Abstract
A wide variety of ototoxic drugs are capable of damaging the sensory hair cells in the mammalian cochlea resulting in permanent hearing. However, the toxic properties of these drugs suggest that some could potentially damage cochlear support cells as well. To test the hypothesis, we treated postnatal day three rat cochlear cultures with toxic doses of gentamicin, cisplatin, mefloquine and cadmium. Gentamicin primarily destroyed the hair cells and disrupted the intercellular connection with the surrounding support cells. Gentamicin-induced hair cell death was initiated through the caspase-9 intrinsic apoptotic pathway followed by activation of downstream executioner caspase-3. In contrast, cisplatin, mefloquine and cadmium initially damaged the support cells and only later damaged the hair cells. Support cells death was initiated through the caspase-8 extrinsic apoptotic pathway followed later by downstream activation of caspase-3. Cisplatin, mefloquine and cadmium significantly reduced the expression of actin and laminin, in the extracellular matrix, leading to significant disarray of the sensory epithelium.
Keywords: hair cells, support cells, cochlea, apoptosis, necrosis, ototoxicity, caspase
Introduction
The cochlea has an exquisite architecture epitomized by three parallel rows of outer hair cells (OHC) and one row of inner hair cells (IHC) surrounded by a diverse array of support cells (SC). This highly organized of matrix of hair cells and SC, which forms the organ of Corti, spirals around the modiolus from the base to the apex of the cochlea. The well-defined cellular architecture of the organ of Corti is disrupted to varying degrees when the sensory hair cells are destroyed by ototoxic drugs and other ototraumatic agents. Because most ototoxic drugs damage the sensory hair cells resulting in permanent hearing loss, the sensory cells are generally considered the most vulnerable and important structures in the cochlea. Consequently, most studies have focused on understanding the mechanisms that result in hair cell death while paying less attention to SC which contribute to the structural and functional integrity of the cochlear sensory epithelium. In recent years, there has been a growing interest in the roles that SC play in the development and maintenance of the cochlea and the extent to which ototoxic drugs damage the SC.
Because cochlear hair cells do not regenerate, there is considerable interest in understanding the biological mechanisms that lead to cell death because this could aid in the development of new therapies to promote the survival of both hair cells and SC. Cochlear hair cells and SC can degenerate in different ways such as through necrosis, apoptosis or anoikis (Alam et al., 2000, He et al., 2017, Hou et al., 2019, Menardo et al., 2012, Nakagawa et al., 1998, Nicotera et al., 1999, Zhang et al., 2018). Necrosis is an energy-independent uncontrolled form of cell death that leads to karyolysis, cell swelling and the eventual rupture of the cell that leads to the release of the cell’s contents into the surrounding extracellular space (Elmore, 2007). This spillage can disrupt the extracellular matrix and cell-cell interconnections which are critically important for reestablishing cochlear homeostasis (Heaney et al., 2002, Milner and Campbell, 2003, Pietrogrande et al., 2018, Raphael, 2002). Apoptosis, by contrast, is a highly orchestrated form of programmed cell death that leads to the removal of damaged or unwanted cells with minimal leakage of the cell’s contents into the surrounding environment (D’Arcy, 2019, Elmore, 2007). Morphologically, apoptosis is characterized by cell shrinkage, membrane blebbing, nuclear condensation and the eventual breakdown of the cell into apoptotic fragments (Kerr et al., 1972). Apoptotic cell death also disrupts intercellular connections and the extracellular environment (Boudreau et al., 1995, Bozzo et al., 2006, Stupack et al., 2001). Apoptosis is triggered by initiator caspase-8 and/or caspase-9. Initiator caspase-9 is a member of the intrinsic cell death pathway activated by intracellular signals that target mitochondria. Mitochondrial dysfunction leads to the opening of the mitochondrial transition pore, loss of mitochondrial membrane potential and release of cytochrome c. Initiator caspase-8, a member of the extrinsic cell death pathway, is activated by death receptors located on the cell’s membrane such as TNFα/TNFR1. Activation of caspase-8 and/or caspase-9 results in the activation of downstream executioner caspase-3 and eventual breakdown of the cell into apoptotic bodies released into the extracellular space.
The hair cells and SC are anchored to one another through the extracellular matrix (ECM) and a variety of cell-cell interconnections (D’Arcangelo et al., 1975, Raphael and Altschuler, 2003, Richardson et al., 1987). The ECM and intercellular contacts play important roles in growth, differentiation, survival and cell death (Chiarugi and Giannoni, 2008, Setz et al., 2011, Shanmugathasan and Jothy, 2000). When the extracellular matrix and intercellular contacts are disrupted, cells become detached from one another resulting in disorganization of the cochlear sensory epithelium. Cellular detachment triggers a unique form of programmed cell death referred to as anoikis (Frisch, 2000, Frisch and Screaton, 2001, Hammar et al., 2004, Miyazaki et al., 2004). Failure of detached cells to undergo anoikis can lead to tumor metastasis (Chun et al., 2013, Taddei et al., 2012).
In the current study, we set out to characterize several aspects of cell death that occurs in hair cells and SC in postnatal cochlear organotypic cultures treated with four different ototoxic compounds, gentamycin (GM) an antibiotic, cisplatin (CIS) an antineoplastic agent, mefloquine (MEF) an anti-malarial, and cadmium (Cd) a neurotoxic metal. These ototoxic compounds are known to damage the hair cells, but the extent to which they damage SC is poorly understood. It is also unclear if ototoxic damage to SC occurs before or after the hair cells are damaged. Because these ototoxic compounds likely disrupt the extracellular matrix (ECM) and intercellular connections between hair cells and SC, laminin labeling was used to assess the effects of these ototoxic drugs on the ECM (Hamill et al., 2009).
Methods
Subjects:
All experiments were performed with postnatal day-3 Sprague-Dawley rat pups (Charles River, Wilmington, MA).
Animal Subject Approval:
This project was approved by the University at Buffalo Institutional Animal Care and Use Committee and carried out in accordance with NIH guidelines.
Cochlear organotypic cultures:
Our procedures for preparing postnatal cochlear organotypic cultures have been described in detail in previous publications (Deng et al., 2013, Ding et al., 2011, Ding et al., 2013b, Qi et al., 2008, Zhang et al., 2018). Briefly, the cochlear basilar membrane was carefully dissected out from the temporal bone and placed on top of a drop of rat collagen gel in serum-free medium. Cochlear explants were cultured in standard serum-free medium overnight and then treated with one of four ototoxic drugs to assess the time course of cell death and the characteristics of the cell death pathway. The numbers of cochlear culture explants used for each experimental condition (e.g., drug, dose, duration, staining method) was n≥ 6. For consistency, photomicrographs are shown for the upper basal turn. The location and numbers of samples evaluated for each condition is indicated in each figure legend.
Ototoxic drugs:
Cochlear cell death was evaluated with four ototoxic compounds using drug doses and exposure durations known to cause hair cell loss. Some explants were treated with 0.25 mM gentamicin (GM, Sigma # G-6896) for 24 h to examine the cell death pathways associated with this aminoglycoside antibiotic. Other were treated with 50 μM cisplatin (CIS, Aldrich #479306) for 24 h or 48 h to identify the programmed cell death pathways associated with this widely used anti-cancer platinum drug. Another group was treated with 50 μM mefloquine (MEF, Sigma #M2319) for 12 h or 24 h to examine programmed cell death with anti-malarial drug with ototoxic properties. Finally, some cultures were treated with 100 μM cadmium chloride (Cd, Sigma #655198) for 24 h or 48 h, an ototoxic heavy metal.
F-Actin and Nuclear Staining:
At the end of the experiment, the cochlear explants were fixed with 10% formalin in 0.1 M phosphate-buffered saline (PBS). To label F-actin, which is heavily expressed in the cuticular plate and stereocilia of hair cells, some samples were labeled with Acti-Stain 488 Phalloidin (1:100, Cat. # PHDG1-A, Cytoskeleton, Inc.) or Acti-Stain Phalloidin 555 (Cat. # PHDH1A, Cytoskeleton). Nuclei were stained with To-Pro-3 (Life Technologies, #T3605).
Immunolabeling:
To identify sensory hair cells, samples were labeled with a rabbit antibody against myosin VI (1:100, Sigma M5187). To recognize SC, samples were labelled with a goat anti- SOX2 monoclonal antibody (1:100, Santa Cruz Biotechnology sc-365823). To assess the extracellular matrix, cultures were labeled with a rabbit anti-laminin antibody (1:100, Abcam; Ab11575). Following fixation, some explants were incubated with one or more primary antibodies (myosin VI, SOX2 and/or laminin) in 1% Triton X-100 and 5% donkey serum in 0.1 M PBS for 24 h. After rinsing 3X with PBS, specimens were incubated with TRITC conjugated donkey anti-rabbit secondary antibody for 2 h (1:200, Jackson ImmunoReseach, #79266) to visualize the myosin VI in hair cells, or Alexa Fluor 488 conjugated donkey anti-goat secondary antibody for 2 h (1:200, Invitrogen 474685) to label SOX2 in SC, and Alexa Fluor 488 conjugated donkey anti-rabbit secondary antibody for 2 h (1:200, Abcam ab150073) to show the laminin in ECM, respectively.
Caspases:
Ototoxic activation of caspase-3, −8 and −9 was carried out using procedures described in our earlier publications (Deng et al., 2013, Ding et al., 2007, Ding et al., 2013a, Ding and Salvi, 2010, Dong et al., 2014, Yu et al., 2015, Zhang et al., 2018). To determine if the intrinsic and/or extrinsic initiator caspase pathways were activated by the ototoxic treatments, samples were evaluated with CaspGLOW Red Active caspase-8 and caspase-9 staining kits (BioVision K188, K118). Ototoxic activation of the downstream executioner pathway was evaluated using a caspase-3 staining kit (BioVision K183). Briefly, the cochlear explants were incubated with CaspGLOW Red Active caspase-8, or CaspGLOW Red Active caspase-9, or CaspGLOW Fluorescein Active caspase-3 for 1 h at the end of experiment. Afterwards, specimens were fixed with 10% formalin in PBS for 3 h.
Microscopy:
As described previously, the surface preparations of the cochlear sensory epithelium were mounted in glycerin on glass slides and evaluated with a confocal microscope (Zeiss LSM-510) (Zhang et al., 2018) using appropriate filters to detect green fluorescence (excitation 488 nm, emission 519 nm), red fluorescent (excitation 550 nm, emission 570 nm), and blue/purple fluorescence (excitation 642 nm, emission 661 nm) respectively. Collected confocal images were further processed with Zeiss LSM Image Examiner, and post-processed with Adobe Photoshop software.
Results
Hair cell and support cell degeneration and disarray:
Cochlear organotypic cultures were labeled with fluorescently labeled phalloidin in order to visualize the surface architecture of the cochlear sensory epithelium. Actin is abundantly expressed in the hair cell stereocilia and cuticular plate and to a lesser extent in the borders of the SC. The surface architecture of a normal cochlea maintained in culture medium for 48 h is shown in Figure 1A1; this representative sample shows robust actin labeling of the stereocilia and cuticular plate in the three orderly, parallel rows of OHC and a single row of IHC (Figure 1). Less intense, but distinct actin labeling was also present along the borders of the hexagonally boundaries of the SC in the inner sulcus (IS) and outer sulcus (OS) (Figure 1 inset). Figure 1A2 show To-Pro-3 labeling of nuclei in the SC and hair cells in deeper layers of the organ of Corti; Figure 1A3 shows the merge of panels A1 and A2.
Figure 1:

Representative photomicrographs illustrating the surface architecture of cochlear organotypic cultures approximately 40% of the distance from the base of the cochlea. Samples labeled with Acti-Stain Phalloidin 555 (Top row) which preferentially labels the actin in the stereocilia and cuticular plate of the three rows of outer hair cells (OHC), one row of inner hair cells (IHC) and the hexagonal borders of the supporting cells (SC) in the inner sulcus (IC) and outer sulcus (OS) region of the epithelium. Nuclei stained with To-Pro-3 (blue, middle row). Merge of phalloidin and To-Pro-3 in bottom row. (A1) Control specimens maintained in culture for 48 h. Note intense labeling of the apical pole of three orderly rows of OHC and single row of IHC and actin labeling of borders of SC in the inner sulcus (IS) and outer sulcus (OS) regions. Inset shows higher magnification view of the hexagonally borders of SC. Note thick actin border surrounding the SC and minimal actin labeling within the border. Scale bar 20 μm. (A2) Note To-Pro-3 of hair cells and support cells in deeper layer of organ of Corti. (A3) Merge of A1 and A2. (B1) Cochlear culture treated with 0.25 mM gentamicin (GM) for 24 h. Note massive loss of stereocilia and cuticular plate of OHC and IHC; missing hair cells surrounded by circumferential ring of actin arranged in orderly rows corresponding to the missing hair cells (inset with dashed border). Inset with solid border shows higher magnification view of apical surface of SC. Note weak labeling of actin borders of SC and diffuse labeling inside the SC border. (B2) Note To-Pro-3 labeled nuclei of SC. (B3) Merge of B1 and B2. (C1) Cochlear culture treated with 50 μM of cisplatin (CIS) for 48 h. Note massive loss of OHC and IHC, disorganized hair cell rows and minimal actin labeling in the SC regions. Cuticular plate of remaining hair cells shrunken or fragmented. (C2) Note paucity of To-Pro-3 labeled nuclei throughout organ of Corti. (C3) Merge of C1 and C2. (D1) Cochlear culture treated with 50 μM mefloquine (MEF) for 24 h. Many OHC and IHC missing; cuticular plate of remaining hair cells shrunken or fragmented and hair cell rows in disarray. Note minimal actin labeling in SC regions. (D2) Note paucity of To-Pro-3 labeled nuclei throughout organ of Corti. (D3) Merge of D1 and D2. (E1) Cochlear culture treated with 100 μM of cadmium (Cd) for 48 h. Hair cell rows in moderate disarray; many OHC and IHC missing. Minimal actin labeling in SC regions. (E2) Note lack of To-Pro-3 nuclear labeling throughout organ of Corti. (D3) Merge of D1 and D2.
When cochlear cultures were treated for 24 h with 0.25 mM GM, many OHC and IHC were destroyed and only a few were still present (Figure 1B1). In the place formerly occupied by a missing hair cells, there was a crisp hexagonal band of phalloidin (Figure 1B1, inset with dashed border). The hair cell lesion reduced the radial width from the row of IHC to the third row of OHC (Figure 1A1 vs 1B1). The IS region was characterized by diffuse actin labeling of SC; the SC borders were blurry and diffuse labeling was present over the surface of the SC. Figure 1B2 shows the To-Pro-3 labeling of nuclei in deeper layers throughout the organ of Corti; Figure 1B3 shows the merge of panels B1 and B2.
The surface architecture of the sensory epithelium was altered in a distinctly different manner when cultures were treated with 50 μM of CIS for 48 h. CIS caused considerable OHC and IHC loss. The vestiges of the remaining hair cells were often shrunken and distorted and the radial distance between IHC and the outermost row OHC seemed to have increased (Figure 1C1). Notably, CIS largely eliminated the phalloidin labeling of the SC in the IS and OS regions. The paucity of To-Pro-3 labeled nuclei in the SC and hair cell regions is clearly evident in Figure 1C2 and the merge of panel C1 and C2 in Figure 1C3.
Treatment with MEF for 24 h disrupted the surface architecture of the sensory epithelium in a manner similar to CIS (Figure 1D1). Many OHC and IHC were missing and remnants of the residual hair cells were shrunken and distorted. There was nearly complete absence of phalloidin labeling of SC and the radial distance between IHC and the outermost OHC appeared larger than normal. Figure 1D2 shows the dearth of To-Pro-3 labeled nuclei in the SC and hair cell region in deeper layers of the organ of Corti; Figure 1D3 shows the merge of panel D1 and D2.
Cd treatment for 48 h destroyed many IHC and OHC (Figure 1E1). The vestiges of the remaining hair cells were shrunken and the hair cell rows were disorganized. Cd largely eliminated phalloidin labeling from SC, much like CIS and MEF. Figure 1E2 shows the To-Pro-3 labeling of nuclei in the deeper layers of the organ of Corti; there was a paucity of nuclei in the SC and hair cell regions. Figure 1E3 shows the merge of panel E1 and E2. Taken together, these results suggest that CIS, MEF and Cd ototoxicity largely abolished the expression of actin, an important structural protein present in most SC whereas GM seemed to alter the distribution and enhance the expression of actin in certain regions.
Ototoxic destruction of SC:
Most ototoxicity studies focus on damage to the sensory hair cells, but the preceding results suggest that these drugs might be toxic SC. To determine the extent to which these four ototoxic drugs damaged the SC and hair cells, cochlear cultures were labeled with antibodies against myosin VI to visualized the hair cells and SOX2 to assess the SC. The surface view (Figure 2A2) and Z-plane images (Figure 2A1) illustrate the location of the SC surrounding the hair cells in a normal control cochlea maintained in culture medium for 48 h. Myosin VI (red) was present throughout the cytoplasm of the three parallel rows of OHC and one row of IHC. SOX2 (green) labeling filled the Hensen cells (HC) alongside the third row of OHC, the Deiters cells (DC) beneath the three rows of OHC; moderate labeling was also present in the pillar cells (PC) and some SC in the IS region. Treatment with 0.25 mM GM for 24 h resulted in significant loss of OHC and IHC (Figure 2). Myosin-labeled remnants of damaged hair cells were present within the sensory epithelium surrounded by rows of HC, DC, PC (Figure 2B1) and SC in the IS region (Figure 2B2). Treatment with 50 μM CIS for 48 h not only damaged or destroyed most IHC and OHC labeled with myosin VI, but almost completely eliminated the SOX2 labeling previously expressed in HC, DC, PC and other SC in the IS region (Figure 2C1–2). Similarly, treatment with 50 μM MEF for 24 h destroyed most myosin VI labeled hair cells and SOX2 labeled HC, DC, PC and other SC in the IS region (Figure 2D1–2). Treatment with 100 μM Cd for 48 also destroyed or damaged most myosin VI labeled hair cells and eliminated many of the SOX2 labeled SC cells throughout the sensory epithelium (Figure 2E1–2).
Figure 2:
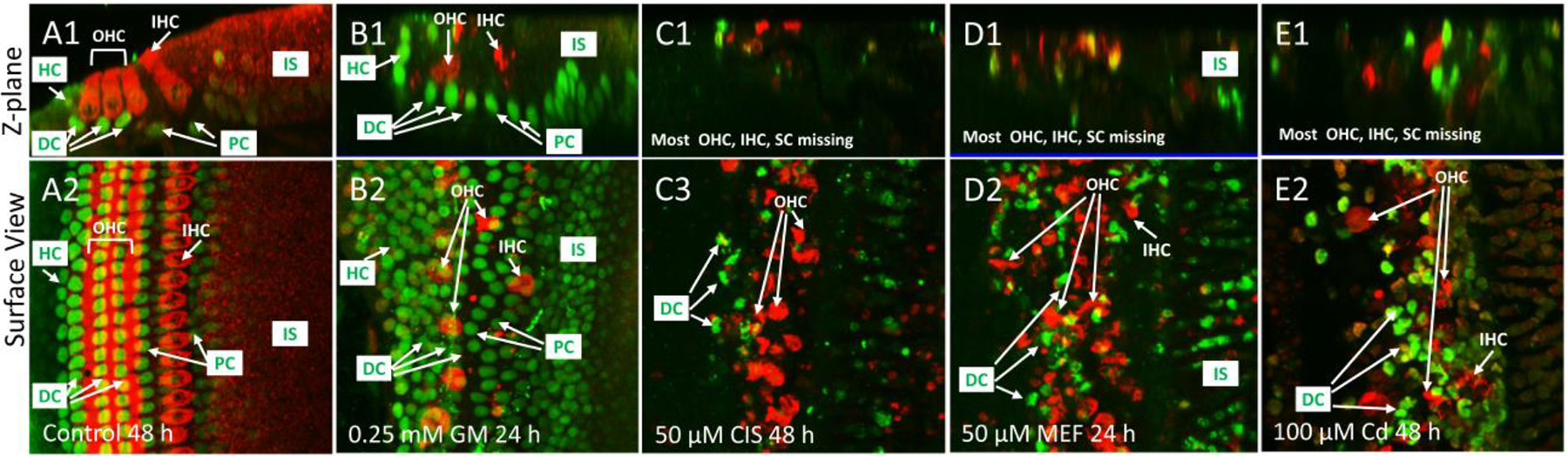
Representative photomicrographs (n ≥6 replicates per condition) from the upper basal turn of cochlear organotypic cultures labeled with myosin VI (red) and SOX2 (green). Representative Z-plane image (A1) and surface view of control cochlear cultured for 48 h. Cytoplasm of three rows of outer hair cells (OHC) and one row of inner hair cells (IHC) labeled with myosin VI. SOX2 expressed in Hensen cells (HC), Deiters cells (DC), pillar cells (PC)) and SC in inner sulcus cells (IS) region. (B1–2) Treatment with 0.25 mM GM for 24 h resulted in loss of most myosin VI expressing OHC and IHC; however, SOX2 labeling was still present in HC, DC, PC and other SC in the IS region. (C1–2) Many myosin VI labeled OHC and IHC and SOX2 labeled SC missing after 48 h treatment with 50 μM CIS. (D1–2) Treatment with 50 μM MEF resulted in significant loss of myosin VI labeled OHC and IHC and SOX2 labeled SC. (E1–2) Many myosin VI labeled OHC and IHC and SOX2 labeled SC missing after treatment with 100 μM Cd.
Ototoxic damage to extracellular matrix:
Laminin, present in the ECM, is involved in cell adhesion and regulating tissue architecture. To determine the extent to which the ECM was disrupted, cochlear organotypic cultures were labeled with antibody against laminin and cell nuclei were labeled with To-Pro-3. In radial sections of normal cochlear explants (Figure 3A1), laminin labeling was most evident in the upper layer of the epithelium above the nuclei of the hair cells and SC (Figure 3A1). Laminin-only-labeled cross sections (To-Pro-3 labeling removed) of cochlear surface preparations were taken at the level of the hair cells or SC. In cross sections in the focal plane of the hair cells (Figure 3A2), highly organized circumferential rings of laminin were evident in the OS, OHC and IHC regions. A strong band of laminin was also present in the PC region whereas regularly spaced patches of laminin labeling occupied the IS area. In confocal images through the support cell layer (Figure 3A3), circumferential rings of laminin were present throughout. Treatment with 0.25 mM GM for 24 h resulted in the loss of many hair cell nuclei, but the nuclei of SC were still present throughout the deeper layers of the epithelium (Figure 3B1). Confocal images taken through the hair cell layer (Figure 3B2) of the epithelium revealed blurred, laminin labeling mainly in the OHC, IHC and PC regions. Images taken in the deeper SC layer (Figure 3B3) revealed thickened circumferential rings of laminin mainly in the OHC, IHC and PC regions. When cultures were treated with 50 μM of CIS for 48 h, there was a large reduction in the number of nuclei seen in radial sections throughout the epithelium (Figure 3C1) and almost complete loss of laminin labeling. There was a large reduction and considerable disarray in laminin staining in cross sections taken through the hair cell layer (Figure 3C2) and SC layer (Figure 3C3). In cultures treated with 50 μM of MEF for 24 h, there was a major loss of hair cell nuclei and SC nuclei and a loss of laminin labeling in radial cross sections of the sensory epithelium (Figure 3D1). Cross sections taken through the hair cell layer (Figure 3D2) and SC layer (Figure 3D3) revealed a significant reduction and considerable disarray in laminin labeling. When cultures were treated with 100 μM of Cd for 48 h, there was major loss of hair cell nuclei and reduction of laminin labeling in radial cross sections of the sensory epithelium (Figure 3E1). Cross sections through the hair cell and SC layers revealed significant loss and disarray in laminin labeling.
Figure 3:
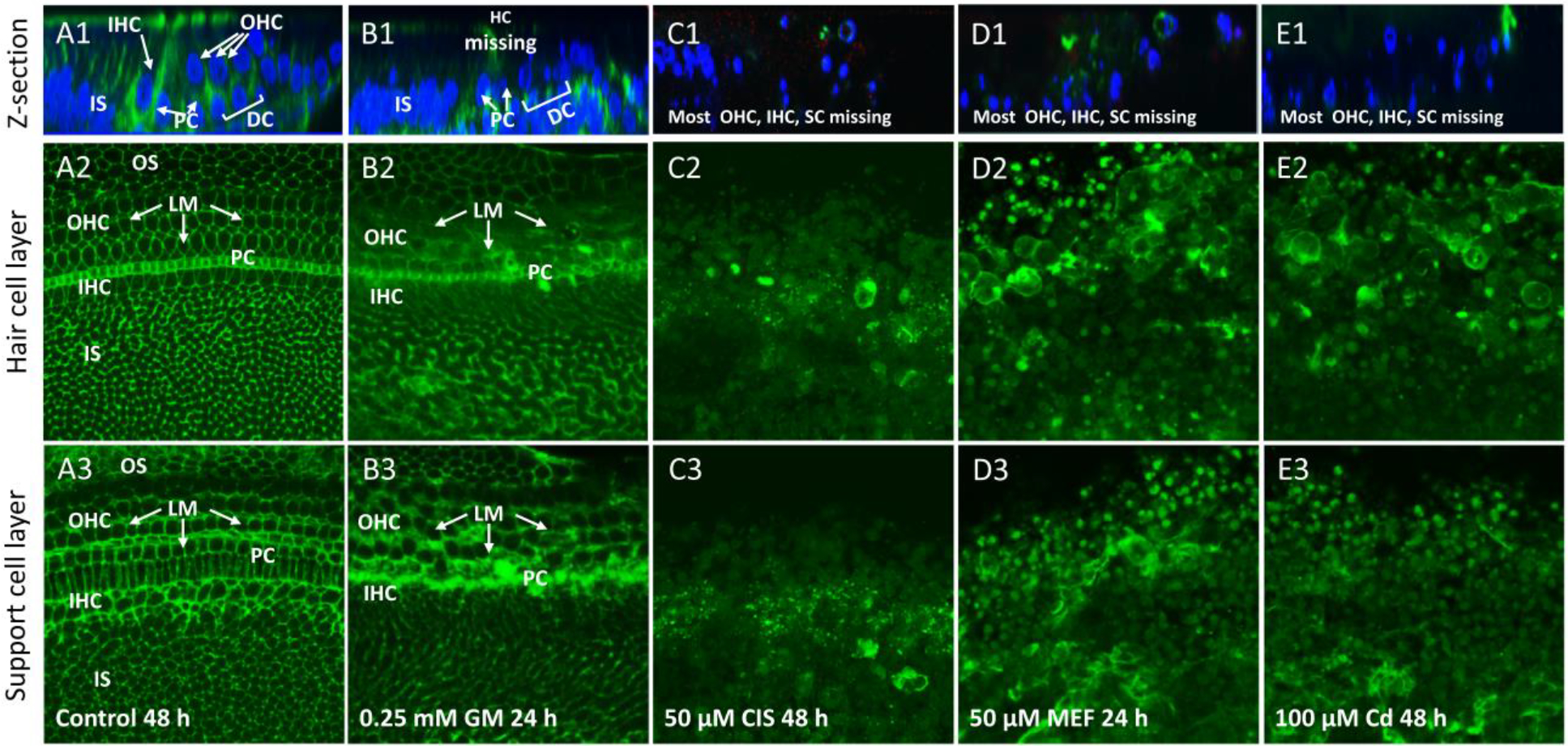
Cochlear organotypic cultures (n ≥6 replicates per condition) from the upper basal turn labeled with an antibody against laminin (LM, green, all rows) and/or with To-Pro-3 (blue, top row only) which labels nuclei. Top row: Representative Z-plane images showing the location of laminin and cell nuclei. Middle row and bottom row only show laminin-labeling of optical sections of surface preparations. Middle row shows sections taken at the level of the hair cells; bottom row shows section from the SC layer. (A1) Z-plane image of control cochlea cultured for 48 h. Strong laminin labeling near and below the surface; nuclear labeling mainly located below the surface in regions occupied by inner hair cells (IHC), outer hair cells (OHC), pillar cells (PC), Deiters cells (DC) and other SC in the inner sulcus (IS) region. (A2) Circumferential rings of LM ln OHC and IHC region and OS region. Strong labeling of laminin in band of PC. Many patches of laminin in IS region. (A3). Circumferential rings of laminin through out the SC layer. (B1–2) Treatment with 0.25 mM GM for 24 h resulted in blurring of circumferential rings of laminin in IHC, OHC and PC regions. (B3) Thickening of circumferential rings of laminin mainly in OHC, IHC and PC regions. (C1–3) Treatment with 50 μM CIS for 48 h reduced the number of cell nuclei; laminin labeling also greatly reduced. (D1–3) Treatment with 50 μM MEF reduced the number of nuclei; laminin labeling in hair cell layer and SC layers reduced and disorganized. (E1–3) Treatment with 100 μM Cd eliminates nuclei; laminin labeling in hair cell and SC layers reduced and greatly disorganized.
The preceding results indicate that GM primarily damages the hair cells and has only a minor influence on the SC and the expression of laminin in the ECM. On the other hand, CIS, MEF and Cd not only caused significant hair cell damage, but also triggered a massive loss of SC and laminin labeling in the ECM which resulted in considerable disorganization of the sensory epithelium.
Caspase-mediated ototoxicity:
Because the structural damage induced by GM differed from that evoked by the other three ototoxins, we expected that there would be major differences in the caspase-mediated cell death pathway triggered GM versus the other three ototoxins. To test this hypothesis, cell permeable carboxy-fluorescein probes were used to evaluate activation of initiator caspase-8 of the extrinsic apoptotic pathway, initiator caspase-9 of the intrinsic apoptotic pathway and downstream executioner caspase-3. (Note: for consistency in caspase image presentation, CaspGLOW active fluorescein, normally green, was presented as red in the confocal images). Assessments were made at an early and a late time point to determine if the processes initiating cell death were triggered by the extrinsic pathway or intrinsic caspase pathway. Cochlear cultures were co-labeled with To-Pro-3 to identify nuclei and with fluorescently-labeled phalloidin to visual actin. As expected, in control cochlea cultured for 48 h, there was no evidence of activated caspase-8, caspase-9 or caspase-3 in Z-plane images of the sensory epithelium (Figure 4A1–3). In cochlear cultures treated with 0.25 mM GM for only 12 h, initiator caspase-9 could be seen in the IHC and OHC region along with less intense staining of downstream executioner caspase-3. There was no evidence of caspase-3, caspase-8 or caspase-9 activation or cell nuclear loss in SC regions, consistent with the SOX2 labeling in Figure 2. These results suggest that GM-induced hair cell loss is initiated through the intrinsic caspase-8 mitochondrial pathway.
Figure 4:
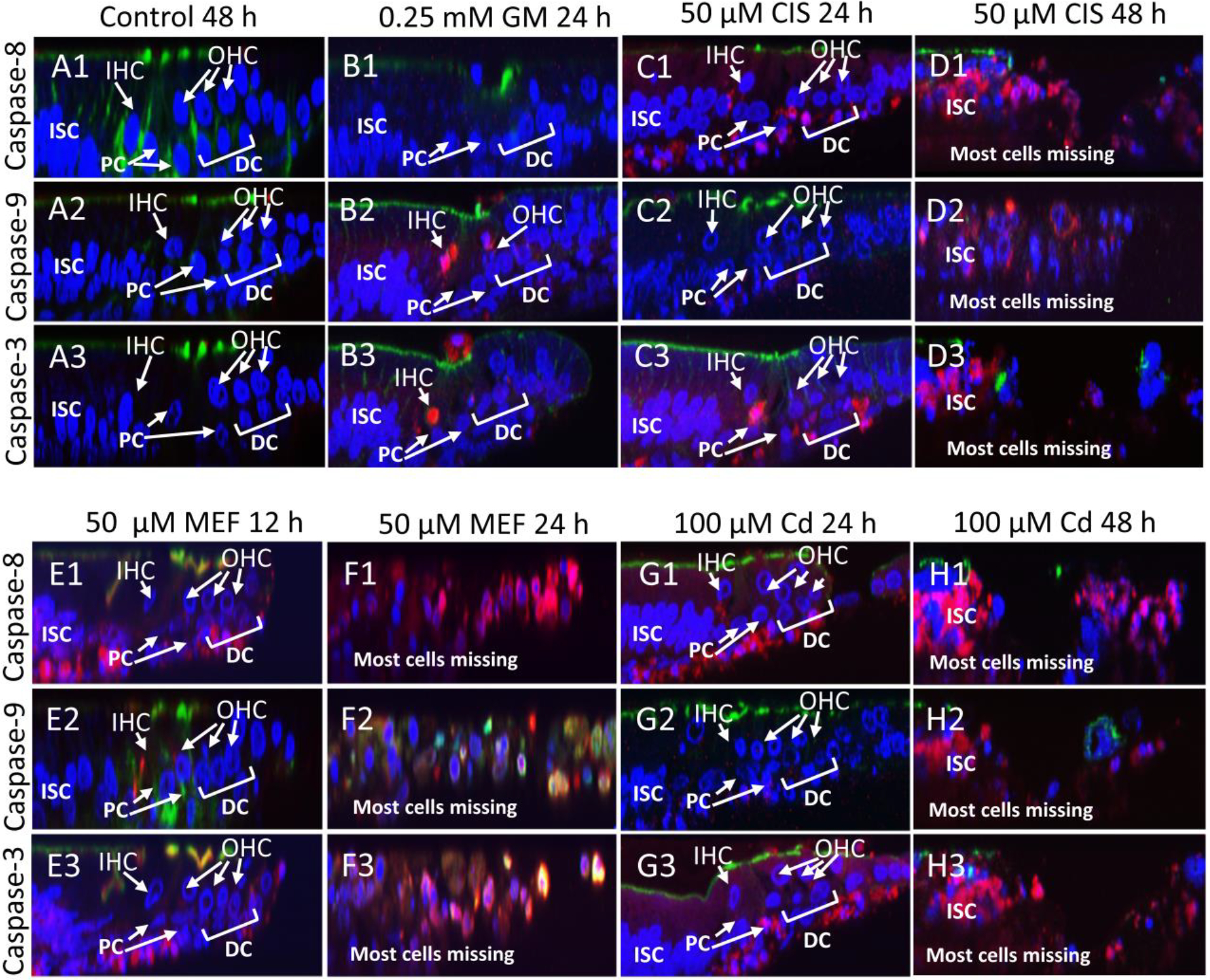
Caspase-mediated cell death in cochlear cultures treated with four different ototoxic drugs. Representative photomicrographs (n ≥6 replicates per condition) from the upper basal turn. Z-plane sections of cochlear cultures labeled with Acti-Stain 488 Phalloidin (green) to label actin, To-Pro-3 (blue) to label nuclei and CaspGLOW Fluorescein Active caspase-3 (red color), and CaspGLOW Red caspase-8 (red color), and CaspGLOW Red Active caspase −9 (red color) to label activated caspases. (A1–3) Control cochlea cultured for 48 h. Actin (green) expressed in stereocilia above inner hair cell (IHC) and outer hair cell (OHC) nuclei. Note absence of activated caspase-3 (A3), caspase-8 (A1) and caspase-9 (A-2). (B1–3) Cochlear cultures treated with 0.25 mM GM for 24 h. (B1) Nuclei (blue) present in pillar cells (PC), Deiters cells (DC) and inner sulcus cells (ISC), but missing from region normally occupied by IHC and OHC. Note absence of extrinsic caspase-8 labeling. (B2) Activated caspase-9 present in region normally occupied by IHC and OHC. (B3). Executioner caspase-3 present in region normally occupied by IHC and OHC. (C1–3) Cochlear cultures treated with 50 μ M CIS for 24 h. (C1) IHC and OHC nuclei still present 24 h after CIS treatment. Note activated caspase-8 around the DC, PC and SC at lower edge of the epithelium. (C2) Note absence of activated caspase-9. (C3) Note activated caspase-3 around PC, DC and lower edge of epithelium. (D1–3) Cochlea cultured with 50 μM of CIS for 48 h. (D1–3) Most hair cell and SC missing. Note presence of activated caspase-8, caspase-9 and caspase-3. (E1–3) Cochlea cultured with 50 μM of MEF for 12 h. Note moderate reduction in hair cell and SC nuclei and strong activation of caspase-8 (E1) and caspase-3 in the region around and below the DC, PC and ISC. Caspase-9 largely absent. (F1–3) Cochlear cultured with 50 μM of MEF for 24 h. Note massive loss of hair cell and SC nuclei and strong labeling of activated caspase-8 and caspase-3. (G1–3) Cochlea cultured with 100 μM of Cd for 24 h. Note moderate loss of hair cell and SC nuclei. Strong labeling of activated caspase-8 and caspase-3 beneath the PC and DC. Note absence of caspase-9. (H1–H3) Cochlea cultured with 100 μM of Cd for 48 h. Most hair cell and supporting cell nuclei missing. Strong labeling of activated caspase-8, caspase-9 and caspase-3 in SC.
A much different picture emerged when cochlear cultures were treated with 50 μM CIS for only 24 h. Under these conditions, labeling of initiator caspase-8 of the extrinsic pathway was observed in the SC (DC, PC, ISC) located underneath the hair cells (Figure 4C1). Executioner caspase-3 labeling was also observed in the same supporting cell region (Figure 4C3); there was no evidence of initiator caspase-9 at this early time point. However, when cochlear were cultured with the same dose of CIS for 48 h, all three caspase appeared (Figure D1–3), but the labeling was stronger and more widespread for caspase-8 than caspase-9. These results suggest that CIS-induced cell death is triggered by death receptors located on the SC in DC, PC, HC and ISC; only later did the hair cells begin to die off.
A similar picture emerged when cochlear cultures were treated with 50 μM of MEF for only 12 h. During the early stage of damage, initiator caspase-8 of the extrinsic pathway only appeared in SC (DC, PC, ISC) below the hair cell layer (Figure 4E1); less intense caspase-3 labeling also appeared in this SC region at this time (Figure 4E3). There was very little labeling of initiator caspase-9 of the intrinsic pathway (Figure 4E2). When cochlear cultures were treated with the same dose of MEF for 24 h, there was extensive loss cell nuclei throughout the sensory epithelium. This was accompanied by strong labeling of initiator caspase-8 and executioner caspase-3 (Figure 4F3) and less intense labeling of initiator caspase-9 in the intrinsic cell death pathway (Figure 4F2). Thus, MEF-induced ototoxicity appears to begin by activating death receptors on the SC leading to activation initiator caspase-8 and then downstream activation of caspase-3.
The cell death pathway elicited with Cd was similar to that for CIS and MEF. When cochlear cultures were treated with 100 μM for 24 h, strong initiator caspase-8 labeling was evident in the SC beneath the hair cells (Figure 4G1); executioner caspase-3 also appeared in this region (Figure 4G3). However, there was little evidence of initiator caspase-9 (Figure 4G2). However, when cochleae were cultured for 48 h with the same dose of Cd, there was robust labeling of caspase-8, caspase-9 and caspase-3 and many missing cell nuclei (Figure 4H1–3). Thus, Cd-ototoxicity also appears to be triggered by membrane damage to SC which leads to the activation of caspase-8 followed by executioner caspase-3.
Discussion
The location, temporal progression and apoptotic cell death programs initiated by CIS, MEF and Cd were strikingly different from those provoked by GM. GM primarily damaged the hair cells and caused mild to moderate disruption to the SC and ECM in the vicinity of the lesion (Figure 1–3). In the vicinity of the hair cell lesion, there was increased expression/thickening of laminin, a component of the ECM suggestive of a homeostatic repair process. GM-induced hair cell death was initiated through the intrinsic mitochondrial pathway, as evidenced by activation of caspase-9, followed by executioner caspase-3 (Figure 4B1–2). However, there was no evidence of extrinsic caspase-8 activation at the time when these other caspases were activated (Figure 4B3). In contrast, CIS, MEF and Cd treatments caused massive loss of both hair cells and SC and major disruption of laminin, a component of the ECM (Figure 1–3). With all three of these ototoxins, the earliest signs of cell death appeared in the SC mainly beneath the hair cells. In the early stages of apoptosis, there were clear signs of initiator caspase-8 activation (Figure 4C1, F1 and G1) and downstream executioner caspase-3 activation, but little evidence of caspase-9. Only later as the lesion evolved and expanded across the epithelium did initiator caspase-9 appear (Figure 4D2, F2, G2).
When SC death comes first:
Our results indicate that CIS, MEF and Cd damage begins in the SC prior to damaging the hair cells, unlike GM which mainly targets the hair cell (Figure 1–2). The early damage to SC from CIS, MEF and Cd was associated with activation of the extrinsic caspase-8 pathway. Cisplatin, which generates damaging reactive oxygen species (Clerici et al., 1996, Gonzalez-Garcia et al., 2010) can initiate caspase-8 mediated apoptosis by acting on the Fas-receptor associated with the FADD adaptor protein (Fas-associated protein with death domain) (Huang et al., 2010, Huang et al., 2003, Micheau et al., 1999). Early activation of caspase-8 has also been seen in the adult cochlea with ethacrynic acid/cisplatin-induced hearing loss (Ding et al., 2007). Mefloquine also caused widespread activation of caspase-8 in vestibular organ culture in both SC and hair cells (Yu et al., 2011). MEF also activated caspase-9, but the labeling was less intense and less widespread consistent with our results. Others have reported that Cd-induced apoptosis in cultured kidney podocytes through the Fas-FADD caspase-8 medicated pathway (Eichler et al., 2006) consistent with our results. However, in proliferating HEI-OC1 cells derived from the organ of Corti, Cd induced greater activation of caspase-9 than caspase-8 (Kim et al., 2008). This difference could be due to the fact that HEI-OC1 cells are proliferating cells lacking the differentiated features of the cells in our cochlear explants.
Extracellular matrix:
Laminin, a secreted glycoprotein, is incorporated into the ECM where it participates in cell adhesion, differentiation and cell migration (Chuang et al., 2014, Davies, 2011, Ishii et al., 1992, Racz et al., 2014, Tsuprun and Santi, 1999, Zhang et al., 2018). GM destroyed the hair cells and altered the general profile of the ECM (Figure 3B1–3) and the pattern of actin labeling mainly in the region of hair cell loss (Figure 1B1); however, the ECM remained largely intact (Figure 3B1–3). These changes are likely related to cell migration, wound healing, structural repair and re-establishment of cell-cell contacts in the organ of Corti (Anttonen et al., 2014, Collado et al., 2011, Hordichok and Steyger, 2007, Hultcrantz and Li, 1995, Raphael and Altschuler, 1991). Maintenance of the ECM and cell-cell contacts likely minimizes the activation of the caspase-8 cell death pathway during GM ototoxicity. Conversely, treatments with CIS, MEF and Cd results in significant disruption of the ECM as reflected in the loss of laminin labeling around both hair cells and SC (Figure 3C–E). Loss of laminin labeling and disruption of the ECM may serve as a trigger for caspase-8 mediated cell death reminiscent of paraquat-induced cell death and anoikis previously observed in the cochlea (Zhang et al., 2018).
Synopsis:
In cochlear organotypic cultures, GM triggered the intrinsic caspase-9 cell death pathway in the hair cells resulting in loss of laminin labeling and mild disorganization of the organ of Corti in the vicinity of the missing hair cells. CIS, MEF and Cd by contrast initially triggered the extrinsic caspase-8 cell death pathway in the SC located beneath the hair cells resulting in massive loss of SC that preceded loss of the sensory hair cells.
Acknowledgements:
Research supported in part by a grant from NIOSH (R01OH010235) and in part by the Foundation of Science and Technology Commission of Shanghai Municipality (#15140900900).
List of Abbreviations:
- Cd
cadmium
- CIS
cisplatin
- DC
Deiters cell
- ECM
extracellular matrix
- GM
gentamycin
- HC
Hensen cells
- IHC
inner hair cells
- IS
inner sulcus
- ISC
inner sulcus cells
- MEF
mefloquine
- OHC
outer hair cells
- OS
outer sulcus
- PC
pillar cell
- PBS
phosphate buffered saline
- SC
support cells
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare they have no competing interests with regards to the findings in this study. RS is a consultant for Auris Medical and CILcare.
References
- Alam SA, Ikeda K, Oshima T, Suzuki M, Kawase T, Kikuchi T, et al. (2000) Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res;141:28–38. [DOI] [PubMed] [Google Scholar]
- Anttonen T, Belevich I, Kirjavainen A, Laos M, Brakebusch C, Jokitalo E, et al. (2014) How to bury the dead: elimination of apoptotic hair cells from the hearing organ of the mouse. J Assoc Res Otolaryngol;15:975–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ (1995) Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science;267:891–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzo C, Sabbatini M, Tiberio R, Piffanelli V, Santoro C, Cannas M (2006) Activation of caspase-8 triggers anoikis in human neuroblastoma cells. Neurosci Res;56:145–53. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Giannoni E (2008) Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol;76:1352–64. [DOI] [PubMed] [Google Scholar]
- Chuang CY, Degendorfer G, Davies MJ (2014) Oxidation and modification of extracellular matrix and its role in disease. Free Radic Res;48:970–89. [DOI] [PubMed] [Google Scholar]
- Chun J, Joo EJ, Kang M, Kim YS (2013) Platycodin D induces anoikis and caspase-mediated apoptosis via p38 MAPK in AGS human gastric cancer cells. J Cell Biochem;114:456–70. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA (1996) Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res;98:116–24. [DOI] [PubMed] [Google Scholar]
- Collado MS, Burns JC, Meyers JR, Corwin JT (2011) Variations in shape-sensitive restriction points mirror differences in the regeneration capacities of avian and mammalian ears. PLoS One;6:e23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen S-C, Soares HD, Morgan JI, Curran T (1975) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature;374:719–23. [DOI] [PubMed] [Google Scholar]
- D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biology International;43:582–92. [DOI] [PubMed] [Google Scholar]
- Davies D (2011) Cell-extracellular matrix versus cell-cell interactions during the development of the cochlear-vestibular ganglion. J Neurosci Res;89:1375–87. [DOI] [PubMed] [Google Scholar]
- Deng L, Ding D, Su J, Manohar S, Salvi R (2013) Salicylate selectively kills cochlear spiral ganglion neurons by paradoxically up-regulating superoxide. Neurotox Res;24:307–19. [DOI] [PubMed] [Google Scholar]
- Ding D, He J, Allman BL, Yu D, Jiang H, Seigel GM, et al. (2011) Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res;282:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Jiang H, Wang P, Salvi R (2007) Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res;226:129–39. [DOI] [PubMed] [Google Scholar]
- Ding D, Qi W, Yu D, Jiang H, Han C, Katsuno K, et al. (2013a) NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Qi W, Yu D, Jiang H, Han C, Kim MJ, et al. (2013b) Addition of exogenous NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis in cochlear organotypic cultures. PLoS One;8:e79817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Salvi R (2010) Paraquat-induced damage to the cochlear hair cells. Dalian Ding Science of the Inner Ear;China Science and Technology Press:325–9. [Google Scholar]
- Dong Y, Ding D, Jiang H, Shi JR, Salvi R, Roth JA (2014) Ototoxicity of paclitaxel in rat cochlear organotypic cultures. Toxicol Appl Pharmacol;280:526–33. [DOI] [PubMed] [Google Scholar]
- Eichler T, Ma Q, Kelly C, Mishra J, Parikh S, Ransom RF, et al. (2006) Single and combination toxic metal exposures induce apoptosis in cultured murine podocytes exclusively via the extrinsic caspase 8 pathway. Toxicol Sci;90:392–9. [DOI] [PubMed] [Google Scholar]
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicologic Pathology;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM (2000) Anoikis. Methods Enzymol;322:472–9. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA (2001) Anoikis mechanisms. Curr Opin Cell Biol;13:555–62. [DOI] [PubMed] [Google Scholar]
- Gao K, Ding D, Sun H, Roth J, Salvi R (2017) Kanamycin Damages Early Postnatal, but Not Adult Spiral Ganglion Neurons. Neurotox Res;32:603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia JA, Nevado J, Garcia-Berrocal JR, Sanchez-Rodriguez C, Trinidad A, Sanz R, et al. (2010) Endogenous protection against oxidative stress caused by cisplatin: role of superoxide dismutase. Acta Otolaryngol;130:453–7. [DOI] [PubMed] [Google Scholar]
- Hamill KJ, Kligys K, Hopkinson SB, Jones JC (2009) Laminin deposition in the extracellular matrix: a complex picture emerges. Journal of Cell Science;122:4409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, et al. (2004) Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes;53:2034–41. [DOI] [PubMed] [Google Scholar]
- He Z, Guo L, Shu Y, Fang Q, Zhou H, Liu Y, et al. (2017) Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy;13:1884–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney DL, Schulte BA, Niedzielski AS (2002) Dystroglycan expression in the developing and senescent gerbil cochlea. Hear Res;174:9–18. [DOI] [PubMed] [Google Scholar]
- Hordichok AJ, Steyger PS (2007) Closure of supporting cell scar formations requires dynamic actin mechanisms. Hear Res;232:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Chen J, Yang J (2019) Autophagy precedes apoptosis during degeneration of the Kolliker’s organ in the development of rat cochlea. Eur J Histochem;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CR, Jin ZX, Dong L, Tong XP, Yue S, Kawanami T, et al. (2010) Cisplatin augments FAS-mediated apoptosis through lipid rafts. Anticancer Research;30:2065–71. [PubMed] [Google Scholar]
- Huang HL, Fang LW, Lu SP, Chou CK, Luh TY, Lai MZ (2003) DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene;22:8168–77. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Li HS (1995) Degeneration patterns of actin distribution in the organ of corti in two genotypes of mice. ORL: Journal of Oto-Rhino-Laryngology and Its Related Specialties;57:1–4. [DOI] [PubMed] [Google Scholar]
- Ishii K, Schroter-Kermani C, Xu D, Merker HJ, Jahnke V (1992) Extracellular matrix in the rat spiral limbus. Eur Arch Otorhinolaryngol;249:224–30. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer;26:239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Jeong HJ, Myung NY, Kim MC, Lee JH, So HS, et al. (2008) The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ Health Perspect;116:854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menardo J, Tang Y, Ladrech S, Lenoir M, Casas F, Michel C, et al. (2012) Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid Redox Signal;16:263–74. [DOI] [PubMed] [Google Scholar]
- Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT (1999) Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem;274:7987–92. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL (2003) The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol;170:3850–8. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Shen M, Fujikura D, Tosa N, Kim HR, Kon S, et al. (2004) Functional role of death-associated protein 3 (DAP3) in anoikis. J Biol Chem;279:44667–72. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamane H, Shibata S, Takayama M, Sunami K, Nakai Y (1997) Two modes of auditory hair cell loss following acoustic overstimulation in the avian inner ear. ORL J Otorhinolaryngol Relat Spec;59:303–10. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamane H, Takayama M, Sunami K, Nakai Y (1998) Apoptosis of guinea pig cochlear hair cells following chronic aminoglycoside treatment. European Archives of Oto-Rhino-Laryngology;255:127–31. [DOI] [PubMed] [Google Scholar]
- Nicotera T, Henderson D, Zheng X-Y, Ding D, McFadden SL (1999) Reactive oxygen species, apoptosis and necrosis in noise-exposed cochleas of chinchillas. Abstracts Association for Research in Otolaryngology;22:626. [Google Scholar]
- Pietrogrande G, Mabotuwana N, Zhao Z, Abdolhoseini M, Johnson SJ, Nilsson M, et al. (2018) Chronic stress induced disturbances in Laminin: A significant contributor to modulating microglial pro-inflammatory tone? Brain Behav Immun;68:23–33. [DOI] [PubMed] [Google Scholar]
- Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res;236:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz E, Gaal B, Kecskes S, Matesz C (2014) Molecular composition of extracellular matrix in the vestibular nuclei of the rat. Brain Struct Funct;219:1385–403. [DOI] [PubMed] [Google Scholar]
- Raphael Y (2002) Cochlear pathology, sensory cell death and regeneration. Br Med Bull;63:25–38. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA (1991) Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton;18:215–27. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA (2003) Structure and innervation of the cochlea. Brain Res Bull;60:397–422. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Crossin KL, Chuong CM, Edelman GM (1987) Expression of cell adhesion molecules during embryonic induction. III. Development of the otic placode. Developmental Biology;119:217–30. [DOI] [PubMed] [Google Scholar]
- Setz C, Brand Y, Radojevic V, Hanusek C, Mullen PJ, Levano S, et al. (2011) Matrix metalloproteinases 2 and 9 in the cochlea: expression and activity after aminoglycoside exposition. Neuroscience;181:28–39. [DOI] [PubMed] [Google Scholar]
- Shanmugathasan M, Jothy S (2000) Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathology International;50:273–9. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA (2001) Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol;155:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei ML, Giannoni E, Fiaschi T, Chiarugi P (2012) Anoikis: an emerging hallmark in health and diseases. J Pathol;226:380–93. [DOI] [PubMed] [Google Scholar]
- Tsuprun V, Santi P (1999) Ultrastructure and immunohistochemical identification of the extracellular matrix of the chinchilla cochlea. Hear Res;129:35–49. [DOI] [PubMed] [Google Scholar]
- Yonovitz A, Fisch JE (1991) Circadian rhythm dependent kanamycin-induced hearing loss in rodents assessed by auditory brainstem responses. Acta Oto-Laryngologica;111:1006–12. [DOI] [PubMed] [Google Scholar]
- Yu D, Ding D, Jiang H, Stolzberg D, Salvi R (2011) Mefloquine damage vestibular hair cells in organotypic cultures. Neurotox Res;20:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ding D, Sun H, Salvi R, Roth J (2015) Neurotoxicity of trimethyltin in rat cochlear organotypic cultures. Neurotoxicity Research;28:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Wang X, Hill K, Chen J, Lemasters J, Yang SM, et al. (2015) Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal;22:1308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sun H, Salvi R, Ding D (2018) Paraquat initially damages cochlear support cells leading to anoikis-like hair cell death. Hear Res. [DOI] [PMC free article] [PubMed] [Google Scholar]


