Abstract
Risk calculators for prediction of conversion of Clinical High-Risk (CHR) individuals to syndromal psychosis have recently been developed and have generated considerable clinical use and research interest. Predictor variables in these calculators have been clinical rather than biological, and our goal was to incorporate a neurochemical imaging measure into this framework and assess its impact on prediction. We combined striatal glutamate 1H MRS data with the SIPS symptoms identified by the Columbia Risk Calculator as having the greatest predictive value in order to develop an imaging-based risk calculator for conversion to psychosis. We evaluated the calculator in 19 CHR individuals, 7 (36.84%) of whom converted to syndromal psychosis during the 2-year follow up. The receiver operating characteristic (ROC) curve for the logistic model including only striatal glutamate and visual perceptual abnormalities showed an AUC=0.869 (95% CI = [0.667, 1.000]) and AUCoa=0.823, with sensitivity of 0.714, specificity of 0.917, positive predictive value of 0.833, and negative predictive value of 0.846. These results represent modest improvements over each of the individual ROC curves based on either striatal glutamate or visual perceptual abnormalities alone. The preliminary model building and evaluation presented here in a small CHR sample suggests that the approach of incorporating predictive imaging measures into risk classification is not only feasible but offers the potential of enhancing risk assessment.
Keywords: Risk calculator, Glutamate, Clinical High-Risk, Conversion to psychosis
1. Introduction
Substantial efforts have been made to develop predictors of which individuals at Clinical High-Risk for psychosis (CHR) will progress to syndromal psychosis. Initial studies focused on psychopathology assessed by symptom rating instruments, while more recent studies have employed multivariate prediction models or risk calculators (Cannon et al., 2016; Ciarleglio et al., 2019; Fusar-Poli et al., 2017). Despite their improvement in predictive validity, these models remain limited by their lack of specificity and principal reliance on clinical or other primarily phenomenological data. Further, clinicians cannot readily use current risk calculators (Cannon et al., 2016; Ciarleglio et al., 2019) because of the substantial training and expertise required to properly administer and score the Structured Interview for Psychosis-Risk Syndromes (SIPS; Miller et al., 2003), the clinical instrument on which they are primarily based.
A recent review demonstrated that combining or sequentially using clinical with neurobiological and physiological variables improves risk prediction in CHR individuals (Schmidt et al., 2017). Therefore, one way of addressing these limitations would be by combining biological measures, such as brain imaging, with clinically-based material in multivariate prediction models or risk calculators. Recent research in CHR individuals has focused on dysregulation of the major excitatory neurotransmitter system, glutamate. Using proton magnetic resonance spectroscopy (1H MRS), a number of studies in CHR subjects have documented these alterations in vivo. Although some cross-sectional studies in CHR subjects have documented increased (de la Fuente-Sandoval et al., 2011; de la Fuente-Sandoval et al., 2015; Grent-’t-Jong et al., 2018; Liemburg et al., 2016), decreased (Shakory et al., 2018; Stone et al., 2009) or unchanged glutamatergic compounds (Demro et al., 2017; Egerton et al., 2014; Liemburg et al., 2016; Modinos et al., 2018; Natsubori et al., 2014; Nenadic et al., 2015) in comparison to healthy subjects, only 3 prospective studies have been published to date. Wood et al. did not find differences in hippocampal Glutamate + Glutamine (Glx) levels between subjects who transitioned to syndromal psychosis and the ones who did not (Wood et al., 2010). In contrast, the other 2 studies showed an association between increased glutamate levels and subsequent conversion to syndromal psychosis: Bossong and colleagues showed that CHR subjects who later developed syndromal psychosis had higher baseline hippocampal glutamate levels compared with CHR individuals who did not transition to syndromal psychosis (Bossong et al., 2019). The other prospective study similarly demonstrated that elevated baseline striatal glutamate in CHR individuals was associated with subsequent conversion to syndromal psychosis (de la Fuente-Sandoval et al., 2013a). The aim of the present, exploratory study was to combine striatal glutamate data that was reported earlier (de la Fuente-Sandoval et al., 2013a) with SIPS-measured symptoms of this same cohort identified by the Columbia Risk Calculator (Ciarleglio et al., 2019) as exhibiting the greatest predictive value.
2. Materials and Methods
2.1. Participants
All procedures involving human subjects/patients who took part in the present study were approved by the Ethics and Scientific Committees of the National Institute of Neurology and Neurosurgery of Mexico (INNN). All subjects over 18 years old provided written informed consent; minors provided assent with written consent by a parent or legal guardian. Participants were CHR individuals recruited from the emergency department, first-episode psychosis clinic, and the Adolescent Program of Neuropsychiatric and Imaging Study (PIENSA), at the INNN in Mexico City. All included individuals (n=19) met criteria for CHR as defined in the SIPS (one met criteria for Brief Intermittent Psychosis Syndrome and 18 for Attenuated Positive Symptom Syndrome) and were screened for drugs of abuse (benzodiazepines, cannabis, cocaine, heroin and opioids) at screening and 1 hour prior to the neuroimaging study. All subjects were antipsychotic naïve, scanned at presentation, and followed clinically for at least 2 years to determine whether they developed syndromal psychosis. The Structured Clinical Interview for DSM-IV (First, 1996) was used to ascertain the transition to syndromal psychosis. The 1H MRS data presented here were published previously (de la Fuente-Sandoval et al., 2013a).
2.2. Magnetic Resonance Neuroimaging Procedures
All neuroimaging studies were conducted at the INNN on a 3T scanner (Signa Excite HDxt, GE Healthcare, USA) with a high-resolution eight-channel head coil. Participants were initially imaged with a T1-weighted spoiled gradient-echo 3-dimensional axial acquisition (SPGR) oriented above and parallel to the anterior commissure- posterior commissure line (TE = 5.7 msec; TR = 13.4 msec; inversion time = 450 msec; flip angle = 20° ; FOV = 25.6 cm; Matrix =256 x 256; slice thickness = 1 mm). These T1-weighted SPGR images were reformatted to sagittal and coronal views for optimal 1H MRS voxel placement. The lower end of the right dorsal caudate voxel was located 3 mm dorsal to the anterior commissure, including the maximum amount of grey matter, with a dorsal extension (thickness) of 2 cm (8 ml − 2 x 2 x 2 cm). The spectra were shimmed to achieve full width at half maximum 12 Hz. 1H MRS spectra were obtained using point-resolved spectroscopy (TE = 35 msec; TR = 2000 msec; spectral width = 5000 Hz; 4096 data points; and 128 water-suppressed averages and 16 water-unsuppressed averages) and analyzed using LCModel software, version 6.1-2T (Provencher, 1993). SPGR scans were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using Statistical Parametric Mapping 8 (Friston et al., 1995) (SPM8, Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm), and voxel location was used to compute the CSF content within each voxel, allowing correction for CSF fraction using the formula
2.3. Clinical Data
Our approach was to evaluate the additional predictive power of Columbia Risk Calculator variables when combined with striatal glutamate. The Columbia Risk Calculator utilized all of the symptoms on the SIPS, including information obtained by the SIPS but not traditionally scored, such as violent ideation and behavior, suicidal ideation and behavior, and subtypes of perceptual experiences (Brucato et al., 2018; Brucato et al., 2019; Ciarleglio et al., 2019). It also utilized scores from the Global Functioning Scale: Social (GF: Social) and Global Functioning Scale: Role (GF: Role) (Cornblatt et al., 2007). While in both the Columbia and NAPLS risk calculators P1 was an important contributor to risk for transition to syndromal psychosis, P4v (Lehembre-Shiah et al., 2017) was evaluated first since it was the most frequently selected variable (based on a lasso-penalized fitting procedure) with the largest standardized effect size in the Columbia Risk Calculator. The narratives of the P4 (perceptual abnormalities) item derived from the SIPS interviews were assessed and rated by two trained clinicians (G Brucato and RR Girgis), blinded to conversion status of the subjects, in order to obtain its visual (P4v) and auditory (P4a) components. Within each group (converters/non-converters to syndromal psychosis), descriptive statistics were computed for a limited set of clinical and demographic variables.
2.4. Statistical Analysis
We compared converters and non-converters on striatal glutamate and P4v using two-sample t-tests (after assessing the normality assumption via quantile plots) and reporting Cohen’s d effect sizes. For each variable, we also computed the area under the receiver operating characteristic (ROC) curve (AUC) and its corresponding 95% bootstrap confidence interval (based on 2000 stratified bootstrap samples). Next, we fit a logistic regression model with conversion status as the outcome and striatal glutamate and P4v as the predictors. Lastly, we evaluated dysphoric mood (SIPS G2) as an additional predictor in our model since it had the second largest standardized effect size in the Columbia Risk Calculator. Both apparent and optimism-adjusted AUC (AUCoa) values were generated for these models. Statistical significance was set at p<0.05.
3. Results
3.1. Demographic and Clinical Information
Baseline imaging was obtained on a total of 19 right-handed CHR individuals, 7 (36.84%) of whom converted to syndromal psychosis. The demographic characteristics of the groups were similar (Gender: non-converters 9 (75%) male; converters 5 (71.43%) male; Age mean (SD): non-converters 20.33 (3.17), converters 18.57 (3.78)).
3.2. Imaging Risk Calculator
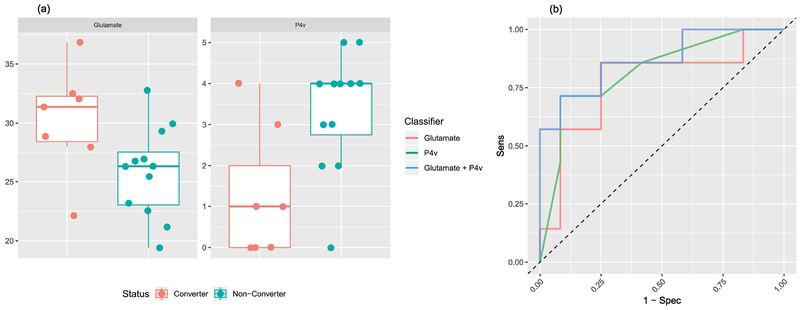
Figure 1 (a) shows boxplots of striatal glutamate and P4v values in the two groups and Figure 1 (b) shows the ROC curves for discrimination based on striatal glutamate alone, P4v alone, or the logistic model with both as predictors. Mean striatal glutamate values were significantly different (p=0.038) between converters (mean = 30.25, SD = 4.59) and non-converters (mean = 25.86, SD = 3.84) with a large effect size (d = 1.07). The ROC curve for striatal glutamate alone has an AUC=0.774 (95% CI = [0.500, 0.976]) and AUCoa=0.764. Mean P4v values were significantly different (p=0.010) between converters (mean = 1.29, SD = 1.60) and non-converters (mean = 3.33, SD = 1.44) with a large effect size (d =−1.37). The ROC curve for P4v alone has an AUC=0.821 (95% CI = [0.589, 1.000]) and AUCoa=0.821. The ROC curve for the logistic model with these two predictors has an AUC=0.869 (95% CI = [0.667, 1.000]) and AUCoa=0.823. The optimal cutoff for the model has a sensitivity of 0.714, specificity of 0.917, positive predictive value of 0.833, and negative predictive value of 0.846. Incorporation of dysphoric mood (SIPS G2, the independent variable with the next-largest standardized effect size in the Columbia Risk Calculator) into the model as an additional predictor added no further predictive ability (AUCoa=0.798). We did not include additional variables since, given the limited sample size, the inclusion of more variables would be detrimental to the model.
Figure 1.
(a) Box plots of striatal glutamate (left) and P4v (right) values by conversion status. (b) ROC curves for striatal glutamate alone, P4v alone, and for the logistic model with striatal glutamate and P4v.
4. Discussion
These findings suggest that adding just one clinical variable (i.e., P4v) to a biological marker (i.e., striatal glutamate), each with substantial predictive properties, modestly improves the performance of either predictor alone and may yield a potentially useful imaging-based risk calculator for the prediction of conversion to syndromal psychosis. The estimated two-predictor model performed well, with an AUCoa of 0.823 and high sensitivity, specificity, positive and negative predictive values. These are substantially greater than the Columbia Risk Calculator that was previously published, on which the current analysis was based (AUCoa = 0.73), and that only incorporated clinical symptoms and phenomenology in a large, independent sample (Ciarleglio et al., 2019). These findings also replicate, in an independent sample, that P4v is a robust negative predictor of conversion to syndromal psychosis, both alone and as part of a risk calculator (Ciarleglio et al., 2019; Lehembre-Shiah et al., 2017). It is possible that the inclusion of information such as P4v, obtained when completing the SIPS but not traditionally scored, conferred the differential predictive components of the NAPLS and Columbia Risk Calculators (Ciarleglio et al., 2019). Importantly, P4v scores are associated with lower risk of conversion to syndromal psychosis (Lehembre-Shiah et al., 2017). That is, presence of a high P4v predicts low likelihood of transition to syndromal psychosis. This is consistent with the fact that visual hallucinations are relatively rare in schizophrenia (Waters et al., 2014) but one of the most common symptoms in the CHR population, occurring in more than 75% of individuals in this sample and others (Marshall et al., 2014). Therefore, it is both the absence of visual perceptual abnormalities that predicts conversion, and their presence that predicts that one will not convert (Ciarleglio et al., 2019; Lehembre-Shiah et al., 2017).
However, it is also possible that our model suffers from overfitting. Although a sample of 19 unmedicated CHR individuals is acceptable for a brain imaging study, this sample size is limited for a study of prediction modeling since the number of events (i.e., conversions) per variable is relatively low. Replication in larger datasets is required to help determine whether the lack of added predictive power from addition of a second clinical variable was attributable mainly to the small sample size, and whether instead synergistic gain in risk prediction might instead be seen in larger samples from adding an imaging measure (or multimodal imaging data) to one or more clinical variables. Although 1H MRS is a non-invasive imaging technique that represents a potential tool for prediction of psychosis (Bustillo et al., 2019), it requires capabilities and resources, limiting its widespread use in standard treatment settings.
Despite these limitations, the results carry several implications. First, although combining striatal glutamate with P4v only modestly improved their predictive value, incorporating a biological measure into the risk calculator substantially decreases the requirement for clinical expertise in CHR and therefore substantially augments symptom-based criteria. In addition, it suggests a utility for risk calculators beyond only predicting conversion to syndromal psychosis – namely, the potential to use imaging-based risk calculators to provide information to patients and clinicians on cost-benefit ratios for specific somatic-based treatments, such as antipsychotic medications, given the evidence that they have been shown to decrease striatal glutamatergic compounds (de la Fuente-Sandoval et al., 2013b; de la Fuente-Sandoval et al., 2018), but also have greater side effects than psychosocial interventions. Finally, these results, taken together with other recent findings on glutamate in CHR individuals (Bossong et al., 2019), support further study of the predictive value of brain imaging biomarkers in psychiatry (First et al., 2018).
Acknowledgements
This project was supported by Consejo Nacional de Ciencia y Tecnología, Mexico, (CONACyT) Grant Nos. 182279 and 261895 to Camilo de la Fuente-Sandoval, CONACyT’s Sistema Nacional de Investigadores to Pablo León-Ortiz and Camilo de la Fuente-Sandoval, and National Institutes of Health Grant No. R01 MH110270 to Lawrence S. Kegeles and Ragy R. Girgis.
Role of the funding source
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
R. Girgis acknowledges receiving research support from Otsuka, Allergan/Forest, BioAvantex, and Genentech. C. de la Fuente-Sandoval has served as a consultant for Janssen (Johnson & Johnson). F. Reyes-Madrigal has served as a speaker for AstraZeneca. J. Lieberman has received support administered through his institution in the form of funding or medication supplies for investigator-initiated research from Lilly, Denovo, Biomarin, Novartis, Taisho, Teva, Alkermes, and Boehringer Ingelheim, and is a member of the advisory board of Intracellular Therapies and Pierre Fabre. He neither accepts nor receives any personal financial remuneration for consulting, advisory board or research activities. He holds a patent from Repligen and receives royalty payments from “SHRINKS: The Untold Story of Psychiatry.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bossong MG, Antoniades M, Azis M, Samson C, Quinn B, Bonoldi I, Modinos G, Perez J, Howes OD, Stone JM, Allen P, McGuire P, 2019. Association of Hippocampal Glutamate Levels With Adverse Outcomes in Individuals at Clinical High Risk for Psychosis. JAMA Psychiatry 76 (2), 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucato G, Appelbaum PS, Lieberman JA, Wall MM, Feng T, Masucci MD, Altschuler R, Girgis RR, 2018. A Longitudinal Study of Violent Behavior in a Psychosis-Risk Cohort. Neuropsychopharmacology 43 (2), 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucato G, Appelbaum PS, Masucci MD, Rolin S, Wall MM, Levin M, Altschuler R, First MB, Lieberman JA, Girgis RR, 2019. Prevalence and phenomenology of violent ideation and behavior among 200 young people at clinical high-risk for psychosis: an emerging model of violence and psychotic illness. Neuropsychopharmacology 44 (5), 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo JR, Gaudiot CE, Lenroot RK, 2019. The Meaning of Glutamate and the Quest for Biomarkers in the Transition to Psychosis. JAMA Psychiatry 76 (2), 115–116. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Jeffries CD, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Kattan MW, 2016. An Individualized Risk Calculator for Research in Prodromal Psychosis. Am J Psychiatry 173 (10), 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio AJ, Brucato G, Masucci MD, Altschuler R, Colibazzi T, Corcoran CM, Crump FM, Horga G, Lehembre-Shiah E, Leong W, Schobel SA, Wall MM, Yang LH, Lieberman JA, Girgis RR, 2019. A predictive model for conversion to psychosis in clinical high-risk patients. Psychol Med 49 (7), 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD, 2007. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin 33 (3), 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff-Guerrero A, 2013a. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int J Neuropsychopharmacol 16 (2), 471–475. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, Alvarado-Alanis P, Ramirez-Bermudez J, Graff-Guerrero A, 2013b. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70 (10), 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, Graff-Guerrero A, 2011. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 36 (9), 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, Solis-Vivanco R, Graff-Guerrero A, Shungu DC, 2018. Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry 83 (6), 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Solis-Vivanco R, Favila R, Graff-Guerrero A, Shungu DC, 2015. Cortico-Striatal GABAergic and Glutamatergic Dysregulations in Subjects at Ultra-High Risk for Psychosis Investigated with Proton Magnetic Resonance Spectroscopy. Int J Neuropsychopharmacol 19 (3), pyv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demro C, Rowland L, Wijtenburg SA, Waltz J, Gold J, Kline E, Thompson E, Reeves G, Hong LE, Schiffman J, 2017. Glutamatergic metabolites among adolescents at risk for psychosis. Psychiatry Res 257, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Stone JM, Chaddock CA, Barker GJ, Bonoldi I, Howard RM, Merritt K, Allen P, Howes OD, Murray RM, McLean MA, Lythgoe DJ, O’Gorman RL, McGuire PK, 2014. Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology 39 (12), 2891–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Drevets WC, Carter C, Dickstein DP, Kasoff L, Kim KL, McConathy J, Rauch S, Saad ZS, Savitz J, Seymour KE, Sheline YI, Zubieta JK, 2018. Clinical Applications of Neuroimaging in Psychiatric Disorders. Am J Psychiatry 175 (9), 915–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, and Williams JBW, 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). American Psychiatric Press, Inc., Washington D.C. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ, 1995. Spatial registration and normalization of images. Human Brain Mapping 3 (3), 165–189. [Google Scholar]
- Fusar-Poli P, Rutigliano G, Stahl D, Davies C, Bonoldi I, Reilly T, McGuire P, 2017. Development and Validation of a Clinically Based Risk Calculator for the Transdiagnostic Prediction of Psychosis. JAMA Psychiatry 74 (5), 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Gross J, Goense J, Wibral M, Gajwani R, Gumley AI, Lawrie SM, Schwannauer M, Schultze-Lutter F, Navarro Schroder T, Koethe D, Leweke FM, Singer W, Uhlhaas PJ, 2018. Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehembre-Shiah E, Leong W, Brucato G, Abi-Dargham A, Lieberman JA, Horga G, Girgis RR, 2017. Distinct Relationships Between Visual and Auditory Perceptual Abnormalities and Conversion to Psychosis in a Clinical High-Risk Population. JAMA Psychiatry 74 (1), 104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg E, Sibeijn-Kuiper A, Bais L, Pijnenborg G, Knegtering H, van der Velde J, Opmeer E, de Vos A, Dlabac-De Lange J, Wunderink L, Aleman A, 2016. Prefrontal NAA and Glx Levels in Different Stages of Psychotic Disorders: a 3T 1H-MRS Study. Sci Rep 6, 21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C, Denny E, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Woods SW, Walker E, Addington J, 2014. The content of attenuated psychotic symptoms in those at clinical high risk for psychosis. Psychiatry Res 219 (3), 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 29 (4), 703–715. [DOI] [PubMed] [Google Scholar]
- Modinos G, Simsek F, Horder J, Bossong M, Bonoldi I, Azis M, Perez J, Broome M, Lythgoe DJ, Stone JM, Howes OD, Murphy DG, Grace AA, Allen P, McGuire P, 2018. Cortical GABA in Subjects at Ultra-High Risk of Psychosis: Relationship to Negative Prodromal Symptoms. Int J Neuropsychopharmacol 21 (2), 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsubori T, Inoue H, Abe O, Takano Y, Iwashiro N, Aoki Y, Koike S, Yahata N, Katsura M, Gonoi W, Sasaki H, Takao H, Kasai K, Yamasue H, 2014. Reduced frontal glutamate + glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull 40 (5), 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic I, Maitra R, Basu S, Dietzek M, Schonfeld N, Lorenz C, Gussew A, Amminger GP, McGorry P, Reichenbach JR, Sauer H, Gaser C, Smesny S, 2015. Associations of hippocampal metabolism and regional brain grey matter in neuroleptic-naive ultra-high-risk subjects and first-episode schizophrenia. Eur Neuropsychopharmacol 25 (10), 1661–1668. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30 (6), 672–679. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Cappucciati M, Radua J, Rutigliano G, Rocchetti M, Dell’Osso L, Politi P, Borgwardt S, Reilly T, Valmaggia L, McGuire P, Fusar-Poli P, 2017. Improving Prognostic Accuracy in Subjects at Clinical High Risk for Psychosis: Systematic Review of Predictive Models and Meta-analytical Sequential Testing Simulation. Schizophr Bull 43 (2), 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakory S, Watts JJ, Hafizi S, Da Silva T, Khan S, Kiang M, Bagby RM, Chavez S, Mizrahi R, 2018. Hippocampal glutamate metabolites and glial activation in clinical high risk and first episode psychosis. Neuropsychopharmacology 43 (11), 2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, O’Gorman RL, Barker GJ, McGuire PK, Oasis, 2009. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry 66 (6), 533–539. [DOI] [PubMed] [Google Scholar]
- Waters F, Collerton D, Ffytche DH, Jardri R, Pins D, Dudley R, Blom JD, Mosimann UP, Eperjesi F, Ford S, Laroi F, 2014. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull 40 Suppl 4, S233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Kennedy D, Phillips LJ, Seal ML, Yucel M, Nelson B, Yung AR, Jackson G, McGorry PD, Velakoulis D, Pantelis C, 2010. Hippocampal pathology in individuals at ultra-high risk for psychosis: a multi-modal magnetic resonance study. Neuroimage 52 (1), 62–68. [DOI] [PubMed] [Google Scholar]