Abstract
Background:
The programmed intermittent epidural bolus (PIEB) technique is widely used in labor analgesia, but the parameter settings of PIEB have not yet been standardized. We designed a study to identify the optimal interval duration for PIEB using 10 mL of ropivacaine 0.08% and sufentanyl 0.3 μg/mL, a regimen commonly used to control labor pain in China, to provide effective analgesia in 90% of women during the first stage of labor without breakthrough pain.
Methods:
We conducted a double-blind sequential allocation trial to obtain the effective interval 90% (EI90%) during the first stage of labor between April 2019 and May 2019. This study included the American Society of Anesthesiologists physical status II–III nulliparous parturients at term, who requested epidural analgesia. The bolus volume was fixed at 10 mL of ropivacaine 0.08% with sufentanyl 0.3 μg/mL. Participants were divided into four groups (groups 60, 50, 40, and 30) according to the PIEB intervals (60, 50, 40, and 30 min, respectively). The interval duration of the first parturient was set at 60 min and that of subsequent parturients varied according to a biased-coin design. The truncated Dixon and Mood method and the isotonic regression analysis method were used to estimate the EI90% and its 95% confidence intervals (CIs).
Results:
Forty-four women were enrolled in this study. The estimated optimal interval was 44.1 min (95% CI 41.7–46.5 min) and 39.5 min (95% CI 32.5–50.0 min), using the truncated Dixon and Mood method and isotonic regression analysis, respectively. The maximum sensory block level above T6 was in nearly 20% of parturients in group 30; however, 5.3%, 0%, and 0% of the parturients presented with sensory block level above T6 in groups 40, 50, and 60, respectively. There were no cases of hypotension and only one parturient complained of motor block.
Conclusion:
With a fixed 10 mL dose of ropivacaine 0.08% with sufentanyl 0.3 μg/mL, the optimal PIEB interval is about 42 min. Further studies are warranted to define the efficacy of this regimen throughout all stages of labor.
Trial registration: Chinese Clinical Trial Registry, ChiCTR1900022199; http://www.chictr.org.cn/com/25/historyversionpuben.aspx?regno=ChiCTR1900022199.
Keywords: Anaesthetic techniques, First stage labor, Labor analgesia, Ropivacaine
Introduction
Epidural anesthesia is a powerful method for relieving pain during labor. Continuous advancements have been made in the epidural technique for labor analgesia, with development of epidural infusion pumps including the high-pressure pump.[1] With a fixed volume of anesthetic automatically pumped at scheduled intervals, the programmed intermittent epidural bolus (PIEB) technique has gradually replaced continuous epidural infusion (CEI) and is now extensively used in obstetric analgesia for superior maternal satisfaction and a lower incidence of adverse events.[2–7]
Regretfully, the parameter settings of PIEB have not been definitively standardized and the settings for an optimal PIEB regimen to improve the effect of labor analgesia remain unclear. Recently, some studies have suggested that the epidural catheter size and type and the flow rate have an effect on epidural analgesia.[8,9] However, other studies have focused more on the pump settings.[1,10,11] For example, a dose-finding study was performed to establish the PIEB pump settings of bupivacaine 0.0625% with fentanyl 2 μg/mL.[11] According to the parameter settings, PIEB can achieve an ideal and desired level of pain relief, without the use of supplementary measures, in 90% of parturients during the first stage of labor. The study suggested that the optimal interval duration for PIEB with bupivacaine and fentanyl was approximately 40 min.
Combined ropivacaine 0.08% and sufentanyl 0.3 μg/mL is a standard regimen to treat labor pain in China, and it is the most commonly used in our hospital. As an amide-type local anesthetic, ropivacaine has pharmacological properties similar to those of bupivacaine. Ropivacaine has increasingly replaced bupivacaine in obstetric anesthesia, because it causes less motor blockade and cardiovascular and central nervous system toxicity.[12,13] In terms of efficacy for labor analgesia, ropivacaine has been determined to be approximately 60% as potent as bupivacaine in a study.[14] However, Wang et al[15] found that the analgesic efficacy of bupivacaine and ropivacaine mainly depends on the concentration rather than the type of anesthetics. It could not be assumed that the optimal interval duration for PIEB with ropivacaine and sufentanyl was the same as that of PIEB with bupivacaine and fentanyl.
Thus, we here investigated the optimal interval duration for PIEB with ropivacaine 0.08% and 0.3 μg/mL sufentanyl to prevent breakthrough pain during the first stage labor in 90% of parturients.
Methods
Ethical approval
This study was undertaken in a tertiary maternity hospital in China. The study was approved by the Ethics Committee of Fujian Provincial Maternity and Children Hospital (No.2019-021). This prospective, double-blind trial was conducted using a biased-coin up-and-down sequential allocation method, from April to May 2019. We obtained written consent from each parturient and their families.
Study design and population
We included the American Society of Anesthesiologists physical status classification II–III primiparous, singleton women in labor at more than 37 weeks of gestation, with cervical dilation of 2 to 5 cm, and a visual analog scale (VAS, 0–10 points, with VAS 0 = no pain and 10 = the worst imaginable pain) score exceeding 5, who requested epidural labor analgesia. We excluded parturients in case of major anomalies of the fetus, intrauterine growth retardation, fetal distress, or non-vertex presentation; maternal factors for exclusion included allergy or hypersensitivity to ropivacaine or sufentanyl, contraindications to epidural analgesia, significant bleeding during pregnancy, significant medical disease, such as cardiopulmonary dysfunction, severe pregnancy-induced hypertension, receipt of parenteral opioids in the previous 4 h, or a body mass index of greater than 35 kg/m2, and unintentional dural puncture.
For a biased-coin up-and-down design, at least 20 to 40 parturients must be enrolled to provide a stable estimate of the target dose in most cases, according to simulation studies.[16,17] Therefore, 44 parturients were enrolled in this study.
Anesthetic procedure
After the eligible parturients arrived in the delivery room, standard monitoring procedures including recording of baseline maternal heart rate, non-invasive blood pressure, type of labor (spontaneous or induced), dosage of oxytocin used, and VAS score were performed. Venous access for intravenous (IV) fluids was established through which Ringer lactate was administered.
Epidural puncture was performed at the L2–3 vertebral interspace, using a loss of resistance technique, and the catheter was inserted into the epidural space with a depth of about 3 to 4 cm. The parturient was placed in the supine position with a left lateral tilt after the catheter was fixed. Then, 3 mL 1.73% lidocaine carbonate (Zhuhai Rundu Pharmaceutical Co., Ltd., China) was injected to rule out the possibility of subarachnoid injection or IV injection in the next 5 min.
Subsequently, a loading dose of 10 mL of ropivacaine (AstraZeneca AB, Sweden) 0.08% with sufentanyl (Yichang Renfu Pharmaceutical Co., Ltd., Hubei, China) 0.3 μg/mL was administered through the catheter. The VAS score was assessed in the following 20 and 60 min, and only parturient whose VAS score was less than 1 continued in the study. A solution of ropivacaine 0.08% with sufentanyl 0.3 μg/mL was administered as a bolus via the PIEB pump 1 h after administration of the loading dose.
The patient-controlled epidural analgesia (PCEA) settings were as follows: 10 mL initial bolus, 5 mL subsequent bolus dose, and 10-min lockout interval. The parturients were assigned to groups 60, 50, 40, and 30 according to the set interval duration. The study was performed using the biased-coin method, with biased-coin allocation based on an Excel-generated list of random responses prepared by the research investigator.[1,10,11] The research investigator would enter the data and unveil the allocated interval for the parturient to a specific nurse. After receiving the information, the nurse would set up the epidural pump and cover the pump with an opaque bag. Neither the parturient nor the anesthetist was aware of the pump settings.
If the parturient felt pain during the labor, the parturient could press the PCEA device button or ask the anesthetist to take measures to relieve her pain. In that case, the analgesic regimen with the given interval duration was considered to be ineffective.
Assessments were conducted by an anesthetist every hour after the initiation of the PIEB until completion of the study. The completion of the study was defined as 6 h after the initiation of PIEB or the end of the first stage of labor, whichever occurred first. The severity of pain is known to become markedly more intense as labor progresses. During the second stage of labor, in particular, labor pain is complex and is influenced by many factors.[18] Thus, to assess the effectiveness of this PIEB regimen, we included only observations of the 6 h in the first stage of labor.
We regarded effective analgesia as the primary outcome, we defined it as no use of requirement for additional measures to relieve labor pain, such as a PCEA or a manual bolus before completion of the study.
Secondary outcomes were as follows: the maximum sensory block level (detected by applying ice in the midclavicular line), motor block degree in the leg (assessed using the modified Bromage score), and non-invasive blood pressure (assessed between uterine contractions).[19,20]
Statistical analysis
The statistic part of our study is instructed by a professional statistician. Parturient characteristics and the secondary outcomes were summarized descriptively between various time interval groups. The primary outcome of effective interval (EI) 90% and its 95% confidence interval (CI) was estimated using the truncated Dixon and Mood method and the isotonic regression analysis method, with the pooled-adjacent-violators algorithm approach. These are two non-parametric methods which have been used and described previously in similar studies.[11] Statistical analyses were performed using the SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA) and the R package (R version 3.1.3; www.r-project.org).[21]
Results
From April 2019 to May 2019, fifty-one parturients participated in the study. Three parturients were excluded from the analysis because they had a VAS score exceeding 1 within 20 min after administration of the loading dose. Two parturients were excluded because their conditions warranted cesarean deliveries. Two parturients were excluded because they suspended the use of the pump due to not feeling uterine contractions. Finally, forty-four parturients were included in the data analysis. Parturient demographics and labor characteristics are analyzed in Table 1. The parturients allocation sequence and response to different PIEB interval times are shown in Figure 1.
Table 1.
Characteristics of parturients included in the study (n = 44).
Figure 1.
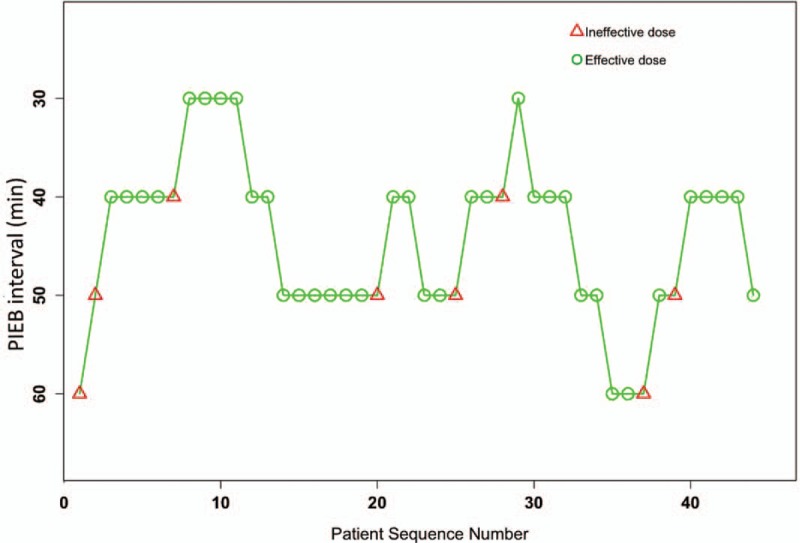
Patient allocation sequence and response to the assigned PIEB interval time. An effective PIEB interval time is expressed by a circle, while an ineffective one is expressed by a triangle. PIEB: Programmed intermittent epidural bolus.
Using the truncated Dixon and Mood method, the estimated effective interval 90% (EI90%) was 44.1 min (95% CI 41.7–46.5 min). Using the isotonic regression analysis, the estimated EI90% was 39.5 min (95% CI 32.5–50.0 min). Considering the two results, we regard approximately 42 min as the optimal interval time.
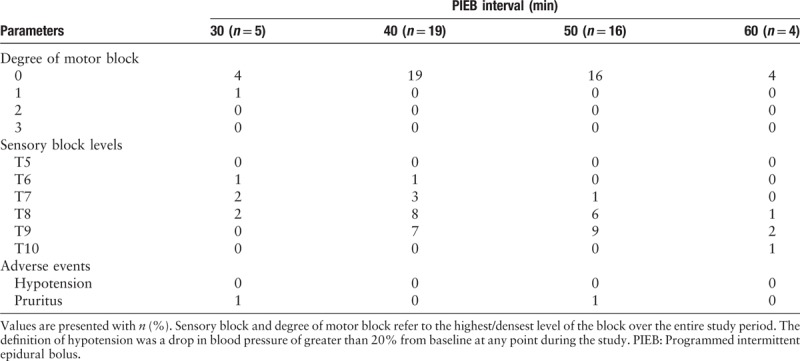
Effective analgesia of 100% and 89% were achieved in groups 30 and 40, respectively. The response rate for each interval time and the adjusted response rates are shown in Table 2. We also summarize the time to PCEA requests after administrating the loading doses in parturients who did not respond to the protocol. Eight parturients failed to reach adequate analgesia with the proposed PIEB regimen. There were two parturients in group 60 who did not achieve adequate analgesia, and the time from PIEB to first bolus rescue was 204 and 98 min, respectively. There were four parturients in group 50 who failed to reach adequate analgesia, and the time from PIEB to first bolus rescue was 120, 197, 105, and 182 min, respectively. There were two parturients in group 40 that did not achieve adequate analgesia, and the time from PIEB to PCEA request was 345 and 240 min, respectively. All the parturients in group 30 achieve adequate analgesia. Parturients in group 30 presented with a higher sensory block level upon ice stimulation than other groups. Almost 20% of the parturients in group 30 presented with sensory blocks above T6, the rate was 5.3%, 0, and 0 in groups 40, 50, and 60, respectively. None of the parturients in group 30 presented with sensory block levels lower than T8. Moreover, no case of hypotension was observed in the study. There was only one parturient in group 30 who presented with motor block. The maximum sensory block level, degree of motor block, and any possible adverse events in each sub-group are shown in Table 3.
Table 2.
Observed response rate of parturients with successful analgesia and PAVA-adjusted response rate.
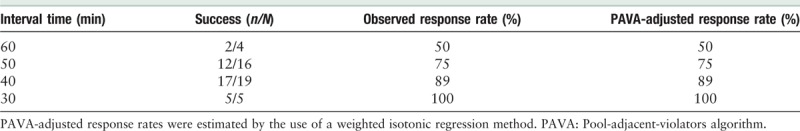
Table 3.
Degree of motor block, sensory block levels, and adverse events.
Discussion
In this study, we obtained the EI90% for PIEB with 10 mL of ropivacaine 0.08% and sufentanyl 0.3 μg/mL in nulliparous parturients during the first stage of labor. Our study showed that the optimal interval time is approximately 42 min. The corresponding hourly consumption of ropivacaine was 12 mg, which was found to be safe for epidural analgesia.
Although ropivacaine has similar pharmacological properties to bupivacaine, it induces less motor blockade and thus lower rates of instrumental vaginal delivery. It has, therefore, largely replaced bupivacaine during labor. Ropivacaine is a long-acting local anesthetic, nevertheless, it has been shown in some studies that ropivacaine is less potent than bupivacaine.[14] We used the same bolus volumes and duration of time intervals as in a study by Epsztein et al[11] where bupivacaine 0.0625% with fentanyl 2 μg/mL was used as the analgesic regimen rather than ropivacaine 0.08% with sufentanil 0.3 μg/mL. In the study, we observed that the incidence of motor blockade was significantly lower than that of the study.[1,11] Only one parturient developed motor blockade, which was assessed as Bromage score 1.
Our study demonstrated that shorter PIEB intervals of 30 to 40 min may induce higher sensory block levels than longer PIEB intervals (50–60 min). In our study, more than 5.3% and 20% of the parturients developed a sensory block above T6 in groups 40 and 30, respectively. Nevertheless, the maximum sensory block level of ropivacaine was found to be lower than that of bupivacaine. Aside from the lower incidence of motor blockade, no cases of hypotension were reported for ropivacaine. While two parturients complained of pruritus, no other occurrences of adverse effects or discomfort in the parturients were observed. PIEB with ropivacaine and sufentanyl was found to be much safer than that of bupivacaine and fentanyl.
We did not use the degree of satisfaction in parturients as an outcome variable in our study. In fact, we assessed the patient satisfaction score in our study. The parturients were instructed to press the PCEA button or requested a physician-delivered top-up if contractions were uncomfortable, so all the parturients were satisfied with labor analgesia.
The analgesia rate during labor in China is still tentatively less than 10% even in more developed areas such as Beijing. The Chinese government is attempting to popularize labor analgesia and increase the labor analgesia rate.[22] However, there is a critical shortage of anesthetists in many hospitals, we thus carried out this study to standardize analgesic protocol for improving safety and efficacy during labor.
Our study had some limitations. First, we only investigated nulliparous women during the first stage of labor with cervical dilation less than 5 cm. Consequently, our results could not apply to more advanced labor stages or multiparous women. Secondly, epidural analgesia administration is not uniform. The anesthetists can choose different types and doses of local anesthetic agents and adjuvant drugs, and different techniques to maintain epidural analgesia for the duration of labor. Evidence suggests that altering these variables may influence the outcomes of labor analgesia. Thus, our results only applied to PIEB with ropivacaine 0.08% and 0.3 μg/mL sufentanyl.
In conclusion, the EI90% for PIEB with 10 mL of ropivacaine 0.08% and sufentanyl 0.3 μg/mL is approximately 42 min. With the recommended PIEB interval time, the parturient can avoid breakthrough pain and make fewer additional requests for PCEA or manual bolus, which will allow the anesthetists to more effectively provide comfort to a greater number of parturients undergoing labor. However, future larger studies are warranted to confirm other PIEB settings and to address the analgesic needs of parturients in all stages of labor.
Acknowledgments
We want to extend our gratitude to statistician Fei Gu and anesthetist Xiao-Fen Chen for their kind help.
Funding
This work was supported by a grant from the Science and Technology Commission of Shanghai Municipality (No. 16411967400)
Conflicts of interest
None.
Footnotes
How to cite this article: Zhou SQ, Wang J, Du WJ, Song YJ, Xu ZD, Liu ZQ. Optimum interval time of programmed intermittent epidural bolus of ropivacaine 0.08% with sufentanyl 0.3 μg/mL for labor analgesia: a biased-coin up-and-down sequential allocation trial. Chin Med J 2020;133:517–522. doi: 10.1097/CM9.0000000000000669
Shuang-Qiong Zhou and Jing Wang contributed equally to this work.
References
- 1.Zakus P, Arzola C, Bittencourt R, Downey K, Ye XY, Carvalho JC. Determination of the optimal programmed intermittent epidural bolus volume of bupivacaine 0.0625% with fentanyl 2 mug.ml(-1) at a fixed interval of forty minutes: a biased coin up-and-down sequential allocation trial. Anaesthesia 2018; 73:459–465. doi: 10.1111/anae.14159. [DOI] [PubMed] [Google Scholar]
- 2.Diez-Picazo LD, Guasch E, Brogly N, Gilsanz F. Is breakthrough pain better managed by adding programmed intermittent epidural bolus to a background infusion during labor epidural analgesia? A randomized controlled trial. Minerva Anestesiol 2019; 85:1097–1104. doi: 10.23736/S0375-9393.19.13470-0. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer LE, Romero DJ, Vasquez OI, Matute EC, Van de Velde M. Effect of programmed intermittent epidural boluses and continuous epidural infusion on labor analgesia and obstetric outcomes: a randomized controlled trial. Arch Gynecol Obstet 2017; 296:915–922. doi: 10.1007/s00404-017-4510-x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Campoo MB, Curto A, Gonzalez M, Aldecoa C. Patient intermittent epidural boluses (PIEB) plus very low continuous epidural infusion (CEI) versus patient-controlled epidural analgesia (PCEA) plus continuous epidural infusion (CEI) in primiparous labour: a randomized trial. J Clin Monit Comput 2019; 33:879–885. doi: 10.1007/s10877-018-0229-x. [DOI] [PubMed] [Google Scholar]
- 5.Leone Roberti Maggiore U, Silanos R, Carlevaro S, Gratarola A, Venturini PL, Ferrero S, et al. Programmed intermittent epidural bolus versus continuous epidural infusion for pain relief during termination of pregnancy: a prospective, double-blind, randomized trial. Int J Obstet Anesth 2016; 25:37–44. doi: 10.1016/j.ijoa.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Ojo OA, Mehdiratta JE, Gamez BH, Hunting J, Habib AS. Comparison of programmed intermittent epidural boluses with continuous epidural infusion for the maintenance of labor analgesia: a randomized, controlled, double-blind study. Anesth Analg 2019; doi: 10.1213/ANE.0000000000004104, [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho B, George RB, Cobb B, McKenzie C, Riley ET. Implementation of programmed intermittent epidural bolus for the maintenance of labor analgesia. Anesth Analg 2016; 123:965–971. doi: 10.1213/ANE.0000000000001407. [DOI] [PubMed] [Google Scholar]
- 8.Krawczyk P, Piwowar P, Salapa K, Lonc T, Andres J. Do epidural catheter size and flow rate affect bolus injection pressure in different programmed intermittent epidural bolus regimens? An in vitro study. Anesth Analg 2018; 129:1587–1594. doi: 10.1213/ANE.0000000000003650. [DOI] [PubMed] [Google Scholar]
- 9.Lange EMS, Wong CA, Fitzgerald PC, Davila WF, Rao S, McCarthy RJ, et al. Effect of epidural infusion bolus delivery rate on the duration of labor analgesia: a randomized clinical trial. Anesthesiology 2018; 128:745–753. doi: 10.1097/ALN.0000000000002089. [DOI] [PubMed] [Google Scholar]
- 10.Bittencourt R, Arzola C, Zakus P, Downey K, Ye XY, Carvalho JCA. A biased coin up-and-down sequential allocation trial to determine the optimum programmed intermittent epidural bolus time interval between 5 mL boluses of bupivacaine 0.125% with fentanyl 2 microg.mL(-1). Can J Anaesth 2019; 66:1075–1081. doi: 10.1007/s12630-019-01407-7. [DOI] [PubMed] [Google Scholar]
- 11.Epsztein Kanczuk M, Barrett NM, Arzola C, Downey K, Ye XY, Carvalho JC. Programmed Intermittent epidural bolus for labor analgesia during first stage of labor: a biased-coin up-and-down sequential allocation trial to determine the optimum interval time between boluses of a fixed volume of 10 mL of bupivacaine 0.0625% with fentanyl 2 mug/mL. Anesth Analg 2017; 124:537–541. doi: 10.1213/ANE.0000000000001655. [DOI] [PubMed] [Google Scholar]
- 12.Riazanova OV, Alexandrovich YS, Guseva YV, Ioscovich AM. A randomized comparison of low dose ropivacaine programmed intermittent epidural bolus with continuous epidural infusion for labour analgesia. Rom J Anaesth Intensive Care 2019; 26:25–30. doi: 10.2478/rjaic-2019-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpern SH, Breen TW, Campbell DC, Muir HA, Kronberg J, Nunn R, et al. A multicenter, randomized, controlled trial comparing bupivacaine with ropivacaine for labor analgesia. Anesthesiology 2003; 98:1431–1435. doi: 10.1097/00000542-200306000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Ngan Kee WD, Ng FF, Khaw KS, Tang SPY, Koo AGP. Dose-response curves for intrathecal bupivacaine, levobupivacaine, and ropivacaine given for labor analgesia in nulliparous women. Reg Anesth Pain Med 2017; 42:788–792. doi: 10.1097/AAP.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 15.Wang LZ, Chang XY, Liu X, Hu XX, Tang BL. Comparison of bupivacaine, ropivacaine and levobupivacaine with sufentanil for patient-controlled epidural analgesia during labor: a randomized clinical trial. Chin Med J 2010; 123:178–183. doi: 10.3760/cma.j.issn.0366-6999.2010.02.010. [PubMed] [Google Scholar]
- 16.Chen L, Wu Y, Cai Y, Ye Y, Li L, Xia Y, et al. Comparison of programmed intermittent bolus infusion and continuous infusion for postoperative patient-controlled analgesia with thoracic paravertebral block catheter: a randomized, double-blind, controlled trial. Reg Anesth Pain Med 2019; 44:240–245. doi: 10.1136/rapm-2018-000031. [DOI] [PubMed] [Google Scholar]
- 17.Bullingham A, Liang S, Edmonds E, Mathur S, Sharma S. Continuous epidural infusion vs programmed intermittent epidural bolus for labour analgesia: a prospective, controlled, before-and-after cohort study of labour outcomes. Br J Anaesth 2018; 121:432–437. doi: 10.1016/j.bja.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Shen X, Li Y, Xu S, Wang N, Fan S, Qin X, et al. Epidural analgesia during the second stage of labor: a randomized controlled trial. Obstet Gynecol 2017; 130:1097–1103. doi: 10.1097/AOG.0000000000002306. [DOI] [PubMed] [Google Scholar]
- 19.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand Suppl 1965; 16:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 20.Graham AC, McClure JH. Quantitative assessment of motor block in labouring women receiving epidural analgesia. Anaesthesia 2001; 56:470–476. doi: 10.1046/j.1365-2044.2001.01524-6.x. [DOI] [PubMed] [Google Scholar]
- 21.Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a precis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 2007; 107:144–152. doi: 10.1097/01.anes.0000267514.42592.2a. [DOI] [PubMed] [Google Scholar]
- 22.Fan ZT, Gao XL, Yang HX. Popularizing labor analgesia in China. Int J Gynaecol Obstet 2007; 98:205–207. doi: 10.1016/j.ijgo.2007.03.007. [DOI] [PubMed] [Google Scholar]


