Abstract
Natural killer (NK) cells are innate immune lymphocytes with a key role in host defense against HIV infection. Recent advances in chimeric antigen receptors (CARs) have made NK cells a prime target for expressing recombinant receptors capable of redirecting NK cytotoxic functions towards HIV-infected cells. In this review, we discuss the role of NK cells in HIV and the mechanisms of actions of HIV-targeting CAR strategies. Furthermore, we also review NK cells signal transduction and its application to CAR NK cell strategies to develop new combinations of CAR intracellular domains and to improve CAR NK signaling and cytotoxic functions.
Introduction
NK cells are lymphocytes that provide an early innate defense against intracellular pathogens and malignant cells. Generally, NK cells specialize in the recognition and rapid lysis of ‘abnormal cells’, such as cells that have been infected by viruses. NK cells, such as B and T cells, differentiate from common lymphoid progenitors in the bone marrow. However, they do not require recombination of the B cell receptor (BCR) and T cell receptor (TCR) because NK cells do not rely on unique antigen receptors. Instead, they rely on a balance between activating and inhibitory receptors to induce signal transduction and promote the cytotoxic functions that kill target cells. Once mature, NK cells circulate in the blood and tissues while surveying for infected or malignant cells. Although NK cells are formidable players in the immune response against viruses, genetically modifying NK cells to express CARs could improve NK cell targeting of infected and malignant cells. In this review, we discuss the role of NK cells during HIV infection, and evaluate how studies focusing on NK cell signal transduction can be utilized to develop novel CAR strategies against HIV. Thus, we also review CAR strategies against HIV and current CAR NK strategies, and compare T cell and NK cell intracellular signaling.
Natural killer cells in HIV pathogenesis
In healthy individuals, NK cells constitute 5–20% of all human peripheral blood mononuclear cells (PBMCs) and can be categorized as either CD56dim CD16+ (the predominant phenotype) or CD56bright CD16neg/dim [1]. During the early stages of viral infection, infected cells release type 1 interferons (IFNs) and other cytokines to recruit NK cells to the site of infection [2]. NK cells are then ‘primed’ by interacting with dendritic cells (DCs), interleukin (IL-12), IL-15, and IL-18 [3]. Although primed NK cells are able to secrete IFN-γ, they are not able to kill until their inhibitory receptors are disengaged and their activating receptors are stimulated. This ‘balance’ between activators and inhibitors has prompted the paradigm that NK cells cannot trigger cytotoxic functions against healthy cells because they express major histocompatibility complex class I (MHC I). The presence MHC I on the cell surface can engage inhibitory killer immunoglobulin (Ig)-like receptors (KIR) on NK cells and promote the transmission of inhibitory signals that block NK cell cytotoxicity [4]. The ability of NK cells to target cells not expressing MHC I is complemented by the ability of viral-specific CD8+ cytotoxic lymphocytes (CTL) to target cells expressing viral antigens presented by MHC I. For example, through the expression of the viral nef gene, HIV-infected cells are able to downregulate MHC I and avoid CTL surveillance [5]. However, by doing so, infected cells become inherently susceptible to killing by NK cells. Hence, this cooperation between NK cells and CTL ensures that viral pathogens are always targeted by cytotoxic cells [6].
Once NK cells are at the site of infection, activating receptors are engaged, and inhibitory receptors are unbound, NK cells can use multiple strategies to fight HIV-infected cells. CD56dim/CD16+ NK cells can kill target cells by releasing lysozymes and cytotoxic granules, such as perforin and granzymes [7]. Perforin is a pore-forming molecule that permeabilizes the membrane and allows granzymes to penetrate the cell, resulting in activation of apoptotic pathways and cell lysis [8]. NK cells can also dispose of target cells by using ‘death ligands’, such as FasL and tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL), to activate receptors on the target cell and induce apoptosis [9]. Furthermore, some NK cells have the ability to specifically lyse target cells coated with antibodies through the process of antibody-dependent cellular cytotoxicity (ADCC). IgG antibodies bound to a target cell have their Fc region exposed and available to interact with Fc receptors, such as CD32 and CD16, on NK cells. Upon receptor activation, NK cells degranulate and release cytotoxic granules against the antibody-coated cell [10,11]. Additionally, NK cells expressing high levels of CD56 and low levels of CD16 have the ability to secrete cytokines, such as tumor necrosis factor (TNF)- and IFN-γ. Production of IFN-γ and TNF- enhances the recruitment of Th1 CD4+ T helper cells and macrophages to the site of infection to promote inflammation and the phagocytosis of infected cells and to control the infection locally [12].
There are many lines of evidence suggesting that NK cells have a crucial role in HIV pathogenesis. For instance, nonhuman primate (NHP) studies have shown that early depletion of NK cells leads to an exponential increase in viral loads during the chronic phase of SIV infection [13]. Furthermore, individuals with slow AIDS progression and individuals protected from seroconversion after multiple exposure to the virus have an effective NK cell population against HIV [14,15]. In addition, Elemans and colleagues showed that NK cells can impose selective pressure on HIV-1 variants, suggesting that NK cells are so effective against the virus that they can induce selection of different mutants [16]. Moreover, because NK cells rely on various germline-encoded receptors that lack the high specificity of TCRs and BCRs, it might be that NK cell receptors are less susceptible to HIV immune-evading mutations because they target different aspects of the viral life cycle.
The role of NK cells in HIV progression has become more apparent with recent studies suggesting that the expression and stimulation of certain NK cell receptors provides better clinical outcomes during HIV infection. For example, large epidemiological studies showed that activating receptor KIR3DS1 and inhibitory receptor KIR3DL1 bind to different alleles of MHC I HLA-B Bw4 that are renowned for their ability to present immunodominant HIV peptides and result in immune responses capable of providing protection against HIV [17,18]. Interestingly, whereas KIR3DS1 is an activating receptor that is likely to mediate direct killing of HIV-infected cells by binding HLA-B Bw4, inhibitory receptor KIR3DL1 also enhances protection against disease progression by providing inhibitory signals during NK cell resting states, which results in a more pronounced NK cell response when the inhibition is abrogated during infection [19,20].
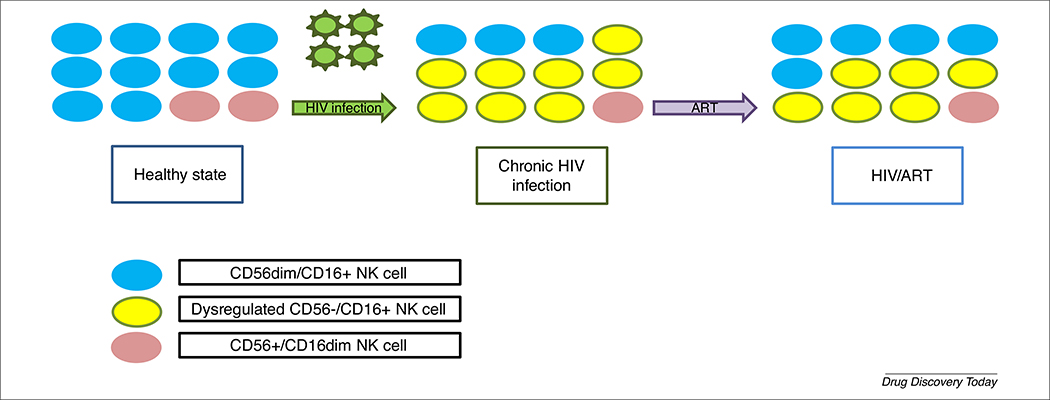
However, several studies have also suggested that HIV infection promotes the dysregulation of NK cells, which results in their inability to help mount a proper immune response against the virus [21]. For instance, investigators observed an early depletion of cytokine-secreting CD56bright CD16 neg/dim NK cells followed by a reduction in cytotoxic CD56dim CD16+ NK cells during the chronic phase of HIV-1 infection [1,22]. This loss of ‘classical’ NK cell phenotypes is predominantly because of the expansion of a pathological NK cell population, termed CD56neg NK cells, which is rarely observed in uninfected individuals [23]. In vitro experiments demonstrated that CD56neg NK cells have limited cytokine-secreting abilities and dramatically reduced cytotoxic functions against K562 erythroleukemia cells compared with CD56+ NK cells isolated from the same HIV donor. Further analysis showed that CD56neg NK cells have higher expression of inhibitory receptors than other NK cell subtypes despite maintaining the same proliferative capacities [23]. Transcriptomic analysis also suggests that these cells have lower expression of genes involved in cytotoxic and cytokine-secreting functions [24]. Therefore, the loss of functional NK cells during HIV infection could explain how HIV evades NK cell antiviral response (Fig. 1). However, functional NK cell phenotypes can partially be recovered with antiretroviral therapy (ART) [25].
FIGURE 1.
Schematics of the transition in natural killer (NK) cell phenotype and functionality. Healthy cells are predominantly CD56dim/CD16+. However, during HIV infection, CD56−/CD16+ cells become dysregulated whereas the population of cytokine-secreting cells, CD56+/CD16dim, is reduced. Effective antiretroviral therapy (ART) treatment can partially recover healthy NK phenotypes.
In addition to dysregulating NK cells, HIV has evolved different strategies to inhibit NK cell activity during pathogenesis. For instance, infected CD4+ T cells downregulate MIC-A, a ligand required for the NKG2D-activating receptor, and cause defective NKG2D-dependent cytotoxic functions [26]. Additionally, aberrant secretion of regulatory cytokines during pathogenesis affects the proper maturation of DCs and leads to dysfunctional cytokine secretions required for NK cell activation and priming [27]. However, these blocks in NK function can be overcome by providing NK cells with additional activating signals, perhaps mediated through a CAR receptor.
CARs for HIV therapy
Advancements in gene therapy have opened the door to genetically modifying NK cells with recombinant receptors capable of activating cells by mimicking the canonical stimulation through germline receptors and cytokines. For immunotherapy against HIV, CARs expressing a CD4 extracellular domain have been linked to different intracellular molecules to redirect cell activation and immunological functions against almost all HIV isolates. These CARs are able to promote NK cell functions against infected cells while circumventing the best attempt of the virus at immune evasion [28].
CAR T cells in HIV
CARs are recombinant fusion proteins that were first designed to mimic T cell receptor signaling by linking an extracellular antigen recognition domain to an intracellular TCRζ chain. The idea was to redirect T cell functions against a specific ligand or target cell, while bypassing canonical MHC presentation and restrictions on T cell activation [29]. In HIV immunotherapy, it was first thought that combining a CD4 extracellular domain to the TCRζ intracellular domain would induce T cell activation upon binding to HIV glycoproteins on the surface of infected cells. Yang and collaborators demonstrated that CD4-TCRζ CAR-transduced T cells were able to bind and induce lysis of GP120-expressing cell lines just as effectively as naturally occurring HIV-1-specific CTL clones. These CAR T cells were also able to inhibit viral replication in HIV-infected cells, suggesting that they had the ability to kill infected cells early during virion production in vitro [30]. However, clinical trials using CD4-TCRζ CAR T cells failed to stop HIV replication. In the first Phase I clinical trial, syngeneic T cells obtained from healthy discordant twins were genetically engineered with CD4-TCRζ CAR and administered to their twin with HIV, but the results showed no significant change in HIV viremia [31]. This lack of clinical success was repeated in a Phase II trial where 24 patients with HIV were randomly assigned to receive CD4-TCRζ CAR T cells, but the treatment failed to induce changes in HIV RNA or proviral DNA loads [32,33]. Overall, CD4-TCRζ CARs were safe to use in patients with HIV and were detectable by PCR 10 years post infusion, but failed to promote any clinical benefit [34].
Many have debated that the lack of CD4-TCRζ CAR efficacy in vivo could be explained by the fact that T cells naturally require at least two signals to fully activate their proliferative abilities and immunological functions. Canonical T cell activation and clonal expansion requires signaling through the TCRζ chain to be complemented by signaling through costimulatory receptors, such as CD28, a molecule activated by binding to CD80/CD86 during antigen presentation [35]. Second-generation CARs were made by linking intracellular domains from CD28, or other costimulators, in tandem to TCRζ chains. Overall, second-generation CARs promoted better T cell activation, clonal expansion, cytokine production, and cytotoxic functions compared with CD4-TCRζ CARs [36]. Furthermore, different T cell responses were observed depending on the costimulatory intracellular domain used. For example, addition of a CD28 intracellular domain improved CAR cytotoxic functions and cell proliferation [37]. Addition of ICOS improved CD8+ persistence and cytokine production [38]. The inclusion of 4–1BB not only similar effects as CD28, but also improved long-term persistence and avoidance of T cell exhaustion [39]. Finally, addition of OX40 repressed secretion of immunosuppressive cytokines [40]. Interestingly, a recent study suggested that the choice of intracellular costimulatory domain used also affects the susceptibility of CAR-expressing T cells to HIV infection. CARs containing 4–1BB, CD27, or ICOS were found to be more susceptible to HIV infection than CARs containing CD28 or OX40 [41]. However, in vivo murine studies suggested that CD4–41BB-TCRζ CAR exhibited the greatest control over HIV replication [41]. These studies also showed that swapping the transgene promoter (in this instance PGK for EF1α) and CAR transmembrane domain from CD4 to CD8 could improve CAR surface expression and decrease CAR susceptibility to HIV infection [41].
Although there now exists a multitude of CAR generations in T cells, each expressing different intracellular domains in tandem or combining cytokine genes to CARs, new strategies have also evolved to target CAR expression on cells from multiple hematopoietic lineages. A recent study showed that transplantation of CAR-expressing autologous HSPC in four male juvenile pigtail macaques differentiated into CD4+ T cells, CD8+ T cells, NK cells, monocytes, and macrophages. These cells were able to mediate killing of HIV-infected cells in vitro, engraft and expand both in the blood and in tissues in vivo, and reduce average rebound viremia in animals withdrawn from antiretroviral therapy. Furthermore, CD4+ T cell percentage and CD4:CD8 ratio were higher in CAR-infused animals than in controls. This strategy provided functional CAR cells capable of protecting immune homeostasis without controlling infection [28].
With the failure of CAR clinical trials to provide clinical benefit and HIV viral suppression, many scientists believe that adoptive transfer of CAR cells should first be tested in a NHP model before being introduced in a clinical setting [42]. The SIV/macaque model for AIDS research is excellent because macaques are physiologically and immunologically similar to humans and develop an AIDS-like disease upon SIV infection. In addition, the NHP model allows for therapeutic approaches that are deemed too ‘risky’ in humans [42]. Furthermore, NHP have a distinct advantage over humans in terms of the ability to control the timing, dose, and route of viral inoculation. CAR T cell work in NHP models has demonstrated that CAR T cells are able to engraft in vivo without systematic life-threatening cytotoxicity, migrate in different tissues, have long-term persistence, and can lower viral rebound after ART withdrawal [43,44].
Although CAR T cells are proving to be a promising model, there are several lines of evidence suggesting that CAR NK cells are a more suitable strategy for immunotherapy. First, NK cells are the most efficient cytolytic cell in the human body because they do not require prior contact stimulation by antigen-presenting cells and yet are regulated by inhibitory receptors before activation [4–7]. Second, the low toxicity, persistence, and reduced risk of graft-versus-host disease (GVHD) make allogeneic CAR NK cells a potential ‘off-the-shelf’ treatment. Several clinical trials have shown that infusions of allogeneic NK cells are safer than T cells and have reduced GVHD [45,46]. In one study, all 14 patients tolerated NK cell infusions without adverse effects (such as fever, headache, nausea/vomiting, or hypotension) that are often associated with CAR T cell infusions [47]. Furthermore, CAR NK cells have limited persistence when infused in vivo, which reduces the risk of prolonged toxic effects observed with CAR T cells [48]. Several studies suggest that the persistence of NK cells 2 weeks post infusion can be considered a successful engraftment [49]. This was showcased in a study where five out of six patients had no donor NK cells persisting after 5 days, although one patient showed persistence for as long as 138 days post infusion [50]. Although NK cell persistence is relatively short, infused NK cells have been found in some studies to expand in vivo, with a patient achieving 100% donor chimerism by day 38 after transplantation [50]. In addition, NK cells secrete fewer proinflammatory cytokines than T cells, which decreases the probability of off-target effects [51]. Taken together, life-threatening toxicities are less likely during CAR NK cell transplantation than during CAR T cell engraftment. Third, CAR NK cells could be a more suitable strategy for immunotherapy because NK cells are inherently capable of ADCC through their germline CD16 receptor. Therefore, CAR-transduced NK cells could promote cytotoxic functions through their artificially acquired receptor or through their natural germline receptor mechanism [51]. Although ex vivo expansion of NK cells via IL-2 has been shown to downmodulate CD16 because of the expression of the matrix metalloproteinase 25 (MMP25), small interfering (si) RNA-mediated disruption of MT6/MMP25 expression can restore CD16 expression on cultured CAR NK cells and reinstate their ADCC potential [52].
CAR NK cells
Initial CAR NK cell strategies have mainly focused on using first-generation CAR vectors with an intracellular TCRζ chain for signal transduction and various extracellular domains involved in the targeting of specific infected or malignant cells, such as CD19, CD20, and Erb2 [53]. For example, Boissel and colleagues investigated the ability of CD20-TCRζ NK cells to kill primary chronic lymphocytic leukemia cells, whereas Esser and colleagues studied the efficacy of GD2-TCRζ NK cells [54,55]. In the early days of anti-HIV CARs, the Roberts and colleagues showed that CD4-TCRζ NK cells can lyse 50% of GP120-expressing cells at an E:T ratio of between 25:1 and 50:1 [56]. However, although most TCRζ-CAR NK cell strategies provide some level of enhanced specific killing in vitro, these CARs succumb to similar issues as TCRζ-CAR T cell strategies and lack enough costimulation to provide effective treatment in vivo [35]. This was evidenced by experiments by Campana et al., where NK cells transduced with a second-generation CD19–41BB-TCRζ CAR reduced the percentage of leukemia cell recovery and enhanced NK cell cytotoxicity significantly more than did CD19-TCRζ NK cells [57]. Furthermore, a humanized ErbB2-CD28-TCRζ CAR was shown to mediate selective killing of target carcinoma cells more efficiently than ErbB2-TCRζ NK cells [58]. Moreover, a NK CAR strategy involving a CS1-CD28-TCRζ CAR suppressed the growth of malignant human IM9 multiple myeloma (MM) cells and significantly prolonged mouse survival in an aggressive orthotopic MM xenograft mouse model [59]. From a clinical standpoint, there are a few ongoing clinical trials using CAR NK cells for cancer immunotherapy. A Phase I clinical trial at St Jude Children’s Research Hospital was completed using CD19–41BB-TCRζ CAR NK cells to target B-lineage acute lymphoblastic leukemia (ALL) malignancy ( NCT00995137). Another Phase II clinical trial with NK cells has been approved for B-lymphoid malignancies using a CD28-TCRζ CAR with an inducible cas-pase-9 suicide gene and IL-15 as an activating cytokine (NCT03056339).
Although CAR T cells strategies in NK cells are effective, initiation of signal transduction in NK cells can involve different pathways and diverge significantly from T cells. Therefore, the systematic application of CAR T cell strategies in NK cells might not be an optimized strategy for NK cells. NK cells expressing a prostate stem cell antigen (PSCA)-DNAX activation protein (DAP) 12 CAR had increased specific cytotoxic abilities compared with NK cells expressing PSCA-TCRζ CAR, suggesting that swapping CAR combinations developed in T cells for intracellular domains specific to NK cell signaling provides better-performing CAR NK strategies [60]. This assumption was further evidenced when Campana et al. tested a NKG2D-DAP10-TCRζ CAR in NK cells and showed increased cytotoxic activity against several leukemia, osteosarcoma, and prostate carcinoma cell lines. In addition, stimulation of the NKG2D extracellular domain also triggered secretion of IFN-γ and TNF-α, and a massive release of cytotoxic granules [61]. Interestingly, swapping intracellular CAR domains for NK cell-specific domains is not always the best answer. When Campana et al. previously compared the first-generation CD19-TCRζ CAR with a CD19-DAP10 CAR, they found that the TCRζ intracellular domain enhanced NK cytotoxicity compared with DAP10 [57].This is likely because NK cell signal transduction follows rigorous rules and, whereas TCRζ alone might be potent enough to induce signal transduction, many NK-specific signaling domains require crosslinking of multiple receptors to acquire a threshold of phosphorylation sufficient to initiate signal transduction.
Potential signaling targets to improve CAR NK activity
The idea that CAR NK cells might behave differently than CAR T cells depending on the combination of intracellular signaling domains chosen is a fascinating hypothesis. Here, we briefly review T cell signaling and contrast NK cell signaling to understand how NK intracellular domains can shape CAR functions.
T cell signal transduction
T cell signal transduction (Fig. 2) is initiated by formation of the immunological synapse, which contemporaneously activates the TCR complex, the CD4/CD8 receptor, and other costimulatory receptors, such as CD28 [46]. Once CD4/CD8 is activated, it induces phosphorylation and activation of Lck, a protein in the Src tyrosine kinase family [63]. In turn, Lck phosphorylates immunoreceptor tyrosine-based activation motifs (ITAM) on the TCRζ intracellular chain, which is then used as ‘docking site’ to recruit and activate zeta-chain associated protein kinase 70 (ZAP-70) via its Src homology 2 (SH2) domain [64]. Activation of ZAP-70 is crucial for T cell signal transduction because ZAP-70 is responsible for the formation of protein scaffolds involved in almost every T cell signaling pathway. For instance, ZAP-70 activates adaptor protein linker for activation (LAT), responsible for recruiting growth factor receptor-bound protein (Grb2) and son of sevenless (Sos, a dual-specific GTP exchange factor) that are crucially involved in the Ras-MAPK pathway and cell proliferation [65,66]. Concurrently, ZAP-70 also activates adaptor protein SLP-76 to promote activation of Itk via the guanine-nucleotide-exchange factor Vav [67]. Itk has a crucial role in the phosphorylation of phospholipase C-γ (PLCγ), a protein involved in the hydrolyzation of phosphatidylinositol 4,5 biphosphate (PIP2) into diaglycerol (DAG) and inositol triphosphate (IP3). IP3 signals through the calcium-calcineurin-nuclear factor of activated T cells (NFAT) pathway to promote the secretion of cytokines, such as IL-2, transcriptional activation, and cytotoxic granule release [68]. DAG mediates activation of protein kinase C-θ (PKCθ), which induces signaling of the NFκB pathway, a crucial mediator of T cell differentiation, activation, and survival [69]. Costimulatory receptors are important in T cell signal transduction because they are involved in the phosphorylation of PI3K, a protein involved in the conversion of PIP2 into PIP3, and activator of Grb2. Through the activation of PI3K, the Akt and mTOR signaling pathways are initiated to enhance cell survival, proliferation, and T cell differentiation [70]. Although we tend to classify signaling pathways as isolated cascades of events, extensive crosstalk occurs between pathways. Therefore, signal transduction is a dynamic and integrative process that might start with the initiation of one pathway but result in activation of multiple signaling events.
FIGURE 2.
Schematic of T cell signal transduction. T cell activation requires two signals; the first is initiated by CD4/8 and T cell receptor (TCR) binding to major histocompatibility complex (MHC)–peptide complexes, which results in the phosphorylation and activation of proteins Lck, TCRζ, and Zap-70. The second signal is initiated by CD28 and costimulatory receptor binding. The combination of both signals drives strong T cell activation, differentiation, and effector immunological functions. The stars indicate direct kinase phosphorylation.
NK cell signal transduction
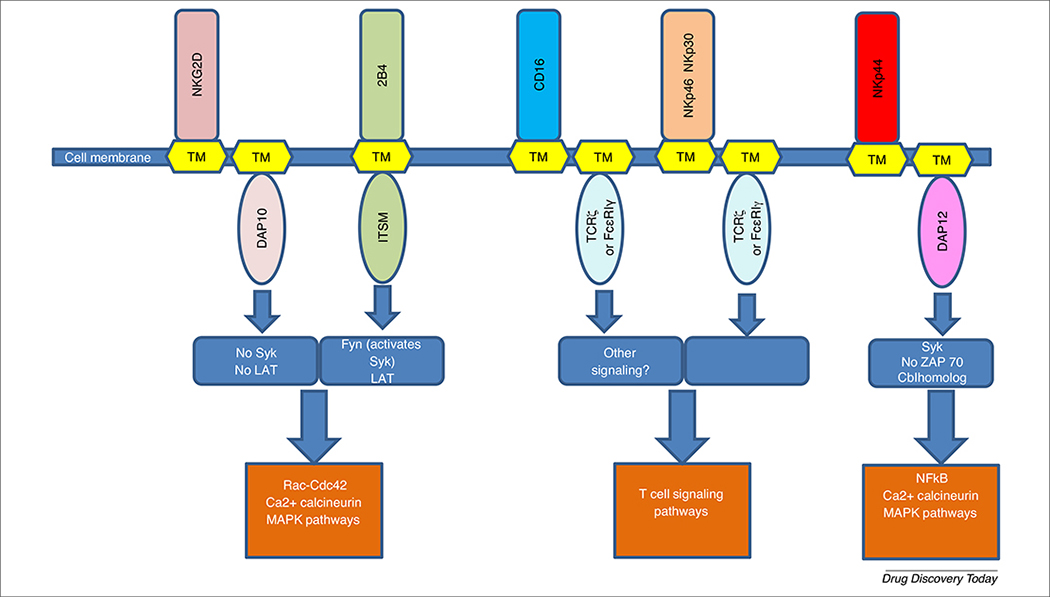
NK cell activation relies on a variety of germline-encoded receptors to initiate signal transduction and promote different cell functions (Fig. 3). Importantly, NK cell signal transduction and activation requires crosslinking of multiple activating receptors to provide the appropriate threshold of intracellular ITAM phosphorylation [71]. This idea is supported by experiments by Long et al. in which intracellular calcium concentrations were measured in resting NK cells, given that calcium (Ca2+) levels in NK cells are associated with cytotoxic degranulation. Results showed that crosslinked stimulation of CD16 with other activating receptors, such as NKG2D, 2B4, or DNAM-1, resulted in higher intracellular Ca2+ levels than CD16 alone. The same Ca2+ increase was observed when NKp46 was crosslinked with 2B4, NKG2D, or DNAM-1, but only slightly enhanced when NKG2D and DNAM-1 were co-cross-linked together [72]. In addition, microscopy experiments using the Ca2+-sensitive dyes Fluo-4 and Fura Red showed that CD16 or 2B4 crosslinking with NKG2D induced a sustained elevation in Ca2+ [72]. Given that Ca2+ mobilization is a crucial step in perforin-dependent NK cell cytotoxicity, these results showed that crosslinking of multiple receptors is required for NK cytotoxic activity and function.
FIGURE 3.
Natural killer (NK) cell signal transduction. Whereas some NK receptors share the downstream signal transduction of with T cells through T cell receptor (TCR)-ζ; other NK cell receptors promote signaling through unique proteins, such as FcεRIγ, DAP10, and DAP12. Therefore, optimizing early signaling events through NK-specific proteins could improve chimeric antigen receptor (CAR) NK cell signaling and function.
Natural cytotoxicity receptors (NCR) were the first NK cell-activating receptors to be studied. They are type 1 transmembrane glycoproteins containing a positively charged amino acid in their transmembrane domain that associates with the negatively charged transmembrane domains of signaling molecules [73]. Two types of NCR expressed on NK cells, NKp46 and NKp30, are also expressed on some subsets of T cells. Upon activation, these receptors associate with TCRζ and/or the FcεRIγ chain to promote signal transduction [74]. Given that TCRζ is canonically used in T cell TCR signaling, stimulation of NKp46 and NKp30 will partly induce similar signal transduction pathways, as in T cells [75].
One receptor expressed on activated NK cells is NKp44, which uses disulfide bonds to associate with DAP12 [76]. In rat studies, immunoprecipitation and blotting of murine Ly49D and DAP12 showed that DAP12 directly phosphorylates Cbl, a protein the homolog of which is involved in the Ras/MAPK pathway. In addition, DAP12 was shown to promote phosphorylation of PLCγ1, Erk, and Erk2, proteins involved in signal transduction via the MAPK and calcium-calcineurin pathways [77]. Although these signaling proteins are also activated by canonical T cell signaling, it does not mean that DAP12 signal transduction mediates the same events as T cell signaling. In fact, further studies demonstrated that DAP12 signaling did not activate ZAP-70, a crucial mediator of T cell signaling, but phosphorylated protein Syk [77]. Therefore, NKp44 signaling through DAP12 might have the same end result as the canonical T cell signaling pathway, but it appears that the upstream signal transduction differs from T cell signaling and is carried out by proteins other than ZAP-70.
Another NK cell receptor of interest is NKG2D, an activating receptor that associates with DAP10 but not with DAP12 [78]. NKG2D-mediated DAP10 signaling was found to activate ZAP-70 but not Syk or LAT (a direct downstream effector of ZAP-70), yet still resulted in the phosphorylation of PLC-γ2 [78]. In addition, NKG2D stimulation induced activation of SLP76 and Vav1, involved in the Rho-Rac/Cdc42 pathway and cell growth and proliferation [78]. These results suggest that NKG2D signals through a pathway similar enough to T cell to activate ZAP-70, Vav1, Rac/Cdc-42, and PLC-γ2, yet is different enough to be Syk and LAT independent.
Other evidence that NK cell signaling can be closely related but differs from T cell signaling is that stimulation of signaling molecules involved in T cell activation can result in inhibitory signaling in NK cell precursors. 2B4 is a member of the SLAM-related receptors containing intracellular immunoreceptor tyrosine-based switch motifs (ITSM) that recruit the adapter proteins SAP and 3BP2 [79]. SAP and 3BP2 signaling is so crucial to the activation of Fyn, LAT, Grb2, Cb1, Vav1, and PLC-γ that mutations in SAP cause the a genetic disorder X-linked lymphoproliferative disease [80,81]. Nonetheless, 2B4 can act as an inhibitory NK cell receptor in certain conditions, especially in NK cell precursors that lack inhibitory receptors for self-MHC class I [82].
The only known receptor capable of initiating NK cell activation and cytotoxic functions without requiring other receptor crosslinking is FcγRIIIA, or CD16, a receptor with low affinity for the Fc region of IgG antibodies [83]. CD16 is perhaps one of the most famous NK cell receptors because of its ability to induce ADCC and killing of target cells coated with IgG antibodies [84]. CD16 associates with not only TCRζ chains to induce signal transduction similar to T cells, but also FcεRIγ chains, a component of the high-affinity IgE receptor FcεRI and structural homolog to TCRζ chain. Site-directed mutagenesis experiments have shown that TCRζ and FcεRIγ were able to signal interchangeably [85]. This was confirmed in mouse large granular lymphocytes, in which NK-like activity against B cell hybridomas producing anti-CD3 and anti-CD16 monoclonal antibodies was rescued when TCRζ chains were depleted but replaced with FcεRIγ [86]. However, several studies suggest that signaling through TCRζ differs from FcεRIγ. Experiments crosslinking the CD16 extracellular domain to FcεRIγ or TCRζ intracellular domains demonstrated that the FcεRIγ chimeras were more efficient at signaling Ca2+ influx and IL-2 production upon CD16 stimulation. In addition, western blot analysis showed that the FcεRIγ chimeras promoted the phosphorylation of phosphoproteins 158, 116, and 101 (probably ubiquitin conjugates [86]) to a greater extent than TCRζ chimeras, suggesting that FcεRIγ induces distinct patterns of tyrosine phosphorylation that differed from CD16-TCRζ phosphorylation [87]. Interestingly, although it was thought that CD16 always associated with FcεRIγ and TCRζ, researchers recently identified a subset of CD56dim NK cells that are deficient in FcεRIγ but express TCRζ at normal levels. The absence of FcεRIγ endows distinct signaling properties to these NK cells, including the lack of signaling proteins SYK, DAB2, and EAT-2 [88]. Although these NK cells are still able to signal through TCRζ and ZAP-70, the decrease in the aforementioned signaling molecules results in abnormal NK cell function [89]. When researchers attempted to stimulate NK cells with target cells and antigens, FcεRIγ-deficient NK cells had reduced cytotoxic abilities and cytokine secretions compared with conventional NK cells [90]. However, in the presence of plasma or purified antibodies specific to the target cells, FcεRIγ-deficient NK cells had exponentially enhanced cytotoxic functions and cytokine-secreting abilities, hinting that these cells work optimally through ADCC. Unexpectedly, FcεRIγ-deficient NK cells could also respond robustly to other pathogens when pathogen-specific antibodies were available. This was seen in an experiment where HCMV-primed FcεRIγ-deficient NK cells could target and degranulate against HSV-1-infected cells upon receiving plasma from HSV-1-seropositive donors [90]. Therefore, the lack of FcεRIγ and SYK-mediated signaling makes direct antigen recognition by NK cells unlikely but enhances cytokine and cytotoxic responses in an antibody-dependent manner [90]. Furthermore, these differences in signaling are also illustrated in the longevity of NK cells. Although conventional NK cells are short lived, increasing evidence shows that FcεRIγ-deficient NK cells are long lived and display memory markers, such as CD44, CD11a, and CCR5 [90]. Based on these findings, CAR NK cells designed with a FcεRIγ intracellular domain could have a different longevity, kinetics of persistence, and cytotoxic behaviors compared with CAR NK cells designed with a TCRζ intracellular domain. However, it remains unclear whether CD16 also induces additional signaling pathways independently of TCRζ and FcεRIγ. This caveat is raised by the fact that, whereas CD16 alone can promote NK cell functions, receptors NKp46 and NKp30 also signal through TCRζ or FcεRIγ but are unable to induce NK cell activation without crosslinking to other receptors. Therefore, elucidating these unknown early signaling events could provide new insights into how to improve CAR strategies.
Given that NK cell signaling differs significantly from T cells, CAR NK cell immunotherapies could be improved by studying interactions between NK cell receptors and signal transduction. For example, studies of the CD16 receptor showed that the transmembrane domain of TCRζ appears to preferentially associate with CD16 rather than with the TCR complex [87]. Whereas most CAR strategies use CD4, TCR, or CD8 transmembrane domains, this finding suggests that using a CD16 transmembrane domain in CAR could allow potential crosstalk between germline TCRζ chains and CAR-recombinant TCRζ chains to enhance cell activation and improve the agglomeration of CARs at the immunological synapse. Furthermore, for CAR NK strategies involving targeted cytokine secretions, studies with the NKG2D receptor using monoclonal antibodies showed that NKG2D signaling alone triggered a cytotoxic response but not an IFN-γ response. These findings rule out the potential for a first-generation CAR-NKG2D to secrete IFN-γ [78]. However, a NKG2D-DAP10-TCRζ CAR was able to increase IFN-γ, GM-CSF, IL-13, MIP-1α, MIP-1β, RANTES, and TNF-α secretion in NK cells [61]. Therefore, the same CAR intracellular domains might vary their effect on NK cell functions depending on the combination used. One strategy to study the efficacy of intracellular CAR combinations in NK cells would involve developing a CAR library with randomly associated intracellular domains and screening for the most efficient combinations. This idea was originally developed in CAR T cells when a laboratory generated a library of >30 000 CARs and tested for CD69 upregulation in response to CAR ligation, IL-2 response to c-myc antibody, and overall response to erbB2-expressing cells [91]. Interestingly, although CD28-TCRζ CARs scored highly, investigators showed that the most functional CAR combination in T cells included NK cell signaling protein DAP10. In fact, the DAP10-TCRζ-CD27 CAR resulted in some of the highest levels of cytotoxic enhancement published in the literature [91].
Concluding remarks
CAR immunotherapy is a promising strategy that can be focused on the elimination of HIV reservoirs and the establishment of a cure against HIV/AIDS. In this review, we have summarized how the adaptation of CAR strategies developed in T cells has proven effective in retargeting NK cells against HIV-infected cells, raising hopes that CAR NK cells could be effective against HIV. Significant evidence suggests that swapping T cell intracellular domains for NK cell signaling domains results in improved cytotoxic killing, improved cytokine secretion, and overall improved NK cell functions. This evidence is supported by the realization that NK cell signal transduction differs from T cell signaling and CAR NK cells strategies should combine intracellular domains pertaining to NK cell signaling. Furthermore, the continued studying of NK cell signal transduction and receptor interactions could hold the key to developing new CAR-NK strategies capable of improving in vivo efficacy and reducing HIV viral loads long term. However, there are still obstacles to overcome before CAR NK cells can become a viable and cost-effective therapy, such as large-scale expansion and cell culturing, transduction efficiency, and safety.
Acknowledgments
This study was partially funded by NIH R01 AI110158 and a Pilot Grant from Tulane University School of Medicine to S.E.B. and R01-AI129745 and MH062261 to S.N.B. All authors contributed to writing and revising the manuscript.
Biography
 Giorgio Zenere is PhD candidate in the Biomedical Sciences program at Tulane University School of Medicine. He is studying the function, mechanism of action, and efficacy of different HIV CAR strategies for T and NK cells both in vitro and in a nonhuman primate (NHP) model at the Tulane National Primate Research Center. During his PhD in the Stephen E. Braun laboratory, Giorgio has trained in immunology, virology, and gene therapy.
Giorgio Zenere is PhD candidate in the Biomedical Sciences program at Tulane University School of Medicine. He is studying the function, mechanism of action, and efficacy of different HIV CAR strategies for T and NK cells both in vitro and in a nonhuman primate (NHP) model at the Tulane National Primate Research Center. During his PhD in the Stephen E. Braun laboratory, Giorgio has trained in immunology, virology, and gene therapy.
 Siddappa N. Byrareddy is an associate professor in the Department of Pharmacology and Experimental Neurosciences, University of Nebraska Medical Center, Omaha. He obtained his PhD from the National Institute of Mental Health and Neurosciences, Bangalore, India and completed postdoctoral training at the Dana-Farber Cancer Institute/Harvard Medical School, Boston, USA. Dr Byrareddy has a strong background in molecular retrovirology, innate immunity, lentiviral evolution, and mucosal virus transmission studies, with 12 years of research experience and over 80 peer-reviewed publications in the field. His laboratory research focuses on the development of effective HIV cure strategies and therapeutics to control HIV infections and emerging infectious diseases.
Siddappa N. Byrareddy is an associate professor in the Department of Pharmacology and Experimental Neurosciences, University of Nebraska Medical Center, Omaha. He obtained his PhD from the National Institute of Mental Health and Neurosciences, Bangalore, India and completed postdoctoral training at the Dana-Farber Cancer Institute/Harvard Medical School, Boston, USA. Dr Byrareddy has a strong background in molecular retrovirology, innate immunity, lentiviral evolution, and mucosal virus transmission studies, with 12 years of research experience and over 80 peer-reviewed publications in the field. His laboratory research focuses on the development of effective HIV cure strategies and therapeutics to control HIV infections and emerging infectious diseases.
 Stephen E. Braun is an assistant professor at Tulane National Primate Research Center within the Tulane University School of Medicine. His background and training are in developing retroviral and lentiviral vectors to genetically modify primary lymphohematopoietic target cells from humans, monkeys, and mice for HIV/SIV infection, genetic diseases, and cancer. After developing novel therapeutic strategies in vitro, he has used immunodeficient mouse models, progressed to large-scale animal studies in rhesus macaque, and advanced preclinical studies to clinical trials. He has more than 25 years of experience in translational research studying gene therapy strategies, with 15 of these years in NHP research using rhesus macaque.
Stephen E. Braun is an assistant professor at Tulane National Primate Research Center within the Tulane University School of Medicine. His background and training are in developing retroviral and lentiviral vectors to genetically modify primary lymphohematopoietic target cells from humans, monkeys, and mice for HIV/SIV infection, genetic diseases, and cancer. After developing novel therapeutic strategies in vitro, he has used immunodeficient mouse models, progressed to large-scale animal studies in rhesus macaque, and advanced preclinical studies to clinical trials. He has more than 25 years of experience in translational research studying gene therapy strategies, with 15 of these years in NHP research using rhesus macaque.
References
- 1.Caligiuri MA (2008) Human natural killer cells. Blood 112, 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh RM and Waggoner SN (2013) NK cells controlling virus-specific T cells: rheostats for acute vs persistent infections. Virology 435, 37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaix J et al. (2008) Priming of natural killer cells by interleukin-18. J. Immunol. 181, 1627–163118641298 [Google Scholar]
- 4.Horowitz A et al. (2012) Activation of natural killer cells during microbial infections. Front. Immunol. 2, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiler AM et al. (2016) Acute viral escape selectively impairs Nef-mediated major histocompatibility complex class I downmodulation and increases susceptibility to antiviral T cells. J. Virol. 90, 2119–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruey-Chyi S et al. (1998) NK cells can recognize different forms of class I MHC. J. Immunol. 161, 755–766 [PubMed] [Google Scholar]
- 7.Cohnen A et al. (2013) Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood 122, 1411–1418 [DOI] [PubMed] [Google Scholar]
- 8.Vivier E et al. (2008) Functions of natural killer cells. Nat. Immunol. 9, 503–510 [DOI] [PubMed] [Google Scholar]
- 9.Colucci S et al. (2007) The death receptor DR5 is involved in TRAIL-mediated human osteoclast apoptosis. Apoptosis 12, 1623. [DOI] [PubMed] [Google Scholar]
- 10.Morel PA et al. (1999) Functional CD32 molecules on human NK cells. Leuk. Lymphoma 35, 47–56 [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL et al. (1988) Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J. Immunol. 141, 3478–3485 [PubMed] [Google Scholar]
- 12.Alter G et al. (2005) Sequential deregulation of NK cell subset distribution and function in acute HIV-1 infection. Blood 106, 3366–3369 [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y et al. (2014) In vivo administration of a JAK3 inhibitor during acute SIV infection leads to significant increases in viral load during chronic infection. PLoS Pathog. 10, e1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker-Sperling VE et al. (2017) Factors associated with the control of viral replication and virologic breakthrough in a recently infected HIV-1 controller. EBioMedicine 16, 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson E et al. (2017) HIV exposed seronegative (HESN) compared to HIV infected individuals have higher frequencies of telomeric killer immunoglobulin-like receptor (KIR) B motifs; contribution of KIR B motif encoded genes to NK cell responsiveness. PLoS One 12, e0185160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elemans M et al. (2017) HIV-1 adaptation to NK cell-mediated immune pressure. PLoS Pathog. 13, e1006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulet S et al. (2008) A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS 22, 1487–1491 [DOI] [PubMed] [Google Scholar]
- 18.Boulet S et al. (2008) Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS 22, 595–599 [DOI] [PubMed] [Google Scholar]
- 19.Martin MP et al. (2002) Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31, 429–434 [DOI] [PubMed] [Google Scholar]
- 20.Martin MP et al. (2007) Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39, 733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolting A et al. (2010) MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology 406, 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elemans M et al. (2017) HIV-1 adaptation to NK cell-mediated immune pressure. PLoS Pathog. 13, e1006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavilio D et al. (2005) Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. U. S. A. 102, 2886–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costanzo MC et al. (2018) Transcriptomic signatures of NK cells suggest impaired responsiveness in HIV-1 infection and increased activity post-vaccination. Nat. Commun. 9, 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juelke K et al. (2010) CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood 116, 1299–1307 [DOI] [PubMed] [Google Scholar]
- 26.Nolting A et al. (2010) MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology 406, 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellegard R et al. (2018) Complement-opsonized HIV-1 alters cross talk between dendritic cells and natural killer (NK) cells to inhibit NK killing and to upregulate PD-1, CXCR3, and CCR4 on T cells. Front. Immunol. 9, 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y et al. (2018) Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol. Sin 39, 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhen A et al. (2017) Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. PLoS Pathog. 13, e1006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang OO et al. (1997) Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc. Natl. Acad. Sci. U. S. A. 94, 11478–11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker RE et al. (2000) Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood 96, 467–474 [PubMed] [Google Scholar]
- 32.Mitsuyasu RT et al. (2000) Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus-infected subjects. Blood 96, 785–793 [PubMed] [Google Scholar]
- 33.Mitsuyasu T et al. (2002) A Phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Cell 6, 788–797 [DOI] [PubMed] [Google Scholar]
- 34.Scholler J et al. (2012) Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 4 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abate-Daga D and Davila ML (2016) CAR models: next-generation CAR modifications for enhanced T-cell function. Mol. Ther. Oncolytics 3, 16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahu GK et al. (2013) Anti-HIV designer T cells progressively eradicate a latently infected cell line by sequentially inducing HIV reactivation then killing the newly Gp120-positive cells. Virology 446, 268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finney HM et al. (1998) Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 161, 2791–2797 [PubMed] [Google Scholar]
- 38.Guedan S et al. (2018) Enhancing CAR T cell persistence through ICOS and 4–1BB costimulation. JCI Insight 3, e96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campana D et al. (2014) 4–1BB chimeric antigen receptors. Cancer J. 20, 134–140 [DOI] [PubMed] [Google Scholar]
- 40.Hombach AA et al. (2012) OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology 1, 458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leibman RS et al. (2017) Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog. 13, e1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans DT and Silvestri G (2013) Nonhuman primate models in AIDS research. Curr. Opin. HIV AIDS 8, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhen A et al. (2017) Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. PLoS Pathog. 13, e1006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H et al. (2018) Therapeutic targeting of HIV reservoirs: how to give T cells a new direction. Front. Immunol. 9, 2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubnitz JE et al. (2010) NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 28, 955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi J et al. (2008) Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br. J. Haematol. 143, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon SR et al. (2010) Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant. 45, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 48.Bonifant CL et al. (2016) Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 3, 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezvani K et al. (2017) Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 25, 1769–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller JS et al. (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3305 [DOI] [PubMed] [Google Scholar]
- 51.Mehta RS and Rezvani K (2018) Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front. Immunol. 9, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peruzzi G et al. (2013) Membrane-type 6 matrix metalloproteinase regulates the activation-induced downmodulation of CD16 in human primary NK cells. J. Immunol. 191, 1883–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermanson DL and Kaufman DS (2015) Utilizing chimeric antigen receptors to direct natural killer cell activity. Front. Immunol. 6, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boissel L et al. (2013) Retargeting NK-92 cells by means of CD19-and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2, e26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esser R et al. (2012) NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J. Cell Mol. Med. 16, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran AC et al. (1995) Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. J. Immunol. 155, 1000–1009 [PubMed] [Google Scholar]
- 57.Imai C et al. (2005) Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106, 376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schönfeld K et al. (2015) Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol. Ther. 23, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu J et al. (2014) CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo anti-tumor activity against human multiple myeloma. Leukemia 28, 917–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Töpfe K et al. (2015) DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J. Immunol. 194, 3201–3212 [DOI] [PubMed] [Google Scholar]
- 61.Chang YH et al. (2013) A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 73, 1777–1786 [DOI] [PubMed] [Google Scholar]
- 63.Molina TJ et al. (1992) Profound block in thymocyte development in mice lacking p56lck. Nature 357, 161–164 [DOI] [PubMed] [Google Scholar]
- 64.Chan AC et al. (1994) The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu. Rev. Immunol. 12, 555–592 [DOI] [PubMed] [Google Scholar]
- 65.Wang H et al. (2010) ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb. Perspect. Biol 2 a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W and Samelson LE (2000) The role of membrane-associated adaptors in T cell receptor signaling. Semin. Immunol. 12, 35–41 [DOI] [PubMed] [Google Scholar]
- 67.Bubeck Wardenburg J et al. (1996) Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J. Biol. Chem. 271, 19641–19644 [DOI] [PubMed] [Google Scholar]
- 68.Gorentla BK and Zhong XP (2012) T cell receptor signal transduction in T lymphocytes. J. Clin. Cell. Immunol. 2012 (Suppl. 12), 005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cantrell DA (2002) T-cell antigen receptor signal transduction. Immunology 105, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung CH et al. (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 20, 1992–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watzl C and Long EO (2010) Signal transduction during activation and inhibition of natural killer cells. Curr. Protoc. Immunol 11 11.9B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bryceson YT et al. (2006) Activation, co-activation, and co-stimulation of resting human NK cells. Immunol. Rev. 214, 73–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kruse PH et al. (2014) Natural cytotoxicity receptors and their ligands. Immunol. Cell Biol. 92, 221–229 [DOI] [PubMed] [Google Scholar]
- 74.Li Y and Sun R (2018) Tumor immunotherapy: new aspects of natural killer cells. Chin. J. Cancer Res. 30, 173–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pende D et al. (1999) Identification and molecular characterization of Nkp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190, 1505–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moretta A et al. (2001) Activating receptors and coreceptors in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19, 197–223 [DOI] [PubMed] [Google Scholar]
- 77.Mcvicar DW et al. (1998) DAP12-mediated signal transduction in natural killer cells. J. Biol. Chem. 273, 32934–32942 [DOI] [PubMed] [Google Scholar]
- 78.Billadeau DD et al. (2003) NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat. Immunol. 4, 557–564 [DOI] [PubMed] [Google Scholar]
- 79.Claus M et al. (2008) Regulation of NK cell activity by 2B4, NTB-A and CRACC. Front. Biosci. 13, 956–965 [DOI] [PubMed] [Google Scholar]
- 80.Morra M et al. (2001) X-linked lymphoproliferative disease: a progressive immunodeficiency. Annu. Rev. Immunol. 19, 657–682 [DOI] [PubMed] [Google Scholar]
- 81.Deckert M and Rottapel R (2006) The adapter 3BP2: how it plugs into leukocyte signaling. Adv. Exp. Med. Biol. 584, 107–114 [DOI] [PubMed] [Google Scholar]
- 82.Sivori S et al. (2002) Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc. Natl. Acad. Sci. U. S. A. 99, 4526–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lanier LL et al. (1991) Analysis of Fc gamma RIII (CD16) membrane expression and association with CD3 zeta and Fc epsilon RI-gamma by site-directed mutation. J. Immunol. 146, 1571–1576 [PubMed] [Google Scholar]
- 84.Yeap WH et al. (2016) CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 6, 34310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koyasu S et al. (1992) T cell receptor complexes containing Fc epsilon RI gamma homodimers in lieu of CD3 zeta and CD3 eta components: a novel isoform expressed on large granular lymphocytes. J. Exp. Med. 175, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Youssef LA et al. (2002) Proteasome-dependent regulation of Syk tyrosine kinase levels in human basophils. J. Allergy Clin. Immunol. 110, 366–373 [DOI] [PubMed] [Google Scholar]
- 87.Vivier E et al. (1992) Signaling function of reconstituted CD16: zeta: gamma receptor complex isoforms. Int. Immunol. 4, 1313–1323 [DOI] [PubMed] [Google Scholar]
- 88.Lee J et al. (2015) Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 42, 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hwang I et al. (2012) Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. Int. Immunol. 24, 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T et al. (2013) Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J. Immunol. 190, 1402–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duong CPM et al. (2013) Engineering T cell function using chimeric antigen receptors identified using a DNA library approach. PLoS One 8, e63037. [DOI] [PMC free article] [PubMed] [Google Scholar]