Abstract
Theory predicts that social interactions can induce an alignment of behavioral asymmetries between individuals (i.e., population-level lateralization), but evidence for this effect is mixed. To understand how interaction with other individuals affects behavioral asymmetries, we systematically manipulated the social environment of Drosophila melanogaster, testing individual flies and dyads (female-male, female-female and male-male pairs). In these social contexts we measured individual and population asymmetries in individual behaviors (circling asymmetry, wing use) and dyadic behaviors (relative position and orientation between two flies) in five different genotypes. We reasoned that if coordination between individuals drives alignment of behavioral asymmetries, greater alignment at the population-level should be observed in social contexts compared to solitary individuals. We observed that the presence of other individuals influenced the behavior and position of flies but had unexpected effects on individual and population asymmetries: individual-level asymmetries were strong and modulated by the social context but population-level asymmetries were mild or absent. Moreover, the strength of individual-level asymmetries differed between strains, but this was not the case for population-level asymmetries. These findings suggest that the degree of social interaction found in Drosophila is insufficient to drive population-level behavioral asymmetries.
Subject terms: Behavioural methods, Genetics of the nervous system, Social behaviour
Introduction
Consistent left-right asymmetries in brain and behavior are widespread among animal species1–4. For instance, the vast majority of people is right handed (a behavior controlled by the left hemisphere), and an advantage of using the left eye (right hemisphere) has been observed in agonistic interactions in chicks5, lizards6, toads7 and baboons8, while in bees the use of left antennae enhances aggressive behavior9 and the use of the right antenna is involved in social behavior10. In these cases, when asymmetries are aligned on the same side in the majority of the population, we consider this population-level lateralization or directional asymmetry. In other cases, individuals exhibit strong and consistent preferences for one side, but these are not aligned at the population level and we define these cases individual-level lateralization or antisymmetry.
How the presence of other individuals influences these asymmetries at the individual and group level remains largely unknown. Mathematical models suggest that selective pressures associated with living in social contexts enhance the alignment of behavioral asymmetries (population-level lateralization), due to the advantages of coordinating between individuals11,12. This hypothesis predicts population-level asymmetries in social contexts more than in individual contexts, for the contexts that occurred repeatedly in the course of evolution (for a recent review see13). The growing evidence on the effect of the social context on asymmetric behavior, though, is mixed9,14. Contexts that are expected to elicit coordinated social behavior are parent-offspring, female-male and agonistic interactions or group movements, in which there are clear advantages for coordination between individuals. Several studies directly support this idea; for instance, gregarious species such as sheep coordinate motor biases within populations and maintain the same side bias across generations15, in many species social interactions between mother and offspring are lateralized at the population level16,17, and population asymmetries have been observed in mating and fighting in several species18–20. In social insects such as honeybees, bumblebees and social stingless bees, a strong population bias in the use of antennae for olfactory learning has been observed, contrary to solitary bees that do not exhibit a population bias21. This evidence suggests that population lateralization might have evolved under the pressure to coordinate between individuals. While this model focuses on explaining the alignment of population-level asymmetries, it does not predict a modulation of individual-level asymmetries as a function of the social context.
To date, individual level lateralization has been explained as the result of advantages that are mostly independent from social interactions13,22, such as avoiding neural reduplication in small nervous systems, simultaneous and parallel processing of information, increased/faster/stronger motor control and cognitive specialization22,23. Data from several taxa are consistent with such advantages, for instance: strongly lateralized chimpanzees are more effective at fishing termites using a stick24, lateralized parrots are better at solving novel problems (string pulling and pebble-seed discrimination) than less lateralized parrots25, lateralized fish perform better in spatial reorientation tasks26, lateralized antlions have advantages in learning27, and lateralized locust perform better crossing a gap28. To our knowledge, no studies have investigated whether individual-level asymmetry is modulated by social context, nor is there theory predicting effects one way or the other.
Population level asymmetries have been identified not only in eusocial or gregarious species, but also in interactions of “solitary” species. Some examples include aggressive/mating contexts in solitary mason bees9, blowfly and tephritid flies19,20, locusts during predator surveillance29. There are also examples of population asymmetries in nominally solitary behaviors in solitary species, including: sensory asymmetries in nematodes30, Drosophila larvae31 and adults32. It is not clear, though, whether these asymmetries are connected to social interactions: larvae and adult flies aggregate and interact during foraging, and locusts exhibit collective migration in their gregarious phase. Moreover, for sexually reproducing animals, it is difficult to rule out the possibility that population-level asymmetries are related to social interactions, since all such animals perform the social behavior of mating. An experimental approach in which the social context is manipulated and the effects on lateralization are monitored might clarify the role of the social context on lateralization.
Here we adopted this approach to understand how social context affects population and individual level lateralization. We use fruit flies (Drosophila melanogaster) to assess how individual-level and population-level asymmetries are affected by social context (two males, two females, one male and one female, one male or one female). As social behaviors, that theory predicts will be modulated by social context, we examined relative position and orientation between individuals. As individual behaviors, that are not expected to be strongly modulated by the social context, we examined circling asymmetry (clockwise/anticlockwise circulation in an arena) and preferential wing use.
The social asymmetry hypothesis predicts: (a) stronger population-level asymmetries in social versus individual contexts, (b) stronger population-level asymmetries in social versus individual behaviors, (c) a modulation of population-level asymmetries with the particular social context, with larger population-level asymmetries in male-female (courtship) and male-male (potentially aggressive rivalry) interactions versus female-female interactions. Lack of these patterns would argue against a causative role of social context in population asymmetries in Drosophila.
Dyadic behaviors
In our investigation of dyadic behaviors, we focused on the relative position between flies, a trait that is lateralized at the population level in different species1. In many vertebrate species, systematic asymmetries in eye use are accompanied by asymmetries in body position, with a preferential use of the left eye for monitoring conspecific in birds, fish and primates33,34. Moreover, several mammals preferentially keep offspring on the left side16. It has been suggested that this reflects an advantage of the right hemisphere in social monitoring34,35. It is not clear, though, whether flies exhibit side biases in their relative position. For instance, the male/female position during courtship systematically differs between species. In most species, such as D. melanogaster, males court females from behind, while in few species males court on the front or side/back, or even circle around the female36–39.
The analysis of social interaction networks between same sex flies40 has shown an effect of sex and genotype in the duration and propensity for interaction. Looking at short interactions between virgin flies of both sexes, a modulation of social context and sex has been observed, with males showing a higher probability of orienting themselves toward females than toward males, and females showing few biases in their position with respect to other flies41. In the same work, interspecific differences between Drosophila species were documented, indicating genetic variation for position asymmetries. The available evidence suggests that the relative position adopted during interactions is genetically modulated, but a systematic investigation of genetic variation for this trait is still lacking.
Individual behaviors
As individual behaviors that do not require the presence of another fly, we studied circling asymmetry (clockwise/anticlockwise circulation in a circular arena) and preferential wing use. Asymmetries at the individual but not the population level have been found in the clockwise/anticlockwise walking preference of individual flies42–44 or male/female dyads45,46 but little is known on the influence of social context.
When not flying, flies use their wings in self grooming (a behavior that is more frequent in social contexts47,48), courtship and aggression. Male flies extend and vibrate wings during courtship in species-specific fashion. For instance, D. melanogaster mainly vibrates one wing, D. suzukii one or two wings, whereas D. biarmipes flutters both wings38. D. melanogaster males make a greater use of wings when they are located behind the female, likely to perform courtship song49. Wing threat is used by both males and females in aggressive contexts directed towards both sexes50. Because flies use wings in social contexts, a modulation of population-level asymmetries in wing use might occur due to evolutionary pressures for coordination. Wing use asymmetry in flies has been studied for several decades45,46 with different outcomes but population-level lateralization has not been observed. Buchanan and colleagues43 found evidence of individual side biases in wing folding (right or left wing closed on top of the other wing). This trait was not correlated to asymmetries in clock-anticlockwise circulation. While some authors45,46 observed individual preferences in wing folding, another51 failed to observe consistent individual preferences. Overall, previous results are not conclusive.
Methods
Drosophila stocks and husbandry
We used five inbred lines of the Drosophila Genetic Reference Panel52 from the Bloomington Drosophila Stock Center. Each line in this collection was started as an isofemale line derived from a single field collection in Raleigh (North Carolina) and then subjected to 20 generations of full-sibling mating, making most loci homozygous within lines53. To assure high genetic diversity among the focal genotypes, we tested lines belonging to different mitochondrial clades: line 69 (clade III), line 136 (clade I), line 338 (clade VI), line 535 (clade I), line 796 (clade I)54. Before the experiments, these lines had been maintained in the same laboratory conditions for at least five generations. Flies were reared on cornmeal/dextrose fly food (made in the Harvard University BioLabs Fly Food Facility) in a single incubator at 25 °C at 30–40% relative humidity with a 12:12-h light:dark cycle. Flies were tested between day 3 and 9 post-eclosion. Before the test, flies were housed in standard fly vials with 30–40 conspecifics of both sexes. Table 1 displays the number of individuals tested in each condition for each line.
Table 1.
Number of individuals tested for each line and sex.
| ♀ | ♂ | ♀-♀ | ♂-♂ | ♀-♂ | |
|---|---|---|---|---|---|
| RAL-69 | 99 | 100 | 152 | 156 | 216 |
| RAL-136 | 100 | 100 | 150 | 196 | 188 |
| RAL-338 | 100 | 100 | 180 | 138 | 200 |
| RAL-535 | 100 | 100 | 160 | 154 | 160 |
| RAL-796 | 100 | 100 | 160 | 160 | 150 |
Apparatus
Behavior was measured in circular acrylic arenas arrayed for simultaneous imaging of 15–20 arenas. See description of imaging set-ups in55. Arenas were illuminated from below by an array of white LEDs (Knema), imaged with digital cameras (Pointgrey Blackfly 1.2 MP Mono GigE PoE) and recorded at a rate of 10 frames per second with Pointgrey FlyCapture software for 60 minutes (36,000 frames). Arenas (2.54 cm in diameter, 1.6 mm deep) were fabricated in black acrylic using a laser cutter. Each arena’s transparent acrylic lid was lubricated with Sigmacote (Sigma) to prevent flies from walking on the underside of the lids. To illuminate the arenas uniformly, we placed a diffuser (two sheets of 3.2 mm-thick transparent acrylic roughened on both sides by orbital sanding) between the LED array and the arena array.
Phenotypic assay
Before the test, flies were lightly anaesthetized with CO2 and placed in the arena using a brush. The trial started after an acclimation of 12–15 minutes and lasted 60 minutes. Flies were tested individually or in dyads that contained two individuals of the same line (but previously kept in separate vials): two females, two males, or one male and one female. Assays were run between 10:00 am and 8 pm. Since preliminary analyses showed that the hours of the day had at most a small effect size on all dependent variables (in line with previous results56), we excluded this variable from further analyses. For each frame, the x and y positions of each fly centroid and the angle of the wings with respect to the body midline were measured with Flytracker57. The released version of the software smooths the computed angles between flies using a spatial kernel of three values from subsequent frames (with weights 1/4, 1/2, 1/4, respectively). When the fly position is expressed in polar coordinates, this produces an error for flies positioned at 180°. To fix this error we modified the code excluding the angle smoothing function.
Analyses
For each individual animal and frame we analyzed individual behaviors and dyadic behaviors.
As individual behaviors, we measured circling asymmetry C and asymmetrical wing use W. To obtain C for each fly, we measured positive and negative deviations in trajectory (degrees) across subsequent frames (see Fig. 1), using the angle between the previous trajectory (vector between time t1 and time t2) and the current trajectory (vector between time t2 and time t3) and calculated the ratio between the sum of positive (anticlockwise) and negative (clockwise) deviations (c) and the overall deviations (sum of the absolute value of all deviations).
Figure 1.
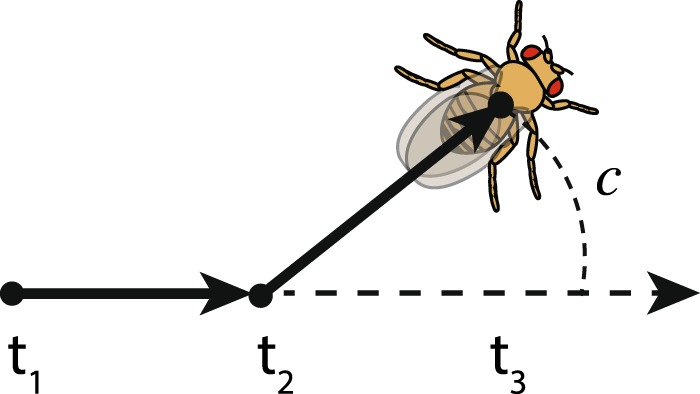
Vectors used to measure circling asymmetry, as the angle between the previous trajectory (vector between t1 and t2) and the current trajectory (vector between t2 and t3). Clockwise deviations are negative, anticlockwise deviations are positive.
Negative C indicates clockwise preferences, positive C indicates anticlockwise preferences. To quantify population-level and individual-level asymmetries we used C and |C| respectively, where significant departures from 0 indicate significant asymmetries.
To obtain W for each fly, we used a similar procedure and measured positive (right wing) and negative (left wing) degrees of wing opening in each frame and calculated the ratio between the sum of positive (left wing) and negative (right wing) opening degrees (w) and the overall wing opening (sum of the absolute value of all wing openings).
Positive W values indicate predominantly right wing use, negative values predominantly left wing use. To quantify population-level and individual-level asymmetries in wing use, we used W and |W| respectively, where significant departures from 0 indicate significant asymmetries.
Dyadic behaviors involved two subjects
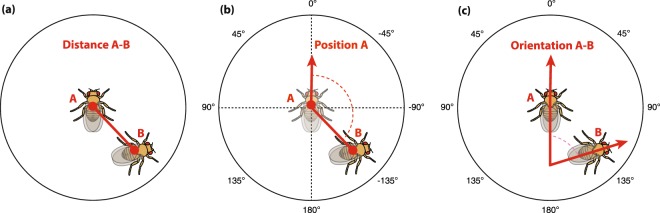
For each frame, the spatial relationship between flies A and B was quantified by calculating: (a) the Distance between the centroids of the two flies [mm], (b) the relative Position as the angle in degrees between back-head vector of the focus fly and the segment vector the centroids of the two flies, (c) the Orientation, as the angle in degrees between the back-head vectors of the two flies. Of these three measures, the Position between the dyad partner fly (B) compared to the focal fly (A) can be lateralized. We scored the relative Position P of a fly (fly B in Fig. 2b) as negative when it was located to the right of the focal fly, and positive when it was located to the left of the focal fly. Values close to +/−180° indicate that the focal fly is in front of the other, while values close to 0 indicate that the focal fly is located behind the other fly.
Figure 2.
Social metrics calculated for dyads of flies. (a) Distance in mm between the centroids of the flies. (b) The Position of fly B is the angle between the vector trajectory of A and the vector between the centroids of A and B. When B is located to the right of A Position is negative, when B is located to the left of A Position is positive. (c) The relative Orientation between A and B is the angle between the facing vectors of the two flies. It ranges between 0°, when flies are parallel, and 180°, when flies are facing opposite directions.
Statistics
We analyzed each variable using an ANOVA with social Context (two females, two males, one male and one female, single fly), Sex (male, female), Strain (RAL-69, RAL-136, RAL-338, RAL-535, RAL-796) and their interactions as independent variables. We set significant results with alpha level of 0.05 and considered to be biologically meaningful factors or interactions that not only were significant but also had a size effect medium or high as defined by ω2 (see58) greater than 0.6. We used one-sample t-tests against the chance level (0) and Cohen’s d as effect size estimate to check for overall asymmetries. Statistical calculations were conducted with R 3.5.2. For the analysis of the variable position we used a bootstrap method (resampling with replacement for 100 replicates) to calculate the standard error around our estimate of the distribution and compare different conditions. These analyses have been performed with MATLAB 2016a.
Results and Discussion
Individual behaviors: circling asymmetry and wing use
To investigate the effect of the social context and strain on the asymmetry of individual behaviors, we investigated clockwise/anticlockwise circling asymmetry, and left/right asymmetry in wing positioning in individual males (M) and females (F) and in dyads with two females (FF), two males (MM), one female and one male (FM). If behavioral asymmetry is enhanced by social context, we would expect greater population-level asymmetries in dyads compared to individual flies, especially in FM and MM dyads, due to the evolutionary importance of mating and aggression. Moreover, we would not necessarily expect modulation of individual-level asymmetries.
Population-level circling asymmetry
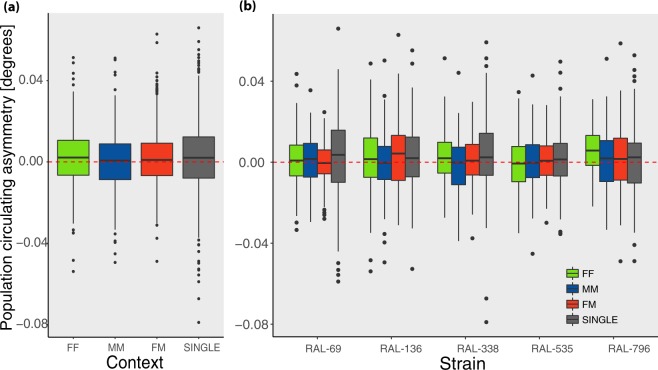
For each fly, strain and context we calculated the circling asymmetry index across the whole trial (60 minutes), which represents each animal’s tendency to turn clockwise or anticlockwise (this is measured as the population circling asymmetry index, Cp, which ranges from −1 to 1). All factors (Context, Sex and Strain) provided only a very small explanatory contribution to the overall variance and there was no significant difference between single flies and dyads (see Fig. 3 and Supplementary Table 1). These results suggest that, in dyads, social context does not modulate population-level circling asymmetry.
Figure 3.
(a) Overall mean population circling asymmetry by Context (FF, MM, FM, SINGLE) and (b) by Context and Strain. The dashed line indicates the absence of population-level circling asymmetry. Here and elsewhere, boxes demarcate the interquartile range, thick horizontal line the median, whiskers minimum/maximum excluding the outliers, and point outside this range outliers, namely points greater/lower than 1.5 times the interquartile range).
We observed a very small but significant preference for the circling anticlockwise asymmetry (t3518 = 6.3, p = 2e-10, Mean = 0.0015, SD = 0.014, d = 0.11, 95% CI = 0.0010–0.0020; see Fig. 3b). In line with this small effect, previous assays did not show population-level asymmetries42,43,45,59. We did not observe any increase in population alignment in dyads compared to individual flies (see Supplementary Table 2), suggesting that the evolutionary pressure for alignment with conspecifics is absent or negligible in circling asymmetry in fruit flies.
Individual-level circling asymmetry
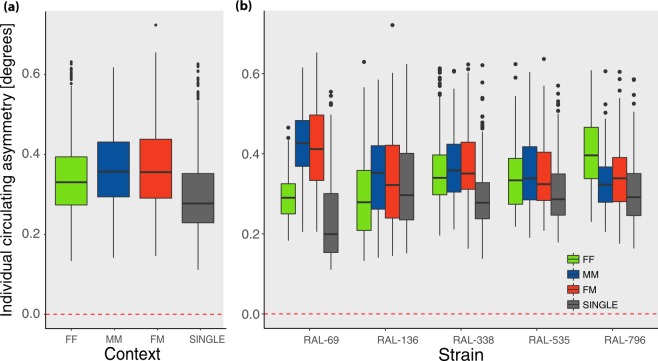
For each fly, strain and context we calculated an absolute circling asymmetry index across the trial (60 minutes). This index ranges from 0 (no individual bias) to 1 (complete preference for either clockwise or anticlockwise circling asymmetry). Exploratory analyses showed a constant level of individual asymmetry during the test. We observed a significant main effect and high ω2 only for Context (F1,3489 = 123.521, p < 2e-16, ω2 = 0.085, Fig. 4a) and Context x Strain (F12,3489 = 35.358, p < 2e-16, ω2 = 0.095, see Fig. 4b), see Supplementary Table 3 for the complete results. Flies in the single context had lower individual asymmetry than flies in dyads (F1,3499 = 319.856, p < 2e-16, ω2 = 0.075), and this factor (Dyad) was the main explanatory factor of the observed variance (Supplementary Table 4). The greater individual asymmetry in dyads suggests that, similarly to what already documented in phototactic behavior in cockroaches60, individual behavioral preferences in insects are modulated by whether a task is performed in isolation or in a group. Overall, looking at the individual circling asymmetry index vs. the absence of asymmetry (0) we observed strong individual-level lateralization (t3518 = 198, p < 2e-16, Mean = 0.340, SD = 0.10, Cohen’s d = 3.3, 95% CI = 0.336, 0.343; see Fig. 4a). This result confirms previous reports of strong individual preferences in locomotor behavior in flies tested individually42,43.
Figure 4.
(a) Overall individual circling asymmetry index by Context (FF, MM, FM, SINGLE) and (b) by Context and Strain. The dashed line indicates the absence of individual-level circling asymmetry.
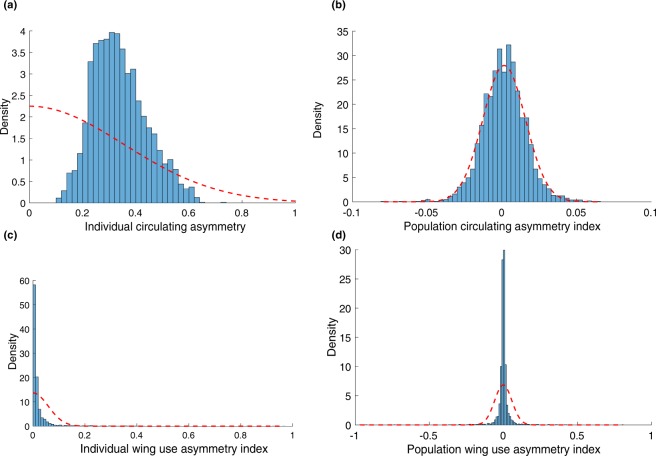
Contrary to our hypothesis, when comparing the data on population- and individual-level circling asymmetries, the effect of the social context (Context), Strain and their interaction appeared much stronger for individual than for population asymmetries. Moreover, while population-level circling asymmetries were small for all genotypes, individual asymmetries were strong for all genotypes (Fig. 4b). This suggests that, in Drosophila melanogaster, circling asymmetry is modulated by the social context mostly at the individual level, whereas circling asymmetry is neither substantial or socially modulated at the population level. The presence of a strong individual level asymmetry and absence of population level asymmetry is clear comparing the histograms of the observed asymmetry indices (individual and population) and their respective expected distributions in the absence of asymmetries (Fig. 5a,b). The social context increases individual but not population circling asymmetries in Drosophila. Interestingly, FM (female-male) dyads and MM (male-male) dyads, in which mating and aggression are expected at high frequencies, did not exhibit greater population asymmetries. Overall, our results on circling asymmetry appear inconsistent with a role of social context as a driver of population motor asymmetries, but indicate a novel role in modulating individual motor asymmetry.
Figure 5.
(a) Distribution of individual-level circling asymmetry values. (b) Distribution of population-level circling asymmetry values. (c) Distribution of individual-level wing use asymmetry values. (d) Distribution of population-level wing-use asymmetry values. Dashed lines indicate the distributions expected in the absence of individual or population level asymmetry (half normal or normal distribution), adjusted on the observed variance.
Population-level and individual-level wing use asymmetry
For each fly, strain and context we calculated the wing use asymmetry index for the whole trial (60 minutes). Exploratory analyses showed that this metric was stable across the experiment. For population-level lateralization there was no significant effect of Context, Sex, Strain or their interaction (Supplementary Table 5), nor a significant difference from the chance level (t3518 = −0.677, p = 0.498, Mean = −0.0007, SD = 0.058). For individual level lateralization we observed significant effect with a very small effect size for Context, Sex, Strain, Condition x Strain, Condition x Sex x Strain (Supplementary Table 6).
Dyadic behaviors
We investigated whether the social context enhances population-level alignments in dyadic behaviors (traits that require the presence of another individual) by looking at the relative position and orientation between two flies. In several vertebrate species (e.g. 5,15–17,61,62.) the right-left relative position between two individuals tends to have a particular bias, likely due to an asymmetrical use of the eye/ear (and contralateral brain hemisphere). This phenomenon is modulated by the social context: chicks show greater asymmetries while interacting with strangers than with familiar individuals5; humans and other mammals prefer to engage with offspring using positions that facilitate the use of the left visual field16,17,63. It is not clear whether insects, and flies in particular, exhibit similar biases.
To assess the effect of social context and genetic background on dyadic behaviors, we looked at Position (defined from −180° to 180°; Fig. 2b) for each fly in dyads of two females (FF), two males (MM), or one female and one male (FM). If increasing social engagement increases population asymmetries of dyadic behaviors, we would expect that FM dyads exhibit the greatest population-level asymmetries, because of the evolutionary importance of male-female interactions for reproduction. The hypothesis that population-level asymmetries are driven by social interaction does not make any prediction about the modulation of individual-level asymmetries (even in social/dyadic behaviors) by social context.
Position
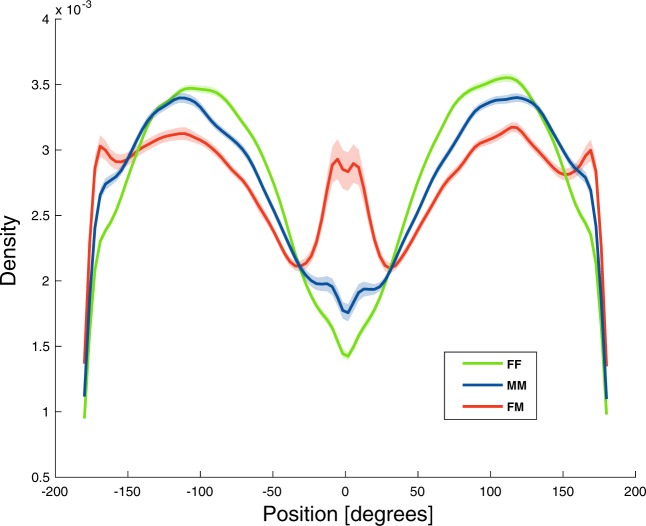
The distributions of Positions (see Fig. 2b) kept by individual flies of all tested genotypes in FF, MM and FM dyads (where 0° indicates a Position in the front of the focal fly) are shown in Fig. 6. The Position of different strains and contexts is shown in Fig. 7. Due to the multimodal distribution of the data and to the different trends observed in different conditions, we used a bootstrap method (resampling with replacement for 100 replicates) to calculate the standard error around our estimate of the distribution and compare different conditions.
Figure 6.
Kernel density distribution of the Position (in degrees) exhibited by the overall sample (all genotypes) during the test in each Context +/− Standard Error around our estimate of the distribution, calculated with bootstrap resampling.
Figure 7.
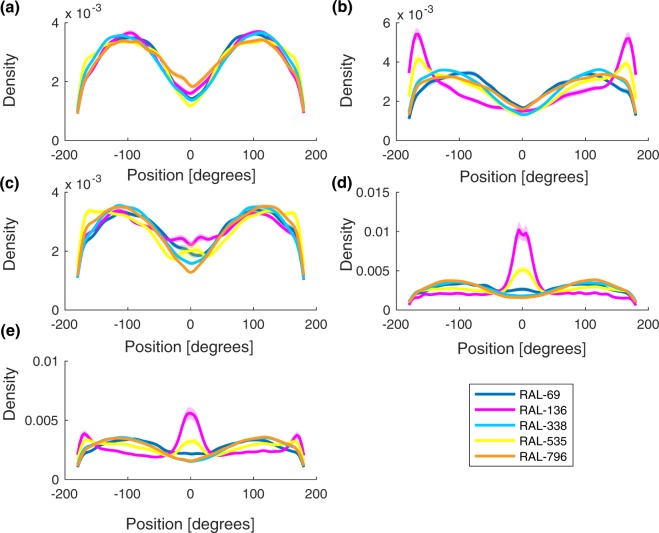
Kernel density distribution of Position (in degrees) kept during the test in each Strain, Context and Sex +/− Standard Error around our estimate of the distribution, calculated with bootstrap resampling: (a) female-female dyads by strain, (b) females of female-male dyads by strain, (c) male-male dyads by strain, (d) males of female-male dyads by strain, (e) female-male dyads.
Overall, same sex dyads (FF and MM) preferentially chose oblique Positions to the other fly (Position ~ 100°) more often than FM dyads. In FM dyads there were additional modes at Position 0° and 180°, which correspond to males positioning females ahead of them and females positioning males behind them. In the genotypes RAL-136 and RAL-338, these relationships were particularly strong, but, surprisingly, RAL-535 and RAL-796 did not show these courtship-associated modes in Position (Fig. 7). These observations suggest a strong interaction between genotype and social context. Further studies could investigate the neurobiological origin of these differences. No side bias in the Position of flies was present in any social context or strain. Hence, in spite of a clear front-back modulation of the Position, the right-left dimension was not modulated by social context or strain.
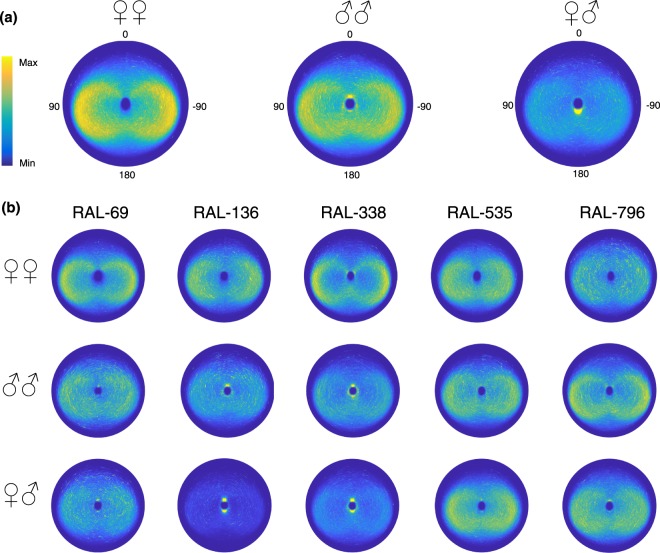
The joint distributions of Position and Distance between focal flies of all tested genotypes and their dyad partners are shown for each context in the polar heatmaps of Fig. 8a. Flies in dyads of the same sex keep their partner to the side and behind them in two ring-shaped regions of density, one on each side (note that this particular pattern likely arises through an interaction of the flies’ behavior and the arena geometry). The main interactions at close Distance are seen in female-male dyads and, to a less extent, male-male dyads. Large differences are observed between genotypes, especially in male-male and female-male dyads (Fig. 8b). These plots confirm the absence of asymmetrical positioning in flies in all social contexts and at the same time the existence of standing genetic variation for other aspects related to the position of flies in different social contexts.
Figure 8.
Heatmap in polar coordinates, showing the joint distribution of Orientation and Distance from the focal fly in each Context: (a) female-female, male-male and female-male dyads overall (b) female-female, male-male and female-male dyads for each strain.
Other behaviors
Although not directly relevant for investigating the role of the social context on behavioral asymmetries, we analyzed the effect of Context and Strain on scalar behavioral measures that cannot be lateralized: Velocity, Distance and Orientation. The analysis of velocity, a measure of general activity, is presented in Supplementary materials 1. Briefly, we observed a strong effect of social Context (slowest speed in FM dyads, faster in single flies) and Strain (Fig. S1.1 and S1.2), and a tendency to increase in velocity over the course of the experiment (Fig. S1.3.)
The Distance between larvae and adult individuals is used as a measure of social interaction in Drosophila64–70 as well as in other species71–75. An effect of strain and sex has been previously found on Distance in the DGRP lines64. In our experiments, Distance between flies was clearly modulated by the social environment, genotype and their interaction (see Supplementary Materials 2: Distance). Briefly, we observed a strong effect of Context, Strain and their interaction (Fig. S2.1 and S2.2). Flies in the FM and MM contexts stayed closer than expected by chance and RAL-136 and RAL-338 had a peak around 3 mm, a distance characteristic of courtship.
General discussion
To date, evidence that social interactions induce and sustain an alignment of behavioral asymmetries between individuals (population-level lateralization) is inconclusive. To shed light to this issue we analyzed individual and population-level asymmetries in Drosophila melanogaster in different social contexts. We systematically manipulated the social environment, observing the spontaneous behavior of individual flies and dyads (male-female, female-female, male-male). We looked at asymmetries in individual behaviors (circling asymmetry and wing use, that do not require partners) and dyadic behaviors (relative position of the partner fly) in five different isogenic strains64. The hypothesis that the social context drives the evolution of an alignment in behavioral asymmetries11,12 predicts a potential increase of population-level lateralization with higher social engagement, likely in an FM > MM > FF trend.
Contrary to this prediction, we observed very little lateralization at the population level in any social context. Instead, individual asymmetries, circling asymmetry in particular, went up in dyads compared to single-fly experiments. Moreover, individual lateralization was generally highest in female-male and male-male dyads (though this was genotype-dependent). These results suggest that the social context affects lateralization in flies, but only at the individual level. The presence of individual-level asymmetries suggests that the absence of population alignments is not due to detrimental effects of individual asymmetries. On the contrary, the large differences between genotypes suggest that variability in individual lateralization is a trait not subject negative selection. Other aspects related to the position of flies and their interaction, such as distance and velocity, were strongly modulated by the genotype and social context, showing that the FM, FF, MM and individual test conditions have biologically significant differences.
Previous reports showed neuroanatomical and sensorimotor population-level asymmetries in this species. For instance, D. melanogaster exhibits a strong population asymmetry in the location of the asymmetrical body in the right part of the brain76,77. This structure is associated with enhanced memory. Moreover, sensory signals coming from the left antenna contribute more to odour tracking than those from the right in adult flies32 and in larvae the right olfactory system performs significantly better chemotaxis than the left31. Besides these asymmetrical traits, flies exhibit visceral asymmetries that include the S shape of the stomach, the left-handed looping of the testes around the vas deferens and the clockwise rotation of the genital plate (see76 for a review). To which extent specific population-level asymmetries derive and are maintained across generations by the advantages of coordinating between individuals is an empirical question. For asymmetries of the viscera that emerge during embryonic development (primary asymmetries) and whose disruption produces pathological conditions78, it seems unlikely that the need for social coordination was the primary evolutionary force in place.
Although D. melanogaster is considered a solitary species (because flies do not form cohesive social groups nor cooperate in rearing offspring79), social habits are nevertheless central40. For instance, Drosophila larvae cooperate in burrowing to dig more effectively80,81 and attract each other through pheromones82. Moreover, adult flies aggregate on food and oviposition sites, select food patches based on the presence of conspecifics83,84, use collective behavioral responses to avoid aversive cues85, interact when competing for resources50 or courtship and mating (reviewed in37), and exhibit social learning86,87. In spite of this, in our assays we did not observe any behavioral alignment at the population level, even in traits such as the right-left position in a dyad, which are lateralized at the population level in other species5,15–17,61,62.
The absence of population-level lateralization we documented is compatible with different scenarios. One possibility is that population-level lateralization of sensorimotor behavior previously documented in Drosophila is driven by social pressures while the behaviors we have investigated are not, and that for this reason we have not observed population asymmetries. It is not obvious, though, that population alignment would be more advantageous in chemosensory tracking than in male-female interactions, given that males and females position themselves in a highly coordinated fashion, at least for some genotypes. So, we think that this scenario is unlikely and incompatible with the lack of response to selection of population asymmetries. Further studies should clarify the developmental origin of these asymmetries, and whether they are related to the primary visceral asymmetries that emerge in early stages of embryonic development.
A possibility is that a selective pressure actively opposes the motor alignment between individuals in Drosophila either because the alignment would directly decrease flies’ fitness or because of pleiotropic effects. This possibility is indirectly supported by several lines of evidence. First, among the many traits that have been subject to artificial selection in Drosophila, population-level asymmetries (also called directional asymmetries) have been suggested to be the only traits that do not respond to selection88,89. This idea is based on selection studies on morphological asymmetries in Drosophila subobscura90, directional wing-folding91 and asymmetry for eye size88 in D. melanogaster, wing-folding and Y-maze choice preference in D. melanogaster and D. paulistorum45, as well as by lack of mutational variance for population-level alignments92.
In contrast with population-level asymmetry, genetic variability for individual-level asymmetry has been consistently observed. Individual-level asymmetries in different Drosophila species have been observed for circling behavior, tapping and wing extension45. More recently, Ayroles and colleagues42 have documented individual biases in Y-maze choices and circling behavior in D. melanogaster. They observed that the average magnitude of the left-right locomotor variability is heritable, unlike the average bias. Although within-genotype variability of individually lateralized behavior in Drosophila is increased by environmental variability, the effect of genotype and genotype x environment interaction has a greater impact on the extent of individual side preferences93. While it is not easy to select for consistent directionality, random asymmetry (the magnitude of difference between right and left bias) responds readily to selection in Drosophila94,95.
While we tried to take a data-first approach to measuring lateralization in different social contexts, it is important to acknowledge some limitations. For example, the degree of social interactions between two flies likely varied substantially over a trial. Two males might be socially interacting when they are close or chasing each other, or fighting, but interacting non-socially when they are far from each other in the arena. We did not explicitly separate out modes of interaction like this in our analyses, though the heatmap analysis did not reveal lateralization at any distance scale. Future investigations might benefit from stratifying the data by high-level behavioral states like “fighting”, “chasing”, or “apart” that vary in their sociality. Supervised machine-learning approaches for automatically classifying behavior (see96 for example) could be employed to segment dyadic behavior within the arenas more granularly, and still provide the throughput needed to screen multiple social contexts and genetic backgrounds.
Using a large sample (above 3500 individuals) and an automated precisely quantitative approach57, we have clarified that individual-level but not population-level behavioral asymmetries are modulated by the social context in a genotype-dependent way in Drosophila. In the light of the available evidence, our findings suggest that there is no genetic variability for population-level behavioral lateralization in Drosophila, although individual asymmetries are not selected against. Moreover, we saw no evidence that the strength of social interactions drove population-level lateralization in either individual or dyadic locomotor behaviors. Although we did not test all potentially lateralized behaviors, and larger social groups should be investigated81, the simultaneous presence of individual lateralization and absence of population-level lateralization in different social contexts in Drosophila argues against the generality of the social lateralization hypothesis.
Data, code and materials
Raw data and scripts are available in Zenodo: 10.5281/zenodo.3268870. The scripts are also archived with a read-me at http://lab.debivort.org/social-asymmetries.
Supplementary information
Acknowledgements
We thank the Research Computing of FAS and the Neuroimaging Core (Ed Soucy, Joel Greenwood and Adam Bercu) at Harvard University for their excellent technical support. E.V. was funded by the Harvard Mind Brain and Behavior Faculty Award. Bd.B. was supported by a Sloan Research Fellowship, a Klingenstein-Simons Fellowship Award, a Smith Family Odyssey Award, and the National Science Foundation under grant no. IOS-1557913.
Author contributions
E.V. conceived the experiments, ran the experiments, analyzed the data, wrote the manuscript; M.C. provided software tools, helped with data analyses and revised the manuscript; Z.W. ran the experiments, analyzed data, and revised the manuscript; Bd.B. conceived the experiments, analyzed data, and revised the manuscript. All authors gave final approval for publication and are accountable for all aspects of their work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61410-7.
References
- 1.Rogers, L. J., Vallortigara, G. & Andrew, R. J. Divided Brains: The Biology and Behaviour of Brain Asymmetries. (Cambridge University Press, 2013).
- 2.Frasnelli E, Vallortigara G, Rogers LJ. Left-right asymmetries of behaviour and nervous system in invertebrates. Neurosci. Biobehav. Rev. 2012;36:1273–91. doi: 10.1016/j.neubiorev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Versace E, Vallortigara G. Forelimb preferences in human beings and other species: Multiple models for testing hypotheses on lateralization. Front. Psychol. 2015;6:233. doi: 10.3389/fpsyg.2015.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallortigara, G. & Versace, E. Laterality at the Neural, Cognitive, and Behavioral Levels. in APA Handbook of Comparative Psychology: Vol. 1. Basic Concepts, Methods, Neural Substrate, and Behavior (ed. Call, J.) (American Psychological Association, 2017).
- 5.Vallortigara G, Cozzutti C, Tommasi L, Rogers LJ. How birds use their eyes: Opposite left-right specialization for the lateral and frontal visual hemifield in the domestic chick. Curr. Biol. 2001;11:29–33. doi: 10.1016/S0960-9822(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 6.Deckel AW. Laterality of aggressive responses in Anolis. J. Exp. Zool. 1995;272:194–200. doi: 10.1002/jez.1402720304. [DOI] [Google Scholar]
- 7.Lippolis G, et al. Lateralisation of predator avoidance responses in three species of toads. Laterality. 2002;7:163–183. doi: 10.1080/13576500143000221. [DOI] [PubMed] [Google Scholar]
- 8.Casperd JM, Dunbar RIM. Asymmetries in the visual processing of emotional cues during agonistic interactions by gelada baboons. Behav. Proc. 1996;37:57–65. doi: 10.1016/0376-6357(95)00075-5. [DOI] [PubMed] [Google Scholar]
- 9.Rogers LJ, Frasnelli E, Versace E. Lateralized antennal control of aggression and sex differences in red mason bees, Osmia bicornis. Sci. Rep. 2016;6:29411. doi: 10.1038/srep29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers LJ, Rigosi E, Frasnelli E, Vallortigara G. A right antenna for social behaviour in honeybees. Sci. Rep. 2013;3:2045. doi: 10.1038/srep02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghirlanda S, Vallortigara G. The evolution of brain lateralization: a game-theoretical analysis of population structure. Proc. R. Soc. B Biol. Sci. 2004;271:853–7. doi: 10.1098/rspb.2003.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghirlanda S, Frasnelli E, Vallortigara G. Intraspecific competition and coordination in the evolution of lateralization. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:861–866. doi: 10.1098/rstb.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasnelli E, Vallortigara G. Individual-Level and Population-Level Lateralization: Two Sides of the Same Coin. Symmetry. 2018;10:739. doi: 10.3390/sym10120739. [DOI] [Google Scholar]
- 14.Niven JE, Bell ATA. Lessons in Lateralisation from the Insects. Trends Ecol. Evol. 2018;33:486–488. doi: 10.1016/j.tree.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Versace, E., Morgante, M., Pulina, G. & Vallortigara, G. Behavioural lateralisation in sheep (Ovis aries). Behav. Brain Res. 184 (2007). [DOI] [PubMed]
- 16.Karenina K, Giljov A, Ingram J, Rowntree VJ, Malashichev Y. Lateralization of mother-infant interactions in a diverse range of mammal species. Nat. Ecol. Evol. 2017;1:1–4. doi: 10.1038/s41559-016-0030. [DOI] [PubMed] [Google Scholar]
- 17.Giljov A, Karenina K, Malashichev Y. Facing each other: mammal mothers and infants prefer the position favouring right hemisphere processing. Biol. Lett. 2018;14:20170707. doi: 10.1098/rsbl.2017.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnell AK, Jozet-Alves C, Hall KC, Radday L, Hanlon RT. Fighting and mating success in giant Australian cuttlefish is influenced by behavioural lateralization. Proceedings. Biol. Sci. 2019;286:20182507. doi: 10.1098/rspb.2018.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benelli G, Romano D, Messing RH, Canale A. First report of behavioural lateralisation in mosquitoes: right-biased kicking behaviour against males in females of the Asian tiger mosquito, Aedes albopictus. Parasitol. Res. 2015;114:1613–1617. doi: 10.1007/s00436-015-4351-0. [DOI] [PubMed] [Google Scholar]
- 20.Romano D, Canale A, Benelli G. Do right-biased boxers do it better? Population-level asymmetry of aggressive displays enhances fighting success in blowflies. Behav. Processes. 2015;113:159–162. doi: 10.1016/j.beproc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Frasnelli E, Vallortigara G, Rogers LJ. Origins of brain asymmetry: Lateralization of odour memory recall in primitive Australian stingless bees. Behav. Brain Res. 2011;224:121–127. doi: 10.1016/j.bbr.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Vallortigara, G. & Rogers, L. J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–89; discussion 589–633 (2005). [DOI] [PubMed]
- 23.MacNeilage PF. Evolution of the strongest vertebrate rightward action asymmetries: Marine mammal sidedness and human handedness. Psychol. Bull. 2014;140:587–609. doi: 10.1037/a0034298. [DOI] [PubMed] [Google Scholar]
- 24.McGrew WC, Marchant LF. Laterality pays off. Primates. 1999;40:509–513. doi: 10.1007/BF02557586. [DOI] [Google Scholar]
- 25.Magat M, Brown C. Laterality enhances cognition in Australian parrots. Proc. R. Soc. B Biol. Sci. 2009;276:4155–4162. doi: 10.1098/rspb.2009.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sovrano VA, Dadda M, Bisazza A. Lateralized fish perform better than nonlateralized fish in spatial reorientation tasks. Behav. Brain Res. 2005;163:122–127. doi: 10.1016/j.bbr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Miler K, Kuszewska K, Woyciechowski M. Larval antlions with more pronounced behavioural asymmetry show enhanced cognitive skills. Biol. Lett. 2017;13:20160786. doi: 10.1098/rsbl.2016.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell, A. T. A. & Niven, J. E. Strength of forelimb lateralization predicts motor errors in an insect. Anim. Behav. 1–4 (2016). [DOI] [PMC free article] [PubMed]
- 29.Romano, D., Benelli, G. & Stefanini, C. Escape and surveillance asymmetries in locusts exposed to a Guinea fowl-mimicking robot predator. Sci. Rep. 1–9, 10.1038/s41598-017-12941-z (2017). [DOI] [PMC free article] [PubMed]
- 30.Alqadah A, Hsieh Y, Xiong R, Chuang C. Stochastic left – right neuronal asymmetry in Caenorhabditis elegans. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150407. doi: 10.1098/rstb.2015.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB. Bilateral olfactory sensory input enhances chemotaxis behavior. Nat. Neurosci. 2008;11:187–199. doi: 10.1038/nn2031. [DOI] [PubMed] [Google Scholar]
- 32.Duistermars BJ, Chow DM, Frye MA. Flies require bilateral sensory input to track odor gradients in flight. Curr. Biol. 2009;19:1301–7. doi: 10.1016/j.cub.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quaresmini C, Forrester GS, Spiezio C, Vallortigara G. Social environment elicits lateralized behaviors in gorillas (Gorilla gorilla gorilla) and Chimpanzees (Pan troglodytes) J. Comp. Psychol. 2014;128:276–284. doi: 10.1037/a0036355. [DOI] [PubMed] [Google Scholar]
- 34.Rosa Salva O, Regolin L, Mascalzoni E, Vallortigara G. Cerebral and Behavioural Asymmetries in Animal Social Recognition. Comp. Cogn. Behav. Rev. 2012;7:110–138. doi: 10.3819/ccbr.2012.70006. [DOI] [Google Scholar]
- 35.MacNeilage, B. P. F., Rogers, L. J. & Vallortigara, G. Origins of Left and Right Brain. Sci. Am. 60–67 (2009). [DOI] [PubMed]
- 36.Markow TA, Hanson SJ. Multivariate analysis of Drosophila courtship. PNAS. 1981;78:430–434. doi: 10.1073/pnas.78.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markow TA, O’Grady PM. Evolutionary Genetics of Reproductive Behavior in Drosophila: Connecting the Dots. Annu. Rev. Genet. 2005;39:263–291. doi: 10.1146/annurev.genet.39.073003.112454. [DOI] [PubMed] [Google Scholar]
- 38.Mazzoni V, Anfora G, Virant-Doberlet M. Substrate vibrations during courtship in three Drosophila species. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spieth HT. Mating Behavior Within the Genus Drosophila (Diptera) Am. Museum Nat. Hist. 1952;99:400–474. [Google Scholar]
- 40.Schneider J, Atallah J, Levine JD. Social structure and indirect genetic effects: Genetics of social behaviour. Biol. Rev. 2017;92:1027–1038. doi: 10.1111/brv.12267. [DOI] [PubMed] [Google Scholar]
- 41.Gowaty PA, Steinichen R, Anderson WW. Indiscriminate Females and Choosy Males: Within- and Between-Species Variation in Drosophila. Evolution. 2003;57:2037–2045. doi: 10.1111/j.0014-3820.2003.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 42.Ayroles JF, et al. Behavioral idiosyncrasy reveals genetic control of phenotypic variability. Proc. Natl. Acad. Sci. 2015;112(21):6706–6711. doi: 10.1073/pnas.1503830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchanan, S. M., Kain, J. S. & de Bivort, B. L. Neuronal control of locomotor handedness in Drosophila. Proc. Natl. Acad. Sci. 112, 201500804 (2015). [DOI] [PMC free article] [PubMed]
- 44.Xiao C, Qiu S, Robertson RM. Persistent One-Way Walking in a Circular Arena in Drosophila melanogaster Canton-S Strain. Behav. Genet. 2018;48:80–93. doi: 10.1007/s10519-017-9881-z. [DOI] [PubMed] [Google Scholar]
- 45.Ehrman L, Thompson, James NJ, Perelle I, Hisey BN. Some approaches to the question of Drosophila laterality. Genet. Res. 1978;32:231–238. doi: 10.1017/S0016672300018723. [DOI] [Google Scholar]
- 46.Perelle IB, Saretsky S, Ehrman L. Lateral consistency in. Drosophila. Anim. Behav. 1978;27:622–623. doi: 10.1016/0003-3472(79)90200-8. [DOI] [Google Scholar]
- 47.Connolly K. The Social Facilitation of Preening Behaviour in Drosophila melanogaster. Anim. Behav. 1968;16:385–391. doi: 10.1016/0003-3472(68)90023-7. [DOI] [PubMed] [Google Scholar]
- 48.Yanagawa A, Guigue AMA, Marion-Poll F. Hygienic grooming is induced by contact chemicals in Drosophila melanogaster. Front. Behav. Neurosci. 2014;8:1–9. doi: 10.3389/fnbeh.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klibaite U, Berman GJ, Cande J, David L, Shaevitz JW. An Unsupervised Method for Quantifying the Behavior of Interacting Individuals. Phys. Biol. 2017;14:015006. doi: 10.1088/1478-3975/aa5c50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsen SP, Chan Y-B, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McManus IC. Wing-Folding in Drosophila. Anim. Behav. 1981;29:626–627. doi: 10.1016/S0003-3472(81)80126-1. [DOI] [Google Scholar]
- 52.Mackay TFC, et al. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang W., Massouras A., Inoue Y., Peiffer J., Ramia M., Tarone A. M., Turlapati L., Zichner T., Zhu D., Lyman R. F., Magwire M. M., Blankenburg K., Carbone M. A., Chang K., Ellis L. L., Fernandez S., Han Y., Highnam G., Hjelmen C. E., Jack J. R., Javaid M., Jayaseelan J., Kalra D., Lee S., Lewis L., Munidasa M., Ongeri F., Patel S., Perales L., Perez A., Pu L., Rollmann S. M., Ruth R., Saada N., Warner C., Williams A., Wu Y.-Q., Yamamoto A., Zhang Y., Zhu Y., Anholt R. R. H., Korbel J. O., Mittelman D., Muzny D. M., Gibbs R. A., Barbadilla A., Johnston J. S., Stone E. A., Richards S., Deplancke B., Mackay T. F. C. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Research. 2014;24(7):1193–1208. doi: 10.1101/gr.171546.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson MF, et al. Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLoS Genet. 2012;8:e1003129. doi: 10.1371/journal.pgen.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Werkhoven, Z., Rohrsen, C., Qin, C., Brembs, B. & de Bivort, B. MARGO (Massively Automated Real-time GUI for Object-tracking), a platform for high-throughput ethology. bioRxiv 593046, 10.1101/593046 (2019). [DOI] [PMC free article] [PubMed]
- 56.Kain JS, Stokes C, de Bivort BL. Phototactic personality in fruit flies and its suppression by serotonin and white. Proc. Natl. Acad. Sci. 2012;109:19834–19839. doi: 10.1073/pnas.1211988109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eyjolfsdottir, E. et al. Detecting social actions of fruit flies. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics)8690 LNCS, 772–787 (2014).
- 58.Albers C, Lakens D. When power analyses based on pilot data are biased: Inaccurate effect size estimators and follow-up bias. J. Exp. Soc. Psychol. 2018;74:187–195. doi: 10.1016/j.jesp.2017.09.004. [DOI] [Google Scholar]
- 59.Qiu S, Xiao C. Walking behavior in a circular arena modified by pulsed light stimulation in Drosophila melanogaster w 1118 line. Physiol. Behav. 2018;188:227–238. doi: 10.1016/j.physbeh.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 60.Crall JD, et al. Social context modulates idiosyncrasy of behaviour in the gregarious cockroach Blaberus discoidalis. Anim. Behav. 2016;111:297–305. doi: 10.1016/j.anbehav.2015.10.032. [DOI] [Google Scholar]
- 61.De Santi A, Bisazza A, Vallortigara G. Complementary left and right eye use during predator inspection and shoal-mate scrutiny in minnows. J. Fish Biol. 2002;60:1116–1125. doi: 10.1111/j.1095-8649.2002.tb01708.x. [DOI] [Google Scholar]
- 62.Walton D., Buchanan J., Murray S.J. Exploring factors distinguishing car-versus-car from car-versus-motorcycle in intersection crashes. Transportation Research Part F: Traffic Psychology and Behaviour. 2013;17:145–153. doi: 10.1016/j.trf.2012.11.001. [DOI] [Google Scholar]
- 63.Forrester Gillian S., Davis Rachael, Mareschal Denis, Malatesta Gianluca, Todd Brenda K. The left cradling bias: An evolutionary facilitator of social cognition? Cortex. 2019;118:116–131. doi: 10.1016/j.cortex.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Anderson BB, Scott A, Dukas R. Social behavior and activity are decoupled in larval and adult fruit flies. Behav. Ecol. 2016;27:820–828. doi: 10.1093/beheco/arv225. [DOI] [Google Scholar]
- 65.Fernandez, R. W. et al. Modulation of social space by dopamine in Drosophila melanogaster, but no effect on the avoidance of the Drosophila stress odorant. Biol. Lett. 13, 20170369 (2017). [DOI] [PMC free article] [PubMed]
- 66.Kaur K, Simon A, Chauhan V, Chauhan A. Effect of bisphenol A on Drosophila melanogaster behavior. A new model for the studies on neurodevelopmental disorders. Behav. Brain Res. 2015;284C:77–84. doi: 10.1016/j.bbr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 67.McNeil, A. R. et al. Conditions affecting social space in Drosophila melanogaster. J. Vis. Exp. e53242, 10.3791/53242 (2015). [DOI] [PMC free article] [PubMed]
- 68.Simon JC, Dickson WB, Dickinson MH. Prior mating experience modulates the dispersal of Drosophila in males more than in females. Behav. Genet. 2011;41:754–67. doi: 10.1007/s10519-011-9470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alisch T, Kao AB, Zucker D, Crall JD, Bivort BLde. MAPLE: a Modular Automated Platform for Large-scale Experiments, a low-cost robot for integrated animal-handling and phenotyping. Elife. 2018;7:e37166. doi: 10.7554/eLife.37166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider, J. & Levine, J. D. Automated identification of social interaction criteria in Drosophila melanogaster. Biol. Lett. 10 (2014). [DOI] [PMC free article] [PubMed]
- 71.Febrer K, Jones TA, Donnelly CA, Stamp Dawkins M. Forced to crowd or choosing to cluster? Spatial distribution indicates social attraction in broiler chickens. Anim. Behav. 2006;72:1291–1300. doi: 10.1016/j.anbehav.2006.03.019. [DOI] [Google Scholar]
- 72.Gribovskiy A, Halloy J, Deneubourg JL, Mondada F. Designing a socially integrated mobile robot for ethological research. Rob. Auton. Syst. 2018;103:42–55. doi: 10.1016/j.robot.2018.02.003. [DOI] [Google Scholar]
- 73.Väisänen J, Jensen P. Social versus exploration and foraging motivation in young red junglefowl (Gallus gallus) and White Leghorn layers. Appl. Anim. Behav. Sci. 2003;84:139–158. doi: 10.1016/j.applanim.2003.07.001. [DOI] [Google Scholar]
- 74.Versace, E., Damini, S., Caffini, M. & Stancher, G. Born to be asocial: newly hatched tortoises avoid unfamiliar individuals. Anim. Behav. 138 (2018).
- 75.Versace, E., Ragusa, M. & Pallante, V. Conserved abilities of individual recognition and genetically modulated social responses in young chicks (Gallus gallus). bioRxiv, 10.1101/743765
- 76.Coutelis JB, Petzoldt AG, Spéder P, Suzanne M, Noselli S. Left-right asymmetry in Drosophila. Semin. Cell Dev. Biol. 2008;19:252–262. doi: 10.1016/j.semcdb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Wolff T, Rubin GM. Neuroarchitecture of the Drosophila central complex: A catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog. J. Comp. Neurol. 2018;526:2585–2611. doi: 10.1002/cne.24512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levin M. Left-right asymmetry in embryonic development: A comprehensive review. Mech. Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Michener, C. The Bees of the World. (Johns Hopkins Univ. Press, 2000).
- 80.Dombrovski Mark, Poussard Leanne, Moalem Kamilia, Kmecova Lucia, Hogan Nic, Schott Elisabeth, Vaccari Andrea, Acton Scott, Condron Barry. Cooperative Behavior Emerges among Drosophila Larvae. Current Biology. 2017;27(18):2821-2826.e2. doi: 10.1016/j.cub.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 81.Durisko Z, Kemp R, Mubasher R, Dukas R. Dynamics of social behavior in fruit fly larvae. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0095495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mast JD, De Moraes CM, Alborn HT, Lavis LD, Stern DL. Evolved differences in larval social behavior mediated by novel pheromones. Elife. 2014;3:e04205. doi: 10.7554/eLife.04205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lihoreau M, Clarke IM, Buhl J, Sumpter DJT, Stephen J. Collective selection of food patches in Drosophila. J. Exp. Biol. 2016;219:668–675. doi: 10.1242/jeb.127431. [DOI] [PubMed] [Google Scholar]
- 84.Tinette S, Zhang L, Robichon A. Cooperation between Drosophila flies in searching behavior. Genes, Brain Behav. 2004;3:39–50. doi: 10.1046/j.1601-183x.2003.0046.x. [DOI] [PubMed] [Google Scholar]
- 85.Ramdya P, et al. Mechanosensory interactions drive collective behaviour in Drosophila. Nature. 2014;519:233–236. doi: 10.1038/nature14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Danchin E, et al. Cultural flies: Conformist social learning in fruitflies predicts long-lasting mate-choice traditions. Science. 2018;362:1025–1030. doi: 10.1126/science.aat1590. [DOI] [PubMed] [Google Scholar]
- 87.Sarin S, Dukas R. Social learning about egg-laying substrates in fruitflies. Proc. Biol. Sci. 2009;276:4323–4328. doi: 10.1098/rspb.2009.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coyne, J. A. Lack of response to selection for directional asymmetry in Drosophlla melanogaster. Heredity119 (1987). [DOI] [PubMed]
- 89.Lewontin, R. C. The Genetic Basis of Evolutionary Change. (Columbia University Press, 1974).
- 90.Maynard Smith J, Sondhi KC. The genetics of a pattern. Genetics. 1960;45:1039–1050. doi: 10.1093/genetics/45.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Purnell DJ, Thompson JN., Jr. Selection for asymmetrical bias in a behavioural character of selection for asymmetrical bias in a behavioural character of Drosophila melanogaster. Heredity. 1973;31:401–405. doi: 10.1038/hdy.1973.94. [DOI] [PubMed] [Google Scholar]
- 92.Monedero JL, Chavarrias D, Lopez-Fanjul C. The lack of mutational variance for fluctuating and directional asymmetry in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 1997;264:233–237. doi: 10.1098/rspb.1997.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akhund-Zade, J., Ho, S., O’Leary, C. & de Bivort, B. The effect of environmental enrichment on behavioral variability depends on genotype, behavior, and type of enrichment. J. Exp. Biol. 222 (2019). [DOI] [PubMed]
- 94.Mather K. Genetical control of stability in development. Heredity. 1953;7:297–336. doi: 10.1038/hdy.1953.41. [DOI] [Google Scholar]
- 95.Thoday JM. Homeostasis in a selection experiment. Heredity. 1958;12:401–415. doi: 10.1038/hdy.1958.41. [DOI] [Google Scholar]
- 96.Kabra, M., Robie, A. A., Rivera-Alba, M., Branson, S. & Branson, K. JAABA: Interactive machine learning for automatic annotation of animal behavior. Nat. Methods10 (2013). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.