Abstract
Background
Esophagectomy is a high-risk surgical procedure. As the population ages, more elderly candidates are being evaluated for esophagectomy. The effects of patient age on outcomes after esophagectomy need to be evaluated.
Study Design
We identified all nonemergent esophagectomies in patients at least 18 years of age within the University HealthSystems Consortium Clinical Database/Resource Manager from 2009 to 2012. Using univariate and multivariate methods, the impact of increasing age on outcomes was analyzed. Additionally, propensity scoring was used to match patients to further investigate the effect of age on the stated outcomes.
Results
Increasing age is associated with increased mortality (p<0.001), length of stay (p<0.001), discharge to rehabilitative care (p<0.001), and cost (p<0.001). The effects of age on mortality (8.0 vs 4.2 %, p=0.03) and discharge to rehabilitative care (44.1 vs 23.4 %, p<0.01) were confirmed using propensity scoring, comparing patients above 80 with those age 70–79.
Conclusions
Increasing age has a significant impact on outcomes following esophagectomy, particularly mortality and discharge disposition. Compared to patients under age 80, patients at least 80 years of age considering esophagectomy should be recognized as a high-risk cohort, and these patients must be carefully risk-stratified, counseled, and selected for surgical intervention to prevent unnecessary hospitalization and mortality.
Keywords: Esophagectomy, Mortality, Geriatric surgery
Introduction
As the population ages and life expectancy continues to increase, more elderly patients are able to undergo major surgery as a part of their cancer therapy.1–3 More than many other surgical interventions, complex gastrointestinal oncologic resections possess a significant morbidity and mortality profile, and the interaction of age and high-risk operative intervention can have profound consequences on perioperative outcomes.3 Optimal patient selection is an ongoing clinical challenge for the surgeon, and identification of risk factors associated with outcomes is an active area of investigation.
Specifically, extirpation of esophageal malignancies has one of the highest rates of morbidity and mortality of all oncologic operations.3 Therefore, it is imperative that both physicians and patients are fully informed about the risks and benefits of esophagectomy, and that providers have access to contemporary outcomes data for esophagectomy in the elderly population as patients are counseled in preparation for surgery. As provider- and system-level care continue to evolve and improve, such data will help guide medical decision-making and resource allocation in an environment of increasing scarcity.
Currently, there are conflicting reports discussing the effect of patient age on outcomes after esophagectomy,3–14 but there is a need to better define predictive factors within a geriatric population using a contemporary dataset. We employed propensity scoring along with univariate and multivariate statistical methods as a way of approximating the results that might have been attained with random assignment. In analyzing a large, national dataset of esophagectomies, we hypothesized that age—when other potentially confounding variables were controlled for—would be a unique clinical indicator of important outcomes following esophagectomy.
Methods
The University HealthSystems Consortium (UHC) Clinical Database/Resource Manager (CDB/RM) was used as our primary dataset. The UHC CDB/RM is a validated administrative database reflecting the experiences of 110 academic medical centers and 130 affiliated hospitals that submit patient- and hospitalization-specific details to a shared database, which is accessed by all participating institutions for operational and clinical performance improvement.15
From the UHC CDB/RM, we identified all nonemergent esophagectomies (ICD9 procedure codes 42.40 [unspecified esophagectomy], 42.42 [total esophagectomy], and 43.99 [Ivor-Lewis esophagectomy]) from 2009 to 2012. Excluding all patients <18 years old, a total of 6,756 cases populated our final dataset. Because provider volume has also been shown to be an important determinant of outcomes in esophagectomy, the patient cohort was stratified into quartiles by both annual center and surgeon volume.16–18 Taking into account yearly variation in hospital volume, the average center volume categories consisted of 1–13 procedures/year (lowest volume), 14–23 procedures/year (low volume), 24–41/year procedures (medium volume), and 43–124 procedures/year (high volume). Patients were also stratified into age groups of 18–60, 60–69, 70–79, and 80+years of age. Severity of illness (SOI) scores were calculated for each patient using a proprietary 18- step algorithm (3M, St. Paul, MN) accounting for primary and secondary diagnoses and comorbidities, classifying patients into minor (1), moderate (2), major (3), and extreme (4) categories. The primary outcomes of interest included in- hospital mortality, length of stay (LOS), discharge disposition, readmission rate, and cost. Discharge disposition was divided into home, home with home health care, rehabilitation (including skilled nursing facilities), and other, with “other” being defined as unknown, discontinuation of care against medical advice, admission to that same hospital as an inpatient, or death. Cost was defined as the total direct cost, calculated by applying an individual hospital’s Medicare cost-to-charge ratio to total hospital charges, and acquired by UHC from Medicare. UHC also adjusts for differences in regional labor costs by applying US Department of Commerce area wage indexes to the labor portion of the cost of a service.
Statistical analysis was performed using SAS 9.e and JMP 9 (SAS Institute, Cary, NC). Baseline continuous variables were compared across age groups using the Kruskal-Wallis test, while categorical variables were compared using Pearson’s chi-squared test. Logistic regression techniques were used to model mortality, readmissions, and discharge disposition, while Poisson regression techniques were used to compare length of stay. Gamma regression techniques were used to compare cost between groups. All multivariate analyses adjusted for gender, race, severity of illness, and center volume.
Propensity scores were created using logistic regression. Specifically, multivariate logistic regression was fitted to model age group in terms of gender, race, severity of illness, surgeon volume, and center volume and their two-way interactions. The predicted probabilities from this model served as propensity scores, which were then used without replacement in an SAS macro to form matched pairs between patients from the 70–79 age group and patients over age 80. These age groups were constructed to ensure clinical relevance (by matching patients close in age to the cutoff suggested by the univariate data). The quality of matches was assessed by testing for within-pair differences in baseline variables. No statistically significant differences were detected, but gender differences were at the borderline of significance (p=0.052). Despite this, only 13 of 288 pairs were discordant for gender. When comparing outcomes for the matched groups, the McNemar test was used to assess within-pair differences in binary outcomes, while Bowker’s test of symmetry was employed for categorical outcomes with more than two levels.
Results
Patient demographics by age group are displayed in Table 1. Older patients were more likely to be female and white (both p<0.001). Severity of illness (SOI), a composite measure of individual comorbidities, was also stratified by age, and in the overall cohort, patients older than 80 were more likely to have an extreme SOI and less likely to have a moderate SOI (p<0.001). Finally, there were no center volume differences regarding where various age groups received surgery (p=0.19).
Table 1.
Patient demographics and perioperative outcomes by age group
| Variable | Age groups | p value | |||
|---|---|---|---|---|---|
| <60 N/median |
60–69 N/median |
70–79 N/median |
80+ N/median |
||
| Gender | |||||
| Male | 1,839 (70.6 %) | 1,871 (77.7 %) | 1,080 (74.4 %) | 202 (69.9 %) | <0.001 |
| Race | |||||
| White | 2,021 (77.6 %) | 2,053 (85.2 %) | 1,243 (85.6 %) | 250 (86.5 %) | <0.001 |
| Black | 221 (8.5 %) | 128 (5.3 %) | 69 (4.8 %) | 11 (3.8 %) | |
| Other | 364 (14.0 %) | 228 (9.5 %) | 140(9.6 %) | 28 (9.7 %) | |
| SOI | |||||
| Minor | 28(1.1 %) | 4 (0.2 %) | 1 (0.1 %) | 1 (0.4 %) | <0.001 |
| Moderate | 469 (18.0 %) | 283 (11.8 %) | 132(9.1 %) | 15(5.2 %) | |
| Major | 1,646 (63.2 %) | 1,563 (64.9 %) | 942 (64.9 %) | 191 (66.1 %) | |
| Extreme | 463 (17.8 %) | 559 (23.2 %) | 377 (26.0 %) | 82 (28.4 %) | |
| CV | |||||
| Lowest | 680 (26.1 %) | 553 (23.0 %) | 357 (24.6 %) | 76 (26.3 %) | 0.19 |
| Low | 602 (23.1 %) | 617(25.6 %) | 372 (25.6 %) | 63(21.8%) | |
| Medium | 657 (25.2 %) | 612(25.4 %) | 342 (23.6 %) | 78 (27.0 %) | |
| High | 667 (25.6 %) | 627 (26.0 %) | 381 (26.2 %) | 72 (24.9 %) | |
| Procedure | |||||
| ICD9–43.99 | 1,653 (63.4 %) | 1,344 (55.8 %) | 844 (58.1 %) | 192 (66.4 %) | <0.001 |
| Other esophagectomy | 953 (36.6 %) | 1,065 (44.2 %) | 608 (41.9 %) | 97 (33.6 %) | |
| Mortality | 55 (2.1 %) | 72 (3.0 %) | 71 (4.9 %) | 23 (8.0 %) | <0.001 |
| LOS (days) | 10 (IQR 8–14) | 10 (IQR 8–15) | 11 (IQR 8–16) | 12 (IQR 9–19) | <0.001 |
| Discharge status | |||||
| Home | 1,215 (46.6 %) | 860 (35.7 %) | 377 (26.0 %) | 44(15.2 %) | <0.001 |
| HHHC | 1,174(45.1 %) | 1,168(48.5 %) | 646 (44.5 %) | 95 (32.9 %) | |
| Rehab | 157 (6.0 %) | 307 (12.7 %) | 357 (24.6 %) | 127 (43.9 %) | |
| Other | 60 (2.3 %) | 74(3.1 %) | 72 (5.0 %) | 23 (8.0 %) | |
| Readmission | 423 (16.2 %) | 406(16.9 %) | 244 (16.8 %) | 57(19.7 %) | 0.50 |
| Total direct cost | $22,792 (IQR $17,036-$33,334) | $24,232 (IQR $17,845-$37,021) | $24,805 (IQR $18,363-$38,908) | $26,470 (IQR $19,168-$43,463) | <0.001 |
SOI severity of illness, CV center volume, ICD 43.99 Ivor-Lewis esophagectomy, LOS length of stay, HHHC home with home health care
Unmatched perioperative outcomes were significantly different between age groups (Table 1). Perioperative mortality was fourfold higher in 80+ year-olds versus those less than 60 years of age (8.0 vs 2.1 %, p<0.001), When comparing the 80+ to <60 cohorts, LOS was longer (median 12 vs 10 days, p<0.001), discharge to rehabilitative care was more common(43.9 vs 6 %, p<0.001), and median cost was higher ($26,470 vs $22,792, p<0.001). Discharge to home decreased as age increased (p<0.001).
Multivariate analysis adjusting for severity of illness, race, gender, and center volume was used to compare patients over age 80 to those below age 80 and confirmed findings of the univariate analysis. Patients above age 80 had a higher risk of mortality (relative risk (RR)=1.68, 95 % confidence interval (CI)=1.00–2.82, p=0.05) and were more likely to be discharged to rehabilitative care (RR=3.47, 95 % CI=2.33–5.16, p<0.001) than younger patients (Table 2).
Table 2.
Adjusted outcomes by age, comparing octogenarians (patients age 80 and above) with all patients under age 80
| Outcomes in octogenarians (80+) | ||
|---|---|---|
| Variable | Odds or risk ratio (95 % CI) | p value |
| Mortality | 1.68(1.00–2.82) | 0.05 |
| LOSa | 0.98(0.92–1.06) | 0.66 |
| Costb | 0.99(0.93–1.07) | 0.88 |
| Readmission | 1.18(0.85–1.63) | 0.32 |
| Discharge HHHC (vs home) | 1.28(0.87–1.87) | 0.21 |
| Discharge rehab (vs home) | 3.47(2.33–5.16) | <0.001 |
Poisson regression
Gamma regression, LOS length of stay, HHHC home with home health care
After matching patients over age 80 with patients age 70–79 via propensity scoring (Table 3), in-hospital mortality (p=0.03) and discharge disposition (p<0.01) remained significantly different between age groups (Table 4). Patients over age 80 were much more likely to be discharged to rehabilitative care (44.1 vs 23.4 %) and much less likely to be discharged home (15.3 vs 26.4 %) than their younger counterparts (Table 4). As demonstrated with univariate and multivariate analyses, perioperative mortality was significantly higher in the octogenarian cohort; compared to those in the 70 to 79-year-old age range (8.0 vs 4.2 %, p=0.03).
Table 3.
Propensity scoring baseline comparison
| Variable | Age group | p value | |
|---|---|---|---|
| 70–79 | 80+ | ||
| Male | 209 (72.6 %) | 202 (70.1 %) | 0.05a |
| Race | |||
| Black | 9 (3.1 %) | 11 (3.8 %) | 0.20b |
| Other | 24 (8.7 %) | 28 (9.7 %) | |
| White | 254 (88.2 %) | 249 (86.5 %) | |
| SOI | |||
| 2 | 12 (4.2 %) | 15 (5.2 %) | 0.10b |
| 3 | 193 (67.0 %) | 191 (66.3 %) | |
| 4 | 83 (28.8 %) | 82 (28.5 %) | |
| ILE | 195 (67.7 %) | 192 (66.7 %) | 0.37a |
| Center volume | |||
| LSTV | 76 (26.4 %) | 76 (26.4 %) | 0.65b |
| LV | 59 (20.5 %) | 63 (21.9 %) | |
| MV | 79 (27.4 %) | 78 (27.1 %) | |
| HV | 74 (25.7 %) | 71 (24.7 %) | |
| Surgeon volume | |||
| LSTV | 82 (28.5 %) | 79 (27.4 %) | 0.61b |
| LV | 79 (27.4 %) | 80 (27.8 %) | |
| MV | 69 (24.0 %) | 67 (23.3 %) | |
| HV | 58 (20.1 %) | 62(21.5 %) | |
McNemar test
Symmetry test, SOI severity of illness, ILE Ivor-Lewis esophagectomy, LSTV lowest volume center, LV low volume center, MV medium volume center, HV high volume center
Table 4.
Propensity scoring outcome comparison
| Variable | Age group | p value | |
|---|---|---|---|
| 70–79 | 80+ | ||
| Mortality | 12 (4.2 %) | 23 (8.0 %) | 0.03a |
| Discharged to | |||
| HHHC | 127 (44.1 %) | 94 (32.6 %) | <0.01b |
| Home | 76 (26.4 %) | 44(15.3 %) | |
| Other | 12 (4.2 %) | 23 (8.0 %) | |
| Rehab | 73 (23.4 %) | 127 (44.1 %) | |
| Readmitted | 48 (16.7 %) | 57 (19.8 %) | 0.34a |
| LOS | Median=11 (IQR 8–17) | Median=12 (IQR 9–19) | 0.57c |
| Total direct cost | Median=$24,855 (IQR $18,408-$40,900) | Median=$26,554 (IQR $19,407-$43,443) | 0.43c |
McNemar test
Symmetry test
Signed rank test, LOS length of stay, HHHC home with home health care
To address the role of center volume and its relationship to age, the four age cohorts were also stratified by center operative experience. Patients over age 80 had a much higher risk of mortality when their esophagectomy was performed at the lowest volume centers compared to the highest volume centers (p=0.04), while none of the other age groups had a statistically significant difference in death rate when comparing the lowest and highest volume centers (Fig. 1).
Fig. 1.
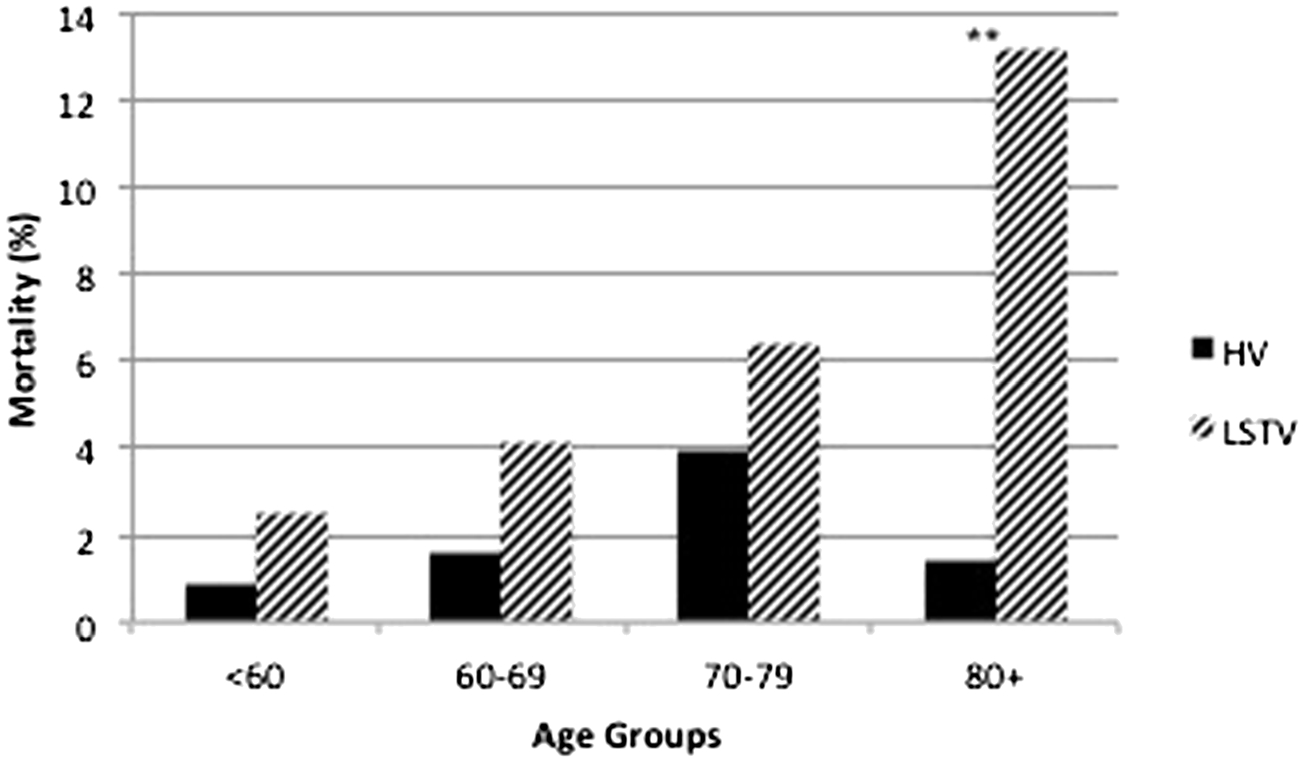
Risk of mortality, age, and center volume. HV highest volume center, LSTV lowest volume center, **p<0.05
Discussion
Using propensity scoring in a large, multi-institutional dataset, this study shows that increasing age is significantly associated with certain adverse outcomes following esophagectomy. In particular, patients over 80 years of age have a higher rate of mortality, longer length of stay, and are more likely to be discharged into rehabilitative care. Furthermore, these elderly patients incur greater costs than patients under 80 years of age. Patients over age 80 that undergo esophagectomy at a low volume center also have a significantly greater risk of mortality when compared to a high volume center, relative to their younger counterparts. Using these data, we can conclude that patients over age 80 are a high risk group that should be carefully evaluated prior to approval for esophageal resection, and if surgical resection takes place, it should absolutely be performed at high volume, experienced centers. For this study, a center that performed (on average) 43 or more esophagectomies per year was considered high volume, and while this is not an absolute minimum case volume, for reasonable clinical outcomes in octogenarians, the trends in our data are clear—high-risk, elderly patients should not undergo esophagectomy at centers that may only perform five per year.
Because there were baseline differences between age groups that may have contributed to the outcomes studied, a case-matched analysis using propensity scoring was performed comparing patients age 70–79 with those over age 80. We chose these groups to determine if relatively small changes in age had significant impact on postoperative outcomes. Instead of comparing patients who may have had a 40- year age difference, this analysis compared patients with a maximum of a 24-year age difference and still showed increased rates of adverse outcomes in the older age group. Even after matching for gender, age, severity of illness, procedure type, and center and surgeon volume, patients over the age of 80 had nearly a twofold increased risk of mortality and were much more likely to be discharged to rehabilitative care than their younger counterparts. The remaining outcomes variables maintained the same trends as seen in the previous analysis, but without statistical significance, likely due to both the matching and the smaller sample size. These patterns shown by propensity score-matched analyses were confirmed using multivariate techniques adjusted for gender, race, severity of illness, and center volume.
This study provides contemporary evidence confirming that age is associated with adverse outcomes, particularly mortality, after esophageal resection.3–5 Other smaller studies contradict many of these findings,6–11; however, many of these studies contain no more than a few hundred patients, and most involve the experiences of a single institution.7–11 Additionally, age is often treated as a binary variable above and below a single cutoff, which may miss more subtle differences between age groups. A common cutoff used has been 70 years of age, which may be too low to identify age-associated increased morbidity and mortality, especially when the median age at diagnosis of esophageal cancer is 67.19 Our study divided patients over age 60 into three age groups to more accurately determine when age becomes an independent predictor of morbidity and mortality and to ensure that differences in subsets of the geriatric population were taken into account.
This study has limitations. The UHC database is skewed with disproportionately higher volume centers (because its membership is primarily large academic centers), and as such not all of our results may be applicable to all US hospitals. However, most esophagectomies are performed at higher volume centers, so this limitation may be less profound. Even among these higher volume centers, volume played a large role in outcomes seen in elderly patients, which implies that the effect of volume across the US hospital system may be even more significant than demonstrated in this study. Secondly, because this was a retrospective observational study, the patient populations being compared had significant differences in multiple baseline variables, some of which may have contributed to the poor outcomes seen in the over 80 age group. Propensity scoring and multivariate regression techniques were used to compensate for these pre-surgery population differences. Additionally, this study lacked long-term outcomes data and the ability to account for readmissions to other hospitals, though we are working with UHC to develop more comprehensive datasets to address these issues. Further studies are needed to investigate the effects of age on esophagectomy, primarily to investigate overall survival and cumulative health-care consumption with age.
Conclusion
This is the most current analysis of outcomes in elderly esophagectomy patients, and the first of its kind to use propensity scoring. These data represent a significant contribution for the ongoing debate on the performance of high-risk surgery in the geriatric population. Based on these findings, age is an important predictor of outcomes and should play a large role in deciding whether or not a patient is an appropriate candidate for esophagectomy. Age should not necessarily be a contraindication for esophagectomy, but for patients over age 80, there is an age-associated increase in mortality and discharge to rehabilitative care, after controlling for other variables. This may be a threshold at which chronological age, and the associated physiologic changes that hinder recovery following high-risk surgery, warrant serious consideration of nonoperative treatment. Perhaps most importantly, if a very elderly patient is to undergo esophagectomy, significant effort should be made to ensure that surgery is performed at the highest volume center feasible. It must be recognized that patients over age 80 are an extremely high-risk cohort, for which surgical intervention must be carefully selected on a case-by-case basis, with appropriate counseling, risk assessment, and center selection.
Footnotes
Conflict of Interest The authors have no financial disclosures to report.
Contributor Information
Christopher C. Stahl, Cincinnati Research in Outcomes and Safety in Surgery, Department of Surgery, University of Cincinnati, 234 Goodman St, ML 0772, Cincinnati, OH 45219, USA
Dennis J. Hanseman, Cincinnati Research in Outcomes and Safety in Surgery, Department of Surgery, University of Cincinnati, 234 Goodman St, ML 0772, Cincinnati, OH 45219, USA
Koffi Wima, Cincinnati Research in Outcomes and Safety in Surgery, Department of Surgery, University of Cincinnati, 234 Goodman St, ML 0772, Cincinnati, OH 45219, USA.
Jeffrey M. Sutton, Cincinnati Research in Outcomes and Safety in Surgery, Department of Surgery, University of Cincinnati, 234 Goodman St, ML 0772, Cincinnati, OH 45219, USA
Gregory C. Wilson, Cincinnati Research in Outcomes and Safety in Surgery, Department of Surgery, University of Cincinnati, 234 Goodman St, ML 0772, Cincinnati, OH 45219, USA
Samuel F. Hohmann, University HealthSystem Consortium, Chicago, IL, USA
Shimul A. Shah, Cincinnati Research in Outcomes and Safety in Surgery, Department of Surgery, University of Cincinnati, 234 Goodman St, ML 0772, Cincinnati, OH 45219, USA
Daniel E. Abbott, Cincinnati Research in Outcomes and Safety in Surgery, Department of Surgery, University of Cincinnati, 234 Goodman St, ML 0772, Cincinnati, OH 45219, USA
References
- 1.“Expectation of life at birth, and projections” No. 104, p. 77 In Statistical Abstract of the United States, 2012. Washington: Government Printing Office, 2012. [Google Scholar]
- 2.Yancik R. Population aging and cancer: a cross-national concern. Cancer J 2005;11(6):437–41. [DOI] [PubMed] [Google Scholar]
- 3.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg 2007;205(6):729–34. [DOI] [PubMed] [Google Scholar]
- 4.Hodari A, Hammoud ZT, Borgi JF, Tsiouris A, Rubinfeld IS. Assessment of morbidity and mortality after esophagectomy using a modified frailty index. Ann Thorac Surg 2013;96(4):1240–5. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, Daley J, Henderson WG, Krasnicka B, Khuri SF. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75(1):217–22. [DOI] [PubMed] [Google Scholar]
- 6.McLoughlin JM, Lewis JM, Meredith KL. The impact of age on morbidity and mortality following esophagectomy for esophageal cancer. Cancer Control 2013;20(2):144–50. [DOI] [PubMed] [Google Scholar]
- 7.Sabel MS, Smith JL, Nava HR, Mollen K, Douglass HO, Gibbs JF. Esophageal resection for carcinoma in patients older than 70 years. Ann Surg Oncol 2002;9(2):210–4. [DOI] [PubMed] [Google Scholar]
- 8.Ruol A, Portale G, Zaninotto G, Cagol M, Cavallin F, Castoro C, Sileni VC, Alfieri R, Rampado S, Ancona E. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome or survival. J Thorac Cardiovasc Surg 2007;133(5): 1186–92. [DOI] [PubMed] [Google Scholar]
- 9.Schweigert M, Solymosi N, Dubecz A, Stadlhuber RJ, Ofner D, Stein HJ. Current outcome of esophagectomy in the very elderly: experience of a German high-volume center. Am Surg 2013;79(8):754–63. [PubMed] [Google Scholar]
- 10.Zehetner J, Lipham JC, Ayazi S, Banki F, Oezcelik A, DeMeester SR, Hagen JA, DeMeester TR. Esophagectomy for cancer in octogenarians. Dis Esophagus 2010;23(8):666–9. [DOI] [PubMed] [Google Scholar]
- 11.Markar SR, Low DE. Physiology, not chronology, dictates outcomes after esophagectomy for esophageal cancer: outcomes in patients 80 years and older. Ann Surg Oncol 2013;20(3):1020–6. [DOI] [PubMed] [Google Scholar]
- 12.Tapias LF, Muniappan A, Wright CD, Gaissert HA, Wain JC, Morse CR, Donahue DM, Mathisen DJ, Lanuti M. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg 2013;95(5):1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonavina L, Incarbone R, Saino G, Clesi P, Peracchia A. Clinical outcome and survival after esophagectomy for carcinoma in elderly patients. Dis Esophagus 2003;16(2):90–3. [DOI] [PubMed] [Google Scholar]
- 14.Markar SR, Karthikesalingam A, Thrumurthy S, Ho A, Muallem G, Low DE. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis Esophagus 2013;26(3):250–62. [DOI] [PubMed] [Google Scholar]
- 15.Sutton JM, Hayes AJ, Wilson GC, Quillin RC, Wima K, Paquette IM, Sussman JJ, Ahmad SA, Shah SA, Abbott DE. Validation of the University HealthSystems Consortium Administrative Dataset: Concordance and Discordance with Patient-Level Institutional Data. J Surg Res 2014, in press 2014. [DOI] [PubMed] [Google Scholar]
- 16.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346(15):1128–37. [DOI] [PubMed] [Google Scholar]
- 17.Wouters MW, Gooiker GA, van Sandick JW, Tollenaar RA. The volume-outcome relation in the surgical treatment of esophageal cancer: a systematic review and meta-analysis. Cancer 2012;118(7): 1754–63. [DOI] [PubMed] [Google Scholar]
- 18.Markar SR, Karthikesalingam A, Thumurthy S, Low DE. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000–2011. J Gastrointest Surg 2012;16(5):1055–63. [DOI] [PubMed] [Google Scholar]
- 19.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2010, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]


