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afilalo 1992.
| Methods | RCT. Method of randomisation: computer‐generated random numbers table. Allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. No ITT analysis. Patients served as their own controls (i.e. one area of the same patient was treated with intervention while another similar area was taken as a control). | |
| Participants | 48 adults (mean age: 38.5 years) with partial thickness burns presenting to an emergency department within 48 hours of injury. Cause of burn ‐ hot liquid, metal, flame or steam. Excluded if previously received treatment other than first aid, had electrical or chemical burns, or burns of the face, hands or perineum, suspected inhalation injury, required hospital admission and had concomitant diseases such as diabetes mellitus. Patients excluded if they could not attend or commit to clinic visits, had poor language and judged to be likely poor compliers with treatment | |
| Interventions | Gp 1: n = 15 Hydrocolloid dressing (DuoDerm, ConvaTec Ltd, Bristol Myer Squibb) Gp 2: n = 15 antiseptic tulle gras dressing with chlorhexidine acetate (Bactigras, Smith & Nephew) plus a layer of SSD | |
| Outcomes | Number of days to complete wound healing Level of pain Number of dressing changes Need for pain medication Ease of application and removal of dressing | |
| Notes | 18 people dropped out from the study and not included in the final analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial described as "open" with different care protocols; no blinding of participants, investigators or assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18 of 48 participants dropped out and were not included in the analyses; also missing data |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Table comparing groups at baseline included only the participants who continued in the study; study funded by pharmaceutical company |
Barret 2000.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 20 children with partial thickness burns (mean %TBSA: 8.4) less than 24 hours old. Excluded if participants greater than 17 years of age, causes other than thermal flame or scald injuries, full‐thickness burns and admission time greater than 24 hours after injury | |
| Interventions | Biosynthetic dressing (Biobrane) versus twice daily application of SSD Control: application of twice‐daily SSD | |
| Outcomes | Time to complete wound healing Pain Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stated as "randomised" but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocols differed. Blinding of participants and investigators not feasible. Pain assessment 'not blinded' and unclear for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It does not appear that there were any withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Patient groups similar at baseline |
Bugmann 1998.
| Methods | RCT. Randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 76 patients (age range 3 months to 15 years, mean age 3.4 years, mean %TBSA 2.1%) with partial thickness burns presenting to an emergency department within 24 hours of injury | |
| Interventions | Silicon‐coated nylon dressing (Mepitel, Molnlycke Health Care, USA) covered with a gauze soaked in chlorhexidine versus SSD (Flamazine, Smith and Nephew) covered by tulle gras and gauze | |
| Outcomes | Time to complete wound healing Number of dressing changes Incidence of wound infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocol differed. Participants and investigators not blinded. Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal greater than 10% but similar between groups and reasons for missing data unlikely to be related to outcomes |
| Selective reporting (reporting bias) | High risk | Pilot study ‐ other outcomes such as pain and adverse events not reported |
| Other bias | Unclear risk | Characteristics at baseline not reported for all participants in the randomised groups |
Caruso 2006.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported | |
| Participants | 84 participants with superficial, mid‐dermal or mixed partial thickness burns at first presentation. Key exclusion criteria included electrical, chemical or frostbite burn, evidence of inhalation injury, treatment of burn with an active agent (i.e. SSD) before study entry and fractures and/or neurological injury | |
| Interventions | Hydrogel (Hydrofiber, ConvaTec, Bristol‐Myers Squibb, USA) dressing versus SSD | |
| Outcomes | Time to complete wound healing Number of dressing changes Cost of dressings Incidence of infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were assigned randomly" but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Stated as "unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 84 patients randomised. Only 2 dropouts in control group because they did not receive the treatment |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Dressings provided by pharmaceutical company and no description of how potential bias was minimised; the company supervised the design of the study, the analyses and the development of the manuscript |
Cassidy 2005.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 72 patients ( age range 3 to 18 years) with superficial or mid‐dermal partial thickness burns less than 10% TBSA. Burns involving face, hands, feet or perineum were excluded. | |
| Interventions | Biosynthetic dressing (Biobrane) versus hydrocolloid dressing | |
| Outcomes | Time to wound healing Level of pain Cost of dressing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as "randomised" but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients completed the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Baseline comparability between groups not reported |
Costagliola 2002.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 59 patients with 73 second degree burns of 50 to 200 cm. Age and gender not provided. | |
| Interventions | Calcium alginate (Algosteril, Smith and Nephew Healthcare Limited) versus SSD | |
| Outcomes | Days to healing Level of pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Potential unit of analysis errors: 59 patients included with 73 burns and it appears that burns, not patients, were randomised to treatment groups |
Curreri 1980.
| Methods | RCT. Allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 18 patients (mean age 34 years) with second‐degree burns (mean %TBSA: 26) | |
| Interventions | Biosynthetic dressing (Hydron) versus SSD | |
| Outcomes | Time to complete wound healing Patient perception/level of satisfaction with application or removal of dressing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "pre‐designed randomised code" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocols were different so blinding of patients and investigators not feasible. Blinding of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Satisfaction reported only for experimental group |
| Other bias | High risk | Patients acted as own control, giving rise to potential unit of analysis errors; although it appear as though there was one site for the "test" dressing and one site for the control dressing per patient (N = 15), the authors stated that 47 "test" dressings were evaluated, giving rise to further potential bias |
Fang 1987.
| Methods | RCT. Method of randomisation, allocation concealment not reported. Blinding of outcome assessors recorded. Patients served as their own controls (i.e. one area of the same patient was treated with intervention while another similar area was taken as a control) | |
| Participants | 27 patients (mean age 18.6 years) with second‐degree burns (mean %TBSA: 24.1) | |
| Interventions | Antimicrobial release biosynthetic dressing (Hydron) versus once or twice‐daily application of SSD | |
| Outcomes | Wound appearance Number of dressing changes Need for surgery Incidence of infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients acted as own control and 2 sites per patient were selected "at random" for the 2 different therapies |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different care protocols ‐ blinding of patients and investigators very unlikely but assessors were reported as blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | (1) Materials for trial provided by pharmaceutical company and no description provided or methods used to prevent bias; (2) patients acted as own control, giving rise to potential unit of analysis errors |
Gerding 1988.
| Methods | RCT with randomisation sequence generated by computer code and allocation concealment achieved by sealed numbered envelopes opened sequentially. Blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 50 wounds in 47 patients (mean age: 19.6 years) with partial thickness burns (mean %TBSA: 6.3) less than 24 hours old. Chemical and electrical burns, grossly contaminated wounds, wounds more than 24 hours old and wounds treated with topical agents were excluded. | |
| Interventions | Biosynthetic dressing (Biobrane) versus twice‐daily application of SSD | |
| Outcomes | Time to complete wound healing Level of pain Need for surgery Cost of dressing Incidence of infection Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated codes" |
| Allocation concealment (selection bias) | Low risk | "Sealed numbered envelopes that were opened sequentially" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants, investigators or assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions (n = 4 of 47) not likely to be related to outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | 50 wounds in 43 patients ‐ wounds randomised instead of patients, but unlikely to bias estimates |
Gerding 1990.
| Methods | RCT with randomisation sequence generated by computer code and allocation concealment achieved by sealed numbered envelopes opened sequentially. No blinding employed. Blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 64 patients (mean age 20.2 years) with partial thickness burns (mean %TBSA: 2.2%) less than 24 hours old. Chemical and electrical burns, grossly contaminated wounds, wounds more than 24 hours old and wounds treated with topical agents were excluded. | |
| Interventions | Biosynthetic dressing (Biobrane) versus twice‐daily application of SSD | |
| Outcomes | Time to complete wound healing Level of pain Incidence of infection | |
| Notes | Withdrawals I: 7/33 (21.2%) C: 5/31 (16.1%) Loss to follow‐up I: 2/33 (6.1%) C: 4/31 (13.0%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | "Computer generated codes within sealed numbered envelopes. . . opened in sequential fashion" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocols were different so blinding of patients and investigators not feasible. Blinding of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/54 (19%) of patients excluded; reasons and group membership reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | (1) Patients acted as own control, giving rise to potential unit of analysis errors; (2) comparison of groups at baseline only made on groups that completed the study |
Gong 2009.
| Methods | Single‐centre, unblinded RCT. Inclusion criteria: 20 to 40 years old; fresh degree II burn wound healing; area of burn wound <10% TBSA; burn caused by fire or hot fluids; no infection on wound surface. Exclusion criteria included serious liver or renal dysfunction; "chronic consumptions"; allergy to sliver dressing or hydrogel; cephalofacial and cervicalis wound surface; patient and family preference for surgery. | |
| Participants | 104 patients (male 62; female 42) recruited from hospital with superficial degree II (n = 56) or deep degree II (n = 48) burns to trunk (n = 38) or extremities (n = 66) | |
| Interventions | Ionic silver pressure dressing for 7 days, followed by hydrogel versus 1% silver sulphadiazine | |
| Outcomes | The detection rate of wound bacteria Wound healing time Speed of wound healing Adverse reactions |
|
| Notes | Abstract and tables in English. Full text translated from Chinese. Intervention and control groups were identical numbers for both superficial and deep degree burns, and paper reports no loss to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants allocated to interventions based on a sequence generated by random number tables." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Unclear risk | Randomised groups appeared comparable at baseline; unknown whether source of funding was from a pharmaceutical company |
Gotschall 1998.
| Methods | RCT. Randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 66 patients (age range 0 to 12 years) with partial thickness scald burns of <15% TBSA | |
| Interventions | Silicon‐coated nylon dressing (Mepitel, Molnlycke Health Care, USA) versus SSD. Wet and dry under cotton gauze dressings applied over both treatment arms | |
| Outcomes | Time to wound healing as measured by number of days until wounds were 25%, 50%, 75% and 100% epithelialised Level of pain Cost of the dressing Incidence of infection Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Treatment assigned randomly" but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocols not identical so participants, investigators and assessors not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | High risk | Outcomes reported incompletely |
| Other bias | Low risk | Children in experimental group had significantly higher total body surface area affected by burns than controls at baseline, but insufficient to lead to bias. |
Grippaudo 2010.
| Methods | Single centre, parallel, RCT | |
| Participants | 80 patients (male 31; female 49) aged between 2 and 65 years with second degree burns to TBSA < 10%. | |
| Interventions | Topical application of ionic hydrogel (Procutase) versus application of silver sulphadiazine (SSD) 1% cream on emergency department presentation | |
| Outcomes | Time to wound healing Wound infection Pain Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..computer random number generator..." Comment: done |
| Allocation concealment (selection bias) | Unclear risk | "Once the patient was found to meet enrolment criteria (...) he or she was assigned to one arm or the other of the treatment tree". Inadequate information provided to ascertain whether allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants would have been aware of treatment group assignment as interventions differed Treating study personnel would have been aware of treatment group assignment as interventions differed Outcome assessor was "unaware which treatment arm was assigned to the subject, and evaluated the wound after the removal of dressing and its cleaning with saline by the nurse" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available for assessment Key outcome data identified in study methods section disclosed Data on adverse events were not completely reported. |
| Other bias | Low risk | None detected |
Guilbaud 1992.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 62 patients (mean age 33 years) with partial thickness burns admitted into a burn centre within 24 hours of injury | |
| Interventions | Hydrogel dressing versus SSD, paraffin gauze or paraffin gauze with antibiotics | |
| Outcomes | Time to complete wound healing Level of pain Number of dressing changes Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised clinical trial" in the abstract only; no description of method. Patients were not randomised but similar sites on each patient were randomised, so the patients had their own control. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description of care protocols. Very unlikely because some of the controls received oral antibiotics. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals not reported |
| Selective reporting (reporting bias) | High risk | Results not clearly reported for any outcomes |
| Other bias | High risk | (1) Patients acted as own control, giving rise to potential unit of analysis errors; (2) control therapy varied and allocation not described. |
Guilbaud 1993.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 93 patients (mean age 35.7 years) with second‐degree burns admitted within 48 hours of injury. Mean %TBSA not described. | |
| Interventions | Hydrogel dressing versus SSD, paraffin gauze or paraffin gauze with antibiotics or topical antibiotics | |
| Outcomes | Time to complete wound healing Level of pain Number of dressing changes | |
| Notes | Withdrawals
I: 8/93 (9%)
C: 8/93 (9%) Loss to follow‐up I: 8/93 (9%) C: 8/93 (9%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial described as "open" with each participant acting as his own control. Investigator and patient not blinded (investigator chose the control treatment for individual patients) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals from each subgroup as follows: (1) second‐degree burns 8/93 (9%); (2) donor sites 29/164 (18%); (3) meshed skin grafts 27/107 (25%); (4) loss of skin substance 20/96 (21%). The majority of withdrawals were from the experimental group, as proscribed in the protocol. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
| Other bias | High risk | (1) Patients acted as own control, giving rise to potential unit of analysis errors; assessment of pain (one of the outcomes) thus not independent; (2) selection of control by investigators; (3) change of dressings at the discretion of the investigators; (4) selection of control sites in same patient made subjectively to ensure similarity with experimental site; (5) care protocols not identical: changes in dressings in experimental group led to withdrawal from the trial, but this was not required in the control group; (6) dressings supplied by company that manufactures them; no guarantees given that results safeguarded from company influence |
Huang 2004.
| Methods | Parallell, RCT in four centres throughout China. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 98 participants (male 79; female 19) aged 18 to 65 years with 166 residual burn wounds (mean %TBSA: 54). Average time since burn was 36 days. Exclusion criteria included serious complications of the heart, liver, kidney or blood system; serious infection; shock; pregnancy or lactation; allergy to sliver ions; diabetic ulceration with associated poor diabetic control; acute metabolic disorder. | |
| Interventions | Nanocrystalline silver dressing (Acticoat) versus silver sulphadiazine (SD‐Ag 1%). Daily topical application of SD‐Ag 1% if the wound was severe (secretion; redness; swelling) or every three days if wound not severe | |
| Outcomes | Time to complete wound healing
Incidence of infection Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The observing doctor hands out the dressing to every patient according to the time that they come to the hospital and to a randomized serial number". It is not clear how time of presentation influenced which treatment group participants were assigned to |
| Allocation concealment (selection bias) | High risk | There is a risk of selection bias: doctor treating the patients allocated dressing |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label treatment protocol The study describes assessment of the wound by two doctors, but it is not clear whether they were aware of treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 participants were withdrawn from the study. The distribution of missing data was not described between the two groups. The analysis of healing time does not take account of the withdrawals; data from participants whose wounds had failed to heal are censored |
| Selective reporting (reporting bias) | Unclear risk | Safety outcomes not reported. Efficacy outcomes were reported, but the authors report no difference in adverse events between groups: no numerical data presented. |
| Other bias | High risk | Patients (n = 98) randomised into groups but outcomes mostly measured effects on wounds (n = 166); unit of analysis errors. Not clear how the wounds distributed in the randomised patients. |
Husain 1983.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. Patients served as their own controls (i.e. one area of the same patient was treated with intervention while another similar area was taken as a control). | |
| Participants | 50 patients (mean age 17.34) with a mean %TBSA: 14.7 | |
| Interventions | Antimicrobial release biosynthetic dressing (Hydron) versus SSD and exposed as routine | |
| Outcomes | Time to complete wound healing Level of pain Incidence of infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each patient used as own control with matched burn ‐ sites not reported as randomised |
| Allocation concealment (selection bias) | High risk | No allocation concealment reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients used as own control; no indication whether investigators/assessors blinded but unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details reported |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
| Other bias | High risk | Potential unit of analysis error as patients used as own control and sites may not be truly independent |
Kumar 2004.
| Methods | RCT. Method of randomisation, allocation concealment and not reported. Described as a non‐blind study. | |
| Participants | 33 participants with a total of 58 wound sites. Patients excluded if the burn injury had occurred > 24 hours prior to commencement of treatment, the wounds identified as full thickness in depth or the wounds exhibited signs of infection. | |
| Interventions | Comparing the effectiveness of biosynthetic dressings (TransCyte and Biobrane) and SSD | |
| Outcomes | Time to complete wound healing Need for surgery Number of dressing changes Narcotic analgesia requirements during dressing change | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients "randomised by lottery" but no further description provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Stated as "unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It does not appear that there were any withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | (1) The unit of randomisation in the study was participants but the measurement of most outcomes were according to burn wound, giving rise to unit of analysis errors. 33 participants were randomised to one of three therapies; the 33 participants had 58 burn wounds and outcomes measured changes to wounds; (2) pharmaceutical company support for study with no description of methods used to minimise the likelihood of bias |
Lal 1999.
| Methods | RCT. No blinding employed. | |
| Participants | 89 children with partial thickness scald burns covering 5% to 25%TBSA treated within 48 hours of injury and showing no initial signs of cellulitis or need for grafting | |
| Interventions | Biosynthetic dressing (Biobrane) versus twice‐daily application of SSD | |
| Outcomes | Time to complete wound healing Hospital length of stay Incidence of infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation table" |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocols not identical so participants, investigators and assessors not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10/89 (11%) of patients dropped out; reasons and group membership reported. Attempts to follow up dropouts by authors but these patients not included in the analyses. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | 7/89 patients in study allocated treatment according to physician preference |
Muangman 2006.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 50 participants with partial thickness burns admitted to a Burns Unit | |
| Interventions | Silver‐impregnated dressing (Acticoat, Smith & Nephew, UK) versus SSD | |
| Outcomes | Level of pain Need for surgery Incidence of infection Hospital length of stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stated as "randomised" but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different care protocols so blinding of patients and investigators not feasible. Not clear whether assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Patient groups similar at baseline; funding not stated |
Muangman 2010.
| Methods | Single‐centre parallel RCT in Thailand. | |
| Participants | 70 participants (male 32; female 38) with a mean age of 38 years presenting within 24 hours prior to study enrolment with superficial second degree burn < 15% TBS (mean %TBSA 2.8). Exclusion criteria included concomitant trauma; chemical/electrical burns; inhalation injuries; facial burns; underlying conditions that could interfere with treatment; restricted availability for out‐patient follow‐up; recent antibiotic use; pregnancy; wound dressing allergy | |
| Interventions | Aquacel‐Ag hydrofibre dressing applied once versus daily application of 1% silver sulphadiazine. | |
| Outcomes | Time to wound healing Pain Total dressing cost Total hospital cost Pain medication Transport cost |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by computer..." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information reported to confirm concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different care protocols so blinding of patients and investigators not feasible. Not clear whether assessor blinded: assumed to be at risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants followed‐up until wound had healed. Assumed that no losses to follow‐up occurred. |
| Selective reporting (reporting bias) | Low risk | The outcomes described in the material and methods section were fully reported Expectation of wound infection would be low so reporting this outcome might not be relevant for this population |
| Other bias | Low risk | None detected |
Neal 1981.
| Methods | RCT. Randomisation and allocation concealment not reported. Blinding at outcome assessment recorded. | |
| Participants | 51 patients (25 children) with small blistered burns seen within 12 hours of injury. Mean 1.7% TBSA for those in the intervention group; mean 1.83% TBSA to those assigned to conventional therapy. | |
| Interventions | Polyurethane film (Op‐site, Smith &Nephew Healthcare Limited) versus chlorhexidine‐impregnated paraffin gauze (Bactigras, Smith and Nephew Healthcare Limited) | |
| Outcomes | Number of days to complete wound healing Level of pain Incidence of infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but no details given |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and investigators not blinded; trial stated that measurement of some outcomes required confirmatory judgement of assessors not involved in the trial but no details provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported with sufficient detail. Three patients in the plastic film group removed their dressing early, it is unclear whether these patients were included in the final analysis or not. |
| Selective reporting (reporting bias) | High risk | Outcomes reported incompletely |
| Other bias | Low risk | None detected |
Noordenbos 1999.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 14 patients (mean age 23.4 years) with moderate to deep partial thickness burns (mean %TBSA: 13.3%). Burn wounds to hands, face, buttocks, feet and genitalia were excluded. | |
| Interventions | Biosynthetic dressing (TransCyte) versus twice daily application of SSD. | |
| Outcomes | Time to complete wound healing Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants acted as own control. Two burned sites on each patient "chosen randomly" but method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocols were different so blinding of patients and investigators not feasible. Not stated whether there were separate assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Patients acted as own control so potential unit of analysis errors |
Opasanon 2010.
| Methods | Single‐centre parallel RCT in Thailand. | |
| Participants | 65 participants (male 36; female 29) with a mean age of 36 years presenting within 24 hours with partial‐thickness burns < 15% TBSA. Exclusion criteria included full thickness burn; pregnancy; compromised immune system; hypersensitivity to alginate silver dressing | |
| Interventions | Ionic silver dressing (Askina Calgitrol Ag) changed every 5 days until wound closure versus daily SSD changes | |
| Outcomes | Time to wound healing Pain Number of dressing changes Nursing time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...were identified and randomised into two groups..." Inadequate information presented to determine whether sequence was unpredictable |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information available to determine whether sequence was concealed from study personnel and participants |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label protocol; wound dressings changed at different times Not clear whether outcome assessors were blind to treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Cannot ascertain whether outcomes were measured but not reported |
| Other bias | Low risk | None detected |
Phipps 1988.
| Methods | RCT. Randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 196 patients with burns (mean %TBSA: 1) presenting to an emergency department. | |
| Interventions | Hydrocolloid dressing (Granuflex, Squibb Surgicare) versus chlorhexidine‐impregnated tulle‐gras (Bactigras, Smith & Nephew Healthcare Limited). Dressings changed at weekly intervals. | |
| Outcomes | Number of days to complete healing Incidence of infection | |
| Notes | Withdrawals: I: 42/92 (45.7%) C: 35/104 (33.7%) Loss to follow‐up I: 42/92 (45.7%) C: 35/104 (33.7%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly" ‐ no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 77 patients were lost to follow‐up. Reasons for leaving early not fully described. Not an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | For the outcome number of days to complete healing means reported, but no SDs |
| Other bias | Low risk | None detected |
Poulsen 1991.
| Methods | RCT. Randomisation produced by computer‐generated random number generator. Allocation concealment recorded. Blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 55 patients with partial thickness burns seen within 6 hours of injury. Patients with burns of the face, hands, feet, axilla and perineum were excluded. | |
| Interventions | Polyurethane film (Op‐site, Smith and Nephew Healthcare Limited) versus paraffin gauze (Jelonet, Smith and Nephew Healthcare Limited) | |
| Outcomes | Number of days to complete wound healing Level of pain Number of dressing changes Incidence of infection Adverse events i.e. skin reactions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on random numbers (Geigy) |
| Allocation concealment (selection bias) | Low risk | Cards drawn from sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different care protocols, so blinding of participants, investigators and assessors unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported with insufficient detail |
| Selective reporting (reporting bias) | High risk | Outcomes reported incompletely |
| Other bias | Low risk | None detected |
Thomas 1995.
| Methods | RCT. Method of randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported | |
| Participants | 50 patients (54 burn sites) with less than 5% TBSA (mean %TBSA 0.83). Excluded if patients had burns which were awkward to dress, such as on the face, neck and axilla, as were those with chemical or electrical burns. | |
| Interventions | Three‐arm study with participants allocated to hydrocolloid dressing, hydrocolloid dressing plus SSD and chlorhexidine‐impregnated paraffin guaze dressing | |
| Outcomes | Time to complete wound healing Level of pain Number of dressing changes Incidence of infections | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "randomly allocated" but no description of the method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Care protocols were different so blinding of patients and investigators not feasible. Blinding of outcome assessors not reported; assessments made by patients and investigators and it is not reported whether separate assessors used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | (1) Financial support received by pharmaceutical company and no description of how potential bias was minimised; (2) 54 burn sites on 50 patients, giving rise of possible unit of analysis errors but these likely to be very minimal as results reported per patient |
Varas 2005.
| Methods | RCT with randomisation technique and allocation concealment described. Blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 14 patients (mean age 41 years) with partial thickness burns (mean %TBSA: 14.6) | |
| Interventions | Silver‐impregnated dressing (Acticoat) versus SSD application and removal twice daily | |
| Outcomes | Level of pain Incidence of infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random drawing of sealed envelopes from a box" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes" in a box ‐ not possible for personnel to guess assignment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different care protocols so blinding of participants and investigators not feasible ‐ not mentioned if assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 of 14 patients were excluded because their burns did not meet eligibility criteria, 6 more patients dropped out (5 because of more pain with control treatment), leaving 4/14 patients remaining in the study. This is likely to cause significant bias because the primary outcome was a comparison of pain scores. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, although not possible to measure healing times because of difficulties in the study. Adverse events also measured. |
| Other bias | Unclear risk | Patients acted as own control, giving rise to potential unit of analysis errors |
Wright 1993.
| Methods | RCT. Randomisation, allocation concealment and blinding of participants and investigators (including outcome assessors) not reported. | |
| Participants | 98 patients (age, gender distribution, %TBSA not provided) with partial thickness burns presenting to an emergency department and seen within 48 hours of injury. Patients with injuries greater than 48 hours old, requiring management other than outpatient treatment such as skin grafting were excluded. | |
| Interventions | Hydrocolloid dressing (Granuflex, ConvaTec Ltd, UK) versus chlorhexidine‐impregnated paraffin gauze (Bactigras, Smith and Nephew Healthcare Limited) | |
| Outcomes | Time to wound healing Level of pain Number of dressing changes Incidence of infection Quality of healing was measured using a 5‐point scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients randomly allocated but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Trial described as "open" and care protocols different. No blinding of either patients or investigators who both assessed outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large dropout from trial or withdrawn because of adverse events 31/98 (32%), leaving 67 evaluable patients |
| Selective reporting (reporting bias) | Unclear risk | Baseline data reported incompletely |
| Other bias | Unclear risk | (1) Authors reported that there were no differences between groups at baseline, but data not displayed; (2) study supported by a company making dressings (ConvaTec) and no assurance given that funding had no influence on the results |
Wyatt 1990.
| Methods | RCT. Method of randomisation and allocation concealment not reported. Blinding at outcome assessment recorded. | |
| Participants | 50 patients with minor second‐degree burns who present to an emergency department and/or occupational medicine clinic. Burns which occurred more than 48 hours before presentation for treatment or burns to face, hands, feet or perineum, electrical and chemical burns and those participants with concomitant disease such as diabetes mellitus were excluded. | |
| Interventions | Hydrocolloid dressing (DuoDerm, ConvaTec, Squibb) versus SSD (Silvadene, Marion Laboratories) | |
| Outcomes | Time to complete wound healing Number of dressing changes Level of pain | |
| Notes | Withdrawals:8/50 (16%) Loss to follow‐up: 8/50 (16%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients "randomly assigned" but no description of method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients and investigators not blinded as the randomised treatments had different care protocols. One of the outcomes, impression of overall healing, was assessed blindly by one investigator. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/50 (16%) patients excluded, 4 because they were lost to follow‐up and 4 because of protocol violations. It is not reported whether the reasons for dropouts were related to group assignment. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Dressing provided by medical equipment company |
C: control ED: emergency department Gp: group I: intervention ITT: intention‐to‐treat NA: not applicable RCT: randomised controlled trial SSD: silver sulphadiazine %TBSA: percentage of total burn surface area
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adly 2010 | Randomisation procedure inadequate (even allocated to Group II, odds to group I); half of patients with 3rd degree burns (12/23 in each group) |
| Allen 1996 | Data for burns inseparable from results for other types of wounds |
| Chang 1995 | Review of pressure garment therapy and of full thickness burns |
| Edstrom 1979 | Includes burns other than superficial or partial thickness burns and non‐dressing interventions |
| Frandsen 1978 | Quasi‐randomisation (alternate allocation by odd/even dates) |
| Hauser 2007 | Trial compared two different topical agents |
| Hermans 1984 | Includes burns other than superficial or partial thickness burns |
| Huang 2010 | Patients with moderate or severe burns were included, however separate outcome data were not provided |
| Kedwards 1993 | Treatment for burn injuries of the hand |
| Kuroyanagi 1995 | There insufficient evidence to determine whether this trial was randomised. The paper is in Japanese with an English abstract only. A Japanese translator advised that the trial author did not report whether or how randomisation occurred. |
| Levine 1976 | No outcome measures defined in the review protocol reported in this study |
| Misterka 1991 | Not a randomised trial. The paper is in Polish with an English abstract only. A Polish translator advised that no randomisation or another method of prospective assignment was mentioned in the trial report |
| Rossbach 1998 | Trial compared two different topical agents |
| Schwarze 2008 | Trial comparing two skin substitutes |
| Sharma 1985 | Quasi‐randomisation |
| Stair 1986 | Results for burn wounds not separable from abrasions |
| Tredget 1998 | Includes burns other than superficial or partial thickness burns |
| Waffle 1988 | Quasi‐randomisation |
| Wayne 1985 | Not randomised trial, matched controls |
| Witchell 1991 | Not a randomised trial |
| Yang 1989 | Unable to separate burn wounds from other burn types |
Characteristics of studies awaiting assessment [ordered by study ID]
Mabrouk 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting document retrieval |
Mostaque 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting document retrieval |
Ostlie 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting document retrieval |
Piatkowski 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting document retrieval |
Silverstein 2011.
| Methods | Randomised controlled trial |
| Participants | 100 participants |
| Interventions | Soft silicone dressing with silver (MAg) versus silver sulphadiazine cream (SSD) |
| Outcomes | Time to complete wound healing Hospital length of stay Number of dressing changes Level of pain Costs of the dressings |
| Notes | Abstract only, locations of burns not described. No relevant data are provided in the published abstract. |
Verbelen 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting document retrieval |
Zhou 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting document retrieval |
Contributions of authors
Jason Wasiak: designed and coordinated the review. Extracted data and checked quality of data extraction. Undertook and checked quality assessment. Performed statistical analysis, interpreted data and checked the analysis. Completed first draft of the review and advised on subsequent drafts. Made an intellectual contribution to the review. Approved final review prior to submission. Performed previous work that was the foundation of the current review. Is guarantor of the review. Appraised search, checked data extraction, contributed guidance on data and analyses, and reviewed changes to the text in the most recent update
Heather Cleland: designed the review. Checked quality of data extraction and interpreted data. Completed first draft of the review. Made an intellectual contribution to the review and approved final review prior to submission. Advised on the review. Performed previous work that was the foundation of the current review. Appraised search, checked data extraction, contributed guidance on data and analyses, and reviewed changes to the text in the most recent update.
Fiona Campbell: designed and coordinated the review. Examined all search results. Extracted data and checked quality of data extraction. Wrote to study author/experts/companies and handsearched journals. Undertook and checked quality assessment. Performed statistical analysis, interpreted data and checked the analysis. Completed first draft of the review and advised on subsequent drafts. Made an intellectual contribution to the review. Approved final review prior to submission. Performed previous work that was the foundation of the current review.
Anneliese Spinks: Made an intellectual contribution to the review and approved the overall review process prior to submission. Advised on the review and reviewed changes to the text in the most recent update.
Contributions of editorial base:
Nicky Cullum: edited the review, advised on methodology, interpretation and review content. Undertook extensive redrafting. Approved the final review prior to submission. Commented on and edited the review update.
Sally Bell‐Syer: co‐ordinated the editorial process. Checked data extraction and quality assessment. Performed part of data analysis and interpretation. Advised on methodology, interpretation and content. Undertook extensive redrafting, editing and copy editing of the review and of the review update.
Ruth Foxlee: designed the search strategy and edited the search methods section.
Rachel Richardson: edited and checked the updated review.
Sources of support
Internal sources
No sources of support supplied
External sources
Royal College of Nursing, UK.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Afilalo 1992 {published data only}
- Afilalo M, Dankoff J, Guttman A, Lloyd J. DuoDERM hydroactive dressing vs silver sulphadiazine/Bactigras in the emergency treatment of partial skin thickness burns. Burns 1992;18(4):313‐6. [DOI] [PubMed] [Google Scholar]
Barret 2000 {published data only}
- Barret JP, Dziewulski P, Ramzy P, Wolf S, Desai MH, Herndon D. Biobrane versus 1% silver sulfadiazine in second‐degree pediatric burns. Plastic and Reconstructive Surgery 2000;105(1):62‐5. [DOI] [PubMed] [Google Scholar]
Bugmann 1998 {published data only}
- Bugmann P, Taylor S, Gyger D, Lironi A, Genin B, Vunda A, et al. A silicone‐coated nylon dressing reduces healing time in burned paediatric patients in comparison with standard sulfadiazine treatment: a prospective randomized trial. Burns 1998;24(7):609‐12. [DOI] [PubMed] [Google Scholar]
Caruso 2006 {published data only}
- Caruso DM, Foster KN, Blome‐Eberwein SA, Twomey JA, Herndon DN, Luterman A, et al. Randomized clinical study of hydrofiber dressing with silver or silver sulfadiazine in the management of partial‐thickness burns. Journal of Burn Care and Research 2006;27(3):298‐309. [DOI] [PubMed] [Google Scholar]
Cassidy 2005 {published data only}
- Cassidy C, Peter SD, Lacey S, Beey M, Ward‐Smith P, Sharp RJ, et al. Biobrane versus Duoderm for the treatment of intermediate thickness burns in children: a prospective randomized trial. Burns 2005;31(7):890‐3. [DOI] [PubMed] [Google Scholar]
Costagliola 2002 {published data only}
- Costagliola M, Castede JC, Chaouat M, Mimoun M, Pannier M, Pellerin Pet al. Algosteril dressing (calcium alginate) versus Flammazine (silver sulphadiazine) in the treatment of second‐degree burns. Fourth Australian Wound Management Association Conference. 2002:53.
Curreri 1980 {published data only}
- Curreri PW, Desai M, Bartlett R, Heimbach D, Parshley P, Trunkey D. Safety and efficacy of a new synthetic burn dressing: a multicenter study. Archives of Surgery 1980;115(8):925‐7. [DOI] [PubMed] [Google Scholar]
Fang 1987 {published data only}
- Fang C, Nathan P, Robb E, Alexander J, MacMillan B. Prospective clinical study of Hydron, a synthetic dressing, in delivery of an antimicrobial drug to second‐degree burns. Journal of Burn Care and Rehabilitation 1987;8(3):206‐9. [DOI] [PubMed] [Google Scholar]
Gerding 1988 {published data only}
- Gerding RL, Imbembo AL, Fratianne RB. Biosynthetic skin substitute vs 1% silver sulfadiazine for treatment of inpatient partial‐thickness thermal burns. Journal of Trauma 1988;28(8):1265‐9. [DOI] [PubMed] [Google Scholar]
Gerding 1990 {published data only}
- Gerding RL, Emerman CL, Effron D, Lukens T, Imbembo AL, Fratianne RB. Outpatient management of partial‐thickness burns: Biobrane versus 1% silver sulfadiazine. Annals of Emergency Medicine 1990;19(2):29‐32. [DOI] [PubMed] [Google Scholar]
Gong 2009 {published data only}
- Gong Z, Yao J, Ji J, Yang J, Xiang T. Effect of ionic silver dressing combined with hydrogel on degree II burn wound healing [zhong guo zu zhi gong cheng yan jiu yu lin chuang kang fu]. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13(42):8373‐6. [Google Scholar]
Gotschall 1998 {published data only}
- Gotschall C, Morrison M, Eichelberger M. Prospective, randomized study of the efficacy of Mepitel on children with partial‐thickness scalds. Journal of Burn Care and Rehabilitation 1998;19(4):279‐83. [DOI] [PubMed] [Google Scholar]
Grippaudo 2010 {published data only}
- Grippaudo FR, Carini L, Baldini R. Procutase versus 1% silver sulphadiazine in the treatment of minor burns. Burns 2010;36(6):871‐5. [DOI] [PubMed] [Google Scholar]
Guilbaud 1992 {published data only}
- Guilbaud J. European comparative clinical study of Inerpan: a new wound dressing in treatment of partial skin thickness burns. Burns 1992;18(5):419‐22. [DOI] [PubMed] [Google Scholar]
Guilbaud 1993 {published data only}
- Guilbaud J, Honde C. Multicentre comparative clinical study of a new wound dressing: PA286 (Inerpan). European Journal of Plastic Surgery 1993;16:73‐6. [Google Scholar]
Huang 2004 {published and unpublished data}
- Huang Y, Li X, Liao Z, Zhang G, Liu Q, Tang J, et al. A randomized comparative trial between Acticoat and SD‐Ag in the treatment of residual burn wounds, including safety analysis. Burns 2007;33(2):161‐6. [DOI] [PubMed] [Google Scholar]
- Huang YS, Li XL, Liao ZJ, Zhang GA, Liu Q, Tang J, et al. Multi‐center clinical study of Acticoat (nanocrystalline silver dressing) for the management of residual burn wounds: summary report. Institute of Burn Research, Southwest Hospital, The Third Military Medical University 2004. [PubMed]
- Li XL, Huang YS, Peng YZ, Liao ZJ, Zhang GA, Liu Q. Multi‐center clinical study of Acticoat (nanocrystalline silver dressing) for the management of residual burn wounds. Chinese Journal of Burns 2006;22(1):15‐8. [PubMed] [Google Scholar]
Husain 1983 {published data only}
- Husain MT, Aktar M, Akhtar N. Report on evaluation of hydron film as burn wound dressing. Burns 1983;9(5):330‐4. [DOI] [PubMed] [Google Scholar]
Kumar 2004 {published data only}
- Kumar RJ, Kimble RM, Boots R, Pegg SP. Treatment of partial thickness burns: a prospective randomised trial using transcyte. ANZ Journal of Surgery 2004;74(8):622‐6. [DOI] [PubMed] [Google Scholar]
Lal 1999 {published data only}
- Lal S, Barrow R, Wolf S, Chinkes D, Hart D, Heggers J, et al. Biobrane improves wound healing in burned children without increased risk of infection. Shock 1999;14(3):314‐9. [DOI] [PubMed] [Google Scholar]
Muangman 2006 {published data only}
- Muangman P, Chuntrasakul C, Silthram S, Suvanchote S, Benjathanung R, Kittidacha S, et al. Comparison of efficacy of 1% silver sulfadiazine and Acticoat for treatment of partial‐thickness burn wounds. Journal of the Medical Association of Thailand 2006;89(7):953‐8. [PubMed] [Google Scholar]
Muangman 2010 {published data only}
- Muangman P, Pundee C, Opasanon S, Muangman S. A prospective, randomized trial of silver containing hydrofiber dressing versus 1% silver sulfadiazine for the treatment of partial thickness burns. International Wound Journal 2010;7(4):271‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neal 1981 {published data only}
- Neal D, Whalley P, Flowers M, Wilson D. The effects of an adherent polyurethane film and conventional absorbent dressing in patients with small partial thickness burns. British Journal of Clinical Practice 1981;35(7‐8):254‐7. [PubMed] [Google Scholar]
Noordenbos 1999 {published data only}
- Noordenbos J, Dore C, Hansbrough JF. Safety and efficacy of TransCyte for the treatment of partial‐thickness burns. Journal of Burn Care and Rehabilitation 1999;20(4):275‐81. [DOI] [PubMed] [Google Scholar]
Opasanon 2010 {published data only}
- Opasanon S, Muangman P, Namviriyachote N. Clinical effectiveness of alginate silver dressing in outpatient management of partial‐thickness burns. International Wound Journal 2010;7(6):467‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opasanon S, Muangman P, Namviriyachote N, Chuntrasakul C. Clinical effectiveness of alginate silver dressing in outpatient management of partial‐thickness burns. EWMA Journal 2010;10(2):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phipps 1988 {published data only}
- Phipps A, Lawrence JB. Comparison of hydrocolloid dressings and medicated tulle‐gras in the treatment of outpatient burns. In: Ryan TJ editor(s). Beyond Occlusion: Wound Care Proceedings, Royal Society of Medicine Services International Congress and Symposium Series No. 136. London: RSM Services Ltd, 1988. [Google Scholar]
Poulsen 1991 {published data only}
- Poulsen T, Freund K, Arendrup K, Nyhuus P, Pedersen O. Polyurethane film vs impregnated gauze in the treatment of outpatient burns: a prospective, randomized study. Burns 1991;17(1):59‐61. [DOI] [PubMed] [Google Scholar]
Thomas 1995 {published data only}
- Thomas SS, Lawrence JC, Thomas A. Evaluation of hydrocolloids and topical medication in minor burns. Journal of Wound Care 1995;4(5):218‐20. [DOI] [PubMed] [Google Scholar]
Varas 2005 {published data only}
- Varas RP, O'Keefe T, Namias N, Pizano LR, Quintanan OD, Tellachea MH, et al. A prospective, randomized trial of Acticoat versus silver sulfadiazine in the treatment of partial thickness burns: which method is less painful?. Journal of Burn Care and Rehabilitation 2005;26(4):344‐7. [DOI] [PubMed] [Google Scholar]
Wright 1993 {published data only}
- Wright A, MacKechnie DW, Paskins JR. Management of partial thickness burns with Granuflex 'E' dressings. Granuflex 'E' vs Bactigras. Burns 1993;19(2):128‐30. [DOI] [PubMed] [Google Scholar]
Wyatt 1990 {published data only}
- Wyatt D, McGowan D, Najarian MP. Comparison of a hydrocolloid dressing and silver sulfadiazine cream in the outpatient management of second‐degree burns. Journal of Trauma 1990;30(7):857‐65. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adly 2010 {published data only}
- Adly OA, Moghazy AM, Abbas AH, Ellabban AM, Ali OS, Mohamed BA. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns 2010;36(5):703‐10. [DOI] [PubMed] [Google Scholar]
Allen 1996 {published data only}
- Allen R, Kerstin M, Klassen H, Friedmann P, Pryce D, Lawrence J, et al. Prospective study of clinical infections in wounds dressed with occlusive versus conventional dressings. 1996 Symposium on Advanced Wound Care and Medical Research Forum on Wound Repair. 1996:115.
Chang 1995 {published data only}
- Chang P, Laubenthal KN, Lewis RW, Rosenquist MD, Lindley Smith P, Kealey GP. Prospective, randomized study of the efficacy of pressure garment therapy in patients with burns. Journal of Burn Care and Rehabilitation 1995;16(5):473‐5. [DOI] [PubMed] [Google Scholar]
Edstrom 1979 {published data only}
- Edstrom LE, Robson MC, Macchiaverna JR, Scala AD. Prospective randomized treatments for burned hands: nonoperative vs. operative. Preliminary report. Scandinavian Journal of Plastic Reconstructive Surgery 1979;13(1):131‐5. [DOI] [PubMed] [Google Scholar]
Frandsen 1978 {published data only}
- Frandsen P, Nielsen O, Sommer J. Treatment of second‐degree burns of the hands. A comparative investigation between glove and occlusive treatment. Ugeskrift for Laeger 1978;140(12):660‐2. [PubMed] [Google Scholar]
Hauser 2007 {published data only}
- Hauser J, Rossbach O, Langer S, Vogt P, Germann G, Steinau HU, et al. Local therapy of grade IIa burns: efficacy and tolerability of a new hydrosome wound gel for the local treatment of grade IIa burns as compared with silver sulfadiazine ointment [German]. Unfallchirurg 2007;110(11):988‐94. [DOI] [PubMed] [Google Scholar]
Hermans 1984 {published data only}
- Hermans M, Hermans R. Preliminary report on the use of a new hydrocolloid dressing in the treatment of burns. Burns 1984;11(2):125‐9. [DOI] [PubMed] [Google Scholar]
Huang 2010 {published data only}
- Huang G‐B, Zhang L, Zhang K‐Y, Zhou L, Wu Q‐H Song G‐D. Therapeutic effects of alginate dressing for refractory burn wound: a randomized controlled study. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14(34):6355‐8. [Google Scholar]
Kedwards 1993 {published data only}
- Kedwards SM, Lawrence JC, Terrill PJ. Assessing PTFE bags for the treatment of hand burns. PTFE bag vs polythene bag. Journal of Wound Care 1993;21(1):10‐2. [DOI] [PubMed] [Google Scholar]
Kuroyanagi 1995 {published data only}
- Kuroyanagi Y, Shioya N, Nakakita N, Nakamura M, Takahashi H, Yasutomi Y, et al. Development of new wound dressing composed of silver sulfadiazine‐impregnated polyurethane membrane laminated with a non‐woven fabric. Japanese Pharmacology and Therapeutics 1995;23(10):383‐408. [Google Scholar]
Levine 1976 {published data only}
- Levine NS, Lindberg RA, Salisbury RE, Mason AD, Pruitt BA. Comparison of coarse mesh gauze with biologic dressings on granulating wounds. American Journal of Surgery 1976;131(6):727‐9. [DOI] [PubMed] [Google Scholar]
Misterka 1991 {published data only}
- Misterka S. Clinical evaluation of hydrogel dressing materials after an 8 year period of their application. Polimery w Medycynie 1991;21(1‐2):23‐30. [PubMed] [Google Scholar]
Rossbach 1998 {published data only}
- Rossbach O, Vogt P, Germann G, et al. Repithel® in comparison with Flammazine® for IIa degree burns [Repithel® im Vergleich zu Flammazine® bei Grad IIa Verbrennungen]. European Wound Management Association Conference; 2005, 15‐17 September; Stuttgart, Germany. 2005; Vol. Posterabstracts P45:226.
Schwarze 2008 {published data only}
- Schwarze H, Kuntscher M, Uhlig C, Hierlemann H, Prantl L, Ottomann C, et al. Suprathel, a new skin substitute, in the management of partial‐thickness burn wounds: results of a clinical study. Annals of Plastic Surgery 2008;60(2):181‐5. [DOI] [PubMed] [Google Scholar]
Sharma 1985 {published data only}
- Sharma SC, Bagree MM, Bhat AL, Banga BB, Singh MP. Amniotic membrane is an effective burn dressing material. Japanese Journal of Surgery 1985;15(2):140‐3. [DOI] [PubMed] [Google Scholar]
Stair 1986 {published data only}
- Stair TO, D'Orta J, Altieri MF, Lippe MS. Polyurethane and silver sulfadiazine dressings in treatment of partial‐thickness burns and abrasions. American Journal of Emergency Medicine 1986;4(3):214‐7. [DOI] [PubMed] [Google Scholar]
Tredget 1998 {published data only}
- Tredget EE, Shankowsky HA, Groeneveld A, Burrell R. A matched‐pair, randomised study evaluating the efficacy and safety of Acticoat silver‐coated dressing for the treatment of burn wounds. Journal of Burn Care and Rehabilitation 1998;19(6):531‐7. [DOI] [PubMed] [Google Scholar]
Waffle 1988 {published data only}
- Waffle C, Simon R, Joslin C. Moisture‐vapour‐permeable film as an outpatient burn dressing. Burns 1988;14(1):66‐70. [DOI] [PubMed] [Google Scholar]
Wayne 1985 {published data only}
- Wayne M. Clinical evaluation of Epi‐Lock ‐ a semiocclusive dressing. Annals of Emergency Medicine 1985;14(1):20‐4. [DOI] [PubMed] [Google Scholar]
Witchell 1991 {published data only}
- Witchell, Crossman C. Dressing burns in children. Nursing Times 1991;87(36):63‐4, 66. [PubMed] [Google Scholar]
Yang 1989 {published data only}
- Yang JY, Tsai YC, Noordhoff MS. Clinical comparison of commercially available Biobrane preparations. Burns 1989;15(3):197‐203. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mabrouk 2012 {published data only}
- Mabrouk A, Boughdadi NS, Helal HA, Zaki BM, Maher A. Moist occlusive dressing (Aquacel Ag) versus moist open dressing (MEBO) in the management of partial‐thickness facial burns: a comparative study in Ain Shams University. Burns 2012;38(3):396‐403. [DOI] [PubMed] [Google Scholar]
Mostaque 2011 {published data only}
- Mostaque AK, Rahman KBMA. Comparisons of the effects of biological membrane (amnion) and silver sulfadiazine in the management of burn wounds in children. Journal of Burn Care and Research 2011;32(2):200‐209. [DOI] [PubMed] [Google Scholar]
Ostlie 2012 {published data only}
- Ostlie DJ, Juang D, Aguayo P, Pettiford‐Cunningham JP, Erkmann EA, Rash DE, et al. Topical silver sulfadiazine vs collagenase ointment for the treatment of partial thickness burns in children: A prospective randomized trial. Journal of Pediatric Surgery 2012;47(6):1204‐1207. [DOI] [PubMed] [Google Scholar]
Piatkowski 2011 {published data only}
- Piatkowski A, Drummer N, Andriessen A, Ulrich D, Pallua N. Randomized controlled single center study comparing a polyhexanide containing bio‐cellulose dressing with silver sulfadiazine cream in partial‐thickness dermal burns. Burns 2011;37(5):799‐803. [DOI] [PubMed] [Google Scholar]
- Piatkowski De Grzymala A, Drummer N, Andriessen A, Ulrich D, Pallua N. RCT comparing a polihexanide containing bio‐cellulose dressing with silver sulfadiazine cream in partial thickness dermal burns. EWMA Journal 2011;11(2 Suppl):63. [DOI] [PubMed] [Google Scholar]
Silverstein 2011 {published data only}
- Silverstein P, Heimbach D, Meites H, Latenser B, Mozingo D, Mullins F, et al. An open, parallel, randomized, comparative, multicenter study to evaluate the cost‐effectiveness, performance, tolerance, and safety of a silver‐containing soft silicone foam dressing (intervention) vs silver sulfadiazine cream. Journal of Burn Care and Research 2011;32(6):617‐26. [DOI] [PubMed] [Google Scholar]
- Silverstein P, Heimbach D, Meites H, Latenser B, Mozingo D, Mullins F, et al. Soft silicone dressing with silver versus silver sulfadiazine cream in the treatment of partial‐thickness burns: a randomised controlled trial. EWMA Journal 2010;10(2):149, Abstract P84. [Google Scholar]
Verbelen 2011 {published data only}
- Verbelen J, Hoeksema H, Heyneman A, Pirayesh A, Monstrey S. Aquacel Ag versus Acticoat: Intermediate results of a prospective, randomized, controlled mono centre study in 100 patients. Burns 2011;37:S8. [DOI] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Zhou P‐Y, Xia Z‐F, Ben D‐F, Ma B, Fang H, Feng P. Clinical application of Aquacel‐Ag dressing in treatment of pediatric superficial I burn wounds. Academic Journal of Second Military Medical University 2011;32(12):1321‐3. [Google Scholar]
Additional references
Burd 2005
- Burd A, Yuen C. A global study of hospitalized paediatric burn patients. Burns 2005;31(4):432‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Demling 2013
- Demling RH, deSanti L, Orgill DP. Skin substitutes. Section 12. http://216.92.122.69/Modules/skinsubstitutes/sec12.htm (accessed 7 February 2013).
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Hettiaratachy 2004
- Hettiaratachy S, Papini R. Initial management of a major burn: II ‐ assessment and resuscitation. BMJ 2004;329(7457):101‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hudspith 2004
- Hudspith S, Rayatt S. First aid and treatment of minor burns. BMJ 2004;328(7454):1487‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrence 1997
- Lawrence FC. Dressing for burns. In: Flanagan M editor(s). Wound Management. Churchill Livingstone, 1997. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Queen 1987
- Queen D, Evans JH, Gaylor JDS, Courtney JM, Reid WH. Burn wound dressings ‐ a review. Burns 1987;13(3):218‐28. [DOI] [PubMed] [Google Scholar]
Quinn 1985
- Quinn KJ, Courtney JH, Evans JH, Gaylor JDS, Reid WH. Principles of burn dressings. Biomaterials 1985;6(6):369‐77. [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 8 April 2010).
Walmsley 2002
- Walmsley S. Advances in Wound Management. PJB Publications Ltd, 2002. [Google Scholar]
Wasiak 2005
- Wasiak J, Cleland H. Minor thermal burns. Clinical Evidence. BMJ Publishing Group, 2005. [PubMed] [Google Scholar]


