Abstract
Purpose
To study longitudinal changes in retinal capillary circulation in eyes treated with iodine 125 (I125) plaque brachytherapy for uveal melanoma using OCT angiography (OCTA).
Design
Longitudinal prospective study of 21 patients undergoing treatment for uveal melanoma with I125 plaque brachytherapy. Eyes with melanoma were imaged with OCTA before treatment and at 12-month intervals until 2 years after brachytherapy.
Participants
After institutional review board approval, participants were enrolled prospectively from an academic ocular oncology clinic.
Methods
Peripapillary (4.5 × 4.5-mm) and macular (3 × 3-mm) OCTA scans were acquired with AngioVue (Optovue, Inc, Fremont, CA).
Main Outcome Measures
The peripapillary nerve fiber layer plexus capillary density (NFLP_CD), macular superficial vascular complex vessel density (mSVC_VD), and foveal avascular zone (FAZ) area were calculated.
Results
Before treatment, no significant difference was found in the NFLP_CD, mSVC_VD, or FAZ area between eyes with melanoma and normal fellow eyes. By 24 months, 11 eyes had developed clinical signs of radiation retinopathy, radiation optic neuropathy, or both. In treated eyes, the NFLP_CD (48.4 ±.44.1%) was reduced at 12 months (46.7±5.0%; P = 0.04, Wilcoxon signed-rank test) and 24 months (44.5±6.1%; P < 0.001). Similarly, the mSVC_VD (48.4 2±3.6%) was reduced in treated eyes at 12 months (43.5±5.9%; P = 0.01) and 24 months (37.4±9.1%; P < 0.001). The FAZ area (0.26±0.11 mm2) increased in treated eyes at 12 months (0.35±0.22 mm2; P = 0.009) and 24 months (0.81±1.03 mm2; P = 0.001). When only eyes with clinically evident radiation changes were evaluated, the changes in NFLP_CD, mSVC_VD, and FAZ area were more pronounced. OCT angiography measurements correlated with both radiation dose and visual acuity. The mSVC_VD measured at 12 months was found to predict the development of clinically apparent radiation retinopathy within 1 year.
Conclusions
OCT angiography demonstrated early emergence of peripapillary and macular capillary vasculature changes after I125 plaque brachytherapy. OCT angiography provided a quantitative measurement of retinal capillary changes associated with ischemia that correlated with visual acuity and radiation dose and may predict future development of radiation-induced retinal toxicity.
Radiation retinopathy and optic neuropathy are vision-threatening complications of radiation treatment for uveal melanoma, the most common primary intraocular tumor in adults.1–4 Vision loss after radiation therapy typically occurs because of radiation optic neuropathy and retinopathy caused by vascular compromise leading to ischemia and edema.5–9. OCT angiography (OCTA) has recently emerged as a method for noninvasively evaluating eyes with radiation-induced vision loss resulting from vascular pathologic features.10–12
Multiple groups have described decreased capillary density in the macula as measured by OCTA after plaque radiotherapy and proton beam radiation for the treatment of melanoma.12–15. Our group demonstrated that the peripapillary microvasculature is impacted similarly by radiation treatment and that decreased peripapillary capillary density as measured by OCTA in eyes with radiation retinopathy correlates with the radiation dose to the nerve and with the vision in the eye.16 In parallel with these discoveries, clinical practice has moved toward the widespread use of antievascular endothelial growth factor (VEGF) agents for the treatment of vision loss attributed to radiation retinopathy, despite limited data to define when treatment is best initiated and the optimal interval for treatment.17–19 A reliable noninvasive method for early detection of radiation retinopathy has the potential to improve clinical outcomes and reduce cost for patients being monitored after radiation treatment.
In this study, we performed longitudinal analyses of macular and peripapillary microvasculature by OCTA in eyes treated with iodine 125 (I125) plaque brachytherapy for uveal melanoma. Changes in the peripapillary nerve fiber layer capillary density (NFLP_CD), macular superficial vascular complex vessel density (mSVC_VD), and foveal avascular zone (FAZ) area were calculated over time from before treatment to 2 years after brachytherapy.
Methods
Patients
After institutional review board approval from the Oregon Health & Science University, informed consent was obtained and patients were enrolled from an academic ocular oncology clinical practice. The study adhered to the tenets of the Declaration of Helsinki and is registered at ClinicalTrials.gov (identifier, ID NCT01955941). All participants provided informed consent. All patients were diagnosed with ciliary body or choroidal melanoma in 1 eye and scheduled to undergo I125 plaque brachytherapy for the treatment of the tumors. Patients were excluded if they had initial vision of worse than 20/200 in the eye with tumor, inability to maintain fixation for OCTA imaging at baseline, or both. Patients with melanoma in any ciliary body or choroidal location were enrolled, including eyes with macular and juxtapapillary tumors. We planned to exclude any patients with blood pressure of more than 180 systolic mmHg and 110 mmHg diastolic at baseline; however, no patients were excluded for meeting these criteria.
Plaque Brachytherapy
Brachytherapy was performed using I125 seeds (IsoAid Model IAI-125A, Port Richey, FL) inserted in a silastic carrier and mounted in gold Collaborative Ocular Melanoma Study-style plaques. Seed activity was calculated to deliver 85 Gy over 100 hours to a prescription depth dependent on tumor thickness. Starting with a minimum prescription depth of 3 mm, the depth was increased by multiples of 0.5 mm for thicker tumors to ensure a margin of 0.5 mm or more but less than 1.0 mm up to tumor thickness of 5 mm. For tumor thicknesses larger than 5 mm, the prescription depth was set to the tumor depth.
The planned seed activity was calculated using Plaque Simulator version 6.4.1 (Eye Physics, LLC, Los Alamitos, CA). The software uses a superposition of a linear source model of all seeds as defined by the consensus data of the American Association of Physicists in Medicine Task Group-40 with corrections for the presence of the plaque, carrier attenuation, and air interface. Some of the plaques were modified in house with cut notches to allow implantation around or near the optic nerve. These were modeled specifically to account for the lack of a lip on the notched edge normally found on commercially available notched Collaborative Ocular Melanoma Study plaques. The size and variation of the plaque were determined by the surgeon based on the limitations of surgical practicality. The dose to 50% of the optic nerve at the retinal surface and 50% of the fovea at the retinal surface was calculated with positioning based on fundus images loaded into the planning software.
OCT Angiography Data Acquisition and Analysis
A spectral-domain 70-kHz AngioVue OCTA system (Optovue, Inc, Fremont, CA) obtained 4.5 × 4.5-mm peripapillary scans centered on the optic disc and 3 × 3-mm macular scans in tumor and fellow eyes. Two repeated B-scans, each consisting of 304 Ascans, were captured at each of 304 locations in 2.9 seconds. One x-fast and 1 y-fast scan were acquired, registered, and merged, minimizing motion artifacts. The commercial version of splitspectrum amplitude-decorrelation angiography algorithm was used to compute OCTA signals.10
The merged volumetric OCTA was quantified by AngioAnalytics version 2018.1.0.22, a quantitative measurement software on AngioVue OCTA system. AngioAnalytics provided an automated algorithm to segment the retina into the nerve fiber layer plexus (also known as the radial peripapillary capillary plexus), superficial vascular complex (from the internal limiting membrane to the inner plexiform layer), and inner retina (from internal limiting membrane to the outer plexiform layer). The segmented slab boundaries were corrected manually if needed. The vessel density was automatically calculated as the percent area occupied by the suprathreshold flow signals within the analyzed area. After the larger vessels were excluded, the remaining angiogram was used to calculate capillary density automatically.
In this study, the NFLP_CD was analyzed within a 1-mm-wide annulus with an outer diameter of 4 mm and inner diameter of 2 mm centered on the optic disc. The mSVC_VD was measured within 1-mm-wide parafoveal circular annulus with an outer diameter of 3 mm and inner diameter of 1 mm centered on the fovea. The foveal avascular zone (FAZ) area (in square millimeters) was calculated on a 3 × 3-mm macular inner retinal angiogram. Trained observers (LL, YJ) reviewed scans, and those with poor image quality, as defined by the following criteria, were excluded: (1) signal strength index less than 50,16,20–22 (2) poor scan alignment or failed motion correction, and (3) focal loss of reflectance signal because of vitreous floaters.
Statistical Analysis
The Wilcoxon signed-rank test was used to compare before and after treatments on tumor eyes. Pearson’s correlation was used to determine the relationship between OCTA parameters and radiation doses. Cox proportional hazards regression was used to investigate the predictive value of OCTA parameters. All statistical analyses were performed with MedCalc software version 18.11.3 (MedCalc Software, Ostend, Belgium).
Results
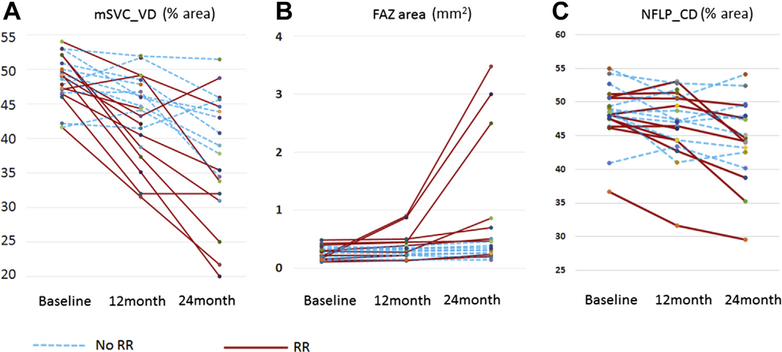
Both eyes of 21 patients recently diagnosed with ciliary body or choroidal melanoma, or both, were imaged with OCTA before planned I125 plaque brachytherapy. Eight patients were men and 13 patients were women, with ages ranging from 41 to 74 years. The affected eye with tumor was the right eye in 8 participants and left eye in 13 participants. Visual acuities in the eye with melanoma ranged from 20/20 to 20/40, and those in the fellow eye were 20/30 or better with the exception of 1 eye whose visual acuity was counting fingers because of amblyopia. Before treatment, no significant difference was found in the NFLP_CD, mSVC_VD, or FAZ area between eyes with melanoma and normal fellow eyes. At 12 months, 4 eyes demonstrated clinical signs of radiation retinopathy or radiation optic neuropathy by ophthalmoscopy or structural OCT.4,23 By 24 months, 11 eyes (52%) demonstrated clinical signs of radiation retinopathy, radiation optic neuropathy, or both (Table 1; Fig 1).
Table 1.
Macular and Peripapillary Retinal Capillary Measurement by OCT Angiography at Baseline, 12 Months, and 24 Months after Plaque Brachytherapy
| All Patients (n = 21) | No Radiation Retinopathy at 24 Months (n = 10) | Radiation Retinopathy at 24 Months (n = 11) | |
|---|---|---|---|
| mSVC_VD (% area) | |||
| Baseline | 48.4±3.6 | 48.1±4.1 | 49.0±4.0 |
| 12 mos | 43.5±5.9 (P = 0.01) | 46.8±3.3 (P = 0.232) | 40.3±6.1 (P = 0.002) |
| 24 mos | 37.4±9.1 (P < 0.001) | 42.4±5.1 (P = 0.02) | 32.5±9.7 (P = 0.002) |
| FAZ area (mm2) | |||
| Baseline | 0.26±0.11 | 0.26±0.09 | 0.26±0.12 |
| 12 mos | 0.35±0.22 (P = 0.009) | 0.27±0.08 (P = 0.375) | 0.42±0.27 (P = 0.10) |
| 24 mos | 0.81±1.03 (P = 0.001) | 0.30±0.10 (P = 0.426) | 1.33±1.29 (P = 0.004) |
| NFLP_CD (% area) | |||
| Baseline | 48.4±4.1 | 49.5±4.2 | 47.3±4.1 |
| 12 mos | 46.7±5.0 (P = 0.04) | 47.4±3.8 (P = 0.131) | 46.0±6.1 (P = 0.275) |
| 24 mos | 44.5±6.1 (P < 0.001) | 46.9±4.6 (P = 0.04) | 41.7±6.7 (P = 0.008) |
FAZ = foveal avascular zone; mSVC_VD = macular superficial vascular complex vessel density; NFLP_CD = peripapillary nerve fiber layer capillary density.
P< 0.05 was used to define significance.
Figure 1.
Graphs showing the (A) macular superficial vascular complex vessel density (mSVC_VD), (B) foveal avascular zone (FAZ) area, and (C) peripapillary nerve fiber layer capillary density (NFLP_CD) for all patients at baseline, 12 months, and 24 months. Each line represents a single eye treated with plaque brachytherapy. Eyes with radiation retinopathy (RR) or papillopathy at 24 months are shown as solid red lines. Eyes without radiation retinopathy or papillopathy at 24 months are shown as dashed blue lines.
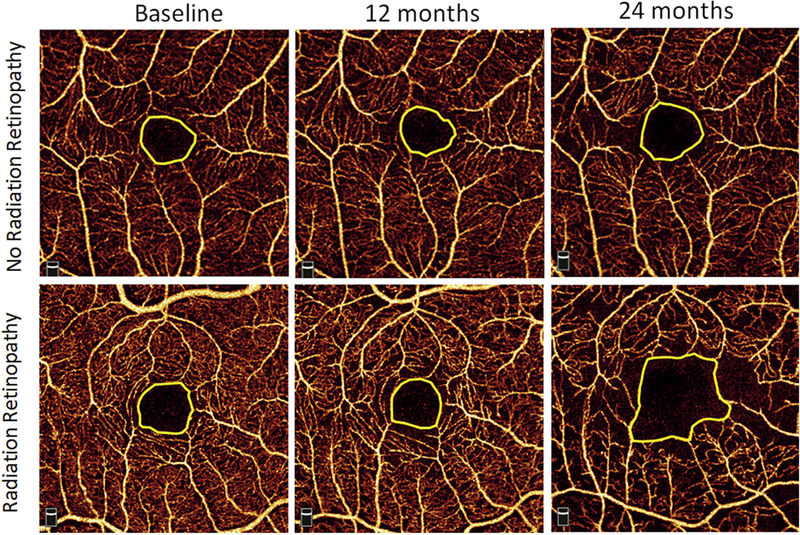
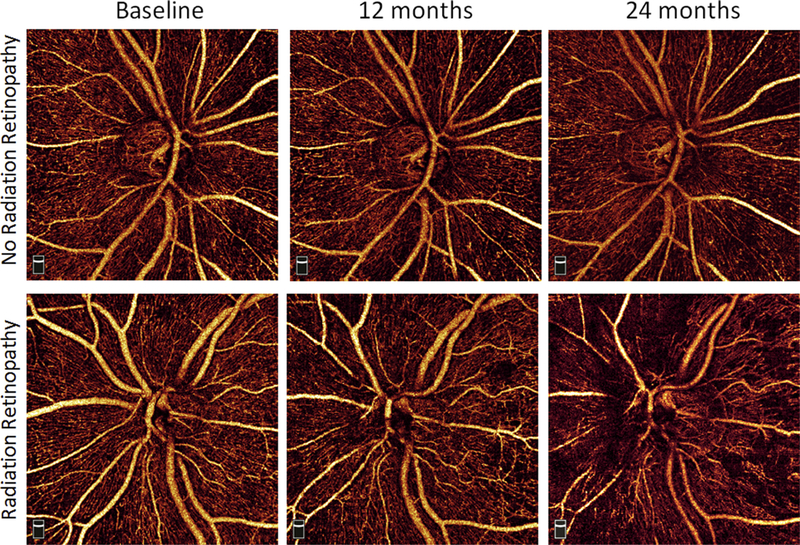
After brachytherapy, the visual acuities in the irradiated eyes ranged from 20/20 to 20/400 at 12 months and from 20/20 to light perception at 24 months. The patient with light perception vision experienced a vitreous hemorrhage at 24 months, and OCTA measurements were not possible, so measurements obtained at 18 months when the vision was counting fingers were used for this patient. Compared with eyes before treatment (48.4±4.1%), the NFLP_CD was reduced significantly in treated eyes at 12 months (46.7±5.0%; P = 0.04) and 24 months (44.5±6.1%; P < 0.001; Table 1; Fig 1). Similarly, the mSVC_VD (48.4±3.6%) was reduced significantly in treated eyes at 12 months (43.5±5.9%; P = 0.01) and 24 months (37.4±9.1%; P < 0.001). The FAZ area increased significantly in eyes treated with radiation at 12 months (0.35±0.22 mm2; P = 0.009) and 24 months (0.81±1.03 mm2; P = 0.001) as compared with that before treatment (0.26±0.11 mm2). When only eyes with clinically evident radiation changes were evaluated, the changes in retinal capillary vasculature were more pronounced, with NFLP_CD measuring 41.7±6.7% at 24 months in eyes with radiation retinopathy or optic neuropathy compared with 47.3±4.1% before treatment (P = 0.008). Macular superficial vascular complex vessel density measured 32.5±9.7% at 24 months after radiation as compared with (49.0±4.0%; P = 0.002) at baseline. Even among eyes without clinically apparent radiation changes, at 24 months, a significant reduction was observed in NFLP_CD (46.9±4.6%) and mSVC_VD (42.4±5.1%) as compared with baseline (49.5±4.2% [P = 0.04] and 48.1±4.1% [P = 0.02], respectively). Whereas in eyes without radiation retinopathy, no significant change was seen at 24 months in FAZ area compared with baseline, eyes with clinical signs of radiation retinopathy demonstrated enlarged FAZ area (1.33±1.29 mm2) as compared with baseline (0.26±0.12 mm2; P = 0.004). Representative examples of the macular and peripapillary OCTA en face images are shown in Figures 2 and 3, respectively.
Figure 2.
Representative example of macular superficial vascular complex and foveal avascular zone (represented by yellow line) at baseline, 12 months, and 24 months for a patient with no radiation retinopathy at 24 months (top row) and a patient who demonstrated radiation retinopathy by 24 months (bottom row).
Figure 3.
Representative example of peripapillary nerve fiber layer capillary density at baseline, 12 months, and 24 months for a patient with no radiation retinopathy at 24 months (top row) and a patient who demonstrated radiation retinopathy by 24 months (bottom row).
We previously demonstrated an inverse linear correlation between radiation dose (radiation dose to 50% of the optic disc) and the peripapillary capillary density (now called the NFLP_CD) among patients treated with I125 plaque brachytherapy for uveal melanoma who demonstrated clinically apparent radiation retinopathy.16
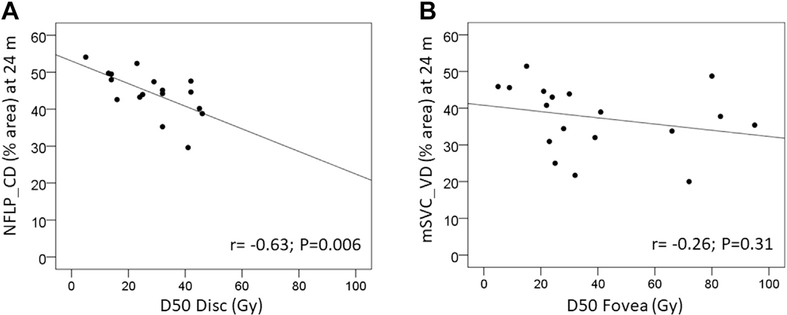
Among the patients within the current cohort, who were followed up longitudinally, again an inverse linear correlation between the radiation dose to the nerve (radiation dose to 50% of the optic disc) and the NFLP_CD at 24 months was demonstrated (Pearson r =−0.63; P = 0.006; Fig 4A). There was no overlap in patients between the prior publication and the current study. A less pronounced inverse linear correlation between the radiation dose to the fovea (radiation dose to 50% of the fovea) and the mSVC_VD was observed. This was not statistically significant (Pearson r = −0.26; P = 0.31; Fig 4B).
Figure 4.
A, Scatterplot showing that peripapillary nerve fiber layer capillary density (NFLP_CD) at 24 months correlates with the radiation dose (Gy) to the optic nerve as measured by radiation dose to 50% of the optic disc (D50 disc). The relationship between NFLP_CD and D50 disc was examined using Pearson’s correlation coefficient. Each eye is indicated with a point on the plot. A best-fit line is shown. B, Scatterplot showing a similar trend between the radiation dose to 50% of the fovea and the macular superficial vascular complex vessel density (mSVC_VD), although this was not statistically significant. P< 0.05 was used to define significance.
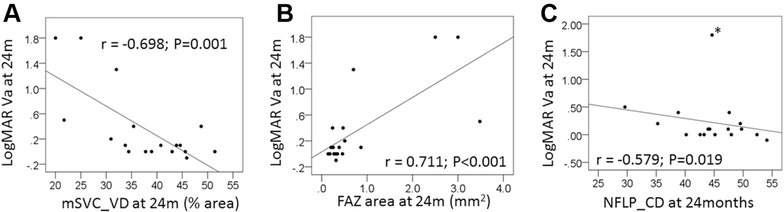
The visual acuity in the treated eyes after brachytherapy was correlated significantly with both the mSVC_VD and the FAZ area (Fig 5). At 24 months (Pearson r =−0.698; P = 0.001), an inverse linear correlation was found between the logarithm of the minimum angle of resolution (logMAR) visual acuity and the mSVC_VD in the treated eye (Fig 5A). A linear correlation was found between the FAZ area and LogMAR visual acuity at 24 months (Pearson r = 0.711; P < 0.001; Fig 5B). Similarly, at 24 months, an inverse linear correlation was found between the NFLP_CD and the logMAR visual acuity when an outlier with severe macular ischemia was not included in the analyses (Pearson r =−0.579; P = 0.019; Fig 5C).
Figure 5.
Correlation between the logarithm of the minimum angle of resolution (logMAR) visual acuity (VA) at 24 months and OCT angiography measurements. Each eye is indicated with a point on the plot. A, The relationship between macular superficial vascular complex vessel density (mSVC_VD) and logMAR VA was examined at 24 months using Pearson’s correlation coefficient. B, The relationship between foveal avascular zone (FAZ) area and logMAR VA was examined at 24 months using Pearson’s correlation coefficient. C, The relationship between peripapillary nerve fiber layer capillary density (NFLP_CD) and logMAR VA was examined at 24 months using Pearson’s correlation coefficient. The asterisks indicate an outlier with severe macular ischemia. This eye was not included in the Pearson’s correlation coefficient presented. P< 0.05 was used to define significance.
We were interested in whether the OCTA measurements at 12 months would be predictive for emergence of clinically apparent radiation retinopathy in the future. When the mSVC_VD values at 12 months for patients who at that time showed no signs of radiation retinopathy, but who would demonstrate signs within the next year, were compared with macular superficial vascular complex values among those who would not demonstrate radiation retinopathy within the year, a predictive relationship was revealed. By Cox proportional hazards regression, a 1% decrease in mSVC_VD was associated significantly with development of radiation retinopathy at 24 months, with a hazard ratio of 1.13 (95% confidence interval, 1.03−1.25; P = 0.01). Comparison of the NFLP_CD and FAZ area did not reveal any predictive capacity in eyes without clinically apparent radiation retinopathy at 12 months in which clinically apparent radiation retinopathy developed by 24 months.
Discussion
Radiation retinopathy and optic neuropathy are vision-threatening complications of radiation treatment for uveal melanoma. OCT angiography provides a unique, quantitative, and highly reproducible method for evaluating regional retinal blood flow and vessel density in regions critical for vision. Herein, we demonstrated that OCTA can be used to monitor vascular compromise longitudinally after radiation therapy in eyes with uveal melanoma, that ischemic changes are observed in both the peripapillary and macular circulations, and that measurement of OCTA macular vascular density may be able to predict the future development of clinically apparent radiation retinopathy.
OCT angiography detected significant changes in the peripapillary and macular microvasculature earlier than clinical examination or structural OCT. Among patients without clinically apparent radiation retinopathy or optic neuropathy, significant reduction in both macular and peripapillary vessel density was measured by OCTA by 24 months after brachytherapy. The FAZ area did not change significantly over this period in eyes without clinically detectable retinopathy, suggesting this is a later finding. The reductions in mSVC_VD and NFLP_CD were greater in eyes with clinical evidence of disease. In these eyes, the FAZ area was also significantly increased after radiation.
Similar to our prior study, an inverse relationship was found between the radiation dose to the disc (radiation dose to 50% of the optic disc) and the peripapillary vessel density (NFLP_CD). A weaker correlation was seen between the radiation dose to the macula (radiation dose to 50% of the fovea) and the macular microvasculature (mSVC_VD). This may reflect that maculopathy is less predictably related to radiation dose or may be related to the small sample size in this study.
The logMAR visual acuity at 24 months was correlated significantly with both the mSVC_VD and the FAZ area. The NFL_CD correlated inversely with LogMAR visual acuity at 24 months as well but only when an outlier with severe macular ischemia was excluded from calculations. These findings are consistent with the concept that preserved macular anatomic features are essential for maintaining vision in eyes treated with radiation.
Data are emerging that demonstrate responses to anti-VEGF therapies for patients with radiation retinopathy, allowing some patients to retain better vision after radiotherapy, particularly those with macular edema. However, the timing for initiation of therapy is a matter of some debate. It is possible that monitoring patients with OCTA after radiation treatment may allow clinicians to identify those patients with subclinical radiation retinopathy not yet detectable by clinical examination or structural OCT who may most benefit from initiation of anti-VEGF treatment.
In the future, a large, multicenter prospective study evaluating peripapillary and macular ischemia with OCTA in patients treated with plaque brachytherapy for uveal melanoma will allow further evaluation of OCTA as a tool for both measuring vascular compromise after radiation treatment and, perhaps more importantly, for predicting the emergence of clinically significant radiation retinopathy and optic neuropathy so that treatment can be initiated. Responses to anti-VEGF agents could also be evaluated. Such a study could be conducted in parallel with one directed at evaluating optimal treatment protocols for radiation retinopathy. The data generated by such a study would improve our understanding of radiation retinopathy and optic neuropathy significantly and how to maintain vision in eyes requiring radiation treatment for uveal melanoma most effectively.
Acknowledgments
The authors thank C. R. Thomas, Jr., MD, Department of Radiation Medicine Chair, Oregon Health & Science University, for support of this work.
Financial Disclosure(s):
The author(s) have made the following disclosure(s): A.H.S.: Consultant, Advisory board, Financial support - Immunocore, Castle Biosciences, Inc. D.H.: Financial support, Patents, Royalties, Equity owner - Optovue, Inc.
Y.J.: Financial support, Patents, Royalties - Optovue, Inc.
Oregon Health & Science University has a significant financial interest in Optovue, Inc, a company that may have a commercial interest in the results of this research and technology. Other authors do not have financial interest in the contents of this article.
Supported by the National Institutes of Health, Bethesda, Maryland (grant nos.: R01 EY027833, R01 EY023285, R01 EY024544, and P30EY010572); a Lloyd Research Endowment Faculty Grant, Portland, Oregon; the Oregon Health & Science University Radiation Medicine Academic Development Fund, Portland, Oregon; Research to Prevent Blindness, New York, New York (unrestricted grant).
Abbreviations and Acronyms
- FAZ
foveal avascular zone
- I125
iodine 125
- logMAR
logarithm of the minimum angle of resolution
- mSVC_VD
macular superficial vascular complex vessel density
- NFLP_CD
peripapillary nerve fiber layer plexus capillary density
- OCTA
OCT angiography
- VEGF
vascular endothelial growth factor
Footnotes
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Oregon Health & Science University approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
References
- 1.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881e1885. [DOI] [PubMed] [Google Scholar]
- 2.Melia BM, Abramson DH, Albert DM, et al. Collaborative Ocular Melanoma Study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years. COMS report no. 16. Ophthalmology. 2001;108:348e366. [DOI] [PubMed] [Google Scholar]
- 3.Gunduz K, Shields CL, Shields JA, et al. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. 1999;117:609e614. [DOI] [PubMed] [Google Scholar]
- 4.Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol. 2005;89:730e738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayreh SS. Post-radiation retinopathy. A fluorescence fundus angiographic study. Br J Ophthalmol. 1970;54:705e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown GC, Shields JA, Sanborn G, et al. Radiation retinopathy. Ophthalmology. 1982;89:1494e1501. [DOI] [PubMed] [Google Scholar]
- 7.Wen JC, Oliver SC, McCannel TA. Ocular complications following I-125 brachytherapy for choroidal melanoma. Eye (Lond). 2009;23:1254e1268. [DOI] [PubMed] [Google Scholar]
- 8.Yousef YA, Finger PT. Optical coherence tomography of radiation optic neuropathy. Ophthalmic Surg Lasers Imaging. 2012;43:6e12. [DOI] [PubMed] [Google Scholar]
- 9.Giuliari GP, Sadaka A, Hinkle DM, Simpson ER. Current treatments for radiation retinopathy. Acta Oncol. 2011;50: 6e13. [DOI] [PubMed] [Google Scholar]
- 10.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710e4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veverka KK, AbouChehade JE, Iezzi R Jr, Pulido JS. Noninvasive grading of radiation retinopathy: the use of optical coherence tomography angiography. Retina. 2015;35: 2400e2410. [DOI] [PubMed] [Google Scholar]
- 13.Shields CL, Say EA, Samara WA, et al. Optical coherence tomography angiography of the macula after plaque radiotherapy of choroidal melanoma: comparison of irradiated versus nonirradiated eyes in 65 patients. Retina. 2016;36: 1493e1505. [DOI] [PubMed] [Google Scholar]
- 14.Matet A, Daruich A, Zografos L. Radiation maculopathy after proton beam therapy for uveal melanoma: optical coherence tomography angiography alterations influencing visual acuity. Invest Ophthalmol Vis Sci. 2017;58:3851e3861. [DOI] [PubMed] [Google Scholar]
- 15.Cennamo G, Breve MA, Velotti N, et al. Evaluation of vascular changes with optical coherence tomography angiography after plaque radiotherapy of choroidal melanoma. Ophthalmic Res. 2018;60:238e242. [DOI] [PubMed] [Google Scholar]
- 16.Skalet AH, Liu L, Binder C, et al. Quantitative OCT angiography evaluation of peripapillary retinal circulation after plaque brachytherapy. Ophthalmol Retina. 2018;2:244e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah SU, Shields CL, Bianciotto CG, et al. Intravitreal bevacizumab at 4-month intervals for prevention of macular edema after plaque radiotherapy of uveal melanoma. Ophthalmology. 2014;121:269e275. [DOI] [PubMed] [Google Scholar]
- 18.Shah NV, Houston SK, Markoe AM, et al. Early SD-OCT diagnosis followed by prompt treatment of radiation maculopathy using intravitreal bevacizumab maintains functional visual acuity. Clin Ophthalmol. 2012;6:1739e1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen JC, McCannel TA. Treatment of radiation retinopathy following plaque brachytherapy for choroidal melanoma. Curr Opin Ophthalmol. 2009;20:200e204. [DOI] [PubMed] [Google Scholar]
- 20.Samara WA, Shahlaee A, Adam MK, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124:235e244. [DOI] [PubMed] [Google Scholar]
- 21.Hollo G Intrasession and between-visit variability of sector peripapillary Angioflow vessel density values measured with the AngioVue optical coherence tomograph in different retinal layers in ocular hypertension and glaucoma. PLoS One. 2016;11:e0161631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiter GS, Told R, Schlanitz FG, et al. Longitudinal association between drusen volume and retinal capillary perfusion in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60:2503e2508. [DOI] [PubMed] [Google Scholar]
- 23.Horgan N, Shields CL, Mashayekhi A, Shields JA. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol. 2010;21:233e238. [DOI] [PubMed] [Google Scholar]