Abstract
This study analyses the changes of moderate-to-vigorous physical activity (MVPA) in a cohort of boys and girls aged 11 (n = 50) and 14 (n = 50). Physical activity was assessed with Bodymedia SenseWear Pro Armband monitor for 6 days in October 2013 and October 2016, considering 90% of daily wear time (21h and 40min). The initial sample (n = 160) included the children who wore the monitors at age 11 but the final analyzed sample included only those children from the initial sample (n = 50), whose data fulfilled the inclusion criteria at age 11 and 14. Physical fitness and somatic characteristics of the final sample (n = 50) were compared to a representative sample of Slovenian schoolchildren at ages 11 (n = 385) and 14 (n = 236) to detect possible bias. Changes in MVPA were controlled for maturity using the timing of adolescent growth spurt as its indicator. The average MVPA decreased more than one quarter (34.96 min) from age 11 to age 14. Children were significantly more active at age 11 than at age 14 (p < 0.01, d = 0.39). The timing of puberty onset in girls was significantly earlier (12.01 ± 1.0 years) (p < 0.01) than in boys (13.2 ± 0.75 years) (p < 0.01, d = 1.35). There was a significant gender difference in moderate-to vigorous physical activity at age 14 (p < 0.05, η2 = 0.12) and between moderate-to vigorous physical activity at age 11 and 14 (η2 = 0.11). After controlling for the timing of adolescent growth spurt the girls at age 11 showed significantly higher level of physical activity than at age 14 (p < 0.01, η2 = 0.17). Early adolescence is crucial for the development of physical activity behaviours, which is especially pronounced in girls. The significant decline of MVPA between ages 11 and 14 in Slovenia are likely influenced by environmental changes since the timing of adolescent growth spurt did not prove as a factor underlying the decline of MVPA.
Introduction
Physical activity (PA) is one of the key health determinants in life [1] and is one of the sources of total energy expenditure, which incorporates active energy expenditure, metabolism in resting state, the thermal effects of food digestion and body growth in children and adolescents [2]. Existing evidence shows that PA has positive effects on psychosocial health, and the functional capacity and wellbeing of people [3], whereas physical inactivity increases health risks [4,5]. Research findings indicate that PA in school-age, and moderate-to-vigorous physical activity (MVPA) in particular, positively influence the public health of the adult population, which resulted in the development of recommendations for policymakers to increase PA of the European population [6]. The first Slovenian PA guidelines for children are based on the World Health Organization (WHO) recommendations of at least 60 minutes of MVPA daily [7]. In order to reach the recommendations, children should undertake various types of PA (regular physical education (PE) in school, organized sport practice, active play, active transportation etc.). The guidelines also define the structure of suitable PA (e.g. warm-up, gradual effort, cool-down, and relaxation), as well as suggest appropriate types (exercises improving aerobic and muscular fitness) and frequency of exercise for children and youth [7,8]. The most elaborated national PA guidelines and WHO recommendations are suggesting for PA volume to decrease with age, which is rather unusual since environmental demands for sedentariness actually increase with age from childhood to adolescence and adulthood [9,10].
In both genders, MVPA levels decrease with age [11], especially during adolescence [12]. Most of the existing studies has found the decline of MVPA in children and adolescents [9,10,13–19], but did not take into account the differences in maturity. The decrease of MVPA may extend into adulthood [12,20,21] and seems to be more associated with biological age than with chronological age [22]. In this regard, we aimed to analyse the changes in habitual PA in a group of children in predominantly pre-adolescent period (at age 11) and predominantly early adolescent period (at age 14) also in relation to maturity. Children and adolescents from different countries experience diverse rhythms of everyday life due to different educational systems, social settings and cultural differences [23]. Considering this, it is important to analyse each national setting separately and adjust national recommendations to the actual needs and possibilities of local populations as well as to the most intensive periods of PA behavioural change [13,24–26], which is also reflected in behavioural patterns of PA in adulthood [27–29].
In addition to the described issues with PA recommendations, also the assessment of PA continues to present a huge research challenge due to multiple research approaches and tools that very often produce incomparable data. In the past, PA of Slovenian children and youth has been assessed predominantly with the use of questionnaires [30–33], but in recent years the development of various wearables [34–38] has enabled the utilization of more objective methods for estimation of PA. Wearable monitors, such as pedometers, load transducers, accelerometers, heart rate (HR) monitors, combined accelerometer/HR monitors and multiple sensor systems [34–36,39], can provide more accurate assessment of mechanical and physiological parameters of PA compared to self-report tools [35]. The most advanced generation of wearable monitors are multisensory devices such as SenseWear Armband Pro (SWA, BodyMedia, Inc., Pittsburgh, PA), used in this study, which provides estimation of EE based on biaxial accelerometer, galvanic skin response and body heat loss [39,40]. SWA has been validated in children [41–43] and adults [44] as well of different intensities of PA [45–47].
Previous studies and comparisons on objectively and subjectively assessed data showed that Slovenian school children and adolescents (6–17 y) are among the most physically active in the world [48–52], with more than 80% reaching the 60 minutes of daily MVPA [49,50]. However, most of the comparisons focused on the preadolescent children [49,50,53,54]. The purpose of this longitudinal study is to analyse the changes in the amount of PA of boys and girls in the early stages of adolescence based on objectively assessed PA data. The monitored period between ages 11 and 14 is characterised by the onset of puberty and intensive somatic development and growth, resulting in increased developmental and behavioural heterogeneity among children within the same chronological age group [12,55]. Namely, differences in PA between pre-adolescent girls and boys can be a result of girls’ typical earlier onset of puberty [56], while at the same time the individual differences in the onset of puberty within boys and girls can very often exceed the mentioned sex differences. We hypothesised that MVPA decreases from age 11 to 14 in boys and girls, that decrease is more pronounced in girls and that maturity negatively affects this decrease.
Methods
Sample
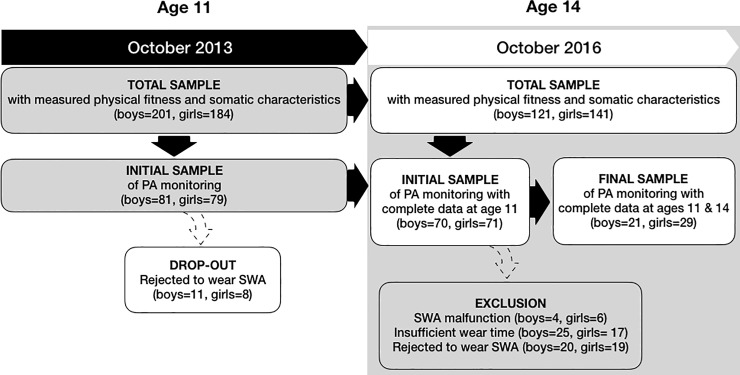
Data for the present study were acquired from a longitudinal cross-sectional study The Analysis of Children’s Development in Slovenia (ACDSi) which has been monitoring physical and motor development of Slovenian children and youth [57,58] and was being carried out every 10 years in the last five decades. The study uses a sentinel approach to provide a nationally representative sample of children from 11 different locations, stratified according to the type of environment (rural, rural-industrial, urban-industrial, urban). The primary sampling unit was school and the secondary one class. In the 2013 ACDSi our sample included 3,476 children and early-adolescents in the 6 to 14 age group. For the purpose of the present study only 11-year-olds (born on October 1, 2002, ± 6 months) of both sexes, who mostly did not yet experience adolescent growth spurt (pre-adolescents), attending primary school Grade 6 at the time of measuring, were included. The total sample of 11-year-olds comprised of 385 children, within which the initial sample of 160 children was invited to wear the SenseWear Pro Armband (SWA). The sample size was dictated by the number of available SWA sensors (n = 50) that still allowed us to monitor their physical activity of children from different localities within one month of the same season and therefore minimise the effects of weather conditions on children’s habitual physical activity. The same schoolchildren wearing SWA at age 11 were measured again at age 14 when the majority already experienced the adolescent growth spurt and were thus considered as early-adolescents. The number of children from the initial sample with sufficient wear time on the required days at age 11 was 141 (boys = 70; girls = 71). The excluded 19 children did not accumulate sufficient wear time due to device malfunction or irregular wearing of the device. The final sample of children at ages 11 and 14 that met the inclusion criteria for both years was 50 (boys = 21). This final sample was included in the final analysis (Fig 1). The average age of the children included in the first round of the PA monitoring at age 11, was 11.3 ± 0.3 years (boys 11.4 ± 0.4; girls 11.3 ± 0.3) and the average age of the children in the second round at age 14 was 14.3 ± 0.3 years (boys 14.3 ± 0.3; girls 14.2 ± 0.3). Due to considerable exclusion and some drop-out from the first to the second round of the PA monitoring, we needed to identify a possible exclusion/drop-out bias (see S1 to S4 Tables) and compared the somatic characteristics and physical fitness of the children in the final sample to the initial sample (see flowchart, Fig 1). In order to investigate possible bias between initial sample and the total sample at age 11 and between the final sample and the non-included children from the initial sample at age 14, we compared their somatic characteristics as well as their physical fitness with the following variables: standing broad jump, obstacle course backwards, 20-s drumming test, flamingo balance test, sit and reach, shoulder circumduction, hand grip strength, bent arm hang, 20-m shuttle-run, height, weight, biceps skinfold, triceps skinfold, subscapular skinfold, suprailiac skinfold, elbow breadth, wrist breadth, calf circumference, mid-thigh circumference, arm length, leg length, shoulder breadth, pelvic breadth, femoral breadth and ankle breadth.
Fig 1. Participants’ flowchart with drop-out and exclusion reasons.
Measuring procedure
PA was measured using a multi-sensor device SWA (Bodymedia SenseWear Pro Armband; BodyMedia Inc., Pittsburgh, USA). SWA device is based on the recognition of energy expenditure patterns and the estimation of PA. It uses several non-invasive biometrical sensors which measure various physical indicators (e.g. thermal conduction rate, galvanic skin response, skin temperature, environmental temperature, and PA measured with 2-axis accelerometer) and calculates energy expenditure via algorithms, which include data from several sensors as well as sex, age, height and weight of a measured subject. SWA was validated against doubly labeled water in children with high correlations for all comparisons (>0.90) [59] and is considered to be a reliable measuring device in children [40,42,47,60]
Children wore the SWA on the triceps of their right arm for one week [61–63], 24 hours per day except when showering, bathing and when practicing sports activities (usually at competitions or swimming), if they were not allowed to wear it due to safety reasons or device limitations (for example water resistance limitations when swimming). Further analysis of PA included only those children who wore the SWA device at least five days in a row (including both weekend days) [61,64,65] and whose wear time exceeded 90%. The SWA device collected data in one-minute epoch intervals. The main reason for our conservative approach was to reduce the error of under- and overestimation of PA due to missing wear-time in a greater extent than in the existing studies.
Body height, sitting height, and body lengths were measured with the use of anthropometers to one-millimetre precision levels (Siber & Hegner, Zurich, Switzerland). Body mass was measured using a portable electronic scale with 100-gram precision (Tanita BWB-800P, Arlington Heights, IL, USA). The precision of measurement was checked every time the scale was moved.
All other anthropometric measurements, physical fitness measurements, protocols, and equipment have been thoroughly described elsewhere [57].
Data collection
All the parents were notified about the purpose and procedures of data collection and their written consent was collected prior to measurements. Data collection was performed in October 2013 and October 2016. A trained team of researchers delivered the SWA devices on a Wednesday morning and collected the device the following Tuesday. The functioning of the SWA was explained to the children prior to the start of the monitoring period. In addition, short written guidelines of the proper use of equipment were prepared and given to the children, their parents, and teachers. Physical fitness tests and anthropometric measurements at ages 11 and 14 were administered by a highly trained team of researchers according to the prescribed protocol [57]. During the measurements, children were barefoot and wore light clothes. Approval of the National Medical Ethics Committee was obtained in June 2013 (ID 138/05/136).
Variables
The analysis of PA included two indicators of PA estimated through the SWA data: total energy expenditure (in calories) and duration of MVPA (in minutes). Although 3 METs (The Metabolic Equivalent of Task) has been widely used as an intensity threshold to distinguish between sedentary and MVPA, there is considerable evidence that 4 MET is a more suitable cut-off point for classifying MVPA in children and adolescents [66,67]; therefore MVPA was defined as a PA ≥ 4 MET. For the assessment of the timing of adolescent growth spurt, we used the anthropometric measurements that included body height, body mass, sitting height, and age at the time of measurement. We used Mirwald’s equation [68] to calculate the timing of adolescent growth spurt of included children. The timing of the adolescent growth spurt provides a benchmark of the maximum growth during early adolescence and provides a common landmark to reflect the occurrence of other body dimension velocities within and between individuals [69]. Adolescent growth spurt typically occurs approximately two years after the onset of puberty [70]. For boys the predictive equation was: maturity offset (years) boys = -9.236 + (0.0002708 * (leg length * sitting height)) + (−0.001663 * (age * leg length)) + (0.007216 * (age * sitting height)) + (0.02292 * (weight ÷ height * 100)). For girls the predictive equation was: maturity offset (years) girls = - 9.376 + (0.0001882 * (leg length * sitting height)) + (0.0022 * (age * leg length)) + (0.005841 * (age * sitting height)) + (- 0.002658 * (age * weight)) + (0.07693 * (weight ÷ height * 100)). The equation estimates the time distance from the adolescent growth spurt at the time of measurement, based on the ratio between the length of the torso and the legs. The growth of leg length precedes the growth of the torso, which changes the body proportions and by subtracting maturity offset from chronological age, age at adolescent growth spurt could be determined. This method was validated in studies of youth PA [71–73] and of young athletes [74–78]. Moreover, this method has been validated in an independent longitudinal samples in boys [79] and girls [80].
Data analysis
PA patterns were analysed with the Bodymedia SenseWear Professional 8.1 software package while all the statistical analyses were calculated by SPSS 25.0 (IBM Inc., Armonk, USA). Data are presented as mean and standard deviation, t-values, F-ratios and effect size (Cohen’s d and η2). Independent T-test was used to check for statistical differences in multiple physical fitness and anthropometric variables (standing broad jump, obstacle course backwards, 20-s drumming test, flamingo balance test, sit and reach, shoulder circumduction, hand grip strength, bent arm hang, 20-m shuttle-run, height, weight, biceps skinfold, triceps skinfold, subscapular skinfold, suprailiac skinfold, elbow breadth, wrist breadth, calf circumference, mid-thigh circumference, arm length, leg length, shoulder breadth, pelvic breadth, femoral breadth and ankle breadth): a) between boys and girls from the total sample in 2013 (n = 385), who were excluded from (n = 244) or included in (n = 141) the initial sample at age 11, and b) between boys and girls from the total sample in 2016, who were excluded from (n = 160) or included in (n = 50) the final sample at age 14 (Fig 1). In this way we were able to determine if the 11- and 14-year-olds who were included in PA analysis in 2013 and 2016, respectively, differed from their excluded peers. Differences in MVPA between boys and girls were then analysed using a paired sampled T-test. Differences in maturity between boys and girls were considered as proposed in other studies [73,81] and were analysed using Independent samples T-test, while normal distribution was checked with Q-Q plots, and the homogeneity of variances with Levene’s test. Repeated Measures Analysis of Covariance (RM ANCOVA) with Bonferroni correction was used to check for differences in longitudinal measures (2 time points, 3 years apart) between boys and girls, controlling for maturity with time distance from adolescent growth spurt (used as a covariate). ES was calculated using Cohen’s d (when t-test was used) and η2 (when RM ANCOVA was used). Alpha was set at 0.05.
Results
Independent samples T-test revealed that there were no statistical differences in physical fitness or somatic characteristics between boys and girls from the total sample (n = 385), who were included in the initial sample (n = 141) or excluded from it (n = 244) at age 11 (see S1 and S2 Tables).
The only small statistically significant difference at age 11 was observed in sit and reach test among boys, included in the initial sample (17.80 cm) and boys, who were excluded from it (15.82 cm) sample (t(190) = -2.03, p = 0.044). At age 14 no statistical differences in physical fitness or somatic characteristics were observed (see S3 and S4 Tables) between boys and girls from the total sample in 2016, who were included in the final sample (n = 50) or excluded from it (n = 160).
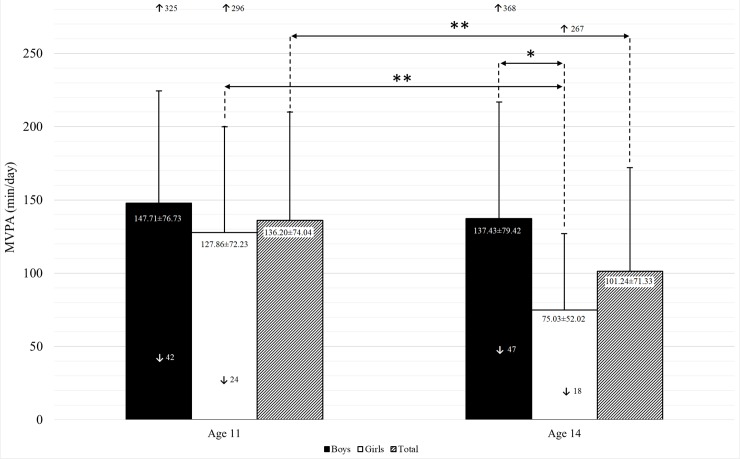
The expressed significant difference in the flexibility of hamstrings muscles and lower back (measured with sit-and-reach test) is probably irrelevant in regard to physical activity and it is unlikely that they influenced the drop-out or irregular wearing of the SWA sensors that lead to exclusion from the analysis of the final sample. Estimated PA values of girls and boys at ages 11 and 14 are shown in Fig 2.
Fig 2. Estimated MVPA of girls and boys at ages 11 and 14.
MVPA–moderate-to-vigorous physical activity; ↑ maximum; ↓ minimum; * p < 0.05; ** p < 0.01; values in the columns as Mean ± SD.
Without controlling for the timing of adolescent growth spurt, total MVPA off boys and girls was significantly higher at age 11 than at age 14 (t(49) = 2.75, p < 0.01, d = 0.39). MVPA in girls at age 11 was statistically higher than three years later (t(28) = 3.18, p < 0.01, d = 0.20), whereas the difference in MVPA between boys at ages 11 and 14 was not statistically significant (t(20) = 0.545, p = 0.592, d = 0.13). MVPA in boys at age 14 was significantly higher than in the girls of the same age (t(32.14) = 3.357, p < 0.05 d = 0.092) by 62.4 min, whereas the differences in MVPA between girls and boys at age 11 was not statistically significant (t(32.14) = 0.935, p = 0.355, d = 0.27).
There was a statistical difference in the timing of adolescent growth spurt between boys and girls. Girls experienced it significantly earlier (12.01 ± 1.0 years old) than boys (13.2 ± 0.75 years old) (t(48) = 4.201, p < 0.01, d = 1.35).
After controlling for the timing of the adolescent growth spurt, results of RM ANCOVA showed that there was a significant difference in average MVPA between boys and girls at age 11(F(1,47) = 6.294, p < 0.05, η2 = 0.12). Total difference of boys’ and girls’ MVPA at age 11 and 14 (F(1,47) = 5.945, p < 0.05, η2 = 0.11) proved to be significant. The amount of MVPA of boys at age 14 did not significantly differ from their MVPA at age 11 (p = 0.60), whereas MVPA of girls at age 14 significantly differed from their MVPA at age 11 (F(1,27) = 9.799, p < 0.01, η2 = 0.17).
Discussion
The aim of this longitudinal study was to analyse the changes in the amount of PA of boys and girls in the early stages of adolescence based on objectively assessed PA data.
The first important finding of the present study is that there is a visible decrease in MVPA between the ages of 11 and 14 which is more pronounced in girls. The average decrease of MVPA in girls was 41% compared to boys, who experienced a 7% decrease. The decrease of MVPA during early adolescence and especially in girls [82,83] was expected since such trends have been observed also in other studies [13,16,84,85]. The findings from the present study concur with a comparative study between Denmark, Portugal, Estonia, and Norway, which discovered decreasing MVPA in pre-adolescents, which was more pronounced in boys (approx. 30%) than in girls (approx. 20%) [86]. Similar findings have also been noticed by Jurak et al. [85] in a comparative study on PA of 11-year-old children from Ljubljana (Slovenia), Zagreb (Croatia) and Ann Arbour (USA). Boys from Ljubljana and Zagreb were physically more active than girls, although more than 90% of adolescents reached the WHO PA recommendations during the weekdays and more than 85% during weekends. Previous studies in Slovenia showed that 86% of the 11-year olds and 66% of 14-year olds is reaching the WHO PA recommendations [49,50,52] which is similar to the findings of the present study, where 84% of 11-year olds (n = 50) and 68% of 14-year olds (n = 50) are reaching the WHO PA recommendations. The significant decrease of MVPA between ages 11 and 14 in Slovenia is likely a combination of several factors. Although some studies found biological development to be related to the drop of PA in adolescence [56], contributing it to various factors such as non-exercise activity thermogenesis [87,88], reduced dopamine release or loss of dopamine receptors [89–91] or influence of biological maturity status, our analysis showed that the drop was evident with or without controlling for the timing of adolescent growth spurt. This leads to the conclusion that in our case the underlying factors of the adolescent drop of PA might not be biological but environmental. In this regard the school environment can be identified as one of the most important factors for this evident change. Namely, between ages 6 and 11 all Slovenian children have 3 classes of PE per week (45 minutes each), while in the last three years of primary school from ages 12 to 14 the number of compulsory PE classes drops to two per week [92] which is a considerable reduction. In the last three years of primary school pupils are also becoming growingly burdened with schoolwork; the number of school subjects increases from 11 to 14 [85] and their weekly workload increases from 25.5 to 28.5 hours, sometimes even to 32 hours [85]. Consequently, the sedentary activities of children at school and at home increase (more homework and studying), which can partly explain the decrease in PA.
As there are no intergenerational comparisons about the objectively evaluated PA of schoolchildren, it is impossible to conclude whether the 14-year-olds today achieve lower levels of PA than their peers in the past in the past. The comparisons of the level of the PA of Slovenian teenagers with their peers from other countries show that the PA of the Slovenian population is higher, particularly during the week [48–51]. Also the comparisons of physical fitness, which is inevitable result of habitual physical activity, show that Slovenian 14-year-old girls today have significantly higher level of physical fitness than the girls of the same age in 1990, whereas the boys still have a slightly lower physical fitness level [93] than their peers from the same year. All of these findings indicate that the decrease of MVPA in this age period is a universal phenomenon [15,86,94,95], although not necessarily influenced by biological changes during the maturation period.
Strengths and limitations
In the existing studies, the duration of wear time was ranging from 1h/day to 16.7 h/day [96], while the most common duration period was ≥ 10 h/day [97]. In order to assess as complete picture of the pre-adolescents’ daily PA patterns as possible one of the most important strengths of the present study is the wear time, which was over 90% (21h and 40min) per day. However, this strong criterion was unfortunately also the main reason for omitting a considerable number of data from the analysis. Further strengths of the present study is the use of the same measuring equipment throughout the study and the inclusion of timing of adolescent growth spurt. However, there are also some limitations: i) as in all PA studies, children were aware that they were being measured and monitored, which might have resulted in the changes of their habitual PA patterns; ii) high measurement and equipment costs limited the availability of SWA sensors and did not allow the measurement of PA of all included children during the same week which means that the PA patterns could be affected by other factors such as different weather conditions; iii) due to the short battery life and recording memory, a 1-minute interval for the collection of signal was used; consequently, not all short-burst PA durations could have been recorded, which might have resulted in underestimating the overall duration and intensity of recorded PA; iv) MVPA was measured only for one week, therefore we cannot interpret our results as behavioural PA; v) biomechanical and physical factors could affect the results as increased mass give lower acceleration and the increased height of children produces longer pendulum [98]; vi) arm worn device is more sensitive to arm movement, therefore results could be overrated [99]; vii) external factors could influence PA between the ages of 11 and 14: family problems, friendship problems, dietary habits and changes of environment/school, socioeconomic status, which have not been controlled for; viii) rigorous inclusion criteria regarding the wear time could have resulted in exclusion of some children who intensively practice sport; for example, children had to remove the measurement equipment during the competitions, in contact sports, and swimming activities; ix) total body mass was used in calculation of energy expenditure, thus it could be confounded by adiposity (a more precise choice would be lean mass proportional measures but it could not be used because the algorithms of the PA analysis software are based on actual body mass); x) anthropometry and equation-based methods like Mirwald et al. equation [69] for predicting the timing of adolescent growth spurt are not the most precise tools [100], therefore radiograph-based methods [80,101] would be recommended in future research; xi) the use of the fixed intensity thresholds in assessment of MVPA during pre-adolescence may not be the most suitable approach during adolescence due to changes in body size, composition and physical fitness, therefore the use of specific cut-off points should be used in the future [102].
Conclusion
This study contributes to the understanding of changes in PA among Slovenian pre-adolescents. Similar to other studies our results confirm the decrease of MVPA with age, but the differences between boys and girls in our study are considerably more pronounced. Since the greater drop of MVPA was observed in girls, future research should aim to explore the use of different approaches in encouraging the PA in boys and girls. Future studies should continue prioritizing longitudinal methodologies with bigger implications on gender differences in MVPA, including information about other PA determinants, such as environment, socioeconomic status and parental education.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability
The data underlying the results presented in the study are included in Supporting information in the xlsx format.
Funding Statement
This research was funded by the Slovenian Research Agency within the Research programme P5-0142 Bio-psycho-social context of kinesiology. The study received invaluable assistance in sports equipment, which was donated to the participating schools from the Olympic Committee of Slovenia and Elan Inventa, a sporting manufacturer and supplier.
References
- 1.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31. [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong N, Welsman JR. The Physical Activity Patterns of European Youth with Reference to Methods of Assessment. Sport Med. 2006;36(12):1067–86. [DOI] [PubMed] [Google Scholar]
- 3.Powell KE, Pratt M. Physical activity and health. Bmj. 1996;313:126 10.1136/bmj.313.7050.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batty GD, Lee I-M. Physical activity and coronary heart disease. Bmj. 2004;328:1089 10.1136/bmj.328.7448.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telama R, Yang X, Viikari J, Välimäki I, Wanne O, Raitakari O. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med. 2005;28(3): 267–273. 10.1016/j.amepre.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838): 247–257. 10.1016/S0140-6736(12)60646-1 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Global recommendations on physical activity for health. Geneva: World Health Organization; 2010. 17 p. Available from https://europepmc.org/books/n/whogophy/pdf [PubMed] [Google Scholar]
- 8.Bratina N, Hadžić V, Batellino T, Pistotnik B, Pori M, Šajber D, et al. Slovenian guidelines for physical activity in children and adolescents in the age group 2–18 years. Slov Med J. 2011;80(12):885–896. [Google Scholar]
- 9.Ortega FB, Konstabel K, Pasquali E, Ruiz JR, Hurtig-Wennlöf A, Mäestu J, et al. Objectively Measured Physical Activity and Sedentary Time during Childhood, Adolescence and Young Adulthood: A Cohort Study. PLoS One. 2013;8(4):e60871 10.1371/journal.pone.0060871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding SK, Page AS, Falconer C, Cooper AR. Longitudinal changes in sedentary time and physical activity during adolescence. Int J Behav Nutr Phys Act. 2015;12(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nader PR, Bradley RH, Houts RM, McRitchie SL, O’Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. Jama. 2008;300(3): 295–305. 10.1001/jama.300.3.295 [DOI] [PubMed] [Google Scholar]
- 12.Bacil EDA, Júnior OM, Rech CR, dos Santos Legnani RF, de Campos W. Physical activity and biological maturation: a systematic review. Rev Paul Pediatr. 2015;33: 114–121. 10.1016/j.rpped.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straatmann VS, Oliveira AJ, Rostila M, Lopes CS. Changes in physical activity and screen time related to psychological well-being in early adolescence: findings from longitudinal study ELANA. BMC Public Health. 2016;16: 977 10.1186/s12889-016-3606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson MC, Gordon-larsen P, Carolina N. Physical Activity and Sedentary Behavior Patterns Are Associated With Selected Adolescent Health Risk. 2006;117(4):1287–1290. [DOI] [PubMed] [Google Scholar]
- 15.Farooq MA, Parkinson KN, Adamson AJ, Pearce MS, Reilly JK, Hughes AR, et al. Timing of the decline in physical activity in childhood and adolescence: Gateshead Millennium Cohort Study. Br J Sports Med. 2018;52(15): 1002–1006. 10.1136/bjsports-2016-096933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corder K, Sharp SJ, Atkin AJ, Griffin SJ, Jones AP, Ekelund U, et al. Change in objectively measured physical activity during the transition to adolescence. Br J Sports Med. 2015;49: 730–736. 10.1136/bjsports-2013-093190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaakkola T, Hakonen H, Kankaanpää A, Joensuu L, Kulmala J, Kallio J, et al. Longitudinal associations of fundamental movement skills with objectively measured physical activity and sedentariness during school transition from primary to lower secondary school. J Sci Med Sport. 2019;22: 85–90. 10.1016/j.jsams.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Dalene KE, Anderssen SA, Andersen LB, Steene-Johannessen J, Ekelund U, Hansen BH, et al. Secular and longitudinal physical activity changes in population-based samples of children and adolescents. Scand J Med Sci Sport. 2018;28: 161–171. [DOI] [PubMed] [Google Scholar]
- 19.Lopes L, Silva Mota JAP, Moreira C, Abreu S, Agostinis Sobrinho C, Oliveira-Santos J, et al. Longitudinal associations between motor competence and different physical activity intensities: LabMed physical activity study. J Sports Sci. 2019;37: 285–290. 10.1080/02640414.2018.1497424 [DOI] [PubMed] [Google Scholar]
- 20.Erlandson MC, Sherar LB, Mosewich AD, Kowalski KC, Bailey DA, Baxter-Jones ADG. Does controlling for biological maturity improve physical activity tracking? MSSE. 2011;43(5):800–807. [DOI] [PubMed] [Google Scholar]
- 21.Cumming SP, Standage M, Gillison FB, Dompier TP, Malina RM. Biological maturity status, body size, and exercise behaviour in British youth: a pilot study. J Sports Sci. 2009;27: 677–686. 10.1080/02640410902725590 [DOI] [PubMed] [Google Scholar]
- 22.Sherar LB, Cumming SP, Eisenmann JC, Baxter-Jones ADG, Malina RM. Adolescent biological maturity and physical activity: biology meets behavior. Pediatr Exerc Sci. 2010;22: 332–349. 10.1123/pes.22.3.332 [DOI] [PubMed] [Google Scholar]
- 23.Heath GW, Parra DC, Sarmiento OL, Andersen LB, Owen N, Goenka S, et al. Evidence-based intervention in physical activity: lessons from around the world. Lancet. 2012;380: 272–281. 10.1016/S0140-6736(12)60816-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twisk JWR. Physical activity guidelines for children and adolescents. Sport Med. 2001;31: 617–627. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez-Pavón D, Kelly J, Reilly JJ. Associations between objectively measured habitual physical activity and adiposity in children and adolescents: Systematic review. Int J Pediatr Obes. 2010;5: 3–18. 10.3109/17477160903067601 [DOI] [PubMed] [Google Scholar]
- 26.Currie C, Molcho M, Boyce W, Holstein B, Torsheim T, Richter M. Researching health inequalities in adolescents: the development of the Health Behaviour in School-Aged Children (HBSC) family affluence scale. Soc Sci Med. 2008;66: 1429–1436. 10.1016/j.socscimed.2007.11.024 [DOI] [PubMed] [Google Scholar]
- 27.Malina RM. Physical activity and fitness: pathways from childhood to adulthood. Am J Hum Biol. 2001;13(2):162–172. [DOI] [PubMed] [Google Scholar]
- 28.Mize TD. Profiles in health: Multiple roles and health lifestyles in early adulthood. Soc Sci Med. 2017;178: 196–205. 10.1016/j.socscimed.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 29.Corder K, Winpenny E, Love R, Brown HE, White M, van Sluijs E. Change in physical activity from adolescence to early adulthood: a systematic review and meta-analysis of longitudinal cohort studies. Br J Sport Med. 2019;53: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strniša K, Planinšec J. Gibalna dejavnost otrok z vidika socialno- ekonomskih razsežnosti Physical Activity for Children in Terms of Socio-Economic Dimensions. Revija za elementarno izobraževanje. 7(1): 99–108. [Google Scholar]
- 31.Zurc J, Pišot R, Strojnik V. Gender Differences in Motor Performance in 5–6 year old children. Kinesiol Slov. 2005;11: 90–104. [Google Scholar]
- 32.Matejek Č, Starc G. The relationship between children’s physical fitness and gender, age and environmental factors. Ann Kinesiol. 2013;4(2):95–108. [Google Scholar]
- 33.Kovač M, Strel J, Jurak G, Leskošek B, Dremelj S. Physical Activity, Physical Fitness Levels, Daily Energy Intake and Some Eating Habits of 11-Year-Old Children. Croatian Journal of Education. 2013;15(1):127–139. [Google Scholar]
- 34.Butte NF, Ekelund U, Westerterp KR. Assessing physical activity using wearable monitors: measures of physical activity. Med Sci Sport Exerc. 2012;44(1S): S5–S12. [DOI] [PubMed] [Google Scholar]
- 35.Ainsworth B, Cahalin L, Buman M, Ross R. The current state of physical activity assessment tools. Prog Cardiovasc Dis. 2015;57(4): 387–395. 10.1016/j.pcad.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 36.Yang C-C, Hsu Y-L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors. 2010;10(8): 7772–7788. 10.3390/s100807772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5(1): 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sport Exerc. 2014;46: 99–106. [DOI] [PubMed] [Google Scholar]
- 39.Casiraghi F, Lertwattanarak R, Luzi L, Chavez AO, Davalli AM, Naegelin T, et al. Energy expenditure evaluation in humans and non-human primates by SenseWear Armband. Validation of energy expenditure evaluation by SenseWear Armband by direct comparison with indirect calorimetry. PLoS One. 2013;8: e73651 10.1371/journal.pone.0073651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stålesen J, Vik FN, Hansen BH, Berntsen S. Comparison of three activity monitors for estimating sedentary time among children. BMC Sports Sci Med Rehabil. 2016;8(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arvidsson D, Slinde F, Larsson S, Hulthen L. Energy cost of physical activities in children: validation of SenseWear Armband. Med Sci Sports Exerc. 2007;39(11): 2076–2084. 10.1249/mss.0b013e31814fb439 [DOI] [PubMed] [Google Scholar]
- 42.Calabró MA, Welk GJ, Eisenmann JC. Validation of the SenseWear Pro Armband algorithms in children. Med Sci Sports Exerc. 2009;41(9): 1714–1720. 10.1249/MSS.0b013e3181a071cf [DOI] [PubMed] [Google Scholar]
- 43.Dorminy CA, Choi L, Akohoue SA, Chen KY, Buchowski MS. Validity of a multisensor armband in estimating 24-h energy expenditure in children. Med Sci Sports Exerc. 2008;40(4): 699–706. 10.1249/MSS.0b013e318161ea8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berntsen S, Hageberg R, Aandstad A, Mowinckel P, Anderssen SA, Carlsen KH, et al. Validity of physical activity monitors in adults participating in free-living activities. Br J Sports Med. 2010;44(9): 657–664. 10.1136/bjsm.2008.048868 [DOI] [PubMed] [Google Scholar]
- 45.Jakicic JM, Marcus M, Gallagher KI, Randall C, Thomas E, Goss FL, et al. Evaluation of the SenseWear Pro ArmbandTM to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36(5): 897–904. 10.1249/01.mss.0000126805.32659.43 [DOI] [PubMed] [Google Scholar]
- 46.King GA, Torres N, Potter C, Brooks TJ, Coleman KJ. Comparison of activity monitors to estimate energy cost of treadmill exercise. Med Sci Sport Exerc. 2004;36(7): 1244–1251. [DOI] [PubMed] [Google Scholar]
- 47.Soric M, Mikulic P, Misigoj-Durakovic M, Ruzic L, Markovic G. Validation of the Sensewear Armband during recreational in-line skating. Eur J Appl Physiol. 2012;112(3): 1183–1188. 10.1007/s00421-011-2045-6 [DOI] [PubMed] [Google Scholar]
- 48.Tremblay MS, Gonzalez SA, Katzmarzyk PT, Onywera VO, Reilly JJ, Tomkinson G. Introduction to the Global Matrix 2. 0: Report Card Grades on the Physical Activity of Children and Youth Comparing 38 Countries. 2016;13(2): 85–86. [DOI] [PubMed] [Google Scholar]
- 49.Sember V, Starc G, Jurak G, Golobič M, Kovač M, Samardžija PP, et al. Results From the Republic of Slovenia’s 2016 Report Card on Physical Activity for Children and Youth. J Phys Act Heal. 2016;13(2): S256–S264. [DOI] [PubMed] [Google Scholar]
- 50.Sember V, Morrison SA, Jurak G, Kovač M, Golobič M, Pavletić Samardžija P, et al. Results from Slovenia’s 2018 report card on physical activity for children and youth. J Phys Act Heal. 2018;15(2): S404–S405. [DOI] [PubMed] [Google Scholar]
- 51.Aubert S, Barnes J, Abdeta C, Abi Nader P, Adeniyi A, Aguilar-Farias N, et al. Global Matrix 3.0 Physical Activity Report Card Grades for Children and Youth: Results and Analysis From 49 Countries. J Phys Act Health. 2018;15(2): S251–S273. [DOI] [PubMed] [Google Scholar]
- 52.Sember V, Morrison SA, Jurak G, Kovac M, Starc G. Differences in physical activity and academic performance between urban and rural schoolchildren in Slovenia. Montenegrin J Sport Sci Med. 2018;7(1): 67–72. [Google Scholar]
- 53.Jurak G, Cooper A, Leskosek B, Kovac M. Long-term effects of 4-year longitudinal school-based physical activity intervention on the physical fitness of children and youth during 7-year follow-up assessment. Cent Eur J Public Health. 2013;21(4): 190–194. [DOI] [PubMed] [Google Scholar]
- 54.Sorić M, Starc G, Borer KT, Jurak G, Kovač M, Strel J, et al. Associations of objectively assessed sleep and physical activity in 11-year old children. Ann Hum Biol. 2014;42(1):31–37. 10.3109/03014460.2014.928367 [DOI] [PubMed] [Google Scholar]
- 55.Bacil EDA, Piola TS, Watanabe PI, Silva MP da, Legnani RFS, Campos W de. Biological maturation and sedentary behavior in children and adolescents: a systematic review. J Phys Educ. 2016;27(e2730):1–10. [Google Scholar]
- 56.Eisenmann JC, Wickel EE. The biological basis of physical activity in children: revisited. Pediatr Exerc Sci. 2009;21(3): 257–272. 10.1123/pes.21.3.257 [DOI] [PubMed] [Google Scholar]
- 57.Jurak G, Kovač M, Starc G. The ACDSi 2013–The Analysis of Children’s Development in Slovenia 2013: Study protocol. Anthropol Notebooks. 2013;19(3): 123–43. [Google Scholar]
- 58.Starc G, Kovač M, Strel J, Bučar Pajek M, Golja P, Robič T, et al. The ACDSi 2014-a decennial study on adolescents’ somatic, motor, psychosocial development and healthy lifestyle: Study protocol. Anthropol Notebooks. 2015;21(3): 107–123. [Google Scholar]
- 59.Calabro MA, Stewart JM, Welk GJ. Validation of pattern-recognition monitors in children using doubly labeled water. Med Sci Sports Exerc. 2013;45(7): 1313–1322. 10.1249/MSS.0b013e31828579c3 [DOI] [PubMed] [Google Scholar]
- 60.Soric M, Turkalj M, Kucic D, Marusic I, Plavec D, Misigoj-Durakovic M. Validation of a multi-sensor activity monitor for assessing sleep in children and adolescents. Sleep Med. 2013;14(2): 201–205. 10.1016/j.sleep.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 61.Cain KL, Sallis JF, Conway TL, Van Dyck D, Calhoon L. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Heal. 2013;10(3): 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25(1): 99–108. 10.1249/00005768-199301000-00014 [DOI] [PubMed] [Google Scholar]
- 63.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sport Exerc. 2000;32(2): 426–431. [DOI] [PubMed] [Google Scholar]
- 64.Freedson P, Bowles HR, Troiano R, Haskell W. Assessment of physical activity using wearable monitors: recommendations for monitor calibration and use in the field. Med Sci Sports Exerc. 2012;44(1): S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc. 2005;37:S582–S588. 10.1249/01.mss.0000185292.71933.91 [DOI] [PubMed] [Google Scholar]
- 66.Saint-Maurice PF, Kim Y, Welk GJ, Gaesser GA. Kids are not little adults: what MET threshold captures sedentary behavior in children? Eur J Appl Physiol. 2016;116(1): 29–38. 10.1007/s00421-015-3238-1 [DOI] [PubMed] [Google Scholar]
- 67.van Loo CMT, Okely AD, Batterham MJ, Hinkley T, Ekelund U, Brage S, et al. Wrist acceleration cut-points for moderate-to-vigorous physical activity in youth. Med Sci Sports Exerc. 2018;50(3):609–616. 10.1249/MSS.0000000000001449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirwald RL, Baxter-Jones ADG, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sport Exerc. 2002;34(4): 689–694. [DOI] [PubMed] [Google Scholar]
- 69.Mirwald RL, Baxter-Jones ADG, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sport Exerc. 2002;34: 689–694. [DOI] [PubMed] [Google Scholar]
- 70.Karlberg J, Kwan C, Gelander L, Albertsson-Wikland K. Pubertal growth assessment. Horm Res Paediatr. 2003;60: 27–35. [DOI] [PubMed] [Google Scholar]
- 71.Beets MW, Vogel R, Forlaw L, Pitetti KH, Cardinal BJ. Social support and youth physical activity: the role of provider and type. Am J Health Behav. 2006;30(3): 278–289. 10.5555/ajhb.2006.30.3.278 [DOI] [PubMed] [Google Scholar]
- 72.Nurmi‐Lawton JA, Baxter‐Jones AD, Mirwald RL, Bishop JA, Taylor P, Cooper C, et al. Evidence of sustained skeletal benefits from impact‐loading exercise in young females: a 3‐year longitudinal study. J Bone Miner Res. 2004;19(2): 314–322. 10.1359/JBMR.0301222 [DOI] [PubMed] [Google Scholar]
- 73.Wickel EE, Eisenmann JC, Welk GJ. Maturity-related variation in moderate-to-vigorous physical activity among 9–14 year olds. J Phys Act Heal. 2009;6(5): 597–605. [DOI] [PubMed] [Google Scholar]
- 74.Malina RM, Coelho E Silva MJ, Figueiredo AJ, Carling C, Beunen GP. Interrelationships among invasive and non-invasive indicators of biological maturation in adolescent male soccer players. J Sports Sci. 2012;30(15): 1705–1717. 10.1080/02640414.2011.639382 [DOI] [PubMed] [Google Scholar]
- 75.Matthys SPJ, Vaeyens R, Coelho-e-Silva MJ, Lenoir M, Philippaerts R. The contribution of growth and maturation in the functional capacity and skill performance of male adolescent handball players. Int J Sports Med. 2012;33(7): 543–549. 10.1055/s-0031-1298000 [DOI] [PubMed] [Google Scholar]
- 76.Sherar LB, Baxter-Jones ADG, Faulkner RA, Russell KW. Do physical maturity and birth date predict talent in male youth ice hockey players? J Sports Sci. 2007;25(8): 879–886. 10.1080/02640410600908001 [DOI] [PubMed] [Google Scholar]
- 77.Till K, Cobley S, Wattie N, O’Hara J, Cooke C, Chapman C. The prevalence, influential factors and mechanisms of relative age effects in UK Rugby League. Scand J Med Sci Sports. 2010;20(2): 320–329. 10.1111/j.1600-0838.2009.00884.x [DOI] [PubMed] [Google Scholar]
- 78.Vandendriessche JB, Vaeyens R, Vandorpe B, Lenoir M, Lefevre J, Philippaerts RM. Biological maturation, morphology, fitness, and motor coordination as part of a selection strategy in the search for international youth soccer players (age 15–16 years). J Sports Sci. 2012;30(15): 1695–1703. 10.1080/02640414.2011.652654 [DOI] [PubMed] [Google Scholar]
- 79.Malina RM, Kozieł SM. Validation of maturity offset in a longitudinal sample of Polish boys. J Sports Sci. 2014;32(5): 424–437. 10.1080/02640414.2013.828850 [DOI] [PubMed] [Google Scholar]
- 80.Kozieł SM, Malina RM. Modified maturity offset prediction equations: validation in independent longitudinal samples of boys and girls. Sport Med. 2018;48(1): 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore JB, Beets MW, Kaczynski AT, Besenyi GM, Morris SF, Kolbe MB. Sex moderates associations between perceptions of the physical and social environments and physical activity in youth. Am J Heal Promot. 2014;29(2): 132–135. [DOI] [PubMed] [Google Scholar]
- 82.Young DR, Cohen D, Koebnick C, Mohan Y, Saksvig BI, Sidell M, et al. Longitudinal associations of physical activity among females from adolescence to young adulthood. J Adolesc Heal. 2018;63(4): 466–473. [DOI] [PubMed] [Google Scholar]
- 83.Morrissey JL, Janz KF, Letuchy EM, Francis SL, Levy SM. The effect of family and friend support on physical activity through adolescence: a longitudinal study. Int J Behav Nutr Phys Act. 2015;12(103):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Booth VM, Rowlands AV, Dollman J. Physical activity temporal trends among children and adolescents. J Sci Med Sport. 2015;18(4): 418–425. 10.1016/j.jsams.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 85.Jurak G, Sorič M, Starc G, Kovač M, Mišigoj-Durakovič M, Borer K, et al. School day and weekend patterns of physical activity in urban 11-year-olds: A cross-cultural comparison. Am J Hum Biol. 2015;27(2):192–200. 10.1002/ajhb.22637 [DOI] [PubMed] [Google Scholar]
- 86.Metcalf BS, Hosking J, Jeffery AN, Henley WE, Wilkin TJ. Exploring the Adolescent Fall in Physical Activity: A 10-yr Cohort Study (EarlyBird 41). Medicine and Science in Sports and Exercise. 2015; 47(10):2084–2092. 10.1249/MSS.0000000000000644 [DOI] [PubMed] [Google Scholar]
- 87.von Loeffelholz C, Birkenfeld A. The role of non-exercise activity thermogenesis in human obesity Endotext [Internet]. MDText. com, Inc; 2018. [Google Scholar]
- 88.Levine JA. Non-exercise activity thermogenesis (NEAT). Best Pract Res Clin Endocrinol Metab. 2002;16(4): 679–702. 10.1053/beem.2002.0227 [DOI] [PubMed] [Google Scholar]
- 89.Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32(9): 1623–1629. 10.1097/00005768-200009000-00016 [DOI] [PubMed] [Google Scholar]
- 90.Sallis JF. Age-related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc. 2000;32(9): 1598–1600. 10.1097/00005768-200009000-00012 [DOI] [PubMed] [Google Scholar]
- 91.Loprinzi PD, Herod SM, Cardinal BJ, Noakes TD. Physical activity and the brain: a review of this dynamic, bi-directional relationship. Brain Res. 2013;1539: 95–104. 10.1016/j.brainres.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 92.Jurak G, Kovač M. (Excusing pupils from physical education lessons in primary school) Opravičevanje med poukom športne vzgoje v osnovni šoli. Didact Slov. 2011;26(4):18–31. [Google Scholar]
- 93.Strel J. Fantje so v povprečju manj gibalno učinkoviti, kot so bili leta 1990, dekleta pa bolj. Sport. 2017;65(3/4): 176–184. [Google Scholar]
- 94.Kimm SYS, Glynn NW, Kriska AM, Barton BA, Kronsberg SS, Daniels SR, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med. 2002;347(10): 709–715. 10.1056/NEJMoa003277 [DOI] [PubMed] [Google Scholar]
- 95.Gordon-Larsen P, Nelson MC, Popkin BM. Longitudinal physical activity and sedentary behavior trends: adolescence to adulthood. Am J Prev Med. 2004;27(4): 277–283. 10.1016/j.amepre.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 96.Masse LC, Fuemmeler BF, Anderson CB, Matthews CE, Trost SG, Catellier DJ, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37(11): S544–54. [DOI] [PubMed] [Google Scholar]
- 97.Trost SG, Mciver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11): S531–43. [DOI] [PubMed] [Google Scholar]
- 98.Fridolfsson J, Börjesson M, Arvidsson D. A Biomechanical Re-Examination of Physical Activity Measurement with Accelerometers. Sensors. 2018;18(10): 3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quante M, Kaplan ER, Rueschman M, Cailler M, Buxton OM, Redline S. Practical considerations in using accelerometers to assess physical activity, sedentary behavior, and sleep. Sleep Heal. 2015;1(4): 275–284. [DOI] [PubMed] [Google Scholar]
- 100.Mills K, Baker D, Pacey V, Wollin M, Drew MK. What is the most accurate and reliable methodological approach for predicting peak height velocity in adolescents? A systematic review. J Sci Med Sport. 2017;20(6): 572–577. 10.1016/j.jsams.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 101.Fransen J, Bush S, Woodcock S, Novak A, Deprez D, Baxter-Jones ADG, et al. Improving the prediction of maturity from anthropometric variables using a maturity ratio. Pediatr Exerc Sci. 2018;30(2): 296–307. 10.1123/pes.2017-0009 [DOI] [PubMed] [Google Scholar]
- 102.Kujala U, Pietilä J, Myllymäki T, Mutikainen S, Föhr T, Korhonen I, et al. Physical activity: Absolute intensity vs. relative-to-fitness-level volumes. Med Sci Sports Exerc. 2017;49(3):474–481. 10.1249/MSS.0000000000001134 [DOI] [PubMed] [Google Scholar]