Abstract
Developments in genetic engineering have allowed researchers and clinicians to begin harnessing viruses to target and kill cancer cells, either through direct lysis or through recruitment of antiviral immune responses. Two powerful viruses in the fight against cancer are the single-stranded RNA viruses vesicular stomatitis virus and Zika virus. Here, we describe methods to propagate and titer these two viruses. We also describe a simple cell-killing assay to begin testing modified viruses for increased potential killing of glioblastoma cells.
Keywords: Oncolytic virus therapy, Vesicular stomatitis virus, Zika virus, Glioblastoma, Immunotherapeutics, Viral titration, Plaque assay, Cell killing
1. Introduction
Oncolytic virus therapy (OVT) is one of the latest additions to the arsenal of immuno-oncology [1]. OVT takes advantage of the ability of viruses to specifically kill tumor cells while leaving the normal cells unharmed. While the idea of using a virus to kill tumor cells or enhance antitumor immunity is not entirely new, most of the path-breaking research has been conducted only in the past two decades owing to significant development in gene transfer technology. Multiple viruses spanning all of the Baltimore classification groups—type I (dsDNA—Herpes simplex virus, adenovirus, vaccinia virus), type II (ssDNA—parvovirus, chicken anemia virus), type III (dsRNA—reovirus), types IV/V (ssRNA—vesicular stomatitis, coxsackie, measles, Newcastle disease, Zika, Seneca Valley)—have been tried, with or without genetic modifications, for stalling the progression of different cancers (melanoma, breast, head, neck, prostate, urothelial, and glioblastoma) [2–10]. One major breakthrough in the clinical use of OVT occurred in 2006 when the State Food and Drug Administration of the People’s Republic of China approved the commercial use of Oncorine™/ H101 (a modified adenovirus developed by Shanghai Sunway Biotech) for treatment of head and neck cancers [11, 12]. Almost a decade later, herpes simplex virus (Talimogene Laherparepvec (T-VEC)/IMLϒGIC™) engineered to upregulate the production of granulocyte-macrophage colony stimulating factor was approved by the FDA for melanoma treatment in the United States. In a follow-up study, T-VEC increased the efficacy of an immune checkpoint (CTLA4) inhibitor, Ipilimumab, when used in combination in patients with advanced, unresectable melanomas [13, 14].
In this review, we shall focus on two single-strand RNA viruses, a (−) sense, Baltimore group V virus, vesicular stomatitis virus (VSV), and a (+) sense, group IV virus, Zika virus (ZIKV), where (−) and (+) refer to the RNA strand encoded by the virus. The genome of a (+) ssRNA virus can be loaded directly onto the ribosome, whereas a (−) ssRNA virus first needs its RNA to be converted into the (+) strand before translation. Both have been used for the treatment of multiple types of cancer, including glioblastoma.
The enveloped virus VSV (Rhabdoviridae family/Vesiculovirus genus) has emerged as one of the most favorable candidates for OVT primarily due to its low pathogenicity in humans, its natural hosts being horses, cattle, and pigs [15]. VSV was identified as an oncolytic virus in the early 2000s when it was found to selectively kill interferon nonresponsive human tumor cells [16]. Researchers demonstrated that this virus was cytotoxic against human melanoma xenografts implanted in mice. Since then, replication-competent strains of VSV have been tested for their efficiency in stopping the progression of numerous tumors—one of its early successes was against Multifocal Glioma and Metastatic Carcinoma in the brain [6, 7]. Studies on the mechanisms behind the antitumor properties of VSV have found that the key processes are the induction of apoptosis (mostly by the viral matrix protein) and a significant reduction in the amount of blood flow (hypoxia) to the tumor cells [17–20].
In addition to the native virus, a wide range of recombinant VSVs that express genes encoding cytokines to stimulate the immune system or encoding proteins that are cytotoxic to the tumor, like thymidine kinase or TP53, are also being tested for potential therapeutic value [6]. A common approach is to engineer a virus overexpressing a proinflammatory cytokine. A recent study using a recombinant VSV expressing interferon-γ shows high potential in the 4T1 mammary adenocarcinoma model [21]. This virus slowed tumor growth in an immune system-dependent manner. Another group reported that VSV engineered to express interferon-β and the sodium iodide symporter (NIS) was nonpathogenic and nontransmissible in a natural VSV host (pig) [8]. This work allays concerns about the potential transmission of VSV OVT from patients to the natural host and raises the possibility that it might be safe within humans as well.
Although identified in the middle of the last century, the neurotropic Zika virus (ZIKV) (Flaviviridae family) garnered widespread attention only in 2007, during an epidemic in the Yap Islands (Micronesia) [22, 23]. Almost a decade later, it was linked to debilitating diseases like Guillain–Barré syndrome and acute disseminated encephalomyelitis (ADEM) in adults. One of the very few flaviviruses capable of vertical transmission (mother-to-child), it is also responsible for microcephaly in infants [24, 25]. Ironically, the ability of ZIKV to trigger apoptotic cell death in neural progenitor cells (NPC)—a possible mechanism of microcephaly—is being used to harness this virus for OV therapies in glioblastoma (GBM) [3–5, 9, 26, 27]. Unlike other flaviviruses such as West Nile virus (WNV), ZIKV triggered apoptosis specifically in glioblastoma stem cells (GSCs) but had minimal effect on differentiated glioma cells (DGC) both in vitro and in brain organoids. Furthermore, intratumoral injection with a ZIKV-Dakar, mouse-adapted strain halted the progression of implanted GBMs in mice [4, 5, 27]. In an attempt to advance this finding, a live attenuated ZIKV vaccine candidate (ZIKV-LAV) was tested for its efficacy against GBMs in mice [9]. While exhibiting a marked decrease in neurotoxicity when compared to a licensed vaccine for another flavivirus, Japanese Encephalitis virus, ZIKV-LAV still effectively halted growth of GBMs. Encouraging findings for ZIKV have been reported for other brain tumors as well, including a recent study showing effectiveness against neuroblastoma [3]. This publication presented data that ZIKV is more likely to bind to neuroblastoma cells expressing the cell surface glycoprotein CD24, as poorly permissive cells lacked CD24 and were less prone to ZIKV-mediated cytopathic effects. In summary, these two viruses hold immense potential for OVT and more studies need to be dedicated to understanding the inherent mechanism.
2. Materials
2.1. VSV Propagation
BHK-21 (ATCC# CCL-10) (see Note 1).
BHK-21 growth medium: Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin solution.
10-cm tissue culture plates.
15-ml conical Falcon tubes.
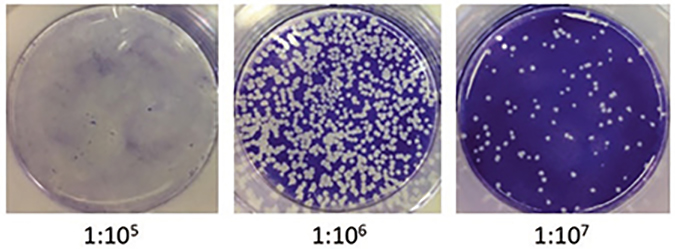
2.2. VSV Titration by Plaque Assay (Fig. 1)
Fig. 1.
Plaque assay of VSV-infected BHK-21. BHK-21 cells were plated in a 6-well plate and left to attach overnight. The following day, serial dilutions of virus were prepared (10−5 through 10−10) and 100 μl added to the monolayer of BHK cells. After 30′ adsorption, the medium was swapped out for methylcellulose and let to solidify. The plates were then incubated at 37 °C for about 2 days. The overlay was then removed, and the cells were stained with 1% crystal violet for 20 min. The number of plaques was then counted after gently washing off the excess stain. The total number of plaque-forming units (PFU) was determined by dividing the average number of plaques by the dilution factor times the volume added
6-Well plates.
2× DMEM.
FBS.
Methylcellulose.
Test tubes.
1× PBS.
Crystal violet solution.
2.3. ZIKV Propagation
C6/36 (ATCC# CRL1660) (see Note 2).
VERO (ATCC# CCL81) (see Note 3).
T-75 flask.
100× calcium–magnesium mix: dissolve 1.327 g CaCl2 2H2O and 2.133 g MgCl2 6H2O in 100 ml of water and autoclave.
Flavivirus dilution media. In a sterile tissue culture hood, mix 437-ml autoclaved dH2O, 50-ml autoclaved 1× PBS, 3 ml of filter-sterilized 35% BSA, 5 ml of autoclaved 1× calcium–magnesium mix, and 5 ml of 100× penicillin–streptomycin solution.
Viral propagation growth media. EMEM with 2-mM L-glutamine, 1-mM sodium pyruvate, 1.5-g/L sodium bicarbonate, 2% FBS, and penicillin–streptomycin.
15- and 50-ml Falcon tubes.
Cryovials.
Dry ice.
Ethanol.
100-kDA cutoff Amicon filter tube.
2.4. ZIKV Titration by Plaque Assay
VERO (ATCC #CCL81).
12-Well plate.
RPMI 1640.
Agarose overlay: 50% low-melting point agarose, 45% 2× MEM, 5% FBS.
Ethanol.
Crystal violet solution.
2.5. Cell Killing Assay for Oncolytic Viruses
U-87 MG (ATCC HTB-14) (see Note 4).
Flat-well bottom 96-well plate.
DMEM.
1× PBS.
FBS.
XTT assay kit.
Absorbance plate reader.
3. Methods
3.1. VSV Propagation
The day before infection split confluent BHK cells 1:10 onto sufficient numbers of 10-cm plates. Cells should be at 30–40% confluency at the time of infection.
On the day of infection inoculate cells at a multiplicity of infection (MOI) of 0.005 (see Note 5).
Incubate until 80–90% cell lysis (~48 h). Harvest supernatant, clarify by low-speed centrifugation (~400 × g), and store at −80 °C.
3.2. VSV Titration by Plaque Assay
The day prior to performing a titration, prepare one 6-well plate per virus preparation to be titrated. Split confluent BHK cells 1:10 to yield wells at 30–40% confluency the next day.
Make 2% methylcellulose by heating ~200 ml of dH2O to 80 °C and then adding 10-g methylcellulose slowly while stirring. Bring the volume up to 500 ml with ice-cold dH2O, continuing to stir. Chill entire mixture to 4 °C with stirring and continue to incubate for about 30 min until all reagent has dissolved. Filter sterilize through a 0.45-μm filter and then place into the freezer to eliminate any bubbles. Store long term at 4 °C.
Mix 12 ml per plate of equal parts 2% methylcellulose with 2× DMEM + 10% FBS.
On the day of the experiment, prewarm the 1% methylcellulose + DMEM and 5% FBS to 37 °C.
For each virus to be tittered, set out nine test tubes in the hood and label them 10−2 through 10−10. Add 0.9 ml of DMEM without serum to each.
Add 100 μl of virus stock to the first tube labeled 10−2. Vortex briefly and transfer 100 μl to the second tube with a fresh tip. Repeat with the remainder of the tubes.
Wash cells in 6-well plates with 1× PBS and add 0.5 ml of serum-free DMEM to each well.
Add 100 μl of the 10−5 dilution to the first well. The second well receives 100 μl of the 10−6 dilution, and so on through the 10−10 dilution.
Incubate the plates at 37 °C for 30 min, rocking every 10 min.
Aspirate the media carefully from each well and then add back 2 ml of the prewarmed 1% methylcellulose solution (from step 4).
Incubate plates at 37 °C for 48 h (see Note 6).
Aspirate media from all wells and carefully add 1% crystal violet stain and incubate at room temperature for 20 min.
Wash away excess stain by carefully immersing plate multiple times in a beaker containing room temperature dH2O.
- The resulting titer can be calculated from the well containing between 10 and 100 plaques using the formula:
3.3. ZIKV Propagation
A day before the scheduled ZIKV infection, plate approximately 106 C6/36 or Vero cells in a T-75 flask (see Note 7).
After overnight incubation, remove growth medium and wash cells with flavivirus dilution media (see Note 8).
Dilute stock virus in 2 ml (see Note 9) of Flavivirus Dilution Media to an appropriate MOI and add this solution to the cells. While MOIs greater than 1 are preferred, we have used MOIs as low as 0.01 successfully.
Incubate the cells with the virus dilution for roughly 2 h at 28 °C (C6/36) or 37 °C (VERO) with constant shaking. If a shaker is not available, swirl the flasks every 15 min (see Note 10).
After the adsorption phase, add 10 ml viral propagation growth medium on top of the initial inoculum and keep the cells in the incubator for 2–4 days, until signs of cytopathic effects are visible (see Note 11).
Collect the supernatant from the flasks and transfer to a Falcon tube. For subsequent steps, solutions should be chilled or kept on ice.
Wash the attached cells with the growth medium in order to collect any viral particles still loosely bound to the surface of the host cells.
Combine the solutions from steps 6 and 7 and spin at 200 × g for 5 min (at 4 °C).
OPTIONAL: Spin this solution at 1200 × g for 10 min (4 °C). After the spin, transfer the supernatant to a fresh Falcon tube and store on ice. Transfer 15 ml from this solution to a 100-kDA cutoff Amicon filter tube. Spin these tubes at 1200 × g for 45 min at 4 °C. After the spin, the supernatant left on top of the filter contains the concentrated virus. Use a P-200 pipette to transfer the concentrate to a fresh cryovial/microcentrifuge. Discard the flow-through. Repeat these steps until all supernatant has been concentrated—each filter can be used for two spins (see Note 12).
After the spin, discard the pellet of dead cells and other debris and aliquot the cleared supernatant into sterile cryovial tubes.
Quick freeze the aliquots of virus stock in dry ice/ethanol (95% ethanol chilled on dry ice).
After the quick freeze step, store these aliquots in −80 °C freezer.
3.4. ZIKV Titration by Plaque Assay
Plate 3 × 105 Vero cells into each well of a 12-well plate and allow attachment overnight (see Note 13).
Prepare tenfold serial dilutions (usually 102–106) of the viral stock in RPMI 1640 without supplements.
Add ~250 μl of each dilution, as well as the undiluted stock, to the corresponding wells.
Incubate the plates at 37 °C for about 2 h. Make sure to swirl the plates every 15 min.
After the adsorption, cover the cells with agarose overlay (see Note 14).
Let the overlay solidify inside the biosafety cabinet and incubate them at 37 °C for 3–4 days.
After 3–4 days, take out the plates and gently remove the overlay by adding a few drops of warm tap water or 1× PBS.
Add 1% crystal violet solution (in 30% ethanol) to the cells and leave them for about 20 min at room temperature.
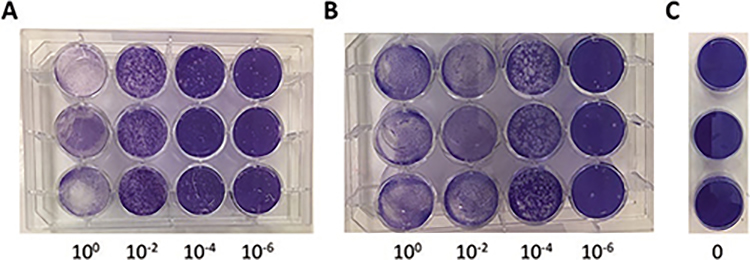
Gently wash the wells with tap water and count plaque formation (as shown in Fig. 2)
Fig. 2.
Plaque assay of ZIKV-infected Vero cells. Vero cells were plated in a 12-well plate and left to attach overnight. The following day, serial dilutions of virus were prepared (10−2, 10−4, 10−6) and 250 μl added to the monolayer of Vero cells (a, b). The increased number of plaques seen in b compared to a, indicates a higher viral titer. As a control, media without virus was added to Vero (c). After 2-h adsorption, agarose overlay medium was added and let to solidify. The plates were then incubated at 37 °C for about 3–4 days. The overlay was then removed, and the cells were stained with 1% crystal violet for about 20 min. The number of plaques was then counted after gently washing off the excess stain. The total number of plaque-forming units (PFU) was determined by dividing the average number of plaques by the dilution factor times the volume added. For example, if there were an average of 20 plaques in the 10−6 wells, the virus titer would be: 20/10−6 * 0.25 ml = 8 * 1 07 PFU/ml
3.5. Cell Killing Assay for Oncolytic Viruses
The day prior to infection, U-87 MG cells are plated at 3 × 104 cells per well of a flat-well bottom 96-well plate (cells should be ~80% confluent at the time of infection) and allowed to adhere overnight. Prepare a total of 12 wells per virus to be tested (six concentrations in duplicate).
The day of infection, prepare a master plate of virus dilutions. Add 100 μl of serum-free DMEM to each well of a 96-well plate, 12 wells per virus to be measured. The typical setup has four different viruses along the short axis diluted in duplicate. Wells in column 1 get no virus and act as the negative control. Wells in column 2 get 0.1 pfu/ml, column 3 get 1 pfu/ml, column 4 get 2.5 pfu/ml, column 5 get 5 pfu/ml, and column 6 get 10 pfu/ml. This can be repeated for the other half of the plate with additional viruses depending on the number needed.
Wash the plate containing the cells with 1× PBS and then add 100 μl of the virus dilution to the corresponding wells and incubate for 1 h.
At the end of the incubation, remove the virus and add 100 μl of DMEM + 2% FBS and then incubate another 72 h.
To determine cell viability, add XTT at 0.5 mg/ml according to the manufacturer’s directions and incubate for 5 h.
Measure optical density at 450 nm. Uninfected cells are set to 100% viability.
Footnotes
These fibroblasts derived from the kidney of a Syrian golden hamster (Mesocricetus auratus) are used for both growth and tittering of VSV. Cells are incubated at 37 °C in 5% CO2. Cultures are typically split two times per week at 1:8.
As the mosquito is the secondary host/vector for arbovirus, these Aedes albopictus clones are widely used for the propagation of ZIKV. Cells are maintained in RPMI 1640 media containing 10% FBS, 1% penicillin–streptomycin solution, 1% nonessential amino acids, 1% sodium pyruvate, 1% L-glutamine, and 3% NaHCO3. As an alternative to RPMI 1640, Eagle’s Minimum Essential Medium (EMEM) can be used as the base media. Cells are incubated at 28 °C in 5% CO2. Cells are typically split twice per week at 1:8.
These epithelial cells isolated from the kidney of an adult African green monkey (Cercopithecus aethiops) are used for the titration of ZIKV. Cells are maintained in EMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin solution. Cells are incubated at 37 °C in 5% CO2. Cultures are typically split two times per week at 1:8. In most cases, VERO has also substituted C6/36 as the preferred host cell for growing ZIKV thus circumventing the issue of slower growth rate which is usually observed in C6/36.
These epithelial cells were isolated from a human brain tumor, most likely a glioblastoma. They provide a useful target for determination of the killing potential of both VSV and ZIKA. Cells are maintained in EMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin solution. Cells are incubated at 37 °C in 5% CO2. Cultures are typically split three times per week at 1:3.
The MOI of infection is calculated by dividing the pfu/ml by the number of cells in the culture. For a typical 10-cm plate of BHK at time of infection, there are approximately 3.5 * 106 cells. So, 0.005 MOI = X pfu/ml/3.5 * 106. X = 17,500 pfu/ml. For 30 ml of medium, add a total of 5.25 * 105 pfu from your stock.
Be careful not to disturb plates during this time or the plaques will become fuzzy and difficult to count.
This is to ensure that the host cell is 70–80% confluent at the time of infection. While C6/36 cells give a higher viral yield, they grow very slowly compared to Vero cells.
This solution facilitates the binding of the viral particles to the host cells.
The volume of flavivirus dilution media should be kept as minimal as possible, in order to avoid dilution of the viral inoculum. The surface area of a T-75 flask is just covered by 2 ml.
Shaking during the adsorption step ensures that the virus inoculum contacts as many cells as possible.
The reduced serum ensures a slow rate of cell proliferation and a more efficient binding of the virus. The former also keeps the virus from being stressed due to overconfluence of host cells. It is advisable to stop the incubation and collect the virus when ~30% host cells are dead.
For selected strains where the titer is less than 105, additional purification and concentration of the viral stocks might be necessary. This is especially relevant when VERO is used instead of C6/36, to grow the virus.
Dispense the cells at the center of the well and gently rock the plate to ensure that the cells form a dispersed monolayer.
As an alternative, 1% carboxymethylcellulose (CMC)/2.5% α-MEM may be used instead of the agarose medium. Both of these have been used equivalently in the literature.
References
- 1.Kamta J, Chaar M, Ande A, Altomare DA, Ait-Oudhia S (2017) Advancing cancer therapy with present and emerging immuno-oncology approaches. Front Oncol 7:64 10.3389/fonc.2017.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fountzilas C, Patel S, Mahalingam D (2017) Review: Oncolytic virotherapy, updates and future directions. Oncotarget 8(60):102617–102639. 10.18632/oncotarget.18309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazar J, Li Y, Rosado A, Phelan P, Kedarinath K, Parks GD, Alexander KA, Westmoreland TJ (2018) Zika virus as an oncolytic treatment of human neuroblastoma cells requires CD24. PLoS One 13(7):e0200358 10.1371/journal.pone.0200358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood H (2017) Neuro-oncology: a new role for Zika virus in glioblastoma therapy? Nat Rev Neurol 13(11):640–641. 10.1038/nrneurol.2017.138 [DOI] [PubMed] [Google Scholar]
- 5.Zhu Z, Gorman MJ, McKenzie LD, Chai JN, Hubert CG, Prager BC, Fernandez E, Richner JM, Zhang R, Shan C, Tycksen E, Wang X, Shi PY, Diamond MS, Rich JN, Chheda MG (2017) Zika virus has oncolytic activity against glioblastoma stem cells. J Exp Med 214(10):2843–2857. 10.1084/jem.20171093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishnoi S, Tiwari R, Gupta S, Byrareddy SN, Nayak D (2018) Oncotargeting by vesicular stomatitis virus (VSV): advances in cancer therapy. Viruses 10(2). 10.3390/v10020090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN (2008) Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci 28(8):1882–1893. 10.1523/JNEUROSCI.4905-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velazquez-Salinas L, Naik S, Pauszek SJ, Peng KW, Russell SJ, Rodriguez LL (2017) Oncolytic recombinant vesicular stomatitis virus (VSV) is nonpathogenic and nontransmissible in pigs, a natural host of VSV. Hum Gene Ther Clin Dev 28(2):108–115. 10.1089/humc.2017.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Wu J, Ye Q, Ma F, Zhu Q, Wu Y, Shan C, Xie X, Li D, Zhan X, Li C, Li XF, Qin X, Zhao T, Wu H, Shi PY, Man J, Qin CF (2018) Treatment of human glioblastoma with a live attenuated Zika virus vaccine candidate. MBio 9(5):e01683–e01618. 10.1128/mBio.01683-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baltimore D (1971) Expression of animal virus genomes. Bacteriol Rev 35(3):235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber K (2006) China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst 98(5):298–300. 10.1093/jnci/djj111 [DOI] [PubMed] [Google Scholar]
- 12.Liang M (2018) Oncorine, the world first oncolytic virus medicine and its update in China. Curr Cancer Drug Targets 18(2):171–176. 10.2174/1568009618666171129221503 [DOI] [PubMed] [Google Scholar]
- 13.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS (2015) Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33(25):2780–2788. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 14.Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, Logan TF, Hauschild A, Lebbe C, Chen L, Kim JJ, Gansert J, Andtbacka RHI, Kaufman HL (2018) Randomized, open-label phase II study evaluating the efficacy and safety of Talimogene Laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol 36(17):1658–1667. 10.1200/JCO.2017.73.7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betancourt D, Ramos JC, Barber GN (2015) Retargeting oncolytic vesicular stomatitis virus to human T-cell lymphotropic virus type 1-associated adult T-cell leukemia. J Virol 89(23):11786–11800. 10.1128/JVI.01356-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC (2000) Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 6(7):821–825. 10.1038/77558 [DOI] [PubMed] [Google Scholar]
- 17.Koyama AH (1995) Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res 37(3):285–290 [DOI] [PubMed] [Google Scholar]
- 18.Gaddy DF, Lyles DS (2005) Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol 79(7):4170–4179. 10.1128/JVI.79.7.4170-4179.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schache P, Gurlevik E, Struver N, Woller N, Malek N, Zender L, Manns M, Wirth T, Kuhnel F, Kubicka S (2009) VSV virotherapy improves chemotherapy by triggering apoptosis due to proteasomal degradation of Mcl-1. Gene Ther 16(7):849–861. 10.1038/gt.2009.39 [DOI] [PubMed] [Google Scholar]
- 20.Qi X, Du L, Chen X, Chen L, Yi T, Chen X, Wen Y, Wei Y, Zhao X (2016) VEGF-D-enhanced lymph node metastasis of ovarian cancer is reversed by vesicular stomatitis virus matrix protein. Int J Oncol 49(1):123–132. 10.3892/ijo.2016.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourgeois-Daigneault MC, Roy DG, Falls T, Twumasi-Boateng K, St-Germain LE, Marguerie M, Garcia V, Selman M, Jennings VA, Pettigrew J, Amos S, Diallo JS, Nelson B, Bell JC (2016) Oncolytic vesicular stomatitis virus expressing interferon-gamma has enhanced therapeutic activity. Mol Ther Oncolytics 3:16001 10.1038/mto.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dick GW (1952) Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 46(5):521–534 [DOI] [PubMed] [Google Scholar]
- 23.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB (2009) Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360(24):2536–2543. 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 24.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP (2016) Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 387(10033):2125–2132. 10.1016/S0140-6736(16)00651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR (2016) Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 374(20):1981–1987. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 26.Souza BS, Sampaio GL, Pereira CS, Campos GS, Sardi SI, Freitas LA, Figueira CP, Paredes BD, Nonaka CK, Azevedo CM, Rocha VP, Bandeira AC, Mendez-Otero R, Dos Santos RR, Soares MB (2016) Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci Rep 6:39775 10.1038/srep39775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubin JA, Zhang RR, Kuo JS (2018) Zika virus has oncolytic activity against glioblastoma stem cells. Neurosurgery 82(5):E113–E114. 10.1093/neuros/nyy047 [DOI] [PMC free article] [PubMed] [Google Scholar]