Abstract
Background
Long‐term drug treatment of schizophrenia with typical antipsychotic drugs has limitations: 25 to 33% of sufferers have illnesses that are treatment resistant. Clozapine is an antipsychotic drug, which is claimed to have superior efficacy and to cause fewer motor adverse effects than typical drugs for people with treatment‐resistant illnesses. Clozapine carries a significant risk of serious blood disorders, which necessitates mandatory weekly blood monitoring at least during the first months of treatment.
Objectives
To evaluate the effects of clozapine compared with typical antipsychotic drugs in people with schizophrenia.
Search methods
For the current update of this review (November 2008) we searched the Cochrane Schizophrenia Group Trials Register.
Selection criteria
All relevant randomised controlled trials (RCTs).
Data collection and analysis
We extracted data independently. For dichotomous data we calculated relative risks (RR) and their 95% confidence intervals (CI) on an intention‐to‐treat basis, based on a fixed‐effect model. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate. For continuous data, we calculated weighted mean differences (WMD) again based on a fixed‐effect model.
Main results
We have included 52 trials (4746 participants) in this review. Forty‐four of the included studies are less than 13 weeks in duration, and, overall, trials were at a significant risk of bias. We found no significant difference in the effects of clozapine and typical neuroleptic drugs for broad outcomes such as mortality, ability to work or suitability for discharge at the end of the study. Clinical improvements were seen more frequently in those taking clozapine (n=1119, 14 RCTs, RR 0.72 CI 0.7 to 0.8, NNT 6 CI 5 to 8). Also, participants given clozapine had fewer relapses than those on typical antipsychotic drugs (n=1303, RR 0.62 CI 0.5 to 0.8, NNT 21 CI 15 to 49). BPRS scores showed a greater reduction of symptoms in clozapine‐treated participants, (n=1205, 17 RCTs, WMD ‐3.79 CI ‐4.9 to ‐2.7), although the data were heterogeneous ( I2=69%). Short‐term data from the SANS negative symptom scores favoured clozapine (n=196, 6 RCTs, WMD ‐7.21 CI ‐8.9 to ‐5.6). We found clozapine to be more acceptable in long‐term treatment than conventional antipsychotic drugs (n=982, 6 RCTs, RR 0.60 CI 0.5 to 0.7, NNT 15 CI 12 to 20). Blood problems occurred more frequently in participants receiving clozapine (3.2%) compared with those given typical antipsychotic drugs (0%) (n=1031, 13 RCTs, RR 7.09 CI 2.0 to 25.6). Clozapine participants experienced more drowsiness, hypersalivation or temperature increase, than those given conventional neuroleptics. However, those receiving clozapine experienced fewer motor adverse effects (n=1495, 19 RCTs, RR 0.57 CI 0.5 to 0.7, NNT 5 CI 4 to 6).
The clinical effects of clozapine were more pronounced in participants resistant to typical neuroleptics in terms of clinical improvement (n=370, 4 RCTs, RR 0.71 CI 0.6 to 0.8, NNT 4 CI 3 to 6) and symptom reduction. Thirty‐four per cent of treatment‐resistant participants had a clinical improvement with clozapine treatment.
Authors' conclusions
Clozapine may be more effective in reducing symptoms of schizophrenia, producing clinically meaningful improvements and postponing relapse, than typical antipsychotic drugs ‐ but data are weak and prone to bias. Participants were more satisfied with clozapine treatment than with typical neuroleptic treatment. The clinical effect of clozapine, however, is, at least in the short‐term, not reflected in measures of global functioning such as ability to leave the hospital and maintain an occupation. The short‐term benefits of clozapine have to be weighed against the risk of adverse effects. Within the context of trials, the potentially dangerous white blood cell decline seems to be more frequent in children and adolescents and in the elderly than in young adults or people of middle age.
The existing trials have largely neglected to assess the views of participants and their families on clozapine. More community‐based long‐term randomised trials are needed to evaluate the efficacy of clozapine on global and social functioning as trials in special groups such as people with learning disabilities.
Plain language summary
Clozapine versus typical neuroleptic medication for schizophrenia
Schizophrenia is a serious, chronic and relapsing mental illness with a worldwide lifetime prevalence of about one per cent. Schizophrenia is characterised by 'positive' symptoms such as hallucinations and delusions and 'negative' symptoms such as emotional numbness and withdrawal. One quarter of those who have experienced an episode of schizophrenia recover and the illness does not recur. Another 25% experience an unremitting illness. Half do have a recurrent illness but with long episodes of considerable recovery from the positive symptoms. The overall cost of the illness to the individual, their carers and the community is considerable.
Antipsychotic medications are classified into typical and atypical drugs. First generation or 'typical' antipsychotic drugs such as chlorpromazine and haloperidol have been the mainstay of treatment, and are effective in reducing the positive symptoms of schizophrenia, but negative symptoms are fairly resistant to treatment. In addition, drug treatments are associated with adverse effects which can often compromise compliance with medication and therefore increase the incidences of relapse.
People who do not respond adequately to antipsychotic medication are sometimes given the 'atypical' antipsychotic drug clozapine, which has been found to be effective for some people with treatment‐resistant schizophrenia. Clozapine is also associated with having fewer movement disorders than chlorpromazine, but may induce life‐threatening decreases in white blood cells (agranulocytosis). We reviewed the affects of clozapine in people with schizophrenia compared with typical antipsychotic drugs drugs.
This review supports the notion that clozapine is more effective than typical antipsychotic drugs for people with schizophrenia in general, and for those who do not improve on typical antipsychotic drugs in particular. Clozapine is associated with less movement adverse effects than typical antipsychotic drugs, but it may cause serious blood‐related adverse effects. White blood cell count monitoring is mandatory for all people taking clozapine. There is a worry, however, that studies are ‐ at the very least ‐ moderately prone to bias favouring clozapine. Better conduct and reporting of trials could greatly have increased our confidence in the results.
Summary of findings
Background
Arguably, the psychopharmacology of schizophrenia has witnessed two major discoveries, the discovery of chlorpromazine and of clozapine. The discovery of chlorpromazine was followed by the introduction of a large family of drugs that are now known as 'typical neuroleptics' or 'first generation antipsychotic drugs'. The putative finding that clozapine is effective in people who have treatment‐resistant schizophrenia (Kane 1988 (CPZ)) stimulated a frantic search for a newer group of drugs known collectively as the 'atypical neuroleptics' or 'second generation antipsychotic drugs'.
Description of the condition
Schizophrenia is a chronic, relapsing mental illness and has a worldwide lifetime prevalence of about 1% irrespective of culture, social class and race. Schizophrenia is characterised by positive symptoms such as hallucinations and delusions and negative symptoms such as emotional numbness and withdrawal. One quarter of those who have experienced an episode of schizophrenia recover and the illness does not recur. Another 25% experience an unremitting illness. Half do have a recurrent illness but with long episodes of considerable recovery from the positive symptoms. Current medication is effective in reducing positive symptoms, but negative symptoms are fairly resistant to treatment. In addition, drug treatments are associated with adverse effects and the overall cost of the illness to the individual, their carers and the community is considerable.
Description of the intervention
Clozapine has been in use for the treatment of schizophrenia since the early 1960s (Hippius 1989). Although its availability has not been interrupted in parts of the world including China and some countries in South America, it was withdrawn from Western markets in 1975 after reports of agranulocytosis (a substantial decline in the white blood cells which made the individuals dangerously susceptible to infection) leading to death in some clozapine‐treated patients (Idänpään‐Heikkilä 1975). More recent studies suggested that clozapine was more effective than other antipsychotic drugs against treatment‐resistant schizophrenia (Kane 1988 (CPZ), Meltzer 1989). Treatment‐resistant schizophrenia is a term generally used for the failure of signs or symptoms to respond satisfactorily to at least two different antipsychotic drugs (Meltzer 1997, Crilly 2007). Health authorities in many countries have approved the use of clozapine only for people with schizophrenia who were (i) resistant to typical neuroleptics and (ii) compliant with blood monitoring. People taking clozapine are required to have their blood sampled at least once a week for the first 18 weeks of treatment and at least once a month thereafter. Another potentially fatal adverse effect of clozapine that has been recently identified is that of myocarditis which usually develops within the first month of commencement and presents with signs of cardiac failure and cardiac arrhythmias (Haas 2007). Echocardiograms are recommended every six months to exclude cardiac damage. People receiving clozapine should also have their fasting blood glucose monitored; in addition to type II diabetes, significant weight gain is frequently experienced by people treated with clozapine (Wirshing 1999). Other adverse effects of clozapine include lowered seizure threshold, hepatic dysfunction and adverse effects associated with its interaction with different neurotransmitters' receptors.
How the intervention might work
Clozapine is a strong antagonist at different subtypes of adrenergic, cholinergic, histaminergic and serotonergic receptors. It may also have a different pattern of adhesion to receptors than other drugs. Clozapine’s common adverse effects are predominantly anticholinergic in nature, with dry mouth, sedation and constipation, drooling, and orthostasis.
Why it is important to do this review
This review represents an important and considerable update of the previous version of this work (Wahlbeck 1999 b).
Objectives
To review the effects of clozapine compared with typical antipsychotic drugs for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
We included relevant randomised controlled trials (RCTs). Where trials are described as 'double‐blind' but are only implied as being randomised, we included these trials in a sensitivity analysis. If there were no substantive differences within primary outcomes (see Types of outcome measures) when these 'implied randomisation' studies were added, then we included these in the final analysis. If there were substantive differences, we only used clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
We included people with schizophrenia and other types of schizophrenia‐like psychosis (e.g. schizophreniform and schizoaffective disorders), irrespective of the diagnostic criteria used. There is no clear evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994). The group of studies dealing with people with illness that had been labelled as 'resistant' were also analysed separately.
Types of interventions
1. Clozapine (trade names Clozaril, Froidir, Leponex, Fazaclo, Klozapol): any dose. 2. Typical antipsychotic drugs: any dose. Another Cochrane systematic review has focused on comparing clozapine to atypical antipsychotic drugs (Lobos 2007).
Types of outcome measures
We grouped outcomes by time ‐ short‐term (up to 12 weeks), medium‐term (13 to 26 weeks) and long‐term (over 26 weeks).
Primary outcomes
Global state, no clinically important change (as defined by individual studies) ‐ medium‐term
Secondary outcomes
1. Death ‐ suicide and natural causes
2. Global state 2.1 Relapse (defined by deterioration in mental state requiring further treatment or hospitalisation) 2.2 Average endpoint global state score 2.3 Average change in global state scores
3. Service outcomes 3.1 Hospitalisation 3.2 Inability to be discharged from hospital
4. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) 4.1 No clinically important change in general mental state 4.2 Average endpoint general mental state score 4.3 Average change in general mental state scores 4.4 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 4.5 Average endpoint specific symptom score 4.6 Average change in specific symptom scores
5. General functioning 5.1 No clinically important change in general functioning including working ability 5.2 Average endpoint general functioning score 5.3 Average change in general functioning scores 5.4 No clinically important change in specific aspects of functioning, such as social or life skills 5.5 Average endpoint specific aspects of functioning, such as social or life skills 5.6 Average change in specific aspects of functioning, such as social or life skills
6. Behaviour 6.1 No clinically important change in general behaviour 6.2 Average endpoint general behaviour score 6.3 Average change in general behaviour scores 6.4 No clinically important change in specific aspects of behaviour 6.5 Average endpoint specific aspects of behaviour 6.6 Average change in specific aspects of behaviour
7. Adverse effects ‐ general and specific (Important adverse effects included movement disorders, weight gain, fits and blood reactions leading to therapy discontinuation) 7.1 Clinically important general adverse effects 7.2 Average endpoint general adverse effect score 7.3 Average change in general adverse effect scores 7.4 Clinically important specific adverse effects 7.5 Average endpoint specific adverse effects 7.6 Average change in specific adverse effects
8. Engagement with services
9. Satisfaction with treatment (including subjective well‐being and family burden) 9.1 Leaving the studies early 9.2 Recipient of care not satisfied with treatment 9.3 Recipient of care average satisfaction score 9.4 Recipient of care average change in satisfaction scores 9.5 Carer not satisfied with treatment 9.6 Carer average satisfaction score 9.7 Carer average change in satisfaction scores
10. Quality of life 10.1 No clinically important change in quality of life 10.2 Average endpoint quality of life score 10.3 Average change in quality of life scores 10.4 No clinically important change in specific aspects of quality of life 10.5 Average endpoint specific aspects of quality of life 10.6 Average change in specific aspects of quality of life
11. Economic outcomes 11.1 Direct costs 11.2 Indirect costs
12. Cognitive functioning 12.1 No clinically important change in cognitive functioning 12.2 Average endpoint cognitive functioning score 12.3 Average change in cognitive functioning scores 12.4 No clinically important change in specific aspects of cognitive functioning 12.5 Average endpoint specific aspects of cognitive functioning 12.6 Average change in specific aspects of cognitive functioning
Search methods for identification of studies
Electronic searches
1. Update of 2009 We searched the Cochrane Schizophrenia Group Trials Register (November 2008) using the phrase:
{[ clozapin* or clozaril* or leponex * in title, abstract, index terms of REFERENCE] or [clozapin* or clozaril* or leponex* in interventions of STUDY]}
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
2. Previous searches for earlier versions of this review Please see Appendix 1.
Searching other resources
Please see Appendix 1.
Data collection and analysis
Selection of studies
Two reviewers independently inspected all study citations identified by the searches and obtained full reports of the studies of agreed relevance. Where disputes arose, we acquired the full report for more detailed scrutiny. The two reviewers inspected these articles independently to assess their relevance to this review. Again, where disagreement occurred we attempted to resolve this through discussion; if doubt still remained we added these trials to the list of those awaiting assessment pending acquisition of further information.
Data extraction and management
1. Extraction We independently extracted data from included studies. Again, any disagreement was discussed, decisions documented and, if necessary, authors of studies contacted for clarification. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
2. Management We extracted the data onto standard, simple forms. Where possible, we entered data into RevMan in such a way that the area to the left of the 'line of no effect' indicates a 'favourable' outcome for clozapine. Where this was not possible, (e.g. scales that calculate higher scores = improvement) we labelled the graphs in RevMan analyses accordingly so that the direction of effects were clear.
3. Scale‐derived data A wide range of instruments are available to measure outcomes in mental health studies. These instruments vary in quality and many are not validated, or are even ad hoc. It is accepted generally that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure) (Rust 1989). Unpublished scales are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore we only included continuous data from rating scales if the measuring instrument had been described in a peer‐reviewed journal. In addition, we set the following minimum standards for instruments: the instrument should either be (a) a self‐report or (b) completed by an independent rater or relative (not the therapist) and (c) the instrument should be a global assessment of an area of functioning.
Assessment of risk of bias in included studies
Again working independently, we assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases. We would not have included studies where sequence generation was at high risk of bias or where allocation was clearly not concealed.
The categories are defined below: YES ‐ low risk of bias NO ‐ high risk of bias UNCLEAR ‐ uncertain risk of bias
If disputes arose as to which category a trial has to be allocated, again, resolution was made by discussion, after working with a third reviewer.
Earlier versions of this review used a different, less well‐developed, means of categorising risk of bias (see Appendix 2).
Measures of treatment effect
1. Binary data For binary outcomes we calculated a standard estimation of the fixed‐effect risk ratio (RR) and its 95% confidence interval (CI). For statistically significant results we calculated the number needed to treat/harm statistic (NNT/H), and its 95% confidence interval (CI) using Visual Rx taking account of the event rate in the control group. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. Where possible, we made efforts to convert outcome measures to binary data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into "clinically improved" or "not clinically improved". It was generally assumed that if there had been a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005a, Leucht 2005b). It was recognised that for many people, especially those with chronic or severe illness, a less rigorous definition of important improvement (e.g. 25% on the BPRS) would be equally valid. If individual patient data were available, the 50% cut‐off was used for the definition in the case of non‐chronically ill people and 25% for those with chronic illness. If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2. Continuous data Continuous data on outcomes in mental health trials are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data we applied the following standards to all endpoint data derived from continuous measures. We applied these criteria before inclusion: (a) standard deviations and means had to be obtainable; and, for finite scores, such as endpoint measures on rating scales, (b) the standard deviation (SD), when multiplied by 2 had to be less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution) (Altman 1996). If a scale starts from a positive value (such as PANSS, which can have values from 30 to 210) the calculation described above in (b) should be modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score.
We did not graphically show skewed endpoint data from studies with less the 200 participants, but added to 'Other data' tables and briefly commented on in the text. However, skewed endpoint data from larger studies (=/>200 participants) pose less of a problem and we entered the data for analysis.
For continuous mean change data (endpoint minus baseline) the situation is even more problematic. In the absence of individual patient data it is impossible to know if change data are skewed. The RevMan meta‐analyses of continuous data are based on the assumption that the data are, at least to a reasonable degree, normally distributed. Therefore we included such data, unless endpoint data were also reported from the same scale.
Unit of analysis issues
1. Cluster trials Studies increasingly employ cluster randomisation (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This can cause Type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a design effect. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect=1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
2. Cross‐over design A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, we will only use data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility At some degree of loss to follow‐up data must lose credibility (Xia 2007). We are forced to make a judgment where this is for the trials likely to be included in this review. Should more than 40% of data be unaccounted for by 8 weeks we did not reproduce these data or use them within analyses.
We attempted to include all people who had been randomised to clozapine or typical treatments. Where possible, we gave cases lost to follow up at the end of the study the worst outcome. For example, we treated those lost to follow up for the outcome of relapse in the analysis as having relapsed. Suicide was also treated as relapse. We agreed these rules before knowing the studies included. We tested the effects of inclusion of this assumption with sensitivity analyses for the primary outcome.
Assessment of heterogeneity
1. Clinical heterogeneity We considered all included studies without any comparison to judge clinical heterogeneity.
2. Statistical 2.1 Visual inspection We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I‐squared statistic This provided an estimate of the percentage of inconsistency thought to be due to chance. I‐squared estimate greater than or equal to 50% was interpreted as evidence of high levels of heterogeneity (Higgins 2003).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook (Higgins 2008). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects (Egger 1997). We did not use funnel plots for outcomes where there were ten or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
Where possible we employed a fixed‐effect model for analyses. We understand that there is no closed argument for preference for use of fixed or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us, however, random‐effects does put added weight onto the smaller of the studies ‐ those trials that are most vulnerable to bias. For this reason we favour using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
When heterogeneous results were found, we investigated the reasons for this. Where heterogeneous data substantially altered the results and the reasons for the heterogeneity were identified, we did not summate these studies in the meta‐analysis, but presented separately and discussed in the text.
Sensitivity analysis
The 2008 update included many studies from China. As there is concern regarding quality of trials from China (Wu 2006) we conducted a sensitivity analysis to investigate whether the findings of these trials substantially differed from other trials.
Results
Description of studies
Results of the search
1. 1997 search During the original 1997 search in Biological Abstracts, EMBASE, MEDLINE, and PsycLIT, we found 139 full reports. We contacted the senior author of each trial published since 1980 and the manufacturer of clozapine (Novartis AG, Switzerland) for additional references, data, and unpublished trials. We also searched the ISI citation index for each selected trial in order to identify further studies, and the reference sections of selected studies were searched for additional trials. We identified 34 additional citations of studies possibly relevant to this review. Novartis AG had agreed to provide additional data on early clozapine studies sponsored by the company, but to date, we have not received any additional data.
Out of these 173 articles 111 were excluded, mostly because they used a non‐controlled design. On inspecting the full papers, relevant references found in these papers, references given by principal authors of recent studies and references provided by the manufacturer of clozapine, 37 separate randomised controlled trials comparing clozapine with typical neuroleptic treatment were found. Two papers were then excluded due to diagnostically mixed study populations (Angst 1971, Van Praag 1976) and three papers due to lack of satisfactory random allocation (Category C) (Bao 1988, Ruiz 1974, Yang 1988). Two papers were excluded due to lack of extractable data (Li 1987, Nahunek 1975).
2. 1999 search For the first update (October 1999) we included one additional trial (Howanitz 1996 (CPZ)) raising the number of included trials to 30 with 80 references. A search of citations listed in SCISEARCH yielded 1094 references, none of which fulfilled the inclusion criteria.
3. 2008 Update (2006 search) The 2008 update of the Cochrane Schizophrenia Group's Register of trials yielded 350 references of which 150 were Chinese. Most Chinese reports included an English abstract. We selected 12 trials for further inspection. We added one Chinese trial (Yang 2004 b) to awaiting assessment and sought further information and excluded (Cui 2002) because it contained no usable data. Ten Chinese trials met the inclusion criteria. Two trials which had been awaiting assessment previously were also included (Lieberman 2003 (CPZ), Volavka 2002 (H)).
4. 2009 Update (2008 search) The 2009 update of the Cochrane Schizophrenia Group's Register of trials yielded 843 references. Fourteen were selected for further inspection and 11 Chinese trials were added to the review as included studies.
Included studies
The current update of this review includes 52 studies. All were described as being randomised.
1. Study length Seven studies were longer than 26 weeks (long‐term) (Tamminga 1994 (H), Kane 1995 (H), Lee 1994 (mainly H), Essock 1996 (H/CPZ/Flu), Rosenheck 1993 (H), Lieberman 2003 (CPZ), Yang 2004 a (CPZ) ); Volavka 2002 (H) reported both short and medium‐term data; the remainder all fall into the 'short‐term' category with a maximum length of 12 weeks.
2. Design There are two cross‐over trials (Gerlach 1974 (H), Gerlach 1975 (H)). We were only able to extract data for mortality and relapse from the first phase of these studies.
3. Participants A total of 4746 participants are included from 52 trials conducted from 1974 and 2007. In the current revision, 11 trials were added and all were conducted in China. Thirty‐six studies involved participants with schizophrenia that had been diagnosed using operationalised criteria (DSM, ICD, CCMD‐2) whilst 16 studies did not report using any diagnostic tool, but only stated the type of illness.
Eight trials include only participants with treatment‐resistant schizophrenia (Klieser 1988 (H), Hong 1997 (CPZ), Kane 1988 (CPZ), Essock 1996 (H/CPZ/Flu), Kumra 1994 (H), Rosenheck 1993 (H), Buchanan 1994 (H), Volavka 2002 (H)). Most studies included participants with mean ages around the late thirties when reported. One trial focused on children or adolescents suffering from schizophrenia (Kumra 1994 (H)), and another trial studied the efficacy of clozapine in elderly people with schizophrenia (Howanitz 1996 (CPZ)).
4. Settings The vast majority of the trials were in‐hospital studies. To our knowledge, only two trials were performed in the community (Kane 1995 (H), Buchanan 1994 (H)). Two long‐term trials were hospital‐based with follow up of discharged participants (Essock 1996 (H/CPZ/Flu), Rosenheck 1993 (H)). One Chinese study (Wang 2001 (CPZ)) used participants who were outpatients, all other Chinese trials when reported were hospital‐based.
5. Interventions The following control treatments were used in different trials: chlorpromazine (29 trials), haloperidol (14 trials), various neuroleptics (two trials), clopenthixol (two trials), loxapine (two trials), perphenazine (one trial) thioridazine (two trials). For clarity we have incorporated these in the study tags: H denotes haloperidol, CPZ chlorpromazine, Clopen clopenthixol, and Thi thioridazine. Five trials used low doses of typical neuroleptic treatment, which may have benefited clozapine results in these studies (Chiu 1976 (CPZ), Leon 1974 (CPZ), Ciurezu 1976 (H), Erlandsen 1977 (H), Honigfeld 1984 (H)). Two of these studies used equal mg doses of clozapine and chlorpromazine (Chiu 1976 (CPZ), Leon 1974 (CPZ)) and the other three used comparatively low doses of haloperidol.
6. Outcomes Many trialists used symptom scales in assessing treatment effects mainly the Brief Psychiatric Rating Scale (BPRS) and its derivative The Positive and Negative Symptom Scale (PANSS) scale. Some studies measured changes in negative symptoms using the Scale for Assessment of Negative Symptoms (SANS), and PANSS negative symptom sub‐score. The use of scoring data were in several cases precluded by the lack of standard deviation figures. Behavioural changes were measured by changes in the Nurse's Observation Scale for In‐patient Evaluation (NOSIE). Assessments of subjective well‐being were determined by authors' own global scales or Heinrichs‐Carpenter Quality of Life Scale.
Definitions of improvement differed across studies. This warranted some caution in drawing conclusions, as it was difficult to decide whether the results concerning clinical improvement were comparable. However, as with a pragmatic approach to diagnosis, it seemed unlikely that those judging improvement would have such dramatically differing criteria as to make summation inappropriate.
6.1 Outcome scales: only details of the scales that provided usable data are shown below. Reasons for exclusions of data are given under 'Outcomes' in the Characteristics of included studies table.
6.1.1 Global state 6.1.1.1 Clinical Global Impression ‐ CGI (Guy 1970) The CGI is a three‐item scale commonly used in studies on schizophrenia that enables clinicians to quantify severity of illness and overall clinical improvement. The items are: severity of illness; global improvement and efficacy index. A seven‐point scoring system is usually used with low scores indicating decreased severity and/or greater recovery. Nine studies reported data from this scale.
6.1.2 Mental state 6.1.2.1 Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) The BPRS is an 18‐item scale measuring positive symptoms, general psychopathology and affective symptoms. The original scale has 16 items, but a revised 18‐item scale is commonly used. Scores can range from 0 to 126. Each item is rated on a seven‐point scale varying from 'not present' to 'extremely severe', with high scores indicating more severe symptoms. Twenty studies reported data from this scale.
6.1.2.2 Mini Mental State Examination ‐ MMSE (Folstein 1975) This clinician‐administered clinical evaluation assesses cognition in five areas: orientation, immediate recall, attention and calculation, delayed recall, and language. The test takes 15 minutes to administer and the score ranges from 0 (severe impairment) to 30 (normal). Volavka 2002 (H) reported data from this scale.
6.1.2.3 Positive and Negative Syndrome Scale ‐ PANSS (Kay 1986) This schizophrenia scale has 30 items, each of which can be defined on a seven‐point scoring system varying from one (absent) to seven (extreme). This scale can be divided into three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity. Nine studies reported data from this scale.
6.1.2.4 Scale for the Assessment of Negative Symptoms ‐ SANS (Andreasen 1983) This scale allows a global rating of the following negative symptoms: alogia (impoverished thinking), affective blunting, avolition‐apathy, anhedonia‐asociality, and attention impairment. Assessments are made on a six‐point scale from zero (not at all) to five (severe). Higher scores indicate more symptoms. Five studies reported data from this scale.
6.1.2.5 Scale for the Assessment of Positive Symptoms ‐ SAPS (Andreasen 1983) This six‐point scale gives a global rating of positive symptoms such as delusions, hallucinations and disordered thinking. Higher scores indicate more symptoms. Wang 2001 (CPZ) reported data from this scale.
6.1.3 Behaviour. 6.1.3.1 Nurses Observational Scale of Inpatients Evaluation ‐ NOSIE (Honigfeld 1965) An 80‐item scale with items rated on a five‐point scale from zero (not present) to four (always present). Ratings are based on behaviour over the previous three days. The seven headings are social competence, social interest, personal neatness, cooperation, irritability, manifest psychosis and psychotic depression. The total score ranges from 0 to 320 with high scores indicating a poor outcome. Two studies reported data from this scale.
6.1.4 Cognitive functioning. 6.1.4.1 Category Instance Generation Test ‐ CIGT (Talland 1965) Higher score indicates a better outcome. Lee 1994 (mainly H) reported data from this scale.
6.1.4.2 Consonant Trigram Test ‐ CTT (Peterson 1959) Higher score indicates a better outcome. Lee 1994 (mainly H) reported data from this scale.
6.1.4.3 Controlled Word Association Test ‐ CWAT (Benton 1983). Higher scores indicate a better outcome. Lee 1994 (mainly H) reported data from this scale.
6.1.4.4 Digit Symbol Substitution Test ‐ DSST (Wechsler 1981) Higher score indicates a better outcome. Lee 1994 (mainly H) reported data from this scale.
6.1.4.5 The Short Cognitive Performance Test ‐ SKT (Lehfeld 1997) This is a psychometric instrument evaluating memory and attention deficits that has been developed and standardised in Germany. The test is useful for staging the severity of cognitive deficits and for assessing the benefits of therapy, especially with people suffering from dementia. Klieser 1990 (H) reported data from this scale.
6.1.4.6 Verbal List Learning Test ‐ VLLT (Buschke 1974) Higher score indicates a better outcome. Lee 1994 (mainly H) reported data from this scale.
6.1.4.7 Wechsler Intelligence Scale for Children Revised ‐ WISC‐R (Wechsler 1974) Higher score indicates a better outcome. Lee 1994 (mainly H) reported data from this scale.
Excluded studies
We excluded over 400 studies from the review ‐ over 180 studies because they were not randomised trials. Many studies were excluded because clozapine had been compared with an atypical antipsychotic or because clozapine had been compared with placebo or with different dosages of clozapine. Forty‐four studies were excluded because no usable data could be extracted from the study report.
Awaiting assessment
Yang 2004 b is awaiting assessment until further information in obtained.
Ongoing studies
We are not aware of any relevant studies that are currently ongoing.
Risk of bias in included studies
Allocation
All 52 studies were stated to be randomised but none provided descriptions of the methods used to generate the sequence or conceal it from those administering the treatment. All studies, therefore, are classified as of unclear quality with a moderate risk of selection bias and of overestimate of positive effect.
Blinding
Thirty‐two trials were stated to have used blinding, although most did not describe the methods used, and none tested the success of blinding for participants or evaluators. The remaining studies did not report whether blinding had been used. Again, this leaves little choice but to rate the risk of observer bias as at best unclear. This gathers further potential for overestimate of positive effects and underestimate of negative ones.
Incomplete outcome data
Many studies did not report the number of people leaving early; bias could be introduced to the final analysis if conducted on those completing the study only. Eleven studies undertook an 'intention‐to‐treat' (ITT) analysis in terms of both efficacy and adverse effects (Leon 1974 (CPZ), Gerlach 1974 (H), Gerlach 1975 (H), Honigfeld 1984 (H), Erlandsen 1977 (H), Ciurezu 1976 (H), Shopsin 1978 (CPZ), Kumra 1994 (H), Rosenheck 1993 (H), Buchanan 1994 (H)). Three studies performed an ITT analysis for adverse effects only (Itoh 1974 (H), Claghorn 1983 (CPZ), Hong 1997 (CPZ)). The two cross‐over trials provided very limited data for the first arm of the study (Gerlach 1974 (H), Gerlach 1975 (H)).
Selective reporting
Rates of attrition in the clozapine group (12%) and typical antipsychotic drugs comparison group (15%) were not excessively high compared with other compounds (Duggan 2005, Hunter 2003, Srisurapanont 2004), nor were they excessively divergent between groups. Attrition from studies involving participants who were treatment‐resistant were also low. Descriptions citing the reasons for leaving the study were not reported in the studies, and we were unable to report whether study attrition were due to protocol violations, withdrawals or drop outs, and therefore it remains unclear whether these trials were affected by attrition bias.
We identified no overt under reporting of outcomes that had been collected by the trialists.
Other potential sources of bias
Many of the trials were supported by the industry which stood to profit from positive results. Overall our judgement regarding the overall risk of bias in the individual studies is illustrated in Figure 1. Not one study had clear descriptions of sequence generation as well as concealment. Blinding was often undertaken but unconvincing and reporting biases common. Studies were often funded by industry with a pecuniary interest in the results. This, along with the other sources of bias outlined above gave us reason to judge, the risk of bias in the studies to be high, and therefore our estimates are likely to be over estimating any true positive effect, and underestimating negative effects.
1.
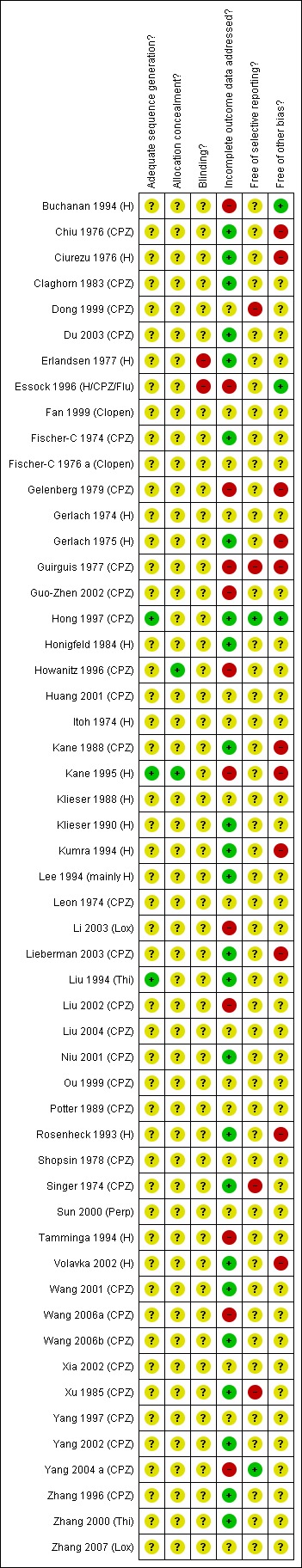
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
Summary of findings for the main comparison. CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL for schizophrenia.
| CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: mostly in hospital Intervention: CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL | |||||
| Relapse ‐ short term | Study population | RR 0.62 (0.45 to 0.84) | 1303 (19 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 130 per 1000 | 81 per 1000 (58 to 109) | |||||
| Medium risk population | ||||||
| 63 per 1000 | 39 per 1000 (28 to 53) | |||||
| Relapse ‐ long term | Study population | RR 0.22 (0.14 to 0.34) | 578 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 354 per 1000 | 78 per 1000 (50 to 120) | |||||
| Medium risk population | ||||||
| 270 per 1000 | 59 per 1000 (38 to 92) | |||||
| Global impression: 1. Not clinically improved ‐ short term | Study population | RR 0.72 (0.66 to 0.79) | 1119 (14 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 661 per 1000 | 476 per 1000 (436 to 522) | |||||
| Medium risk population | ||||||
| 556 per 1000 | 400 per 1000 (367 to 439) | |||||
| Unable to work | Study population | RR 0.87 (0.75 to 1) | 416 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 622 per 1000 | 541 per 1000 (467 to 622) | |||||
| Medium risk population | ||||||
| 687 per 1000 | 598 per 1000 (515 to 687) | |||||
| Adverse effects: 1. Blood problems ‐ decreased white cell count | Study population | RR 7.09 (1.96 to 25.62) | 1031 (13 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse effects: 4. Salivation ‐ too much | Study population | RR 2.25 (1.96 to 2.58) | 1479 (17 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 216 per 1000 | 486 per 1000 (423 to 557) | |||||
| Medium risk population | ||||||
| 105 per 1000 | 236 per 1000 (206 to 271) | |||||
| Adverse effects: 5a. Weight gain | Study population | RR 1.28 (1.07 to 1.53) | 590 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 394 per 1000 | 504 per 1000 (422 to 603) | |||||
| Medium risk population | ||||||
| 364 per 1000 | 466 per 1000 (389 to 557) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation not well described; blinding not likely, nor tested
Summary of findings 2. CLOZAPINE versus TYPICAL ANTIPSYCHOTICS for people with schizophrenia whose illness has proved resistant to treatment.
| CLOZAPINE versus TYPICAL ANTIPSYCHOTICS for people with schizophrenia whose illness has proved resistant to treatment | ||||||
| Patient or population: patients with schizophrenia Settings: mostly in hospital Intervention: CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA | |||||
| Relapse ‐ short term | Study population | RR 1.04 (0.61 to 1.78) | 396 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 117 per 1000 | 122 per 1000 (71 to 208) | |||||
| Medium risk population | ||||||
| 103 per 1000 | 107 per 1000 (63 to 183) | |||||
| Relapse ‐ long term | Study population | RR 0.17 (0.1 to 0.3) | 423 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 367 per 1000 | 62 per 1000 (37 to 110) | |||||
| Medium risk population | ||||||
| 367 per 1000 | 62 per 1000 (37 to 110) | |||||
| Global impression: 1. Not clinically improved ‐ short term | Study population | RR 0.71 (0.64 to 0.79) | 370 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 932 per 1000 | 662 per 1000 (596 to 736) | |||||
| Medium risk population | ||||||
| 957 per 1000 | 679 per 1000 (612 to 756) | |||||
| Global impression: 1. Not clinically improved ‐ long term | Study population | RR 0.83 (0.76 to 0.91) | 648 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 837 per 1000 | 695 per 1000 (636 to 762) | |||||
| Medium risk population | ||||||
| 836 per 1000 | 694 per 1000 (635 to 761) | |||||
| Adverse effects 1. Blood problems | Study population | RR 1.9 (0.97 to 3.71) | 827 (5 studies) | |||
| 26 per 1000 | 49 per 1000 (25 to 96) | |||||
| Medium risk population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse effects 4. Salivation ‐ too much | Study population | RR 2.01 (1.74 to 2.32) | 827 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 290 per 1000 | 583 per 1000 (505 to 673) | |||||
| Medium risk population | ||||||
| 182 per 1000 | 366 per 1000 (317 to 422) | |||||
| Adverse effects 5. Weight gain | Study population | RR 1.33 (1.11 to 1.59) | 484 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 431 per 1000 | 573 per 1000 (478 to 685) | |||||
| Medium risk population | ||||||
| 421 per 1000 | 560 per 1000 (467 to 669) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation not well described; blinding not likely nor tested
1. COMPARISON 1. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ OVERALL
All data are derived from 50 studies.
1.1 Death Four deaths occurred in 614 people treated with typical neuroleptics compared with three deaths in 629 people treated with clozapine. There were no significant differences in mortality between groups (n=1243, 12 RCTs, RR 0.56 CI 0.1 to 2.3).
1.2 Relapse rate We included 19 short‐term studies, and found incidences of relapse were lower in the clozapine group (n=1303, RR 0.62 CI 0.5 to 0.8, NNT 21 CI 15 to 49) compared with typical antipsychotic drugs. Long‐term data (4 RCTs, n=578) also favoured clozapine but data were heterogeneous (I‐squared =76%) (RR 0.22 CI 0.1 to 0.3).
1.3 Global impression 1.3.1 Clinical improvement as defined by study authors We found that the number of participants who had not improved were lower in the clozapine group (n=1119, 14 RCTs, RR 0.72 CI 0.7 to 0.8, NNT 6 CI 5 to 8). Three long‐term studies also favoured clozapine (n=719, RR 0.81 CI 0.7 to 0.9) but the data are heterogeneous (I‐squared statistic 81%).
1.3.2 Readiness for hospital discharge There were no significant differences between treatment groups for the number of participants who were judged to be not ready for discharge (short‐term, n=447, 5 RCTs, RR 0.88 CI 0.8 to 1.0). Long‐term data also failed to show a significant difference (n=648, 2 RCTs, RR 0.82 CI 0.6 to 1.1).
1.4 Hospitalisation ‐ Not discharged or readmitted within one year after discharge Data were available from two long‐term studies (Essock 1996 (H/CPZ/Flu), Rosenheck 1993 (H)), and we found no significant advantage for clozapine (n=648, RR 0.94 CI 0.9 to 1.0) compared with typical antipsychotic drugs.
1.5 Unable to work We found no significant difference in the number of participants who were assessed as being unable to work (n=416, 4 RCTs, RR 0.87 CI 0.8 to 1.0), although the data suggested a trend favouring clozapine (p=0.06).
1.6 Participant dissatisfaction No significant differences were found for dissatisfaction with treatment in two short‐term studies (n=114, RR 0.72 CI 0.4 to 1.3). We found longer‐term data in (Rosenheck 1993 (H)). These favoured the clozapine group who were less dissatisfied with their treatment compared with conventional antipsychotic drugs (n=423, RR 0.45 CI 0.3 to 0.8, NNT 13 CI 9 to 37).
1.7 Leaving the study early ‐ acceptability of treatment We used leaving the study early data as a proxy measure for the acceptability of treatment. Short‐term data from 32 studies involving 2316 participants indicated that significantly more participants given clozapine found treatment acceptable (RR 0.81 CI 0.7 to 1.0, NNT 35 CI 20 to 217). Longer‐term data from six studies showed a significant benefit in the clozapine group (n=982, RR 0.60 CI 0.5 to 0.7, NNT15 CI 12 to 20). The long‐term attrition rate from clozapine treatment is approximately 33% and 56% when treated with typical antipsychotic drugs.
1.8 Mental state 1.8.1 BPRS and PANSS We found BPRS mental state scores favoured clozapine during short‐term assessment in 16 studies (n=1205, WMD ‐3.79 CI ‐4.9 to ‐2.7), although the data were heterogeneous (I squared=69%). Longer‐term BPRS data (Lee 1994 (mainly H)) were equivocal (n=52, WMD 0.80 CI ‐5.7 to 7.3). PANSS scores from three Chinese trials favoured the clozapine groups (n=163, WMD ‐3.82 CI ‐7.4 to ‐0.3) during short‐term analysis. Also, PANSS scores assessed over the long‐term favoured clozapine (Rosenheck 1993 (H), n=235, WMD ‐6.90 CI ‐10.7 to ‐3.1).
1.8.2 Negative symptoms Short‐term continuous data on negative symptom scores from six short‐term trials with 236 participants favoured clozapine (SANS, WMD ‐7.12 CI ‐8.8 to ‐5.5) but these are heterogeneous data (I squared=92%). Longer‐term negative symptoms scores assessed with the PANSS negative sub score were not significantly different (Rosenheck 1993 (H), n=235, WMD ‐0.90 CI ‐6.6 to 4.8).
1.8.3 Positive symptoms We found short‐term SAPS scores were equivocal (Wang 2001 (CPZ), n=60, WMD 4.39 CI ‐12.2 to 20.9). Longer‐term data from the PANSS positive scores favoured clozapine (Rosenheck 1993 (H), n=235, WMD ‐2.20 CI ‐3.3 to ‐1.1).
1.9 Cognitive function Cognitive impairment from one small study (n=82) favoured the clozapine group who experienced less impairment (Klieser 1990 (H), RR 0.56 CI 0.3 to 0.9, NNT 4 CI 3 to 21) when assessed with the SKT scale compared with those given typical antipsychotic drugs. One small study (Lee 1994 (mainly H), n=54) reported data on a series of cognitive functioning tests (verbal, memory or executive functions etc.) and we found outcomes to be equivocal, except for 'psychomotor speed and attention' scores which favoured the clozapine‐treated participants over the three pre‐stated cut‐off points (short‐term, WMD 1.40 CI 0.2 to 2.6, medium‐term, WMD 1.30 CI 0.01 to 3.0, long‐term, WMD 2.10 CI 0.8 to 3.4).
1.10 Behaviour We found behavioural scores from the NOSIE scale favoured clozapine (n=40, 2 RCTs, RR 0.36 CI 0.2 to 0.9, NNT 4 CI 3 to 20) compared with typical antipsychotic drugs, during short‐term analysis.
1.11 Adverse effects 1.11.1 Blood problems We defined blood problems as (a) any blood problem requiring withdrawal of participants from trials, or (b) leukopenia, defined as a white cell count <3000 per cubic mm, or (c) neutropenia, defined as granulocyte count <1500 per cubic mm. Blood problems occurred more frequently in participants receiving clozapine (3.2%) compared with those given typical antipsychotic drugs (0%) in short‐term studies (n=1031, 13 RCTs, RR 7.09 CI 2.0 to 25.6). We found two long‐term studies had a much higher incidence of blood problems in both clozapine (7%) and control group (haloperidol) (5.2%), but no significant differences were found (n=462, RR 1.35 CI 0.7 to 2.8) (Rosenheck 1993 (H), Tamminga 1994 (H)). We found incidences of abnormal ESR were higher in the clozapine group (Dong 1999 (CPZ), n=62, RR 10.78 CI 2.8 to 41.9, NNH 2 CI 2 to 9). Also, Dong 1999 (CPZ) and Li 2003 (Lox) reported changes in white blood cell count, and we found that the clozapine group had a significant increase in white cells (n=122, RR 13.02 CI 2.6 to 65.5, NNH 5 CI 2 to 38) compared with the typical antipsychotic group.
1.11.2 Other adverse effects Clozapine commonly caused drowsiness (n=1527, 16 RCTs, RR 1.23 CI 1.1 to 1.3, NNH 11 CI 7 to 18). No significant differences were found in the incidences of low blood pressure/dizziness (n=1478, 14 RCTs, RR 1.13 CI 1.0 to 1.3) between clozapine and the typical neuroleptic drugs. Salivation occurred more frequently in the clozapine group (n=1479, 17 RCTs, RR 2.25 CI 2.0 to 2.6), but data were heterogeneous (I squared=68%). Dry mouth occurred more frequently in the typical antipsychotic group (n=859, 9 RCTs, RR 0.38 CI 0.3 to 0.5, NNH 7 CI 6 to 8), compared with clozapine. We found participants given clozapine gained weight significantly more than those given typical antipsychotic drugs (n=590, 5 RCTs, RR 1.28 CI 1.1 to 1.5, NNH 10 CI 5 to 37). We found continuous data for weight gain (n=58, MD ‐0.17 CI ‐3.1 to 2.8) to be equivocal.
Extrapyramidal movement disorders were more frequent in those who were treated with conventional neuroleptics (n=1495, 19 RCTs, RR 0.57 CI 0.5 to 0.7, NNT 5 CI 4 to 6). No significant differences were found between clozapine and the typical neuroleptic drugs for fits (n=1157, 9 RCTs, RR 1.51 CI 0.8 to 2.8). Increases in body temperature were more frequent in the clozapine group (n=1147, 9 RCTs, RR 1.57 CI 1.3 to 2.0, NNH 12 CI 7 to 23).
We found oral glucose tolerance tests data (Liu 2004 (CPZ)) were equivocal (WMD 0.30 CI ‐0.16, 0.76). One study from China reported continuous data for fasting blood glucose over different time cut‐off points, with most data being equivocal (Yang 2004 a (CPZ)). Long‐term data, however, revealed more participants in the clozapine group had abnormal blood glucose (n=87, WMD 1.00 CI 0.4 to 1.6). One study (Li 2003 (Lox)) reported data for tachycardia and electro‐cardiogram tests and we found no significant differences between clozapine and loxapine. We found no significant difference in TESS scores (n=50, 1 RCT, WMD ‐0.90 CI ‐1.93 to 0.13).
1.12 Sensitivity Analysis In the 2008 update we included ten trials that were conducted in China. Some differences in effect size were observed, but sensitivity analyses demonstrated that the results of Chinese trials followed the same general affect as trials conducted in western countries. The most meaningful comparisons were those related to short‐term studies. The overall reduction in clinical symptoms, for instance, in ten short‐term non‐Chinese studies were (n=828, BPRS, WMD ‐6.32 CI ‐8.1 to ‐4.6). Whilst, the overall reduction in clinical symptoms in six short‐term Chinese studies were (n=317, BPRS, WMD ‐2.56 CI ‐4.1 to ‐1.0). Similarly, the relative risk for leaving the study early in 21 short‐term non‐Chinese studies were (n=1553, RR 0.84 CI 0.7 to 1.0) compared with five short‐term Chinese studies (n=278, RR 0.61 CI 0.3 to 1.4).
2. COMPARISON 2. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ TREATMENT‐RESISTANT SCHIZOPHRENIA
All data were derived from eight studies (Buchanan 1994 (H)), Essock 1996 (H/CPZ/Flu), Hong 1997 (CPZ), Kane 1988 (CPZ), Klieser 1988 (H), Kumra 1994 (H), Rosenheck 1993 (H), Volavka 2002 (H)) 2.1 Death We found no significant difference in mortality rates in four studies that included 939 participants with treatment‐resistant schizophrenia (RR 0.64 CI 0.1 to 3.1).
2.2 Relapse rate Analysis of four short‐term studies (396 people) did not reveal any significant difference in relapse rates between treatment groups (RR 1.04 CI 0.6 to 1.8), but we found data from one longer‐term study did favour clozapine (n=423, RR 0.17 CI 0.1 to 0.3, NNT 4 CI 4 to 4).
2.3 Global impression 2.3.1 Clinical improvement as defined by study authors We found that participants allocated to clozapine had greater clinical improvement than the typical antipsychotic group (n=370, 4 RCTs, RR 0.71 CI 0.6 to 0.8, NNT 4 CI 4 to 4). Similarly, longer‐term data also favoured clozapine (n=648, 2 RCTs, RR 0.83 CI 0.8 to 0.9, NNT 8 CI 5 to 14).
2.3.2 Readiness for hospital discharge No significant differences were found between clozapine and typical antipsychotic drugs when assessed on dischargeability in two longer‐term studies (n=648, RR 0.82 CI 0.6 to 1.1).
2.4 Hospitalisation The numbers of participants who were either not discharged from hospital or were readmitted revealed no significant differences (n=648, RR 0.94 CI 0.9 to 1.0) between intervention groups over one year's treatment.
2.5 Leaving the study early ‐ acceptability of treatment The acceptability of treatment as measured by the number of people leaving the study revealed no significant difference between treatment groups (n=436, 5 RCTs, RR 1.19 CI 0.7 to 1.9) during short‐term analyses with 14% attrition in clozapine group and 12% in the typical antipsychotic drugs group. Longer‐term data, however, significantly favoured clozapine (n=648, RR 0.57 CI 0.5 to 0.7, NNT 4 CI 3 to 5) compared with typical antipsychotic drugs, with 39% attrition in the clozapine group compared with 70% leaving from the typical antipsychotic drugs group.
2.6 Participant satisfaction Only one long‐term study provided data on patient satisfaction and we found that more participants given clozapine were satisfied with their treatment (n=423, RR 0.45 CI 0.3 to 0.8, NNT 13 CI 9 to 37) than those allocated to typical antipsychotic drugs (Rosenheck 1993 (H)).
2.7 Mental state We found BPRS endpoint scores favoured clozapine (n=429, 5 RCTs, WMD ‐7.83 CI ‐10.0 to ‐5.6) compared with the typical antipsychotic group during short‐term analyses. Longer‐term data were equivocal (Rosenheck 1993 (H), n=235, WMD ‐6.90 CI ‐10.7 to ‐3.1). We found medium‐term data by Volavka 2002 (H) (n=77) were not significantly different for PANSS total, negative or positive symptom scores. We found negative symptoms score data from four short‐term studies favoured the clozapine group (n=164, SMD ‐0.44 CI ‐0.8 to ‐0.1) but these data contain the skewed Kumra 1994 (H) figures.
2.8. Adverse effects 2.8.1 Blood problems The differences in the number of participants with blood problems failed to reach statistical significance (p=0.06) although a trend could be seen which suggests blood problems were less frequent in the typical antipsychotic group (n=827, 5 RCTs, RR 1.90 CI 1.0 to 3.7).
2.8.2 Drowsiness Participants given clozapine experienced significantly more incidences of drowsiness (n=827, 5 RCTs, RR 1.22 CI 1.1 to 1.3, NNH 10 CI 7 to 20) compared with participants given typical antipsychotic drugs.
2.8.3 Low blood pressure/dizziness We found no significant difference in incidences of dizziness between groups (n=806, 4 RCTs, RR 1.08 CI 0.9 to 1.2).
2.8.4 Salivation Short‐term data indicated that participants given clozapine experience more hypersalivation (n=827, 5 RCTs, RR 2.01 CI 1.7 to 2.3) than those receiving typical antipsychotic drugs, but data were heterogeneous (I‐squared 78%). We found incidences of dry mouth were significantly lower in the clozapine group (n=383, 4 RCTs, RR 0.27 CI 0.2 to 0.5, NNT 5 CI 5 to 7) compared with typical drugs.
2.8.5 Weight gain The number of participants who gained weight were found to be higher in the clozapine group (n=484, 3 RCTs, RR 1.33 CI 1.1 to 1.6, NNH 8 CI 4 to 24) compared with those given typical antipsychotic drugs.
2.8.6 Movement disorder Incidences of movement disorder were significantly less frequent in the clozapine group (n=521, 4 RCTs, 0.77 CI 0.7 to 0.9, NNT 8 CI 6 to 17).
2.8.7 High temperature We found that the participants allocated to clozapine had more incidences of raised temperatures than the typical antipsychotic group (n=766, 3 RCTs, RR 1.36 CI 1.0 to 1.8), but data were heterogeneous (I‐squared 51%).
2.8.8 Fits No significant differences were found in the incidences of fits between groups (n=784, 5 RCTs, RR 1.75 CI 0.9 to 3.4).
3. COMPARISON 3. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ CHILDREN OR ADOLESCENTS
All data derived from one study (Kumra 1994 (H). 3.1 Death No deaths occurred in the (Kumra 1994 (H)) study (n=21).
3.2 Relapse No relapses occurred during the six weeks.
3.3 Global impression Data for 'not clinically improved' were equivocal (Kumra 1994 (H), n=21, RR 0.82 CI 0.2 to 2.8).
3.4 Leaving the study early We found no significant difference in the number of participants leaving the study early (n=11, RR 3.30 CI 0.4 to 26.8).
3.5 Mental state We found no significant difference in BPRS scores or SANS scores in the small study by (Kumra 1994 (H), n=21).
3.6 Adverse effects In the single study (Kumra 1994 (H)) dealing with adolescents or children, 40% (4/10) of the clozapine‐treated group developed blood problems (RR 9.82 CI 0.6 to 162.2) but the differences were not statistically significant. Drowsiness occurred more frequently in the clozapine group (n=21, RR 3.30 CI 1.2 to 8.9, NNH 2 CI 2 to 16). Hypersalivation also occurred more frequently in the clozapine group (n=21, RR 3.85 CI 1.0 to 14.4, NNH 2 CI 2 to 185), compared with typical antipsychotic drugs. No statistically significant differences were found for weight gain, movement disorders or fits.
4. COMPARISON 4. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ ELDERLY PEOPLE
All data derived from one study (Howanitz 1996 (CPZ). 4.1 Death No deaths occurred in the Howanitz 1996 (CPZ) study (n=42).
4.2 Leaving the study early We found no significant differences in rates of attrition (n=42).
4.3 Blood problems We found that elderly people were also at risk of developing blood problems with 8% affected in the clozapine group, but the differences were not statistically significant (n=42, RR 3.8 CI 0.2 to 74.6).
4.4 Other adverse effects The occurrence of drowsiness, dizziness, hypersalivation, weight gain, movement disorders or fits revealed no significant difference between groups.
5. Publication bias To look for a possible publication bias (that is, the possibility that studies with negative findings have not reached full publication) funnel graphs for clinical improvement, relapse frequency and number of people leaving early (acceptability) were constructed by plotting number of study participants (on the 'y' axis) against the log odds ratios (on the 'x' axis). No 'gap' in the funnel indicating a publication bias affecting the results were found.
Discussion
Summary of main results
1. COMPARISON 1. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ OVERALL (Table 1) 1.1 Death This review did not reveal any difference in mortality between clozapine and the typical neuroleptic treatment groups. This would be a rare adverse event and the short duration of the included studies means that these trials would be unlikely to highlight any differences there may be.
1.2 Global impression 1.2.1 Relapse We found clozapine to be more advantageous than conventional neuroleptics in avoiding, or postponing, psychotic relapses at least in the short term. The relapse‐preventing effect of clozapine were heterogeneous in the long‐term studies, but favoured clozapine. However, the relapse‐preventing effect of clozapine were not seen in treatment‐resistant participants during short‐term analyses, but were effective in one longer‐term study (Rosenheck 1993 (H), n=423, RR 0.17 CI 0.1 to 0.3, NNT 4 CI 4 to 4).
1.2.2 Global impression ‐ clinical improvement Treating six people with schizophrenia with clozapine instead of typical neuroleptics resulted in one additional person showing a clinical improvement. This may be a real finding and, if so, important. However, as has been said many times above, there are reasons to consider that bias may also influence the research and therefore even this modest result my over estimate clozapine's effects.
Global impression was chosen as the primary outcome of this review. There is some suggestion of a real effect of clozapine over and above that of haloperidol, chlorpromazine and a few other typical drugs ‐ but the data are not strong and may include important biases in favour of clozapine.
1.3 Discharge ‐ not ready We found both short and long‐term studies failed to show any advantage for participants given clozapine compared with typical antipsychotic drugs or for hospital readmission in long‐term studies.
1.4 Unable to work About half of all participants in the four included studies were judged as being unable to work. The outcome data between groups were not significantly different but the graph revealed a trend favouring clozapine (p=0.06). More studies may have produced a significant outcome.
1.5 Participant dissatisfaction Clozapine participants were more satisfied with their treatment in one long‐term study (Rosenheck 1993 (H)). This was an important finding as participant satisfaction may enhance compliance. Two short‐term studies were equivocal, and more studies measuring satisfaction with treatment are needed to substantiate the long‐term benefit.
1.6 Leaving the study early Using study attrition as a proxy measure of acceptability, the results favoured clozapine in short‐term studies. Longer‐term studies revealed a larger advantage for clozapine over typical antipsychotic drugs (n=982, RR 0.60 CI 0.5 to 0.7, NNT 15 CI 12 to 20), and as with participant dissatisfaction the benefits are more apparent during the longer durations of treatment.
1.7 Mental state 1.7.1 BPRS/PANSS We found short‐term studies indicated a greater improvement for participants on clozapine compared with typical antipsychotic drugs, but the data were heterogeneous, the differences in overall reduction were small, and the clinical significance of a difference of this magnitude may be questioned. We found long‐term BPRS data (Lee 1994 (mainly H)) from a single small study (n=52) to be equivocal and without much larger data sets it is impossible to interpret such findings. PANSS scores which are based upon the BPRS scale were found to favour clozapine in a single long‐term study, and three short‐term Chinese trials, which included two studies (Li 2003 (Lox), Zhang 2007 (Lox)) that used loxapine as the comparator drug, and is thought to have drug profile similar to atypical antipsychotic drugs.
1.7.2 Negative symptoms Negative symptom score from the SANS scale favoured clozapine group with about a seven point advantage. Again, the clinical significance of such a shift of this magnitude is questionable. We were able to include data from one long‐term study (Rosenheck 1993 (H)) and found that negative symptoms were not significantly different when assessed using the PANSS negative symptoms sub‐score.
1.7.3 Positive symptoms SAPS scores in a single short‐term study (Wang 2001 (CPZ)) failed to reveal any significant differences, although small participant numbers (n=60) limited the possibility of detecting a real treatment effect. Longer‐term PANSS positive data from a larger study (n=235) did favour clozapine in a group of participants who were treatment‐resistant, although the points difference was only about two and the clinical relevance of this is unclear.
1.8 Cognitive functioning Few studies measured cognitive functioning and outcome data were complicated by the use of different scales by single studies making meta‐analyses impossible. We found participants given clozapine had less cognitive impairment (n=82, RR 0.56 CI 0.3 to 0.9, NNT 4 CI 3 to 21). Most other cognitive outcomes were equivocal and were based on small numbers of participants.
1.9 Behaviour Few studies reported usable data for behavioural changes. We were only able to report two studies that measured behaviour using the NOSIE scale and data favoured clozapine. However, only 40 participants were included and more robust data are needed.
1.10 Adverse effects 1.10.1 Blood problems The reason for clozapine being taken off the international market in 1978 had been due to a fatal or potentially fatal drop in white cells in the blood of 16 people in Finland (Idänpään‐Heikkilä 1975). The rate of white blood cell problems (leukopenia) seems to be agreed to be 1.5 to 2% with an increased risk in females and the elderly (Alvir 1993). In the short‐term studies included in this review 16 cases of blood problems from 507 clozapine‐treated individuals were reported, which indicates a blood problem frequency of 3.2%. A relatively high effect size is observed in one Chinese study contributing data to the meta‐analysis on blood problems (Ou 1999 (CPZ)). If this study is excluded, the frequency of blood problems is reduced to 1.9%. Also, twelve of the cases of blood problems occurred in adults (frequency 2.4%) and four occurred in children or adolescents (frequency 40%). Two of the adult cases were in a study of elderly people (frequency 8%). Based on these small but important numbers, clozapine‐induced leukopenia seemed to be more frequent in children and adolescents, and in elderly people. Furthermore, incidences of blood problems were even higher in long‐term clozapine treatment (7%) but were not significantly more frequent than the control group receiving haloperidol (5%). Limited data also suggests that clozapine produces more problems with erythrosedimentation rate and initial increases in white cells.
1.10.2 Other adverse effects People on clozapine experienced significantly more hypersalivation, temperature increase, and drowsiness than those given conventional neuroleptics, but less uncomfortable dry mouth. Over half of those allocated clozapine experienced hypersalivation compared with about one fifth of participants in the control groups. Hypersalivation is a real problem with use of clozapine. It is remarkable in its quantity and very socially disabling. The management of this problem is the focus of another review (Syed 2008).
In assessing the frequency of extrapyramidal adverse effects one has to remember that some trialists (Kane 1988 (CPZ), Lee 1994 (mainly H), Tamminga 1994 (H), Kumra 1994 (H), Rosenheck 1993 (H), Buchanan 1994 (H)) used anticholinergic add‐on medication in the control group to alleviate neurological adverse effects. To this extent the comparisons were biased in favour of the typical neuroleptics. Even with this the clozapine group displayed fewer movement disorders than those treated with the typical neuroleptic drugs.
1.11 Sensitivity analysis The 2008 update of this review included ten trials conducted in China. Chinese trials were not different from other trials in terms of efficacy and adverse effects, despite observed differences in effect size.
2. COMPARISON 2. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ TREATMENT‐RESISTANT SCHIZOPHRENIA (Table 2) 2.1 Overall Most findings are based on trials with a total number of participants of 400 to 600. These studies are most difficult to undertake. Nevertheless, that millions of people are now, or have been, treated with this potent drug on the back of data from a few industry‐funded trials reporting limited data for use in everyday life is not ideal and more replication is indicated.
In neuroleptic‐resistant patients, clozapine shows a somewhat greater advantage in controlling symptoms. Participants classified as treatment‐resistant evidenced a significantly better improvement rate with clozapine than with typical pharmacological treatment. The weighted mean difference between short‐term treatments in BPRS total score is about eight points, which probably is a clinically meaningful difference. Treatment‐resistant people did not show any beneficial effect of clozapine on relapse frequency or attrition rates in the short‐term, but the outcomes of long‐term clozapine treatment were more beneficial. The newly included Chinese trials provided no data regarding treatment‐resistant schizophrenia.
3. COMPARISON 3. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ CHILDREN OR ADOLESCENTS 3.1 Overall From the limited data we found no differences between clozapine and typical antipsychotic drugs in children for the outcomes of death, relapse, global impression, leaving the study early and mental state. The adverse effects, drowsiness, hypersalivation occurred more frequently in the clozapine group. No significant differences were found for weight gain, movement disorders or fits. All data came from a single study (Kumra 1994 (H)) involving just 21 people. Issues with the frequency of blood dyscrasias in this subgroup are discussed above (1.10.1).
4. COMPARISON 4. CLOZAPINE versus TYPICAL ANTIPSYCHOTIC DRUGS ‐ ELDERLY PEOPLE 4.1 Overall We found no significant differences in the outcomes of death, leaving the study early or adverse effects in elderly people. All data came from a single study, Howanitz 1996 (CPZ), which randomised just 42 participants. Issues with the frequency of blood dyscrasias in this subgroup are discussed above (1.10.1).
Overall completeness and applicability of evidence
Randomised clozapine studies have been published since 1974, but the majority have a duration of only four to eight weeks. The severe adverse effect of loss of white blood cells (agranulocytosis) may occur later than during the first four to eight weeks of treatment and may therefore be under‐reported in short‐term studies. Also, deficiencies of global and social functioning caused by schizophrenia may take much longer to improve and any beneficial effect of clozapine may thus be underestimated in short‐term studies.
Data on mortality were missing in a majority of the studies. Outcome reporting were mainly symptom and physician‐oriented. Participant‐oriented global and functional outcomes, such as readiness for discharge and ability to work were seldom reported. Participant satisfaction was only reported in four studies and no studies reported family burden. There is clearly a need for studies focusing not only on symptoms, but also on general and social functioning, family burden and participant acceptability.
The setting also compromises the applicability of the studies. Most of the included studies were undertaken in hospital, whereas the majority of people with schizophrenia are treated in the community. Our data, therefore, on the important aspects of functions of normal living are remarkably limited. It is surprising that after over three decades of research we still do not have much long‐term, community‐based data. It is not that these data are impossible to generalise ‐ just that, considering the enormous change use of clozapine has wrought, we had expected much more easily applicable data to routine community care.
Quality of the evidence
The quality of reporting in most studies was poor. There are likely to be significant biases in the results favouring clozapine. The magnitude of the effects of these biases on, for example, the primary outcome, could be considerable with an overestimate of 30% being entirely credible (Jüni 2001). This shocking finding, combined with the wide use of clozapine, must mean that better independent studies are a matter of urgency.
Potential biases in the review process
We have just worked with published reports and by doing this we may be perpetuating a reporting and publishing bias. This version of the review includes many trials from the People's Republic of China. These trials have been the focus of specific research and it has been found that many that are stated to be randomised are not (Wu 2006). We have not contacted the authors of these trials but did not identify any overt bias in the results and have left these trials in.
Agreements and disagreements with other studies or reviews
This review substantially updates and improves past work. However, it largely agrees with findings from previous versions but, perhaps puts less emphasis on the positive findings because of the new Risk of Bias table function of this version of RevMan.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia Clozapine demonstrated some advantage over typical antipsychotic drugs in terms of clinical improvement with one person improving for every six people treated over and above those given typical drugs. Also, the incidences of relapse are likely to be postponed in some people, but this advantage is less apparent with about one relapse prevented for every 21 people given clozapine. Clozapine reduced the number of people dropping out of studies and as a consequence people with schizophrenia may find clozapine more tolerable than typical drugs. However, this effect is to be expected only when compared with low potency drugs such as chlorpromazine; when compared with high potency drugs such as haloperidol no difference in attrition rates are detected. Furthermore, data suggests that people receiving clozapine will express more satisfaction with their drug treatment, although this is based on only one trial. With clozapine treatment there will probably be a slight improvement in negative symptoms and general behaviour/functioning may improve but there is no evidence that a person's ability to be discharged from hospital or to work will be changed. Clozapine is likely to cause more drowsiness and weight gain than typical drugs, but less movement disorders. People with schizophrenia will also need to base their decisions upon their own personal response to treatment and consider that these data are statistical outcomes that are not universally applicable.
2. For clinicians Clozapine appears to be as effective as typical antipsychotic drugs. It presents an advantage in clinical improvement and relapse prevention but studies are at risk of bias. It may provide greater compliance with treatment since both participant satisfaction and attrition rates are improved. Clozapine may be effective in inducing clinical improvement in the long‐term, and acceptability of treatment is significantly improved in the long‐term. Long‐term relapse prevention may also be improved compared with typical antipsychotic drugs but heterogeneity leaves doubt. More studies are needed to replicate and validate these findings.
Non‐trial studies suggest that one to two of every 100 people receiving clozapine will have serious white blood cell problems that will necessitate a precipitous withdrawal of medication, careful monitoring for infection, and even specialised nursing and hospitalisation should infection occur (Alvir 1993). Everyone taking clozapine must have weekly blood monitoring during the first 18 weeks of treatment and at least monthly thereafter. In one small study (Kumra 1994 (H)) almost half of the clozapine‐treated children and adolescents developed blood problems so use of clozapine in this subgroup must be of overriding necessity and undertaken with extreme caution. Similar precautions are warranted when prescribing clozapine to elderly people. Treatment‐resistant people appear to respond better to clozapine than typical antipsychotic drugs when evaluated on global clinical improvement measures and negative symptoms scores. Clozapine may also be more tolerable over the long term, as indicated by significantly fewer attrition from studies.
3. For managers / policy makers We found no data for service outcomes, or economic outcomes. People given clozapine do require monitoring for potential blood disorders, which may influence the decision for countries with limited resources.
Implications for research.
1. General The majority of existing randomised studies on clozapine are short‐term in‐hospital trials focusing on clinical outcomes. Short studies may underestimate both adverse effects and global efficacy. The effects of clozapine in comparison to typical neuroleptics in hospitalised adult patients would have been more clear if studies had been better reported. Compliance with CONSORT (Moher 2001) by both authors and editors would ensure that the methods used were accessible to the readership and would also avoid the loss of valuable data.
The outcomes currently described are largely disease‐oriented and may not be very relevant for measuring the global functioning level of a person with schizophrenia. Several important treatment aspects, such as social functioning and family burden, have not been assessed in any of the trials. Some highly relevant outcomes, such as the person's own satisfaction with the treatment, their ability to be discharged from hospital, and to work for a living, were reported in only a small minority of studies. The typically reported outcomes were heterogeneous rating scales. The non‐reporting of participant‐oriented data and the reporting of heterogeneous scales of sometimes questionable validity are major obstacles when summing up data for assessment of clozapine effectiveness. There is a compelling need for an internationally agreed set of standardised outcomes for schizophrenia trials.
Data for assessing the effectiveness of clozapine in the long term are only beginning to accumulate. No good quality data are available for assessing the effectiveness of clozapine in special groups, such as those with learning disabilities. Also data on effects in elderly people are scarce, and further randomised studies are needed in this group of people.
2. Specific The large collection of trials reviewed here still leave many questions unanswered. Trials are not of high quality and are prone to considerable bias. Large pragmatic randomised, community‐based trials that measure global outcomes such as healthy days, social functioning, satisfaction with treatment, ability to live and work in the community, and compliance are required, especially focusing on those whose illness is clearly difficult to treat. One suggested design is presented in Table 8.
1. Suggested design for future study.
| Methods | Allocation: randomised, clearly described. Blinding: double, tested. Duration: one year. |
| Participants | Diagnosis: schizophrenia. N=400‐500.* Age: adults. Sex: both. History: severely ill, no clear response to at least two antipsychotic drugs given continuously in adequate doses for at least 12 weeks. |
| Interventions | 1. Clozapine: dose flexible within recommended limits. N=200. 2. Typical drug not before given to patient: dose flexible within recommended limits. N=200 |
| Outcomes | Death. Adverse effects: list, including weight change, hypersalivation, blood dyscrasia. Service outcomes: admitted, ready for discharge. Social functioning: working, trouble with family, trouble with police. Satisfaction with treatment: binary outcome, family, clinician and patient. Healthy days. Compliance: attending follow up, taking medication, blood testing. |
| Notes | * Powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. |
Feedback
Results
Summary
The 'Results' section states there is no significant difference in weight gain between clozapine and typical neuroleptics. This is contrary to the meta‐analysis ('Adverse effects 5. Weight gain') which reveals a significant difference.
Reply
The weight gain data from four trials are heterogeneous. Thus we have employed random‐effects statistics to include between‐study variance in the pooled estimate. The pooled estimate and its 95% confidence interval using random‐effects odds ratio is 1.07 (CI 0.37 to 3.10), which is not a significant difference. Due to the heterogeneity it would be incorrect to apply the Peto odds ratio (which the author of the comment appears to have done).
Contributors
Comment received from JM van Bruggen, Amsterdam. Reply from Kristian Wahlbeck, Helsinki, October 1999
What's new
| Date | Event | Description |
|---|---|---|
| 16 March 2009 | New search has been performed | Inclusion of 11 new Chinese studies |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 29 July 2008 | Amended | Converted to new review format. |
| 24 June 2008 | New citation required and conclusions have changed | Substantive amendment |
Notes
Cochrane Schizophrenia Group internal peer review complete (see Module). External peer review complete.
Acknowledgements
We would like to thank the substantial contribution of Kristian Wahlbeck and Maxim V Chein to the earlier versions of this review.
Also, the staff at the Cochrane Schizophrenia Group (CSG) in Nottingham, UK, and Dr Paul White, Brisbane, Australia, are acknowledged for all their practical help and spiritual support during our review process. Dr Emtithal Rezk, Damascus, Syria, participated actively in trial searches for the first version of this review. Librarian Anja Roilas at the Department of Psychiatry, University of Helsinki, Finland, is gratefully acknowledged for her enduring efforts to retrieve the full papers for the use of the reviewers. Jack Barkman and Outi Wilén of Novartis Finland Oy are acknowledged for providing us with information on published clozapine trials. Dr Michael Phillips, Beijing Hui Long Guan Hospital, Beijing, People's Republic of China and Dr Min Jiang, University of Turku, Turku, Finland, are acknowledged for helping us identify trials in Chinese language and Dr Katariina Lahtinen for translating the Czech trials. The following authors who provided us with further information on their trials, are acknowledged: Xi‐qing Bao, Jeffrey Bedwell/Sanjiv Kumra, Susan Essock, Freddy Howard/John M. Kane, Eckhardt Klieser, Ann Mortimer, and Robert Rosenheck. Fabio Lawson participated in the searches. Jun Xia translated Chinese papers.
Previous versions of this review (Essali 1997, Essali 1997 b, Essali 1998, Wahlbeck 1999 a, Wahlbeck 1999 b) have been supported by 1. Helsinki University Central Hospital (HUCH), Finland; 2. National Institute on Drug Abuse (NIDA), USA; 3. National Research and Development Centre for Welfare and Health (STAKES), Finland; 4. Medicinska Understödsföreningen Liv och Hälsa r.f., Helsinki, Finland; and 5. Finska Läkaresällskapet (Finnish Medical Society), Finland. This version of the review rests on the past considerable contribution of both authors and funders but did not receive support from these sources for the 2008 and 2009 updates.
Appendices
Appendix 1. Search strategies for earlier versions of this review
1. Electronic search strategies 1.1 Update of 2008 1.1 Cochrane Schizophrenia Group Trials Register (March 2006) We searched using the phrase:
[(clozapin* or clozaril* or leponex *) in REFERENCE TI/AB/IN fields and (clozapin* or clozaril* or leponex*) in STUDY Intervention fields]
1.2 Update of 2004 1.2.1 Cochrane Schizophrenia Group Trials Register (August 2003) We searched using the phrase:
[(clozapin* or clozaril* or leponex *) in REFERENCE TI/AB/IN fields and (clozapin* or clozaril* or leponex*) in STUDY Intervention fields]
1.2.2 LILACS (August 2003) We searched using the phrase:
[RANDOM$ or ALEATORI$ or CASUAL or ACASO or AZAR or SINGLE‐MASKED STUDY/ or DOUBLE‐MASKED STUDY/ or PROPHYLATIC CONTROLLED TRIALS/ or (PLACEBO$ and CONTROL$) or (CLINICAL$ and TRIAL$) or ((DUPLO or DOBLE or SIMPLE or TRIPLO or TRIPLE) and (CEGO or CIEGO)) or ((DOUBL$ or SINGL$ or TRIPL$ or TREBL$) and (BLIND$ or MASK$)) AND (clozapine or clozaril or leponex)]
1.3 Earliest versions of this review (Essali 1997 b, Wahlbeck 1999 b) 1.3.1 Cochrane Schizophrenia Group Trials Register (July 1999) We searched using the phrase:
[clozapine or clozaril or leponex or #42=28 ] (#42 is the intervention field of the register and 28 is the code for clozapine)
1.3.2 CENTRAL of The Cochrane Library (1998, Issue 2) We searched using the phrase:
[clozapi* or leponex or clozaril]
1.3.3 MEDLINE (January 1966 to June 1999) We searched using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and (clozapine or clozaril or leponex)]
1.3.4 EMBASE (January 1980 to July 1998) We searched using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and (clozapine or clozaril or leponex)]
1.3.5 BIOLOGICAL ABSTRACTS (January 1982 to July 1998) We searched using the Cochrane Schizophrenia Group's phrase for randomised controlled trials combined with the phrase:
[and (clozapine or clozaril or leponex)]
1.3.6 LILACS (January 1982 to July 1997) We searched using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and (clozapine or clozaril or leponex)]
1.3.7 PsycLIT (January 1974 to July 1998) We searched using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with the phrase:
[and (clozapine or clozaril or leponex)]
1.3.8 Science Citation Index (1998) We searched using the 27 included studies that were first identified from the earlier versions of this review (Wahlbeck 1999b). We inspected reports of articles that had cited these studies in order to identify further trials.
2. Searching other sources 2.1 References We searched the references of all identified studies for more studies.
2.2 Personal contact We contacted the first author of each study published since 1980 for information regarding unpublished trials.
2.3 Drug company We contacted the manufacturer of clozapine (Novartis AG, Switzerland) for additional data.
Appendix 2. Methods of assessing risk of bias in past versions of the review
We assessed the methodological quality of included studies using the criteria described in the Cochrane Handbook (Higgins 2008). These criteria, which is based on the degree of allocation concealment. Poor concealment has been associated with overestimation of treatment effect (Schulz 1995). Category A includes studies in which allocation has been randomised and concealment is explicit. Category B studies are those which have randomised allocation but in which concealment is not explicit. Category C studies are those in which allocation has neither been randomised nor concealed. Only trials that are stated to be randomised (categories A or B of the handbook) will be included in this review. The categories are defined below:
A. Low risk of bias (adequate allocation concealment). B. Moderate risk of bias (some doubt about the results). C. High risk of bias (inadequate allocation concealment).
Additionally, we assessed the methodological quality of included trials in this review using the Jadad Scale (Jadad 1996). The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scale includes three items:
A. Was the study described as randomised? B. Was the study described as double‐blind? C. Was there a description of withdrawals and drop outs?
Each item receives one point if the answer is positive. In addition, a point can be deducted if either the randomisation or the blinding/masking procedures described are inadequate. For this review we used a cut‐off of two points on the Jadad scale to check the assessment made by the handbook criteria. However, we did not use the Jadad Scale to exclude trials.
When disputes arose as to which category a trial should be allocated, again resolution was attempted by discussion. When this was not possible we did not enter the data and the trial was added to the list of those awaiting assessment until further information could be obtained.
Data and analyses
Comparison 1. CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 12 | 1243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.27] |
| 2 Relapse | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 short term | 19 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.84] |
| 2.2 long term | 4 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.14, 0.34] |
| 3 Global impression: 1. Not clinically improved | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 short term | 14 | 1119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.66, 0.79] |
| 3.2 long term | 3 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.88] |
| 4 Global impression: 2. Not ready for discharge | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 short term | 5 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 4.2 long term | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.62, 1.08] |
| 5 Hospitalisation: 1. Not discharged or readmitted within 1 year after discharge (long term) | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 6 Unable to work | 4 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.00] |
| 7 Participant dissatisfaction | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 short term | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.40, 1.30] |
| 7.2 long term | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.25, 0.82] |
| 8 Leaving the study early | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 short term | 32 | 2316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.97] |
| 8.2 long term | 6 | 982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.52, 0.69] |
| 9 Mental state: 1. Overall clinical symptoms | 22 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 short term (end point BPRS, low score = best) | 17 | 1205 | Mean Difference (IV, Fixed, 95% CI) | ‐3.79 [‐4.90, ‐2.68] |
| 9.2 long term (end point BPRS, low score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐5.70, 7.30] |
| 9.3 short term (endpoint PANSS, low score = best) | 3 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐3.82 [‐7.36, ‐0.28] |
| 9.4 long term (end point PANSS, low score = best) | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐6.90 [‐10.66, ‐3.14] |
| 10 Mental state 2. Negative symptoms | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 short term (SANS scale, low score = best) | 5 | 215 | Mean Difference (IV, Fixed, 95% CI) | ‐7.12 [‐8.78, ‐5.46] |
| 10.2 long term (PANSS negative symptoms, low score = best) | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐6.63, 4.83] |
| 11 Mental state 3: Positive symptoms | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 short term (end point SAPS low score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.39 [‐12.15, 20.93] |
| 11.2 long term (end point PANSS positive symptoms, low score = best) | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐3.27, ‐1.13] |
| 12 Cognitive functioning: impairment ‐short term (SKT) | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.34, 0.92] |
| 13 Cognitive functioning: Various scales | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Mini mental state ‐medium term (MMSE, high score = best) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.70, 0.90] |
| 13.2 psychomotor speed and attention ‐short term (DSST, high score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.18, 2.62] |
| 13.3 psychomotor speed and attention ‐medium term (DSST, high score = best) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.01, 2.59] |
| 13.4 psychomotor speed and attention ‐long term (DSST, high score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.79, 3.41] |
| 13.5 verbal working memory ‐short term (CTT, high score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐5.80, 2.60] |
| 13.6 verbal working memory ‐medium term (CTT, high score = best) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐4.43, 4.43] |
| 13.7 verbal working memory ‐long term (CTT, high score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐6.81, 2.21] |
| 13.8 verbal fluency ‐short term (CIGT, high score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐3.86, 11.46] |
| 13.9 verbal fluency ‐medium term (CIGT, high score = best) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐4.99, 11.19] |
| 13.10 verbal fluency ‐long term (CIGT, high score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐6.35, 10.15] |
| 13.11 verbal fluency ‐short term (CWAT, high score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.40 [‐2.36, 11.16] |
| 13.12 verbal fluency ‐medium term (CWAT, high score = best) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 6.5 [‐0.64, 13.64] |
| 13.13 verbal fluency ‐long term (CWAT, high score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐1.08, 13.48] |
| 13.14 immediate recall memory ‐short term (VLL‐IR, high score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐2.10, 0.70] |
| 13.15 immediate recall memory ‐medium term (VLL‐IR, high score = best) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.98, 0.98] |
| 13.16 immediate recall memory ‐long term (VLL‐IR, high score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.31, 1.71] |
| 13.17 delayed recall memory ‐short term (VLL‐DR, high score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.42, 1.22] |
| 13.18 delayed recall memory ‐medium term (VLL‐DR, high score = best) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.72, 2.12] |
| 13.19 delayed recall memory ‐long term (VLL‐DR, high score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.56, 1.36] |
| 13.20 executive functions ‐short term (WISC‐R‐Maze, high score = best) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.58, 0.38] |
| 13.21 executive functions ‐medium term (WISC‐R‐Maze, high score = best) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐0.99, 3.19] |
| 13.22 executive functions ‐long term (WISC‐R‐Maze, high score = best) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.93, 2.33] |
| 14 Behaviour: 1. No change/deterioration ‐ short term (NOSIE) | 2 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.90] |
| 15 Adverse effects: 1. Blood problems | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Blood abnormal | 13 | 1031 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.09 [1.96, 25.62] |
| 15.2 Blood problems ‐ long term | 2 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.66, 2.79] |
| 15.3 Abnormal ESR | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.78 [2.78, 41.85] |
| 15.4 White blood cell count increase | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.02 [2.59, 65.51] |
| 16 Adverse effects: 2. Drowsiness | 16 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.13, 1.34] |
| 17 Adverse effects: 3. Low blood pressure /dizziness | 14 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.98, 1.29] |
| 18 Adverse effects: 4. Salivation | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 too much | 17 | 1479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.96, 2.58] |
| 18.2 too little | 9 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.28, 0.52] |
| 19 Adverse effects: 5a. Weight gain | 5 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.07, 1.53] |
| 20 Adverse effects: 5b.Weight gain | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐3.12, 2.78] |
| 21 Adverse effects: 6. Movement disorder | 19 | 1495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.50, 0.65] |
| 22 Adverse effects: 7. Fits | 9 | 1157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.82, 2.78] |
| 23 Adverse effects: 8. High temperature | 9 | 1147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.25, 1.98] |
| 24 Adverse effects: 9. OGTT | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.16, 0.76] |
| 25 Adverse effects: 10. Fasting blood sugar (high score = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 25.1 Baseline | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 25.2 Short‐term, 12 weeks | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.45, 0.45] |
| 25.3 long‐term, 26 weeks | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.41, 1.59] |
| 25.4 Long‐term, 52 weeks | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.82, 0.82] |
| 26 Adverse effects: 11. Blood suger (high score = worse) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [0.66, 1.50] |
| 27 Adverse effects: 12. Cardiovacular | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.74, 9.61] |
| 27.1 tachycardia | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.62, 40.28] |
| 27.2 abnormal ECG | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.34] |
| 28 Adverse effects: 13. TESS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.93, 0.13] |
1.1. Analysis.
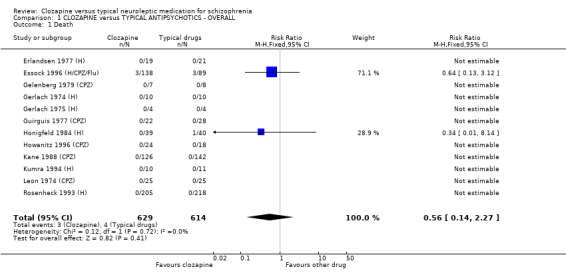
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 1 Death.
1.2. Analysis.
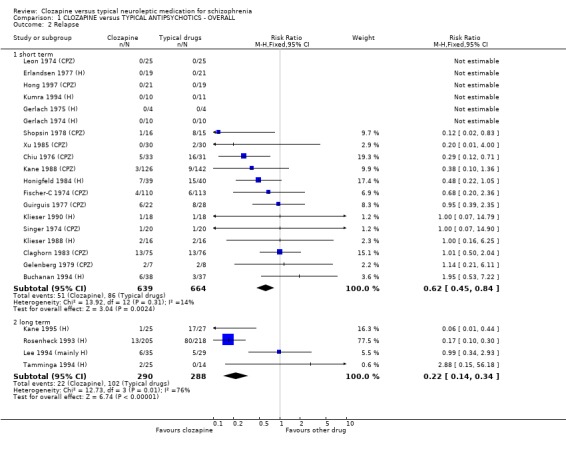
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 2 Relapse.
1.3. Analysis.
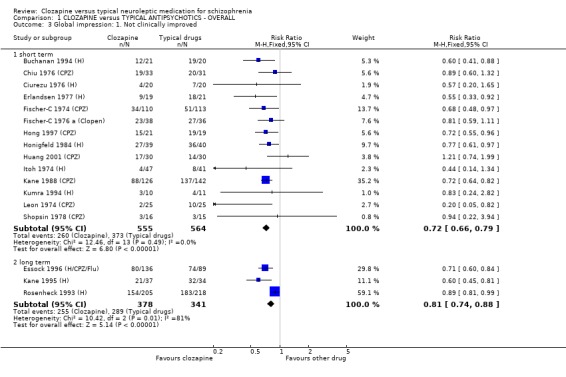
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 3 Global impression: 1. Not clinically improved.
1.4. Analysis.
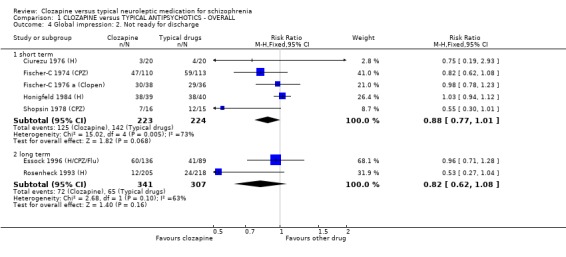
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 4 Global impression: 2. Not ready for discharge.
1.5. Analysis.
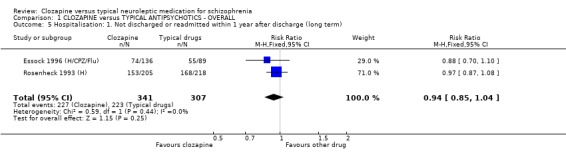
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 5 Hospitalisation: 1. Not discharged or readmitted within 1 year after discharge (long term).
1.6. Analysis.
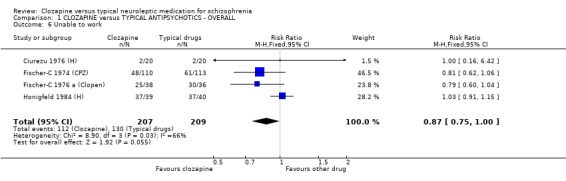
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 6 Unable to work.
1.7. Analysis.
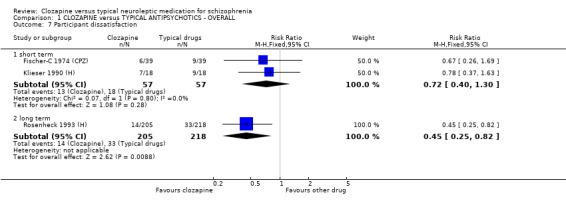
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 7 Participant dissatisfaction.
1.8. Analysis.
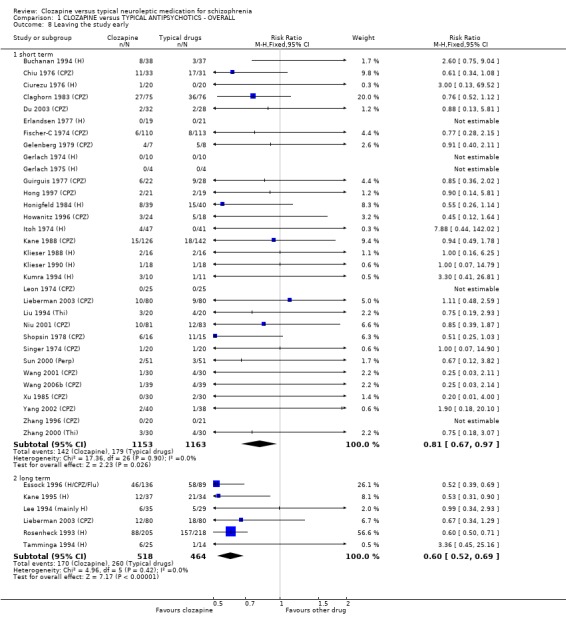
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 8 Leaving the study early.
1.9. Analysis.
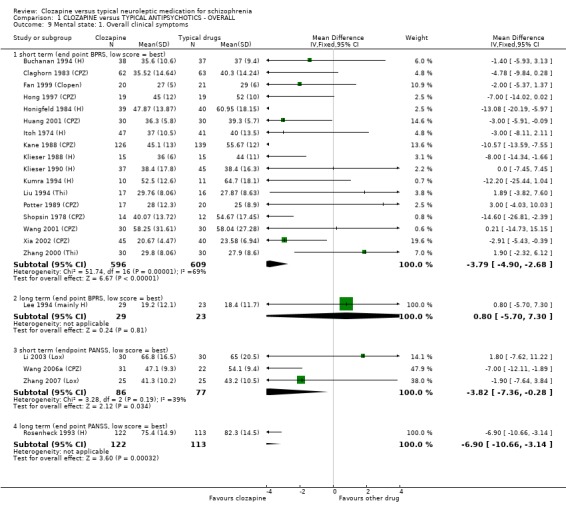
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 9 Mental state: 1. Overall clinical symptoms.
1.10. Analysis.
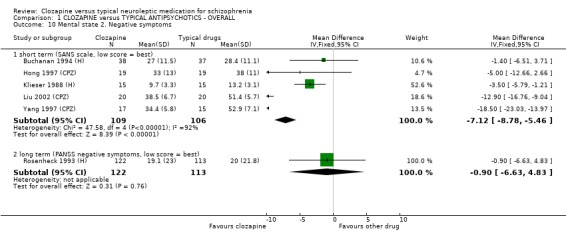
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 10 Mental state 2. Negative symptoms.
1.11. Analysis.
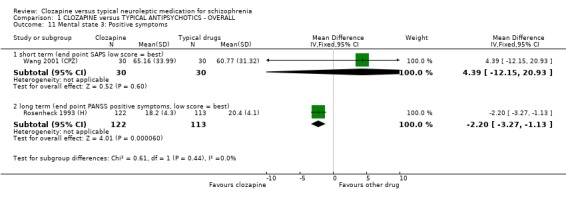
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 11 Mental state 3: Positive symptoms.
1.12. Analysis.
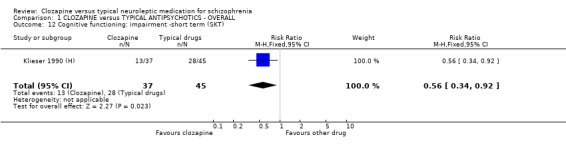
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 12 Cognitive functioning: impairment ‐short term (SKT).
1.13. Analysis.
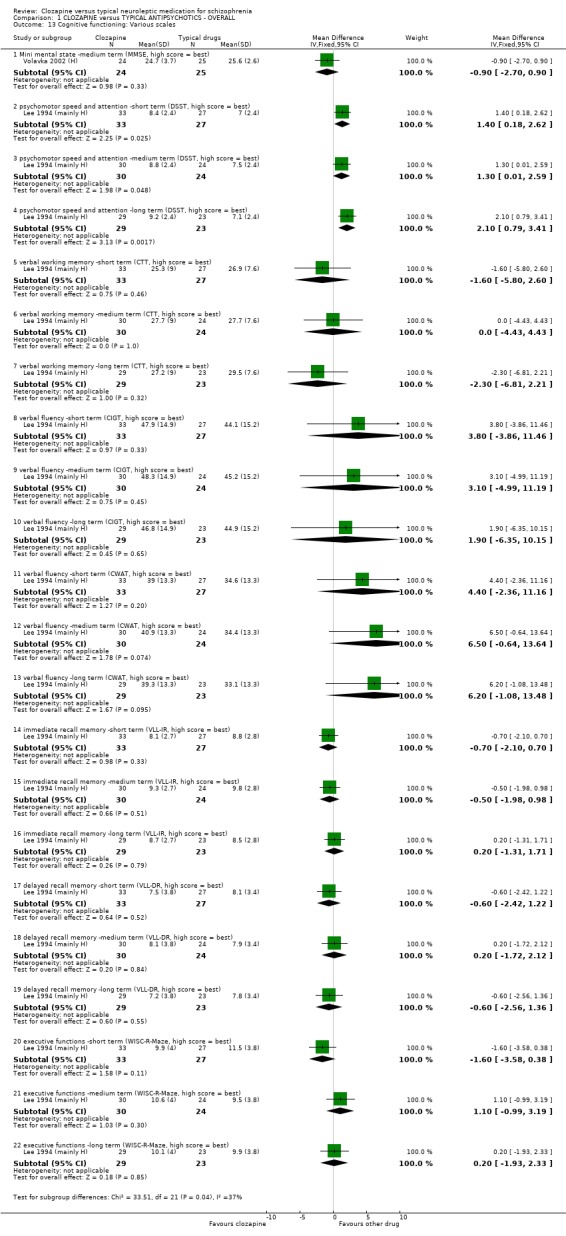
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 13 Cognitive functioning: Various scales.
1.14. Analysis.
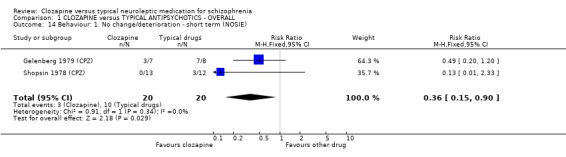
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 14 Behaviour: 1. No change/deterioration ‐ short term (NOSIE).
1.15. Analysis.
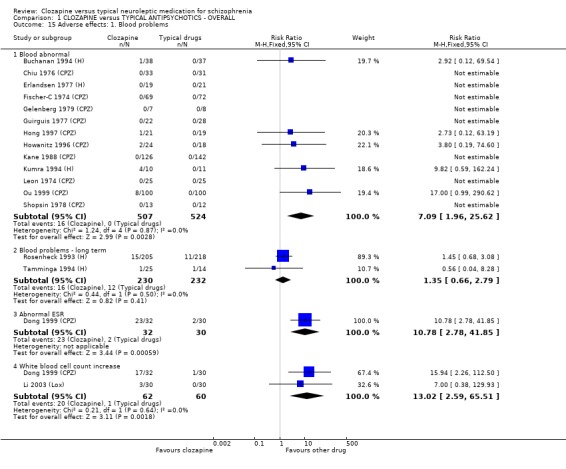
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 15 Adverse effects: 1. Blood problems.
1.16. Analysis.
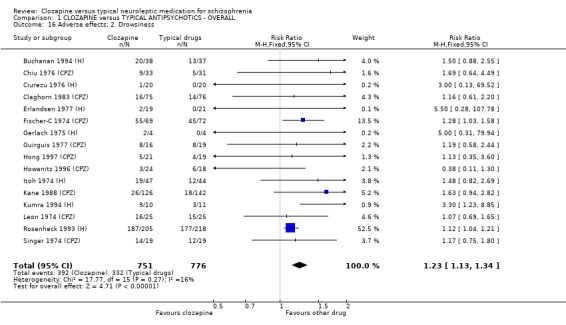
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 16 Adverse effects: 2. Drowsiness.
1.17. Analysis.
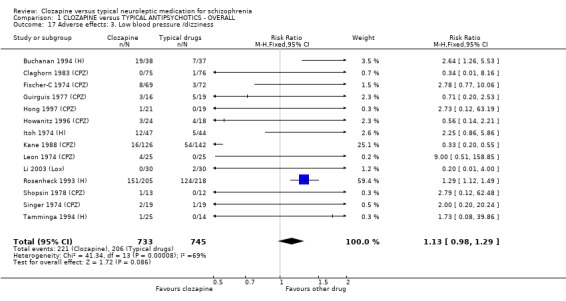
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 17 Adverse effects: 3. Low blood pressure /dizziness.
1.18. Analysis.
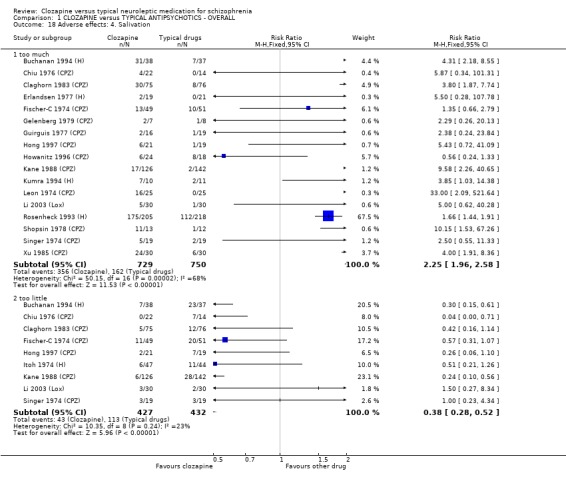
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 18 Adverse effects: 4. Salivation.
1.19. Analysis.
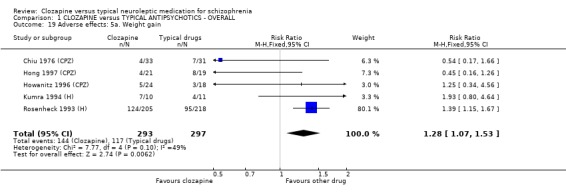
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 19 Adverse effects: 5a. Weight gain.
1.20. Analysis.
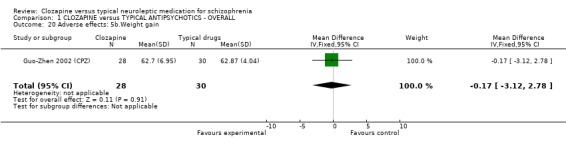
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 20 Adverse effects: 5b.Weight gain.
1.21. Analysis.
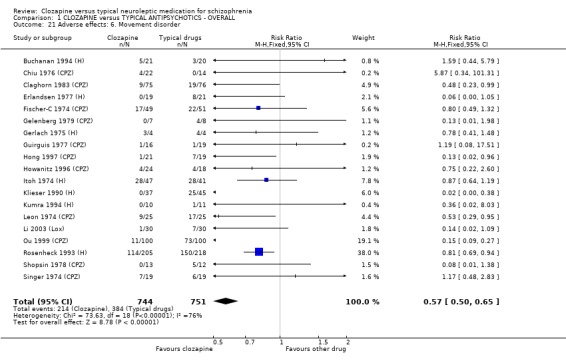
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 21 Adverse effects: 6. Movement disorder.
1.22. Analysis.
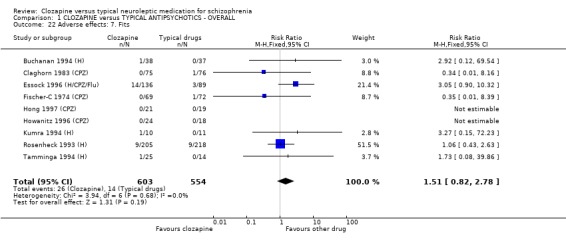
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 22 Adverse effects: 7. Fits.
1.23. Analysis.
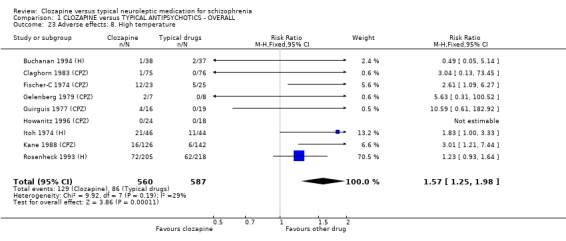
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 23 Adverse effects: 8. High temperature.
1.24. Analysis.
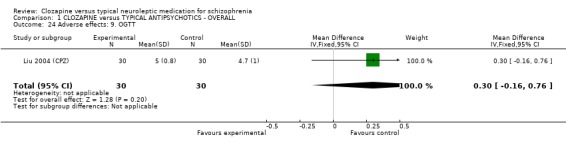
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 24 Adverse effects: 9. OGTT.
1.25. Analysis.
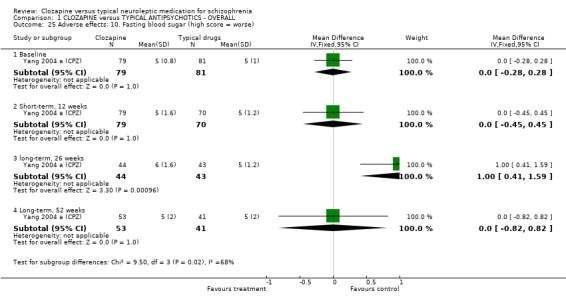
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 25 Adverse effects: 10. Fasting blood sugar (high score = worse).
1.26. Analysis.
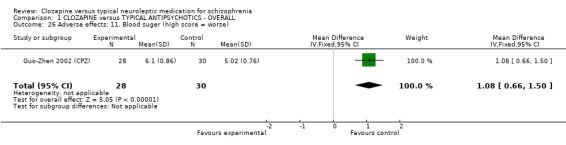
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 26 Adverse effects: 11. Blood suger (high score = worse).
1.27. Analysis.
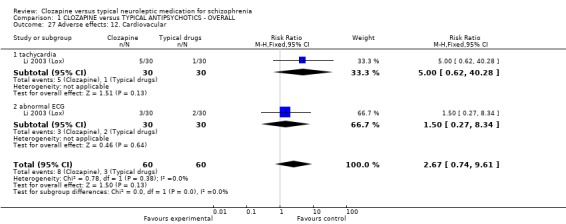
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 27 Adverse effects: 12. Cardiovacular.
1.28. Analysis.
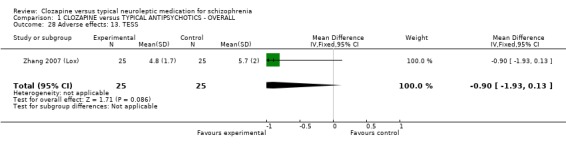
Comparison 1 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ OVERALL, Outcome 28 Adverse effects: 13. TESS.
Comparison 2. CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.13, 3.12] |
| 2 Relapse | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 short term | 4 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.61, 1.78] |
| 2.2 long term | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.10, 0.30] |
| 3 Global impression: 1. Not clinically improved | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 short term | 4 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.64, 0.79] |
| 3.2 long term | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.76, 0.91] |
| 4 Global impression: 2. Not ready for discharge ‐ long term | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.62, 1.08] |
| 5 Hospitalisation: 1. Not discharged or readmitted within 1 year after discharge (long term) | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 6 Leaving the study early | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 short term | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.94] |
| 6.2 long term | 2 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.49, 0.66] |
| 7 Participant dissatisfaction ‐ long term | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.25, 0.82] |
| 8 Mental state: 1. Various scales | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 short term (end point BPRS, low score = best) | 5 | 429 | Mean Difference (IV, Fixed, 95% CI) | ‐7.83 [‐10.01, ‐5.64] |
| 8.2 long term (end point PANSS, low score = best) | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐6.90 [‐10.66, ‐3.14] |
| 8.3 medium term, end point (PANSS‐total, low score = best) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐5.05, 9.45] |
| 8.4 medium term, end point (PANSS‐negative symptoms, low score = best) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.46, 3.26] |
| 8.5 medium term, end point (PANSS‐positive symptoms, low score = best) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.44, 3.64] |
| 9 Mental state: 2. Negative symptoms ‐ short term (low score = best) | 4 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.75, ‐0.13] |
| 10 Adverse effects 1. Blood problems | 5 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.97, 3.71] |
| 11 Adverse effects 2. Drowsiness | 5 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.11, 1.34] |
| 12 Adverse effects 3. Low blood pressure /dizziness | 4 | 806 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.24] |
| 13 Adverse effects 4. Salivation | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 too much | 5 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.74, 2.32] |
| 13.2 too little | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.16, 0.45] |
| 14 Adverse effects 5. Weight gain | 3 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.11, 1.59] |
| 15 Adverse effects 6. Movement disorder | 4 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.90] |
| 16 Adverse effects 7. High temperature | 3 | 766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.04, 1.77] |
| 17 Adverse effects 8. Fits | 5 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.90, 3.43] |
2.1. Analysis.
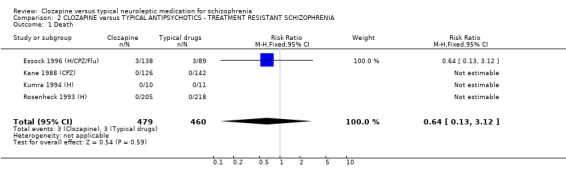
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 1 Death.
2.2. Analysis.
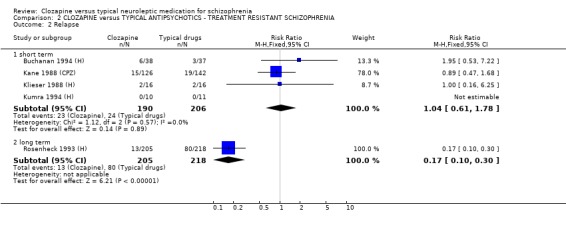
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 2 Relapse.
2.3. Analysis.
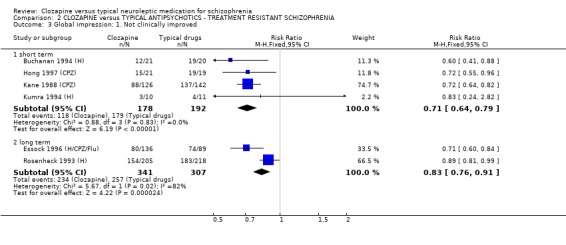
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 3 Global impression: 1. Not clinically improved.
2.4. Analysis.
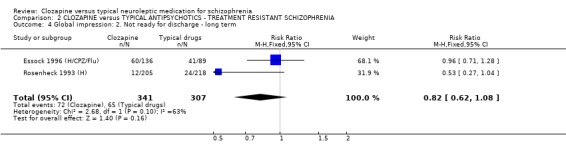
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 4 Global impression: 2. Not ready for discharge ‐ long term.
2.5. Analysis.
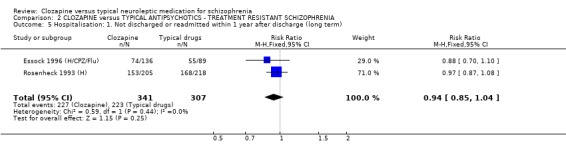
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 5 Hospitalisation: 1. Not discharged or readmitted within 1 year after discharge (long term).
2.6. Analysis.
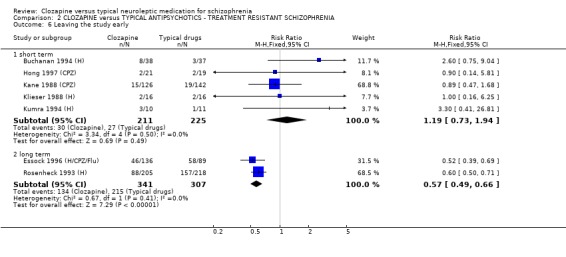
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 6 Leaving the study early.
2.7. Analysis.
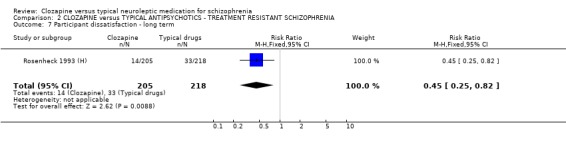
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 7 Participant dissatisfaction ‐ long term.
2.8. Analysis.
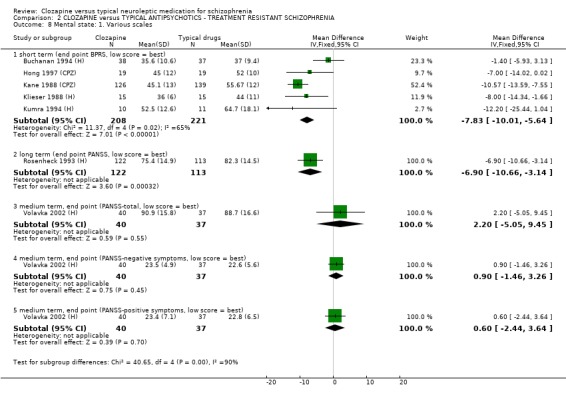
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 8 Mental state: 1. Various scales.
2.9. Analysis.
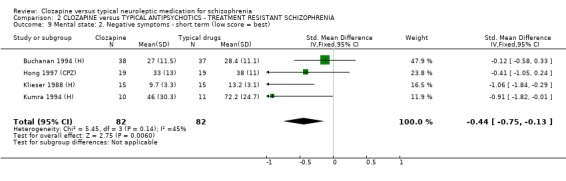
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 9 Mental state: 2. Negative symptoms ‐ short term (low score = best).
2.10. Analysis.
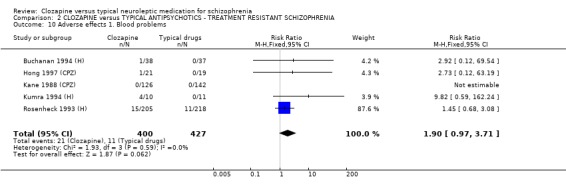
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 10 Adverse effects 1. Blood problems.
2.11. Analysis.
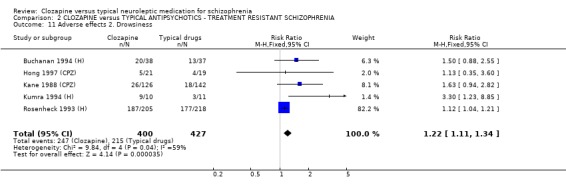
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 11 Adverse effects 2. Drowsiness.
2.12. Analysis.
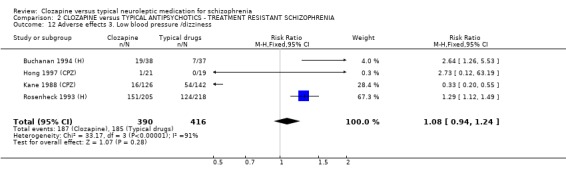
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 12 Adverse effects 3. Low blood pressure /dizziness.
2.13. Analysis.
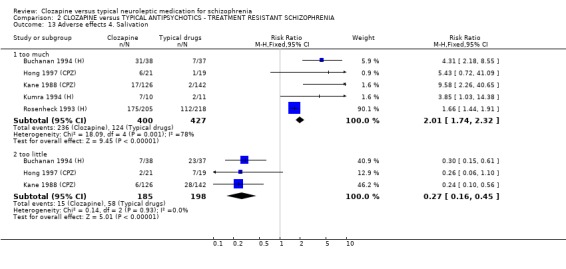
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 13 Adverse effects 4. Salivation.
2.14. Analysis.
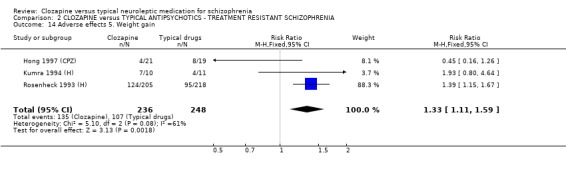
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 14 Adverse effects 5. Weight gain.
2.15. Analysis.
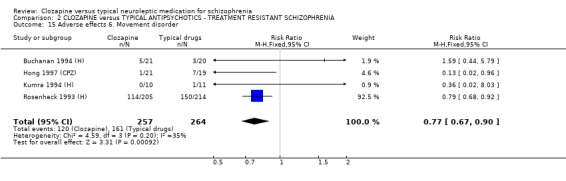
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 15 Adverse effects 6. Movement disorder.
2.16. Analysis.
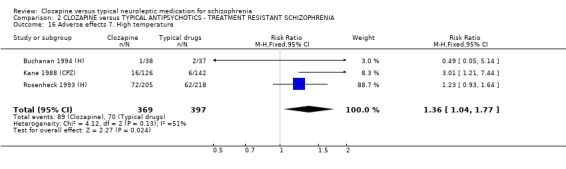
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 16 Adverse effects 7. High temperature.
2.17. Analysis.
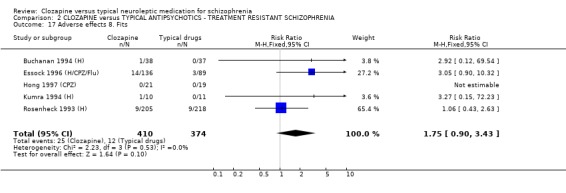
Comparison 2 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ TREATMENT RESISTANT SCHIZOPHRENIA, Outcome 17 Adverse effects 8. Fits.
Comparison 3. CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Relapse | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Global impression: 1. Not clinically improved | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.82] |
| 4 Leaving the study early | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.3 [0.41, 26.81] |
| 5 Mental state: 1. End point BPRS (low score = best) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐12.20 [‐25.44, 1.04] |
| 6 Mental state: 2. Negative symptoms (end point SANS, low score = best) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐26.20 [‐49.99, ‐2.41] |
| 7 Adverse effects: 1. Blood problems | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.82 [0.59, 162.24] |
| 8 Adverse effects: 2. Drowsiness | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.3 [1.23, 8.85] |
| 9 Adverse effects: 3. Too much salivation | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [1.03, 14.38] |
| 10 Adverse effects: 4. Weight gain | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.80, 4.64] |
| 11 Adverse effects: 5. Movement disorder | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.03] |
| 12 Adverse effects: 6. Fits | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.15, 72.23] |
3.1. Analysis.
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 1 Death.
3.2. Analysis.
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 2 Relapse.
3.3. Analysis.
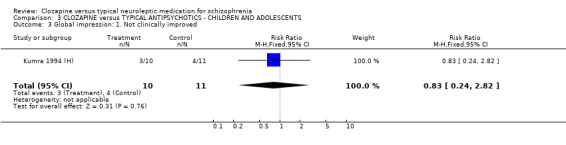
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 3 Global impression: 1. Not clinically improved.
3.4. Analysis.
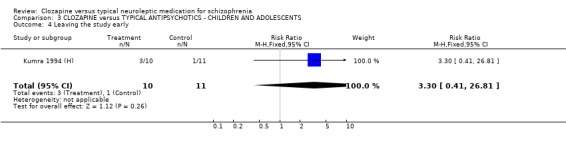
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 4 Leaving the study early.
3.5. Analysis.
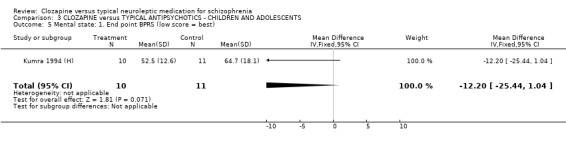
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 5 Mental state: 1. End point BPRS (low score = best).
3.6. Analysis.
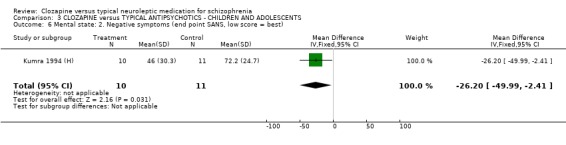
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 6 Mental state: 2. Negative symptoms (end point SANS, low score = best).
3.7. Analysis.
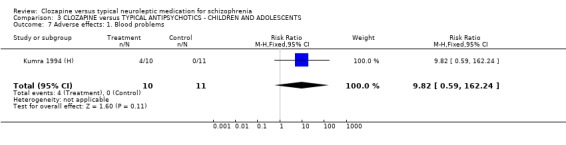
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 7 Adverse effects: 1. Blood problems.
3.8. Analysis.
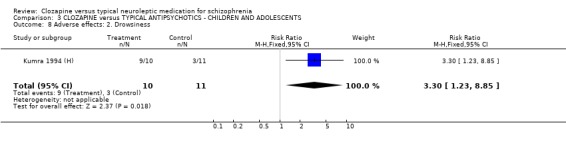
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 8 Adverse effects: 2. Drowsiness.
3.9. Analysis.
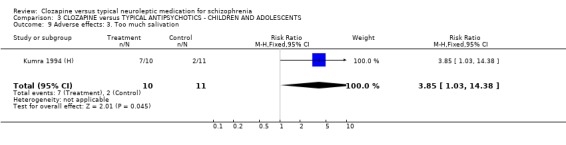
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 9 Adverse effects: 3. Too much salivation.
3.10. Analysis.
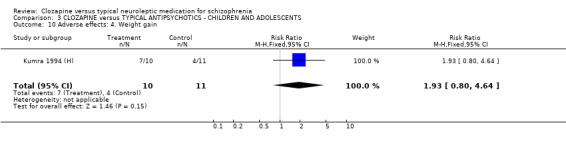
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 10 Adverse effects: 4. Weight gain.
3.11. Analysis.
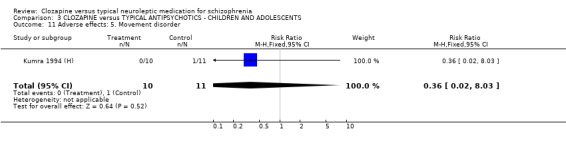
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 11 Adverse effects: 5. Movement disorder.
3.12. Analysis.
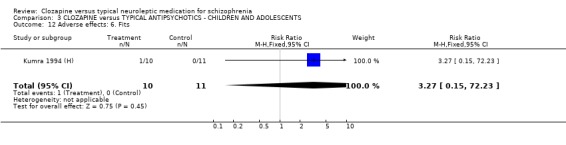
Comparison 3 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ CHILDREN AND ADOLESCENTS, Outcome 12 Adverse effects: 6. Fits.
Comparison 4. CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Leaving the study early | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.64] |
| 3 Adverse effects: 1. Blood problems | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.8 [0.19, 74.60] |
| 4 Adverse effects: 2. Drowsiness | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.30] |
| 5 Adverse effects: 3. Low blood pressure /dizziness | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.21] |
| 6 Adverse effects: 4. Too much salivation | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.33] |
| 7 Adverse effects: 5. Weight gain | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.56] |
| 8 Adverse effects: 6. Movement disorder | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.22, 2.60] |
| 9 Adverse effects: 7. Fits | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse effects 8. High temperature | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
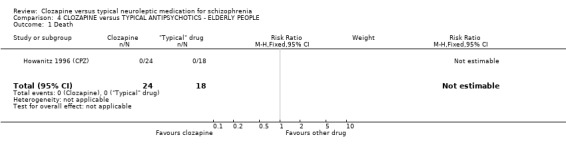
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 1 Death.
4.2. Analysis.
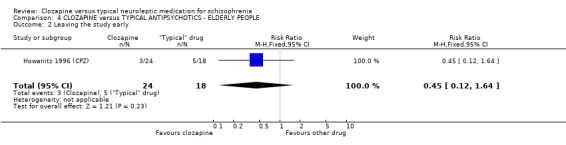
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 2 Leaving the study early.
4.3. Analysis.
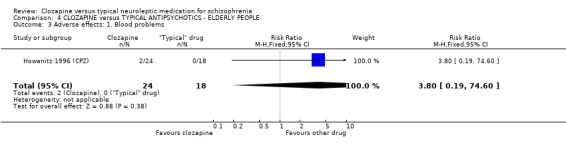
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 3 Adverse effects: 1. Blood problems.
4.4. Analysis.
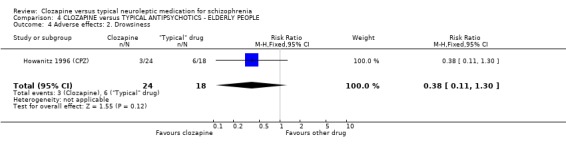
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 4 Adverse effects: 2. Drowsiness.
4.5. Analysis.
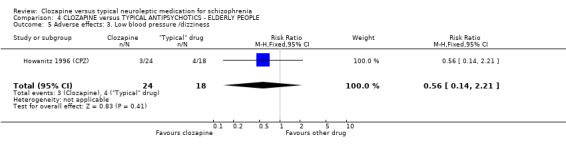
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 5 Adverse effects: 3. Low blood pressure /dizziness.
4.6. Analysis.
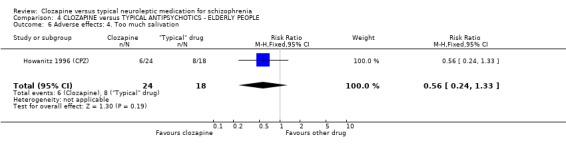
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 6 Adverse effects: 4. Too much salivation.
4.7. Analysis.
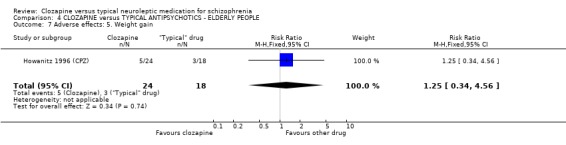
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 7 Adverse effects: 5. Weight gain.
4.8. Analysis.
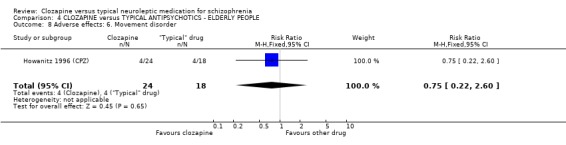
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 8 Adverse effects: 6. Movement disorder.
4.9. Analysis.
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 9 Adverse effects: 7. Fits.
4.10. Analysis.
Comparison 4 CLOZAPINE versus TYPICAL ANTIPSYCHOTICS ‐ ELDERLY PEOPLE, Outcome 10 Adverse effects 8. High temperature.
Comparison 5. SENSITIVITY ANALYSIS ‐ CHINESE TRIALS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 short term | 21 | 1533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.68, 1.03] |
| 1.2 Chinese trials | 5 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.27, 1.39] |
| 2 Mental state: 1. Overall clinical symptoms | 16 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 short term (end point BPRS, low score = best) | 10 | 828 | Mean Difference (IV, Fixed, 95% CI) | ‐6.32 [‐8.06, ‐4.58] |
| 2.2 Chinese trials | 6 | 317 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐4.10, ‐1.01] |
5.1. Analysis.
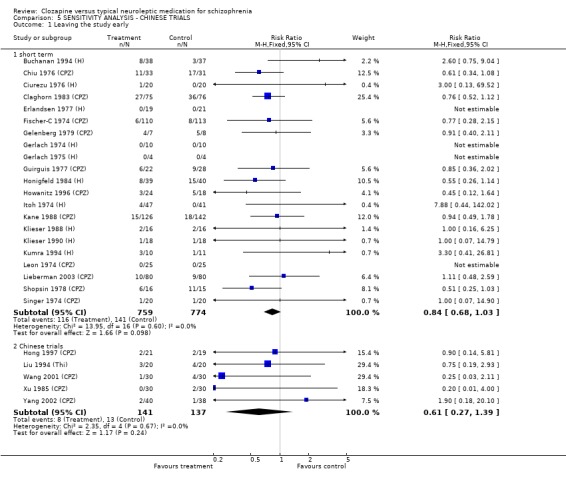
Comparison 5 SENSITIVITY ANALYSIS ‐ CHINESE TRIALS, Outcome 1 Leaving the study early.
5.2. Analysis.
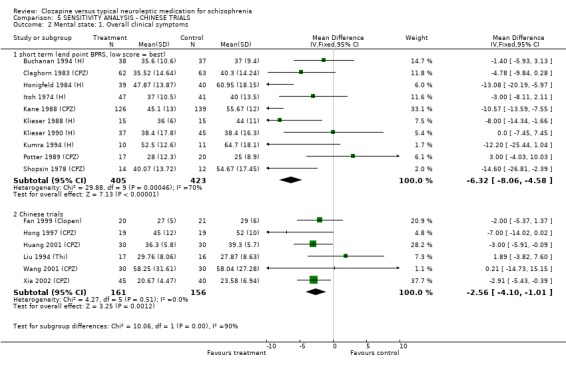
Comparison 5 SENSITIVITY ANALYSIS ‐ CHINESE TRIALS, Outcome 2 Mental state: 1. Overall clinical symptoms.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Buchanan 1994 (H).
| Methods | Allocation: randomised. Blindness: double. Duration: 10 weeks (no wash‐out). Setting: outpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R & SCID). N=75. Sex: 23 F, 52 M. Age: 18‐55 years, mean 35 years. History: non‐complete response to at least two trials of therapeutic doses of neuroleptics for at least six weeks; less than 30% improvement in prospective six week trial of fluphenazine 10 to 30 mg/day; chronic illness. | |
| Interventions | 1. Clozapine: dose increased to 400 mg/day weeks one to four, 200 to 600 mg/day weeks five to six, fixed dose weeks seven to ten, average dose at study end 413 ±SD 60 mg/day + placebo. N=38. 2. Haloperidol: dose increased to 20 mg/day weeks one to four, 10‐30 mg/day weeks five to six, fixed dose weeks seven to ten, average dose at study end 26 ±SD 7 mg/day + benztropine 4 mg/day. N=37. | |
| Outcomes | Relapse.
Leaving the study early.
Mental state: 18‐item BPRS, SANS.
Quality of life: QOLS.
Global functioning: Level of Functioning Scale.
Adverse effects: SAI, Maryland Psychiatric Research Centre Involuntary Movement Scale.
Compliance. Unable to use ‐ Clinical improvement: 20% reduction in BPRS (data not reported). |
|
| Notes | Jadad score 4. Drop‐outs (N=2) excluded from results in original report have been included in present meta‐analysis. Benztropine medication in group two may have affected results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | High risk | Reasons for loss to follow up not described by group |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Low risk | Grant from NIMH; medicines supplied by Sandoz Pharmaceutical Corp |
Chiu 1976 (CPZ).
| Methods | Allocation: randomised. Blindness: double (medication in identical capsules). Duration: six weeks (preceded by five day washout). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (no diagnostic criteria). N=64. Sex: not reported. Age: <60 years. History: acutely ill. | |
| Interventions | 1. Clozapine: dose initially 150 mg/day, increased by 50 mg/day to 300 mg/day. N=33. 2. Chlorpromazine: dose initially 150 mg/day, increased by 50 mg/day to 300 mg/day. N=31. | |
| Outcomes | Relapse.
Leaving the study early.
Behaviour: NOSIE.
Adverse effects.
Laboratory tests. Unable to use ‐ Mental state: BPRS (mean total scores not reported). Global effect: CGI (mean total scores not reported). |
|
| Notes | Jadad score 4. A matching procedure may have resulted in a selection bias in the outcomes presented in the original paper. High drop‐out rate. Equal mg‐doses may have benefited clozapine outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, identical capsules, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Withdrawn participants accounted for, with reasons for their withdrawal reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Sandoz Pharmaceuticals provided material support |
Ciurezu 1976 (H).
| Methods | Allocation: randomised. Blindness: double (medication in identical preparations). Duration: 40 days. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (paranoid, n=16, simple n=11, hebephrenic n=11 & other n=2, no diagnostic criteria). N=40. Sex: 25 F, 15 M. Age: range 16 to 45 years, average 25 years. History: not reported. | |
| Interventions | 1. Clozapine: dose average 402 mg/day, range 100 to 900 mg/day. N=20. 2. Haloperidol: dose average 9 mg/day, range 4 to 20 mg/day. N=20. | |
| Outcomes | Global effect: ability to work.
Adverse effects.
Leaving the study early.
Discharge‐ability. Unable to use ‐ Mental state: BPRS (no data reported). |
|
| Notes | Jadad score 4. Low haloperidol doses may not have been comparable to clozapine doses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants reported in outcome data |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Sandoz Pharmaceuticals employee involved with administration of results |
Claghorn 1983 (CPZ).
| Methods | Allocation: randomised. Blindness: double (identical tablets). Design: multi‐centre. Duration: four to eight weeks (preceded by two weeks wash‐out). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐II). N=151. Sex: 59 F, 92 M. Age: 18 to 65 years, median 30 years. History: intolerant to at least two prior neuroleptics. | |
| Interventions | 1. Clozapine: dose initially 25 mg/day; one‐week build‐up to 300 mg/day; days 8 to 28 150 to 900 mg/day, average 417 mg/day. N=75. 2. Chlorpromazine: dose initially 50 mg/day; one‐week increased to 600 mg/day; days 8 to 28, 300 to 1800 mg/day, average 795 mg/day. N=76. | |
| Outcomes | Relapse. Global effect: CGI. Leaving the study early. Mental state:18‐item BPRS. Behaviour: 30‐item NOSIE. Adverse effects: AIMS, SAS (not blind). | |
| Notes | Jadad score 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants not completing trial were accounted for, reasons for attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Dong 1999 (CPZ).
| Methods | Allocation: randomised. Blindness: not stated. Duration: five weeks. Setting: not reported. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2R). N=62. Sex: 35 F, 27 M. Age: mean 32 yrs. History: illness 10 yrs (SD 8), ESR (male 0˜15 mm/hour; female 0˜20 mm/hour), WBC: 4*109/L˜10*109/L. | |
| Interventions | 1. Clozapine: dose 225 to 500 mg/day. N=32. 2. Chlorpromazine: dose 400 to 700 mg/day. N=30. | |
| Outcomes | Clinical tests: ESR, WBC. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | High risk | Only raised white blood cells reported |
| Free of other bias? | Unclear risk | Unclear |
Du 2003 (CPZ).
| Methods | Allocation: randomised ‐ no further details. Blindness: not reported. Duration: 12 weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N=81. Sex: male and female. Age: 18 to 45 years. Excluded: organic psychotic patients; allergic to experiment drugs; with severe physical illness; pregnant or breast feeding women. | |
| Interventions | 1. Clozapine: dose not reported. N=32. 2. Chlorpromazine: dose not reported. N=28. 3. Risperidone: dose not reported. N=21 | |
| Outcomes | Leaving the study early. Unable to use ‐ Mental state: BPRS (group numbers not reported). Global state: GAS (group numbers not reported). Adverse events: TESS (group numbers not reported). |
|
| Notes | CSG: 8466 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Erlandsen 1977 (H).
| Methods | Allocation: randomised. Blindness: double. Duration: 40 days. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (no diagnostic criteria). N=40. Sex: male. Age: range 22 to 75 years, average 43 years. History: mean duration of illness 15 years. | |
| Interventions | 1. Clozapine: dose 50 to 400 mg/day. N=19. 2. Haloperidol: dose 1 to 8 mg/day. N=21. | |
| Outcomes | Leaving the study early.
Global assessment.
Laboratory tests. Unable to use ‐ Mental state: BPRS (no SDs). |
|
| Notes | Jadad score 1. No wash‐out period before trial reported. Low haloperidol doses may not have been comparable to clozapine doses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | High risk | Double blind, but investigators were able to guess correctly in all patients after two to three days, due to the sedating and relaxing effect of clozapine |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Essock 1996 (H/CPZ/Flu).
| Methods | Allocation: randomised. Blindness: not blind. Duration: 24 months. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia/schizoaffective disorder. N=227*. Age: mean 41 years. Sex: 90 F, 137 M. History: severely ill, unresponsive to two treatment trials/ unacceptable side effects with conventional neuroleptics. SCID interview performed on 173 participants. | |
| Interventions | 1. Clozapine: dose average 496 mg/day. N=136. 2. "Usual care": dose average chlorpromazine equivalents 1,386 mg/day. N=89. The most frequent control treatments were haloperidol, chlorpromazine and fluphenazine; atypical neuroleptics were used almost not at all. | |
| Outcomes | Leaving the study early. Mental state: BPRS. Quality of life: QOLI. Adverse effects: AIMS. Clinical improvement: at least 20% improvement in BPRS total score or at least 20% improvement in BPRS psychotic item subscale. Discharge. Readmission. | |
| Notes | *Two participants not accounted for. Jadad score 2 Two people randomised to clozapine did not begin the trial. During trial some patients in usual care group began clozapine treatment (10 people at 6 months, 59 people at 24 months). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | High risk | Open label |
| Incomplete outcome data addressed? All outcomes | High risk | Reasons for loss to follow up not described |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Low risk | Grant from NIHM |
Fan 1999 (Clopen).
| Methods | Allocation: randomised. Blindness: double, no further detail. Duration: six weeks. Setting: not reported. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N=41. Age: mean 28. Sex: not reported. History: naive antipsychotics; mean duration of illness ˜ 13 yrs. | |
| Interventions | 1. Clozapine: dose average 188 mg/day. N=20. 2. Clopenthixol: dose average 36 mg/day. N=21. | |
| Outcomes | Mental state: BPRS. Adverse effects: TESS, SAS, Myotonia, Akathisia, Tachycardia, Blood cell counting, Salivation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Two raters did not conduct treatment |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | No details |
Fischer‐C 1974 (CPZ).
| Methods | Allocation: randomised. Blindness: double (medication in identical capsules). Design: five multi‐centre (Czechoslovakia, Finland, Netherlands, Sweden, Switzerland). Duration: 40 days (preceded by washout of at least four days). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia ‐ paranoid (62%), moderate to severe (˜50%) symptoms (no diagnostic criteria). N=223. Sex: 67 F, 155 M (one sex unknown). Age: average 34 years, range 15‐68 years. History: not reported. | |
| Interventions | 1. Clozapine: dose initially 75 to 200 mg/day, average dose at study end 310 mg/day; range 50 to 1000 mg/day. N=110. 2. Chlorpromazine: dose initially 75 to 200 mg/day, average dose at study end 360 mg/day; range 25 to 900 mg/day. N=113. | |
| Outcomes | Global effect.
Leaving the study early.
Adverse effects: Sandoz Side Effect Check List. Unable to use ‐ Mental state: BPRS (no SD). |
|
| Notes | Jadad score 4. Data on patient dissatisfaction reported only in Czech and Swiss studies. Side effect frequency reports available only from Finnish (and partly Czech as well as Swedish) study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Fischer‐C 1976 a (Clopen).
| Methods | Allocation: randomised. Blindness: double (medication in identical capsules). Duration: 42 days (preceded by at least seven day washout) Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia ‐ moderate to severe (no diagnostic criteria). N=74. Sex: not reported. Age: not reported. History: not reported. | |
| Interventions | 1. Clozapine: dose initially 200 mg/day, median 300 mg/day. N=38. 2. Clopenthixol: dose initially 100 mg/day, median 100 mg/day. N=36. | |
| Outcomes | Global effect.
Adverse effects: Sandoz Side Effect Check List.
Laboratory tests and ECG. Unable to use ‐ Mental state: BPRS (no data reported). |
|
| Notes | Jadad score 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, identical capsules, untested |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Gelenberg 1979 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: four to eight weeks (preceded by >two day washout). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐II). N=15. Sex: 7 F, 8 M. Age: range 18 to 43 years, mean 30 years. History: not reported. | |
| Interventions | 1. Clozapine: dose initially 25 mg/day; average dose 279 mg/day, range 125 to 525 mg/day. N=7. 2. Chlorpromazine: dose initially 50 mg/day; average 606 mg/day, max 1050 mg/day. N=8. Amobarbital, chloral hydrate & paraldehyde as needed. | |
| Outcomes | Death.
Relapse.
Behaviour: NOSIE.
Leaving the study early.
Adverse effects: AIMS, SAI (not blind). Unable to use ‐ Mental state: BPRS (no SDs reported). |
|
| Notes | Jadad score 3. Trial terminated prematurely due to reports of clozapine‐related agranulocytosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | High risk | Attrition not fully reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Funded in part by Sandoz Pharmaceuticals |
Gerlach 1974 (H).
| Methods | Allocation: randomised. Blindness: varied with outcome. Design: cross‐over. Duration: 28 weeks; first arm 82 days (preceded by 5 to 51 day washout). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia ‐ paranoid (N=11), hebephrenic (N=7), catatonic (N=2). No diagnostic criteria. N=20. Sex: male. Age: range 18 to 60 years. History: illness duration 2 to 33 years. | |
| Interventions | 1. Clozapine: dose initially 50 mg/day, median at day 82, 200 mg/day; followed by haloperidol after a second wash‐out period. N=10.
2. Haloperidol: dose initially 1 mg/day, median at day 82, 10 mg/day; followed by clozapine after second wash‐out period. N=10. Procyclidine, biperiden & nitrazepam as needed. |
|
| Outcomes | Death.
Relapse.
Leaving the study early.
Global effect: non‐blind clinical evaluation.
Adverse effects: non‐blind check list.
ECG & laboratory tests. Unable to use ‐ Mental state: blind 18‐item BPRS (no SDs). |
|
| Notes | Jadad score 1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Participants aware of treatment; assessors were blind, untested |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Gerlach 1975 (H).
| Methods | Allocation: randomised. Blindness: double (medication in identical capsules). Design: cross‐over. Duration: nine weeks, first arm three weeks (preceded by three week wash‐out). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (no diagnostic criteria). N=8. Sex: male. Age: range 24 to 66 years. History: not reported. | |
| Interventions | 1. Clozapine: dose 225 mg/day; followed by haloperidol after second 21 day wash‐out. N=4. 2. Haloperidol: dose 9 mg/day; followed by clozapine after second 21 day wash‐out. N=4. Biperiden injections for acute dystonias. | |
| Outcomes | Death. Relapse. Leaving the study early. Adverse effects. Neurological condition (authors´ rating scale). | |
| Notes | Jadad score 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Support provided by Sandoz pharmaceuticals who supplied clozapine and Jansen Pharma who supplied haloperidol |
Guirguis 1977 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: seven weeks. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (no diagnostic criteria). N=50. Sex: 15 F, 35 M. Age: average 38.5 years. History: acute illness; age of onset clozapine group, 33 years; CPZ group, 26 years. | |
| Interventions | 1. Clozapine: dose range 75 to 450 mg/day. N=22. 2. Chlorpromazine: dose range 150 to 900 mg/day. N=28. | |
| Outcomes | Death.
Relapse.
Leaving the study early.
Adverse effects: checklist. Unable to use ‐ Mental state: BPRS (mean total scores not reported). Behaviour: NOSIE (mean total scores not reported). Global effect: CGI (no data). |
|
| Notes | Jadad score 3. The clozapine patients had a significantly higher age of onset, were significantly older and had almost significantly worse NOSIE ratings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | High risk | Reasons for attrition not described |
| Free of selective reporting? | High risk | Not all assessment scale data were reported |
| Free of other bias? | High risk | Funded by Sandoz Pharmaceuticals |
Guo‐Zhen 2002 (CPZ).
| Methods | Allocation: randomised. Blindness: not reported. Duration: eight weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N=84. Sex: male and female. Age: 19 to 38. History: length of illness 1.7± 1.5 years. | |
| Interventions | 1. Clozapine: dose 300 to 500 mg/d. N=28. 2. Chlorpromazine: dose 50 to 600 mg/d. N=30. 3. Risperidone: dose 1.5 to 5 mg/d. N=26. | |
| Outcomes | Physiological: weight change. | |
| Notes | CSG: 8389 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | High risk | Study attrition not reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Hong 1997 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: 12 weeks (preceded by a 60 mg/day haloperidol baseline period lasting up to six weeks). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=40. Sex: 26 F, 14 M. Age: average 38.5 years. History: treatment‐refractory*. | |
| Interventions | 1. Clozapine: dose initially 25 mg/day for one week, mean dose 543 mg/day, max dose 900 mg/day. N=21. 2. Chlorpromazine capsules: initial dose 50 mg/day for one week, mean dose 1163 mg/day, max dose 1800 mg/day. N=19. Fixed‐flexible dose schedule. | |
| Outcomes | Leaving the study early. Mental state: PANSS, BPRS. Global effect: CGI. Improvement: decrease at least 20% in BPRS total score. Adverse effects. | |
| Notes | Jadad score 5. *Treatment‐refractory=severe psychotic symptoms according to BPRS item scores for >six months despite treatment with neuroleptics from at least two different classes at dosages of at least 1000 mg chlorpromazine equivalents. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised ‐ by using a table of random numbers |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Single blinding, both medications were identical in appearance and package but two raters were also researchers, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Low risk | Reported all listed measures |
| Free of other bias? | Low risk | Grants from national science committee of ROC and hospital funds |
Honigfeld 1984 (H).
| Methods | Allocation: randomised. Blindness: double. Design: two centres. Duration: 40 days. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (no diagnostic criteria). N=79. Sex: not reported. Age: not reported. History: not reported. | |
| Interventions | 1. Clozapine: dose average 397 mg/day. N=39. 2. Haloperidol: dose average 7.6 mg/day. N=40. | |
| Outcomes | Death. Relapse. Global effect: ability to work. Leaving the study early. Mental state: BPRS. Discharge‐ability. | |
| Notes | Jadad score 2. Low haloperidol doses may not have been comparable to clozapine doses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Details of attrition described |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | No details |
Howanitz 1996 (CPZ).
| Methods | Allocation: randomised. Blindness: double (medication in identical capsules). Duration: 12 weeks (preceded by one to seven days wash‐out). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=42. Sex: 3 F, 39 M. Age: >55 years; average 66.75 years. History: average duration of illness ‐ 39 years; average length of hospitalisation ‐ 36.9 years; symptoms PANSS>60. | |
| Interventions | 1. Clozapine: dose initially 12.5 mg/day, max 300 mg/day. N=24. 2. Chlorpromazine: dose initially 25 mg/day, max 600 mg/day. N=18. Benztropine and chloral hydrate as needed. | |
| Outcomes | Adverse effects: AIMS.
Leaving the study early. Unable to use ‐ Global effect: CGI (participant numbers not reported). Mental state: PANSS (participant numbers not reported). |
|
| Notes | Jadad score 3. Trialists did not perform an intention‐to‐treat analysis with regard to efficacy. Eight patients were excluded from the efficacy analysis in the original paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Low risk | Randomised by hospital pharmacist under "double blind conditions" |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | High risk | Attrition not fully reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Huang 2001 (CPZ).
| Methods | Allocation: randomised. Blindness: not stated. Duration: eight weeks. Setting: not stated. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2R). N=90. Sex: not reported. Age: 22 yrs (SD5). History: average length of illness 10 months. | |
| Interventions | 1. Clozapine: dose 400 mg/day. N=30. 2. Chlorpromazine: dose 500 mg/day. N=30. 3. Risperidone: dose 4 to 6 mg/day. N=30. | |
| Outcomes | Mental state: BPRS. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Itoh 1974 (H).
| Methods | Allocation: randomised. Blindness: double. Design: multi‐centre. Duration: 12 weeks. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia ‐ hebephrenic (N=46), paranoid (N=22), undifferentiated (N=19), catatonic (N=4) (no diagnostic criteria). N=91. Age: not reported. History: not reported. | |
| Interventions | 1. Clozapine: dose initially 75 mg/day, max 500 mg/day. N=47. 2. Haloperidol: dose initially 2.25 mg/day, max 15 mg/day. N=44. |
|
| Outcomes | Global effect. Mental state: BPRS & Keio University Psychiatric Rating Scale for Schizophrenia. Adverse effects: Keio University´s Extrapyramidal Symptoms Rating Scale. Leaving the study early. Behavioural rating: two scales. | |
| Notes | Jadad score 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, not tested |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Kane 1988 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Design: multi‐centre. Duration: six weeks. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐III), undifferentiated ˜50%, paranoid ˜33%. N=268. Sex: 54 F, 214 M. Age: mean 36 years. History: treatment‐resistant*; unresponsive/intolerant to six weeks haloperidol & benztropine period. | |
| Interventions | 1. Clozapine: dose up to 500 mg/day weeks one to two, flexible dose thereafter, max 900 mg/day. N=126. 2. Chlorpromazine: dose up to 1000 mg/day weeks one to two, flexible dose thereafter, max 1800 mg/day; also benztropine 6 mg/day. N=142. | |
| Outcomes | Death. Relapse. Leaving the study early. Improvement: decrease of >20% in BPRS total score & CGI score of <3 or BPRS total score <35. Global effect: CGI. Mental state: BPRS. Behaviour: NOSIE. Adverse effects: AIMS, SAS. | |
| Notes | Jadad score 5. * Treatment resistant = 3+ periods of neuroleptic treatment, 1000 mg/day of chlorpromazine equivalents without significant symptomatic relief & BPRS total score of at least 45. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested, adverse effects (Table 7) clearly different making blinding problematic |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Funded by Sandoz Pharmaceuticals |
Kane 1995 (H).
| Methods | Allocation: randomised. Blindness: double. Design: multi‐centre. Duration: 29 weeks. Setting: outpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R & SCID). N=71. Sex: 21 F, 50 M. Age: mean 40 ± 9 years. History: symptomatic, but not profoundly treatment‐refractory. | |
| Interventions | 1. Clozapine: dose not reported. N=37. 2. Haloperidol: dose 10 mg/day. N=34. | |
| Outcomes | Relapse. Mental state: BPRS, SANS. Neuropsychological tests. Improvement: >20% reduction in BPRS psychosis factor score. | |
| Notes | Jadad score 3. Data extracted from abstracts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated block randomisation |
| Allocation concealment? | Low risk | Sealed envelopes, identical capsules |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | High risk | Attrition details not all reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Novartis Pharmaceuticals provided financial support, and grant received from NIMH. |
Klieser 1988 (H).
| Methods | Allocation: randomised. Blindness: double. Duration: six weeks (preceded by 14‐day washout). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia ‐ chronic treatment‐resistant (no diagnostic criteria). N=32. Sex: 19 F, 11 M. Age: average 48 years. History: duration of illness average 17 years. | |
| Interventions | 1. Clozapine: dose 400 mg/day. N=16. 2. Haloperidol: dose 20 mg/day. N=16. Biperiden & chloral hydrate as needed. | |
| Outcomes | Relapse. Leaving the study early. Global effect: CGI. Mental state: BPRS, AMDP & SANS. | |
| Notes | Jadad score 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | No details |
Klieser 1990 (H).
| Methods | Allocation: randomised. Blindness: double (single blind for side effects). Duration: 28 days. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia ‐ acute paranoid (ICD‐9). N=180. Sex: 96 F, 84 M. Age: average 34 years. History: not reported. | |
| Interventions | 1. Clozapine: dose 400 mg/day, average 350 mg/day. N=37.
2. Remoxipride: dose 400 mg/day, average 375 mg/day. N=38.
3. Haloperidol: dose 15 mg/day, average 16 mg/day. N=45.
4. Risperidone: dose 4/8 mg/day. N=40.
5. Zotepine: dose 225 mg/day. N=20. Biperiden and chloral hydrate as needed. |
|
| Outcomes | Relapse. Mental state: BPRS & AMDP. Adverse effects: SAI. Leaving the study early. Global effect: CGI, Global Tolerance. General intelligence: KAI. Cognitive functioning: SKT. Patient satisfaction. | |
| Notes | Jadad score 2. No intention‐to‐treat analysis performed. Drop outs not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind mostly, single blind for adverse events, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Kumra 1994 (H).
| Methods | Allocation: randomised. Blindness: double. Duration: six weeks (preceded by 4 week wash‐out). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R), disorganised (N=10), undifferentiated (N=10), paranoid (N=1). N=21. Age: range 6 to 18 years average 14 years. Sex: 10 girls, 11 boys. History: onset by age 12 years; neuroleptic‐resistant. | |
| Interventions | 1. Clozapine + placebo: dose initially 6.25 to 25 mg/day (depending on weight); dose average 176 mg/day, range 25 to 525 mg/day. N=10. 2. Haloperidol: dose initially 0.25 to 1 mg/day (depending on weight); dose average 16 mg/day, range 7 to 27 mg/day & benztropine as needed 6 mg/day. N=11. | |
| Outcomes | Mental state: BPRS, SANS, SAPS, Bunney‐Hamburg Rating Scale. Global effect: CGI, Children´s global assessment scale. Leaving the study early. Adverse effects: SAI, AIMS, Subjective Treatment Emergent Symptoms Scale. EEG, EKG and laboratory tests including CSF sampling. | |
| Notes | Jadad score 5. Dispersion of Bunney‐Hamburg ratings greater in haloperidol group, which means that groups were not comparable in this respect. Haloperidol doses seem high when compared with clozapine doses. Benztropine medication in group two may have affected results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double mostly but single for adverse events, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons for study attrition described |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Funded by Sandoz Pharmaceuticals |
Lee 1994 (mainly H).
| Methods | Allocation: randomised. Blindness: not blind. Duration: 12 months. Setting: not stated. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐III‐R & SADS). N=64. Sex: 17 F, 47 M. Age: average 25.5 years. History: duration of illness <5 years, average 3.3 years; not treatment‐resistant. Symptoms: average BPRS at baseline ‐ clozapine 25 ±SD 12, typical drugs 24 ±SD 12; minimal positive symptoms during prior neuroleptic treatment. | |
| Interventions | 1. Clozapine: dose average at 12 months 344 mg/day. N=35. 2. Various typical neuroleptics: dose average 522 mg/day of chlorpromazine equivalents (mainly haloperidol) + benztropine as needed. N=29. | |
| Outcomes | Mental state: BPRS.
Leaving the study early.
Neuropsychological function: Digit Symbol Substituent Test, Category Instance Generation Test, Controlled Word Association Test, Verbal List Learning Test, WISC.
Adverse effects: AIMS, SAI. Unable to use ‐ Neuropsychological function: Wisconsin Card Sorting Test. |
|
| Notes | Jadad score 2. Benztropine medication in group two may have affected results. Assessment bias is possible due to non‐blind conditions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Single, assessors blind to drug allocation, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | No details |
Leon 1974 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: six weeks, three and four year naturalistic follow‐up. Setting: inpatients, some patients were discharged to family care during study. | |
| Participants | Diagnosis: schizophrenia; heterogenous subtypes (DSM‐II). N=50. Sex: 21 F, 29 M. Age: average 28.5 years. History: not reported. | |
| Interventions | 1. *Clozapine dose average 600 mg/day, max 1600 mg/day. N=25. 2. Chlorpromazine capsules: dose average 600 mg/day. N=25. | |
| Outcomes | Death. Global effect: clinical evaluation. Leaving the study early. Mental state: symptom check‐list. Adverse effects. Hospital admission. Length of hospital stay. Out‐patient visits. | |
| Notes | *Clozapine not given during follow‐up. Jadad score 2. Two schizoaffective people, both in clozapine group. By mistake clozapine patients throughout the trial received twice the dose that was intended to be given. This may have benefited the clozapine group outcomes and may have resulted in more adverse effects in the clozapine group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double, untested |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Li 2003 (Lox).
| Methods | Allocation: randomised ‐ no details. Blindness: not reported. Duration: eight weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N=60. Age 16 to 60 years. Sex: male and female. Excluded: children, pregnant or breast feeding women, received anti‐psychotic medication a week prior to study, severe physical illness, organic mental disorder, alcohol, drug dependence. | |
| Interventions | 1. Clozapine: dose 25 to 600 mg/d. N=30. 2. Loxapine: dose 34 to 340 mg/d. N=30. | |
| Outcomes | Mental state: PANSS. Adverse events. | |
| Notes | CSG: 10103 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | High risk | Study attrition not reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Lieberman 2003 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: 52 weeks. Setting: not stated. | |
| Participants | Diagnosis: people with schizophrenia (DSM IV). N=160*. Age= 28.6 years. Sex: 77 F, 83 M. Setting: inpatients. History: naive first episode patients. | |
| Interventions | 1. Clozapine + BZ placebo: dose mean 292 mg/day, max 400 mg/day. N=80. 2. Chlorpromazine + BZ: dose mean 319 mg/day, max 600 mg/day; 2 mg/bid (BZ). N=80. | |
| Outcomes | Leaving the study early. Unable to use ‐ Remission 52 weeks. Mental state: BPRS, SANS (no usable data). Global state: CGI, GAF (no usable data). Adverse effects: COSTART, SAESS (no usable data). Physiological measurements: ECG, WBC, serum glucose (no usable data). |
|
| Notes | 164 patients were randomised. Four of them withdrew before the first administration of medication, thus they report as randomised 160 patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Numbers reported but reasons for attrition not described |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Funded by Novartis Pharmaceuticals |
Liu 1994 (Thi).
| Methods | Allocation: randomised. Blindness: double. Duration: six weeks. Setting: not stated. | |
| Participants | Diagnosis: schizophrenia. N=40. Age: mean 25.5 years. Sex: 16 F, 24 M. Setting: inpatients. History: not reported. | |
| Interventions | 1. Clozapine. dose no further details. N=20. 2. Thioridazine: dose no further details. N=20. | |
| Outcomes | Leaving the study early. Mental state: BPRS. Global effect: CGI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised ‐ by using table of random numbers |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | Reported part of TESS |
| Free of other bias? | Unclear risk | Clozapine was provided by Huizhou (Guangdong Province) Pharmaceutical Corp; Thioridazine was provided by Dongting (Hunan Province) Pharmaceutical Corp. |
Liu 2002 (CPZ).
| Methods | Allocation: randomised ‐ no further details. Blindness: not reported. Duration: eight weeks. | |
| Participants | Diagnosis: schizophrenia (DSM‐ III‐R). N=40. Sex: all male. Age: 17 to 42 years. History: length of illness 2 to 17years. | |
| Interventions | 1. Clozapine: dose 325 mg/d. N=20. 2. Chlorpromazine: dose 430 mg/d. N=20. | |
| Outcomes | Mental state: SANS. Unable to use ‐ Adverse events: no usable data |
|
| Notes | CSG:10502 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | High risk | Study attrition not reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Liu 2004 (CPZ).
| Methods | Allocation: randomised ‐ no further info details. Blindness: not reported. Duration: six weeks. Exclusion: pregnant or breast feeding women, patients with organic disease. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N=90. Sex: male and female. Age: 27 years. Exclusion: pregnant or breast feeding women, patients with organic disease. History: length of illness mean five months. | |
| Interventions | 1. Clozapine: dose 306 mg/d. N=30. 2. Chlorpromazine: dose 412 mg/d. N=30. 3. Risperidone: dose 4.05 mg/d. N=30. | |
| Outcomes | Adverse effects: blood tests (OGTT). | |
| Notes | CSG: 08358 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Niu 2001 (CPZ).
| Methods | Allocation: randomised ‐ no further details. Blindness: double. Duration: 12 weeks. | |
| Participants | Diagnosis: first onset schizophrenia (CCMD‐2‐R). N=164. Age: 16 to 40 years. Sex: male and female. History: length of illness median 11 months. | |
| Interventions | 1. Clozapine: dose 392 mg/d. N=81. 2. Chlorpromazine: dose 551 mg/d. N=83. | |
| Outcomes | Leaving the study early | |
| Notes | CSG: 9979 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Ou 1999 (CPZ).
| Methods | Allocation: randomised, no further details. Blindness: not stated. Duration: eight weeks. Setting: not stated. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R), BPRS total score greater than 35, at least two positive symptoms of SAPS, inpatients. N=200. Age: not reported. Sex male and female. History: not reported. | |
| Interventions | 1. Clozapine: dose mean 361 mg/day. N=100. 2. Chlorpromazine: dose mean 445 mg/day. N=100. | |
| Outcomes | Global state. Service utilisation. Clinical global response. Social functioning. Quality of life. Satisfaction with treatment. Adverse effects/events. Extrapyramidal side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Potter 1989 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: eight weeks. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐III and Chinese criteria). N=57. Sex: 22 F, 35 M. Age: mean 32 years. History: mean duration of illness six years. | |
| Interventions | 1. Clozapine: dose 50 to 600 mg/day. N=20. 2. Chlorpromazine: dose 100 to 600 mg/day. N=17. 3. Clozapine: dose 50 to 400 mg/day + chlorpromazine 100 to 400 mg/day. N=20. | |
| Outcomes | Mental state: BPRS. | |
| Notes | Jadad score 2. Average doses not reported, and may not have been equivalent to each other. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised, no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Rosenheck 1993 (H).
| Methods | Allocation: randomised. Blindness: double. Design: multi‐centre. Duration: one year. Setting: inpatients and outpatient services. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R & SCID). N=423. Sex: 10 F, 413 M. Age: average 43.5 years. History: mean age onset 22 years, treatment‐resistant*, high level use of inpatient services (30 to 364 days of hospitalisation in preceding year). | |
| Interventions | 1. Clozapine: dose 100 to 900 mg/day, average dose at week 26, 552 mg/day; also placebo benztropine. N=205. 2. Haloperidol: dose 5 to 30 mg/day, average dose at week 26, 28 mg/day; also benztropine 2 to 10 mg/day. N=218. | |
| Outcomes | Leaving the study early. Mental state: PANSS. Improvement: decrease of >20% in PANSS total. Quality of life: Heinrichs‐Carpenter Quality of Life Scale. Adverse effects: AIMS, Barnes Akathisia Scale, SAI, adverse effects checklist. Use of services: days of hospitalisation (skewed data), outpatient visits (no SD given). Costs: medication, health care, estimated non‐health care costs. | |
| Notes | Jadad score 4. *Treatment‐resistant = persisting psychotic symptoms despite treatment with >one antipsychotic drugs at 1000 mg chlorpromazine equivalents. During trial 83 patients assigned to clozapine switched to conventional antipsychotic drugs and 49 patients assigned to haloperidol switched to clozapine for at least four weeks. Cross‐over cases are excluded from continuous data on mental state (PANSS). Benztropine medication in group two may have affected results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised, no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported, reasons for attrition not described |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Clozapine provided by Novartis Pharmaceuticals |
Shopsin 1978 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: five weeks (preceded by three to seven day washout). Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia ‐ floridly psychotic; authors´ checklist, no widely used diagnostic criteria not reported). N=39. Age: range 21 to 55 years. Sex: not reported. History: not reported. | |
| Interventions | 1. Clozapine: dose initially 25 mg/day, one‐week build‐up to 300 mg/day, mean dose at week four, 800 mg/day, max dose 900 mg/day. N=16. 2. Chlorpromazine: dose initially 150 mg/day, one‐week build‐up to 600 mg/day, mean dose at week four , 1333 mg/day, max dose 1800 mg/day. N=15. 3. Placebo. N=8. Chloral hydrate and/or paraldehyde as needed. | |
| Outcomes | Relapse. Leaving the study early. Global effect: CGI. Mental state: BPRS. Behaviour: NOSIE. Adverse effects: modified SAI. Discharge‐ability. ECG, blood pressure, ophthalmological examination. | |
| Notes | Jadad score 4. Number of drop‐outs not specifically reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, identical capsules, untested |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Singer 1974 (CPZ).
| Methods | Allocation: randomised (systematised random order). Blindness: double. Duration: 40 days (preceded by two week washout). Setting: not reported. | |
| Participants | Diagnosis: schizophrenia (no diagnostic criteria reported). N=40. Sex: 22 F, 8 M. Age: range 16 to 61 years; mean 32 years. History: acute illness. | |
| Interventions | 1. Clozapine: dose initially 50 to 100 mg/day, dose average 155 mg/day, range 50 to 300 mg/day. N=20. 2. Chlorpromazine: dose initially 50 to 100 mg/day, dose average 196 mg/day, range 75 to 600 mg/day. N=20. Dose adjusted according to need. | |
| Outcomes | Relapse.
Adverse effects.
Leaving the study early. Unable to use ‐ Mental state: 18‐item BPRS (no SD). Global effect: Global Clinical Scale (authors own, no data). |
|
| Notes | Jadad score 3. Drop‐outs excluded from analyses of results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons for attrition reported |
| Free of selective reporting? | High risk | Not all outcome data reported |
| Free of other bias? | Unclear risk | Unclear |
Sun 2000 (Perp).
| Methods | Allocation: randomised ‐ no further details. Blindness: not reported. Duration: six weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N=153. Sex: male and female. Age: mean ˜31 years. | |
| Interventions | 1. Clozapine: dose 50 to 600mg/d. N=51. 2. Perphenazine: dose 8 to 60 mg/d. N=51. 3. Clozapine: dose 100 to 300 mg/d + perphenazine 32 to 50mg/d. N=51. | |
| Outcomes | Leaving the study early. Unable to use ‐ Mental state: BPRS (no usable data). Adverse events: TESS (no usable data). |
|
| Notes | CSG: 10621 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Tamminga 1994 (H).
| Methods | Allocation: randomised. Blindness: double. Duration: 12 months (preceded by one to six month stabilization with haloperidol & one month drug‐free/fixed low‐dose). Setting: not reported. | |
| Participants | Diagnosis: schizophrenia with tardive dyskinesia (no diagnostic criteria reported). N=43*. Sex: not reported. History: not reported. | |
| Interventions | 1. Clozapine: dose initially 50 mg/day, final dose average 294 mg/day + placebo. N=25. 2. Haloperidol: dose initially 5 mg/day, final dose average 28.5 mg/day + benztropine. N=14. | |
| Outcomes | Relapse. Leaving the study early. Mental state: BPRS. Adverse effects: Maryland Psychiatric Research Centre Involuntary Motor Scale, videotape evaluation. | |
| Notes | Jadad score 3. *Four patients had not completed the protocol when report was written. Haloperidol doses seem high when compared with clozapine doses. Benztropine medication in group 2 may have affected results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested |
| Incomplete outcome data addressed? All outcomes | High risk | Data for 32 participants, 4 others' data missing |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Volavka 2002 (H).
| Methods | Allocation: randomised. Blindness: double. Duration: 14 weeks. Setting: inpatients. Design: multi‐centre. | |
| Participants | Diagnosis: people with schizophrenia or schizoaffective disorder (DSM‐IV). N=167* Sex: 133 M, 24 F. Age: range 18 to 60 years; mean 40.8 years. History: patients had suboptimal response to previous treatment. | |
| Interventions | 1. Clozapine: dose period one 401 mg/day; period two 526 mg/day + placebo td. N=40. 2. Olanzapine: dose period one 19 mg/day; period two 30 mg/day + placebo td. N=39. 3. Risperidone: dose period one 8 mg/day; period two 12 mg/day + placebo td. N=41. 4. Haloperidol: dose period one 20 mg/day;period two 26 mg/day + benztropine 2 mg td. N=37. | |
| Outcomes | Mental state: PANSS total; PANSS positive and Negative subscale.
Hopkins Verbal Learning Test.
Cognitive functioning: MMSE. Unable to use ‐ Quality of Life Scale: QLS. Behaviour: NOSIE. WCST. Side effects: ESRS / EPS / FTT. Drop out*. |
|
| Notes | *167 patients were randomly assigned to a treatment group, but then 10 terminated the study before receiving study medication. Thus, the study is based on data from 157 subjects. Note: Period One (eight weeks): the doses were escalated to their target levels clozapine 500 mg/d; olanzapine 20 mg/d; risperidone 8 mg/d; haloperidol 20 mg/d. Period Two (six weeks): the doses were titrated within dose ranges clozapine 200 to 800 mg/d; olanzapine 10 to 40 mg/d; risperidone 4 to 16 mg/d; haloperidol 10 to 30 mg/d. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, identical tablets, untested |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons for attrition described |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | High risk | Grant from NIHM, UNC‐MHNCRC, Foundation of Hope, Raleigh Pharmaceutical companies (Jansen, Eli Lilly, Novartis, Merck) provided medications and Eli Lilly provided 18% of total study costs |
Wang 2001 (CPZ).
| Methods | Allocation: randomised. Blinding: not reported. Duration: eight weeks. Setting: outpatients. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). N=85. Age: mean 36 years. Sex: M 33, F 52. History: discharged patients, who were 'much improved by clozapine treatment'. | |
| Interventions | 1. Clozapine: dose 300 mg/day. N=45. 2. Chlorpromazine: dose 500 mg/day. N=40. | |
| Outcomes | Mental state: BPRS, SAPS. Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Wang 2006a (CPZ).
| Methods | Allocation: randomised ‐ no further details. Blindness: not reported. Duration: 12 weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N=105. Sex: male and female. Age: average 29 years. History: length of illness three months to three years. | |
| Interventions | 1. Clozapine: dose 25 to 600 mg/d. N=35. 2. Chlorpromazine: dose 25 to 600 mg/d. N=35. 3. Risperidone: dose 1 to 7 mg/d. N=35. | |
| Outcomes | Mental state: PANSS. Adverse events: TESS | |
| Notes | outcome: PANSS endpoint score, change score, curative effect, adverse events. CSG: 10006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | High risk | Study attrition not reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Wang 2006b (CPZ).
| Methods | Allocation: randomised ‐ no further details. Blindness: not reported. Duration: three months + one year follow up (only those with PANSS score decreased rate >50% are followed up, chlorpromazine = 20, clozapine = 26, risperidone = 27). | |
| Participants | Diagnosis: first onset schizophrenia (CCMD‐2‐R). N=117. Sex: male and female. Age: 18 to 50 years. History: length of illness three months to three years Excluded: patients with organic mental disorder or pregnant or breast feeding. | |
| Interventions | 1. Clozapine: dose 25 to 600 mg/d. N=39. 2. Chlorpromazine: 25 to 600 mg/d. N=39. 3. Risperidone: 1 to 7mg/d. N=39. | |
| Outcomes | Leaving the study early. | |
| Notes | Chlorpromazine group: four dropped out due to EPS. Clozapine group:one dropped out due to white cell decrease. CSG: 10008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Xia 2002 (CPZ).
| Methods | Allocation: randomised. Blindness: not reported. Duration: two months. Setting: not reported. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N=85. Age: mean 47 yrs, range 18‐65. Sex: M 44, F 41. History: mean length of illness five years. | |
| Interventions | 1. Clozapine: dose 480 mg/day. N=45. 2. Chlorpromazine: dose 570 mg/day. N=40. | |
| Outcomes | Mental state: BPRS. Adverse effects: weight gain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | No details |
Xu 1985 (CPZ).
| Methods | Allocation: randomised. Blindness: double. Duration: eight weeks. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐III), BPRS total score>38. N=60. Age: range 18 to 55 years. Sex: female and male. History: not reported. | |
| Interventions | 1. Clozapine: mean dose 400 mg/day. N=30. 2. Chlorpromazine: mean dose 693 mg/day. N=30. | |
| Outcomes | Leaving the study early. Unable to use ‐ Mental state: BPRS (no data reported). Global functioning: GAS (no data reported). |
|
| Notes | Jadad score: assessment ongoing. Paper reports higher rates of leucopenia (16/30 vs 10/30) in chlorpromazine group. Because data may be mistakenly reversed in the paper, they have not been included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Double blind, untested; two independent raters gave the assessment; all tablets were indistinguishable in all aspects of their appearance |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons for loss to follow up described by group |
| Free of selective reporting? | High risk | No data for BPRS and GAS |
| Free of other bias? | Unclear risk | Unclear |
Yang 1997 (CPZ).
| Methods | Allocation: randomised. Blindness: not reported. Duration: three months. Setting: not reported. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). N=32. Sex: M 17, F 15. Age: 27 yrs (SD 7). History: first episode, illness <five years. | |
| Interventions | 1. Clozapine: dose range 400 to 600 mg/day. N=17. 2. Chlorpromazine: dose range 500 to 700 mg/day. N=15. | |
| Outcomes | Mental state: SANS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Yang 2002 (CPZ).
| Methods | Allocation: randomised. Blinding: double. Duration: 12 weeks. Setting: not reported. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2 and DSM‐IV). N=78. Sex: M 43, F35. Age: 28 yrs. History: illness 14 months (SD 6), first episode, antipsychotic naive, education for 10 yrs (SD 4.8), course of positive symptoms >=1 month; BPRS>35 (18 items, rank: 1˜7). | |
| Interventions | 1. Clozapine: dose 400 mg/day. N=40. 2. Chlorpromazine: dose 600 mg/day. N=38. | |
| Outcomes | Mental state: BPRS.
Leaving the study early. Unable to use ‐ Adverse events: SANS, GAF, EEG (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons for loss to follow up were described |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Yang 2004 a (CPZ).
| Methods | Allocation: randomised. Blindness: not reported. Duration: 52 weeks. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=164*. Sex: not reported. Age: 16 to 40 yrs. History: first episode, age of onset <=35 years old, BPRS at least two items of the five psychotic symptoms items >=4, no antipsychotics treatment or the antipsychotics treatment was less than 14 days and no drug treatment before admission. | |
| Interventions | 1. Clozapine: dose not reported. N=79. 2. Chlorpromazine: dose not reported. N=81. | |
| Outcomes | Leaving the study early. Fasting blood sugar (FBS). Adverse events. | |
| Notes | *Four participants not accounted for after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no further details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | High risk | Study attrition not reported |
| Free of selective reporting? | Low risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Zhang 1996 (CPZ).
| Methods | Allocation: randomised. Blindness: no details. Duration: five weeks. Setting: no details. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N=41. Sex: male and female. Age: mean ˜28 years. History: length of illness ˜8±7.5 years. | |
| Interventions | 1. Clozapine: dose 225 to 450 mg/d. N=20. 2. Chlorpromazine: dose 400‐750 mg/d. N=21. | |
| Outcomes | Leaving the study early. | |
| Notes | CSG: 8366 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | No details |
| Incomplete outcome data addressed? All outcomes | Low risk | No participants left the study early |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Zhang 2000 (Thi).
| Methods | Allocation: randomised. Blindness: not reported. Duration: six weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N=60. Sex: male and female. Age: mean ˜25 ±7. History: length of illness* 25.4±23.9 (thioridazine group), 19.8±10.8(clozapine group). | |
| Interventions | 1. Clozapine: dose 200 to 500 mg/d. N=30. 2. Thioridazine: dose 300 to 800mg/d. N=30. | |
| Outcomes | Leaving the study early. Mental state: BPRS. | |
| Notes | *Did not report if length of illness is month or years. CSG 8405 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | Low risk | Study attrition reported |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
Zhang 2007 (Lox).
| Methods | Allocation: randomised ‐ no further details. Blindness: not reported. Duration: six weeks. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N=50. Sex: all female. Age: mean 34±15 years. History: average length of illness ˜4.5±2.5 years. Included: clean of anti‐psychotics prior to hospital admission, without severe physical illnesses. | |
| Interventions | 1. Clozapine: dose 25 to 300 mg/d. N=25. 2. Loxapine: dose 34 to 204 mg/d. N=25. | |
| Outcomes | Mental state: PANSS
Adverse effects: TESS. Unable to use ‐ Adverse events: miscellaneous (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomised ‐ no details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No details |
| Free of selective reporting? | Unclear risk | No details |
| Free of other bias? | Unclear risk | Unclear |
AIMS ‐ Abnormal Involuntary Movement Scale AMDP ‐ Arbeitsgemeinschaft für Methodik und Dokumentation in der Psychiatrie BZ ‐ benztropine BPRS ‐ symptom rating scale (Brief Psychiatric Rating Scale) CGI ‐ global rating scale (Clinical Global Impressions) CSF ‐ cerebrospinal fluid COSTART‐ coding symbol and thesaurus for adverse event terminology. DM ‐ diabetes mellitus DSM ‐ diagnostic sets of operational criteria (Diagnostic and Statistical Manual of Mental Disorders) DSM‐II ‐ second edition, 1968 DSM‐III ‐ third edition, 1980 DSM‐III‐R ‐ third edition, revised, 1987 DSM‐IV ‐ fourth edition, 1994 ESR‐ erythrocyte sedimentation rate FBS ‐ fasting blood sugar FTT‐ finger tapping test GAF ‐ Global assessment of function scale. ICD ‐ International Classification of Diseases Jadad score ‐ an instrument measuring risk of bias in trial reports in the range 0 (bad) to 5 (good). KAI ‐ test for measuring general intelligence MRI ‐ magnetic resonance imaging NOSIE ‐ behaviour rating scale (Nurses´ Observation Scale for Inpatient Evaluation) OGTT ‐ oral glucose tolerance test PANSS ‐ symptom rating scale (Positive And Negative Syndrome Scale) QOLI ‐ rating scale for objective functional state and subjective quality of life (Quality of Life Inventory) QOLS ‐ rating scale for deficit symptoms in schizophrenia (Quality of Life Scale) SAI ‐ rating scale for evaluating neurological side effects (Simpson‐Angus Index) SADS ‐ schedule for reliably making a diagnosis (Schedule for Affective Disorders and Schizophrenia) SANS ‐ symptom rating scale (Scale for the Assessment of Negative Symptoms) SAPS ‐ symptom rating scale (Scale for the Assessment of Positive Symptoms) SCID ‐ schedule for reliably making a diagnosis SD ‐ a measure of dispersion (standard deviation) SAS ‐ Simpson‐Angus scale ‐ a scale for assessing neurological adverse effects SKT ‐ a test of cognitive functioning (Syndrome Kurz Test) TESS ‐ Treatment Emergent Signs and Symptoms WBC ‐ white blood cells WCST ‐ Wisconsin card sorting test WISC ‐ a test measuring executive function (Wechsler Intelligence Scale for Children)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine (100, 300 or 600 mg/day). |
| Adams 1991 | Allocation: not randomised, case series. |
| Adityanjee 1995 | Allocation: not randomised, case series. |
| Agelink 1998 | Allocation: not randomised. |
| Aitchison 1997 | Allocation: not randomised, case series. |
| Allison 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: olanzapine versus clozapine versus haloperidol versus risperidone. |
| Altamura 1999a | Allocation: unclear. Participants: people with schizophrenia. Interventions: risperidone versus clozapine versus olanzapine versus haloperidol. Outcomes: no usable data. |
| Altamura 1999b | Allocation: randomised. Participants: people with paranoid schizophrenia. Interventions: olanzapine versus haloperidol. |
| Alvarez 1997 | Allocation: not randomised, case series. |
| Ames 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| An 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Angst 1971 | Allocation: randomised. Participants: people with schizophrenia and other diagnoses. |
| Anil 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol versus other typicals. Outcomes: no usable data. |
| Arango 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: olanzapine versus haloperidol. Outcomes: no usable data. |
| Atmaca 2003 | Allocation: randomised. Participants: people with schizophrenia. Intervention: clozapine versus quetiapine versus olanzapine versus risperidone versus no psychopharmacologic treatment. |
| Bao 1988 | Allocation: randomised, allocation concealment quality category C (correspondence with author). |
| Battegay 1977 | Allocation: not randomised. |
| Baymiller 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Beasley 2001 | Allocation: not randomised. |
| Bellack 2004 | Allocation: randomised. Participants: people with schizophrenia or schizo‐affective disorder. Interventions: clozapine versus risperidone. |
| Berardi 1998 | Allocation: not randomised, case series. |
| Beuzen 1998 | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia. Interventions: clozapine versus olanzapine. |
| Bian 2003 | Allocation: not randomised. |
| Birmaher 1992 | Allocation: not randomised, case report. |
| Bitter 2004 | Allocation: randomised. Participants: people with treatment‐resistant or treatment‐intolerant schizophrenia. Interventions: clozapine versus olanzapine. |
| Blum 1972 | Allocation: only retrospective part of study was controlled, prospective study not controlled. |
| Boehle 1995 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus fluphenazine. Outcomes: no usable data. |
| Borovicka 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine + placebo versus clozapine + phenylpropanolamine. |
| Bourgeois 2004 | Allocation: randomised. Participants: people with schizophrenia and schizo‐affective disorder. Intervention: clozapine versus olanzapine versus no treatment. |
| Brandt‐Christensen 1998 | Allocation: not randomised, case series. |
| Brankovic 1998 | Allocation: not randomised. |
| Brar 1997 | Allocation: not randomised, case series. |
| Breier 1993 | Allocation: not randomised, case series. |
| Broich 1998 | Allocation: not randomised. |
| Buchanan 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: olanzapine versus haloperidol. Outcomes: no useable data. |
| Buchsbaum 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: sertindole versus haloperidol. |
| Buchsbaum 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: sertindole versus haloperidol. |
| Buchsbaum 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: olanzapine versus haloperidol. Outcomes: no usable data. |
| Cai 2000 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Cao 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Cao 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Cassano 1997 | Allocation: not randomised, case series. |
| Cavazzoni 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: olanzapine versus haloperidol versus risperidone versus clozapine. Outcomes: no usable data. |
| Cha 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Chakos 1995 | Allocation: not randomised. |
| Chen 1998a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Chen 1998b | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine given at two dosages, no comparator. |
| Chen 1999a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Chen 1999b | Allocation: unclear. Participants: unclear. Interventions: clozapine versus typical drugs. Outcomes: no usable data. |
| Chen 2001a | Allocation: not randomised. |
| Chen 2001b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus lithium carbonate. |
| Chen 2002 | Allocation: not randomised, blinding not reported. |
| Chen 2003a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Chen 2003b | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Chen 2005 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Chengappa 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol versus conventional antipsychotics. Outcomes: no usable data. |
| Chengappa 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: haloperidol versus placebo. |
| Choc 1990 | Allocation: not randomised, case series. |
| Chong 1997 | Allocation: not randomised, case series. |
| Chou 1999 | Allocation: controlled study. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Chouinard 1976 | Allocation: not randomised, case series. |
| Cohen 1991 | Allocation: not randomised. |
| Conley 1997 | Allocation: not randomised, case series. |
| Conley 2003 | Allocation: randomised. Participants: people with treatment resistant schizophrenia. Interventions: clozapine versus olanzapine. |
| Cosar 1999 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus sulpiride versus chlorpromazine versus haloperidol. Outcomes: no usable data. |
| Covell 1999 | Allocation: randomised. Participants: unclear. Interventions: haloperidol versus clozapine. Outcomes: no usable data. |
| Covington 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Cramer 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Cui 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus chlorpromazine versus risperidone. Outcome: no usable data ‐ EKG. |
| CUTLASS 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: typical antispychotics versus atypical antispychotics, clozapine not used. |
| Dai 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Davidson 1993 | Allocation: not randomised, case series. |
| Davies 1991 | Allocation: not randomised, case series. |
| Davies 1993 | Allocation: not randomised, case series. |
| De 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine. |
| De 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine, no comparator. |
| Dejanovic 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus fluphenazine versus haloperidol. Outcomes: no usable data. |
| Deng 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Diamond 1986 | Allocation: not randomised. |
| Diaz 2005 | Allocation: randomised. Particioants: people with schizophrenia or schizo‐affective disorder. Interventions: clozapine, no comparator. |
| Dickson 1998 | Allocation: not randomised. |
| Dittmann‐Balcar 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Drew 1994 | Allocation: not randomised, case series. |
| Drummond 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: ICI 204,636 versus haloperidol. |
| Du 2004a | Allocation: not randomised. |
| Du 2004b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidal. |
| Dye 1996 | Allocation: not randomised. |
| Earnst 1999 | Allocation: not randomised. |
| Edwards 1999 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus CBT. |
| Elman 1997 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus fluphenazine versus placebo. Outcomes: no usable data. |
| Elman 1999 | Allocation: not randomised. |
| Faltus 1973 | Allocation: not randomised, case series. |
| Faltus 1974 | Allocation: not randomised, case series. |
| Fan 2003 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Finzen 2002 | Allocation: not randomised. |
| Frazier 1994 | Allocation: not randomised, case series. |
| Fremont 1996 | Allocation: not randomised, case series. |
| Friedman 2003 | Allocation: not randomised. |
| Gallhofer 1996 | Allocation: not randomised. |
| Gan 1996 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine, no comparator. |
| Gan 1999 | Allocation: not randomised. Interventions: clozapine versus risperidone. |
| Ganguli 2005 | Allocation: unclear. Participants: people with schizophrenia. Interventions: behavioral interventions + novel antipsychotics. |
| Gao 2003 a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Gao 2003 b | Allocation: cohort study. |
| Ge 2004 | Allocation: quasi‐randomisation, according to the date of admission. |
| Gekiere 1996 | Allocation: not randomised, case series. |
| Gerlach 1977 | Allocation: randomised. Participants: people with schizophrenia. Interventions: G31,406 versus orphenadrine versus placebo. |
| Gerlach 1978 | Allocation: not randomised. |
| Glick 2004 | Allocation: randomised. Particioants: people with schizophrenia or schizoaffective disorder. Interventions: clozapine versus olanzapine. |
| Goff 1996 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine + placebo verus clozapine + D‐cycloserine 5 mg/d versus clozapine + D‐cycloserine 15mg/d versus clozapine + D‐cycloserine 50 mg/d versus clozapine + D‐cycloserine 250mg/d). |
| Goldberg 1993 | Allocation: not randomised, case series. |
| Goldberg 2000 | Allocation: not randomised. |
| Gordon 1996 | Allocation: not randomised, retrospective case series. |
| Gray 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine + patient education versus clozapine. |
| Gross 1969 | Allocation: not randomised, case series. |
| Gross 1970 | Allocation: not randomised, case series. |
| Gross 1974 | Allocation: not randomised, case series. |
| Guo 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Guo 2003a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine. |
| Guo 2003b | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Hagger 1993 | Allocation: not randomised, case control study. |
| Hammock 1995 | Allocation: not randomised, case report. |
| Hao 2004 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Haring 1994 | Allocation: not randomised, case series. |
| Hasegawa 1993 | Allocation: not randomised, case series. |
| He 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Heim 1987 | Allocation: unclear. Diagnosis: schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Hemphill 1975 | Allocation: not randomised, case series. |
| Herst 1997 | Allocation: not randomised, case series. |
| Hinze‐Selch 1997 | Allocation: not randomised, case series. |
| Honer 1995 | Allocation: not randomised, case series. |
| Honer 2004 | Allocation: randomised. Participants: people with schizophrenia with incomplete response to clozapine. Interventions: clozapine versus risperidone versus clozapine + placebo. |
| Honigfeld 1990 | Allocation: not randomised. |
| Hou 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Huang 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Hummer 1995 | Allocation: not randomised. |
| Hummer 1996 | Allocation: not randomised. |
| Hummer 1997 | Allocation: not randomised. |
| Hussain 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: rivastigmine and galanthamine treatment for cognitive impairment. |
| Hussain 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: rivastigmine and galanthamine treatment for cognitive impairment. |
| Jalenques 1992 | Allocation: not randomised, case series. |
| Jeste 1993 | Allocation: not randomised, case series. |
| Jia 2000 | Allocation: controlled study. |
| Jin 2002 | Allocation: randomised. Participants: people with schizophrenia treated with clozapine. Interventions: fluoxitine versus placebo. |
| Joffe 1996 | Allocation: not randomised, case series. |
| Jones 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: atypicals other than clozapine risperidone typicals. |
| Josiassen 2003 | Allocation: randomised. Participants: people with schizophrenia and schizo‐affective disorder who are receiving clozapine. Interventions: risperidone versus placebo. |
| Josiassen 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine augmented with risperidone. |
| Juul‐Povlsen 1985 | Allocation: not randomised, case series. |
| Kahn 1993 | Allocation: not randomised, case series. |
| Kahn 1994 | Allocation: not randomised. |
| Kane 1993 | Allocation: not randomised. |
| Keefe 2004 | Allocation: randomised. Participants: people with schizophrenia. Intervention: olanzapine versus haloperidol. |
| Kelly 2003 | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia. Interventions: clozapine versus olanzapine. |
| Kenny 1992 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus standard neuroleptics. Outcomes: no usable data. |
| Kiejna 1993 | Allocation: not randomised, case series. |
| Kilian 2004 | Allocation: not randomised. |
| Knegtering 2002 | Allocation: randomised. Participants: unclear. Interventions: quetiapine versus risperidone. |
| Ko 1995 | Allocation: unclear. Participants: people with schizophrenia. Interventions: ziprasidone versus haloperidol. |
| Kogeorgos 1995 | Allocation: randomised. Participants: people with schizophrenia. Interventions: sulpiride versus risperidone versus classical neuroleptics. |
| Koukkou 1979 | Allocation: not randomised. |
| Krakowski 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine versus haloperidol. Outcomes: no usable data. |
| Kronig 1995 | Allocation: not randomised, case series. |
| Kufferle 1997 | Allocation: not randomised. |
| Kuha 1986 | Allocation: not randomised, case series. |
| Kuoppasalmi 1993 | Allocation: not randomised, case series. |
| Kurz 1995 | Allocation: not randomised. |
| Lacro 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: haloperidol versus risperidone. |
| Lahti 2003 | Allocation: not randomised. |
| Laker 1998 | Allocation: not randomised, case series. |
| Lapierre 1980 | Allocation: not randomised, case series. |
| Lei 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Leon 1995 | Allocation: not randomised. |
| Leppig 1989 | Allocation: not randomised, case series. |
| Levkovitch 1995 | Allocation: not randomised, case series. |
| Levkowitz 1994 | Allocation: not randomised, case series. |
| Levy 2004 | Allocation: review. |
| Lewis 2004 | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia. Interventions: clozapine versus non‐clozapine atypical antipsychotics |
| Li 1987 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine, chlorpromazine or penflurodil. Outcomes: no usable data. |
| Li 2001 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone versus sulpiride. |
| Li 2001 b | Allocation: randomised. Participants: people with schizophrenia treated with clozapine. Interventions: Chinese herb versus no treatment. |
| Li 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Li 2003 a | Allocation: unclear. Participants: people with schizophrenia receiving clozapine. Interventions: venlafaxine versus sulpiride. |
| Li 2003 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Li 2003 c | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Li 2003 d | Allocation: controlled study. Participant: unclear. Interventions: risperidone + low dosage of haloperidol. |
| Li 2004 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Li 2004 b | Allocation: not randomised. Participants: people with schizophrenia. Interventions: no clozapine. |
| Li 2004 c | Allocation: controlled study. |
| Li 2004 d | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine+doxepin. |
| Liang 2002 | Allocation: randomised. Prticipants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Liao 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Lieberman 1989 | Allocation: not randomised, case series. |
| Lieberman 2001 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: olanzapine versus perphenazine versus quetiapine versus risperidone versus ziprasidone versus clozapine. Outcomes: no usable data. |
| Lieberman 2001 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine; olanzapine; haloperidol; risperidone. Outcomes: no usable data. |
| Lin 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus zotepine. |
| Lindenmayer 1994 a | Allocation: not randomised, case series. |
| Lindenmayer 1994 b | Allocation: not randomised, case series. |
| Lindström 1988 | Allocation: not randomised, case series. |
| Lingjærde 1996 | Allocation: not randomised, case series. |
| Litman 1996 | Allocation: not randomised, case series. |
| Liu 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine + sulpiride versus clozapine versus sulpiride. |
| Liu 1996 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus tiapride. |
| Liu 1997 | Allocation: not randomised. Participants: people with schizophrenia. Interventions: no clozapine. |
| Liu 1999 a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Liu 1999 b | Allocation: not randomised. |
| Liu 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone versus clozapine+ risperidone. |
| Liu 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Liu 2003 a | Allocation: not randomised. Participants: people with mental disorders. Interventions: different psycholeptics. |
| Liu 2003 b | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Liu 2003 c | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine + imipramine. |
| Liu 2004 a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Liu 2004 b | Allocation: controlled study. Participants: people with refractory schizophrenia. Interventions: clozapine versus olanzapine. |
| Liu 2004 c | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Liu 2004 d | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Liu 2005 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine with different dosages. |
| Liu 2005 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine with different dosages. |
| Louwerens 2000 | Allocation: not randomised. |
| Lu 1998 | Allocation: randomised. Participants: people with schizophrenia treated with clozapine. Interventions: diphenhydramine versus placebo. |
| Lu 2002 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine + Shu Xuening |
| Lu 2002 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Lu 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus loxapine succants. |
| Lu 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus fluvoxamine. |
| Lu 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone versus quetiapine. |
| Luo 1994 | Allocation: cohort study. |
| Luo 2001 | Allocation: cohort study. |
| Lv 2004 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Lü 2002 | Allocation: randomised. Participants: people with schizophrenia and healthy. Interventions: clozapine in single dose. |
| Ma 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine + ECT versus chlorpromazine + ECT. Outcomes: serum levels of prolactin. |
| Malykhin 2003 | Allocation: randomised. Participants: people with schizo‐affective disorder. Interventions: clozapine versus risperidone versus haloperidol. Outcomes: no usable data. |
| Mao 2000 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Marchesi 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Marder 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Markianos 2001 | Allocation: not randomised. |
| Matejcek 1984 | Allocation: randomised. Participants: healthy people. |
| Mattes 1989 | Allocation: not randomised, case series. |
| Matz 1974 | Allocation: not randomised, case series. |
| Mazurek 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: atypicals. |
| McAllister 1989 | Allocation: not randomised, case series. |
| McEvoy 1995 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine in different serum ranges. |
| McGurk 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Meehan 2000 | Allocation: not randomised. |
| Mei 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Meltzer 1989 | Allocation: not randomised, case series. |
| Meltzer 1996 | Allocation: unclear (drug withdrawal study). |
| Meltzer 1999 | Allocation: not randomised. |
| Meltzer 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine |
| Meltzer 2004 | Allocation: randomised. Participants: people with schizophrenia or schizo‐affective disorder. Intervention: placebo versus haloperidol |
| Meng 2002 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Miller 1994 | Allocation: not randomised, case series. |
| Miller 1998 | Allocation: not randomised. |
| Milton 1978 | Allocation: randomised. Participants: unclear. Interventions: clozapine versus chlorpromazine. Outcomes: no usable data. |
| Molcan 1974 | Allocation: not randomised, case series. |
| Moller 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: zotepine versus placebo. |
| Moresco 2004 | Allocation: randomised. Participants: people with refractory schizophrenia. Interventions: clozapine versus olanzapine. |
| Mortimer 1994 | Allocation: controlled, not randomised. |
| Mulqueen 2000 | Allocation: unclear. Participants: people with schizophrenia. Interventions: haloperidol versus clozapine versus olanzapine. Outcomes: no usable data. |
| Muñecas 1975 | Allocation: not randomised, case series. |
| Naber 1989 | Allocation: not randomised, case series. |
| Naber 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Nahunek 1975 | Allocation: unclear, double‐blind cross‐over study. Participants: people with schizophrenia. Interventions: clozapine versus perphenazine. Outcomes: no usable data. |
| Nahunek 1976 | Allocation: unclear. Participants: people with schizophrenia. Interventions: chlorpromazine versus clozapine versus flupenthixol/flupentixol versus pimozide/R.6238 versus thioridazine versus thiothixene. Outcomes: no usable data. |
| Nair 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine. |
| Nan 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Nemeroff 1996 | Allocation: not randomised. |
| Niu 2001 | Allocation randomised. Participants: people with schizophrenia. Interventions: clozapine versus chlorpromazine. Outcomes: no usable data. |
| Oliemeulen 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Owen 1989 | Allocation: not randomised, case series. |
| Owen 1993 | Allocation: not randomised. |
| Pang 2002 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Panteleeva 1987 | Allocation: not randomised, case series. |
| Panteleeva 1991 | Allocation: not randomised, case series. |
| Paunovic 1991 | Allocation: not randomised. |
| Peacock 1996 | Allocation: not randomised. |
| Peet 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: ethyl eicosapentaenoate placebo + clozapine versus ethyl eicosapentaenoate placebo + new atypical versus ethyl eicosapentaenoate 1g + new atypical versus ethyl eicosapentaenoate 2g + new atypical versus ethyl eicosapentaenoate 4g + new atypical. Outcomes: no usable data. |
| Peng 2001 a | Allocation: randomised. Participants: unclear. Interventions: clozapine versus risperidone. |
| Peng 2001 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Peng 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Percudani 1998 | Allocation: not randomised. |
| Perez 2003 | Allocation: randomised. Participants: people with non affective psychosis. Interventions: olanzapine versus risperidone versus haloperidol. Outcomes: no usable data. |
| Petit 1992 | Allocation: not randomised. |
| Pickar 1992 | Allocation: not randomised. |
| Pickar 1994 | Allocation: not randomised, case series. |
| Pickar 1994 1 | Allocation: not randomised. |
| Pickar 1995 | Allocation: not randomised, case report. |
| Pickar 2003 | Allocation: cohort study. |
| Pinto 1999 | Allocation: randomised. Participants: people with schizophrenia. Interventions: CBT + social skills training versus supportive therapy. |
| Pollack 1998 | Allocation: not randomised. |
| Pollmächer 1995 | Allocation: not randomised. |
| Potkin 1993 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine, no comparator. |
| Potkin 1994 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine in different serum ranges. |
| Potkin 1994 b | Allocation: not randomised, case series. |
| Potkin 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: sertindole versus haloperidol. |
| Potkin 1997 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Potkin 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol versus placebo. Outcomes: no usable data. |
| Potkin 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Potkin 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Povlsen 1985 | Allocation: not randomised, retrospective case series. |
| Preiningerová 1974 | Allocation: not randomised, case series. |
| Preussler 1995 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus fluphenazine. Outcomes: no usable data. |
| Preussler 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus fluphenazine. Outcomes: no usable data. |
| Purdon 2003 | Allocation: randomised Participants: people with schizophrenia. Interventions: risperidone versus haloperidol. |
| Qian 2004 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone versus quetiapine. |
| Raja 2000 | Allocation: not randomised. |
| Rajarethinam 2003 | Allocation: not randomised. |
| Rao 1994 | Allocation: not randomised, case control study. |
| Ratey 1993 | Allocation: not randomised, case series. |
| Remschmidt 1994 | Allocation: not randomised, case control. |
| Ren 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidal. |
| Ren 2004 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Ren 2004 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine+ fluoxetine. |
| Rettenbacher 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus amisulpride. |
| Rodova 1973 | Allocation: not randomised. |
| Rosenberg 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: unclear. Outcomes: no usable data. |
| Rossger 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus fluphenazine. Outcomes: no usable data. |
| Ruiz 1974 | Allocation: quasi randomised; "in accordance with the ordinal randomisation number" open list or sequential randomisation (category C). |
| Rüther 1979 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Safferman 1993 | Allocation: not randomised, case series. |
| Salganik 1998 | Allocation: randomised. Participants: people with schizophrenia. Interventions: haloperidol versus clozapine. Outcomes: no usable data. |
| Schmauss 1989 | Allocation: not randomised, case series. |
| Schulz 1997 | Allocation: not randomised. |
| Shalev 1993 | Allocation: randomised. Participants: people with schizophrenia. Interventions: haloperidol versus levopromazine versus perphenazine. |
| Shen 2002 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine. |
| Shen 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Shi 2000 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Shi 2000 c | Allocation: not randomised. |
| Shi 2004 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Shi 2004 a | Allocation: randomised. Participants: people with schizophrenia and healthy participants. Interventions: unclear. Outcomes: no usable data. |
| Shirakawa 1996 | Allocation: not randomised, case series. |
| Shopsin 1978 a | Allocation: not randomised. |
| Shun 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine + health‐education. |
| Siefen 1986 | Allocation: not randomised, case control. |
| Simpson 1974 | Allocation: not randomised, case series. |
| Simpson 1978 | Allocation: not randomised. |
| Singer 1973 | Allocation: randomised. Participants: people with schizophrenia Intervention: clozapine versus placebo. |
| Small 1987 | Allocation: not randomised, case series. |
| Small 2003 | Allocation: randomised. Participants: people with schizophrenia and schizo‐affective disorder. Intervention: clozapine + lithium versus clozapine + placebo. |
| Speer 1997 | Allocation: not randomised. |
| Spivak 1997 a | Allocation: not randomised, case series. |
| Spivak 1997 b | Allocation: not randomised, case series. |
| Spivak 1998 | Allocation: not randomised, case control. |
| Stankovska 1999 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus haloperidol. Outcomes: no usable data. |
| Stone 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine + glucose versus clozapine + saccharine. |
| Strejilevich 2004 a | Allocation: not randomised. |
| Stroup 2003 | Allocation: not randomised. Participants: not stated. Interventions: clozapine given in third phase, no further details. |
| Stryjer 2004 | Allocation: randomised. Particioants: people with schizophrenia or schizo‐affective disorder. Intervention: clozapine + donepezil versus clozapine + placebo. |
| Sumiyoshi 2003 | Allocation: randomised. Participants: schizophrenia or schizo‐affective disorder. Intervention: buspirone versus placebo. |
| Sun 2000 | Allocation: controlled study. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Sun 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Sun Lm2 | Allocation: quasi‐randomisation. |
| Suppes 1999 | Allocation: randomised. Participants: schizo‐affective disorder bipolar type or bipolar I disorder. |
| Szymanski 1994 | Allocation: not randomised, case series. |
| Tandon 1993 | Allocation: not randomised, case series. |
| Tang 2002 a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Tang 2002 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Tang 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Tauscher 1999 | Allocation: not randomised. |
| Tiihonen 2003 | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia. Intervention: clozapine + lamotrigine versus clozapine + placebo. |
| Tiihonen 2004 | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia. Intervention: clozapine + lamotrigine versus clozapine + placebo. |
| Tong 2001 | Allocation: randomised. Participants: people with enuresis. |
| Trichard 1998 | Allocation: not randomised. |
| Turner 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: modafinil versus placebo. |
| Turpeinen 1996 | Allocation: not randomised, case series. |
| UK Study 1993 | Allocation: not randomised, case series. |
| Van Praag 1976 | Allocation: randomised. Participants: heterogeneous diagnoses. |
| VanderZwaag 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine in different serum ranges. |
| Vass 2004 | Allocation: randomised. Participants: people with resistance schizophrenia. Interventions: lamotrigine as adjuvant drug to antipsychotics. |
| Vinar 1976 | Allocation: not randomised. |
| Vlokh 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus fluanxol. Outcomes: no usable data. |
| Wang 1994 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus sulpiride. |
| Wang 1995 | Allocation: randomised Participants: people with schizophrenia. Interventions: clozapine versus chlorpromazine. Outcomes: no data reported. |
| Wang 1999 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Wang 2000 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus sulpiride. |
| Wang 2000 b | Allocation: randomised. Participants: people with schizophrenia receiving clozapine. Interventions: fluoxetine versus placebo. |
| Wang 2001 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Wang 2002 a | Allocation: unclear. Participants: people with refractory schizophrenia. Interventions: clozapine versus risperidone. |
| Wang 2002 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Wang 2002 c | Allocation: not randomised, controlled study. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Wang 2002 d | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia. Intervention: clozapine versus risperidone. |
| Wang 2003 a | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine + euvifor. |
| Wang 2003 b | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Wang 2003 c | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Wang 2004 a | Allocation: controlled study. Participants: people with schizophrenia. Interventions: clozapine versus olanzapine. |
| Wang 2004 b | Allocation: unclear. Participants: people with refractory schizophrenia. Interventions: clozapine versus clozapine+diazepam. |
| Wang 2004 c | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Wang 2004 d | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Wang 2004 e | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine+ yinxing leaf versus clozapine + placebo. |
| Wei 1996 | Allocation: randomised. Participants: people with schizophrenia. Interventions: treated versus drug free, no further details. Outcome: EEG changes. |
| Weickert 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine versus risperidone versus olanzapine versus placebo. |
| Weiser 1975 | Allocation: not randomised. |
| Welbel 1980 | Allocation: not randomised. |
| Weng 1998 | Allocation: randomised. Participants: people with schizophrenia. Intervention: clozapine versus risperidone. |
| Wiholm 1989 | Allocation: not randomised. |
| Williams 1993 | Allocation: not randomised. |
| Wilson 1994 | Allocation: not randomised, case series. |
| Wirshing 1990 | Allocation: not randomised, case report. |
| Wirshing 1999 a | Allocation: not randomised. |
| Woggon 1978 | Allocation: not randomised. |
| Wu 2000 | Allocation: controlled study. Participants: people with schizophrenia. Interventions:clozapine versus chlorpromazine. Outcomes: no usable data. |
| Wu 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone at two dosages. |
| Wu 2002 | Allocation: unclear. Participants: people with refractory schizophrenia. |
| Wudarsky 1999 | Allocation: not randomised. |
| Xiang 2005 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Xie 1998 | Allocation: not randomised. |
| Xie 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine + risperidone versus risperidone. |
| Xin 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Xing 2002 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus Chinese herb. |
| Xu 1997 | Allocation: unclear. Participants: people with schizophrenia treated with clozapine. Interventions: diphenhydramine versus no additional drugs. |
| Xu 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Xu 2002 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Xu 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Yagcioglu 2005 | Allocation: randomised. Participants: people with schizophrenia partially responsive to clozapine. Interventions: risperidone versus placebo. |
| Yan 1984 | Allocation: not randomised, case series. |
| Yang 1988 | Allocation: quasi‐randomised, sequentially assigned. |
| Yang 1998 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Yang 1999 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus chlorpromazine. Outcomes: no usable data. |
| Yang 2004a | Allocation: randomised. Participants: people with refractory schizophrenia. Interventions: clozapine versus quetiapine. |
| Yao 1999 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine + sulpride. |
| Yen 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: risperidone versus haloperidol. Outcomes: no usable data. |
| Yin 2002 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone versus clozapine + risperidone. |
| Yu 2002 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine. |
| Yu 2002 b | Allocation: unclear. Participants: people with treatment‐resistant schizophrenia. Interventions: clozapine versus risperidone. |
| Yu 2004 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine + psychological and social intervention. |
| Yu 2005 a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone |
| Yu 2005 b | Allocation: unclear. Participants: people with schizophrenia. |
| Yue 2004 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone versus clozapine + risperidone. |
| Zahn 1993 | Allocation: not randomised. |
| Zahn 1994 | Allocation: not randomised. |
| Zapletálek 1974 | Allocation: not randomised, case series. |
| Zapletálek 1980 | Allocation: not randomised, case series. |
| Zeng 2001 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Zeng 2002 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Zeng 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus clozapine + psychological education. |
| Zhang 1997 | Allocation: controlled study. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Zhang 2000 | Allocation: unclear. Participants: people with refractory schizophrenia. Interventions: clozapine versus risperidone. |
| Zhang 2002 a | Allocation: cohort study. Participants: people with schizophrenia. Interventions: starting with risperidone or switching from clozapine to risperidone. |
| Zhang 2002 b | Allocation: not randomised. Participants: people with schizophrenia on maintenance doses of clozapine and sulpiride. Interventions: clozapine versus sulpiride. |
| Zhang 2002 c | Allocation: not randomised. |
| Zhang 2002 d | Allocation: randomised. Participants: people with schizophrenia treated with clozapine. Interventions: paroxetine versus placebo. |
| Zhang 2002 e | Allocation: randomised. Participants: people with first‐onset schizophrenia. Interventions: clozapine versus risperidone. |
| Zhang 2002 f | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine. |
| Zhang 2002 h | Allocation: unclear. Participants: people with schizophrenia versus healthy participants. |
| Zhang 2002 i | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Zhang 2004 g | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus sulpiride versus chlorpromazine. |
| Zheng 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Zhou 1997 | Allocation: randomised. Participants: people with schizophrenia. Interventions: electroacupuncture and reduced doses of antipsychotics. |
| Zhou 2000 a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Zhou 2000 b | Allocation: randomised. Participants: people with schizophrenia. Interventions: clozapine versus risperidone. |
| Zhou 2003 a | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Zhou 2003 b | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. |
| Zhu 1999 a | Allocation: randomised. Participants: people with schizophrenia. Intervention: clozapine versus risperidone. |
| Zhu 1999 b | Allocation: not randomised. |
| Zhu 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine. |
| Zhu 2002 a | Allocation: randomised. Participants: people with schizophrenia treated with clozapine. Interventions: music versus no music. |
| Zhu 2002 b | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia Interventions: clozapine versus clozapine + pipotiazine palmitate. |
| Zhu 2002 c | Allocation: quasi‐randomisation, according to the date of admission. |
| Zhu 2003 | Allocation: randomised. Participants: people with treatment‐resistant schizophrenia. Interventions: clozapine versus risperidone. |
| Zimmermann 1996 | Allocation: not randomised. |
| Zito 1993 | Allocation: not randomised, case series. |
| Zoccali 2003 | Allocation: randomised. Participants: people with chronic schizophrenia. Interventions: clozapine versus risperidone versus olanzapine or mirtazepine. |
| Zoccali 2004 | Allocation: randomised. Participants: people with schizophrenia and schizo‐affective disorder who are receiving clozapine. Interventions: mirtazepine versus placebo. |
| Zou 2001 | Allocation: unclear. Participants: people with schizophrenia. Interventions: clozapine, no comparator. |
| Zuo 2002 | Allocation: unclear. Participants: people with schizophrenia, first‐episode recovered on clozapine or risperidone. Interventions: clozapine versus risperidone. |
FKP ‐ a symptom rating scale for evaluation of pharmacotherapy in psychoses Serejskij scale ‐ a global rating scale VP ‐an adverse effect rating scale
Characteristics of studies awaiting assessment [ordered by study ID]
Yang 2004 b.
| Methods | Allocation: randomised. |
| Participants | Diagnosis: schizophrenia. |
| Interventions | 1. Clozapine. 2. Typical antipsychotic drugs. |
| Outcomes | Unclear ‐ are being sought. |
| Notes | None. |
Differences between protocol and review
We have added to the review a primary outcome ‐ "no clinically important change in global state" as defined by the individual studies ‐ this had not been clearly defined in the first versions of the review.
We have substantially reformatted this review in light of changes to software (RevMan 5).
We have analysed outcomes using relative risk (see Methods) ‐ rather than odds ratios.
Contributions of authors
Adib Essali ‐ initiated the review and participated in literature searches, selected studies and extracted data, wrote report. N Haj‐hasan ‐ selected studies. Chunbo Li ‐ participated in data extraction. John Rathbone ‐ selected studies, extracted data and helped writing the report during the 2009 update.
Sources of support
Internal sources
Al‐tal General Hospital, Damascus, Syrian Arab Republic.
Shanghai Jiaotong University, China.
Department of Health, England and Wales, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Buchanan 1994 (H) {published data only}
- Arango C, Breier A, McMahon R, Carpenter WT Jr, Buchanan RW. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. American Journal of Psychiatry 2003;160(8):1421‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Arango C, Buchanan RW, Breier A, MacMahon R, Carpenter JWT. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. Schizophrenia Research 2003;60(1):189. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Carpenter W T, Davies O, Moricle LA, Irish D. Clozapine treatment in schizophrenic outpatients: Preliminary results from a double‐blind efficacy study. Schizophrenia Research 1991;4(3):315. [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT Jr. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. American Journal of Psychiatry 1994;151:20‐6. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Waltrip RW II, Listwak S, Holmes C, Goldstein DS. The effect of clozapine on plasma norepinephrine: relationship to clinical efficacy. Neuropsychopharmacology 1994;10:1‐7. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT. Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. American Journal of Psychiatry 1998;155:751‐60. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Holstein C, Breier A. The comparative efficacy and long‐term effect of clozapine treatment on neurological test performance. Biological Psychiatry 1994;36:717‐25. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Koeppl P, Breier A. Stability of neurological signs with clozapine treatment. Biological Psychiatry 1994;36:198‐200. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Buchanan RW, Irish D, Breier A. Differential effect of clozapine on weight: a controlled study. American Journal of Psychiatry 1996;153:817‐9. [DOI] [PubMed] [Google Scholar]
Chiu 1976 (CPZ) {published data only}
- Chiu E, Burrows G, Stevenson J. Double‐blind comparison of clozapine with chlorpromazine in acute schizophrenic illness. Australian and New Zealand Journal of Psychiatry 1976;10:343‐7. [DOI] [PubMed] [Google Scholar]
Ciurezu 1976 (H) {published data only}
- Ciurezu T, Lonescu R, Nica‐Udangiu S, Niturad D, Oproiu L, Tudorache D, et al. Double‐blind clinical study of HF 1854 (LX 100‐129, clozapine or leponex) as compared with haloperidol [Étude clinique en "double blind" du HF 1854 (=LX 100‐129 =clozapine =Leponex) comparé à l´halopéridol]. Neurologie et psychiatrie 1976;14:29‐34. [PubMed] [Google Scholar]
Claghorn 1983 (CPZ) {published data only}
- Claghorn J, Honigfeld G, Abuzzahab FS Sr, Wang R, Steinbook R, Tuason V, Klerman G. The risks and benefits of clozapine versus chlorpromazine. Journal of Clinical Psychopharmacology 1987;7:377‐84. [PubMed] [Google Scholar]
- Claghorn JL, Abuzzahab FS Sr, Wang R, Larson C, Gelenberg AJ, Klerman GL, et al. The current status of clozapine. Psychopharmacology Bulletin 1983;19:138‐40. [Google Scholar]
- Honigfeld G, Patin J, Singer J. Clozapine antipsychotic activity in treatment‐resistant schizophrenics. Advances in Therapy 1984;1:77‐97. [Google Scholar]
Dong 1999 (CPZ) {published data only}
- Dong Z, Liu C, Feng C, Wang C, Gan J. Experimental study the effect of clozapine on ESR and WBC. Medical Journal of Chinese Civil Administration 1999;11(2):82‐3, 125. [Google Scholar]
Du 2003 (CPZ) {published data only}
- Du Wen‐Jia, Yu Jin‐Long, Zheng Hong‐Bo, Zhong Xiao‐Qi, Ma Cui. Chlorpromazine, clozapine and risperidone in the treatment of cognitive function in schizophrenia. International Medicine & Health Guidance News 2003;9(16):86‐8. [Google Scholar]
Erlandsen 1977 (H) {published data only}
- Erlandsen C. Trial of a new neuroleptic drug, Leponex (clozapine) in long‐standing schizophrenia [Utprøving av et nytt nevroleptikum, Leponex (clozapin) hos schizofrene med lang sykehistorie]. Nordic Journal of Psychiatry 1981;35:248‐53. [Google Scholar]
- Erlandsen C. Trial of clozapine in chronic schizophrenics. Proceedings of the 6th World Congress of Psychiatry. Honolulu, USA. 1977:266.
Essock 1996 (H/CPZ/Flu) {published and unpublished data}
- Covell NH, Weissman EM, Essock SM. Weight gain with clozapine compared to first generation antipsychotic medications. Schizophrenia Bulletin 2004;30(2):229‐40. [EMBASE: 2004274059; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Essock SM, Frisman LK, Covell NH, Hargreaves WA. Cost‐effectiveness of clozapine compared with conventional antipsychotic medication for patients in state hospitals. Archives of General Psychiatry 2000;57(10):987‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Essock SM, Hargreaves WA, Covell NH, Goethe J. Clozapine's effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacology Bulletin 1996;32:683‐97. [PubMed] [Google Scholar]
- Essock SM, Hargreaves WA, Dohm FA, Goethe J, Carver L, Hipshman L. Clozapine eligibility among state hospital patients. Schizophrenia Bulletin 1996;22:15‐25. [DOI] [PubMed] [Google Scholar]
- Jackson CT, Covell NH, Essock SM. Differential effectiveness of clozapine for patients nonresponsive to or intolerant of first generation antipsychotic medications. Schizophrenia Bulletin 2004;30(2):219‐27. [EMBASE: 2004274058] [DOI] [PubMed] [Google Scholar]
Fan 1999 (Clopen) {published data only}
- Fan S, Sun ZG, Li J. The comparison of effects of clopenthixal and clozapine in the treatment of schizophrenia. Acta Acadimae Medicinae Qingdao 1999;35(2):125‐7. [Google Scholar]
Fischer‐C 1974 (CPZ) {published data only}
- Dick P, Rémy M, Rey‐Bellet JJ. Comparison of two antipsychotic drugs: chlorpromazine and clozapine [Essai de comparaison de deux antipsychotiques: La chlorpromazine et la clozapine]. Therapeutische Umschau 1975;32:497‐500. [PubMed] [Google Scholar]
- Ekblom B, Haggstrom JE. Clozapine (Leponex) compared with chlorpromazine: a double‐blind evaluation of pharmacological and clinical properties. Current Therapeutic Research and Clinical Experience 1974;16:945‐57. [PubMed] [Google Scholar]
- Fischer‐Cornelssen K, Ferner U. Results of European multi‐center double‐blind studies with clozapine (Leponex) [Ergebnisse Europäischer Multicenter‐doppelblindstudien mit Clozapin (Leponex)]. Clozapin: Zweites Symposium. Wien, 1976.
- Fischer‐Cornelssen K, Ferner U, Steiner H. Multihospital Trial [Multifokale Psychopharmakaprüfung]. Arzneimittelforschung 1974;24:1706‐24. [PubMed] [Google Scholar]
- Fischer‐Cornelssen K, Ferner U, Steiner H. Multispectral investigation of psychotropic drugs [Multifokale Psychopharmakaprüfung]. Arzneimittelforschung 1974;24:1006‐7. [PubMed] [Google Scholar]
- Fischer‐Cornelssen KA, Ferner UJ. An example of European multicenter trials: multispectral analysis of clozapine. Psychopharmacology Bulletin 1976;12:34‐9. [PubMed] [Google Scholar]
- Honigfeld G, Patin J, Singer J. Clozapine antipsychotic activity in treatment‐resistant schizophrenics. Advances in Therapy 1984;1:77‐97. [Google Scholar]
- Niskanen P, Achté K, Jaskari M, Karesoja M, Melsted B, Nilsson LB. Results of a comparative double‐blind study with clozapine and chlorpromazine in the treatment of schizophrenic patients. Psychiatria Fennica 1974;5:307‐13. [Google Scholar]
- Vencovsky E, Peterova E, Baudis P. Comparison of the therapeutic effect of clozapine and chlorpromazine [Srovnáni terapeutického úcinku clozapinu s chlorpromazinem]. Ceskoslovenska Psychiatrie 1975;71:21‐6. [PubMed] [Google Scholar]
Fischer‐C 1976 a (Clopen) {published data only}
- Fischer‐Cornelessen KA, Ferner UJ. An example of European multicenter trials: multi spectral analysis of clozapine. Psychopharmacology Bulletin 1976;12:34‐9. [PubMed] [Google Scholar]
Gelenberg 1979 (CPZ) {published data only}
- Gelenberg AJ, Doller JC. Clozapine versus chlorpromazine for the treatment of schizophrenia: preliminary results from a double‐blind study. Journal of Clinical Psychiatry 1979;40:238‐40. [PubMed] [Google Scholar]
Gerlach 1974 (H) {published data only}
- Gerlach J, Koppelhus P, Helweg E, Monrad A. Clozapine and haloperidol in a single‐blind cross‐over trial: therapeutic and biochemical aspects in the treatment of schizophrenia. Acta Psychiatrica Scandinavica 1974;50:410‐24. [DOI] [PubMed] [Google Scholar]
- Gerlach J, Munkvad I. Clozapine and haloperidol: clinical and biochemical aspects in the treatment of schizophrenia [Clozapin og haloperidol: Kliniske og biokemiske aspekter i skizofrenibehandlingen]. Nordisk Psykiatrisk Tidsskrift 1974;28:463. [Google Scholar]
Gerlach 1975 (H) {published data only}
- Gerlach J, Thorsen K, Fog R. Extrapyramidal reactions and amine metabolites in cerebrospinal fluid during haloperidol and clozapine treatment of schizophrenic patients. Psychopharmacologia 1975;40:341‐50. [DOI] [PubMed] [Google Scholar]
Guirguis 1977 (CPZ) {published data only}
- Guirguis E, Voineskos G, Gray J, Schlieman E. Clozapine (Leponex) versus chlorpromazine (Largactil) in acute schizophrenia (a double‐blind controlled study). Current Therapeutic Research 1977;21:707‐19. [Google Scholar]
Guo‐Zhen 2002 (CPZ) {published data only}
- Yuan Guo‐Zhen, Huang Yan‐Ping, Li Xiang. Antipsychotics on weight and blood glucose. Journal of Clinical Psychological Medicine 2002;12(6):369‐70. [Google Scholar]
Hong 1997 (CPZ) {published data only}
- Hong CJ, Chen JY, Chiu HJ, Sim CB. A double‐blind comparative study of clozapine versus chlorpromazine on Chinese patients with treatment‐refractory schizophrenia. International Clinical Psychopharmacology 1997;12:123‐30. [DOI] [PubMed] [Google Scholar]
Honigfeld 1984 (H) {published data only}
- Honigfeld G, Patin J, Singer J. Clozapine antipsychotic activity in treatment‐resistant schizophrenics. Advances in Therapy 1984;1:77‐97. [Google Scholar]
Howanitz 1996 (CPZ) {published data only}
- Horananitz E, Pardo M, Smelson DA, Engelhart C, Eisenstein N, Losonczy MF. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia: erratum. Journal of Clinical Psychiatry 1999;60(5):341. [PsycINFO 1999‐05378‐017] [DOI] [PubMed] [Google Scholar]
- Howanitz E, Pardo M, Smelson DA, Engelhart C, Eisenstein N, Losonczy MF. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. Journal of Clinical Psychiatry 1999;60:41‐4. [DOI] [PubMed] [Google Scholar]
- Howanitz EM, Pardo M, Litwin P, Stern RG, Wainwright KM, Losonczy MF. Efficiacy of clozapine versus chlorpromazine in geriatric schizophrenia. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; 1996 May 4‐9; New York. New York: American Psychiatric Association, 1996.
Huang 2001 (CPZ) {published data only}
- Huang A. A comparative analysis of eeg changes after taking risperidone, chlorpromazine and clozapine. Medical Journal of Chinese Civil Administration 2001;13(1):19, 20. [Google Scholar]
Itoh 1974 (H) {published data only}
- Itoh H, Miura S, Yagi G, Sakurai S, Ohtsuka N. Some methodological considerations for the clinical evaluation of neuroleptics ‐ comparative effects of clozapine and haloperidol on schizophrenics. Folia Psychiatrica Neurologica Japonica 1977;31:17‐24. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yagi G, Ogita K, Itoh H, Miura S. Some methodological considerations for demonstrating differential effects of neuroleptics / Comparison of clozapine with haloperidol in schizophrenia. Journal of Pharmacology 1974;5:S98. [Google Scholar]
Kane 1988 (CPZ) {published and unpublished data}
- Anon. Study demonstrates efficacy of clozapine in treatment‐resistant schizophrenia. Clinical Pharmacy 1988;7(12):858. [MEDLINE: ] [PubMed] [Google Scholar]
- Borison RL, Diamond BI, Sinha D, Gupta RP, Ajiboye PA. Clozapine withdrawal rebound psychosis. Psychopharmacology Bulletin 1988;24:260‐3. [PubMed] [Google Scholar]
- Conley RR, Schulz SC, Baker RW, Collins JF, Bell JA. Clozapine efficacy in schizophrenic nonresponders. Psychopharmacology Bulletin 1988;24:269‐74. [PubMed] [Google Scholar]
- Heh CW, Herrera J, DeMet E, Potkin S, Costa J, Sramek J Hazlett E, Buchsbaum MS. Neuroleptic‐induced hypothermia associated with amelioration of psychosis in schizophrenia. Neuropsychopharmacology 1988;1:149‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herrera JM, Costa J, Sramek J, Heh C. Clozapine in refractory schizophrenia. Preliminary findings. Schizophrenia Research 1988;1:305‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herrera JN, Sramek JJ, Costa JF, Roy S, Heh CW, Hguyen BH. High potency neuroleptics and violence in schizophrenics. Journal of Nervous and Mental Diseases 1988;176:558‐61. [DOI] [PubMed] [Google Scholar]
- Honigfeld G, Patin J. Predictors of response to clozapine therapy. Psychopharmacology 1989;99:S64‐7. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment‐resistant schizophrenic. A double‐blind comparison with chlorpromazine. Archives of General Psychiatry 1988;45(9):789‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine in treatment‐resistant schizophrenics. Psychopharmacology Bulletin 1988;24:62‐7. [PubMed] [Google Scholar]
- Kane JM, Honigfeld G, Singer J, Meltzer H, Clozaril Collaborative Study Group. Clozapine for the treatment of treatment‐resistant schizophrenia: a double‐blind comparison with chlorpromazine. Archives of General Psychiatry 1988;45:789‐96. [DOI] [PubMed] [Google Scholar]
- Kane JM, Honigfeld G, Singer J, Meltzer HY, Clozaril Collaborative Study Group. Clozapine for the treatment‐resistant schizophrenic: results of a US multicenter trial. Psychopharmacology 1989;99:S60‐3. [Google Scholar]
- Small JG, Milstein V, Small IF, Miller MJ, Lellams JJ, Corsaro CJ. Computerized EEG profiles of haloperidol, chlorpromazine, clozapine and placebo in treatment‐resistant schizophrenia. Clinical Electroencephalography 1987;18:124‐35. [PubMed] [Google Scholar]
Kane 1995 (H) {published and unpublished data}
- Bellack AS, Schooler NR, Kane JM, Marder SR. The impact of clozapine on psychosocial competence. Schizophrenia Research 1995;15:143. [Google Scholar]
- Kane JM, Marder SR, Schooler NR, Wirshing WC, Umbricht D, Baker RW, Wirshing DA, Safferman A, Ganguli R, McMeniman M, Borenstein M. Clozapine and haloperidol in moderately refractory schizophrenia: A 6‐month randomized double‐blind comparison [Clozapin und haloperidol bei maessig resistenter schizophrenie]. Archives of General Psychiatry 2001;58(10):965‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kane JM, Schooler NR, Marder S, Wirshing W, Ames D, Umbricht D, et al. Efficacy of clozapine versus haloperidol in a long‐term clinical trial. Schizophrenia Research 1996;18:127. [Google Scholar]
- Marder SR. Antipsychotics in treatment‐refractory schizophrenia. Journal of Clinical Psychiatry 1998;59:259‐65. [Google Scholar]
- Marder SR, Kane JM, Schooler NR, Wirshing WC, Baker R, Ames D, et al. Effectiveness of clozapine in treatment resistant schizophrenia. Schizophrenia Research 1997;24:187. [Google Scholar]
- Schooler N, Borenstein M, Ames D, Baker R, Umbritch D, Wirshing W, Kane J, Marder S. First improvement with clozapine: How patient should we be?. Schizophrenia Research 1997;24(1,2):188. [Google Scholar]
- Schooler N, Kane J, Marder S, Baker R, Safferman A, Wirshing W. Efficacy of clozapine versus haloperidol in a long‐term clinical trial: preliminary findings. Schizophrenia Research 1995;15:165. [Google Scholar]
- Umbricht D, Ames D, Wirshing WC, Baker R, Chengappa R, Borenstein M, et al. Predictors of response to clozapine in a long‐term double blind treatment study. Schizophrenia Research 1997;24:189. [Google Scholar]
- Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J. Effects of clozapine on auditory event‐related potentials in schizophrenia. Biological Psychiatry 1998;44:716‐25. [DOI] [PubMed] [Google Scholar]
- Umbricht DS, Wirshing WC, Wirshing DA, McMeniman M, Schooler NR, Marder SR, Kane JM. Clinical predictors of response to clozapine treatment in ambulatory patients with schizophrenia. Journal of Clinical Psychiatry 2002;63(5):420‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wirshing WC, Baker R, Umbricht D, Ames D, Schooler N, Kane J, Marder SR, Borenstein D. Clozapine vs haloperidol:drug intolerance in a controlled six month trial. Schizophrenia Research 1997;24(1,2):268. [Google Scholar]
Klieser 1988 (H) {published data only}
- Klieser E, Schönell H. Clinical pharmacological studies for the treatment of negative symptoms in schizophrenia [Klinisch‐pharmakologische studien zur behandlung schizophrener minussymptomatik]. In: Möller HJ, Pelzer E editor(s). Neuere Ansätze zur Diagnostik und Therapie schizophrener Minussymptomatik. Berlin: Springer‐Verlag, 1990, 1990. [Google Scholar]
- Schönell H, Klieser E. Comparison of the effects of standardized doses of haloperidol and clozapine on systematic delusions of chronic schizophrenics. Psychopharmacology 1988;96(Suppl):186. [Google Scholar]
- Stein D, Schönell H, Klieser E. Haloperidol for the treatment of systematised delusions of chronic schizophrenic patients [Haloperidol zur Behandlung des systematisierten Wahns chronisch schizophrener Patienten]. In: Saletu B editor(s). Biologische Psychiatrie. 2. Drei‐Länder‐Symposium für Biologische Psychiatrie. Stuttgart: Georg Thieme Verlag, 1989. [Google Scholar]
Klieser 1990 (H) {published data only}
- Klieser E, Lehmann E, Heinrich K. Risperidone in comparison with various treatments of schizophrenia. In: Kane JM, Möller HJ, Awouters editor(s). Medical Psychiatry. Vol. 3 Serotonin in antipsychotic treatment: mechanisms and clinical practice, New York: Marcel Dekker, 1996. [Google Scholar]
- Klieser E, Schönell H. Clinical pharmacological studies for the treatment of negative symptoms in schizophrenia [Klinisch‐pharmakologische studien zur behandlung schizophrener minussymptomatik]. In: Möller HJ, Pelzer E editor(s). Neuere Ansätze zur Diagnostik und Therapie schizophrener Minussymptomatik. Berlin: Springer‐Verlag, 1990. [Google Scholar]
- Klieser E, Strauss WH, Burtscheid W. Double‐blind comparative study of haloperidol, remoxipride and clozapine in schizophrenia. Proceedings of the 15th Congress of the Collegium Internationale Neuro‐Psychopharmacologicum; 1986 Dec 14‐17; Puerto Rico. Puerto Rico: Neuro‐Psychopharmacologicum, 1986:111.
- Klieser E, Strauss WH, Lemmer W. The tolerability and efficacy of the atypical neuroleptic remoxipride compared with clozapine and haloperidol in acute schizophrenia. Acta Psychiatrica Scandinavica 1994;89:S68‐73. [DOI] [PubMed] [Google Scholar]
Kumra 1994 (H) {published and unpublished data}
- Gordon CT, Frazier JA, McKenna K, Gledd J, Zametkin A, Zahn T. Childhood‐onset schizophrenia: an NIMH study in progress. Schizophrenia Bulletin 1994;20:697‐712. [DOI] [PubMed] [Google Scholar]
- Huffman GB. Efficacy of clozapine for schizophrenia in children. American Family Physician 1997;55(4):1356. [Google Scholar]
- Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, Hamburger SD, Smith AK, Albus KE, Alaghband‐Rad J, Rapoport JL. Childhood‐onset schizophrenia. A double‐blind clozapine‐haloperidol comparison. Archives of General Psychiatry 1996;53:1090‐7. [DOI] [PubMed] [Google Scholar]
- Kumra S, Jacobsen LK, Rapoport JL. Childhood‐onset schizophrenia: a double‐blind clozapine trial. Proceedings of the American Psychiatric Association Annual Meeting; 1996 May 8; New York. New York: American Psychiatric Association, 1996.
- Piscitelli SC, Frazier JA, McKenna K, Albus KE, Grothe DR, Gordon CT. Plasma clozapine and haloperidol concentrations in adolescents with childhood‐onset schizophrenia: association with response. Journal of Clinical Psychiatry 1994;55:SB94‐7. [PubMed] [Google Scholar]
- Rapoport J, Kumra S, Jacobsen LK. The spectrum of extrapyramidal symptoms in children and young adults. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego. San Diego: American Psychiatric Association, 1997.
- Sanjiv K, Leslie KJ, Judith LR. Childhood‐onset schizophrenia ‐ a double‐blind clozapine trial. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; 1996 May 4‐9; New York. New York: American Psychiatric Association, 1996.
Lee 1994 (mainly H) {published data only}
- Lee MA, Jayathilake K, Meltzer HY. A comparison of the effect of clozapine with typical neuroleptics on cognitive function in neuroleptic‐responsive schizophrenia. Schizophrenia Research 1999;37:1‐11. [DOI] [PubMed] [Google Scholar]
- Lee MA, Thompson PA, Meltzer HY. Effects of clozapine on cognitive function in schizophrenia. Journal of Clinical Psychiatry 1994;55:82‐7. [PubMed] [Google Scholar]
- Meltzer HY. Clozapine withdrawal: serotonergic or dopaminergic mechanisms?. Archives of General Psychiatry 1997;54:760‐3. [DOI] [PubMed] [Google Scholar]
Leon 1974 (CPZ) {published data only}
- Leon CA. Efficiacy of clozapine. Archives of General Psychiatry 1978;35:905. [DOI] [PubMed] [Google Scholar]
- Leon CA. Therapeutic effects of clozapine. A four‐year follow‐up study of a controlled clinical trial. Acta Psychiatrica Scandinavica 1979;59:471‐80. [DOI] [PubMed] [Google Scholar]
- Leon CA, Estrada H. The therapeutic effects of clozapine on psychotic symptoms (a double‐blind study) [Efectos terapeuticos de la Clozapina sobre los sintomas de Psicosis]. Revista Colombiana Psiquiatria 1974;3:309‐18. [Google Scholar]
Li 2003 (Lox) {published data only}
- Li X, Zou Z, Wang Y. Controlled study of loxapine and clozapine in the treatment of schizophrenia. International Chinese Neuropsychiatry Medicine Journal 2003;4(1):1‐2. [Google Scholar]
Lieberman 2003 (CPZ) {published data only}
- Lieberman JA, Phillips M, Gu H, Stroup S, Zhang P, Kong L, Ji ZKG, Hamer RM. Atypical and conventional antipsychotic drugs in treatment‐naive first‐episode schizophrenia: a 52‐week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology 2003;28(5):995‐1003. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Phillips M, Kong L, Gu H, Koch G. Efficacy and safety of clozapine versus chlorpromazaine in first‐episode psychosis: results of a 52 week randomized double blind trial. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000 Dec 10‐14; San Juan. San Juan: American College of Neuropsychopharmacology, 2000.
- Lieberman JA, Phillips M, Kong L, Gu H, Koch G. Efficacy and safety of clozapine versus chlorpromazine in first episode psychosis: results of a 52‐week randomized double‐blind trial. Schizophrenia Research 2001;49(1,2):236. [Google Scholar]
Liu 1994 (Thi) {published data only}
- Liu BL, Chen YY, Yang DS. Effects of thioridazine on schizophrenics and clinical utility of plasma levels. Chinese Journal of Neurology and Psychiatry 1994;27:364‐7. [Google Scholar]
Liu 2002 (CPZ) {published data only}
- Liu J. Clozapine in the treatment of negative symptoms of schizophrenia‐control study. Guizhou Medical Journal 2002;26(6):547‐49. [Google Scholar]
Liu 2004 (CPZ) {published data only}
- Liu Jun‐Biao, Wu Jing‐Hua. Chlorpromazine, clozapine, risperidone on glucose metabolism in patients with first‐episode schizophrenia, the effects of blood lipids and weight. Chinese Journal of Nervous and Mental Diseases 2004;30(4):293‐95. [Google Scholar]
Niu 2001 (CPZ) {published data only}
- Niu Y, Phillips M, Ji Z. A contrast study of the effect of chlorpromazine and clozapine on the cognitive function in non‐system therapeutical schizophrenic patients. Chinese Journal of Psychiatry 2001;34(4):197‐200. [Google Scholar]
Ou 1999 (CPZ) {published data only}
- Ou G. Comparison between chlorpromazine and clozapine in the treatment of positive symptoms in schizophrenia. Journal of Clinical Psychological Medicine 1999;9(3):155‐6. [Google Scholar]
Potter 1989 (CPZ) {published data only}
- Potter WZ, Ko GN, Zhang LD, Yan W. Clozapine in China: a review and preview of US/PRC collaboration. Psychopharmacology 1989;99(Suppl):87‐91. [DOI] [PubMed] [Google Scholar]
Rosenheck 1993 (H) {published and unpublished data}
- Charney DS, Rosenheck RA, Cramer J, Xu W, Jonathan T. The efficacy and cost‐effectiveness of clozapine. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego. San Diego: American Psychiatric Association, 1997.
- Cramer JA, Rosenheck R, Xu W, Thomas J, Henderson W, Charney DS. Quality of life in schizophrenia: a comparison of instruments. Schizophrenia Bulletin 2000;26(3):659‐66. [EMBASE: 2000317440; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Chang S, Choe Y, Cramer J, Xu W, Thomas J, Henderson W, Charney D. Medication continuation and compliance: a comparison of patients treated with clozapine and haloperidol. Journal of Clinical Psychiatry 2000;61(5):382‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Rosenheck R, Charney D. The clinical and economic impact of clozapine treatment on refractory schizophrenia. Controlled Clinical Trials 1993;14:419. [Google Scholar]
- Rosenheck R, Charney D, Cramer J, Xu W, Thomas J. A randomized, double‐blind trial of the efficacy and cost‐effectiveness of clozapine. Schizophrenia Research 1997;24:188. [Google Scholar]
- Rosenheck R, Cramer J, Allan E, Erdos J, Frisman LK, Xu W. Cost‐effectiveness of clozapine in patients with high and low levels of hospital use. Archives of General Psychiatry 1999;56:565‐72. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Cramer J, Jurgis G, Perlick D, Xu W, Thomas J, Henderson W, Charney D. Clinical and psychopharmacologic factors influencing family burden in refractory schizophrenia. Journal of Clinical Psychiatry 2000;61(9):671‐6. [EMBASE: 2000343433] [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Cramer J, Xu W, Grabowski J, Douyon R, Thomas J. Multiple outcome assessment in a study of the cost‐effectiveness of clozapine in the treatment of refractory schizophrenia. Health Services Research 1998;33:1237‐61. [PMC free article] [PubMed] [Google Scholar]
- Rosenheck R, Cramer J, Xu W, Thomas J, Henderson W, Frisman L, Fye C, Charney D. A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. New England Journal of Medicine 1997;337:809‐15. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Doyle J, Leslie D, Fontana A. Changing environments and alternative perspectives in evaluating the cost‐effectiveness of new antipsychotic drugs. Schizophrenia Bulletin 2003;29(1):81‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Dunn L, Peszke M, Cramer J, Xu W, Thomas J, et al. Impact of clozapine on negative symptoms and on the deficit syndrome in refractory schizophrenia. American Journal of Psychiatry 1999;156:88‐93. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Evans D, Herz L, Cramer J, Xu W, Thomas J, Henderson W, Charney D. How long to wait for a response to clozapine: a comparison of time course of response to clozapine and conventional antipsychotic medication in refractory schizophrenia. Schizophrenia Bulletin 1999;25(4):709‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Lawson W, Crayton W, Cramer J, Xu W, Thomas J. Predictors of differential response to clozapine and haloperidol. Biological Psychiatry 1998;44:475‐82. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Tekell J, Peters J, Cramer J, Fontana A, Xu W. Does participation in psychosocial treatment augment the benefit of clozapine? Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia. Archives of General Psychiatry 1998;55:618‐25. [DOI] [PubMed] [Google Scholar]
Shopsin 1978 (CPZ) {published data only}
- Honigfeld G, Patin J, Singer J. Clozapine antipsychotic activity in treatment‐resistant schizophrenics. Advances in Therapy 1984;1:77‐97. [Google Scholar]
- Klein H, Aronson M, Shopsin B. Clozapine: double‐blind controlled trial in the treatment of schizophrenia. Psychopharmacology Bulletin 1978;14:12‐5. [PubMed] [Google Scholar]
- Shopsin B, Klein H, Aaronsom M, Collora M. Clozapine, chlorpromazine, and placebo in newly hospitalized, acutely schizophrenic patients: a controlled, double‐blind comparison. Archives of General Psychiatry 1979;36:657‐64. [DOI] [PubMed] [Google Scholar]
Singer 1974 (CPZ) {published data only}
- Singer K, Law S. Comparative double‐blind study with clozapine (Leponex) and chlorpromazine in acute schizophrenia. Journal of International Medical Research 1974;2:433‐5. [Google Scholar]
Sun 2000 (Perp) {published data only}
- Sun Z. Clozapine combined with perphenazine in the treatment of schizophrenia. Hebei Mental Health 2000;13(2):142‐4. [Google Scholar]
Tamminga 1994 (H) {published data only}
- Tamminga CA, Thaker GK, Moran M, Kakigi T, Gao XM. Clozapine in tardive dyskinesia: observations from human and animal model studies. Journal of Clinical Psychiatry 1994;55(Suppl B):102‐6. [PubMed] [Google Scholar]
Volavka 2002 (H) {published data only}
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer J‐P, Citrome L, McEvoy J, Kunz M, Chakos M, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment‐resistant patients with schizophrenia and schizoaffective disorder. Schizophrenia Research 2002;53(3 Suppl 1):194. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. American Journal of Psychiatry 2002;159(6):1018‐28. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman J‐PLB, Citrome L, McEvoy J, Kunz M, Chakos M, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment‐resistant patients with schizophrenia and schizoaffective disorder. Proceedings of the 14th Congress of the European College of Neuropsychopharmacology; 2001 Oct 13‐17; Istanbul. Istanbul: European College of Neuropsychopharmacology, 2001, issue 3:256.
- Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer J‐P, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatric Services 2001;52(11):1510‐4. [CINAHL 2002032396] [DOI] [PubMed] [Google Scholar]
- Citrome LL, Volavka J, Czobor P, Nolan K, Lieberman JA, Lindenmayer J‐P, Sheitman BB. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco. San Francisco: American Psychiatric Association, 2003.
- Citrome LL, Volavka J, Czobor P, Sheitman BB, Lindenmayer J‐P, McEvoy JP, Lieberman JA. Atypical antipsychotics and hostility in schizophrenia: a double‐blind study. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. Marathon Multimedia, 2001.
- Citrome LL, Volavka J, Czobor P, Sheitman BB, Lindenmayer J‐P, McEvoy JP, Lieberman JA. Atypical antipsychotics and hostility in schizophrenia: a double‐blind study. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia. Philadelphia: American Psychiatric Association, 2002.
- Lindenmayer J‐P, Czobor P, Volavka J, Citrome LL, Sheitman BB, McEvoy JP, Cooper TB. Changes in glucose and cholesterol in schizophrenia treated with atypicals. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia. Philadelphia: American Psychiatric Association, 2002.
- Lindenmayer J‐P, Czobor P, Volavka J, Lieberman JA, Citrome LL, Sheitman BB, McEvoy JP. Effects of atypicals on the syndromal profile in treatment‐resistant schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco. San Francisco: American Psychiatric Association, 2003.
- Lindenmayer J‐P, Czobor P, Yolayka J, Lieberman JA, McEvoy JP, Citrome LL, Sheitman BB. Do atypicals change the syndrome profile in treatment‐resistant schizophrenia?. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. Marathon Multimedia, 2001.
- Lindenmayer J‐P, Czobor P, Yolayka J, Lieberman JA, McEvoy JP, Citrome LL, Sheitman BB. Do atypicals change the syndrome profile in treatment‐resistant schizophrenia?. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia. Philadelphia: American Psychiatric Association, 2002.
- Lindenmayer J‐P, Volavka J, Lieberman JA, Citrome LL, Sheitman B, McEvoy JP, Cooper T. Do atypicals change the syndromal profile in treatment‐resistant schizophrenia?. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia. Philadelphia: American Psychiatric Association, 2002.
- Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Changes in glucose and cholesterol levels in schizophrenia patients treated with typical and atypical antipsychotics. Proceedings of the 23rd Congress of the Collegium Internationale Neuro‐Psychopharmacologicum; 2002 Jun 23‐27; Montreal. Montreal: International Journal of Neuropsychopharmacology, 2002, issue Suppl 1:S169.
- Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. American Journal of Psychiatry 2003;160(2):290‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mohr P, Volavka J, Lieberman JA, Czobor P, McEnvoy J, Lindenmayer J‐P, Citrome L, Sheitman B. Clozapine, olanzapine, risperidone, and haloperidol in refractory schizophrenia. Proceedings of the 10th Congress of the Association of European Psychiatrists (AEP); 2000 Oct 28 ‐ Nov 1; Prague. Prague: European Psychiatry, 2000; Vol. Suppl 2:284.
- Volavka J, Czobor P, Cooper TB, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Lieberman JA. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychiatry 2004;65(1):57‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome LMJP, Cooper TB, Lieberman JA. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychopharmacology 2004;24(2):225‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Sheitman B, Lindenmayer J‐P, Citrome L, McEvoy J, Cooper TB, Chakos M, Kline JALN. Clozapine, olanzapine, risperidone, and haloperidol in refractory schizophrenia. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; Dec 10‐14; San Juan. San Juan: Neuropsychopharmacology, 2000.
- Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. American Journal of Psychiatry 2002;159(2):255‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2001 (CPZ) {published data only}
- Wang C, Xiang Y, Weng Y. The drug‐compliance, dose and concentration for the maintenance treatment of clozapine in schizophrenic patients. Chinese Journal of Psychiatry 2001;34(3):138‐41. [Google Scholar]
Wang 2006a (CPZ) {published data only}
- Wang C, Li Y, Pan M. A clinical study of risperidone, clozapine and chlorpromazine in patients with first‐episode schizophrenia. Journal of Clinical Psychosomatic Diseases 2006;12(1):7‐9. [Google Scholar]
Wang 2006b (CPZ) {published data only}
- Wang C, Li Y, Li Y. Comparative study on the life quality of first‐episode schizophrenics treated with risperidone, clozapine and chlorpromazine. Journal of Clinical Psychosomatic Diseases 2006;12(2):87‐9. [Google Scholar]
Xia 2002 (CPZ) {published data only}
- Xia Y, Wang D. A control study of putting on weight for taking clozapine and chlorpromazine in schizophrenic patients. Health Psychology Journal 2002;10(2):137‐9. [Google Scholar]
Xu 1985 (CPZ) {published data only}
- Xu WR, Bao‐long Z, Qui C, Mei‐fang G. A double‐blind study of clozapine and chlorpromazine treatment in the schizophrenics. Chinese Journal of Nervous Mental Diseases 1985;11:222‐4. [Google Scholar]
Yang 1997 (CPZ) {published data only}
- Yang F. A control study of clozapine and chlorpromazine in the treatment of the negative symptoms of schizophrenia. Journal of Clinical Psychological Medicine 1997;7(1):6‐8. [Google Scholar]
Yang 2002 (CPZ) {published data only}
- Yang FD, Ji ZF. Effects of chlorpromazine and clozapine on the cognitive function in first‐episode schizophrenia patients. Chinese New Drugs Journal 2002;11(2):152‐5. [Google Scholar]
Yang 2004 a (CPZ) {published data only}
- Yang F, Zhang P. Blood glucose in first‐episode schizophrenia with chlorpromazine and clozapine treatment. Shanghai Archives of Psychiatry 2004;16(1):1‐3. [Google Scholar]
Zhang 1996 (CPZ) {published data only}
- Zhang Yan‐Jun, Xu Xi‐Yun, Tian Mao‐Sheng, Liu Ai‐Jun, Zhao Feng‐Yu, Kong Ling‐Sheng, Gong Fu‐Min, Bai Chang‐Lin, Ren Xian‐Feng. The effects of clozapine on the sedimentation rate‐control study. Shandong Archives of Psychiatry 1996;9(4):22‐4. [Google Scholar]
Zhang 2000 (Thi) {published data only}
- Dian‐Zhang, Yi Yan, Jiang Kun‐Huo, Zhou Min. Domestic thioridazine and clozapine treatment of schizophrenia randomized controlled study. Journal of Yichun Medical College 2000;12(4):243‐45. [Google Scholar]
Zhang 2007 (Lox) {published data only}
- Zhang L. Loxapine succinate women and clozapine in the treatment of schizophrenia. Medical Journal of Chinese People's Health 2007;19(17):768. [Google Scholar]
References to studies excluded from this review
Abraham 1997 {published data only}
- Abraham G, Nair C, Tracy JI, Simpson GM, Josiassen RC. The effects of clozapine on symptom clusters in treatment‐refractory patients. Journal of Clinical Psychopharmacology 1997;17:49‐53. [DOI] [PubMed] [Google Scholar]
- Abraham G, Tracy JI, Stanilla JK, Verghese C, Simpson GM, Josiassen RC. The relationship of extrapyramidal symptoms and schizophrenia psychopathology. Schizophrenia Research 1997;24:263. [Google Scholar]
Adams 1991 {published data only}
- Adams CE, Essali A. Working with clozapine. Psychiatric Bulletin 1991;3:231‐2. [Google Scholar]
Adityanjee 1995 {published data only}
- Adityanjee, Pandurangi AK, Pelonero AL. M‐CPP challenge response as predictor of response to clozapine in neuroleptic resistant schizophrenia patients. Schizophrenia Research 1995;15:141. [Google Scholar]
Agelink 1998 {published data only}
- Agelink M W, Kamcili E, Malessa R, Zeit T, Klieser E. Autonomic effects of standard and atypical neuroleptics in schizophrenia: comparison of clozapine vs. haloperidol. Pharmacopsychiatry 1997;30:144. [Google Scholar]
- Agelink MW, Kamcili E, Malessa R, Zeit T, Klieser E. Effects of clozapine and haloperidol on cardiovascular autonomic reactivity in schizophrenic patients: can autonomic function tests predict the response to neuroleptic treatment?. Proceedings of the 10th European College of Neuropsychopharmacology Congress; 1997 Sept 13‐17; Vienna. Vienna: European College of Neuropsychopharmacology, 1997.
- Agelink MW, Malessa R, Kamcili E, Zeit T, Lemmer W, Bertling R. Cardiovascular autonomic reactivity in schizophrenics under neuroleptic treatment: a potential predictor of shot‐term outcome?. Neuropsychobiology 1998;38:19‐24. [DOI] [PubMed] [Google Scholar]
Aitchison 1997 {published data only}
- Aitchison KJ, Kerwin RW. Cost‐effectiveness of clozapine. A UK cost‐based study. British Journal of Psychiatry 1997;171:125‐30. [DOI] [PubMed] [Google Scholar]
Allison 2001 {published data only}
- Allison D, Cavazzoni P, Beasley C, Holcombe J, Buse J. Analysis of random glucose concentration data from patients with schizophrenia treated with typical and atypical agents during double‐blind, randomized, controlled clinical trials. Proceedings of the 7th World Congress of Biological Psychiatry; 2001 Jul 1‐6; Berlin. Berlin: Biological Psychiatry, 2001; Vol. Suppl 1. [7th World Congress of Biological Psychiatry [CD‐ROM]: Conifer, Excerpta Medica Medical Communications BV, 2001 P010‐25]
- Allison DB, Cavazzoni P, Beasley Jr CM, Berg PH, Mukhopadhyay N, Mallinckrodt C, Baker RW, Holcombe J, Taylor CC, Breier A, Buse JB. Random blood glucose levels in patients with schizophrenia treated with typical and atypical antipsychotic agents: an analysis of data from double‐blind, randomized, controlled clinical trial. Proceedings of the 14th Congress of the European College of Neuropsychopharmacology; 2001 Oct 13‐17; Istanbul. Istanbul: European Neuropsychopharmacology, 2001; Vol. 3:280.
Altamura 1999a {published data only}
- Altamura AC, Cao A, Croce ML, Serri L, Soddu A, Laddomada A, Percudani M. Are atypical antipsychotics less depressogenic than typical compounds?. Journal of the European College of Neuropsychopharmacology 1999;9:S168. [Google Scholar]
Altamura 1999b {published data only}
- Altamura AC, Velonà I, Curreli R, Bravi D. Olanzapine in the treatment of paranoid schizophrenia. Journal of the European College of Neuropsychopharmacology 1999;9:S297. [Google Scholar]
Alvarez 1997 {published data only}
- Alvarez E, Barón F, Perez‐Blanco J, Soriano DPJ, Masip C, Perez‐Solá V. Ten years' experience with clozapine in treatment‐resistant schizophrenic patients: factors indicating the therapeutic response. European Psychiatry 1997;12:S343‐6. [DOI] [PubMed] [Google Scholar]
Ames 1996 {published data only}
- Ames D, Wirshing WC, Baker RW, Umbricht DSG, Sun AB, Carter J, Schooler NR, Kane JM, Marder SR. Predictive value of eosinophilia for neutropenia during clozapine treatment. Journal of Clinical Psychiatry 1996;57:579‐81. [DOI] [PubMed] [Google Scholar]
An 2003 {published data only}
- An BF, Liu XQ, Chen JX. A comparative study between quetiapine and clozapine in the treatment of schizophrenia and the relation with the plasman levels of IL‐2, SIL‐2R. Schuan Mental Health 2003;16(3):152‐4. [Google Scholar]
Angst 1971 {published data only}
- Angst J, Bente D, Berner P, Heimann H, Helmchen H, Hippius H. The clinical effect of clozapine [Das klinische Wirkungsbild von Clozapin]. Pharmakopsychiatrie Neuro‐Psychopharmakologie 1971;4:201‐11. [Google Scholar]
- Angst J, Jaenicke U, Padrutt A, Scharfetter C. Results of a double‐blind trial of HF 1854 (8‐chloro‐11‐(4 methyl‐1‐piperazinyl)‐5H‐dibenzo (b, e) (1.4) diazepin) compared to levomepromazine [Ergebnisse eines Doppelblindversuches von HF 1854 (8‐Chlor‐11‐(4 methyl‐1‐piperazinyl)‐5H‐dibenzo (b,e) (1,4) diazepin) im Vergleich zu Levomepromazin]. Pharmakopsychiatrie Neuro‐Psychopharmakologie 1971;4:192‐200. [Google Scholar]
Anil 2001 {published data only}
- Anil AE, Lee M, Jayathilake K, Meltzer HY. Clozapine's effect on primary negative symptoms and the initial level of symptom severity as a predictor of response. Proceedings of the 14th Congress of the European College of Neuropsychopharmacology; 2001 Oct 13‐17; Istanbul. Istanbul: European Neuropsychopharmacology, 2001; Vol. 3:267.
Arango 2001 {published data only}
- Arango c, Summerfelt A. Buchanan R. Lack of P50 changes in a randomised double‐blind study of olanzapine and haloperidol. Proceedings of the VIII International Congress on Schizophrenia Research; 2001 April 28‐May 2; British Columbia. British Columbia: Schizophrenia Research, 2001.
Atmaca 2003 {published data only}
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. Journal of Clinical Psychiatry 2003;64(5):598‐604. [DOI] [PubMed] [Google Scholar]
Bao 1988 {published and unpublished data}
- Bao XG. A double‐blind study on the effect of clozapine penfluridol and chlorpromazine in the treatment of schizophrenia. Chung Hua Shen Ching Ching Shen Ko Tsa Chih 1988;21:274‐6. [PubMed] [Google Scholar]
Battegay 1977 {published data only}
- Battegay R, Coter B, Fleischhauer J, Rauchfleisch U. Results and side effects of treatment with clozapine (Leponex R). Comprehensive Psychiatry 1977;18:423‐8. [DOI] [PubMed] [Google Scholar]
Baymiller 2002 {published data only}
- Baymiller SP, Ball P, McMahon RP, Buchanan RW. Serum glucose and lipid changes during the course of clozapine treatment: the effect of concurrent beta‐adrenergic antagonist treatment. Schizophrenia Research 2002;59:49‐57. [DOI] [PubMed] [Google Scholar]
Beasley 2001 {published data only}
- Beasley CM, Dananberg, Kwong KC, Taylor CCM, Breier A. Treatment‐emergent potential impaired glucose tolerance and potential diabetes with olanzapine compared to other antipsychotic agents and placebo. Biological Psychiatry 2001;49:121S. [Google Scholar]
Bellack 2004 {published data only}
- Bellack AS, Schooler NR, Marder SR, Kane JM, Brown CH, Yang Y. Do clozapine and risperidone affect social competence and problem solving?. American Journal of Psychiatry 2004;161(2):364‐7. [DOI] [PubMed] [Google Scholar]
Berardi 1998 {published data only}
- Berardi D, Troia M, Dell'Atti M, Bartoletti C, Cantaroni C, Ferrari G. Clozapine effectiveness in a psychiatric service in Italy. European Psychiatry 1998;13:317‐9. [DOI] [PubMed] [Google Scholar]
Beuzen 1998 {published data only}
- Beuzen JN, Birkett M, Kiesler G, Wood A. Olanzapine vs. clozapine in resistant schizophrenic patients ‐ results of an international double‐ blind randomised clinical trial. Proceedings of the 21st Collegium Internationale Neuro‐Psychopharmacologicum Congress;1998 Jul 12‐16; Glasgow. Glasgow: Neuro‐Psychopharmacologicum, 1998.
Bian 2003 {published data only}
- Bian QT, Xie GR. A comparative study of quality of life in schizophrenia treated with chlorpromazine, clozapine or risperidone. Chinese Journal of Clinical Psychology 2003;7472(2):125‐7. [Google Scholar]
Birmaher 1992 {published data only}
- Birmaher B, Baker R, Kapur S, Quintana H, Ganguli R. Clozapine for the treatment of adolescents with schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31:160‐4. [DOI] [PubMed] [Google Scholar]
Bitter 2004 {published data only}
- Bitter I, Dossenbach MRK, Brook S, Feldman PD, Metcalfe S, Gagiano CA, Furedi J, Bartko G, Janka Z, Banki CM, Kovacs G, Breier A. Olanzapine versus clozapine in treatment‐resistant or treatment‐intolerant schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2004;28:173‐180. [DOI] [PubMed] [Google Scholar]
Blum 1972 {published data only}
- Blum A, Mauruschat W. Temperature increases and changes in white blood therapy with neuroleptics, with particular attention to the novel Dibenzodiazepin derivative clozapine [Temperaturanstiege und Blutweissveränderungen unter der Therapie mit Neuroleptika ‐unter besonderer Berücksichtigung des neuartigen Dibenzodiazepin‐Derivates Clozapin]. Pharmakopsychiatrie Neuro‐Psychopharmakologie 1972;5:155‐69. [Google Scholar]
Boehle 1995 {published data only}
- Boehle C, Volz H P, Hornstein C, Preussler B, Kunze M, Rauch J, Sauer H. Neuropsychological performance of clozapine treated schizophrenics. Pharmacopsychiatry 1995;28:166. [Google Scholar]
Borovicka 1997 {published data only}
- Borovicka MC, Fuller MA, Konicki PE, Brescan D, Popli A, Jurjus G, Kwon K, Steele V, White J, Jaskiw GE. Treatment of Clozapine‐induced weight gain with phenylpropanolamine. Schizophrenia Research 1997;24(1, 2):185‐6. [Google Scholar]
Bourgeois 2004 {published data only}
- Bourgeois M, Swendsen J, Young F, Amador X, Pini S, Cassano GB, Lindenmayer JP, Hsu C, Alphs L, Meltzer HY, =InterSePT Study Group. Awareness of disorder and suicide risk in the treatment of schizophrenia: results of the international suicide prevention trial. American Journal of Psychiatry 2004;161(8):1494‐6. [DOI] [PubMed] [Google Scholar]
Brandt‐Christensen 1998 {published data only}
- Brandt‐Christensen M, Peacock L, Gerlach J. Clozapine versus typical antipsychotics in schizophrenia: EPS retrospectively (1‐20 years) and prospectively (five years). Proceedings of the 9th Congress of Association of European Psychiatrists; 1998 September 20‐24; Copenhagen. Copenhagen: Association of European Psychiatrists, 1998.
Brankovic 1998 {published data only}
- Brankovic S, Milovanovic S, Damjanovic A, Paunovic VR. No difference between the effects of clozapine and fluphenazine on probabilistic reasoning in paranoid schizophrenia. Proceedings of the 21st Congress of the Collegium Internationale Neuro‐psychopharmacologicum; 1998 Jul 12‐16; Glasgow. Glasgow: Neuro‐psychopharmacologicum, 1998.
- Ravanic DB, Djukic‐Dejanovic SM, Stojiljkovic M, Jankovic S, Paunovic VR, Bankovic D. Antipsychotic efficacy of clozapine vs fluphenazine in positive and negative schizophrenia syndrome.. Journal of Neural Transmission 1996;103:XLVI. [Google Scholar]
Brar 1997 {published data only}
- Brar JS, Chengappa KN, Parepally H, Sandman AR, Kreinbrook SB, Sheth SA, Ganguli R. The effects of clozapine on negative symptoms in‐patients with schizophrenia with minimal positive symptoms. Annals of Clinical Psychiatry 1997;9(4):227‐34. [DOI] [PubMed] [Google Scholar]
Breier 1993 {published data only}
- Breier A, Buchanan RW, Irish D, Carpenter WT Jr. Clozapine treatment of outpatients with schizophrenia: outcome and long‐term response pattern. Hospital and Community Psychiatry 1993;44:1145‐9. [DOI] [PubMed] [Google Scholar]
Broich 1998 {published data only}
- Broich K, Grünwald F, Kasper S, Klemm E, Biersack H‐J, Möller H‐J. D2‐dopamine receptor occupancy measured by IBZM‐SPECT in relation to extrapyramidal side effects. Pharmacopsychiatry 1998;31:159‐62. [DOI] [PubMed] [Google Scholar]
Buchanan 2003 {published data only}
- Buchanan RW, Ball MP, Weiner E, Kirkpatrick B, Gold J, Carpenter WT. Olanzapine treatment of residual positive and negative symptoms. Schizophrenia Research 2003;60(1):275. [DOI] [PubMed] [Google Scholar]
Buchsbaum 1996 {published data only}
- Buchsbaum M, Hazlett E, Haznedar M. Positron emission tomography in schizophrenics treated with sertindole and haloperidol. Proceedings of the 10th World Congress of Psychiatry; 1996 Aug 23‐28; Madrid. Madrid: World Congress of Psychiatry, 1996.
Buchsbaum 1997 {published data only}
- Buchsbaum M, Hazlett E, Bark N, Gupta A, Fallon J, Guich S, Haznedar M. Positron emission tomography in schizophrenics treated with atypical and typical neuroleptics. Schizophrenia Research 1997;24(1,2):163. [Google Scholar]
Buchsbaum 2004 {published data only}
- Buchsbaum MS, Haznedar MM, Hazlett E. Antipsychotic response prediction with fdg‐pet in schizophrenia. Proceedings of the Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence. Florence: World Psychiatric Association, 2004. [WO32.1]
Cai 2000 {published data only}
- Cai C, Wan C, Cheng F. The controlled investigation of risperidone and clozapine in the treatment of schizophrenia. Health Psychology Journal 2000;8(2):152‐4. [Google Scholar]
Cao 2001 {published data only}
- Cao D, Xie S. Different effects of clozapine and risperidone on levels of blood glucose in patients with schizophrenia. Modern Rehabilitation 2001;5(12):144‐5. [Google Scholar]
Cao 2003 {published data only}
- Cao W, Liu X‐L, Wei H‐Y. A comparative study of the therapeutic effects of clozapine and risperidone in the treatment of schizophrenia. Herald of Medicine 2003;22(10):679‐680. [Google Scholar]
Cassano 1997 {published data only}
- Cassano GB, Ciapparelli A, Villa M. Clozapine as a treatment tool: only in resistant schizophrenic patients?. European Psychiatry 1997;12:S347‐51. [DOI] [PubMed] [Google Scholar]
Cavazzoni 2002 {published data only}
- Cavazzoni P, Berg PH, Millikan M, Carlson C, Beasley CM. An integrated analysis of treatment‐emergent extrapyramidal syndrome in schizophrenic patients during olanzapine clinical trials versus placebo, haloperidol, risperidone or clozapine. Schizophrenia Research 2002;53(3 Suppl1):171. [DOI] [PubMed] [Google Scholar]
- Cavezzoni P, Berg P, Millikan M, Carlson C, Beasley C. An integrated analysis of treatment‐emergent extrapyramidal syndrome in schizophrenic patients during olanzapine clinical trials versus placebo, haloperidol, risperidone. Proceedings of the 23rd Congress of the Collegium Internationale Neuro‐Psychopharmacologicum; 2002 Jun 23‐27; Montreal. Montreal: International Journal of Neuropsychopharmacology, 2002; Vol. Suppl 1:S164. [P.3.W.026]
Cha 2002 {published data only}
- Cha A, Xu Y, Zhu XH. The curative effect comparison of risperidone and clozapine to treat recurrent schizophrenia. Chinese Medical Journal of Metallurgical Industry 2002;19(3):143‐4. [Google Scholar]
Chakos 1995 {published data only}
- Chakos MH, Liebermann JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet 1995;345:456‐7. [DOI] [PubMed] [Google Scholar]
Chen 1998a {published data only}
- Chen S, Yang Y, Deng X. Comparison of efficacy and side effects between risperidone and clozapine on negative symptoms of schizophrenia. Sichuan Mental Health 1998;11(4):254‐6. [Google Scholar]
Chen 1998b {published data only}
- Chen YG, Zhao JP, Xie G. A study on serum concentration and clinical response of clozapine with different dose administration for treatment of schizophrenia. Chinese Journal of Psychiatry 1998;31(2):104‐7. [Google Scholar]
Chen 1999a {published data only}
- Chen ZA, Lan LM, Zhu PJ. Risperidone vs clozapine in treatment of schizophrenia. Chinese Journal of New Drugs And Clinical Remedies 1999;18(5):277‐80. [Google Scholar]
Chen 1999b {published data only}
- Chen D, Yu J, Li G. A randomized controlled trial of antipsychotics in type ? And type ? Schizophrenia. Sichuan Mental Health 1999;12(1):16‐8. [Google Scholar]
Chen 2001a {published data only}
- Chen GY, Li B, Li C. Clinical observation on schizophrenic patients treated with risperidone. Journal of Clinical Psychological Medicine 2001;11(6):325‐8. [Google Scholar]
Chen 2001b {published data only}
- Chen L, Zhang X, Li J. A controlled study between clozapine and lithium carbonate in treatment‐refractory schizophrenia. Sichuan Mental Health 2001;14(4):213‐4. [Google Scholar]
Chen 2002 {published data only}
- Chen L. A comparative study of EEG changes in patients treated by risperidone, chlorpromazine and clozpine. Journal of Qiqihar Medical 2002;23(5):485‐6. [Google Scholar]
Chen 2003a {published data only}
- Chen F, Liang L, Zhu XH. A control study of elderly patients with schizophrenia treated with olanzapine or clozapine. Journal of Clinical Psychological Medicine 2003;13(5):298‐9. [Google Scholar]
Chen 2003b {published data only}
- Chen Z, Wang G, Wang H. Comparative study on the quality of life of schizophrenia treated with risperidone and clozepine. Journal of Hubei Medical University 2003;24(2):197‐9. [Google Scholar]
Chen 2005 {published data only}
- Chen Q, Tang Y‐L, Mao P‐X, Cai Z‐J. Effects of clozapine and risperidone on the glucose‐regulating mechanism of patients with schizophrenia. Chinese Journal of Clinical Rehabilitation 2005;9(4):116‐118. [Google Scholar]
Chengappa 2001 {published data only}
- Chengappa KN. New antipsychotics‐clinical trials and followup. www‐commons.cit.nih.gov/crisp/index.html (accessed 19th February 2001).
Chengappa 2003 {published data only}
- Chengappa KNR, Goldstein JM, Greenwood M, John V, Levine J. A post hoc analysis of the impact on hostility and agitation of quetiapine and haloperidol among patients with schizophrenia. Clinical Therapeutics 2003;25(2):530‐41. [DOI] [PubMed] [Google Scholar]
Choc 1990 {published data only}
- Choc MG, Hsuan F, Honigfeld G, Robinson WT, Ereshefsky L, Crismon ML, Saklad SR, Hirschowitz J, Wagner R. Single‐ versus multiple‐dose pharmacokinetics of clozapine in psychiatric patients. Pharmaceutical Research 1990;7:347‐51. [DOI] [PubMed] [Google Scholar]
Chong 1997 {published data only}
- Chong SA, Tan CH, Khoo YM, Lee HS, Wong KE, Ngui F, Winslow M. Clinical evaluation and plasma clozapine concentrations in Chinese patients with schizophrenia. Therapeutic Drug Monitoring 1997;19:219‐23. [DOI] [PubMed] [Google Scholar]
Chou 1999 {published data only}
- Chou X, Chen Q, Li J. A clinical controlled study between risperidone and clozapine in the treatment of chronic schizophrenia. Sichuan Mental Health 1999;12(4):244‐6. [Google Scholar]
Chouinard 1976 {published data only}
- Chouinard G, Annable L. Clozapine in the treatment of newly admitted schizophrenic patients. A pilot study. Journal of Clinical Pharmacology 1976;16:289‐97. [DOI] [PubMed] [Google Scholar]
Cohen 1991 {published data only}
- Cohen BM, Keck PE, Satlin A, Cole JO. Prevalence and severity of akathisia in patients on clozapine. Biological Psychiatry 1991;29:1215‐9. [DOI] [PubMed] [Google Scholar]
Conley 1997 {published data only}
- Conley RR, Carpenter WT Jr, Tamminga, CA. Time to clozapine response in a standardized trial. American Journal of Psychiatry 1997;154:1243‐7. [DOI] [PubMed] [Google Scholar]
Conley 2003 {published data only}
- Conley RR, Kelly DL, Richardson CM, Tamminga CA, Carpenter Jr WT. The efficacy of high‐dose olanzapine versus clozapine in treatment‐resistant schizophrenia: a double‐blind, crossover study. Journal of Clinical Psychopharmacology 2003;23(6):668‐71. [DOI] [PubMed] [Google Scholar]
Cosar 1999 {published data only}
- Cosar B, Candansayar S, Taner E, Isik E. Comparison of efficacy of clozapine, sulpiride, chlorpromazine and haloperidol in chronic schizophrenic patients therapy. Journal of the European College of Neuropsychopharmacology 1999;9:S287. [Google Scholar]
Covell 1999 {published data only}
- Covell NH, Essock SM. Weight with clozapine as compared to conventional antipsychotic medications. Schizophrenia Research 1999;36:354. [Google Scholar]
Covington 2000 {published data only}
- Covington L, Cola PA. Clozapine vs. Haloperidol: Antipsychotic effects on sexual function in schizophrenia. Sexuality and Disability 2000;18(1):41‐8. [Google Scholar]
Cramer 2001 {published data only}
- Cramer J, Rosenheck R, Xu W, Henderson W, Thomas J, Charney D. Detecting improvement in quality of life and symptomatology in schizophrenia. Schizophrenia Bulletin 2001;27(2):227‐34. [DOI] [PubMed] [Google Scholar]
Cui 2002 {published data only}
- Cui B, Shen J, Tian H, Wang Y, Guo X. A comparative study of the effects of risperidone, chlorpromazine and clozapine to patients with schizophrenia by the electrocardiograms. Tianjin Pharmacy 2002;3:54‐5. [Google Scholar]
CUTLASS 2003 {published data only}
- Brownlee MM. The CUTLASS study: a multi‐centre randomised controlled trial of atypical anti‐psychotic drugs in severe schizophrenia. National Research Register 2000.
- Jones PB. Cost utility of the latest antipsychotics in severe schizophrenia (CUTLASS). National Research Register 2001.
- Kerwin R. Cost utility of the latest antipsychotics in severe schizophrenia (CUTLASS) ‐ a multi‐centre, randomised controlled trial. National Research Register 2000.
- Lewis S. The CUTLASS study: a multi‐centre randomised controlled trial of atypical anti‐psychotic drugs in severe schizophrenia. National Research Register 2000.
- Lewis Shon. Cost utility of the latest antipsychotics in severe schizophrenia (CUTLASS): a multi‐centre, randomised, controlled trial. National Research Register 2000.
- Marshall M. Cost utility of the latest anti psychotics in severe schizophrenia (CUTLASS). National Research Register 2000.
- Spurrell M. Cost utility of the latest antipsychotics in severe schizophrenia (CUTLASS).. National Research Register 2002.
Dai 2004 {published data only}
- Dai J‐P, Zhao Z‐H, Mai G‐Y. Comparative study on the influence of olanzapine and clozapine on efficacy and quality of life in schizophrenia. Chinese Journal of Behavioral Medical Science 2004;13(4):396‐98. [Google Scholar]
Davidson 1993 {published data only}
- Davidson M, Kahn RS, Stern RG, Hirschowitz J, Apter S, Knott P, Davis KL. Treatment with clozapine and its effect on plasma homovanillic‐acid and norepinephrine concentrations in schizophrenia. Psychiatric Research 1993;46:151‐63. [DOI] [PubMed] [Google Scholar]
Davies 1991 {published data only}
- Davies MA, Conley RR, Schulz SC, Bell Delaney J. One‐year follow‐up of 24 patients in a clinical trial of clozapine. Hospital and Community Psychiatry 1991;42:628‐9. [DOI] [PubMed] [Google Scholar]
Davies 1993 {published data only}
- Davies LM, Drummond MF. Assessment of costs and benefits of drug‐therapy for treatment‐resistant schizophrenia in the United Kingdom. British Journal of Psychiatry 1993;162:38‐42. [DOI] [PubMed] [Google Scholar]
De 2003 {published data only}
- Leon J, Odom‐White A, Josiassen RC, Diaz FJ, Cooper TB, Simpson GM. Serum antimuscarinic activity during clozapine treatment. Journal of Clinical Psychopharmacology 2003;23(4):336‐41. [DOI] [PubMed] [Google Scholar]
De 2004 {published data only}
- Leon J, Diaz FJ, Josiassen RC, Simpson GM. Possible individual and gender differences in the small increases in plasma prolactin levels seen during clozapine treatment. European Archives of Psychiatry and Clinical Neuroscience 2004;254(5):318‐25. [DOI] [PubMed] [Google Scholar]
Dejanovic 2002 {published data only}
- Dejanovic SM Djukic, Pantovic MM, Alexopulos C, Milovanovic DR, Paunovic VR, Ravanic DB. Clozapine vs. Classical antipsychotics in schizophrenia. Proceedings of the XIIth World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama. Yokohama: World Congress of Psychiatry, 2002.
- Djukic‐Dejanovic SM, Ravanic DB, Jankovic S, Stojiljkovic M, Bankovic D, Paunovic VR. Efficacy of neuroleptic drugs in schizophrenia during long‐term posthospital period: clozapine vs haloperidol. Journal of Neural Transmission 1996;103:XXIX. [Google Scholar]
Deng 2000 {published data only}
- Deng H, Zheng H, He Z. A comparative trial of olanzapine versus clozapine in the treatment of schizophrenia. Shanghai Archives of Psychiatry 2000;12(3):143‐45. [Google Scholar]
Diamond 1986 {published data only}
- Diamond B, Borison R. Basic and Clinical Studies of Neuroleptic‐Induced Supersensitivity Psychosis and Dyskinesia. Psychopharmacology Bulletin 1986;22:900‐5. [PubMed] [Google Scholar]
Diaz 2005 {published data only}
- Diaz FJ, Leon J, Josiassen RC, Cooper TB, Simpson GM. Plasma clozapine concentration coefficients of variation in a long‐term study. Schizophrenia Research 2005;72(2‐3):131‐5. [DOI] [PubMed] [Google Scholar]
Dickson 1998 {published data only}
- Dickson RA, Dalby TJ, Williams R, Warden SJ. Hospital days in clozapine‐treated patients. Canadian Journal of Psychiatry 1998;43:945‐8. [DOI] [PubMed] [Google Scholar]
Dittmann‐Balcar 2003 {published data only}
- Dittmann‐Balcar A, Bender S, Schall U, Klimke A, Mueller N, Vorbach U, Kuehn KU, Dittmann RW, Naber D. Effects of olanzapine versus clozapine on executive functions in schizophrenia. Schizophrenia Research 2003;60(1):131. [Google Scholar]
Drew 1994 {published data only}
- Drew LRH, Wood MM, Way RT. Initial experiences with clozapine at Kenmore Psychiatric Hospital. Medical Journal of Australia 1994;161:199‐201. [DOI] [PubMed] [Google Scholar]
Drummond 1996 {published data only}
- Drummond M, Knapp M, Burns T, Miller K, Ruiz R. Methodological issues in the outcomes assessment of a new, atypical antipsychotic. Proceedings of the 9th Congress of the European College of Neuropsychopharmacology; 1996 Sep 21‐25; Amsterdam. Amsterdam: Neuropsychopharmacology, 1996.
Du 2004a {published data only}
- Du Z‐H, Wu H‐L. Comparison of chlorpromazine, clozapine and risperidone in the effect on indices of liver function. Journal of Practical Medical Techniques 2004;11(6A):885‐6. [Google Scholar]
Du 2004b {published data only}
- Du Z‐H. A study on the change of serum thyroxin treated with risperdal or clozapine. Journal of Practical Medical Techniques 2004;11(68):1020‐21. [Google Scholar]
Dye 1996 {published data only}
- Dye SM, Mortimer AM. The neuropsychology of clozapine treatment in schizophrenia. Proceedings of the 8th Biennial Winter Workshop on Schizophrenia; 1996 Mar 16‐22; Crans Montana, Switzerland. Crans Montana: Schizophrenia Research, 1996:1‐12.
- Dye SM, Mortimer AM, Lock M. Clozapine versus treatment as usual in schizophrenia. Schizophrenia Research 1996;18(2,3):126. [Google Scholar]
Earnst 1999 {published data only}
- Earnst KS, Taylor SF, Smet IC, Goldman RS, Tandon R, Berent S. The effects of typical antipsychotics, clozapine, and risperidone on neuropsychological test performance in schizophrenia. Schizophrenia Research 1999;40(3):255‐6. [DOI] [PubMed] [Google Scholar]
Edwards 1999 {published data only}
- Edwards J, Maude D, McGorry P, Cocks J, Burnett P, Davern M, Bennett C, Harrigan S, Herman T, Wade D, Bell R. Treatment of enduring positive symptoms in first‐episode psychosis: a randomised controlled trial of cbt and clozapine. Schizophrenia Research 1999;1,2 & 3:278. [Google Scholar]
- Edwards J, Wong L, Burnett P, Harrigan SM, McGorry PD, Wade D, Murphy B, Drew L, Albiston D. Enduring positive symptoms in first episode psychosis: A randomised controlled trial of clozapine and CBT. Schizophrenia Research 2003;60(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elman 1997 {published data only}
- Elman I, Goldstein DS, Holmes C, Adler C, Pickar D, Breier A. The effects of clozapine on plasma norepinephrine kinetics in schizophrenic patients. Schizophrenia Research 1997;24(1,2):214. [Google Scholar]
Elman 1999 {published data only}
- Elman I, Goldstein D, Eisenhofer G, Folio J, Malhotra A, Adler C, Pickar D, Breier A. Mechanism of perihepal noradrenegic Stimulation by Clozapine. Neuropsychopharmacology 1999;20:29‐34. [DOI] [PubMed] [Google Scholar]
Faltus 1973 {published data only}
- Faltus F, Hynek K, Dolezalová V, Kmunicková Z, Zemek P. Experience in the treatment of schizophrenia with clozapine. Activitas Nervosa Superior 1973;15:95. [PubMed] [Google Scholar]
Faltus 1974 {published data only}
- Faltus F. Clozapine in the maintenance therapy of schizophrenia. Activitas Nervosa Superior 1974;16:205‐6. [PubMed] [Google Scholar]
Fan 2003 {published data only}
- Fan C, Leiying, Wang K. The effect of clozapine and risperidone on blood sugar of patients with schizophrenia. Shandong Archives of Psychiatry 2003;16(3):131‐2. [Google Scholar]
Finzen 2002 {published data only}
- Finzen A. Changing neuroleptics: From new to conventional ‐ And vice versa [Neuroleptikawechsel: von atypischen zu konventionellen und zuruck ‐ und umgekehrt]. Psychiatrische Praxis 2002;29(8):445‐6. [Google Scholar]
Frazier 1994 {published data only}
- Frazier JA, Gordon CT, McKenna K, Lenane MC, Jih D, Rapoport JL. An open trial of clozapine in 11 adolescents with childhood onset schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry 1994;33:658‐63. [DOI] [PubMed] [Google Scholar]
Fremont 1996 {published data only}
- Fremont P, Bazin N, Pillot B, Lochu A, Colen Demelo P, Pastol I, Zanata G. Four years using clozapine in community psychiatry. Encephale 1996;22:24‐7. [PubMed] [Google Scholar]
Friedman 2003 {published data only}
- Friedman JH. Atypical antipsychotics in the EPS‐vulnerable patient. Psychoneuroendocrinology 2003;28(Suppl 1):39‐51. [DOI] [PubMed] [Google Scholar]
Gallhofer 1996 {published data only}
- Gallhofer, Bauer, Lis, Krieger, Gruppe. Cognitive dysfunction in schizophrenia: comparison of treatment with atypical antipsychotic agents and conventional neuroleptic drugs. European Neuro‐psychopharmacology 1996;S2:13‐20. [DOI] [PubMed] [Google Scholar]
Gan 1996 {published data only}
- Gan J, Lu C. Influence of smoking on dosage, therapeutic efficacy and side effects of clozapine in schizophrenic. Chinese Journal of Behavioral Medical Science 1996;5(4):195‐6. [Google Scholar]
Gan 1999 {published data only}
- Gan JL. A control study of risperidone and clozapine in the freatment of first episode schizophrenia. Medical Journal of Chinese Civil Administration 1999;11(1):4‐7, 61. [Google Scholar]
Ganguli 2005 {published data only}
- Ganguli R, Brar JS. Prevention of weight gain, by behavioral interventions, in patients starting novel antipsychotics. Schizophrenia Bulletin 2005;31:561‐2. [Google Scholar]
Gao 2003 a {published data only}
- Gao CZ, Gao Z. A study of quetiapine in the treatment of first‐onset schizophrenia. Journal of Clinical Psychological Medicine 2003;13(4):221‐2. [Google Scholar]
Gao 2003 b {published data only}
- Gao X, Yu D, Zen Y. It was decreased for thyroxine in schizophrenics taking clozapine. Sichuan Mental Health 2003;16(2):68‐70. [Google Scholar]
Ge 2004 {published data only}
- Ge Q, Xiong L, Liang X. A comparative study of risperidone, clozapine and haloperidol in the treatment of patients with schizophrenia. Sichuan Mental Health 2004;17(2):79‐81. [Google Scholar]
Gekiere 1996 {published data only}
- Gekiere F, Dessalles MC, Poisson N, Maitre L. Clinical follow‐up ‐ EEG ‐ serum determinations: therapeutic experience with clozapine. Encephale 1996;22:16‐23. [PubMed] [Google Scholar]
Gerlach 1977 {published data only}
- Gerlach J, Rasmussen PT, Hansen L, Kristjansen P. Antiparkinsonian agents and long‐term neuroleptic treatment. Effect of G 31406, orphenadrine, and placebo on parkinsonism, schizophrenic symptoms, depression and anxiety. Acta Psychiatrica Scandinavica 1977;55(4):251‐60. [DOI] [PubMed] [Google Scholar]
Gerlach 1978 {published data only}
- Gerlach J, Simmelsgaard H. Tardive dyskinesia during and following treatment with haloperidol, haloperidol+biperiden, thioridazine, and clozapine. Psychopharmacology 1978;59:105‐12. [DOI] [PubMed] [Google Scholar]
Glick 2004 {published data only}
- Glick ID, Zaninelli R, Hsu C, Young FK, Weiss L, Gunay I, Kumar V. Patterns of concomitant psychotropic medication use during a 2‐year study comparing clozapine and olanzapine for the prevention of suicidal behavior. Journal of Clinical Psychiatry 2004;65(5):679‐85. [DOI] [PubMed] [Google Scholar]
Goff 1996 {published data only}
- Goff DC, Tsai G, Amico E, Coyle J. Trials of d‐cycloserine added to conventional antipsychotics and clozapine in schizophrenia. Schizophrenia Research 1999;36:280. [Google Scholar]
- Goff DC, Tsai G, Manoach DS, Flood J, Darby DG, Coyle JT. D‐cycloserine added to clozapine for patients with schizophrenia. American Journal of Psychiatry 1996;153(12):1628‐30. [MEDLINE: ; PMID 8942463] [DOI] [PubMed] [Google Scholar]
Goldberg 1993 {published data only}
- Goldberg TE, Greenberg RD, Griffin SJ, Gold JM, Kleinman JE, Pickar D, Schulz SC, Weinberger DR. The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. British Journal of Psychiatry 1993;162:43‐8. [DOI] [PubMed] [Google Scholar]
Goldberg 2000 {published data only}
- Goldberg TE, Dodge M, Aloia M, Egan MF, Weinberger DR. Effects of neuroleptic medications on speech disorganization in schizophrenia: biasing associative networks towards meaning. Psychological Medicine 2000;30:1123‐30. [DOI] [PubMed] [Google Scholar]
Gordon 1996 {published data only}
- Gordon BJ, Milke DJ. Dose related response to clozapine in a state psychiatric hospital population: a naturalistic study. Psychiatric Quarterly 1996;67:65‐74. [DOI] [PubMed] [Google Scholar]
Gray 2000 {published data only}
- Gray R. Research in brief. Does patient education enhance compliance with clozapine? A preliminary investigation. Journal of Psychiatric and Mental Health Nursing 2000;7(3):285‐6. [DOI] [PubMed] [Google Scholar]
Gross 1969 {published data only}
- Gross H, Langner E. Clinical qualification of Neurolepticums from Dibenzodiazepin series [Klinische Qualifikation eines Neurolepticums aus der Dibenzodiazepin‐Reihe]. Arzneimittelforschung 1969;19:496‐8. [Google Scholar]
Gross 1970 {published data only}
- Gross H, Langner E. The neuroleptikum 100‐129/HF‐1854 (clozapine) in the psychiatrie [Das neuroleptikum 100‐129/HF‐1854 (Clozapin) in der psychiatrie]. International Pharmacopsychiatry 1970;4:220‐30. [Google Scholar]
Gross 1974 {published data only}
- Gross H, Langner E, Pfolz H. Clozapine in the long‐term treatment of chronic schizophrenia [Clozapin in der Langzeittherapie der Chronischen Schizophrenie]. Arzneimittelforschung 1974;24:987‐9. [PubMed] [Google Scholar]
Guo 2001 {published data only}
- Guo HR, Zhang SR, Sun FG. Clinical controlled study of risperidone and clozapine in treatment of schizophrenia. Journal of Xinxiang Medical College 2001;18(3):174‐6. [Google Scholar]
Guo 2003a {published data only}
- Guo JY, Du QX. A study of beam changes after taking the different dosages of clozapine with schizophrenic patients. Medical Journal of Chinese People Health 2003;15(11):655‐6. [Google Scholar]
Guo 2003b {published data only}
- Guo S, Lu L, Cheng W. Effect of clozapine and risperidone on interleukin‐2 on schizophrenia. Shanghai Archives of Psychiatry 2003;15(1):35‐8. [Google Scholar]
Hagger 1993 {published data only}
- Hagger C, Buckley P, Kenny JT, Friedman L, Ubogy D, Meltzer HY. Improvement in cognitive functions and psychiatric symptoms in treatment ‐refractory schizophrenic patients receiving clozapine. Biological Psychiatry 1993;34:702‐12. [DOI] [PubMed] [Google Scholar]
Hammock 1995 {published data only}
- Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self‐injurious behavior. Journal of Autism and Developmental Disorders 1995;25:611‐26. [DOI] [PubMed] [Google Scholar]
Hao 2004 {published data only}
- Hao H, Liu J, Huang S. Cost‐effectiveness analysis of risperidone and clozapine in the treatment of schizophrenia. China Pharmacy 2004;15(12):733‐4. [Google Scholar]
Haring 1994 {published data only}
- Haring C, Neudorfer C, Schwitzer J, Hummer M, Saria A, Hinterhuber H, Fleischhacker WW. EEG alterations in patients treated with clozapine in relation to plasma levels. Psychopharmacology 1994;114:97‐100. [DOI] [PubMed] [Google Scholar]
Hasegawa 1993 {published data only}
- Hasegawa M, Gutierrez‐Esteinou R, Way L, Meltzer HY. Relationship between clinical efficacy and clozapine concentrations in plasma in schizophrenia: effect of smoking. Journal of Clinical Psychopharmacology 1993;13:383‐90. [PubMed] [Google Scholar]
He 2003 {published data only}
- He J, Chen YX. A controlled study on quetiapine and clozapine in the treatment of schizophrenic patients. Medical Journal of Chinese Civil Administration 2003;15(6):335‐6. [Google Scholar]
Heim 1987 {published data only}
- Heim M. Ranking of neuroleptic therapy in the self‐evaluations of schizophrenic patients [Zum Stellenwert neuroleptischer Therapie in der Selbstbeurteilung schizophren Erkrankter]. Psychiatrie Neurologie und Medizinische Psychologie 1987;39(8):487‐91. [PubMed] [Google Scholar]
Hemphill 1975 {published data only}
- Hemphill RE, Pascoe FD, Zabow T. An investigation of clozapine in the treatment of acute and chronic schizophrenia and gross behaviour disorders. South African Medical Journal 1975;49:2121‐5. [PubMed] [Google Scholar]
Herst 1997 {published data only}
- Herst L, Powell G. Is clozapine safe in the elderly?. Australian and New Zealand Journal of Psychiatry 1997;31:411‐7. [DOI] [PubMed] [Google Scholar]
Hinze‐Selch 1997 {published data only}
- Hinze‐Selch D, Mullington J, Orth A, Lauer CJ, Pollmacher T. Effects of clozapine on sleep: a longitudinal study. Biological Psychiatry 1997;42:260‐6. [DOI] [PubMed] [Google Scholar]
Honer 1995 {published data only}
- Honer WG, MacEwan GW, Kopala L, Altman S, Chisholm‐Hay S, Singh K. A clinical study of clozapine treatment and predictors of response in a Canadian sample. Canadian Journal of Psychiatry 1995;40:208‐11. [DOI] [PubMed] [Google Scholar]
Honer 2004 {published data only}
- Honer W, MacEwan GW, Williams R, Falkai P, McKenna PJ, Pomarol‐Clotet E, Chen EY, Leung SP, Wong J, Stip E. A randomized, placebo ‐ controlled, double ‐ blind trial of augmentation of clozapine with risperidone. Schizophrenia Bulletin 2005;31:487. [Google Scholar]
- Honer WG. The care‐study: initial data from a double‐blind randomised controlled study of augmenting clozapine with risperidone. Proceedings of the thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence. Florence: World Psychiatric Association, 2004. [WO24.1.]
Honigfeld 1990 {published data only}
- Honigfeld G, Patin J. A two‐year clinical and economic follow‐up of patients on clozapine. Hospital and Community Psychiatry 1990;41:882‐5. [DOI] [PubMed] [Google Scholar]
Hou 2001 {published data only}
- Hou J, Xu G, Ma C. The effects of clozapine and risperidone on serum prolactin levels of female schizophrenia. Journal of Clinical Psychological Medicine 2001;11(1):1‐3. [Google Scholar]
Huang 2003 {published data only}
- Huang S‐P, Ma Z‐W, Guo B‐Y. Effectiveness of quetiatine vs clozapine in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases 2003;9(4):206‐7. [Google Scholar]
Hummer 1995 {published data only}
- Hummer M, Kemmler G, Kurz M, Kurzthaler I, Oberbauer H, Fleischhacker WW. Weight gain induced by clozapine. European Neuropsychopharmacology 1995;5:437‐40. [DOI] [PubMed] [Google Scholar]
Hummer 1996 {published data only}
- Hummer M, Unterweger BS, Kemmler G, Falk M, Kurz M, Oberbauer H, Fleischhacker WW. Does eosinophilia predict clozapine induced neutropenia?. Psychopharmacology 1996;124(1‐2):201‐4. [DOI] [PubMed] [Google Scholar]
Hummer 1997 {published data only}
- Hummer M, Kurz M, Kurzthaler I, Oberbauer H, Miller C, Fleischhacker W. Hepatotoxicity of clozapine. Journal of Clinical Psychopharmacology 1997;17:314‐7. [DOI] [PubMed] [Google Scholar]
Hussain 2003 {published data only}
- Hussain MZ, Chaudhry ZA. Rivastigmine and galantamine treatment for schizophrenic cognitive impairment. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco. San Francisco: American Psychiatric Association, 2003.
Hussain 2004 {published data only}
- Hussain MZ, Chaudhry ZA. Rivastigmine and Galantamine Treatment of Schizophrenic Cognitive Impairment. Proceedings of the 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York. New York: American Psychiatric Association, 2004.
Jalenques 1992 {published data only}
- Jalenques I, Coudert AJ. A new therapeutic approach to drug‐resistant schizophrenia: clozapine. Long‐term prespective study in 16 patients [Une nouvelle approche medicamenteuse des schizophrenies resistantes: la clozapine. Etude prospective a long terme chez 16 patients]. Acta Psychiatrica Belgica 1992;92:323‐38. [PubMed] [Google Scholar]
Jeste 1993 {published data only}
- Jeste DV, Lacro JP, Gilbert PL, Kline J, Kline N. Treatment of late‐life schizophrenia with neuroleptics. Schizophrenia Bulletin 1993;4:817‐30. [DOI] [PubMed] [Google Scholar]
Jia 2000 {published data only}
- Jia Z, Zhang Z, Jin S. A controlled trial for comparing clozapine combined with pipotiazine palmitate to clozapine alone in the treatment of negative symptoms in schizophrenic patients. Herald of Medicine 2000;19(2):142‐3. [Google Scholar]
Jin 2002 {published data only}
- Jin Z, Chen W, Yuan B. A clinical observation of treatment of negative symptoms in schizophrenia with a combination of fluoxetine and clozapine. Journal of Clinical Psychological Medicine 2002;12(2):76‐7. [Google Scholar]
Joffe 1996 {published data only}
- Joffe G, Venäläinen E, Tupala J, Hiltunen O, Wahlbeck K, Gadeke R, et al. The effect of clozapine on the course of illness in chronic schizophrenia: focus on treatment outcome in out‐patients. International Clinical Psychopharmacology 1996;11:265‐72. [DOI] [PubMed] [Google Scholar]
Jones 2005 {published data only}
- Jones PB, Davies L, Barnes TR, Murray RM, Dunn G, Hayhurst KP, Markwick A, Lloyd H, Lewis SW. Randomised controlled trial of effect on quality of life of prescription of second generation ( atypical ) versus first generation antipsychotic drugs in schizophrenia. Schizophrenia Bulletin. 2005; Vol. 31:489. [DOI] [PMC free article] [PubMed]
Josiassen 2003 {published data only}
- Josiassen R, Joseph A, Kohegyi E, Paing W. Clozapine augmentation with risperidone in refractory schizophrenia. Schizophrenia Research 2003;60(1):288. [Google Scholar]
Josiassen 2005 {published data only}
- Josiassen RC, Joseph A, Kohegyi E, Stokes S, Dadvand M, Paing WW, Shaughnessy RA. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double‐blind, placebo‐controlled trial. American Journal of Psychiatry 2005;162(1):130‐6. [DOI] [PubMed] [Google Scholar]
Juul‐Povlsen 1985 {published data only}
- Juul‐Povlsen U, Noring U, Fog R, Gerlach J. Tolerability and therapeutic effect of clozapine. A retrospective investigation of 216 patients treated with clozapine for up to 12 years. Acta Psychiatrica Scandinavica 1985;71:176‐85. [DOI] [PubMed] [Google Scholar]
Kahn 1993 {published data only}
- Kahn RS, Davidson M, Siever L, Gabriel S, Apter S, Davis KL. Serotonin function and treatment response to clozapine in schizophrenic patients. American Journal of Psychiatry 1993;150:1337‐42. [DOI] [PubMed] [Google Scholar]
Kahn 1994 {published data only}
- Kahn. Serotonin challenge tests in schizophrenia. Proceedings of the XX1st Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1998 Jul 12‐16; Glasgow. Glasgow: Neuro‐Psychopharmacologicum, 1998.
- Kahn, Davidson, Siever, Sevy, Davis. Clozapine treatment and its effect on neuroendocrine responses induced by serotonin agonist , ‐Chlorophenylpiperazine. Society of Biological Psychiatry 1994;35:909‐12. [DOI] [PubMed] [Google Scholar]
Kane 1993 {published data only}
- Kane JM, Woerner MG, Pollack S, Safferman AZ, Lieberman JA. Does clozapine cause tardive dyskinesia?. Journal of Clinical Psychiatry 1993;54:327‐30. [PubMed] [Google Scholar]
Keefe 2004 {published data only}
- Keefe RSE, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RRJ, Yurgelun‐Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first‐episode psychosis: a randomized, double‐blind trial of olanzapine versus low doses of haloperidol. American Journal of Psychiatry 2004;161(6):985‐95. [DOI] [PubMed] [Google Scholar]
Kelly 2003 {published data only}
- Kelly DL, Conley RR, Richardson CM, Tamminga CA, Carpenter Jr WT. Adverse effects and laboratory parameters of high‐dose olanzapine vs. clozapine in treatment‐resistant schizophrenia. Annals of Clinical Psychiatry 2003;15(3‐4):181‐6. [DOI] [PubMed] [Google Scholar]
Kenny 1992 {published data only}
- Kenny JT, Meltzer HY. Effect of atypical and typical antipsychotic drugs on neuropsychological functions in early‐ stage schizophrenic patients. Proceedings of the 7th Biennial European Winter Workshop on Schizophrenia; 1994 Jan 23‐28; Les Diablerets. Les Diablerets: Schizophrenia Research, 1992; Vol. 2:162.
Kiejna 1993 {published data only}
- Kiejna A, Borys J, Hein AK, Baranowski P. Clinical evaluation of Clozapol in the treatment of schizophrenia [Ocena kliniczna Klozapolu w leczeniu schizofrenii]. Psychiatria Polska 1993;26:563‐74. [PubMed] [Google Scholar]
Kilian 2004 {published data only}
- Kilian R, Angermeyer MC. The impact of antipsychotic medication on the incidence and the costs of inpatient treatment in people with schizophrenia: results from a prospective observational study [Der Einfluss der Neuroleptikabehandlung auf die Inzidenz und die Kosten stationarer psychiatrischer Behandlungen bei schizophren Erkrankten: Ergebnisse einer prospektiven Beobachtungsstudie]. Psychiatrische Praxis 2004;31(3):138‐46. [DOI] [PubMed] [Google Scholar]
Knegtering 2002 {published data only}
- Knegtering H, Castlelein S, Linde J V D, Bous J. Sexual dysfunctions and serum prolactine levels in patients using risperidone or quetiapine: a randomised trial. Schizophrenia research 2002;53(3 Suppl 1):163. [Google Scholar]
Ko 1995 {published data only}
- Ko G, Goff D, Herz H, Wilner K, Posever T, Howard H, Heym J, Wong D, Etienne P. Status report: ziprasidone. Schizophrenia Research 1995;15(1):154. [Google Scholar]
Kogeorgos 1995 {published data only}
- Kogeorgos J, Kanellos P, Michalakeas A, Ioannidis J. Sulpiride and risperidone vs. "classical neuroleptics" in schizophrenia: a follow‐up study. Proceedings of the 8th Congress of the European College of Neuropsychopharmacology; 1995 Sep 30 ‐ Oct 4; Venice. Venice: Neuropsychopharmacology, 1995.
Koukkou 1979 {published data only}
- Koukkou M, Angst J, Zimmer D. Paroxysmal EEG activity and psychopathology during the treatment with clozapine. Pharmakopsychiatrie Neuro‐psychopharmakologie 1979;12:173‐83. [DOI] [PubMed] [Google Scholar]
Krakowski 2001 {published data only}
- Krakowski MI. Clozapine and olanzapine in violent schizophrenics. www‐commons.cit.nih.gov/crisp/index.html (accessed 19th February 2001).
Kronig 1995 {published data only}
- Kronig MH, Munne RA, Szymanski S, Safferman AZ, Pollack S, Cooper T, Kane JM, Lieberman JA. Plasma clozapine levels and clinical response for treatment‐refractory schizophrenic patients. American Journal of Psychiatry 1995;152:179‐82. [DOI] [PubMed] [Google Scholar]
Kufferle 1997 {published data only}
- Kufferle B, Tauscher J, Asenbaum S, Vesely C, Podreka I, Brucke T, Kasper S. IBZM SPECT imaging of striatal dopamine‐2 receptors in psychotic patients treated with the novel antipsychotic substance quetiapine in comparison to clozapine and haloperidol. Psychopharmacology 1997;133:323‐28. [DOI] [PubMed] [Google Scholar]
Kuha 1986 {published data only}
- Kuha S, Mittinen E. Long‐term effect of clozapine in schizophrenia. A retrospective study of 108 chronic schizophrenics treated with clozapine for up to seven years. Nordisk Psykiatrisk Tidsskrift 1986;40:225‐30. [Google Scholar]
Kuoppasalmi 1993 {published data only}
- Kuoppasalmi K, Rimón R, Naukkarinen H, Lang S, Sandqvist A, Leinonen E. Clozapine decreases the level of anxiety and aggressive behaviour in patients with therapy‐refractory schizophrenia. Psychiatria Fennica 1993;24:153‐62. [Google Scholar]
- Kuoppasalmi K, Rimón R, Naukkarinen H, Lang S, Sandqvist A, Leinonen E. The use of clozapine in treatment‐refractory schizophrenia. Schizophrenia Research 1993;10:29‐32. [DOI] [PubMed] [Google Scholar]
Kurz 1995 {published data only}
- Kurz M, Hummer M, Kurzthaler I, Oberbauer H, Fleischhacker WW. Efficacy of medium‐dose clozapine for treatment‐resistant schizophrenia. American Journal of Psychiatry 1995;152:1690‐1. [DOI] [PubMed] [Google Scholar]
- Kurz M, Hummer M, Oberbauer H, Fleischhacker WW. Extrapyramidal side effects of clozapine and haloperidol. Psychopharmacology Berlin 1995;118:52‐6. [DOI] [PubMed] [Google Scholar]
Lacro 2001 {published data only}
- Lacro J. Antipsychotic treatment in late life schizophrenia. www‐commons.cit.nih.gov/crisp/index.html (accessed 19th February 2001).
Lahti 2003 {published data only}
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: Comparing clozapine with haloperidol. Biological Psychiatry 2003;53(7):601‐8. [DOI] [PubMed] [Google Scholar]
Laker 1998 {published data only}
- Laker MK, Duffett RS, Cookson JC. Long‐term outcome with clozapine: comparison of patients continuing and discontinuing treatment. International Clinical Psychopharmacology 1998;13:75‐8. [DOI] [PubMed] [Google Scholar]
Lapierre 1980 {published data only}
- Lapierre YD, Ghadirian A, St‐Laurent J, Chaudhry RP. Clozapine in acute schizophrenia ‐ efficacy and toxicity. Current Therapeutic Research 1980;27:391‐400. [Google Scholar]
Lei 2002 {published data only}
- Lei X, Liang T, Zhang X. A controlled study on risperidone and clozapine in treatment of schizophrenia. China Pharmacist 2002;5(3):169‐71. [Google Scholar]
Leon 1995 {published data only}
- Leon Jde, Odom‐White A, Stanilla J, Joiassen R, Simpson GM. Does clozapine induce akathisia?. Schizophrenia Research 1995;15(1,2):207. [Google Scholar]
Leppig 1989 {published data only}
- Leppig M, Bosch B, Naber D, Hippius H. Clozapine in the treatment of 121 out‐patients. Psychopharmacology 1989;99:S77‐9. [DOI] [PubMed] [Google Scholar]
Levkovitch 1995 {published data only}
- Levkovitch Y, Kronenberg J, Kayser N, Zvyagelski M, Gaoni B, Gadoth N. Clozapine for tardive‐dyskinesia in adolescents. Brain and Development 1995;17:213‐5. [DOI] [PubMed] [Google Scholar]
Levkowitz 1994 {published data only}
- Levkowitz Y, Kronnenberg Y, Kaysar N, Harari H, Gaoni B. Clozapine in adolescence onset schizophrenia. Harefuah 1994;127:16‐8. [PubMed] [Google Scholar]
Levy 2004 {published data only}
- Levy DE, O'Malley AJ, Normand SL. Covariate adjustment in clinical trials with non‐ignorable missing data and non‐compliance. Statistics in Medicine 2004;23(15):2319‐39. [DOI] [PubMed] [Google Scholar]
Lewis 2004 {published data only}
- Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, Taylor D, Hayhurst KP, Lloyd H, Markwick A. A randomised, controlled trial of new atypical drugs versus clozapine in treatment‐resistant schizophrenia. Schizophrenia Research 2004;67(1):6‐7. [DOI] [PubMed] [Google Scholar]
Li 1987 {published data only}
- Li Y. Application of NOSIE in the study of neuroleptic treatment. Chung Hua Shen Ching Ching Shen Ko Tsa Chih 1987;20:325‐7. [PubMed] [Google Scholar]
Li 2001 a {published data only}
- Li J, Dai X. A comparative study of rispredone and sulpride in treating schizophrenia. Medical Journal of Chinese Civil Administration 2001;13(3):136‐8. [Google Scholar]
Li 2001 b {published data only}
- Li K, He X, Xu Z. A clinic study on therapeutic effect of syr anshenjianpi for digestion system side effects caused by antipsychotic drugs. Medical Journal of Chinese Civil Administration 2001;13(6):326‐30. [Google Scholar]
Li 2002 {published data only}
- Li D, Zheng M, Zheng J. A comparative study between quetiapine and clozapine in the treatment of schizophrenia. Journal of Clinical Psychological Medicine 2002;12(5):267‐8. [Google Scholar]
Li 2003 a {published data only}
- Li C. A controlled trial comparing venlafaxine versus sulpiride as adjunct in the treatment of negative symptoms of schizophrenia. Shanghai Archives of Psychiatry 2003;15(1):39‐41. [Google Scholar]
Li 2003 b {published data only}
- Li C‐M, Guo L. A comparison of cognitive function in the first‐onset schizophrenia treated with quetiapine and clozapine. Medical Journal of Chinese People Health 2003;15(12):718, 721. [Google Scholar]
Li 2003 c {published data only}
- Li T, Xue XM, Gong CF. A comparison study on the efficacy of risperidone vs dozapine in cognition of schizophrenia. Medical Journal of Chinese People Heacth 2003;15(11):653‐6. [Google Scholar]
Li 2003 d {published data only}
- Li Y, Li S, Cheng Y. A comparative efficacy between risperdone combining little dosage haloperidol on short time and clozapine alone in the treatment of schizophrenia.. Sichuan Mental Health 2003;16(4):196‐8. [Google Scholar]
Li 2004 a {published data only}
- Li Z. A comparative study of clozapine and risperdone lead to putting on weight.. Heath Psychology Journal 2004;12(5):396‐7. [Google Scholar]
Li 2004 b {published data only}
- Li X‐H, Wan J. Effects of the nursing care of mutual participation model on the rehabilitation of inpatients with early schizophrenia. Zhongguo Linchuang Kangfu 2004;8(24):4958‐9. [Google Scholar]
Li 2004 c {published data only}
- Li Y, Shang J, Li C. Control study of loxapine succinate and clozapine on curative effects in treatment of schizophrenics. Heath Psychology Journal 2004;12(3):202. [Google Scholar]
Li 2004 d {published data only}
- Li Zy. Doxepin auxiliary treatment in negative symptoms of schizophrenia. Chinese Journal of Rehabilitation 2004;19(3):192‐3. [Google Scholar]
Liang 2002 {published data only}
- Liang S, Yu G, Ding G. Controlled study of olanzapine and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry 2002;15(4):193‐4. [Google Scholar]
Liao 2004 {published data only}
- Liao C, Yang H, Gao H. Effects of clozapine and risperidone on blood routine examinations of patients with schizophrenia. Journal of Clinical Psychosomatic Diseases 2004;10(3):156‐7. [Google Scholar]
Lieberman 1989 {published data only}
- Lieberman JA, Saltz BL, Johns CA, Pollack S, Kane JM. Clozapine effects on tardive dyskinesia. Psychopharmacology Bulletin 1989;25:57‐62. [PubMed] [Google Scholar]
Lieberman 2001 a {published data only}
- Lieberman JA, Schneider LS, McEroy J, Pariot P, Stroup S, Adiao J, Lebowitz BD. Effectiveness trials of antipsychotic drugs. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. Marathon Multimedia, 2001.
- Lieberman JA, Schneider LS, McEroy J, Pariot P, Stroup S, Adiao J, Lebowitz BD. Effectiveness trials of antipsychotic drugs. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia. Philadelphia: American Psychiatric Association, 2002. [No. 103D]
Lieberman 2001 b {published data only}
- Lieberman JA. Risperidone and clozapine in chronic schizophrenia. www‐commons.cit.nih.gov/crisp/index.html (accessed 19th February 2001).
Lin 2003 {published data only}
- Lin C‐C, Bai Y‐M, Chen J‐Y, Wang Y‐C, Liou Y‐J, Chao C‐H, Lai I‐C, Tsai K‐Y, Chiu H‐J. Switching from clozapine to zotepine in schizophrenic patients: a randomized, single‐blind controlled study. Journal of the European College of Neuropsychopharmacology 2003;13(4):S318. [Google Scholar]
Lindenmayer 1994 a {published data only}
- Lindenmayer JP, Grochowski S, Mabugat L. Clozapine effects on positive and negative symptoms: a six‐month trial in treatment‐refractory schizophrenics. Journal of Clinical Psychopharmacology 1994;14:201‐4. [PubMed] [Google Scholar]
Lindenmayer 1994 b {published data only}
- Lindenmayer JA, Bernstein‐Hyman B, Grochowski S. A new five‐factor model of schizophrenia. Psychiatric Quarterly 1994;65:299‐322. [DOI] [PubMed] [Google Scholar]
Lindström 1988 {published data only}
- Lindstrom LH. A retrospective study on the long‐term efficacy of clozapine in 96 schizophrenic and schizoaffective patients during a 13‐year period. Psychopharmacology 1989;99:S84‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lindström L. The effect of long‐term treatment with clozapine in schizophrenia; a retrospective study in 96 patients treated with clozapine for up to 13 years. Acta Psychiatria Scandinavica 1988;77:524‐9. [DOI] [PubMed] [Google Scholar]
- Lindström LH, Lundberg T. Long‐term effect on outcome of clozapine in chronic therapy‐resistant schizophrenic patients. European Psychiatry 1997;12:S353‐5. [DOI] [PubMed] [Google Scholar]
Lingjærde 1996 {published data only}
- Lingjærde O, Bela M, Krüger MB, Dahle LG, Fossheim I, Helle J. Improvement patterns in schizophrenic "nonresponders" treated with clozapine. Nordic Journal of Psychiatry 1996;50:457‐67. [Google Scholar]
Litman 1996 {published data only}
- Litman RE, Su TP, Potter WZ, Hong WW, Pickar D. Idazoxan and response to typical neuroleptics in treatment‐resistant schizophrenia. Comparison with the atypical neuroleptic, clozapine. British Journal of Psychiatry 1996;168:571‐9. [DOI] [PubMed] [Google Scholar]
Liu 1996 {published data only}
- Liu QH, Li XL, Zhang YQ, Jin SL, Li ZC, Wang NS, Chu JF, Ma SX. A control study of clozapine in combination with sulpiride in alleviating the negative symptoms of schizophrenia. Chinese Journal of Psychiatry 1996;29(2):87‐90. [Google Scholar]
Liu 1996 b {published data only}
- Liu BW, Wang YM, Yu FP, Jiang DF, Sun YX, Tang ZC. Tiapride vs clozapine in treatment of schizophrenia in 153 patients. Chinese Journal of New Drugs and Chinical Remedies 1996;3(2):79, 80. [Google Scholar]
Liu 1997 {published data only}
- Liu P, Luo HC, Shen YC, Guo H, Zhao XY, Yu J, Yin JL, Liu GZ, Zhong HW, Zhang B, Lu LL, Yang FS, Zhang SQ, Ma QM, Yan XX, Zhu LC, Zhu SM, Nie XW. Combined use of ginkgo biloba extracts on the efficacy and adverse reactions of various antipsychotics. Chinese Journal of Clinical Pharmacology 1997;13(4):193‐8. [Google Scholar]
Liu 1999 a {published data only}
- Liu Q, Man C, Li X. A control study of risperidone and colzapine to treating the positive symptoms of schizophrenia. Sichuan Mental Health 1999;12(2):98‐9, 100. [Google Scholar]
Liu 1999 b {published data only}
- Liu S. Two year followup observations on treatments of type schizophrenia with maintenance dose of clozapine or fluanxol. Acta Medicinae Sinica 1999;12(3):266‐7. [Google Scholar]
Liu 2001 {published data only}
- Liu Q, Li X. A comparative study on the efficacy of combining risperidone and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry 2001;14(1):28‐30. [Google Scholar]
Liu 2003 {published data only}
- Liu F, Dai M. The effects of risperidone and clozapine to patients with schizophrenia's life quality. Sichuan Mental Health 2003;16(4):203‐4. [Google Scholar]
Liu 2003 a {published data only}
- Liu T, Li L, Zuo F. A clinical analysis of pharmacotherapies in inpatients with mental disorders. Journal of Clinical Psychological Medicine 2003;13(4):219‐20. [Google Scholar]
Liu 2003 b {published data only}
- Liu Y, Li H, Wang H. Effect and related factors of clozapine and risperidone on glucose‐insulin homeostasis in schizophrenic patients. Shanghai Archives of Psychiatry 2003;15(4):257‐59, 266. [Google Scholar]
Liu 2003 c {published data only}
- Liu X, Lin L, Wang D. The effect of clozapine combined with imipramine on negative symptoms of deteriorated schizophrenia. Shandong Archires of Psychiatry 2003;16(3):153‐4. [Google Scholar]
Liu 2004 a {published data only}
- Liu TF, Yang JZ. Risperidone vs clozapine for treatment of schizophrenia in 30 patients each. Chinese Journal of New Drugs and Clinical Remedies 2004;3(3):147‐9. [Google Scholar]
Liu 2004 b {published data only}
- Liu W, Li H, Zheng L. A controlled study on olanzapine and clozapine in the treatment of the acute phase of schizophrenia. Shanghai Archives of Psychiatry 2004;16(5):282‐4. [Google Scholar]
Liu 2004 c {published data only}
- Liu Y, Li H‐F, Wang H‐F, Wang K‐X, Gao Z‐S, Gu N‐F. Influence of clozapine and risperidone on body weight, leptin level and lipid metabolism in schizophrenic patients. Chinese Journal of New Drugs and Clinical Remedies 2004;9(23):579‐82. [Google Scholar]
Liu 2004 d {published data only}
- Liu Y, Xu M, Chen X. A controlled study of quetiapine and clozapine in the treatment of schizophrenia with predominantly negative symptoms. Shandong Archires of Psychiatry 2004;17(1):6‐8. [Google Scholar]
Liu 2005 {published data only}
- Liu S‐F, Mu J‐L, Zhang Y‐J, Wang S‐G, Wei P, Wang D‐P, Wang Y, Ma H‐J, Jia F‐J. Effect of clozapine of different dosages on the cognitive function in patients with schizophrenia assessed by the changes of p300 potentials. Chinese Journal of Clinical Rehabilitation 2005;9(8):56‐7. [Google Scholar]
Liu 2005 b {published data only}
- Liu S‐F, Wang Y, Wang D‐P, Wei P, Wang S‐G, Ma H‐J, Jia F‐J. Effects of different doses clozapine on glucose metabolism in male schizophrenics. Chinese Journal of New Drugs and Clinical Remedies 2005;24(2):98‐101. [Google Scholar]
Louwerens 2000 {published data only}
- Louwerens JW, vdMeij APM, Slooff CJ. Therapy resistance: the effectiveness of the second antipsychotic drug, a multicentre double blind comparative study ('switch study'). Schizophrenia Research 2000;41:183. [Google Scholar]
Lu 1998 {published data only}
- Lu X, Xue S, Yang M. A double‐blind comparative study of diphenhydramine and placebo in the treatment of sialorrhea caused by clozapine. Sichuan Mental Health 1998;11(1):35‐7. [Google Scholar]
Lu 2002 a {published data only}
- Lu H, Yang X. Observation of therapeutic effect of shu xuening and clozapine on chronic schizophrenia. Guangxi Journal of Traditional Chinese Medicine 2002;25(1):9‐10. [Google Scholar]
Lu 2002 b {published data only}
- Lu Y, Ren Q, Tian M. A comparison of cognitive function in the first‐onset schizophrenia treated with risperidone and clozapine. Shandong Archives of Psychiatry 2002;15(4):206‐7. [Google Scholar]
Lu 2003 {published data only}
- Lu X‐Q, Zhu G‐L. Control studies of loxapine succinate and clozapine in treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases 2003;9(3):157‐8. [Google Scholar]
Lu 2004 {published data only}
- Lu M‐L, Lane H‐Y, Lin S‐K, Chen K‐P, Chang W‐H. Adjunctive fluvoxamine inhibits clozapine‐related weight gain and metabolic disturbances. Journal of Clinical Psychiatry 2004;65(6):766‐71. [DOI] [PubMed] [Google Scholar]
Lu 2005 {published data only}
- Lu S, Zhang S‐A, Ren Y. Comparative study on the psychopathology and the quality of life of schizophrenia treated with risperidone, clozapine and quetiapine. Journal of Nursing Science 2005;20(3):57‐9. [Google Scholar]
Luo 1994 {published data only}
- Luo H, Wan X, Zhao Y. Efficacy of clozapine ill the treatment positive and negative symptoms of schizophrenia. Chinese Journal of Neurology 1994;27(1):9‐13. [Google Scholar]
Luo 2001 {published data only}
- Luo W, Wang J, Deng Z. Preliminary study on the property of clozaping with traditional chinese medicine theory. Sichuan Mental Health 2001;14(1):12‐3. [Google Scholar]
Lv 2004 {published data only}
- Lv L‐X, Guo S‐Q, Chen W, Li Q, Cheng J, Zhang H‐Y, Shi T‐Y, Shi Y. Effects of clozapine and risperidone on serum interleukin‐2,‐10,‐18 in adolescent schizophrenia patients. Journal of Applied Clinical Pediatrics 2004;19(11):983‐86. [Google Scholar]
Lü 2002 {published data only}
- Lü L, Guo S, Ji M. A control study of the effect of one single dosage of clozapine on eeg in schizophrenia. Journal of Clinical Psychological Medicine 2002;12(3):131‐2. [Google Scholar]
Ma 2001 {published data only}
- Ma W, Yang L, Li S. Comparative study on serum prolactin change during ECT at the basis of chlorpromazine or clozapine treatment. Chinese Mental Health Journal 2001;15(5):349‐50. [Google Scholar]
Malykhin 2003 {published data only}
- Malykhin N. Comparative efficacy of risperidone, clozapine and haloperidol in the treatment of schizoaffective disorders with manic symptoms. Journal of the European College of Neuropsychopharmacology. 2003; Vol. 13, issue 4:S304.
Mao 2000 {published data only}
- Mao Y, Da Z. Study of cost‐effectiveness on risperidone vs clozapine in the treatment of inpatients with schizophrenia. Shanghai Archives of Psychiatry 2000;12(4):211‐13. [Google Scholar]
Marchesi 1996 {published data only}
- Marchesi GF, Nardi B, Pannelli G, Santone G, Ianni P, Brandoni M. Acute CEEG modifications after clozapine and haloperidol administration in schizophrenics. Proceedings of the 20th Congress of the Collegium Internationale Neuro‐psychopharmacologicum; 1996 Jun 23‐27; Melbourne. Melbourne: Neuro‐psychopharmacologicum, 1996.
- Marchesi GF, Nardi B, Pannelli G, Santone G, Ianni P, Brandoni M. Acute pharmacodynamical effects of clozapine and haloperidol ‐ ceeg investigation in schizophrenics. Proceedings of the 10th World Congress of Psychiatry; 1996 Aug 23‐28; Madrid. Madrid: World Congress of Psychiatry, 1996.
Marder 2003 {published data only}
- Marder SR, Schooler NR, Kane JM, Petrides G, Chengappa KN, Wirshing WC, Wirshing DA, Umbricht D, Parapelli H. Tolerability of clozapine and risperidone during a twenty nine week trial. Schizophrenia Research 2003;60(1):293‐4. [Google Scholar]
Markianos 2001 {published data only}
- Markianos M, Hatzimanolis J, Lykouras L. Neuroendocrine responsivities of the pituitary dopamine system in male schizophrenic patients during treatment with clozapine, olanzapine, risperidone, sulpiride, or haloperidol. European Archives of Psychiatry & Clinical Neuroscience 2001;251(3):141‐6. [DOI] [PubMed] [Google Scholar]
Matejcek 1984 {published data only}
- Matejcek M, Neff G, Tjeerdsma H, Krebs E. Pharmaco‐EEG studies with fluperlapine. Arzneimittel Forschung 1984;34(1):114‐20. [PubMed] [Google Scholar]
Mattes 1989 {published data only}
- Mattes JA. Clozapine for refractory schizophrenia: an open study of 14 patients treated up to two years. Journal of Clinical Psychiatry 1989;50:389‐91. [PubMed] [Google Scholar]
Matz 1974 {published data only}
- Matz R, Rick W, Thompson H, Gershon S. Clozapine ‐ a potential antipsychotic agent without extrapyramidal manifestations. Current Therapeutic Research, Clinical and Experimental 1974;16:687‐95. [PubMed] [Google Scholar]
Mazurek 2003 {published data only}
- Mazurek I, Loza B, Lecyk A. Atypical versus typical antipsychotic treatment prognosis based on veps and wcst scores in paranoid schizophrenia. Journal of the European College of Neuropsychopharmacology 2003;13(4):S347. [Google Scholar]
McAllister 1989 {published data only}
- McAllister CG, Rapaport MH, Pickar D, Paul SM. Effects of short‐term administration of antipsychotic drugs on lymphocyte subsets in schizophrenic patients. Archives of General Psychiatry 1989;46:956‐7. [DOI] [PubMed] [Google Scholar]
McEvoy 1995 {published data only}
- McEvoy J, Freudenreich O, McGee M, VanderZwaag C, Levin E, Rose J. Clozapine decreases smoking in patients with chronic schizophrenia. Biological Psychiatry 1995;37:550‐2. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, VanderZwaag C, McGee M, Freudenreich O, Wilson WH, Cooper TB. A double blind, randomized trial comparing clozapine treatment within three distinct serum level ranges in patients with refractory chronic schizophrenia. Schizophrenia Research 1996;22:127. [DOI] [PubMed] [Google Scholar]
McGurk 2005 {published data only}
- McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H, Schooler NR, Kane JM. The effects of clozapine and risperidone on spatial working memory in schizophrenia. American Journal of Psychiatry 2005;162(5):1013‐6. [DOI] [PubMed] [Google Scholar]
Meehan 2000 {published data only}
- Meehan KM, David SR, Taylor CC, Sutton VK. Change in extrapyramidal symptoms with olanzapine in comparison with other antipsychotic agents. Schizophrenia Research 2000;41:192. [Google Scholar]
Mei 2001 {published data only}
- Mei Q, Zhu X, Shen J. A comparative study of social function in schizophrenic patients treated with risperidone or clozapine. Journal of Clinical Psychological Medicine 2001;11(2):83‐5. [Google Scholar]
Meltzer 1989 {published data only}
- Meltzer HY, Bastani B, Young Kwon K, Ramirez LF, Burnett S, Sharpe J. A prospective study of clozapine in treatment‐resistant schizophrenic patients. I. Preliminary report. Psychopharmacology 1989;99:S68‐72. [DOI] [PubMed] [Google Scholar]
Meltzer 1996 {published data only}
- Meltzer HY, Lee MA, Ranjan R, Mason EA, Cola PA. Relapse following clozapine withdrawal ‐ effect of neuroleptic drugs and cyproheptadine. Psychopharmacology 1996;124(1‐2):176‐87. [DOI] [PubMed] [Google Scholar]
Meltzer 1999 {published data only}
- Meltzer HY. Suicide and schizophrenia: clozapine and the Intersept study. Journal of Clinical Psychiatry 1999;60(Suppl 12):47‐50. [PubMed] [Google Scholar]
Meltzer 2003 {published data only}
- Meltzer HY. Reducing risk of suicide in schizophrenia. Journal of the European College of Neuropsychopharmacology 2003;13(4):S163. [Google Scholar]
- Meltzer HY. Reducing suicidality in schizophrenia and schizoaffective disorder. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco. San Francisco: American Psychiatric Association, 2003.
- Meltzer HY, Alphs L, Greem AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer J‐P, Potkin S, InterSePT Study Group. Clozapine treatment for suicidality in schizophrenia. Archives of General Psychiatry 2003;60:82‐91. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Baldessarini RJ. Reducing the risk for suicide in schizophrenia and affective disorders. Journal of Clinical Psychiatry 2003;64(9):1122‐9. [DOI] [PubMed] [Google Scholar]
Meltzer 2004 {published data only}
- Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta‐Trial Study Group. Placebo‐controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. American Journal of Psychiatry 2004;161(6):975‐84. [DOI] [PubMed] [Google Scholar]
Meng 2002 {published data only}
- Meng J, Tang MQ, Huang JS. Long‐time following study on quality of life in 204 schizophrenics treatd with risperidone or clozapine. Chinese Journal of Behavioral Medical Science 2002;11(4):397‐9. [Google Scholar]
Miller 1994 {published data only}
- Miller DD, Fleming F, Holman TL, Perry PJ. Plasma clozapine concentrations as a predictor of clinical response: a follow‐up study. Journal of Clinical Psychiatry 1994;55:S117‐21. [PubMed] [Google Scholar]
Miller 1998 {published data only}
- Miller CH, Mohr F, Umbricht D, Woerner M, Fleischhacker WW, Lieberman J. The prevalence of acute extrapyramidal signs and symptoms in patients treated with clozapine, risperidone, and conventional neuroleptics. Journal of Clinical Psychiatry 1998;59:69‐75. [DOI] [PubMed] [Google Scholar]
Milton 1978 {published data only}
- Milton F, Patwa VK, Hafner RJ. Confrontation vs. belief modification in persistently deluded patients. British Journal of Medical Psychology 1978;51:127‐30. [DOI] [PubMed] [Google Scholar]
Molcan 1974 {published data only}
- Molcan J, Novotny V, Schulpkova L. Our experience with clozapine treatment. Activitas Nervosa Superior 1974;16:200‐1. [PubMed] [Google Scholar]
Moller 2004 {published data only}
- Moller HJ, Riedel M, Muller N, Fischer W, Kohnen R. Zotepine versus placebo in the treatment of schizophrenic patients with stable primary negative symptoms: a randomized double‐blind multicenter trial. Pharmacopsychiatry 2004;37(6):270‐8. [DOI] [PubMed] [Google Scholar]
Moresco 2004 {published data only}
- Moresco RM, Cavallaro R, Messa C, Bravi D, Gobbo C, Galli LLG, Colombo C, Rizzo G, Velona I, Smeraldi E, Fazio F. Cerebral D2 and 5‐HT2 receptor occupancy in Schizophrenic patients treated with olanzapine or clozapine. Journal of Psychopharmacology 2004;18(3):355‐65. [DOI] [PubMed] [Google Scholar]
Mortimer 1994 {published and unpublished data}
- Mortimer AM, Lech S, Lock M, Smith A. The neuropsychology of clozapine treatment. A pilot controlled study. Neuropsychopharmacology 1994;10:S206. [Google Scholar]
- Mortimer AM, Smith A, Lock M, Lekh S, Rooke‐Ley S. Clozapine and neuropsychological function. Preliminary report of a controlled study. Human Psychopharmacology 1995;10:157‐8. [Google Scholar]
Mulqueen 2000 {published data only}
- Mulqueen AW, Wudarsky M, Nicolson RJ, Gochman P, Hamburger S, Lenane M, Rapoport JHL. Weight gain in pediatric patients on typical and atypical antipsychotics. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago. Chicago: American Psychiatric Association, 2000.
- Mulqueen AW, Wudarsky M, Nicolson RJ, Gochman P, Hamburger S, Lenane M, Rapoport JHL. Weight gain in pediatric patients on typical and atypical antipsychotics. Proceedings of the 55th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA, USA. 2002.
Muñecas 1975 {published data only}
- Arranz‐Muñecas T, Lopez‐Beorlegui T, Cavallos M, Sanz JA. Clinical trials of clozapine (Leponex) [Ensayo clinico de la clozapina (Leponex)]. Actas Luso Espanolas de Neurologia Psquiatria Y Ciencias Afines 1975;3:103‐10. [PubMed] [Google Scholar]
Naber 1989 {published data only}
- Naber D, Lepping M, Grohmann R, Hippius H. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia ‐ a retrospective study of 387 patients. Psychopharmacology 1989;99:S73‐6. [DOI] [PubMed] [Google Scholar]
Naber 2001 {published data only}
- Naber D, Bandelow B, Bender S, Klimke A, Ku"hn K, Lambert M, Lemmer W, Dittmann M, Riedel ER. Subjective well‐being under neuroleptic treatment with olanzapine versus clozapine: first results from a double‐blind clinical trial using the swn self‐rating scale. Schizophrenia Research 2001;49:240. [Google Scholar]
- Naber D, Degner D, Bender S, Klimke A, Kuhn KU, Lambert M, Lemmer W, Riedel M, Vorbach EU, Dittmann RW. Olanzapine vs. clozapine: findings on subjective well‐being from a double‐blind clinical trial. Proceedings of the 11th Biennial Winter Workshop on Schizophrenia; 2002 Feb 24‐Mar 1; Davos. Davos: Schizophrenia Research, 2002.
Nahunek 1975 {published data only}
- Nahunek K, Svestka J, Misurec J, Rodova A. Clinical experience with clozapin [Klinické zkusenosti s clozpinem]. Ceskoslovenska Psychiatrie 1975;71:11‐6. [PubMed] [Google Scholar]
Nahunek 1976 {published data only}
- Nahunek K, Rodova A, Svestka J. Outline classification of neuroleptic drugs based on results of short‐term control crossed studies in schizophrenia. Ceskoslovenska Psychiatrie 1976;72(2):104‐14. [Google Scholar]
- Nahunek K, Svestka J, Ceskova E. On the question of differences between therapeutic responses after particular neuroleptics in comparison to perphenazine in schizophrenics. Ceskoslovenska Psychiatrie 1981;77(1):25‐30. [PubMed] [Google Scholar]
Nair 1997 {published data only}
- Nair C, Abraham G, Stanilla JK, Simpson GM, Josiassen RC. Tardive dyskinesia and extrapyramidal symptoms in treatment‐resistant schizophrenics treated with clozapine. Schizophrenia Research 1997;24(1,2):272. [Google Scholar]
Nan 2001 {published data only}
- Nan Z, Wang J, Ji H. A controlled study of risperidone and clozapine both influences congnitive function of patients with schizophrenia. Sichuan Mental Health 2001;14(4):198‐200. [Google Scholar]
Nemeroff 1996 {published data only}
- Nemeroff. Quality of life and new antipsychotics. Proceedings of the 10th World Congress of Psychiatry; 1996 Aug 23‐28; Madrid. Madrid: World Congress of Psychiatry, 1996.
Niu 2001 {published data only}
- Niu Y, Phillips M, Ji Z. A contrast study of the effect of chlorpromazine and clozapine on the cognitive function in non‐system therapeutical schizophrenic patients. Chinese Journal of Psychiatry 2001;34(4):197‐200. [Google Scholar]
Oliemeulen 2000 {published data only}
- Oliemeulen EAP, Hoof JJM, Jogem‐Kosterman BJM, Hulsttijn W, Tuynman‐Qua HG. Is olanzapine a substitute for clozapine? The effects on psychomotor performance. Schizophrenia Research 2000;41(1):187. [Google Scholar]
Owen 1989 {published data only}
- Owen RR Jr, Beake BJ, Marby D, Dessain EC, Cole JO. Response to clozapine in chronic psychotic patients. Psychopharmacology Bulletin 1989;25:253‐6. [PubMed] [Google Scholar]
Owen 1993 {published data only}
- Owen RR Jr, Gutierrez‐Esteinou R, Hsiao J, Hadd K, Benkelfat C, Lawlor BA, Murphy DL, Pickar D. Effects of clozapine and fluphenazine treatment on responses to m chlorophenylpiperazine infusions in schizophrenia. Archives of General Psychiatry 1993;50(8):636‐44. [DOI] [PubMed] [Google Scholar]
Pang 2002 {published data only}
- Pang D, Wang C, Cui A. Effect of quetiapine fumarate and clozapine on clinical rehabilitation of schizophrenia:a controlled study. Chinese Journal of Clinical Rehabilitation 2002;6(7):1007. [Google Scholar]
Panteleeva 1987 {published data only}
- Panteleeva GP, Kovskaya MY, Belyaev BS, Minsker EI, Vynar O, Ceskova E. Clozapine in the treatment of schizophrenic patients: an international multicenter trial. Clinical Therapy 1987;10:57‐68. [PubMed] [Google Scholar]
Panteleeva 1991 {published data only}
- Panteleeva GP, Gamkrelidze SA, Dikaia VI, Magalif AA, Okuneva TP, Perevozniuk AG. Problem of side effects of leponex on blood (multicenter international study) [K voprosu o pobochnom deistvii leponeksa na sostoianie krovi (mnogotsentrovoe mezhdunarodnoe issledovanie)]. Zhurnal Nevropatologii I Psikhiatrii Imeni S S Korsakova 1991;91:70‐3. [PubMed] [Google Scholar]
Paunovic 1991 {published data only}
- Paunovic VR, Timotijevic I, Marinkovic D. Neuroleptic actions on the thyroid axis: different effects of clozapine and haloperidol. International Clinical Psychopharmacology 1991;6(3):133‐9. [PubMed] [Google Scholar]
Peacock 1996 {published data only}
- Peacock L, Solgaard T, Lublin H, Gerlach J. Clozapine versus typical antipsychotics. A retro‐ and prospective study of extrapyramidal side effects. Psychopharmacology 1996;124:188‐96. [DOI] [PubMed] [Google Scholar]
Peet 2002 {published data only}
- Horrobin DF, Bennett CN, Peet M. Correlation between clinical improvement and red cell fatty acid changes when treating schizophrenia with eicospentaenoic acid. Schizophrenia Research 2001;49(12):232. [Google Scholar]
- Peet M, Horrobin DF. A dose‐ranging exploratory study of the effects of ethyl‐eicosapentaenoate in patients with persistent schizophrenic symptoms. Journal of Psychiatric Research 2002;36:7‐18. [DOI] [PubMed] [Google Scholar]
Peng 2001 a {published data only}
- Peng H, Kuang Y, Huang X. A control study of risperidone in combination with clozapine in treating refractory schizophrenia. Journal of Modern Clinical Medical Bioengineering 2001;7(2):100‐2. [Google Scholar]
Peng 2001 b {published data only}
- Peng J, Liu T, Wang Y. Effects of clozapine and risperidone treatment on the serum prolactin, t3 and t4 levels. Journal of Clinical Psychological Medicine 2001;11(5):259‐60. [Google Scholar]
Peng 2004 {published data only}
- Peng J‐F, Xu Y‐M, Wang Y‐B. Effects of clozapine and risperidone on the serum prolactin levels and body weight regulation. Journal of Clinical Psychological Medicine 2004;14(5):268‐9. [113903] [Google Scholar]
Percudani 1998 {published data only}
- Percudani M, Fattore G, Galletta J, Contini A, Altamura AC. Clozapine treatment in therapy‐refractory schizophrenia: an economic analysis. Proceedings of the 9th Congress of Association of European Psychiatrists; 1998 Sep 20‐24; Copenhagen. Copenhagen: Association of European Psychiatrists, 1998.
Perez 2003 {published data only}
- Perez R, Gonzalez‐Blanch C, Sierra‐Biddle D, Martinez I, Vazquez‐Barquero JL, Crespo‐Facorro B. Efficacy and safety of olanzapine, risperidone and haloperidol in acute treatment of patients with first episode psychosis. Schizophrenia Research 2003;60(1):298‐9. [Google Scholar]
Petit 1992 {published data only}
- Petit M, Dollfus S. Critical study of the conditions of prescription and evaluation criteria of neuroleptic treatment in resistant schizophrenia [Etude critique des conditions de prescription et des criteres d'evaluation d'un traitement neuroleptique dans les schizoprenies resistantes]. Encephale 1992;18:447‐51. [PubMed] [Google Scholar]
Pickar 1992 {published data only}
- Litman RE, Hommer DW, Radant A, Clem T, Pickar D. Quantitative effects of typical and atypical neuroleptics on smooth pursuit eye tracking in schizophrenia. Schizophrenia Research 1994;12:107‐20. [DOI] [PubMed] [Google Scholar]
- Pickar D, Owen RR, Litman RE, Konicki PE, Gutierrez R, Rapaport MH. Clinical and biologic responses to clozapine in patients with schizophrenia: crossover comparison with fluphenazine. Archives of General Psychiatry 1992;49:345‐53. [DOI] [PubMed] [Google Scholar]
Pickar 1994 {published data only}
- Pickar D, Owen RR, Litman RE, Hsiao JK, Su TP. Predictors of clozapine response in schizophrenia. Journal of Clinical Psychiatry 1994;55:S129‐32. [PubMed] [Google Scholar]
Pickar 1994 1 {published data only}
- Pickar D, Litman RE, Hong WW, Su TP, Weissman EM, Hsiao JK, Potter WZ. Clinical response to clozapine in patients with schizophrenia. Archives of General Psychiatry 1994;51(2):159‐60. [DOI] [PubMed] [Google Scholar]
Pickar 1995 {published data only}
- Pickar D, Hsiao JK. Clozapine treatment of schizophrenia. JAMA 1995;274:981‐3. [PubMed] [Google Scholar]
Pickar 2003 {published data only}
- Pickar D, Bartko JJ. Effect size of symptom status in withdrawal of typical antipsychotics and subsequent clozapine treatment in patients with treatment‐resistant schizophrenia. American Journal of Psychiatry 2003;160(6):1133‐8. [DOI] [PubMed] [Google Scholar]
Pinto 1999 {published data only}
- Pinto A, La‐Pia S, Mennella R, Giorgio D, DeSimone L. Cognitive behavioral therapy and clozapine for clients with treatment refractory schizophrenia. Psychiatric Services 1999;50(7):901‐4. [DOI] [PubMed] [Google Scholar]
Pollack 1998 {published data only}
- Pollack S, Woerner MG, Howard A, Fireworker RB, Kane JM. Clozapine reduces rehospitalization among schizophrenia patients. Psychopharmacology Bulletin 1998;34:89‐92. [PubMed] [Google Scholar]
Pollmächer 1995 {published data only}
- Pollmächer T, Hinze‐Selch D, Mullington J, Holsboer F. Clozapine‐induced increase in plasma levels of soluble interleukin‐2 receptors. Archives of General Psychiatry 1995;52:877‐8. [DOI] [PubMed] [Google Scholar]
Potkin 1993 {published data only}
- Potkin SG, Bera R, Gulasekaram B. High and low dose of clozapine compared in a double ‐ blind study. Schizophrenia Research 1993;9:246‐7. [Google Scholar]
Potkin 1994 a {published data only}
- Potkin SG, Bera R, Gulasekaram B, Costa J, Hayes S, Jin Y. Plasma clozapine concentrations predict clinical response in treatment‐resistant schizophrenia. Journal of Clinical Psychiatry 1994;55:133‐6. [PubMed] [Google Scholar]
Potkin 1994 b {published data only}
- Potkin SG, Buchsbaum MS, Jin Y, Tang C, Telford J, Friedman G, Lottenberg S, Najafi A, Gulasekaram B, Costa J. Clozapine effects on glucose metabolic rate in striatum and frontal cortex. Journal of Clinical Psychiatry 1994;55:SB63‐6. [PubMed] [Google Scholar]
Potkin 1996 {published data only}
- Potkin SG, Zborowski JN, Wu JC, Mack JC, Sebree TB, Wallin BC. Brain imaging to determine the effects of sertindole in schizophrenic patients. Proceedingd of the 149th Annual Meeting of the American Psychiatric Association; 1996 May 4‐9; New York. New York: American Psychiatric Association, 1996.
Potkin 1997 {published data only}
- Potkin SG, Jin Y, Bunney B, Gulasekaram B, Costa J, Keator DB, Telford J, Wu JC, Najafi A, Bunney WE Jr. Clinical and brain imaging effects of adjunctive high dose glycine with clozapine in schizophrenia. Schizophrenia Research 1997;24(1, 2):187. [Google Scholar]
Potkin 2000 {published data only}
- Potkin SG, Basile VS, Badri F, Keator D, Wu JC, Alva G, Doo M, Bunney Jr WE, Kennedy JL. D1 receptor alleles predict PET metabolic correlates of clinical response to clopazine. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S6. [Google Scholar]
Potkin 2001 {published data only}
- Potkin SG, Fleming K, Jin Y, Gulasekaram B. Clozapine enhances neurocognition and clinical symptomatology more than standard neuroleptics. Journal of Clinical Psychopharmacology 2001;21(5):479‐83. [DOI] [PubMed] [Google Scholar]
Potkin 2003 {published data only}
- Potkin SG, Alphs L, Hsu C, Krishn n K, Ranga Rama, Anand R, Young FK, Meltzer H, Green A. Predicting Suicidal Risk in Schizophrenic and Schizoaffective Patients in a Prospective Two‐Year Trial. Biological Psychiatry 2003;54(4):444‐52. [DOI] [PubMed] [Google Scholar]
Povlsen 1985 {published data only}
- Povlsen UJ, Noring U, Fog R, Gerlach J. Tolerability and therapeutic effect of clozapine. A retrospective investigation of 216 patients treated with clozapine for up to 12 years. Acta Psychiatrica Scandinavica 1985;71:176‐85. [DOI] [PubMed] [Google Scholar]
Preiningerová 1974 {published data only}
- Preiningerová O, Hanus H, Zapletálek M. Clozapine in outpatient practice. Activitas Nervosa Superior Praha 1974;16:204‐5. [PubMed] [Google Scholar]
Preussler 1995 {published data only}
- Preussler B, Bohle C, Jeschke G, Volz H P, Sauer H. Psychometric performance of clozapine and fluphenazine treated schizophrenics. Pharmacopsychiatry 1995;28:204. [Google Scholar]
Preussler 1997 {published data only}
- Preussler B, Hubner G, Rossger G, Jeschke G, Lorenz S, Volz H P, Sauer H. Psychometric performance of chronic schizophrenics treated with a typical neuroleptic (fluphenazine) or an atypical neuroleptic drug (clozapine) ‐ a double‐blind controlled clinical trial. Pharmacopsychiatry 1997;30:207. [Google Scholar]
Purdon 2003 {published data only}
- Purdon SE, Woodward N, Lindborg SR, Stip E. Procedural learning in schizophrenia after 6 months of double‐blind treatment with olanzapine, risperidone, and haloperidol. Psychopharmacology 2003;169(3‐4):390‐7. [DOI] [PubMed] [Google Scholar]
Qian 2004 {published data only}
- Qian D, Pan B, Yang G. Cost‐effectiveness analysis of 3 kinds of therapeutic schemes for schizophrenia. Evaluation and Analysis of Drug‐use in Hospital of China 2004;4(2):110‐111. [Google Scholar]
Raja 2000 {published data only}
- Raja M, Azzoni A. Second generation antipsychotics in the emergency care setting. A prospective naturalistic study. General Hospital Psychiatry 2000;22(2):107‐14. [DOI] [PubMed] [Google Scholar]
Rajarethinam 2003 {published data only}
- Rajarethinam R, Gilani S, Tancer M, DeQuardo J. Augmentation of clozapine partial responders with conventional antipsychotics [3]. Schizophrenia Research 2003;60(1):97‐8. [DOI] [PubMed] [Google Scholar]
Rao 1994 {published data only}
- Rao PA, Pickar D, Gejman PV, Ram A, Gershon ES, Gelernter J. Allelic variation in the D4 dopamine receptor (DRD4) gene does not predict response to clozapine. Archives of General Psychiatry 1994;51:912‐7. [DOI] [PubMed] [Google Scholar]
Ratey 1993 {published data only}
- Ratey JJ, Leveroni C, Kilmer D, Gutheil C, Swartz B. The effects of clozapine on severely aggressive psychiatric inpatients in a state hospital. Journal of Clinical Psychiatry 1993;54:219‐23. [PubMed] [Google Scholar]
Remschmidt 1994 {published data only}
- Remschmidt H, Schulz E, Martin M. An open trial of clozapine in thirty six adolescents with schizophrenia. Journal of Child and Adolescent Psychopharmacology 1994;4:31‐4. [Google Scholar]
Ren 2002 {published data only}
- Ren Q, Lu Y. Comparison of quality of life of schizophrenic outpatients treated with risperdal or clozapine. Chinese Mental Health Journal 2002;16(3):198‐9. [Google Scholar]
Ren 2004 a {published data only}
- Ren K, Zhao X, Jiang X. Effects of risperidone and clozapine on life quality of schizophrenics. Journal of Clinical Psychosomatic Diseases 2004;10(1):3‐4. [Google Scholar]
Ren 2004 b {published data only}
- Ren XF, Ma ZH, Zheng SJ. The effect of negative symptoms of chronic schizophrenia with clozapine combination fluoxetine. Medical Journal of Chinese People Health 2004;16(7):391‐3. [Google Scholar]
Rettenbacher 2004 {published data only}
- Rettenbacher Ma, Baumgartner S, Ebenbichler C, Edlinger M, Hofer a, Hummer M, Kemmler G, Lechleitner M, Fleischhacker W. Alterations of glucose metabolism under treatment with clozapine vs. amisulpride. Schizophrenia Research 2004;67(1):191. [Google Scholar]
Rodova 1973 {published data only}
- Rodova A, Svestka J, Nahunek K, Ceskova E. A blind comparison of clozapine and perphenazine in schizophrenics. Activitas Nervosa Superior 1973;15:94‐5. [PubMed] [Google Scholar]
Rosenberg 2002 {published data only}
- Rosenberg KP, Bleiberg K, Kocsis JH. Psychotropic‐induced sexual dysfunction among outpatients.. Proceedings of the 155th Anual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia. Philadelphia: American Psychiatric Association, 2002.
- Rosenberg, Kenneth P, Bleiberg K, Kocsis J. Psychotropic‐induced sexual dysfunction among outpatients. Proceedings of the 155th Annual Meeting of the American Psychiatric Association 2002 May 18‐23, Philadelphia. Philadelphia: American Psychiatric Association, 2002.
Rossger 1997 {published data only}
- Rossger G, Preussler B, Rauch J, Kunze M, Lorenz S, Harting J, Volz H P, Sauer H. Neuropsychological test performance of chronic schizophrenics treated with clozapine or fluphenazine ‐ a double‐blind, controlled clinical trial. Pharmacopsychiatry 1997;30:212. [Google Scholar]
Ruiz 1974 {published data only}
- Ruiz Ruiz M. A clinical study with pimozide in chronic schizophrenics [Estudio doble ciego comparativo entre clozapina y clorpromacina en las esquizofrenias]. Archives of Neurobiology 1974;37:169‐80. [PubMed] [Google Scholar]
Rüther 1979 {published data only}
- Rüther E. Anti‐psychotic therapy with haloperidol and clozapine [Antipsychotische therapie mit haloperidol und clozapin]. Fortschritte der Medizin 1979;97:1372. [PubMed] [Google Scholar]
Safferman 1993 {published data only}
- Safferman AZ, Lieberman JA, Pollack S, Kane JM. Akathisia and clozapine treatment. Journal of Clinical Psychopharmacology 1993;13:286‐7. [DOI] [PubMed] [Google Scholar]
Salganik 1998 {published data only}
- Salganik I, Modai I, Bercovici BR, Kutzuk D, Weizman A. Clozapine vs haloperidol therapy in elderly chronic schizophrenic inpatients: preliminary results. A double blind, cross over randomized study. International Journal of Geriatric Psychopharmacology 1998;1(4):185‐7. [Google Scholar]
Schmauss 1989 {published data only}
- Schmauss M, Wolff R, Erfurth A, Rüther E. Tolerability of long‐term clozapine treatment. Psychopharmacology 1989;99:S105‐8. [DOI] [PubMed] [Google Scholar]
Schulz 1997 {published data only}
- Schulz E, Fleischhaker C, Remschmidt HE. Correlated changes in symptoms and neurotransmitter indices during maintenance treatment with clozapine or conventional neuroleptics in adolescents and young adults with schizophrenia. Journal of Child and Adolescent Psychopharmacology 1996;6(2):119‐31. [DOI] [PubMed] [Google Scholar]
- Schulz, Fleischhaker, Clement, Remschmidt. Blood biogenic amines during clozapine treatment of early‐onset schizophrenia.. Jounal of Neural Transmition 1997;104:1077‐89. [DOI] [PubMed] [Google Scholar]
Shalev 1993 {published data only}
- Shalev A, Hermesh H, Rothberg J, Munitz H. Poor neuroleptic response in acutely exacerbated schizophrenic patients. Acta Psychiatrica Scandinavica 1993;87(2):86‐91. [DOI] [PubMed] [Google Scholar]
Shen 2002 {published data only}
- Shen W, Ling T. Analysis of correlation between therapeutic effect and eeg changes in the treatment of schizophrenia with clozapine. Sichuan Mental Health 2002;15(4):206‐8. [Google Scholar]
Shen 2004 {published data only}
- Shen J, Feng Z, Guo J, Wang W, Wang Z. Comparative study on therapy of schizophrenia with quetiapine and clozapine. Tianjin Pharmacy 2004;16(1):38‐9. [Google Scholar]
Shi 2000 b {published data only}
- Shi F, Cheng X, Zhang Y. Controlled study of risperidone and clozapine in the treatment of schizophrenia. Shanghai Archives of Psychiatry 2000;12(1):27‐29. [Google Scholar]
Shi 2000 c {published data only}
- Shi X, Cheng X, Yao S. Compared analysis of blood glucose between schizophrenic inpatients treated with clozapine and conventional neuroleptic medications. Sichuan Mental Health 2000;13(3):174‐5. [Google Scholar]
Shi 2004 {published data only}
- Shi T, Zhang R, Guo X. Influences of risperidone and clozapine on plasma levels of cytokine in first‐episode schizophrenics. Chinese Journal of Nervous and Mental Diseases 2004;30(5):339‐41. [Google Scholar]
Shi 2004 a {published data only}
- Shi Y, Wang Z, Lv L. Changes of IL‐6 in plasma and cerebrospinal fluid of schizophrenics with clozapine. Journal of Clinical Psychosomatic Diseases 2004;10(3):153‐5. [Google Scholar]
Shirakawa 1996 {published data only}
- Shirakawa I, Chaves AC. One‐year experience with clozapine in the treatment of chronic schizophrenic patients [Um ano de experiência com clozapina no tratamento de pacientes esquizofrênicos crônicos]. Jornal Brasileiro de Psiquiatria 1996;45:23‐6. [Google Scholar]
Shopsin 1978 a {published data only}
- Shopsin B, Klein H, Aronson M. Clozapine: double‐blind control trial in the treatment of acute schizophrenia. Psychopharmacology Bulletin 1978;14(2):12‐5. [PubMed] [Google Scholar]
Shun 2005 {published data only}
- Shun S, Zhang Y, Liu M. Effects of health‐education on recovery of insight and treatment compliance in schizophrenics. Journal of Clinical Psychosomatic Diseases 2005;11(1):37‐38, 47. [Google Scholar]
Siefen 1986 {published data only}
- Siefen G, Remschmidt H. Results treatment with clozapine in schizophrenic adolescents [Behandlungsergebnisse mit Clozapin bei schizophrenen Jugendlichen]. Zeitschrift fur Kinder und Jugendpsychiatrie 1986;14:245‐57. [PubMed] [Google Scholar]
Simpson 1974 {published data only}
- Simpson GM, Varga E. Clozapine ‐ a new antipsychotic agent. Current Therapeutic Research 1974;16:679‐86. [PubMed] [Google Scholar]
Simpson 1978 {published data only}
- Simpson GM, Lee HJ, Shrivastava RK. Clozapine in tardive dyskinesia. Psychopharmacology 1978;56:75‐80. [DOI] [PubMed] [Google Scholar]
Singer 1973 {published data only}
- Singer K, Lam CM. Evaluation of Leponex (clozapine) in schizophrenia with acute symptomatology. Journal of International Medical Research 1973;1:627. [Google Scholar]
Small 1987 {published data only}
- Small JG, Milstein V, Marhenke JD, Hall DD, Kellams JJ. Treatment outcome with clozapine in tardive dyskinesia, neuroleptic sensitivity, and treatment‐resistant psychosis. Journal of Clinical Psychiatry 1987;48:263‐7. [PubMed] [Google Scholar]
Small 2003 {published data only}
- Small JG, Klapper MH, Malloy FW, Steadman TM. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. Journal of Clinical Psychopharmacology 2003;23(3):223‐8. [DOI] [PubMed] [Google Scholar]
Speer 1997 {published data only}
- Speer AM, Risch SC, Hamner MB, Molloy M, Ulmer HG, Vane CL, Vincent DJ, George MS. The effect of an atypical antipsychotic (risperidone) on temporal lobe and prefrontal cortex activity in schizophrenia. Schizophrenia Research 1997;24:173. [Google Scholar]
Spivak 1997 a {published data only}
- Spivak B, Mester R, Abesgaus J, Wittenberg N, Adlersberg S, Gonen N, Weizman A. Clozapine treatment for neuroleptic‐induced tardive dyskinesia, parkinsonism, and chronic akathisia in schizophrenic patients. Journal of Clinical Psychiatry 1997;58:318‐22. [DOI] [PubMed] [Google Scholar]
Spivak 1997 b {published data only}
- Spivak B, Mester R, Wittenberg N, Maman Z, Weizman A. Reduction of aggressiveness and impulsiveness during clozapine treatment in chronic neuroleptic‐resistant schizophrenic patients. Clinical Neuropharmacology 1997;20:442‐6. [DOI] [PubMed] [Google Scholar]
Spivak 1998 {published data only}
- Spivak B, Roitman S, Vered Y, Mester R, Graff E, Talmon Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic‐resistant schizophrenic patients maintained on clozapine. Clinical Neuropharmacology 1998;21(4):245‐50. [PubMed] [Google Scholar]
Stankovska 1999 {published data only}
- Stankovska G, Kirovski P. Pharmacotherapy in acute schizophrenia. Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg. Hamburg: World Congress of Psychiatry, 1999:171.
Stone 2003 {published data only}
- Stone WS, Seidman LJ, Wojcik JD, Green AI. Glucose effects on cognition in schizophrenia. Schizophrenia Research 2003;62(1‐2):93‐103. [DOI] [PubMed] [Google Scholar]
Strejilevich 2004 a {published data only}
- Strejilevich SA, Palatnik A, Bustin J, Cassone J, Gimenez M, Figueroa S, Erausquin G. Lack of EPS predicts QoL in schizophrenics treated with clozapine or atypicals. Proceedings of the 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York. New York: American Psychiatric Association, 2004.
Stroup 2003 {published data only}
- Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: Schizophrenia trial design and protocol development. Schizophrenia Bulletin 2003;29(1):15‐31. [DOI] [PubMed] [Google Scholar]
Stryjer 2004 {published data only}
- Stryjer R, Strous R, Bar F, Shaked G, Shiloh R, Rozencwaig S, Grupper D, Buchman N, Kotler M, Rabey JM, Weizman A. Donepezil augmentation of clozapine monotherapy in schizophrenia patients: a double blind cross‐over study. Human Psychopharmacology 2004;19(5):343‐6. [DOI] [PubMed] [Google Scholar]
Sumiyoshi 2003 {published data only}
- Sumiyoshi T, Park S, Ertugrul A, Clemmons FC, Jayathilake K, Meltzer HY. The effect of buspirone, a serotonin1A agonist, on cognitive function in schizophrenia. Schizophrenia Research 2003;60(1):304. [DOI] [PubMed] [Google Scholar]
Sun 2000 {published data only}
- Sun T. A controlled study comparing risperidone and clozapine in the treatment of schizophrenia. Heath Psychology Journal 2000;8(3):290‐2. [Google Scholar]
Sun 2004 {published data only}
- Sun DJQSS. Effect of risperidone and clozapine on the blood sugar and blood fat in patients with schizophrenia. Journal of Hebei Medical College for Continuing Education 2004;21(2):8‐10. [Google Scholar]
Sun Lm2 {published data only}
- Sun Lm. A comparative analysis of leukocyte affected by clozapine. Journal of Taishan Medical College 2000;21(3):225‐6. [Google Scholar]
Suppes 1999 {published data only}
- Suppes T, Webb A, Paul B, Carmody T, Kraemer H, Rush AJ. Clinical outcome in a randomized 1 year trial of clozapine versus treatment as usual for patients with treatment resistant illness and a history of mania. American Journal of Psychiatry 1999;156(9):1164‐9. [DOI] [PubMed] [Google Scholar]
Szymanski 1994 {published data only}
- Szymanski S, Masiar S, Mayerhoff D, Loebel A, Geisler S, Pollack S, Kane J, Lieberman J. Clozapine response in treatment‐refractory first episode schizophrenia. Biological Psychiatry 1994;35:278‐80. [DOI] [PubMed] [Google Scholar]
Tandon 1993 {published data only}
- Tandon R, Goldman RS, DeQuardo JR, Goldman M. Positive and negative symptoms covary during clozapine treatment in schizophrenia. Journal of Psychiatric Research 1993;27:341‐7. [Google Scholar]
Tang 2002 a {published data only}
- Tang M, Wang H, Huang J. Influences of treating schizophrenics with risperidone and clozapine on the quality of life of their spouses and parents. Nervous Diseases and Mental Hygjene 2002;2(2):67‐9. [Google Scholar]
Tang 2002 b {published data only}
- Tang M, Zhao G, Wang H. Comparison of quality of life of family with schizophrenic outpatients treated with risperidone or clozapine. Chinese Mental Health Journal 2002;16(5):350‐3. [Google Scholar]
Tang 2003 {published data only}
- Tang Y. A controlled study of schizophrenia treated with quetiapine and clozapine. Shanghai Archives of Psychiatry 2003;15(1):27‐9. [Google Scholar]
Tauscher 1999 {published data only}
- Tauscher J, Küfferle B, Asenbaum S, Fischer P, Pezawas L, Barnas C. In vivo 123I IBZM SPECT imaging of striatal dopamine‐2 receptor occupancy in schizophrenic patients treated with olanzapine in comparison to clozapine and haloperidol. Psychopharmacology 1999;141:175‐81. [DOI] [PubMed] [Google Scholar]
Tiihonen 2003 {published data only}
- Tiihonen J, Hallikainen T, Ryynanen OP, Repo‐Tiihonen E, Kotilainen IEM, Toivonen P, Wahlbeck K, Putkonen A. Lamotrigine in treatment‐resistant schizophrenia: a randomized placebo‐controlled crossover trial. Biological Psychiatry 2003;54(11):1241‐8. [DOI] [PubMed] [Google Scholar]
Tiihonen 2004 {published data only}
- Tiihonen J, Hallikainen T, Ryynanen OP, Repo‐Tiihonen E, Kotilainen I, Eronen M, Toivonen P, Wahlbeck K, Putkonen A. Lamotrigine in clozapine treatment‐resistant schizophrenia [Lamotrigiini klotsapiiniresistentissa skitsofreniassa]. Duodecim 2004;120(1):85‐6. [PubMed] [Google Scholar]
Tong 2001 {published data only}
- Tong Z, Du WLX. Amitriptyline in the treatment of enuresis induced by clozapine. Sichuan Mental Health 2001;14(2):92‐3. [Google Scholar]
Trichard 1998 {published data only}
- Trichard C, Paillere Martinot ML, Attar Levy D, Recassens C, Monnet F, Martinot J L. Binding of antipsychotic drugs to cortical 5‐HT(2A) receptors: A PET study of chlorpromazine, clozapine, and amisulpride in Schizophrenic patients. American Journal of Psychiatry 1998;155(4):505‐8. [DOI] [PubMed] [Google Scholar]
Turner 2004 {published data only}
- Turner DC, Clark L, Pomarol‐Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropyschopharmacology 2004;29(7):1363‐73. [DOI] [PubMed] [Google Scholar]
Turpeinen 1996 {published data only}
- Turpeinen P. Clozapine in adolescent psychiatric patients. CNS Drugs 1996;6:339‐40. [Google Scholar]
UK Study 1993 {published data only}
- Clozapine Study Group. The safety and efficacy of clozapine in severe treatment‐resistant schizophrenic patients in the UK. British Journal of Psychiatry 1993;163:150‐4. [DOI] [PubMed] [Google Scholar]
Van Praag 1976 {published data only}
- Praag HM, Korf J, Dols LCW. Clozapine versus perphenazine: the value of the biochemical mode of action of neuroleptics in predicting their therapeutic activity. British Journal of Psychiatry 1976;129:547‐55. [DOI] [PubMed] [Google Scholar]
VanderZwaag 1996 {published data only}
- Freudenreich O, Weiner RD, McEvoy JP. Clozapine‐induced electroencephalogram changes as a function of clozapine serum levels. Biological Psychiatry 1997;42:132‐7. [DOI] [PubMed] [Google Scholar]
- VanderZwaag C, McGee M, McEvoy JP, Freudenreich O, Wilson WH, Cooper TB. Response of patients with treatment‐refractory schizophrenia to clozapine within three serum level ranges. American Journal of Psychiatry 1996;153:1579‐84. [DOI] [PubMed] [Google Scholar]
Vass 2004 {published data only}
- Vass A, Kremer I, Gurelik I, Blenaru M, Bar G, Javitt D, Heresco‐Levy U. Pilot‐controlled trial of lamotrigine adjuvant treatment in schizophrenia. Proceedings of the 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York. New York: American Psychiatric Association, 2004.
Vinar 1976 {published data only}
- Vinar O, Taussigova D. Schizophrenic syndromes improving with neuroleptic drug treatment. Ceskoslovenska psychiatrie 1976;3:176‐181. [PubMed] [Google Scholar]
Vlokh 2002 {published data only}
- Vlokh IY, Moroz OU, Mikhnyak SI. Treatment with fluanxol and associated changes in atp‐ase activity of red blood cells membranes. Schizophrenia Research 2002;53(3 Suppl 1):205. [Google Scholar]
Wang 1994 {published data only}
- Wang CH, Qin TF, Lin YL, Zhao XF. A clinical effect and following‐up study about sulpiride and clozapine for 105 cases of the schizophrenia type?. Journal of Xinxiang Medical College 1994;11(2):148‐51. [Google Scholar]
Wang 1995 {published data only}
- Wang CH. Clinical effect and follow‐up observation of type I schizophrenia. Medical Journal of Chinese Civil Administration 1995;7(4):204‐6. [Google Scholar]
Wang 1999 {published data only}
- Wang J, Wang Y, Yu J, Zhu Z. A cross‐check analysis of risperidone and clozapine in the treatment of schizophrenia. Journal of Taishan Medical College 1999;20(4):334‐6. [Google Scholar]
Wang 2000 a {published data only}
- Wang G‐P, Zhong X‐J, Ji R. The control observation of effects of clozapine and sulpiride on weights of schizophrenics. Journal of Clinical Psychosomatic Diseases 2000;6(4):206‐7. [Google Scholar]
Wang 2000 b {published data only}
- Wang LZ, Zhu S. Fluoxctine augmentation of clozapine treatment in patients with chronic schizophrenia. Shanghai Archives of Psychiatry 2000;12(2):66‐8. [Google Scholar]
Wang 2001 b {published data only}
- Wang J, Nan Z, Bai J. The relationship of schizophrenic cognitive function impairment with positive and negative symptoms. Journal of Clinical Psychological Medicine 2001;11(6):346‐8. [Google Scholar]
Wang 2002 a {published data only}
- Wang R, Geng Y, Pan D, Zhang S. A Comparative Trial of Efficacy of Risperidone vs Clozapine in Treatment of Refractory Schizophrehia. Chinese Journal of Pharmaco Epiolemiology 2002;11(5):230‐1. [Google Scholar]
Wang 2002 b {published data only}
- Wang C, Feng Y, Wang L. A double‐blind randomized controlled study of olanzapine and clozapine on treatment of schizophrenia. Shanghai Archives of Psychiatry 2002;14(3):143‐5. [MEDI0312] [Google Scholar]
Wang 2002 c {published data only}
- Wang G, Chen Z, Wang H. Comparative study on quality of life of schizophrenics treated with risperidone or clozapine. Chinese Mental Health Journal 2002;16(3):200‐2. [Google Scholar]
Wang 2002 d {published data only}
- Wang R, Geng Y, Pan D. A comparative trial on the efficacy of risperidone and clozapine in treatment‐resistant schizophrenia. Shandong Archives of Psychiatry 2002;15(4):221‐2. [Google Scholar]
Wang 2003 a {published data only}
- Wang G‐P, Xie R, Ma X‐Z. Clozapine combined with euvifor in the treatment of negative symptoms of schizophrenia. Journal of Clinical Psychosomatic Diseases 2003;9(4):218‐19. [Google Scholar]
Wang 2003 b {published data only}
- Wang K, Zhang K. A study of olanzapine and clozapine in the treatment of schizophrenia. Shandong Archires of Psychiatry 2003;16(3):141‐3. [Google Scholar]
Wang 2003 c {published data only}
- Wang Y‐B, Wang D‐P, Yan C‐L. A control study of quetiapine and clozapine in the treatment of first ‐ episode schizophrenia. Journal of Clinical Psychosomatic Diseases 2003;9(3):154‐6. [Google Scholar]
Wang 2004 a {published data only}
- Wang H. Controlled study of the effect of olanzapine and clozapine on electroencephalogram of schizophrenic patients. Journal of North China Coal Medical College 2004;6(3):289‐90. [Google Scholar]
Wang 2004 b {published data only}
- Wang L, Li B, Zhao Z. A controlled study of compound diazepam in adjunctive treatment for schizophrenia. Journal of Binzhou Medical College 2004;27(2):111‐2. [Google Scholar]
Wang 2004 c {published data only}
- Wang M, Han F, Ma H. Comparation of quetiapine and clozapine in treatment of patients with first‐onset schizophrenia. Chinese Journal of Clinical Pharmacology and Therapeutics 2004;9(5):551‐54. [Google Scholar]
Wang 2004 d {published data only}
- Wang X. Comparative study of effects of quetiapine and clozapine on quality of life of schizophrenics. Journal of Clinical Psychosomatic Diseases 2004;10(3):167‐8. [Google Scholar]
Wang 2004 e {published data only}
- Wang Y, Jin S, Yan M. Effect of yinxing leaf and clozapine in the treatment of first onset schizophrenia. Shandong Archives of Psychiatry 2004;17(4):213‐14. [Google Scholar]
Wei 1996 {published data only}
- Wei Q. Effect of antipsychotic drugs on EEG. Journal of Clinical Electroencephalogy 1996;5(2):92‐4. [Google Scholar]
Weickert 2003 {published data only}
- Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of cognitive performances during a placebo period and an atypical antipsychotic treatment period in schizophrenia: critical examination of confounds. Neuropyschopharmacology 2003;28(8):1491‐00. [DOI] [PubMed] [Google Scholar]
Weiser 1975 {published data only}
- Weiser G, Tahedl A, Reisecker F, Meyer H. Initial benefits of the treatment of acute schizophrenia with high droperidol. A comparative study [Vorteile der Initialbehandlung akuter Schizophrenien mit hochdosiertem Droperidol. Eine Vergleichsstudie]. Arzneimittel Forschung 1975;25(11):1845‐8. [PubMed] [Google Scholar]
Welbel 1980 {published data only}
- Welbel L. Differences in the clinical effect of various neuroleptics [Roznice w dziallaniu klinicznym niektórych neuroleptyków]. Psychiatria Polska 1980;14:113‐8. [PubMed] [Google Scholar]
Weng 1998 {published data only}
- Weng Y. A controlled trial of risperidone versus clozapine in the treatment of schizophrenia. Journal of Clinical Psychological Medicine 1998;8(2):83‐5. [Google Scholar]
Wiholm 1989 {published data only}
- Wiholm BE, Myrhed M. Clozapine‐new antipsychotics in therapy resistance. Good power but a high risk of side effects [Klozapin‐nytt antipsykotikum vid terapiresistens. God effekt men stor risk för biverkningar]. Läkartidningen 1989;86:2508‐9. [PubMed] [Google Scholar]
Williams 1993 {published data only}
- Williams R, Baillie P, Dickson RA, Dalby JT. Cognitive and behavioural efficacy of clozapine in clinical trials. Canadian Journal of Psychiatry 1993;38:522. [DOI] [PubMed] [Google Scholar]
Wilson 1994 {published data only}
- Wilson WH. Open clozapine treatment following a controlled clinical trial of lithium augmentation of haloperidol for refractory schizophrenia. Lithium 1994;5:113‐4. [Google Scholar]
Wirshing 1990 {published data only}
- Wirshing WC, Phelan CK, Vanputten T, Marder SR, Engel J. Effects of clozapine on treatment‐resistant akathisia and concomitant tardive‐dyskinesia. Journal of Clinical Psychopharmacology 1990;10:371‐3. [PubMed] [Google Scholar]
Wirshing 1999 a {published data only}
- Wirshing DA, Vincenzo P, Marder SR, Wirshing WC. Sexual side effects of atypical antipsychotic medications. Proceedings of the 152nd annual meeting of the American Psychiatric Association; 1999 May 15‐20; Washington D C. Washington D C: American Psychiatric Association, 1999.
Woggon 1978 {published data only}
- Woggon B. Effects and side‐effects of bromperidol in comparison with other antipsychotic drugs. Acta Psychiatrica Belgica 1978;78(1):155‐72. [PubMed] [Google Scholar]
Wu 2000 {published data only}
- Wu Y. A control study on effects of chlorpromazine and clozapine on serum prolactin in schizophrenia patients. Health Psychology Journal 2000;8(2):148‐4. [Google Scholar]
Wu 2001 {published data only}
- Wu T, Li Y, Li M. Efficacy of very low dosage of risperidone in the treatment of first‐episode schizophrenia. Journal of Clinical Psychological Medicine 2001;11(3):152‐4. [Google Scholar]
Wu 2002 {published data only}
- Wu L. A control study of risperidone and clozapine combination for the treatment of refractory schizophrenia. Health Psychology Journal 2002;10(2):135‐7. [Google Scholar]
Wudarsky 1999 {published data only}
- Wudarsky M, Nicolson R, Hamburger SD, Spechler L, Gochman P, Bedwell J, Lenane MC, Rapoport JL. Elevated prolactin in pediatric patients on typical and atypical antipsychotics. Journal of Child and Adolescent Psychopharmacology 1999;9(4):239‐45. [DOI] [PubMed] [Google Scholar]
Xiang 2005 {published data only}
- Xiang D‐F, Liu X‐L. Treatment of 31 cases of schizophrenia with quetiapine. Herald of Medicine 2005;24(1):40‐2. [Google Scholar]
Xie 1998 {published data only}
- Xie Q, Li J, Li Z. Clozapine versus chlorpromazine in the treatment of schizophrenia. Sichuan Mental Health 1998;11(1):18‐20. [Google Scholar]
Xie 2001 {published data only}
- Xie C, Ni X. The compared study of treating schizophrenia with risperidone combining clozapine. Journal of Preventive Medicine Information 2001;17(4):245‐6. [Google Scholar]
Xin 2001 {published data only}
- Xin X, Du B, Zeng Z. A controlled clinical study of risperidone and low dose of clozapine in treating schizophrenia. Herald of Medicine 2001;20(8):501‐2. [Google Scholar]
Xing 2002 {published data only}
- Xing X‐A, Xie Cg, Wu DC. A double ‐ blind comparison study of l ‐ stepholidine and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry 2002;15(2):79‐80. [Google Scholar]
Xu 1997 {published data only}
- Xu HD, Dong AZ, Zhang TT, Shu WJ. Diphenhydramine for clozapine‐induced salivation at various dosage levels. Chinese Journal of New Drugs and Chinical Remedies 1997;7(4):205‐7. [Google Scholar]
Xu 2001 {published data only}
- Xu C, Tang J, Zhang X. The effect of risperidone and clozapine treatment on serum interleukin 8, interleukin 15 in schizophrenia.. Medical Journal of Chinese Civil Administration 2001;13(3):132‐5. [Google Scholar]
Xu 2002 {published data only}
- Xu D, Zhang Y, Huang W. The analysis of costs on risperidone versus clozapine during schizophrenia's hospitalisation. Medical Journal of Chinese Civil Administration 2002;14(1):13‐4. [Google Scholar]
Xu 2003 {published data only}
- Xu LZ, Ouyang JL, Gao SZ. Effects of domestic quetiapine vs clozapine in treatment of schizophrenia. Chinese Journal of New Drugs and Clinical Remedies 2003;7477(9):542‐5. [Google Scholar]
Yagcioglu 2005 {published data only}
- Yagcioglu AEA, Akdede BBK, Turgut TI, Tumuklu M, Yazici MK, Alptekin K, Ertugrul A, Jayathilake K, Gogus A, Tunca Z, Meltzer HY. A double‐blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. Journal of Clinical Psychiatry 2005;66(1):63‐72. [DOI] [PubMed] [Google Scholar]
Yan 1984 {published data only}
- Yan WW. A double‐blind cross‐over controlled study on clozapine and chlorpromazine in the treatment of chronic schizophrenia. New Drugs Clinical Remedies 1984;3:15‐7. [Google Scholar]
Yang 1988 {published data only}
- Yang WJ. Three antipsychotic drugs in the treatment of schizophrenia ‐ a controlled and double‐blind study. Chung Hua Shen Ching Ching Shen Ko Tsa Chih 1988;21:277‐80. [PubMed] [Google Scholar]
Yang 1998 {published data only}
- Yang Z, Xu H, Song Y. A controlled study of efficacy of risperidone and clozapine on treating schizophrenia whose predominant clinical features were negative symptoms. Sichuan Mental Health 1998;11(3):151‐2, 159. [Google Scholar]
Yang 1999 {published data only}
- Yang F, Ji Z. Relationships of cognitive function, positive or negative symptoms, and response to antipsychotic in first‐episode schizophrenic patients. Sichuan Mental Health 1999;12(4):223‐6. [Google Scholar]
Yang 2004a {published data only}
- Yang F‐S, Yang Y‐L, Zhang Z‐H. Control study on quetiapine and clozapine in treatment of refractory schizophrenia. Medical Journal of Chinese People Health 2004;16(1):12‐13. [Google Scholar]
Yao 1999 {published data only}
- Yao H. A double blind randomized study comparing clozapine and clozapine combination with sulpiride in the treatment of schizophrenia. Sichuan Mental Health 1999;12(4):250‐1. [Google Scholar]
Yen 2004 {published data only}
- Yen YC, Lung FW, Chong MY. Adverse effects of risperidone and haloperidol treatment in schizophrenia. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2004;28(2):285‐90. [DOI] [PubMed] [Google Scholar]
Yin 2002 {published data only}
- Yin Y, Son L. The impact of clozapine and risperidone on qtc. Shanghai Archives of Psychiatry 2002;14(3):154‐5. [Google Scholar]
Yu 2002 {published data only}
- Yu DS, Zhang XB, Jiang XJ, Sun DH, Xiao H, Zhang SN. The correlations between effects in patients with schizophrenia receiving clozapine:same root correlation hypothesis. Modern Rehabilitation 2002;6(21):3305‐6. [Google Scholar]
Yu 2002 b {published data only}
- Yu GH, Ding GA, Li X. Comparison of compliance between risperidone and clozapine while treating‐resistant schizophrenia: a three year follow‐up. Chinese Journal of Misdiagnostics 2002;2(8):1143‐4. [Google Scholar]
Yu 2004 {published data only}
- Yu EL, Xiu YL, Zhao XZ. One‐year follow‐up study of systemic early intervention to first episode schizophrenia. Chinese Journal of Clinical Rehabilitation 2004;8(36):8178‐81. [Google Scholar]
Yu 2005 a {published data only}
- Yu D‐S, Xu J, Sun D‐H, Zeng Y‐Y, Gao X‐N, Jiang X‐J. A comparative study of clozapine vs risperidone on body posture balance. Chinese Journal of New Drugs and Clinical Remedies 2005;24(2):125‐8. [Google Scholar]
Yu 2005 b {published data only}
- Yu J, Hao Z, Sun Y. Control study on quetiapine and clozapine in the treatment of refractory schizophrenia. Journal of Clinical Psychosomatic Diseases 2005;11(1):31‐2. [Google Scholar]
Yue 2004 {published data only}
- Yue Y, Song L, Xu Y. A comparative study on risperidone in the two‐year treatment of schizophrenia. Shanghai Archives of Psychiatry 2004;16(3):165‐7. [Google Scholar]
Zahn 1993 {published data only}
- Zahn TP, Pickar D. Autonomic effects of clozapine in schizophrenia: comparison with placebo and fluphenazine. Biological Psychiatry 1993;34:3‐12. [DOI] [PubMed] [Google Scholar]
Zahn 1994 {published data only}
- Zahn TP, Pickar D, Haier RJ. Effects of clozapine, fluphenazine and placebo on reaction time measures of attention and sensory dominance in schizophrenia. Schizophrenia Research 1994;13:133‐44. [DOI] [PubMed] [Google Scholar]
Zapletálek 1974 {published data only}
- Zapletálek M, Pazdirek S, Hübsch T, Strnad D. Clinical experience with clozapine in psychoses. Activitas Nervosa Superior 1974;16:203‐4. [PubMed] [Google Scholar]
Zapletálek 1980 {published data only}
- Zapletálek M, Preiningerova O, Hanus H. Clozapine caused a dependency? [Verursacht Clozapin eine Abhängigkeit?]. Agressologie 1980;21:19‐21. [PubMed] [Google Scholar]
Zeng 2001 {published data only}
- Zeng ZX, Han MF. A comparison of the effects of risperidone and clozapine on electroencephalogram. Herald of Medicine 2001;20(8):499, 500. [Google Scholar]
Zeng 2002 {published data only}
- Zeng Y, Fang Q, Gao X. The 4th day serum prolactin level predicting the effective dose taking risperidone or clozapine. Journal of Clinical Psychological Medicine 2002;12(5):277‐9. [Google Scholar]
Zeng 2003 {published data only}
- Zeng Z, Zeng Z. An extended two‐year follow up of psychological education on insight recovery and drug therapy compliance and recurrence in schizophrenic patients. Modern Rehabilitation 2003;7(12):1774‐5. [Google Scholar]
Zhang 1997 {published data only}
- Zhang C. A controlled study comparing risperidone and clozapine in the treatment of schizophrenia. Journal of Clinical Psychological Medicine 1997;7(2):74‐5. [Google Scholar]
Zhang 2000 {published data only}
- Zhang Y, Song L. Combined treatment of refractory schizophrenia with clozapine and risperidone. Journal of Clinical Psychological Medicine 2000;10(2):87‐8. [Google Scholar]
Zhang 2002 a {published data only}
- Zhang Y. Efficacy and side effect analysis of risperidone in the treatment of schizophrenia.. Health Psychology Journal 2002;10(2):126‐9. [Google Scholar]
Zhang 2002 b {published data only}
- Zhang C, Cui C, Si S. A follow‐up study of the maintenance treatment on the schizophrenia with clozapine and sulpiride. Sichuan Mental Health 2002;15(4):220‐1. [Google Scholar]
Zhang 2002 c {published data only}
- Zhang L. The investigation and analysis of weight gain in schizophrenia patients after taking antipsychotic drugs. Journal of Practical Nursing 2002;18(7):47‐8. [Google Scholar]
Zhang 2002 d {published data only}
- Zhang Q. A clinical study of treatment of negative symptoms in schizophrenia with a combination of clozapine and paroxetine. Journal of Qiqihar Medical 2002;23(7):731‐2. [Google Scholar]
Zhang 2002 e {published data only}
- Zhang Q. A comparison of cognitive function in the first‐onset schizophrenia treated with risperidone and clozapine. Journal of Qiqihar Medical 2002;23(8):852‐3. [Google Scholar]
Zhang 2002 f {published data only}
- Zhang W, Wang X, Tao J. The effect of clozapine on the insulin sensitivity. Journal of Clinical Psychological Medicine 2002;12(4):196‐8. [Google Scholar]
Zhang 2002 h {published data only}
- Zhang X, Pang L, Huang Y. Sexual dysfunction of 220 schizophrenics. Health Psychology Journal 2002;10(4):247‐8. [Google Scholar]
Zhang 2002 i {published data only}
- Zhang X‐Z, Yu J‐L. Control studies of relapses between risperidone and clozapine in schizophrenia. Journal of Clinical Psychosomatic Diseases 2002;8(3):145‐7. [Google Scholar]
Zhang 2004 g {published data only}
- Zhang Y, Zhang G, Tang Q. A control study of weight gain caused by chlorpromazine, clozapine and sulpiride in schizophrenics.. Journal of Clinical Psychosomatic Diseases 2004;10(3):182‐4. [Google Scholar]
Zheng 2003 {published data only}
- Zheng YJ, Wang GH, Cheng ZL. Effects of clozapine and risperidone on the glucose metabolism in first‐episode schizophrenic patients. Chinese Journal of Psychiatry 2003;36(4):207‐10. [Google Scholar]
Zhou 1997 {published data only}
- Zhou G, Jin SB, Zhang LD. Comparative clinical study on the treatment of schizophrenia with electroacupuncture and reduced doses of antipsychotic drugs. American Journal of Acupuncture 1997;25(1):25‐31. [Google Scholar]
Zhou 2000 a {published data only}
- Zhou Y, Zhao J. The relationship of schizophrenic symptoms with cognitive function impairment. Journal of Clinical Psychological Medicine 2000;10(5):260‐2. [Google Scholar]
Zhou 2000 b {published data only}
- Zhou Z, Shu Z, Yu Y. A control study of risperidone and clozapine in the treatment of the negative symptoms of schizophrenia. Aerospace Medicine 2000;11(2):69‐71. [Google Scholar]
Zhou 2003 a {published data only}
- Zhou P, Gong F, Fan C. A control of treating chronic schizophrenia with seroquel or clozapine. Jiangxi Medical Journal 2003;38(6):395‐6. [Google Scholar]
Zhou 2003 b {published data only}
- Zhou R, Dai X. A comparative study of quetiapine and clozapine in the treatment of patients with schizophrenia. Shanghai Archives of Psychiatry 2003;15(4):215‐7. [Google Scholar]
Zhu 1999 a {published data only}
- Zhu X, Mei Q. A controlled study comparing drug compliance with risperidone and clozapine in treatment of schizophrenia. Journal of Clinical Psychological Medicine 1999;9(3):151‐2. [Google Scholar]
Zhu 1999 b {published data only}
- Zhu Y, Zhang S, Zhang D. A controlled trial comparing chlorimipramine and sulpiride as adjunct to clozapine in the treatment of negative symptoms of schizophrenia. Journal of Clinical Psychological Medicine 1999;9(4):204‐5. [Google Scholar]
Zhu 2001 {published data only}
- Zhu F, Ji Z, Fie L. The pharmacokinetics of clozapine and it's clinical utilization. Journal of Clinical Psychological Medicine 2001;11(1):12‐5. [Google Scholar]
Zhu 2002 a {published data only}
- Zhu Y, Jin X. Effect of music treatment on memory decrease of patients with schizophrenia caused by clozapine. Chinese Journal of Rehabilitation Theory and Practice 2002;8(11):684‐6. [Google Scholar]
Zhu 2002 b {published data only}
- Zhu H, Yu G, Deng D. A study of clozapine combined with or without pipotiazine palmitate in refractory schizophrenia. Journal of Clinical Psychological Medicine 2002;12(1):15‐7. [Google Scholar]
Zhu 2002 c {published data only}
- Zhu H, Ma C, Hou J, Yin Q, Hu H, Guo Y. Study of serum il‐6 and a‐ifn in the pre‐and post‐treatment of 68 patients with first episode schizophrenia.. Chinese Journal of Nervous and Mental Diseases 2002;28(5):324‐6. [Google Scholar]
Zhu 2003 {published data only}
- Zhu F, Lin R, Zhang J. Risperidone versus clozapine in treatment‐resistant schizophrenia:a randomized controlled study. Shanghai Archives of Psychiatry 2003;15(3):168‐71. [Google Scholar]
Zimmermann 1996 {published data only}
- Zimmermann U, REchlin T, Kaschka WP. Heart rate variability in schizophrenic patients: effects of clozapine. Schizophrenia Research 1996;12(18):128. [Google Scholar]
Zito 1993 {published data only}
- Zito JM, Volavka J, Craig TJ, Czobor P, Banks S, Vitrai J. Pharmacoepidemiology of clozapine in 202 inpatients with schizophrenia. Annals of Pharmacotherapy 1993;27:1262‐9. [DOI] [PubMed] [Google Scholar]
Zoccali 2003 {published data only}
- Zoccali R, Muscatello MR, Torre DL, Malara G, Canale A, Crucitti DDC, Spina E. Lack of a pharmacokinetic interaction between mirtazapine and the newer antipsychotics clozapine, risperidone and olanzapine in patients with chronic schizophrenia. Pharmacological Research 2003;48(4):411‐4. [DOI] [PubMed] [Google Scholar]
Zoccali 2004 {published data only}
- Zoccali R, Muscatello MR, Cedro C, Neri P, Torre D, Spina E, Rosa AE, Meduri M. The effect of mirtazapine augmentation of clozapine in the treatment of negative symptoms of schizophrenia: a double‐blind, placebo‐controlled study. International Clinical Psychopharmacology 2004;19(2):71‐6. [DOI] [PubMed] [Google Scholar]
Zou 2001 {published data only}
- Zou D, Zhao J. The efficacy of clozapine treatment on cognitive impairments of patients with type I, II schizophrenia. Chinese Journal of Clinical Psychology 2001;9(3):210‐1. [Google Scholar]
Zuo 2002 {published data only}
- Zuo J, Xun Z, Wang M. A comparison of social ability of patients with schizophrenia treated with risperidone and those treated with clozapine. Tianjin Pharmacy 2002;20(5):54‐5. [Google Scholar]
References to studies awaiting assessment
Yang 2004 b {published data only}
- Yang B, Wang YD, Zhang L. Effects of clozapine, risperidone and haloperidol on plasma leptin in first‐episode schizophrenic patients. Journal of The Fourth Military Medical University 2004;25(14):1323‐25. [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvir 1993
- Alvir JMJ, Jeffrey PH, Lieberman A, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine‐induced agranulocytosis: incidence and risk factor in the United States. New England Journal of Medicine 1993;329:162‐7. [DOI] [PubMed] [Google Scholar]
Andreasen 1983
- Andreasen NC. Negative symptoms in schizophrenia. Archives of General Psychiatry 1983;39:784‐8. [DOI] [PubMed] [Google Scholar]
Benton 1983
- Benton Al, Hamsher K, Verney NR, Spreen D. Contributions to Neuropsychological Assessment. New York: Oxford University Press, 1983. [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Buschke 1974
- Buschke H, Fuld PA. Evaluation storage, retention and retrieval in disordered memory and learning. Neurology 1974;11:1019‐25. [DOI] [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. [DOI] [PubMed] [Google Scholar]
Crilly 2007
- Crilly J. The history of clozapine and its emergence in the US market a review and analysis. History of Psychiatry 2007;18:39‐60. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town. Cape Town: Cochrane Collaboration, 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Duggan 2005
- Duggan L, Fenton M, Rathbone J, Dardennes R, El‐Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001359.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein NF, Folstein SE, McHugh PR. Mini‐Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1970
- Guy W, Bonato RR, eds. Clinical Global Impressions. Manual for the ECDEU Assessment Battery 2. Rev ed. National Institute of Mental Health, 1970. [Google Scholar]
Haas 2007
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green, (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd., 2008.
Hippius 1989
- Hippius H. The history of clozapine. Psychopharmacology 1989;99:3‐5. [DOI] [PubMed] [Google Scholar]
Honigfeld 1965
- Honigfeld G, Klett CJ. The nurses' observation scale for inpatient evaluation. A new scale for measuring improvement in chronic schizophrenia. Journal of Clinical Psychology 1965;21:65‐71. [DOI] [PubMed] [Google Scholar]
Hunter 2003
- Hunter RH, Joy CB, Kennedy E, Gilbody SM, Song F. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000440] [DOI] [PubMed] [Google Scholar]
Idänpään‐Heikkilä 1975
- Idänpään‐Heikkilä J, Alhava E, Olkinuora M. Clozapine and agranulocytosis. Lancet 1975;2:611. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. British Medical Journal 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986. [Google Scholar]
Lehfeld 1997
- Lehfeld H, Erzigkeit H. The SKT‐a short cognitive performance test for assessing deficits of memory and attention. International Psychogeriatrics 1997;9(1):115‐21. [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. British Journal of Psychiatry 2005;187:366‐71. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. [DOI] [PubMed] [Google Scholar]
Lobos 2007
- Lobos CA, Komossa K, Leucht S, Hunger H, Schmidt F, Schwarz S, Rummel‐Kluge C. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Meltzer 1997
- Meltzer HY. Treatment‐resistant schizophrenia‐‐the role of clozapine. Current Medical Research and Opinion: 1997;14:1‐20. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Peterson 1959
- Peterson LR, Peterson MJ. Short term rentention of individual verbal items. Journal of Experimental Psychology 1959;58:193‐8. [DOI] [PubMed] [Google Scholar]
Rust 1989
- Rust J, Golombok S. Modern Psychometrics. London: Routledge, 1989. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Srisurapanont 2004
- Srisurapanont M, Maneeton B, Maneeton N. Quetiapine for schizophrenia. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000967.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Syed 2008
- Syed R, Au K, Cahill C, Duggan L, He Y, Udu V, Xia J. Pharmacological interventions for clozapine‐induced hypersalivation. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD005579.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talland 1965
- Talland GA. Deranged Memory. New York: Academic Press, 1965. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
Wechsler 1974
- Wechsler D. Wechsler Intelligence Scale for Children Revised. New York: Psychological Corporation, 1974. [Google Scholar]
Wechsler 1981
- Wechsler D. Wechsler Adult Intelligence Scale Revised. New York: Psychological Corporation, 1981. [Google Scholar]
Wirshing 1999
- Wirshing DA, Wirshing WC, Kysar L, Berisford MA. Novel antipsychotics: comparison of weight gain liabilities. Journal of Clinical Psychology 1999;60:358‐63. [PubMed] [Google Scholar]
Wu 2006
- Wu T, Li Y, Liu G, Bian Z, Li J, Zhang J, Xie L, Ni J. Investigation of authenticity of 'claimed' randomized controlled trials (RCTs) and quality assessment of RCT reports published in China. Proceedings: 14th Cochrane Colloquium, Dublin, 23‐26 October 2006. 2006.
Xia 2007
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, Pinfold V, Takriti Y. The Leeds Outcomes Stakeholders Survey (LOSS) Study. In: 15th Cochrane Colloquium, Sao Paulo, 23‐27 October 2007. 2007.
References to other published versions of this review
Essali 1997
- Essali A, Rezk E, Wahlbeck K, Cheine M. Review: clozapine reduces relapse and symptoms compared with typical neuroleptic drugs in schizophrenia. Evidenced‐Based Medicine 1997;2:182. [Google Scholar]
Essali 1997 b
- Essali MA, Rezk E, Wahlbeck K, et al. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews 1997, Issue 2. [DOI: 10.1002/14651858.CD000059] [DOI] [PubMed] [Google Scholar]
Essali 1998
- Essali A, Rezk E, Wahlbeck K, Cheine M. Review: clozapine reduces relapse and symptoms compared with typical neuroleptic drugs in schizophrenia. Evidence‐Based Mental Health 1998;1:17. [Google Scholar]
Wahlbeck 1999 a
- Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine effectiveness in schizophrenia: a systematic review and meta‐analysis of randomized trials. American Journal of Psychiatry 1999;156:990‐9. [DOI] [PubMed] [Google Scholar]
Wahlbeck 1999 b
- Wahlbeck K, Cheine M, Essali A. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000059.] [DOI] [PubMed] [Google Scholar]


