Abstract
We examined the effects of silver island films on multiphoton excitation of rhodamine derivatives and biochemical fluorophores. The fluorophores were studied in approximately 1 μm thick samples between two quartz slides coated with silver islands. Intensity and lifetime measurements of rhodamine B demonstrated enhanced two-photon excitation near the metal particles. Time-resolved measurements showed a decreased lifetime of RhB with two-photon excitation as compared to unsilvered quartz slides, which indicates the two-photon excitation was localized near the metal particles. Additionally, rhodamine B showed increased photostability with two-photon excitation near metal particles, which is consistent with the shorter lifetime. For lower quantum yield fluorophores such as rose bengal, eosin, 1-anilinonaphthalene-8-sulfonic acid, and coumarin 152, we found the emission from the micron thick sample was almost completely due to the fluorophores near the silver particles, perhaps to within 300 Å of the metal surfaces. These results suggest the use of metal particles for localized multiphoton excitation of biological macromolecules or cells. Additionally, one can imagine metal colloids with bound fluorophores being selectively excited when placed in highly fluorescent samples such as tissue samples.
Introduction
Fluorescence measurements are typically performed in the free-space conditions, in which the fluorophores radiate into an essentially unbounded vacuum. However, it is known that the emission spectral properties of fluorophores can be dramatically altered by the presence of nearby metallic surfaces.1,2 As examples, the lifetimes of lanthanide chelates are known to oscillate with distance from a plane metal surface,3 and proximity to colloid-size silver particles can result in dramatic decreases in the decay times.4,5 In recent publications we summarized the effects of metal particles on fluorescence from the perspective of using these interactions for new types of fluorescence experiments.6 We noted that proximity to metal particles can greatly increase the radiative decay rates, and thus increase the quantum yield of weakly fluorescent species.7,8 Metal particles can also increase the rate of resonance energy transfer.9 Surprisingly, the increased quantum yields are accompanied by decreased lifetimes. This latter effect is expected to result in enhanced photostability since the fluorophore spends less time in the excited state for each absorption–emission cycle. It seems likely that the interaction of fluorophores with metallic surfaces and particles will result in new classes of clinical assays.10 For clarity we note that we are considering the emission of fluorophores near metal surfaces, and not the effects of biochemical species on surface plasmon resonance11,12 or surface-enhanced Raman scattering.13,14
In addition to increasing the emissive rate, metallic surfaces and particles are known to concentrate the incident light.15,16 The maximum enhancement in the incident electric field has been calculated to be a factor of 140 near appropriately sized metallic ellipsoids.2 Since the incident intensity is the square of the incident field, this effect could result in enhanced excitation near metal particles by a factor of nearly 20 000-fold. Even more remarkable is the possible enhancement for multiphoton excitation. For simultaneous absorption of two photons the rate of excitation is proportional to the square of the incident intensity. This suggests that two-photon excitation could be enhanced by a factor of 3.8 × 108. Such an enhancement in the excitation would provide selective excitement of fluorophores bound to metal colloids even if the solution contained a considerable concentration of other fluorophores that could undergo two-photon excitation at the same wavelength but that are distant from the metallic surface. While a 108 enhancement in excitation may sound impossible, we note that Raman scatter is typically enhanced by a factor of 106 for molecules absorbed on rough silver surfaces.17 It is now thought that this enhancement may be due to a small fraction of the molecules near hot particles or particle clusters where the Raman scattering signal was enhanced by 1014-fold.18–20 One recent report suggested that enhanced Raman scatter is accompanied by enhanced fluorescence.21
The use of metallic structures to enhance multiphoton excitation has already been considered for use in aperatureless near-field scanning optical microscopy.22,23 In this approach the metallic scanning probe is illuminated with laser pulses, resulting in a high local electric field near the tip and two or three-photon excitation. Multiphoton excitation has been observed from the evanescent wave near a surface plasmon resonance.24 However, to the best of our knowledge, multiphoton excitation has not been reported near metal colloids. In the present paper we describe our initial studies of two-photon excitation near silver island films. We show that multiphoton excitation (MPE) is greatly enhanced for fluorophores near silver island films. This phenomenon suggests the use of metallic particles to enhance the emission from nearby fluorophores in solutions, cells or tissues.
Materials and Methods
Procedure for Making Silver Nanoparticle Films.
Silver islands were formed on quartz microscope slides.25 The use of quartz provided UV transmission and less autofluorescence than glass. The quartz slides were first soaked in a 10:1 (v/v) mixture of H2SO4 (95–98%) and H2O2 (30%) overnight before the deposition. The slides were washed with distilled water and air-dried prior to use. Silver deposition was carried out in a clean 30 mL beaker equipped with a Teflon-coated stir bar. To a fast stirring silver nitrate solution (0.22 g in 26 mL of Millipore water), eight drops of fresh 5% NaOH solution was added. Dark-brownish precipitates were formed immediately. Less than 1 mL of ammonium hydroxide was then added drop by drop to redissolve the precipitates. The clear solution was cooled to 5°C by placing the beaker in an ice bath, followed by soaking the cleaned and dried quartz slides in the solution. At 5°C, a fresh solution of D-glucose (0.35 g in 4 mL of water) was added. The mixture was stirred for 2 min at that temperature. Subsequently, the beaker was removed from the ice bath and allowed to warm to 30°C. As the color of the mixture turned from yellow-greenish to yellow-brown, the color of the slides became greenish. The slides were then removed, washed with water and bath sonicated for 1 min at room temperature. After the slides were rinsed with water, they were stored in water for several hours prior to the experiments.
Spectroscopic Measurements.
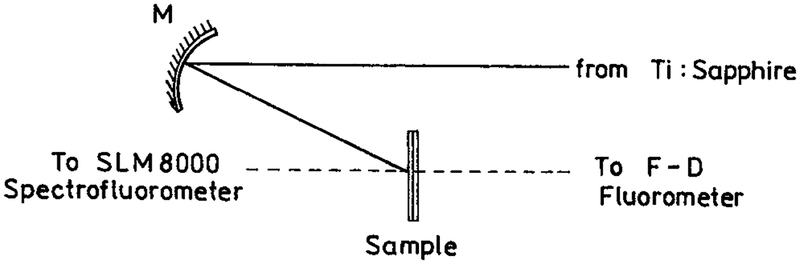
Rhodamine B (RhB) was from Lambda Physik. Eosin sodium salt, rose bengal and coumarin 152 were from Aldrich and 1-anilinonaphthalene-8-sulfonic acid (ANS) was from Sigma. The experimental sample geometry is shown in Scheme 1. Two-photon excitation of RhB, eosin and rose bengal was accomplished with the 852 nm output of a Tsunami mode-locked Ti:sapphire laser, 80 MHz repetition rate, 90 fs pulse, about 0.5 W average power. For coumarin 152 (C152) and ANS the multiphoton excitation wavelength was near 800 nm. The excitation was focused on the sample with a 15 cm radius concave mirror. The solution was placed between two high-quality quartz plates, λ/4 flatness (Starna, Inc.). The plates were half uncoated and half coated with silver islands, as described above. From absorption measurements the thickness of the samples between the plates was found to be near 1 μm. This sandwich sample was mounted on an x−y positioner. The focused spot of the laser was about 4 mm in length and 30 μm in diameter. The x−y positioner was used to move the sample so the laser illuminated regions with or without silver islands. This position change was accomplished without any change in the experimental geometry. Scattered excitation was eliminated with a combination of a heat filter and BG-38 glass filter for the emission spectra and a BG-38 and a 580 nm interference filter for time-resolved measurements.
SCHEME 1:
Experimental Geometry for Studies of Fluorophores between Silver Island Films
Intensity decays were measured in the frequency domain using instrumentation described previously.26,27 For the frequency-domain measurements the emission was observed through a 580 interference filter. For all steady state and frequency-domain measurements the excitation was vertically polarized and the emission observed through a horizontally oriented polarizer to minimize scattered light. The FD intensity decay were analyzed in terms of the multiexponential model
| (1) |
where τi are the lifetimes with amplitudes αi and Σαi = 1.0. Fitting to the multiexponential model was performed as described previously.28 The contribution of each component to the steady-state intensity is given by
| (2) |
The mean decay time is given by
| (3) |
Results
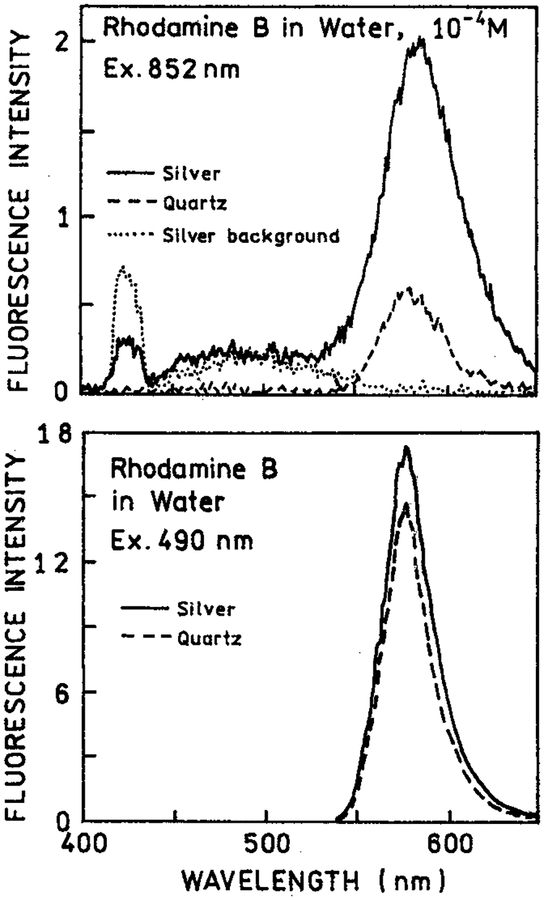
We examined the emission spectra of RhB between silver island films with two-photon excitation at 852 nm (Figure 1, top). The emission intensity for RhB between the metal particles (−) was increased about 4-fold relative to RhB between uncoated quartz plates (- − -). When the sample was first exposed to the focused 852 nm light we could visibly detect some white light from the illuminated region. This “spark” decayed in less than 1 s, but some white light background remained. This white light was seen from the silver islands alone without RhB (⋯). Such a white continuum emission for illuminated metal probes has been reported previously in near-field microscopy.29 Importantly, the RhB signal remained stable following the initial white light transient. We also examined RhB with one-photon excitation of 490 nm (Figure 1, bottom). In this case there was almost no effect of the silver islands as compared to the uncoated quartz plates.
Figure 1.
Top: Emission spectra of 10−4 M rhodamine B between silver island films with two-photon excitation at 852 nm (−). Also shown are the signals observed for uncoated quartz slides (---) or silver islands alone without rhodamine B (⋯). Bottom: RhB between silver islands (−) or quartz plates (---) with one-photon excitation at 490 nm, observed through a long-pass 540 nm and BG-38 emission filter.
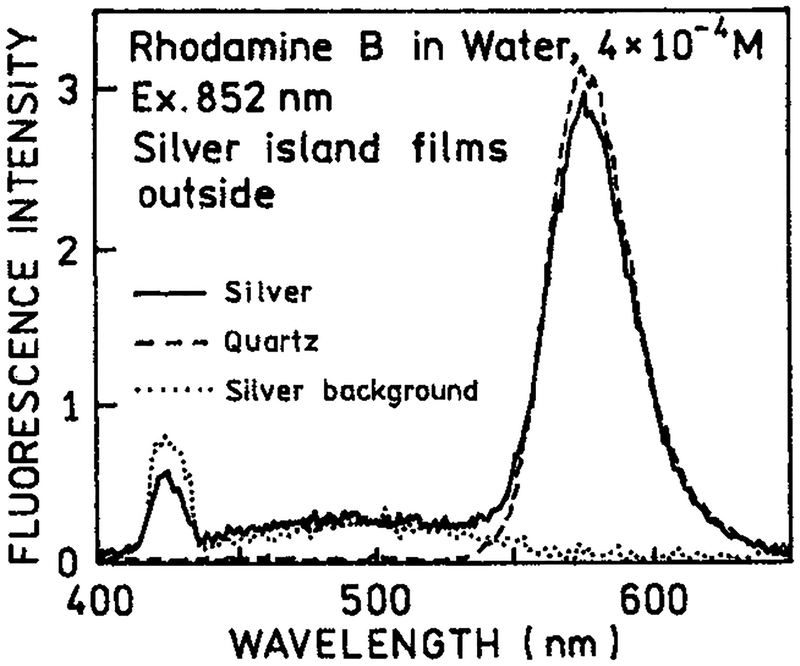
As a control experiment we examined RhB with two-photon excitation in the presence of silver islands, but with the plates rotated so that the islands were on the outer surface not in contact with RhB (Figure 2). In this case we found no difference between the silvered and unsilvered plates. The white continuum emission was still observed from the silver islands. This result demonstrated that the enhanced emission of RhB seen by Figure 1 requires close proximity of the RhB and metallic silver surfaces.
Figure 2.
Emission spectra of rhodamine B with two-photon excitation at 852 nm between quartz slides with silver islands on the outer surface (−), between uncoated quartz slides (---) and for the silver islands alone without RhB (⋯).
The enhanced emission of RhB near silver island films (Figure 1) can be understood by considering the nature of our layered sample (Scheme 1). The fluorophore is uniformly distributed in the 1 μm thick sample between two slides. The region affected by the metal islands is expected to extend about 250 Å in the solution. Recalling that there were two silver island surfaces, we estimated that only about 5% of the solution is within the active area. In fact, even this percentage is probably too high because the fluorophores within 50 Å of the metal surface are typically quenched. Assuming 5% of the sample is affected by the metal. The 4-fold enhancement for RhB (Figure 1, top) suggests an 80-fold enhancement of two-photon excitation near the metal particles. The high quantum yield of RhB and the small fraction of fluorophores near the metal particles explains the absence of a significant effect with one-photon excitation (Figure 1, bottom). In this case the majority of the emission occurs from RhB molecules distant from the silver islands.
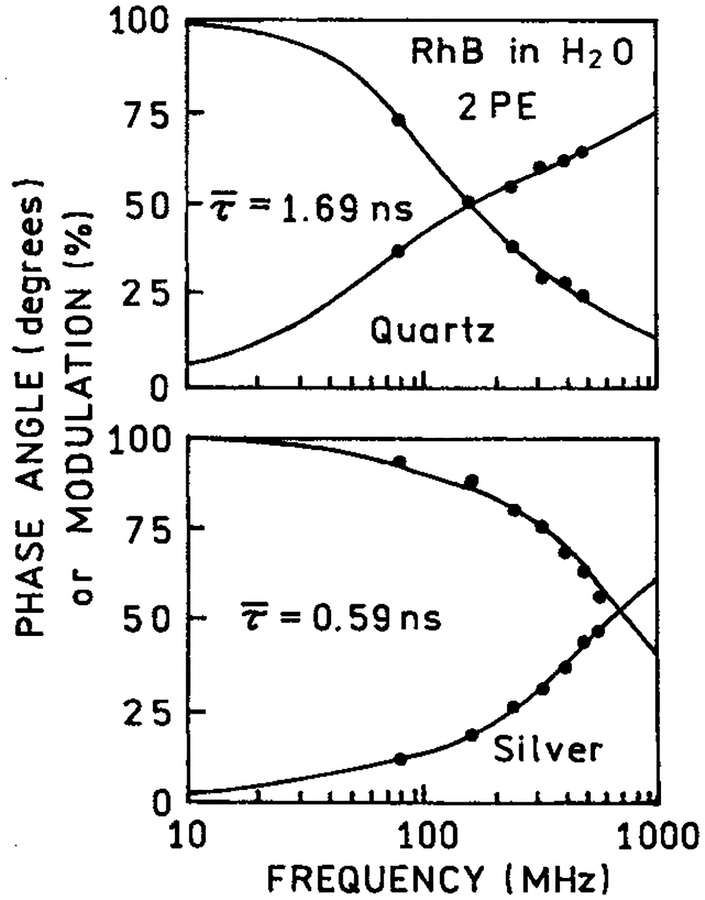
We examined the frequency-domain intensity decays of RhB between coated and uncoated quartz plates with one and two-photon excitation. For one-photon excitation the mean lifetime was essentially unchanged between the coated or uncoated plates (Table 1). This result is consistent with Figure 1 (bottom), which showed that most of the one-photon individual emission of RhB occurred from the bulk sample. Contrasting results were found for the intensity decay of RhB with two-photon excitation (Figure 3). In this case the mean lifetime is dramatically shortened by the silver island films (Table 1). Previous reports showed that lifetimes are reduced for fluorophores near silver islands.7 We interpret the reduced RhB lifetime with 852 nm excitation as the result of localized two-photon excitation of RhB molecules near the metal particles. The reduced RhB lifetime also demonstrated that the excitation is not due to second harmonic generation by the metal islands. The lifetime of RhB resulting from excitation by the second harmonic would be the same as that found in the bulk solution.
TABLE 1:
Intensity Decays of Rhodamine B with One- and Two-Photon Excitation, in the Presence and Absence of Silver-island Films
| excitation/conditions | τ1 (ns) | τ2 (ns) | α1 | α2 | f1 | f2 | χR2 | |
|---|---|---|---|---|---|---|---|---|
| one-photon, Q | 1.59a | 0.201 | 1.66 | 0.428 | 0.572 | 0.083 | 0.917 | 1.8 |
| one-photon, S | 1.65 | 0.23 | 1.89 | 0.575 | 0.425 | 0.140 | 0.860 | 2.5 |
| two-photon, Q | 1.69 | 0.35 | 1.98 | 0.544 | 0.456 | 0.175 | 0.825 | 1.4 |
| two-photon, S | 0.59 | 0.30 | 2.47 | 0.982 | 0.018 | 0.870 | 0.130 | 2.4 |
.
Figure 3.
Frequency-domain intensity decay of RhB between quartz slides (top) and silver island films (bottom) with two-photon excitation at 852 nm observed at 580 nm.
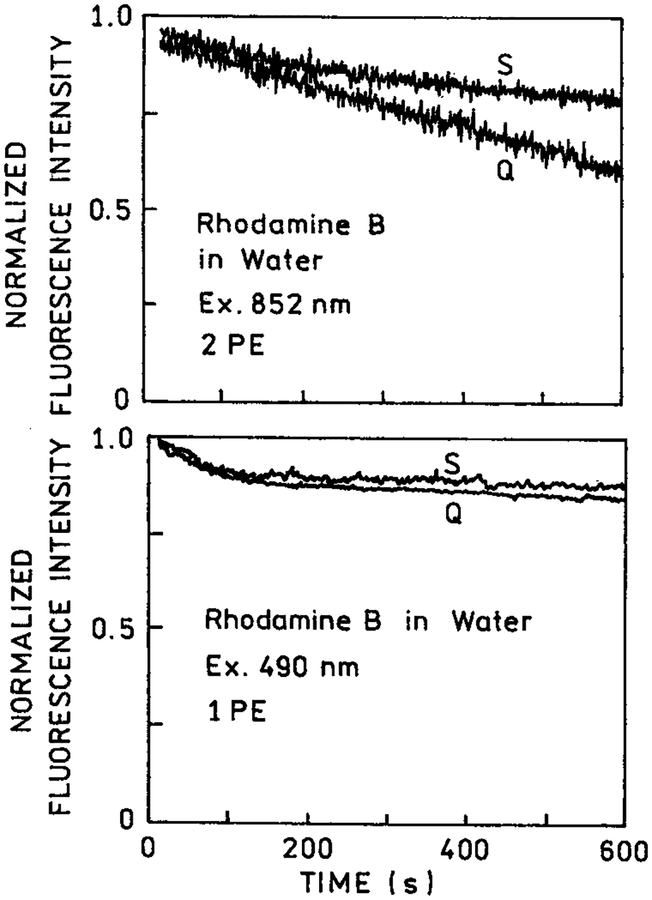
In many applications of fluorescence, photostability of the fluorophore is a primary consideration. This is particularly true in single molecule detection where it has been estimated that approximately 1000 photons can be observed from a highly stable fluorophore like rhodamine prior to photodestruction.30 Since photochemistry occurs in the excited state, a reduction in the fluorescence lifetime is expected to result in increased photostability. We examined the photostability of rhodamine B between coated and uncoated quartz slides with one- and two-photon excitation (Figure 4). For one-photon excitation, the photostability was unaffected by the presence or absence of silver islands (bottom). For two-photon excitation, we noticed increased photostability in the presence of silver islands (top). These results are consistent with the shorter lifetime observed for rhodamine B between silver islands and with our assertion that two-photon excitation occurs preferentially near the silver island films.
Figure 4.
Photostability of rhodamine B between quartz slides (Q) and silver island films (S) with two-photon excitation at 852 nm (top) and with one-photon excitation at 490 nm (bottom). The 490 nm excitation was from an argon ion laser attenuated to about 10 mW. The observation wavelength was 580 nm.
In the preceding experiments, we used rhodamine B, which displays a high quantum yield (Q = 0.48) in bulk solution. As a result, much of the emission occurred from the bulk solution in regions unaffected by the silver islands. In a previous report,7 we showed that silver islands increased the quantum yield of low quantum yield fluorophores. Hence, we expected multiphoton excitation of fluorophores near silver island films to result in less photobleaching. More specifically, we expected multiphoton excitation to occur near the silver islands and the silver islands to increase the quantum yield of the nearby fluorophores. Simultaneously, these higher quantum yield fluorophores have a shorter lifetime and thus greater photostability. This interpretation is consistent with the increased photostability of RhB observed near silver island films (Figure 4, top).
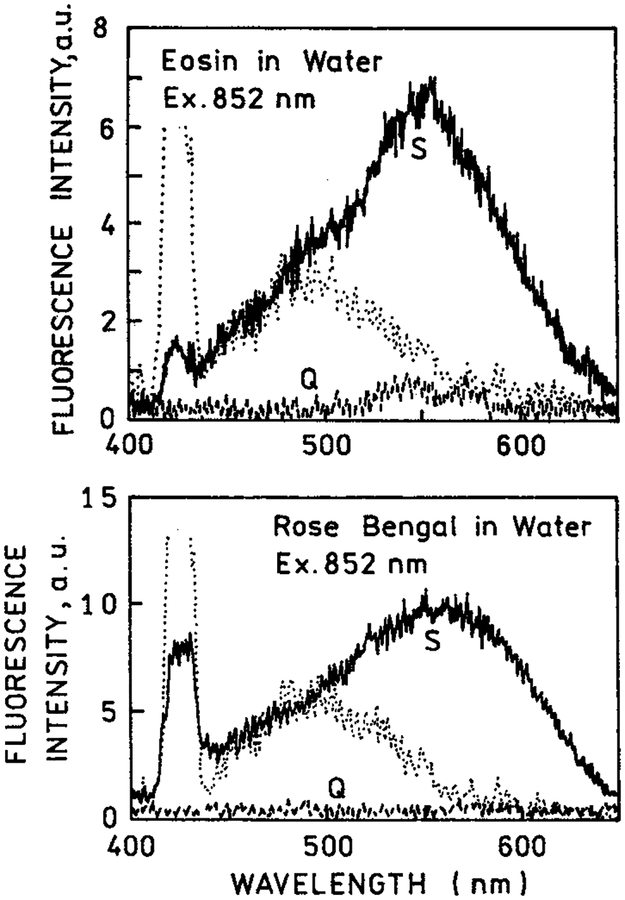
We examined other fluorophores with multiphoton excitation near silver island films. We chose eosin (Q = 0.24) and rose bengal (Q = 0.02) for their lower quantum yields but spectral similarity to rhodamine B. Figure 5 shows emission spectra for eosin and rose bengal between quartz plates and between silver island films. In these spectra, the white light continuum resulting from the silver island films is more evident because of the lower overall signal. Because of the lower quantum yields in bulk solution a larger fraction of the total emission is due to eosin and rose bengal near the metal surfaces than with rhodamine B. Importantly, with two-photon excitation, there is essentially no emission from eosin or rose bengal under conditions where there is substantial emission from the fluorophores between the silver islands. This result suggested selective and localized two-photon excitation near metal particles.
Figure 5.
Emission spectra of eosin (top) and rose bengal (bottom) between quartz slides and between silver island films, using two-photon excitation at 852 nm (−). Also shown are the signals observed for the fluorophores between quartz slides or (---) the silver island films without eosin or rose bengal (⋯).
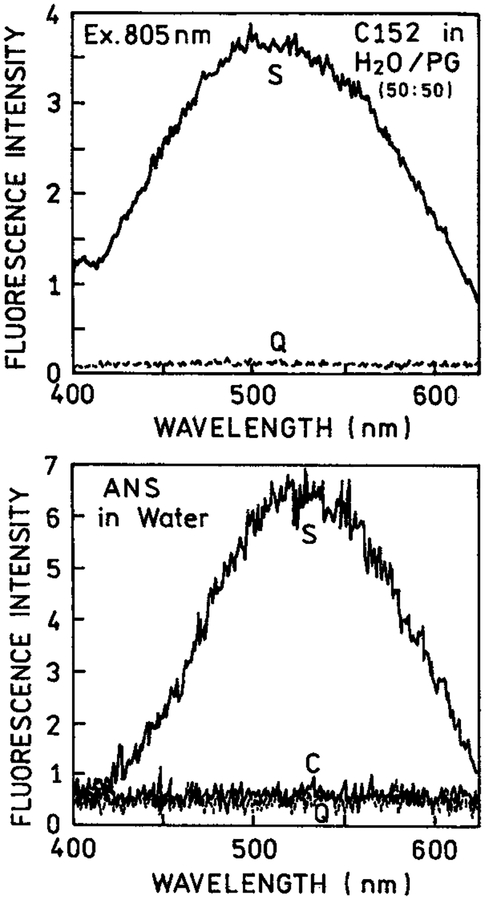
We pursued the concept of selective excitation further using biochemically relevant fluorophores such as coumarin 152 (Q = 0.011) and ANS (Figure 6). Because of the lower quantum yields in bulk solution, a larger fraction of the total emission is due to eosin and rose bengal near the metal surfaces than with rhodamine B. We observed a remarkable enhancement of the two-photon induced emission for these fluorophores between silver island films. In the case of ANS with a very low quantum yield in water (Q < 0.01) essentially no signal was seen for ANS between the uncoated slides. Furthermore, the ANS signal observed from a bulk solution in a cuvette was insignificant compared to the two-photon induced emission in the presence of silver particles. The results shown in Figures 1, 5, and 6 suggest that multiphoton excitation near silver particles is a general phenomenon which can result in highly localized excitation in regions near the metal particles.
Figure 6.
Emission spectra of coumarin 152 (top) in water-propylene glycol, 50–50 vv and ANS in water (bottom) in a 0.1 mm cuvette (C), between quartz slides (Q) and between silver island films (S).
Discussion
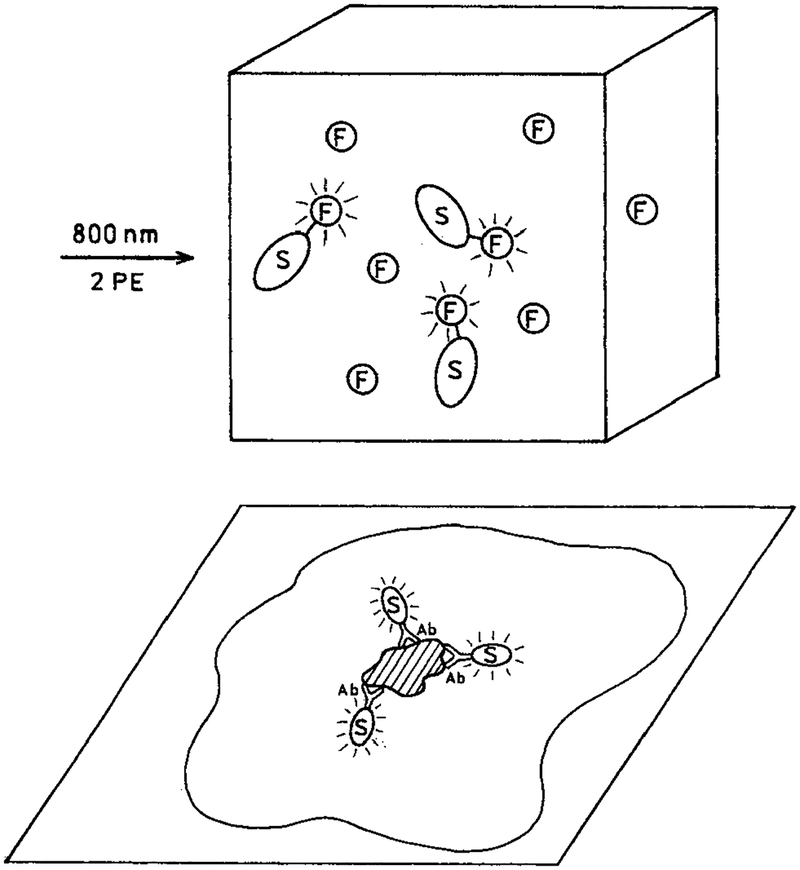
In our opinion, enhanced multiphoton excitation by metal particles can have numerous applications in the biochemical and biological applications of fluorescence. For example, Scheme 2 (top) shows a sample containing fluorophores that are either free in solution or covalently linked to a metal particle at an appropriate distance where the fluorescence would be enhanced and not quenched. Our results suggest that illumination of such a solution will result in emission only from those fluorophores attached to the silver particles and not from the fluorophores free in solution. This effect would be enhanced further by using fluorophores with low quantum yields when not adjacent to a metallic surface. One can also imagine the use of metal particles to selectively enhance the emission from localized regions of cells or tissues. Scheme 2 (bottom) shows the expected result when metal particles are targeted to a cell membrane by the presence of a covalently bound antibody. In this case we expect multiphoton excitation to excite cellular autofluorescence only from regions in close proximity to the metal particles. If the enhancement of multiphoton excitation by metallic particles is adequately large, one could imagine their use in tissues where scattering reduces the peak intensity of the excitation pulses.
SCHEME 2:
(Top) Selective Multiphoton Excitation of Fluorophores on Metal Colloids in the Presence of Free Fluorophore and (Bottom) Localized Multiphoton Excitation of Intracellular Autofluorescence by Metal Colloids
Acknowledgment.
This work was supported by the National Institute of Health, National Center for Research Resources, RR-08119.
References and Notes
- (1).Chance RR; Prock A; Silbey R Molecular fluorescence and energy transfer near interfaces. Adv. Chem. Phys 1978, 37, 1–65. [Google Scholar]
- (2).Kümmerlen J; Leitner A; Brunner H; Aussenegg FR; Wokaun A Enhanced dye fluorescence over silver island films: Analysis of the distance dependence. Mol. Phys 1993, 80(5), 1031–1046. [Google Scholar]
- (3).Barnes WL Topical review: Fluorescence near interfaces: The role of photonic mode density. J. Modern Opt 1998, 45, 661–699. [Google Scholar]
- (4).Weitz DA; Garoff S; Hanson CD; Gramila TJ Fluorescent lifetimes of molecules on silver-island films. Opt. Lett 1982, 7 (2), 89–91. [DOI] [PubMed] [Google Scholar]
- (5).Leitner A; Lippitsch ME; Draxler S; Riegler M; Aussenegg FR Fluorescence properties of dyes adsorbed to silver islands, investigated by picosecond techniques. Appl. Phys. B 1985, 36, 105–109. [Google Scholar]
- (6).Lakowicz JR Radiative decay engineering: Biophysical and biomedical applications. Anal. Biochem 2001, 298, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lakowicz JR; Shen Y; D’Auria S; Malicka J; Gryczynski Z; Gryczynski I 2001. Radiative decay engineering 2: Effects of silver island films on fluorescence intensity, lifetimes and resonance energy transfer. Anal. Biochem in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lakowicz JR; Shen Y; Gryczynski Z; D’Auria S; Gryczynski I Intrinsic fluorescence from DNA can be enhanced by metallic particles. Biochem. Biophys. Res. Commun 2001, 286, 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lakowicz JR; Shen Y; Malicka J; D’Auria S; Gryczynski Z; Gryczynski I 2001. Effects of metallic silver particle on resonance energy transfer between fluorophores bound to DNA. Nucl. Acid Res (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Schalkhammer T; Aussenegg FR; Leitner A; Brunner H; Hawa G; Lobmaier C; Pittner F Detection of fluorophore-labeled antibodies by surface-enhanced fluorescence on metal nanoislands. SPIE 1997, 2976, 129–136. [Google Scholar]
- (11).Kooyman RPH; Kolkman H; Van Gent J; Greve J Surface plasmon resonance immunosensors: Sensitivity considerations. Anal. Chim. Acta 1988, 213, 35–45. [Google Scholar]
- (12).Hendrix M; Priestley ES; Joyce GF; Wong C-H Direct observation of aminoglycoside-RNA interactions by surface plasmon resonance. J. Am. Chem. Soc 1997, 119 (16), 3641–3648. [DOI] [PubMed] [Google Scholar]
- (13).Vo-Dinh T Surface-enhanced Raman spectroscopy using metallic nanostructures. Trends Anal. Chem 1998, 17 (8–9), 557–582. [Google Scholar]
- (14).Kneipp M; Kneipp H; Itzkan I; Dasari RR; Feld MS Surface-enhanced Raman scattering: A new tool for biomedical spectroscopy. Curr. Sci 1999, 77 (7), 915–924. [Google Scholar]
- (15).Chen CJ; Osgood RM Direct observation of the local field enhanced surface photochemical reactions. Phys. Rev. Lett 1983, 50 (21), 1705–1708. [Google Scholar]
- (16).Link S; El-Sayed MA Shape and size dependence of radiative, nonradiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem 2000, 19 (3), 409–453. [Google Scholar]
- (17).Vo-Dinh T; Stokes DL; Griffin GD; Volkan M; Kim UJ; Simon MI Surface-enhanced Raman scattering (SERS) method and instrumentation for genomics and biomedical analysis. J. Raman Spectrosc 1999, 30, 785–793. [Google Scholar]
- (18).Nie S; Emory SR Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [DOI] [PubMed] [Google Scholar]
- (19).Kneipp K; Kneipp H; Kartha VB; Manoharan R; Deinum G; Itzkan I; Dasari RR; Feld MS Detection and identification of a single DNA base molecule using surface-enahnced Raman scattering (SERS). Phys. Rev. E 1998, 57 (6), R6281–R6284. [Google Scholar]
- (20).Michaels AM; Nirmal M; Brus LE Surface-enhanced Raman spectroscopy of individual rhodamine 6G molecules on large Ag nanocrystals. J. Am. Chem. Soc 1999, 121, 9932–9939. [Google Scholar]
- (21).Eggeling C; Schaffer J; Seidel CAM; Korte J; Brehm G; Schneider S; Schrof W Homogeneity, transport, and signal properties of single Ag particles studied by single-molecule surface-enhanced resonance Raman scattering. J. Phys. Chem. A 2001, 105 (15), 3673–3679. [Google Scholar]
- (22).Kawata Y; Xu C; Denk W Feasibility of molecular-resolution fluorescence near-field microscopy using multiphoton absorption and field enhancement near a sharp tip. J. Appl. Phys 1999, 85 (3), 1294–1301. [Google Scholar]
- (23).Sanchez EJ; Novotny L; Xie XS Near-field fluorescence microscopy based on two-photon excitation with metal tips. Phys. Rev. Lett 1999, 82 (20), 4014–4017. [Google Scholar]
- (24).Kano H; Kawata S Two-photon-excited fluorescence enhanced by a surface plasmon. Opt. Lett 1996, 21 (22), 1848–1850. [DOI] [PubMed] [Google Scholar]
- (25).Ni F; Cotton TM Chemical procedure for preparing surface-enhanced Raman scattering active silver films. Anal. Chem 1986, 58, 3159–3163. [DOI] [PubMed] [Google Scholar]
- (26).Lakowicz JR; Maliwal BP Construction and performance of a variable-frequency phase modulation fluorometer. Biophys. Chem 1985, 21, 61–78. [DOI] [PubMed] [Google Scholar]
- (27).Laczko G; Gryczynski I; Gryczynski Z; Wiczk W; Malak H; Lakowicz JRA 10-Ghz frequency-domain fluorometer. Rev. Sci. Instrum 1990, 61, 2331–2337. [Google Scholar]
- (28).Lakowicz JR; Laczko G; Cherek H; Gratton E; Limkeman M Analysis of fluorescence decay kinetics from variable-frequency phase shift and modulation data. Biophys. J 1994, 46, 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sanchez EJ; Novotny L; Xie S Near-field fluorescence microscopy based on two-photon excitation with metal tips. Phys. Rev. Lett 1999, 82 (20), 4014–4017. [Google Scholar]
- (30).Ambrose WP; Goodwin PM; Jett JH; Van Orden A; Wemer JH; Keller RA Single molecule fluorescence spectroscopy at ambient temperature. Chem. Rev 1999, 99, 2929–2956. [DOI] [PubMed] [Google Scholar]