Abstract
Real‐time assessment of excised tissue may help to improve surgical results in breast tumor surgeries. Here, as a step towards this purpose, the potential of second and third harmonic generation (SHG, THG) microscopy is explored. SHG and THG are nonlinear optical microscopic techniques that do not require labeling of tissue to generate 3D images with intrinsic depth‐sectioning at sub‐cellular resolution. Until now, this technique had been applied on fixated breast tissue or to visualize the stroma only, whereas most tumors start in the lobules and ducts. Here, SHG/THG images of freshly excised unprocessed healthy human tissue are shown to reveal key breast components—lobules, ducts, fat tissue, connective tissue and blood vessels, in good agreement with hematoxylin and eosin histology. DNA staining of fresh unprocessed mouse breast tissue was performed to aid in the identification of cell nuclei in label‐free THG images. Furthermore, 2‐ and 3‐photon excited auto‐fluorescence images of mouse and human tissue are collected for comparison. The SHG/THG imaging modalities generate high quality images of freshly excised tissue in less than a minute with an information content comparable to that of the gold standard, histopathology. Therefore, SHG/THG microscopy is a promising tool for real‐time assessment of excised tissue during surgery.
Keywords: 3‐D imaging, biomedical technology, breast, second harmonic generation microscopy, third harmonic generation microscopy
Most breast cancers are carcinomas, a type of cancer that is derived from epithelial cells, aligning ducts and lobules. This study shows that second and third harmonic generation (SHG/THG) microscopy reveals the morphology of those key structures in freshly excised, unprocessed human breast tissue, including their cells and cell nuclei. The SHG/THG images have an information content comparable to that of the gold standard, (H&E histology) but are generated much faster, in less than a minute.
1. INTRODUCTION
For cancer instant pathology it is important to visualize the full morphology of healthy and diseased tissue. Relevant histopathological features include cell and nuclear pleomorphism, nuclear‐to‐cytoplasmic ratio, growth pattern of the cells and fibrosis 1, 2, 3, 4. Breast tissue is composed of glandular tissue (lobules and ducts) and stroma (mainly white fat and fibrous tissue). Most breast cancers are carcinomas, a type of cancer that is derived from epithelial cells, cells that align the ducts and lobules, that is, within the glandular tissue 5. In the breast stroma, information on the collagen orientation and distribution can be helpful for breast tumor diagnosis 6, 7, 8, but clearly it is important to be able to identify cellular features in the lobules and ducts. The gold standard for diagnosis, histopathology, involves formalin‐fixation, paraffin‐embedding, slicing and hematoxylin and eosin (H&E) staining of the tissue 9. However, this process is time‐consuming and makes intra‐operative feedback to the surgeon during surgery difficult. Therefore, developing a high quality, real‐time and label‐free method to visualize the morphology of key tissue components is of great significance for the diagnosis and investigation of breast cancer.
A novel imaging technique that meets these requirements is higher harmonic generation microscopy (HHGM). This technique is label‐free and provides 3D images with a high, sub‐cellular resolution within seconds 10. HHGM requires no external contrast agents and reveals tissue contrast provided by interfaces and inhomogeneities, and non‐centrosymmetric molecular structures via generation of respectively third 11, 12, 13, 14, 15, 16, 17, 18, 19 and second 20, 21, 22, 23, 24, 25 optical harmonics of the incident light (third and second harmonic generation [THG and SHG]). HHGM has been applied to several biological tissues, such as zebrafish embryos 26, 27, 28, vocal folds 29, retina 30, 31, oral mucosa 32, 33, bone 34, 35, fat tissue 36, skin tissue 37, 38, 39, 40, 41, 42, 43 and brain tissue 10, 44, 45, 46. HHGM has been used to identify erythrocytes 16, 47, 48, 49, and several types of leukocytes 50, 51, 52, 53. HHGM has been suggested as a promising clinical tool, for example for atherosclerosis diagnosis 54 and atopic dermatitis 40, but mainly for cancer diagnosis 10, 37, 38, 39, 42, 43, 55, 56, 57, 58, 59. In particular, we showed that with SHG/THG images of tissue from glioma patients, we could successfully discriminate normal and malignant tissue, based on the classical histopathological hallmarks such as increased cellularity and nuclear pleomorphism 10. For the assessment of breast cancer alternative optical techniques have been investigated, such as Raman spectroscopy and microscopy, which provide molecular information. Using the spectral information cancerous breast tissue can be distinguished from normal and benign tissue, both ex‐vivo 60, 61, 62, 63, 64, 65 and in‐vivo 66. Studies using SHG/THG microscopy to image breast tissue have also shown very promising results, but were applied on stained and fixated breast tissue 59, unstained but fixated breast biopsies 67 or breast cell lines 57, 68. Tu et al 69, 70 applied label‐free multimodal multi‐photon microscopy (including SHG/THG microscopy) to unprocessed breast tissue to visualize the micro‐environment of mammary tumors induced in rats, and to visualize extra‐cellular vesicles in the stroma tissue of a human tumor specimen. The ability of SHG/THG microscopy to visualize the morphology of key structural components such as the lobules and ducts of fresh unprocessed healthy breast tissue has until now not been demonstrated.
Here, we applied SHG/THG microscopy to fresh unprocessed healthy human tissue and compared with the gold standard histological images to identify the observed structures. In addition, we performed a DNA staining experiment on mouse breast tissue to identify cell nuclei in the label‐free THG images. Furthermore, we compared SHG/THG images with 2‐photon (2PF), and 3‐photon (3PF) excited auto‐fluorescence (3PF) images to determine the most information‐rich imaging modality.
2. MATERIALS AND METHODS
2.1. Ethics statement
All experimental procedures on mouse breast tissues were carried out in accordance with the European Council Directive (2010/63/EU) by permission of the Animal Research Law of the Netherlands. All procedures on human breast tissue were performed with the approval of the Biobank Pathology Unit of the Amsterdam Universitair Medische Centra (Amsterdam UMC) and in accordance with Dutch license procedures and the declaration of Helsinki. All patients undergoing surgery gave a written informed consent to use their biopsy specimens in scientific research.
2.2. Sample preparation
Healthy human breast samples were obtained from two patients with breast carcinoma undergoing breast mastectomy surgery in the Amsterdam UMC. The resected breast tissues were transported in a standard pathology transport container to the pathology department of Amsterdam UMC. There, a small piece of the tissue (maximum 1 × 1 × 1 cm3) was cut, far enough away from the tumor, which was immediately transported in phosphate‐buffered saline (PBS) to the laboratory.
Ten mouse breast tissue samples were excised from leftover female wild type mice provided by Neuroscience Amsterdam (CNCR, VU Amsterdam, Amsterdam, The Netherlands). Two mice were not pregnant and 2 months old when sacrificed. It was difficult to find fully developed lobules in those mouse breast samples, and therefore also breast samples of eight mice that were 18 days pregnant when sacrificed were imaged. The samples were rinsed with PBS solution to remove blood and hairs and were cut in samples of maximum 1 × 1 cm2 with varying thicknesses in the order of 0.2 cm.
Mouse and human tissue samples were placed in a plastic Petri Dish (diameter 50 mm) that was filled with agar, covered with PBS and flattened with a 0.17 mm thick glass cover slip (diameter 25 mm, Menzel‐Gläser, Braunschweig, Germany) (Figure 1C), and were imaged without further processing.
Figure 1.
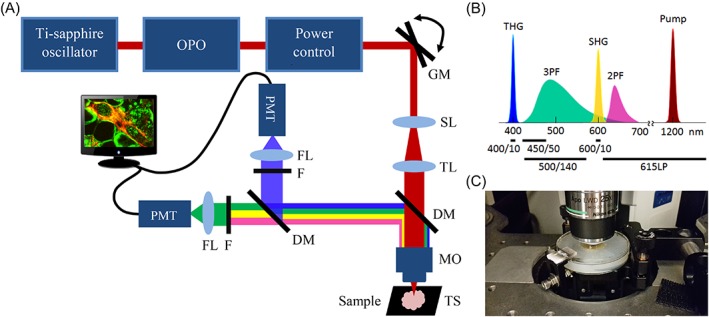
HHG microscopy for breast tissue imaging. A, Multi‐photon microscope setup: A laser source produces pulses of 200 fs at 1200 nm. The laser beam passes a pair of galvo‐scanner mirrors (GM), scan lens (SL), tube lens (TL) and is focused into the sample with a microscope objective (MO). The first dichroic mirror reflects backscattered HHG signals, and a second one splits them into a first channel (THG) and a second (SHG/2PF/3PF) channel. The signals are filtered by interference filters (F) and focused on the photomultiplier tube detectors (PMT) with lenses (FL). Sample can be moved with a motorized translation stage (TS). B, The light spectrum of the incoming laser beam and generated signals by the tissue, and types of filters used (indicated with black lines) to detect these signals. C, Photograph of a breast sample embedded in agar under a 0.17 mm glass cover slip in the middle of a plastic disk and the microscope objective on top
After imaging with the multi‐photon microscope, the human breast tissue samples were fixated in 4% formaldehyde, taken back to the pathology department (Amsterdam UMC), and subjected to the standard processing procedure, resulting in 3 μm thick H&E stained and unstained slices. The H&E stained slices were imaged using a Leica DM4000B microscope equipped with a Leica DC500 digital camera. IM50 imaging software was used to record and store low‐ and high‐magnification images, which were processed with “ImageJ” software (ver. 1.51d; National Institute of Health (NIH), Bethesda, Maryland). The unstained slices were again imaged with SHG/THG microscopy, to determine whether there are any differences with the fresh tissue. This might be of relevance for analysis without the risk of tissue disintegration with time.
DNA staining on the healthy fresh mouse breast tissue was performed with the dye Hoechst‐33342 (NucBlue Live ReadyProbes Reagent R37605, Life Technologies Europe B.V., Bleiswijk, The Netherlands), which is a cell‐permeable nuclear counterstain for live and fixed cells and tissue sections and used for both two‐photon and three‐photon fluorescence imaging 71, 72, 73. Mouse breast tissue was placed in a Petri Dish with 3 mL PBS and 15 droplets dye at 20°C and incubated for 30 minutes. Thereafter, the sample was rinsed with PBS, embedded in agar and placed under the microscope.
2.3. Image acquisition
For the HHGM imaging we used a modified commercial setup described before (Figure 1A) 10, 46. In brief, HHGM images were generated using a commercial two‐photon laser‐scanning microscope (TriMScope I; LaVision BioTec GmbH, Bielefeld, Germany). The light source was an optical parametric oscillator (Mira‐OPO, APE, Berlin, Germany), which generated 200 fs pulses at 1200 nm with linear polarization and repetition rate of 80 MHz. The OPO was pumped at 810 nm by a Ti‐sapphire oscillator (Coherent Chameleon Ultra II Coherent Inc., Santa Clara, California).
The laser beam was focused onto the sample using a water‐dipping objective (25×/1.10, Nikon APO long working distance, Nikon Instruments Europe B.V., Amsterdam, The Netherlands), which provided a lateral resolution of 0.5 μm in the THG images. The average power of the laser at the sample was approximately 100 mW. The signals generated by the sample were detected in epi‐direction with two high‐sensitivity GaAsP photomultiplier tubes (PMT, Hamamatsu H7422‐40, Hamamatsu Photonics K.K., Shizuoka, Japan), as a function of position of the focus in the sample. The generated signals were filtered from the incoming laser beam using a dichroic mirror (Chroma T800LPXRXT, Chroma Technology Corp, Bellows Falls, Vermont), split into two channels by a second dichroic mirror (Chroma T425LPXR), and filtered by narrow‐band interference filters (Figure 1B) for the detection of THG (400 ± 5 nm, Chroma Z400/10×) and SHG (600 ± 5 nm, Chroma D600/10×). For acquisition of multi‐photon auto‐fluorescence images, the SHG filter was interchanged with a filter for the detection of 3PF (500 ± 70 nm, HQ500/140M) or 2PF (615‐800 nm, ET615LP) signals. For the DNA staining experiment the SHG filter was replaced by an appropriate 3PF filter (450 ± 25 nm, ET450/50M).
Single images were made by transversely scanning of the laser beam over the sample using a pair of galvo‐scanner mirrors. Mosaic imaging was performed by transverse (xy) scanning of the sample with a motorized translation stage. The intrinsically confocal properties of HHGM provide direct depth sectioning. Full 3D images were obtained by moving the microscope objective in vertical (z) direction with a stepper motor.
The higher harmonic generation (HHG) images were acquired using the TriMScope I software (“Imspector Pro”), stored in 16‐bit tiff format, and processed and analyzed with “ImageJ” software (ver. 1.51d, NIH, Bethesda, Maryland). Logarithmic contrast enhancement was used to better visualize the weaker generated signals. 2D images are made within seconds and 3D scans within minutes. An overview of the image dimensions and acquisitions time for each figure can be found in Table S1, Supporting Information in the supplementary information.
3. RESULTS
In all images in this manuscript THG signals are depicted in green and SHG signals in red, resulting in a yellow color where they overlap. THG is an interface sensitive technique, based on spatial variations in the third‐order non‐linear susceptibility, refractive index and dispersion intrinsic to the tissue 14. Examples of THG sources are lipid‐water and protein fibers‐water interfaces. SHG is a non‐linear optical process including generating signals by non‐centrosymmetric molecular structures, such as structural protein arrays. Example of SHG sources are microtubule arrays and collagen fibers 21.
3.1. HHG imaging of human breast structures compared to standard histology
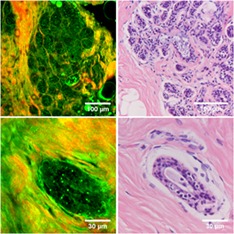
Figure 2 shows SHG/THG images of various healthy human breast structures: lobules (A,B), ducts (C,D), connective tissue (E), fat tissue (F) and blood vessels (G,H). After SHG/THG imaging the tissue was fixated, sliced and stained with H&E to compare with the gold standard of histopathology. Figure 2 shows also these H&E stained images. Video S1 to S3 in the supplementary information show SHG/THG 3D depth scans of the areas corresponding to Figures 2A,D,H, and Figure 3 shows their orthogonal views. There were no relevant differences in THG/SHG results between the imaged human samples.
Figure 2.
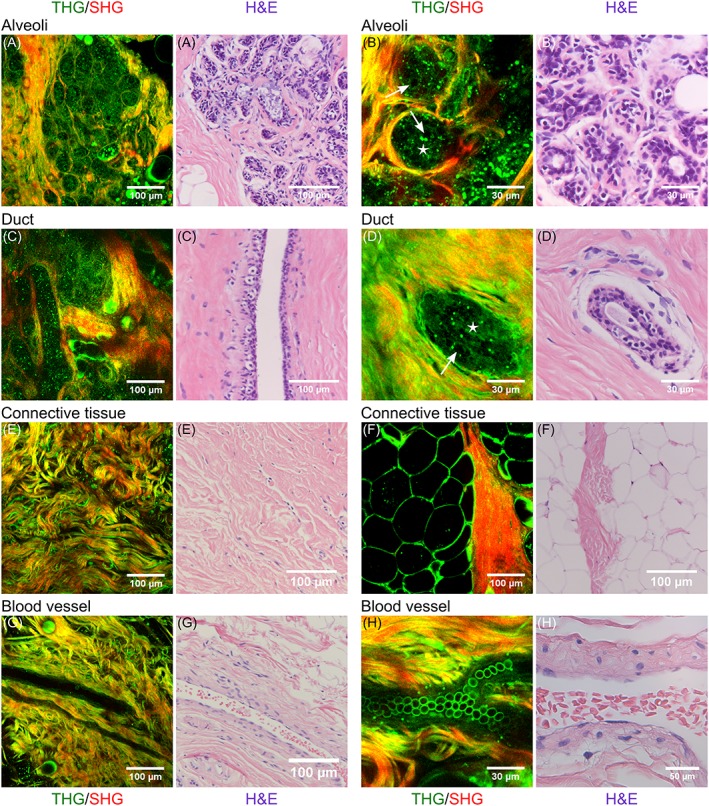
Combined THG (green) and SHG (red) images of normal human breast tissue (left) compared to the H&E histology of similar structures in the same samples (right). A‐B, alveoli; C‐D, ducts; E, connective tissue; F, fat tissue; G‐H, blood vessels. Epithelial cells and luminal substance inside alveoli and ducts are indicated with arrows and stars respectively. A,D,H, are depth scans of respectively 130, 50 and 30 μm deep with depth steps of 2 μm, and a total acquisition time of respectively 2.6 minutes, 61.6 seconds and 38.4 seconds
Figure 3.
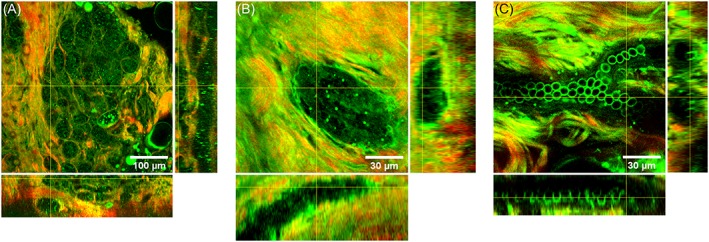
3D information of human breast structures. Combined THG (green) and SHG (red) images of a lobule (A) duct (B) and blood vessel (C) in human breast tissue. At the sides are orthogonal views of the depth scans of 130 μm (A), 50 μm (B) and 30 μm (C) with steps of 2 μm, and a total acquisition time of respectively 2.6 minutes, 61.6 seconds and 38.4 seconds. THG signals were generated by the epithelial cells in alveoli (A) and duct (B), by endothelial cells in the blood vessel wall and by erythrocytes (C). The connective tissue surrounding the alveoli, duct and blood vessel consist of collagen revealed with SHG, and elastin interfaces revealed with THG
A lobule consists of alveoli, both alveoli and ducts consist of epithelial cell layers surrounded by a basement membrane 2. All these components are visible in the HHG images of the lobules (Figure 2A,B) and ducts (Figure 2C,D). Epithelial cells are visualized by granular structures in the cytoplasm, with an apparent black hole at the position of the nucleus (indicated with arrows). Also, the luminal substance between the cells is visible (indicated with stars). Both each alveolus (Figure 2B) and the whole lobule (Figure 2A) are surrounded by SHG signals generated by collagen. Also in ducts the epithelial cells and luminal substance are visible, surrounded by collagen. The 3D depth scan of 130 μm deep (shown in Video S1) shows that the duct in Figure 2C is located underneath the alveoli in Figure 2A. This is also clearly visible in the orthogonal views (Figure 3A).
The breast stroma, supporting the lobules and ducts, consists mainly of fibrous (collagen and elastin) tissue and fat cells. The collagen (SHG and THG) and elastin (THG) bundles are clearly visible in the HHGM image (Figure 2E). Also, fat cells were easily identified with THG microscopy, because strong THG signals generated by the lipid‐water interface revealed their cellular contours (Figure 2F).
Breast stroma also contains blood vessels, which were easily identified in the HHGM images (Figure 2G,H) as hollow tubes surrounded by collagen and elastin fiber containing erythrocytes. In these examples, it is difficult to distinguish stroma from vessel wall. The endothelial cells that line the inside of the vessel wall and erythrocytes at the bottom of the vessel generate THG signals (Figure 2G,H, Video S3 and orthogonal views in Figure 3C).
All SHG/THG images of the breast structures show good agreement with the H&E stained images. Because of the distortion of tissue in the fixation process, one‐to‐one comparison was not directly possible, though every component could still be identified in the H&E images.
The 3 μm fixated and embedded, but not H&E stained, slices were also imaged with THG/SHG microscopy, and showed similar results compared to the fresh tissue (Figure S1), except that the SHG and THG signals were better separated, and produced less of the “yellow” overlap signal. Possibly, because the fixation and/or embedding processes have changed some of the (optical) properties of the tissue. Most likely, an increase in the relative THG signal from inside the duct, necessitated a downscaling of the THG signal relative to the SHG signal in Figure S1B,E. The increase in THG signal might also be due to change of interface properties—we filled the sample voids or substituted water‐filled areas with paraffin, which has a high index of refraction (1.45).
3.2. DNA staining of mouse breast tissue
To investigate if the black holes in the epithelial cells are indeed nuclei (Figure 2A‐D) we performed a DNA staining experiment on mouse breast tissue and co‐recorded SHG/THG signals with 3‐photon excited fluorescence signals (3PF) from the Hoechst DNA staining dye.
Figure 4 shows a lobule, two ducts and a blood vessel aligned with a peripheral nerve. From these images one can see that the alveoli and ducts contain a lot of cells and that indeed the DNA is co‐localized with the black holes in the SHG/THG images, which can therefore be identified as the cell nuclei (Figure 4A‐L). Similar to the human breast tissue, epithelial cells inside the mouse alveoli and ducts were visualized with THG microscopy as granular spheres surrounding a THG silent nucleus. In Figure 4E the epithelial cells are less granular.
Figure 4.
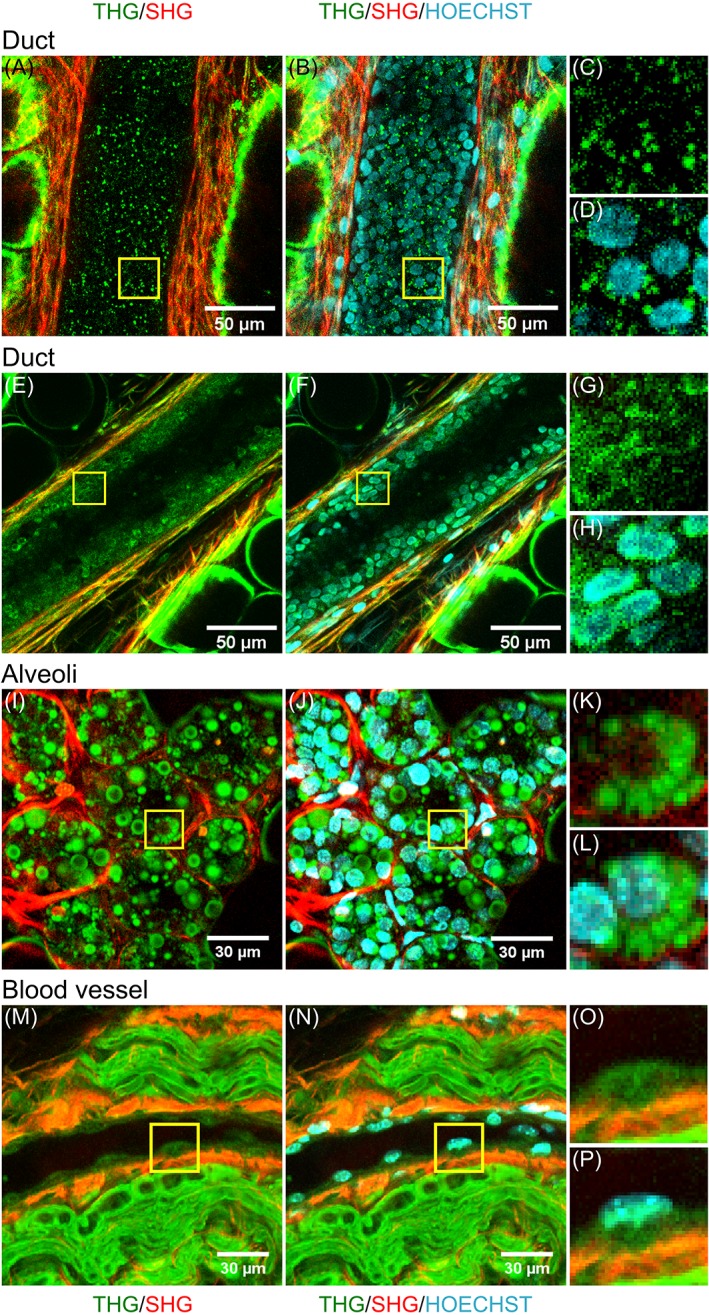
Cell nuclei in mouse breast tissue visualized with the DNA dye Hoechst‐33342. For two breast ducts of a non‐lactating mouse (A‐D, E‐H), and a lobule (I‐L) and a blood vessel surrounded by a peripheral nerve of a lactating mouse (M‐P) the combined THG (green) and SHG (red) images are shown (left), and the THG/SHG images combined with the 3PF signals from the DNA dye revealing the cell nuclei (middle). Magnified images of cells (marked with yellow squares in left and middle images) show that in the THG image the black holes in the epithelial cells of alveoli and ducts, and the thicker parts of the endothelial cells in the blood vessel contain DNA
The blood vessel contains endothelial cells, clearly visible at the inside of the wall as flattened cells, with the nucleus, as indicated by the 3PF signal, in the thicker part of the cell (Figure 4M‐P). For these cells the structures within the nuclei or the nuclear membrane did produce signals detected in the THG channel, instead of being silent. 3PF signals were also visualized in the breast stroma, most likely from the nuclei of fibroblasts or macrophages (Figure 4B,F).
The ducts were from non‐pregnant mice, while the lobule was from an 18‐day pregnant mouse. In contrast to the non‐pregnant mice and human breast tissues, the tissue from the pregnant, and therefore lactating, mouse also contained little fat cells in the alveoli visible as small round circles, which do not contain DNA (indicated with arrows). Furthermore, the mouse breast stroma (from both pregnant and non‐pregnant mice) consists primarily of fat cells 74, of which the cellular contours were clearly visible around the ducts by the generated THG signals (Figure 4A,E).
3.3. Imaging breast structures with different imaging modalities
In addition to THG and SHG signals, the breast tissue also generated auto‐fluorescent signals. We compared the different imaging modalities by collecting emission at 500 and 615 nm through broad band interference filters, to detect 3‐photon excited fluorescence (3PF, 500 ± 70 nm) and 2‐photon excited fluorescence (2PF, 615‐800 nm) respectively, using an excitation wavelength of 1200 nm. Figure 5 compares several breast structures imaged with THG, SHG, 3PF and 2PF microscopy.
Figure 5.
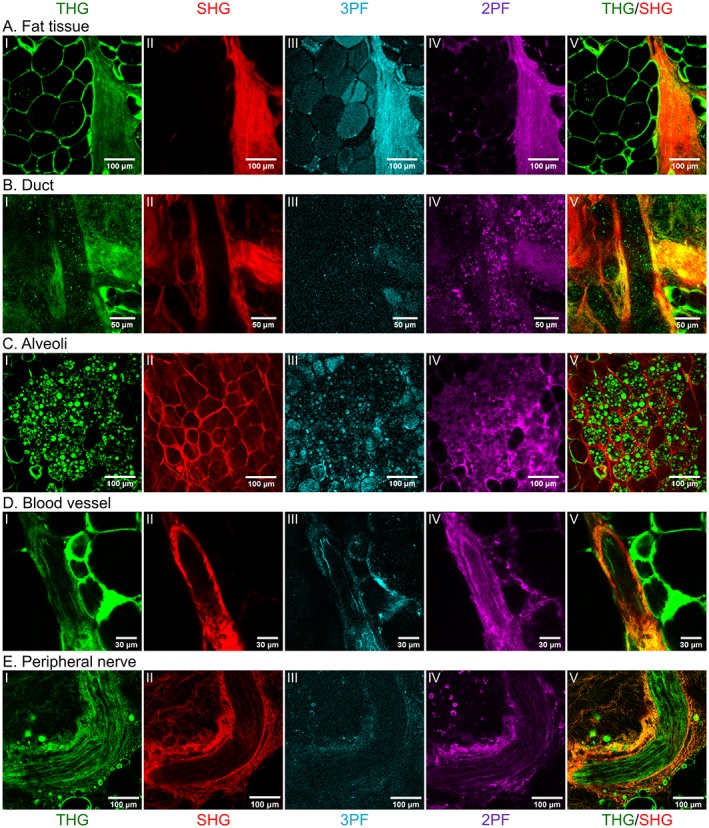
Comparing different imaging modalities. Human (A,B), lactating mouse (C) and non‐lactating mouse (D,E) breast tissue structures imaged with several imaging modalities upon excitation at 1200 nm: THG (I, green), SHG (II, red), 3PF (III, blue), and 2PF (IV, purple). The most right panel (V) shows the combined THG/SHG images of each structure. A, Fat tissue; B, Duct; C, Lobule; D, Blood vessel; E, Peripheral nerve. THG signals are generated by interfaces in the tissue, which reveal fat cells, epithelial cells, elastin and axons. SHG signals are generated mostly by collagen. Because the tissue is not stained the 3PF and 2PF signals are generated by auto‐fluorescence molecules
As described in the previous sections, THG signals are generated on interfaces, revealing fat cells, epithelial cells including their cell nuclei, elastin fibers, endothelial cells and erythrocytes, while SHG signals are generated by connective tissue, mostly by collagen fibers in the stroma, and surrounding alveoli, ducts and blood vessels. The interfaces of the elastin layer in the blood vessel wall (generating THG signals) is here clearly visible as a stripe aligned with the blood vessel (Figure 5DI). Another component of the breast stroma that we did not describe before are peripheral nerves (Figure 5E). Nerves contain axons, of which the myelin sheets generate strong THG signals, and which are aligned by connective tissue (including collagen) generating SHG signals.
Collagen and elastin are also intrinsic fluorophores that generate 2PF signals and weaker 3PF signals. Compared to SHG, the 3PF and 2PF signals were weaker and no fibers could be distinguished. 3PF signals were mostly generated inside fat cells (in stroma and the small cells inside pregnant mouse alveoli), by the blood vessel wall (including elastin), and surrounding the nerves. 2PF signals were generated on and nearby the edge of fat cells, at the boundaries and inside blood vessels and nerves, and inside alveoli, except for the little fat cells and cell nuclei, probably generated by cell cytoplasm. Compared to THG and SHG microscopy, multi‐photon auto‐fluorescent signals were less specific and tissue boundaries were less well revealed.
4. DISCUSSION AND CONCLUSION
We investigated which pathological relevant tissue components can be visualized by SHG/THG microscopy in human and mouse breast tissue by imaging fresh unprocessed healthy breast samples and comparing the resulting images with the histological images. Our experiments show that HHG microscopy can identify microscopic breast structures without the need of processing the tissue (no labeling and fixation) within a short time span of a few minutes. Lobules, including alveoli with cells and cell nuclei, ducts, connective tissue, fat tissue, blood vessels and nerves were identified, comparable to the structures that are seen in microscopic images of H&E stained slices, the gold standard.
Ductular structures in mouse and human breasts were the most difficult structures to identify, because in some cases the ducts were completely filled with cells and therefore might be mistaken for invasive tumor. Furthermore, alveoli and ducts were sometimes hard to distinguish. This also holds for H&E slides, because alveoli and ducts have the same cellular composition, and therefore are very similar in 2D images. The advantage of HHG microscopy is that it can provide 3D information because of optical sectioning, making it easier to determine whether the structure is a tube, hence a duct, or a cluster of cells, hence an alveolus.
For the diagnosis of breast cancer the morphology of the cells especially in lobules and ducts is important, because histological features that the pathologist looks at include abnormal cellularity, nuclear‐to‐cytoplasmic ratio, nuclear pleomorphism and the growth pattern of cells 1, 2, 3, 4. The HHG images show important details of the healthy equivalents of these breast structures, the epithelial cells including their cell nuclei inside lobules and ducts. The DNA staining experiment showed that epithelial cells in alveoli and ducts are visualized in the THG images by bright granular spheres around a THG‐silent cell nucleus. In contrast, the nuclear structures of endothelial cells in blood vessels did generate THG signals. In previous work on the brain, we also showed that in some cells the nucleus generates a brighter THG signal than in other cells (neurons, glial cells, tumor cells), whereas sometimes only the nucleus boundary is visible 10, 75. Factors that may influence the THG generated within the nucleus may be chromatin condensation in the cell nucleus or the environment of the cell, because THG microscopy is an interface sensitive technique 14. Epithelial cells in mammary glands store fat and may contain other substances in the cytoplasm in the form of granules, which explains the THG‐bright granular spheres.
In this paper we demonstrated that THG microscopy reveals the morphology of the key structural components, lobules and ducts, and their corresponding cells and cell nuclei, in healthy breast tissue. The next step will be to determine whether this morphological information revealed with SHG/THG microscopy will contribute to distinguishing normal from cancerous breast tissue, by investigating tumor tissue. We expect that areas of high cell density and pathological cell morphology will be recognizable like we demonstrated for brain tumors 10, 75. Furthermore, the increased vascularity, typical for tumor areas should be easily identifiable from the well‐resolved blood vessels in the SHG/THG images. Part of these components is visualized with THG and others with SHG. Therefore, the combination of these two imaging modalities provides an optimal information density.
Compared to the multi‐photon auto‐fluorescent signals, THG and SHG microscopy were more specific and tissue boundaries were better revealed. Other studies explored the auto‐fluorescence signals from fresh breast tissue, using shorter excitation wavelengths (740‐880 nm) 76, 77, 78, 79. They showed that epithelial cells are clearly visualized by the 2PF signals from nicotinamide adenine dinucleotide hydrogen (NADH) and flavin adenine dinucleotide (FAD) fluorescence, with the non‐autofluorescent nuclei appearing as black holes. We expected to also observe NADH and FAD auto‐fluorescence signals (maximum λex/λem 350/450 nm and 450/535 nm respectively) as 3PF signals, with our excitation wavelength of 1200 nm. Possibly, this may have been observed in the 3PF image of the lobule (Figure 5CIII), but not in the duct (Figure 5BIII). This might be due to the fact that the 3PF are weaker because it involves a three‐photon process instead of a two‐photon process. However, excitation at 1200 nm is preferred in a clinical setting, because of the deeper penetration depth, which enables imaging more cell layers below the cutting surface, eliminating the need to create a laborious smooth surface.
In this paper we demonstrated that HHG microscopy has the ability to reveal the morphology of key structural components of the breast, without any processing of the tissue involved, by generation high quality images in 3D, within a few minutes, or 2D within approximately 10 seconds, with sub‐cellular resolution. This makes HHG microscopy a promising technique for application during breast surgery, either ex‐vivo or in‐situ. Ex‐vivo tissue analysis within the operating room can be achieved by developing transportable, ex‐vivo SHG/THG microscopes. Indeed, such devices start to appear on the market. Our next step is to apply such a machine in an operating room, in close collaboration with the surgeons and pathologists. Decision making will be greatly aided by automated image analysis 80, 81, initially to be offset against the diagnosis made by pathologists. Furthermore, in our and other labs, micro‐endoscopes are in development 82, 83, 84 to enable in‐situ multi‐photon imaging, including SHG/THG, and aid a surgeon in determining which tissue to excise. In future, SHG/THG in‐situ and ex‐vivo analysis may well be valuable additions to the medical imaging instrumentarium of the surgeon.
CONFLICTS OF INTEREST
M.L. Groot is a cofounder of Tritos Diagnostics.
AUTHOR BIOGRAPHIES
Please see Supporting Information online.
Supporting information
Table S1 Image dimensions and acquisition time of figures in the article.
Figure S1: Fresh and fixated/embedded human breast sample imaged with SHG (red) and THG (green) compared to H&E histology. A‐C, Duct; D‐F, Lobule. The fresh breast tissue is fixated in 4% formaldehyde, sliced in 3 μm thick slices, and paraffin‐embedded. The SHG/THG images of the unstained slices (B,E) show similar results to the fresh tissue (A,D), and are comparable with the H&E histology (C,F).
Video S1 Depth scan of alveoli in human breast tissue, with underneath a blood vessel and a duct, imaged with THG (green) and SHG (red) microscopy. The depth scan is 130 μm deep with depth steps of 2 μm and a total acquisition time of 2.6 minutes. Logarithmic contrast enhancement is applied to enhance weaker signals.
Video S2 Depth scan of a duct in human breast tissue imaged with THG (green) and SHG (red) microscopy. The scan is 50 μm deep with depth steps of 2 μm, and a total acquisition time of 61.6 seconds. Logarithmic contrast enhancement is applied to enhance weaker signals.
Video S3 Depth scan of a blood vessel in human breast tissue imaged with THG (green) and SHG (red) microscopy. The scan is 30 μm deep with depth steps of 2 μm, and a total acquisition time of 38.4 seconds. Logarithmic contrast enhancement is applied to enhance weaker signals.
ACKNOWLEDGMENTS
We thank J. Wortel for the availability of the mouse tissue, and P. Scholten for her help with setting up the logistics for the human breast tissue. This research is supported by the NWO domain Applied and Engineering Sciences (which is part of the Netherlands Organization for Scientific Research [NWO], and which is partly funded by the Ministry of Economic Affairs, Agriculture and Innovation), project OBAMA (No. 12708) and InstantPathology (No. 15825) Furthermore, this research has support from Laserlab‐Europe (EU‐H2020 654148).
van Huizen LMG, Kuzmin NV, Barbé E, van der Velde S, te Velde EA, Groot ML. Second and third harmonic generation microscopy visualizes key structural components in fresh unprocessed healthy human breast tissue. J. Biophotonics. 2019;12:e201800297. 10.1002/jbio.201800297
Funding information Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Number: 15825; Stichting voor de Technische Wetenschappen, Grant/Award Number: 12708; Laserlab‐Europe, Grant Number: 654148
REFERENCES
- 1. Millis R. R., Atlas of Breast Pathology, MTP Press Limited, Lancaster, England: 1984. [Google Scholar]
- 2. Moinfar F., Essentials of Diagnostic Breast Pathology, Springer, Heidelberg, Germany: 2007. [Google Scholar]
- 3. Ellis I. O., Pinder S. E., Bobrow L., Buley I. D., Coyne J., J J., Going S. H., Jasani B., Lakhani S., Lowe J., Miller K., Rhodes A., Walker R. A., Wells C. A., Pathology Reporting of Breast Disease, NHS Cancer Screening Programmes and Royal College of Pathologists, Sheffield and London: 2005. [Google Scholar]
- 4. O'Malley F. P., Pinder S. E., Mulligan A. M., Breast Pathology, Elsevier Saunders, Philadelphia, PA: 2011. [Google Scholar]
- 5. Lester S. C., in The Breast (Eds: Kuman V., Abbas A. K., Aster J. C.), Elsevier, Philadephia, PA: 2015, p. 1043. [Google Scholar]
- 6. Golaraei A., Kontenis L., Cisek R., Tokarz D., Done S. J., Wilson B. C., Barzda V., Biomed. Opt. Express 2016, 7, 4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burke K., Tang P., Brown E., J. Biomed. Opt. 2013, 18, 31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ambekar R., Lau T.‐Y., Walsh M., Bhargava R., Toussaint K. C., Biomed. Opt. Express 2012, 3, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nascimento A. F., in Other Malignant Lesions of the Breast (Eds: O'Malley F. P., Pinder S. E., Mulligan A. M.), Elsevier Saunders, Philadelphia, PA: 2011, p. 326. [Google Scholar]
- 10. Kuzmin N. V., Wesseling P., Hamer P. C., Noske D. P., Galgano G. D., Mansvelder H. D., Baayen J. C., Groot M. L., Biomed. Opt. Express 2016, 7, 1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barad Y., Eisenberg H., Horowitz M., Silberberg Y., Appl. Phys. Lett. 1997, 70, 922. [Google Scholar]
- 12. Debarre D., Supatto W., Beaurepaire E., Opt. Lett. 2005, 30, 2134. [DOI] [PubMed] [Google Scholar]
- 13. Cheng J.‐X., Sunney Xie X., J. Opt. Soc. Am. B 2002, 19, 1604. [Google Scholar]
- 14. Debarre D., Beaurepaire E., Biophys. J. 2007, 92, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahou P., Olivier N., Labroille G., Duloquin L., Sintes J. M., Peyrieras N., Legouis R., Debarre D., Beaurepaire E., Biomed. Opt. Express 2011, 2, 2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Müller M., Squier J., Wilson K. R., Brakenhoff G. J., J. Microsc. 1998, 191, 266. [DOI] [PubMed] [Google Scholar]
- 17. Oron D., Yelin D., Tal E., Raz S., Fachima R., Silberberg Y., J. Struct. Biol. 2004, 147, 3. [DOI] [PubMed] [Google Scholar]
- 18. Squier J., Müller M., Brakenhoff G., Wilson K. R., Opt. Express 1998, 3, 315. [DOI] [PubMed] [Google Scholar]
- 19. Yelin D., Silberberg Y., Opt. Express 1999, 5, 169. [DOI] [PubMed] [Google Scholar]
- 20. Chu S. W., Chen I. H., Liu T. M., Sun C. K., Lee S. P., Lin B. L., Cheng P. C., Kuo M. X., Lin D. J., Liu H. L., J. Microsc. 2002, 208, 190. [DOI] [PubMed] [Google Scholar]
- 21. Campagnola P. J., Millard A. C., Terasaki M., Hoppe P. E., Malone C. J., Mohler W. A., Biophys. J. 2002, 82, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams R. M., Zipfel W. R., Webb W. W., Curr. Opin. Chem. Biol. 2001, 5, 603. [DOI] [PubMed] [Google Scholar]
- 23. Verbiest T., Elshocht S. V., Kauranen M., Hellemans L., Snauwaert J., Nuckolls C., Katz T. J., Persoons A., Science 1998, 282, 913. [DOI] [PubMed] [Google Scholar]
- 24. Freund I., Deutsch M., Opt. Lett. 1986, 11, 94. [DOI] [PubMed] [Google Scholar]
- 25. Hellwarth R., Christensen P., Opt. Commun. 1974, 12, 318. [Google Scholar]
- 26. Chen S.‐Y., Hsieh C.‐S., Chu S.‐W., Lin C.‐Y., Ko C.‐Y., Chen Y.‐C., Tsai H.‐J., Hu C.‐H., Sun C.‐K., JBO 2006, 11, 054022. [DOI] [PubMed] [Google Scholar]
- 27. Olivier N., Luengo‐Oroz M. A., Duloquin L., Faure E., Savy T., Veilleux I., Solinas X., Debarre D., Bourgine P., Santos A., Peyriéras N., Beaurepaire E., Science 2010, 329, 967. [DOI] [PubMed] [Google Scholar]
- 28. Chu S.‐W., Chen S.‐Y., Tsai T.‐H., Liu T.‐M., Lin C.‐Y., Tsai H.‐J., Sun C.‐K., Opt. Express 2003, 11, 3093. [DOI] [PubMed] [Google Scholar]
- 29. Yildirim M., Durr N., Ben‐Yakar A., JBO 2015, 20, 096013. [DOI] [PubMed] [Google Scholar]
- 30. Karunendiran A., Cisek R., Tokarz D., Barzda V., Stewart B. A., Biomed. Opt. Express 2017, 8, 4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Masihzadeh O., Lei T. C., Domingue S., Kahook M. Y., Bartels R. A., Ammar D. A., 2015, 21, 538–547. [PMC free article] [PubMed] [Google Scholar]
- 32. Tai S. P., Lee W. J., Shieh D. B., Wu P. C., Huang H. Y., Yu C. H., Sun C. K., Opt. Express 2006, 14, 6178. [DOI] [PubMed] [Google Scholar]
- 33. Tsai M.‐R., Chen S.‐Y., Shieh D.‐B., Lou P.‐J., Sun C.‐K., Biomed. Opt. Express 2011, 2, 2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Genthial R., Beaurepaire E., Schanne‐Klein M.‐C., Peyrin F., Farlay D., Olivier C., Bala Y., Boivin G., Vial J.‐C., Débarre D., Gourrier A., Sci. Rep. 2017, 7, 1. 10.1038/s41598-017-03548-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tokarz D., Cisek R., Wein M. N., Turcotte R., Haase C., Yeh S.‐C. A., Bharadwaj S., Raphael A. P., Paudel H., Alt C., Liu T.‐M., Kronenberg H. M., Lin C. P., PLoS One 2017, 12, e0186846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai C.‐K., Wang T.‐D., Lin J.‐W., Hsu R.‐B., Guo L.‐Z., Chen S.‐T., Liu T.‐M., Biomed. Opt. Express 2013, 4, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen S. Y., Chen S. U., Wu H. Y., Lee W. J., Liao Y. H., Sun C. K., IEEE J. Sel. Top. Quant. Electron. 2010, 16, 478. [Google Scholar]
- 38. Weigelin B., Bakker G.‐J., Friedl P., IntraVital 2012, 1, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee G. G., Lin H. H., Tsai M. R., Chou S. Y., Lee W. J., Liao Y. H., Sun C. K., Chen C. F., IEEE Trans. Biomed. Circuits Syst. 2013, 7, 158. [DOI] [PubMed] [Google Scholar]
- 40. Lee J. H., Chen S. Y., Yu C. H., Chu S. W., Wang L. F., Sun C. K., Chiang B. L., J. Biomed. Opt. 2009, 14, 014008. [DOI] [PubMed] [Google Scholar]
- 41. Tai S. P., Tsai T. H., Lee W. J., Shieh D. B., Liao Y. H., Huang H. Y., Zhang K., Liu H. L., Sun C. K., Opt. Express 2005, 13, 8231. [DOI] [PubMed] [Google Scholar]
- 42. Tsai M. R., Cheng Y. H., Chen J. S., Sheen Y. S., Liao Y. H., Sun C. K., J. Biomed. Opt. 2014, 19, 36001. [DOI] [PubMed] [Google Scholar]
- 43. Wu P. C., Hsieh T. Y., Tsai Z. U., Liu T. M., Sci. Rep. 2015, 5, 8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleinfeld D., Mitra P. P., Helmchen F., Denk W., Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim H., Sharoukhov D., Kassim I., Zhang Y., Salzer J. L., Melendez‐Vasquez C. V., Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Witte S., Negrean A., Lodder J. C., de Kock C. P., Testa Silva G., Mansvelder H. D., Louise Groot M., Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clay G. O., Schaffer C. B., Kleinfeld D., J. Chem. Phys. 2007, 126, 025102. [DOI] [PubMed] [Google Scholar]
- 48. Chen C. K., Liu T. M., Biomed. Opt. Express 2012, 3, 2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saytashev I., Glenn R., Murashova G. A., Osseiran S., Spence D., Evans C. L., Dantus M., Biomed. Opt. Express 2016, 7, 3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsai C.‐K., Chen Y.‐S., Wu P.‐C., Hsieh T.‐Y., Liu H.‐W., Yeh C.‐Y., Lin W.‐L., Chia J.‐S., Liu T.‐M., Biomed. Opt. Express 2012, 3, 2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gavgiotaki E., Filippidis G., Kalognomou M., Tsouko A. A., Skordos I., Fotakis C., Athanassakis I., J. Struct. Biol. 2015, 189, 105. [DOI] [PubMed] [Google Scholar]
- 52. Gavgiotaki E., Filippidis G., Zerva I., Agelaki S., Georgoulias V., Athanassakis I. E. D. B. E., Nonlinear Microscopy as Diagnostic Tool for the Discrimination of Activated T Cells, Vol. 10414, Optical Society of America, Munich, Germany: 2017, p. 1041406. [Google Scholar]
- 53. Wu C.‐H., Wang T.‐D., Hsieh C.‐H., Huang S.‐H., Lin J.‐W., Hsu S.‐C., Wu H.‐T., Wu Y.‐M., Liu T.‐M., Sci. Rep. 2016, 6, 37210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Small D. M., Jones J. S., Tendler I. I., Miller P. E., Ghetti A., Nishimura N., Biomed. Opt. Express 2018, 9, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehravar S., Banerjee B., Chatrath H., Amirsolaimani B., Patel K., Patel C., Norwood R. A., Peyghambarian N., Kieu K., Biomed. Opt. Express 2015, 7, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harpel K., Baker R. D., Amirsolaimani B., Mehravar S., Vagner J., Matsunaga T. O., Banerjee B., Kieu K., Biomed. Opt. Express 2016, 7, 2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gavgiotaki E., Filippidis G., Markomanolaki H., Kenanakis G., Agelaki S., Georgoulias V., Athanassakis I., J. Biophotonics 2017, 10, 1152. [DOI] [PubMed] [Google Scholar]
- 58. Adur J., Pelegati V. B., de Thomaz A. A., Baratti M. O., Almeida D. B., Andrade L. A., Bottcher‐Luiz F., Carvalho H. F., Cesar C. L., PLoS One 2012, 7, e47007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Adur J., Pelegati V. B., de Thomaz A. A., D'Souza‐Li L., Assuncao Mdo C., Bottcher‐Luiz F., Andrade L. A., Cesar C. L., J. Biomed. Opt. 2012, 17, 081407. [DOI] [PubMed] [Google Scholar]
- 60. Abramczyk H., Brozek‐Pluska B., Surmacki J., Jablonska‐Gajewicz J., Kordek R., Prog. Biophys. Mol. Biol. 2012, 108, 74. [DOI] [PubMed] [Google Scholar]
- 61. Depciuch J., Kaznowska E., Zawlik I., Wojnarowska R., Cholewa M., Heraud P., Cebulski J., Appl. Spectrosc. 2016, 70, 251. [DOI] [PubMed] [Google Scholar]
- 62. Haka A. S., Shafer‐Peltier K. E., Fitzmaurice M., Crowe J., Dasari R. R., Feld M. S., Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kong K., Zaabar F., Rakha E., Ellis I., Koloydenko A., Notingher I., Phys. Med. Biol. 2014, 59, 6141. [DOI] [PubMed] [Google Scholar]
- 64. Li Q., Gao Q., Zhang G., Biomed. Opt. Express 2014, 5, 2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu C. H., Zhou Y., Sun Y., Li J. Y., Zhou L. X., Boydston‐White S., Masilamani V., Zhu K., Pu Y., Alfano R. R., Technol. Cancer Res. Treat. 2013, 12, 371. [DOI] [PubMed] [Google Scholar]
- 66. Haka A. S., Volynskaya Z., Gardecki J. A., Nazemi J., Lyons J., Hicks D., Fitzmaurice M., Dasari R. R., Crowe J. P., Feld M. S., Cancer Res. 2006, 66, 3317. [DOI] [PubMed] [Google Scholar]
- 67. Lee W., Kabir M. M., Emmadi R., Toussaint K. C. Jr., J. Microsc. 2016, 264, 175. [DOI] [PubMed] [Google Scholar]
- 68. Gavgiotaki E., Filippidis G., Psilodimitrakopoulos S., Markomanolaki H., Kalognomou M., Agelaki S., Georgoulias V., Athanassakis I. E. D. B. E. S. P. P. F., Hillman E., Third Harmonic Generation Microscopy as a Diagnostic Tool for the Investigation of Microglia BV‐2 and Breast Cancer Cells Activation, Vol. 9536, Optical Society of America, Munich, Germany: 2015, p. 953614. [Google Scholar]
- 69. Tu H., Liu Y., Marjanovic M., Chaney E. J., You S., Zhao Y., Boppart S. A., Sci. Adv. 2017, 3, e1600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tu H., Liu Y., Turchinovich D., Marjanovic M., Lyngso J., Laegsgaard J., Chaney E. J., Zhao Y., You S., Wilson W. L., Xu B., Dantus M., Boppart S. A., Nat. Photonics 2016, 10, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bestvater F., Spiess E., Stobrawa G., Hacker M., Feurer T., Porwol T., Berchner‐Pfannschmidt U., Wotzlaw C., Acker H., J. Microsc. 2002, 208, 108. [DOI] [PubMed] [Google Scholar]
- 72. Chu S. W., Tai S. P., Ho C. L., Lin C. H., Sun C. K., Microsc. Res. Tech. 2005, 66, 193. [DOI] [PubMed] [Google Scholar]
- 73. Gryczynski I., Malak H., Lakowicz J. R., Bioimaging 1996, 4, 138. [Google Scholar]
- 74. Hovey R., McFadden T., Akers R., J. Mammary Gland Biol. Neoplasia 1999, 4, 53. [DOI] [PubMed] [Google Scholar]
- 75. Kuzmin N. V., Idema S., Aronica E., de Witt Hamer P. C., Wesseling P., Groot M. L., in Higher Harmonic Generation Imaging for NeuroPathology (Eds: Pavone F. S., Shoham S.), CRC Press, Boca Raton, FL: 2018. [Google Scholar]
- 76. Nie Y. T., Wu Y., Fu F. M., Lian Y. E., Zhuo S. M., Wang C., Chen J. X., J. Microsc. 2015, 258, 79. [DOI] [PubMed] [Google Scholar]
- 77. Provenzano P. P., Eliceiri K. W., Yan L., Ada‐Nguema A., Conklin M. W., Inman D. R., Keely P. J., Microsc. Microanal. 2008, 14, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu X., Chen G., Qiu J., Lu J., Zhu W., Chen J., Zhuo S., Yan J., Oncol. Lett. 2016, 11, 3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu Y., Fu F., Lian Y., Chen J., Wang C., Nie Y., Zheng L., Zhuo S., Lasers Med. Sci. 2015, 30, 1109. [DOI] [PubMed] [Google Scholar]
- 80. Zhang Z., Kuzmin N. V., Groot M. L., de Munck J. C., Bioinformatics 2017, 33, 1712. [DOI] [PubMed] [Google Scholar]
- 81. Zhang Z., Kuzmin N. V., Groot M. L., de Munck J. C., J. Biophotonics 2017, 11, e201600256. [Google Scholar]
- 82. Akhoundi F., Qin Y., Peyghambarian N., Barton J. K., Kieu K., Biomed. Opt. Express 2018, 9, 2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lombardini A., Mytskaniuk V., Sivankutty S., Andresen E. R., Chen X., Wenger J., Fabert M., Joly N., Louradour F., Kudlinski A., Rigneault H., Light Sci. Appl. 2018, 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zong W., Wu R., Li M., Hu Y., Li Y., Li J., Rong H., Wu H., Xu Y., Lu Y., Jia H., Fan M., Zhou Z., Zhang Y., Wang A., Chen L., Cheng H., Nat. Methods 2017, 14, 713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Image dimensions and acquisition time of figures in the article.
Figure S1: Fresh and fixated/embedded human breast sample imaged with SHG (red) and THG (green) compared to H&E histology. A‐C, Duct; D‐F, Lobule. The fresh breast tissue is fixated in 4% formaldehyde, sliced in 3 μm thick slices, and paraffin‐embedded. The SHG/THG images of the unstained slices (B,E) show similar results to the fresh tissue (A,D), and are comparable with the H&E histology (C,F).
Video S1 Depth scan of alveoli in human breast tissue, with underneath a blood vessel and a duct, imaged with THG (green) and SHG (red) microscopy. The depth scan is 130 μm deep with depth steps of 2 μm and a total acquisition time of 2.6 minutes. Logarithmic contrast enhancement is applied to enhance weaker signals.
Video S2 Depth scan of a duct in human breast tissue imaged with THG (green) and SHG (red) microscopy. The scan is 50 μm deep with depth steps of 2 μm, and a total acquisition time of 61.6 seconds. Logarithmic contrast enhancement is applied to enhance weaker signals.
Video S3 Depth scan of a blood vessel in human breast tissue imaged with THG (green) and SHG (red) microscopy. The scan is 30 μm deep with depth steps of 2 μm, and a total acquisition time of 38.4 seconds. Logarithmic contrast enhancement is applied to enhance weaker signals.


