Abstract
Treatments that target alterations in gut microbiota may be beneficial for patients with irritable bowel syndrome (IBS). A systematic review and meta-analysis was conducted of randomised clinical trials (RCTs) evaluating the efficacy and safety of probiotics, prebiotics and synbiotics. Factors considered in the analysis included global IBS symptoms and/or abdominal pain, secondary symptoms and the frequency of adverse events. A total of 33 RCTs involving 4,321 patients were identified. Overall, probiotics significantly improved global IBS symptoms compared to placebos (standardised mean difference = −0.32, 95% confidence interval: −0.48 to −0.15; P <0.001), with significant heterogeneity between studies (I2 = 72%; P <0.001). This remained apparent in both single- and multi-strain probiotic interventions as well as synbiotic formulations. However, evidence regarding prebiotics was scarce. There were no significant inter-group differences in terms of the frequency of adverse events. Future RCTs should address methodological limitations, including short follow-up periods and patient adherence.
Keywords: Irritable Bowel Syndrome, Gastrointestinal Microbiome, Dietary Supplements, Probiotics, Prebiotics, Synbiotics, Meta-Analysis, Systematic Review
Irritable bowel syndrome (IBS) is a disorder which manifests as a set of chronic gastrointestinal (GI) symptoms and changes in bowel habits in the absence of evident structural and biochemical abnormalities. 1,2 Overall, IBS is the most commonly diagnosed GI disorder with a global prevalence of 10–15% and is more frequent among individuals aged <50 years old.3,4 Altered bowel habits are the most commonly reported clinical feature, with the syndrome predominantly associated with constipation (IBS-C), diarrhoea (IBS-D) or a mixture of both conditions (IBS-M).1 In addition, patients with IBS often experience abdominal pain, which can be provoked by emotional stress or eating and is usually alleviated by the passing of stool.1,2
A diagnosis of IBS is confirmed according to the latest version of the Rome criteria based on the clinical experience and consensus of a committee of multinational experts.2,5–7 The role of radiological imaging in the diagnosis of IBS is still limited to those patients with ‘red flag’ symptoms, such as rectal bleeding, iron-deficiency anaemia and weight loss, in order to exclude other underlying diseases.8 However, a recent study indicated that diffusion-weight imaging can accurately assess disease activity among patients with Crohn’s disease, a GI condition with similar symptoms to IBS.9
Despite extensive research, the typical mechanistic pathways of IBS have not yet been clearly elucidated. It has been postulated that enteric infections, immunomodulation, visceral hypersensitivity and an imbalance in neurotransmitters may all play a role in the development of IBS.10–12 Importantly, alterations in the gut microbiota can induce changes in gut motility, permeability, food processing and visceral perception which eventually leads to the occurrence of IBS-related symptoms.13,14 Multiple studies have shown that IBS patients experience bacterial overgrowth in the small intestine or altered GI microbes.15–18 A recent meta-analysis observed that patients with IBS (particularly IBS-D) have significantly reduced GI colonies of Bifidobacterium, Lactobacillus and Faecalibacterium prausnitzii bacteria compared to healthy individuals.19 Furthermore, the link between GI microbial disruption and IBS is corroborated by the fact that 10–53% of patients are diagnosed with IBS following a GI infection.20
Such findings have opened a new avenue of treatment to control IBS symptoms, namely the manipulation of gut microbiota. Potential therapies to modulate the microbial composition of the GI environment include dietary supplements incorporating prebiotics, probiotics or synbiotics. Prebiotics are non-digestible dietary compounds that stimulate the growth and activity of specific bacterial populations, while probiotics are live microorganisms that can be supplemented in adequate amounts to induce therapeutic benefits.21 Synbiotics, the combination of both prebiotics and probiotics, can provide beneficial effects to the host and improve the viability of its constituents.22 Nevertheless, the effects of such therapeutic approaches in the treatment of IBS are questionable, particularly with regards to using single or several variations or combinations of probiotics and prebiotics. Therefore, a comprehensive evaluation of the efficacy and safety of prebiotics, probiotics and synbiotics in the management of patients with IBS is necessary.
Methods
All procedures were conducted according to the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.23 Only prospective randomised clinical trials (RCTs) published in Englishlanguage peer-reviewed journals between 2000 and 2019 that compared the effects of prebiotics, probiotics and synbiotics on adult IBS patients (aged ≥18 years) were included in the analysis. Trials including children or patients with other GI disorders were excluded. The diagnosis of IBS was confirmed according to any version of the Rome criteria in order to ensure minimal heterogeneity if other diagnostic criteria or basic physician opinions were used initially.2,5–7
In order to be eligible for inclusion, the RCTs had to involve the administration of at least one of three therapeutic interventions (prebiotics, probiotics and/or synbiotics) to a specific cohort of IBS patients and compare outcomes with another group receiving a placebo. The minimum sample size was 50 patients. Trials using probiotics could include either single-or multi-strain preparations. If a trial incorporated multiple intervention groups with different doses, the group with the highest dose was included in the analysis in order to avoid any overlap that might result from multiple analyses of placebo outcomes. Trials employing a cross-over design were excluded.24 In addition, narrative reviews, case reports, conference proceedings, retrospective studies and systematic reviews were excluded.
The primary outcomes of the meta-analysis included the efficacy of the therapeutic interventions on global IBS symptoms and/or abdominal pain. These outcomes were presented as continuous variables in terms of mean differences in scores at the end of the follow-up period. Additionally, secondary outcomes included the effects of the interventions on the scores of other symptoms (i.e. bloating/distension, flatulence and urgency), along with impact on quality of life (QOL). In terms of safety, the reported frequencies of adverse events at the end of the follow-up period were analysed.
A comprehensive literature search was performed of various databases, including MEDLINE® (National Library of Medicine, Bethesda, Maryland, USA), Embase (Elsevier, Amsterdam, The Netherlands) Cochrane Library (Cochrane, London, UK) and Google Scholar (Google LLC, Mountain View, California, USA). The search was conducted in June 2019 using the following keywords combined as appropriate using Boolean operators (e.g. “or” and “and”): “irritable bowel syndrome”, “irritable bowel”, “probiotic”, “Bacillus”, “Bifidobacterium”, “Lactobacillus”, “Streptococcus”, “Enterococcus”, “Propionibacterium”, “Saccharomyces”, “Clostridium”, “synbiotic”, “prebiotic”, “fructooligosaccharide”, “inulin”, “randomized/randomised” and “trial”.
Two researchers independently screened the titles and abstracts of identified articles to determine their eligibility for inclusion in the analysis. The reference lists of the articles were also screened for any additional publications. Any disagreements concerning eligibility were discussed until a consensus was reached. Information concerning all eligible articles was uploaded to a reference management software (EndNote, Version X7, Clarivate Analytics, Philadelphia, Pennsylvania, USA) to check for any potential duplication. Subsequently, all non-full-text articles were excluded from the final analysis.
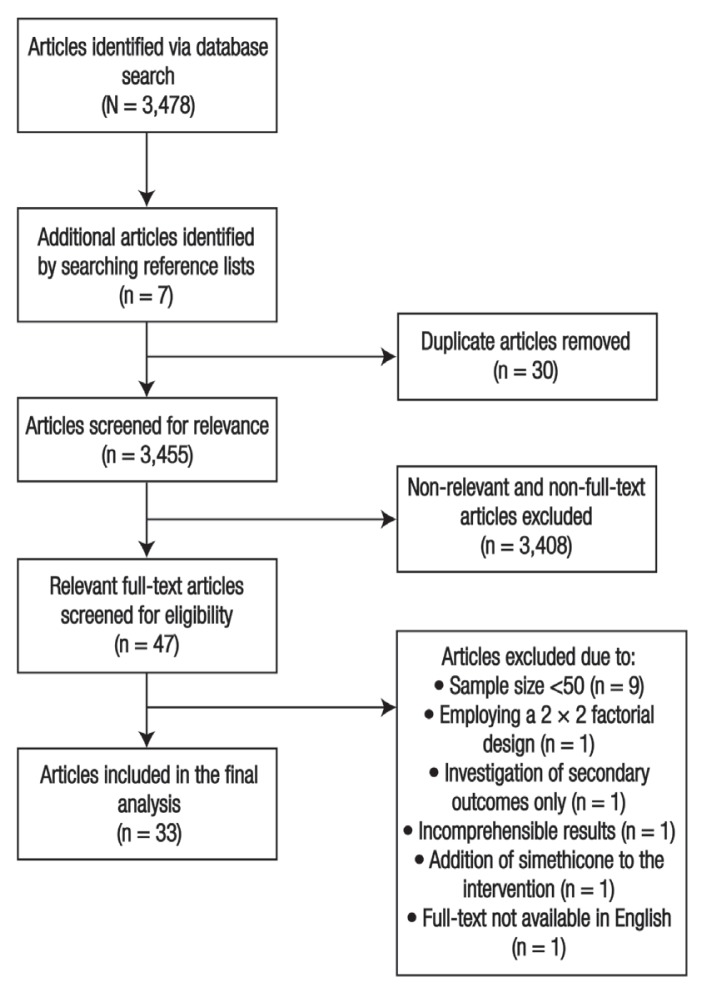
An initial literature search revealed a total of 3,478 publications across the databases, of which 30 were duplicates. In addition, seven eligible articles were identified from reference lists. After the exclusion of 3,408 irrelevant publications, a total of 47 full-text RCTs were assessed for eligibility. During the assessment, 14 trials were excluded for various reasons, including having <50 patients in both groups, presenting outcomes in an uninterpretable manner, employing a 2 × 2 factorial design with changes in diet, investigating QOL as the primary outcome without focusing on IBS symptoms, adding simethicone to the intervention or for not being written in English. Ultimately, a total of 33 RCTs were included in the final analysis [Figure 1].
Figure 1.
Flow chart showing the search process used to identify articles included in this study’s systematic review and meta-analysis.
Information concerning each of these RCTs was recorded in an Excel spreadsheet, Version 2016 (Microsoft Corp., Redmond, Washington, USA). The name of the first author, year of publication, country, study duration and sample size of the study was recorded as well as the gender distribution of the patients and the number of patients allocated to the study groups. Regarding disease-specific data, the distribution of IBS subtypes, version of Rome criteria utilised and data collection instrument was noted. In terms of intervention-related data, the type of intervention (i.e. prebiotic, probiotic or synbiotic), use of single- or multi-strain probiotics and the dosage and form of the intervention was documented, as well as outcome data with regards to scores for global IBS symptoms, abdominal pain, bloating/distension, flatulence, urgency and QOL and the frequency of adverse events at the end of the follow-up period.
Each trial underwent quality assessment using the Cochrane Risk of Bias Tool which assesses processes of random sequence generation and blinding of outcomes, participant/personnel data and intervention allocation, among other measurements of bias.25 The results were presented graphically using RevMan software, Version 5.3 (Cochrane), with each domain interpreted as being either low-risk, high-risk or unclear. With regards to statistical analysis, continuous variables (i.e. symptom and QOL scores) were presented as standardised mean differences (SMDs) with 95% confidence intervals (CIs), while dichotomous variables (i.e. frequencies of adverse events) were expressed as relative risks (RRs) with 95% CIs. Overall effects were analysed using z-statistics. Inter-study heterogeneity was assessed using the I2 test, with a random effect model applied in the event of significant heterogeneity (I2 ≥50%). A subgroup analysis was performed based on the sample size, type of therapeutic intervention and the version of Rome criteria utilised. A P value of <0.05 was considered statistically significant.
Results
The general characteristics of the RCTs are outlined in Table 1.26–58 All of the RCTs were published between 2000 and 2018, with the duration of the intervention ranging between 2–24 weeks. Overall, there were a total of 4,321 patients with IBS, of which 59.5% were female.26–58 In terms of IBS subtypes, six RCTs included patients with IBS-D, one with IBS-C and one with both IBS-D and IBS-M.26–33 The remaining trials included patients with all subtypes.34–58 With regards to location, the majority of the trials were conducted in Europe (n = 18) followed by Asia (n = 13).26–33,36–58 The remaining two RCTs were based in South Africa and the USA, respectively.34,35
Table 1.
Summary of randomised clinical trials assessing the efficacy and safety of probiotics, prebiotics and synbiotics in the treatment of irritable bowel syndrome26–58
| Author and year of study | Country | Study duration in weeks | Sample size (male/female) | Mean age in years ± SD | Group allocation | Diagnostic criteria | IBS subtypes | Intervention | Strain or type of intervention | Dosage and form of intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Azpiroz et al.51 (2017) | Spain | 4 | 79 (48/31) | I: 41.0 ± 11.1 P: 42.4 ± 10.6 |
I: 41 P: 38 |
Rome III | All | Prebiotic | FOO | Twice daily in powder sachets |
| Niv et al.50 (2016) | Israel | 12 | 108 (37/71) | I: 46.2 ± 19.2 P: 40.8 ± 15.6 |
I: 49 P: 59 |
Rome III | All | Prebiotic | PHGG | Once daily in powder sachets |
| Olesen et al.42 (2000) | Denmark | 12 | 63 (11/52) | I: 45.1 ± 13.1 P: 45.1 ± 13.1 |
I: 30 P: 32 |
Rome I | All | Prebiotic | FOO | Once daily in powder sachets |
| Abbas et al.27 (2014) | Pakistan | 6 | 72 (53/19) | I: 37.7 ± 11.6 P: 33.0 ± 12.0 |
I: 37 P: 35 |
Rome III | IBS-D | Probiotic SS | S. boulardii | Once daily in syrup |
| Amirimani et al.39 (2013) | Iran | 4 | 92 (36/56) | I: 44.9 ± 13.0 P: 37.7 ± 10.5 |
I: 41 P: 31 |
Rome III | All | Probiotic SS | L. reuteri | Once daily |
| Begtrup et al.54 (2013) | Denmark | 24 | 131 (97/34) | I: 31.6 ± 10.1 P: 29.4 ± 8.6 |
I: 54 P: 44 |
Rome III | All | Probiotic MS | L. paracasei, L. acidophilus and B. lactis | Twice daily in capsules |
| Choi et al.33 (2011) | Korea | 4 | 74 (37/37) | I: 40.2 ± 13.1 P: 40.6 ± 12.9 |
I: 34 P: 33 |
Rome II | IBS-D and IBS-M | Probiotic SS | S. boulardii | Twice daily in capsules |
| Drouault-Holowacz et al.44 (2008) | France | 4 | 100 (24/76) | I: 47.0 ± 14.0 P: 44.0 ± 14.0 |
I: 48 P: 52 |
Rome II | All | Probiotic MS | B. longum, L. acidophilus, Lactococcus lactis and Streptococcus thermophilus | Once daily in powder sachets |
| Ducrotté et al.36 (2012) | India | 4 | 214 (151/63) | I: 36.5 ± 12.1 P: 38.4 ± 13.1 |
I: 108 P: 106 |
Rome III | All | Probiotic SS | L. plantarum | Once daily in capsules |
| Guglielmetti et al.56 (2011) | Italy | 4 | 122 (40/82) | I: 36.7 ± 12.4 P: 40.9 ± 12.8 |
I: 60 P: 62 |
Rome III | All | Probiotic SS | B. bifidum | Once daily in capsules |
| Guyonnet et al.32 (2007) | France | 6 | 267 (199/68) | I: 49.4 ± 11.4 P: 49.2 ± 11.4 |
I: 135 P: 132 |
Rome II | IBS-C | Probiotic MS | B. animalis, S. thermophilus and L. delbrueckii | Twice daily in yoghurt |
| Hod et al.29 (2017) | Israel | 8 | 107 (0/107) | I: 29.0 ± 4.0 P: 30.0 ± 6.0 |
I: 54 P: 53 |
Rome III | IBS-D | Probiotic MS | L. rhamnosus, L. paracasei, L. plantarum, L. acidophilus, L. bulgaricus, L. lactis, B. bifidum, B. longum, B. breve, B. infantis and S. thermophilus | Twice daily in capsules |
| Ishaque et al.31 (2018) | Bangladesh | 16 | 360 (281/79) | I: 32.2 ± 10.1 P: 31.7 ± 9.7 |
I: 181 P: 179 |
Rome III | IBS-D | Probiotic MS | Bacillus subtilis, B. bifidum, B. breve, B. infantis, B. longum, L. acidophilus, L. delbrueckii, L. casei, L. plantarum, L. rhamnosus, L. helveticus, L. salivarius, L. lactis and S. thermophilus | Twice daily in capsules |
| Jafari et al.37 (2014) | Iran | 4 | 108 (43/65) | I: 36.6 ± 12.1 P: 36.8 ± 11.0 |
I: 54 P: 54 |
Rome III | All | Probiotic MS | B. animalis, L. acidophilus, L. delbrueckii and S. thermophilus | Twice daily in capsules |
| Kajander et al.49 (2008) | Finland | 20 | 86 (6/80) | I: 50.0 ± 13.0 P: 46.0 ± 13.0 |
I: 43 P: 43 |
Rome II | All | Probiotic MS | L. rhamnosus, Propionibacterium freudenreichii and B. animalis | Once daily in a milk product |
| Ki Cha et al.30 (2012) | Korea | 10 | 50 (26/24) | I: 37.9± 12.4 P: 40.3± 11.2 |
I: 25 P: 25 |
Rome III | IBS-D | Probiotic MS | L. acidophilus, L. plantarum, L. rhamnosus, B. breve, B. lactis, B. longum and S. thermophilus | Once daily in capsules |
| Lyra et al.55 (2016) | Finland | 12 | 262 (64/198) | I: 47.2 ± 12.5 P: 49.4 ± 12.9 |
I: 131 P: 131 |
Rome III | All | Probiotic SS | L. acidophilus | Once daily in powder |
| Nobaek et al.41 (2000) | Sweden | 4 | 51 (15/36) | I: 51.0 ± 22.0 P: 46.0 ± 19.0 |
I: 25 P: 26 |
Rome I | All | Probiotic SS | L. plantarum | Once daily in a rosehip drink |
| Pineton de Chambrun et al.57 (2015) | France | 8 | 179 (25/154) | I: 42.5 ± 12.5 P: 45.4 ± 14 |
I: 86 P: 93 |
Rome III | All | Probiotic SS | S. cerevisiae | Once daily in capsules |
| Preston et al.35 (2018) | USA | 12 | 113 (45/68) | I: 40.6 ± 13.4 P: 39.9 ± 14.0 |
I: 76 P: 37 |
Rome III | All | Probiotic MS | L. acidophilus and L. rhamnosus | Twice daily in capsules |
| Roberts et al.52 (2013) | UK | 4 | 179 (30/149) | I: 44.7 ± 11.9 P: 43.7 ± 12.8 |
I: 88 P: 91 |
Rome III | All | Probiotic MS | B. lactis, S. thermophilus and L. delbrueckii | Twice daily in a milk product |
| Shin et al.28 (2018) | Korea | 8 | 51 (22/29) | I: 35.0 ± 5.0 P: 38.0 ± 8.0 |
I: 24 P: 27 |
Rome III | IBS-D | Probiotic SS | L. gasseri | Twice daily in capsules |
| Simrén et al.47 (2010) | Sweden | 8 | 74 (52/22) | I: 42.0 ± 15.0 P: 44.0 ± 16.0 |
I: 37 P: 37 |
Rome II | All | Probiotic MS | L. paracasei, L. acidophilus and B. lactis | Once daily in fermented milk |
| Sisson et al.53 (2014) | UK | 12 | 186 (129/57) | I: 39.6 ± 10.5 P: 36.8 ± 10.8 |
I: 124 P: 62 |
Rome III | All | Probiotic MS | L. rhamnosus, L. plantarum, L. acidophilus and Enterococcus faecium | Once daily in syrup |
| Søndergaard et al.48 (2011) | Denmark | 8 | 52 (13/39) | I: 53.9 ± 14.0 P: 48.5 ± 13.7 |
I: 27 P: 25 |
Rome II | All | Probiotic MS | L. paracasei, L. acidophilus and B. lactis | Once daily in fermented milk |
| Spiller et al.58 (2016) | UK | 12 | 379 (62/317) | I: 45.3 ± 15.7 P: 45.4 ± 14.1 |
I: 192 P: 187 |
Rome III | All | Probiotic SS | S. cerevisiae | Twice daily in capsules |
| Stevenson et al.34 (2014) | South Africa | 8 | 81 (2/79) | I: 48.1 ± 13.5 P: 47.3 ± 12.1 |
I: 54 P: 27 |
Rome II | All | Probiotic SS | L. plantarum | Once daily in capsules |
| Sun et al.26 (2018) | China | 4 | 200 (116/84) | I: 43.0 ± 12.5 P: 44.9 ± 13.0 |
I: 105 P: 95 |
Rome III | IBS-D | Probiotic SS | Clostridium butyricum | rice daily in capsules |
| Whorwell et al.43 (2006) | UK | 4 | 182 (0/182) | I: 41.8 ± 1.1 P: 42.4 ± 1.1 |
I: 90 P: 92 |
Rome II | All | Probiotic SS | B.infantis | Once daily in capsules |
| Williams et al.46 (2009) | UK | 8 | 52 (7/45) | I: 40.0 ± 12.0 P: 38.0 ± 11.0 |
I: 28 P: 24 |
Rome II | All | Probiotic MS | L. acidophilus, B.lactis and B. bifidum | Once daily in capsules |
| Cappello et al.45 (2013) | Italy | 4 | 62 (21/41) | I: 36.6 ± 2.2 P: 40.8 ± 2.2 |
I: 32 P: 32 |
Rome II | All | Synbiotic | L. plantarum, L. rhamnosus, L. gasseri, L. acidophilus, L. salivarius, L. sporogenes, B. infantis, B. longum, S. termophilus and inulin | Twice daily in powder sachets |
| Rogha et al.38 (2014) | Iran | 12 | 56 (12/44) | I: 42.6 ± 12.8 P: 37.7 ± 12.4 |
I: 23 P: 33 |
Rome III | All | Synbiotic | B. coagulans and FOO | Once daily in tablets |
| Shavakhi et al.40 (2014) | Iran | 2 | 129 (44/85) | I: 36.1 ± 7.9 P: 36.4 ± 10.5 |
I: 66 P: 63 |
Rome II | All | Synbiotic | L. casei, L. rhamnosus, L. acidophilus, L. delbrueckii ssp. bulgaricus, B. breve, B. longum, S. thermophilus and FOO | Twice daily in capsules |
SD = standard deviation; IBS = irritable bowel syndrome; I = intervention group; P = placebo group; FOO = fructooligosaccharides; PHGG = partially hydrolysed guar gum; D = diarrhoea; SS = single-strain; S. = Saccharomyces; L. = Lactobacillus; MS = multi-strain; B = Bifidobacterium; M = mixed condition; C = constipation.
The Rome I criteria were used for diagnosis in two trials, while the Rome II criteria were used in 11 trials.32–34,40–49 The rest of the trials utilised the Rome III criteria.26–31,35–39,50–58 In terms of intervention, three trials investigated prebiotics (partially-hydrolysed guar gum and fructooligosaccharides) and three investigated synbiotics.38,40,42,50,51 The remaining 27 RCTs evaluated probiotics.26–37,39,41,43,44,46–49,52–58 Just over half of the probiotic trials contained multiple bacterial strains (n = 14).29–32,35,37,44,47–49,52–54 The other 13 trials contained single strains, comprising of Lactobacillus, Bifidobacterium and Saccharomyces species as well as Clostridium butyricum.26–28,33,34,36,39,41,43,55–58
Only one of the RCTs was single-blinded.39 The remaining trials employed a double-blinded design.26–38,40–58 The method of randomisation was not explicitly mentioned in five trials (15.2%); this was therefore categorised as an unclear risk in the risk of bias assessment under the random sequence generation domain.32,35,41,43,46 Block randomisation and a random allocation table was used in 12 RCTs each.26,28–40,43,45,47–52,54–58 Computer-based or online randomisation so ftware was used in four trials.27,31,34,53 For all studies, the primary outcome analysis was based on the intention-to-treat paradigm, apart from two studies investigating probiotic products and one study investigating a synbiotic intervention.31,39,40
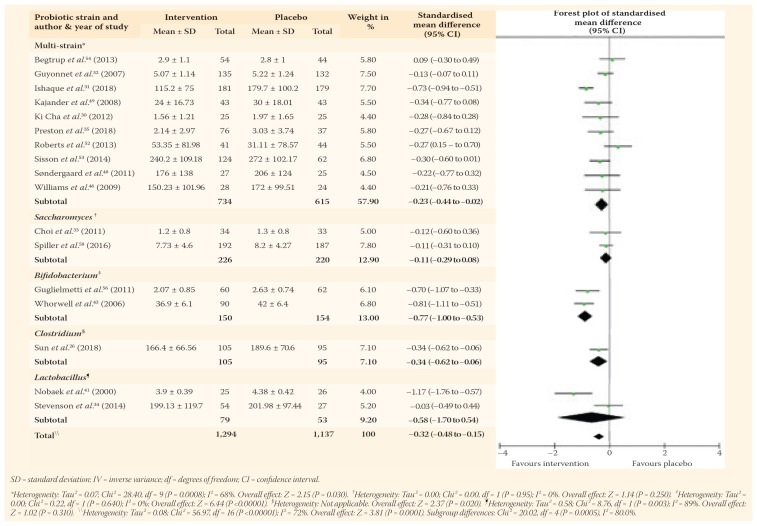
The efficacy results of the interventions versus a placebo were presented in 17 probiotic trials involving 2,431 patients for probiotics and three prebiotic trials involving 250 patients, while none of the synbiotic trials investigated effects on global symptoms scores.26,30–35,41–43,46,48–54,56,58 The pooled outcomes of the probiotic RCTs indicated significant improvements in global symptoms scores (SMD = −0.32, 95% CI: −0.48 to −0.15; P <0.001). However, significant heterogeneity was observed between studies (I2 = 72%; P <0.001). This improvement remained significant with probiotics containing multi-strains (SMD = −0.23, 95% CI: −0.44 to −0.02; P = 0.030) and those using species of Bifidobacterium (SMD = −0.77, 95% CI: −1.00 to −0.53; P <0.001) and Clostridium (SMD = −0.34, 95% CI: −0.62 to −0.06; P = 0.020) [Table 2].
Table 2.
Additionally, probiotics significantly improved global IBS symptom scores in studies with a sample size of <150 patients (SMD = −0.31, 95% CI: −0.52 to −0.10; P = 0.004) and >150 patients (SMD = −0.32, 95% CI: −0.57 to −0.06; P <0.001) as well as studies utilising Rome I (SMD = −1.17, 95% CI: −1.76 to −0.57; P <0.001), Rome II (SMD = −0.29, 95% CI: −0.53 to −0.05; P = 0.020) and Rome III (SMD = −0.28, 95% CI: −0.50 to −0.06; P = 0.010) diagnostic criteria. As for prebiotics, there was no significant effect on IBS symptoms using a random effects model (SMD = 0.59, 95% CI: −0.01 to 1.19; P = 0.050). This lack of significance was also apparent in the subgroup analyses.
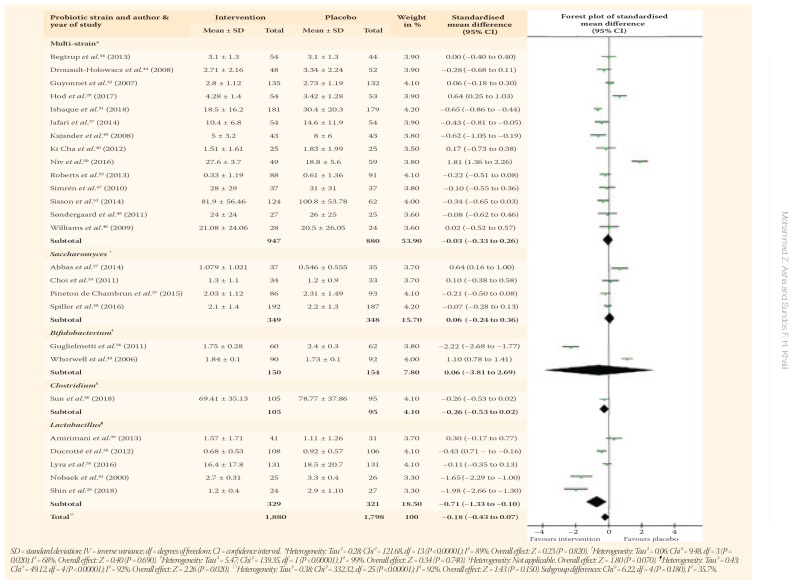
Abdominal pain scores were assessed in 29 RCTs, including 25 probiotics, one prebiotic and three synbiotic trials.26–33,36–41,43–50,52–58 In a pooled analysis, there were no significant differences concerning abdominal pain scores with probiotics as compared to a placebo (SMD = −0.18, 95% CI: −0.43 to 0.07; P = 0.150) with significant heterogeneity between studies (I2 = 92%; P <0.001). However, abdominal pain scores were reduced significantly with probiotics containing Lactobacillus species (SMD = −0.71, 95% CI: −1.33 to −0.10; P = 0.020) and when the Rome I criteria were utilised (SMD = −1.65, 95% CI: −2.29 to −1.00; P <0.001) [Table 3].
Table 3.
The one prebiotic RCT assessing abdominal pain scores compared partially-hydrolysed guar gum to a placebo.47 No significant improvement was observed in abdominal pain (SMD = 0.69, 95% CI: −0.28 to 1.36; P = 0.810). However, different combinations of probiotics and prebiotics in the synbiotic RCTs resulted in significant abdominal pain amelioration compared to a placebo (SMD = −4.27, 95% CI: −7.73 to −0.80; P = 0.020); in addition, the difference remained significant in trials employing the Rome III diagnostic criteria (SMD = −11.24, 95% CI: −13.46 to −9.01; P <0.001).
Regarding other IBS symptoms, the pooled effects of the probiotic trials showed no significant improvements in bloating and urgency scores. However, there was a trend of flatulence alleviation, with the effects nearing statistical significance (SMD = −0.68, 95% CI: −1.38 to 0.01; P = 0.050). Furthermore, a subgroup analysis revealed promising outcomes for distinct symptoms with certain single-strain probiotics. Specifically, probiotics containing Saccharomyces improved bloating (SMD = −0.20, 95% CI: −0.37 to −0.03; P = 0.020), while those containing Lactobacillus improved flatulence (SMD = −1.84, 95% CI: −2.43 to −1.25; P <0.001) and those containing Bifidobacterium improved urgency (SMD = −0.55, 95% CI: −0.85 to −0.26; P <0.001) in several RCTs.27,33,41,43,58 In one trial, a synbiotic intervention incorporating inulin and several probiotic strains significantly improved scores for both flatulence (SMD = −1.84, 95% CI: −2.43 to −1.25; P <0.001) and urgency (SMD = −0.70, 95% CI: −1.21 to −0.20; P = 0.006).45
In general, the use of probiotics did not significantly improve QOL scores, except in two trials involving Lactobacillus strains (SMD = 0.57, 95% CI: 0.21 to 0.94; P = 0.020).28,34 Similarly, most of the prebiotic and synbiotic interventions did not affect QOL, although one prebiotic trial noted improvements following a 12-week regimen of partially-hydrolysed guar gum.50 As for the frequency of adverse events, a pooled risk analysis revealed no significant differences between patients receiving different types of interventions and those receiving a placebo (RR = 1.17, 95% CI: 0.98 to 1.40; P = 0.080; I2 = 0%).
Discussion
While various pharmalogical treatments are available to alleviate the symptoms of IBS, such as tricyclic antidepressants, antispasmodics and selective serotonin reuptake inhibitors, non-pharmalogical options are needed in order to improve efficacy of treatment and mitigate the risk of adverse events.59 Probiotics, prebiotics and synbiotics are potentially promising approaches of altering gut microbiota and alleviating symptoms of functional bowel disorders. The current article presents a systematic review and meta-analysis of recent RCTs evaluating the efficacy and safety of probiotics, prebiotics and synbiotics in the context of IBS.
The findings of the present analysis indicate that probiotics had the most robust effect on improving global IBS symptoms, particularly those containing multi-strains and Bifidobacterium species. Additionally, Lactobacillus-containing probiotic products helped to significantly reduce specific symptoms (i.e. abdominal pain and flatulence) and improve the QOL of patients. Intriguingly, when probiotics were combined with prebiotics in synbiotic formulations, they also exhibited beneficial effects on urgency, abdominal pain and flatulence.
Probiotics have significant effects on the integrity of the GI epithelium, which is maintained via tight junction (TJ) proteins. Zyrek et al. observed that probiotics containing the Escherichia coli strain Nissle 1917 promoted the expression and redistribution of the ZO-2 protein to cellular contact sites in order to ultimately stabilise TJ proteins and preserve cellular morphology.60 Another species, L. plantarum, utilised in various RCTs as a component of single- and multi-strain probiotic formulations, stimulates the expression of important TJ proteins (ZO-1, ZO-2, cingulin and occludin), leading to a remarkable enhancement in intestinal barrier functionality.29–31,34,36,41,45,53 These regulatory mechanisms eventually stabilise GI functions against pathogenic bacteria and limit the development of increased intestinal permeability, a factor likely involved in the pathogenesis of IBS.61
Bowel movement, another relevant target for IBS patients, could also be regulated via probiotic-containing products. In a recent meta-analysis of RCTs involving patients with constipation, Miller et al. found that probiotics containing Lactobacillus or Bifidobacterium species increased stool frequency and reduced intestinal transit time.62 In the present analysis, Lactobacillus-containing products resulted in significant pain reduction, which may have contributed to QOL improvement. Indeed, Lactobacillus species are often reported as beneficial for abdominal pain in functional GI disorders.63,64 Although the exact mechanism of pain in IBS is as yet unclear, it is possible this symptom is mediated via persistent low-grade intestinal inflammation and changes in the quantity of gut microbiota.65
Overall, the act of altering the gut microbiota via the administration of probiotics seems to yield beneficial results for general IBS symptoms. This could be further supported by the use of synbiotics; in the present analysis, this type of intervention resulted in significant improvements in abdominal pain, urgency and flatulence. The synbiotics were primarily composed of the most common probiotic strains (Lactobacillus and Bifidobacterium).40,45 On the other hand, the benefits of prebiotics (e.g. fructooligosaccharides and partially-hydrolysed guar gum) seem to be less apparent.42,50,51 Indeed, the role of prebiotics in alleviating IBS symptoms is controversial since most of them are fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Such compounds, including fructans and fructose, are poorly absorbed in the small intestine and undergo fermentation, exacerbating IBS symptoms.66,67
In terms of methodologies, the RCTs included in the current analysis were of moderate-to-high quality, with the majority employing a double-blind design. Moreover, a rigorous search strategy was used based on several keyword combinations in order to identify the most relevant studies. Minimal limits for sample sizes and methodological considerations were based on previous recommendations so that the findings would be reliable.24 Importantly, despite variations in the version used and the lack of trials utilising the latest criteria (Rome IV), all trials employed a unified diagnostic tool.
Previous systematic reviews and meta-analyses have confirmed the effectiveness and safety of probiotics for IBS patients.66 However, such analyses have failed to provide reliable recommendations regarding specific bacterial strains. In the present analysis, Lactobacillus and Bifidobacterium species resulted in significant benefits; these strains are therefore recommended by the authors. Supporting evidence exists, indicating that alterations in these species have been previously reported in IBS patients.68–70 Moreover, unlike other recently-published meta-analyses on this topic, the current article presents insight into the effect of synbiotics, showing that this type of intervention results in a significant ameliorating effect on IBS symptoms.71,72 In addition, to the best of the authors’ knowledge, this is the first meta-analysis to assess the effects of these formulations on QOL.
Nonetheless, the present analysis was not without limitations. The majority of the RCTs included in the analysis assessed adherence to regimens qualitatively via verbal questioning. In addition, while patients were instructed to maintain their usual dietary patterns, no formal dietary assessment was performed; as such, the confounding effect of nutritional variables was not taken into consideration. Moreover, although it is the authors’ belief that the duration of interventions should be greater than four weeks, 13 trials (39.4%) did not meet this threshold.26,33,36,37,39–41,43–45,51,52,56 Therefore, longer follow-up periods are warranted in future studies. Furthermore, the effects of probiotics on specific subgroups of patients are unclear; for instance, the impact on those with IBS-D is conflicting, while little is known about the outcomes on patients with other IBS subtypes.26–31 Hence, future trials incorporating specific IBS subtypes are recommended.
Another important limitation of the present meta-analysis was the failure to determine the exact sources of heterogeneity between studies. The authors suggest that consistent methodological designs, such as unified symptomatic assessment scores, should be used in future studies on this topic. Moreover, it is vital to highlight the low number of prebiotic studies identified in this review, since this limitation may interfere with the interpretation of pertinent outcomes. Finally, in terms of safety outcomes, it was difficult to determine whether repeatedly-reported side-effects (such as abdominal pain, diarrhoea, nausea, flatulence and heartburn) were due to the interventions or the disease itself because of symptom overlap. Therefore, the exact relationship between the interventions and such symptoms was unclear.
Conclusion
The findings of this systematic review and meta-analysis indicate that probiotics and synbiotics have the potential to alleviate global IBS symptoms. More specifically, products containing Lactobacillus species significantly reduced abdominal pain and flatulence scores and improved QOL, while urgency and other general symptoms were alleviated by Bifidobacterium-containing formulations. Therefore, preparations containing multi-strains of these bacterial species might be beneficial. However, there was significant inter-study heterogeneity, which warrants cautious interpretation of these findings. Future studies on this topic should employ longer follow-up periods, unify symptomatic assessment scores, monitor dietary patterns and clinically assess patient adherence to the interventions.
References
- 1.Koloski NA, Talley NJ. Irritable bowel syndrome. In: Goldman MB, Troisi R, Rexrode KM, editors. Women and Health. 2nd ed. Cambridge, Massachusetts, USA: Academic Press; 2012. pp. 1353–66. [Google Scholar]
- 2.Drossman DA, Richter JE, Talley N, Thompson G, Corazziari E, Whitehead W. The functional gastrointestinal disorders: Diagnosis, pathophysiology and treatment - A multinational consensus. New York, USA: Little, Brown and Co; 1994. [Google Scholar]
- 3.Defrees DN, Bailey J. Irritable bowel syndrome: Epidemiology, pathophysiology, diagnosis, and treatment. Prim Care. 2017;44:655–71. doi: 10.1016/j.pop.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA, Corazziari E, Talley N, Thompson W, Whitehead W, Rome II. The functional gastrointestinal disorders. 2nd ed. McLean, Virginia, USA: Degnon Associates, Inc; 2000. [Google Scholar]
- 6.Drossman DA, Corazziari E, Delvaux M, editors. Rome III: The functional gastrointestinal disorders. 3rd ed. McLean, Virginia, USA: Degnon Associates Inc; 2006. [Google Scholar]
- 7.Drossman DA, Chang L, Chey WD, Kellow J, Tack J, Whitehead WE, editors. Rome IV: Functional gastrointestinal disorders - Disorders of gut-brain interaction. 4th ed. Raleigh, North Carolina, USA: Rome Foundation; 2016. [Google Scholar]
- 8.Kavanagh RG, O’Grady J, Carey BW, O’Connor OJ, Maher MM. Review of the role of abdominal imaging in irritable bowel syndrome. World J Radiol. 2018;10:143–9. doi: 10.4329/wjr.v10.i11.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abd-El Khalek Abd-ALRazek A, Fahmy DM. Diagnostic value of diffusion-weighted imaging and apparent diffusion coefficient in assessment of the activity of Crohn disease: 1.5 or 3 T. J Comput Assist Tomogr. 2018;42:688–96. doi: 10.1097/RCT.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Malley D. Immunomodulation of enteric neural function in irritable bowel syndrome. World Journal of Gastroenterology: WJG. 2015 Jun 28;21(24):7362. doi: 10.3748/wjg.v21.i24.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome—a meta-analysis. American Journal of Gastroenterology. 2006 Aug 1;101(8):1894–9. doi: 10.1111/j.1572-0241.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 12.Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome–A Review on the Pathophysiology, Current Research and Future Therapy. Frontiers in microbiology. 2019;10:1136. doi: 10.3389/fmicb.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringel Y, Ringel-Kulka T. The intestinal microbiota and irritable bowel syndrome. J Clin Gastroenterol. 2015;49:S56–9. doi: 10.1097/mcg.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 14.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giamarellos-Bourboulis EJ, Pyleris E, Barbatzas C, Pistiki A, Pimentel M. Small intestinal bacterial overgrowth is associated with irritable bowel syndrome and is independent of proton pump inhibitor usage. BMC Gastroenterol. 2016;16:67. doi: 10.1186/s12876-016-0484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoshal UC, Shukla R, Ghoshal U, Gwee KA, Ng SC, Quigley EM. The gut microbiota and irritable bowel syndrome: Friend or foe? Int J Inflam. 2012;2012 doi: 10.1155/2012/151085. 151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimura S, Ishimura N, Mikami H, Okimoto E, Uno G, Tamagawa Y, et al. Small intestinal bacterial overgrowth in patients with refractory functional gastrointestinal disorders. J Neurogastroenterol Motil. 2016;22:60–8. doi: 10.5056/jnm15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, Koussoulas V, Barbatzas C, Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: Relationship with irritable bowel syndrome. Dig Dis Sci. 2012;57:1321–9. doi: 10.1007/s10620-012-2033-7. [DOI] [PubMed] [Google Scholar]
- 19.Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017;49:331–7. doi: 10.1016/j.dld.2017.01.142. [DOI] [PubMed] [Google Scholar]
- 20.Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: A systematic review and meta-analysis. Gastroenterology. 2017;152:1042–54.e1. doi: 10.1053/j.gastro.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floch MH. Probiotics and prebiotics. Gastroenterol Hepatol (N Y) 2014;10:680–1. [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics-A review. J Food Sci Technol. 2015;52:7577–87. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazurak N, Broelz E, Storr M, Enck P. Probiotic therapy of the irritable bowel syndrome: Why is the evidence still poor and what can be done about it? J Neurogastroenterol Motil. 2015;21:471–85. doi: 10.5056/jnm15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun YY, Li M, Li YY, Li LX, Zhai WZ, Wang P, et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci Rep. 2018;8:2964. doi: 10.1038/s41598-018-21241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas Z, Yakoob J, Jafri W, Ahmad Z, Azam Z, Usman MW, et al. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: A randomized trial. Eur J Gastroenterol Hepatol. 2014;26:630–9. doi: 10.1097/meg.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 28.Shin SP, Choi YM, Kim WH, Hong SP, Park JM, Kim J, et al. A double blind, placebo-controlled, randomized clinical trial that breast milk derived-Lactobacillus gasseri BNR17 mitigated diarrhea-dominant irritable bowel syndrome. J Clin Biochem Nutr. 2018;62:179–86. doi: 10.3164/jcbn.17-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hod K, Sperber AD, Ron Y, Boaz M, Dickman R, Berliner S, et al. A double-blind, placebo-controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea-predominant IBS. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.13037. [DOI] [PubMed] [Google Scholar]
- 30.Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–7. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 31.Ishaque SM, Khosruzzaman SM, Ahmed DS, Sah MP. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018;18:71. doi: 10.1186/s12876-018-0788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: A multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–86. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 33.Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: Effect on quality of life. J Clin Gastroenterol. 2011;45:679–83. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson C, Blaauw R, Fredericks E, Visser J, Roux S. Randomized clinical trial: Effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition. 2014;30:1151–7. doi: 10.1016/j.nut.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Preston K, Krumian R, Hattner J, de Montigny D, Stewart M, Gaddam S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: A double-blind, randomised, placebo-controlled study. Benef Microbes. 2018;9:697–706. doi: 10.3920/bm2017.0105. [DOI] [PubMed] [Google Scholar]
- 36.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–18. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jafari E, Vahedi H, Merat S, Momtahen S, Riahi A. Therapeutic effects, tolerability and safety of a multi-strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med. 2014;17:466–70. [PubMed] [Google Scholar]
- 38.Rogha M, Esfahani MZ, Zargarzadeh AH. The efficacy of a synbiotic containing Bacillus coagulans in treatment of irritable bowel syndrome: A randomized placebo-controlled trial. Gastroenterol Hepatol Bed Bench. 2014;7:156–63. [PMC free article] [PubMed] [Google Scholar]
- 39.Amirimani B, Nikfam S, Albaji M, Vahedi S, Nasseri-Moghaddam S, Sharafkhah M, et al. Probiotic vs. placebo in irritable bowel syndrome: A randomized controlled trial. Middle East J Dig Dis. 2013;5:98–102. [PMC free article] [PubMed] [Google Scholar]
- 40.Shavakhi A, Minakari M, Farzamnia S, Peykar MS, Taghipour G, Tayebi A, et al. The effects of multi-strain probiotic compound on symptoms and quality-of-life in patients with irritable bowel syndrome: A randomized placebo-controlled trial. Adv Biomed Res. 2014;3:140. doi: 10.4103/2277-9175.135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–8. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 42.Olesen M, Gudmand-Hoyer E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am J Clin Nutr. 2000;72:1570–5. doi: 10.1093/ajcn/72.6.1570. [DOI] [PubMed] [Google Scholar]
- 43.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 44.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–52. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Cappello C, Tremolaterra F, Pascariello A, Ciacci C, Iovino P. A randomised clinical trial (RCT) of a symbiotic mixture in patients with irritable bowel syndrome (IBS): Effects on symptoms, colonic transit and quality of life. Int J Colorectal Dis. 2013;28:349–58. doi: 10.1007/s00384-012-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, et al. Clinical trial: A multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 47.Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, et al. Clinical trial: The effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - A randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–27. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 48.Søndergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: A randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–72. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 49.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, et al. Clinical trial: Multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 50.Niv E, Halak A, Tiommny E, Yanai H, Strul H, Naftali T, et al. Randomized clinical study: Partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr Metab (Lond) 2016;13:10. doi: 10.1186/s12986-016-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azpiroz F, Dubray C, Bernalier-Donadille A, Cardot JM, Accarino A, Serra J, et al. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.12911. [DOI] [PubMed] [Google Scholar]
- 52.Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome--A 12 week double-blind study. Aliment Pharmacol Ther. 2014;40:51–62. doi: 10.1111/apt.12787. [DOI] [PubMed] [Google Scholar]
- 54.Begtrup LM, de Muckadell OB, Kjeldsen J, Christensen RD, Jarbøl DE. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--A randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48:1127–35. doi: 10.3109/00365521.2013.825314. [DOI] [PubMed] [Google Scholar]
- 55.Lyra A, Hillilä M, Huttunen T, Männikkö S, Taalikka M, Tennilä J, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22:10631–42. doi: 10.3748/wjg.v22.i48.10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--A double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–32. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 57.Pineton de Chambrun G, Neut C, Chau A, Cazaubiel M, Pelerin F, Justen P, et al. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig Liver Dis. 2015;47:119–24. doi: 10.1016/j.dld.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Spiller R, Pélerin F, Cayzeele Decherf A, Maudet C, Housez B, Cazaubiel M, et al. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United European Gastroenterol J. 2016;4:353–62. doi: 10.1177/2050640615602571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munjal A, Dedania B, Cash B. Update on pharmacotherapy for irritable bowel syndrome. Curr Gastroenterol Rep. 2019;21:25. doi: 10.1007/s11894-019-0692-7. [DOI] [PubMed] [Google Scholar]
- 60.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–16. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 61.Slyepchenko A, Maes M, Jacka FN, Köhler CA, Barichello T, McIntyre RS, et al. Gut microbiota, bacterial translocation, and interactions with diet: Pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother Psychosom. 2017;86:31–46. doi: 10.1159/000448957. [DOI] [PubMed] [Google Scholar]
- 62.Miller LE, Ouwehand AC, Ibarra A. Effects of probioticcontaining products on stool frequency and intestinal transit in constipated adults: Systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol. 2017;30:629–39. doi: 10.20524/aog.2017.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33:1302–10. doi: 10.1111/j.1365-2036.2011.04665.x. [DOI] [PubMed] [Google Scholar]
- 64.Jadrešin O, Hojsak I, Mišak Z, Kekez AJ, Trbojević T, Ivković L, et al. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children: RCT study. J Pediatr Gastroenterol Nutr. 2017;64:925–9. doi: 10.1097/mpg.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 65.Chichlowski M, Rudolph C. Visceral pain and gastrointestinal microbiome. J Neurogastroenterol Motil. 2015;21:172–81. doi: 10.5056/jnm15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ooi SL, Correa D, Pak SC. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome - What is the current evidence? Complement Ther Med. 2019;43:73–80. doi: 10.1016/j.ctim.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Yan YL, Hu Y, Gänzle MG. Prebiotics, FODMAPs and dietary fiber — Conflicting concepts in development of functional food products? Curr Opin Food Sci. 2018;20:30–7. doi: 10.1016/j.cofs.2018.02.009. [DOI] [Google Scholar]
- 68.Lacy BE. Hot topics in primary care: Role of the microbiome in disease - Implications for treatment of irritable bowel syndrome. J Fam Pract. 2017;66:S40–5. [PubMed] [Google Scholar]
- 69.Principi N, Cozzali R, Farinelli E, Brusaferro A, Esposito S. Gut dysbiosis and irritable bowel syndrome: The potential role of probiotics. J Infect. 2018;76:111–20. doi: 10.1016/j.jinf.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 70.Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7:F1000. doi: 10.12688/f1000research.14592.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–61. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 72.Ford AC, Harris LA, Lacy BE, Quigley EM, Moayyedi P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044–60. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]