Abstract
Purpose/Objectives
The Gamma-Knife radiosurgery (GKRS) (Elekta AB, Stockholm) platform delivers highly conformal and precise radiation; however, intracranial displacement during treatment allows for the potential of a marginal target-miss. Frameless (mask-based) GKRS using the Gamma Knife Icon system monitors nasal tip motion as a surrogate for intracranial motion by tracking an infrared marker using a high-definition motion management (HDMM) system. To date, there is limited data available regarding the incidence and severity of motion and factors that impact intrafraction motion when treating with frameless GKRS.
Materials/Methods
A retrospective study was performed to evaluate patients with brain tumors who were treated with frameless GKRS using the Gamma Knife Icon between May and December 2018. All patients underwent mask-based immobilization using a thermoplastic mask. Data on patient demographics, mask type, use of bite block, and number of treatments received, use of anxiolytics, treatment time, and whether a physics clearance check was performed prior to treatment were collected. For each treatment session, average displacement (mm), maximum displacement (mm) and total treatment time (min) were recorded and logistic regression analyses were performed.
Results
Data was collected for 89 consecutive treatments (38 patients). Of these, an anxiolytic was used in 61 treatments and a physics clearance check was performed for 45 treatments. The median average and maximum displacement was 0.60 mm and 1.22 mm, respectively. An average displacement greater than 0.60 mm was seen with Eastern Cooperative Oncology Group performance status (ECOG) > 1, male gender, and malignant tumors (p < 0.05). Anxiolytic use prior to treatment was associated with a significant reduction in average displacement (p < 0.05). Significantly greater odds of observing a maximum displacement over 1.22 mm was seen with patients with ECOG > 1, male gender, and increased treatment time (p < 0.05). Age > 65 and anxiolytic use were associated with a significant reduction in maximum displacement (p < 0.05). Performance of clearance checks and use of bite block use did not impact average or maximum patient displacement.
Conclusions
This is the first study to evaluate patient and treatment-related factors that influence intrafraction motion during GKRS with mask-based immobilization through HDMM tracking. Increased intracranial displacement during frameless GKRS was associated with higher ECOG, male gender, increased treatment time and malignant tumors, while anxiolytics were shown to mitigate excessive motion. Radiosurgery teams should consider these patient factors when treating patients with mask immobilization.
Keywords: Frameless, mask-based Gamma-Knife, intrafraction motion, intracranial displacement
Introduction
The use of targeted radiation via Gamma Knife (Elekta AB, Stockholm) or linear-accelerator based radiosurgery has fundamentally changed the treatment paradigm of primary and metastatic brain tumors as these techniques allow for precise irradiation of tumors while largely sparing normal tissues (1). The Gamma Knife treatment platform utilizes either 192 or 201 cobalt-60 radiation sources that are aligned with a collimator system to direct individual beams to a specific point. This juxtaposition of low dose beams permits high-dose radiation delivery to the target site with minimal impact on tissues located outside the target volume (2). Fixation during Gamma Knife radiosurgery (GKRS) using a rigid stereotactic titanium-alloy frame allows for reduced patient motion; however, the invasiveness and discomfort of securing 4 screws to the patient’s skull makes performing fractionated treatments using this system impractical (3, 4). Less invasive mask-based treatment modalities have been garnering interest and allow for fractionated radiosurgery (5-8). Gamma Knife Icon allows for frameless radiosurgery with thermoplastic mask immobilization, has on-board cone-beam computed tomography (CBCT) capability, and contains an automated collimator setting adjustment process within the GammaPlan software which has the capability to adapt to slight inter-fraction positional changes. Intrafraction motion monitoring capabilities are carried out on the Gamma Knife Icon via the a high-definition motion management (HDMM) system with nasal tip marker infrared tracking (9).
Despite offering a less invasive and more flexible dose-fractionation schedule, the use of frameless Gamma Knife is still limited by concerns regarding its ability to deliver radiation in an accurate and reproducible manner. Although preliminary data suggest increased motion variations with mask-based Gamma Knife (10), there remains a paucity of data regarding the magnitude of motion variation and patient factors leading to increased movement. The purpose of this study was to assess the magnitude of intrafraction displacement and identify which patient and treatment associated factors significantly influence motion susceptibility during frameless GKRS treatments.
Methods and Materials
Inclusion criteria
Data collection and analysis were approved by our Institutional Review Board. Adult patients with primary or metastatic intracranial tumors treated with frameless GKRS from May to December 2018 were included in the study. All patients underwent thermoplastic mask-based immobilization and were treated with one to five fractions. Mask-based radiosurgery immobilization was chosen based on a variety of clinical factors, including proximity to critical structures, use of fractionation, expected treatment time, and patient preference.
Workflow
On the day prior to treatment delivery, patients underwent a thin-slice magnetic resonance imaging (MRI) of the brain for pretreatment planning purposes. In the treatment suite, a customized thermoplastic mask was created to fit the shape and contours of the face. Patients were fitted with either a green mask produced by the Klarity corporation or a yellow mask produced by the Elekta corporation.
An oral cavity device, also known as a “bite block”, was utilized in most patients to presumably aid with immobilization. A CBCT of the head was also obtained at this time to determine the stereotactic coordinates. Subsequently, a simulation CT scan of the head was performed for better image quality and co-registration to the MRI and CBCT. The MRI and simulation CT were initially co-registered and the simulation CT was then fused with the CBCT scan (stereotactic reference). MRI and simulation CT were used to identify critical structures and a conformal radiation dose plan was developed to target the intracranial disease sites.
On the day of treatment, the patient was immobilized and a reflective marker was placed to the patient’s nose tip to serve as a stable anatomical reference point for motion tracking throughout treatment. A pre-treatment CBCT was obtained and co-registered to the stereotactic reference CBCT from the prior day. Image registration was used to assess patient anatomy and positioning. During treatment, the patient position was monitored by the HDMM system via infrared camera tracking of the reflective nasal tip marker. If motion beyond the preset threshold (1.5 mm) was detected, treatment was paused In such an instance when treatment was withdrawn or paused due to unacceptable degree of motion, patient position was subsequently corrected. A new CBCT was then obtained and registered to the stereotactic CBCT to ensure accuracy of position prior to restarting treatment.
Data collection and statistical analysis
Data on patient demographics including age, sex, body mass index (BMI), Eastern Cooperative Oncology Group performance status (ECOG), anxiolytic use prior to treatment, and nature of lesion (malignant or benign) were obtained through retrospective chart review. Information regarding the type of mask (green mask from Klarity corporation vs. yellow mask from Elekta corporation), use of bite block, and whether a clearance check was performed, were documented prior to treatment.
Intrafraction average displacement and maximum displacement in relation to the pretreatment CBCT were recorded. Additionally, total treatment time and number of treatment withdrawals requiring repositioning were recorded during radiation delivery.
Logistic regression analysis was used to identify patient and treatment factors associated with increased average displacement and increased maximum displacement. As information regarding mask type and whether anxiolytic medication was administered was not available for all patients, these factors were analyzed separately using univariate logistic regression analysis. All other factors were analyzed using multivariate logistic regression. Results with p < 0.05 were considered to be statistically significant. SPSS statistical package (Version 25; IBM ® ) was used for statistical analysis.
Results
Clinical Characteristics
During the period between May to December 2018, 38 unique patients underwent a total of 89 treatments with frameless GKRS for various intracranial lesions. Of these patients, 18 (47%) were female and 20 (52%) were male. Median age was 61 years and age range was 26-78 years. ECOG was 0 in 20 cases (52.6%), 1 in 10 cases (26.3%), 2 in 7 cases (18.4%), and unknown in 1 case (2.6%). Fourteen patients (36.8%) had a normal BMI, while 24 patients (63.1%) had BMIs in the overweight range. Of the 38 patients, 5 (13.1%) had benign tumors and 33 (86.8%) had malignant lesions. The clinical characteristics of the patient cohort are outlined in Table 1.
Table 1.
Summary of clinical characteristics in patients who underwent GKRS
| Parameter | No. of patients (N=38) |
| Type of lesion | |
| Non-malignant | 5 |
| Malignant | 33 |
| Age | |
| <65 | 18 |
| >65 | 20 |
| Median age - 61 yrs | |
| BMI | |
| < 24.9 (normal) | 14 |
| >24.9 (overweight) | 24 |
| ECOG status | |
| ECOG<1 | 20 |
| ECOG>1 | 18 |
| Gender | |
| Female | 18 |
| Male | 20 |
Treatment characteristics
A bite block for immobilization was used in 84 treatments (94.3%). With regard to mask type, 62 treatments (69.7%) were performed using the yellow mask and 27 treatments (30.3%) were performed using the green mask.
Physics clearance checks were performed prior to radiation delivery during 45 treatments (50.5%). Anxiolytics were used during 61 treatments (68.5%). Withdrawal of treatment due to unacceptable motion occurred during 28 treatments (31.4%) and involved 16 patients. The number of interruptions during therapy ranged from 1 to 5 times per treatment. Median total treatment time was 23 minutes (range: 2-84 minutes). The treatment characteristics are outlined in Table 2.
Table 2.
Summary of treatment characteristics in patients who underwent GKRS
| Parameter | No of treatments (N=89) |
| Thermoplastic mask type | |
| Yellow (Elekta) | 62 |
| Green (Klarity) | 27 |
| Anxiolytic use prior to treatment | 61 |
| Clearance check prior to treatment | 45 |
| Withdrawal during treatment | 28 |
| Median total treatment time (min) | 23 |
Analysis of intrafraction motion.
Intrafraction motion was measured as displacement from the designated pretreatment stereotactic coordinates. Examples of two patients’ intrafraction motion as recorded by the HDMM during frameless GKRS are depicted in Figure 1. Average intrafraction displacement ranged from 0.1 mm to 1.25 mm with a median of 0.6 mm. Maximum intrafraction displacement ranged from 0.07 mm to 3.16 mm with a median of 1.22 mm. The mean average displacement was 0.65 mm while the mean maximum displacement was 1.29 mm. Intrafraction displacement data is displayed in Table 3.
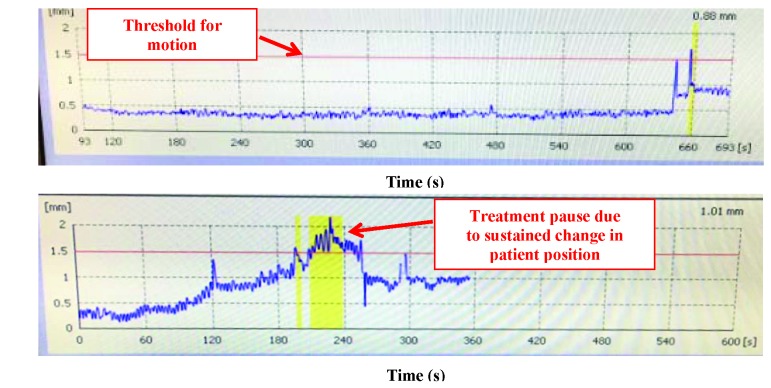
Figure 1.
Patient examples of intrafraction motion as detected by the HDMM system.
This figure demonstrates intrafraction motion as detected by the HDMM system for two patients undergoing gamma knife treatment on the GK icon. The designated threshold for motion is indicated by the red line at 1.50 mm. The top panel shows a patient with minimal intrafraction motion. When there is sustained intracranial motion above the threshold, treatment is paused (as noted by the yellow region on the bottom panel).
Table 3.
Summary of intrafraction displacement
| Displacement Type | Median | Mean | Standard Deviation | Range |
| Average displacement (mm) | 0.60 | 0.65 | 0.46 | 0.1 to 1.25 |
| Maximum displacement (mm) | 1.22 | 1.29 | 0.67 | 0.07 to 3.16 |
The median, mean, standard deviation, and range of average intrafraction displacement and maximum intrafraction displacement as determined by differences between designated pretreatment patient position coordinates and intratreatment patient position coordinates.
Analysis of factors associated with motion
Logistic regression analysis was used to identify factors associated with increased average and maximum displacement. Greater odds of observing an average displacement greater than 0.6 mm (median) were seen with ECOG > 1, male gender, and malignant tumors (p < 0.05). Anxiolytic use prior to treatment was associated with a significant reduction in average displacement (p < 0.05), while performance of physics clearance checks, use of bite blocks and increased treatment time did not appear to influence average displacement. Factors associated with increased average displacement on logistic regression analysis are summarized in Table 4 and Figure 2a.
Table 4.
Analysis of factors associated with a greater than median average displacement (0.6 mm)
| Characteristic | Category | Odds ratio (95% CI) | P-value |
| Age | <65 | Ref | |
| >65 | 0.722 (0.207-2.512) | 0.608 | |
| Bite block | No | Ref | |
| Yes | 0.144 (0.008-2.568) | 0.187 | |
| Mask Type | Green | Ref | |
| Yellow | 1.004 (0.310-3.247) | 0.995 | |
| Number of Treatments | 1 | Ref | |
| >1 | 0.901 (0.305-2.664) | 0.851 | |
| BMI | <24.9 | Ref | |
| >24.9 | 1.805 (0.593-5.489) | 0.298 | |
| ECOG | <1 | Ref | |
| >1 | 10.982 (2.472-39.051) | 0.001 | |
| Gender | Female | Ref | |
| Male | 5.609 (1.392-22.610) | 0.015 | |
| Tumor pathology | Benign | Ref | |
| Malignant | 11.051 (2.071-58.983) | 0.005 | |
| Treatment time (minutes) | <23 | Ref | |
| >23 | 0.936 (0.304-2.881) | 0.909 | |
| Anxiolytic use | No | Ref | |
| Yes | 0.156 (0.037-0.662) | 0.012 | |
| Clearance check | No | Ref | |
| Yes | 2.737 (0.499-15) | 0.246 |
Logistic regression analysis of average displacement > 0.6 mm with regard to patient and treatment characteristics. ECOG >1, male gender, malignant tumor pathology, and lack of anxiolytic use (noted in bold font) showed significant association with increased average displacement.
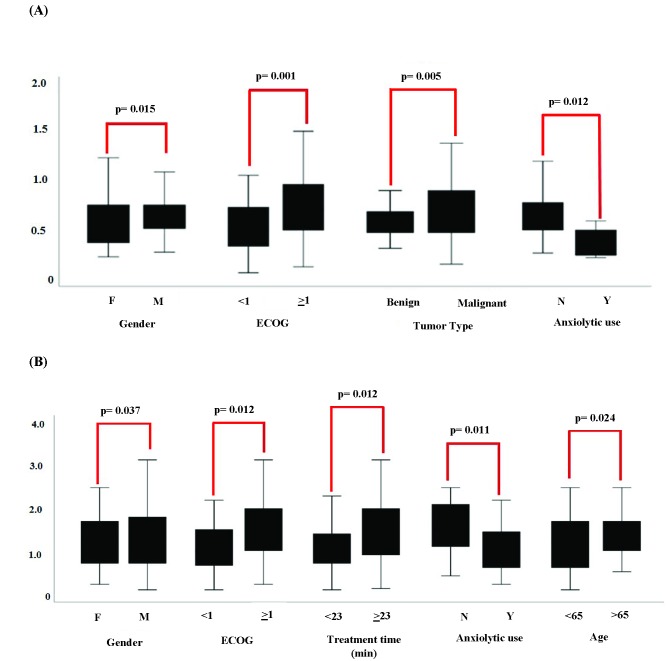
Figure 2.
Box plots demonstrate median and range of average displacement with regard to various to patient and treatment characteristics; p values designate results from logistic regression analysis.
Demonstrates factors that showed a significant association with increased average displacement.
Demonstrates factors that showed a significant association with increased maximum displacement.
Significantly greater odds of observing a maximum displacement over 1.22 mm (median) were seen with ECOG > 1, male gender, and increased treatment time (p < 0.05), while age > 65 and anxiolytic use were associated with a significant reduction in maximum displacement (p < 0.05). Performance of clearance checks and absence of bite block use did not appear to impact maximum patient displacement. Factors associated with increased maximum displacement on logistic regression analysis are summarized in Table 5 and Figure 2b.
Table 5.
Analysis of factors associated with a greater than median maximum displacement (1. 22 mm)
| Characteristic | Category | Odds ratio (95% CI) | P-value |
| Age | <65 | Ref | |
| >65 | 0.207 (0.052-0.815) | 0.024 | |
| Bite block | No | Ref | |
| Yes | 0.075 (0.005-1.219) | 0.069 | |
| Mask Type | Green | Ref | |
| Yellow | 1.842 (0.555-6.110) | 0.318 | |
| Number of Treatments | 1 | Ref | |
| >1 | 1.127 (0.382-3.321) | 0.828 | |
| BMI | <24.9 | Ref | |
| >24.9 | 0.852 (0.284-2.562) | 0.776 | |
| ECOG | <1 | Ref | |
| >1 | 4.936 (1.416-17.209) | 0.012 | |
| Gender | Female | Ref | |
| Male | 4.220 (1.092-16.300) | 0.037 | |
| Tumor pathology | Benign | Ref | |
| Malignant | 3.194 (0.719-14.180) | 0.127 | |
| Treatment time (minutes) | <23 | Ref | |
| >23 | 4.507 (1.389-12.426) | 0.012 | |
| Anxiolytic use | No | Ref | |
| Yes | 0.171 ( 0.044-0.673) | 0.011 | |
| Clearance check | No | Ref | |
| Yes | 7.778 (0.887- 68.191) | 0.064 |
Logistic regression analysis of maximum displacement > 1.22 mm with regard to patient and treatment characteristics. ECOG >1, increased treatment time, and lack of anxiolytic use (noted in bold font) showed significant association with increased maximum displacement.
Discussion
Stereotactic radiosurgery mandates highly accurate and precise radiation delivery to a specific target while minimizing impact on adjacent normal tissues and critical structures. This was traditionally achieved by rigid frame-based fixation, which minimizes intracranial motion while also providing a stereotactic coordinate system for treatment localization. Frame-based immobilization has been the cornerstone for treatment of intracranial lesions with GKRS. More recently, radiosurgery via mask-based Gamma Knife has increased considerably due to its convenience, minimally invasive nature, and the ability to fractionate treatments for larger tumors (7). Studies have also shown that frameless fractionated GKRS of large tumors (volumes > 10 cm3) produce satisfactory tumor control rates that are comparable to frame-based systems (7) .
Despite the many benefits of frameless GKRS, recent studies have suggested that mask-based immobilization may be associated with increased motion error during treatment (5, 6, 10). Carminucci and colleagues examined the accuracy of frame-based and frameless fixation using the GK Icon system in a retrospective cohort study. In this study, patients were immobilized via stereotactic head frame or a noninvasive thermoplastic mask. The positioning of the skull in stereotactic space as noted on CBCT was compared to the positioning on the original planning MRI to assess for set up error. CBCT imaging prior to treatment and during treatment were used to assess intrafraction motion although continuous monitoring was not studied. Their results demonstrated that although motion error is small for both treatment systems, mask-based immobilization led to significantly larger setup and intrafraction motion error in comparison to patients treated with frame fixation (10).
The current study aimed to validate these findings in our patient cohort using the onboard continuous monitoring system on the GK Icon device. We postulated that mask-based systems have a higher propensity for intrafraction displacement and increased motion is likely associated with certain patient and treatment factors. We found the mean average displacement was 0.65 mm and the mean maximum displacement was 1.29 mm (Table 3). These results suggest that although intrafraction motion with frameless GKRS is relatively small, it may be clinically significant, particularly when treating lesions in close proximity to certain critical, radiosensitive neuroanatomical structures. The magnitude of motion error demonstrated in our study is comparable to those reported in other studies involving radiosurgery using mask immobilization. For instance, Tryggestad et al. demonstrated a mean intrafraction motion of 0.71 ± 0.8 mm for Linac based SRS, while Li and colleagues reported a mean displacement measure of 0.56 ± 0.51 mm for frameless GK based SRS (5, 11). When interpreting this data, it is, however, important to recognize that motion error may be detected with greater sensitivity in mask-based treatments due to the availability of real time monitoring of patient motion via the HDMM system. As frame-based treatments only allow for determination of intrafraction motion via pre and post treatment CBCTs, the magnitude of intrafraction motion between frame and mask based systems may not be directly comparable.
Our study also examined whether increased motion error was associated with various patient and treatment based factors. Dutta and collegues previously examined the relationship between displacement and various extrinsic factors for frame-based treatments and noted larger intrafraction displacements were associated with lower Karnofsky performance status (KPS) and longer total frame pin lengths (12). To our knowledge, the current study is the first to identify potential factors associated with increased intrafraction motion shifts in patients treated with frameless GKRS. Our study found that greater average displacement for masked treatments was associated with ECOG > 1, male gender, and malignant tumors while increased maximum displacement was associated with ECOG > 1 and increased treatment time. We hypothesized that poorer functional status likely contributed to increased motion error due to physical limitations that reduced patient ability to remain immobile during treatment. Similarly, patients with malignant brain tumors were more likely to have additional systemic disease and/or cancer related pain, which could contribute to greater intrafraction motion. It is unclear why male gender was associated with increased motion error. Initially, we hypothesized that higher BMI of males could contribute to increased motion error due to increased variability associated with positioning of larger patients on the treatment couch. However, on logistic regression analysis, BMI did not correlate with average or maximum displacement. Therefore, the association of gender with increased displacement may be due to certain confounding factors that were not identified in this study.
Interestingly, one of the strongest associations noted in our study was the reduction of average and maximum displacement with anxiolytic use. This finding suggests that patients’ psychiatric state and any angst regarding treatment delivery should be addressed adequately prior to starting treatment, and that patients should be offered the use of anxiolytic medications immediately prior to treatment, if appropriate.
Another concept that is often discussed when delivering radiosurgery is the necessity of adding margins to the target volume to ensure adequate coverage. The aim of Gamma Knife radiosurgery is to completely treat the target with a high degree of accuracy and precision while limiting the damage to normal tissues. Therefore, the addition of margins > 1 mm is not typical when performing SRS using the GK system. Ma et al. assessed the impact of adding margins during GK radiosurgery on peripheral normal brain sparing via a mathematical model. In this study, the authors added margins ranging from 0.5-3.0 mm, and found that on average, a 2 mm margin led to an increase of approximately 55% of the prescription dose volume and a predicted symptomatic necrosis rate of 6-25% (13).
Our current study did not directly address whether adding margins would have improved target coverage and therefore limited the possibility of a target miss due to intracranial motion. Future directions for the current study could involve an analysis to determine whether margin expansions during GK are associated with the development of symptomatic radiation-necrosis.
The limitations of our study include the fact that it is a retrospective study with a limited sample size. Additionally, we did not compare motion shifts in the mask based system to frame-based systems, which could also have subtle shifts during treatment. Our analysis focused on intrafraction motion; however there are other factors such as set up errors, image registration inaccuracies or software calculation errors that can add to uncertainty in accurate treatment delivery. Chung et al. examined the accuracy of image co-registration in frameless GKRS and found image co-registration errors with mask based treatments were similar in magnitude to imaging errors observed with frame-based GKRS (14). Additionally, our sample size was limited and we did not perform subgroup analysis to assess whether the association of various factors (male gender, higher ECOG, malignant tumors, anxiolytic use) with increased/decreased displacement was due to the presence of other confounders such as increased medical comorbidities, pain from metastatic disease, and preexisting psychiatric issues.
Conclusions
In summary, our study demonstrates that various patient associated factors contribute to small yet possibly clinically significant displacements during thermoplastic mask-based GKRS. Therefore, intrafraction motion during treatment delivery must be considered, particularly for patients who are male, individuals with malignant tumors, those with lower functional status, and those with plans involving longer treatment times. Additionally, medication use to reduce anxiety prior to treatment should be considered when appropriate as a means to reduce intrafraction motion and improve targeting during radiosurgery delivery.
Acknowledgements
Authors disclosure of potential conflicts of interest: Dr. Trifiletti receives unrelated clinical trial research funding from Novocure and publishing fees from Springer. The remaining authors have nothing to disclose.
Author contributions
Conception and design: Danushka S. Seneviratne, Laura A.Vallow, Austin Hadley, Steven Herchko, Timothy D. Malouff, William C. Stross, Daniel M. Trifiletti, Jennifer L. Peterson
Data collection: Danushka S. Seneviratne, Austin Hadley, Steven Herchko, Jennifer L. Peterson, Daniel M. Trifiletti
Data analysis and interpretation: Danushka S. Seneviratne, Austin Hadley, Daniel M. Trifiletti
Manuscript writing: Danushka S. Seneviratne, Timothy D. Malouff, William C. Stross
Final approval of manuscript: Danushka S. Seneviratne, Laura A.Vallow, Austin Hadley, Timothy D. Malouff, William C. Stross, Daniel M. Trifiletti, Deanna H. Pafundi, Jennifer L. Peters
References
- 1. Trifiletti DM, Sheehan JP, Grover S, Dutta SW, Rusthoven CG, Kavanagh BD, et al. National trends in radiotherapy for brain metastases at time of diagnosis of non-small cell lung cancer. J Clin Neurosci. 2017;45:48-53. Epub 2017/09/04. doi: 10.1016/j.jocn.2017.08.028. PubMed PMID: 28866073. [DOI] [PubMed] [Google Scholar]
- 2. Leksell L, Jernberg B. Stereotaxis and tomography. A technical note. Acta Neurochir (Wien). 1980;52(1-2):1-7. Epub 1980/01/01. PubMed PMID: 6990697. [DOI] [PubMed] [Google Scholar]
- 3. Lunsford LD, Flickinger J, Lindner G, Maitz A. Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery. 1989;24(2):151-9. Epub 1989/02/01. doi: 10.1227/00006123-198902000-00001. PubMed PMID: 2645538. [DOI] [PubMed] [Google Scholar]
- 4. Ruschin M, Nayebi N, Carlsson P, Brown K, Tamerou M, Li W, et al. Performance of a novel repositioning head frame for gamma knife perfexion and image-guided linac-based intracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(1):306-13. Epub 2010/04/14. doi: 10.1016/j.ijrobp.2009.11.001. PubMed PMID: 20385456. [DOI] [PubMed] [Google Scholar]
- 5. Li W, Bootsma G, Von Schultz O, Carlsson P, Laperriere N, Millar BA, et al. Preliminary Evaluation of a Novel Thermoplastic Mask System with Intra-fraction Motion Monitoring for Future Use with Image-Guided Gamma Knife. Cureus. 2016;8(3):e531. Epub 2016/04/16. doi: 10.7759/cureus.531. PubMed PMID: 27081592; PubMed Central PMCID: PMCPMC4829406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lunsford LD, Niranjan A, Fallon K, Kim JO. Frame versus Frameless Leksell Stereotactic Radiosurgery. Prog Neurol Surg. 2019;34:19-27. Epub 2019/05/17. doi: 10.1159/000493046. PubMed PMID: 31096212. [DOI] [PubMed] [Google Scholar]
- 7. Park HR, Park KW, Lee JM, Kim JH, Jeong SS, Kim JW, et al. Frameless Fractionated Gamma Knife Radiosurgery with ICON for Large Metastatic Brain Tumors. J Korean Med Sci. 2019;34(8):e57. Epub 2019/03/06. doi: 10.3346/jkms.2019.34.e57. PubMed PMID: 30833881; PubMed Central PMCID: PMCPMC6393762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, et al. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J Radiat Oncol Biol Phys. 2019;103(3):618-30. Epub 2018/11/06. doi: 10.1016/j.ijrobp.2018.10.038. PubMed PMID: 30395902. [DOI] [PubMed] [Google Scholar]
- 9. Wright G, Harrold N, Hatfield P, Bownes P. Validity of the use of nose tip motion as a surrogate for intracranial motion in mask-fixated frameless Gamma Knife((R)) Icon therapy. J Radiosurg SBRT. 2017;4(4):289-301. Epub 2018/01/04. PubMed PMID: 29296453; PubMed Central PMCID: PMCPMC5658824. [PMC free article] [PubMed] [Google Scholar]
- 10. Carminucci A, Nie K, Weiner J, Hargreaves E, Danish SF. Assessment of motion error for frame-based and noninvasive mask-based fixation using the Leksell Gamma Knife Icon radiosurgery system. J Neurosurg. 2018;129(Suppl1):133-9. Epub 2018/12/14. doi: 10.3171/2018.7.GKS181516. PubMed PMID: 30544303. [DOI] [PubMed] [Google Scholar]
- 11. Tryggestad E, Christian M, Ford E, Kut C, Le Y, Sanguineti G, et al. Inter- and intrafraction patient positioning uncertainties for intracranial radiotherapy: a study of four frameless, thermoplastic mask-based immobilization strategies using daily cone-beam CT. Int J Radiat Oncol Biol Phys. 2011;80(1):281-90. Epub 2010/10/19. doi: 10.1016/j.ijrobp.2010.06.022. PubMed PMID: 20951506. [DOI] [PubMed] [Google Scholar]
- 12. Dutta SW, Kowalchuk RO, Trifiletti DM, Peach MS, Sheehan JP, Larner JM, et al. Stereotactic Shifts During Frame-Based Image-Guided Stereotactic Radiosurgery: Clinical Measurements. Int J Radiat Oncol Biol Phys. 2018;102(4):895-902. Epub 2018/09/02. doi: 10.1016/j.ijrobp.2018.05.042. PubMed PMID: 30170871. [DOI] [PubMed] [Google Scholar]
- 13. Ma L, Sahgal A, Larson DA, Pinnaduwage D, Fogh S, Barani I, et al. Impact of millimeter-level margins on peripheral normal brain sparing for gamma knife radiosurgery. Int J Radiat Oncol Biol Phys. 2014;89(1):206-13. Epub 2014/04/15. doi: 10.1016/j.ijrobp.2014.01.011. PubMed PMID: 24725703. [DOI] [PubMed] [Google Scholar]
- 14. Chung HT, Kim JH, Kim JW, Paek SH, Kim DG, Chun KJ, et al. Assessment of image co-registration accuracy for frameless gamma knife surgery. PLoS One. 2018;13(3):e0193809. Epub 2018/03/03. doi: 10.1371/journal.pone.0193809. PubMed PMID: 29499061; PubMed Central PMCID: PMCPMC5834193. [DOI] [PMC free article] [PubMed] [Google Scholar]