Abstract
Objectives
Infant formulas are useful alternatives to breast milk in many circumstances but may pose health risks to infants and children due to contamination by potentially toxic metals. This study aimed to determine the aluminium, arsenic and mercury concentrations and carry out an exposure health risk assessment in commonly consumed infant formulas in Nigeria.
Methods
Different brands of both locally manufactured and imported infant formulas were purchased in March 2017 from stores in Port Harcourt, Nigeria. Analysis of metals in the samples was performed by atomic absorption spectrophotometry. The health risk was assessed by comparing estimated daily intake of aluminium, arsenic and mercury with the provisional tolerable daily intake acceptable by the Joint Food and Agricultural Organization/World Health Organization Expert Committee on Food Additives (JECFA).
Results
A total of 26 infant formulas were analysed. The levels of arsenic were higher in cereal-based formulas compared to milk-based formulas, but the difference was not significant (P >0.05). The intake levels of aluminium, arsenic and mercury in infant formulas were found to be 8.02–14.2%, 437.1–771% and 23.7–41.8% of the provisional tolerable daily intake JECFA threshold values, respectively.
Conclusion
Commonly consumed infant formulas in Nigeria may add to the body burden of arsenic in children.
Keywords: Infant Formulas, Toxicity Test, Aluminum, Arsenic, Mercury, Health Risk Appraisal, Child Health, Nigeria
Advances in Knowledge
- Infant formula consumption in Nigeria may add to children’s body burden of arsenic.
- Food regulatory agencies in developing nations should maintain regular and periodic checks of foods to ensure permissible limits of contaminants.
Application to Patient Care
- Heavy metals may be implicated in the aetiology of certain diseases.
- Physicians should be mindful of dietary sources of heavy metals in the diagnosis and management of diseases.
Breastfeeding is the best adapted-food for infants and imparts health benefits due to its composition which reduces the risk of disease and strengthens the infant’s immune system.1 Infant formulas are alternatives to breast milk specially produced to completely satisfy the nutritional needs of infants during their first months of life and until appropriate complementary feeding can be introduced.2 Although infant formulas are beneficial and generally considered safe, they may be a source of contaminants, including heavy metals, which bioaccumulate to pose health risks to infants.3 As infancy is characterised by high growth and development rates, infants are susceptible to noxious chemical contaminants due to their special physiology, toxicokinetics and body weight (BW) ratio. Earlier reports on dairy-based milk powder and infant formulas have cited contamination by toxic metals.4 To ensure safety, various regulatory agencies have set threshold intake levels of heavy metals for infants, including aluminium, arsenic and mercury.5
Aluminium is a widely distributed element in the Earth’s crust and was long considered safe for humans because of its relatively low bioavailability.6 This safety has been questioned, however, by epidemiological reports that link chronic aluminium exposure to Alzheimer’s disease.7 Chronic dietary aluminium exposure, especially in susceptible individuals with impaired renal function, often leads to adverse neurologic, skeletal, haematopoietic, immunologic and other health effects.7 The Joint Food and Agricultural Organization/World Health Organization (WHO) Expert Committee on Food Additives (JECFA), based on experimental bioavailability and toxicological data, established a provisional tolerable weekly intake (PTWI) of aluminium at 2 mg/kg BW/week.8
The use of arsenic in agriculture has led to severe soil contamination, thus constituting immediate human health risks due to contaminated dust, soil particles or water.9 In particular, rice has high grain arsenic but is still used to fortify many infant foods due to its high fibre content.10 Despite the wide use of rice in infant formulas, arsenic levels in baby foods has received little attention.11 Arsenic’s classification as a human carcinogen correlates with skin, lung, bladder, kidney and liver cancers and is capable of influencing neurological, respiratory, cardiovascular, immunological and endocrine systems. As a result, the JECFA estimated that the benchmark dose lower confidence limit for inorganic arsenic species should be no more than 2.1 μg/kg BW/day.12,13
The toxicity of mercury depends on its chemical form. Inorganic mercury is mainly associated with renal damage, but methylmercury crosses the placenta and blood-brain barriers to cause irreversible neuronal damage in the fetus and growing children.14 Even organic produce cannot confer protection, as reports have found that both organic and conventional produce can be prone to heavy metal contamination.15 The upper limits of estimates of average dietary exposure to total mercury from foods other than fish and shellfish for children was set at 4 μg/kg BW/week by JECFA.13
The actual dietary intake of aluminium, arsenic and mercury can be estimated and compared with corresponding toxicological reference intakes such as the provisional tolerable daily intake (PTDI) and PTWI to assess the risk to children’s health arising from the presence of contaminants in food. There is a paucity of information on children’s exposure to metals through food because of a scarcity of child-specific data on food consumption. In developing countries, including Nigeria, there is lean data on contamination of infant food.4
This study aimed to determine the aluminium, arsenic and mercury concentrations and conduct an exposure health risk assessment in commonly consumed infant formulas in Nigeria. The infant’s aluminium, arsenic and mercury daily intakes were calculated to provide context of such exposure to both the international dietary aluminium, arsenic and mercury regulations and the values reported in the literature from similar studies. This study also employed principal component analyses (PCA) of heavy metal concentrations in three types of infant formulas: milk-based (M), cereal-based (C) and a mixture of cereal and milk-based (CM).
Methods
This study was conducted on samples of both locally manufactured and imported brands of commonly consumed formulas for infants ranging in age from birth to beyond the first year of life. This sample represented approximately 75% of infant formulas available on the Nigerian market and were purchased from stores in Port Harcourt, Rivers State, Nigeria, in March 2017. The infant formulas were divided into three groups: M1–9, C1–7 and CM1–10 [Table 1]. These products consisted of infant formulas and follow-on formula samples which were soy-based; milk and rice-based; rice, wheat and mixed cereal gruel (all sold as powder); vegetable meals; and fruit-based desserts.
Table 1.
Concentrations of aluminium, arsenic and mercury found in different brands of infant formulas in Nigeria (N = 26)
| Sample code | Brand name | Manufacturer (Country) | Packaging type | Aluminium in mg/kg | Arsenic in mg/kg | Mercury in mg/kg |
|---|---|---|---|---|---|---|
| M1 | Pre NAN | Nestle (Nigeria) | Aluminium tin | 1.92 | 0.83 | 0.020 |
| M2 | Sma | Wyeth Nutritionals (South Africa) | Aluminium tin | 1.39 | 0.05 | 0.010 |
| M3 | Peak | Baby Friesland Campina (Nigeria) | Aluminium tin | 1.02 | 0.29 | 0.000 |
| M4 | Lactogen | 1 Nestle (Nigeria) | Aluminium tin | 2.47 | 0.16 | 0.020 |
| M5 | Nutristart | Laffort (France) | Hard cardboard exterior with formula in aluminium foil | 1.86 | 0.03 | 0.001 |
| M6 | Sma Pro | Nestle (Nigeria) | Aluminium tin | 1.08 | 0.39 | 0.050 |
| M7 | Sma Pro | Wyeth Nutritionals (South Africa) | Aluminium tin | 2.07 | 0.24 | 0.001 |
| M8 | Cowbell | Tina Cowbell (Nigeria) | Aluminium tin | 1.43 | 0.34 | 0.003 |
| M9 | My Boy Eldorin | Pocket Friendly (Nigeria) | Aluminium tin | 2.03 | 0.61 | 0.020 |
| C1 | Nestle Cerelac | Nestle (Nigeria) | Aluminium tin | 1.53 | 1.50 | 0.010 |
| C2 | Nestum Baby | Cereal Nestle (Nigeria) | Hard cardboard exterior with formula in aluminium foil | 1.97 | 1.56 | 0.001 |
| C3 | Golden Country Baby Cereal | Sun Mark Ltd. (England) | Aluminium tin | 1.43 | 0.08 | 0.010 |
| C4 | Friso Gold | Friso Gold (Singapore) | Aluminium tin | 1.54 | 0.26 | 0.000 |
| C5 | Cerelac Infant Cereal | NA | Aluminium tin | 0.41 | 0.15 | 0.010 |
| C6 | Pediasure: Grow and Gain | NA | Hard cardboard exterior with formula in aluminium foil | 2.35 | 1.02 | 0.003 |
| C7 | Aptamil: Organic Rice | NA | Hard cardboard exterior with formula in aluminium foil | 1.39 | 0.17 | 0.002 |
| CM1 | Nutriban | Nutrimental (Brazil) | Hard cardboard exterior with formula in aluminium foil | 0.73 | 1.27 | 0.014 |
| CM2 | Ridielac (Vina Milk) | Vietnam Dairy Products (Vietnam) | Hard cardboard exterior with formula in aluminium foil | 2.07 | 0.02 | 0.003 |
| CM3 | Nutriben | Alter Farmacia (Spain) | Hard cardboard exterior with formula in aluminium foil | 0.45 | 0.33 | 0.000 |
| CM4 | Ninolac | Ninolac Maroc SARL (Luxembourg) | Hard cardboard exterior with formula in aluminium foil | 0.58 | 0.02 | 0.002 |
| CM5 | Gerber | Nestle (Nigeria) | Plastic | 2.04 | 0.02 | 0.003 |
| CM6 | Heinz Dinners | Heinz (USA) | Hard cardboard exterior with formula in aluminium foil | 1.52 | 0.10 | 0.030 |
| CM7 | Gerber Oatmeal | Gerber (USA) | Plastic | 1.16 | 0.11 | 0.000 |
| CM8 | Heinz Summer Fruits | Nestle (Nigeria) | Plastic with aluminium lining | 1.26 | 0.79 | 0.004 |
| CM9 | Nutrilac Infant Cereal | Wyeth Nutritionals (South Africa) | Hard cardboard exterior with formula in aluminium foil | 0.92 | 1.32 | 0.000 |
| CM10 | Cerelac Infant Cereal | Friesland Campina (Nigeria) | Aluminium tin | 0.99 | 0.60 | 0.012 |
M = milk-based sample; C = cereal-based sample; CM = a mixture of cereal and milk-based sample; NA = not available.
The infant formula samples (1–2 g) were weighed with plastic materials to prevent contamination with metals. The samples were then digested using hot-block digestion as in a previous procedure.15 Approximately 9 mL of 65% concentrated nitric acid and 3 mL of perchloric acid were added in a ratio of 3:1 prior to heating and the solution was transferred to a hot plate and heated to 120°C for approximately five hours. The samples were then placed in an oven where the temperature was gradually increased by 10°C every 60 minutes until reaching 450°C. After 18 hours, the samples had been converted to white ash and were left to cool. The white ash was then dissolved in 5 mL of 1.5% nitric acid and a final volume of 25 mL was made by the addition of deionised water. Metal concentrations were assayed with atomic absorption spectroscopy (Model 205, Buck Scientific, East Norwalk, Connecticut, USA). Samples were analysed in triplicate.16
The instrument was recalibrated after every 10 runs. The analytical procedure was checked using the spike recovery method. A known standard of the metals was introduced into already-analysed samples and reanalysed. Results of recovery studies for aluminium, arsenic and mercury found a recovery rate of more than 97%.16 The relative standard deviation (SD) between replicate analyses was less than 4%. The limit of detection for aluminium, arsenic and mercury were 0.005, 0.001 and 0.001 mg/kg, respectively, with blank values reading as 0.00 mg/kg for all the metals in deionised water with an electrical conductivity of less than 5 μS/cm. The limit of quantification was 0.04 mg/kg.
The JECFA PTDI values were used as threshold values for the consumption of aluminium, arsenic and mercury.8,13 To assess aluminium exposure in children through the consumption of commonly used infant formulas in Nigeria, a JECFA PTDI of 286 μg/kg BW/day and a PTWI of 2,000 μg/kg BW/week were considered threshold values. In assessing infants’ arsenic intake, a PTDI of 2.1 μg/kg BW/day and a PTWI of 15 μg/kg BW/week was considered acceptable. The threshold values for children’s exposure to mercury was set at a PTDI of 0.57 μg/kg BW/day and a PTWI of 4.0 μg/kg BW/week.
The exposure health risks of aluminium, arsenic and mercury from consumption of infant formulas were based on estimated daily intake (EDI) and compared with the JECFA PTDI.17,18
| [Equation 1] |
Where EDI is the estimated daily intake of aluminium, arsenic and mercury (mg/kg/day), C is the mean concentration of aluminium, arsenic or mercury in infant formula samples (mg/kg), IR is the intake of infant formula (kg/BW/day) and BW is the infant’s body weight. The EDI of aluminium, arsenic and mercury in different infant formulas was calculated using the actual aluminium, arsenic and mercury levels from this study to multiply the recommended consumption/intake rate by manufacturers divided by BW [Equation 1]. The EDI was calculated for 0–12 month old infants with a BW of 3.5–10.5 kg.19 The daily consumed powder formula, according to the infants’ mean BW, were obtained from feeding tables and dosages recommended by manufacturers. For an exposure assessment of aluminium, arsenic and mercury in infant formulas, the percentage of aluminium, arsenic and mercury according to PTDI was calculated using the lowest and highest EDI [Equation 2]. All the calculated PTDIs were based on data appropriate for infants of various ages and BWs. The EDI was compared with the JECFA PTDI for aluminium, arsenic and mercury. The highest and lowest EDI values from all three types of infant formula were used to calculate the percentages of aluminium, arsenic and mercury that contributed to PTDI.
Percentages of aluminium, arsenic and mercury in infant formulas were calculated using the following equation:
| [Equation 2] |
Statistical analysis was carried out using Graphpad Prism, Version 6.5 (GraphPad Software Inc., San Diego, California, USA). All results were expressed as mean ± SD. The data were analysed using one-way analysis of variance and Tukey’s post-hoc test at a 95% confidence level. A P value of <0.05 was considered statistically significant. PCA was applied to reduce the number of variables and extract as much information as possible.
This study was approved by the Ethics Committee on Research at the University of Port Harcourt, Rivers State, Nigeria.
Results
A total of 26 infant formulas were analysed in this study. The concentrations of aluminium, arsenic and mercury varied from 0.41–2.47, 0.02–1.56 and 0.000–0.050 mg/kg, respectively. Mercury was not detected in 19.2% of the tested formulas. The highest and lowest detected values of mercury were observed in M6 (0.050 mg/kg) and C2 (0.001 mg/kg), respectively. Arsenic was detected in all analysed formulas, with the highest value detected in C2 (1.56 mg/kg) and the lowest values in CM2, 4 and 5 (0.02 mg/kg each). Aluminium was found to be present in all analysed formulas, with the highest and lowest values observed in M4 (2.47 mg/kg) and C5 (0.41 mg/kg), respectively [Table 1].
The mean mercury concentration in all three types of infant formula was 0.01 mg/kg. The mean arsenic concentration in cereal-based infant formula was higher compared to milk-based and a mixture of cereal and milk-based formula (0.68 ± 0.67 versus 0.33 ± 0.26 and 0.46 ± 0.52 mg/kg, respectively); these differences were not statistically significant (P >0.05). The highest mean concentration of aluminium was found in the milk-based infant formula compared to cereal-based and a mixture of cereal and milk-based formula (1.70 ± 0.49 versus 1.52 ± 0.6 and 1.17 ± 0.56 mg/kg, respectively); these differences were also not statistically significant (P >0.05) [Table 2].
Table 2.
Mean concentrations of aluminium, arsenic and mercury in three different groups of infant formulas in Nigeria (N = 26)
| Infant formula type | Mean concentration ± SD in mg/kg (range) | ||
|---|---|---|---|
| Aluminium | Arsenic | Mercury | |
| M1–9 | 1.70 ± 0.49 (1.02–2.47) | 0.33 ± 0.26 (0.03–0.83) | 0.01 ± 0.02 (0.00–0.05) |
| C1–7 | 1.52 ± 0.6 (0.41–2.35) | 0.68 ± 0.67 (0.08–1.56) | 0.01 ± 0.00 (0.00–0.01) |
| CM1–10 | 1.17 ± 0.56 (0.45–2.07) | 0.46 ± 0.52 (0.02–1.32) | 0.01 ± 0.01 (0.00–0.03) |
SD = standard deviation; M = milk-based sample; C = cereal-based sample; CM = a mixture of cereal and milk-based sample.
The EDI of aluminium, arsenic and mercury in milk-based infant formula ranged from 0.023–0.040, 0.005–0.008 and 1.4 × 104–2.4 × 104 mg/kg BW/day, respectively. In cereal-based infant formula, the EDI of aluminium, arsenic and mercury ranged from 0.021–0.036, 0.009–0.016 and 1.4 × 104–2.4 × 104 mg/kg BW/day, respectively. The EDI of aluminium, arsenic and mercury in a mixture of cereal and milk-based infant formula ranged from 0.016–0.025, 0.006–0.011 and 1.4 × 104–2.4 × 104 mg/kg BW/day, respectively [Table 3].
Table 3.
Estimated daily intake of aluminium, arsenic and mercury in different groups of infant formulas in Nigeria
| Infant formula type and infant age | DIR in kg | BW in kg | EDI in mg/kg BW/day | ||
|---|---|---|---|---|---|
| Aluminium | Arsenic | Mercury | |||
| Milk-based | |||||
| Age | |||||
| 0–2 weeks | 0.075 | 3.5 | 0.036 | 0.007 | 2.1 × 104 |
| 2–4 weeks | 0.100 | 4.2 | 0.040 | 0.008 | 2.4 × 104 |
| 2 months | 0.110 | 4.7 | 0.040 | 0.008 | 2.3 × 104 |
| 4 months | 0.145 | 6.5 | 0.038 | 0.007 | 2.2 × 104 |
| 6 months | 0.135 | 7.5 | 0.031 | 0.006 | 1.8 × 104 |
| 6–12 months | 0.135 | 10 | 0.023 | 0.005 | 1.4 × 104 |
| Cereal-based | |||||
| Age | |||||
| 0–2 weeks | 0.075 | 3.5 | 0.032 | 0.015 | 2.1 × 104 |
| 2–4 weeks | 0.100 | 4.2 | 0.036 | 0.016 | 2.4 × 104 |
| 2 months | 0.110 | 4.7 | 0.035 | 0.016 | 2.3 × 104 |
| 4 months | 0.145 | 6.5 | 0.033 | 0.015 | 2.2 × 104 |
| 6 months | 0.135 | 7.5 | 0.027 | 0.012 | 1.8 × 104 |
| 6–12 months | 0.135 | 10 | 0.021 | 0.009 | 1.4 × 104 |
| Mixture of cereal and milk-based | |||||
| Age | |||||
| 0–2 weeks | 0.075 | 3.5 | 0.021 | 0.010 | 2.1 × 104 |
| 2–4 weeks | 0.100 | 4.2 | 0.025 | 0.011 | 2.4 × 104 |
| 2 months | 0.110 | 4.7 | 0.020 | 0.011 | 2.3 × 104 |
| 4 months | 0.145 | 6.5 | 0.023 | 0.010 | 2.2 × 104 |
| 6 months | 0.135 | 7.5 | 0.016 | 0.008 | 1.8 × 104 |
| 6–12 months | 0.135 | 10 | 0.016 | 0.006 | 1.4 × 104 |
DIR = daily intake rate; BW = body weight; EDI = estimated daily intake.
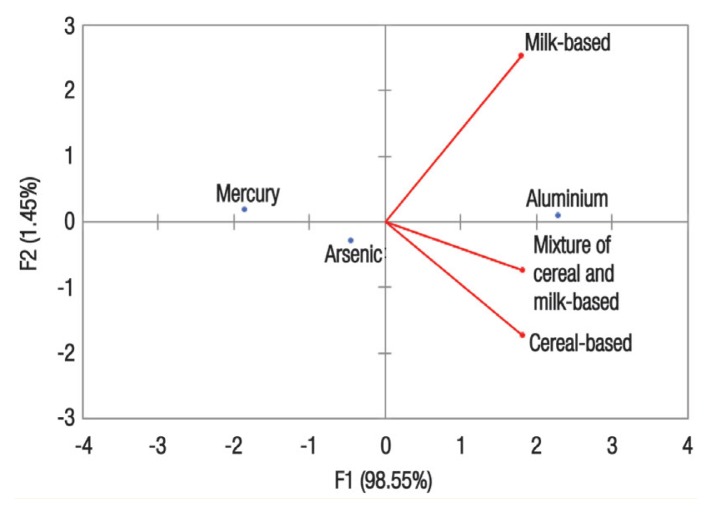
Compared to the PTDI JECFA threshold values there was an intake range of 8.02–14.2% for aluminium, 437.1–771% for arsenic and 23.7–41.8% for mercury [Table 4]. The PCA for the three variables (aluminium, arsenic and mercury) resulted in a biplot with aluminium in the first quadrant alongside milk-based formulas [Figure 1]. This finding suggests that aluminium is the principal component with an eigenvalue of 2.956 and corresponding variability of 98.55%.
Table 4.
Percentage of the Joint Food and Agricultural Organization/ World Health Organization Expert Committee on Food Additives provisional tolerable daily intake of aluminium, arsenic and mercury found in analysed infant formulas in Nigeria
| Aluminium | Arsenic | Mercury | |
|---|---|---|---|
| JECFA PTWI in μg/kg BW/week | 2000 | 15 | 4.0 |
| JECFA PTDI in μg/kg BW/day | 286 | 2.1 | 0.57 |
| Age | Percentage of JECFA PTDI | ||
| 0–2 weeks | 12.7 | 693.9 | 37.6 |
| 2–4 weeks | 14.2 | 771 | 41.8 |
| 2 months | 13.9 | 757.9 | 41.1 |
| 4 months | 13.3 | 722.3 | 39.1 |
| 6 months | 10.7 | 582.9 | 31.6 |
| 6–12 months | 8.02 | 437.1 | 23.7 |
JECFA = Joint Food and Agricultural Organization/World Health Organization Expert Committee on Food Additives; PTWI = provisional tolerable weekly intake; BW = bodyweight; PTDI = provisional tolerable daily intake.
Figure 1.
Biplot of principal component analysis.
Discussion
The present study showed the presence of aluminium, arsenic and mercury in infant formulas sold in Nigeria. Aluminium and mercury levels were within permissible limits, but arsenic concentrations in the infant formulas exceeded established safe limits. The mean concentrations of aluminium and mercury were highest in milk-based infant formulas compared to other types of infant formulas, whereas arsenic was lowest in milk-based infant formulas compared to other analysed types. The mean arsenic concentration in this study was higher than the threshold value of 0.14 mg/kg.20
The detected aluminium concentrations in the current study was higher than those reported earlier in Nigeria but this level of contamination is not considered a health concern.21 Aluminium in infant formulas marketed in Nigeria may be considered safe because, contrary to expectations and given the ubiquity of aluminium in the environment and fortification of formulas with iron and other ingredients, the aluminium in all the infant-based formulas were under the PTDI threshold value and the EDI for children aged 0–12 month. In this study, aluminium levels in milk-based infant formulas were higher compared to cereal-based formulas (1.70 ± 0.49 versus 1.52 ± 0.6 mg/kg, respectively). These aluminium levels were also higher than those reported by Koo et al. for both milk-based and soy-based infant formulas (14–565 μg/L and 455–2346 μg/kg, respectively).22 Aluminium can have plant and animal origins, with the former reflecting the content in the soil and in the water resulting from carry-over or run-off. The produce used as raw materials for these infant formulas may have been grown in contaminated soil or produced with aluminium-contaminated water.23
Baxter et al. reported that soya-based formulas usually contained higher concentrations of aluminium than milk-based formulas although there is evidence that some manufacturers can lower concentrations of aluminium in soy-based formulas.23 High levels of aluminium disrupt enzyme activities and impairs mitochondrial functions in many organs, especially the haemopoietic system, nervous system and bones, which are especially important in growing infants and children.24
According to the Committee on Nutrition of the American Academy of Pediatrics, aluminium content should not exceed 30,000 ng/kg of BW/day for infants and children, with chronic renal failure requiring phosphate binders.25 The JECFA, based on the experimental bio-availability and toxicological data, reduced the PTWI of aluminium from 7,000 to 2,000 μg/kg of BW for humans. Interestingly, according to the same committee, estimates of dietary exposure of children to aluminium containing food additives, including high dietary exposures, can exceed PTWI by up to two-fold.14 This study found an aluminium intake range of 8.02–14.2% for full-term 0–12-month-old infants (3.5–10 kg BW) of the JECFA PTDI for the analysed infant formulas. These levels are within the PTWI of aluminium established by the JECFA.8 However, this aluminium content of infant formulas is higher than the aluminium content of breast milk, which is usually approximately 15–30 μg/L.26 This may be of concern in infants with impaired renal function and premature infants who should be on infant formula with an aluminium level of less than 100 ng/g.27 Fat concentrates, lactose and mineral mixtures have been reported as contributing to the total aluminium content in infant formulas and could be reduced by over 70% by replacing these components with low-aluminium equivalents.27 More than 90% of the infant formulas in this study were packaged in some form of aluminium. Migration of aluminium from the packaging may have contributed to the presence of aluminium in the infant formulas in this study.25
Arsenic can cause cancer in many organs, including the skin, lungs, bladder, kidney and liver; it is also capable of influencing the neurological, respiratory and cardiovascular systems.13 Arsenic has also been implicated in diabetic pathophysiology and reproductive toxicity.13 This study found an arsenic intake range of 437.1–771% of the JECFA PTDI for infant formulas. Recent research has shown that infant formulas, specifically rice-based infant food, contains arsenic which can be traced to the natural raw materials used for processing.28,29 Currently, there is no guideline for arsenic content in baby food, including infant formulas, but the food industry has been advised to adhere to a 0.2 mg/kg arsenic level to ensure the safety of infants and young children.29,30 Infant formulas derived from rice have been shown to contain arsenic, which potentially has health risks for infants due to long-term exposure starting at a young age.30 In this study, the cereal-based infant formulas showed the highest levels of arsenic at 0.68 ± 0.67 mg/kg. Although some work has reported that infant formula contributes minimally to overall inorganic arsenic exposure in children and does not pose a significant public health concern, the EDI of arsenic in all infant formulas in the current study exceeded the infant age group PTDI of 2.1 μg/kg BW/day set by the JECFA.30 The arsenic levels in the present study are general measurements and not representative of any specific type of arsenic.
Although the main dietary source of mercury in humans is fish, non-fish sources can also contain mercury.24 This study found an intake range of 23.7–41.8% of the PTDI for the analysed infant formulas. EDI of mercury in infant formula was found to be under the PTDI value recommended by the JECFA.31 Mercola showed that high-fructose corn syrups may be a source of mercury in infant formulas.32 In addition, there are many instances at which metals can enter infant formulas, such as through metal, plastic or glass containers used for storing formula, through washing or leaching from lids and/or through equipment and processes used for filling these containers.
This study was subject to some limitations. Due to financial constraints, this study only focused on a small sample. It is possible that the levels of metals in the samples may be due to batch contamination. In addition, metal speciation could not be performed. Further studies with larger sample sizes including metal speciation analyses will shed more light on the public health impact of infant formula consumption in Nigeria
Conclusion
Feeding infants and children in Nigeria with commonly available infant formulas may pose a risk to infants and add to their body burden of arsenic. Although exceeding the PTWI occasionally may not indicate a health risk, the assessed content of arsenic in the infant formulas was much higher than the PTDI and PTWI recommended by the JECFA, suggesting a cause for public health concern. Notwithstanding the lack of legislation on mercury content in infant and baby foods, manufacturers are advised to be more circumspect in their choice of raw materials, and more studies should be conducted to justify the need for legislation concerning levels of total mercury (metal speciation) in infant and baby foods. Food regulatory agencies in developing nations should periodically conduct targeted analyses of all infant formula types to ensure that the permissible limits of contaminants are not exceeded and no processes or ingredients have been inadvertently introduced which would accidentally contaminate the product.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
No funding was received for this study.
References
- 1.Hörnell A, Lagström H, Lande B, Thorsdottir I. Breastfeeding, introduction of other foods and effects on health: A systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr Res. 2013;57:1–27. doi: 10.3402/fnr.v57i0.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Codex Alimentarius Commission. Standard for infant formula and formulas for special medical purposes intended for infants. Rome: Food and Agriculture Organization of the United Nations and World Health Organization; 1981. Codex Stan 72-1981. [Google Scholar]
- 3.Domínguez A, Paz S, Rubio C, Gutiérrez A, González-Weller D, Revert C, et al. Essential and toxic metals in infant formula from the European Community. Open Access J Toxicol. 2017;2:1–8. doi: 10.19080/OAJT.2017.02.555585. [DOI] [Google Scholar]
- 4.European Environment and Health Information System. Exposure of children to chemical hazards in food. [Accessed: Aug 2019]. From: www.euro.who.int/__data/assets/pdf_file/0003/97446/4.4.pdf.
- 5.ChemLinked. GB 2762-2012 National Food Safety Standard Maximum Levels of Contaminants in Foods. [Accessed: Aug 2019]. From: https://food.chemlinked.com/node/3441.
- 6.Gupta N, Gaurav SS, Kumar A. Molecular basis of aluminium toxicity in plants: A review. Am J Plant Sci. 2013;4:21–37. doi: 10.4236/ajps.2013.412A3004. [DOI] [Google Scholar]
- 7.Bondy SC. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology. 2016;52:222–9. doi: 10.1016/j.neuro.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Osman NA, Zaki A, Agmay NF, Shehata GM. Aluminum in food: Dietary exposure among adolescent residents in the food catering establishments in Alexandria, Egypt. Glob J Pharm Educ Res. 2017;6:1–6. [Google Scholar]
- 9.Rintala EM, Ekholm P, Koivisto P, Peltonen K, Venäläinen ER. The intake of inorganic arsenic from long grain rice and rice-based baby food in Finland - Low safety margin warrants follow up. Food Chem. 2014;150:199–205. doi: 10.1016/j.foodchem.2013.10.155. [DOI] [PubMed] [Google Scholar]
- 10.Jackson BP, Taylor VF, Punshon T, Cottingham KL. Arsenic concentration and speciation in infant formulas and first foods. Pure Appl Chem. 2012;84:215–23. doi: 10.1351/PAC-CON-11-09-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljung K, Palm B, Grandér M, Vahter M. High concentrations of essential and toxic elements in infant formula and infant foods - A matter of concern. Food Chem. 2011;127:943–51. doi: 10.1016/j.foodchem.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 12.International Agency for Research on Cancer. Arsenic and arsenic compounds. [Accessed: Aug 2019]. From: https://www.ncbi.nlm.nih.gov/books/NBK304380.
- 13.Food and Agriculture Organization of the United Nations, World Health Organization. Safety evaluation of certain contaminants in food: Prepared by the Seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) [Accessed: Aug 2019]. From: https://apps.who.int/iris/handle/10665/44520.
- 14.Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B. Mercury exposure and children’s health. Curr Probl Pediatr Adolesc Health Care. 2010;40:186–215. doi: 10.1016/j.cppeds.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobin R, Larkin T, Moane S. The Irish organic food market: Shortfalls, opportunities and the need for research. J Sci Food Agric. 2011;91:2126–31. doi: 10.1002/jsfa.4503. [DOI] [PubMed] [Google Scholar]
- 16.Orisakwe OE, Igweze ZN, Udowelle NA. Candy consumption may add to the body burden of lead and cadmium of children in Nigeria. Environ Sci Pollut Res Int. 2019;26:1921–31. doi: 10.1007/s11356-018-3706-3. [DOI] [PubMed] [Google Scholar]
- 17.Poitevin E. Official methods for the determination of minerals and trace elements in infant formula and milk products: A review. J AOAC Int. 2016;99:42–52. doi: 10.5740/jaoacint.15-0246. [DOI] [PubMed] [Google Scholar]
- 18.Bargellini A, Venturelli F, Casali E, Ferrari A, Marchesi I, Borella P. Trace elements in starter infant formula: Dietary intake and safety assessment. Environ Sci Pollut Res Int. 2018;25:2035–44. doi: 10.1007/s11356-016-8290-9. [DOI] [PubMed] [Google Scholar]
- 19.Onayade AA, Abiona TC, Abayomi IO, Makanjuola RO. The first six month growth and illness of exclusively and non-exclusively breast-fed infants in Nigeria. East Afr Med J. 2004;81:146–53. doi: 10.4314/eamj.v81i3.9145. [DOI] [PubMed] [Google Scholar]
- 20.Bratakos SM, Lazou AE, Bratakos MS, Lazos ES. Aluminium in food and daily dietary intake estimate in Greece. Food Addit Contam Part B Surveill. 2012;5:33–44. doi: 10.1080/19393210.2012.656289. [DOI] [PubMed] [Google Scholar]
- 21.Ikem A, Nwankwoala A, Odueyungbo S, Nyavor K, Egiebor N. Levels of 26 elements in infant formula from USA, UK, and Nigeria by microwave digestion and ICP-OES. Food Chem. 2002;77:439–47. doi: 10.1016/S0308-8146(01)00378-8. [DOI] [Google Scholar]
- 22.Koo WW, Kaplan LA, Krug-Wispe SK. Aluminum contamination of infant formulas. JPEN J Parenter Enteral Nutr. 1988;12:170–3. doi: 10.1177/0148607188012002170. [DOI] [PubMed] [Google Scholar]
- 23.Baxter MJ, Burrell JA, Massey RC. The aluminium content of infant formula and tea. Food Addit Contam. 1990;7:101–7. doi: 10.1080/02652039009373826. [DOI] [PubMed] [Google Scholar]
- 24.Bundesministerium für Gesundheit. Aluminium: Toxikologie und gesundheitliche Aspekte körpernaher Anwendungen. [Accessed: Aug 2019]. From www.sozialministerium.at/Themen/Gesundheit/Verbrauchergesundheit/Studie---Aluminium-Toxikologie-undgesundheitliche-Aspekte-k%C3%B6rpernaher-Anwendungen-.html.
- 25.American Academy of Pediatrics Committee on Nutrition. Aluminum toxicity in infants and children. Pediatrics. 1986;78:1150–4. [PubMed] [Google Scholar]
- 26.Fernandez-Lorenzo JR, Cocho JA, Rey-Goldar ML, Couce M, Fraga JM. Aluminum contents of human milk, cow’s milk and infant formulas. J Pediatr Gastroenterol Nutr. 1999;28:270–5. doi: 10.1097/00005176-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Dabeka R, Fouquet A, Belisle S, Turcotte S. Lead, cadmium and aluminum in Canadian infant formulae, oral electrolytes and glucose solutions. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:744–53. doi: 10.1080/19393210.2011.571795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carignan CC, Cottingham KL, Jackson BP, Farzan SF, Gandolfi AJ, Punshon T, et al. Estimated exposure to arsenic in breastfed and formula-fed infants in a United States cohort. Environ Health Perspect. 2015;123:500–6. doi: 10.1289/ehp.1408789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata T, Meng C, Umoren J, West H. Risk assessment of arsenic in rice cereal and other dietary sources for infants and toddlers in the US. Int J Environ Res Public Health. 2016;13:361. doi: 10.3390/ijerph13040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cubadda F, Jackson BP, Cottingham KL, Van Horne YO, Kurzius-Spencer M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci Total Environ. 2017;579:1228–39. doi: 10.1016/j.scitotenv.2016.11.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joint FAO/WHO Expert Committee on Food Additives. Safety evaluation of certain food additives and contaminants: prepared by the Seventy fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives ( JECFA) [Accessed: Aug 2019]. From: https://apps.who.int/iris/handle/10665/44813. [PubMed]
- 32.Mercola J. This common food ingredient can really mess up your metabolism. [Accessed: Aug 2019]. From: https://clareswinney.wordpress.com/2010/01/28/this-common-food-ingredient-can-really-mess-upyour-metabolism/