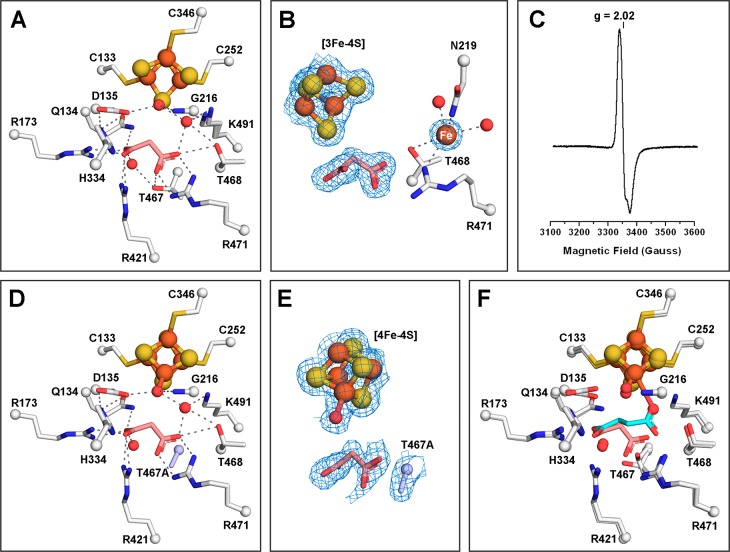
Figure 4.
Malonate binds to the active site of LmFH-2 but not to the Fe–S cluster. (A) LmFH-2 in a complex with malonate (salmon). This structure contains an inactive [3Fe-4S] cluster. (B) Snapshot of the Fe–S cluster destruction by oxidation. LmFH-2 with malonate was exposed to molecular oxygen in the anaerobic chamber, which oxidizes the [4Fe-4S]2+ cluster to [3Fe-4S]+ and releases Fe (orange sphere; labeled in white). The 2Fo – Fc electron density map (blue mesh) is contoured at 1.5 RMSD for malonate (salmon), [3Fe-4S] cluster, and Fe. (C) EPR spectrum of oxygen-exposed LmFH-2 showing a signal characteristic of a [3Fe-4S]+ cluster with a g value of 2.02. (D) LmFH-2-T467A in a complex with malonate (salmon). This structure contains an active [4Fe-4S] cluster. The T467A substitution is colored light blue. (E) The 2Fo – Fc electron density map contoured at 1.5 RMSD (blue mesh) for malonate (salmon), [4Fe-4S] cluster, water molecule, and T467A substitution (light blue). (F) Superposition of LmFH-2-T467A in a complex with malonate (salmon) and LmFH-2 in a complex with succinate (cyan). The [3Fe-4S] cluster and [4Fe-4S] cluster are shown as orange (Fe) and yellow (S) spheres. The water molecules are shown as red spheres. The hydrogen bonds are shown as gray dashed lines.