Abstract
Background
Withdrawal (detoxification) is necessary prior to drug‐free treatment or as the end point of long‐term substitution treatment.
Objectives
To assess the effectiveness of opioid antagonists to induce opioid withdrawal with concomitant heavy sedation or anaesthesia, in terms of withdrawal signs and symptoms, completion of treatment and adverse effects.
Search methods
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 3, 2009), Medline (January 1966 to 11 August 2009), Embase (January 1985 to 2009 Week 32), PsycINFO (1967 to July 2009), and reference lists of articles.
Selection criteria
Controlled studies of antagonist‐induced withdrawal under heavy sedation or anaesthesia in opioid‐dependent participants compared with other approaches, or a different regime of anaesthesia‐based antagonist‐induced withdrawal.
Data collection and analysis
One reviewer assessed studies for inclusion, undertook data extraction and assessed quality. Inclusion decisions and the overall process were confirmed by consultation between all authors.
Main results
Nine studies (eight randomised controlled trials) involving 1109 participants met the inclusion criteria for the review.
Antagonist‐induced withdrawal is more intense but less prolonged than withdrawal managed with reducing doses of methadone, and doses of naltrexone sufficient for blockade of opioid effects can be established significantly more quickly with antagonist‐induced withdrawal than withdrawal managed with clonidine and symptomatic medications. The level of sedation does not affect the intensity and duration of withdrawal, although the duration of anaesthesia may influence withdrawal severity. There is a significantly greater risk of adverse events with heavy, compared to light, sedation (RR 3.21, 95% CI 1.13 to 9.12, P = 0.03) and probably with this approach compared to other forms of detoxification.
Authors' conclusions
Heavy sedation compared to light sedation does not confer additional benefits in terms of less severe withdrawal or increased rates of commencement on naltrexone maintenance treatment. Given that the adverse events are potentially life‐threatening, the value of antagonist‐induced withdrawal under heavy sedation or anaesthesia is not supported. The high cost of anaesthesia‐based approaches, both in monetary terms and use of scarce intensive care resources, suggest that this form of treatment should not be pursued.
Plain language summary
The potential risks and high cost of using opioid blocking drugs during heavy sedation or anaesthesia to bring on withdrawal outweigh the benefits
Drugs that block opioids are sometimes given to opioid dependent people while they are under heavy sedation or anaesthesia to speed up withdrawal. The review of trials shows that this sort of withdrawal treatment is quicker than withdrawal managed with reducing doses of methadone or clonidine plus symptomatic medications. The intensity of withdrawal experienced with anaesthesia‐based approaches is similar to that experienced with approaches using only minimal sedation, but there is a significantly increased risk of serious adverse events with anaesthesia‐assisted approaches. The lack of additional benefit, and increased risk of harm, suggest that this form of treatment should not be pursued.
Summary of findings
Background
Description of the condition
The signs and symptoms of the opioid withdrawal syndrome include irritability, anxiety, apprehension, muscular and abdominal pains, chills, nausea, diarrhoea, yawning, lacrimation, sweating, sneezing, rhinorrhoea, general weakness and insomnia. Symptoms of the opioid withdrawal syndrome usually begin two to three half‐lives after the last opioid use, i.e. 6 to 12 hours for short half‐life opioids such as heroin and morphine, and 36 to 48 hours for long half‐life opioids such as methadone. Following cessation of a short half‐life opioid, symptoms reach peak intensity within two to four days, with most of the obvious physical withdrawal signs no longer observable after seven to 14 days (Jaffe 1997; Mattick 1996). The opioid withdrawal syndrome is rarely life‐threatening (Jaffe 1997; Mattick 1996) or associated with significant aberrations of mental state (Farrell 1994). However, completion of withdrawal is difficult for most people (Mattick 1996).
The first, or acute, phase of withdrawal is followed by a period of six months or so of a secondary or protracted withdrawal syndrome. This protracted syndrome is characterised by a general feeling of reduced well‐being which is reflected in measurable abnormal physiological functioning. During this period, strong cravings for opioids may be experienced periodically. The malaise associated with protracted abstinence is thought to be a major factor in relapse (Satel 1993). The protracted nature of withdrawal makes the period of recovery from dependence typically lengthy and influenced by a range of factors, both social and treatment related. The types of intervention offered following the acute phase of withdrawal to promote recovery and prevent relapse are substantially different to those offered in the management of acute withdrawal and may include psychological and lifestyle counselling, support groups, and pharmacological and medical treatment. This long‐term aspect of treatment of opioid dependence was excluded from this review because of its substantially different nature.
This review is one of a series of Cochrane reviews relating to the management of opioid withdrawal. Other reviews consider the use of opioid antagonists with minimal sedation (Gowing 2009b); alpha2‐adrenergic agonists (Gowing 2009); buprenorphine (Gowing 2009a); reducing doses of methadone (Amato 2005); inpatient versus other settings (Day 2005), and psychosocial and pharmacological treatments for opoid detoxification (Amato 2008).
Description of the intervention
For many years routine procedures involved suppression of withdrawal with methadone and gradual reduction of the methadone dose (Kleber 1982). This approach derived from observations that the withdrawal syndrome from methadone was milder, although of longer duration, than that from morphine. Methadone's high oral bioavailability, efficacy and long duration of withdrawal relief (24 to 36 hours) were additional factors that have contributed to it being the main medication used in specialist withdrawal programs for most of the past three decades.
Ambivalence to the use of a drug of dependence to treat opioid dependence, government restrictions on the prescription of methadone, and consumer dislike of the protracted nature of methadone withdrawal (Farrell 1994) have, to some extent, limited the use of methadone in this way. Discovery of the capacity of the alpha2‐adrenergic agonist, clonidine, to ameliorate some signs and symptoms of withdrawal led to widespread use of this drug as a non‐opioid alternative for managing withdrawal (Gossop 1988). However, the use of clonidine has been hampered by side effects of sedation and hypotension. Alternative pharmacotherapies that have been explored recently for the management of opioid withdrawal include lofexidine, an analogue of clonidine that has less effect on blood pressure (Gowing 2009) and buprenorphine (Gowing 2009a)
How the intervention might work
The rationale underlying antagonist‐induced withdrawal is that a more rapid transition from dependence to abstinence might increase rates of completion of withdrawal. Initial experiments with the use of opioid antagonists to induce withdrawal date back to the 1970s (Bearn 1999). While initial studies showed that the objective signs of withdrawal reduce rapidly with successive injections of naloxone (the only opioid antagonist available at that time), there was little further interest in the approach until clonidine became available. It seems likely that the use of antagonists alone was limited by poor acceptability to opioid users. Experience with the capacity of clonidine to ameliorate the signs and symptoms of opioid withdrawal led to studies to investigate clonidine (and subsequently other alpha2‐adrenergic agonists as well as other medications) in combination with opioid antagonists to manage opioid withdrawal.
The approach that is the focus of this review is the use of opioid antagonists (naloxone, naltrexone or nalmefene) to induce withdrawal with concomitant heavy sedation or anaesthesia that is intended to both reduce the severity of some withdrawal symptoms and reduce the subjective experience of acute signs and symptoms of withdrawal (Bearn 1999; Brewer 1997; Simon 1997). The approach of administering opioid antagonists to induce withdrawal, in combination with medication to ameliorate withdrawal symptoms but with minimal sedation, is the subject of a separate Cochrane review (Gowing 2009b).
Why it is important to do this review
Dependence on opioid drugs is a major health and social issue in most societies. Although the prevalence of opioid use is low ‐ the United Nations Office on Drugs and Crime (World Drug Report 2007) estimates that 0.4% of the global population abuses opioid drugs ‐ the burden of disease is substantial. The burden to the individual user and the community of opioid dependence arises from mortality (NIH 1997), which is most marked in the 15 to 34 year age group (Hall 1998), transmission of HIV and hepatitis C, health care costs, crime and law enforcement costs as well as the less tangible costs of family disruption and lost productivity (Mark 2001).
Treatment is central to the reduction of the harms incurred by individuals and the community from opioid dependence. Managed withdrawal, or detoxification, by itself is not an effective treatment for dependence (Lipton 1983; Mattick 1996). Rates of completion of withdrawal tend to be low, and rates of relapse to opioid use following detoxification are high (Broers 2000; Gossop 1989; Vaillant 1988), but withdrawal remains a required first step for many forms of longer‐term treatment (Kleber 1982). It may also represent the end point of an extensive period of substitution treatment such as methadone maintenance. As such, the availability of managed withdrawal is essential to an effective and comprehensive treatment system.
There has been considerable controversy surrounding the administration of opioid antagonists under anaesthesia or heavy sedation for the purposes of inducing opioid withdrawal (Dyer 1998; Mayor 1997; Stephenson 1997). The controversy relates in part to the commercialisation of the technique without its efficacy having been accepted as proven according to standard scientific conventions. However, controversy regarding the technique also relates to the exposure of patients to potentially life‐threatening risks that are not normally associated with opioid withdrawal, including aspiration pneumonia and seizures. Because of this controversy, attention is given in this review to adverse events that could potentially compromise patient safety. These adverse events comprise aspects of withdrawal that place the patient at risk of harm, particularly vomiting during sedation, as well as incidents that are not typically part of the opioid withdrawal syndrome.
There is a complex range of variables that can potentially influence the course and subjective severity (or intensity) of withdrawal, including the type of opioid used, dose taken, duration of use, general physical health, and psychological factors, such as the reasons for undertaking withdrawal and fear of withdrawal (Farrell 1994; Phillips 1986; Preston 1985). Outcomes of a withdrawal episode may also be influenced by a prior period of maintenance treatment, since such treatment is likely to result in a degree of stabilisation in health and social functioning that may facilitate successful withdrawal. Where information is available, the influence of these variables is considered.
Objectives
To assess the effectiveness of interventions involving the administration of opioid antagonists to induce opioid withdrawal with concomitant heavy sedation or anaesthesia, relative to other approaches to detoxification (reducing doses of methadone, adrenergic agonists, buprenorphine, antagonist‐induced withdrawal with minimal sedation, symptomatic medications) or placebo, or with comparison of different regimes of antagonist‐induced withdrawal under heavy sedation or anaesthesia. Outcomes include the intensity and duration of withdrawal signs and symptoms, duration of withdrawal treatment, completion of treatment, and occurrence of adverse events.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled clinical trials. Included studies provided detailed information on the type and dose of drugs used and the characteristics of patients treated, as well as the nature of withdrawal signs and symptoms experienced, the occurrence of adverse events OR rates of completion of treatment.
Types of participants
We included studies that involved participants who were primarily opioid dependent and underwent managed withdrawal.
Types of interventions
Experimental interventions involved the administration of an opioid antagonist (naloxone, naltrexone, nalmefene), with the aim of inducing withdrawal, in conjunction with heavy sedation or anaesthesia.
Sedation is distinguished from anaesthesia by the patient continuing to be able to be roused. In addition, the pharmacological agent used to induce sedation will generally differ from anaesthetising agents. To be considered for inclusion, interventions were required to use a level of sedation that substantially limits the patient's awareness and memory of withdrawal. Interventions where light sedation is administered as an aid to sleep or to reduce anxiety, as adjuncts to treatment, were excluded. Interventions involving the use of opioid antagonists with minimal sedation are reviewed separately (Gowing 2009b).
Comparison interventions involved the use of reducing doses of methadone, an alpha2‐adrenergic agonist, buprenorphine, symptomatic medications, opioid antagonists with minimal sedation, or placebo to manage withdrawal, or a different regime of antagonist‐induced withdrawal with concomitant heavy sedation or anaesthesia. For the purpose of this review, symptomatic medications are defined as benzodiazepines, anti‐emetics, anti‐diarrhoeals, anti‐psychotics, anti‐spasmodic, muscle relaxants or non‐opioid analgesics, administered in combination as needed, or according to a defined regime.
Types of outcome measures
Primary outcomes
The studies included were assessed on the basis of a number of measures:
intensity of withdrawal;
duration of treatment (as an indication of the duration of withdrawal, and retention in treatment);
nature and incidence of adverse events, and
completion of withdrawal treatment.
We defined adverse effects as clinically significant signs and symptoms of opioid withdrawal (such as vomiting and diarrhoea) plus any incidents that are not typical components of the opioid withdrawal syndrome (delirium, hypotension).
Secondary outcomes
We also sought to assess data on the number of participants engaged in further treatment, particularly naltrexone maintenance treatment, following completion of the withdrawal intervention. As indicated in the background, managed withdrawal by itself is not an effective treatment for dependence. Hence we consider engagement in further treatment to be an outcome of interest.
Search methods for identification of studies
All searches included non‐English language literature. We assessed studies with English abstracts on the basis of the abstract. If it was thought the study was likely to meet inclusion criteria we translated it sufficiently to extract study methods and results.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library, which includes the Cochrane Drugs and Alcohol Group Trials Register, Issue 3, 2009), Medline (January 1966 to 11 August 2009), Embase (January 1966 to 2009 week 32), and PsycINFO (1967 to July 2009).
We developed a search strategy to retrieve references for all the Cochrane reviews relating to the management of opioid withdrawal in one operation. This strategy was adapted to each of the major databases and the supporting platform as indicated in Appendix 1; Appendix 2; Appendix 3; Appendix 4.
Searching other resources
We handsearched the reference lists of retrieved studies, reviews and conference abstracts.
Data collection and analysis
Selection of studies
One author (LG) assessed each potentially relevant study for inclusion according to the identified inclusion and exclusion criteria. Inclusion and exclusion decisions were confirmed by consultation with other authors.
Data extraction and management
One author (LG) extracted key information and this was confirmed by consultation with the other authors. Key findings of studies were summarised descriptively in the first instance and the capacity for quantitative meta‐analysis was considered. We contacted study authors if we required additional information to include these studies in meta‐analyses.
Assessment of risk of bias in included studies
In this version of the review, the approach to assessing methodological quality of included studies has been changed in line with the approach recommended in the Cochrane Handbook 2008, the requirements of RevMan 5, and criteria developed by the Cochrane Drugs and Alcohol Review Group for the assessment of prospective observational studies (Appendix 5).
The recommended approach for assessing risk of bias in studies included in Cochrane Reviews is based on the evaluation of six specific methodological domains (namely, sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and "other issues"). For each study the six domains are analysed, described as reported in the study and a final judgement on the likelihood of bias is provided. This is achieved by answering a pre‐specified question about the adequacy of the study in relation to each domain, such that a judgement of "yes" indicated low risk of bias, "no" indicates high risk of bias, and "unclear" indicates unclear or unknown risk of bias. To make these judgements we used the criteria indicated by the Cochrane Handbook 2008, as adapted by the Cochrane Drugs and Alcohol Group for applicability to the addiction field and incorporating criteria for observational studies.
We considered blinding separately for subjective and objective outcomes. Lack of blinding is a source of serious risk of bias for subjective outcomes, but is less significant with objective outcomes, such as completion of treatment and duration of treatment. Incomplete outcome data is considered only for intensity of withdrawal and nature and incidence of adverse effects. Retention in treatment (duration of treatment) and completion of treatment are frequently primary outcome measures in addiction research.
Details of the assessments of risk of bias are included in the Characteristics of included studies.
Unit of analysis issues
Where all arms in a multi‐arm trial were to be included in the meta‐analysis, and one treatment arm was to be included in more than one of the treatment comparisons, we used subcategories to allow presentation of information from each arm of the trial.
Data synthesis
Statistical analyses were undertaken using Review Manager 5.0.15. For dichotomous data (eg. number completing treatment), we calculated risk ratios, and for continuous data (eg. withdrawal scores), we calculated standardised mean differences. Where significant statistical heterogeneity was detected (based on the Chi square statistic and associated P‐value), we applied a random‐effects model. This review included eight randomised controlled trials and one quasi‐randomised study. The calculation of overall effects was suppressed where it would have involved combination of results from the cohort study and randomised controlled trials as the differing level of risk associated with quasi‐randomised studies raises questions about the validity of calculating overall effects in this way.
Subgroup analysis and investigation of heterogeneity
This review also aimed to consider the following potential sources of heterogeneity through subgroup analyses: (1) drug of dependence and severity of dependence (as indicated by duration and level of use), (2) poly‐drug use, (3) concurrent physical and psychiatric illness, (4) precipitants to the withdrawal episode, (5) the nature of the treatment setting and (6) the nature of adjunct treatment, including other medications to manage symptoms. Insufficient studies met the inclusion criteria to support such analyses.
Sensitivity analysis
Methodological quality was not used as a criterion for inclusion in the review. We had intended to judge the impact of methodological quality through sensitivity analysis. This would have involved considering the overall estimate of effect with studies with a high risk of bias included or excluded, but there were insufficient studies judged to have low or uncertain risk of bias for such analyses to be undertaken.
Results
Description of studies
Results of the search
Our search strategy identified 60 reports, relating to 52 different studies, with treatment regimes for opioid withdrawal involving the administration of opioid antagonists, that were potentially relevant to this review.
Included studies
Nine studies (12 articles) involving 1109 participants, met the inclusion criteria for this review (seeCharacteristics of included studies). In total, 654 participants were treated with opioid antagonists administered under heavy sedation or anaesthesia.
Eight of the studies were randomised controlled trials. In one study (Krabbe 2003) participants were allocated consecutively to the interventions being compared.
Comparisons
Four studies compared antagonist‐induced withdrawal under anaesthesia with conventional approaches: in Krabbe 2003 the comparison was tapered methadone; in McGregor 2002 and Favrat 2006 the comparison was inpatient detoxification managed with clonidine and symptomatic medications; in Collins 2005 there were two comparison groups, one treated with buprenorphine, the other with clonidine.
Two studies compared regimes of antagonist‐induced withdrawal with differing levels of sedation: Seoane 1997 compared light and heavy sedation; de Jong 2005 compared minimal sedation and anaesthesia‐assisted regimes.
Three studies compared different regimes of antagonist‐induced withdrawal under anaesthesia: in Kienbaum 2000 propofol and methohexital were compared as the anaesthetic agents; in Huang 2002 ketamine and tramadol were compared as adjunct medications, and in Jovaisa 2006, ketamine was compared with placebo as an adjunct medication.
Treatment setting
In all studies antagonist‐induced withdrawal was administered in a hospital setting with intensive care facilities. Comparison treatments were provided on an inpatient basis in specialist drug and alcohol clinics.
Participant characteristics
In five of the nine studies that met the inclusion criteria all participants were withdrawing from heroin or other short‐acting opioids. In Seoane 1997, 63% were injecting users; in McGregor 2002, 94% were injecting users; in Collins 2005, 29% of the antagonist‐induced withdrawal group were injecting users, in Huang 2002 79% were using by inhalation only. Jovaisa 2006 did not report drug use history, but use of long‐acting opioids was an exclusion criterion and all participants were stabilised on morphine for two days prior to detoxification.
In Krabbe 2003 and de Jong 2005, participants were using heroin and/or methadone. All were stabilised on methadone (doses not reported) prior to detoxification. In Favrat 2006, 34% of participants were withdrawing following methadone maintenance treatment.
All participants in Kienbaum 2000 were withdrawing following methadone maintenance treatment. Mean doses of methadone for the propofol and methohexital groups, respectively, were 89 ± 23 and 106 ± 19mg/day, with the last dose of methadone administered 24 hours prior to antagonist‐induced withdrawal.
Treatment regimes
The dose and type of opioid antagonist used to induce withdrawal in the studies included in this review varied. Three studies (de Jong 2005; Favrat 2006; Krabbe 2003) used naltrexone; two (Kienbaum 2000; McGregor 2002) used naloxone; three (Huang 2002; Jovaisa 2006; Seoane 1997) used both naloxone and naltrexone; and one (Collins 2005) used nalmefene followed by naltrexone.
In seven studies anaesthesia was induced and maintained with propofol, although Seoane 1997, Huang 2002 and Collins 2005 also used midazolam. Jovaisa 2006 used isoflurane, and Kienbaum 2000 compared propofol and methohexital as anaesthetic agents in the context of antagonist‐induced withdrawal. The duration of anaesthesia was at least three hours in all studies.
All nine studies reported using a range of adjunct medications.
The diversity of treatment regimes is a reflection of the general area of antagonist‐induced withdrawal, with different clinical and research teams developing different approaches based on clinical experience and knowledge of the pathophysiology of dependence and withdrawal. Regimes of antagonist‐induced withdrawal, with concomitant heavy sedation or anaesthesia, that have been reported in the literature, are summarised in Table 5. The nine studies that met the inclusion criteria for this review are listed first.
1. Summary of treatment regimes.
| Study ID | Opioid antagonist | Anaesthesia/Sedation | Other medication |
| Collins 2005 | Nalmefene 4mg iv over 30 minutes, naltrexone 50mg via nasogastric tube. | Propofol anaesthesia. Intubated and ventilated. Duration 4‐6 hours. Propofol and midazolam to maintain anaesthesia. | Pre‐med: sodium citrate, ranitidine, clonidine, heparin. During procedure: lidocaine, tubo‐curarine, succinylcholine, isoflurane, vercuronium, esmolol, labetalol, nitroglycerine as needed. Recovery: ketorolac, ondansetron, neostigmine, glycopyrrolate. |
| de Jong 2005 | Naltrexone 100mg (oral) prior to anaesthesia. Further 100mg via nasogastric tube at end anaesthesia. | Propofol anaesthesia induced when withdrawal evident. Bispectral Index maintained at 40‐50. Intubated and ventilated. Duration 4 hours. | Pre‐med: clonidine, diclofenac, ondansetron, diazepam, nicotine (smokers only). Tropisetron, octreotide, gallamine, succinylcholine during procedure. |
| Favrat 2006 | Naltrexone 100mg (oral) prior to anaesthesia. | Propofol anaesthesia induced when withdrawal evident. Bispectral Index maintained at 45‐60. Intubated. Duration 5‐6 hours. | Laxative, H2 anti‐histamines prior evening (pre‐admission). Sodium citrate with naltrexone. During procedure: lidocaine, clonidine, octreotide as needed. Recovery: ketorolac, glycopyrrolate‐neostigmine if needed. |
| Huang 2002 | Naloxone 0.03mg/kg iv, naltrexone 0.6mg/kg via nasogastric tube 15 minutes later. | Propofol and midazolam. Intubated, ventilated. Duration 3 hours. | Ketamine compared with tramadol as adjuncts during procedure. Unclear whether other medications used. |
| Jovaisa 2006 | Naloxone 1.6mg iv, then 0.8mg/hour iv infusion. Naltrexone 100mg via orogastric tube. | Propofol and isoflurane. Intubated, ventilated. Duration 3 hours. | Pre‐med: clonidine, octreotide, heparin. During procedure: lidocaine, pipecuronium; comparison of ketamine and placebo as adjuncts. Recovery: clonidine, carbamazepine, clonazepam. |
| Kienbaum 2000 | Naloxone 12.4mg iv over 60 minutes, then 0.8mg/hour for 24 hours. | Propofol or methohexital. Intubated and ventilated. | Flunitrazepam, clonidine, midazolam, ceftriaxone, famotidine, heparin, potassium chloride |
| Krabbe 2003 | Naltrexone 100mg (oral) prior to anaesthesia. Naloxone 0.8mg every 20 minutes until no withdrawal. Naltrexone 100mg via nasogastric tube at end anaesthesia. | Propofol anaesthesia induced when withdrawal evident. Bispectral Index <50. Intubated and ventilated. | Purge; pre‐med (including clonidine); tropisetron, gallamine, succinylcholine. |
| McGregor 2002 | Naloxone, 4 or 5 boluses at 30 minute intervals to 10 or 12 mg. Naltrexone, 50mg (oral) after recovery from anaesthesia. | Propofol anaesthesia. Intubated, spontaneous ventilation. Propofol titrated against withdrawal signs. Duration of anaesthesia about 4 hours. | Clonidine, octreotide, other symptomatic medications. |
| Seoane 1997 | Naloxone 60‐80mcg/kg over 5‐10 minutes. Naltrexone 50mg oral. | "Light" or "heavy" sedation by propofol and midazolam for 6‐8 hours. | Clonidine, metoclopramide, diazepam |
| Albanese 2000 | Naltrexone, 4‐5 doses of 12.5‐25mg via gastric tube. Naloxone, 0.8mg as test. | Propofol. Intubated with spontaneous respiration. Duration not reported. | Clonidine, diazepam, midazolam |
| Allhoff 1999 | Naloxone iv, 12.4mg over 60 minutes. Naloxone infusion 0.8mg/h for 24h. Naltrexone 50mg via gastric tube 12h after 1st naloxone. | Propofol or methohexital. | Clonidine infusion then oral. Flunitrazepam, diclofenac, timipramine. |
| Cucchia 1998 | Naltrexone 50mg oral. | Midazolam sedation (arousal possible). | Clonidine, ondansetron, loperamine, butylscopolamine. |
| Elman 2001 | Nalmefene 2mg iv. Naltrexone 200mg via gastric tube 30 minutes later. | Induced with propofol and ketamine, maintained with propofol. Intubated and ventilated. Mean duration 4.7 hours. | Octreotide, glycopyrrolate, reglan, baclofen, ondansetron, tylenol, midazolam. |
| Gold 1999 | Naloxone 0.4mg iv as test. Nalmefene, infusion, 4mg over 2‐3 hours. Naloxone 0.4mg iv, naltrexone 50 mg oral, post anaesthesia. | Propofol. Intubated and ventilated. | Glycopyrrolate, clonidine, ondansetron, ketorolac, midazolam. |
| Hensel 2000a | Naltrexone 1.5mg/kg via nasogastric tube. | Propofol anaesthesia controlled by observed clinical signs or EEG threshold. | Clonidine infusion. |
| Hensel 2000b | Naltrexone 1.5mg/kg via nasogastric tube. Naloxone to test completion. | Propofol. Intubated and ventilated. Duration 310‐350 minutes. | Midazolam, clonidine, ranitidine, loperamide, ondansetron, diclofenac. |
| Legarda 1994 | Naltrexone 50mg oral. | Midazolam sedation. | Guanfacine, loperamide, ondansetron. |
| Loimer 1989 | Naloxone, 10mg iv, 0.8mg/hour 24 hours, (1) 0.4mg iv every 2 hours for 24 hours or (2) 0.4mg/hour until no opiates in urine. | Methohexitone for 30‐60 minutes. | None reported. |
| Loimer 1990 | Naloxone, 10mg iv, 2mg challenge, 0.8mg/hour 48 hours. | Methohexitone for 30‐40 minutes. | None reported. |
| Loimer 1991a | Naloxone, 4mg/200ml infusion. | Midazolam; duration not reported. | None reported. |
| Loimer 1991b | 72 hours.Naloxone, 10mg iv, 0.8mg/hour | Methohexitone; duration not reported. | None reported. |
| Loimer 1991c | Naloxone, 10mg iv, 0.8mg/hour 72 hours. | Methohexitone; duration not reported. | None reported. |
| Loimer 1993 | Naltrexone 50mg oral. Naloxone 4mg nasal spray. | Midazolam sedation | Clonidine, ondansetron |
| Lorenzi 1999 | Naloxone 4mg infused over 5 hours. Naloxone 0.8mg as test next day before naltrexone 10mg. | Midazolam, propofol. Intubated and mechanically ventilated. | Atracurium, diazepam, atropine, clonidine |
| Ma 2003 | Naltrexone, up to 400mg via nasogastric tube, or 300‐350mg via tube and/or naloxone 5‐15mg iv over 20‐30 minutes, followed by nalmefene (4‐12mg iv) or nalmefene (4mg iv) and naloxone (25mg iv) infused over 8 hours. Naloxone test before stopping anaesthesia. | Induced with propofol and succinylcholine, maintained with propofol to Bispectral Index 55‐60. Intubated. Duration around 6 hours. | Midazolam or diazepam pre‐med. Glycopyrrolate, ondansetron, droperidol, sandostatin, clonidine during procedure. |
| McDonald 2000 | Naltrexone via oral gastric tube in doses of 12.5mg, 25mg, 50mg and 50mg every 90 minutes with stomach drained at 45 minutes. | Propofol anaesthesia to Bispectral Index 40‐60. Intubated and ventilated. | Midazolam pre‐med. Rocuronium as muscle relaxant. |
| Pfab 1999 | Naloxone, 0.2mg/kg iv over 1 hour, naltrexone 100‐150mg oral. | Midazolam then propofol anaesthesia, 11‐22 hours. | Clonidine, heparin, omeprazole. |
| Presslich 1989b | Naloxone, 10mg iv in 1 hour, then 0.4mg/hour 24 hours. | Thiopentone. Duration not reported. | None reported. |
| Scherbaum 1998 | Naloxone 12.4mg in 60 minutes, then 0.8mg/hour overnight. Naltrexone 50mg oral at end anaesthesia. | Methohexital or propofol, dose to suppress reflexes. Patients intubated. Duration about 6 hours. | Pipercuronium, trimipramine, diclofenac. |
| Tornay 2003 | Naltrexone 50mg oral when sleepy. | Midazolam 60‐135mg. Intubation available but not required. | Buprenorphine for 7 days prior to antagonist procedure. Clonidine, ondansetron, loperamide as adjuncts to antagonist. |
| Tretter 1998 | Naloxone maximum 10mg over 2 hours. Naltrexone, 50mg oral. | Propofol anaesthesia for 5‐6 hours. | Omeprazol, cephalosporine, vercuronium, midazolam, perazine. |
For 12 of the 31 studies listed in Table 5, the primary antagonist administered under sedation or anaesthesia was naloxone (with six of these studies being undertaken by Loimer and colleagues); six studies used both naltrexone and naloxone; nine studies primarily used naltrexone; and four studies used nalmefene alone or in combination with naltrexone and/or naloxone.
Doses of naloxone administered during the period of sedation or anaesthesia ranged from 4 to 15mg (i.v.), naltrexone from 50 to 350mg (oral), and nalmefene from 2 to 12mg (i.v.). In the studies where more than one opioid antagonist was administered, the effective dose range was even greater. One of the lowest dose regimes was used by Lorenzi 1999: 4mg naloxone under anaesthesia, followed the next day by a challenge test with 0.8mg naloxone prior to 10mg naltrexone. Loimer 1991a also used a low dose regime for patients withdrawing from methadone: 4mg naloxone under anaesthesia followed by 50mg naltrexone post‐anaesthesia. At the other end of the range, Pfab 1999 administered 0.2mg/kg naloxone (equating to 14mg for a 70kg person) plus 100 to 150mg naltrexone during anaesthesia; Elman 2001 administered 2mg nalmefene followed 30 minutes later by 200mg naltrexone; and Ma 2003 administered naltrexone, nalmefene and naloxone.
Loimer and colleagues used barbiturate (methohexitone or thiopentone) or benzodiazepine (midazolam) anaesthetic agents while later studies tended to use propofol (see Table 5). In these later studies there also appears to be a trend towards longer duration of anaesthesia ‐ Loimer and colleagues reported durations of 30 to 60 minutes, compared to more than four hours in studies published after 1998, with the longest period of anaesthesia (11 to 22 hours) reported by Pfab 1999.
Loimer and colleagues generally do not report using adjunct medications, with the exception of Loimer 1993 in which clonidine and ondansetron were used. However, later studies report the use of up to five adjunct medications including alpha2 adrenergic agonists (clonidine, guanfacine); anti‐emetic agents (ondansetron, loperamide, metoclopramide); muscle relaxants and anti‐spasmodic (butylscopolamine, baclofen); antidepressants (trimipramine, trazodone); non‐opioid analgesics (diclofenac, ketorolac, paracetamol); agents to reduce stomach acidity or secretions (omeprazole, famotidine, glycopyrrolate, octreotide, atropine, ranitidine); anxiolytics or sedatives (diazepam, midazolam, flunitrazepam); an antipsychotic (perazine); antibiotics (cephalosporine, ceftriaxone); an anticoagulant (heparin); and a vasodilator (nitroglycerine).
It is clear from general literature (eg. Brewer 1997; Brewer 1998b; Bulthuis 2000; Chanmugam 2000; Foster 2003; Gooberman 1998) that some practitioners providing rapid detoxification are using naltrexone implants or depot preparations to achieve a more sustained opioid antagonist effect in the immediate post‐anaesthesia phase. However, none of the studies considered for this review used such formulations in their protocols.
Excluded studies
Forty‐three studies (48 articles) did not meet the criteria for inclusion in this review (seeCharacteristics of excluded studies).
Risk of bias in included studies
For summary results of the judged risk of bias across the included studies for each domain, see Figure 1 and Figure 2.
1.
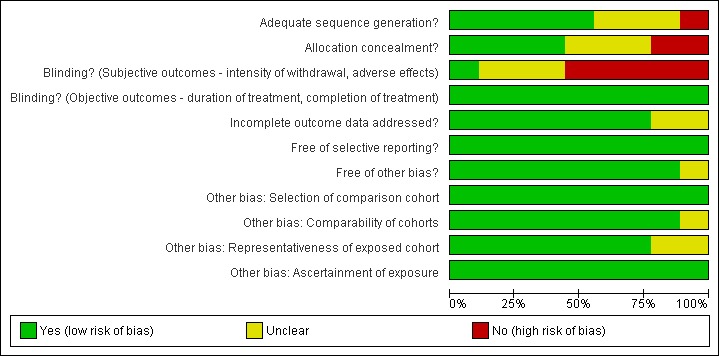
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
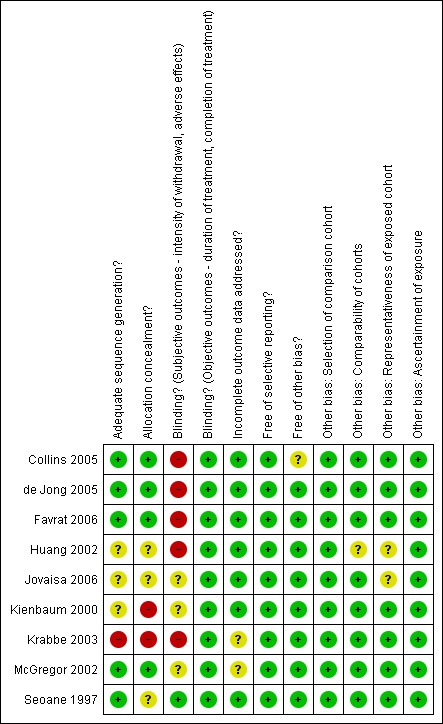
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Two of the nine studies (Kienbaum 2000, Krabbe 2003) were judged to have a high risk of allocation bias, in three studies (Huang 2002, Jovaisa 2006, Seoane 1997) the risk of bias was considered to be unclear, and four studies (Collins 2005, de Jong 2005, Favrat 2006, McGregor 2002) were judged to have a low risk of allocation bias.
Blinding
The risk of assessment bias for objective outcomes (duration and completion of treatment) was judged to be low for all studies as these outcomes are considered unlikely to be affected by an awareness of group allocation. This section therefore applies only to the risk of assessment bias in relation to subjective outcomes (intensity of withdrawal, occurrence and severity of adverse effects).
Five studies (Collins 2005, de Jong 2005, Favrat 2006, Huang 2002 and Krabbe 2003) were judged to have a high risk of assessment bias due to non‐blinding of participants potentially affecting ratings of withdrawal symptoms and adverse effects that are largely undertaken by participants. It is impossible (and unethical) to blind participants to an anaesthetic procedure making it difficult to control this potential risk of bias.
Incomplete outcome data
Retention (duration of treatment) and completion of treatment are primary outcome measures for opioid withdrawal interventions. Hence the risk of bias due to incomplete data was considered only for the outcomes of intensity of withdrawal and adverse effects.
Two studies (Krabbe 2003, McGregor 2002) were judged to have an unknown risk of bias due to incomplete outcome data, due to significant differences in dropout or loss to follow‐up between the treatment groups. In all other studies the risk of bias due to incomplete outcome data was considered to be low.
Selective reporting
None of the studies were judged to be at risk of reporting bias.
Other potential sources of bias
One study (Collins 2005) was judged to have an unknown risk of bias due to enrollment in the study being stopped early. Huang 2002 reported minimal information on participant characteristics preventing assessment of the comparability of the groups in this study. The method for recruiting participants was not reported for two studies (Huang 2002; Jovaisa 2006). It is unclear whether the participants in these studies were typical of dependent heroin users.
Effects of interventions
for the main comparison.
| Antagonist‐induced compared to conventional for opioid withdrawal | ||||||
|
Patient or population: patients with opioid withdrawal Settings: Intervention: Antagonist‐induced Comparison: conventional | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| conventional | Antagonist‐induced | |||||
| Number completing detoxification | Medium risk population | RR 1.42 (1.09 to 1.84) | 100 (2) | ⊕⊕⊝⊝ low1,2 | ||
| 576 per 1000 | 818 per 1000 (628 to 1060) | |||||
| Number commencing naltrexone maintenance treatment ‐ Clonidine comparison | Medium risk population | RR 4.28 (2.91 to 6.3) | 240 (3) | ⊕⊕⊕⊝ moderate2 | ||
| 177 per 1000 | 758 per 1000 (515 to 1115) | |||||
| Number commencing naltrexone maintenance treatment ‐ Buprenorphine comparison | Medium risk population | RR 1.29 (1.04 to 1.6) | 72 (1) | ⊕⊕⊕⊝ moderate2 | ||
| 730 per 1000 | 942 per 1000 (759 to 1168) | |||||
| Retained in naltrexone maintenance treatment or abstinent at 12 weeks ‐ Tapered methadone comparison | Medium risk population | RR 2 (0.9 to 4.45) | 30 (1) | ⊕⊕⊝⊝ low1,2 | ||
| 333 per 1000 | 666 per 1000 (300 to 1482) | |||||
| Retained in naltrexone maintenance treatment or abstinent at 12 weeks ‐ Clonidine comparison | Medium risk population | RR 2.77 (1.37 to 5.61) | 240 (3) | ⊕⊕⊕⊝ moderate2 | ||
| 88 per 1000 | 244 per 1000 (121 to 494) | |||||
| Retained in naltrexone maintenance treatment or abstinent at 12 weeks ‐ Buprenorphine comparison | Medium risk population | RR 0.82 (0.34 to 1.97) | 72 (1) | ⊕⊕⊕⊝ moderate2 | ||
| 243 per 1000 | 199 per 1000 (83 to 479) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study at high risk of allocation bias.
2 Less than 300 events
2.
| Heavy compared to light sedation for opioid withdrawal | ||||||
|
Patient or population: patients with opioid withdrawal Settings: Intervention: Heavy Comparison: light sedation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| light sedation | Heavy | |||||
| Participants experiencing adverse effects | Medium risk population | RR 3.21 (1.13 to 9.12) | 572 (2) | ⊕⊕⊕⊝ moderate1 | ||
| 13 per 1000 | 42 per 1000 (15 to 119) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Less than 300 events
Results are presented in three parts. The first part considers the comparison of antagonist‐induced withdrawal, with heavy sedation or anaesthesia, and conventional approaches to opioid withdrawal (tapered methadone: Krabbe 2003; clonidine plus symptomatic medications: McGregor 2002, Collins 2005; Favrat 2006; and buprenorphine: Collins 2005). The second part considers the comparison of regimes of antagonist‐induced withdrawal with differing levels of sedation (Seoane 1997; de Jong 2005). The third part considers the comparison of different anaesthetic agents and adjunct medications in the context of antagonist‐induced withdrawal (Kienbaum 2000; Huang 2002; Jovaisa 2006). Within each part the four types of outcome measures identified as being of interest are addressed: (a) intensity and duration of withdrawal; (b) duration of treatment; (c) nature and incidence of adverse effects; and (d) completion of withdrawal and post‐detoxification outcomes.
(1) Comparison with conventional approaches
(a) Intensity and duration of withdrawal signs and symptoms
Using the Subjective Opioid Withdrawal Scale (SOWS, which has a maximum score of 64), Krabbe 2003 reported a mean score of 11.5 before anaesthesia, 20 on the day after anaesthesia, declining to pre‐treatment levels in four days. The pattern of scores from the Objective Opioid Withdrawal Scale (OOWS, which has a maximum score of 13) was similar: 1.9 on the day before anaesthesia, 3.9 on the day after anaesthesia, and declining to pre‐treatment levels in three days. Withdrawal scores for the tapered methadone group were lower than the peak scores for the group receiving antagonist‐induced withdrawal. In the tapered methadone group peak withdrawal occurred much later, on day 18, some six days after cessation of methadone and commencement of naltrexone. Based on SOWS and OOWS scores, the duration of withdrawal was four days in the antagonist‐induced withdrawal group, and 20 days in the tapered methadone group.
In Collins 2005, withdrawal was assessed by participants and observers using the SOWS and OOWS, and the Clinical Institute Narcotic Assessment. Withdrawal scores for the anaesthesia group were significantly higher prior to anaesthesia. The authors attributed this to anticipatory anxiety and less use of available clonazepam. There were no significant differences in withdrawal scores for the three groups on days two and three. Withdrawal was not assessed after discharge on day three.
McGregor 2002 did not directly compare withdrawal scores for the groups receiving antagonist‐induced withdrawal or standard inpatient treatment. Favrat 2006 did not assess withdrawal severity.
(b) Duration of withdrawal treatment
Duration of withdrawal treatment was not directly reported by any of the four studies. The closest data reported by McGregor 2002 was the mean (± SE) time to first dose of naltrexone to be 0.1 ± 0.3 days for antagonist‐induced withdrawal, and 4.2 ± 0.6 days for standard inpatient withdrawal managed with clonidine and symptomatic medications.
Data from Collins 2005 that was most relevant to duration was the mean (± SE) weeks in treatment, combining detoxification and naltrexone aftercare: 2.83 ± 0.47 weeks for the anaesthesia group, 3 ± 0.45 weeks for the buprenorphine group, and 2.47 ± 0.58 weeks for the clonidine group (difference not significant).
(c) Nature and incidence of adverse effects
Krabbe 2003 and Favrat 2006 reported no adverse effects.
McGregor 2002 reported no serious adverse effects associated with the anaesthetic procedure. The authors noted that three of 37 (8.1%) who received octreotide during the anaesthetic procedure, compared with seven of 11 (64%) who did not receive octreotide, experienced vomiting and/or diarrhoea.
Collins 2005 reported three potentially life‐threatening adverse events, all in the anaesthesia group: pulmonary edema 14 hours after extubation; mixed bipolar state with suicidal ideation five days after anaesthesia; and diabetic ketoacidosis two days after discharge.
(d) Completion of withdrawal and post‐detoxification outcomes
Three studies (Collins 2005; Favrat 2006; McGregor 2002) reported data on the number of participants who refused group allocation or failed to attend to commence detoxification. In McGregor 2002 significantly more participants allocated to standard inpatient detoxification with clonidine failed to attend, but the overall effect for the three studies is not significant (Figure 3: RR 0.51; 95% CI 0.15 to 1.68; P=0.03)
3.
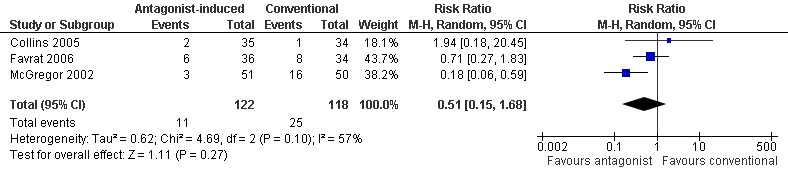
Forest plot of comparison: 1 Antagonist‐induced vs conventional, outcome: 1.1 Number refusing group allocation or failing to attend.
Data suitable for inclusion in meta‐analyses is limited, but the four studies suggest that:
completion of withdrawal is significantly more likely with antagonist‐induced withdrawal compared to tapered methadone (Figure 4: risk ratio 1.82; 95% confidence interval 1.14 to 2.91, P=0.01);
there may be no significant difference in completion of withdrawal with antagonist‐induced withdrawal compared to inpatient withdrawal managed with clonidine (Figure 4: risk ratio 1.26; 95% confidence interval 0.92 to 1.73, P=0.15);
commencement of maintenance doses of naltrexone (50mg/day) is significantly more likely with antagonist‐induced withdrawal under anaesthesia compared to clonidine (Figure 5: risk ratio 4.28; 95% confidence interval 2.91 to 6.30, P<0.01) and buprenorphine (Figure 5: risk ratio 1.29; 95% confidence interval 1.04 to 1.60, P=0.02).
4.
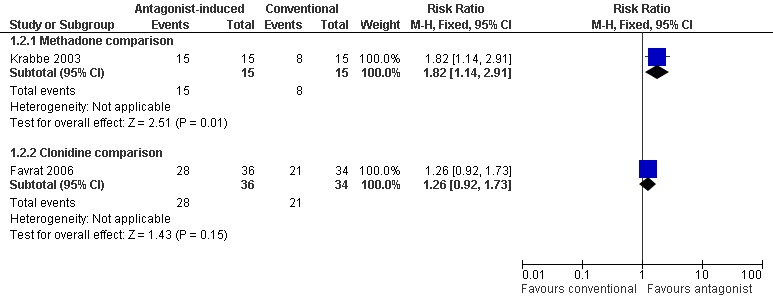
Forest plot of comparison: 1 Antagonist‐induced vs conventional, outcome: 1.2 Number completing detoxification.
5.
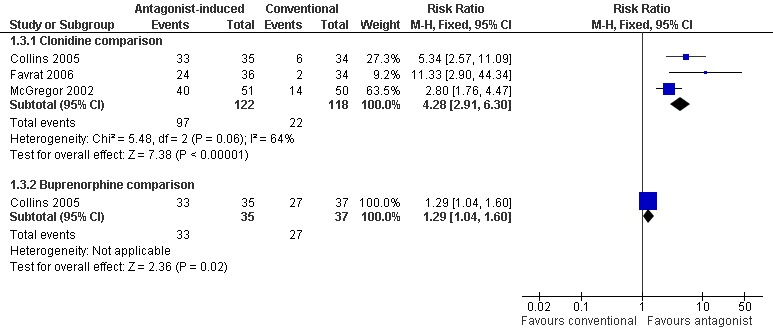
Forest plot of comparison: 1 Antagonist‐induced vs conventional, outcome: 1.3 Number commencing naltrexone maintenance treatment.
Data at three months follow‐up were reported as either the number of participants abstinent from opioids (Favrat 2006, Krabbe 2003) or the number of participants retained in naltrexone maintenance treatment (McGregor 2002 and Collins 2005). These data suggest that significantly more participants withdrawing under anaesthesia were retained in treatment at three months, compared to those whose withdrawal was managed with clonidine (Figure 6: risk ratio 2.77; 95% confidence interval 1.37 to 5.61, P=0.005). In Krabbe 2003 somewhat more participants in the tapered methadone group were abstinent at 3 months, but the difference was not statistically significant (Figure 6: risk ratio 2.00; 95% confidence interval 0.90 to 4.45, P=0.09). In Collins 2005, there was no significant difference between antagonist‐induced withdrawal and withdrawal managed with buprenorphine in terms of retention in naltrexone maintenance treatment at 3 months (Figure 6: risk ratio 0.82; 95% confidence interval 0.34 to 1.97, P=0.66).
6.
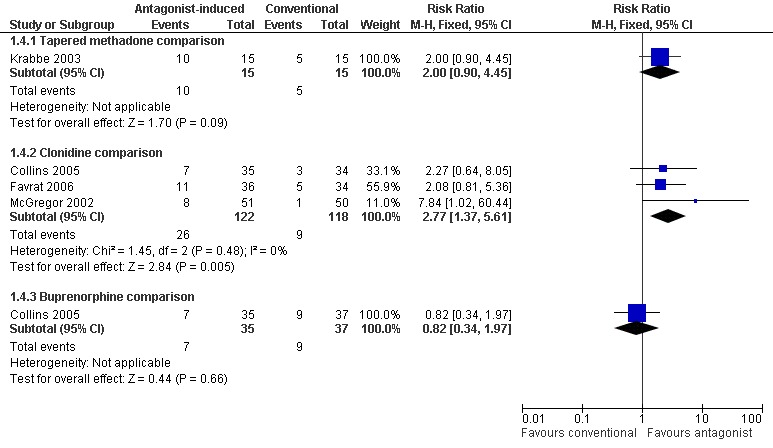
Forest plot of comparison: 1 Antagonist‐induced vs conventional, outcome: 1.4 Retained in naltrexone maintenance treatment or abstinent at 12 weeks.
(2) Comparison of different levels of sedation
Two studies compared regimes of antagonist‐induced withdrawal with differing levels of sedation. Seoane 1997 compared light and heavy sedation; de Jong 2005 compared minimal sedation and anaesthesia‐assisted regimes.
(a) Intensity and duration of withdrawal signs and symptoms
Seoane 1997 reported that the peak score by a modified Wang scale (maximum score 29) occurred five minutes after the end of naloxone infusion, and was 4.8 ± 2.9 in the light sedation group, and 4.9 ± 3.0 in the heavy sedation group. A second peak of withdrawal was observed at the end of sedation: 3.4 ± 1.9 in the light sedation group and 3.9 ± 2.0 in the heavy sedation group. The frequency of individual withdrawal signs was said to be similar in the two groups.
In de Jong 2005, the score by the SOWS (maximum score 64) in the evening of day 1 was reported to be 24.4 in the anaesthesia group and 20.8 in the group receiving antagonist‐induced withdrawal with minimal sedation. These were around the maximum withdrawal scores recorded. Craving in the morning of day two was rated using a visual analogue scale (details of which were not reported) at 26.4 in the anaesthesia group, and 18.1 in the comparison minimal sedation group. Graphs of withdrawal scores and craving show the intensity of withdrawal to be similar in the two groups. SOWS and craving scores returned to baseline after one week, objective ratings in three days.
(b) Duration of withdrawal treatment
No data reported.
(c) Adverse effects
Seoane 1997 reported that two of 150 in the light sedation group and four of 150 in the heavy sedation group had respiratory depression due to excessive sedation requiring intubation (five of the six were heroin smokers). One participant in the heavy sedation group developed aspirative pneumonia requiring antibiotics (discharged on 5th day, whereas the majority were discharged after 24 hours). Two of 150 in the light sedation group and four of 150 in the heavy sedation group had other minor complications (bradycardia, fever). Overall, four of 150 (3%) in the light sedation group and nine of 150 (6%) in the heavy sedation group experienced adverse effects.
In de Jong 2005 it was reported that five in the anaesthesia group, and none in the comparison minimal sedation group, experienced adverse effects (extreme drowsiness; agitation; persistent hypoxia; pneumonia; fever; pulmonary complication from aspiration during anaesthesia). All adverse effects resolved with treatment.
Overall there was a significantly greater risk of adverse events with heavy, compared to light, sedation (Figure 7: risk ratio 3.21; 95% confidence interval 1.13 to 9.12, P = 0.03).
7.
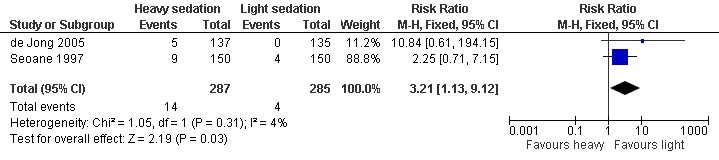
Forest plot of comparison: 2 Heavy vs light sedation, outcome: 2.1 Participants experiencing adverse effects.
(3) Comparison of anaesthetic agent
Kienbaum 2000 compared antagonist‐induced withdrawal with either propofol or methohexital as the anaesthetic agents. Huang 2002 compared ketamine and tramadol as adjuncts to propofol anaesthesia; Jovaisa 2006 compared ketamine and placebo as adjuncts to propofol anaesthesia.
(a) Intensity and duration of withdrawal signs and symptoms
Kienbaum 2000 administered naloxone under propofol or methohexital anaesthesia and assessed withdrawal using the Short Opiate Withdrawal Scale (ShOWS, 10 items each rated 0‐3) four days before admission, at admission, daily for one week, then at 10, 14 and 28 days after detoxification. Scores rose from baseline levels of 2.5‐4.5 to maximums (on the day after anaesthesia) of 16.3 ± 2.1 in the group anaesthetised with propofol and 18.2 ± 2.0 in the group administered methohexital. Withdrawal scores then steadily decreased, with scores reducing to a level that was not significantly above baseline on day six for the propofol group and day 14 for the methohexital group. Thus, Kienbaum 2000 noted that withdrawal symptoms decreased significantly more rapidly in the propofol group. They noted that participants in this group could also be extubated significantly earlier than participants anaesthetised with methohexital.
In Jovaisa 2006, the use of ketamine was associated with significantly less increase in blood pressure and heart rate, compared to placebo, following administration of opioid antagonist. In Huang 2002, heart rate and blood pressure increased to a greater extent in the group administered ketamine following administration of naloxone and naltrexone, compared to those administered tramadol.
Jovaisa 2006 reported that withdrawal scores were significantly less in the two hours after administration of opioid antagonist for the ketamine group compared to the placebo group, and the ketamine group required significantly less carbamazepine (473±335 vs 957±423 mg) and clonazepam (5.0±2.7 vs 8.6±3.7 mg) in the first 48 hours following opioid antagonist.
Huang 2002 reported that mean scores for the withdrawal symptoms of anxiety, "tearing", diarrhoea and nausea were significantly higher in the group treated with ketamine, compared to the group treated with tramadol.
(b) Duration of withdrawal treatment
Data not reported.
(c) Adverse effects
Kienbaum 2000 noted large amounts of gastric and rectal discharge after naloxone administration, with high fluid requirements in both groups. One patient required an additional two weeks treatment because of partial subclavian vein thrombosis presumed related to a central venous catheter. It was not reported which anaesthetic this patient had received.
Huang 2002 and Jovaisa 2006 reported there were no complications.
(d) Completion of withdrawal and post‐detoxification outcomes
In Kienbaum 2000, one participant in each group used heroin in the two to three weeks after detoxification. Two in the propofol group did not complete follow‐up interviews at 14 and 28 days. No other data were reported.
Huang 2002 did not report any data.
Jovaisa 2006 reported that participants in the ketamine group were opiate free for 9.4±6.6 weeks, compared to 8.0±7.0 weeks for the placebo group (difference not significant). There were no significant differences in the numbers retained in aftercare treatment.
Discussion
Summary of main results
The nine studies that met the criteria for inclusion in this review are diverse in the nature of the comparison intervention, and the particular regime of antagonist‐induced withdrawal used. The diversity and small number of studies limits the strength of conclusions that can be drawn, from these studies alone, as to the the effectiveness of anaesthesia‐assisted, antagonist‐induced withdrawal. This discussion section therefore considers relevant information from other studies, in addition to the results of the included studies, against each of the identified outcomes of interest: (a) intensity and duration of withdrawal signs and symptoms; (b) duration of withdrawal treatment; (c) adverse effects; and (d) completion of treatment and post‐detoxification outcomes.
(a) Intensity and duration of withdrawal signs and symptoms
The results of Krabbe 2003 indicate that antagonist‐induced withdrawal is more intense, but less prolonged than withdrawal managed with reducing doses of methadone. Favrat 2006 did not assess withdrawal severity, and McGregor 2002 did not directly compare withdrawal signs and symptoms in the group receiving antagonist‐induced withdrawal and those treated with clonidine and symptomatic medications. Collins 2005 found no significant difference in withdrawal severity following anaesthesia compared to withdrawal managed with either buprenorphine or clonidine.
The findings of Seoane 1997 and de Jong 2005 indicate that the level of sedation does not affect the intensity and duration of withdrawal, although it remains possible that duration of anaesthesia may influence withdrawal severity.
The findings of Kienbaum 2000 indicate that withdrawal is affected by the anaesthetic agent, with more intense and more prolonged withdrawal associated with methohexital compared with propofol anaesthesia. Jovaisa 2006 and Huang 2002 indicate that adjunct medications can significantly moderate the reaction to the initial administration of opioid antagonist, and the severity of withdrawal post‐anaesthesia.
Early studies of anaesthesia‐assisted, antagonist‐induced withdrawal reported minimal withdrawal post‐anaesthesia, or at least no significant change in severity compared to pre‐anaesthesia (eg. Brewer 1998a; Legarda 1994; Loimer 1989; Loimer 1990). However, the studies included in this review indicate that while antagonist‐induced withdrawal is less prolonged than withdrawal managed with conventional approaches, it is nonetheless associated with significant symptoms. Other more recent studies (eg. Cucchia 1998; Elman 2001; Pfab 1999; Scherbaum 1998; Tretter 1998) support this conclusion, with the authors of these studies reporting that most participants experienced moderate withdrawal lasting at least a few days following the anaesthetic procedure.
A number of factors could be contributing to the divergence between early and later studies in terms of the reported severity of withdrawal.
Firstly, the means by which withdrawal is assessed may be important. This is highlighted by the study of Loimer 1991b, in which no correlation was found between participant and observer ratings of withdrawal, with observer ratings significantly under‐estimating withdrawal compared to participant ratings. Hence, studies that rely only on observer ratings, such as the CINA (Peachey 1988) and Wang (Wang 1974) scales, may be biased towards lower ratings of withdrawal. Furthermore, these scales place an emphasis on physical signs, such as changes in heart rate and blood pressure, piloerection and rhinorrhea, which tend to resolve more quickly than other symptoms. Assessment methods that incorporate participant ratings and which include more subjective symptoms, such as anxiety, depression, body aches and sleep disturbances, can be expected to more accurately reflect participants' withdrawal experience. The Short Opiate Withdrawal Scale (Gossop 1990), or the combination of the Objective and Subjective Opiate Withdrawal Scales (Handelsman 1987) provide for incorporation of participant ratings. Several of the studies considered for this review used these scales (Collins 2005; Hensel 2000b; Kienbaum 2000; Krabbe 2003; Loimer 1991b; McGregor 2002; Pfab 1999; Scherbaum 1998). The data reported for these studies indicate that subjective symptoms were significantly elevated for several days following detoxification.
A second factor that may be impacting on the severity of withdrawal reported is the nature of opioid used by participants prior to detoxification. Pfab 1999 and Hensel 2000b both report outcomes according to the type of opioid participants had been using. Data from these studies indicate that withdrawal from methadone is more severe and more prolonged than withdrawal from heroin. This finding is also supported by Bell 1999 for antagonist‐induced withdrawal with minimal sedation. In this regard it is interesting to note that the treatment regimes used by Loimer's group, for which withdrawal was reported as minimal, generally involved participants being stabilised on morphine for two to three days prior to anaesthesia.
Data from McGregor 2002 also indicate that the severity of withdrawal is influenced by the length of time between last heroin use and induction of anaesthesia.
While some factors have been identified, insufficient data are available to determine the extent to which these factors may contribute to the intensity and duration of withdrawal signs and symptoms experienced by patients undergoing antagonist‐induced withdrawal.
(b) Duration of withdrawal treatment
McGregor 2002 reported a significantly shorter time between admission and first dose of naltrexone for participants receiving naloxone‐induced withdrawal compared to those treated with clonidine and symptomatic medications. In Krabbe 2003, participants receiving antagonist‐induced withdrawal achieved maintenance doses of naltrexone in a significantly shorter period than those whose withdrawal was managed with reducing doses of methadone. The treatment regimes used for Collins 2005 were designed with different time intervals to the first dose of naltrexone: on day 1 in the anaesthesia group, day 2 in the buprenorphine group, and day 7 in the clonidine group.
Hence, the studies included in this review support a conclusion that antagonist‐induced withdrawal reduces the time period between opioid use and establishment of an antagonist blockade of opioid receptors. However, insufficient data are available to determine whether, and for how long, withdrawal symptoms continued after commencement of naltrexone maintenance.
(c) Adverse events
McGregor 2002, Krabbe 2003 and Favrat 2006 reported no serious adverse events associated with antagonist‐induced withdrawal or conventional approaches, while Collins 2005 reported three potentially life‐threatening adverse events in the anaesthesia group, but none in the buprenorphine or clonidine groups. The findings of Seoane 1997 and de Jong 2005 indicate significantly greater risk of adverse events with heavy, compared to light, sedation (Figure 7: risk ratio 3.21; 95% confidence interval 1.13 to 9.12, P=0.03). The adverse effects in participants treated with heavy sedation or anaesthesia comprised respiratory depression, pneumonia arising from aspiration during sedation or anaesthesia, fever, bradycardia, agitation, pulmonary edema, mixed bipolar state with suicidal ideation, and diabetic ketoacidosis.
Kienbaum 2000 noted high fluid requirements after administration of opioid antagonist, and reported one serious adverse event. Huang 2002 and Jovaisa 2006 reported there were no complications.
Other studies have reported significant adverse events associated with antagonist‐induced withdrawal.
Elman 2001 reported episodes of irregularities in respiratory pattern during withdrawal induced by nalmefene and naltrexone under propofol anaesthesia, with one of seven participants having a self‐limited episode of respiratory arrest during the acute post‐anaesthetic phase.
Cucchia 1998 reported vomiting in 67% of participants (the level of sedation was reported to be such that they were able to be woken when vomiting occurred). Tretter 1998 reported vomiting in three of 14 during anaesthesia.
In 123 treatments, Albanese 2000 reported one transient psychotic episode.
In one of 22 participants treated by Scherbaum 1998, orotracheal intubation was maintained for 13 hours because of a prolonged recovery from anaesthesia. Scherbaum 1998 also commented on the occurrence of bradycardia and/or hypokalaemia in most participants following the anaesthetic procedure.
Pfab 1999 decided to abandon anaesthesia‐based detoxification after two of 12 patients had transient renal insufficiency and one suffered pulmonary dysfunction that required 35 days of mechanical ventilation and 51 days of hospitalisation before the patient recovered.
Brewer 1998a, reporting on a series of 510 cases, noted one instance of bradycardia and first degree heart block which resolved spontaneously within a few minutes.
Allhoff 1999 retrospectively reviewed ECG tracings for 22 patients undergoing anaesthetic‐based withdrawal. They found the heart rate was significantly lowered and the cQT interval significantly lengthened after detoxification. Modest hypokalaemia was linked to cQT prolongation in 10 ECG tracings. Furthermore, 12 tracings from 10 patients showed T‐wave inversion after detoxification, with sinus rhythm being turned into a rhythm arising from the AV node in two cases.
Albanese 2000 reported one case of ventricular bigeminy (premature beat following normal beat) in 123 treatments.
Hensel 2000a reported that of 30 participants treated with naltrexone under propofol anaesthesia, eight developed bradycardia and first degree AV block, and six experienced mild but persistent hypotension. Using a similar treatment protocol, Hensel 2000b reported six cases of bradycardia and nine of persistent hypotension amongst 72 participants.
Vomiting during sedation or anaesthesia is significant because of the potential for aspiration of stomach contents if the airway is not adequately protected. This can result in aspiration pneumonia, as occurred in one of 150 participants in the heavy sedation group of Seoane 1997. Approaches taken to minimise the risks of vomiting during anaesthesia include intubation to protect the airway (adopted by the majority of studies, but used for only a proportion of participants in Seoane 1997), administration of prophylactic antibiotics to prevent infection in the event of aspiration (Kienbaum 2000, Tretter 1998), and the use of medications to reduce the amount and acidity of gastric secretions, thereby reducing the incidence of vomiting (Elman 2001; Gold 1999; Hensel 2000b; Kienbaum 2000;Tretter 1998).
Data from McGregor 2002 support the value of medications to control gastric secretions, with 64% of participants not receiving octreotide as an adjunct medication, compared to 8% who did receive octreotide, experiencing vomiting and/or diarrhoea during withdrawal.
The basis of respiratory dysfunction is unclear, particularly given that antagonist‐induced withdrawal is typically associated with increases in spontaneous ventilation (Hoffman 1998). Nonetheless, it points to the need for anaesthesia‐assisted withdrawal to be limited to facilities with the capacity for adequate monitoring of patients undergoing withdrawal, and for provision of mechanical ventilation as necessary.
One death under anaesthesia has been reported (Bearn 1999; Dyer 1998). In addition, there has been one death reported in the USA due to respiratory arrest within hours of treatment (Stephenson 1997) and another death in Australia of apparent cardiac arrest, less than 24 hours after anaesthesia (Blake 1999). Only one of the studies considered for this review (Gold 1999) reported a death temporally proximate to anaesthetic‐assisted withdrawal. In this case the cause of death remains uncertain as the family refused a post‐mortem, although based on serum levels, a drug overdose was excluded.
There is evidence of cardiac irregularities associated with anaesthesia‐assisted withdrawal which might underlie some of the life‐threatening adverse events. Cardiovascular effects reported include bradycardia and first degree heart block (Brewer 1998a; Hensel 2000a; Scherbaum 1998), prolongation of the QT interval (Allhoff 1999) and other abnormalities of heart rhythm (Albanese 2000; Allhoff 1999). Allhoff 1999 linked the cardiovascular effects to modest hypokalaemia. Scherbaum 1998 also commented on the occurrence of hypokalaemia in participants after anaesthesia‐assisted withdrawal. Kienbaum 1998, in a study of the pathophysiology of anaesthesia‐assisted withdrawal, reported significant increases in plasma epinephrine and cardiac index associated with administration of naloxone (12.4mg) under methohexitone anaesthesia. This increase in circulating catecholamines and the likelihood of fluid and electrolyte loss during antagonist‐induced withdrawal have been identified as creating the potential for significant arrhythmia (Whittington 2000). The use of additional drugs, particularly alpha2 adrenergic agonists such as clonidine, has been identified as important to reduce the risk of hyperadrenergic crisis and pulmonary oedema arising from the surge in catecholamines (Gevirtz 1999). The findings of Huang 2002 and Jovaisa 2006 suggest that tramadol and ketamine may also have some effectiveness in moderating increases in heart rate and blood pressure. The importance of monitoring cardiac function and electrolyte levels during and after anaesthesia‐assisted withdrawal has also been stressed (Whittington 2000). The use of medications to reduce vomiting and diarrhoea (eg. ondansetron, octreotide) might also be expected to be beneficial by reducing fluid loss associated with antagonist‐induced withdrawal.
The diversity of the adverse effects indicates the basis of adverse effects is complex. As has been suggested by others (Collins 2005; Kienbaum 2000), it may be that the rapid precipitation of withdrawal that is possible under anaesthesia imposes a level of physiological stress that significantly increases the risk of adverse effects, with the nature of the adverse effect dependent on the physical and mental health status of the individual.
The discussion above identifies the nature of opioid drugs used prior to detoxification and the use of adjunct medications as factors that might influence the severity of anaesthesia‐assisted withdrawal and the risk of adverse effects. Other possible factors include the anaesthetic agent used, the duration of anaesthesia, as well as the nature, dose and route of administration of the opioid antagonist used to induce withdrawal.
The findings of Huang 2002 and Jovaisa 2006 indicate that the potential influence of adjunct medications on outcome also needs to be considered. However, the adjunct medications used in the studies considered for this review are too diverse and too numerous to be able to identify specific effects at this time.
The three opioid antagonists used in the studies considered for this review ‐ naloxone, naltrexone, and nalmefene ‐ differ in their duration of action and also in the route of administration. Naloxone and nalmefene are typically administered by injection, usually intravenously, whereas naltrexone has been administered orally, usually as a slurry via a gastric tube for anaesthesia‐assisted withdrawal. Again, because of the diversity of dose regimes it is not possible at this time to identify any effect on outcome arising from the nature, route of administration or dose of the opioid antagonist used to induce withdrawal. However, an indication of the potential effect is provided by a study in which plasma naltrexone was measured during withdrawal induced by naltrexone (given via gastric tube in repeated doses of 12.5, 25, 50 and 50mg) under propofol anaesthesia (McDonald 2000). It was found that increases in plasma naltrexone were variable. Factors suggested as influencing absorption included positioning of the gastric tube, and attenuated blood flow to the stomach due to anaesthesia or sympathetic nervous system activation. This finding suggests that the choice of opioid antagonist may influence outcomes, and also that injected opioid antagonists may have an advantage over orally administered antagonists in terms of more predictable bioavailability, and hence better management of withdrawal severity and risk of adverse events.
(d) Completion of treatment and post‐detoxification outcomes
Data are limited, but it appears that antagonist‐induced withdrawal under anaesthesia, compared to withdrawal managed with clonidine and possibly tapered methadone, is associated with increased rates of commencement of naltrexone maintenance treatment, and increased rates of retention in naltrexone maintenance treatment for up to three months. However, there are no significant differences in these outcomes for antagonist‐induced withdrawal under anaesthesia compared to withdrawal managed with buprenorphine.
Both Krabbe 2003 and McGregor 2002 report higher rates of dropout, particularly early in treatment, from conventional compared to antagonist‐induced withdrawal. Some of the early dropout may be attributable to participants' disappointment on being allocated to standard treatment rather than a novel approach. Collins 2005 and McGregor 2002 report low rates of retention in naltrexone maintenance treatment following detoxification.
The level of sedation during withdrawal has no effect on completion of detoxification or abstinence at one month post‐detoxification (de Jong 2005).
It is clear from the above studies, and others considered for this review, that not all patients who undergo anaesthesia‐assisted withdrawal continue with naltrexone maintenance treatment, and a significant proportion relapse to opioid use. However, the rates of engagement in naltrexone maintenance treatment and abstinence from opioid use in the post‐detoxification period cannot be accurately quantified at this time.
There have been reports of deaths in participants subsequent to detoxification under anaesthesia or heavy sedation (Bearn 1999; Brewer 1997; Dyer 1998; Stephenson 1997). The point of highest risk is probably the time of relapse to opioid use post‐detoxification. Not only will there be decreased tolerance to opioids at this time because of cessation of opioid use, but the response to opioids may be enhanced because of opioid receptor up regulation (White 1999) resulting in a high risk of overdose.
Overall completeness and applicability of evidence
The information on the effectiveness of antagonist‐induced withdrawal under heavy sedation or anaesthesia is sparse. The studies that met the criteria for inclusion in this review varied considerably in treatment regimes, the nature of the comparison modality, and the nature of outcome data reported and this limited the nature and extent of the analyses that were able to be undertaken.
There is little information on the nature of withdrawal signs and symptoms experienced by patients, the duration of significant symptoms, the overall severity, or the acceptability to patients of this approach to the management of opioid withdrawal. It remains uncertain to what extent the nature of the opioid used prior to withdrawal, the length of time between last opioid use and administration of opioid antagonist, the anaesthetic agent used, the dose of opioid antagonist and the duration of anaesthesia may contribute to both withdrawal severity and adverse effects. The use of adjunct medications appears to be important, but the evidence on type and amount of adjunct medications remains limited.
Quality of the evidence
The quality of the evidence for the analyses that were undertaken is considered to be moderately strong.
Potential biases in the review process
The review draws together studies of diverse design and some risk of internal bias. The review itself is consequently exposed to some risk of bias.
Authors' conclusions
Implications for practice.
The treatment regimes for the administration of opioid antagonists under anaesthesia or heavy sedation vary in the opioid antagonist used, the dose and mode of administration, the anaesthetic agent, duration of anaesthesia, and other medications employed (seeTable 5). The dose and half‐life of the opioid used prior to withdrawal also varied. It is not possible to identify "standard" treatment regimes for antagonist‐induced withdrawal in conjunction with heavy sedation or anaesthesia.
Antagonist‐induced withdrawal is more intense but less prolonged than withdrawal managed by tapered methadone or clonidine plus symptomatic medications, and is associated with significant reductions in the time between opioid use and commencement of naltrexone treatment.
The severity of antagonist‐induced withdrawal is probably influenced by the nature of opioid used prior to withdrawal, the length of time between last opioid use and administration of opioid antagonist, the anaesthetic agent used, the dose of opioid antagonist and the duration of anaesthesia. However, the extent to which these factors influence outcomes is unclear.
The use of adjunct medications including alpha2‐adrenergic agonists (e.g. clonidine), anti‐emetic and anti‐diarrhoeal agents appears to be important in reducing vomiting and diarrhoea during anaesthesia as well as controlling the effects of a surge in catecholamines triggered by administration of opioid antagonists. Reducing vomiting and diarrhoea will help to control fluid loss which carries with it the risk of hypokalaemia, which in turn can trigger cardiac arrhythmias. Controlling vomiting and diarrhoea can be expected to also contribute significantly to patient comfort in the post‐anaesthesia recovery period.
The reported occurrence of vomiting during sedation, respiratory depression and cardiac irregularities point to the approach being limited to facilities equipped for intubation, assisted ventilation and a high level of monitoring, and with the capacity to respond to the adverse events that might occur.
The increased risk of clinically significant adverse events associated with withdrawal under heavy sedation or anaesthesia make the value of anaesthesia‐assisted antagonist‐induced withdrawal questionable. Given that the intensity and duration of withdrawal, and rates of completion of withdrawal, are similar for antagonist‐induced withdrawal with minimal sedation, or withdrawal managed with buprenorphine, these would appear to be preferable approaches to managing withdrawal in those wishing to transfer to naltrexone maintenance treatment.
Implications for research.
The lack of additional benefit, and increased risk of harm associated with antagonist‐induced withdrawal under heavy sedation or anaesthesia, as compared to approaches with minimal sedation, suggests that this form of treatment should not be pursued. Research resources would be better directed towards assessment and development of minimal sedation approaches, or the use of buprenorphine to facilitate transition to naltrexone maintenance treatment.
However, if undertaken, any research should explore factors that might influence outcomes. These factors include the nature, dose and route of administration of opioid antagonist; the anaesthetic agent, depth and duration of anaesthesia; the type, dose and timing of adjunct medications; and the nature, dose and timing of last opioid use.
The signs and symptoms of withdrawal should be assessed across a time course, from prior to commencement of the intervention through to subsidence of signs and symptoms, which may be some weeks after cessation of opioid use. Assessments should incorporate both objective signs and subjective symptoms.
Consideration could also be given to participant characteristics, such as severity of dependence, psychological and health status, duration of use, previous treatment history, employment status and family support, and the extent to which these contribute to the outcome of withdrawal.
What's new
| Date | Event | Description |
|---|---|---|
| 5 October 2009 | New search has been performed | new trials founded needs new citation |
| 17 August 2009 | New citation required but conclusions have not changed | New search, new trials, new assessment of included studies |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 26 March 2008 | Amended | Converted to new review format. |
| 24 November 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Fabrizio Faggiano was contact editor for the review.
Appendices
Appendix 1. CENTRAL search strategy via Ovid Online
1 exp opioid‐related disorders/ 2 (opiat$ or opioid$ or heroin$ or narcot$).ti,ab 3 exp substance withdrawal syndrome/ 4 (detoxifi$ or desintoxi$ or disintoxi$ or disintossi$ or withdrawal).ti,ab 5 exp metabolic detoxication,drug/ 6 (1 or 2) and (3 or 4 or 5)
Appendix 2. MEDLINE search strategy via Ovid Online
1 exp opioid‐related disorders/ 2 (opiat$ or opioid$ or heroin$ or narcot$).ti,ab 3 exp substance withdrawal syndrome/ 4 (detoxifi$ or desintoxi$ or disintoxi$ or disintossi$).ti,ab 5 exp metabolic detoxication, drug/ 6 (1 or 2) and (3 or 4 or 5) 7 limit 6 to human
Appendix 3. EMBASE search strategy via Ovid Online
1 exp opiate addiction/ 2 (opiat$ or opioid$ or heroin$ or narcot$).ti,ab 3 exp withdrawal syndrome/ 4 (detoxifi$ or desintoxi$ or disintoxi$ or disintossi$).ti,ab 5 *drug detoxification/ 6 (1 or 2) and (3 or 4 or 5) 7 limit 6 to human
Appendix 4. PsycINFO search strategy via EBSCO Host
1 MM "Opiates" 2 (opiat$ or opioid$ or heroin$ or narcot$) 3 MM "Drug Withdrawal" 4 detoxification or detoxify or detoxified 5 MM "Detoxification" 6 S1 or S2 7 S3 or S4 or S5 8 S6 and S7 (Population group: Human selected for final line)
Appendix 5. Criteria for assessing risk of bias in RCTs, CCTs and observational studies
| Item | Judgment | Description |
| 1. Was the method of randomization adequate? | Yes | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization |
| No | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention Observational prospective study |
|
| Unclear | Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’ | |
| 2. Was the treatment allocation concealed? | Yes | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomization); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| No | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. Observational prospective study |
|
| Unclear | Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |
| 3. Was knowledge of the allocated interventions adequately prevented during the study? (blinding of patients, provider, outcome assessor) Objective outcomes |
Yes | Blinding of participants, providers and outcome assessor and unlikely that the blinding could have been broken; Either participants or providers were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. No blinding, but the objective outcome measurement are not likely to be influenced by lack of blinding |
| 4. Was knowledge of the allocated interventions adequately prevented during the study? (blinding of patients, provider, outcome assessor) Subjective outcomes |
Yes | Blinding of participants, providers and outcome assessor and unlikely that the blinding could have been broken; Either participants or providers were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. |
| No | No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; Either participants or outcome assessor were not blinded, and the non‐blinding of others likely to introduce bias |
|
| Unclear | Insufficient information to permit judgement of ‘Yes’ or ‘No’; | |
| 5. Were incomplete outcome data adequately addressed? For all outcomes except retention in treatment or drop out |
Yes |
No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods All randomized patients are reported/analyzed in the group they were allocated to by randomization irrespective of non‐compliance and co‐interventions (intention to treat) |
| No | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; |
|
| Unclear | Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomized not stated, no reasons for missing data provided; number of drop out not reported for each group) | |
| 6. Free of other bias: Comparability of cohorts on the basis of the design or analysis |
yes | Exposed and non exposed individuals are matched in the design for most important confounding factors Analysis are adjusted for most important confounding factors |
| no | No matching or no adjustment for most important confounding factor | |
| unclear | No information about comparability of cohort | |
| 7. Free of other bias: Representativeness of the exposed cohort |
yes | The sample is representative of the average population receiving the intervention in clinical practice |
| no | The sample is a selected group of population not representative of the average population | |
| unclear | no description of the derivation of the cohort | |
| Free of other bias: Selection of the non exposed cohort |
Yes | the sample has been drawn from the same community as the exposed cohort |
| no | the sample has been drawn from a different source | |
| unclear | no description of the derivation of the non exposed cohort | |
| Free of other bias: Ascertainment of exposure |
yes | Information in the study was obtained from a secure record (eg clinical records or structured interview) |
| no | Self report | |
| unclear | no description |
Data and analyses
Comparison 1. Antagonist‐induced vs conventional.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number refusing group allocation or failing to attend | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.15, 1.68] |
| 2 Number completing detoxification | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Methadone comparison | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.14, 2.91] |
| 2.2 Clonidine comparison | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.92, 1.73] |
| 3 Number commencing naltrexone maintenance treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Clonidine comparison | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.28 [2.91, 6.30] |
| 3.2 Buprenorphine comparison | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.04, 1.60] |
| 4 Retained in naltrexone maintenance treatment or abstinent at 12 weeks | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Tapered methadone comparison | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.45] |
| 4.2 Clonidine comparison | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.37, 5.61] |
| 4.3 Buprenorphine comparison | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.34, 1.97] |
1.1. Analysis.
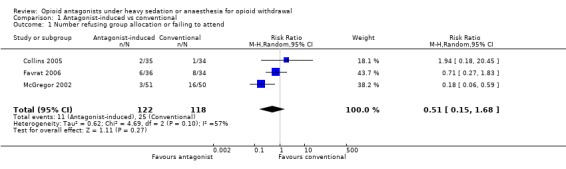
Comparison 1 Antagonist‐induced vs conventional, Outcome 1 Number refusing group allocation or failing to attend.
1.2. Analysis.
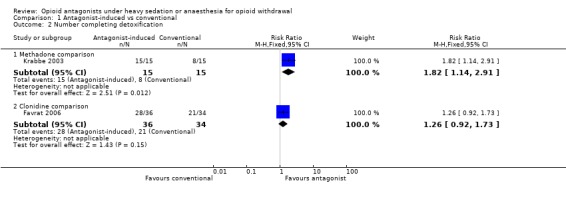
Comparison 1 Antagonist‐induced vs conventional, Outcome 2 Number completing detoxification.
1.3. Analysis.
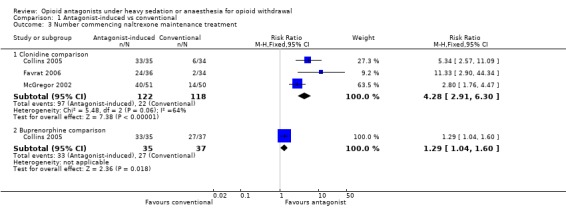
Comparison 1 Antagonist‐induced vs conventional, Outcome 3 Number commencing naltrexone maintenance treatment.
1.4. Analysis.
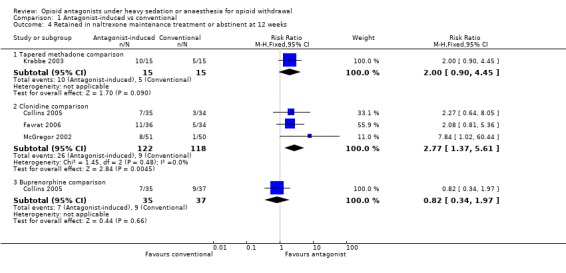
Comparison 1 Antagonist‐induced vs conventional, Outcome 4 Retained in naltrexone maintenance treatment or abstinent at 12 weeks.
Comparison 2. Heavy vs light sedation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants experiencing adverse effects | 2 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [1.13, 9.12] |
2.1. Analysis.
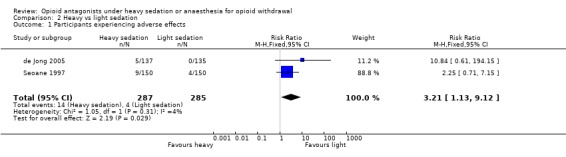
Comparison 2 Heavy vs light sedation, Outcome 1 Participants experiencing adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Collins 2005.
| Methods | Random allocation in blocks of 12, computer generated. No blinding. Groups similar on demographic and clinical characteristics. | |
| Participants | 106 heroin dependent by DSM‐IV. 36% iv users. Group sizes (1) 35 (2) 37 (3) 34. 72% male. Mean age 36. Major psychiatric illness, active medical illness, dependence on other drugs or alcohol exclusion criteria. 36% currently married or cohabiting. 56% currently employed. | |
| Interventions | (1) Nalmefene 4mg iv over 30 minutes, naltrexone 50mg via nasogastric tube, under propofol anaesthesia (4‐6 hours). Various adjunct medications, including octreotide. (2) Buprenorphine, 8mg (single dose) day 1, naltrexone 12.5mg afternoon of day 2, 25mg 12 hours later, then 50mg/day. (3) Clonidine, max 1.2mg/day, discharged day 3, naltrexone 12.5mg day 7, 25mg day 8, then 50mg/day.. Clonidine and various adjunct medications available to all groups. All received 72 hours inpatient care, followed by 12 weeks outpatient naltrexone maintenance with relapse prevention psychotherapy as aftercare. | |
| Outcomes | Graphs of withdrawal severity. Mean weeks in treatment. Number of participants completing inpatient phase, receiving at least one dose of naltrexone, receiving full 50mg dose of naltrexone. Number retained in treatment over 12 weeks. Number retained 12 weeks who provided 2 or less opiate positive urine samples. Number experiencing serious adverse events. | |
| Notes | Withdrawal assessed by Subjective & Objective Opiate Withdrawal Scales, and Clinical Institute Narcotic Assessment, four times a day during inpatient phase. Country: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "...using random, computer‐generated assignments with stratification by sex. ... In addition, the Berger‐Exner test was used to confirm that no selection bias in enrollment occurred." |
| Allocation concealment? | Low risk | Quote: "All staff remained unaware of the randomization sequence..." |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | Patients were not blinded to treatment ‐ sham anaesthesia not ethical. Unclear whether observers were blinded to treatment. |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes unlikely to be affected by knowledge of treatment. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow‐up similar in three groups. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Unclear risk | Enrollment stopped at 106 participants (aim 150) "because actual differences in withdrawal severity scores and treatment retention were smaller than anticipated, leading to an impractically large recalculated sample size..." Comment: It seems unlikely that the stopping of enrolment resulted in bias. |
| Other bias: Selection of comparison cohort | Low risk | Experimental and control groups drawn from same population. |
| Other bias: Comparability of cohorts | Low risk | No significant differences in demographics of three groups. |
| Other bias: Representativeness of exposed cohort | Low risk | Participants drawn from population seeking heroin detoxification. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
de Jong 2005.
| Methods | Random allocation in pairs by project data manager. Groups similar. No blinding. 20% did not complete one‐month follow‐up. | |
| Participants | 272 opioid‐dependent by DSM‐IV. Group sizes (1) 137 (2) 135. 82% male. Mean age 36. Mean 12 years heroin use, 7.4 years methadone use. All stabilised on methadone (dose not reported) prior to detoxification. Mean 8 previous detoxification treatments. (1) 10.6% (2) 18% married. (1) 42% (2) 35.9% unemployed. Inpatient treatment in addiction centre and general hospital. | |
| Interventions | (1) Naltrexone 100mg oral. Anaesthesia induced with propofol when withdrawal apparent. Duration anaesthesia 4 hours, with intubation and ventilation. Further 100mg naltrexone via nasogastric tube at end anaesthesia. (2) Naltrexone, 12.5mg day 1, 25mg day 2, 50mg day 3. Range of adjunct medications in both groups. Naltrexone 50mg/day and relapse prevention treatment as follow‐up. | |
| Outcomes | Graphs of withdrawal scores and craving. Proportion abstinent and proportion using naltrexone at 1‐month follow‐up. Cost of procedure. Participants experiencing adverse events. | |
| Notes | Withdrawal assessed by Subjective and Objective Opiate Withdrawal Scales. Self‐report drug use by European version of Addiction Severity Index. Craving by vas. Country: The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomization assignments (block sizes of two) were generated ... using SAS System for Windows version 8.0." |
| Allocation concealment? | Low risk | Quote: "Randomization assignments (block sizes of two) were generated centrally by the project data manager..." |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | Quote: "A case record form (CRF) was completed for each patient by non‐blinded nursing staff." |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes considered unlikely to be affected by lack of blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out and missing data similar in two groups. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent |
| Other bias: Selection of comparison cohort | Low risk | Experimental and control groups drawn from same population. |
| Other bias: Comparability of cohorts | Low risk | No significant differences in demographics of two groups. |
| Other bias: Representativeness of exposed cohort | Low risk | Participants drawn from clients of four addiction treatment centres. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
Favrat 2006.
| Methods | Random allocation by senior pharmacist, computer generated numbers. Treatment and outcome assessment not blinded. Groups similar on demographics. | |
| Participants | 70 opioid dependent by DSM‐IV, (1) 14 (2) 10 in methadone treatment. Group sizes (1) 36 (2) 34. Treatment commenced by (1) 26 (2) 21. 77% male, mean age 30, 62% single, 28% employed. Exclusion criteria included severe psychiatric or medical condition, pregnancy, dependence on alcohol, cocaine or benzodiazepines. | |
| Interventions | (1) Naltrexone 100mg; propofol anaesthesia when withdrawal apparent; intubated. Overnight in intensive care unit; 50mg naltrexone next day before transfer to inpatient substance abuse clinic. (2) Clonidine (divided doses) 0.6mg/day for 3 days, then tapered and ceased after day 7. Both groups had 1 week inpatient care in substance use clinic following detoxification. | |
| Outcomes | Number completing detoxification (defined as 3 days of retention in anaesthesia treatment or 7 days in standard inpatient treatment, without drug use). Self‐report abstinence at 3, 6, 12 months. Number commencing naltrexone. Mean days in treatment. | |
| Notes | Withdrawal severity not assessed. No urine screening reported. Country: Switzerland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Participants ... were randomly assigned by computer‐generated numbers..." |
| Allocation concealment? | Low risk | Quote: "The senior pharmacist of the psychiatric teaching hospital was responsible for the centralised randomisation process and remained unaware of the participants' characteristics or identities." |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | No blinding, and no assessment of withdrawal severity. |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes considered unlikely to be affected by lack of blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropout similar in two groups. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent. |
| Other bias: Selection of comparison cohort | Low risk | Experimental and control groups drawn from same population. |
| Other bias: Comparability of cohorts | Low risk | No significant differences in demographics of two groups. |
| Other bias: Representativeness of exposed cohort | Low risk | Participants drawn from patients admitted to substance abuse detoxification unit. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
Huang 2002.
| Methods | Random allocation. Blinding not reported. | |
| Participants | 160 opioid dependent by ICD‐10; Group sizes (1) 78 (2) 82. Duration of drug abuse 15 months to 11 years. 79% using by inhalation. 91% male, mean age 30. | |
| Interventions | Naloxone 0.03mg/kg iv followed by naltrexone 0.6mg/kg by nasogastric tube under anaesthesia with propofol, midazolam and (1) ketamine or (2) tramadol. Anaesthesia maintained for 3 hours, iv tranquillizers for 2 hours. Inpatient treatment, general hospital. | |
| Outcomes | Heart rate and blood pressure before and after anaesthesia, after each dose of naloxone, and after naltrexone. Main withdrawal symptoms before and after treatment. | |
| Notes | Ten signs and symptoms of withdrawal assessed; scoring method not reported. Country: China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "...volunteers were divided at random into two groups". Method of sequence generation not reported. |
| Allocation concealment? | Unclear risk | Method not reported. |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | No blinding reported. |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes not reported, and considered unlikely to be affected by lack of blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | No drop out reported. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent |
| Other bias: Selection of comparison cohort | Low risk | Experimental and comparison groups drawn from same population. |
| Other bias: Comparability of cohorts | Unclear risk | Comparability of group demographics not reported. |
| Other bias: Representativeness of exposed cohort | Unclear risk | Participants described as volunteers, but means of recruiting participant and source population not reported. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
Jovaisa 2006.
| Methods | Random allocation (method not reported). Double‐blind stated. 8/58 failed to comply with protocol and were excluded. Groups similar on demographics and drug use. | |
| Participants | 58 opioid dependent by ICD‐10 and DSM‐IV. Eight excluded for failure to comply with study protocol and incomplete data collection. Group sizes (1) 22 (2) 28. Use of long‐acting opioids an exclusion criterion. Mean age 23, 84% male, mean 4 years of opiate abuse, mean 2.3 previous medical detoxifications. | |
| Interventions | Stabilised on morphine 2 days (inpatient). Naloxone 1.6mg iv, 0.8mg/h iv infusion, naltrexone 100mg via oro‐gastric tube under isoflurane anaesthesia with intubation. Prior to opioid antagonist treated with (1) ketamine, subanaesthetic, 0.5mg/kg/h or (2) placebo ‐ normal saline. Inpatient treatment, general hospital. | |
| Outcomes | Mean arterial pressure, heart rate, opiate withdrawal during anaesthesia. Number entering post‐detoxification treatment. Status at 4 month follow‐up. | |
| Notes | Withdrawal severity by modified Wang scale during anaesthesia, and by Subjective and Objective Withdrawal Scales following anaesthesia. Country: Lithuania | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Patients were randomly assigned ... on the day of procedure." Method of sequence generation not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | Unclear risk | Double‐blind stated and placebo used, but unclear whether treating staff were blind. |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | Double‐blind stated and these outcomes considered unlikely to be affected by knowledge of treatment method. |
| Incomplete outcome data addressed? All outcomes | Low risk | Eight participants excluded prior to randomisation. No dropout during detoxification; loss to follow‐up after detoxification similar for the two groups. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent |
| Other bias: Selection of comparison cohort | Low risk | Experimental and comparison groups drawn from same population. |
| Other bias: Comparability of cohorts | Low risk | Groups similar on demographics and drug use. |
| Other bias: Representativeness of exposed cohort | Unclear risk | Means of recruiting participant and source population not reported. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
Kienbaum 2000.
| Methods | Random allocation, method not reported. Withdrawal symptoms for 11 in each group, biochemistry & haemodynamics for 10 in each group. Participants blind to anaesthetic used. | |
| Participants | 25 (8 female, 17 male) in MMT. Mean (±SE) (1) 89±23 (2) 106 ± 19 mg/day. Last dose 24h before naloxone. Mean age 29 years. Duration of addiction 90 mo, MMT 20 mo. Inpatient treatment ‐ intensive care unit and psychiatric ward. | |
| Interventions | General anaesthesia with (1) propofol (2) methohexital. Naloxone iv 0.4, 0.8, 1.6, 3.2 and 6.4mg at 15min intervals, then 0.8mg/h for 24h. Intubated and ventilated. Naltrexone 50mg/d, oral, for at least 4 weeks following detox. | |
| Outcomes | Mean daily withdrawal score. Time for score to return to baseline. Levels of catecholamines in plasma. Cardiovascular parameters. Number relapsing to heroin and completing follow‐up. | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale. Country: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Two groups ... each received different aesthetics according to a randomisation list." Method of sequence generation not reported. |
| Allocation concealment? | High risk | Patients blinded to allocation, but it appears unlikely that allocation was concealed from treating staff. |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | Unclear risk | Patients blind to allocation, but unclear whether observers and treating staff also blind. |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes considered unlikely to be affected by lack of blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | Exclusions and dropouts similar in two groups. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent |
| Other bias: Selection of comparison cohort | Low risk | Experimental and comparison groups drawn from same population. |
| Other bias: Comparability of cohorts | Low risk | Demographics of groups similar. |
| Other bias: Representativeness of exposed cohort | Low risk | Participants drawn from methadone substitution program. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
Krabbe 2003.
| Methods | Consecutive assignment to treatment. Groups similar except duration of methadone significantly less in methadone group. Dropouts considered as relapsed. Intention to treat analysis. No blinding reported. | |
| Participants | 30 opioid‐dependent by DSM‐IV. 15 in each group. 80% male. Mean age (1) 34.9 (2) 31.4. Duration of heroin use (1) 11.1 ± 7 (2) 6.3 ± 6.2 years. Duration of methadone use (1) 9.4 ± 6.7 (2) 3.5 ± 5.2 years. 20% employed. Mean previous treatments (1) 9.6 ± 7.5 (2) 6.9 ± 5.8. Inpatient treatment (1) hospital and addiction clinic (2) addiction clinic only. | |
| Interventions | Methadone during waiting period (dose not reported). (1) Naltrexone 100mg oral. Propofol anaesthesia induced when withdrawal evident. Mechanical ventilation. 0.8mg naloxone test every 20 min until no withdrawal. 100mg naltrexone via nasogastric tube. Range of adjunct medications. (2) Methadone tapered over 1‐2 weeks. Naltrexone maintenance as aftercare, commenced (1) immediately (2) 6 days after last methadone. | |
| Outcomes | Graph of mean withdrawal scores. Number of participants with opiate‐free urine samples during follow‐up. | |
| Notes | Withdrawal assessed by Subjective and Objective Opiate Withdrawal Scales. Country: The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Consecutive allocation. |
| Allocation concealment? | High risk | Treatments undertaken consecutively. |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | High risk | No blinding |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes considered unlikely to be affected by lack of blinding. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | (1) 0/15 (2) 9/15 dropped out in first week, but data on severity of withdrawal able to be compared only for first four days. Completion of withdrawal and duration of treatment is primary outcome measure. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent |
| Other bias: Selection of comparison cohort | Low risk | Experimental and control cohorts selected from same population. |
| Other bias: Comparability of cohorts | Low risk | Groups similar except duration of methadone significantly less in methadone group. |
| Other bias: Representativeness of exposed cohort | Low risk | Both groups drawn from population of opioid‐dependent clients with a clear wish to attain abstinence. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
McGregor 2002.
| Methods | Random allocation by researcher. Groups similar on demographics and drug use history except recent amphetamine use (higher in antagonist‐induced withdrawal group). Intention to treat analysis. Higher dropout from standard detoxification group. Observers and treating clinician post‐detoxification blind to group allocation. | |
| Participants | 101 heroin users, 94% injectors, dependent by DSM‐IV. Group sizes (1) 51 (2) 50. 60% male. Mean age 31 years. Mean 10 years heroin use. 77% single. 48% unemployed. Inpatient treatment in (1) intensive care unit of general hospital, and substance use clinic if required, (2) substance use clinic only. | |
| Interventions | (1) Naloxone, 4 or 5 boluses to 10 or 12 mg, under propofol anaesthesia. Intubated, spontaneous ventilation. Duration of anaesthesia about 4 hours. Range of adjunct medications. (2) Clonidine and symptomatic medications. Both groups given 400ug naloxone challenge (1) after recovery from anaesthesia (2) after at least 3 days. If no significant response, given 50mg naltrexone. Aftercare naltrexone (50mg/day) and supportive counselling. | |
| Outcomes | Number completing treatment; number commencing naltrexone; days between admission and naltrexone induction; number experiencing adverse events; number abstinent at follow‐up by self‐report and hair analysis. | |
| Notes | Withdrawal assessed by Subjective and Objective Opiate Withdrawal Scales, and modified Objective Opiate Withdrawal Scale during anaesthesia. Country: Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomisation was carried out in blocks of four...". Random numbers table used. |
| Allocation concealment? | Low risk | Quote: "Randomisation was carried out ... by a member of the research team blind to participants' identity or history." |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | Unclear risk | Participants and staff of detoxification unit aware of treatment. Medical officer providing follow‐up naltrexone maintenance treatment and research staff blinded to group allocation, but adequacy of blinding uncertain. |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes considered unlikely to be affected by awareness of treatment group. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Retention in treatment, and completion of treatment primary outcome measures. Significant differences in dropout rates for two groups resulted in data on severity of withdrawal being at risk of bias, but these data not used. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent |
| Other bias: Selection of comparison cohort | Low risk | Experimental and control groups drawn from same population. |
| Other bias: Comparability of cohorts | Low risk | Groups similar on demographics. |
| Other bias: Representativeness of exposed cohort | Low risk | Participants drawn from general opioid‐dependent population wishing to undergo detoxification. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
Seoane 1997.
| Methods | Random allocation by computer generated numbers in sealed envelopes. Groups similar. Intention to treat analysis. Participants blind. | |
| Participants | 300 heroin users, 150 in each group. 210 male, 90 female. (1) 54 (2) 58 used by non‐injecting routes only. Mean age 29 years. Duration of addiction 7.6 years. Mean 4.5 previous detox attempts. Inpatient care ‐ intensive care unit. Discharged to supervision of relative. | |
| Interventions | Naloxone infusion (0.06‐0.08 mg/kg) 5‐10min, then 50mg naltrexone, oral, under (1) light or (2) heavy sedation by propofol and midazolam for 6‐8 hours. Naltrexone 50mg/d, oral, for 1 year after detox. | |
| Outcomes | Withdrawal score. Number experiencing adverse effects. Sedation time. Duration in intensive care unit and time to discharge. Status at 1mo follow‐up. | |
| Notes | Withdrawal assessed by modified Wang scale. Country: Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "The randomisation process was done before initiating the study, according to a table of three‐digit random numbers generated by computer." |
| Allocation concealment? | Unclear risk | Quote: "Three hundred envelopes were created ... containing the document establishing the sedation randomly assigned". Comment: It is not reported whether the envelopes were opaque or consecutively numbered. |
| Blinding? Subjective outcomes ‐ intensity of withdrawal, adverse effects | Low risk | Patients blind to treatment group. |
| Blinding? Objective outcomes ‐ duration of treatment, completion of treatment | Low risk | These outcomes considered unlikely to be affected by lack of blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | No dropout during detoxification. |
| Free of selective reporting? | Low risk | None apparent |
| Free of other bias? | Low risk | None apparent |
| Other bias: Selection of comparison cohort | Low risk | Experimental and comparison groups drawn from same population. |
| Other bias: Comparability of cohorts | Low risk | Characteristics of two groups similar. |
| Other bias: Representativeness of exposed cohort | Low risk | Participants drawn from patients referred for new detoxification treatment. |
| Other bias: Ascertainment of exposure | Low risk | Data collection established by study protocol. |
h: hour(s) MMT: methadone maintenance treatment min: minute(s) mo: month(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albanese 2000 | Follow‐up study of 120 opioid users undergoing antagonist‐induced withdrawal (123 procedures). No treatment comparison. Insufficient outcome data. |
| Allhoff 1999 | Retrospective case review of electrocardiographic abnormalities associated with antagonist‐induced withdrawal. No treatment comparison. Limited outcome data. |
| Arnold‐Reed 2005 | Antagonist‐induced withdrawal with minimal sedation. |
| Bartter 1996 | Discusses series of approaches to opioid withdrawal leading to development of a regime of antagonist‐induced withdrawal. Case series, not a controlled study. Insufficient detail on treatment protocols and outcomes. |
| Bell 1999 | Comparison of outcomes for cohorts of heroin‐dependent and methadone‐dependent clients receiving antagonist‐induced withdrawal. No treatment comparison. Low level sedation. |
| Boehle 2000 | Discusses clinical experience with antagonist‐induced withdrawal in 5 opioid‐dependent patients. No treatment comparison. |
| Brewer 1998a | Describes method of anaesthesia‐assisted antagonist‐induced withdrawal. Case series, not controlled study. Variable treatment protocol. Insufficient detail on outcomes. |
| Cucchia 1998 | Describes method of antagonist‐induced opioid withdrawal and outcomes for 20 patients. No treatment comparison. |
| Dettling 1996 | No English abstract. Likely to overlap with Tretter 1998. |
| E. Berenguel 2001 | Reports outcomes for 20 opioid‐dependent patients treated with antagonist‐induced withdrawal. No treatment comparison. |
| Elman 2001 | Follow‐up study of acute and long‐term outcomes for 7 opioid‐dependent patients treated with antagonist‐induced withdrawal and naltrexone maintenance. No treatment comparison. |
| Foster 2003 | Reports outcomes for two cohorts (n1 = 55, n2 = 46) receiving naltrexone implants during antagonist‐induced withdrawal under general anaesthesia or sedation. Not controlled study. No treatment comparison. |
| Gold 1999 | Reports outcomes for 20 opioid‐dependent patients treated with antagonist‐induced withdrawal. No treatment comparison. |
| Hensel 2000a | Randomised controlled trial comparing methods of monitoring anaesthesia during antagonist‐induced withdrawal. Insufficient information on participant characteristics. Limited outcome data. |
| Hensel 2000b | Follow‐up study of 72 opioid‐dependent patients treated with antagonist‐induced withdrawal. No treatment comparison. |
| Ivaskevicius 2005 | Reports use of antagonist‐induced withdrawal under anaesthesia for management of opioid withdrawal. No treatment comparison. |
| Kasvikis 1997 | Reports outcomes of antagonist‐induced withdrawal for management of opioid withdrawal. No treatment comparison. |
| Lawental 2000 | Compares outcomes for 139 opioid‐dependent participants treated with antagonist‐induced withdrawal and historical cohort receiving standard inpatient detoxification. Retrospective data collection. Insufficient outcome data. Unclear (probably variable) treatment regimes. |
| Legarda 1994 | Describes outcomes for 11 opioid‐dependent males receiving antagonist‐induced withdrawal. No treatment comparison. |
| Loimer 1989 | Reports treatment of 12 opioid‐dependent males with antagonist‐induced withdrawal. No treatment comparison. |
| Loimer 1990 | Initiated as randomised controlled trial comparing effects of naloxone and placebo when administered under anaesthesia to opioid‐dependent patients. Placebo group switched to naloxone, negating treatment comparison. Insufficient outcome data. |
| Loimer 1991a | Reports use of antagonist‐induced withdrawal under anaesthesia to treat 7 opioid‐dependent patients. No treatment comparison. |
| Loimer 1991b | Comparison of observer‐ and subject ratings of withdrawal in context of antagonist‐induced opioid withdrawal. No treatment comparison. |
| Loimer 1991c | Compares outcomes for antagonist‐induced withdrawal and methadone detoxification. Insufficient data on outcomes during acute withdrawal. Tapered methadone group probably not recruited and treated concurrently. |
| Loimer 1993 | Reports outcomes of antagonist‐induced withdrawal in 20 opioid‐dependent men with naloxone administered nasally. No treatment comparison. |
| London 1999 | Reports outcomes of antagonist‐induced withdrawal for 20 patients. Level of sedation unclear ‐ participants probably conscious. No treatment comparison. Variable adjunct medications. |
| Lorenzi 1999 | Evaluation of protocol for antagonist‐induced withdrawal in terms of haemodynamic status and withdrawal signs. No treatment comparison. |
| Ma 2003 | Retrospective case review relating dose of clonidine to severity of symptoms during antagonist‐induced withdrawal under anaesthesia. |
| McDonald 2000a | Assesses absorption of naltrexone during anaesthesia and consequent effectiveness of antagonist‐induced withdrawal. No treatment comparison. |
| McDonald 2001 | Investigates EEG as means of evaluating opioid detoxification during anaesthesia. No treatment comparison. |
| Pereira 1999 | Reports use of antagonist‐induced withdrawal during pregnancy. No treatment comparison. |
| Pfab 1999 | Compares outcomes of antagonist‐induced withdrawal for cohorts withdrawing from methadone, heroin and codeine. No treatment comparison. |
| Pinto 2001 | Follow‐up study of 45 opioid‐dependent patients treated with antagonist‐induced withdrawal under anaesthesia. No treatment comparison. |
| Presslich 1989a | Reports treatment of 15 opioid‐dependent patients with antagonist‐induced withdrawal. No treatment comparison. Insufficient detail of treatment outcomes. |
| Presslich 1989b | Reports treatment of 6 opioid‐dependent patients with antagonist‐induced withdrawal. No comparison treatment. Insufficient detail on outcomes. |
| Rabinowitz 2002 | Follow‐up study of relapse following antagonist‐induced withdrawal and naltrexone maintenance or standard inpatient detoxification and counselling. Not controlled study. Post‐detoxification focus. |
| Saunders 2002 | Randomised controlled trial of antagonist‐induced withdrawal, with or without sedation, or advice to commence methadone maintenance treatment, followed by naltrexone maintenance treatment. Conference abstract only. Insufficient outcome data. |
| Scherbaum 1998 | Single group study assessing effectiveness and adverse effects of antagonist‐induced opioid withdrawal. No comparison treatment. |
| Teplin 2005 | Reports withdrawal severity for opioid dependent patients in 24 hours after antagonist‐induced withdrawal under anaesthesia. No treatment comparison. |
| Tornay 2003 | Follow‐up study of 16 opioid‐dependent participants treated with buprenorphine for 7 days prior to antagonist‐induced withdrawal. No comparison treatment. |
| Tretter 1998 | Reports outcomes of antagonist‐induced withdrawal for 14 of 88 opioid‐dependent patients. Case series, not controlled study. No treatment comparison. |
| Zemtsovski 2005 | Reports adverse effects during antagonist‐induced withdrawal under anaesthesia. No treatment comparison. |
| Zimmermann 2003 | Reports method of antagonist‐induced withdrawal for opioid dependence. No treatment comparison. |
Contributions of authors
Linda Gowing assessed each potentially relevant study according to identified inclusion and exclusion criteria. Inclusion and exclusion decisions were confirmed by discussion with co‐authors. Linda Gowing extracted key information and compiled a first draft of the review. Robert Ali and Jason White confirmed and commented on review content.
Sources of support
Internal sources
Drug and Alcohol Services South Australia, Australia.
External sources
Commonwealth Department of Health and Aging, Australia.
Declarations of interest
Two of the authors of this review (LG, RA) were involved in one of the studies (McGregor 2002) included in this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Collins 2005 {published data only}
- Collins ED, Kleber HD, Whittington RA, Heitler NE. Anaesthesia‐assisted vs buprenorphine‐ or clonidine‐assisted heroin detoxification and naltrexone induction. A randomised trial. JAMA 2005;294(8):903‐13. [DOI] [PubMed] [Google Scholar]
- Collins ED, Whittington RA, Heitler NE, Kleber HD. A randomised comparison of anaesthesia‐assisted heroin detoxification with buprenorphine and clonidine assisted detoxifications. Drug and Alcohol Dependence 2002;66(Supplement):35. [Google Scholar]
de Jong 2005 {published data only}
- Jong CAJ, Laheij RJF, Krabbe PFM. General anaesthesia does not improve outcome in opioid antagonist detoxification treatment: a randomised controlled trial. Addiction 2005;100:206‐15. [DOI] [PubMed] [Google Scholar]
Favrat 2006 {published data only}
- Favrat B, Zimmermann G, Zullino D, Krenz S, Dorogy F, Muller J, et al. Opioid antagonist detoxification under anaesthesia versus traditional clonidine detoxification combined with an additional week of psychosocial support: A randomised clinical trial. Drug & Alcohol Dependence 2006;81(2):109‐16. [DOI] [PubMed] [Google Scholar]
Huang 2002 {published data only}
- Huang W‐Y, Xiao X‐S, Liu Y, Liao X‐Q, Zhou D‐W, Dai H. The comparison of effects of rapid opiate detoxification with ketamine complex and with tramadol and naltrexone under general anesthesia with propofol. Chinese Journal of Clinical Rehabilitation 2002;6(23):3625‐6. [Google Scholar]
Jovaisa 2006 {published data only}
- Jovaisa T, Laurinenas G, Vosylius S, Sipylaite J, Badaras R, Ivaskevicius J. Effects of ketamine on precipitated opiate withdrawal. Medicina (Kaunas) 2006;42(8):625‐34. [PubMed] [Google Scholar]
Kienbaum 2000 {published data only}
- Kienbaum P, Scherbaum N, Thurauf N, Michel MC, Gastpar M, Peters J. Acute detoxification of opioid‐addicted patients with naloxone during propofol or methohexital anaesthesia: a comparison of withdrawal symptoms, neuroendocrine, metabolic, and cardiovascular patterns. Critical Care Medicine 2000;28(4):969‐76. [DOI] [PubMed] [Google Scholar]
Krabbe 2003 {published data only}
- Krabbe PFM, Koning JPF, Heinen N, Laheij RJF, Cauter RMV, Jong CAJ. Rapid detoxification from opioid dependence under general anaesthesia versus standard methadone tapering: abstinence rates and withdrawal distress experience. Addiction Biology 2003;8(3):351‐8. [DOI] [PubMed] [Google Scholar]
- Laheij RJF, Krabbe PFM, Jong CAJ. Rapid heroin detoxification under general anaesthesia. JAMA 2000;283(9):1143. [DOI] [PubMed] [Google Scholar]
McGregor 2002 {published data only}
- Ali R, Thomas P, White J, McGregor C, Danz C, Gowing L, et al. Antagonist‐precipitated heroin withdrawal under anaesthetic prior to maintenance naltrexone treatment: determinants of withdrawal severity. Drug and Alcohol Review 2003;22(4):425‐31. [DOI] [PubMed] [Google Scholar]
- McGregor C, Ali R, White JM, Thomas P, Gowing L. A comparison of antagonist‐precipitated withdrawal under anaesthesia to standard inpatient withdrawal as a precursor to maintenance naltrexone treatment in heroin users: Outcomes at 6 and 12 months. Drug and Alcohol Dependence 2002;68(1):5‐14. [DOI] [PubMed] [Google Scholar]
Seoane 1997 {published data only}
- Seoane A, Carrasco G, Cabré L, Puiggrós A, Hernández E, Álvarez M, et al. Efficacy and safety of two new methods of rapid intravenous detoxification in heroin addicts previously treated without success. British Journal of Psychiatry 1997;171:340‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albanese 2000 {published data only}
- Albanese AP, Gevirtz C, Oppenheim B, Field JM, Abels I, Eustace JC. Outcome and six month follow up of patients after ultra rapid opiate detoxification (URODSM). Journal of Addictive Diseases 2000;19(2):11‐28. [DOI] [PubMed] [Google Scholar]
Allhoff 1999 {published data only}
- Allhoff T, Renzing‐Kohler K, Kienbaum P, Sack S, Scherbaum N. Electrocardiographic abnormalities during recovery from ultra‐short opiate detoxification. Addiction Biology 1999;4(3):337‐44. [DOI] [PubMed] [Google Scholar]
Arnold‐Reed 2005 {published data only}
- Arnold‐Reed DE, Hulse GK. A comparison of rapid (opioid) detoxification with clonidine‐assisted detoxification for heroin‐dependent persons. Journal of Opioid Management 2005;1(1):17‐23. [DOI] [PubMed] [Google Scholar]
Bartter 1996 {published data only}
- Bartter T, Gooberman LL. Rapid opiate detoxification. American Journal of Drug and Alcohol Abuse 1996;22(4):489‐95. [DOI] [PubMed] [Google Scholar]
Bell 1999 {published data only}
- Bell JR, Young MR, Masterman SC, Morris A, Mattick RP, Bammer G. A pilot study of naltrexone‐accelerated detoxification in opioid dependence. Medical Journal of Australia 1999;171:26‐30. [DOI] [PubMed] [Google Scholar]
Boehle 2000 {published data only}
- Boehle C, Kindgen Milles D, Burtscheidt W, Tarnow J, Gaebel W. Antagonist‐induced opiate detoxification. Report on clinical experience as well as on short and intermediate range course [Antagonisteninduzierte Opiatentgiftung. Erfahrungsbericht zur klinischen Anwendung sowie zum kurz‐ und mittelfristigen Verlauf]. Nervenarzt 2000;71(9):745‐50. [DOI] [PubMed] [Google Scholar]
Brewer 1998a {published data only}
- Brewer C, Laban M, Schmulian C, Gooberman L, Kasvikis Y, Maksoud NA. Rapid opiate detoxification and naltrexone induction under general anaesthesia and assisted ventilation: experiences with 510 patients in four different centres. Acta psychiatrica belgica 1998;98:181‐9. [Google Scholar]
Cucchia 1998 {published data only}
- Cucchia AT, Monnat M, Spagnoli J, Ferrero F, Bertschy G. Ultra‐rapid opiate detoxification using deep sedation with oral midazolam: short and long‐term results. Drug and Alcohol Dependence 1998;52:243‐50. [DOI] [PubMed] [Google Scholar]
Dettling 1996 {published data only}
- Dettling M, Tretter F. Opiate withdrawal in anaesthesia (forced narcotic withdrawal, "turbo withdrawal") in narcotic dependence. Changes in withdrawal treatment‐‐wishes and reality [Der Opiatentzug in Narkose (forcierter Narkoseentzug, "Turboentzug") bei Opiatabhangigkeit. Entzugsbehandlungen im Wandel der Zeit‐‐Wunsch und Wirklichkeit]. Nervenarzt 1996;67(9):805‐10. [DOI] [PubMed] [Google Scholar]
E. Berenguel 2001 {published data only}
- Espinosa Berenguel JL, Palazon Sanchez C, Felices Abad F, Garcia Basterrechea JM, Gil Rueda B, Blanco Molina TB, et al. Study of an ultrashort opiate detoxification protocol in an intensive care unit: Preliminary results. Medicina Intensiva 2001;25(6):217‐22. [Google Scholar]
Elman 2001 {published data only}
- Elman I, D'Ambra MN, Krause S, Breiter H, Kane M, Morris R, et al. Ultrarapid opioid detoxification: effects on cardiopulmonary physiology, stress hormones and clinical outcomes. Drug and Alcohol Dependence 2001;61(2):163‐72. [DOI] [PubMed] [Google Scholar]
Foster 2003 {published data only}
- Foster J, Brewer C, Steele T. Naltrexone implants can completely prevent early (1‐month) relapse after opiate detoxification: A pilot study of two cohorts totaling 101 patients with a note on naltrexone blood levels. Addiction Biology 2003;8(2):211‐7. [DOI] [PubMed] [Google Scholar]
Gold 1999 {published data only}
- Gold CG, Cullen DJ, Gonzales S, Houtmeyers D, Dwyer MJ. Rapid opioid detoxification during general anaesthesia: a review of 20 patients. Anesthesiology 1999;91(6):1639‐47. [DOI] [PubMed] [Google Scholar]
Hensel 2000a {published data only}
- Hensel M, Wolter S, Kox WJ. EEG controlled rapid opioid withdrawal under general anaesthesia. British Journal of Anaesthesia 2000;84(2):236‐8. [DOI] [PubMed] [Google Scholar]
Hensel 2000b {published data only}
- Hensel M, Kox WJ. Safety, efficacy, and long‐term results of a modified version of rapid opiate detoxification under general anaesthesia: a prospective study in methadone, heroin, codeine and morphine addicts. Acta Anaesthesiologica Scandinavica 2000;44(3):326‐33. [DOI] [PubMed] [Google Scholar]
Ivaskevicius 2005 {published data only}
- Ivaskevicius J, Jovaisa T, Laurinenas G, Vosylius S, Sipylaite J, Badaras R. [Safety and effectiveness of opiate antagonist detoxification under general anesthesia]. Medicina (Kaunas) 2005;41(12):1011‐8. [PubMed] [Google Scholar]
Kasvikis 1997 {published data only}
- Kasvikis Y, Mitskidou P, Brewer C, Siafaka A, Anagnostakos A. Opiate withdrawal under general anaesthesia and naltrexone induction in 24 hours: A clinical report on 25 cases from Athens, Greece, and a short literature review. Psychiatriki 1997;7(3):191‐8. [Google Scholar]
Lawental 2000 {published data only}
- Lawental E. Ultra rapid opiate detoxification as compared to 30‐day inpatient detoxification program ‐ a retrospective follow‐up study. Journal of Substance Abuse 2000;11(2):173‐81. [DOI] [PubMed] [Google Scholar]
Legarda 1994 {published data only}
- Legarda JJ, Gossop M. A 24‐h inpatient detoxification treatment for heroin addicts: a preliminary investigation. Drug and Alcohol Dependence 1994;35(2):91‐3. [DOI] [PubMed] [Google Scholar]
Loimer 1989 {published data only}
- Loimer N, Schmid R, Presslich O, Lenz K. Continuous naloxone administration suppresses opiate withdrawal symptoms in human opiate addicts during detoxification treatment. Journal of Psychiatric Research 1989;23(1):81‐6. [DOI] [PubMed] [Google Scholar]
Loimer 1990 {published data only}
- Loimer N, Schmid R, Lenz K, Presslich O, Grünberger J. Acute blocking of naloxone‐precipitated opiate withdrawal symptoms by methohexitone. British Journal of Psychiatry 1990;157:748‐52. [DOI] [PubMed] [Google Scholar]
Loimer 1991a {published data only}
- Loimer N, Lenz K, Schmid R, Presslich O. Technique for greatly shortening the transition from methadone to naltrexone maintenance of patients addicted to opiates. American Journal of Psychiatry 1991;148(7):933‐5. [DOI] [PubMed] [Google Scholar]
Loimer 1991b {published data only}
- Loimer N, Linzmayer L, Grunberger J. Comparison between observer assessment and self rating of withdrawal distress during opiate detoxification. Drug and Alcohol Dependence 1991;28:265‐8. [DOI] [PubMed] [Google Scholar]
Loimer 1991c {published data only}
- Loimer N, Linzmayer L, Schmid R, Grunberger J. Similar efficacy of abrupt and gradual opiate detoxification. American Journal of Drug and Alcohol Abuse 1991;17(3):307‐12. [DOI] [PubMed] [Google Scholar]
Loimer 1993 {published data only}
- Loimer N, Hofmann P, Chaudhry H. Ultrashort noninvasive opiate detoxification. American Journal of Psychiatry 1993;150(5):839. [DOI] [PubMed] [Google Scholar]
London 1999 {published data only}
- London M, Paul E, Gkolia I. Ultra‐rapid opiate detoxification in hospital. Psychiatric Bulletin 1999;23(9):544‐6. [Google Scholar]
Lorenzi 1999 {published data only}
- Lorenzi P, Marsili M, Boncinelli S, Fabbri LP, Fontanari P, Zorn AM, et al. Searching for a general anaesthesia protocol for rapid detoxification from opioids. European Journal of Anaesthesiology 1999;16(10):719‐27. [DOI] [PubMed] [Google Scholar]
Ma 2003 {published data only}
- Ma H, Tang J, White PF, Wender RH, Leverone T, Quon R, et al. The effect of clonidine on gastrointestinal side effects associated with ultra‐rapid opioid detoxification. Anaesthesia and Analgesia 2003;96(5):1409‐12. [DOI] [PubMed] [Google Scholar]
McDonald 2000a {published data only}
- McDonald T. Plasma naltrexone during opioid detoxification. Journal of Addictive Diseases 2000;19(4):59‐64. [DOI] [PubMed] [Google Scholar]
McDonald 2001 {published data only}
- McDonald T, Hoffman WE, Berkowitz R. Combining median electroencephalography frequency and sympathetic activity in an index to evaluate opioid detoxification in patients. Journal of Neurosurgical Anesthesiology 2001;13(2):74‐8. [DOI] [PubMed] [Google Scholar]
Pereira 1999 {published data only}
- Pereira C, Brewer C. Rapid opiate detoxification under sedation during pregnancy : a report on 14 cases with one ten‐year follow‐up. Addiction Biology 1999;4(2):239‐40. [Google Scholar]
Pfab 1999 {published data only}
- Pfab R, Hirtl C, Zilker T. Opiate detoxification under anaesthesia: no apparent benefit but suppression of thyroid hormones and risk of pulmonary and renal failure. Journal of Toxicology . Clinical Toxicology 1999;37(1):43‐50. [DOI] [PubMed] [Google Scholar]
Pinto 2001 {published data only}
- Pinto E, Reggers J, Delhez M, Fuchs S, Venneman I, Lamy M, Ansseau M. Ultra‐fast opiate detoxification under general anaesthesia: preliminary results of the Liege protocol. Revue Medicale de Liege 2001;56(8):572‐6. [PubMed] [Google Scholar]
Presslich 1989a {published data only}
- Presslich O, Loimer N, Aschauer G, Fodor G, Pfersmann D, Muller K, et al. Staggered opiate withdrawal via tiapride and naloxone, as a new therapeutic approach [Fraktionierter opiatentzug mit tiaprid und naloxon, ein neuer therapieansatz]. Psychiatrische Praxis 1989;16(5):179‐81. [PubMed] [Google Scholar]
Presslich 1989b {published data only}
- Presslich O, Loimer N, Lenz K, Schmid R. Opiate detoxification under general anaesthesia by large doses of naloxone. Journal of Toxicology. Clinical Toxicology 1989;27(4‐5):263‐70. [DOI] [PubMed] [Google Scholar]
Rabinowitz 2002 {published data only}
- Rabinowitz J, Cohen H, Atias S. Outcomes of naltrexone maintenance following ultra rapid opiate detoxification versus intensive inpatient detoxification. American Journal on Addictions 2002;11(1):52‐6. [DOI] [PubMed] [Google Scholar]
Saunders 2002 {published data only}
- Saunders JB, Jones R, Dean A, Connor J, Young R, Keen L, et al. Comparison of rapid opiate detoxification and naltrexone with methadone maintenance in the treatment of opiate dependence: A randomised controlled trial. Drug and Alcohol Dependence 2002;66(Suppl 1):156. [Google Scholar]
Scherbaum 1998 {published data only}
- Scherbaum N, Klein S, Kaube H, Kienbaum P, Peters J, Gastpar M. Alternative strategies of opiate detoxification: evaluation of the so‐called ultra‐rapid detoxification. Pharmacopsychiatry 1998;31(6):205‐9. [DOI] [PubMed] [Google Scholar]
Teplin 2005 {published data only}
- Teplin D, Raz B, Daiter J, Varenbut M, Zachos CT, Whang P, et al. Measurement of symptom withdrawal severity in a 24‐hour period after the anesthesia‐assisted rapid opiate detoxification procedure. American Journal of Drug & Alcohol Abuse 2005;31(2):327‐35. [PubMed] [Google Scholar]
Tornay 2003 {published data only}
- Tornay CB, Favrat B, Monnat M, Daeppen JB, Schnyder C, Bertschy G, et al. Ultra‐rapid opiate detoxification using deep sedation and prior oral buprenorphine preparation: long‐term results. Drug and Alcohol Dependence 2003;69(3):283‐8. [DOI] [PubMed] [Google Scholar]
Tretter 1998 {published data only}
- Tretter F, Burkhardt D, Bussello‐Spieth B, Reiss J, Walcher S, Büchele W. Clinical experience with antagonist‐induced opiate withdrawal under anaesthesia. Addiction 1998;93(2):269‐75. [DOI] [PubMed] [Google Scholar]
Zemtsovski 2005 {published data only}
- Zemtsovski MI, Kipriianov VS, Tros'ko OU, Brazhnikov AV, Strashnov VI. [Complications of accelerated opioid detoxification]. Anesteziologiia i Reanimatologiia 2005;3:11‐5. [PubMed] [Google Scholar]
Zimmermann 2003 {published data only}
- Zimmermann G, Favrat B, Muller J, Zullino D, Krenz S, Deyras E, et al. [Ultrafast opiate detoxification under general anesthesia: the St. Loup Hospital experience]. Revue Medicale de la Suisse Romande 2003;123(2):97‐9. [PubMed] [Google Scholar]
Additional references
Amato 2005
- Amato L, Davoli M, Ferri M, Ali R. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858] [DOI] [Google Scholar]
Amato 2008
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD005031.pub2] [DOI] [PubMed] [Google Scholar]
Bearn 1999
- Bearn J, Gossop M, Strang J. Rapid opiate detoxification treatments. Drug and Alcohol Review 1999;18(1):75‐81. [Google Scholar]
Blake 1999
- Blake S. Addict robbed of her new life. The Sunday Telegraph (Australia) 1999, issue 6 May.
Brewer 1997
- Brewer C. Ultra‐rapid, antagonist‐precipitated opiate detoxification under general anaesthetic or sedation. Addiction Biology 1997;2(3):291‐302. [DOI] [PubMed] [Google Scholar]
Brewer 1998b
- Brewer C, Gastfriend DR. Rapid opioid detoxification. JAMA 1998;279(23):1872. [PubMed] [Google Scholar]
Broers 2000
- Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in Geneva: follow‐up at 1 and 6 months. Drug and Alcohol Dependence 2000;58(1):85‐92. [DOI] [PubMed] [Google Scholar]
Bulthuis 2000
- Bulthuis D, Diaz JE. Ultrarapid opiate detoxification. Annals of Emergency Medicine 2000;35(1):100‐1. [DOI] [PubMed] [Google Scholar]
Chanmugam 2000
- Chanmugam AS, Hengeller M, Ezenkwele U. Development of rhabdomyolysis after rapid opioid detoxification with subcutaneous naltrexone maintenance therapy. Academic Emergency Medicine 2000;7(3):303‐5. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, [updated February 2008]. [Google Scholar]
Day 2005
- Day E, Ison J, Strang J. Inpatient versus other settings for detoxification for opioid dependence. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004580.pub2] [DOI] [PubMed] [Google Scholar]
Dyer 1998
- Dyer C. Addict died after rapid opiate detoxification. BMJ 1998;316(7126):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 1994
- Farrell M. Opiate withdrawal. Addiction 1994;89(11):1471‐5. [DOI] [PubMed] [Google Scholar]
Gevirtz 1999
- Gevirtz C, Subhedar DV, Choi CS. Catecholamine surge in opioid‐addicted patients undergoing detoxification under general anaesthesia. Anesthesiology 1999;90(1):334‐5. [DOI] [PubMed] [Google Scholar]
Gooberman 1998
- Gooberman LL, Bradway DW. Depot naltrexone vs oral naltrexone post‐detoxification. Journal of Addictive Diseases 1998;17(2):150. [Google Scholar]
Gossop 1988
- Gossop M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug and Alcohol Dependence 1988;21(3):253‐9. [DOI] [PubMed] [Google Scholar]
Gossop 1989
- Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow‐up study. British Journal of Psychiatry 1989;154:348‐53. [DOI] [PubMed] [Google Scholar]
Gossop 1990
- Gossop M. The development of a short opiate withdrawal scale (SOWS). Addictive Behaviours 1990;15(5):487‐90. [DOI] [PubMed] [Google Scholar]
Gowing 2009
- Gowing L, Farrell M, Ali R, White J. Alpha2 adrenergic agonists for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858] [DOI] [Google Scholar]
Gowing 2009a
- Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858] [DOI] [Google Scholar]
Gowing 2009b
- Gowing L, Ali R, White J. Opioid antagonists with minimal sedation for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858] [DOI] [Google Scholar]
Hall 1998
- Hall W, Darke S. Trends in opiate overdose deaths in Australia 1979‐1995. Drug and Alcohol Dependence 1998;52:71‐7. [DOI] [PubMed] [Google Scholar]
Handelsman 1987
- Handelsman L, Cochrane KJ, Aronson MJ, Ness RA, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. American Journal of Drug and Alcohol Abuse 1987;13(3):293‐308. [DOI] [PubMed] [Google Scholar]
Hoffman 1998
- Hoffman WE, Berkowitz R, McDonald T, Hass F. Ultra‐rapid opioid detoxification increases spontaneous ventilation. Journal of Clinical Anaesthesia 1998;10(5):372‐6. [DOI] [PubMed] [Google Scholar]
Jaffe 1997
- Jaffe JH, Knapp CM, Ciraulo DA. Opiates: Clinical Aspects. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG editor(s). Substance Abuse: A comprehensive textbook. 3rd Edition. Baltimore: Williams & Wilkins, 1997. [Google Scholar]
Kienbaum 1998
- Kienbaum P, Thurauf N, Michel MC, Scherbaum N, Gastpar M, Peters J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu‐opioid receptor blockade in opioid‐addicted patients during barbiturate‐induced anaesthesia for acute detoxification. Anesthesiology 1998;88(5):1154‐61. [DOI] [PubMed] [Google Scholar]
Kleber 1982
- Kleber HD, Riordan CE. The treatment of narcotic withdrawal: a historical review. Journal of Clinical Psychiatry 1982;43(6):30‐4. [PubMed] [Google Scholar]
Lipton 1983
- Lipton D, Maranda M. Detoxification from heroin dependency: An overview of method and effectiveness. Advances in Alcohol and Substance Abuse 1983;2(1):31‐55. [Google Scholar]
Mark 2001
- Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug and Alcohol Dependence 2001;61(2):195‐206. [DOI] [PubMed] [Google Scholar]
Mattick 1996
- Mattick RP, Hall W. Are detoxification programmes effective?. Lancet 1996;347:97‐100. [DOI] [PubMed] [Google Scholar]
Mayor 1997
- Mayor S. Specialists criticise treatment for heroin addiction. BMJ 1997;314:1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2000
- McDonald T. Plasma naltrexone during opioid detoxification. Journal of Addictive Diseases 2000;19(4):59‐64. [DOI] [PubMed] [Google Scholar]
NIH 1997
- NIH Consensus Development Statement. Effective Medical Treatment of Heroin Addiction. National Institutes of Health. National Institutes of Health, 1997, November 17‐19.
Peachey 1988
- Peachey JE, Lei H. Assessment of opioid dependence with naloxone. British Journal of Addiction 1988;83:193‐201. [DOI] [PubMed] [Google Scholar]
Phillips 1986
- Phillips GT, Gossop M, Bradley B. The influence of psychological factors on the opiate withdrawal syndrome. British Journal of Psychiatry 1986;149:235‐8. [DOI] [PubMed] [Google Scholar]
Preston 1985
- Preston KL, Bigelow GE. Pharmacological advances in addiction treatment. International Journal of the Addictions 1985;20(6&7):845‐67. [DOI] [PubMed] [Google Scholar]
Satel 1993
- Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM‐IV?. American Journal of Psychiatry 1993;150(5):695‐704. [DOI] [PubMed] [Google Scholar]
Simon 1997
- Simon DL. Rapid opioid detoxification using opioid antagonists: history, theory and the state of the art. Journal of Addictive Diseases 1997;16(1):103‐22. [DOI] [PubMed] [Google Scholar]
Stephenson 1997
- Stephenson J. Opiate detoxification under anaesthetic. JAMA 1997;278(16):1319. [DOI] [PubMed] [Google Scholar]
Vaillant 1988
- Vaillant GE. What can long‐term follow‐up teach us about relapse and prevention of relapse in addiction?. British Journal of Addiction 1988;83(10):1147‐57. [DOI] [PubMed] [Google Scholar]
Wang 1974
- Wang RI, Wiesen RL, Lamid S, Roh BL. Rating the presence and severity of opiate dependence. Clinical Pharmacology and Therapeutics 1974;16:653‐8. [DOI] [PubMed] [Google Scholar]
White 1999
- White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction 1999;94(7):961‐72. [PubMed] [Google Scholar]
Whittington 2000
- Whittington A, Collins ED, Kleber HD. Rapid opioid detoxification during general anaesthesia: is death not a significant outcome?. Anesthesiology 2000;93(5):1363‐4. [DOI] [PubMed] [Google Scholar]
World Drug Report 2007
- United Nations Office on Drugs and Crime. World Drug Report 2007. Available from www.unodc.org.


