Abstract
Perineuronal nets (PNN) of the extracellular matrix are dense aggregations of chondroitin-sulfate proteoglycans that usually surround fast-spiking parvalbumin-expressing inhibitory interneurons (PV). The development of PNN around PV appears specifically at the end of sensitive periods of visual learning and limits the synaptic plasticity in the visual cortex of mammals. Seasonal songbirds display a high level of adult neuroplasticity associated with vocal learning, which is regulated by fluctuations of circulating testosterone concentrations. Seasonal changes in testosterone concentrations and in neuroplasticity are associated with vocal changes between the non-breeding and breeding seasons. Increases in blood testosterone concentrations in the spring lead to the annual crystallization of song so that song becomes more stereotyped. Here we explore whether testosterone also regulates PNN expression in the song control system of male and female canaries. We show that, in both males and females, testosterone increases the number of PNN and of PV neurons in the three main telencephalic song control nuclei HVC, RA (nucleus robustus arcopallialis) and Area X and increases the PNN localization around PV interneurons. Singing activity was recorded in males and quantitative analyses demonstrated that testosterone also increased male singing rate, song duration and song energy while decreasing song entropy. Together, these data suggest that the development of PNN could provide the synaptic stability required to maintain the stability of the testosterone-induced crystallized song. This provides the new evidence for a role of PNN in the regulation of adult seasonal plasticity in seasonal songbirds.
Keywords: Testosterone, Vocal learning, PNN, Parvalbumin, Song System, Adult Seasonal Plasticity
INTRODUCTION
The acoustic structure of song in songbirds is socially learned during ontogeny (Catchpole and Slater, 2008; Marler and Peters, 1987; Thorpe, 1958; Waser and Marler, 1977). After a period of sensory learning during which the tutor song is memorized as a template, birds start producing unstructured vocalizations similar to human babies’ babbling: the subsong. This is followed by a period of plastic song, which resembles more closely the typical adult song with clearly detectable syllables, but these syllables are still poorly structured and quite variable between successive renditions. In adult birds, song then acquires a precisely defined structure with identifiable and very stable syllables. This is the crystallized song which is used by adult males to attract females and repel competitive males (Brainard and Doupe, 2002; Collins, 2004; Doupe and Kuhl, 1999; Williams, 2004). During adulthood, many aspects of song, including singing motivation, song stereotypy and other aspects of song structure (Alward et al., 2017b; Madison et al., 2015), are modulated by the action of testosterone and its metabolites in the brain (Ball and Balthazart, 2016, 2007; Schlinger and Brenowitz, 2016). Day length along with supplementary factors such as temperature and social context regulate seasonal reproductive cycles in birds (Dawson et al., 2001; Dawson and Sharp, 2007; Nicholls and Storey, 1977). These environmental cues modulate the timing of the endogenous release of testosterone and ultimately song behavior (Nottebohm et al., 1987; Smith et al., 1997a).
In the spring, the progressive increase in day length stimulates the regrowth of the gonads and plasma testosterone concentrations increase. This increase in turn stimulates singing behavior and induces the annual song crystallization (Marler et al., 1988; Schlinger and Brenowitz, 2016). After a long period of photostimulation, during the summer, seasonal songbirds become photorefractory, they molt and their gonads regress (Dawson et al., 2001). This period is associated with a marked decrease of the singing rate and changes in multiple features of song (Ball, 1999; Schlinger and Brenowitz, 2016). In some species, including domesticated canaries, males even stop singing until the end of the molting period. In the fall, birds progressively regain their photosensitivity under the influence of shorter day lengths so they can be photostimulated again at the end of the next winter. These changes in photoperiodic condition are paralleled by brain changes in gonadotropin-releasing hormone gene expression (Stevenson et al., 2012).
During the late summer and fall, some species of songbirds enter in a new period of sensorimotor learning during which their song becomes plastic again (Brainard and Doupe, 2002). Part of the developmental song learning process is thus recapitulated. Whether adult songbirds are able to learn new songs (i.e. have a new sensory phase) or simply modify their song based on the expression of different parts of the template they had acquired during their first year of life may vary widely among species (Beecher and Brenowitz, 2005; Brenowitz and Beecher, 2005). In canaries there are seasonal recapitulations of song even in deafened birds (Mori et al., 2018). Testosterone also appears to induce this sensorimotor learning process in adult female canaries (Rouse and Ball, 2016; Vellema et al., 2019). Birds displaying this form of adult song plasticity are mainly seasonal birds and are called open-ended learners as opposed to close-ended learner species, such as zebra finches (Taeniopygia guttata), that do not change their song at all during adult life. However even among open-ended learners, the plasticity of adult song is variable. Some species, such as the European starling (Sturnus vulgaris), are able as adults to learn new song elements and incorporate them in their repertoire (Böhner et al., 1990), while other species, such as canaries (Serinus canaria), incorporate each year new elements in their song, but we do not known whether birds express on different years a subset of song elements that were learned during early ontogeny or actually acquire new elements as adults (Beecher and Brenowitz, 2005; Brenowitz and Beecher, 2005).
The seasonality of song production and learning is correlated with neural changes occurring mainly between fall and the onset of the breeding season (late winter/early spring) (Brenowitz, 2004; Smith et al., 1997b; Tramontin et al., 2003). Briefly, the vernal increase in testosterone before the onset of the breeding season dramatically changes the morphology of neurons in the song control system (SCS), a set of interconnected brain nuclei that mediates the learning and production of song and that includes HVC (used as a proper name), RA (robust nucleus of the arcopallium) and Area X (Brainard and Doupe, 2002; Mooney, 2009; Nottebohm, 2005) (Tramontin et al., 2001; Tramontin and Brenowitz, 2000). These three nuclei increase in volume by the end of winter and continue to grow into spring. In canaries these seasonal changes in volume can be induced by photoperiodic manipulations that mimic the seasonal states (Hurley et al., 2008). In HVC, the increase in volume is due in part to the recruitment of new neurons and their enhanced survival driven by the increase of testosterone (Alvarez-Buylla and Kirn, 1997; Balthazart et al., 2008; Rasika et al., 1994; Tramontin and Brenowitz, 1999). In RA and Area X, the volume increase is accounted for by an augmentation of neuronal size and spacing partly due to trans-synaptic trophic effects driven by changes in HVC (Brenowitz, 2004; Tramontin and Brenowitz, 2000; Wissman and Brenowitz, 2009). Additionally, in RA, there is an increased spine density directly regulated by testosterone (DeVoogd et al., 1985; Hill and DeVoogd, 1991).
The perineuronal nets (PNN) of the extracellular matrix are another important aspect of the neural plasticity. They have been linked to the closing of the sensitive period for visual learning in mammals, but their role is not well understood in the context of vocal learning. These PNN are aggregations of chondroitin sulfate proteoglycans, tenascin R, hyaluronic acid and binding proteins that form a scaffold, mainly around fast-spiking interneurons expressing parvalbumin (Deepa et al., 2006; Wang and Fawcett, 2012). They are found in large numbers in the somatosensory cortex of the mammalian brain at the end of the sensitive period for sensory learning (Liu et al., 2013; Nakamura et al., 2009). Their expression in the visual cortex of mammals increases during development in an experience-dependent manner following visual stimulation (Liu et al., 2013; Ye and Miao, 2013). Additionally, their degradation in the visual cortex restores some of the associated visual plasticity (Pizzorusso et al., 2002). Their main function is to stabilize the synaptic connectivity by creating a physical barrier preventing new synaptic contacts (Karetko and Skangiel-Kramska, 2009). They also increase the fast-spiking activity of parvalbumin interneurons necessary for a precise timing of neural inhibition (Balmer, 2016) and they regulate ion exchanges (Härtig et al., 1999; Maroto et al., 2013). It is assumed that they play an important role in the closing of sensitive periods for sensory learning by limiting the associated synaptic plasticity (Hensch, 2004; Werker and Hensch, 2014).
Recent studies point out that PNN could also be involved in the closing of the sensitive periods of neural plasticity associated with sensory and/or sensorimotor learning of vocalizations in songbirds. Interestingly, a high density of PNN is found in the SCS of songbirds. It was previously shown that the number of PNN and the proportion of them localized around PV cells increase during developmental song learning in zebra finches, a closed-ended learning species (Balmer et al., 2009; Cornez et al., 2018). PNN densities are also higher in adult zebra finch males compared to females (females do not sing in this species) in the key telencephalic song control nuclei HVC, RA and Area X (Cornez et al., 2015; Meyer et al., 2014).
PNN expression does not seem to vary as a function of the photoperiodic condition in European starlings, a seasonal open-ended learning species, but it varies widely in adult males between species with different learning abilities (Cornez et al., 2017). European starlings express PNN at very low densities in the SCS as compared to zebra finches and it was hypothesized that this might explain the marked behavioral plasticity across seasons in this species (Cornez et al., 2017). It has indeed been clearly demonstrated that male European starlings can learn new songs in adulthood during all phases of their reproductive cycle, even when they are photorefractory (Böhner et al., 1990). In contrast, domesticated canaries, which also display seasonal cycles are able to modify their song each year, but it is not clear whether they actually learn new syllables as adults (Leitner et al., 2001b). Correlatively, canaries have a level of PNN expression in the SCS that is intermediate between European starlings and zebra finches. Canaries may thus provide a better model to explore the role of PNN in vocal learning associated with seasonal fluctuations of testosterone.
In this study, we tested whether the testosterone-induced song crystallization correlates with an increase of PNN expression in the SCS of male canaries. Additionally, a time-course experiment was performed in females to explore how quickly testosterone affects the SCS morphology and, in particular, the development of PNN around PV interneurons. Both studies were performed on adult photosensitive birds that were treated with exogenous testosterone or received a sham treatment and were then exposed to a long-day photoperiod mimicking what happens at the onset of the spring during song crystallization (Marler et al., 1988).
METHODS
Animals
Two-year-old male and female canaries of the Fife fancy breed were obtained from a colony maintained at the University of Antwerp, Belgium. They were born and had gone through a full breeding cycle in this colony. All subjects had been on a natural daylight cycle during the months preceding their arrival in the laboratory. Females (n=58) arrived in January and males (n=11) arrived in April at the University of Liège. Upon arrival, all birds were housed in single-sex collective cages with food and water provided ad libitum. Cuttlebones, anise-scented sand, perches and baths were provided as environmental enrichment. Egg food was provided once a week. All experimental procedures complied with Belgian laws concerning the Protection and Welfare of Animals and the Protection of Experimental Animals, and experimental protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège (Protocol 926).
Experimental Design
Experiment 1: effect of testosterone in adult males
Males arriving in the laboratory during the spring were photosensitive and slightly photostimulated. They were directly transferred to a short-day photoperiod (8L:16D) to retain their photosensitive condition and decrease their gonadal size until the onset of the experiment. All birds were castrated in late October/early November under isoflurane gas anesthesia. In late November each bird was transferred to an individual cage placed within a sound-attenuated recording chamber and his baseline level of singing behavior was recorded for 2 days. Birds were then randomly assigned to a testosterone treatment group (T-treated, n=6) or a control group (controls, n=5) and they received a 10 mm long Silastic™ implant (Dow Corning reference no. 508–004; inner diameter 0.76 mm, outer diameter 1.65 mm) filled with either crystalline testosterone (Fluka Analytical, Sigma-Aldrich) or left empty as a control. Implants were inserted subcutaneously in the back of the birds after being checked under a stereo-microscope to make sure they were completely sealed; they were incubated in 0.9% NaCl at 37°C overnight before being inserted. On the same day, the photoperiod was changed to 16L:8D to optimize the effect of testosterone and a blood sample was taken from the wing vein in the early morning just after the light went on. After these treatments, birds were transferred back to the recording chambers and their singing behavior was recorded daily for 2 hours starting immediately after lights on until brain collection, 24 days later.
Experiment 2: time course of testosterone action in adult females
Upon arrival in January, females were housed on a short-day photoperiod (8L:16D) for at least 11 days. Four to seven days before the beginning of the experiment, a baseline blood sample was collected from the wing vein of each female. Because these birds were used in parallel to study the effects of testosterone (T) on neurogenesis in the song control nucleus HVC (Shevchouk et al., 2017), on day −1 birds were injected, 5 times with 2 hours between each injection, with 100 μl of a 10 mg/ml Bromodeoxyuridine (BrdU) solution dissolved in 0.9% saline with 28 mg/L NaOH (approximately 50 mg/kg for 20 grams birds). Birds were then randomly allocated to one of 4 control groups (6–7 subjects per group) or 4 Testosterone-treated groups (8 subjects per group). At the start of the experiment (day 0), each bird received a 10 mm long Silastic™ implant filled with either crystalline testosterone or left empty as a control (see detail of procedures in the previous section). On the same day, the photoperiod was changed to 14L:10D and birds were moved to individual cages. The brains of all females from one control and one T-treated group were collected 1, 2, 9 or 21 days after receiving the implants. Due to limitations in the number of available housing cages, the experiment was performed in two successive cohorts with experimental manipulations of birds from the longest time points (days 9 and 21) started with a delay of 7 days after birds from the two shortest time points (days 1 and 2). All other aspects of the procedures were identical in the two batches. No song recording was made during this experiment since sound-attenuated boxes and recording equipment used in experiment 1 were not yet available in our laboratory.
All procedures described in the following sections were then similar for both experiments unless otherwise stated.
Blood collection
50–150 μl of blood was collected from the wing vein of all subjects before the initiation of T treatments and on the day of brain collection. Blood collection was always performed within 3 minutes after catching the birds in their cage during the first hour after lights on for males and between 2.5 and 6 hours after lights on for females. The blood was collected into Na-heparinized micropipettes (Hirschmann; 9000250) and any further blood flow was stopped by pressing cotton on the vein puncture after a maximum of 150 μl was collected. Blood was centrifuged at 9000 g for 9 minutes and the supernatant plasma was collected and stored at −80° C until further use.
Testosterone Enzyme Immunoassay
10 μl of plasma from each sample was diluted in 150 μl of ultra-pure water. Recovery samples were spiked with 20,000 CPM of tritiated-testosterone (Perkin-Elmer). All samples were extracted twice with 2 ml of dichloromethane. The organic phase was eluted into clean tubes, dried with nitrogen gas and stored at −20°C until further use. Average recovery was 92.7%.
Extracted samples were re-suspended in 400 μl Enzyme Immunoassay (EIA) buffer by vortexing for 30 seconds and shaking for 90 min at 1350 rpm. Re-suspended samples were either left undiluted or diluted to a total of 1600 μl depending on expected testosterone concentrations, in order to fall within the detection range of the assay curve. They were then assayed for testosterone concentrations using a Cayman Chemicals testosterone EIA kit following manufacturer’s instructions. All male samples were assayed in a single plate in triplicates. The minimum and maximum detection limits of the EIA, as determined by the lowest and highest concentration detected, were 4.07 pg/ml and 522.59 pg/ml respectively. The intra-assay coefficient of variation varied between 0.10 and 10.9% (mean = 3.96%). All female samples were assayed on the same day using 4 separate plates in triplicates. The minimum and maximum detection limits of the EIA were 5.02 pg/ml and 305.39 pg/ml, respectively. The intra-assay variation varied between 6.19 and 8.59% (mean = 7.63%) and the inter-assay variation between plates ranged from 8.96% to 18.47% (mean = 13.97%).
Analysis of male songs
All males from experiment 1 were individually housed in sound-attenuated chambers throughout the experiment and their singing behavior was recorded everyday during 2 consecutive hours starting directly after lights on. Sounds from all chambers were acquired simultaneously via custom-made microphones (microphone from Projects Unlimited/Audio Products Division, amplifier from Maxim Integrated) through an Allen & Heath ICE-16 multichannel recorder connected to a computer. The sound files were 16-bit acquired at a frequency of 44100 Hertz (Hz) which translates to a frequency range of 0–22,050 Hz and saved as 1 minute .wav files sequences using Raven Pro v1.4 software (Bioacoustics Research Program 2011; Raven Pro: Interactive Sound Analysis Software, Version 1.4, Ithaca, NY: The Cornell Lab of Ornithology).
The sound analyses were performed with the same software. The daily 2 hours sound recordings were first reassembled for each channel corresponding to each experimental bird. Spectrogram views of these files were constructed with a direct Fourier transform (DFT) size of 256 samples (172 Hz per sample) and a temporal frame overlap of 50% with a hop size of 128 samples. These parameters were automatically determined by the software to provide an optimized frequency/time resolution for the spectrographic analysis and were identical for all recordings analyzed in the study.
The first hour of recordings of each bird was analyzed in detail for days −2, +3, +10, +17, +24 considering day 0 as the day of implant insertion. One hour of recording was sufficient to obtain at least 240 seconds of songs for each bird (except on the first few days when birds barely started singing), the duration of vocalizations necessary and sufficient to identify the complete repertoire of the canary, as estimated in our preliminary analyses confirming a previously published study (Halle et al., 2003).
Songs were considered as vocalizations of at least one second in duration separated by a gap of at least 0.5 seconds (Alward et al., 2017b, 2013). All songs corresponding to these criteria were manually selected through the entire one hour-long recording and counted. The duration of each song was determined by the software and was averaged for each bird and each day analyzed. This measure was also summed to obtain the total duration (in seconds) of singing during one hour and divided by 3600 to obtain the percentage of time spent singing (% time singing).
One control bird started singing at a low rate one week after the beginning of the experiment, but the other control birds never sang. As observed in previous work on male canaries (Alward et al., 2013; Madison et al., 2015; Sartor et al., 2005; Shevchouk et al., 2018) all T-treated birds sang within one week after the treatment initiation, 4 of them already sang on day 3, but 2 of them already sang at low rate before the beginning of the experiment. The number of songs and the % time spent singing could thus be analyzed for all birds, but it was not possible to compare song quality between T-treated and control males. The 4 testosterone-treated birds that started singing quickly after the T implantation were used to explore the development in time of song parameters defining its quality.
For the analysis of song quality, each individual song was processed through the automated sound analysis of the Raven software and results were averaged for each bird and each day. The derived measures thus refer to entire songs not to individual syllables. The additional automated measurements characterized the song “loudness” (energy in decibels or dB), average and maximum power (dB), root mean squared and maximum amplitude (arbitrary units U), the energy distribution across frequencies (5%, 1st quartile, center, 3rd quartile and 95% frequencies in Hz), the bandwidth (Hz) of this energy distribution between the 1st and 3rd quartile (inter-quartile range bandwidth) and between 5% and 95% (90% bandwidth), the frequency at which the maximum power occurs (maximum frequency in Hz) and the average entropy. The entropy associated with the distribution of power across frequencies was measured at each sampling time point across the entire song and averaged to provide a single measure for each song. These measures were then averaged for the entire recording of the day for each bird and they provided a measure of disorder within the energy distribution.
Brain collection and processing
After the selected duration for each experimental group (males: 24 days; females: 1, 2, 9 or 21 days), subjects were weighed, their cloacal protuberance was measured, a blood sample was taken from the wing vein and then birds were anaesthetized with 0.03 ml of Nembutal. Once reflexes had stopped, birds were intracardially perfused with phosphate-buffered saline (PBS; 1.43 g/L Na2HPO4, 0.48 g/L KH2PO4, 7.2 g/L NaCl) to remove blood, immediately followed by 4% paraformaldehyde (PFA; 4.3 g/L NaOH, 40 g/L paraformaldehyde, 18.8 g/L NaH2PO4.H20) to fix the brain. After perfusion, the brain was immediately extracted from the skull and post-fixed overnight in 15 ml PFA.
The syrinx was extracted and weighed. The presence of the implant was confirmed and the testosterone-filled implants were checked for the presence of remaining hormone inside the implant. On the following day, brains were transferred to 15 ml of 30% sucrose solution (15.6 g/L Na2HPO4, 1.5 g/L KH2PO4, 300g/L sucrose). Once brains had sunk to the bottom of the vial, they were frozen on dry ice and stored at −80°C until used.
The two hemispheres of the male brains were first separated and each hemisphere was cut sagitally in 4 series of 3 wells. Female brains were cut coronally into 4 series of 4 wells. We showed previously that there is no difference in the density of parvalbumin-immunoreactive cells and of perineuronal nets counted in sagittal or coronal sections in the zebra finch brain (Cornez et al., 2017). All brains were cut in 30 μm thick sections on a Leica CM 3050S cryostat and stored in anti-freeze (0.01 M PBS with 10 g/L polyvinyl pyrrolidone, 300 g/L sucrose, and 300 ml/L ethylene glycol) at −20°C.
Immunohistochemistry
Half a series of female sections (2 wells; 240 μm between sections) and a complete series of male sections from both the left and right hemispheres (3 wells: 120 μm between sections) were double-labeled for parvalbumin and chondroitin sulfate, one of the main components of the perineuronal nets, following a previously described protocol (Cornez et al., 2015). Briefly, sections were blocked in 5% Normal Goat Serum (NGS) diluted in Tris-buffered Saline (TBS) with 0.1% Triton-X-100 (TBST) for 30 minutes. They were incubated overnight at 4°C in a mixture of 2 primary antibodies diluted in TBST: a mouse monoclonal anti-chondroitin sulfate antibody (CS-56, 1:500; C8035, Sigma Aldrich) specific for the glycosaminoglycan portion of the chondroitin sulfate proteoglycans that are the main components of the PNN, and a polyclonal rabbit anti-parvalbumin antibody (1:1000; ab11427, Abcam).
On the next day, sections were incubated at room temperature in a mixture of secondary antibodies diluted in TBST. A goat anti-mouse IgG coupled with Alexa488 (green, 1:100, Invitrogen) was used to visualize PNN staining and a goat anti-rabbit IgG coupled with Alexa 546 (red, 1:200, Invitrogen) was used to visualize PV cells. Finally, sections were mounted on slides using TBS with gelatin and coverslipped with Vectashield containing DAPI (H-1500, Vector laboratories) that was used to confirm that PNN that were not surrounding PV-positive cells were localized around a cell nucleus.
The other half series of female brains was double-labeled for parvalbumin and BrdU to test whether the observed increase of PV labeling in HVC resulted from the neurogenesis of new PV cells or simply from an increased expression of the protein in a stable number of neurons. DNA was first denaturated in a bath of 2N HCl solution during 20 minutes at 37°C followed by a bath in 0.1M sodium borate buffer to neutralize the acid. Sections were then blocked in 5% Normal Goat Serum (NGS) diluted in TBST for 30 minutes. They were incubated overnight at 4°C in a mixture of two primary antibodies diluted in TBST: a rat monoclonal anti-BrdU antibody (1:300; OBT0030, ABD Serotec) and a polyclonal rabbit anti-parvalbumin antibody (1:1000; ab11427, Abcam). Sections were then incubated at room temperature in a mixture of secondary antibodies diluted in TBST. A goat anti-rat IgG coupled with Alexa488 (green, 1:100, Invitrogen) was used to visualize BrdU staining and a goat anti-rabbit IgG coupled with Alexa 546 (red, 1:200, Invitrogen) was used to visualize PV cells. Finally, sections were mounted on slides using TBS with gelatin and coverslipped with Vectashield containing DAPI (H-1500, Vector laboratories).
Measure of nuclei volume
The dense parvalbumin and chondroitin sulfate staining was used to quantify the volume of HVC, RA and Area X (see examples in Fig. 5). Photomicrographs of all stained sections containing at least one of these nuclei were acquired at 5X magnification (recorded field equal to 1.43 by 1.92 mm) and the volume of these nuclei was quantified as previously described (Cornez et al., 2017). First, the area of the Regions of Interest (ROIs in mm2) within each section was measured using Image J (NIH, https://imagej.nih/ij). The volume of each ROI was then estimated by multiplying the measured surface in each section by the distance between sections (240 μm for females and 120 μm for males) and then summing the results for all the sections. Finally, the mean of volumes in the left and right hemispheres was calculated. The volume of HVC and RA was also measured using a Hu staining performed in another series of sections for the purpose of a parallel previously published experiment (Shevchouk et al., 2017). The absolute values of these volumes slightly differ between these two methods, but are significantly correlated (HVC: Pearson R=0.766, p<0.001; RA: Pearson R= 0.900, p<0.001) and the pattern of result is similar.
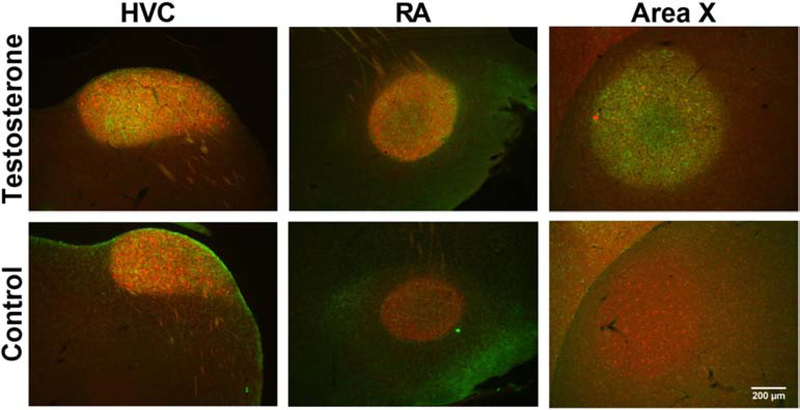
Figure 5.
Representative photomicrographs at low magnification illustrating the specific labeling of HVC, RA and Area X by the dense clusters of PV and PNN and the increased volume of these nuclei in testosterone treated males by comparison with control birds who received empty Silastic™ implants.
PNN & PV quantification
The numbers of PV-positive cells (PV), of cells surrounded by PNN (PNN) and of PV-positive cells surrounded by PNN (see examples in Fig. 8) were counted in photomicrographs of the 3 song control nuclei HVC, RA and Area X on both brain sides of all birds. The boundaries of the ROIs were determined based on the bright PV and/or PNN staining. In each bird, two photomicrographs were acquired on each brain side in 2 sections equally spaced in the rostrocaudal axis for each ROI. These 4 photomicrographs documenting each nucleus were obtained with a Leica fluorescence microscope with a 40X objective (recorded field equal to 0.18 × 0.24 mm) and fixed settings. The numbers of PV, of PNN and of PV-positive cells surrounded by PNN (PV+PNN) were counted in all photomicrographs with Image J software (NIH, https://imagej.nih/ij) as previously described (Cornez et al., 2017).
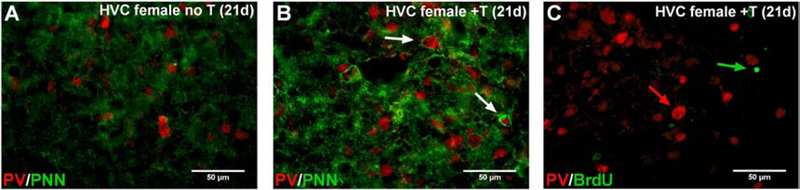
Figure 8.
(A-B) Representative PV and PNN co-labeling in the brain of control (A) and T-treated (B) females 21 days after the implants. PV-expressing cells appear in red, PNN appear in green. (C) PV and BrdU labeling in the brain of a T-treated female 21 days after the implantation, showing no colocalization of the two labeling. PV-expressing cells appear in red, BrdU-positive cell nuclei appear in green.
Each photomicrograph contained only the ROIs, so that quantifying the entire image always sampled a similar area. For each ROI, the mean of left and right data for each section was calculated and this result was then averaged between sections to obtain the number of stained structures per counted surface in a given ROI. These numbers were converted in densities/mm2 and also used to compute the % PV surrounded by PNN (%PVwithPNN) and the % PNN surrounding PV (%PNNwithPV). Finally, the volume of each nucleus of each bird was used to estimate the number of counted objects in the entire nuclei using the following formula: (number of counted object)*(nuclei volume/(counted area*section thickness))mm3. This allowed us to obtain the total number of PV, PNN and PV+PNN per nucleus.
BrdU & PV quantifications
The quantification of PV and BrdU positive cells were made the same way in HVC only because this is the only song control nucleus where adult neurogenesis is present and changing seasonally. The number of PV+, BrdU+ and PV+BrdU+ cells were quantified to calculate the % of PV cells colocalized with BrdU.
Statistics
In males PNN, PV and nuclei volumes were quantified separately in the two hemispheres and each set of data was analyzed by a 2-Way repeated measures ANOVAs to explore the possibility of lateralization and the effects of testosterone. Only 2 effects with p<0.05 were detected (one main effect and one interaction) out of 54 results ([3 total counts + 5 densities or ratio+1 volume per nucleus] X 3 nuclei with a main and an interaction effect in each case), which is essentially below what would be expect by chance (5 effects out of 100 tests). These results are therefore no discussed further. In females we could not reliably identify the brain side in all sections. Therefore all structures were quantified in both hemispheres but average results for both sides were calculated and used in all analyses presented in this paper.
In males, testosterone concentration in the blood before treatment and at brain collection in both groups was analyzed using a 2-Way repeated measure ANOVA (time*treatment). The song numbers and % time singing on days −2, +3, +10, +17, +24 in all birds were analyzed with a 2-Way Repeated measure ANOVA (time*treatment). The other song parameters quantified at days +3, +10, +17, +24 only in the 4 testosterone-treated males that sang regularly after day 3 were analyzed with a 1-Way repeated measure ANOVA. Significant interactions and time effects were analyzed using Tukey post-hoc tests. The syrinx mass, cloacal protuberance area and the brain measurements differences between groups were analyzed with unpaired t-tests.
In females, testosterone and morphological measures were already reported in another publication focused on neurogenesis and plasticity in song control nuclei (Shevchouk et al 2017) and will only be briefly summarized here. The densities and total numbers of PV, PNN and BrdU alone or combined were analyzed with a 2-Way ANOVA (brain collection day*treatment). Significant interactions were analyzed using Tukey post-hoc tests.
Differences between groups and effects in the ANOVA were considered significant for p<0.05. All data are summarized by their mean and standard error of the mean (SEM) but individual data points are also presented in most graphs. Effect sizes are presented as Cohen’s d for comparisons between 2 groups and partial eta square (ηp2) for two factors ANOVAs.
RESULTS
Experiment 1: effects of testosterone in adult males
The treatment of castrated males with testosterone for 24 days induced profound changes in physiology, singing behavior and PNN expression in the brain.
Physiological and morphological changes
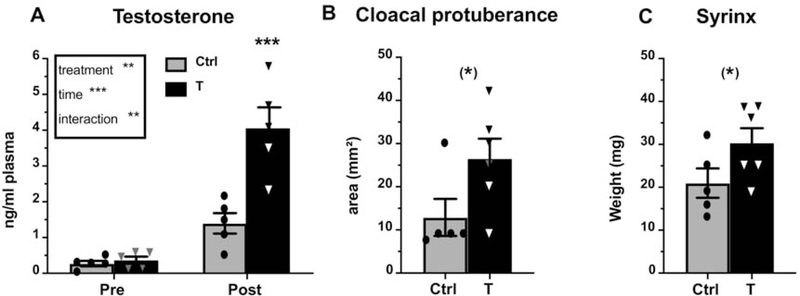
The testosterone plasma concentration increased following T treatment and the ANOVA of these data identified a significant main effect of treatment (F1,8=23.11, p<0.01, ηp2=0.74) and time (F1,8=43.70, p<0.001, ηp2=0.85) as well as a significant interaction between these two factors (F1,8=12.37, p<0.01, ηp2=0.61). The post-hoc tests confirmed that the T implants combined with the photoperiodic change had increased the plasma testosterone concentration by the end of the experiment (Fig. 1A; T post vs. T pre: p=0.0006, DF=16, d=1.73; T post vs. Control post: p=0.0004, DF=16, d=1.57). This measure did not differ between the two groups before the treatment (p=0.99, DF=16, d=0.52) nor within the control group before and after the treatment (p=0.21, DF=16, d=1.52), suggesting that photostimulation alone was not sufficient to significantly increase testosterone concentrations in castrated males. Additionally, the cloacal protuberance area, an androgen-dependent structure, was numerically larger in the T-treated birds although the effect was not significant (Fig. 1B, t9=2.117, p=0.063, d=1.10). Finally, the syrinx weight was also numerically larger in T-treated birds at the end of the experiment (Fig. 1C, t9=1.905, p=0.089, d=1.03). In both cases the effect did not reach statistical significance but the large effect size suggests the existence of an effect that would be worth analyzing in future work.
Figure 1.
(A) Plasma testosterone concentration (ng/ml) before the treatment (Pre) and at brain collection (Post) as well as cloacal protuberance size (B) and syrinx weight (C) at brain collection in control (light grey) and T-treated (black) castrated male canaries. Results of the 2-Way repeated measures ANOVA are summarized in the insert in panel A. Significant differences between treatments at each time point as determined by Tukey post hocs (panel A) or by unpaired t-test (panels B and C) are indicated above the corresponding bars. (*) =p<0.10, **= p<0.01, ***= p<0.001.
Testosterone increases singing motivation and song quality and decreases entropy
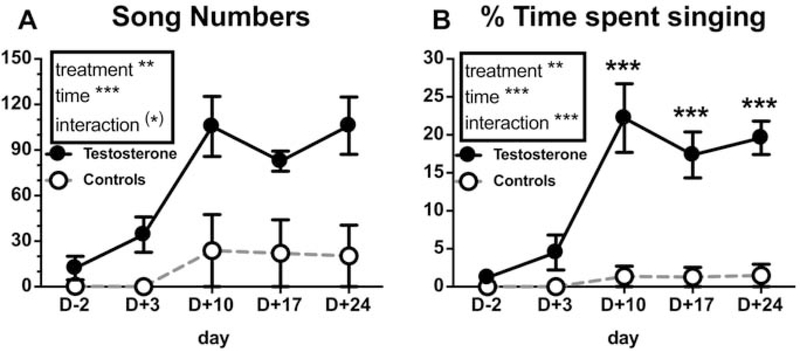
Exogenous testosterone progressively increased the singing motivation as measured by the number of songs produced (Fig. 2A; treatment: F1,8=19.13, p<0.01, ηp2=0.71; time: F4,32=7.05, p<0.001, ηp2=0.65; interaction: F4,32=2.37, p=0.073, ηp2=0.39) and the % time spent singing (Fig. 2B; treatment: F1,8=20.09, p<0.01, ηp2=0.72; time: F4,32=13.88, p<0.001, ηp2=0.59; interaction: F4,32 =10.16, p<0.001, ηp2=0.51) while little or no change was detected in controls. Post-hoc tests of the main time effect revealed that the number of songs and the % time spent singing were significantly increased as compared with the pre-experimental values starting on day 10 post-treatment (Song number: D-2 vs. D10 p<0.001, d=1.18, vs. D17 p<0.01, d=1.26, vs. D24 p<0.001, d=1.18; % time singing: D-2 vs. D10 p<0.0001, d=1.30, vs. D17 p<0.0001, d=1.65, vs. D24 p<0.0001, d=1.24, DF=40 for all comparisons). Interaction post-hoc tests showed that T-treated birds spent a higher proportion of time singing compared to controls from day 10 post-treatment onward (see Fig. 2B, DF=32, d= 1.53–1.75).
Figure 2.
(A) Number of songs produced and (B) percentage of time spent singing during the first hour of recording in control (dotted line; only one bird sang at low rate, others had zero scores) and T-treated (continuous line) castrated male canaries at days −2, +3, +10, +17 and +24 (day 0 being the day of implant insertion). Results of the 2-Way repeated measures ANOVA are summarized in the inserts. When the interaction is significant, significant difference between treatments within a given time point as determined by Tukey post-hoc tests is shown. Statistical significance is indicated as follows: (*) p<0.10, ** p<0.01, *** p<0.001.
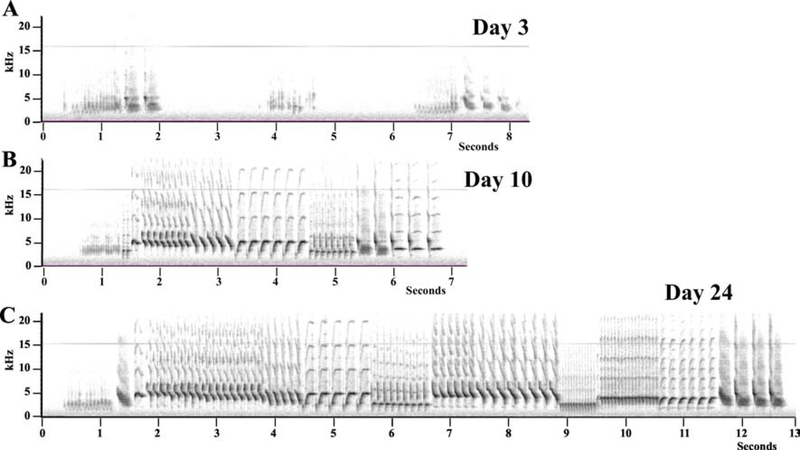
Only 4 T-treated birds sang sufficiently after 3 days of treatment to allow an analysis of changes in time of their song quality. Representative spectrograms of the song produced by one of these males at three different time points during the activation by testosterone are shown in figure 3.
Figure 3.
Representative spectrograms of the songs produced by a same testosterone-treated male after 3, 10 and 24 days of exposure to the steroid. Panel A shows three very short songs (1–2 sec) produced after only 3 days of exposure to testosterone by a male who was not signing at all before. Panels B and C illustrate the fact that already after 10 days complex well-structured songs are produced by the castrated males treated with testosterone.
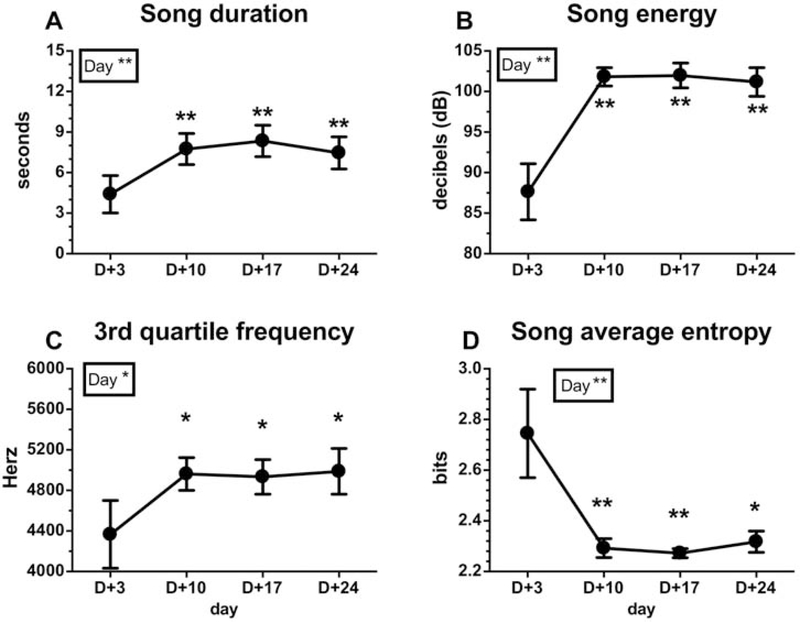
Analysis of the song parameters of these 4 birds revealed a global increase of song quality and a decrease in entropy over time. The song duration increased significantly with time (Fig. 4A; F3,9=11.84, p<0.01, ηp2=0.80). Post-hoc tests revealed that T-treated birds sang significantly longer songs from day 10 until day 24 compared to day 3 post-treatment (Fig. 4A, DF=9, d=1.06–1.31). T also induced an overall increase of the song loudness as revealed by a significant increase over time of the song energy (Fig. 4B; F3,9=12.99, p<0.01, ηp2=0.81) as well as average power, maximum power, RMS amplitude and maximum amplitude (Table 1). The post-hoc tests revealed again that all these measures were significantly higher on day 10 and subsequently than on day 3 (see Table 1 and Fig. 4B, DF=9, d=1.53–1.74). Additionally, the energy distribution across frequencies (center frequency, frequency 5%, frequency 95%, 1st quartile frequency, maximum frequency (Table 1) and 3rd quartile frequency (Fig. 4C)) increased with time after the testosterone treatment. There was a main effect of time or a tendency for all these measurements to vary with time (Table 1 and Fig. 4C; F=5.44, p<0.05, DF=3, ηp2=0.64). The 3rd quartile frequency was significantly larger on day 10 and subsequently than on day 3 as confirmed by post-hoc tests (Fig. 4C, DF=9, d=0.98–1.03). Together these measures indicated a displacement of the song energy distribution towards higher frequencies, although there was no change in the song bandwidth as revealed by the inter-quartile range bandwidth and the 90 % bandwidth (Table 1; see methods for the definition of all these measures). Finally, the average song entropy, a measure of disorder in song structure, decreased significantly with time (Fig. 4D; F3,9=9.12, p<0.01, ηp2=0.75), with post-hoc tests revealing again a significant change occurring on day 10 and after (Fig. 4D, DF=9, d=1.30–1.38).
Figure 4.
(A) Song duration (seconds), (B) song energy (dB), (C) 3rd Quartile frequency (Hz) and (D) song average entropy (bits) at days +3, +10, +17 and +24 in 4 T-treated castrated male canaries that had started singing 3 days after the treatment. These song characteristics could obviously not be evaluated in control males that did not sing, with only one exception. The results of the repeated measure ANOVA are indicated in the insert. When the ANOVA is significant, significant differences with day +3 as determined by Tukey post hoc are shown. Statistical significance is indicated as follows: * p<0.05, ** p<0.01.
Table 1:
Changes with time of song parameters in 4 T-treated male canaries that started singing 3 days after the beginning of the treatment.
| Singing behavior | statistics (F) | Effect size | ||||||
|---|---|---|---|---|---|---|---|---|
| MALES + T (n=4) | H+3 | H+10 | H+17 | H+24 | HF | hay | ηp2 | |
| 1st quartile frequency (Hz) | 3408.90 ± 238.59 | 3972.00 ± 174.84 | 3938.09 ± 158.34 | 3959.94 ± 174.67 | 3,9 | 4.599* | 0.61 | |
| 5% frequency (Hz) | 2678.48 ± 211.49 | 3151.92 ± 134.95 | 3104.71 ± 116.64 | 3110.98 ± 117.10 | 3,9 | 3.041(*) | 0.50 | |
| 95% frequency (Hz) | 5144.30 ± 379.10 | 5657.25 ± 269.51 | 5704.06 ± 282.06 | 5804.64 ± 299.91a | 3,9 | 4.970* | 0.62 | |
| Center frequency (Hz) | 3885.87 ± 288.02 | 4471.15 ± 160.92 | 4443.06 ± 163.51 | 4504.88 ± 199.00 | 3,9 | 4.365* | 0.59 | |
| Maximum frequency (Hz) | 4076.81 ± 247.94 | 4600.31 ± 150.85 | 4517.86 ± 102.38 | 4630.25 ± 124.46 | 3,9 | 3.375(*) | 0.53 | |
| IQR bandwidth (Hz) | 957.58 ± 108.04 | 990.28 ± 109.09 | 995.41 ± 70.72 | 1027.85 ± 98.30 | 3,9 | 0.192 | 0.06 | |
| 90% bandwidth (Hz) | 2465.81 ± 261.59 | 2505.31 ± 263.77 | 2599.33 ± 185.48 | 2693.64 ± 226.66 | 3,9 | 0.584 | 0.16 | |
| Average power (dB) | 39.04 ± 2.20 | 50.08 ± 0.75aa | 50.10 ± 0.96aa | 49.72 ± 1.13aa | 3,9 | 14.690*** | 0.83 | |
| Maximum power (dB) | 71.80 ± 2.15 | 81.93 ± 0.70aa | 82.06 ± 0.96aa | 81.39 ± 1.15aa | 3,9 | 13.700** | 0.82 | |
| RMS amplitude (U) | 76.91 ± 15.65 | 236.71 ± 20.59aaa | 242.19 ± 24.27aaa | 236.98 ± 29.44aaa | 3,9 | 21.42*** | 0.88 | |
| Maximum amplitude (U) | 681.08 ± 117.49 | 1877.33 ± 162.70aaa | 1973.63 ± 214.13aaa | 1920.89 ± 256.68aaa | 3,9 | 20.62*** | 0.87 | |
The mean ± SEM of each measure is presented for each time point. Last columns indicate the statistical results (F) of the one-way repeated measures ANOVA analyzing changes in time for each song parameter, the corresponding degrees of freedom (DF) and effect sizes.
p<0.10
p<0.05
p<0.01
p<0.001
Significant differences compared to day 3 revealed by the Tukey post hoc tests are indicated in bold with a p<0.05, aa p<0.01, aaa p<0.001.
Testosterone increases the number of PNN around PV interneurons in the SCS
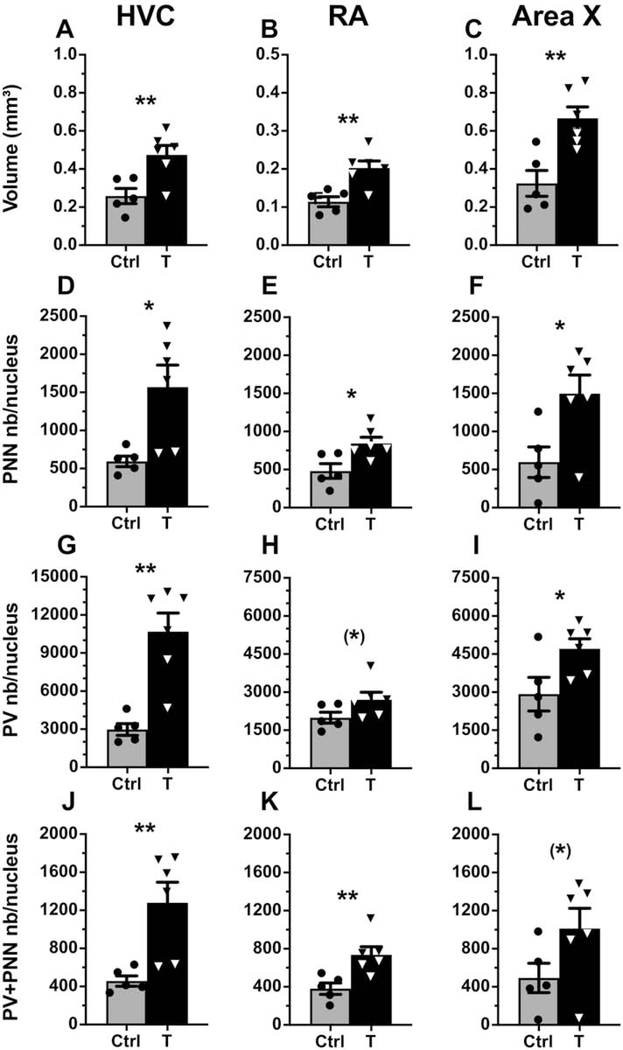
As shown in previous studies, systemic T treatment significantly increased the volume of the 3 song control nuclei (HVC: t9=3.25, p=<0.01, d=1.41; RA: t9=3.68, p=<0.01, d=1.48; Area X: t9=3.77, p=<0.01, d=1.50) as compared to controls (see representative photomicrographs in Fig. 5 and their quantification in Fig. 6A–C).
Figure 6.
(A-C) Nucleus volume (mm3), (D-F) total number of PNN/nucleus, (G-I) total number of PV/nucleus and (J-L) total number of PV+PNN/nucleus in HVC, RA and Area X of control (light grey) and T-treated (black) male canaries. Significant differences between groups identified by unpaired T-tests are indicated as follows: (*) p<0.10, * p<0.05, ** p<0.01.
The total number of PNN in these 3 song control nuclei increased significantly with the testosterone treatment (Fig. 6D–F; HVC: t9=2.983, p<0.05, d=1.35; RA: t9=2.911, p<0.05, d=1.33; Area X: t9=2.760, p<0.05, d=1.30). Testosterone also increased the number of parvalbumin-expressing neurons significantly in HVC and Area X and a non significant statistical trend in the same direction, still associated with a large effect size (d=1.00), was observed in RA (Fig. 6G–I; HVC: t9=4.597, p<0.01, d=1.60; RA: t9=1.840, p=0.099, d=1.00; Area X: t9=2.408, p<0.05, d=1.99). Additionally, the number of PV-positive neurons surrounded by PNN (PV+PNN) increased in HVC and RA and a non significant change in the same direction was observed in Area X (Fig. 6J–L; HVC: t9=3.366, p<0.01, d=1.43; RA: t9=3.302, p<0.01, d=1.42; Area X: t9=1.902, p=0.090, d=1.03). Although smaller than in the other two nuclei, the effect size of the increase was still large in Area X.
In HVC, the density (number/mm2) of PV interneurons increased significantly in T-treated birds, the overall density of PNN did not change, but the density of PNN surrounding PV (PV+PNN) increased significantly (Table 2). This demonstrates that the increase in the total number of PV and PV+PNN cells in HVC is proportionally larger than the increase in volume of the nucleus. In contrast, in RA, the density of PV cells decreased significantly, so that the nearly significant overall increase in total number is presumably accompanied by an increased cell spacing (Table 2). In Area X, no changes in densities were observed suggesting that the overall increase in PV, PNN and PV+PNN total numbers is simply proportional to the increase in volume of the nucleus (Table 2).
Table 2:
Densities of PV, PNN, PV+PNN, % PV with PNN and % PNN around PV in HVC, RA and Area X of male canaries.
| H+24 | statistics (t) | Effect size | |||
|---|---|---|---|---|---|
| MALES | Ctrl (n=5) | T (n=6) | treatment | DF | Cohen’s d |
| HVC | |||||
| PNN density (/mm2) | 71.98 ± 6.66 | 96.75 ± 12.84 | 1.61 | 9 | 0.90 |
| PV density (/mm2) | 354.49 ± 34.89 | 665.63 ± 46.40 | 5.170*** | 9 | 1.66 |
| PV+PNN density (/mm2) | 54.95 ± 3.95 | 79.33 ± 8.81 | 2.351* | 9 | 1.18 |
| % PV with PNN | 16.00 ± 1.62 | 12.47 ± 1.98 | −1.34 | 9 | 0.78 |
| % PNN with PV | 78.12 ± 6.82 | 83.12 ± 2.31 | 0.75 | 9 | 0.46 |
| RA | |||||
| PNN density (/mm2) | 138.54 ± 37.87 | 129.00 ± 13.94 | −0.255 | 9 | 0.16 |
| PV density (/mm2) | 534.05 ± 40.68 | 402.47 ± 25.16 | −2.856* | 9 | 1.32 |
| PV+PNN density (/mm2) | 109.13 ± 27.13 | 112.23 ± 13.33 | 0.108 | 9 | 0.07 |
| % PV with PNN | 21.02 ± 5.17 | 28.38 ± 3.45 | 1.22 | 9 | 0.72 |
| % PNN with PV | 82.52 ± 5.34 | 86.79 ± 2.79 | 0.747 | 9 | 0.46 |
| Area X | |||||
| PNN density (/mm2) | 49.54 ± 10.84 | 68.37 ± 13.09 | 1.078 | 9 | 0.65 |
| PV density (/mm2) | 267.03 ± 23.28 | 214.78 ± 14.85 | −1.959 | 9 | 1.05 |
| PV+PNN density (/mm2) | 41.80 ± 8.69 | 46.44 ± 11.65 | 0.308 | 9 | 1.96 |
| % PV with PNN | 14.99 ± 2.85 | 21.75 ± 5.37 | 1.045 | 9 | 0.63 |
| % PNN with PV | 87.70 ± 5.32 | 61.55 ± 10.57 | −2.069 | 9 | 1.09 |
The mean ± SEM of each measure is presented for each group. The last column indicates the statistical value (t) of the unpaired T-test comparison between groups for each brain measurement analyzed, the corresponding degrees of freedom (DF) and effect sizes.
p<0.10
p<0.05
p<0.01
p<0.001.
The patterns of changes in total numbers of PNN, PV and PV+PNN numbers as observed in figure 6 were very similar, suggesting that the PNN increase mainly reflected their development around an increasing number of PV interneurons. This was confirmed by the absence of difference between groups in the percentage of PV surrounded by PNN in the 3 nuclei and the high percentage of PNN surrounding PV interneurons in both groups in HVC and RA (Table 2, between 78% and 86%). Nevertheless, the lower, but non-significant, % PNN around PV in Area X of T-treated birds (Table 2, controls: 87.7%, testosterone: 61.55%) suggests that some PNN develop following T treatment around other cell types specifically in Area X.
Experiment 2: time course of testosterone action in adult females
Females treated with testosterone for various durations (1, 2, 9 or 21 days) also experienced major changes in physiology and in the expression of PNN in the song control nuclei.
Physiological and morphological changes after testosterone treatment
The brain sections used here came from birds previously used to analyze the effects of T on the plasticity in HVC and compare it to the plasticity in the preoptic area (Shevchouk et al., 2017). As previously reported plasma testosterone concentration rapidly increased in T-treated females. There was also a minor and transient increase in plasma testosterone in the control females presumably resulting from the transfer of birds to a long day photoperiod.
The cloacal protuberance length and the syrinx weight, that are both androgen-dependent, also increased with time in T-treated females (see (Shevchouk et al., 2017) for details). Interestingly these structures also displayed a moderate increase in the control females again suggesting an effect of photostimulation alone.
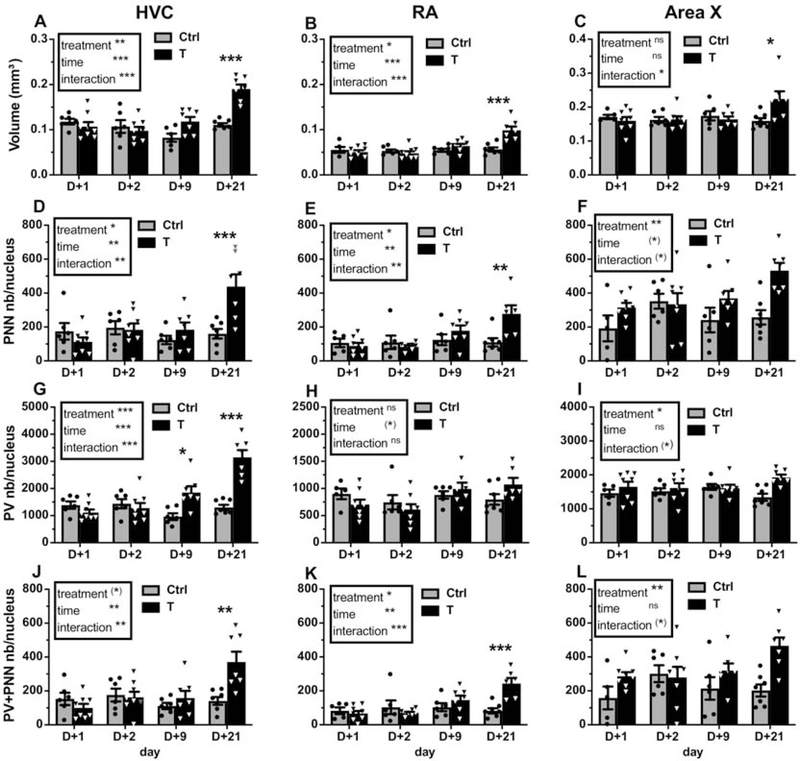
The previously published volumes of HVC, RA and Area X are illustrated here again in figure 7A–C since these data were used to compute the total numbers of PNN and PV-positive cells. There was a main effect of treatment and time and a significant interaction between these factors in the analysis of the volumes of HVC (Fig.7A; treatment: F1,47=12.2, p<0.01, ηp2=0.21; time: F3,47=12.09, p<0.001, ηp2=0.44; interaction: F3,47=10.32, p<0.001, ηp2=0.40) and RA (Fig. 7B; treatment: F1,45=6.75, p<0.05, ηp2=0.13; time: F3,45=9.81, p<0.001, ηp2=0.40; interaction: F3,45=8.06, p<0.001, ηp2=0.35). In both nuclei the volume was significantly higher on day 21 compared to the other time points (p<0.001 for all comparisons except day 21 vs. day 9 in RA: p<0.05). In Area X, there was no main effect of treatment and time, but there was an interaction between these factors (Fig. 7C; F3,46=4.21, p<0.05, ηp2=0.22). The post-hoc analyses of these interactions confirmed that the volume of HVC, RA and Area X was significantly increased in T-treated females compared to control females at day 21 only (Fig. 7A–C).
Figure 7.
(A-C) Volume of song control nuclei (mm2), (D-F) total number of PNN/nucleus, (G-I) total number of PV/nucleus and (J-L) total number of PV+PNN/nucleus in HVC, RA and Area X of control (light grey) and T-treated (black) female canaries. Results of the 2-Way ANOVA of these data are summarized in the inserts. Significant interactions were further analyzed by Tukey post-hoc tests whose results are indicated as follows: (*) p<0.10, * p<0.05, ** p<0.01, *** p<0.001 by comparison with the control group at the same time point.
Testosterone increases PV and PNN expression in the female song control nuclei
Testosterone increased the total numbers of PV-positive neurons (Fig. 7G; treatment: F1,47=20.93, p<0.001, ηp2=0.31; time: F3,47=13.01, p<0.001, ηp2=0.45; interaction: F3,47=16.04, p<0.001, ηp2=0.51) and of PNN (Fig. 7D; treatment: F1,47=4.82, p<0.05, ηp2=0.09; time: F3,47=5.69, p<0.01, ηp2=0.27; interaction: F3,47=6.36, p<0.01, ηp2=0.29) in the female HVC as it did in males. The availability of results at different time points revealed however a difference in the rate of development of PV and of PNN. Post-hoc analyses of the interactions indeed supported the conclusion that the number of PV-expressing neurons had already increased significantly as compared with control birds after 9 days of treatment with T, whereas a significant increase in PNN numbers was only observed after 21 days of treatment (see details in Fig. 7D and G). In parallel, the number of PV+PNN in HVC increased with time, but only became significantly different between groups on day 21 (Fig. 7J; treatment: F1,47=3.95, p=0.053, ηp2=0.07; time: F3,47=5.14, p<0.01, ηp2=0.25; interaction: F3,47=5.78, p<0.01, ηp2=0.27, see figure 8A–B for representative photomicrographs). There was, as observed in males, no change in the % PV with PNN (Table 3), suggesting that the increase of PNN and PV+PNN numbers specifically reflects the development of PNN around the PV interneurons that start developing after 9 days of treatment.
Table 3:
Changes in time of the densities of PV, PNN, PV+PNN, % PV with PNN and % PNN around PV in HVC, RA and Area X of female canaries treated or not with testosterone.
| D+1 | D+2 | D+9 | D+21 | statistics (F value) - effect size (ηp2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FEMALES | Ctrl | T | Ctrl | T | Ctrl | T | Ctrl | T | Treatment (DF=1,49) | Time (DF=3,49) | Interaction (DF=3,49) |
| HVC | |||||||||||
| PNN density (/mm2) | 43.5 ± 10.2 | 31.2 ± 6.3 | 55.1 ± 9.8 | 56.6 ± 10.3 | 44.8 ± 6.545 | 50.1 ± 8.8 | 43.1 ± 7.3 | 71.8 ± 9.4 | 0.87 (0.02) | 2.18 (0.12) | 1.92 (0.10) |
| PV density (/mm2) | 358.9 ± 39.1 | 315.6 ± 16.6 | 412.1 ± 36.1 | 395.5 ± 46.5 | 325.9 ± 43.4 | 489.1 ± 38.16* | 354.1 ± 28.4 | 483.3 ± 18.9 | 5.53* (0.10) | 2.18 (0.11) | 4.37** (0.21) |
| PV+PNN density (/mm2) | 38.7 ± 8.0 | 27.6 ± 5.8 | 48.4 ± 7.6 | 50.1 ± 8.8 | 41.5 ± 5.143 | 44.3 ± 9.4 | 38.1 ± 6.2 | 55.9 ± 6.5 | 0.28 (0.01) | 1.77 (0.10) | 1.26 (0.07) |
| % PV with PNN | 12.2 ± 3.2 | 9.4 ± 2.3 | 12.7 ± 2.5 | 14.7 ± 3.0 | 14.9 ± 3.4 | 8.6 ± 1.4 | 11.5 ± 2.3 | 11.5 ± 1.2 | 1.03 (0.02) | 0.50 (0.03) | 1.08 (0.06) |
| % PNN with PV | 87.2 ± 8.6 | 90.9 ± 6.1 | 91.7 ± 7.0 | 90.7 ± 5.7 | 94.3 ± 3.7 | 86.6 ± 6.6 | 90.6 ± 3.6 | 81.9 ± 6.8 | 0.60 (0.01) | 0.25 (0.02) | 0.45 (0.03) |
| RA | |||||||||||
| PNN density (/mm2) | 50.3 ± 10.5 | 49.3 ± 8.2 | 59.0 ± 18.4 | 55.1 ± 7.6 | 58.9 ± 17.5 | 90.0 ± 14.9 | 57.2 ± 10.0 | 83.8 ± 9.9 | 2.22 (0.04) | 1.69 (0.09) | 1.06 (0.06) |
| PV density (/mm2) | 474.1 ± 29.1 | 412.1 ± 30.3 | 417.9 ± 56.6 | 382.4 ± 53.0 | 444.5 ± 43.6 | 486.2 ± 42.8 | 429.6 ± 53.2 | 349.9 ± 25.6 | 1.2 (0.02) | 1.35 (0.08) | 0.78 (0.05) |
| PV+PNN density (/mm2) | 35.8 ± 10.0 | 37.0 ± 7.1 | 55.1 ± 18.9 | 46.4 ± 6.1 | 48.9 ± 13.4 | 72.6 ± 11.7 | 43.9 ± 7.0 | 75.5 ± 6.3 | 2.61 (0.05) | 2.32(*) (0.12) | 1.61 (0.09) |
| % PV with PNN | 7.2 ± 1.9 | 8.8 ± 1.7 | 12.1 ± 2.5 | 12.9 ± 1.6 | 10.0 ± 2.6 | 15.1 ± 2.2 | 11.0 ± 2.2aa | 22.4 ± 2.9aa | 8.91** (0.15) | 4.95** (0.23) | 2.32 (0.12) |
| % PNN with PV | 65.7 ± 14.5 | 71.1 ± 11.2 | 92.5 ± 4.8a | 86.5 ± 5.1a | 85.1 ± 3.5 | 82.8 ± 4.6 | 77.5 ± 6.1 | 92.3 ± 3.7 | 0.31 (0.01) | 2.90* (0.15) | 0.73 (0.04) |
| Area X | |||||||||||
| PNN density (/mm2) | 33.7 ± 13.3 | 61.7 ± 6.8 | 65.8 ± 9.1 | 60.2 ± 11.5 | 43.9 ± 20.7 | 69.7 ± 7.1 | 48.1 ± 6.7 | 68.2 ± 6.7 | 7.06* (0.13) | 0.92 (0.05) | 1.42 (0.08) |
| PV density (/mm2) | 257.7 ± 21.0 | 309.8 ± 16.6 | 280.6 ± 6.3 | 296.0 ± 23.7 | 292.7 ± 16.6 | 298.2 ± 8.8 | 255.4 ± 20.3 | 273. 6 ± 18.2 | 3.26 (0.06) | 1.18 (0.07) | 0.60 (0.04) |
| PV+PNN density (/mm2) | 27.9 ± 11.7 | 55.9 ± 6.6 | 56.1 ± 9.3 | 50.1 ± 11.3 | 39.8 ± 10.2 | 61.7 ± 7.2 | 38.1 ± 6.2 | 60.2 ± 6.5 | 7.08* (0.13) | 0.56 (0.03) | 1.49 (0.08) |
| % PV with PNN | 12.5 ± 6.0 | 18.6 ± 2.6 | 19.8 ± 3.0 | 16.0 ± 3.2 | 13.1 ± 3.1 | 20.7 ± 2.5 | 15.0 ± 2.3 | 22.6 ± 2.7 | 3.95 (0.07) | 0.38(*) (0.02) | 1.57 (0.09) |
| % PNN with PV | 76.4 ± 8.8 | 91.4 ± 4.4 | 84.1 ± 6.7 | 76.4 ± 11.4 | 89.0 ± 6.9 | 87.6 ± 4.5 | 77.5 ± 4.2 | 88.6 ± 4.5 | 0.74 (0.02) | 0.49 (0.03) | 1.11 (0.06) |
The mean ± SEM of each measure is presented for each time point and each group. The last column indicates the statistical value (F) of the two-way ANOVA for the main effects of treatment, time and interaction for each brain measure, the degrees of freedom (DF) and effects sizes in parentheses. Significant differences between treatment groups within a specific time point as revealed by Tukey post hoc tests are shown in bold.
p<0.10
p<0.05
p<0.01
p<0.001.
Significant differences compared to day 1 following post hoc analysis of the time effect are shown in bold with a p<0.05, aa p<0.01.
We also analyzed the density of PV, PNN and PV+PNN in HVC. There was no significant change in the PNN and PV+PNN density (Table 3). These values numerically increased on day 21, but the increase was not sufficient to compensate for the increase in the total volume of the nucleus. The overall density of PV interneurons in HVC was significantly higher in T-treated birds towards the end of the experiment, but this difference did not change significantly over time, although there was a significant interaction of time with treatment (Table 3). The post-hoc analyses identified a significant difference between groups on day 9 only (p<0.05) that was no longer significant on day 21 suggesting that the increase in volume occurring at that time compensated the increase in PV density occurring at day 9. Finally the % PNN surrounding PV was not affected by time or treatment and was higher than 80% in all groups (Table 3) confirming that a majority of PNN develops around PV interneurons in HVC.
In RA, the total number of PNN (Fig. 7E) and of PV+PNN (Fig. 7K) was also increased by the T-treatment (PNN: F1,45=4.38, p<0.05, ηp2=0.09; PV+PNN: F1,45=5.33, p<0.05, ηp2=0.11) and they also significantly changed with time (PNN: F3,45=5.02, p<0.01, ηp2=0.25; PV+PNN: F3,45=5.74, p<0.01, ηp2=0.28). There was in addition a significant interaction between these two factors (PNN: F3,45=4.60, p<0.01, ηp2=0.23; PV+PNN: F3,45=6.99, p<0.001, ηp2=0.32) and post-hoc analyses of these interactions indicated significant differences between T-treated and control females on day 21 only. There was in contrast no change in the total number of PV cells in RA (no effect of treatment and no interaction; marginal change with time, F3,45=2.79, p=0.051, ηp2=0.16).
The % of PV with PNN in RA was also larger in T-treated females compared to control birds and progressively increased with time (see Table 3), but there was no significant interaction between these factors, although the magnitude of the difference between the two groups tended to increase with time. As is the case in males a large proportion of PNN were located around PV cells in all groups (between 65% and 92%) and surprisingly there was a significant change of the % PNN around PV over time (Table 3). Post-hoc tests showed that this proportion was larger on day 2 compared to day 1 (Table 3) suggesting a rapid reorganization of these structures. No changes in PV or PNN or PV+PNN densities were observed in RA (Table 3) indicating that changes in total numbers mostly reflected the increase in volume of the entire nucleus.
In Area X, the general pattern of changes in the number of PNN, PV and PV+PNN in females was similar to what had been observed in males. There was in each case an overall main effect of treatment indicating that the total number of PNN (Fig. 7F; F1,46=11.48, p<0.01, ηp2=0.20), PV (Fig. 7I; F1,46=6.08, p<0.05, ηp2=0.12) and PV+PNN (Fig. 7L; F1,46=10.99, p<0.01, ηp2=0.19) was increased in T-treated females. However, there were only trends of interaction effects (PNN: F3,46=2.68, p=0.058, ηp2=0.15; PV: F3,46=2.67, p=0.059, ηp2=0.15; PV+PNN: F3,xx=2.80, p=0.051, ηp2=0.15), so that the specific time point when differences between groups first appeared could not be statistically confirmed. There was no significant effect of time, except for a non significant trend affecting the total number of PNN (F3,46=2.52, p=0.069, ηp2=0.14).
Interestingly, there was also a main effect of the T-treatment on the density of PNN and PV+PNN (Table 3), suggesting that these T-induced increases are, contrary to what was observed in males, larger than the increase in volume of the nucleus. Finally, the % PNN around PV did not change over time and was not affected by T, although it was higher in all groups of females (76%−91%) than in T-treated males of experiment 1 (61%), suggesting that PNN surround partly different cells types in males and in females.
Increase in PV expression does not reflect neurogenesis
As females had been injected with BrdU at the beginning of the experiment and neurogenesis is known to occur seasonally in the HVC of canaries, we also tested whether the large increase in the number of PV interneurons reflected neurogenesis of this specific cell type or only an increased expression of the protein in a stable number of interneurons. Detailed analysis of sections double-labeled for PV and BrdU failed to identify any double-labeled cells, while the two labels were clearly present in these sections, but always in different cells (see Figure 8C). The increased number of PV cells in HVC is thus likely due to an increased expression of the protein in neurons that were already present before the treatment.
DISCUSSION
Testosterone induces song crystallization and increases the number of PNN in the SCS of males
We confirmed here that a systemic treatment with testosterone associated with photostimulation mimics what happens at the onset of the breeding season in terms of physiology by increasing plasma testosterone and the cloacal protuberance area. Correlatively, there is a large increase in the volume of song control nuclei as previously shown in canaries both in response to photoperiod changes and/or testosterone treatment (Boseret et al., 2006; Hurley et al., 2008; Sartor and Ball, 2005), reflecting the extensive seasonal neuroplasticity that has been observed these brain structures (Nottebohm, 1981; Nottebohm et al., 1987). Moreover, testosterone increased in less than a week the motivation to sing as indicated by a higher singing rate and percentage of time spent singing. This replicates effects detected multiple times in similar studies from our laboratories (Alward et al., 2013; Madison et al., 2015; Sartor et al., 2005; Shevchouk et al., 2018) as well as similar effects induced by testosterone in female canaries (Bottjer and Dignan, 1988; Fusani et al., 2003; Hartog et al., 2009; Madison et al., 2015; Nottebohm, 1980).
Additionally, testosterone induced a change in song structure reflected by longer song durations, an increased sound energy and a displacement of vocalizations towards higher frequencies. Testosterone also decreased song entropy. The majority of these changes are observed when canaries crystallize their song and correspond to previous findings regarding the effects of testosterone on singing behavior (Alliende et al., 2010; Alward et al., 2017a, 2016; Madison et al., 2015; Sartor et al., 2005).
It has also been shown in two songbird species that treatment of juveniles with exogenous testosterone produces an early crystallization of song. This means that the song learning process is stopped at an early stage which in turn results in adults producing an impoverished song (in zebra finches: (Korsia and Bottjer, 1991) and in white-crowned sparrows: (Whaling et al., 1995). Treatment with testosterone in adulthood seems to have a similar effect on crystallization but the song expressed here by the treated males appeared quite normal. It would however be interesting to compare in gonadally intact males the features of song produced during the breeding period in males that have or have not been treated with exogenous testosterone during the previous autumnal phase of plastic song to investigate whether the steroid has a similar effect on song structure in adult birds.
These findings clearly indicate that the treatment with exogenous testosterone mimics what happens at the onset of breeding at the physiological, behavioral and neurobiological levels. This study also demonstrates that testosterone increases in adult male canaries the number of PNN in the three key song control nuclei: HVC, RA and Area X. This increase is systematically accompanied by an increased colocalization around parvalbumin expressing neurons. These data suggest that the development of PNN around PV-interneurons could represent a novel mechanism controlling the testosterone-induced annual song crystallization typical of adult seasonal open-ended learning songbird species, similar to what happens during development.
Testosterone induces a similar increase in the number of PNN in the SCS of females
In females, there was, as in males, an overall main effect of testosterone on the number of PNN and of PV+PNN (a trend for PV+PNN in HVC). This suggests that this aspect of neuroplasticity can also be studied in the brain of females who possess the same interconnected SCS nuclei as males and produce vocalizations closely resembling male-typical song when exposed to exogenous testosterone (Madison et al., 2015; Nottebohm, 1980). Interestingly, when the interaction of this treatment with time was significant (in HVC and RA), the post-hoc tests showed that the effect of testosterone on PNN and PV+PNN only became significant after 21 days in females. No significant effect was detected on day 9, except for an increase on PV neurons in HVC (see below), suggesting that the development of PNN in song control nuclei is a relatively slow process. The specific duration of exposure to T required for this development cannot however be precisely determined since no brains were collected between day 9 and 21. It is also difficult to compare these latencies in males and females since methodologies were slightly different and only a single time point (at 24 days) is available for males.
When we performed this experiment on females, we were unable, due to lack of suitable equipment (see methods), to record their singing behavior. It is nevertheless interesting to compare the time course of PNN and PV development in the brain of females with the rate of changes in singing rate and quality of the T-treated males as female canaries treated with testosterone display similar, though not identical, behavioral and neural changes as males (Madison et al., 2015). This could provide some information on whether the PNN increase is the result of, or a prerequisite, for song crystallization.
In females, testosterone increased the volume of HVC, RA and Area X as well as the number of PNN around PV cells only between 9 and 21 days, while in males, the singing motivation (song numbers and percentage time spent singing) rapidly increased to reach its highest level between 3 and 10 days after T-treatment. This increase in singing motivation was also paralleled by changes in song structure that reached their plateau on day 10, suggesting that the song became crystallized at that time. If the timing of these mechanisms is similar in males and females, these data could suggest that the increase in singing motivation and song crystallization precedes the growth of song nuclei and the increase in the number of PNN in those nuclei. It is however likely, based on previous work (e.g. Madison et al., 2015) that singing activity and song structure develop more slowly after exposure to exogenous testosterone in females than in males. Comparisons between sexes are therefore extremely hazardous.
It has been shown previously that the motivation to sing is controlled in male and female canaries by testosterone action in the medial preoptic nucleus (POM), and not by direct action in the SCS (Alward et al., 2016, 2013; Vandries et al., 2019), and that, accordingly testosterone induces morphological changes more rapidly in the POM than in the song control nuclei (Shevchouk et al., 2019, 2017). The present findings as they relate to song motivation were therefore expected. However, the fact that PNN development might follow the changes in song structure would counter-intuitively suggest that PNN development around PV neurons is a consequence rather than a cause of song crystallization. This conclusion must however remain tentative because a) it is unlikely that the male and female brain and behavior react to testosterone at the exact same speed, and b) there is no guarantee that the changes we observed here in male song quality (e.g., decrease in entropy) represent a full crystallization of the song. A detailed time course experiment analyzing changes in both brain and behavior in the same male subjects would be needed to reach a final conclusion.
In HVC, testosterone induces the development of PNN around additional PV-expressing interneurons
Interestingly, in male canaries, there was a large increase (more than three times) in the number of parvalbumin expressing neurons in HVC as well as an increase in the density of these cells. These increased numbers thus proportionally exceed the growth of HVC volume induced by testosterone. As parvalbumin characterizes fast-spiking GABAergic interneurons (Hu et al., 2014), this increase could presumably constitute the cellular basis for the overall increase of local neural inhibition in HVC that is necessary to maintain a stable crystallized song (Kosche et al., 2015; Liberti et al., 2016; Vallentin et al., 2016). The rapid inhibition of specific projection neurons in HVC could be provided by these fast-spiking interneurons. These cells might also facilitate the production of specific phrases or syllables, like the so-called sexy or special syllables, which are produced at a high repetition rate and are particularly attractive for females (Vallet and Kreutzer, 1995). As those syllables are produced in higher proportions during the breeding season (Voigt and Leitner, 2008), a higher local inhibition provided by this testosterone-induced increased number and density of parvalbumin expressing interneurons in HVC is a potential mechanism that would support this breeding-specific pattern of singing behavior. In addition to the sexy syllable-type phrases, the canary crystallized song displays a high level of stereotypy that is reflected in the very accurate and fast syllable repetition (Leitner et al., 2001a; Maddison et al., 2015).
We also asked whether testosterone induced an increase in the tempo of singing in the present experiment but this could only be assessed by comparing songs in the 4 males that started singing reliably on day 3. We analyzed within the first 50 trills recorded for each male on day 3 and 24 the rate (number per sec) of repetition of short syllables (>0.15 sec) within trills and the number of these syllables per trill. The number of syllables per trill tended to increase (7.6±0.8 vs.9.9±1.2, means ± SEM; t3=3.116, p=0.053) but unexpectedly the rate at which they were produced (number per sec) actually decreased (14.1±0,8 vs. 12.3±0.7; t3=3.326, p=0.045). No definitive conclusion should however be drawn from these limited data that need be confirmed by a study with a large number of subjects.
The increased number of PNN and of PV+PNN in HVC without changes in the percentage of PV interneurons surrounded by PNN strongly suggests that the T-induced addition of PNN in HVC is causally related to the development of new parvalbumin expressing neurons. As PNN increase the fast-spiking activity of PV-interneurons (Balmer, 2016), it is likely that concomitant PV and PNN increase in HVC supports some aspects of the song crystallization occurring in the spring.
The lower song entropy observed in this study could also be promoted by this increase of PV interneurons surrounded by PNN in HVC. Indeed, individual RA-projecting neurons in HVC are activated at the same time as specific elements of the songs are produced (Fee et al., 2004; Yu and Margoliash, 1996). The control of the neural activity of such neurons could be provided upstream by inhibitory interneurons within HVC. It has been previously shown that the local inhibitory dynamics in HVC controls the stability of crystallized song in adult zebra finches (Kosche et al., 2015; Liberti et al., 2016). Fast-spiking activity of PV-expressing interneurons surrounded by PNN could enhance local inhibition to monitor the production of syllables repeated at a high rate and with good accuracy. This hypothesis should nevertheless be confirmed with the use of in-vivo electrophysiological tools coupled with the subsequent immunohistochemical analysis of the recorded neurons.
It should be noted that no PV neuron was co-labeled with BrdU in the female experiment strongly suggesting that the increase in PV neurons does not reflect neurogenesis of this cell type, but rather the induction of parvalbumin synthesis in neurons that were previously in place, but did not express this calcium-binding protein. This is in agreement with previous work in songbirds indicating that neurogenesis in HVC mostly, if not exclusively, concerns the RA-projecting neurons and there is no replacement in adulthood of Area X-projecting neurons and of interneurons (Alvarez-Buylla et al., 1988; Balthazart and Ball, 2016; Kirn et al., 1999, 1991; Scharff et al., 2000; Scott and Lois, 2007; Scotto-Lomassese et al., 2007). A similar phenomenon (testosterone-induced increase in number of neurons expressing a protein in the absence of neurogenesis) has been reported in other experimental systems, namely in the preoptic area and hypothalamus of various species. We showed for example that the number of aromatase-expressing neurons in the quail preoptic area is increased 4–5 fold when castrated males are treated with testosterone (Balthazart et al., 1996; Foidart et al., 1994) but essentially no neurogenesis can be detected in this brain area (Bardet et al., 2012; Mouriec and Balthazart, 2013).
Nevertheless neurogenesis occurred in the HVC of these females following testosterone-treatment as reported in a previous publication based on the same subjects (Shevchouk et al., 2017) and it also reached its plateau at 9 days after the beginning of the testosterone treatment. This suggests that the increased survival of new born neurons, which are mainly RA-projecting neurons in males (Kirn and Nottebohm, 1993), accompanied by an increased expression of PV inhibitory interneurons occurs more or less at the same time as the T-induced song crystallization, which occurs in physiological conditions at the onset of the male breeding season. The subsequent development of PNN observed here between 9 and 21 days in females would stabilize the connectivity of these new PV-expressing interneurons to sustain the stable production of the newly crystallized song. Indeed, it was shown that an important role of the PNN is to act as a physical barrier and, as a scaffold that binds specific proteins, limits the synaptic plasticity around the fast-spiking PV interneurons (van ‘t Spijker and Kwok, 2017).
Mechanisms underlying effects of testosterone on PNN
The cellular and molecular mechanisms mediating these effects of testosterone on PNN development remain poorly understood. Studies in mammals clearly indicate that the formation of PNN is activity- or experience dependent (Nowicka et al., 2009; Ye and Miao, 2013) and the blockade of calcium or sodium channels prevents their accumulation (Dityatev et al., 2007). For example in rats and cats, PNN increase with age in the visual cortex but this increase is inhibited by raising the animals in the dark (Lander et al., 1997; Liu et al., 2013; Pizzorusso et al., 2002; Ye and Miao, 2013). Similarly PNN development is inhibited following destruction of a few vibrissae in the corresponding barrel cortex of mice and rats (Bahia et al., 2008; McRae et al., 2007; Nakamura et al., 2009; Nowicka et al., 2009). Similar mechanisms are probably playing a role in the context of vocal learning as suggested by a study in zebra finches showing that tutor deprivation during development decreases the number of PNN located around PV cells in the HVC of fully grown males (Balmer et al., 2009). PNN density also seems to be regulated up-stream by enzymes called metalloproteinases that are able to degrade several components of the extracellular matrix (Mott and Werb, 2004; Wen et al., 2018) even if the direct causal link with this enzyme remains a bit uncertain
The effects of testosterone observed here following exposure to testosterone could therefore be activity-dependent and result from changes in neuronal firing within the song control nuclei related to the increased singing activity. Alternatively, testosterone could regulate the metalloproteinase expression or activity or regulate in a more direct manner the synthesis of some of the components of the PNN by a local action on genes expression. To discriminate between these two broad types of action, one could implant testosterone directly in HVC. Because this would not induce any singing activity per se (Alward et al., 2016), this manipulation would directly test the activity-dependent aspect of the induction.
Possible role of testosterone-induced PNN development in RA and Area X
In RA, there was a trend for an increase of the total number of PV-interneurons in males but not in females. The density of PV-interneurons was however decreasing in males, but not in females, suggesting that in males the total number of PV interneurons in this nucleus remains constant and there is an increased cell-spacing that matches the previously reported increased cell-spacing in RA during the breeding season based on studies of sparrows (Brenowitz, 2004). There was an increase of total PNN and PV+PNN in the RA of both males and females as well as an increased percentage of PV-interneurons surrounded by PNN in females. These data suggest that the development of PNN in RA occurs around PV interneurons that were already present in this nucleus in females, but may be partly around new PV-expressing interneurons in males.
The seasonal increase in RA volume is due to increased cell spacing partly under the influence of new projections coming from HVC (DeVoogd et al., 1985). When the song crystallizes, the development of PNN around PV-interneurons could participate to the stabilization of these new connections. This would however require that the new HVCRA projecting neurons terminate their axon, at least in part, on inhibitory neurons (that express parvalbumin) in RA. Nevertheless, an increased number of PNN in RA as possibly induced by testosterone could stabilize inhibitory microcircuits and participate to the production of a stable song. Indeed, implanting an androgen receptor blocker in RA of male canaries has been shown to increase the variability of syllables from one rendition to the other (Alward et al., 2017a).
In Area X, testosterone increased the total number of PV-interneurons in both males and females. This increase had however a lower magnitude than in HVC (+60% in males and even less in females) and was not accompanied by an increase in density in males. This change is thus proportional to the growth of the nucleus volume. In contrast, there was an increased density of these measures in females, so that the increase in the number of PNN exceeded the growth of the nucleus. There was again an increased number of PNN and PV+PNN in the Area X of both males and females, suggesting a possible involvement in the testosterone-induced seasonal plasticity.
Area X is at the origin of the anterior forebrain pathway: it projects to the medial portion of the dorsolateral thalamus (DLM) which in turn projects to the lateral part of the magnocellular nucleus of the anterior nidopallium (LMAN) which then projects to RA (Nordeen and Nordeen, 2008). This pathway is implicated in song learning but also in the generation of song variability that is important for sensorimotor learning during ontogeny and also for the maintenance of a stable song in adulthood (Andalman and Fee, 2009; Kao et al., 2005; Tumer and Brainard, 2007). LMAN expresses a high density of androgen receptors (Balthazart et al., 1990) and it is also a potentially important site of androgen action on song crystallization in that LMAN lesions reduce the song variability of T-induces song in adult female canaries (Rouse and Ball, 2016). Similarly, lesions of Area X are known to eliminate within-syllable variation in fundamental frequency, which potentially plays a role in the motor exploration that enables reinforcement-driven song learning (Kojima et al., 2018).
One of the main functions of Area X is to monitor through its action via DLM the neural activity of LMAN (Brainard, 2008, 2004) and it is thus possible that PNN development in this nucleus could decrease the song variability induced by LMAN. Area X sends inhibitory projections to DLM which in turn excites LMAN (Luo and Perkel, 1999). A higher inhibitory signal provided by Area X would consequently decrease DLM and LMAN activity and the song variability that correlates positively with LMAN activation. Moreover, after T treatment, a lower percentage of PNN are found around PV-expressing cells in Area X (61.55%) compared to other SCS nuclei and compared to other SCS nuclei of adult zebra finches (Cornez et al., 2015). PNN in Area X could thus surround projection neurons that do not express parvalbumin. PNN could potentially develop around inhibitory projection neurons (expressing or not parvalbumin) to limit the synaptic plasticity and stabilize the inhibitory signals sent to DLM after the learning of the newly crystallized songs. Another possible function of PNN in Area X would be the stabilization of local inhibitory circuits as in HVC and RA. In both cases, knowing that Area X is important for song learning and for the control of song variability (Brainard, 2004), the development of PNN in Area X probably plays an important role in the testosterone-induced stabilization of newly crystallized songs that are produced by adult open-ended seasonal songbirds at the onset of the breeding season.
CONCLUSIONS
Together, the present data suggest a clear correlation between the increase of PV-interneurons in HVC and Area X, the development of PNN around these neurons in HVC, RA and Area X and the song crystallization that occurs at the onset of the breeding season in males. PNN development is probably an important mechanism of the testosterone-induced neuroplasticity that controls the seasonal changes in vocal behavior. If PNN limit the synaptic plasticity in the SCS as was shown in the mammalian visual cortex (Liu et al., 2013; Pizzorusso et al., 2002), one would expect a lower number and density of PNN in the SCS of adult open-ended learning species during the fall when the song is more variable. In a previous study, we were not able to detect any variation in PNN expression in starlings that were exposed to various photoperiodic treatments placing them in the physiological conditions corresponding to the different phases of the annual cycle (Cornez et al., 2017). In contrast, we showed here that treating male or female canaries with exogenous testosterone increases PNN expression in the 3 main telencephalic song control nuclei. As previously mentioned, it is probable that the very low level of PNN in starlings relates to their capacity to learn new songs as adults at any time during the annual cycle. The more limited song plasticity in canaries who can only modify their song during autumn suggests that they might be a better experimental model for the study of the expression and role of PNN in adult song plasticity. The present study of the effects of testosterone confirms this suggestion.
Highlights.
Perineuronal nets are present in high numbers in song control nuclei of canaries
Testosterone stimulates development of perineuronal nets in song control nuclei
Testosterone also increase parvalbumin expression in these nuclei
These brain changes occur in parallel with the activation of singing activity
These brain and behavioral effect of testosterone are observed in both sexes
Acknowledgements
This work was supported by a grant from the Interuniversity Attraction Pole (IAP P7/17) to CAC and JB and from the National Institute of Neurological Disorders and Stroke (RO1NS104008) to GFB, JB and CAC. CAC is FRS-FNRS Research Associate and GC is Research Fellow of the FRS-FNRS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alliende JA, Méndez JM, Goller F, Mindlin GB, 2010. Hormonal acceleration of song development illuminates motor control mechanism in canaries. Dev. Neurobiol 70, 943–960. 10.1002/dneu.20835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, 1997. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J.Neurobiol 33, 585–601. 10.1002/(SICI)1097-4695(19971105) [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F, 1988. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc. Natl. Acad. Sci 85, 8722–8726. 10.1073/pnas.85.22.8722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF, 2017a. Dissociable Effects on Birdsong of Androgen Signaling in Cortex-Like Brain Regions of Canaries. J. Neurosci 37, 8612–8624. 10.1523/JNEUROSCI.3371-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF, 2013. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc. Natl. Acad. Sci 110, 19573–19578. 10.1073/pnas.1311371110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Madison FN, Parker SE, Balthazart J, Ball GF, 2016. Pleiotropic Control by Testosterone of a Learned Vocal Behavior and Its Underlying Neuroplasticity. eNeuro 3, 1–17. 10.1523/ENEURO.0145-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Rouse ML, Balthazart J, Ball GF, 2017b. Testosterone regulates birdsong in an anatomically specific manner. Anim. Behav 124, 291–298. 10.1016/j.anbehav.2016.09.013 [DOI] [Google Scholar]
- Andalman AS, Fee MS, 2009. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc. Natl. Acad. Sci 106, 12518–12523. 10.1073/pnas.0903214106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia CP, Houzel JC, Picanço-Diniz CW, Pereira A, 2008. Spatiotemporal distribution of proteoglycans in the developing rat’s barrel field and the effects of early deafferentation. J. Comp. Neurol 510, 145–157. 10.1002/cne.21781 [DOI] [PubMed] [Google Scholar]
- Ball GF, 1999. Neuroendocrine basis of seasonal changes in vocal behavior among songbirds, in: Hauser M, Konishi M (Eds.), The Design of Animal Communication. MIT Press, Cambridge,MA, pp. 213–253. [Google Scholar]
- Ball GF, Balthazart J, 2016. Neuroendocrine Regulation of Reproductive Behavior in Birds, in: Pfaff DW, Joels M (Eds.), Hormones, Brain and Behavior. Academic Press, San Diego, CA, pp. 217–254. 10.1016/B978-0-12-803592-4.00029-8 [DOI] [Google Scholar]
- Ball GF, Balthazart J, 2007. The neuroendocrinology and neurochemistry of birdsong, in: Lajtha A (Ed.), Handbook of Neurochemistry and Molecular Neurobiology, 3rd Edition, Blaustein JD (Volume Ed.). Springer, New York, pp. 419–457. [Google Scholar]
- Balmer TS, 2016. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro 3, 1–13. 10.1523/ENEURO.0112-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer TS, Carels VM, Frisch JL, Nick TA, 2009. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci 29, 12878–12885. 10.1523/JNEUROSCI.2974-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF, 2016. Endocrine and social regulation of adult neurogenesis in songbirds. Front. Neuroendocrinol 10.1016/j.yfrne.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Boseret G, Konkle AT, Hurley LL, Ball GF, 2008. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci 27, 801–817. https://doi.org/EJN6059 [pii]10.1111/j.1460-9568.2008.06059.x [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Wilson EM, Ball GF, 1992Immunocytochemical Localization of Androgen Receptors in the Male Songbird and Quail Brain. Journal of Comparative Neurology 317, 407–420. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Tlemçani O, Harada N, 1996. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J.Chem.Neuroanat. 11, 147–171. [DOI] [PubMed] [Google Scholar]
- Bardet SM, Mouriec K, Balthazart J, 2012. Birth of neural progenitors during the embryonic period of sexual differentiation in the Japanese quail brain. J. Comp. Neurol 520, 4226–4253. 10.1002/cne.23153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher MD, Brenowitz EA, 2005. Functional aspects of song learning in songbirds. Trends Ecol. Evol. 20, 143–149. 10.1016/j.tree.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J, 2006. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria). J Neurobiol 66, 1044–1060. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Dignan TP, 1988. Joint hormonal and sensory stimulation modulate neuronal number in adult canary brains. J.Neurobiol 19, 624–635. [DOI] [PubMed] [Google Scholar]
- Brainard MS, 2008. The anterior forebrain pathway and vocal plasticity, in: Zeigler HP, Marler P (Eds.), Neuroscience of Birdsong. Cambridge University Press, Cambridge, pp. 240–255. [Google Scholar]
- Brainard MS, 2004. Contributions of the anterior forebrain pathway to vocal plasticity. Ann N Y Acad Sci 1016, 377–394. 10.1196/annals.1298.042 [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ, 2002. What songbirds teach us about learning. Nature 417, 351–358. 10.1038/417351a [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, 2004. Plasticity of the adult avian song control system. Ann N Y Acad Sci 1016, 560–585. 10.1196/annals.1298.006 [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Beecher MD, 2005. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci. 28, 127–132. 10.1016/j.tins.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB, 2008. Bird song Biological themes and Variations. Cambridge University Press, Cambridge UK. [Google Scholar]
- Collins S, 2004. Vocal fighting and flirting: the functions of birdsong, in: Marler P, Slabbekoorn H (Eds.), Nature’s Lusic, The Science of Birdsong. Elsevier, Amsterdam, pp. 39–79. [Google Scholar]
- Cornez G, Jonckers E, ter Haar SM, Van der Linden A, Cornil CA, Balthazart J, 2018. Timing of perineuronal net development in the zebra finch song control system correlates with developmental song learning. Proc. R. Soc. B Biol. Sci 285, 20180849 10.1098/rspb.2018.0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornez G, Madison FN, Van der Linden A, Cornil C, Yoder KM, Ball GF, Balthazart J, 2017. Perineuronal nets and vocal plasticity in songbirds: A proposed mechanism to explain the difference between closed-ended and open-ended learning. Dev. Neurobiol 77, 975–994. 10.1002/dneu.22485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornez G, ter Haar SM, Cornil CA, Balthazart J, 2015. Anatomically Discrete Sex Differences in Neuroplasticity in Zebra Finches as Reflected by Perineuronal Nets. PLoS One 10, e0123199 10.1371/journal.pone.0123199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF, 2001. Photoperiodic control of seasonality in birds. J Biol Rhythm 16, 365–380. 10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Dawson A, Sharp PJ, 2007. Photorefractoriness in birds-photoperiodic and non-photoperiodic control. Gen. Comp. Endocrinol 153, 378–384. 10.1016/j.ygcen.2007.01.043 [DOI] [PubMed] [Google Scholar]
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW, 2006. Composition of perineuronal net extracellular matrix in rat brain: A different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem 281, 17789–17800. 10.1074/jbc.M600544200 [DOI] [PubMed] [Google Scholar]
- DeVoogd TJ, Nixdorf B, Nottebohm F, 1985. Synaptogenesis and changes in synaptic morphology related to acquisition of a new behavior. Brain Res. 329, 304–308. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, Schachner M, 2007. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev. Neurobiol 67, 570–588. 10.1002/dneu.20361 [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK, 1999. Birdsong and human speech: Common themes and mechanisms. Annu.Rev.Neurosci 22, 567–631. [DOI] [PubMed] [Google Scholar]
- Fee MS, Kozhevnikov AA, Hahnloser RH, 2004. Neural mechanisms of vocal sequence generation in the songbird. Ann N Y Acad Sci 1016, 153–170. 10.1196/annals.1298.0221016/1/153 [pii] [DOI] [PubMed] [Google Scholar]
- Foidart A, De Clerck A, Harada N, Balthazart J, 1994. Aromatase-immunoreactive cells in the quail brain: Effects of testosterone and sex dimorphism. Physiol.Behav 55, 453–464. [DOI] [PubMed] [Google Scholar]
- Fusani L, Metzdorf R, Hutchison JB, Gahr M, 2003. Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries. J Neurobiol 54, 370–379. [DOI] [PubMed] [Google Scholar]
- Halle F, Gahr M, Kreutzer M, 2003. Impaired recovery of syllable repertoires after unilateral lesions of the HVC of male domesticated canari[dummy]es. Anim. Biol 53, 113–128. 10.1163/157075603769700322 [DOI] [Google Scholar]
- Härtig W, Derouiche A, Welt K, Brauer K, Grosche J, Mäder M, Reichenbach A, Brückner G, 1999. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 842, 15–29. 10.1016/S0006-8993(99)01784-9 [DOI] [PubMed] [Google Scholar]
- Hartog TE, Dittrich F, Pieneman AW, Jansen RF, Frankl-Vilches C, Lessmann V, Lilliehook C, Goldman SA, Gahr M, 2009. Brain-derived neurotrophic factor signaling in the HVC is required for testosterone-induced song of female canaries. J Neurosci 29, 15511–15519. https://doi.org/29/49/15511 [pii]10.1523/JNEUROSCI.2564-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, 2004. Critical Period Regulation. Annu. Rev. Neurosci 27, 549–579. 10.1146/annurev.neuro.27.070203.144327 [DOI] [PubMed] [Google Scholar]
- Hill KM, DeVoogd TJ, 1991. Altered daylength affects dendritic structure in a song-related brain region in red-winged blackbirds. Behav. Neural Biol 56, 240–250. 10.1016/0163-1047(91)90379-5 [DOI] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P, 2014. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 345, 1255263–1255263. 10.1126/science.1255263 [DOI] [PubMed] [Google Scholar]
- Hurley LL, Wallace AM, Sartor JJ, Ball GF, 2008. Photoperiodic induced changes in reproductive state of border canaries (Serinus canaria) are associated with marked variation in hypothalamic gonadotropin-releasing hormone immunoreactivity and the volume of song control regions. Gen Comp Endocrinol 158, 10–19. https://doi.org/S0016-6480(08)00200-1 [pii]10.1016/j.ygcen.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS, 2005. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433, 638–643. [DOI] [PubMed] [Google Scholar]
- Karetko M, Skangiel-Kramska J, 2009. Diverse functions of perineuronal nets. Acta Neurobiol. Exp. (Wars). 69, 564–577. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Alvarez-Buylla A, Nottebohm F, 1991. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J. Neurosci 11, 1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Fishman Y, Sasportas K, Alvarez-Buylla A, Nottebohm F, 1999. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J Comp Neurol 411, 487–494. [PubMed] [Google Scholar]
- Kirn JR, Nottebohm F, 1993. Direct evidence for loss and replacement of projection neurons in adult canary brain. J.Neurosci 13, 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Kao MH, Doupe AJ, Brainard MS, 2018. The Avian Basal Ganglia Are a Source of Rapid Behavioral Variation That Enables Vocal Motor Exploration. J. Neurosci 38, 9635–9647. 10.1523/JNEUROSCI.2915-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsia S, Bottjer SW, 1991. Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. J.Neurosci 11, 2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosche G, Vallentin D, Long MA, 2015. Interplay of Inhibition and Excitation Shapes a Premotor Neural Sequence. J. Neurosci 35, 1217–1227. 10.1523/JNEUROSCI.4346-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander C, Kind P, Maleski M, Hockfield S, 1997. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J. Neurosci 17, 1928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Gahr M, 2001a. Seasonal changes in the song pattern of the non-domesticated canary (Serinus canaria), a field study. Behaviour 138, 885–904. 10.1163/156853901753172700 [DOI] [Google Scholar]
- Leitner S, Voigt C, Garcia-Segura LM, Van’t Hof T, Gahr M, 2001b. Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm. Behav 40, 160–168. 10.1006/hbeh.2001.1700 [DOI] [PubMed] [Google Scholar]
- Liberti WA, Markowitz JE, Perkins LN, Liberti DC, Leman DP, Guitchounts G, Velho T, Kotton DN, Lois C, Gardner TJ, 2016. Unstable neurons underlie a stable learned behavior. Nat. Neurosci 19, 1665–1671. 10.1038/nn.4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xu H, Yu T, Yao J, Zhao C, Yin ZQ, 2013. Expression of perineuronal nets, parvalbumin and protein tyrosine phosphatase σ in the rat visual cortex during development and after BFD. Curr. Eye Res 38, 1083–94. 10.3109/02713683.2013.803287 [DOI] [PubMed] [Google Scholar]
- Luo MM, Perkel DJ, 1999. A GABAergic, strongly inhibitory projection to a thalamic nucleus in the zebra finch song system. J.Neurosci 19, 6700–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison FN, Rouse ML, Balthazart J, Ball GF, 2015. Reversing song behavior phenotype: Testosterone driven induction of singing and measures of song quality in adult male and female canaries (Serinus canaria). Gen. Comp. Endocrinol 215, 61–75. 10.1016/j.ygcen.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P, Peters S, Ball GF, Dufty AM, Wingfield JC, 1988. The role of sex steroids in the acquisition and production of birdsong. Nature 336, 770–772. [DOI] [PubMed] [Google Scholar]
- Marler PA, Peters S, 1987. A sensitive period for song acquisition in the song sparrow, Melospiza melodia: a case of age-limited learning. Ethology 76, 89–100. [Google Scholar]
- Maroto M, Fernández-Morales J-C, Padín JF, González JC, Hernández-Guijo JM, Montell E, Vergés J, de Diego AMG, García AG, 2013. Chondroitin sulfate, a major component of the perineuronal net, elicits inward currents, cell depolarization, and calcium transients by acting on AMPA and kainate receptors of hippocampal neurons. J. Neurochem 125, 205–213. 10.1111/jnc.12159 [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT, 2007. Sensory Deprivation Alters Aggrecan and Perineuronal Net Expression in the Mouse Barrel Cortex. J. Neurosci. 27, 5405–5413. 10.1523/JNEUROSCI.5425-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CEA, Boroda E, Nick TA, 2014. Sexually dimorphic perineuronal net expression in the songbird. Basal Ganglia 3, 229–237. 10.1016/j.baga.2013.10.002 [DOI] [Google Scholar]
- Mooney R, 2009. Neurobiology of song learning. Curr. Opin. Neurobiol 19, 654–660. 10.1016/j.conb.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori C, Liu WC, Wada K, 2018. Recurrent development of song idiosyncrasy without auditory inputs in the canary, an open-ended vocal learner. Sci. Rep 8, 1–10. 10.1038/s41598-018-27046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JD, Werb Z, 2004. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol 16, 558–564. 10.1016/j.ceb.2004.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouriec K, Balthazart J, 2013. Peripubertal proliferation of progenitor cells in the preoptic area of Japanese quail (Coturnix japonica). Brain Res. 1516, 20–32. 10.1016/j.brainres.2013.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nakano K, Morita S, Nakashima T, Oohira A, Miyata S, 2009. Expression of chondroitin sulfate proteoglycans in barrel field of mouse and rat somatosensory cortex. Brain Res. 1252, 117–129. 10.1016/j.brainres.2008.11.022 [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Storey CR, 1977. The effect of duration of the daily photoperiod on recovery of photosensitivity in photorefractory canaries (Serinus canarius). Gen.Comp.Endocrinol 31, 72–74. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, 2008. Circuits and cellular mechanisms of sernsory acquisition, in: Zeigler HP, Marler P (Eds.), Neuroscience of Birdsong. Cambridge University Press, New York, pp. 256–270. [Google Scholar]
- Nottebohm F, 2005. The neural basis of birdsong. PLoS Biol 3, e164 10.1371/journal.pbio.0030164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, 1981. A brain for all seasons: Cyclical anatomical changes in song-control nuclei of the canary brain. Science 214, 1368–1370. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, 1980. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 189, 429–436. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane LA, Wingfield JC, 1987. Seasonal changes in gonadal hormone levels of adult male canaries and their relation to song. Behav.Neural Biol 47, 197–211. [DOI] [PubMed] [Google Scholar]
- Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S, 2009. Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur. J. Neurosci 30, 2053–2063. 10.1111/j.1460-9568.2009.06996.x [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L, 2002. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–51. 10.1126/science.1072699 [DOI] [PubMed] [Google Scholar]
- Rasika S, Nottebohm F, Alvarez-Buylla A, 1994. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proc. Natl. Acad. Sci. 91, 7854–7858. 10.1073/pnas.91.17.7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse ML, Ball GF, 2016. Lesions targeted to the anterior forebrain disrupt vocal variability associated with testosterone-induced sensorimotor song development in adult female canaries, S erinus canaria. Dev. Neurobiol 76, 3–18. 10.1002/dneu.22295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF, 2005. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris). Behav Neurosci 119, 233–244. 10.1037/0735-7044.119.1.233 [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF, 2005. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria). Horm Behav 47, 467–476. 10.1016/j.yhbeh.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F, 2000. Targeted neuronal death affects replacement and vocal behavior in adult songbirds. Neuron 25, 481–492. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Brenowitz EA, 2016. Neural and hormonal control of birdfsong, in: Pfaff DW, Joels M (Eds.), Hormones, Brain and Behavior 3rd Edition. Academis press, San Diego, CA, pp. 255–290. [Google Scholar]
- Scott BB, Lois C, 2007. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J. Comp. Neurol 502, 202–214. 10.1002/cne.21296 [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Rochefort C, Nshdejan A, Scharff C, 2007. HVC interneurons are not renewed in adult male zebra finches. Eur J Neurosci 25, 1663–1668. https://doi.org/EJN5418 [pii]10.1111/j.1460-9568.2007.05418.x [DOI] [PubMed] [Google Scholar]
- Shevchouk OT, Ball GF, Cornil CA, Balthazart J, 2019. Rapid testosterone-induced growth of the medial preoptic nucleus in male canaries. Physiol. Behav 204, 20–26. 10.1016/j.physbeh.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchouk OT, Ghorbanpoor S, Ball GF, Cornil CA, Balthazart J, 2017. Testosterone-induced neuroendocrine changes in the medial preoptic area precede song activation and plasticity in song control nuclei of female canaries. Eur. J. Neurosci 45, 886–900. 10.1111/ejn.13530 [DOI] [PubMed] [Google Scholar]
- Shevchouk OT, Ghorbanpoor S, Smith E, Liere P, Schumacher M, Ball GF, Cornil CA, Balthazart J, 2018. Behavioral evidence for sex steroids hypersensitivity in castrated male canaries. Horm. Behav 103, 80–96. 10.1016/j.yhbeh.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz E. a, Beecher MD, Wingfield JC, 1997a. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J.Neurosci 17, 6001–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC, 1997b. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J. Neurobiol 32, 426–442. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Bernard DJ, McCarthy MM, Ball GF, 2012. Photoperiod-dependent regulation of gonadotropin-releasing hormone 1 messenger ribonucleic acid levels in the songbird brain. Gen. Comp. Endocrinol 190, 81–87. 10.1016/j.ygcen.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe WH, 1958. The learning of song patterns by birds, with especial reference to the song of the chaffinch, Fringilla coelebs. Ibis (Lond. 1859). 100, 535–570. [Google Scholar]
- Tramontin AD, Brenowitz EA, 2000. Seasonal plasticity in the adult brain. TINS 23, 251–258. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA, 1999. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J.Neurobiol 40, 316–326. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Perfito N, Wingfield JC, Brenowitz EA, 2001. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. Gen Comp Endocrinol 122, 1–9. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA, 2003. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol 57, 130–140. [DOI] [PubMed] [Google Scholar]
- Tumer EC, Brainard MS, 2007. Performance variability enables adaptive plasticity of “crystallized” adult birdsong. Nature 450, 1240–1244. 10.1038/nature06390 [DOI] [PubMed] [Google Scholar]
- Vallentin D, Kosche G, Lipkind D, Long MA, 2016. Inhibition protects acquired song segments during vocal learning in zebra finches. Science 351, 267–271. 10.1126/science.aad3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet E, Kreutzer M, 1995. Female canaries are sexually responsive to special song phrases. Anim. Behav 49, 1603–1610. 10.1016/0003-3472(95)90082-9 [DOI] [Google Scholar]
- van ‘t Spijker HM, Kwok JCF, 2017. A Sweet Talk: The Molecular Systems of Perineuronal Nets in Controlling Neuronal Communication. Front. Integr. Neurosci 11, 1–10. 10.3389/fnint.2017.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandries LM, Ghorbanpoor S, Cornez G, Shevchouk OT, Ball GF, Cornil CA, Balthazart J, 2019. Testosterone or Estradiol When Implanted in the Medial Preoptic Nucleus Trigger Short Low-Amplitude Songs in Female Canaries. Eneuro 6, ENEURO.0502–18.2019. 10.1523/eneuro.0502-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellema M, Diales Rocha M, Bascones S, Zsebők S, Dreier J, Leitner S, Van der Linden A, Brewer J, Gahr M, 2019. Accelerated redevelopment of vocal skills is preceded by lasting reorganization of the song motor circuitry. Elife 8 10.7554/eLife.43194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt C, Leitner S, 2008. Seasonality in song behaviour revisited: Seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria). Horm. Behav 54, 373–378. 10.1016/j.yhbeh.2008.05.001 [DOI] [PubMed] [Google Scholar]
- Wang D, Fawcett J, 2012. The perineuronal net and the control of cns plasticity. Cell Tissue Res. 349, 147–160. 10.1007/s00441-012-1375-y [DOI] [PubMed] [Google Scholar]
- Waser MS, Marler P, 1977. Song learning in canaries. J. Comp. Physiol. Psychol 91, 1–7. 10.1037/h0077299 [DOI] [PubMed] [Google Scholar]
- Wen TH, Afroz S, Reinhard SM, Palacios AR, Tapia K, Binder DK, Razak KA, Ethell IM, 2018. Genetic Reduction of Matrix Metalloproteinase-9 Promotes Formation of Perineuronal Nets Around Parvalbumin-Expressing Interneurons and Normalizes Auditory Cortex Responses in Developing Fmr1 Knock-Out Mice. Cereb. Cortex 28, 3951–3964. 10.1093/cercor/bhx258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, Hensch TK, 2014. Critical Periods in Speech Perception: New Directions. Annu. Rev. Psychol 1–24. 10.1146/annurev-psych-010814-015104 [DOI] [PubMed] [Google Scholar]
- Whaling CS, Nelson DA, Marler P, 1995. Testosterone-induced shortening of the storage phase of song development in birds interferes with vocal learning. Dev. Psychobiol 28, 367–376. 10.1002/dev.420280703 [DOI] [PubMed] [Google Scholar]
- Williams H, 2004. Birdsong and Singing Behavior. Ann. N. Y. Acad. Sci 1016, 1–30. 10.1196/annals.1298.029 [DOI] [PubMed] [Google Scholar]
- Wissman AM, Brenowitz EA, 2009. The role of neurotrophins in the seasonal-like growth of the avian song control system. J Neurosci 29, 6461–6471. https://doi.org/29/20/6461 [pii]10.1523/JNEUROSCI.0638-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Miao Q. long, 2013. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 32, 352–363. 10.1016/j.matbio.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Yu AC, Margoliash D, 1996. Temporal hierarchical control of singing in birds. Science 273, 1871–1875. [DOI] [PubMed] [Google Scholar]