Abstract
Ex-vivo gene therapy using stem cells or T cells transduced by retroviral or lentiviral vectors has shown remarkable efficacy in the treatment of immunodeficiencies and cancer. However, the process is expensive, technically challenging, and not readily scalable to large patient populations, particularly in underdeveloped parts of the world. Direct in vivo gene therapy would avoid these issues, and such approaches with adeno-associated virus (AAV) vectors have been shown to be safe and efficacious in clinical trials for diseases affecting differentiated tissues such as the liver and CNS. However, the ability to transduce lymphocytes with AAV in vivo after systemic delivery has not been carefully explored. Here, we show that both standard and exosome-associated preparations of AAV8 vectors can effectively transduce a variety of immune cell populations including CD4+ T cells, CD8+ T cells, B cells, macrophages, and dendritic cells after systemic delivery in mice. We provide direct evidence of T cell transduction through the detection of AAV genomes and transgene mRNA, and show that intracellular and transmembrane proteins can be expressed. These findings establish the feasibility of AAV-mediated in vivo gene delivery to immune cells which will facilitate both basic and applied research towards the goal of direct in vivo gene immunotherapies.
Subject terms: Gene delivery, Viral vectors
Introduction
Gene therapy using ex-vivo transduction of patient-derived cells has revolutionized medicine, particularly in the case of readily accessible blood hematopoietic stem cells or lymphocytes to treat immunodeficiencies or cancer1–3. However, the laborious workflow and infrastructure required makes these therapies expensive, time-consuming, and unscalable. Moreover, the manipulation of cells outside the body may introduce undesirable phenotypic changes4. Administering vectors directly into patients would solve most of these problems, potentially expanding the reach of gene therapies to large patient populations anywhere in the world, but unfortunately, such therapies have not advanced due to the absence of a proven and effective delivery system.
AAV vectors were thoroughly developed over the past 35 years and are now demonstrating remarkable, life-changing efficacy in clinical trials for several diseases, including recently FDA approved treatments for a form of hereditary blindness and spinal muscular atrophy5–9. The majority of AAV-mediated gene therapies have focused on differentiated cells such as muscle10,11, neurons, astrocytes12, and liver13. AAV has generally been considered inefficient at transducing T cells; however, a recent study revealed that CD3+ T cells are a natural reservoir for multiple wild type AAV serotypes14. Here we demonstrate that AAV8 vectors can mediate transgene expression in multiple immune cell types after systemic delivery in mice, providing an important proof of concept for developing these vectors for use in in vivo therapies.
Results
GFP expression in immune cells after systemic delivery of AAV8 and exo-AAV8 vectors in adult mice
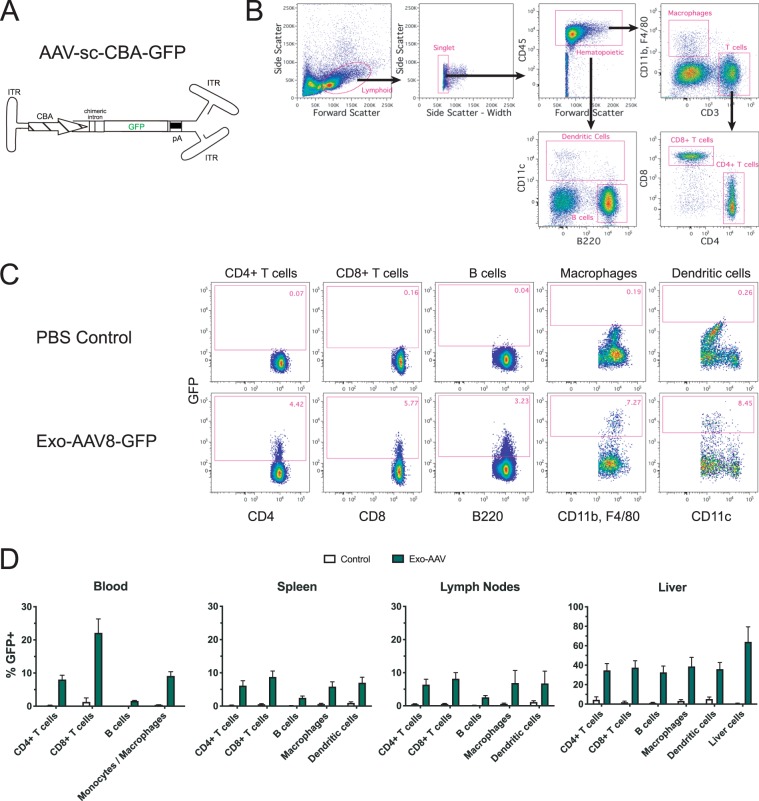
We previously demonstrated that exosome-associated AAV (exo-AAV) vectors mediate higher transduction efficiencies of target organs compared to conventional AAV vectors when delivered into mice15–19. To determine whether these vectors could mediate in vivo transduction of immune cells when delivered systemically, we injected C57BL/6 mice intravenously with 3 × 1012 genome copies (gc, 1.2 × 1014 gc/kg) of an exo-AAV8 vector encoding a self-complementary (sc) green fluorescent protein (GFP) transgene expression cassette under control of the strong, ubiquitous chicken beta actin (CBA) promoter (AAV-sc-CBA-GFP) (Fig. 1A), or PBS as a control. Twelve days post injection, we isolated immune cells from the spleen, lymph nodes, and liver, and assessed GFP expression in CD4+ T cells, CD8+ T cells, B cells, macrophages, and dendritic cells by flow cytometry (Fig. 1B–D).
Figure 1.
GFP is detected in multiple immune cell types after systemic injection of exo-AAV8-GFP in adult mice. (A) Schematic of self-complementary (sc) AAV construct used in this study. CBA, hybrid CMV immediate early, chicken beta actin promoter; GFP, green fluorescent protein; pA, polyadenylation signal; ITR, inverted terminal repeat. Adult male C57BL/6 mice were injected with PBS or 3 × 1012 gc (1.2 × 1014 gc/kg) exo-AAV8-CBA-GFP and euthanized on day 12 for analysis of tissues. (B) Representative gating strategy used to analyze different immune cell populations from the spleen. (C) Representative flow cytometry data showing GFP expression in indicated immune cell populations from the spleen at day 12 post-injection. (D) Quantitative summary of GFP expression in indicated cell populations in indicated tissues at day 12 post-injection. Data represent mean values ± SEM from n = 6 mice for PBS and n = 7 mice for exo-AAV8-GFP groups collected across three independent experiments.
GFP expression was detected in all cell types in all tissues studied, demonstrating the ability of exo-AAV vectors to transduce immune cells in vivo. In the spleen and lymph nodes, GFP was expressed in 6.2–6.4% of CD4+ T cells, 8.2–8.8% of CD8+ T cells, 2.5–2.6% of B cells, 5.9–6.9% of macrophages, and 6.8–7.0% of dendritic cells. Strikingly, the extent of expression was substantially higher for all 5 cell types in the liver, with percentages of GFP-positive cells ranging from 32.6–38.7% (Fig. 1D). As expected, Non-hematopoietic liver cells (defined as CD45-negative) were also efficiently transduced, with 64.0% of the cells expressing GFP.
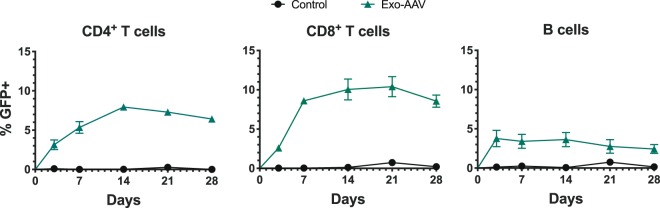
To investigate the kinetics of transgene expression, we injected mice with 9.1 × 1011 gc (3.64 × 1013gc/kg) of exo-AAV8-sc-CBA-GFP and analyzed immune cells from the blood at days 3, 7, 14, 21, and 28 post-injection. For CD4+ and CD8+ T cells, the percentage of GFP positive cells peaked at day 14 and slowly declined through 28 days. The peak of transduction efficiency in B cells was day 3 which remained relatively steady through two weeks and then showed a minor decline through 28 days (Fig. 2).
Figure 2.
GFP is maintained in circulating blood lymphocytes for several weeks after systemic injection of exosome-associated AAV8-GFP in adult mice. Adult male C57BL/6 mice were injected intravenously with PBS or 9.1 × 1011 gc (3.64 × 1013gc/kg) of exo-AAV8-CBA-GFP and bled on days 3, 7, 14, 21, and 28. GFP expression in indicated cell populations is shown quantitatively over time. Data represent mean values ± SEM from n = 3 mice for PBS and n = 3 mice for exo-AAV8-GFP groups.
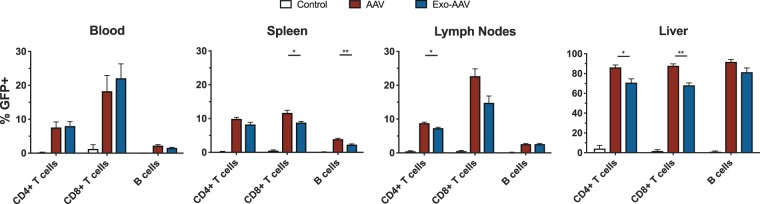
To directly compare the transduction efficiency of conventional AAV and exo-AAV vectors, we injected mice with equal doses of these vectors containing the same sc-CBA-GFP construct. As shown in Fig. 3, both vectors mediated similar transduction levels in the selected immune cells, with conventional AAV yielding slightly higher percentages in some cell populations.
Figure 3.
GFP is detected in multiple lymphocyte populations after systemic injection of conventional or exosome-associated AAV8-GFP in adult mice. Adult male C57BL/6 mice were injected intravenously with PBS or 1 × 1012 gc (4 × 1013 gc/kg) of either standard AAV8-CBA-GFP or exo-AAV8-CBA-GFP and euthanized on day 12. Flow cytometric analysis of GFP expression in indicated cell populations in indicated tissues is summarized. Data represent mean values ± SEM from n = 3 mice for PBS and n = 3 mice for each vector group. *p < 0.05, **p < 0.01 for unpaired t-tests between indicated groups.
To provide additional evidence for the direct transduction of immune cells by exo-AAV8, we sorted CD4+ and CD8+ T cells from the spleens of day 12 mice and isolated DNA to detect the GFP cassette from AAV genomes by qPCR, and RNA to detect GFP mRNA by qRT-PCR. For CD4+ and CD8+ T cells respectively, we found 6.4 and 5.8-fold higher levels of AAV genomes in exo-AAV8-GFP injected mice over PBS-injected controls (i.e. background levels of detection) (Fig. 4A). Similarly, we detected 6.0 and 3.4-fold higher levels, respectively, of GFP cDNA in exo-AAV8-GFP injected mice (Fig. 4B). These findings support our flow cytometry data, and collectively demonstrate that systemically administered exo-AAV8 vectors can mediate detectable transduction of immune cells in vivo.
Figure 4.
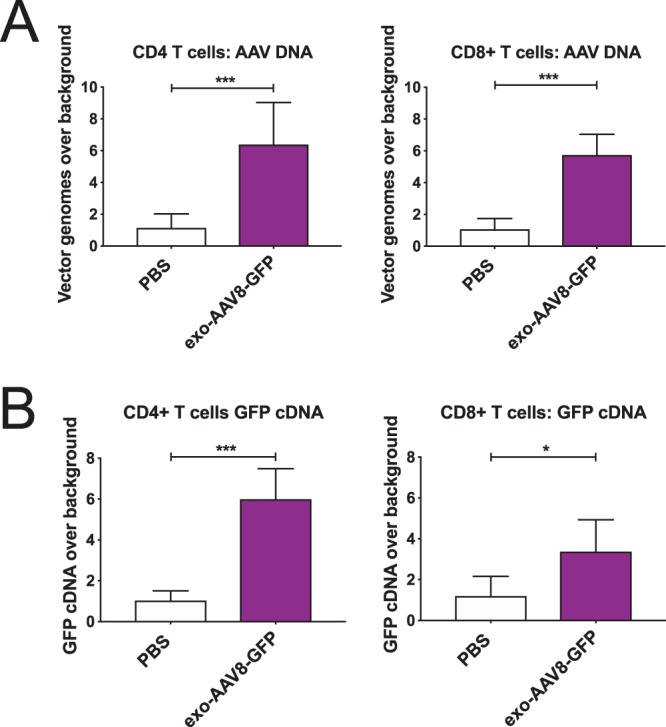
GFP vector transgene and mRNA are detected in T cells after systemic injection of exo-AAV8-CBA-GFP in adult mice. CD4+ and CD8+ T cells were flow-sorted to detect (A) AAV genomes by qPCR and (B) vector expressed GFP mRNA by RT-qPCR. All Ct values were normalized to GAPDH values and normalized values for exo-AAV8-GFP injected mice compared to background levels in PBS-injected mice. For (A), average Ct values with GFP probe in PBS-treated mice was cycle 32.99 * for CD8 T cells, cycle 33.49 for CD4 T cells. For (B), average Ct values with GFP probe in PBS-treated mice was cycle 34.36 for CD8 T cells, cycle 34.97 for CD4 T cells. n = 4 for PBS, n = 6 for exo-AAV8-GFP; * p < 0.05, *** p ≤ 0.005.
Expression of human IL-2Rα on the surface of lymphocytes after systemic delivery of exo-AAV8 vector
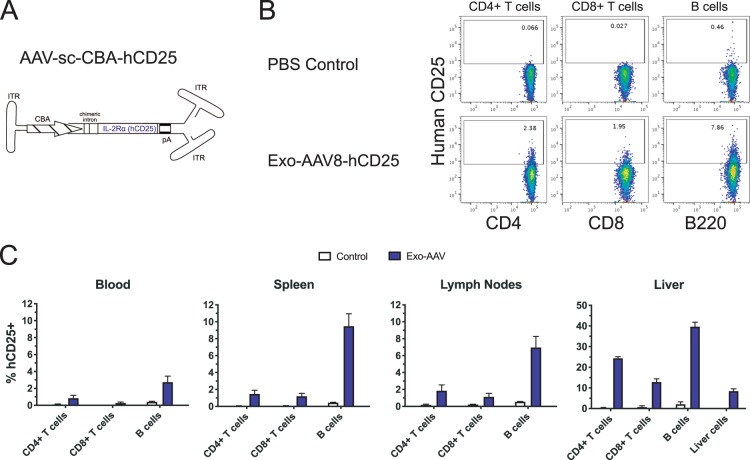
As many immune cell therapies (e.g. CAR T cells) modify or express ligands on the surface of lymphocytes, we tested the feasibility of expressing a human receptor on the surface of mouse immune cells using AAV-mediated in vivo transduction. We constructed a sc AAV plasmid encoding human interleukin-2 receptor alpha chain (IL-2Rα), also known as CD25, and produced exo-AAV8-sc-CBA-hCD25 vector (Fig. 5A). We injected mice intravenously with 1 × 1012 gc (4 × 1013 gc/kg) of exo-AAV8-sc-CBA-hCD25 or PBS as a control. At day 12 post-injection, CD4+ T cells, CD8+ T cells, and B cells from the blood, spleen, lymph nodes, and liver were analyzed for hCD25 (hCD25) expression by flow cytometry (Fig. 5B,C).
Figure 5.
Human IL-2Rα (hCD25) is detected in multiple lymphocyte populations after systemic injection of exo-AAV8-hCD25 in adult mice. (A) Schematic of self-complementary (sc) AAV construct used in this study, consisting of the same elements as for Fig. 1A except that the GFP cassette is replaced with hCD25. Mice were injected i.v. with PBS or 1 × 1012 gc of exo-AAV8-hCD25 (4 × 1013 gc/kg) and 12 days later mice were euthanized for analysis of cells in different tissues. (B) Representative flow cytometry data showing hCD25 expression in indicated lymphocyte populations in the spleen using the gating strategy outlined in Fig. 1B. (C) Quantitative summary of hCD25 expression in each cell population in each tissue. Data represent mean values ± SEM from n = 3 mice for PBS and n = 3 mice for exo-AAV8-hCD25 groups.
We found detectable expression of hCD25 on all three lymphocyte populations, with 0.8–1.9% of CD4+ T cells, 0.3–1.2% of CD8+ T cells, and 2.7–9.5% of B cells in the blood, spleen, and lymph nodes expressing hCD25. Again, these levels were substantially higher in the liver, with 24.4% of CD4 T cells, 12.9% of CD8+ T cells, and 39.7% of B cells expressing hCD25. Interestingly, the transduction of liver cells was much lower, with only 8.4% of these cells expressing hCD25. These results demonstrate the ability of AAV vectors to mediate in vivo transduction and expression of a surface receptor on immune cells.
Discussion
In this study, we explored the use of AAV vectors to mediate expression of transgene products in lymphocytes after systemic delivery in mice. We observed readily detectable expression of two genes of interest (GFP, and a cell surface receptor) out to at least 28 days post injection.
We initiated our study with exosome-associated AAV (exo-AAV) vectors as we have previously demonstrated higher levels of transduction of target organs compared to standard AAV vectors15–21. After our initial observation that we could transduce lymphocytes with systemically injected exo-AAV8-GFP we performed a dose-matched study with conventional AAV8. Surprisingly, similar levels of transduction were observed between exo-AAV8 and AAV8, with conventional AAV8 transducing slightly more cells. In this study we used self-complementary (sc) vectors as well as relatively high doses of AAV (4 × 1013 gc/kg), both factors which may have minimized any boost in transduction observed with exo-AAV. For example, our earlier study demonstrating superior hepatocyte transduction by exo-AAV815 was achieved at much lower doses (~4 × 1010 gc/kg vs. 4 × 1013 gc/kg) and also with a single stranded AAV genome. This suggests that the enhanced transduction efficiency of exo-AAV may be limited to certain cell types or lower doses of vector.
Since we observed transduction of lymphocytes using either conventional AAV8 and exo-AAV8 vectors, both types could be pursued for clinical development. Since conventional AAV8 vectors are already in clinical trials, there are currently less regulatory and manufacturing hurdles to overcome than for exo-AAV vectors. Systemically injected AAV8 vectors are currently used in clinical trials for hemophilia B22, so the same clinical protocols and limitations should apply for the transduction of lymphocytes using AAV8. Some of these protocols use short-term, high-dose corticosteroid treatments to limit development of anti-AAV8 CTL responses. However, patients with pre-existing anti-AAV8 neutralizing antibodies are often excluded. The use of exo-AAV vectors, which can shield AAV capsids from host antibodies, may provide an attractive alternative option.
While the doses in the current study are high (up to 1.2 × 1014 gc/kg tested), these are clinically-relevant doses used for systemically delivered AAV vectors8. Importantly, all treated mice (total of 19 exo-AAV/AAV-injected animals in these experiments) survived the duration of the study with no signs of toxicity. Future preclinical toxicology studies will further validate the safety of this approach. Currently, the high dose required to achieve appreciable transduction of lymphocytes by the exo-AAV8/AAV8 vectors is a weakness of the system. At these doses, the ubiquitous CBA promoter in the transgene cassette results in high levels of collateral expression in peripheral organs (heart, liver, muscle). However, this can be ameliorated in the future at two levels. The first is by directly targeting the exo-AAV vector to lymphocyte ligands or selecting new AAV capsids with improved transduction efficiencies for these cells, enabling us to use much lower doses. The second is by transcriptional/post-transcriptional targeting/detargeting transduced cells. This can be done by using tissue-specific promoters that express well in lymphocytes but not in peripheral organs. Additionally, detargeted expression by vectors can be achieved by placing microRNA (miR) recognition sites in the AAV vector of miRs highly expressed in organs such as liver, but not the target cells23.
Another surprising finding of our study was the higher percentages of GFP expressing liver resident lymphocytes compared to spleen or lymph nodes. This raises the possibility that accumulation of vector in the liver may contribute to higher transduction efficiencies in resident immune cell populations. Alternatively, some GFP protein/mRNA may be transferred from highly-transduced hepatocytes to local lymphocytes24. Future studies employing AAV transgene cassettes which allow detargeting from hepatocytes with miR seed sequences25 or T cell specific promoters26, may help delineate this phenomenon.
We assessed the kinetics of transgene expression by performing serial bleeds of mice and assessing lymphocytes for GFP expression throughout a monthlong timecourse. While expression in CD4+ T cells and CD8+ T cells peaked at day 14 and declined out to day 28, it peaked at day 3 in B cells with a slower decline over time. This may reflect cell-type differences in vector uptake and/or kinetics of transgene expression. The decline over time suggests that expression is largely mediated by episomally-maintained AAV that is diluted out over time upon cell division rather than the small percentage of AAV that stably integrates into the genome in vivo (~0.1%)27–29.
We observed an apparent discrepancy between the transduction profiles between the cytoplasmic reporter gene GFP and the transmembrane protein hCD25. CD8+ T cells exhibited the lowest percentages of hCD25 expression and B cells the highest, which was reversed from our findings with GFP. Moreover, a lower percentage of liver cells exhibited surface expression of hCD25 than T and B cells, in contrast to the experiment with GFP transgene, in which liver cells had higher percentages of GFP compared to T and B cells. The discrepancy in transgene expression may be due to differences between these cell types in their ability to process and translocate transmembrane receptors to the cell surface, or differential effects of the transgenes on the survival and/or proliferation of these cell types. Alternatively, since hCD25 is a transmembrane protein, it is likely present on the surface of the exo-AAV which may have altered the target cell tropism of the particle compared to exo-AAV-GFP without surface modification.
Collectively, our findings demonstrate that AAV vectors can mediate transduction and expression of both intracellular and transmembrane proteins in lymphocytes following systemic administration in mice. These results establish early feasibility of direct in vivo gene modification of immune cells. In support of this, a very recent publication utilizing a very sensitive Cre recombinase-based reporter system demonstrated that a systemically injected AAV8 vector could transduce lymphocytes in the spleens of immune competent mice, albeit at efficiencies much lower than what we obtained30. Moreover, Münch et al. expressed a CD4-binding DARPin molecule on the AAV capsid to specifically target human CD4+ T cells in a humanized mouse model, and showed that very low doses (5 × 109 gc/mouse) could deliver a luciferase transgene that generated a bioluminescence signal localized precisely to the spleen31. Finally, the Zimmermann group has shown that intrathymic (i.t.) injection of AAV vectors into mice can achieve up to 5% thymocyte transduction efficiency32, and this can be used to correct immunodeficiency in ZAP70-deficient mice.
Lentivirus vectors have demonstrated remarkable efficacy in the transduction of peripheral blood mononuclear cells (PBMC) ex-vivo for the generation of CAR T cells33, and more recently, two studies from the Buchholz group demonstrated their ability to mediate direct in vivo modification of CD4+ and CD8+ T cells in humanized mouse models when used in conjunction with cell targeting strategies34,35. Transduction levels were in the range of 0.5–5%, similar to the transduction efficiencies we obtained with exo-AAV in the spleen, lymph nodes, and blood. Notably, in both lentivirus studies, efficient transduction required activation of the PBMCs. Jamali et al. used a transduction-enhancing cationic peptide to further increase the efficiency of the targeted lentivirus system in cultured cells36.
Both the lentivirus and AAV-based vectors have unique advantages and caveats for in vivo gene delivery. One obvious advantage of lentivirus vectors over AAV is the packaging capacity (~9 kb for lentivirus and 4.7 and 2.3 kb for single-stranded and self-complementary AAV genomes, respectively). Also, lentivirus integrates into the chromosome and in general, will mediate stable transduction of cells. In cases where long-term expression is desired, this is an important positive feature. In contrast, AAV vector genomes mainly persist episomally as concatamers37, so AAV genomes will be diluted in expanding T and B cell populations, resulting in waning transgene expression over time38. It is possible, however, that over time, low-frequency integration events lead to stable lymphocyte clones. We expect this to be an infrequent event; however future preclinical studies performed over longer intervals can assay for an increase in percentage of transduced cells at later timepoints and look at whether these cells are clonal and contain integration of the full transgene cassette necessary for expression. In applications such as CRISPR/Cas9 editing, short-term expression is desired to reduce potential off-target editing. Moreover, many T cell engineering strategies (e.g. CAR T cells) may also benefit from transient expression to limit adverse events from unregulated immune responses. From a practical standpoint, AAV vectors have already established an extensive safety profile for in vivo gene therapy in humans compared to lentivirus vectors, which have mostly been used for ex-vivo gene therapy thus far.
Our validation of exo-AAV vectors for in vivo delivery introduces additional potentialities, including reduced host antibody recognition of AAV particles15,18, and the prospect of cell targeting strategies through decoration of exosomes with relevant ligands18, similar to the work done with targeted lentivirus vectors39.
Methods
All methods and experiments were approved by the Partners Institutional Biosafety Committee of Partners Healthcare (Massachusetts General Hospital).
AAV constructs
AAV transgene plasmid (AAV2 inverted terminal repeat (ITR)-flanked) encoding green fluorescent protein (GFP) under the hybrid CMV immediate-early/chicken beta actin (CBA) promoter, AAV-CBA-GFP, was kindly provided by Dr. Miguel Sena-Esteves (UMass Medical Center). AAV-CBA-GFP encodes a self-complementary (sc) AAV genome.
exo-AAV and AAV preparations
exo-AAV were produced in 293 T cells as previously described21. Briefly, a triple transfection of AAV plasmid, rep/cap plasmid (AAV8 serotype used) and helper plasmids (pAdΔF6) was performed using the calcium phosphate method in ten 15 cm dishes. Media was changed to 2% FBS in DMEM the day after transfection and to exosome-free 2% FBS at 48 h post transfection. At 72 h post transfection, media was harvested. Cell debris and apoptotic bodies were removed by sequential, 10 min 300 x g and 2000 x g centrifugations, respectively. The supernatant containing exo-AAV was then centrifuged at 20,000 x g for 1 h to deplete larger microvesicles. Next, the remaining media was centrifuged at 100,000 x g for 1 hour using a Type 70 Ti rotor in an Optima™ L-90K ultracentrifuge (both Beckman Coulter, Inc., Indianapolis IN). The resulting pelleted material was resuspended in serum-free, antibiotic-free DMEM. Conventional AAVs were purified from the cell lysate using iodixanol-gradient ultracentrifugation. Vectors were stored at −80 °C until use. Before titration, exo-AAV sample was treated with DNase to remove plasmid DNA from the transfection by mixing 5 µl of the sample with 1 µl DNase I, 5 µl 10x buffer, and 39 µl water. Samples were incubated 1 h at 37 °C and then Dnase I was inactivated at 75 °C for 15 min. We purified AAV genomes using High Pure Viral Nucleic Acid Kit (Roche, Indianapolis, IN). Next, exo-AAV preparations were titered using a quantitative TaqMan PCR that detects AAV genomes (polyA region of the transgene cassette) as previously described40.
Mice
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Blood, spleens, lymph nodes, and livers were harvested from 8–12 week old male and female mice. All animal experiments were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital. We confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Flow cytometry
Blood samples were collected in microfuge tubes containing EDTA and subjected to red cell lysis with ACK buffer (Corning). Single cell suspensions were prepared from spleen, lymph node, and liver samples by mechanical disruption. All samples were stained with antibodies at 4 °C for 30 min. Flow cytometry was performed on the LSRII, Fortessa (both BD Immunocytometry Systems), or Cytoflex S (Beckman Coulter). Cell sorting was performed on the FACSAria II (BD Immunocytometry Systems). Flow data was analyzed with FlowJo software (Treestar). All antibodies were purchased from BioLegend or Life Technologies.
RNA isolation and GFP cDNA synthesis
Total RNA was isolated from 104 sorted GFP+ T cells using the RNeasy® Plus Micro Kit (Qiagen) according to manufacturer’s instructions. RNA was eluted in nuclease-free water and stored at −80 °C until use. cDNA was synthesized using 3 µl of RNA with the SuperScript® VILO™ cDNA synthesis kit (Invitrogen) according to manufacturer’s protocol.
DNA isolation
Total genomic DNA and viral DNA were purified from approximately 105 sorted T cells using the DNeasy® Blood and Tissue Kit (Qiagen) according to manufacturer’s instructions. DNA was stored at −20 °C until use.
GFP qPCR
To detect GFP cDNA in T cells and liver samples we used TaqMan® Gene Expression Assays with two probe and primer sets, one for enhanced GFP (Mr04329676_mr Enhance) and mouse GAPDH (Mm99999915_g1) (ThermoFisher). We used 1 ul of the cDNA reaction in a qPCR mastermix using TaqMan™ Fast Universal PCR Master Mix (2x), no AmperErase™ UNG (ThermoFisher). Each cDNA sample was run separately in both a GAPDH and GFP Taqman probe and primer reaction in an ABI7500 Fast Thermalcycler.
Statistics
Statistical analysis was performed using GraphPad Prism (v6.01; LaJolla, CA). A p-value ≤ 0.05 was considered statistically significant. For analysis between two groups, an unpaired t test was utilized.
Acknowledgements
We thank the MGH Quantitative Real-Time PCR Core Facility for use of the quantitative PCR equipment for AAV quantitation and RT-qPCR analysis of transgene expression, and the MGH Pathology Flow Cytometry Core Facility for staff-assisted cell sorting. This work was supported by the National Institutes of Health (R01-AI107020, R21-AI124143 to J.J.M., and R01-DC017117 to C.A.M.) and the European Research Council (Consolidator Grant 617432 to F.M.).
Author contributions
J.J.M. and C.A.M. designed the project, supervised and performed research, analyzed data, and wrote the manuscript with input from all the authors. C.B.B. performed research and analyzed data. F.M. analyzed data. K.S.H., J.N., A.V. and A.M. performed research. B.P.K. designed research.
Data availability
Raw data that support the findings of this study are available from the corresponding authors. Plasmid constructs described in this publication are available upon completion of a standard Material Transfer Agreement with The Massachusetts General Hospital.
Competing interests
C.A.M. has an issued patent as well as patent applications involving the exo-AAV platform. C.A.M. has a competing financial interest in Chameleon Biosciences, Inc., a company developing an enveloped Adeno Associated Virus (AAV) vector platform technology for repeated dosing of systemic gene therapy. C.A.M.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. B.P.K. is a co-inventor on various patents and patent applications that describe gene editing and epigenetic editing technologies. F.M. is an employee of and holds equity in Spark Therapeutics. C.A.M, B.P.K. and F.M. declare no competing non-financial interests. C.B.B., J.J.M., KSH, JN, AV, and AM declare no competing financial and non-financial interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: James J. Moon and Casey A. Maguire.
Contributor Information
James J. Moon, Email: jjmoon@mgh.harvard.edu
Casey A. Maguire, Email: cmaguire@mgh.harvard.edu
References
- 1.Eichler F, et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacein-Bey-Abina S, et al. A modified gamma-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2014;371:1407–1417. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster Stephen J., Svoboda Jakub, Chong Elise A., Nasta Sunita D., Mato Anthony R., Anak Özlem, Brogdon Jennifer L., Pruteanu-Malinici Iulian, Bhoj Vijay, Landsburg Daniel, Wasik Mariusz, Levine Bruce L., Lacey Simon F., Melenhorst Jan J., Porter David L., June Carl H. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. New England Journal of Medicine. 2017;377(26):2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire AM, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rangarajan Savita, Walsh Liron, Lester Will, Perry David, Madan Bella, Laffan Michael, Yu Hua, Vettermann Christian, Pierce Glenn F., Wong Wing Y., Pasi K. John. AAV5–Factor VIII Gene Transfer in Severe Hemophilia A. New England Journal of Medicine. 2017;377(26):2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 7.George LA, et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendell JR, et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 9.Smalley E, First AAV. gene therapy poised for landmark approval. Nat. Biotechnol. 2017;35:998–999. doi: 10.1038/nbt1117-998. [DOI] [PubMed] [Google Scholar]
- 10.Tarantal AF, Lee CCI, Martinez ML, Asokan A, Samulski RJ. Systemic and Persistent Muscle Gene Expression in Rhesus Monkeys with a Liver De-Targeted Adeno-Associated Virus Vector. Hum. gene Ther. 2017;28:385–391. doi: 10.1089/hum.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortolanza S, et al. AAV6-mediated systemic shRNA delivery reverses disease in a mouse model of facioscapulohumeral muscular dystrophy. Mol. Ther. 2011;19:2055–2064. doi: 10.1038/mt.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watakabe A, et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci. Res. 2015;93:144–157. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Li S, et al. Efficient and Targeted Transduction of Nonhuman Primate Liver With Systemically Delivered Optimized AAV3B Vectors. Mol. Ther. 2015;23:1867–1876. doi: 10.1038/mt.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huser, D. et al. High Prevalence of Infectious Adeno-associated Virus (AAV) in Human Peripheral Blood Mononuclear Cells Indicative of T Lymphocytes as Sites of AAV Persistence. Journal of virology91, 10.1128/JVI.02137-16 (2017). [DOI] [PMC free article] [PubMed]
- 15.Meliani A, et al. Enhanced liver gene transfer and evasion of preexisting humoral immunity with exosome-enveloped AAV vectors. Blood Adv. 2017;1:2019–2031. doi: 10.1182/bloodadvances.2017010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudry E, et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016;23:380–392. doi: 10.1038/gt.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wassmer SJ, Carvalho LS, Gyorgy B, Vandenberghe LH, Maguire CA. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci. Rep. 2017;7:45329. doi: 10.1038/srep45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyorgy B, Fitzpatrick Z, Crommentuijn MH, Mu D, Maguire CA. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomater. 2014;35:7598–7609. doi: 10.1016/j.biomaterials.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gyorgy B, et al. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol. Ther. 2017;25:379–391. doi: 10.1016/j.ymthe.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.György Bence, Maguire Casey A. Extracellular vesicles: nature's nanoparticles for improving gene transfer with adeno-associated virus vectors. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2017;10(3):e1488. doi: 10.1002/wnan.1488. [DOI] [PubMed] [Google Scholar]
- 21.Maguire CA, et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012;20:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani AC, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol. Ther. 2011;19:526–535. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu D, Walker CM. Continuous CD8(+) T-cell priming by dendritic cell cross-presentation of persistent antigen following adeno-associated virus-mediated gene delivery. J. virology. 2011;85:12083–12086. doi: 10.1128/JVI.05375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed SS, et al. A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS Gene therapy in Canavan mice. Mol. Ther. 2013;21:2136–2147. doi: 10.1038/mt.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marodon G, et al. Specific transgene expression in human and mouse CD4+ cells using lentiviral vectors with regulatory sequences from the CD4 gene. Blood. 2003;101:3416–3423. doi: 10.1182/blood-2002-02-0578. [DOI] [PubMed] [Google Scholar]
- 27.McCarty DM, Young SM, Jr., Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 28.Russell DW, Miller AD, Alexander IE. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc. Natl Acad. Sci. U S Am. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penaud-Budloo M, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang JF, Toulmin SA, Brida KL, Eisenlohr LC, Davidson BL. Standard screening methods underreport AAV-mediated transduction and gene editing. Nat. Commun. 2019;10:3415. doi: 10.1038/s41467-019-11321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munch RC, et al. Off-target-free gene delivery by affinity-purified receptor-targeted viral vectors. Nat. Commun. 2015;6:6246. doi: 10.1038/ncomms7246. [DOI] [PubMed] [Google Scholar]
- 32.Moreau A, et al. Efficient intrathymic gene transfer following in situ administration of a rAAV serotype 8 vector in mice and nonhuman primates. Mol. Ther. 2009;17:472–479. doi: 10.1038/mt.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chono H, et al. Optimization of lentiviral vector transduction into peripheral blood mononuclear cells in combination with the fibronectin fragment CH-296 stimulation. J. Biochem. 2011;149:285–292. doi: 10.1093/jb/mvq135. [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer, A. et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol Med10, 10.15252/emmm.201809158 (2018). [DOI] [PMC free article] [PubMed]
- 35.Zhou Q, et al. Exclusive Transduction of Human CD4+ T Cells upon Systemic Delivery of CD4-Targeted Lentiviral Vectors. J. Immunol. 2015;195:2493–2501. doi: 10.4049/jimmunol.1500956. [DOI] [PubMed] [Google Scholar]
- 36.Jamali A, et al. Highly Efficient and Selective CAR-Gene Transfer Using CD4- and CD8-Targeted Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2019;13:371–379. doi: 10.1016/j.omtm.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakai H, et al. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. virology. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadaczek P, Mirek H, Berger MS, Bankiewicz K. Limited efficacy of gene transfer in herpes simplex virus-thymidine kinase/ganciclovir gene therapy for brain tumors. J. Neurosurg. 2005;102:328–335. doi: 10.3171/jns.2005.102.2.0328. [DOI] [PubMed] [Google Scholar]
- 39.Frank AM, Buchholz CJ. Surface-Engineered Lentiviral Vectors for Selective Gene Transfer into Subtypes of Lymphocytes. Mol. Ther. Methods Clin. Dev. 2019;12:19–31. doi: 10.1016/j.omtm.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra115. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data that support the findings of this study are available from the corresponding authors. Plasmid constructs described in this publication are available upon completion of a standard Material Transfer Agreement with The Massachusetts General Hospital.