Abstract
Nanoliposome is a useful dosage form to increase solubility and absorption of simvastatin (SMV), and consequently improves its therapeutic effects. However, in vivo toxicity of SMV could also be elevated accompanied by the absorption enhancement, which is a decisive factor for the clinical application of SMV nanoliposome (SMV-Lipo), but has not been studied systematically and reported so far. In this study, organ toxicity of SMV-Lipo was evaluated in mice in the presence and absence of isoproterenol and compared to those of free SMV. Results demonstrated that compared to free SMV, the SMV-Lipo administrated at an equal dose of 25 mg/kg/d led to severe myocardiotoxicity, hepatotoxicity at baseline and more pronounced liver injury with elevation of alanine aminotransferase. In addition, muscular adverse effect was also observed in SMV-Lipo treated group but not in SMV group. Pharmacokinetic studies revealed that compared to free SMV, the SMV-Lipo administration significantly improved the plasma SMV concentration, and the oral bioavailability was 6.5 times of free SMV. Notably, when the dosage of free SMV increased to 50 mg/kg/d, yielding the comparable plasma concentration as SMV-Lipo given at 25 mg/kg/d, the myocardiotoxicity was observed in free SMV treated mice as well, which further confirmed that the enhanced absorption of SMV by the nanoliposomal formulation resulted in more severe myocardiotoxicity than the equal dose of free SMV.
Keywords: Simvastatin, Nanoliposome, Myocardiotoxicity, Muscular toxicity, Hepatotoxicity
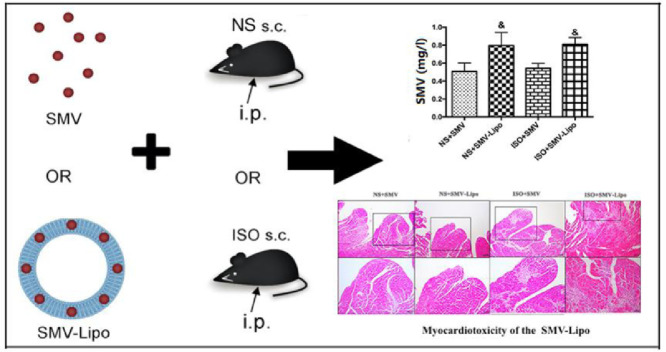
Graphical abstract
When given at the same dosage and administration regimen, the SMV-Lipo presented significant myocardiotoxicity due to the significant enhancement of the plasma SMV concentration by nanoliposomes.
1. Introduction
Simvastatin (SMV) is the most frequently prescribed statin for hypercholesterolemia [1], [2], which blocks 3-hydroxy-3-methyl coenzyme A (HMG-CoA) reductase, a rate-limiting enzyme of the cholesterol biosynthetic pathway [3]. SMV can effectively lower total and low-density lipoprotein (LDL) cholesterol in blood and alleviate hypercholesterolemia, which is crucial to prevention of atherosclerosis and coronary heart diseases. Multiple large cardiovascular disease outcome trials such as PROVE-IT, TNT and IDEAL corroborated the clinical value of statin therapy in prevention of atherosclerotic cardiovascular disease [4], [5], [6].
Isoproterenol (ISO) is a non-selective β-adrenergic receptor (β-AR) agonist. Excessive β-AR stimulation is closely related to cardiac remodeling which is characterized by hypertrophy and fibrosis both in vivo and in vitro [7], [8]. In clinic, excessive activation of the sympathetic nerve is one of the common pathological features of many patients with cardiovascular diseases such as hypertension, coronary heart disease, arrhythmias and cardiomyopathy. SMV has been reported to have protective effects on ISO-induced cardiac hypertrophy [9].
With the wide use in clinic, the adverse effects of statins, such as gastrointestinal disturbance, hepatotoxicity and myopathy, are reported increasingly [10]. It was reported that the rate of statin-related events was nearly 18% and musculoskeletal symptoms predominated accounting for 40% of statin-related events, which is manifested in different forms [11]. According to ACC/AHA/NHLBI and FDA [12], definitions of muscular adverse effects of statin therapy include asymptomatic Creatine Kinase (CK, biochemical marker enzyme of myocardial injury) elevation, myopathy (every kinds of muscular complaints), myalgia (muscular complaints combined with CK elevation) and Rhabdomyolysis (muscular complaints with pronounced CK elevation more than 10 times the upper limit of normal).
SMV is known to be lipophilic statin, limited solubility and irregular intestinal absorption are important causes of its low bioavailability less than 5% [13]. Thus, many various drug delivery systems such as polymeric micro/nanoparticle, dendrimer and liposome have been designed for improvement of SMV delivery [14], [15], [16]. Among those, nanoliposome is of great interest since it has been regarded as an efficient dosage form to increase the pharmacodynamics of the hydrophobic drugs through increasing solubility and absorption of the loaded drugs by encapsulating which within the bilayers of the liposomes [17], [18]. In previous publication, we reported that the SMV-Lipo remarkably increased the plasma concentration of SMV, compared to the free SMV, which result from the great solubilization and high efficient clathrin-mediated transepithelial transport of the SMV nanoliposome [19]. In addition, we proved the much greater inhibitory effect of SMV-Lipo than free SMV on ISO-induced cardiac hypertrophy and fibrosis, when given at the same dose of 10 mg/kg/d and same administration regimen (i.p.) [20].
It has been commonly accepted that within the appropriate dose range, therapeutic effect of statin is dose-dependent and high-dose statin therapy could more efficiently prevent cardiovascular events than standard-dose therapy [4]. So, based on the results of SMV given at 10 mg/kg/d (i.p.), a 2.5 times higher dosage of 25 mg/kg/d (i.p.) was also used to evaluate their pharmacodynamics. Unexpectedly, the HE staining showed that the SMV-Lipo at this high dose produced a pronounced myocardiotoxicity, which evidenced by severe inflammatory cells infiltration and cell death, which has not been reported as an in vivo side effect of SMV so far. There are a few studies implied that statins can induce cardiomyocytes death or have toxic effects on cardiomyocytes, but the results are all from cellular experiments [21], [22], [23]. Hence, it is important to unveil the potential risk of systemic application of the SMV or SMV-Lipo and bring attention of people who use this medicine in clinic. Based on these, we selected 25 mg/kg/d as the dosage applied in this study to evaluate and compare the toxicities of the SMV and the SMV-Lipo on cardiomyocytes, muscle, liver and kidney.
Based on the preliminary work, the myocardiotoxicity of SMV-Lipo were observed in the mice which were subjected to isoproterenol (ISO) and SMV-Lipo simultaneously. In addition, it is not clear whether the SMV-Lipo presents myocardiotoxicity without ISO stimulation per se. Therefore, in this study, the myocardiotoxicity of the SMV and the SMV-Lipo were evaluated in the presence or absence of ISO stimulation. Besides, the toxicities of SMV and SMV-Lipo on muscle, liver and kidney were also compared, since muscular toxicity, hepatotoxicity and nephrotoxicity are the major SMV-related toxicities reported in clinic. In addition, the plasma SMV concentrations were determined by HPLC to confirm the improvement of SMV absorption and the potential aggravation of the nanoliposomal formulation on the SMV-related myocardiotoxicity and hepatotoxicity. Besides, to clarify whether the myocardiotoxicity observed in the SMV-Lipo treated mice arise from the enhanced absorption of SMV by the nanoliposomal formulation or from the SMV-Lipo itself, a high dose of the free SMV (50 mg/kg/d) was administrated to mice by i.p. to achieve a close plasma SMV concentration of the toxic SMV-Lipo, and the myocardiotoxicity of the free SMV was evaluated.
2. Material and methods
2.1. Materials
All the materials and reagents are detailed in the supplementary material.
2.2. Animals
Male BALB/c mice of 10 weeks old, weighting from 24 to 25 g were obtained from SPF Biotechnology Co., Ltd. (Beijing, China). The ARRIVE guidelines were followed and all animal studies were performed based on the approval by Animal Care Committee, Peking University Health Science Center (LA2010-059). All mice were acclimatized for one week prior to experimentation. This article does not contain any studies with human participants performed by any of the authors.
2.3. SMV-Lipo preparation and in vivo studies
We prepared the SMV-Lipo as we described in our previously published paper, and achieved comparable characteristic parameters of the SMV-Lipo (supplementary materials) as we reported before [19].
To evaluate the toxicities of the SMV-Lipo, 48 male BALB/c mice were randomly assigned to six groups. The group assignment and drug treatment are shown in Table 1. Briefly, the mice were subjected to daily subcutaneous (s.c.) injection of normal saline (NS) or ISO (5 mg/kg/d) for 7 d. The mice received intraperitoneal (i.p.) injection of SMV (25 mg/kg/d), SMV-Lipo (at an SMV dose of 25 mg/kg/d); The mice in the control group were treated with the blank nanoliposome to evaluate its toxicity; treatment began on the same day when ISO inducement or vehicle administration started and lasted for 7 d. After 7 d, cardiac function of the mice was evaluated using echocardiography (detailed in supplementary material), toxicities including myocardiotoxicity, muscular toxicity, hepatotoxicity and nephrotoxicity were evaluated in the presence or absence of ISO. Myocardiotoxicity was evaluated by HE staining (histological analysis is available in supplementary material), serum lactate dehydrogenase (LDH) activity, serum creatine kinase MB isoenzyme (CK-MB) concentration and the mRNA expression of fetal cardiac genes. Muscular toxicity was detected by serum creatine kinase (CK) activity and potassium concentration. Hepatotoxicity was evaluated by HE staining and serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity. Nephrotoxicity was evaluated by serum urea concentration and the ratio of N-acetyl-β-d-glucosaminidase (NAG) and creatinine (Cr) in urine. Real-time PCR was conducted for detecting the expressions of the genes involved.
Table 1.
The group assignment and drug treatment for toxicities studies of the SMV and the SMV-Lipo (SMV dose was 25 mg/kg/d).
| Group | Model (s.c.) |
Drug administration regimen(i.p.) |
||||
|---|---|---|---|---|---|---|
| Saline | ISO | Blank Lipo | SMV | SMV-Lipo | ||
| Basal | Control | + | – | + | – | – |
| NS+SMV | + | – | – | + | – | |
| NS+SMV-Lipo | + | – | – | – | + | |
| ISO | ISO | – | + | + | – | – |
| ISO+SMV | – | + | – | + | – | |
| ISO+SMV-Lipo | – | + | – | – | + | |
In addition, to verify whether the myocardiotoxicities would also occur in the free SMV treated mice at a high SMV dose (50 mg/kg), 16 male BALB/c mice were randomly divided into four groups: NS, NS+SMV (50 mg/kg i.p.), ISO, ISO+SMV (50 mg/kg i.p.). The group assignment and drug treatment are shown in are shown in Table 2.
Table 2.
The group assignment and drug treatment for investigation of myocardiotoxicities of the free SMV (SMV dose was 50 mg/kg/d).
| Group | Model (s.c.) |
Drug administration (i.p.) |
|||
|---|---|---|---|---|---|
| Saline | ISO | vehicle | SMV | ||
| Basal | Control | + | – | – | – |
| NS+SMV | + | – | – | + | |
| ISO | ISO | – | + | + | – |
| ISO+SMV | – | + | – | + | |
2.4. Real-time quantitative PCR
The method of real-time quantitative PCR is detailed in supplementary material and the primer sequence is shown in Table 3.
Table 3.
Primer sequence.
| Genes | Forward (5′−3′) | Reverse (5′−3′) |
|---|---|---|
| F4/80 | TGACTCACCTTGTGGTCCTAA | CTTCCCAGAATCCAGTCTTTCC |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| ANF | GCTTCCAGGCCATATTGGAG | GGGGGCATGACCTCATCTT |
| BNP | AGGACCAAGGCCTCACAAAA | ACTTCAAAGGTGGTCCCAGAG |
| β-actin | GAGACGGAGCTGTTGGTAAAA | TCTTGCTCAGTGTCCTTGCTGG |
Abbreviations: ANF, Atrial Natriuretic Peptide; BNP, Brain Natriuretic Peptide.
2.5. Serum biomarkers determination
For determination of LDH activity and CK-MB concentration, blood samples were collected and centrifuged to obtain serum. LDH and CK-MB were spectrophotometrically assayed using the kits as the manufacturer's protocol.
To determine activities of CK, ALT, AST and potassium level, blood samples from each group were collected and centrifuged (Dragon Lab DM1424, Beijing, China) at 1672 g and 4 °C for 10 min to separate plasma. Blood samples were assayed using 7020 Automatic analyzer (Hitachi High-Technologies, Japan).
2.6. Pharmacokinetics studies and determination of plasma SMV concentrations
To determine the formulation effects of the nanoliposome on the pharmacokinetics of SMV, 16 BALB/c mice were randomly divided to two groups (8 mice in each group) and subjected to a single i.p. administration of SMV (25 mg/kg) or SMV-liposomes (equivalent to 25 mg/kg SMV). The blood samples were collected at time points of 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.75, 5.5, 7, and 10 h. Plasma SMV concentrations were determined by HPLC (supplementary material).
2.7. Statistical analysis
All data were presented as mean ± SD and analyzed with SPSS19.0. One-way analysis of variance (ANOVA) were used to compare difference and P < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Effects of the SMV and the SMV-Lipo on cardiac functions
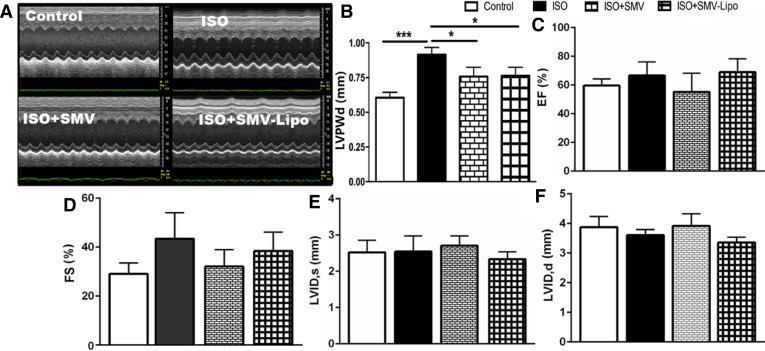
As cardiac function maintenance is crucial for the patients taking cholesterol-lowering agents to prevent the cardiovascular events. We first investigated the mice cardiac function. Echocardiography revealed that compared to the healthy animal, the mice subjected to ISO exhibited a marked increase in diastolic left ventricle posterior wall thickness (LVPW, d) (0.61 ± 0.03 vs. 0.89 ± 0.09, P < 0.001), suggesting that ISO administration result in cardiac hypertrophy in the model group. Treatment with the SMV or SMV-Lipo significantly decreased this parameter (P < 0.05), suggesting that the SMV and SMV-Lipo at 25 mg/kg/d significantly inhibited ISO-induced cardiac hypertrophy (Fig. 1A and 1B), which is consistent with our previous results [20]. Other parameters including Ejection fraction (EF%), percent fractional shortening (FS%), left ventricle internal diameter at end-systole (LVID, s), left ventricle internal diameter at end-diastole (LVID, d) had no significant difference among all the groups (Fig. 1C–1F), indicating that the ISO, SMV and SMV-Lipo with the experimental regimen would not affect cardiac function significantly, due to the powerful compensatory mechanism of the heart.
Fig. 1.
Cardiac functions of the mice. (A) Images of M-mode of LV; (B) LVPWd; (C) EF%; (D) FS%; (E) LVID, s; (F) LVID, d; * P < 0.05, *** P < 0.001.
3.2. Myocardiotoxicity evaluation of the SMV and SMV-Lipo
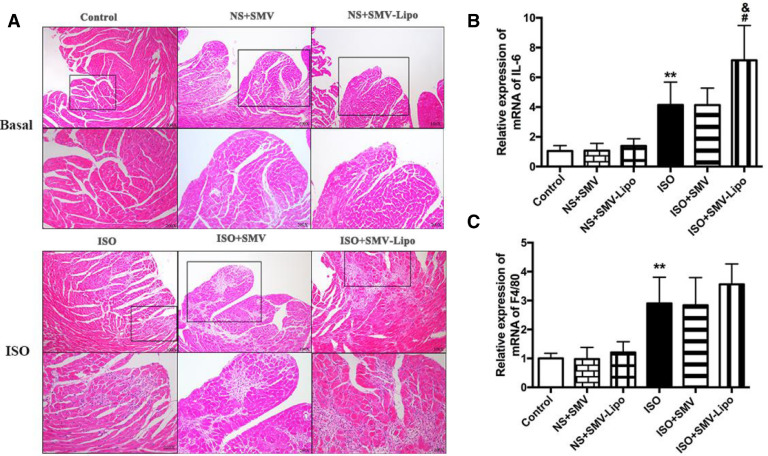
Myositis, which commonly affects skeletal muscle, is well characterized by an acute muscle ache, pain or other muscular complaints and myocyte necrosis allied with infiltration of inflammatory cells in biopsy [24], [25], [26]. HE staining of the heart sections (Fig. 2A) showed that at baseline without ISO stimulation, the mice subjected to SMV or SMV-Lipo alone were basically normal compared to the healthy animals. Mice in the ISO model group showed inflammatory cells infiltration and myocardiocytes necrosis. ISO+SMV-Lipo treatment remarkably deteriorated the ISO-induced inflammatory response which proved by the more diffused myocardiocytes loss and numerus inflammatory cells infiltrated in the interstitium. The extensive lesions in the SMV-Lipo group indicated that the SMV-Lipo triggered excessive cardiomyocytes loss and inflammatory response. The pathological changes displayed in the SMV-Lipo group highly suggested that statin related myositis could affect cardiomyocytes as well. It is reported that Lovastatin lead to the reduction of cardiomyocyte viability in a dose-dependent manner and part of this cardiotoxicity may involve oncotic and apoptotic cell death [27], [23], which can be partly blocked by caspase-3 inhibitor. This might be an explanation for severe inflammatory cells infiltration in the SMV-Lipo group.
Fig. 2.
Myocardiotoxicity of the SMV-Lipo. (A) Representative images of HE staining, original magnification: × 100 (upper); × 200 (bottom); The mRNA expression of (B) IL-6 and (C) F4/80. * P < 0.05, ** P < 0.01.
Similarly, ISO stimulation significantly upregulated the expressions of IL-6 and F4/80 (Fig. 2B and 2C). The mice subjected to SMV only had no difference with those in the ISO model group. However, the mice in the ISO+SMV-Lipo group displayed a more pronounced increase in the relative expressions of IL-6 mRNA compared to the ISO model group, which indicated that the SMV-Lipo exacerbated the ISO-induced inflammation. Notably, under ISO stimulation, compared to the SMV group, the mice received SMV-Lipo showed significantly higher mRNA expression of IL-6, suggesting that when administrated at same dosage, the SMV-Lipo exhibited toxic effects on cardiomyocytes while SMV crude drug has no toxicity. ISO has been reported to impair the free fatty acid uptake in cardiomyocytes [28], and it is reported that atorvastatin could inhibited the insulin-mediated glucose uptake [29]. Therefore, the metabolic disorder may be the reason of the SMV-Lipo related cardiomyocytes death.
3.3. Effects of the SMV and SMV-Lipo on serum biochemical parameters
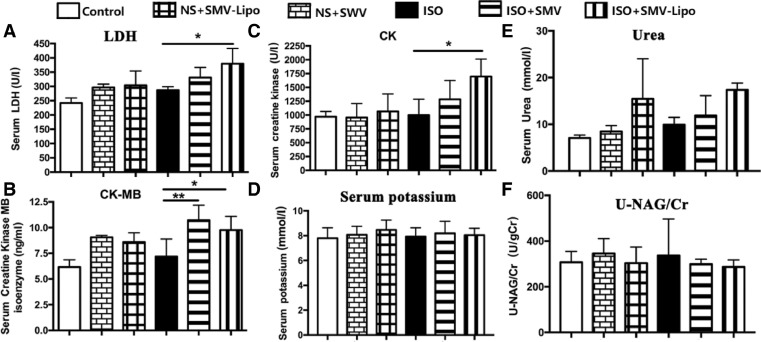
The serum activity of LDH, which is a crucial enzyme for energy metabolism in heart and is generally used as a laboratory index for myocardial injuries, was significantly elevated by the SMV-Lipo (Fig. 3A). Compared to the healthy animals, ISO stimulation increased the LDH by 22.4% (242.1 ± 17.72 vs. 296.4 ± 22.54, P > 0.05). Under ISO stimulation, the SMV-Lipo further elevated the serum LDH level (379.8 ± 52.97 vs. 296.4 ± 22.54, P < 0.05) while SMV had no difference with ISO group, which indicated that the SMV-Lipo aggravated ISO induced myocardiocytes injury. To exclude the elevation of LDH due to other types of muscle injuries, we tested cardiac specific CK-MB (Fig. 3B). The levels of released CK-MB in the blood stream were comparable between the SMV and SMV-Lipo group at the basal line without ISO stimulation or after ISO stimulation. Since SMV blocks the cholesterol synthesis and membrane cholesterol modulates membrane fluidity in various tissues. The SMV may disrupt the membrane fluidity and integrity of cardiomyocytes, which eventually lead to leakage of cellular content and cell death, which is deteriorated by the SMV-Lipo, as evidenced by LDH activity and CK-MB. At the same time, the blank Lipo is nontoxic to cardiomyocytes because the lipids commonly used in preparation of liposomes are found biocompatible, biodegradable and less immunogenic in the human body [30], [31]. The observed more severe toxicity in SMV-Lipo group possibly arises from the loaded SMV.
Fig. 3.
The serum biochemical parameters. (A) Serum LDH activity; (B) Serum CK-MB concentration; (C) Serum activity of CK (U/l); (D) Serum concentrations of potassium; (E) Serum concentration of urea; (F) Serum urinary N-acetyl-β-glucosaminidase (NAG)/creatinine concentration (Cr) ratio. * P < 0.05, ** P < 0.01.
Skeletal muscular adverse effect is the most commonly reported side effect of statins. According to ACC/AHA/NHLBI and FDA definitions [12], the muscular adverse effects of statin are characterized by muscle pain or weakness combined with increased concentration of CK in blood, which is a protein biomarker of the damaged myocytes. The results showed that compared to the healthy animals in control group, ISO, SMV, SMV-Lipo treated mice showed comparable levels of serum CK. ISO had no direct effect on CK. Compared to ISO model group, SMV administration elevated the CK level by 17.1% and SMV-Lipo elevated the CK level by 69.4% (1696 ± 316.8 vs. 1001 ± 284.6, P < 0.05), Which suggested that the mice subjected to ISO+SMV-Lipo displayed muscular disorder (Fig. 3C). Among all the groups, when administrated at the equal dosage and regimen, the SMV-Lipo but not the SMV led to a significant CK elevation. Since the blank liposome had no muscular toxicity, indicating that the SMV related muscular toxicity might be amplified by the SMV-Lipo. Potassium elevation, which is an important indication for rhabdomyolysis, was not observed in all mice groups (Fig. 3D). These results could be explained as that the short period of drug administration and the dosage used in this study induced muscular disorder, but without life-threatening rhabdomyolysis.
Some of breakdown products of the damaged skeletal muscles, such as the protein myoglobin may lead to kidney failure. Therefore, we next evaluated the nephrotoxicity of SMV-Lipo. At basal condition, there were no significance among the control, SMV and SMV-Lipo groups. But the level of serum urea in SMV-Lipo treated mice had an upward tendency compared to their respective control, which indicated the SMV-Lipo may affect the kidney function (Fig. 3E). To determine whether the SMV-Lipo injured the kidney structure, the ratio between the NAG and creatinine in urine was investigated (Fig. 3F), as it is a sensitive biomarker of renal tubular injury in clinic. However, NAG/Cr ratio showed no difference among all these groups, indicating that there was no organic lesion in renal tubules under SMV or SMV-Lipo treatment with or without ISO inducement.
3.4. Hepatotoxicity evaluation of the SMV and SMV-Lipo
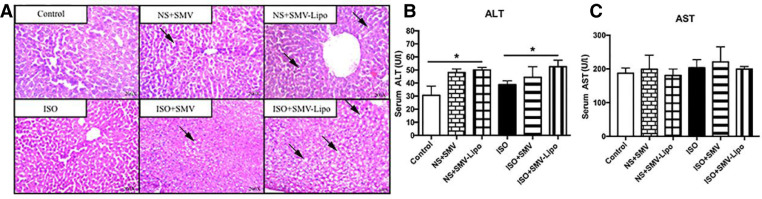
Another commonly reported adverse effect of statin is hepatotoxicity, which is characterized by the elevated plasma concentrations of transaminases. The incidence of liver toxicity is far less than that of the muscular adverse effects [32]. At the basal condition without ISO inducement, HE staining (Fig. 4A) showed that without ISO induction, the lobular structures and liver cells are basically normal in the SMV group, while liver cells in the SMV-Lipo group arranged in disorder and centrilobular hepatocytes showed microvesicular steatosis (accumulation of small lipid droplets in cytoplasm) compared with the liver of healthy animals. ISO stimulation did not affect the liver morphology compared to the healthy animals. Compared to the ISO model, the liver cells in SMV-Lipo groups were severely damaged and showed diffused microvesicular steatosis, indicating the injury of liver cells caused by SMV-Lipo administration. In both condition with or without ISO stimulation, the liver enzyme ALT were significantly elevated by SMV-Lipo treatment compared with their respective controls (Fig. 4B). The AST level were comparable among all groups (Fig. 4C), which may because the ALT is more sensitive indicator than AST for early and acute hepatic damage. Taken together, the hepatotoxicity of SMV were aggravated by the SMV-Lipo. The mechanisms may involve several aspects. It is reported that SMV inhibits not only the cholesterol biosynthesis but also the lipophilic side chain of ubiquinone, which is very important for the electron transport chain (ETC) in mitochondria [33]. The disruption of mitochondrial respiratory function could be responsible for the observed organ toxicities, particularly in heart (Fig. 2, Fig. 3A and B) and liver (Fig. 4). As the metabolism in these two organs is exuberant and they are rich in mitochondria. Besides, SMV is hydrolyzed in the liver, the increased accumulation of the SMV in the hepatocytes enhanced by liposomal formulation may be another reason of the SMV-Lipo-induced hepatocytes toxicity.
Fig. 4.
Hepatotoxicity of the SMV-Lipo. (A) HE staining of the liver; SMV-Lipo‐induced vacuolar degeneration of cells is indicated by arrows; Serum activity of (B) ALT (U/l) and (C) AST (U/l); * P < 0.05.
3.5. Plasma concentrations and pharmacokinetics of the SMV and SMV-Lipo
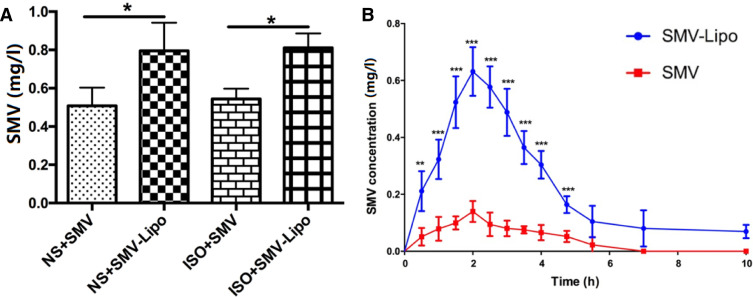
In our previous work, we have proved that the blank Lipo had no toxicity in the presence or absence of ISO [19]. The pronounced myocardiotoxicity, muscular and hepatotoxicity of the SMV-Lipo drew our attention to the plasma SMV concentration. The results of HPLC analysis showed that compared to the SMV group, the stable plasma SMV concentration was significantly higher in the SMV-Lipo group, when they were administrated to the mice at the equal dosage of 25 mg/kg/d and regimen (i.p.) (Fig. 5A).
Fig. 5.
Plasma concentrations and Pharmacokinetics of the SMV and SMV-Lipo. (A) Stable plasma SMV concentrations of SMV groups (25 mg/kg) and SMV-Lipo groups (25 mg SMV/kg) after i.p. administration for 5 d; (B) Plasma concentrations of SMV after a single i.p. injection of SMV (25 mg/kg) and SMV-Lipo (25 mg SMV/kg); * P < 0.05, ** P < 0.01, *** P < 0.001.
Besides, the results of the pharmacokinetics study revealed that the maximum plasma concentrations (Cmax) of SMV and the SMV-Lipo treated groups were 145.83 ± 34.2 and 653.64 ± 74.1 (P < 0.001), respectively. The Cmax of the SMV-Lipo treated group was 4.48 times higher than the free SMV (P < 0.001) (Fig. 5B). The key parameters of pharmacokinetics are shown in Table 4. The results demonstrated that compared to free SMV, the liposome formulation remarkably promoted the bioavailability of SMV to 6.5 times. These results further proved that SMV-Lipo significantly increased the absorption and bioavailability of SMV.
Table 4.
Main pharmacokinetics parameters of SMV and the SMV-Lipo.
| Parameters | SMV | SMV-Lipo |
|---|---|---|
| Cmax(µg/l) | 145.83 ± 34.2 | 653.64 ± 74.1⁎⁎⁎ |
| Tmax(h) | 2.00 ± 0.0 | 2.00 ± 0.5 |
| T1/2(h) | 1.75 ± 0.4 | 2.11 ± 0.4 |
| AUC0-10(µg•h/l) | 385.07 ± 65.9 | 2246.84 ± 292.2⁎⁎⁎ |
| AUC0-∞(µg•h/l) | 454.14 ± 161.7 | 2930.00 ± 387.6⁎⁎ |
| Fr(%) | 100 | 645 |
P < 0.01,.
P < 0.001 vs. the SMV group.
3.6. Myocardiotoxicities of the free SMV
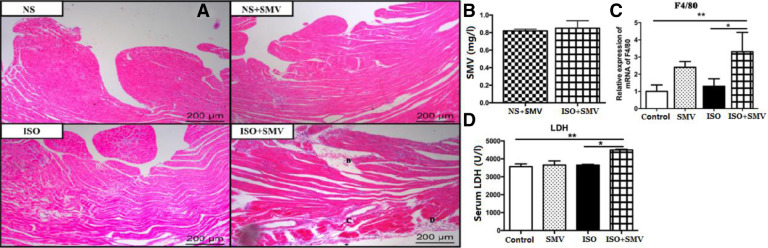
To further clarify that the myocardiotoxicities of the SMV-Lipo did arise from the enhanced absorption of SMV but not from nanoliposome itself, we increased the dosage of free SMV to 50 mg/kg/d (i.p.). The results of HPLC showed that the stable plasma SMV concentration of the free SMV treated mice group yielded about 0.8 mg/L, which was at the comparable plasma SMV concentration of the toxic SMV-Lipo given at 25 mg/kg (Fig. 6B). HE staining of the heart sections (Fig. 6A) showed that at baseline without ISO stimulation, the mice subjected to SMV alone had no obvious abnormality compared to the healthy animals. Mice in the ISO model group showed inflammatory cells infiltration. Notably, the inflammatory response was aggravated in the mice subjected to ISO and SMV simultaneously. Consistently, the mRNA expression of biomarker of the macrophage F4/80 was significantly up-regulated (Fig. 6C) and myocardiocytes injury indicator LDH in serum was also significantly increased in the ISO+SMV group (Fig. 6D), which indicated that when the plasma concentration of SMV is equivalent to the SMV-Lipo, the same myocardiotoxicities could also occur in the free SMV treated mice.
Fig. 6.
Myocardiotoxicity of the free SMV. (A) Representative images of HE staining; (B) Stable plasma SMV concentrations of the SMV groups (50 mg/kg); The mRNA expression of (C) F4/80 and (D) LDH. * P < 0.05, ** P < 0.01.
These results further confirmed that the SMV-Lipo induced significant myocardiotoxicity, as well as the muscular toxicity and hepatotoxicity, due to the absorption enhancement of SMV by nanoliposomal formulation. So, the toxicities of SMV, especially the myocardiotoxicity, demonstrated in this study are not only crucial for the SMV-Lipo, but also cannot be neglected for the all commercialized SMV formulations aimed to improve the bioavailability or for long-term application of SMV. The latter might also lead to the accumulation of SMV and their plasma concentrations of SMV could be elevated either in some circumstances (eg. drug interactions or some drugs inhibiting the activity of liver drug-metabolizing enzymes), and subsequently the myocardiotoxicity of SMV would occur unavoidably.
4. Conclusion
In this study, we demonstrated that when given at the same dosage and administration regimen, the SMV-Lipo presented significant myocardiotoxicity, muscular toxicity and hepatotoxicity due to the absorption enhancement of SMV by nanoliposomal formulation.
Acknowledgments
Declaration of interest
The authors declare no conflicts of interest with other organizations or individuals regarding the content or publication of this paper.
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (No. 81770268) and the National Basic Research Program of China (No. 2015CB932100).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2019.02.002.
Appendix. Supplementary materials
References
- 1.Jackevicius C.A., Mamdani M., Tu J.V. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen T.R., Kjekshus J., Berg K., Haghfelt T., Faergeman O., Faergeman G. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the scandinavian simvastatin survival study (4S).1994. Atheroscler Suppl. 2004;5(3):81–87. doi: 10.1016/j.atherosclerosissup.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Corsini A., Bellosta S., Baetta R., Fumagalli R., Paoletti R., Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84(3):413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Cannon C.P., Braunwald E., McCabe C.H., Rader D.J., Rouleau J.L., Belder R. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 5.Waters D.D., Guyton J.R., Herrington D.M., McGowan M.P., Wenger N.K., Shear C. Treating to new targets (TNT) study: does lowering low-density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit? Am J Cardiol. 2004;93(2):154–158. doi: 10.1016/j.amjcard.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Scheen A.J. The IDEAL study comparing simvastatin 20-40mg versus atorvastatin 80mg for secondary prevention after myocardial infarction: between two ideas of the ideal. Rev Med Liege. 2006;61(1):53–59. [PubMed] [Google Scholar]
- 7.Lohse M.J., Engelhardt S., Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93(10):896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 8.Xiao H., Li H., Wang J.J., Zhang J.S., Shen J., An X.B. IL-18 cleavage triggers cardiac inflammation and fibrosis upon beta-adrenergic insult. Eur Heart J. 2018;39(1):60–69. doi: 10.1093/eurheartj/ehx261. [DOI] [PubMed] [Google Scholar]
- 9.Al-Rasheed N.M., Al-Oteibi M.M., Al-Manee R.Z., Al-Shareef S.A., Al-Rasheed N.M., Hasan I.H. Simvastatin prevents isoproterenol-induced cardiac hypertrophy through modulation of the JAK/STAT pathway. Drug Des Devel Ther. 2015;9:3217–3229. doi: 10.2147/DDDT.S86431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J., Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Plutzky J., Skentzos S., Morrison F., Mar P., Shubina M. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasternak R.C., Smith S.C., Jr., Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–572. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 13.Tubic-Grozdanis M., Hilfinger J.M., Amidon G.L., Kim J.S., Kijek P., Staubach P. Pharmacokinetics of the CYP 3A substrate simvastatin following administration of delayed versus immediate release oral dosage forms. Pharm Res. 2008;25(7):1591–1600. doi: 10.1007/s11095-007-9519-6. [DOI] [PubMed] [Google Scholar]
- 14.Pouponneau P., Leroux J.C., Soulez G., Gaboury L., Martel S. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials. 2011;2(13):3481–3486. doi: 10.1016/j.biomaterials.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 15.Al-Jamal W.T., Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44(10):1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 16.Li C., Zhang X., Huang X., Wang X., Liao G., Chen Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int J Nanomedicine. 2013;8:1285–1292. doi: 10.2147/IJN.S41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikhaylov G., Mikac U., Magaeva A.A., Itin V.I., Naiden E.P., Psakhye I. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat Nanotechnol. 2011;6(9):594–602. doi: 10.1038/nnano.2011.112. [DOI] [PubMed] [Google Scholar]
- 18.Ullrich K.J., Papavassiliou F. Contraluminal transport of small aliphatic carboxylates in the proximal tubule of the rat kidney in situ. Pflugers Arch. 1986;407(5):488–492. doi: 10.1007/BF00657505. [DOI] [PubMed] [Google Scholar]
- 19.Qi R., Zhang H., Xu L., Shen W., Chen C., Wang C. G5 PAMAM dendrimer versus liposome: a comparison study on the in vitro transepithelial transport and in vivo oral absorption of simvastatin. Nanomedicine. 2015;11(5):1141–1151. doi: 10.1016/j.nano.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Tuerdi N., Xu L., Zhu B., Chen C., Cao Y., Wang Y. Preventive effects of simvastatin nanoliposome on isoproterenol-induced cardiac remodeling in mice. Nanomedicine. 2016;12(7):1899–1907. doi: 10.1016/j.nano.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Costa R.A., Fernandes M.P., de Souza-Pinto N.C., Vercesi A.E. Protective effects of l-carnitine and piracetam against mitochondrial permeability transition and PC3 cell necrosis induced by simvastatin. Eur J Pharmacol. 2013;701(1–3):82–86. doi: 10.1016/j.ejphar.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Rabkin S.W., Kong J.Y. Lovastatin-induced cardiac toxicity involves both oncotic and apoptotic cell death with the apoptotic component blunted by both caspase-2 and caspase-3 inhibitors. Toxicol Appl Pharmacol. 2003;193(3):346–355. doi: 10.1016/j.taap.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Rabkin S.W., Lodha P., Kong J.Y. Reduction of protein synthesis and statin-induced cardiomyocyte cell death. Cardiovasc Toxicol. 2007;7(1):1–9. doi: 10.1007/s12012-007-0003-7. [DOI] [PubMed] [Google Scholar]
- 24.Staffa J.A., Chang J., Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346(7):539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 25.Thompson P.D., Clarkson P., Karas R.H. Statin-associated myopathy. JAMA. 2003;289(13):1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 26.Ballantyne C.M., Corsini A., Davidson M.H., Holdaas H., Jacobson T.A., Leitersdorf E. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163(5):553–564. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- 27.Demyanets S., Kaun C., Pfaffenberger S., Hohensinner P.J., Rega G., Pammer J. Hydroxymethylglutaryl-coenzyme A reductase inhibitors induce apoptosis in human cardiac myocytes in vitro. Biochem Pharmacol. 2006;71(9):1324–1330. doi: 10.1016/j.bcp.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Heather L.C., Catchpole A.F., Stuckey D.J., Cole M.A., Carr C.A., Clarke K. Isoproterenol induces in vivo functional and metabolic abnormalities: similar to those found in the infarcted rat heart. J Physiol Pharmacol. 2009;60(3):31–39. [PubMed] [Google Scholar]
- 29.Jiang Z., Yu B., Li Y. Effect of three statins on glucose uptake of cardiomyocytes and its mechanism. Med Sci Monit. 2016;22:2825–2830. doi: 10.12659/MSM.897047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goyal P., Goyal K., Vijaya Kumar S.G., Singh A., Katare O.P., Mishra D.N. Liposomal drug delivery systems–clinical applications. Acta Pharm. 2005;55(1):1–25. [PubMed] [Google Scholar]
- 31.Mugabe C., Halwani M., Azghani A.O., Lafrenie R.M., Omri A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(6):2016–2022. doi: 10.1128/AAC.01547-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiner Z., Catapano A.L., De Backer G., Graham I., Taskinen M.R., Wiklund O. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 33.Satoh K., Yamato A., Nakai T., Hoshi K., Ichihara K. Effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on mitochondrial respiration in ischaemic dog hearts. Br J Pharmacol. 1995;116(2):1894–1898. doi: 10.1111/j.1476-5381.1995.tb16679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.