Abstract
Objectives
Chlamydia trachomatis (CT) and Mycoplasma genitalium (MG) cause the majority of non-gonococcal urethritis (NGU). The role of Ureaplasma urealyticum (UU) in NGU is unclear. Prior case–control studies that examined the association of UU and NGU may have been confounded by mixed infections and less stringent criteria for controls. the objective of this case–control study was to determine the prevalence and aetiology of mixed infections in men and assess if UU monoinfection is associated with NGU.
Methods
We identified 155 men with NGU and 103 controls. Behavioural and clinical information was obtained and men were tested for Neisseria gonorrhoeae and CT, MG, UU and Trichomonas vaginalis (TV). Men who were five-pathogen negative were classified as idiopathic urethritis (IU).
Results
Twelve per cent of NGU cases in which a pathogen was identified had mixed infections, mostly UU coinfections with MG or CT; 27% had IU. In monoinfected NGU cases, 34% had CT, 17% had MG, 11% had UU and 2% had TV. In controls, pathogens were rarely identified, except for UU, which was present in 20%. comparing cases and controls, NGU was associated with CT and MG monoinfections and mixed infections. UU monoinfection was not associated with NGU and was almost twice as prevalent in controls. Men in both the case and control groups who were younger and who reported no prior NGU diagnosis were more likely to have UU (Or 0.97 per year of age, 95% CI 0.94 to 0.998 and OR 6.3, 95% CI 1.4 to 28.5, respectively).
Conclusions
Mixed infections are common in men with NGU and most of these are UU coinfections with other pathogens that are well-established causes of NGU. UU monoinfections are not associated with NGU and are common in younger men and men who have never previously had NGU. almost half of NGU cases are idiopathic.
BACKGROUND
Urethritis is the most common genitourinary syndrome in sexually active men less than 50 years of age,1 with an estimated prevalence of 2.8 million cases in the USA annually.2 Urethritis is classified, based on Gram stain smear (GSS) testing of urethral secretions, into gonococcal urethritis (caused by Neisseria gonorrhoeae (NG)) and non-gonococcal urethritis (NGU).3 NGU is more common than gonococcal urethritis in men and has been associated with multiple pathogens, including Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), Trichomonas vaginalis (TV) and Ureaplasma urealyticum (UU).2 Rare causes of NGU may include viruses, like herpes simplex virus and adenovirus, and oropharyngeal organisms.1 Prior studies have reported that 29%–48% of NGU diagnoses have no identifiable aetiology,4–6 a syndrome termed idiopathic urethritis (IU).
CT and MG are strongly associated with NGU,1 6 but the association of Ureaplasma sp with NGU remains controversial.7–9 Conflicting findings from early studies may be explained, in part, by the discovery that at least two different Ureaplasma sp, UU and U. parvum, commonly colonise the male urethra.10 11 A recent meta-analysis found that UU, but not undifferentiated Ureaplasma infections or U. parvum, was associated with NGU.12 Case–control studies of the association of UU with NGU have produced disparate results; about half found that UU was associated with NGU.1 13–17 Prior case–control studies may have had several limitations. First, some studies tested for a limited number of pathogens and their results may have been confounded by coinfection with UU by another untested infection. To evaluate this will require data on the prevalence or composition of mixed infections in NGU. Second, the control group definitions differed in prior studies. Some studies defined controls as asymptomatic men only (ie, no urethral Gram stains were performed), whereas others defined controls as men with <5 polymorphonuclear leucocytes per high-power field (PMN/HPF) by urethral swab Gram stain. Using a cut-off of <5 PMN/HPF risks categorising men with low-level inflammation (ie, 2–4 PMN/HPF) as controls. The US Centers for Disease Control (CDC) 2015 STD Treatment Guidelines categorise men with 2–4 PMN/HPF as having NGU, regardless of symptoms,18 as >16% of symptomatic men with confirmed CT had <5 PMN/HPF on Gram stain.19 To minimise the risk of misclassifying men with low-level inflammation from NGU-associated pathogens as controls, case–control studies are needed that use the stricter CDC-recommended ≤1 PMN/HPF Gram stain cut-off.
We conducted a case–control study that tested men with and without NGU for multiple NGU-associated pathogens. We defined controls as asymptomatic men with ≤1 PMN/HPF to align with the contemporary NGU definition. Our primary objective was to compare the prevalence and composition of mixed and monopathogen infections in well-defined groups of men with NGU and asymptomatic controls. Our secondary objectives were to assess if UU monoinfections were associated with NGU and identify factors associated with UU infection.
METHODS
Study population and procedures
The study population was men >18 years of age with and without urethritis symptoms who presented to the Marion County Public Health Department Bell Flower STD Clinic in Indianapolis, Indiana, and enrolled in the Idiopathic Urethritis Men’s Project (IUMP) study.20 IUMP is a prospective study that aims to identify pathogens associated with IU in men with NGU. A urethral swab was obtained and GSS testing was performed on urethral secretions. Men with Gram-negative intracellular diplococci present on GSS testing were excluded. Men were diagnosed with NGU if they had ≥5 PMN/HPF with symptoms of discharge or dysuria and/or urethral discharge on physical exam. Men were asked if they had urethritis symptoms, a physical exam was performed to assess for urethral discharge and first-catch urine was collected for pathogen testing. Demographic and sexual behavioural data were also collected using a computer-assisted survey. Asymptomatic men without urethral discharge on physical examination and ≤1 PMN/HPF were identified as controls. All study subjects provided written consent prior to study enrolment.
Five-pathogen testing
First-catch urine from the enrolment visit was tested for NG, CT, MG, TV and UU by the Indiana University School of Medicine Infectious Diseases Research Laboratory. CT and NG nucleic acid amplification testing (NAAT) was performed as previously reported.21 TV, MG and UU NAAT testing was performed using an in-house quantitative PCR assay as previously described.22–24 Men negative for all five pathogens were considered to have IU.
Statistical analysis
Demographic characteristics were collected at baseline. Descriptive statistics including medians, ranges, frequencies and percentages were computed to assess variables’ distributional properties and to describe the sample. The baseline demographics were then compared between case and control groups with the Kruskal-Wallis test for continuous variables and the χ2 test or Fisher’s exact test (where the distribution warranted) for categorical variables. Using monoinfection status for those with only a single infection as compared with those with multiple infections, case versus control were compared for each monoinfection and again for multiple infections using the χ2 test or Fisher’s exact test. Multivariable logistic regression was used to assess the association of various demographic factors for those with UU monoinfection controlling for case versus control. Additional multivariable logistic regression models were fit using NGU (case or control) as the dependent variable to examine the relationship of UU with NGU controlling for demographic factors. All analyses were performed using SAS V.9.3 statistical software.
RESULTS
Study population
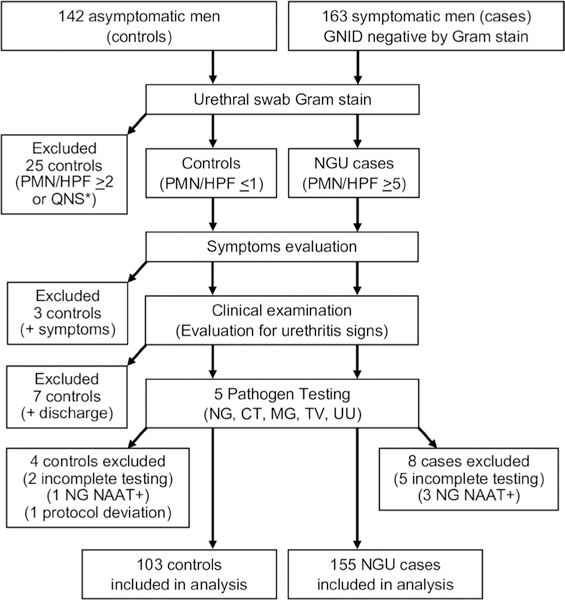
We evaluated 305 men (142 controls and 163 cases) enrolled in the IUMP study between 4 August 2016 and 24 May 2018, of which 103 controls and 155 NGU cases are included in this analysis (figure 1). Thirty-nine controls were excluded: 25 had evidence of urethral inflammation (≥2 PMN/HPF) or unsatisfactory Gram stains; 3 had urethritis symptoms; 7 had a discharge on physical examination; 1 tested positive for NG; 2 had incomplete five-pathogen testing; and 1 had a protocol deviation. In the NGU group, we excluded five men with incomplete five-pathogen testing and three who tested positive for NG.
Figure 1.
Study population inclusion and exclusion flow chart. GNID, gram negative intracellular diplococci; QNS, quantity not sufficient; CT, Chlamydia trachomatis; MG, Mycoplasma genitalium; NAAT, nucleic acid amplification testing; NG, Neisseria gonorrhoeae; NGU, non-gonococcal urethritis; PMN/HPF, polymorphonuclear leucocytes per high-power field; TV, Trichomonas vaginalis; UU, Ureaplasma urealyticum.
Study participant characteristics are shown in table 1. In men with NGU, the median age was 28 (range 18–64), 65% were African-American, 90% were non-Hispanic and 87% identified as heterosexual. Reported lifetime sexual behaviours were receptive oral sex in 97%, vaginal sex in 95% and insertive anal sex in 59%. The majority of cases (87%) presented to clinic for evaluation of urethritis symptoms. NGU had been previously diagnosed in 46%. On physical examination, 90% of cases were circumcised, a discharge was present in 98% and meatal erythema was observed in 8%.
Table 1.
Study participant characteristics
| Characteristic | NGU cases (n=155) |
Controls (n=103) |
P value | |
|---|---|---|---|---|
| Age, median (range) | 28 (18–64) | 29 (19–69) | 0.66 | |
| Race, n (%) | <0.0001 | |||
| African-American | 101 (65%) | 50 (49%) | ||
| Caucasian | 29 (19%) | 42 (41%) | ||
| Other | 25 (16%) | 10 (10%) | ||
| Ethnicity* | 0.96 | |||
| Hispanic | 14 (10%) | 10 (10%) | ||
| Non-Hispanic | 129 (90%) | 90 (90%) | ||
| Sexual orientation | 0.0003 | |||
| Homosexual | 9 (6%) | 22 (21%) | ||
| Heterosexual | 135 (87%) | 69 (67%) | ||
| Bisexual/other | 11 (7%) | 12 (12%) | ||
| Reason for visit | - | |||
| Having symptoms | 134 (86%) | 0 | ||
| Worried have STI | 16 (10%) | 21 (20%) | ||
| Contact to STI | 2 (1%) | 7 (7%) | ||
| Routine check-up | 3 (2%) | 73 (71%) | ||
| Other | 0 | 2 (2%) | ||
| Reported sexual history/behaviours | ||||
| Lifetime partners, median (IQR) | 13.5 (1–300) | 13 (1–400) | 0.73 | |
| Received oral sex† | 149 (97%) | 100 (99%) | 0.41 | |
| Vaginal sex‡ | 146 (95%) | 87 (85%) | 0.0092 | |
| Insertive anal sex§ | 89 (59%) | 75 (74%) | 0.015 | |
| Prior STI diagnosis | ||||
| Gonorrhoea¶ | 58 (40%) | 24 (24%) | 0.0013 | |
| Chlamydia** | 88 (59%) | 32 (33%) | 0.0001 | |
| Trichomoniasis†† | 16 (11%) | 5 (5%) | 0.097 | |
| NGU‡‡ | 67 (46%) | 10 (10%) | <0.0001 | |
| Physical exam | - | |||
| Circumcised§§ | 139 (90%) | 86 (90%) | ||
| Discharge | 152 (98%) | 0 | ||
| Meatal erythema | 12 (8%) | 0 | ||
Missing 12 in cases and 3 in controls.
Missing 1 in cases and 2 in controls.
Missing 1 in cases and 1 in controls.
Missing 5 in cases and 2 in controls.
Missing 8 in cases and 4 in controls.
Missing 5 in cases and 5 in controls.
Missing 13 in cases and 5 in controls.
Missing 8 in cases and 7 in controls.
Missing 1 in cases and 7 in controls.
NGU, non-gonococcal urethritis.
Compared with men with NGU, the control men were of a similar age and ethnicity, but were significantly less likely to identify as African-American (65% vs 49%, p<0.0001) and heterosexual (87% vs 67%, p=0.0003). Also, the controls were more likely to report insertive anal sex (59% vs 74%, p=0.015) and less likely to report vaginal sex (95% vs 85%, p=0.0092) than the NGU cases. The majority of the control men presented to the clinic for a routine check-up and significantly fewer had been previously diagnosed with gonorrhoea (24% vs 40%, p=0.0013), chlamydia (33% vs 59%, p=0.0001) and NGU (10% vs 46%, p<0.0001), compared with men with NGU. None of the control men had discharge or meatal erythema on physical examination (exclusion criteria).
Prevalence and composition of monoinfections and mixed infections in men with NGU and controls
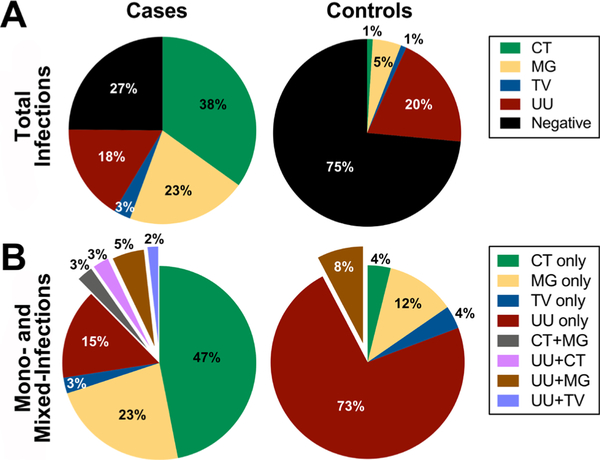
Of the 155 men with NGU, a pathogen was identified in 73%; IU was diagnosed in 27% (table 2, figure 2). CT was the most common pathogen, and was found in 38% (n=59) of the cases; 53/59 (89.8%) were monoinfections and 6/59 (10.2%) were mixed infections. MG was the second most common pathogen, occurring in 23% (n=35) of the cases; 26/35 (74.3%) were monoinfections and 9/35 (25.7%) were mixed infections. UU was present in 18% (n=28) of the cases; 17/28 (60.7%) were monoinfections and 11/28 (39.3%) were mixed infections. TV was uncommon and only identified in five NGU cases (three monoinfections, two mixed infections).
Table 2.
Composition and association of total monoinfections and mixed infections with NGU
| Pathogens | NGU cases (n=155) | Controls (n=103) | OR (95% CI) | P value |
|---|---|---|---|---|
| Negative/IU | 27% (42/155) | 75% (77/103) | – | |
| Total (mono+mixed) | 73% (113/155) | 25% (26/103) | ||
| NG | Excluded | Excluded | – | |
| CT | 38% (59/155) | 1% (1/103) | 63 (8.52 to 461.38) | <0.0001 |
| MG | 23% (35/155) | 5% (5/103) | 5.72 (2.16 to 15.15) | 0.0001 |
| TV | 3% (5/155) | 1% (1/103) | 3.4 (0.39 to 29.53) | 0.41 |
| UU | 18% (28/155) | 20% (21/103) | 0.86 (0.46 to 1.62) | 0.64 |
| Monoinfections only | 88% (99/113) | 92% (24/26) | ||
| CT only | 34% (53/155) | 1% (1/103) | 53 (7.19 to 390.57) | <0.0001 |
| MG only | 17% (26/155) | 3% (3/103) | 6.7 (1.98 to 22.83) | 0.0006 |
| TV only | 2% (3/155) | 1% (1/103) | 2.0 (0.21 to 19.62) | >0.9999 |
| UU only | 11% (17/155) | 18% (19/103) | 0.54 (0.27 to 1.11) | 0.090 |
| Mixed infections only | 12% (14/113) | 8% (2/26) | 5.0 (1.11 to 22.55) | 0.021 |
| CT+MG | 2% (3/155) | 0 | ||
| UU+CT | 2% (3/155) | 0 | ||
| UU+MG | 4% (6/155) | 2% (2/103) | ||
| UU+TV | 1% (2/155) | 0 | ||
CT, Chlamydia trachomatis; IU, idiopathic urethritis; MG, Mycoplasma genitalium; NG, Neisseria gonorrhoeae; NGU, non-gonococcal urethritis; TV, Trichomonas vaginalis; UU, Ureaplasma urealyticum.
Figure 2.
Five-pathogen testing results from men with non-gonococcal urethritis (NGU) and controls. (A) Distribution of four NGU-associated pathogens and idiopathic urethritis in men with NGU (n=155), compared with controls (n=103). Neisseria gonorrhoeae (NG)-positive specimens were excluded. Five-pathogen negative men with NGU were classified as idiopathic urethritis. (B) Contribution of monoinfections and mixed infections in men with NGU with an identified pathogen (n=113), compared with controls (n=26). Mixed infections are denoted by the exploded slices. CT, Chlamydia trachomatis; MG, Mycoplasma genitalium; TV, Trichomonas vaginalis; UU, Ureaplasma urealyticum.
In the 103 control men, most NGU-associated pathogens were uncommon. Detected infections included one CT, five MG (three monoinfections, two mixed infections) and one TV. In contrast, UU was detected in 20% (n=21); 19 (90.5%) were monoinfections and 2 (9.5%) were mixed infections.
Mixed infections were detected in both men with NGU and controls. Mixed infections occurred in 12% (n=14/113) of cases and in 8% (n=2/24) of the control men in whom at least one pathogen was detected. In mixed infections, UU was present in 79% (n=11/14) of cases and 100% (n=2) of controls, most frequently with MG (43% of cases (n=6/14), 100% (n=2) of controls).
Comparing cases and controls, CT and MG monoinfections and mixed infections were significantly associated with NGU (p<0.0001, p=0.0006 and p=0.021, respectively). In contrast, the total UU prevalence (monoinfections and mixed infections) was similar in the cases compared with the controls (18% vs 20%, p=0.64). UU monoinfections trended towards being associated with controls (11% vs 18%, p=0.090).
Risk factors for UU infections
Given that UU monoinfection was not associated with NGU but trended towards being associated with controls, we used logistic regression models to assess whether UU monoinfections were associated with specific factors, controlling for case versus control (online supplementary table 1). No association with UU monoinfection was seen when adjusting for race, education, sexual orientation, a self-reported history of NG, CT, MG, syphilis, or human papillomavirus, duration of sexual activity, or giving or receiving oral sex, vaginal sex, or anal sex within the past 60 days or 1 year. However, an increased risk of prevalent UU monoinfection was associated with younger age (OR 0.966 per year of age, 95% CI 0.935 to 0.998) and no history of self-reported NGU (OR 6.33, 95% CI 1.41 to 28.5). Additional multivariable logistic regression was performed to further explore the relationship between UU and the clinical group (cases vs controls). After controlling for multiple demographic factors which were individually associated with differences in the clinical groups, no association between UU and clinical group was found.
DISCUSSION
Our primary objective was to define the proportion and composition of monoinfections and mixed infections with four NGU-associated pathogens and IU in men with NGU and controls. We found that mixed infections were common, present in 12% (n=14/113) of cases with an identified pathogen. Importantly, of the 14 mixed infections in men with NGU, the majority (79%) were coinfected with UU; most frequently with MG as the copathogen (n=6). The two mixed infections in controls were also MG and UU coinfections.
By identifying monoinfections in cases and controls, we were able to assess for pathogen-specific associations with NGU. As expected, CT and MG monoinfections were strongly associated with NGU and, together, comprised approximately 50% of cases. TV infections were rare and the study design was likely statistically underpowered to detect associations with TV. UU monoinfections trended towards being associated with controls (11% vs 18%); not NGU. Further, total UU prevalence was similar in the cases and controls (18% vs 20%). Importantly, mixed infections, consisting primarily of UU coinfections, were associated with NGU. The observation that mixed infections, but not UU monoinfections, are associated with NGU is significant as it highlights that prior studies that observed associations between UU and NGU might be explained by the presence of undetected coinfecting pathogens.
Our observations that monoinfection UU was more prevalent in controls, and that men with UU were younger and more likely to have never had NGU, suggest that UU readily colonises the urethra, but does not necessarily elicit urethritis. Substantial evidence supports the idea that UU is sexually transmitted. One study found that the prevalence of urogenital UU in virginal girls was 33%, but increased to 75% in sexually active girls.25 Also, UU has been associated with a recent change in sex partner.26 27 Our study confirms prior observations that UU is more common in younger men.26 27 Together, the observations above suggest that UU readily colonises immune-susceptible hosts. A possible explanation for our observation that UU was more prevalent in men who reported that they had not previously been diagnosed with NGU is that NGU treatment may clear UU. Indeed, our preliminary findings from a 1-month test-of-cure visit in UU-infected men with NGU indicate a high cure rate with azithromycin (manuscript in preparation). Our UU prevalence of 18%–20% in cases and controls is similar to the pooled prevalence from seven other case–control studies of 14%–18%.12 This is remarkable given that our study used a strict PMN definition of ≤1 PMN/HPF in controls and the high UU prevalence indicates that UU colonisation elicits little to no urethral inflammation in men.
It is unclear why some, but not all, men with UU monoinfection develop urethritis and we propose several hypotheses. First, it is possible that UU monoinfection NGU actually represents UU coinfected with unknown pathogens associated with IU, the latter driving urethritis. Second, UU may be an opportunistic pathogen which overgrows when urethral microbiome perturbation occurs, supported by prior studies that found a correlation between urethritis symptoms and increased UU or U. parvum organism load.28 29 Third, urethritis may indicate the presence of virulent UU strains or specific host factors (eg, proinflammatory genetic determinants). Indeed, Ureaplasma quasispecies exist due to horizontal gene transfer.30 Also, UU-specific immunity may occur, which is suggested by the observation that UU has been associated with fewer lifetime sexual partners.16 To our knowledge, no study has used large-scale comparative genomics techniques to assess if virulent UU species exist in men with NGU due to monoinfection UU.
IU accounted for 27% of NGU cases in our study, which is somewhat lower compared with other contemporary studies.4–6 However, considering that UU is not associated with NGU, appears to readily colonise the urethra without eliciting inflammation, and frequently coexists with other urethral pathogens, the true contribution of IU may be as high as 43% (including the 18% UU monoinfections). Further studies are underway using next-generation sequencing to identify pathogens associated with IU in men with and without UU monoinfection.
Screening studies have demonstrated that the majority of CT infections in men are asymptomatic. However, only a single CT infection (1%) was identified in controls in our study. We hypothesise that the majority of CT-infected asymptomatic men had objective evidence of urethral inflammation on Gram stain (≥2 PMN/HPF) and were excluded by our stringent inclusion criteria. Indeed, a study of Gram stain PMN counts in men with symptomatic urethritis found that 15% of CT infections had a Gram stain PMN/HPF 2–5; only <7% had a PMN/HPF ≤1.19 Our data support this observation and suggest that, although men commonly may be asymptomatic, urogenital CT infection rarely causes little to no urethral inflammation (≤1 PMN/HPF) when assessed by Gram stain. It is likely that urethral inflammation influences the survival and transmission of coinfections, like UU and MG. Studies are underway to further understand how CT influences the male urethral microbiome and metabolome.
A major strength in our study was the use of five-pathogen testing to identify monoinfections and mixed infections in men with and without NGU. Another strength is that our study design incorporated a strict definition for negative control men (asymptomatic men without discharge and ≤1 PMN/HPF). Our study does have limitations. This was a single-centre study of men presenting to an STD clinic, which may make the results less generalisable to other populations and clinical locations. In addition, we used strict inclusion criteria to define NGU (≥5 PMN/HPF) and negative controls (≤1 PMN/HPF) and our results may not be generalisable to populations that were excluded from our analyses, such as NGU cases with 2–4 PMN/HPF, or asymptomatic men with urethritis. Also, our study did not quantify UU load in cases and controls, and we cannot exclude that men with cases had higher UU load.
In conclusion, mixed infections occurred in 12% of pathogen-positive NGU, were predominantly coinfected with UU and were associated with NGU. Approximately 50% of NGU is associated with CT and MG. UU monoinfections were more common in controls, in younger men and in men with no history of NGU. Together, these data suggest that UU is not associated with NGU, but frequently colonises the urethra with other NGU pathogens, especially MG.
Supplementary Material
Key messages.
► Mixed infections with Ureaplasma urealyticum and other pathogens are common in men with non-gonococcal urethritis (NGU).
► U. urealyticum monoinfections are not associated with NGU and are more common in young men and men who have never had NGU.
► Given that U. urealyticum is not associated with NGU, approximately half of NGU cases have no known cause.
Acknowledgements
The authors are grateful to Sara Hanson, Christina Davis, Janet Arno, Virginia Caine, the Bell Flower Clinic staff and the IUMP study participants.
Funding This work was funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health grant R01 AI116706 to DEN and the Indiana Clinical and Translational Sciences Institute (UL1TR002529) KL2 training award to SJJ from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Footnotes
Handling editor Henry John christiaan de Vries
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The study was approved by the Indiana University Institutional Review Board (Protocol no 1510663888) and Marion County Public Health Department Research Review Committee.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information.
► Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/sextrans-2019-054121).
REFERENCES
- 1.Bradshaw CS, Tabrizi SN, Read TRH, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis 2006;193:336–45. [DOI] [PubMed] [Google Scholar]
- 2.Martin DH. Nongonococcal urethritis: new views through the prism of modern molecular microbiology. Curr Infect Dis Rep 2008;10:128–32. [DOI] [PubMed] [Google Scholar]
- 3.Martin D Urethritis in Males In: Holmes KKSP, Stamm WE, eds. Sexually transmitted diseases. 4th edn New York: McGraw-Hill, 2008: 1107–26. [Google Scholar]
- 4.Manhart LE, Gillespie CW, Lowens MS, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis 2013;56:934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis 2011;52:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Totten PA, Schwartz MA, Sjöström KE, et al. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J Infect Dis 2001;183:269–76. [DOI] [PubMed] [Google Scholar]
- 7.Ford DK. Culture of human genital “T-strain” pleuropneumonia-like organisms. J Bacteriol 1962;84:1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YH, Tarr PI, Schumacher JR, et al. Reevaluation of the role of T-mycoplasmas in nongonococcal urethritis. J Am Vener Dis Assoc 1976;3:25–8. [PubMed] [Google Scholar]
- 9.O’leary WM. Ureaplasmas and human disease. Crit Rev Microbiol 1990;17:161–8. [DOI] [PubMed] [Google Scholar]
- 10.kong F, James G, Ma Z, et al. Phylogenetic analysis of Ureaplasma urealyticum-support for the establishment of a new species, Ureaplasma parvum. Int J Syst Bacteriol 1999;49:1879–89. [DOI] [PubMed] [Google Scholar]
- 11.Robertson JA, Stemke GW, Davis JW, et al. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol 2002;52:587–97. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Wang R, Li X, et al. Are Ureaplasma spp. a cause of nongonococcal urethritis? A systematic review and meta-analysis. PLoS One 2014;9:e113771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Povlsen K, Bjørnelius E, lidbrink P, et al. Relationship of Ureaplasma urealyticum biovar 2 to nongonococcal urethritis. Eur J Clin Microbiol Infect Dis 2002;21:97–101. [DOI] [PubMed] [Google Scholar]
- 14.Deguchi T, Yoshida T, Miyazawa T, et al. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex Transm Dis 2004;31:192–5. [DOI] [PubMed] [Google Scholar]
- 15.Ondondo RO, Whittington WLH, Astete SG, et al. Differential association of Ureaplasma species with non-gonococcal urethritis in heterosexual men. Sex Transm Infect 2010;86:271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wetmore CM, Manhart LE, lowens MS, et al. Ureaplasma urealyticum is associated with nongonococcal urethritis among men with fewer lifetime sexual partners: a case-control study. J Infect Dis 2011;204:1274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frølund M, Lidbrink P, Wikström A, et al. Urethritis-associated pathogens in urine from men with non-gonococcal urethritis: a case-control study. Acta Derm Venereol 2016;96:689–94. [DOI] [PubMed] [Google Scholar]
- 18.Workowski KA, Bolan GA, Papp JR. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 19.Rietmeijer CA, Mettenbrink CJ. Recalibrating the gram stain diagnosis of male urethritis in the era of nucleic acid amplification testing. Sex Transm Dis 2012;39:18–20. [DOI] [PubMed] [Google Scholar]
- 20.Batteiger TA, Jordan SJ, Toh E, et al. Detection of rectal Chlamydia trachomatis in heterosexual men who report cunnilingus. Sex Transm Dis 2019;46:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Pol B, liesenfeld O, Williams JA, et al. Performance of the COBAS CT/NG test compared to the Aptima AC2 and viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 2012;50:2244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JA, Eddleman L, LeMonte A, et al. Evaluation of a laboratory developed test for detection of Trichomonas vaginalis using a modification of the Abbott m2000 RealTime system. Poster session presented at: STI & AIDS World Congress 2013. Sex Transm Infect 2013;89. [Google Scholar]
- 23.Svenstrup HF, Jensen JS, Björnelius E, et al. Development of a quantitative real-time PCR assay for detection of Mycoplasma genitalium. J Clin Microbiol 2005;43:3121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao L, Glass JI, Paralanov V, et al. Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J Clin Microbiol 2010;48:2715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bump RC, Sachs LA, Buesching WJ. Sexually transmissible infectious agents in sexually active and virginal asymptomatic adolescent girls. Pediatrics 1986;77:488–94. [PubMed] [Google Scholar]
- 26.Jensen AJ, Kleveland CR, Moghaddam A, et al. Chlamydia trachomatis, Mycoplasma genitalium and Ureaplasma urealyticum among students in northern Norway. J Euro Acad Derm Venereol 2013;27:e91–6. [DOI] [PubMed] [Google Scholar]
- 27.Horner P, Thomas B, Gilroy CB, et al. role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis 2001;32:995–1003. [DOI] [PubMed] [Google Scholar]
- 28.Shimada Y, Ito S, Mizutani K, et al. Bacterial loads of Ureaplasma urealyticum contribute to development of urethritis in men. Int J STD AIDS 2014;25:294–8. [DOI] [PubMed] [Google Scholar]
- 29.Deguchi T, Shimada Y, Horie K, et al. Bacterial loads of Ureaplasma parvum contribute to the development of inflammatory responses in the male urethra. Int J STD AIDS 2015;26:1035–9. [DOI] [PubMed] [Google Scholar]
- 30.Xiao L, Paralanov V, Glass JI, et al. Extensive horizontal gene transfer in ureaplasmas from humans questions the utility of serotyping for diagnostic purposes. J Clin Microbiol 2011;49:2818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.