Abstract
Purpose
Paclitaxel (PTX) is a first-line chemotherapeutic agent for treating ovarian cancer. However, PTX resistance has become a major obstacle in ovarian cancer therapy. The underlying mechanism associated with PTX resistance is still unclear.
Patients and Methods
We used qPCR to detect taurine up-regulated 1 (TUG1) expression in normal ovarian tissues and ovarian tumor tissues. A combination of small interfering RNA (siRNA), cell counting kit 8 (CCK8), colony formation assay and nude mouse model were used to detect the effect of TUG1 on ovarian cancer cell PTX-resistance. Autophagy/cytotoxicity dual staining assay, luciferase reporter assay, Western blot and RNA-binding protein immunoprecipitation assay were used for further mechanistic studies.
Results
TUG1 is highly expressed not only in ovarian tumor tissues compared with normal ovarian tissues but also in the chemo-resistant group compared with the sensitive group. Knockdown of TUG1 by siRNA decreased ovarian cancer cell and xenograft tumor PTX resistance with or without PTX treatment. Moreover, deletion of TUG1 in ovarian cancer cells decreased autophagosome formation and increased apoptosis as demonstrated by autophagy/cytotoxicity dual staining and Western blot assays. Furthermore, microRNA-29b-3p (miR-29b-3p) was found as the direct target of TUG1. Additionally, TUG1 could directly bind Ago2, a key protein of the RNA-induced silencing complex.
Conclusion
Our findings suggest that TUG1, through targeting miR-29b-3p, induces autophagy and consequently results in PTX resistance in ovarian cancer.
Keywords: ovarian cancer, lncRNA, TUG1, autophagy, sponge, miR-29b-3p
Introduction
Ovarian cancer is the most common gynecological cancer and is the most deadly female tumor in the developed world.1 According to the American Cancer Society, in 2018, there were 22,240 new diagnosed cases of ovarian cancer and 14,070 ovarian cancer deaths in the United States.2 Ninety percent of ovarian cancers are epithelial ovarian cancers (EOC), and most women are diagnosed at an advanced stage (International Federation of Gynecology and Obstetrics [FIGO] stage III).3 The standard of therapy for EOC patients remains surgery and cytotoxic chemotherapy. Although with modern management, most EOC patients are curative at an early stage, most women with advanced disease will develop recurrences with progressively shorter disease-free intervals due to ovarian cancer cell resistance to chemotherapy drugs. The 5-year survival for EOC patients is approximately 40%.2 Therefore, a better understanding of the molecular mechanisms underlying chemotherapy resistance will lead to the development of better diagnostic approaches and more effective treatments for EOC.
Paclitaxel (PTX) is a first-line chemotherapeutic agent against ovarian cancer,4 which binds and stabilizes microtubules, resulting in an impairment of the metaphase to anaphase transition during mitosis that leads to cell arrest at the G2/M phase and subsequent apoptosis.5 The emergence of drug resistance is a major limitation of its clinical success. Because there are many mechanisms of PTX resistance, including pumping intracellular PTX out of the cancer cell,6 overexpression of anti-apoptotic proteins,7 and overriding the spindle assembly checkpoint,8 reversing drug resistance is limited to those mechanisms. Recently, accumulating evidence has revealed that treatment with chemotherapy agents, including PTX, can lead to an autophagy response.9,10 Autophagy, a favored survival strategy, can help cancer cells overcome starvation or cellular stresses such as chemotherapeutic agents.11 Autophagy is thought to confer stress tolerance and maintain tumor cell survival. Therefore, a better understanding of the molecular events contributing to autophagy during PTX resistance would be beneficial to ovarian cancer chemotherapy.
With the development of whole-genome sequencing technology, it has been revealed that only ~2% of the human genome is translated into proteins, whereas the vast majority of transcripts are noncoding RNAs.12 Among them are long noncoding RNAs (lncRNAs), which are more than 200 nt in length and unable to be translated into proteins.13 A growing body of evidence has suggested that a number of lncRNAs are dynamically expressed in tissue- or cell-type-specific manner, especially in cancer.14 Aberrant expression of some lncRNAs has also been found in cancer drug resistance, including ovarian cancer. For example, HOTAIR upregulation induced platinum resistance in ovarian cancer though DNA methylation15 or NF-κB activation;16 UCA1 promoted PTX resistance in ovarian cancer cells through sponging miR-129;17 H19 contributed to cisplatin resistance in high-grade serous ovarian cancer by regulating glutathione metabolism.18 Therefore, lncRNAs play a major role in regulating chemotherapy drug resistance in ovarian cancer patients.
The lncRNA taurine-upregulated gene 1 (TUG1), located in chromosome 22q12, was initially found to have important functions in the differentiation of the murine retina, and knockdown of TUG1 blocked retinal development in the developing eye.19 Growing evidence has shown that TUG1 can promote the progression of various cancers including ovarian cancer.20 TUG1 was also associated with cancer chemoresistance. For example, TUG1 sponged miR-197 to enhance drug sensitivity in breast cancer,21 TUG1 regulated LIMK2b via EZH2 playing an important role in small cell lung cancer chemoresistance,22 and TUG1 was also associated with chemoresistance in esophageal squamous cell carcinoma.23 However, the role of TUG1 in autophagy and PTX resistance in ovarian cancer has not been reported until now.
In this study, we first validated TUG1 expression in ovarian cancer tissues (sensitive and resistant) and demonstrated a significant association of TUG1 overexpression with chemoresistance. Based on these results, we next deleted TUG1 and found that the depletion of TUG1 enhanced PTX sensitivity in SK-OV-3 and A2780/R ovarian cancer cells. Furthermore, our results indicated that TUG1 could modulate autophagy through sponging miR-29b-3p thereby resulting in PTX resistance. Thus, our study is the first to suggest that TUG1 plays a key role in PTX resistance through the induction of autophagy. Our study can provide clinical guidance for ovarian cancer patient chemotherapy.
Materials and Methods
Tissue Samples
Ovarian cancer samples (N = 41) and benign ovarian tumor samples (N = 26) were collected at Shanghai Pudong New Area People’s Hospital (Shanghai, China) from patients treated with the standard of care paclitaxel-platinum therapy after surgery. The experimental protocols were approved by the Ethics Committee of the Shanghai Pudong New Area People’s Hospital. The methods were performed in accordance with the approved guidelines by the Ethics Committee of the Shanghai Pudong New Area People’s Hospital, and all patients provided written provided informed consent in accordance with the Declaration of Helsinki. The ovarian cancer samples were divided into two groups: chemosensitive and chemoresistant, according to new guidelines to evaluate the response to treatment in solid tumors.24 The clinicopathological characteristics of ovarian cancer patients are summarized in Table 1.
Table 1.
The Clinicpathological Characteristics of Ovarian Cancer Patients
| Characteristics | Resistance (n=20) | Sensitive (n=21) |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 50.1 ± 7.8 | 51.1 ± 8.1 |
| Range | 35–71 | 31–73 |
| FIGO stage | ||
| II | 1 | 5 |
| III-IV | 19 | 16 |
| Histology | ||
| Serous | 15 | 13 |
| Nonserous | 3 | 6 |
| Clear cell carcinoma | 1 | 1 |
| Unspecifed adnocarcinomas | 2 | 1 |
| Tumor grade (FIGO) | ||
| Well diff | 1 | 3 |
| Moderately and poorly diff | 19 | 18 |
Abbreviations: FIGO, Federation International of Gynecology and Obstetrics; SD, Standard Deviation; Diff, Different.
Cell Culture
The human ovarian cancer cell line A2780, two normal ovarian epithelial cell lines (IOSE80 and IOSE386, Canadian Ovarian Tissue Bank, British Columbia Cancer Research Centre, Canada) and HEK293 cells (Chinese Academy of Sciences, Shanghai, China) were cultured using Dulbecco’s Modified Eagle Media (DMEM) (Thermo Scientific HyClone, MA, USA), supplied with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C and 5% CO2. SK-OV-3 cells (Chinese Academy of Sciences, Shanghai, China) were cultured in McCoy’s 5A (Thermo Scientific Gibco, MA, USA) under the same culture conditions. A2780/R cells, which are PTX-resistant, were generously donated by Prof. Jia and cultured in the presence of 2 µg/mL PTX under the same conditions as above. The use of A2780/R cells was approved by the Ethics Committee of the Shanghai Pudong New Area People’s Hospital and Jiangsu Cancer Hospital.
Quantitative Real-Time PCR (qPCR) Analysis of lncRNA and miRNA Expression
Total RNA was extracted using TRIzol reagent (Invitrogen, CA, USA). Additionally, 1 μg of total RNA was reverse-transcribed to cDNA using a RevertAid First-strand cDNA Synthesis Kit (Thermo Scientific, MA, USA) according to the manufacturer’s instructions. To detect lncRNA expression, qPCR was carried out using the PrimeScript SYBR Green Master Mix (Takara, Takara Island, Japan) and a 7500 real-time PCR system (Applied Biosystems, CA, USA). To detect miRNA expression, qPCR was performed with reagents for stem-loop miRNA primer (RiboBio, Guangzhou, China) using a 7500 real-time PCR system. The primers used were listed as follows: TUG1, forward: 5ʹ-CTGAAGAAAGGCAACATC-3ʹ, reverse: 5ʹ-GTAGGCTACTACAGGATTTG-3ʹ; miR-29b-3p, forward: 5′-ACACTCCAGCTGGGTAGCACCATTTGAAATC-3ʹ, reverse: 5ʹ-GTAGGCTACTACAGGATTTG-3ʹ; RT primer: 5ʹ-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAACACTGA-3ʹ; GAPDH, forward: 5ʹ- GGTATCGTGGAAGGACTCATGAC-3ʹ, reverse: 5ʹ- ATGCCAGTGAGCTTCCCGTTCAG-3ʹ; U6, forward: 5′-CTCGCTTCGGCAGCACA-3ʹ, reverse: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ; RT primer: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ. The GAPDH and U6 levels were used as the internal normalization control. TUG1 and miR-29b-3p expression were calculated with the comparative threshold cycle (2−ΔΔCt) approach.
Transfection of TUG1 siRNA and Anti-miR Inhibitors
siRNA specifically targeting lncRNA TUG1 (Si-TUG1-1, Si-TUG1-2) and anti-miR-29b-3p inhibitors were from RiboBio (Guangzhou, China) and were used for inhibition of lncRNA or miRNA activity in cells. The sequences used were listed as follows Si-TUG1-1: 5ʹ-CAGUCCUGGUGAUUUAGACAGUCUU-3ʹ, Si-TUG1-2: 5ʹ-CCCAGAAGUUGUAAGUUCACCUUGA-3ʹ, anti-miR-29b-3p inhibitors:5′–AACACUGAUUUCAAAUGGUGCUA–3′. Scrambled siRNA negative control (Si-NC) was purchased from Invitrogen (Invitrogen, USA). Then, 100 nM miR-29b-3p inhibitor or 50 nM TUG1 siRNA was transfected using Lipofectamine 2000 (Invitrogen, CA, USA) as described by the manufacturer.
Cell Counting Kit 8 (CCK8) Assay
Cell viability against PTX–induced death was determined using the CCK8 assay. In brief, ovarian cancer cells were plated and transfected with lncRNA-specific siRNA or inhibitor followed were incubated for 48 h with exposing to various concentration of PTX (2, 4, 8, 16, 32 μM for A2780/R; 0.5, 1, 2, 4, 8 nM for SK-OV-3). Then, 10 μL CCK8 solution (Dojindo, Japan) was added for 2 h, and the absorbance was measured at 490 nm by ELX800 (Biotek, VT, USA) according to manufacturer’s instructions. All experiments were carried out in triplicate.
Colony Formation Assay
Cells were seeded at 105 cells per well in six-well plates. Cells were grown to approximately 80% confluence in 6-well plates and transfected with 100 nM miRNA inhibitor or 50 nM lncRNA-specific siRNA was transfected using Lipofectamine 2000 (Invitrogen, CA, USA) in the presence or absence of PTX for 48 h. Then, 500 cells were cultured per well in a twenty-four-well plate. After two weeks, colonies were fixed for 10 min and then visualized using Crystal Violet Staining Solution (Beyotime, China).
Autophagy/Cytotoxicity Dual Staining Assay
Cells were cultured and transfected with 100 nM miRNA inhibitor or 50 nM lncRNA-specific siRNA using Lipofectamine 2000 (Invitrogen, CA, USA). After drug treatment, the cells were fixed, permeabilized and incubated with 100 μL Cell-Based Propidium Iodide (PI) Solution, followed by Cell-Based Monodansylcadaverine (MDC) Solution (Abcam, Cambridge, UK). MDC, a fluorescent compound that is incorporated into multilamellar bodies by both an ion trapping mechanism and the interaction with membrane lipids, as a probe for detection of autophagic vacuoles in cultured cells.25 Apoptotic cells are characterized by DNA fragmentation and, consequently, loss of nuclear DNA content. PI is capable of binding and labeling DNA makes it possible used as a marker of cell death.26 Images were taken with a DMI3000 B microscope (Leica, Wetzlar, Germany). Then, the cells were harvested and disrupted, followed by determining the immunofluorescence intensity of MDC and PI with excitation/emission wavelengths at 335/512 nm and 520/600 nm by EnSpire 2300 (PerkinElmer, MA, USA), respectively. The fluorescence intensity was normalized to the total protein concentration.
Luciferase Reporter Assay
The wild type (WT) and mutant (MT) TUG1 luciferase reporter vectors were constructed by subcloning the WT sequences (CACAACCATTTTGAAGCCCTGTT) and MT sequences (CTTGGGGTCAGTTATTGACATCGA) into pGL3 Luciferase Reporter Vectors (Promega, Madison, WI, USA). HEK293 cells were transfected with 80 ng luciferase reporter plasmid and miR-29b-3p mimics (final concentration, 50 nM) (RiboBio, Guangzhou, China) using Lipofectamine 2000 (Invitrogen, CA, USA). After 24 h, luciferase activity was measured using the Dual-Luciferase Reporter System (Berthold Technologies, Stuttgart, Germany) according to the manufacturer’s instructions.
Preparation of Cell Lysates and Western Blotting Analysis
For preparation of cell lysates and Western blotting analysis, cells were lysed in RIPA lysis buffer (1% Triton X-100, 50 mM Tris/HCl pH 7.4, 1 mM DTT, 150 mM NaCl and 1% protease inhibitor cocktail) and incubated on ice for 40 min, followed by centrifugation at 12,000g to remove cellular debris. The protein concentration was quantified by a BCA Protein assay kit (Thermo Scientific, MA, USA). 50 μg of total protein was resolved by SDS-PAGE, followed by transferring onto a Polyvinylidenefluoride membrane (Millipore Billerica, USA). The membranes were blocked in TBST (Tris-buffered saline with 0.1% tween 20) containing 5% non-fat milk and immunoblotted with LC3B(1:500, Proteintech 14600-1-AP, Chicago, USA), Beclin1 antibody (1:1000, Proteintech 11306-1-AP, Chicago, USA), cleaved caspase 3 (1:1000, Cell Signaling #9661, Beverly, USA), cleaved caspase 7 (1:1000, Cell Signaling #8438, Beverly, USA), and GAPDH (1:5000, Proteintech 10494-1-AP, Chicago, USA) and detected using HRP-labeled secondary antibody and enhanced chemiluminescence reagent (Pierce, Rockford, USA).
RNA-Binding Protein Immunoprecipitation (RIP) Assay
Following the manufacturer’s instructions, RIP assays were performed using the Magna RIP Kit (Millipore, Bedford, MA, USA). In brief, cells were lysed in RIP lysis buffer and incubated with magnetic beads conjugated with human Ago2 antibody or negative control normal IgG. Then, qPCR was used to amplify the retrieved RNA, while U6 was used as a nonspecific control.
Nude Mouse Model
Twelve female BALB/c athymic nude mice were randomly divided into two groups. All animal experiments were carried out in accordance with guidelines evaluated and approved by approved by the Committee on the Ethics of Animal Experiments of the Nanjing Medical University. A total of 1×107/mL A2780/R cells transfected with or without TUG1 siRNA were collected and hypodermically injected into the upper left flank region with 0.1 mL PBS per mouse.27,28 When the tumor size reached a volume of approximately 50 mm3,29 the mice were treated with paclitaxel therapy at a dose of 10 mg/kg twice per week.27,28,30 The tumor size of the mice was monitored every four days, and the tumors were harvested 3 weeks after therapy. Then, macroscopic photographs of tumors were captured, and the tumors were weighed.
Statistical Analysis
Student’s t test (two tails) and one-way ANOVA were used to analyze the differences between groups. Data are presented as the means ± S.D. The general acceptance level of significance was p<0.05. Computer-based calculations were conducted using SPSS version 20 (SPSS Inc., Chicago, IL, USA).
Results
TUG1 Is Upregulated in Ovarian Cancer Tissues and Cells, and Overexpression of TUG1 Is Associated with Chemoresistance
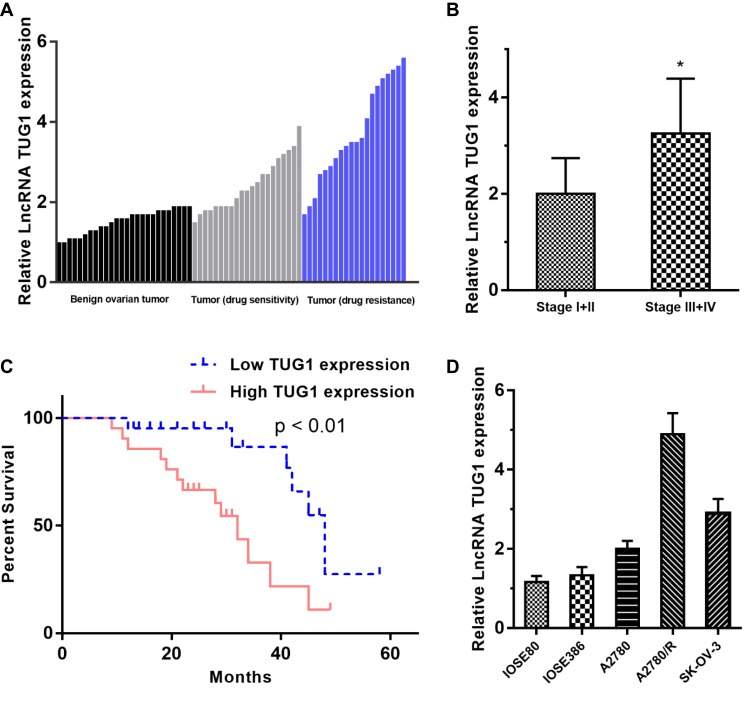
TUG1 expression was significantly upregulated in the 41 ovarian cancer samples compared to the 26 benign ovarian tissues. We next divided the 41 ovarian cancer patients into a drug resistance group (n = 20) and a drug sensitive group (n = 21) according to NCCN guidelines and found that the expression of TUG1 was significantly overexpressed in patients with drug resistance compared with drug sensitivity (Figure 1A). Furthermore, we also analyzed the association of TUG1 expression and the clinicopathological characteristics of the cancer patients. Interestingly, the advanced tumor node metastasis (TNM) stage group (stage III+IV) showed a higher level of TUG1 (p = 0.0132) than the earlier period group (stage I+II) using student’s t test analysis (Figure 1B). In addition, we divided the 41 ovarian cancer patients into a relatively high TUG1 expression group (n = 21) and a relatively low TUG1 expression group (n = 20) according to qPCR results and next analyzed the relationship between TUG1 expression and patient overall survival (OS). The results suggested that high TUG1 expression in ovarian cancer patients indicated poorer prognosis (Figure 1C). We also detected TUG1 expression in normal ovarian epithelial cells (IOSE80 and IOSE386) and ovarian cancer cells (A2780, A2780/R and SK-OV-3). Compared with normal ovarian epithelial cells, ovarian cancer cells had significantly higher expression of TUG1 (Figure 1D). Importantly, the drug-resistant A2780/R cells showed the highest expression of TUG1, which was also higher than the expression in the corresponding drug-sensitive A2780 cells (Figure 1D).
Figure 1.
TUG1 expression in ovarian cancer tissues and cells and patient clinical parameters. (A) TUG1 overexpression in ovarian cancer tissues, especially in drug-resistant tissues (P < 0.001). (B) The expression of TUG1 was higher in the advanced TNM stage group (stage III/IV) than in the earlier period group (stage I/II) using student’s t test analysis (p =0.0132). (C) Ovarian cancer patients with high TUG1 expression showed poorer prognosis. (D) TUG1 expression in normal ovarian cells and ovarian cancer cells. *p<0.05.
Abbreviations: TUG1, taurine up-regulated 1; TNM, tumor node metastasis; lncRNA, long noncoding RNAs.
Knockdown of TUG1 Inhibits Ovarian Cancer Cell Chemoresistance and Autophagy
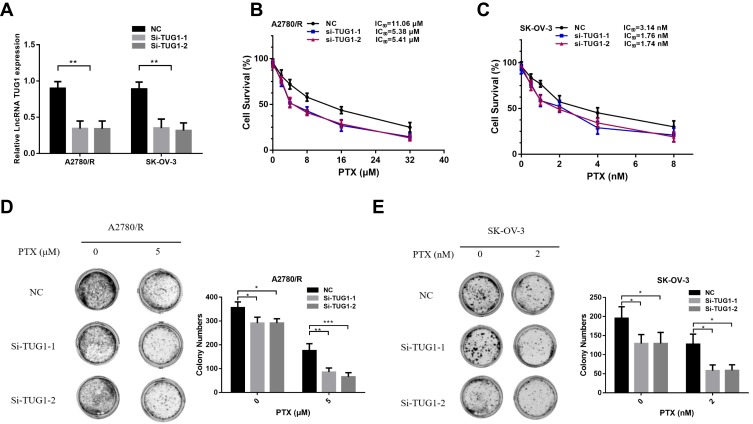
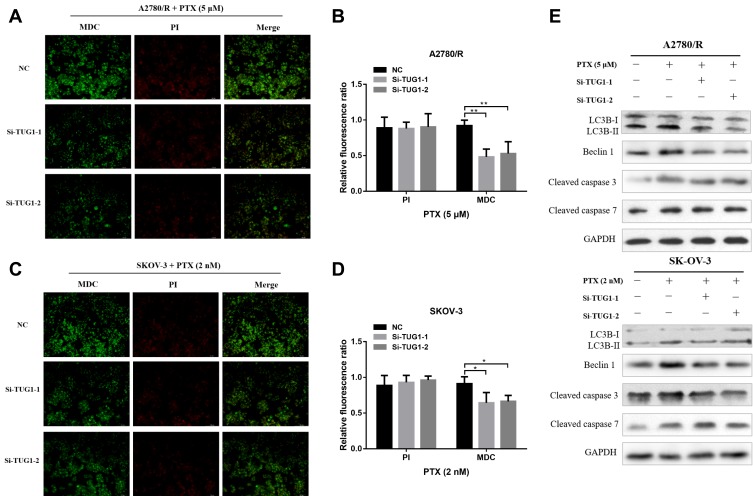
To further elucidate the role of TUG1 in ovarian cancer chemoresistance, we downregulated TUG1 levels in two ovarian cancer cells with high TUG1 expression using two siRNAs targeting TUG1. TUG1 was significantly inhibited in A2780/R and SK-OV-3 cells (Figure 2A). The CCK8 assay showed that TUG1 downregulation enhanced PTX sensitivity in A2780/R and SK-OV-3 cells. The IC50 values of TUG1 siRNA-treated cells decreased by approximately one half compared to the corresponding negative control group (Figure 2B and C). Consistent with these results, colony formation assays revealed that the number of cell colonies generated in the Si-TUG1-1 and Si-TUG1-2 groups obviously decreased compared with the si-NC group (Figure 2D and E). Interestingly, statistical analysis showed that colony numbers were reduced in the Si-TUG1-1 and Si-TUG1-2 groups in the presence of PTX compared to without PTX treatment (Figure 2D and E). In addition, using MDC staining (green fluorescence) assay, we observed that the number of autophagosome cells decreased in Si-TUG1-1- and Si-TUG1-2-transfected compared with Si-NC treated A2780/R and SK-OV-3 cells (Figure 3A–D). However, there was no obvious change in the number of apoptotic cells between the Si-TUG1-1- and Si-TUG1-2-treated groups and the Si-NC-transfected group with the PTX addition with PI staining (red fluorescence) assay, which also was confirmed quantitatively (Figure 3A–D). Next, the apoptosis- and autophagy-related protein levels were determined in TUG1 downregulated cells. As shown in Figure 3E, a remarkably higher levels of the conversion of LC3B-I to LC3B-II and Beclin1 were detected following treatment with PTX; however, depletion of TUG1 decreased the expression of Beclin-1 and the conversion of LC3B-I to LC3B-II. In addition, significantly increased expression levels of cleaved caspase-3 and caspase 7 were detected in PTX-treated and TUG1 knockout A2780/R and SK-OV-3 cells. The results revealed that TUG1 could affect PTX sensitivity in cancer cells by regulating autophagy.
Figure 2.
Silencing of TUG1 suppresses ovarian cell chemoresistance. (A) qRT-PCR was conducted to detect the effect of the TUG1 siRNA transfection of A2780/R and SK-OV-3 cells using one-way ANOVA analysis. (B) The cell viability of A2780/R cells was detected by CCK8 assay after TUG1 knockdown during PTX treatment. (C) The cell viability of SK-OV-3 cells was detected by CCK8 assay after knockdown of TUG1 during PTX treatment. (D) The colony assay was also used to detect the viability of A2780/R cells with or without PTX. (E) The colony assay was also used to detect the viability of A2780/R cells with or without PTX using one-way ANOVA analysis (p<0.05). *p<0.05; **p<0.01 and ***p<0.001.
Abbreviations: TUG1, taurine up-regulated 1; lncRNA, long noncoding RNAs; qRT-PCR, qualitative real-time polymerase chain reaction; PTX, Paclitaxel; CCK8, cell counting kit 8; NC, negative control; Si, Small interfering RNA.
Figure 3.
Depletion of TUG1 inhibited cell autophagy. (A and B) Autophagy/Cytotoxicity dual staining assay was used to test cell autophagy and apoptosis of A2780/R cells after silencing TUG1 in the presence of PTX using one-way ANOVA analysis. (C and D) Autophagy/Cytotoxicity dual staining assay was used to test cell autophagy and apoptosis of SK-OV-3 cells after silencing TUG1 under PTX conditions using one-way ANOVA analysis (p<0.05). (E) Autophagy proteins (Beclin-1, LC3B-I and LC3B-II) and apoptosis proteins (cleaved caspase 3 and 7) were detected through Western blot assay after inhibiting TUG1 under PTX and no PTX conditions. *p<0.05; **p<0.01.
Abbreviations: TUG1, taurine up-regulated 1; PTX, Paclitaxel; NC, negative control; MDC, Monodansylcadaverine; PI, Propidium Iodide; LC-3B, microtubule associated protein 1 light chain 3 beta; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Si, Small interfering RNA.
TUG1 Serves as a Sponge for miR-29b-3p, and the miR-29b-3p Inhibitor Reverses the Effect of TUG1 Deletion on Ovarian Cancer PTX Resistance
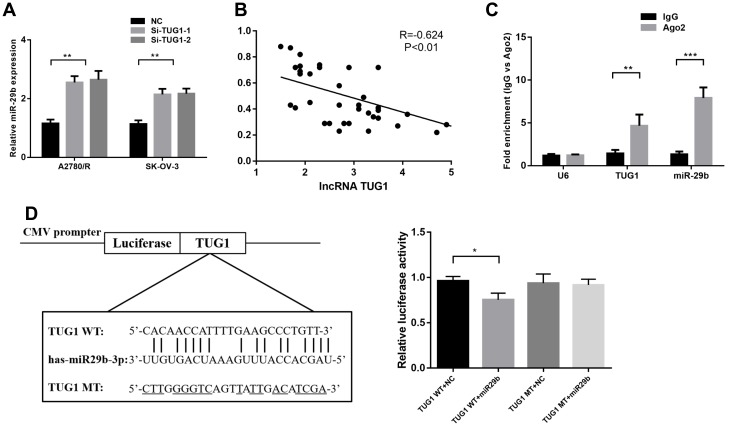
The potential target miRNAs of TUG1 were predicted by miRcode (http://mircode.org/). miR-29b-3p has been associated with chemoresistance31 and autophagosome formation,32 and TUG1 depletion increased miR-29b-3p expression in A2780/R and SK-OV-3 cells (Figure 4A). Moreover, qPCR analysis of TUG1 and miR-29b-3p showed a significant inverse correlation in the 41 ovarian cancer tissues (Figure 4B). We next conducted anti-Ago2 RIP to explore whether TUG1 and miR-29b-3p are in the same RNA-induced silencing complex. The results in Figure 4C show that TUG1 and miR-29b-3p were highly enriched with the Ago2 antibody in A2780/R cells (Figure 4C). Dual-luciferase reporter assays were performed to detect the binding of TUG1 and miR-29b-3p. The luciferase activity of the TUG1 + miR-29b-3p group was attenuated compared with the TUG1-WT + NC group, but the luciferase activity of the TUG1-MUT group was not impacted in transfected 293T cells (Figure 4D). Thus, our results suggest that TUG1 functions as a miR-29b-3p sponge in ovarian cancer cells.
Figure 4.
TUG1 acts as a molecular sponge for miR-29b-3p. (A) miR-29b-3p expression increased when TUG1 was depleted in A2780/R and SK-OV-3 cells as measured by qRT-PCR assay using one-way ANOVA analysis. (B) The correlation between TUG1 and miR-29b-3p levels in ovarian cancer tissues was measured using Pearson correlation analysis. (C) RIP experiments were performed in A2780/R cells with an Ago2 antibody, and the co-precipitated RNA was subjected to qPCR for TUG1 using one-way ANOVA analysis. (D) Putative miR-29b-3p-binding sequence of TUG1. A mutation was generated in the TUG1 sequence in the complementary site for the seed region of miR-29b-3p. The luciferase activity was measured using a dual-luciferase reporter assay in A2780/R cells transfected with empty vector, wild-type TUG1 (TUG1 WT) vector or mutant TUG1 (TUG1 MT) vector with a mutation in the miRNA binding site using one-way ANOVA analysis (p<0.05). *p<0.05; **p<0.01 and ***p<0.001.
Abbreviations: TUG1, taurine up-regulated 1; lncRNA, long noncoding RNAs; NC, negative control; IgG, immunoglobulin G; WT, wild type; MT, mutant type; RIP, RNA-binding protein immunoprecipitation; qRT-PCR, qualitative real-time polymerase chain reaction; U6, RNU6; AGO2, Argonaute RISC Catalytic Component 2; CMV, Cytomegalovirus; Si, Small interfering RNA.
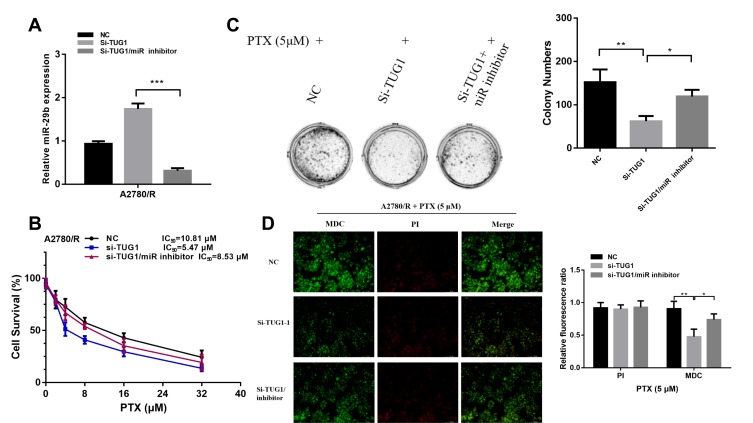
We used a miR-29b-3p inhibitor to reverse the increase in miR-29b-3p levels caused by TUG1 knockdown (Figure 5A) and then performed CCK8 (Figure 5B) and colony formation assays (Figure 5C) to measure the chemoresistance of A2780/R cells to PTX. The results showed that the miR-29b-3p inhibitor partially abrogated TUG-1 knockout-induced PTX sensitivity. In addition, compared with the TUG1 downregulation group, the autophagy/apoptosis cell images revealed that autophagosome formation was upregulated in the si-TUG1/miR-29b-3p inhibitor group (Figure 5D).
Figure 5.
The miR-29b-3p inhibitor reverses the PTX resistance effect of TUG1 deletion in ovarian cancer cells. (A) qRT-PCR was used to detect miR-29b-3p expression after inhibition of TUG1 and treatment with si-TUG1/miR-29b-3p inhibitor using one-way ANOVA analysis. (B) The miR-29b-3p inhibitor remitted the chemosensitivity of TUG1 knockdown measured by CCK8 assay. (C) The miR-29b-3p inhibitor remitted the chemosensitivity of TUG1 knockdown measured by colony assay using one-way ANOVA analysis. (D) The miR-29b-3p inhibitor remitted the autophagy of TUG1 knockdown measured by autophagy/cytotoxicity dual staining assay using one-way ANOVA analysis. *p<0.05; **p<0.01 and ***p<0.001.
Abbreviations: TUG1, taurine up-regulated 1; PTX, Paclitaxel; NC, negative control; MDC, Monodansylcadaverine; PI, Propidium Iodide; qRT-PCR, qualitative real-time polymerase chain reaction; Si, Small interfering RNA.
TUG1 Alleviates the Sensitivity of Ovarian Cancer Cells to Antitumor Reagents in vivo
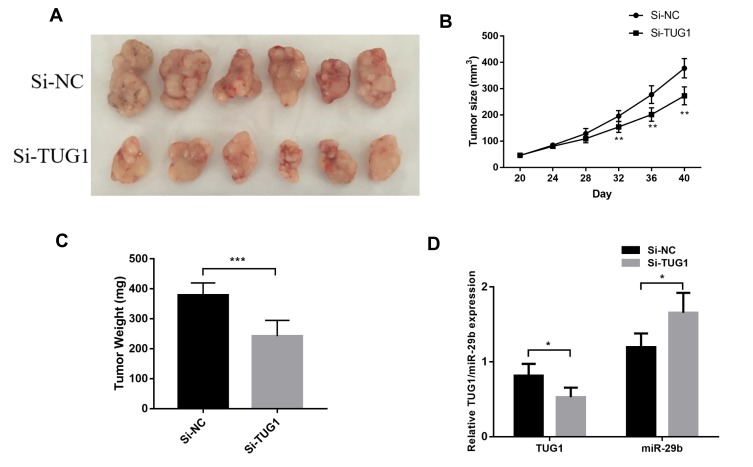
To further evaluate the effect of TUG1 on the chemoresistance of ovarian cancer cells, we established a xenograft tumor model in nude mice using A2780/R cells with or without knockdown of TUG1. The results showed that TUG1 promoted tumor growth to resist antitumor reagents in the xenograft model (Figure 6A). With PTX treatment, the tumors generated by cells that were transfected with a TUG1 interfere reagent grew slower than the tumors of NC-transfected cells, suggesting that TUG1 promoted chemotherapy resistance in vivo (Figure 6B). In addition, the size and weight of the tumors generated by A2780/R cells with TUG-1 downregulation were significantly smaller than the ones generated by the control cells (Figure 6C). qPCR analysis confirmed that the average expression of TUG1 was lower in the TUG-1-silenced tumor tissues compared with control tumors, whereas miR-29b-3p was upregulated (Figure 6D).
Figure 6.
TUG1 regulates ovarian cancer PTX resistance in vivo. (A) NC or si-TUG1 was transfected into A2780/R cells, which were injected into nude mice (n = 6). When the tumor size reached a volume of approximately 50 mm3, the mice were treated with PTX twice per week. (B) The tumor size of the mice was monitored every four days using student’s t test analysis. (C) Tumor weights are represented as the means of tumor weight per group using student’s t test analysis (p=0.0005). (D) qPCR was performed to detect the average expression of TUG1 and miR-29b-3p using one-way ANOVA analysis (p<0.05). *p<0.05; **p<0.01 and ***p<0.001.
Abbreviations: TUG1, taurine up-regulated 1; PTX, Paclitaxel; Si-NC, small interfering RNA negative control; qRT-PCR, qualitative real-time polymerase chain reaction; Si, Small interfering RNA; NC, negative control.
Discussion
TUG1 is involved in the biological processes of various cancer cells, including the development of chemoresistance.23,31 However, to date, no information is available on the role of TUG1 on ovarian cancer chemoresistance. Reportedly, TUG1 could promote ovarian cancer cell proliferation, invasion and metastasis.20 In our study, we found that TUG1 expression was not only high in ovarian tumor tissues compared with normal ovarian tissues but was also higher in the chemoresistance group compared with the sensitive group. TUG1 overexpression in ovarian cancer indicated an unfavorable prognosis, which was consistent with the results of a previous study.33 Deletion of TUG1 inhibited ovarian cancer cell activity. Furthermore, our study also revealed that TUG1 enhanced ovarian cancer cell resistance to PTX though inducing an increase in autophagy. To the best of our knowledge, this is the first report on the role of TUG1-regulated autophagy in the chemoresistance of ovarian cancer cells.
LncRNAs, which are located in the cytoplasm, can interact with miRNAs by miRNA response elements (MREs).34 The consequence of competition for miRNA binding is a decreased miRNA expression and thus an impairment of miRNA activity. TUG1, a 7.1 kb lncRNA, was reported to regulate cancer progression by sponging miRNAs. For example, TUG1 can interact with miR-144-3p,35 miR-335-5p,36 miR-212-3p37 and miR-132-3p38 to promote osteosarcoma cancer development. In hepatocellular carcinoma, TUG1 regulates cancer cell proliferation and metastasis by sponging miR-14439 and miR-142-3p.40 In addition, TUG1 also acted as a competing endogenous RNAs (ceRNA) of miR-26a,41 miR-600,42 miR-29c,43 miR-384,44 miR-196a45 and miR-19721 to promote cancer progression. In our study, we found that TUG1 enhanced ovarian cancer cell chemoresistance to PTX by acting as a ceRNA and sponging miR-29b-3p. This finding is consistent with a previous study indicating that TUG1 could sponge miR-29b-3p.46,47 Downregulation of miR-29b-3p was associated with DFS of ovarian cancer patients.48 The MUC1 aptamer-miR-29b-3p chimera played an important role not only in inhibiting49 but also in reducing the chemoresistance50 of ovarian cancer through targeted delivery of miR-29b-3p. In addition, miR-29b-3p deletion inhibited the sensitivity to chemotherapy in patients with ovarian carcinoma.31 These previous studies were consistent with our study, suggesting that TUG1 sponged and decreased miR-29b-3p expression in ovarian cancer and enhanced ovarian cancer cell chemoresistance to drugs.
To demonstrate prove whether miR-29b-3p is the pivotal target of TUG1 in ovarian cancer cell chemoresistance, we used three different methods. First, a luciferase reporter assay was used to detect whether TUG1 binds to miR-29b-3p directly via sequence complementation. Ago2 protein, a key component of the RNA-induced silencing complex, has been shown to be important in the progression of lncRNA sponging of miRNAs. Thus, we next used an Ago2 antibody to pull down TUG1 by RIP assay. In addition, we interfered with the expression of miR-29b-3p by deleting TUG1. The results showed that miR-29b-3p interference alleviated ovarian cancer cell chemosensitivity caused by TUG1 deletion. These results indicated that TUG1 served as a ceRNA by sponging miR-29b-3p.
Most previous studies indicated that TUG1 promotes cancer cell progression and chemoresistance mainly through the epithelial-mesenchymal transition,40,44,51,52 a phenotypic alteration in which epithelial cells acquire invasive mesenchymal characteristics. EMT plays an important role not only in cancer metastasis but also in chemoresistance. In addition, TUG1 also promotes cancer progression by regulating glycolysis in osteosarcoma and hepatocellular carcinoma.53,54 Our study indicated that TUG1 also enhanced ovarian cancer cell chemoresistance by increasing autophagy, which is also recognized as a vital element of tumor cell proliferation, metastasis and chemoresistance. This is the first study to suggest that TUG1 could regulate autophagy and promote cancer development. Meanwhile, our study also improved our understanding of additional regulatory mechanisms of TUG1 in the development of various tumors.
Autophagy and apoptosis are two closely related biological processes, especially in cancer. In our study, autophagy decreased in ovarian cancer cells after TUG1 deletion with PTX by labeling autophagic vesicles with MDC. However, ovarian cancer cell apoptosis, measured by staining with PI, indicated that cell apoptosis decreased after interference with TUG1 expression only in the absence of PTX treatment. Under PTX exposure, TUG1 deletion did not increase ovarian cancer cell apoptosis. These different effects are likely due to different autophagy responses to different cell stresses. A decrease in autophagy, a favored survival strategy, would lead to increased apoptosis when the cells are not stressed (for example, without PTX treatment). However, after PTX exposure, in order to survive, cancer cells did not significantly increase apoptosis when TUG1-associated autophagy was decreased.
In summary, TUG1 was not only overexpressed ovarian tumor tissues compared with normal ovarian tissues but was also higher in the chemoresistant group compared with the sensitive group, and higher TUG1 was associated with poorer prognosis in ovarian cancer patients. Knockdown of TUG1 decreased the autophagic response and chemoresistance in ovarian cancer cells by sponging miR-29b-3p. Thus, our findings indicated that TUG1 maybe plays a vital role in autophagy and chemoresistance and could be used as a therapeutic target for PTX resistance in ovarian cancer patients.
Data Sharing Statement
All relevant data are within the paper.
Funding
This project was supported by the Science and Technology Development Fund of Shanghai Pudong New Area (grant no. PKJ2016-Y32) awarded to Dr. Li, China Postdoctoral Science Foundation Grant (2018M640464) and The young talents program of Jiangsu Cancer Hospital (QL201805) awarded to Dr. Zhu.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi: 10.1016/S0140-6736(13)62146-7 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Archiv. 2012;460(3):237–249. doi: 10.1007/s00428-012-1203-5 [DOI] [PubMed] [Google Scholar]
- 4.Morgan RJ, Alvarez RD, Armstrong DK, et al. Ovarian cancer, version 3.2012. J Natl Compr Canc Netw. 2012;10(11):1339–1349. doi: 10.6004/jnccn.2012.0140 [DOI] [PubMed] [Google Scholar]
- 5.Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10(1):123–130. doi: 10.1016/S0955-0674(98)80095-1 [DOI] [PubMed] [Google Scholar]
- 6.Dong X, Mattingly CA, Tseng MT, et al. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009;69(9):3918–3926. doi: 10.1158/0008-5472.CAN-08-2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wertz IE, Kusam S, Lam C, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471(7336):110–114. doi: 10.1038/nature09779 [DOI] [PubMed] [Google Scholar]
- 8.Fu Y, YE D, CHEN H, et al. Weakened spindle checkpoint with reduced BubR1 expression in paclitaxel-resistant ovarian carcinoma cell line SKOV3-TR30. Gynecol Oncol. 2007;105(1):66–73. doi: 10.1016/j.ygyno.2006.10.061 [DOI] [PubMed] [Google Scholar]
- 9.Xi G, Hu X, Wu B, et al. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011;307(2):141–148. doi: 10.1016/j.canlet.2011.03.026 [DOI] [PubMed] [Google Scholar]
- 10.Zhang SF, Wang X-Y, Fu Z-Q, et al. TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy. 2015;11(2):225–238. doi: 10.1080/15548627.2014.998931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z, Zhou L, Chen Z, et al. Stress management by autophagy: implications for chemoresistance. Int J Cancer. 2016;139(1):23–32. doi: 10.1002/ijc.29990 [DOI] [PubMed] [Google Scholar]
- 12.Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–1563. [DOI] [PubMed] [Google Scholar]
- 13.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145(2):178–181. doi: 10.1016/j.cell.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 14.Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teschendorff AE, Lee S-H, Jones A, et al. HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome Med. 2015;7:108. doi: 10.1186/s13073-015-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozes AR, Miller DF, Özeş ON, et al. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. 2016;35(41):5350–5361. doi: 10.1038/onc.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Ye C, Liu J, et al. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem Biophys Res Commun. 2018;501(4):1034–1040. doi: 10.1016/j.bbrc.2018.05.104 [DOI] [PubMed] [Google Scholar]
- 18.Zheng ZG, Xu H, Suo -S-S, et al. The essential role of H19 contributing to cisplatin resistance by regulating glutathione metabolism in high-grade serous ovarian cancer. Sci Rep. 2016;6:26093. doi: 10.1038/srep26093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15(6):501–512. doi: 10.1016/j.cub.2005.02.027 [DOI] [PubMed] [Google Scholar]
- 20.Kuang D, Zhang X, Hua S, et al. Long non-coding RNA TUG1 regulates ovarian cancer proliferation and metastasis via affecting epithelial-mesenchymal transition. Exp Mol Pathol. 2016;101(2):267–273. doi: 10.1016/j.yexmp.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 21.Tang T, Cheng Y, She Q, et al. Long non-coding RNA TUG1 sponges miR-197 to enhance cisplatin sensitivity in triple negative breast cancer. Biomed Pharmacother. 2018;107:338–346. doi: 10.1016/j.biopha.2018.07.076 [DOI] [PubMed] [Google Scholar]
- 22.Niu Y, Ma F, Huang W, et al. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer. 2017;16(1):5. doi: 10.1186/s12943-016-0575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Wang W, Li G, et al. High TUG1 expression is associated with chemotherapy resistance and poor prognosis in esophageal squamous cell carcinoma. Cancer Chemother Pharmacol. 2016;78(2):333–339. doi: 10.1007/s00280-016-3066-y [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 25.Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66(1):3–14. [PubMed] [Google Scholar]
- 26.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1(3):1458–1461. doi: 10.1038/nprot.2006.238 [DOI] [PubMed] [Google Scholar]
- 27.Jeong JY, Kim K-S, Moon J-S, et al. Targeted inhibition of phosphatidyl inositol-3-kinase p110β, but not p110α, enhances apoptosis and sensitivity to paclitaxel in chemoresistant ovarian cancers. Apoptosis. 2013;18(4):509–520. doi: 10.1007/s10495-013-0807-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017;10:5377–5390. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopper O, de Witte CJ, Lõhmussaar K, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25(5):838–849. doi: 10.1038/s41591-019-0422-6 [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Li Y, Xie C, et al. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer. 2014;135(6):1286–1296. doi: 10.1002/ijc.v135.6 [DOI] [PubMed] [Google Scholar]
- 31.Dai F, Zhang Y, Chen Y. Involvement of miR-29b signaling in the sensitivity to chemotherapy in patients with ovarian carcinoma. Hum Pathol. 2014;45(6):1285–1293. doi: 10.1016/j.humpath.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 32.Zhou S, Lei D, Bu F, et al. MicroRNA-29b-3p targets SPARC gene to protect cardiocytes against autophagy and apoptosis in hypoxic-induced H9c2 cells. J Cardiovasc Transl Res. 2018. [DOI] [PubMed] [Google Scholar]
- 33.Li TH, Zhang -J-J, Liu S-X, et al. Long non-coding RNA taurine-upregulated gene 1 predicts unfavorable prognosis, promotes cells proliferation, and inhibits cells apoptosis in epithelial ovarian cancer. Medicine (Baltimore). 2018;97(19):e0575. doi: 10.1097/MD.0000000000010575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Han X, Qi X, et al. TUG1 promotes osteosarcoma tumorigenesis by upregulating EZH2 expression via miR-144-3p. Int J Oncol. 2017;51(4):1115–1123. doi: 10.3892/ijo.2017.4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Yang T, Zhang Z, et al. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017;108(5):859–867. doi: 10.1111/cas.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Tian G, Tian F, et al. Long non-coding RNA TUG1 promotes osteosarcoma cell proliferation and invasion through inhibition of microRNA-212-3p expression. Exp Ther Med. 2018;16(2):779–787. doi: 10.3892/etm.2018.6216 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Li G, Liu K, Du X. Long non-coding RNA TUG1 promotes proliferation and inhibits apoptosis of osteosarcoma cells by sponging miR-132-3p and upregulating SOX4 expression. Yonsei Med J. 2018;59(2):226–235. doi: 10.3349/ymj.2018.59.2.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv J, Kong Y, Gao Z, et al. LncRNA TUG1 interacting with miR-144 contributes to proliferation, migration and tumorigenesis through activating the JAK2/STAT3 pathway in hepatocellular carcinoma. Int J Biochem Cell Biol. 2018;101:19–28. doi: 10.1016/j.biocel.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 40.He C, Liu Z, Jin L, et al. lncRNA TUG1-Mediated Mir-142-3p downregulation contributes to metastasis and the epithelial-to-mesenchymal transition of hepatocellular carcinoma by targeting ZEB1. Cell Physiol Biochem. 2018;48(5):1928–1941. doi: 10.1159/000492517 [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Tang X, Wang Z, et al. TUG1 promotes prostate cancer progression by acting as a ceRNA of miR-26a. Biosci Rep. 2018;38(5):BSR20180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Hu J, Wang G, et al. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J Exp Clin Cancer Res. 2018;37(1):106. doi: 10.1186/s13046-018-0771-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Lu Y, Tang L, Zhang Z, et al. Long noncoding RNA TUG1/miR-29c axis affects cell proliferation, invasion, and migration in human pancreatic cancer. Dis Markers. 2018;2018:6857042. doi: 10.1155/2018/6857042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian W, Ren Z, Lu X. Knockdown of long non-coding RNA TUG1 suppresses nasopharyngeal carcinoma progression by inhibiting epithelial-mesenchymal transition (EMT) via the promotion of miR-384. Biochem Biophys Res Commun. 2019;509(1):56–63. doi: 10.1016/j.bbrc.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Sun D-M, Yu J-F, et al. Long noncoding RNA TUG1 promotes renal cell carcinoma cell proliferation, migration and invasion by downregulating microRNA‑196a. Mol Med Rep. 2018;18(6):5791–5798. doi: 10.3892/mmr.2018.9608 [DOI] [PubMed] [Google Scholar]
- 46.Han X, Hong Y, Zhang K. TUG1 is involved in liver fibrosis and activation of HSCs by regulating miR-29b. Biochem Biophys Res Commun. 2018;503(3):1394–1400. doi: 10.1016/j.bbrc.2018.07.054 [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Li H, Ge A, et al. Long non-coding RNA TUG1 inhibits apoptosis and inflammatory response in LPS-treated H9c2 cells by down-regulation of miR-29b. Biomed Pharmacother. 2018;101:663–669. doi: 10.1016/j.biopha.2018.02.129 [DOI] [PubMed] [Google Scholar]
- 48.Flavin R, Smyth P, Barrett C, et al. miR-29b expression is associated with disease-free survival in patients with ovarian serous carcinoma. Int J Gynecol Cancer. 2009;19(4):641–647. doi: 10.1111/IGC.0b013e3181a48cf9 [DOI] [PubMed] [Google Scholar]
- 49.Dai F, Zhang Y, Zhu X, et al. Anticancer role of MUC1 aptamer-miR-29b chimera in epithelial ovarian carcinoma cells through regulation of PTEN methylation. Target Oncol. 2012;7(4):217–225. doi: 10.1007/s11523-012-0236-7 [DOI] [PubMed] [Google Scholar]
- 50.Dai F, Zhang Y, Zhu X, et al. The anti-chemoresistant effect and mechanism of MUC1 aptamer-miR-29b chimera in ovarian cancer. Gynecol Oncol. 2013;131(2):451–459. doi: 10.1016/j.ygyno.2013.07.112 [DOI] [PubMed] [Google Scholar]
- 51.Madaan A, Nadeau-vallée M, Rivera JC, et al. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1). Am J Obstet Gynecol. 2017;216(1):60 e1–60 e17. doi: 10.1016/j.ajog.2016.09.072 [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Sun H, Kong H, et al. The Lncrna-TUG1/EZH2 axis promotes pancreatic cancer cell proliferation, migration and EMT phenotype formation through sponging Mir-382. Cell Physiol Biochem. 2017;42(6):2145–2158. doi: 10.1159/000479990 [DOI] [PubMed] [Google Scholar]
- 53.Han X, Yang Y, Sun Y, et al. LncRNA TUG1 affects cell viability by regulating glycolysis in osteosarcoma cells. Gene. 2018;674:87–92. doi: 10.1016/j.gene.2018.06.085 [DOI] [PubMed] [Google Scholar]
- 54.Lin YH, Wu M-H, Huang Y-H, et al. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67(1):188–203. doi: 10.1002/hep.29462 [DOI] [PubMed] [Google Scholar]