Abstract
Background
With increased rates of obesity and insulin resistance in youth, development of postprandial dyslipidemia, an important cardiovascular disease risk factor, is a concern. Glucagon-like peptides (ie, GLP-1 and GLP-2) and bile acids have been shown to regulate dietary fat absorption and postprandial lipids in animal models and humans. We hypothesize that the physiological response of GLPs and bile acids to dietary fat ingestion is impaired in adolescents with obesity and this associates with marked postprandial dyslipidemia and insulin resistance.
Methods
In this cross-sectional study, normal weight adolescents and adolescents with obesity underwent a 6-hour oral fat tolerance test. The postprandial lipoprotein phenotype profile was determined using various assays, including nuclear magnetic resonance spectroscopy, to characterize lipoprotein particle number, size, lipid content, and apolipoproteins. GLP-1 and GLP-2 were quantified by electrochemiluminescent immunoassays. Total bile acids were measured by an automated enzymatic cycling colorimetric method and the bile acid profile by mass spectrometry.
Results
Adolescents with obesity exhibited fasting and postprandial dyslipidemia, particularly augmented postprandial excursion of large triglyceride-rich lipoproteins. Postprandial GLPs were reduced and inversely correlated with postprandial dyslipidemia and insulin resistance. Postprandial bile acids were also diminished, particularly lithocholic acid, a potent stimulator of GLP-1 secretion.
Conclusion
Blunted postprandial GLP and bile acid response to dietary fat ingestion strongly associates with marked postprandial dyslipidemia. Further investigation is needed to assess their potential utility as early biomarkers for postprandial dyslipidemia in adolescents with obesity.
Keywords: dyslipidemia, Glucagon-Like Peptide 1, pediatric obesity, insulin resistance, bile acids
The prevalence of pediatric dyslipidemia has increased concurrently with rising rates of pediatric obesity and insulin resistance. Dyslipidemia in adolescence strongly tracks into adulthood (1) and the pathogenesis of atherosclerosis begins early in life (2). While cardiovascular disease (CVD) risk is traditionally assessed using fasting lipid measurements, the postprandial state predominates over the course of a day and nonfasting triglycerides (TGs) independently predict CVD events (3). Overproduction of intestinal apolipoprotein B48 (apoB48)-containing triglyceride-rich lipoproteins (TRLs) (ie, chylomicrons [CMs]) and atherogenic CM remnant accumulation occur in insulin-resistant states (4). Indeed, adolescents with central obesity (5) and insulin resistance (6) exhibit elevated postprandial TGs. The fasting lipoprotein phenotype (as assessed by nuclear magnetic resonance [NMR] spectroscopy) in adolescents more strongly associates with vascular damage than traditional fasting lipid parameters (7,8), yet postprandial lipoprotein abnormalities have yet to be characterized in adolescents with obesity and insulin resistance.
Intestinal lipid handling is governed by a complex neuroendocrine network involving gut peptides (eg, glucagon-like peptide 1 [GLP-1] and 2 [GLP-2]). GLPs are cosecreted from enteroendocrine L-cells in response to nutrient ingestion, including dietary fat. Acute administration of the GLP-1R agonist exendin-4 attenuated postprandial TRL-TG and TRL-apoB48 in healthy mice and hamsters (9), further confirmed with the GLP-1 receptor (GLP-1R) agonist exenatide in healthy men (10). Conversely, intraperitoneal GLP-2 injections increased postprandial TRL-TG and TRL-apoB48 in hamsters and mice (11), also confirmed in healthy men (12). In addition to regulating cholesterol catabolism and emulsifying dietary lipid to enhance digestion, bile acids are metabolic signaling molecules that activate the nuclear farnesoid X receptor (FXR) and membrane-bound Takeda G-protein-coupled receptor 5 (TGR5) receptor. Fxr–/– mice exhibit a pro-atherogenic lipid profile and elevated plasma and hepatic TGs (13). TGR5 agonism improves dyslipidemia in high-fat diet-fed mice (14) and stimulates GLP-1 secretion (14,15).
Given the ability of exogenous administration of GLPs and bile acids to modulate postprandial lipemia, we hypothesized that postprandial impairment of these regulators is an early pathological manifestation in adolescents with obesity and insulin resistance and they associate with metabolic dyslipidemia in the postprandial state. Therefore, we performed a cross-sectional study to assess postprandial lipids and lipoproteins, GLPs, and bile acids in healthy adolescents and adolescents with obesity and varying degrees of insulin resistance to determine the impact of insulin resistance on postprandial dyslipidemia and associated intestinal dysregulation as a potential mechanism. Specifically, we (1) characterized the postprandial lipid/lipoprotein phenotype profile in adolescents with obesity, (2) determined if the GLP response to dietary fat is altered in adolescents with obesity and associates with postprandial dyslipidemia and/or insulin resistance, and (3) assessed postprandial impairments in the bile acid pool in adolescents with obesity and their association with GLPs, postprandial dyslipidemia, and insulin resistance. Our findings contribute to a better understanding of the early pathophysiological manifestations of obesity, postprandial dyslipidemia, and insulin resistance in a young population.
Patients and Methods
Participant recruitment
For this cross-sectional study, 15 normal weight adolescents (NW; 8M/7F) and 30 adolescents with obesity (OB; 15M/15F) aged 12 to <19 years were recruited with informed consent through the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) project and the SickKids Team Obesity Management Program (STOMP). NW was defined as a BMI > 3rd and <85th age- and sex-specific percentile and OB as a BMI > 97th percentile, according to the World Health Organization (WHO) growth reference standards (16). Exclusion criteria included pregnancy, liver disease (except nonalcoholic fatty liver disease), diagnosed type 2 diabetes (T2D), taking medication that influences lipids (eg, statins), previous bariatric surgery, or development delay precluding assent/consent. Two participants prescribed metformin stopped taking their medication at least 3 days prior to the study day. A cohort subset (ie, NW; n = 6, OB; n = 6) was selected for postprandial serum bile acid profile analysis. OB adolescents with severe obesity (ie, 120% of the 95th percentile based on Centers for Disease Control and Prevention (CDC) reference growth charts) and postprandial dyslipidemia (ie, postprandial TG > 2.0 mmol/L), were selected.
Oral fat tolerance test
Participants were asked to restrain from intense physical activity 48 hours prior. Following an overnight (minimum 10 hour) fast, an intravenous catheter was inserted, and a fasting blood sample was taken. Participants consumed a standardized high-fat liquid meal (macronutrient composition consistent with recommendations (17)) comprising 150 mL of 35% whipping cream, 9.2 g of Nesquik chocolate syrup, 4.7 g of Beneprotein unflavored instant protein powder, and 50 g of ice cubes (542 kcal: 83.0% fat, 11.8% carbohydrates, 5.2% protein) within 15 minutes. Subsequent blood draws were taken 1, 2, 4, and 6 hours after the start of consumption. Participants did not consume any food or drink, except water, during the 6 hours.
Anthropometric, body composition, and blood pressure measurements
Measurements were made in triplicate and the calculated mean was used as the final measure. Height was measured using a stadiometer (Holton Ltd. Crymych, Dyfed, UK). Body weight was measured with a stand-on scale (ScaleTronix Model No. 5002, Carol Stream, IL). Waist circumference (WC) was measured in accordance with the NIH protocol using a measuring tape with a tension indicator device (Hoechstmass Balzer GmbH, Sulzbach, Germany). BMI was calculated as weight (in kg)/(height [in m])2. Waist-to-height ratio (WHtR) was calculated as WC (in cm)/height (in cm). BMI z-scores were determined based on the WHO growth reference data (16). WC and WHtR z-scores were determined based on the US National Health and Nutrition Survey cycle III (NHANES III) (18). Body fat percentage was determined using bioelectrical impedance analysis (BIA) (IMP SFB7 Impedimed Multifrequency Bioelectrical Impedance Analyzer, Pinkenba, Australia), calculated using the Gray equation (19) for OB participants and the Deurenberg equation (20) for NW participants. Systolic and diastolic blood pressure (BP) were measured using the General Electric Dash 3000 (General Electric Healthcare, Milwaukee, WI).
Quantification of glycemic, lipid, hepatic, and inflammatory parameters
Serum alanine aminotransferase (ALT), apolipoprotein A1 (apoA1), apolipoprotein B (apoB), aspartate aminotransferase (AST), C-reactive protein (CRP), glucose, high-density lipoprotein cholesterol (HDL-C), insulin, low-density lipoprotein cholesterol (LDL-C), TGs, total cholesterol, and whole blood Hemoglobin A1c (HbA1c) were measured on the ARCHITECT Plus ci4100. Non-HDL-C was calculated as total cholesterol – HDL-C and remnant cholesterol was calculated as total cholesterol – HDL-C – LDL-C (21). Plasma apoB48 was measured using the Human apoB48 enzyme-linked immunosorbent assay (ELISA) kit (FUJIFILM Wako Shibayagi Corporation, Richmond, VA). Serum adiponectin and leptin were measured using the Human Adiponectin ELISA and Human Leptin “Dual Range” ELISA (EMD Millipore Corporation, Billerica, MA), respectively.
Quantification of GLP-1, GLP-2, and DPP-IV
Plasma collected in BD™ P800 Blood Collection and Preservation System tubes was used to measure active and total GLP-1 by active and total GLP-1 (ver.2) electrochemiluminescent sandwich immunoassay kits (Meso Scale Diagnostics, LLC., Rockville, MD), respectively. Plasma active GLP-2 was quantified by radioimmunoassay using antiserum no. 92160, which detects the N-terminus of GLP-2 (1-33) and does not cross-react with GLP-2 degradation products or the major proglucagon fragment (MPGF). Plasma Dipeptidyl Peptidase IV (DPP-IV) was measured using the Human DPPIV/CD26 Quantikine ELISA Kit (R&D Systems, Inc. Minneapolis, MN).
Lipoprotein phenotype profile quantification
The serum lipoprotein phenotype profile was determined using a 400-MHz proton NMR analyzer (LipoScience, Inc., Raleigh, NC) at the NIH. The particle number for 4 TRL (very large/large, medium, small, very small), 3 LDL (large, medium, small), and 3 HDL subclasses (large, medium, small) were measured (all supplementary material and figures are located in a digital research materials repository; see ref. (22)). Average TRL-particle (P), LDL-P, and HDL-P sizes (ie, diameter in nanometers) were determined by summing the known diameter of each subclass multiplied by its relative mass percentage as estimated from the intensity of its NMR signal (23). Very large and very small subclasses are excluded from the TRL-P size calculation. Estimates of TRL-TG and TRL-C levels were derived from NMR spectra using conversion factors that estimate the lipid content based on size (23).
Bile acid quantification
Serum total bile acids were measured on the ARCHITECT Plus ci4100 (see ref. (22) for details of total bile acid reagent change during period of analysis). Ultrahigh-performance liquid chromatography electrospray ionization-mass spectrometry in multiple reaction monitoring (MRM) mode (UHPLC-(-)ESI-MRM/MS) was used to quantify the bile acid profile and 3 metabolic intermediates of bile acid synthesis in serum samples at the University of Victoria Genome British Columbia (BC) Proteomics Centre (Victoria, BC). See ref. (22) for procedure details. Detailed LC and mass spectrometry operation parameters and analytical sensitivities are described elsewhere (24).
Statistical analysis
Statistical analyses were performed using R software, version 3.5.1 and plots were created using GraphPad Prism 7, version 7.04 (GraphPad Software, Inc., La Jolla, CA). All figures show data as mean ± standard error of the mean (SEM). The trapezoidal method was used to approximate the area under the curve (AUC) and delta AUC (∆AUC). This method calculates the area of a trapezoid as (t2–t1) × (([analyte]t1 + [analyte]t2)/2), where t = time point (hours) and [analyte] = analyte concentration at the indicated time point. After calculating the trapezoid area between each time point, the AUC was calculated as the sum of all trapezoids. To calculate the ∆AUC, the analyte concentration at each time point was first subtracted from the analyte concentration at fasting, providing an estimated of the AUC above the fasting value. Continuous variables were compared between 2 groups (eg, NW and OB) using independent samples t-test and among 3 groups (eg, NW, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (L), and HOMA-IR (H)) using ANOVA (ref. (22)). Two-way mixed ANOVA was used to analyze the postprandial response with 2 groups (eg, NW and OB (Fig. 2 in (22)) 3 groups (eg, NW, HOMA-IR (L), and HOMA-IR (H) (Fig. 3 in (22)) as the between-subject variable and time (ie, fasting, 1, 2, 4, and 6 hours) as the within-subject variable. Using G*Power software, the statistical power of the 2-way mixed ANOVA for comparing between 2 groups was determined to be 0.994 (moderate effect size [ie, 0.25], α of 0.05, ε of 1, and sample size of 45) using the full cohort and 0.518 for the bile acid profile subanalysis (moderate effect size [ie, 0.25], α of 0.05, ε of 1, and sample size of 12). Monotonous relationships between continuous variables were assessed by Spearman’s rank correlation. All statistical tests were 2-sided and P < .05 was used to indicate statistically significant differences.
Results
Cohort characteristics
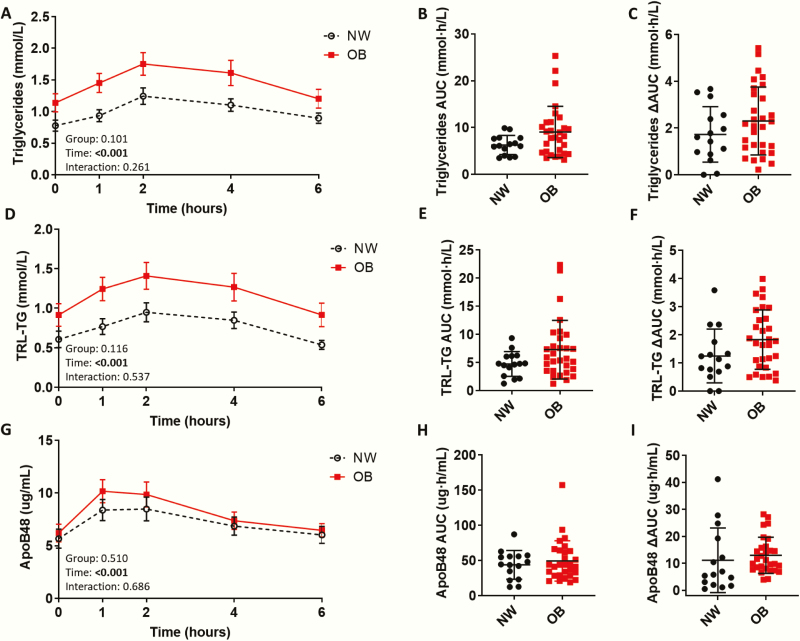
Sex, age, and pubertal status (ie, Tanner stage) did not significantly differ between NW and OB groups (Table 1). However, the ethnic composition did differ between groups, primarily due to a higher proportion of individuals classified as “other” in the OB group, which included all ethnicities other than Caucasian, East Asian, and South Asian. Fasting insulin and HOMA-IR were significantly higher in the OB than in the NW group. Postprandial insulin (ie, all postprandial time points, AUC, ∆AUC) was also significantly elevated in the OB compared with the NW group (Fig. 4 and Table 2 in (22)). All adiposity measures were significantly lower in NW adolescents. Fasting ALT, AST/ALT ratio, CRP, and leptin were higher and adiponectin was significantly lower in the OB than in the NW group (Fig. 5 and Table 3 in (22)). OB adolescents exhibited elevated fasting LDL-C and reduced fasting HDL-C (Table 4 in (22). Fasting total atherogenic lipoprotein cholesterol (ie, non-HDL-C) and particle number (ie, apoB) were significantly elevated in the OB group. Total cholesterol, LDL-C, HDL-C, non-HDL-C, apoB, and apoAI only changed minimally postprandially (Fig. 6 in (22)), while TG peaked 2 hours postprandially in both groups (Fig. 1A–C). Fasting and postprandial TG did not significantly differ between NW and OB groups.
Table 1.
Cohort characteristics.
| Parameter | NW (n = 15) | OB (n = 30) | P value | |
|---|---|---|---|---|
| Sex (M/F) | 8/7 | 15/15 | 1.00 | |
| Age (years) | 15.3 ± 1.80 | 15.6 ± 1.79 | .635 | |
| Tanner stage 1,2,3,4,5, n (%)a | 0(0), 2(13), 3(20), 8(53), 2(13) | 0(0), 1(4), 7(25), 8(29), 12(43) | .100 | |
| Ethnicity | .010 | |||
| Caucasian, n (%) | 9 (60) | 10 (33) | ||
| East Asian, n (%) | 2 (13) | 2 (7) | ||
| South Asian, n (%) | 4 (27) | 6 (20) | ||
| Other, n (%) | 0 (0) | 12 (40) | ||
| Anthropometric measures | ||||
| SBP (mmHg) | 114 ± 8.37 | 124 ± 10.8 | .003 | |
| DBP (mmHg) | 60.9 ± 4.99 | 66.8 ± 8.36 | .015 | |
| Weight (kg) | 53.6 (49.4–65.5) | 99.1 (87.1–116) | <.001 | |
| BMI | kg/m2 | 18.8 (17.5–21.4) | 34.8 (31.5–39.8) | <.001 |
| z-score | –0.32 (–0.74 to 0.18) | 2.98 (2.53–3.73) | <.001 | |
| WC | cmb | 74.8 ± 7.54 | 119 ± 20.3 | <.001 |
| z-score | 0.18 ± 0.46 | 2.08 ± 0.40 | <.001 | |
| WHtR | Ratio (cm/cm)b | 0.44 ± 0.04 | 0.70 ± 0.11 | <.001 |
| z-score | -0.33 ± 0.61 | 2.01 ± 0.44 | <.001 | |
| Body fat percentage (%) | 21.5 ± 7.10 | 41.9 ± 6.88 | <.001 | |
| Glycemic parameters | ||||
| Fasting glucose (mmol/L) | 4.92 (4.85–5.27) | 4.98 (4.83–5.26) | .952 | |
| HbA1c (%) | 5.14 ± 0.22 | 5.22 ± 0.29 | .370 | |
| Fasting insulin (pmol/L) | 58.5 (47.6–68.8) | 98.9 (66.1–204) | <.001 | |
| HOMA-IR | 1.81 (1.54–2.10) | 3.06 (1.99–6.94) | <.001 | |
| SPISE | 10.3 ± 1.96 | 4.26 ± 1.48 | <.001 |
Abbreviations: BMI, body mass index; DBP, diastolic BP; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; NW, normal weight adolescents; OB, adolescents with obesity; SBP, systolic BP; SPISE, single point insulin sensitivity estimator; WC, waist circumference; WHtR, waist-to-height ratio
Analytes indicated as mean ± SD were compared between NW and OB groups using independent samples t-test or Welch’s t-test if unequal variance. Analytes indicated as median (IQR) were compared between NW and OB groups using Mann Whitney U-test. Categorical variables were compared using the chi square or Fisher exact test. Significant P values (P < .05) are bolded.
aOB group n = 28 for Tanner stage.
bThese variables were log-transformed prior to analysis.
Figure 1.
Postprandial excursion of triglycerides (TGs), triglyceride-rich lipoprotein (TRL)-TG, and apoB48 in normal weight adolescents and adolescents with obesity. (A) Postprandial serum TGs in normal weight adolescents (NW; n = 15) and adolescents with obesity (OB; n = 30), (B) TG area under the curve (AUC), and (C) TG ∆AUC (∆AUC). (D) Postprandial serum triglyceride-rich lipoprotein TG (TRL-TG) in NW and OB groups, (E) TRL-TG AUC, and (F) TRL-TG ∆AUC. (D) Postprandial serum apolipoprotein B48 (apoB48) in NW and OB (n = 29) groups, (E) apoB48 AUC, and (F) apoB48 ∆AUC. Postprandial response analyzed by 2-way mixed ANOVA: P values for main effect of group, main effect of time, and group × time interaction shown on graph. AUC and ∆AUC compared using the independent sample t-test, Welch’s t-test, or Mann–Whitney U test. (A,D,G) Mean ± standard error of the mean shown. (B,C, E,F, H,I) Mean ± standard deviation shown. *P < .05; **P < .01; ***P < 0.001.
As a secondary analysis for a subset of analytes, adolescents were divided into groups based on surrogate measures of hepatic and whole-body insulin sensitivity: the HOMA-IR and the Single Point Insulin Sensitivity Estimator (SPISE) index, respectively. These secondary analyses were simply performed to be hypothesis generating due to the limitations of using surrogate markers of insulin sensitivity, as discussed later in the Discussion. For HOMA-IR, adolescents with obesity were divided into those with a HOMA-IR value below (HOMA-IR (L); 10M/10F) and above (HOMA-IR (H); 5M/5F) sex-specific cut-offs that were developed using pubertal adolescents with obesity (ie, 5.22 [males] and 3.82 [females]) (25). HOMA-IR was calculated as fasting glucose (mmol/L) × fasting insulin (µU/mL)/22.5. For the SPISE index, adolescents were divided into those above (8M/7F) and below (14M/15F) the cutoff value for insulin resistance (ie, <6.61 indicates insulin resistance) (26). All NW subjects had a SPISE value >6.61 and all subjects with obesity had a SPISE value <6.61, except for 1 subject, who was removed from the analysis. The SPISE index was calculated as 600 × (fasting HDL-C (mg/dL))0.185/(fasting TG (mg/dL)0.2 × BMI (kg/m2)1.338). When comparing TG between groups based on HOMA-IR, fasting and postprandial (ie, all postprandial time points and AUC) were highest in HOMA-IR (H) adolescents, although the postprandial increase (ie, ∆AUC) did not differ between groups (Fig. 7 in (22)). Subjects with a SPISE index value <6.61 had elevated TG concentration at 1 hour postprandially compared with those with a value >6.61 (Fig. 8 in (22)).
Exaggerated postprandial TRLs and their remnants in adolescents with obesity and insulin resistance
Fasting and postprandial TRL-TG showed clear trends toward higher levels in the OB adolescents than in the NW controls but the differences did not reach statistical significance (Fig. 1D–F). However, when divided by HOMA-IR, fasting and postprandial TRL-TG, including the postprandial rise (ie, ∆AUC), were elevated in HOMA-IR (H) adolescents (Fig. 7 in (22)). Similarly, fasting and postprandial apoB48 did not differ between NW and OB adolescents (Fig. 1G–I), while the postprandial apoB48 excursion was significantly higher in HOMA-IR (H) than in HOMA-IR (L) adolescents (ie, 1 hour, 4 hours, ∆AUC) (Fig. 7 in (22)). Subjects with a SPISE index value <6.61 had higher TRL-TG concentration at 1, 2, 4, and 6 hours postprandially and higher fasting apoB48 than those with a value >6.61 (Fig. 8 in (22)). While fasting and postprandial TRL cholesterol (TRL-C) did not differ between NW and OB adolescents, fasting and postprandial (ie, all postprandial time points and AUC) remnant cholesterol were significantly higher in OB than NW adolescents, respectively (Fig. 9 in (22)).
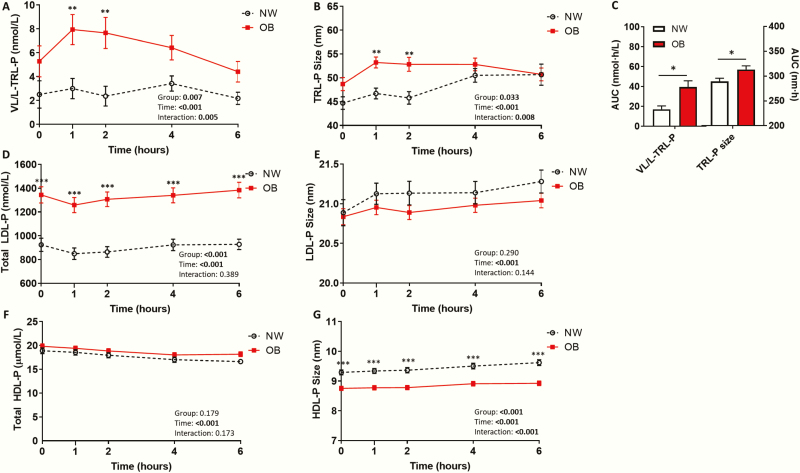
While total TRL-P did not significantly differ between groups (Fig. 10A in (22)), fasting and postprandial very large/large TRL-P and average TRL-P size were significantly higher in OB than in NW adolescents (Fig. 2A–C). Fasting and postprandial total LDL-P were also higher in OB than in NW (Fig. 2D). However, small LDL-P was not significantly different between NW and OB adolescents (Fig. 11C in (22)). Total HDL-P did not differ between groups, although HDL-P size was significantly smaller in OB than in NW adolescents (Fig. 2F–G). Large HDL-P number was significantly lower and small HDL-P number was significantly higher in OB than in NW adolescents (Fig. 11D and 11F in (22)).
Figure 2.
Postprandial lipid/lipoprotein phenotype characterization in normal weight adolescents and adolescents with obesity. Postprandial serum (A) very large/large , triglyceride-rich lipoprotein (TRL) particle number (VL/L-TRL-P), (B) mean TRL-P size, (C) VL/L-TRL-P and mean TRL-P size area under the curve (AUC) in normal weight adolescents (NW; n = 15) and adolescents with obesity (OB; n = 30). Postprandial serum (D) total low-density lipoprotein (LDL) particle number (LPL-P), (E) mean LDL-P size, (F) total high-density lipoprotein (HDL) particle number (HDL-P), and (G) mean HDL-P size in NW and OB groups. Postprandial response analyzed by two-way mixed ANOVA: P-values for main effect of group, main effect of time, and group*time interaction shown on graph. AUC compared using the independent sample t-test, Welch’s t-test, or Mann–Whitney U test. Mean ± standard error of the mean shown. *P < .05; **P < .01; ***P < .001.
Blunted postprandial, but elevated fasting, GLPs in adolescents with obesity and insulin resistance
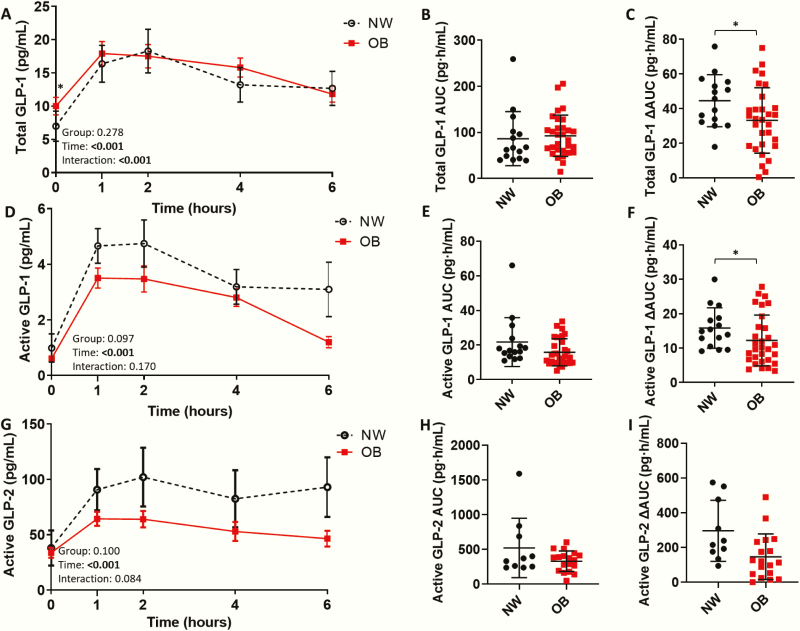
Total and active GLP-1 response to dietary fat (ie, ∆AUC) were significantly lower in OB than in NW adolescents (Fig. 3A–F) (Table 5 in (22)). However, postprandial active GLP-2 did not significantly differ between NW and OB adolescents (Fig. 3G–I). When divided by HOMA-IR, total and active GLP-1 response to dietary fat (ie, ∆AUC) were significantly lower in HOMA-IR (H) than in NW and HOMA-IR (L) adolescents (Fig. 7 in (22)). Furthermore, postprandial active GLP-2 was blunted in HOMA-IR (H) compared with NW adolescents (Fig. 7 in (22)). Active GLP-1 and GLP-2, but not total GLP-1, exhibited a blunted postprandial response (ie, ∆AUC) in subjects with a SPISE index value <6.61 compared with those with a value >6.61 (Fig. 8 in (22)). OB adolescents exhibited higher fasting total GLP-1 compared to NW adolescents, while fasting active GLP-1 and GLP-2 did not significantly differ between groups.
Figure 3.
Impaired total and active glucagon-like peptide (GLP)-1 and active GLP-2 response to dietary fat ingestion. (A) Postprandial total glucagon-like peptide 1 (GLP-1) in normal weight adolescents (NW; n = 15) and adolescents with obesity (OB; n = 30), (B) total GLP-1 area under the curve (AUC), and (C) ∆AUC. (D) Postprandial active GLP-1 in NW and OB groups, (E) active GLP-1 AUC, and (F) ∆AUC. (G) Postprandial active GLP-2 in NW (n = 10) and OB (n = 18) groups, (H) active GLP-2 AUC, and (I) ∆AUC. Postprandial response analyzed by 2-way mixed ANOVA: P values for main effect of group, main effect of time, and group×time interaction shown on graph. AUC and ∆AUC compared using the independent sample t-test, Welch’s t-test, or Mann–Whitney U test. (A,D,G) Mean ± standard error of the mean shown. (B,C,E,F,H,I) Mean ± standard deviation shown. *P < .05; **P < .01; ***P < .001.
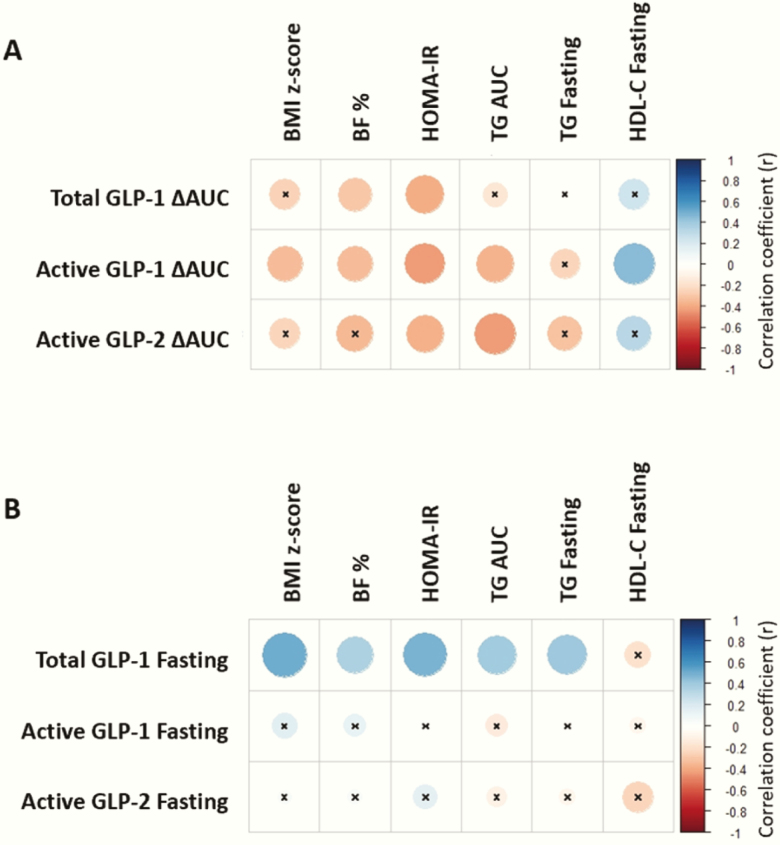
Postprandial (ie, ∆AUC) GLPs inversely correlated with HOMA-IR (Fig. 4A). Postprandial active GLP-1 also inversely correlated with BMI z-score, body fat percentage, TG AUC, and positively correlating with fasting HDL-C. Postprandial total GLP-1 and active GLP-2 also inversely correlated with body fat percentage and TG AUC, respectively. Conversely, fasting total GLP-1 positively correlated with all metabolic parameters, except HDL-C (Fig. 4B). Fasting active GLP-1 and GLP-2 did not significantly correlate with any metabolic parameters.
Figure 4.
Postprandial glucagon-like peptide (GLP)-1 and GLP-2 negatively correlate, while fasting GLP-1 positively correlates with adiposity insulin resistance, and dyslipidemia. (A) Spearman’s rank correlation heatmap between postprandial (ie, ∆AUC) total and active glucagon-like peptide 1 (GLP-1) and active GLP-2 and adiposity (ie, body mass index (BMI) z-score, body fat percentage (BF %), homeostatic model assessment for insulin resistance (HOMA-IR), dyslipidemia (ie, area under the curve (AUC) triglycerides (TG), fasting TG, and fasting high-density lipoprotein cholesterol (HDL-C). (B) Spearman correlation heatmap between fasting total and active GLP-1 and active GLP-2 and adiposity, HOMA-IR, and dyslipidemia. The strength of the correlation between two variables is represented by the color of the square at the intersection of those variables. Colors range from bright blue (strong positive correlation; ie, r = 1.0) to bright red (strong negative correlation; ie, r = -1.0). x, correlation coefficient not significant (P-value ≥ 0.05). Total and active GLP-1 (n = 45), active GLP-2 (n = 28).
DPP-IV concentration does not explain altered GLP levels
DPP-IV concentration did not change postprandially in a cohort subset, and thus only fasting DPP-IV was analyzed in all subjects (Fig. 12 in (22)). Fasting DPP-IV concentration did not significantly differ between groups, although levels were significantly lower in OB groups combined than in NW. DPP-IV concentration negatively correlated WHtR z-score (r = –0.31, P = .042), BMI z-score (r = –0.32, P = .030) and body fat percentage (r = –0.43, P = .003), but did not correlate with HOMA-IR (P = .087).
Sex differences in postprandial TRLs and GLPs
In males, TG and TRL-TG were significantly higher in the OB than in the NW group (main effect of group: P = .031 and P = .034, respectively) (Fig. 13 in (22)). However, TG and TRL-TG levels did not differ between NW and OB female adolescents. Male OB adolescents exhibited an exaggerated postprandial apoB48 response (ie, ∆AUC) compared with NW subjects, although not significant in female adolescents. Male OB adolescents had higher TG, TRL-TG, and apoB48 concentrations than females, while NW adolescents did not exhibit a sex difference (Fig. 14 in (22)).
Only male OB adolescents exhibited a blunted postprandial active GLP-1 response (ie, AUC, ∆AUC) to dietary fat (Fig. 15 in (22)). However, neither male or female adolescents, when analyzed separately exhibited differences in total GLP-1 or active GLP-2 between NW and OB groups. NW and OB males had significantly higher fasting total GLP-1 than females, and OB males only had higher postprandial (ie, AUC) total GLP-1 (Fig. 16 in (22)). However, fasting and postprandial active GLP-1 and GLP-2 did not differ between sexes for either NW or OB groups (22).
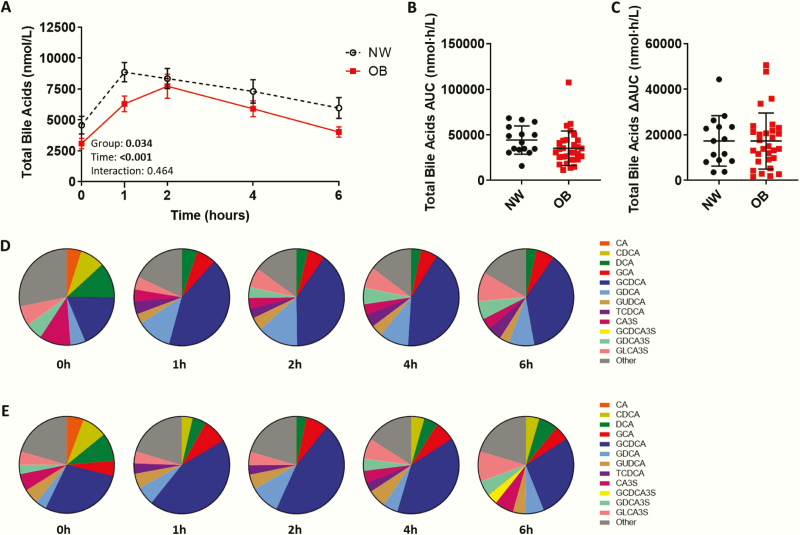
Altered total bile acids in adolescents with obesity
Postprandial, but not fasting, total bile acids were significantly lower in OB adolescents (main effect of group: P = .034) in the total adolescent cohort (NW, n = 15; OB, n = 30), although AUC and ∆AUC were not significantly different (Fig. 5) (Table 6 in (22)). Neither fasting nor postprandial total bile acids significantly differed between NW, HOMA-IR (L), and HOMA-IR (H) groups (fasting (Kruskal Wallis P = .089); AUC (ANOVA P = .175)). The bile acid profile was quantified by mass spectrometry in a smaller cohort subset (NW (n = 6); OB (n = 6)). In this cohort subset, age and sex did not differ between groups, while OB adolescents exhibited excess adiposity, insulin resistance, and dyslipidemia (Table 7 in (22)). In this cohort subset, postprandial, but not fasting, total bile acids were lower in OB adolescents (Table 6 in (22)), regardless of the quantification method used (Fig. 17 in (22)). Total bile acid concentration obtained using mass spectrometry and the Abbott ARCHITECT correlated strongly (Pearson correlation; r = 0.84, P < .001).
Figure 5.
Adolescents with obesity have a lower total bile acids pool and altered bile acid composition postprandially. (A) Postprandial total bile acids in normal weight adolescents (NW; n = 15) and adolescents with obesity (OB; n = 30) measured on the Abbott ARCHITECT, (B) total bile acid area under the curve (AUC) and (C) ∆AUC. Postprandial response analyzed by two-way mixed ANOVA: P values for main effect of group, main effect of time, and group×time interaction shown on graph. AUC and ∆AUC compared using the independent sample t-test, Welch’s t-test, or Mann–Whitney U test. (A) Mean ± standard error of the mean shown. (B,C) Mean ± standard deviation shown. *P < .05. Comparison of bile acid composition expressed as a percentage of total bile acids in (D) NW (n = 6) and (E) OB (n = 6) adolescents at fasting (0h) and 1, 2, 4, and 6 hours after ingestion of a high-fat liquid meal. Only bile acid species >3% of total bile acids are shown. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid, GCA, glycine-conjugated CA; GCDCA, glycine-conjugated CDCA; GDCA, glycine-conjugated DCA; GUDCA, glycine-conjugated ursodeoxycholic acid; TCDCA, taurine-conjugated CDCA; CA3S, CA-3-sulfate; GCDCA3S, GCDCA-3-sulfate; GDCA3S, GDCA-3-sulfate; GLCA3S, glycine-conjugated lithocholic acid-3-sulfate.
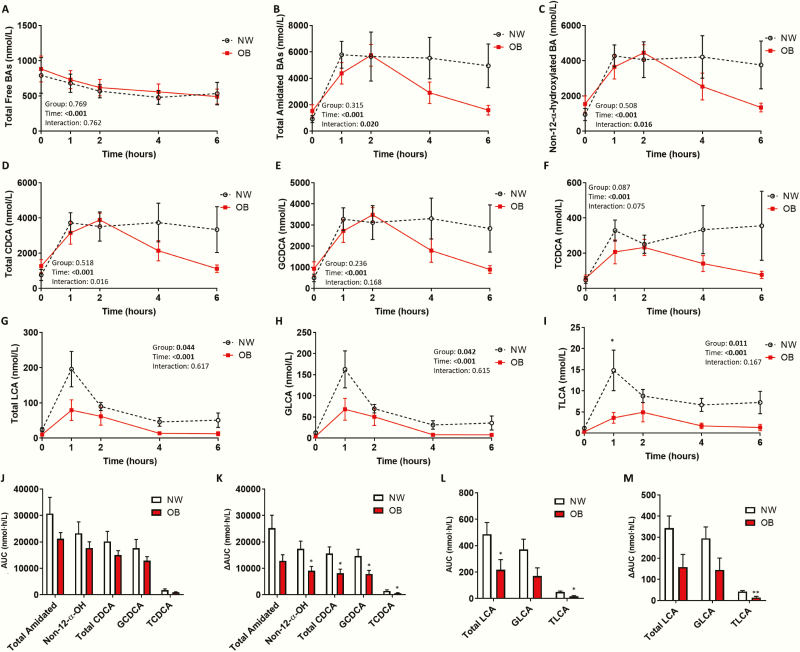
Adolescents with obesity exhibit lower non-12α-hydroxylated bile acids, CDCA, and LCA
The postprandial bile acid pool was dominated by amidated (ie, primarily glycine-conjugated) bile acids, while the fasting bile acid pool had a greater diversity of bile acid species (ie, free, amidated, and sulfated) (Fig. 5D–E). The postprandial bile acid pool in OB adolescents returned to a characteristic fasting composition more quickly than in NW adolescents. All bile acid groups, except free bile acids, increased postprandially (Fig. 6), (Fig. 18 in (22)). OB adolescents exhibited a steeper postprandial decline in total and primary amidated bile acids than NW (Fig. 6B) (Fig. 18C in (22)). Similarly, 12α-hydroxylated (Fig. 18G in (22)) and non-12α-hydroxylated (Fig. 6C) bile acids declined more rapidly in OB adolescents. This was more evident with non-12α-hydroxylated bile acids, evident by a significantly lower ∆AUC in OB compared to NW adolescents (P = .040). Specifically, the postprandial excursion of non-12α-hydroxylated bile acids, chenodeoxycholic acid (CDCA), and lithocholic acid (LCA), were significantly blunted in OB adolescents (Fig. 6).
Figure 6.
Impaired postprandial response of amidated non-12α-hydroxylated bile acids, chenodeoxycholic acid (CDCA) and LCA, in adolescents with obesity. Postprandial serum concentration of (A) total free bile acids, (B) total amidated bile acids, (C) non-12α-hydroxylated bile acids, (D) total CDCA, (E) glycine-conjugated CDCA (GCDCA), (F) taurine-conjugated CDCA (TCDCA), (G) total lithocholic acid (LCA), (H) glycine-conjugated LCA (GLCA), and (I) taurine-conjugated LCA (TLCA) in normal weight adolescents (NW; n = 6) and adolescents with obesity (OB; n = 6) measured by mass spectrometry. (J) AUC and (K) ∆AUC for total amidated bile acids, non-12α-hydroxylated bile acids, total CDCA, GCDCA, and TCDCA. (L) AUC and (M) ∆AUC for total LCA, GLCA, and TLCA. Postprandial response analyzed by two-way mixed ANOVA: P values for main effect of group, main effect of time, and group×time interaction shown on graph. AUC and ∆AUC compared using the independent sample t-test, Welch’s t-test, or Mann–Whitney U test. *P < 0.05; **P < 0.01 compared with NW. Mean ± standard error of the mean shown.
Adolescents with obesity have reduced metabolic intermediates of bile acid synthesis
Three metabolic intermediates of bile acid synthesis decreased postprandially, returning to baseline by 4 hours (Fig. 19 in (22)). Fasting and postprandial 3β-OH-5-cholestenoic acid (3β-C5) and 3β-7α-diOH-5-cholestenoic acid (3β,7α-C5) were significantly lower in OB than in NW adolescents. However, a downstream intermediate, 7α-OH-3-oxo-4-cholestenoic acid (7α-C4), did not differ between groups.
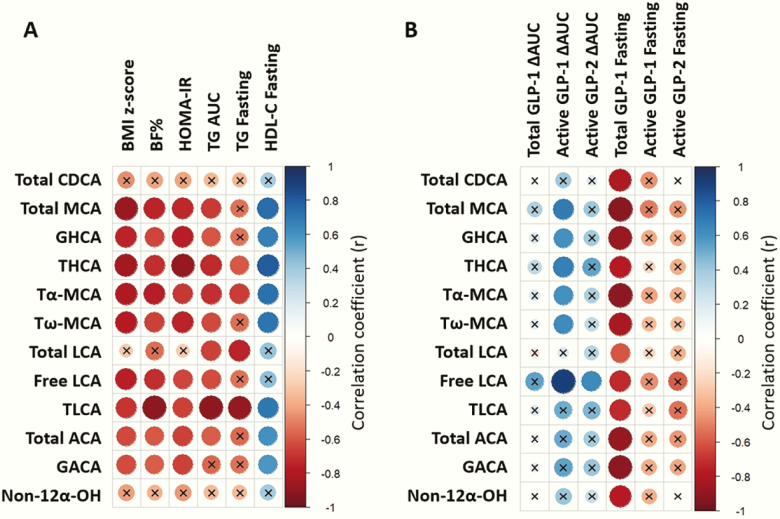
Bile acids associate with metabolic abnormalities and gut peptide dysregulation
Of selected bile acid species (ie, those that differed between NW and OB adolescents), all except total CDCA and non-12-α-hydroxylated bile acids, significantly negatively correlated with BMI z-score, body fat percentage, HOMA-IR, TG AUC, and/or fasting TG, and positively correlated with fasting HDL-C (Fig. 7). Postprandial bile acids species directly correlated with postprandial active GLP-1, while they inversely correlated with fasting total GLP-1.
Figure 7.
Postprandial bile acids correlate with adiposity, insulin resistance, dyslipidemia, glucagon-like peptide (GLP-1), and GLP-2. (A) Spearman’s rank correlation heatmap between postprandial (ie, ΔAUC) bile acid species and adiposity (ie, body mass index (BMI) z-score, body fat percentage (BF %), homeostatic model assessment for insulin resistance (HOMA-IR), dyslipidemia (ie, area under the curve (AUC) triglycerides (TG), fasting TG, and fasting high-density lipoprotein cholesterol (HDL-C). (B) Spearman correlation heatmap between bile acid species and total glucagon-like peptide 1 (GLP-1) ΔAUC, active GLP-1 ΔAUC, active GLP-2 ΔAUC, fasting total GLP-1, fasting active GLP-1, and fasting active GLP-2. The strength of the correlation between two variables is represented by the color of the square at the intersection of those variables. Colors range from bright blue (strong positive correlation; ie, r = 1.0) to bright red (strong negative correlation; ie, r = –1.0). x, correlation coefficient not significant (P ≥ .05); n = 12 (n = 10 for active GLP-2 ΔAUC and fasting).
Discussion
The above findings support our overall hypothesis that postprandial impairment of GLPs and bile acids are early pathological manifestations in a young population with obesity and they associate with the degree of adiposity, postprandial metabolic dyslipidemia, and insulin resistance. Specifically, we demonstrated an augmented excursion of large TRL particles following ingestion of a high-fat meal, paralleled by impaired postprandial GLPs and bile acids in adolescents with obesity. We found that both very large/large TRL-P number and TRL-P size were elevated in adolescents with obesity, suggesting adiposity is associated with the formation of a greater number of larger TRL-P. Elevated fasting apoB48 in female adolescents (27) with obesity has previously been reported and kinetic studies have shown increased CM production rate in adults with insulin resistance (4) and T2D (28). We did not find a significant difference in fasting or postprandial apoB48 between NW and OB adolescents. However, when dividing groups by HOMA-IR, a surrogate marker of hepatic insulin resistance, those with obesity and high HOMA-IR showed an elevated postprandial excursion of apoB48, suggesting hepatic insulin resistance in adolescents with obesity may be associated with an exaggerated postprandial rise of intestinally derived TRLs. On the other hand, adolescents with a low SPISE index value, a surrogate marker of whole-body insulin resistance, had elevated fasting, but not postprandial apoB48. While these findings would need to be confirmed with more robust measures of insulin sensitivity, these findings suggest a potential difference between the association of hepatic and whole-body insulin resistance with CM production. By extending these observations to youth, our data highlights the early manifestations of postprandial lipid abnormalities in adolescents with obesity. Elevated atherogenic remnant cholesterol, total LDL-P, and lower HDL-P size, were also apparent and likely stemmed from TRL accumulation. Elevated postprandial TRLs may result from reduced clearance via diminished LDL receptor expression (29) and/or increased competition for clearance (30) in insulin-resistant states. CM overproduction is also an important contributor, partly due to loss of the inhibitory action of insulin on CM output in insulin-resistant states (31). Increased plasma free fatty acids (FFAs) from the diminished inhibitory effect of insulin on adipose tissue lipolysis (32) and/or enhanced CM assembly (33) may also contribute. Postprandial regulators, including GLPs and bile acids, have also been implicated in regulating intestinal lipid handling and were thus investigated further in the present study.
Total GLP-1 response to a high-fat liquid meal (ie, ΔAUC) was blunted in OB adolescents and inversely correlated with the degree of adiposity, insulin resistance and dyslipidemia. These findings are further supported by a previous report of blunted GLP-1 secretion following an oral glucose tolerance test (OGTT) in adolescents with overweight/obesity (34). Furthermore, we showed diminished GLP levels in response to a high-fat meal in adolescents with obesity and a high HOMA-IR value. Reduced sensitivity of FFA sensing receptors (eg, GPR120) may play a role in impaired GLP secretion (35). Leptin resistance may be involved as L-cell leptin receptor activation promoted GLP-1 secretion and high-fat diet-fed mice exhibited hyperleptinemia, leptin resistance, and reduced GLP-1 response to oral glucose (36). This is consistent with our observations that OB adolescents exhibit elevated leptin that inversely correlated with postprandial GLPs. Insulin resistance may also directly attenuate GLP-1 secretion, as L-cell insulin resistance associates with impaired GLP-1 secretion in vitro and in vivo (37). Conversely, fasting total GLP-1 directly associated with the degree of adiposity, insulin resistance, and dyslipidemia, suggesting a potential compensatory increase to maintain fasting levels of biologically active peptides in a state of attenuated postprandial levels. Previous studies reported elevated fasting GLP-1 in insulin-resistant mice, despite impaired GLP-1 secretion following an OGTT (37) and higher fasting GLP-1 in adolescents with obesity compared with lean controls (34).
Our observations of blunted active GLPs following a high-fat meal (ie, ΔAUC) in OB adolescents is consistent with previous studies in adolescents with overweight/obesity following an OGTT (34) and mixed meal (38). However, we found that the concentration of DPP-IV, the main degradative enzyme of GLPs, was reduced in OB compared with NW adolescents and therefore its levels does not explain the lower active GLPs observed. Given similar alterations in active GLP-1 and GLP-2 in OB adolescents, a differential responsiveness to their actions may explain their association with postprandial dyslipidemia. GLP-1 and GLP-2 co-infusion in insulin-resistant hamsters exhibited a preferential response to hyperlipidemic GLP-2 effects, suggesting GLP-2 hypersensitivity and/or GLP-1 insensitivity (39). Differential responsiveness to GLP-1 and GLP-2 may also explain their association with adiposity and insulin resistance given the ability of GLP-1 to increase satiety (40), while GLP-2 promotes nutrient absorption (41). Gut microbiota dysbiosis, which has been extensively reported in obese and insulin-resistant states, has been shown to impair GLP-1 signaling in mice through enteric neurons (42).
Bile acids are known to regulate energy expenditure, glucose, and lipid metabolism via activating FXR and TGR5. We found that the postprandial bile acid response to dietary fat ingestion was impaired in OB adolescents, consistent with previous studies showing reduced postprandial, but not fasting, total bile acids in adults with obesity (43). Prolonged hyperinsulinemia, as observed in insulin-resistant states, has been shown to suppress hepatic transcription of Cyp7a1, the rate-limiting enzyme in bile acid synthesis (44). Liver-specific insulin receptor knockout mice and high-fat diet-fed mice also exhibit reduced Cyp7a1 expression (45). Fasting and postprandial bile acid synthesis intermediates (ie, 3β-C5 and 3β,7α-C5) were reduced in OB adolescents. However, serum 7α-C4, a downstream intermediate of bile acid synthesis that correlates with CYP7A1 enzymatic activity, did not differ between groups. Thus, it remains inconclusive whether reduced bile acid synthesis contributes to the observed lower bile acid levels in obesity. Reduced postprandial bile acids may also be due to impaired enterohepatic circulation due to reduced bile acid transporter expression (46) or impaired gallbladder emptying (47).
The primary non-12α-hydroxylated bile acid, CDCA, and its downstream secondary bile acid, LCA, were blunted postprandially. A shift towards 12α-hydroxylated bile acids is consistent with previous findings in adults with T2D (48). Mice lacking the hepatic sterol 12α-hydroxylase, CYP8B1, exclusively contain non-12α-hydroxylated bile acids and exhibit lower body weight, increased GLP-1, and improved glucose tolerance (49). The ability of FXR to reduce Cyp8b1 expression is abolished in a mouse model of hepatic insulin resistance (45). Preferential reduction of non-12α-hydroxylated bile acids in OB adolescents may be due to CYP8B1 upregulation. As FXR activation promotes an antiatherogenic lipid profile (50), postprandial impairment of CDCA, a potent FXR ligand, may contribute to postprandial dyslipidemia. Impaired LCA, a natural TGR5 agonist, may contribute to weight gain via reduced energy expenditure, explaining the inverse correlation of TLCA with BMI z-score and body fat percentage. Reduced TGR5 activation may also impair GLP-1 secretion (14,15), and improve dyslipidemia and glycemia (14).
A main limitation of our study is the surrogate measures of insulin sensitivity that were used, namely HOMA-IR and the SPISE index. Surrogate measures have the advantage of being less invasive, inexpensive, and less labor-intensive than the gold standard test of insulin sensitivity, the hyperinsulinemic euglycemic clamp test. However, it is important to recognize that surrogate measures are not equivalent to the gold standard clamp test. Firstly, HOMA-IR solely depends on fasting glucose and insulin concentrations and is based physiologically on the feedback loop between hepatic glucose production and pancreatic β-cell insulin production, which functions to maintain euglycemia. Therefore, HOMA-IR is largely a reflection of hepatic insulin sensitivity. HOMA-IR was originally determined based on the hyperinsulinemic–euglycemic clamp (51), although the correlation between HOMA-IR and hyperinsulinemic–euglycemic clamp in pediatric populations vary between studies (eg, r = 0.56–0.91) (52–55). In contrast to HOMA-IR, the SPISE index is a surrogate marker of whole-body insulin sensitivity determined from fasting TG and HDL-C concentrations and BMI. The SPISE index only moderately correlated with the hyperinsulinemic euglycemic clamp (ie, r = 0.474 in adults, r = 0.561 in pediatrics) in the population from which the SPISE index was derived (26). Furthermore, only 29 pediatric patients were included in the aforementioned analysis and this index has yet to be validated in a separate pediatric population.
Adolescents with obesity exhibited postprandial dyslipidemia, primarily exaggerated postprandial large TRL particles following dietary fat ingestion. Dyslipidemia and insulin resistance associated with diminished metabolic regulators, GLPs and bile acids, which may contribute to metabolic impairments associated with excess adiposity. However, it remains unknown whether GLPs or bile acids are initially impaired, potentially contributing to the subsequent dysregulation of the other, given their complex interplay. While bile acids promote GLP secretion (14,15), GLP-1R agonism has also been shown to reduce gallbladder emptying in humans (56) and increase hepatic Cyp7a1 mRNA expression in mice (57). Gallbladder GLP-2R activation has also been shown to mediate bile acid-induced gallbladder filling (58). Overall, blunted postprandial GLPs and bile acids are an early manifestation in adolescents with obesity and correlate with abnormal postprandial lipid and lipoprotein metabolism and insulin resistance. These observations are foundational for future investigation into their potential clinical utility as early biomarkers of the development of postprandial dyslipidemia and insulin resistance in obesity.
Acknowledgments
We would like to thank all the participants and their families for their participation, without whom this study would not have been possible. The MSD instrument used to measure intensity of emitted light for the active and total GLP-1 assay kits was supported by the 3D (Diet, Digestive Tract and Disease) Centre funded by the Canadian Foundation for Innovation and Ontario Research Fund, project number 19442 and 30961. We would like to thank Jun Han and Christoph Borchers from the University of Victoria—Genome BC Proteomics Centre (PC) UVic node of the Metabolomics Innovation Centre for bile acid profile analysis by mass spectrometry. Dr. Khosrow Adeli is the guarantor of this manuscript.
Financial Support: This study was supported by a Foundations Grant from the Canadian Institutes of Health Research (CIHR) to K.A. V.H. was supported by a CIHR doctoral award.
Author Contributions: V.H. and K.A. designed the research study. V.H. conducted clinical study days, performed sample and statistical analysis, data interpretation, and manuscript writing. J.K.H. assisted with participant recruitment and manuscript writing. A.W. and A.R. analyzed lipoprotein profile by NMR spectroscopy. B.H. and J.J.H. analyzed intact GLP-2. S.A. assisted with development of statistical code. KA assisted with data interpretation and manuscript writing.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- apoB
apolipoprotein B
- AST
aspartate aminotransferase
- AUC
area under the curve
- BIA
bioelectrical impedance analysis
- BP
blood pressure
- C
cholesterol
- CALIPER
Canadian Laboratory Initiative on Pediatric Reference Intervals
- CDC
Centers for Disease Control and Prevention
- CDCA
chenodeoxycholic acid
- CM
chylomicron
- CRP
C-reactive protein
- CVD
cardiovascular disease
- DPP-IV
dipeptidyl peptidase IV
- ELISA
enzyme-linked immunosorbent assay
- FXR
farnesoid X receptor
- GLP
glucagon-like peptide
- HDL
high-density lipoprotein
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- LCA
lithocholic acid
- LDL
low-density lipoprotein
- MRM
multiple reaction monitoring
- NHANES
National Health and Nutrition Examination Survey
- NMR
nuclear magnetic resonance
- NW
normal weight
- OB
obese
- OGTT
oral glucose tolerance test
- P
particle
- SEM
standard error of the mean
- STOMP
SickKids Team Obesity Management Program
- SPISE
Single Point Insulin Sensitivity Estimator
- T2D
type 2 diabetes
- TG
triglyceride
- TGR5
Takeda G-protein-coupled receptor 5
- TRL
triglyceride-rich lipoprotein
- WHO
World Health Organization
- WHtR
waist-to-height ratio
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Juhola J, Magnussen CG, Viikari JS, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159(4):584–590. [DOI] [PubMed] [Google Scholar]
- 2. Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–1656. [DOI] [PubMed] [Google Scholar]
- 3. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. Jama. 2007;298(3):309–316. [DOI] [PubMed] [Google Scholar]
- 4. Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler Thromb Vasc Biol. 2006;26(6):1357–1363. [DOI] [PubMed] [Google Scholar]
- 5. Sahade V, França S, Adan LF. The influence of weight excess on the postprandial lipemia in adolescents. Lipids Health Dis. 2013;12:(17):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umpaichitra V, Banerji MA, Castells S. Postprandial hyperlipidemia after a fat loading test in minority adolescents with type 2 diabetes mellitus and obesity. J Pediatr Endocrinol Metab. 2004;17(6):853–864. [DOI] [PubMed] [Google Scholar]
- 7. Urbina EM, McCoy CE, Gao Z, et al. Lipoprotein particle number and size predict vascular structure and function better than traditional lipids in adolescents and young adults. J Clin Lipidol. 2017;11(4):1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah AS, Davidson WS, Gao Z, Dolan LM, Kimball TR, Urbina EM. Superiority of lipoprotein particle number to detect associations with arterial thickness and stiffness in obese youth with and without prediabetes. J Clin Lipidol. 2016;10(3):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsieh J, Longuet C, Baker CL, et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia. 2010;53(3):552–561. [DOI] [PubMed] [Google Scholar]
- 10. Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol. 2012;32(6):1513–1519. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh J, Longuet C, Maida A, et al. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137(3):997–1005, 1005.e1. [DOI] [PubMed] [Google Scholar]
- 12. Meier JJ, Nauck MA, Pott A, et al. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130(1):44–54. [DOI] [PubMed] [Google Scholar]
- 13. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–744. [DOI] [PubMed] [Google Scholar]
- 14. Zambad SP, Tuli D, Mathur A, et al. TRC210258, a novel TGR5 agonist, reduces glycemic and dyslipidemic cardiovascular risk in animal models of diabesity. Diabetes Metab Syndr Obes. 2013;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng C, Zhou W, Wang T, et al. A novel TGR5 activator WB403 promotes GLP-1 secretion and preserves pancreatic β-cells in type 2 diabetic mice. Plos One. 2015;10(7):e0134051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kolovou GD, Mikhailidis DP, Kovar J, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9(3):258–270. [DOI] [PubMed] [Google Scholar]
- 18. Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5-19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 2015;78(6):723–729. [DOI] [PubMed] [Google Scholar]
- 19. Gray DS, Bray GA, Gemayel N, Kaplan K. Effect of obesity on bioelectrical impedance. Am J Clin Nutr. 1989;50(2):255–260. [DOI] [PubMed] [Google Scholar]
- 20. Deurenberg P, Kusters CS, Smit HE. Assessment of body composition by bioelectrical impedance in children and young adults is strongly age-dependent. Eur J Clin Nutr. 1990;44(4):261–268. [PubMed] [Google Scholar]
- 21. Nordestgaard BG, Langsted A, Mora S, et al. ; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative . Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cutpoints—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2016;62(7):930–946. [DOI] [PubMed] [Google Scholar]
- 22. Higgins V, Asgari S, Hamilton JK, et al. Impaired postprandial response of glucagon-like peptides and bile acids concomitant with postprandial dyslipidemia in adolescents with obesity and insulin resistance—Supplemental Files. TSpace; 2019. Deposited 23 July 2019. http://hdl.handle.net/1807/95925
- 23. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–870. [DOI] [PubMed] [Google Scholar]
- 24. Han J, Liu Y, Wang R, Yang J, Ling V, Borchers CH. Metabolic profiling of bile acids in human and mouse blood by LC-MS/MS in combination with phospholipid-depletion solid-phase extraction. Anal Chem. 2015;87(2):1127–1136. [DOI] [PubMed] [Google Scholar]
- 25. Kurtoğlu S, Hatipoğlu N, Mazıcıoğlu M, Kendirici M, Keskin M, Kondolot M. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J Clin Res Pediatr Endocrinol. 2010;2(3):100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paulmichl K, Hatunic M, Højlund K, et al. ; Beta-JUDO Investigators; RISC Investigators . Modification and validation of the triglyceride-to-HDL cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: the Single Point Insulin Sensitivity Estimator (SPISE). Clin Chem. 2016;62(9):1211–1219. [DOI] [PubMed] [Google Scholar]
- 27. Vine DF, Wang Y, Jetha MM, Ball GD, Proctor SD. Impaired ApoB-lipoprotein and triglyceride metabolism in obese adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):970–982. [DOI] [PubMed] [Google Scholar]
- 28. Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagné C, Couture P. Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J Lipid Res. 2007;48(6):1336–1342. [DOI] [PubMed] [Google Scholar]
- 29. Mamo JC, Watts GF, Barrett PH, Smith D, James AP, Pal S. Postprandial dyslipidemia in men with visceral obesity: an effect of reduced LDL receptor expression? Am J Physiol Endocrinol Metab. 2001;281(3):E626–E632. [DOI] [PubMed] [Google Scholar]
- 30. Brunzell JD, Hazzard WR, Porte D Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973;52(7):1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nogueira JP, Maraninchi M, Béliard S, et al. Absence of acute inhibitory effect of insulin on chylomicron production in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2012;32(4):1039–1044. [DOI] [PubMed] [Google Scholar]
- 32. Panarotto D, Rémillard P, Bouffard L, Maheux P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur J Clin Invest. 2002;32(2):84–92. [DOI] [PubMed] [Google Scholar]
- 33. Haidari M, Leung N, Mahbub F, et al. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem. 2002;277(35):31646–31655. [DOI] [PubMed] [Google Scholar]
- 34. Stenlid R, Manell H, Halldin M, et al. High DPP-4 concentrations in adolescents are associated with low intact GLP-1. J Clin Endocrinol Metab. 2018;103(8):2958–2966. [DOI] [PubMed] [Google Scholar]
- 35. Ichimura A, Hirasawa A, Poulain-Godefroy O, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. [DOI] [PubMed] [Google Scholar]
- 36. Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52(2):252–259. [DOI] [PubMed] [Google Scholar]
- 37. Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, Brubaker PL. Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology. 2009;150(2):580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chanoine JP, Mackelvie KJ, Barr SI, Wong AC, Meneilly GS, Elahi DH. GLP-1 and appetite responses to a meal in lean and overweight adolescents following exercise. Obesity. 2008;16(1):202–204. [DOI] [PubMed] [Google Scholar]
- 39. Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62(2):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520.9449682 [Google Scholar]
- 41. Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120(4):806–815. [DOI] [PubMed] [Google Scholar]
- 42. Grasset E, Puel A, Charpentier J, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017;25(5):1075–1090.e5. [DOI] [PubMed] [Google Scholar]
- 43. Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond). 2013;37(12):1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park WH, Pak YK. Insulin-dependent suppression of cholesterol 7α-hydroxylase is a possible link between glucose and cholesterol metabolisms. Exp Mol Med. 2011;43(10):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14(7):778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haeusler RA, Camastra S, Nannipieri M, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J Clin Endocrinol Metab. 2016;101(5):1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Potthoff MJ, Potts A, He T, et al. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol. 2013;304(4):G371–G380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sonne DP, van Nierop FS, Kulik W, Soeters MR, Vilsbøll T, Knop FK. Postprandial plasma concentrations of individual bile acids and FGF-19 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101(8):3002–3009. [DOI] [PubMed] [Google Scholar]
- 49. Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2015;64(4):1168–1179. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 52. Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25(11):2081–2087. [DOI] [PubMed] [Google Scholar]
- 53. George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab. 2011;96(7):2136–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89(3):1096–1101. [DOI] [PubMed] [Google Scholar]
- 55. Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. [DOI] [PubMed] [Google Scholar]
- 56. Keller J, Trautmann ME, Haber H, et al. Effect of exenatide on cholecystokinin-induced gallbladder emptying in fasting healthy subjects. Regul Pept. 2012;179(1-3):77–83. [DOI] [PubMed] [Google Scholar]
- 57. Farr S, Stankovic B, Hoffman S, et al. Bile acid treatment and FXR agonism lower postprandial lipemia in mice. [Published online ahead of print January 31, 2020]. Am J Physiol Gastrointest Liver Physiol. 2020. Doi: 10.1152/ajpgi.00386.2018. [DOI] [PubMed] [Google Scholar]
- 58. Yusta B, Matthews D, Flock GB, et al. Glucagon-like peptide-2 promotes gallbladder refilling via a TGR5-independent, GLP-2R-dependent pathway. Mol Metab. 2017;6(6):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.