Abstract
In past decades, alginate-based multilayer microcapsules have been given important attention in various pharmaceutical investigations. Alginate-poly l lysine-alginate (APA) is studied the most. Due to the similarity between the structure of polyethyleneimine (PEI) and poly-L-lysine (PLL) and also lower price of PEI than PLL, this study was conducted to compare the efficacy of linear (LPEI) and branch (BPEI) forms of PEI with PLL as covering layers in fabrication of microcapsules. The microcapsules were fabricated using electrostatic bead generator and their shape/size, surface roughness, mechanical strength, and interlayer interactions were also investigated using optical microscopy, AFM, explosion test and FTIR, respectively. Furthermore, cytotoxicity was evaluated by comparing the two anionic final covering layers alginate (Alg) and sodium cellulose sulphate (NCS) using MTT test. BPEI was excluded from the rest of the study due to its less capacity to strengthen the microcapsules and also the aggregation of the resultant alginate-BPEI-alginate microcapsules, while LPEI showed properties similar to PLL. MTT test also showed that NCS has no superiority over Alg as final covering layer. Therefore, it is concluded that, LPEI could be considered as a more cost effective alternative to PLL and a promising subject for future studies.
Keywords: Microencapsulation, Polyethylene imine, Poly l-lysine, Alginic acid, Poly electrolyte complexes, Extrusion
Graphical abstract
1. Introduction
Since the last century, design and fabrication of alginate microcapsules is considered to be an interesting method to entrap drugs, enzymes, proteins, probiotics, live mammalian cells, etc. Microencapsulation can be used to achieve very different goals such as controlled-manner release of drugs, protection of certain labile materials from harsh conditions (e.g. acidic pH of GI tracts), formulation of insoluble, and volatile or hygroscope drugs or immunoisolation of live cells [[1], [2], [3], [4], [5]].
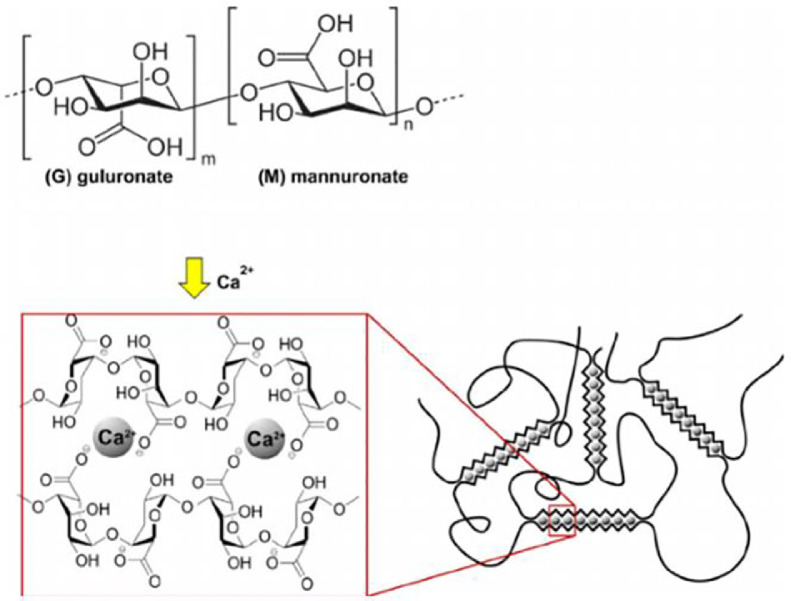
Different categories of materials are used as building units of microcapsules, namely, carbohydrates (carrageenan, agarose, gellan gum, alginate, chitosan, and hyaluronic acid), proteins (gelatin, collagen, fibrin, and elastin), and synthetic polymers (poly hydroxyl ethyl methacrylate-methyl methacrylate and poly acrylonitrile-co-polyethylene glycol). Among natural materials, alginic acid has been the most used, because of its high biocompatibility, mild production processes, high gel-forming capacity, and safe in vivo properties. Alginate is a poly anionic linear polysaccharide made from residues of β-D mannuronic acid and α-L guluronic acid which occur in blocks (M blocks and G blocks) and in less regularly distributed mixed region. It is obtained from different types of brown seaweed and also produced by two types of bacteria (Pseudomonas and Azotobacter) [6].
Capsule-making materials should be able to imitate the extracellular matrix properties and their preparing process should be mild and live cells compatible. Hydrogels are extremely hydrated hydrophilic polymers [6], [7]. Interaction among multivalent cations, such as Ca2+, Sr2+, Ba2+, and alginic acid is known as the mechanism of alginate hydrogel production. These ionic interactions lead to the formation of interchains bridges between alginate chains and production of cross-linked hydrogel; this structure is called egg-box (Fig. 1) [8], [9], [10], [11]
Fig. 1.
Structure of alginic acid and egg-box model (Reproduced with permission from [51]. Copyright 2002 John Wiley and Sons).
In order to improve the mechanical stability [12], [13], reduction of pore size and prevention of alginate microcapsules from being solubilized, covering of microcapsules with a cationic layer such as PLL [14], PLO [15], chitosan [16], etc., is recommended. However, till date, despite lots of studies, scientists were unable to come to a conclusion on which cation is the best option for covering the alginate microcapsules [17].
PLL is a homopolymer composed of l-lysine, a cationic amino acid. It contains lots of primary amines which in protonated form are capable of forming electrostatic interactions with anionic sites [18], [19], [20]. Since cytotoxicity and inflammatory effects of positive charge on biological systems have been proven, there is therefore a great concern among scientists about covering the unbounded part of PLL; therefore, using another anionic layer (e.g. alginate) to cover the positive charge of PLL has been suggested. As such, APA microcapsules have been fabricated [21], [22], [23], [24], [25], [26].
From the beginning of the multilayer microcapsules’ development, APA has been used in achieving very different goals as described earlier. Even though few studies have introduced other cationic polymers (e.g. PLO) and their preferences toward PLL [27], there are more studies which supported PLL against other cationic materials [28]. However, PLL is still studied and used the most, because there is a long history of research behind it.
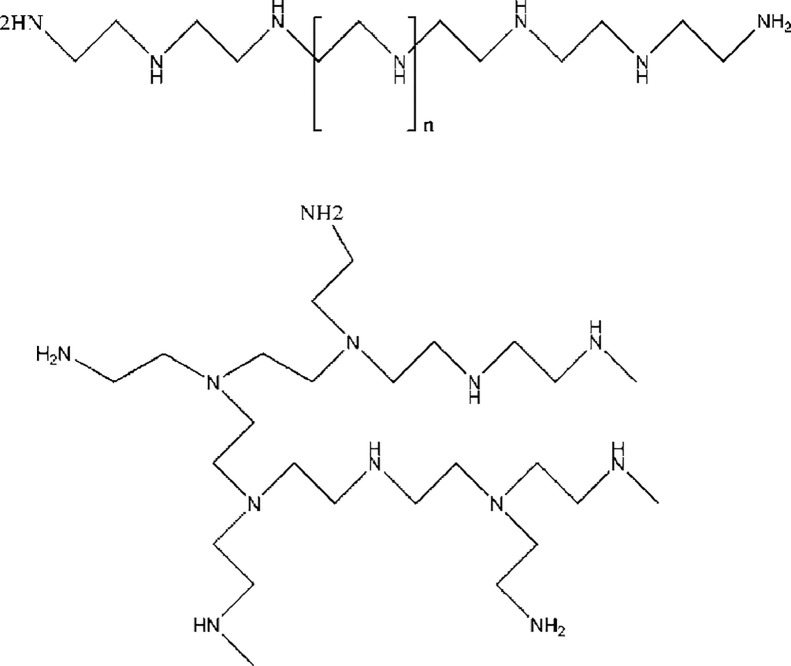
Polyethylene imine is a cationic polymer which is compared a lot with PLL, mostly in the field of gene delivery and has shown higher efficiency than PLL in that area [29]. In its structure, one in every third atom is an amino nitrogen which could be protonated and interacted with negative ions [30]. Two common structures of PEI are BPEI and LBPEI forms (Fig. 2) and they are synthesized through acidic polymerization of aziridine and cationic ring-opening polymerization of ethyl oxazoline, followed by acidic hydrolysis of the corresponding substituted polyamine, respectively. The physical form of BPEI is liquid while that of the LPEI is solid in room temperature [31], [32], [33].
Fig. 2.
Structure of PEI; up, linear, down, branched (Reproduced with permission from [52]. Copyright 2008 Elsevier B.V.).
Different types of techniques are being used for the fabrication of microcapsules; but the desired characteristics of microcapsules dictate the method to be used. In this study, electrostatic bead generator was used to fabricate the microcapsules.
Complete coverage of positive charges on cationic polymers could be achieved using materials with higher anionic charge density like sulphate ion [34]. In this study, in order to investigate this, the efficiency of two anionic polymers, alginic acid and NCS, as final covering layers was compared to see if the induced cytotoxicity would change or not. NCS, an anionic derivative of cellulose is a biocompatible and nontoxic compound, which causes no inflammatory responses and is stable in physiologic condition [35], [36].
According to the literature, PLL is the most used polycation in fabrication of multilayer microcapsules due to its desirable properties. Since there is a good similarity between the structure of PEI (especially LPEI) and PLL, it could be assumed that, these two polymers would show similar properties in covering of microcapsules. To the best of our knowledge, there has not been a thorough comparison between these polymers as covering layers of microcapsules. Therefore, considering the lower cost of PEI, the aim of this study was to investigate PEI as a potential alternative to PLL. Comprehensive comparison was made in terms of shape, size, surface morphology, mechanical stability, cytotoxicity, and long-term (in vitro) stability.
2. Materials and methods
2.1. Materials
High G content alginic acid (MW, 100 000–200 000, G content, 70%), calcium chloride, branched and linear PEI (Mn ∼10 000), PLL (MW 70 000–150 000, 0.01%), HEPES, MTT reagent, and DMSO were purchased from Sigma-Aldrich (USA). Cell culture media (RPMI), antibiotic solution of penicillin/streptomycin, and trypsin 10X were obtained from Biosera Co. (England). Fetal bovine serum was purchased from Gibco Co. (USA). HepG2 cell line (NCBI Code: C158) was obtained from Pasteur Institute of Iran.
2.2. Fabrication of the multilayer alginate microcapsules
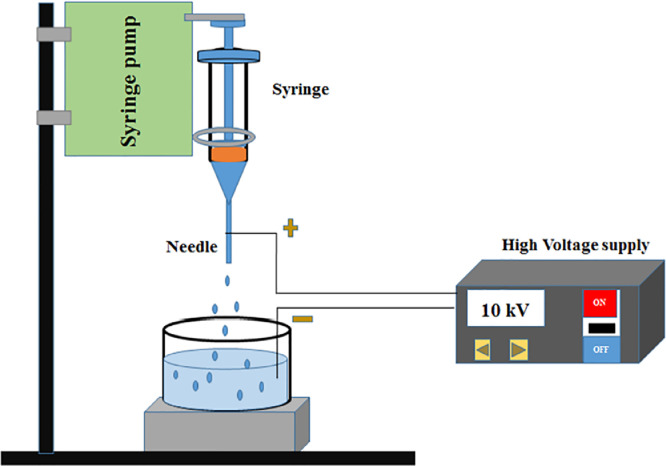
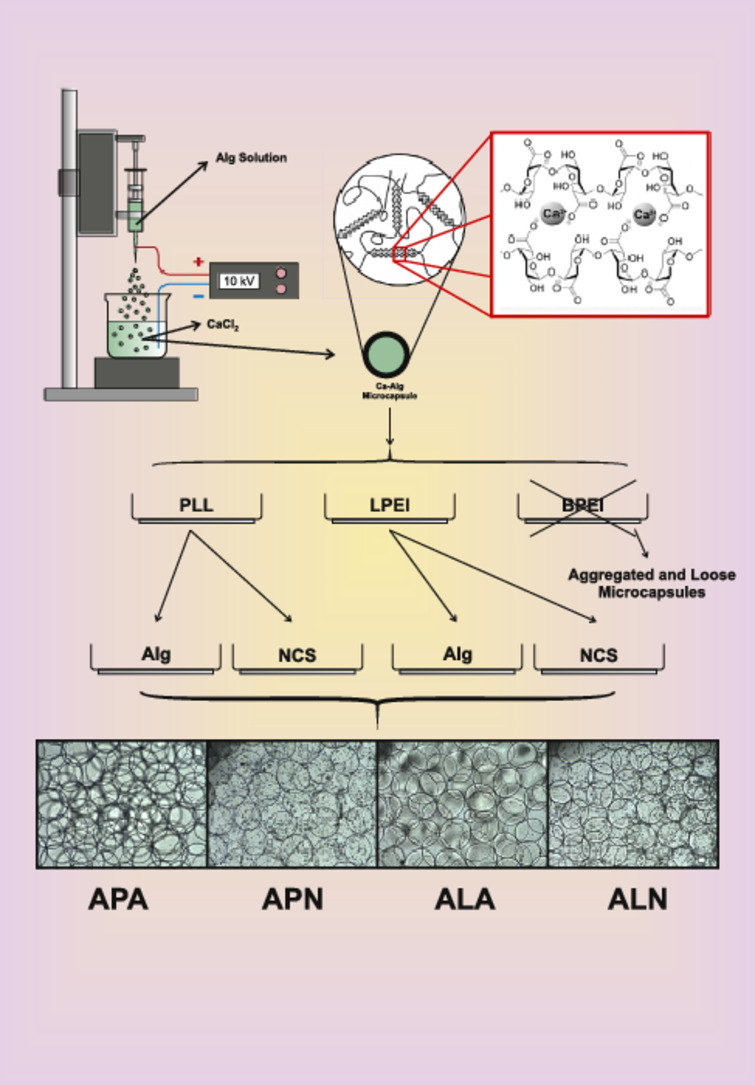
The alginate microcapsules were fabricated using electrostatic bead generator, according to a three-step procedure. Briefly as shown schematically in Fig. 3, the device established electrostatic potential of 10 kV between the needle feeding the alginate solution and the surface of CaCl2 (120 mM) solution, as cross-linker. Sterile alginate solution (5 ml) with concentration of 1%, 1.5%, 2% or 2.5% was dropped into the CaCl2. The distance between the needle and surface of the CaCl2 solution in a beaker was approximately 4 to 5 cm and alginate solution was pumped into the CaCl2 solution with a rate of 10 ml/h. The Ca-alg microcapsules were allowed to harden in the CaCl2 solution for 15 min while magnetically stirred. In the next step, to form the microcapsules coating layers, the Ca-alg microcapsules were successively incubated in a cationic solution (PLL, BPEI or LPEI) with different concentrations (0.01%, 0.03% or 0.06%) for 5 min, then rinsed with a saline solution and incubated in 0.2% alginate or NCS solution for 5 min. It should be noted that NCS solution was used as the final coating layer of the microcapsules only after selection of the suitable cationic polymers. Finally, microcapsules were filtered and kept for further investigations. Alginate and the other polymers were dissolved in a solution, pH 7.2 to 7.4, containing 0.9% NaCl and 0.24% HEPES. Except otherwise stated, all the concentrations are shown in percentage of weight to volume (W/V). All solutions were kept at 4 °C until they were used [37]. Alginate and cationic polymer solutions were sterilized by filter 0.22 µm, CaCl2 and NaCl/HEPES via autoclave.
Fig. 3.
Schematic of electrostatic bead generator used for fabrication of alginate microcapsules.
2.3. Evaluation of the microcapsules
2.3.1. Shape and size
Shape and size of the microcapsules were evaluated using inverted microscope (OPTIKA, XDS-2FL) equipped with a digital camera. To do this, microcapsules from each batch were placed in a plate and observed under the microscope at 4X magnification. The program installed on the camera measured the microcapsules diameter through three points on their perimeter. The diameter (µm) of 30 microcapsules per batch was assessed in each experiment. All the experiments were done three times and the results were reported as mean ± SD [38].
2.3.2. FTIR studies
FTIR studies were conducted to investigate the interactions of different cationic layers with alginate layer in ALA, ABA, and APA microcapsules. FTIR spectra of the microcapsules were recorded in a FTIR spectrometer (Jasco-FTIR, model 8300, Japan) between 4000 and 400 cm–1 and scanning speed of 2 mm/s. A fixed amount of sample was mixed with dry potassium bromide and ground into a fine powder, and then it was compressed into a KBr disk by applying a pressure of 300 kg/cm2 with a hydraulic press [39].
2.3.3. Mechanical stability
The mechanical stability of the microcapsules was evaluated using explosion test. In this assay, different types of microcapsules (ALA0.01%, ALA0.03%, ALA0.06%, ABA0.01%, ABA0.03%, ABA0.06%, APA0.01%, APA0.03%, and APA0.06%, where subscripts indicate the cationic layer concentration) were suspended in DI water and incubated in shaker incubator (JAL TAJHIZ LABTECH, JTSDL40), at 37 °C and 60 rpm for 24 h. The size of hydrogel microcapsules will increase as a result of water uptake in contact with hypo osmotic medium. After incubation, the amount of size growth (Eq. 1) and also the number of broken microcapsules in ten randomly selected microcapsules were measured. The lower the increase in the size of the microcapsules, the higher the mechanical stability [28]. Each experiment was done three times and the results were reported as mean ± SD.
| (1) |
Where, D0 is the initial size of the microcapsules and D is the size of the microcapsules after 24 h incubation in DI water.
2.3.4. Surface roughness
The surface roughness of microcapsules is one of the influential factors in their in-vivo effectiveness. The more the surface roughness is the more the accumulation of immune cells. Quantitative analysis of surface roughness of the microcapsules was carried out by AFM, using NanoWizard II (JPK-Germany) apparatus, operating in the intermittent contact mode at room temperature. One of the most common parameter to report the surface roughness is Peak-to-Valley. It describes the distance between the lowest valley and the highest peak on the measured surface [40]. Peak to valley was measured using JPK data processing program.
In order to prepare the samples, the microcapsules were washed with 0.9% NaCl solution, filtered, and dried on a glass plate at 37 °C. Then, the glass plate was transferred directly onto the AFM platform for imaging. Three images from each sample were taken and analyzed and the results were reported as mean ± SD.
2.3.5. Long-term stability
Long-term stability is considered as one of the most important parameter in the design and fabrication of various delivery systems based on the aim and route of administration. Since the ion composition in the medium strongly affects the maintenance of polyelectrolyte complexes and as a consequence on stability of microcapsules, therefore, long-term stability of the multilayer microcapsules was investigated in 0.9% NaCl at two temperature conditions: 2 to 8 °C and also in 37 °C [41]. Microcapsules were transferred into two plates 12-well and 3 ml of saline was added to each well. One plate was kept at 2 to 8 °C and the other one kept at 37 °C. Size and integrity of the microcapsules were monitored weekly, using inverted microscope while the saline solution was changed every second day.
2.3.6. Cytotoxicity
Cytotoxicity of different multilayer microcapsules was evaluated using colorimetric MTT assay on HepG2 cell line. Briefly, 15 000 cells/well in plate 96-well were seeded. The cells were incubated at 37 °C, 5% CO2 concentration, and 95% relative humidity (RH), for 24 h. After the incubation, cells were treated using different types of microcapsules for 24 h, except one column as control. After the treatment, microcapsules were removed and cells were washed with PBS, then 20 µl MTT reagent (5 mg/ml) was added to each well and plate was covered with aluminum foil and was kept in incubator for 4 h. Then, MTT reagent was removed and 200 µl DMSO was added to each well. The plate was kept in a dark environment for about 30 min. Color intensity was read using ELISA plate reader (SYNERGY, BioTek, USA) at 580 nm. The percentage of cell viability was calculated using Eq. 2 [42]. MTT experiment was done three times and the results were reported as mean ± SD.
| (2) |
Where Mean OD is the average OD for each column in relation to each type of microcapsule and Control OD is the average OD for the control column.
2.3.7. Statistical analysis
The statistical analysis was performed using SPSS version 17.0. One-way analysis of variance (ANOVA) and post-hoc Tukey test were used to investigate the differences between groups. Differences were considered significant when P < 0.05.
3. Results and discussion
3.1. Fabrication of the alginate microcapsules
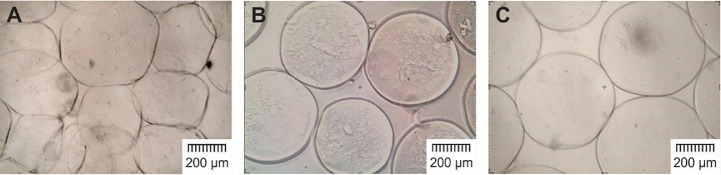
Alginate solution has been used in many studies frequently with concentration of 1% to 3%. The optimum concentration of alginate solution was selected from 1%, 1.5%, 2% or 2.5%, according to the shape and size of the resultant microcapsules. As shown in Fig. 4 by applying alginate solution 1% irregular-shaped and loose microcapsules were fabricated while 1.5% and 2% alginate solutions led to the fabrication of the microcapsules with similar shape and size. The use of 2.5% alginate solution caused needle to clog. Since the strength of the microcapsules is directly related to the concentration of the alginate solution, that is, between 1.5% and 2%, the latter one was selected for fabrication of the microcapsules.
Fig. 4.
Microscopic images of alginate microcapsules made from different concentrations of alginate solution. (A) 1%, (B) 1.5%, and (C) 2% (magnification, 4×).
3.2. Evaluation of the microcapsules
3.2.1. Shape and size
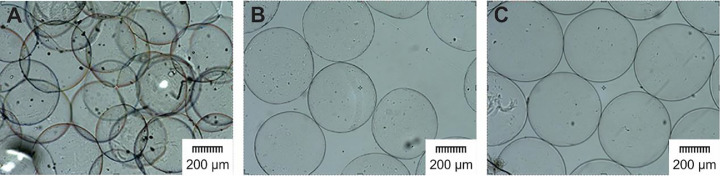
Different types and concentrations of cationic polymers (PLL, BPEI, and LPEI) have different effect on the properties of the microcapsules. As shown in Fig. 5 the application of PLL and LPEI in all the three concentrations: 0.01%, 0.03% and 0.06% resulted in spherical and separated microcapsules, while coating by BPEI led to the formation of the undesirable and aggregated microcapsules. Since BPEI contrary to PLL and LPEI has branched structure, aggregated ABA microcapsules could be formed as a result of the interactions between several positive charges on the side chains of BPEI in one microcapsule and negative charges of close microcapsules and as a result of this, the nearby microcapsules would be stuck together.
Fig. 5.
Different appearance of microcapsules as a result of the application of different cationic polymers. (A) ABA, (B) ALA, and (C) APA(magnification, 4×).
Evaluation of 30 microcapsules per each batch showed that the diameter of the single layer microcapsules was 491.32 ± 5.87 µm. As shown in Table 1 the size of different multilayer microcapsules was measured between 500 and 1000 µm. For each certain cationic polymer, applying the highest concentration resulted in the smallest microcapsules. This shows that the higher the concentration of cationic polymers, the more rigid the microcapsules that will be produced and this could withstand the size growth more strongly during coating process. Hyun Joon [43] came up with the same conclusion that the application of higher concentration of PEI leads to hydrogels with higher elastic moduli.
Table 1.
The size distribution (µm) of different types of microcapsules.
| Type of microcapsule | Size range (µM) |
|---|---|
| ABA0.06* | 520–600 |
| ABA0.03 | 650–760 |
| ABA0.01 | 700–780 |
| ALA0.06 | 630–750 |
| ALA0.03 | 850–900 |
| ALA0.01 | 850–1000 |
| APA0.06 | 600–730 |
| APA0.03 | 770–850 |
| APA0.01 | 840–900 |
Subscripts indicate the cationic layer concentration (%, w/v).
Since, all the microcapsules showed spherical shape with a narrow size distribution of less than 10% CV, reproducibility of our fabrication method was proven.
3.2.2. FTIR studies
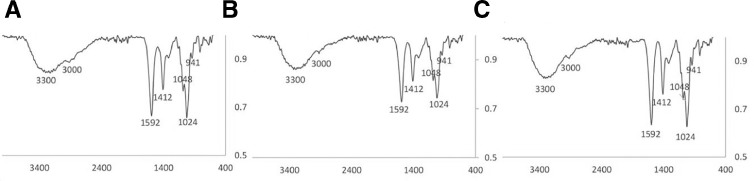
In order to improve the microcapsules strength, cationic layers were used to cover them by ionic interaction between their protonated amine and carboxylic groups of alginate microcapsules. Fig. 6 shows the FTIR spectrum of the different multilayer microcapsules. The FTIR results were in complete agreement with Tam and Susan [44]; there are absorbance bands in these spectrums which are related to alginate: broad peak near 3300 cm−1 shows the stretching vibrations of hydrogen-bonded OH groups; two peaks near 1412 and 1592 cm−1 show the symmetric and asymmetric stretching vibrations of the coo-, respectively. There are also three peaks in the 1100 to 940 cm−1 region, showing various vibrations of the carbohydrate ring. Aside the alginate related bands, all the three spectrums show a small peak, near 3000 cm−1 that is related to Amide band. The similarity of the spectrums in Fig. 6 shows that the between layer interactions of these microcapsule are the same as each other. In other word, BPEI, LPEI, and PLL interacted with alginate microcapsules in the same way.
Fig. 6.
FTIR spectra of multilayer microcapsules, (A) ABA, (B) ALA and (C) APA.
3.2.3. Mechanical stability
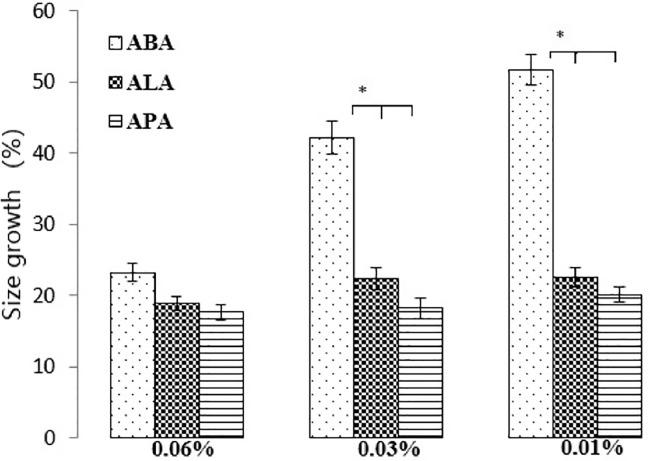
The mechanical stability of the microcapsules covered with different concentrations of PLL, BPEI or LPEI was investigated using explosion test. As stated earlier, more stable microcapsules will show less size growth. Results of size growth evaluation in the microcapsules in Fig. 7 showed that increasing the concentration of cationic polymers from 0.01% to 0.06%, led to the higher mechanical stability in the microcapsules [45], [46], [47]. However, as shown in Fig. 7 the difference between the efficiency of different concentrations in covering of the microcapsules is significant only for BPEI (P < 0.05), but it is not significant for LPEI and PLL. This could be due to the spatial limitation in branched structure of BPEI to interact properly with anionic sites of the microcapsules. This limitation is significant when low concentrations of BPEI (0.01% and 0.03%) were used, while when the highest concentration (0.06%) is used, this limitation is not an issue anymore, and in this concentration, the positive charge density is enough to interact with carboxylic groups as linear polymers; LPEI and PLL. Hyun [43], reported similar idea about limited mobility of high MW PEI which resulted in incomplete interaction between cationic amine and alginate carboxylic groups. In another study, Darrabi et al. [27] reported that polymer with shorter structure could cover the microcapsules better than polymer with longer chain structure.
Fig. 7.
Microcapsules size growth (%), after explosion test. Comparison of different types and concentrations of cationic polymers (*P < 0.05, n = 3).
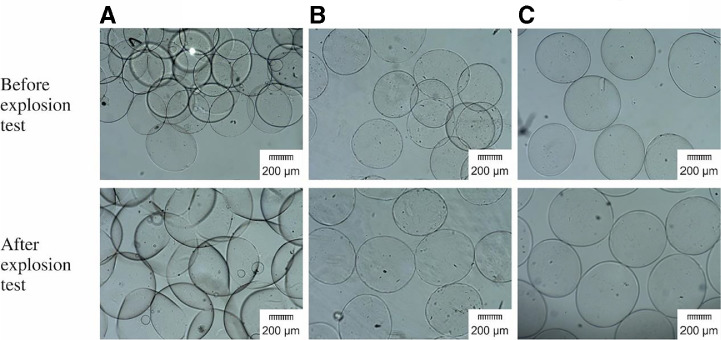
According to the present results, it could be concluded that, the concentration of 0.06% would be better as compared to lower concentrations, since it leads to the fabrication of the smaller and spherical microcapsules. The explosion test also showed that LPEI and PLL display similar mechanical stability in each of the three concentrations (P > 0.05), due to their similar conformation which resulted in the same interactions. In addition, during the explosion test, no broken capsule was found in ALA and APA microcapsules and all of them remained intact, while some ABA microcapsules revealed some irregularity in their shape and were ruptured (Fig. 8). Finally, amongst the three polycations, BPEI was abandoned from the rest of the study due to its less efficiency as compared to LPEI and PLL as a covering layer when used in low and middle concentrations and also production of undesirable aggregated microcapsules when used in high concentration.
Fig. 8.
Microscopic images of multilayer microcapsules. (A) ABA, (B) ALA and (C) APA (magnification, 4×).
3.2.4. Cytotoxicity test
Since the cytotoxicity of positive charges of carriers on biological systems is proven, covering of the alginate microcapsules with an anionic layer following by the cationic layering is suggested. To investigate the effect of anionic charge density on the layering efficiency, the cytotoxicity of the microcapsules covered with alginate or NCS as final layers, was evaluated using MTT assay on HepG2 cell line. Table 2 shows the quantitative values of cell viability. According to International Organization for Standardization (ISO), decrease in cell viability by 30%, might mean that the material could be considered toxic and hence not biocompatible [48]. The MTT result showed that NCS and alginate showed similar efficiency in the covering of un-bounded positively-charged sites (P > 0.05). Since all the reported cell viability values are more than 70%, all the different types of microcapsules: ALA, ALN, APA, and APN, were considered safe and therefore can further be studied for their in vivo applications. This is in line with Jinchen et al. [49] who announced that in vitro cytotoxicity evaluation of alginate based microcapsules showed negligible toxicity. Since, NCS showed similar result to the alginate, it has been involved in the remaining steps of study for further investigations.
Table 2.
Percentage of cell (HepG2) viability after exposure to ALA, ALN, APA and APN (P > 0.05) (mean ± SD, n = 3).
| Cell viabili (%) | Type of microcaps |
|---|---|
| ALA | 73.36 ± 6.18 |
| ALN | 88.33 ± 4.99 |
| APA | 80.02 ± 15.73 |
| APN | 75.44 ± 6.23 |
3.2.5. Surface roughness
Surface roughness of microcapsules is an important factor in their in vivo effectiveness. As stated by Genaro Alberto Paredes Juárez [50], one of the most important factors to be considered in the design of safe and biocompatible systems is to fabricate smooth surfaces which do not cause immune cells adhesion and activation. Therefore, surface roughness of ALA, APA, ALN, APN, and Ca-alg microcapsules was considered as an important parameter to be compared.
As earlier stated, P/V, has been used as a parameter to evaluate the microcapsules surface roughness. Table 3 shows the values obtained from P/V measurement. The surface roughness between the different types of microcapsules is not statistically different (P > 0.05).
Table 3.
Peak to valley measurement in different types of microcapsules (P > 0.05) (mean ± SD, n = 3).
| Type of microcapsule | P/V (ΜM) |
|---|---|
| ALA | 0.35 ± 0.03 |
| ALN | 0.12 ± 0.04 |
| APA | 0.22 ± 0.12 |
| APN | 0.18 ± 0.03 |
| CA-ALG | 0.45 ± 0.18 |
3.2.6. Long-term stability
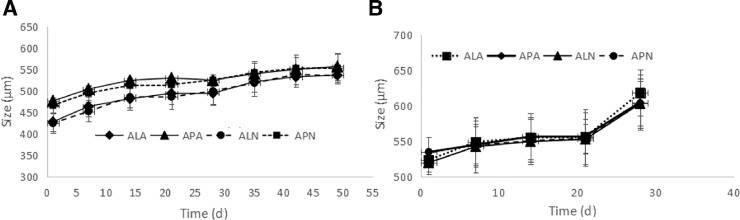
Since the maintenance of polyelectrolyte complexes in multilayer microcapsules is strongly affected by the ion composition in the medium, therefore, the stability of the microcapsules in 0.9% NaCl was investigated. Fig. 9A, shows that the size of all the different types of microcapsules gradually increased up to the 6th week and then the plateau could be seen in the following days. Fig. 10 shows that the microcapsules were stable in NaCl medium in 7 weeks, as they all remained intact. It could be concluded that different types of these microcapsules showed similar and suitable long-term stability properties in both temperature conditions (Fig. 9A and 9B).
Fig. 9.
Size growth in different types of microcapsules. Incubation temperature, (A) 2 to 8 °C and (B) 37 °C (mean ± SD, n = 3).
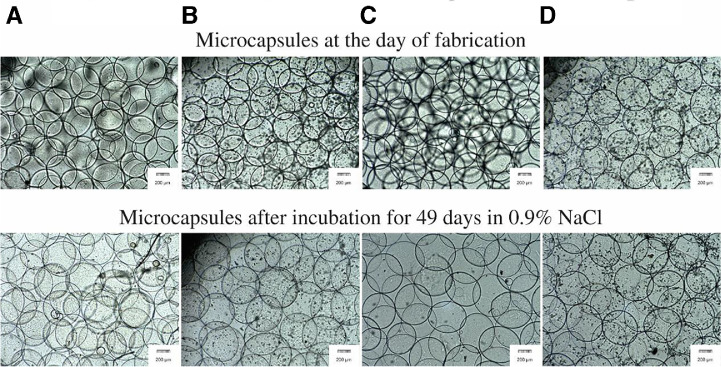
Fig. 10.
Microscopic images of the multilayer microcapsules. Upper panel shows the microcapsules at the day of fabrication, lower panel shows the microcapsules after incubation for 49 days in 0.9% NaCl. (A) ALA, (B) ALN, (C) APA and (D) APN (magnification, 4×). Black spots in B and D series are NCS precipitates.
4. Conclusion
The comparison between three cationic polymers: LPEI, BPEI and PLL, as covering layers of alginate microcapsules through the evaluation of the shape, size, and mechanical stability of the microcapsules led to the exclusion of BPEI from the rest of the study owing to the formation of the undesirable, aggregated, and looser ABA microcapsules. It seems that similar and linear structure of LPEI and PLL led to the similar interaction with alginate and consequently similar behavior in covering of the microcapsules. Investigation of the microcapsules cytotoxicity using MTT test showed that NCS and Alg behaved similarly in covering of the positive surface charges of the microcapsules and the cell viability post exposure to ALA, APA, ALN, and APN was not significantly different. At different steps of this research, such as surface roughness study, long-term stability investigation, and cytotoxicity evaluation, NCS showed no superiority over alginate and it is also more costly than alginate, therefore, it does not seem logical to use it as the final covering layer. These microcapsules could be used as carrier systems in different areas depending on the encapsulated substance. It may be concluded that ALA showed similar in vitro properties to APA and could be considered as the more cost-effective alternative to APA and also a subject for further research in future studies.
Declaration of interest
The authors declare that there is no conflict of interest.
Acknowledgement
This study was fully funded and supported by Shahid Beheshti University of Medical Sciences (Research grant number. 7026).
The authors would like to acknowledge Mr. F. Rafraf and Mr. S.M. Foroutanfar for their valuable supports in design of encapsulator device and the graphical parts of this project.
Contributor Information
Arash Mahboubi, Email: a.mahboubi@sbmu.ac.ir.
Leila Nematollahi, Email: Leila.nematollahi@pasteur.ac.ir.
References
- 1.Tomaro-Duchesneau C., Saha S., Malhotra M., Kahouli I., Prakash S. Microencapsulation for the therapeutic delivery of drugs, live mammalian and bacterial cells, and other biopharmaceutics: Current status and future directions. J Pharm. 2013;2013 doi: 10.1155/2013/103527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidebach T., Först P., Kulozik U. Microencapsulation of probiotic cells for food applications. Crit Rev Food Sci Nutr. 2012;52(4):291–311. doi: 10.1080/10408398.2010.499801. [DOI] [PubMed] [Google Scholar]
- 3.Onwulata C.l. Encapsulation of new active ingredients. Annu Rev Food Sci Technol. 2012;3:183–202. doi: 10.1146/annurev-food-022811-101140. [DOI] [PubMed] [Google Scholar]
- 4.Farhadnejad H., Mortazavi S.A., Erfan M., Darbasizadeh B., Motasadizadeh H., Fatahi Y. Facile preparation and characterization of pH sensitive Mt/CMC nanocomposite hydrogel beads for propranolol controlled release. Int J Biol Macromol. 2018;111:696–705. doi: 10.1016/j.ijbiomac.2018.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Hossieni-Aghdam S.J., Foroughi-Nia B., Zare-Akbari Z., Mojarad-Jabali S., Farhadnejad H. Facile fabrication and characterization of a novel oral pH-sensitive drug delivery system based on CMC hydrogel and HNT-AT nanohybrid. Int J Biol Macromol. 2018;107:2436–2449. doi: 10.1016/j.ijbiomac.2017.10.128. [DOI] [PubMed] [Google Scholar]
- 6.Gasperini L., Mano J.F., Reis R.L. Natural polymers for the microencapsulation of cells. J R Soc Interface. 2014 Nov6;11(100) doi: 10.1098/rsif.2014.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vos P., Lazarjani H.A., Poncelet D., Faas M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev. 2014;67-68:15–34. doi: 10.1016/j.addr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Donati I., Holtan S., Mørch Y.A., Borgogna M., Dentini M., Skjåk-Bræk G. New hypothesis on the role of alternating sequences in calcium−alginate gels. Biomacromolecules. 2005;6(2):1031–1040. doi: 10.1021/bm049306e. [DOI] [PubMed] [Google Scholar]
- 9.Grant G.T., Morris E.R., Rees D.A., Smith P.J., Thom D. Biological interactions between polysaccharides and divalent cations: the egg‐box model. FEBS Lett. 1973;32(1):195–198. [Google Scholar]
- 10.Rees D.A. Polysaccharide shapes and their interactions-some recent advances. Pure Appl Chem. 1981;53(1):1–14. [Google Scholar]
- 11.Kuo C.K., Ma P.X. Maintaining dimensions and mechanical properties of ionically crosslinked alginate hydrogel scaffolds in vitro. J Biomed Mater Res A. 2008;84(4):899–907. doi: 10.1002/jbm.a.31375. [DOI] [PubMed] [Google Scholar]
- 12.de Vos P., Haan B.D., Schilfgaarde R.V. Effect of the alginate composition on the biocompatibility of alginate-polylysine microcapsules. Biomaterials. 1997;18(3):273–278. doi: 10.1016/s0142-9612(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 13.Juste S., Lessard M., Henley N., Ménard M., Hallé J.P. Effect of poly‐L‐lysine coating on macrophage activation by alginate‐based microcapsules: Assessment using a new in vitro method. J Biomed Mater Res A. 2005;72(4):389–398. doi: 10.1002/jbm.a.30254. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Wang W., Ma J., Guo X., Yu X., Ma X. Proliferation and differentiation of mouse embryonic stem cells in APA microcapsule: A model for studying the interaction between stem cells and their niche. Biotechnol prog. 2006;22(3):791–800. doi: 10.1021/bp050386n. [DOI] [PubMed] [Google Scholar]
- 15.Thanos C., Calafiore R., Basta G., Bintz B., Bell W., Hudak J., Vasconcellos A. Formulating the alginate–polyornithine biocapsule for prolonged stability: Evaluation of composition and manufacturing technique. J Biomed Mater Res A. 2007;83(1):216–224. doi: 10.1002/jbm.a.31472. [DOI] [PubMed] [Google Scholar]
- 16.Baruch L., Machluf M. Alginate–chitosan complex coacervation for cell encapsulation: Effect on mechanical properties and on long‐term viability. Biopolymers. 2006;82(6):570–579. doi: 10.1002/bip.20509. [DOI] [PubMed] [Google Scholar]
- 17.Santos E., Zarate J., Orive G., Hernández R.M., Pedraz J.L. Biomaterials in cell microencapsulation. In: Pedraz J.L., Orive G., editors. Therapeutic applications of cell microencapsulation. Springer; 2010. pp. 5–21. [Google Scholar]
- 18.Sela M., Arnon R., Jacobson I. Synthesis of poly‐L‐lysine and poly‐L‐lysyl albumin via ϵ, N‐trifluoroacetyl‐α, N‐carboxy‐L‐lysine anhydride. Biopolymers. 1963;1(6):517–525. [Google Scholar]
- 19.Ahn C.H., Chae S.Y., Bae Y.H., Kim S.W. Synthesis of biodegradable multi-block copolymers of poly (L-lysine) and poly (ethylene glycol) as a non-viral gene carrier. J Control Release. 2004;97(3):567–574. doi: 10.1016/j.jconrel.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Fuller W.D., Verlander M.S., Goodman M. A procedure for the facile syntheis of amino‐acid N‐carboxyanhydrides. Biopolymers. 1976;15(9):1869–1871. doi: 10.1002/bip.1976.360150922. [DOI] [PubMed] [Google Scholar]
- 21.Strand B.L., Ryan L., Veld P.I., Kulseng B., Rokstad A.M., Skjåk-Bræk G., Espevik T. Poly-l-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10(3):263–275. doi: 10.3727/000000001783986800. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S.R., Khattak S.F., Roberts S.C. Polyelectrolytes for cell encapsulation. COCIS. 2005;10(1):45–51. [Google Scholar]
- 23.Tuch B., Vaithilingam V., Foster J. Human trials with microencapsulated insulin-producing cells: Past, present and future. In: Brandtner E.M., Dangerfield J.A., editors. Bioencapsulation of living cells for diverse medical applications. Bentham Science; 2013. pp. 40–69. [Google Scholar]
- 24.Jacobs-Tulleneers-Thevissen D., Chintinne M., Ling Z., Gillard P., Schoonjans L., Delvaux G. Beta cell therapy consortium EU-FP7 sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56(7):1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- 25.Acarregui A., Murua A., Pedraz J.L., Orive G., Hernández R.M. A perspective on bioactive cell microencapsulation. BioDrugs. 2012;26(5):283–301. doi: 10.1007/BF03261887. [DOI] [PubMed] [Google Scholar]
- 26.Spasojevic M., Paredes-Juarez G.A., Vorenkamp J., de Haan B.J., Schouten A.J., de Vos P. Reduction of the inflammatory responses against alginate-poly-l-lysine microcapsules by anti-biofouling surfaces of PEG-b-PLL diblock copolymers. PloS one. 2014;9(10) doi: 10.1371/journal.pone.0109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darrabie M.D., Kendall W.F., Opara E.C. Characteristics of poly-l-ornithine-coated alginate microcapsules. Biomaterials. 2005;26(34):6846–6852. doi: 10.1016/j.biomaterials.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Ponce S., Orive G., Hernández R., Gascón A.R., Pedraz J.L., de Haan B.J. Chemistry and the biological response against immunoisolating alginate–polycation capsules of different composition. Biomaterials. 2006;27(28):4831–4839. doi: 10.1016/j.biomaterials.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Farrell L.L., Pepin J., Kucharski C., Lin X., Xu Z., Uludag H. A comparison of the effectiveness of cationic polymers poly-l-lysine (PLL) and polyethylenimine (PEI) for non-viral delivery of plasmid DNA to bone marrow stromal cells (BMSC) Eur J Pharm Biopharm. 2007;65(3):388–397. doi: 10.1016/j.ejpb.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Cortes S., Berenguel R.M., Madejon A., Perez-Mendez M. Adsorption of polyethyleneimine on silver nanoparticles and its interaction with a plasmid DNA: A surface-enhanced Raman scattering study. Biomacromolecules. 2002;3(4):655–660. doi: 10.1021/bm015640o. [DOI] [PubMed] [Google Scholar]
- 31.Weyts K.F., Goethals E.J. New synthesis of linear polyethyleneimine. Polymer Bull. 1988;19(1):13–19. [Google Scholar]
- 32.Brissault B., Kichler A., Guis C., Leborgne C., Danos O., Cheradame H. Synthesis of linear polyethylenimine derivatives for DNA transfection. Bioconjugate Chem. 2003;14(3):581–587. doi: 10.1021/bc0200529. [DOI] [PubMed] [Google Scholar]
- 33.Samal S.K., Dash M., Van Vlierberghe S., Kaplan D.L., Chiellini E., Van Blitterswijk C. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41(21):7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- 34.Hadjialirezaei S. Coating of alginate capsules. Institutt for Bioteknologi. 2013 [Google Scholar]
- 35.Schaffellner S., Stadlbauer V., Stiegler P., Hauser O., Halwachs G., Lackner C. Porcine islet cells microencapsulated in sodium cellulose sulfate. Transplant Proc. 2005;37(1):248–252. doi: 10.1016/j.transproceed.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Abastado J.S., Gunzburg W.H., Brandtner E.M. The diversity of uses for cellulose sulphate encapsulation. Brandtner E.M., Dangerfield J.A., editors. Bioencapsulation of living cells for diverse medical applicationsBentham Science. 2013:70–92. [Google Scholar]
- 37.Stucky E.C., Schloss R.S., Yarmush M.L., Shreiber D.I. Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response. Cytotherapy. 2015;17(10):1353–1364. doi: 10.1016/j.jcyt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breguet V., Gugerli R., Stockar U.V., Marison I.W. CHO immobilization in alginate/poly-l-lysine microcapsules: An understanding of potential and limitations. Cytotechnology. 2007;53(1-3):81–93. doi: 10.1007/s10616-007-9045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal T., Paul S., Sa B. Polymethylmethacrylate coated alginate matrix microcapsules for controlled release of diclofenac sodium. Pharmacol Pharm. 2011;2(2):56–66. [Google Scholar]
- 40.Boussu K., van der Bruggen B., Volodin A., Snauwaert J., van Haesendonck C., Vandecasteele C. Roughness and hydrophobicity studies of nanofiltration membranes using different modes of AFM. J Colloid Interface Sci. 2005;286(2):632–638. doi: 10.1016/j.jcis.2005.01.095. [DOI] [PubMed] [Google Scholar]
- 41.Gåserød O., Sannes A., Skjåk-Bræk G. Microcapsules of alginate–chitosan. II. A study of capsule stability and permeability. Biomaterials. 1999;20(8):773–783. doi: 10.1016/s0142-9612(98)00230-0. [DOI] [PubMed] [Google Scholar]
- 42.Senthilraja P., Kathiresan K. In vitro cytotoxicity MTT assay in Vero, HepG2 and MCF-7 cell lines study of Marine Yeast. J Appl Pharm Sci. 2015;5(3):80–84. [Google Scholar]
- 43.Kong H.J., Mooney D.J. The effects of poly (ethyleneimine)(PEI) molecular weight on reinforcement of alginate hydrogels. Cell Transplant. 2003;12(7):779–785. doi: 10.3727/000000003108747253. [DOI] [PubMed] [Google Scholar]
- 44.Tam S.K., Dusseault J., Polizu S., Ménard M., Hallé J.P., Yahia L.H. Physicochemical model of alginate–poly-l-lysine microcapsules defined at the micrometric/nanometric scale using ATR-FTIR, XPS, and ToF-SIMS. Biomaterials. 2005;26(34):6950–6961. doi: 10.1016/j.biomaterials.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Thu B., Bruheim P., Espevik T., Smidsrød O., Soon-Shiong P., Skjåk-Bræk G. Alginate polycation microcapsules: I. Interaction between alginate and polycation. Biomaterials. 1996;17(10):1031–1040. doi: 10.1016/0142-9612(96)84680-1. [DOI] [PubMed] [Google Scholar]
- 46.Thu B., Bruheim P., Espevik T., Smidsrød O., Soon-Shiong P., Skjåk-Bræk G. Alginate polycation microcapsules: II. Some functional properties. Biomater. 1996;17(11):1069–1079. doi: 10.1016/0142-9612(96)85907-2. [DOI] [PubMed] [Google Scholar]
- 47.Buitelaar R., Bucke C., Tramper J., Wijffels R. Elsevier; 1996. Immobilized cells: Basics and applications. [Google Scholar]
- 48.ISO . International Organization for Standardization; Geneva: 2009. Biological evaluation of medical devices–part 5: Tests for in vitro cytotoxicity. [Google Scholar]
- 49.Sun J., Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials. 2013;6(4):1285–1309. doi: 10.3390/ma6041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paredes J.G.A., Spasojevic M., Faas M.M., de Vos P. Immunological and technical considerations in application of alginate-based microencapsulation systems. Front Bioeng Biotechnol. 2014;2:26. doi: 10.3389/fbioe.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris E.R., Grant G.T., Rees D.A., Smith P.J.C., Thom D. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett. 1973;32(1):195–198. [Google Scholar]
- 52.Intra J., Salem A.K. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J Control Release. 2008;130(2):129–138. doi: 10.1016/j.jconrel.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]