Abstract
Telomeres ensure chromosome length homeostasis and protection from catastrophic end-to-end chromosome fusions. All eukaryotes require this essential, strictly conserved telomere-dependent genome preservation. However, recent evolutionary analyses of mammals, plants, and flies report pervasive rapid evolution of telomere proteins. The causes of this paradoxical observation – that unconserved machinery underlies an essential, conserved function – remain enigmatic. Indeed, these fast-evolving telomere proteins bind, extend, and protect telomeric DNA, which itself evolves slowly in most systems. We hypothesize that the universally fast-evolving subtelomere – the telomere-adjacent, repetitive sequence – is a primary driver of the ‘telomere paradox’. Under this model, radical sequence changes in the subtelomere perturb subtelomere-dependent, telomere functions. Compromised telomere function then spurs adaptation of telomere proteins to maintain telomere length homeostasis and protection. We propose an experimental framework that leverages both protein divergence and subtelomeric sequence divergence to test the hypothesis that subtelomere sequence evolution shapes recurrent innovation of telomere machinery.
Keywords: telomere, rapid evolution, positive selection, paradox, subtelomere, selfish genetic elements
Telomeres preserve genome integrity and transmission
More than 1.5 billion years ago, linear chromosomes emerged from their circular chromosome ancestors [1–5]. Encoding DNA in a linear arrangement conferred myriad evolutionary advantages, including meiotic chromosome segregation [6]. However, linear chromosomes also posed a new threat to genome integrity—a chromosome end. Incomplete lagging strand synthesis at terminal ends erodes unique genetic elements, i.e., the ‘end-replication problem’ [7, 8]. Additionally, naked chromosome ends appear as double-stranded breaks to DNA repair machinery. These mis-identified chromosome termini trigger cellular senescence and/or end-to-end chromosome fusions [9–11]. Fused chromosomes break during mitosis, further imperiling genome integrity, transmission, and ultimately, cell viability and development.
The nucleoprotein structure found at contemporary eukaryotic chromosome ends—telomeres—counteract these threats to genome integrity [10, 12–14].Telomeres are universally comprised of non-unique sequence complexed with specialized and non-specialized packaging proteins. The recurrently refreshed telomeric DNA serves as a template on which an RNA primer assembles, ensuring complete synthesis of the unique sequence on the lagging strand [15]. Specialized proteins that bind this repetitive DNA regulate telomere length homeostasis [16] and effectively ‘hide’ chromosome ends from DNA repair machinery [10, 17]. Together, telomeric DNA and telomeric proteins preserve genome integrity.
All eukaryotes depend on chromosome elongation and protection of chromosome ends from fusion [18]. Indeed, preservation of unique DNA and the resolution of chromosome ends are pervasive, vital functions. However, recent studies of telomere evolution across closely related species suggest that telomere proteins diversify over remarkably short evolutionary timescales [19–21]. Such rapid evolutionary change is unexpected. Maintenance of linear chromosome ends is essential and conserved. Reminiscent of the ‘centromere paradox’ (see Glossary) articulated two decades ago [22], pervasive evolution beneath an ostensibly static chromosomal function implicates an ever-present pressure on telomeres to constantly change (Box 1). However, unlike the fast-evolving centromeric DNA proposed to drive rapid centromere protein evolution [22], telomeric DNA evolves comparatively slowly across eukaryotes (www.telomerase.asu.edu/sequences_tr.html). We hypothesize instead that the universally fast-evolving subtelomere – the telomere-adjacent, satellite-rich sequence – is a primary driver of this paradox [21, 23]. Consistent with this possibility, the short timescale of subtelomeric sequence divergence mirrors the short timescale of telomere protein divergence[24–27]. Moreover, subtelomere chromatin packaging and transcriptional regulation have profound effects on adjacent telomere function[28]. We conclude with calls to improve subtelomere assembly and annotation to facilitate subtelomere evolutionary analysis. We propose evolution-guided experiments both to detect the functional consequences of telomere protein evolution and to evaluate the interactions among heterologous proteins and subtelomere variants. These experiments will elucidate the forces shaping recent telomere protein innovations. Moreover, appreciating the causes and consequences of telomere protein evolution between close relatives might offer insights into the well-described radical differences in telomere protein identity, sequence, and function across anciently diverged model systems [18, 29–32].
Text Box 1: Lessons from the centromere paradox for conceptualizing telomere evolution.
Centromeres direct chromosome segregation during meiosis and mitosis. Despite strict conservation of most chromosome segregation machinery, the underlying DNA and chromatin proteins of centromeres instead evolve rapidly. Twenty years ago, Henikoff, Ahmad, and Malik named this surprising observation, the ‘Centromere Paradox’ [22]. The authors conjectured that selfish, centromeric satellite sequence gains preferential access to the oocyte during female meiosis, causing the rapid rise and replacement of satellite variants. This turnover, which would result in different centromeric sequences between even close relatives, was proposed to drive the signatures of adaptive evolution at centromere proteins. Under this model, centromere proteins evolve to suppress the incidental deleterious consequence of heterogeneous centromere pairing during male meiosis. Empirical evidence of such ‘selfish centromeres’ soon followed [95, 96]. Inspired by several features of the centromere paradox, we propose that telomeres also harbor this paradoxical evolution.
At first glance, the evolution of centromeres and telomeres is fundamentally different. The key observation underlying the centromere paradox is fast-evolving centromeric DNA. Telomerase-dependent species encode a telomeric repeat that instead evolves slowly (see main text). Also, the localization of the histone variant that defines where centromere proteins assemble (CenpA in mammals) is largely sequence-independent [97] whereas such telomere machinery as TRF1 and TRF2 are sequence-dependent [10]. Despite these important differences, centromeres and telomeres harbor several strikingly analogous patterns of evolution.
Centromere proteins undergo dramatic turnover across evolution. The proteins CenpT, CenpX, CenpH, and CenpK, among many others, appear at human and budding yeast kinetochores (the complex to which microtubules attach to the centromere) but are absent from D. melanogaster kinetochores [98]. Whole-protein turnover is also pervasive at diverged telomeres across similar timescales [18, 29–32]. Additionally, rapid evolution driven by positive selection shapes both centromere proteins [99–101] and telomere proteins (see main text), rendering orthologous genes wildly divergent across close relatives. Finally, evidence of recurrent gene duplication from parent genes such as the CenpA ortholog (e.g.,[58]) mirror duplications from such telomere integrity genes like Pot1, hiphop, and caravaggio (see main text).
In the original centromere paradox formulation, rapid evolution of not only centromeric DNA but also pericentromeric DNA was invoked to explain fast evolution of centromere proteins [22]. Indeed, the pericentric heterochromatic sequence also evolves rapidly and disruption of pericentric chromatin packaging results in centromere instability and dysfunction [102]. This coupling of pericentromeric and centromeric biology parallels the coupling of subtelomeric and telomeric biology. By analogy to the centromere paradox, the subtelomeric sequences, which evolve at a similarly rapid pace to the pericentromeric DNA, might be an important driver of telomere protein evolution. Functional crosstalk between the subtelomere and the telomere establishes the possibility of co-evolution between subtelomere sequence and telomere proteins. Specifically, we propose that new variants arising in the subtelomere can perturb telomere length homeostasis, protection, and nuclear positioning, spurring telomere protein evolution to maintain canonical telomere function. Meiotic drive, the proposed cause of the centromere paradox, is one of several evolutionary pressures that we envisage selects for new subtelomeric variants (Box 2).
Telomere elongation mechanisms across eukaryotes
In most eukaryotes, the telomerase holoenzyme catalyzes DNA repeat addition via reverse transcription of the TERC (‘TElomerase RNA Component’) non-coding RNA [13–15, 33]. Telomerase appears in fungal, plant, ciliate, invertebrate, and vertebrate genomes, among others [3, 34], pointing to its ancient origins and conserved terminal repeat-addition function. All vertebrates sampled thus far share the (TTAGGG)n terminal repeat[35]. This repeat is also found in corals, sponges, flatworms and some fungal species (e.g., Neurospora crassa) [36, 37]. Such deep homology points to an ancient origin not only of telomerase but also our contemporary telomeric DNA repeat sequence. Eukaryotes that do not have (TTAGGG)n typically have instead only slight deviations, such as Tetrahymena thermophila (TTGGGG)n [12], Caenorhabditis elegans (TTAGGC)n [38] and Arabidopsis thaliana (TTTAGGG)n [39]. Extreme deviations appear across distant fungal species such as Candida albicans (ACGGATGTCTAACTTCTTGGTGT)n, Saccharomyces cerevisiae (C1–3A/TG1–3)n and Schizosaccharomyces pombe (TTAC(A)G2–5)n [37].
Even more extreme deviations from the ancient (TTAGGG)n-based telomeres are found in rare species that do not encode telomerase at all. Species from the onion genus, Allium, and non-Drosophilids from the insect order, Diptera, encode no telomerase and instead harbor long arrays of simple and complex satellites at chromosome termini [40]. These unusual telomeres are thought to depend instead on an ancient, strictly conserved recombination-based elongation mechanism found in flies, worms, and mammals [30, 41–43]. In humans, this alternative elongation pathway, called ALT (‘Alternative Lengthening of Telomeres’), is most prominent in the 10–15% of cancers that fail to reactivate telomerase [42].
Arguably the most deviant telomeres are those of Drosophila melanogaster. D. melanogaster has neither telomerase-added repeats nor satellite sequence at its telomeres. Instead, D. melanogaster harbors arrays of specialized retrotransposons [44]. These retrotransposons belong to a sub-family of mobile elements from the ‘jockey’ non-LTR family and insert almost exclusively at the terminal ends. This mobile element-dependent mechanism of telomere elongation is also found in a distant relative D. virilis, consistent with a single retrotransposon ‘domestication event’ that has been vertically transmitted for over 40 million years [45]. This elongation mechanism, however, can be lost as well. A study this year uncovered the recent collapse of the retrotransposon-based mechanism along the lineage leading to D. biarmipes, a species that ostensibly relies now only on an ALT-like mechanism to elongate its telomeres [46].
Such ancient and radical changes in telomere elongation mechanisms likely precipitated radical changes to the telomere proteins required to maintain length homeostasis, end-protection, and nuclear positioning. Indeed, proteins that make up the mammalian end-protection complex, Shelterin, are not found in the Drosophila end-protection complex, Terminin [47]. However, new analyses of telomere proteins along recent evolutionary timescales suggest that radical transitions of telomere elongation mechanisms cannot account for observed turnover of telomere protein residues, whole proteins, and protein functions across eukaryotes. Below we review rapid telomere evolution across species that share either the transposon-based or telomerase-based elongation mechanisms.
Rapid and recurrent telomere protein evolution across eukaryotes
The recent explosion of publicly available genome sequences of individuals from natural populations or from closely related species offers unprecedented power to examine recent and recurrent adaptive telomere protein evolution [19, 20, 48]. Several new studies have leveraged these resources and discovered pervasive signals of rapid evolution driven by positive selection at essential telomere proteins as well as recurrent gene duplication (Figure 1A).
Figure 1. Telomere proteins evolve rapidly between closely related species, possibly driven by subtelomere evolution.
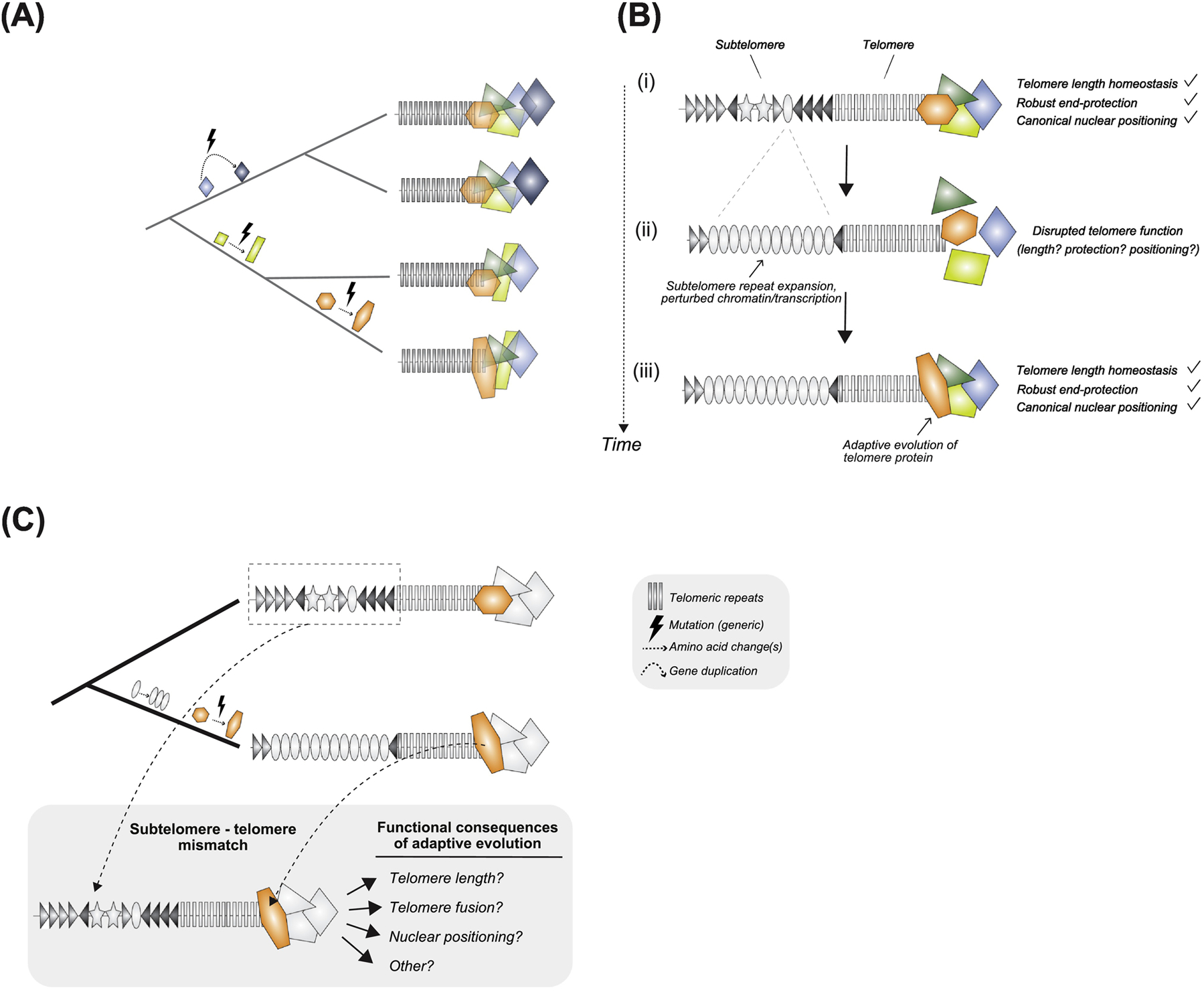
(A) Mutations arise over evolutionary time that, if retained, result in codon divergence (orange, yellow shapes) or whole gene presence/absence (blue diamonds). (B) Telomere proteins adapted to telomere-adjacent subtelomere sequence confers canonical telomere function (top). Under the proposed model, transient disruption to telomere function due to expansion of a particular subtelomere variant (oval, middle) compromises fitness. Natural selection restores canonical telomere function via telomere protein adaptation (orange shape, bottom). (C) Engineering an evolutionary mismatch between the contemporary subtelomeric sequence of one species and the contemporary but divergent telomere protein of a sister species. Experimental introduction of co-evolved subtelomeric variants into these mismatched genotypes might rescue the disrupted telomere function.
One recent study conducted a comprehensive population genetic, molecular evolution, and phylogenomic analysis of genes required for telomere integrity in Drosophila [20]. These genes, when depleted or mutated, cause telomere length changes or telomere fusions. The authors detected statistical enrichment of rapid protein evolution in both mutant phenotype classes compared to genome-wide averages. These data suggest that, like classically fast-evolving immune [49, 50] and reproduction factors [51–53], telomere integrity genes as a group are unusual for their rapid rates of evolution over short stretches of evolutionary time. The study next delineated those genes specifically evolving under positive selection, i.e., genes that rapidly (and non-randomly) accumulate amino acid-changing mutations that result in species differences. The authors detected this signature using both population genetic data from D. melanogaster individuals compared to the closest relative, D. simulans, and phylogenetic data spanning 15 million years of Drosophila evolution. These signatures of adaptation appear in genes required for telomere length homeostasis and telomere end-protection from chromosome fusions [20], suggesting evolutionary pressure on both functions to change over time. These data are reminiscent of evolutionary rates documented across centromere proteins cited in the original formulation of the centromere paradox (Box 1).
In addition to adaptively evolving telomere proteins, the study also documented recent and rampant gene birth, death, and turnover within gene families founded by telomere integrity genes. Previous work had uncovered K81, a young, sperm-specific protein duplicated from a ubiquitously expressed parent gene HipHop [54]. In D. melanogaster, HipHop protects both somatic and germline chromosome ends from fusions [55, 56]. K81 replaces HipHop at telomeres during late sperm development and travels into the egg where it protects paternal telomeres from end-to-end fusions. The more recent study documented numerous k81-like daughter genes across 15 million years of Drosophila evolution. Most daughter genes were expressed primarily in testis and/or ovaries. There was even one case of hiphop degeneration and putative replacement by a daughter hiphop gene (in a new location). In addition to hiphop-derived duplications, the authors also documented duplications from the end-protection gene caravaggio, among others [57]. Recurrent duplication offers yet another parallel to the centromere paradox – the centromeric histone duplicates recurrently along distinct but closely related lineages [58] (Box 1).
Recent adaptive telomere protein evolution has been detected not only across telomeric retrotransposon-dependent Drosophila species but also telomerase-dependent species including Arabidopsis [19] and mammals [21, 48]. Importantly, within Arabidopsis and within mammals, the telomerase-added telomeric repeat is static. A. thaliana encodes two full-length, ancient Pot1 gene duplicates, AtPot1a and AtPot1b[59]. AtPOT1a interacts with telomerase to promote repeat addition processivity [60]. AtPOT1b, in contrast, negatively regulates telomerase enzyme activity [61] through its interaction with a different factor (TER2). Leveraging a phylogenetic framework to analyze AtPot1a and AtPot1b alleles from 11 closely related species, the authors detected a statistically significant excess of amino acid-changing mutations in AtPot1a only. The authors went on to show that, in vitro, positively selected sites determine binding affinity of AtPOT1a to CTC1, a member of the telomeric CST complex that likely serves an end-protection function in Arabidopsis [62]. The discovery of adaptive telomere protein evolution in Arabidopsis suggest that even telomerase-dependent telomeres are associated with proteins under pressure to recurrently change. However, the underlying causes of the recent rapid evolution remain enigmatic.
Using the same phylogenetic framework in mammals, another recent study also found pervasive signatures of positive selection at telomere integrity genes across telomerase– dependent species [21]. Sampling spanned humans to armadillos, yet dense sampling within subclades such as primates, rodents, and bats allowed the authors to detect statistically significant accumulation of amino acid-changing mutations at many telomere integrity genes, including those involved in length regulation and end-protection. Signatures of positive selection were particularly striking at three telomere integrity genes specialized for female meiosis – MAJIN, TERB1, and TERB2. These proteins replace the Shelterin components during meiosis I, tethering telomeres to the nuclear membrane [63]. Germline specialization of these fast evolving telomere proteins is reminiscent of germline specialization of the young gene duplicates of Terminin members in Drosophila [20] as well as young duplicates associated with centromeres (Box 1).
What is the universal force shaping telomeres across eukaryotes?
Why hasn’t natural selection honed a single set of unchanging telomere proteins that preserve chromosome ends from erosion and protect chromosome ends from fusions? The answer to this question remains enigmatic. However, the apparent universality of recent telomere protein evolution implicates a universal evolutionary pressure that transcends telomeric sequence identity and elongation mechanism, both of which change only sporadically. Drawing inspiration from the ‘centromere paradox’ ([22], Box 1), we envisage rapidly evolving repetitive sequence shaping and re-shaping telomere proteins over time. This repetitive sequence cannot be the telomeric repeat itself – species with the same telomerase-added repeat unit undergo rapid telomere protein diversification [19, 21]. We support instead the possibility that the subtelomere – the transition zone between telomeric DNA and the unique chromosome-specific DNA – triggers rapid evolutionary change at telomere proteins [21, 23]. To our knowledge, Anderson, Gilliland, and Langley were the first to propose that subtelomeric sequence variation might select for adaptive variation at telomere proteins ([23],Figure 1B).
Subtelomeres are repeat-rich, gene-poor, and dominated by both heterochromatin proteins and silent histone marks [64–68]. This heterochromatin packaging is expected to suppress mobile element insertions and excisions [69] and to engage specialized repair pathways that limit ectopic recombination [70]. Minimized ectopic recombination and suppressed mobile element activity should constrain subtelomere sequence change, yet this genomic region is universally fast-evolving. High rates of non-allelic recombination dominate subtelomeres: interchromosomal telomere exchange is pervasive and intra-telomere recombination causes expansions and contractions of repetitive sequence [25–27]. Even base substitution rates are extremely high [25, 71]. These extreme sequence changes between close relatives have been documented across eukaryotes, including in yeast [71, 72], worms [27], flies [25], and primates [73, 74]. The timescale of such changes is remarkably short—massive structural diversity is detectable even within populations [24–27, 75].
Despite the extreme evolutionary plasticity of subtelomeric sequence, decades of work have established robust functional links between the subtelomere and conserved telomere biology. In multiple systems, the lincRNA transcript encoded by telomeric repeats, called TERRA, initiates transcription in the subtelomere [76]. Both DNA methylation of the subtelomeric CpG island promoters of TERRA [77] and subtelomeric H3K9me3 [78] negatively regulate TERRA in mammalian cells. TERRA transcript accumulation promotes telomere shortening in telomerase-positive cells [79] but promotes elongation of both especially short telomeres [80] and telomeres that depend only on recombination-based elongation [81]. In budding yeast, two subtelomeric proteins (Tbf1 and Reb1) promote telomere shortening [82]. Subtelomere regulation has been linked not only to telomere length but also to telomere stability. In humans, depletion of CTCF at subtelomeres reduces TERRA transcription, destabilizes the binding of telomere proteins TRF1 and TRF2, and elevates telomere damage signaling [83]. In D. melanogaster, depletion of the telomere protein PEO causes end-to-end fusions only of telomeres adjacent to a particular class of subtelomeric chromatin [84]. Finally, accumulating evidence suggests that telomere perturbations affect subtelomere regulation. For example, in budding yeast, depletion of telomere protein Rap1 elevates TERRA transcription [85] and in fission yeast, Shelterin recruits the H3K9 methyltransferase complex to establish heterochromatin in the subtelomere [86].
The functional crosstalk described above raises the possibility that subtelomere sequence and telomere proteins might co-evolve. In general, variation in repeat sequence and array size can impact local chromatin marks, chromatin-bound proteins, and heterochromatin spreading [87, 88]. We predict that particular subtelomere repeat variants, e.g., the presence/absence or expansion/contraction of a satellite array, perturb subtelomere chromatin organization, ncRNA transcription, or nuclear localization. Under the proposed model, subtelomere sequence changes that compromise adjacent telomere function trigger rapid evolution of telomere binding proteins to restore telomere homeostasis and end-protection (Figure 1B). Consistent with the possibility of subtelomere sequence evolution shaping telomere biology, distal cis-acting elements near telomeres determine structural stability and chromosome-specific telomere length in humans [89]. Whether such perturbations select for telomere protein innovation remains to be tested (see future directions).
What is the primary force shaping rapid subtelomeric sequence diversification? The contribution of subtelomeric mutations – expansions and contractions of particular elements due to non-allelic recombination, gene conversion, transposition, replication slippage, and excision – undoubtedly contribute to radical alterations to the underlying sequence. Shaped by neutral processes alone (e.g., genetic drift), slightly deleterious structural changes can rise to high frequency, fix, and ultimately spur adaptation of the proteins that mediate the relevant telomere biology. Alongside such purely stochastic forces, directional selection likely plays an important, if not primary, role in shaping subtelomere sequence evolution. Directional selection on subtelomere-embedded genes and regulatory instructions [90] might inadvertently perturb telomeric processes coupled to the subtelomere; however, we favor the role of selfish genetic elements in shaping the subtelomere landscape (Box 2). The centromere paradox also invoked selfish elements to explain how a conserved, essential function might be supported by fast-evolving machinery (Box 1).
Text Box 2: Selfish elements predicted to shape subtelomere sequence evolution.
We hypothesize that three types of subtelomere-embedded selfish elements – biased gene converters, segregation distorters, or mobile elements — shape subtelomeric sequence turnover. Under this genetic conflict model, telomere proteins evolve rapidly to mediate the incidental deleterious consequences of sequence changes shaped by the rise and fall of these selfish, subtelomere-embedded, elements.
The discovery of rapid evolution at several repair proteins that act on Drosophila telomeres motivated the hypothesis that subtelomere-embedded selfish elements might preferentially recruit proteins that mark the template strand during repair [20]. Biased gene conversion could spread the element onto both homologous and non-homologous chromosomes or even expand in cis if strand invasion occurs via looping. Intriguingly, the positively selected, meiosis-specific telomere proteins MAJIN, TERB1, and TERB2 [21] are present at telomeres precisely during the developmental moment when crossovers are formed and resolved. Indeed, these proteins are required for bouquet formation [63], a structure comprised of clustered telomere ends poised for chromosomal exchange. Adaptive evolution of these proteins along mammalian lineages might shape bouquet architecture and dynamics to suppress the collateral damage of selfish subtelomere repeat expansions. Alternatively, these proteins might evolve to directly suppress episodes of selfish gene conversion.
Another study examining the molecular population genetics of D. melanogaster subtelomeres proposed that ‘meiotic drivers’ embedded in this dynamic region can increase their own likelihood of inclusion into the oocyte rather than into the other meiotic products, called ‘polar bodies’ [92, 93]. The polar bodies represent evolutionary dead ends. Detailed molecular and cell biological analyses in mouse have revealed that manipulation of spindle microtubule attachments to chromosomes mediate selfish chromosome movement toward the oocyte and away from the polar body [95, 103, 104]. In this mouse model, the locus responsible for this biased segregation is an expanded centromere, satisfying a major prediction of the centromere drive model proposed to explain the centromere paradox. Subtelomeric loci might behave like neocentromeres during meiosis II, recruiting microtubules and preferentially gaining access to the oocyte [104, 105]. Under this model, telomere proteins suppress directly selfish subtelomeric variants or instead the collateral damage of selfish subtelomere repeat expansions.
Finally, selfish mobile elements accumulate at subtelomeres. The freshwater crustacean Daphnia has non-long terminal repeat (non-LTR) Hebe elements [106], D. melanogaster has solo-LTR invader elements [66, 107], S. cerevisiae has Ty5 elements [108], Bombyx has the non-LTR SARTBm element, and humans have LTR10, MER61, and Alu elements [109]. These dynamic entities also reorganize subtelomeric DNA as they increase their own genomic copy number [110, 111]. Telomere proteins might evolve to mediate the consequences of evolution (sequence and/or chromatin changes) upon mobile element invasions.
The above pressures sourced in the subtelomere are expected to be universal. However, some telomere evolution must be driven by pressures that are restricted only to specific clades. For example, the retrotransposon-based telomere elongation mechanism in Drosophila raises the possibility that some Drosophila telomere proteins evolve rapidly to police selfish retrotransposons that evolve to escape host control [20, 46]. Similarly, the rapidly-evolving human telomere protein TPP1 restricts herpes virus, which hijacks the telomere to enhance its own replication [112]. Evolution at TPP1 might be required to keep up with viral evolution. Finally, the plasticity of the telomeric repeat sequence across yeast might shape the evolution of sequence-specific telomere binding proteins [113].
Selfish elements enhance their own transmission relative to other elements in the genome from one generation to the next. This transmission advantage arises from non-Mendelian segregation or over-replication in the germline, where selfish elements gain access to the next generation [91]. Gene poor, repetitive genomic regions are especially hospitable habitats for these elements, where copy number expansions are expected to have modest effects on fitness compared to those in the gene-rich euchromatin. Intriguingly, telomere protein novelty is repeatedly associated with the germline, from the young telomere integrity gene duplicates in Drosophila [20] to the meiosis-specific telomere proteins in mammals [21]. These empirical observations raise the possibility that subtelomeric-embedded selfish elements, akin to pathogens, engage the host in genetic conflict. Indeed, like pathogens, selfish elements evolve rapidly to proliferate in host genomes and host genomes evolve rapidly to contain them [92, 93]. We hypothesize that three types of subtelomere-embedded selfish elements—biased gene converters, segregation distorters, and mobile elements—might rapidly and profoundly alter the subtelomeric DNA and organization, shaping directly or indirectly the telomeric proteins and elongation mechanisms that represent key observations of the telomere paradox (Box 2). An experimental approach described below promises to uncover the subtelomere-mediated evolution of telomere proteins.
Concluding remarks and future directions
Recent population genetic and molecular evolution analyses of telomere proteins across closely related species suggest that, independent of telomere elongation mechanism, telomere machinery must recurrently innovate. How do we evaluate the functional consequences of telomere protein evolution and the importance of the subtelomere? Rapid protein evolution offers critical information for uncovering the cryptic evolutionary pressure on telomeres. New publicly available genomes that densely sample individual clades facilitate ‘evolutionary screens’ for these fast-evolving genes. We propose leveraging the diverse alleles from close relatives as an evolution-generated ‘allelic series’. In a focal model system, CRISPR/Cas9-mediated transgenesis can be used to swap heterologous alleles into the native location. If telomere proteins recurrently innovate to optimize species-specific telomere requirements, then heterologous alleles from close relatives should compromise the specific telomere biology shaped by recent evolution (e.g., newly acquired amino acid-changing mutations or the birth of a new gene). The approach aims to disrupt subtelomere-telomere protein co-evolution by engineering an evolutionary ‘mismatch’ between a contemporary telomere protein from one species and the telomeres and subtelomeres of a close relative (Figure 1C). To preserve protein:protein interactions, engineering mosaic proteins or swapping of additional protein partners might be required. We expect such ‘mismatched’ genotypes to exhibit telomere length changes, chromosome fusions, and/or aberrant telomere nuclear positioning.
To determine the role of the subtelomere in shaping these telomere functions and ultimately, telomere protein evolution, we first need to define subtelomere sequence variation. This is a significant challenge. Assembling complete subtelomere sequence is still a major effort. Like centromeric DNA, subtelomeric DNA is often omitted from genome assemblies [67]. Early work that defined subtelomere sequence relied on Southern blots, careful assembly of ‘orphan clones’, and cytogenetics [67]. Fortunately, long read-based assemblies from diverse organisms are rapidly accumulating, ushering forth a new era for dissecting the evolution and function of repetitive genome regions (e.g., [27, 75, 94]). Once the subtelomere of a single representative individual is defined, then sampling individuals from within populations, between populations, or between closely related species will be important for honing in on the sequences that diverge rapidly – both in sequence identity and quantity.
Under the proposed model, particular subtelomere variants affect subtelomere biology, such as subtelomere chromatin organization, transcription, and nuclear positioning. This assumption remains largely untested. Once defined, subtelomere sequence variants that modulate subtelomere functions can be combined with the heterologous alleles to test for genetic interactions between particular variants from the subtelomere and different versions of telomere proteins. Discovery of genetic interactions between heterologous alleles and particular subtelomere variants would be consistent with the potential for subtelomere sequence evolution to shape telomere protein evolution. Depending on the system, testing for genetic interactions might be feasible with CRISPR/Cas9-mediated deletions or additions of particular subtelomere variants or instead, heterologous allele introgression into alternative subtelomeric genetic backgrounds. Given the chromosome-specificity of particular subtelomere variants [94], we expect heterogeneity in the functional readout across different chromosome ends. Experimental evolution can also be leveraged. The compromised fitness of the ‘mismatched’ genotypes (Figure 1C) described above might select for particular subtelomere variants over experimental evolution. Model systems with short generation times are well-suited for whole genome sequencing of experimentally evolved populations to detect such sequence turnover. These manipulated backgrounds are expected to unleash the cryptic pressure of subtelomeres on basic telomere biology. We anticipate that a new focus on recent evolutionary timescales of both telomere proteins and subtelomeric DNA, combined with experimental approaches, will define the ever-changing molecular players, mechanisms, and selection regimes shaping eukaryotic telomeres.
Outstanding Questions.
What are the functional consequences of rapid telomere protein evolution on telomere length homeostasis, end-protection, and nuclear positioning?
What are the evolutionary pressures on telomere proteins to change? Does subtelomeric sequence divergence shape telomere protein divergence?
Which telomere proteins recurrently undergo adaptive protein evolution (or loss of constraint) along multiple independent lineages? ssDNA binding, dsDNA binding, or protein:protein interactors? Which domains undergo adaptive evolution? Are certain residues recurrently selected along independent lineages? Are end-protectors or telomere length regulators more often subjected to positive selection?
What role might neutral processes like genetic drift play in shaping contemporary telomere protein repertoires?
What are the forces that shape subtelomere sequence evolution? Are biased gene converters, meiotic drivers, and/or mobile elements contributing to subtelomere sequence turnover?
What are the functional consequence of subtelomeric sequence evolution on subtelomeric chromatin organization, transcription, and nuclear positioning?
Highlights.
Telomeres preserve genome integrity by ensuring complete replication of unique sequence and protection against end-to-end chromosome fusions.
Although telomere integrity is strictly conserved, telomere proteins evolve rapidly across flies, across plants, and across mammals.
Rapid evolution of the telomere-adjacent subtelomeric sequence, combined with functional crosstalk between telomeres and subtelomeres, raises the possibility that subtelomere sequence evolution drives telomere protein evolution.
We propose that selfish genetic elements shape some subtelomere sequence evolution and ultimately, adaptive telomere protein evolution across eukaryotes.
Acknowledgements
The authors thank the Levine Lab, D. Dudka, I. Drinnenberg, D. Shippen, G. Cenci, H. Malik and two anonymous reviewers for profoundly helpful comments on earlier versions of the manuscript. We apologize to those authors whose work we were unable to include due to space constraints. This work was supported by NIH NIGMS R35GM124684 to MTL.
Glossary
- bouquet formation
the clustering of telomeres at the nuclear periphery during meiotic prophase
- centromere paradox
the observation that faithful chromosome segregation is a strictly conserved cellular process yet the underlying DNA and packaging proteins evolve rapidly (see Box 1)
- CST complex
conserved complex across eukaryotes made up of proteins CTC1, STN1, and TEN1 in mammals (or Cdc13, Stn1, and Ten1 in yeast). Depending on the species, CST supports chromosome end-protection or telomere length regulation.
- fitness
an individual’s ability to survive and reproduce, which translates into its genetic contribution to the next generation
- genetic conflict
the competitive interaction between two genetic parties over evolutionary time, wherein the success of one compromises the success of the other; might be inter-genomic as seen in host-pathogen interactions or intra-genomic as seen in host – selfish element interactions
- genetic drift
the evolutionary force that results in allele frequency changes due to random sampling (rather than adaptation); a population ‘bottleneck’ is a classic example of genetic drift shaping population variation
- orthologous genes
genes with a single common origin, related only through a speciation event
- phylogenomics
the combined use of whole genome sequence data with phylogenetic tree building to infer multigene family evolution; allows delineation of orthologs and paralogs as well as gene losses and gene gains
- positive selection
the directional force shaping the evolutionary trajectory of adaptive mutations
- Shelterin
the protein complex found at the termini of mammalian chromosomes, protects chromosome ends of inappropriate repair and regulates telomerase access
- Terminin
the protein complex found at the termini of Drosophila chromosomes, protects chromosome ends of inappropriate repair, proposed functional analog to the Shelterin complex but derived from distinct origins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Lange T (2004) T-loops and the origin of telomeres. Nat Rev Mol Cell Biol 5 (4), 323–9. [DOI] [PubMed] [Google Scholar]
- 2.French KL et al. (2015) Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc Natl Acad Sci U S A 112 (19), 5915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garavis M et al. (2013) On the origin of the eukaryotic chromosome: the role of noncanonical DNA structures in telomere evolution. Genome Biol Evol 5 (6), 1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koonin EV (2006) The origin of introns and their role in eukaryogenesis: a compromise solution to the introns-early versus introns-late debate? Biol Direct 1, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosek J et al. (2006) On the origin of telomeres: a glimpse at the pre-telomerase world. Bioessays 28 (2), 182–190. [DOI] [PubMed] [Google Scholar]
- 6.Goodenough U and Heitman J (2014) Origins of eukaryotic sexual reproduction. Cold Spring Harb Perspect Biol 6 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olovnikov AM (1973) A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41 (1), 181–90. [DOI] [PubMed] [Google Scholar]
- 8.Watson JD (1972) Origin of concatemeric T7 DNA. Nat New Biol 239 (94), 197–201. [DOI] [PubMed] [Google Scholar]
- 9.Cenci G et al. (1997) UbcD1, a Drosophila ubiquitin-conjugating enzyme required for proper telomere behavior. Genes Dev 11 (7), 863–75. [DOI] [PubMed] [Google Scholar]
- 10.de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & Development 19 (18), 2100–2110. [DOI] [PubMed] [Google Scholar]
- 11.van Steensel B et al. (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92 (3), 401–13. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn EH and Gall JG (1978) A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol 120 (1), 33–53. [DOI] [PubMed] [Google Scholar]
- 13.Greider CW and Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43 (2 Pt 1), 405–13. [DOI] [PubMed] [Google Scholar]
- 14.Greider CW and Blackburn EH (1987) The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51 (6), 887–98. [DOI] [PubMed] [Google Scholar]
- 15.Greider CW (1991) Telomeres. Curr Opin Cell Biol 3 (3), 444–51. [DOI] [PubMed] [Google Scholar]
- 16.Vega LR et al. (2003) Getting to the end: telomerase access in yeast and humans. Nat Rev Mol Cell Biol 4 (12), 948–59. [DOI] [PubMed] [Google Scholar]
- 17.de Lange T (2010) How shelterin solves the telomere end-protection problem. Cold Spring Harb Symp Quant Biol 75, 167–77. [DOI] [PubMed] [Google Scholar]
- 18.Fulcher N et al. (2014) If the cap fits, wear it: an overview of telomeric structures over evolution. Cell Mol Life Sci 71 (5), 847–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beilstein MA et al. (2015) Evolution of the Telomere-Associated Protein POT1a in Arabidopsis thaliana Is Characterized by Positive Selection to Reinforce Protein-Protein Interaction. Mol Biol Evol 32 (5), 1329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YC et al. (2017) Recurrent Innovation at Genes Required for Telomere Integrity in Drosophila. Mol Biol Evol 34 (2), 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontremoli C et al. (2018) Evolutionary rates of mammalian telomere-stability genes correlate with karyotype features and female germline expression. Nucleic Acids Res 46 (14), 7153–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henikoff S et al. (2001) The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293 (5532), 1098–102. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JA et al. (2009) Molecular population genetics and evolution of Drosophila meiosis genes. Genetics 181 (1), 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosini A et al. (2007) Human subtelomeric duplicon structure and organization. Genome Biol 8 (7), R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JA et al. (2008) Molecular population genetics of Drosophila subtelomeric DNA. Genetics 178 (1), 477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linardopoulou EV et al. (2005) Human subtelomeres are hot spots of interchromosomal recombination and segmental duplication. Nature 437 (7055), 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim C et al. (2019) Long-read sequencing reveals intra-species tolerance of substantial structural variations and new subtelomere formation in C. elegans. Genome Res 29 (6), 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalo S et al. (2006) DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol 8 (4), 416–24. [DOI] [PubMed] [Google Scholar]
- 29.Cervenak F et al. (2017) Double-stranded telomeric DNA binding proteins: Diversity matters. Cell Cycle 16 (17), 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fajkus J et al. (2005) Telomeres in evolution and evolution of telomeres. Chromosome Res 13 (5), 469–79. [DOI] [PubMed] [Google Scholar]
- 31.Lewis KA and Wuttke DS (2012) Telomerase and telomere-associated proteins: structural insights into mechanism and evolution. Structure 20 (1), 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linger BR and Price CM (2009) Conservation of telomere protein complexes: shuffling through evolution. Crit Rev Biochem Mol Biol 44 (6), 434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greider CW and Blackburn EH (1989) A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337 (6205), 331–7. [DOI] [PubMed] [Google Scholar]
- 34.Blackburn EH et al. (2006) Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 12 (10), 1133–8. [DOI] [PubMed] [Google Scholar]
- 35.Silvestre DC and Londono-Vallejo A (2012) Telomere dynamics in mammals. Genome Dyn 7, 29–45. [DOI] [PubMed] [Google Scholar]
- 36.Gomes NM et al. (2010) Telomere biology in Metazoa. FEBS Lett 584 (17), 3741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teixeira MT and Gilson E (2005) Telomere maintenance, function and evolution: the yeast paradigm. Chromosome Res 13 (5), 535–48. [DOI] [PubMed] [Google Scholar]
- 38.Cangiano G and La Volpe A (1993) Repetitive DNA sequences located in the terminal portion of the Caenorhabditis elegans chromosomes. Nucleic Acids Res 21 (5), 1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards EJ and Ausubel FM (1988) Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell 53 (1), 127–36. [DOI] [PubMed] [Google Scholar]
- 40.Mason JM et al. (2016) Telomerase lost? Chromosoma 125 (1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capkova Frydrychova R et al. (2008) Regulation of telomere length in Drosophila. Cytogenet Genome Res 122 (3–4), 356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobinoff AP and Pickett HA (2017) Alternative Lengthening of Telomeres: DNA Repair Pathways Converge. Trends Genet 33 (12), 921–932. [DOI] [PubMed] [Google Scholar]
- 43.Lackner DH et al. (2012) Organismal propagation in the absence of a functional telomerase pathway in Caenorhabditis elegans. EMBO J 31 (8), 2024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardue ML and DeBaryshe PG (2011) Retrotransposons that maintain chromosome ends. Proc Natl Acad Sci U S A 108 (51), 20317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casacuberta E and Pardue ML (2003) Transposon telomeres are widely distributed in the Drosophila genus: TART elements in the virilis group. Proc Natl Acad Sci U S A 100 (6), 3363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saint-Leandre B et al. (2019) Diversification and collapse of a telomere elongation mechanism. Genome Res 29 (6), 920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raffa GD et al. (2011) Terminin: a protein complex that mediates epigenetic maintenance of Drosophila telomeres. Nucleus 2 (5), 383–91. [DOI] [PubMed] [Google Scholar]
- 48.Morgan CC et al. (2013) Molecular adaptation of telomere associated genes in mammals. BMC Evol Biol 13, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shultz AJ and Sackton TB (2019) Immune genes are hotspots of shared positive selection across birds and mammals. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Lee R et al. (2017) Genome-scale detection of positive selection in nine primates predicts human-virus evolutionary conflicts. Nucleic Acids Res 45 (18), 10634–10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark NL et al. (2006) Evolution of reproductive proteins from animals and plants. Reproduction 131 (1), 11–22. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen R et al. (2005) A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol 3 (6), e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panhuis TM et al. (2006) Rapid evolution of reproductive proteins in abalone and Drosophila. Philos Trans R Soc Lond B Biol Sci 361 (1466), 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loppin B et al. (2005) Origin and neofunctionalization of a Drosophila paternal effect gene essential for zygote viability. Curr Biol 15 (2), 87–93. [DOI] [PubMed] [Google Scholar]
- 55.Dubruille R et al. (2010) Specialization of a Drosophila capping protein essential for the protection of sperm telomeres. Curr Biol 20 (23), 2090–9. [DOI] [PubMed] [Google Scholar]
- 56.Gao G et al. (2011) Paternal imprint essential for the inheritance of telomere identity in Drosophila. Proc Natl Acad Sci U S A 108 (12), 4932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cenci G et al. (2003) The Drosophila HOAP protein is required for telomere capping. Nat Cell Biol 5 (1), 82–4. [DOI] [PubMed] [Google Scholar]
- 58.Kursel LE and Malik HS (2017) Recurrent Gene Duplication Leads to Diverse Repertoires of Centromeric Histones in Drosophila Species. Mol Biol Evol 34 (6), 1445–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumann P et al. (2002) Human Pot1 (protection of telomeres) protein: cytolocalization, gene structure, and alternative splicing. Mol Cell Biol 22 (22), 8079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Renfrew KB et al. (2014) POT1a and components of CST engage telomerase and regulate its activity in Arabidopsis. PLoS Genet 10 (10), e1004738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cifuentes-Rojas C et al. (2012) An alternative telomerase RNA in Arabidopsis modulates enzyme activity in response to DNA damage. Genes Dev 26 (22), 2512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surovtseva YV et al. (2009) Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol Cell 36 (2), 207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shibuya H et al. (2015) MAJIN Links Telomeric DNA to the Nuclear Membrane by Exchanging Telomere Cap. Cell 163 (5), 1252–1266. [DOI] [PubMed] [Google Scholar]
- 64.Riethman H et al. (2005) Human subtelomere structure and variation. Chromosome Res 13 (5), 505–15. [DOI] [PubMed] [Google Scholar]
- 65.Blasco MA (2007) The epigenetic regulation of mammalian telomeres. Nat Rev Genet 8 (4), 299–309. [DOI] [PubMed] [Google Scholar]
- 66.Karpen GH and Spradling AC (1992) Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics 132 (3), 737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mefford HC and Trask BJ (2002) The complex structure and dynamic evolution of human subtelomeres. Nat Rev Genet 3 (2), 91–102. [DOI] [PubMed] [Google Scholar]
- 68.Jezek M and Green EM (2019) Histone Modifications and the Maintenance of Telomere Integrity. Cells 8 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grewal SI and Jia S (2007) Heterochromatin revisited. Nat Rev Genet 8 (1), 35–46. [DOI] [PubMed] [Google Scholar]
- 70.Amaral N et al. (2017) Nuclear Dynamics of Heterochromatin Repair. Trends Genet 33 (2), 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teytelman L et al. (2008) Silent but not static: accelerated base-pair substitution in silenced chromatin of budding yeasts. PLoS Genet 4 (11), e1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yue JX et al. (2017) Contrasting evolutionary genome dynamics between domesticated and wild yeasts. Nat Genet 49 (6), 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudd MK et al. (2009) Comparative sequence analysis of primate subtelomeres originating from a chromosome fission event. Genome Res 19 (1), 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ventura M et al. (2012) The evolution of African great ape subtelomeric heterochromatin and the fusion of human chromosome 2. Genome Res 22 (6), 1036–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Audano PA et al. (2019) Characterizing the Major Structural Variant Alleles of the Human Genome. Cell 176 (3), 663–675 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azzalin CM and Lingner J (2015) Telomere functions grounding on TERRA firma. Trends Cell Biol 25 (1), 29–36. [DOI] [PubMed] [Google Scholar]
- 77.Nergadze SG et al. (2009) CpG-island promoters drive transcription of human telomeres. RNA 15 (12), 2186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnoult N et al. (2012) Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1alpha. Nat Struct Mol Biol 19 (9), 948–56. [DOI] [PubMed] [Google Scholar]
- 79.Pfeiffer V and Lingner J (2012) TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet 8 (6), e1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cusanelli E et al. (2013) Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol Cell 51 (6), 780–91. [DOI] [PubMed] [Google Scholar]
- 81.Balk B et al. (2013) Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat Struct Mol Biol 20 (10), 1199–205. [DOI] [PubMed] [Google Scholar]
- 82.Berthiau AS et al. (2006) Subtelomeric proteins negatively regulate telomere elongation in budding yeast. EMBO J 25 (4), 846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng Z et al. (2012) A role for CTCF and cohesin in subtelomere chromatin organization, TERRA transcription, and telomere end protection. EMBO J 31 (21), 4165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cenci G et al. (2015) The Analysis of Pendolino (peo) Mutants Reveals Differences in the Fusigenic Potential among Drosophila Telomeres. PLoS Genet 11 (6), e1005260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iglesias N et al. (2011) Subtelomeric repetitive elements determine TERRA regulation by Rap1/Rif and Rap1/Sir complexes in yeast. EMBO Rep 12 (6), 587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J et al. (2016) The proper connection between shelterin components is required for telomeric heterochromatin assembly. Genes Dev 30 (7), 827–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dorer DR and Henikoff S (1994) Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77 (7), 993–1002. [DOI] [PubMed] [Google Scholar]
- 88.Sentmanat MF and Elgin SC (2012) Ectopic assembly of heterochromatin in Drosophila melanogaster triggered by transposable elements. Proc Natl Acad Sci U S A 109 (35), 14104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Britt-Compton B et al. (2006) Structural stability and chromosome-specific telomere length is governed by cis-acting determinants in humans. Hum Mol Genet 15 (5), 725–33. [DOI] [PubMed] [Google Scholar]
- 90.Brown CA et al. (2010) Rapid expansion and functional divergence of subtelomeric gene families in yeasts. Curr Biol 20 (10), 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurst GD and Werren JH (2001) The role of selfish genetic elements in eukaryotic evolution. Nat Rev Genet 2 (8), 597–606. [DOI] [PubMed] [Google Scholar]
- 92.Burt A and Trivers R (2006) Genes in conflict: The biology of selfish genetic elements, Harvard University Press. [Google Scholar]
- 93.Werren JH (2011) Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc. Natl. Acad. Sci. U. S. A 108 Suppl 2, 10863–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCaffrey J et al. (2017) High-throughput single-molecule telomere characterization. Genome Res 27 (11), 1904–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chmatal L et al. (2014) Centromere strength provides the cell biological basis for meiotic drive and karyotype evolution in mice. Curr Biol 24 (19), 2295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fishman L and Willis JH (2005) A novel meiotic drive locus almost completely distorts segregation in mimulus (monkeyflower) hybrids. Genetics 169 (1), 347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Black BE and Bassett EA (2008) The histone variant CENP-A and centromere specification. Curr Opin Cell Biol 20 (1), 91–100. [DOI] [PubMed] [Google Scholar]
- 98.Drinnenberg IA et al. (2016) Evolutionary Turnover of Kinetochore Proteins: A Ship of Theseus? Trends Cell Biol 26 (7), 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malik HS and Henikoff S (2001) Adaptive evolution of Cid, a centromere-specific histone in Drosophila. Genetics 157 (3), 1293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schueler MG et al. (2010) Adaptive evolution of foundation kinetochore proteins in primates. Mol Biol Evol 27 (7), 1585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cooper JL and Henikoff S (2004) Adaptive evolution of the histone fold domain in centromeric histones. Mol Biol Evol 21 (9), 1712–8. [DOI] [PubMed] [Google Scholar]
- 102.Janssen A et al. (2018) Heterochromatin: Guardian of the Genome. Annu Rev Cell Dev Biol 34, 265–288. [DOI] [PubMed] [Google Scholar]
- 103.Akera T et al. (2017) Spindle asymmetry drives non-Mendelian chromosome segregation. Science 358 (6363), 668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dawe RK et al. (2018) A Kinesin-14 Motor Activates Neocentromeres to Promote Meiotic Drive in Maize. Cell 173 (4), 839–850 e18. [DOI] [PubMed] [Google Scholar]
- 105.Axelsson E et al. (2010) Segregation distortion in chicken and the evolutionary consequences of female meiotic drive in birds. Heredity (Edinb) 105 (3), 290–8. [DOI] [PubMed] [Google Scholar]
- 106.Gladyshev EA and Arkhipova IR (2010) A subtelomeric non-LTR retrotransposon Hebe in the bdelloid rotifer Adineta vaga is subject to inactivation by deletions but not 5’ truncations. Mob DNA 1 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asif-Laidin A et al. (2017) Short and long-term evolutionary dynamics of subtelomeric piRNA clusters in Drosophila. DNA Res 24 (5), 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zou S et al. (1996) The Saccharomyces retrotransposon Ty5 influences the organization of chromosome ends. Nucleic Acids Res 24 (23), 4825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujiwara H (2014) Accumulation of Telomeric-Repeat-Specific Retrotransposons in Subtelomeres of Bombyx mori and Tribolium castaneum In Subtelomeres (Louis EJ and Becker MM eds), pp. 227–241, Springer Berlin Heidelberg. [Google Scholar]
- 110.Doolittle WF and Sapienza C (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284 (5757), 601–3. [DOI] [PubMed] [Google Scholar]
- 111.Kidwell MG and Lisch DR (2001) Perspective: transposable elements, parasitic DNA, and genome evolution. Evolution 55 (1), 1–24. [DOI] [PubMed] [Google Scholar]
- 112.Deng Z et al. (2014) HSV-1 remodels host telomeres to facilitate viral replication. Cell Rep 9 (6), 2263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steinberg-Neifach O and Lue NF (2015) Telomere DNA recognition in Saccharomycotina yeast: potential lessons for the co-evolution of ssDNA and dsDNA-binding proteins and their target sites. Front Genet 6, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]


