Abstract
Characterizing the adhesive dynamics of Plasmodium falciparum infected erythrocytes (IEs) to different endothelial cell receptors (ECRs) in flow is a big challenge considering available methods. This study investigated the adhesive dynamics of IEs to five ECRs (CD36, ICAM-1, P-selectin, CD9, CSA) using simulations of in vivo-like flow and febrile conditions. To characterize the interactions between ECRs and knobby and knobless IEs of two laboratory-adapted P. falciplarum isolates, cytoadhesion analysis over time was performed using a new tracking bioinformatics method. The results revealed that IEs performed rolling adhesion exclusively over CD36, but exhibited stationary binding to the other four ECRs. The absence of knobs affected rolling adhesion both with respect to the distance travelled by IEs and their velocity. Knobs played a critical role at febrile temperatures by stabilizing the binding interaction. Our results clearly underline the complexity of the IE-receptor interaction and the importance of knobs for the survival of the parasite at fever temperatures, and lead us to propose a new hypothesis that could open up new strategies for the treatment of malaria.
Subject terms: Parasite immune evasion, Parasite biology
Introduction
Cytoadhesion of Plasmodium falciparum to human endothelial cell receptors (ECRs) causes complications and deaths following malaria infection. In 2018, 405,000 malaria-related deaths were registered (61% of which were of children younger than 5 years old)1. Cytoadhesion leads to accumulation of infected erythrocytes (IEs) within the microvascular bed of vital organs such as the brain, lungs, and kidneys. Death can eventually occur due to decreased blood supply and organ failure2,3. Cytoadhesion results from interactions between members of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family and different ECRs4–8. About 60 var genes per parasite genome encode PfEMP1 family members. Only one PfEMP1 variant is located on the membrane of IEs at the trophozoite stage, but the corresponding var gene is already expressed at the ring stage in a mutually exclusive pattern9. The most studied interaction partners are the ECRs CD36, intracellular adhesion molecule 1 (ICAM-1), endothelial protein C receptor, and chondroitin sulfate A (CSA)6,7. In general, PfEMP1 molecules cluster on nanoscale protrusions, called knobs, located on the membrane of IEs. Knobs consist of various submembranous structural proteins, predominantly knob-associated histidine-rich protein (KAHRP)10. KAHRP contains several binding domains that interact with both parasite and host factors. Knobs begin to appear on the surface of IEs at 16 h post-invasion (hpi). The density of knobs increases from 20 to 60/µm2 with parasite development from the trophozoite stage to the schizont stage respectively11. The presence of knobs on the surface of IEs leads to increase their stiffness, which in turn increases their binding ability11,12.
Many aspects of IE-ECR interactions have not been clarified. The leukocyte cytoadhesion model of recruitment and extravasation to the site of inflammation has been proposed. This model states that adhesion of leukocytes to various ECRs during inflammation can be divided into three main phases called tethering, rolling, and immobilization. During the tethering phase, leukocytes contact three selectin receptors. Thereafter, rolling movement is enhanced by α4β7 and α4β1. Finally, firm immobilization to ICAM-1 or ICAM-2 occurs13–15. It is uncertain whether IEs use the same cytoadhesive mechanics as leukocytes. Controversial data regarding IE-ECR cytoadhesive dynamics under flow conditions have been reported. For example, IE-ICAM-1 adhesive mechanics were described by some authors as rolling adhesion and by others as static binding, similar to IE-CD36 adhesive mechanics16–19.
Cytoadhesive dynamics are dependent on cell properties, ligand-receptor bond dynamics, and flow conditions14,20. The membranes of IEs are 10-fold stiffer than those of non-IEs due to the presence of knobs. In addition, trophozoite-stage IEs are biconcave and parasites localize to the periphery of these cells, while schizont-stage IEs are spherical and parasites localize throughout these cells21,22. These changes alter the adhesive properties of IEs20,23. In terms of adhesive bond dynamics between IEs’ ligands and ECRs, there are two types of bonds, termed slip and catch bonds, which are formed by non-covalent interactions. Life time of slip bonds is decreased in the presence of an external tensile force. Conversely, that of catch bonds increase with increasing external force24. However, catch bonds can decrease with very high external force and this is called catch-slip bonds25. In terms of flow conditions, the arterial and venous vasculature is exposed to different shear stresses, which correspond with differences in the blood flow rate/velocity between arteries and veins. Normal physiological shear stress ranges from 10 to 70 dyn/cm2 in arteries, but from 1 to 6 dyn/cm2 in veins26. Shear stress affects the stabilities of different types of bonds, which in turn affects IE-ECR adhesive dynamics24–28.
A cardinal host response to P. falciparum infection is fever at irregular intervals, and IEs are important in the febrile state. Fever during infection modulates the immune response to the pathogen. In vitro experiments demonstrated the effect of fever on protein expression and parasite survival29,30. However, the effects of fever on the adhesive dynamics and binding affinities of IEs are poorly described in the literature. This study describes the cytoadhesive behavior of IEs to a variety of ECRs (CD36, ICAM-1, P-selectin, CD9, and CSA). We simulated the dynamic forces acting on IEs in the human circulation using a laminar flow system and various shear stresses. We also investigated the effect of febrile temperature (40 °C) on the binding dynamics and affinities of IEs to different ECRs. The results led us to formulate a new hypothesis regarding IE-ECR interactions during infection.
Results
Experimental setup
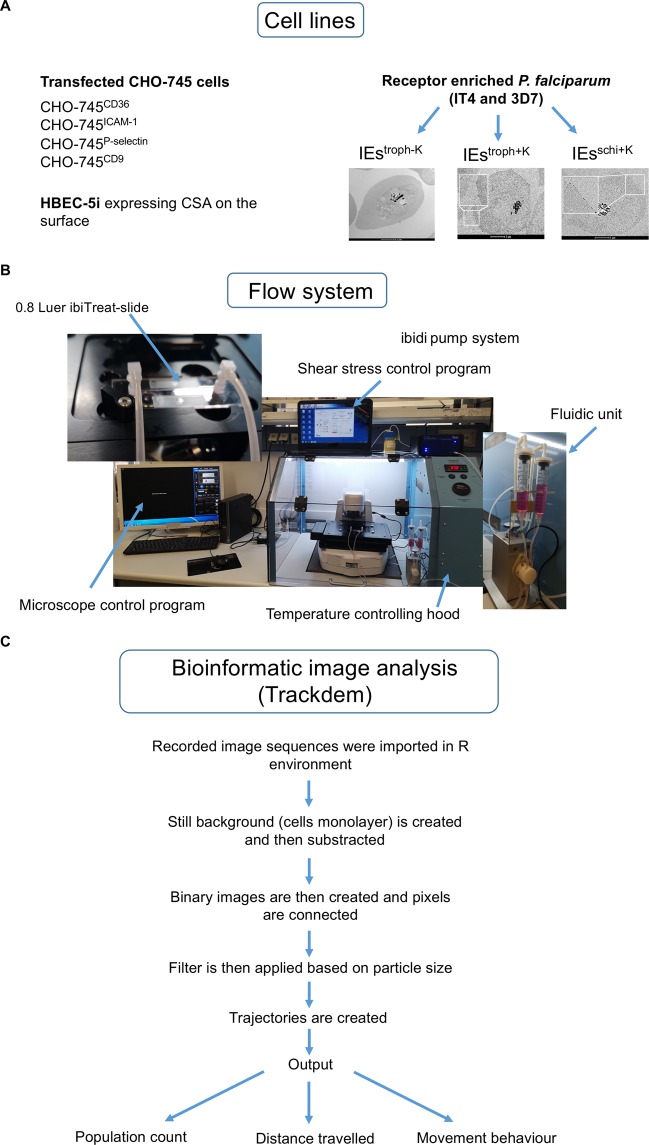
Endothelial cytoadhesion and the binding capacities of various laboratory strains and patient isolates of P. falciparum have been mainly investigated under static conditions6,31,32. This is classically achieved by directly incubating IEs with cells or recombinant receptors of interest8,16,33,34. Finally, adherent IEs are counted manually. These experiments demonstrated that laboratory-adapted trophozoite-stage IT4 and 3D7 isolates show different binding capacities to various ECRs under static conditions31,33. The main objective of this study was to characterize the binding behavior of erythrocytes infected with P. falciparum to the ECRs CD36, ICAM-1, P-selectin, CD9, and CSA under controlled flow and temperature conditions. For this purpose, we used transfected CHO cells expressing GFP fusions of the receptors of interest on the cell surface33. P. falciparum populations with increased binding for ICAM-1, P-selectin and CD9 receptors were obtained by performing at least seven rounds of enrichment, as described previously33. Whereas the IEs populations used for characterizing the binding over CD36 were obtained from the starting cultures without any enrichment, since over 80% of PfEMP1 proteins contain CIDRα2-6 domains, which are involved in CD36 binding35. All the IEs populations were tested for the presence or absence of knobs on their cell surfaces by scanning electron microscopy (SEM) (Fig. 1A). The HBEC-5i cell line was used as a model of endothelial cell cytoadhesion to CSA. Transcriptome profiling showed that IEs populations enriched for binding to HBEC-5i showed exclusive expression of var2csa and the HBEC-5i enriched population bind exclusively to CSA on the surface of HBEC-5i cells (Fig. S6A)36. A laminar flow system was used to imitate the hydrodynamic forces exerted on IEs in blood microvessels (Fig. 1B). In all experiments, different wall shear stresses (6, 4, 3, 2, 1.5, and 0.9 dyn/cm2) were applied, which resemble the range of shear stress exerted on ECs lining blood microvessels26,37. Each magnitude of shear stress was applied for 10 min, except for 0.9 dyn/cm2, which was applied for 30 min. Bioinformatics image analysis was performed using the trackdem R package38. The IEs number was adjusted to 1 × 107 before the start of each experiment. Individual IEs were tracked through the entire sequence of images, and then trajectories were constructed and plotted over the static background. The accumulated distance traveled by IEs was defined as the length of each trajectory during the entire 10 min tracking time and was obtained from the output of trackdem analysis (Fig. 1C).
Figure 1.
Scheme for the analysis processes. Cell lines used in this study. (A) IEstroph−K stands for knobless trophozoites (85–100% without knobs), IEstroph+K stands for knobby trohozoites (99–100% with knobs) and IEsschi+K stands for knobby schizonts (99–100% with knobs). Flow system for controlling shear stress as well as temperature. (B) Bioinformatics scheme for tracking analysis (C).
Binding behavior of trophozoite-stage IEs to CHO-745CD36 cells
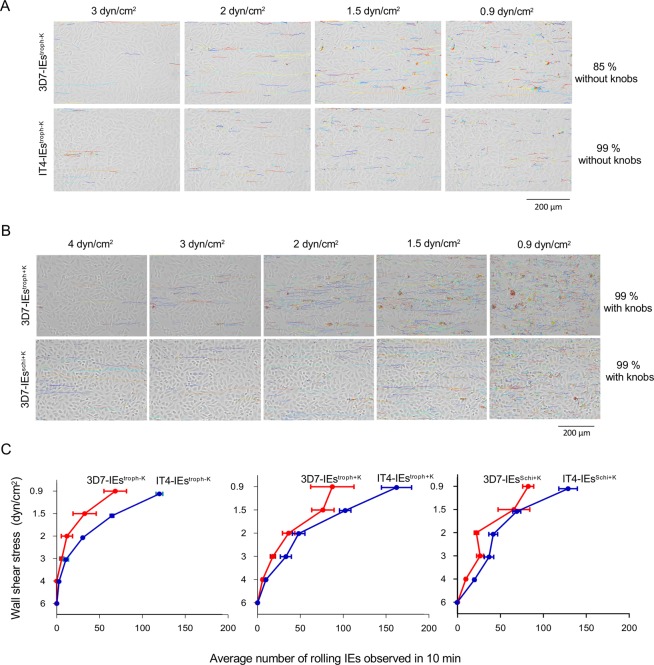
A sequential binding pattern was observed upon exposure of knobless trophozoite-stage IEs of the two isolates IT4 and 3D7 (IT4-IEstroph−K and 3D7-IEstroph−K, respectively) to CHO-745 cells expressing CD36 (CHO-745CD36). IEs were initially captured by cells and were then tethered to cells via flipping binding. IT4-IEstroph−K and 3D7-IEstroph−K exhibited no or very low adhesion at 6 and 4 dyn/cm2, meaning no trajectories were created. This reflects the inability of IEs to be captured from the flow under high shear stress. Tracking of knobless IEs of the two isolates at 3 dyn/cm2 revealed generation of a few trajectories (Fig. 2A,C and S1 Movie). Lowering the shear stress to 2 dyn/cm2 increased the number of trajectories (12 ± 6 tracks/0.2 mm2 for 3D7-IEstroph−K and 31 ± 1 tracks/0.2 mm2 for IT4-IEstroph−K) (S2 Movie). The number of trajectories was even higher at 1.5 dyn/cm2 (32 ± 13 tracks/0.2 mm2 for 3D7-IEstroph−K and 66 ± 2 tracks/0.2 mm2 for IT4-IEstroph−K) and highest at 0.9 dyn/cm2 (68 ± 13 tracks/0.2 mm2 for 3D7-IEstroph−K and 124 ± 4/0.2 tracks/mm2 for IT4-IEstroph−K) (Fig. 2A,C).
Figure 2.
Cell trajectories showing the rolling binding behavior of IEs to CHO-745CD36. Trajectories of 3D7-IEstroph−K and IT4-IEstroph−K. (A) Trajectories of 3D7-IEstroph+K and 3D7-IEsschi+K under various shear stresses. (B) Average number of rolling IEs at applied shear stresses. (C) Images were analyzed using the trackdem (0.4.2) R package38,58. The static cell background was subtracted from all images and then moving particles (i.e., IEs) were detected. The Shiny R package38,58 was used with thresholding of 0.1. The PixelRange of moving particles was set to 30–500. Individual trajectories were subsequently reconstructed based on a minimum present (incThres) equal to six frames (i.e., 30 sec). Finally, trajectories were plotted over the still background. Examples of trajectories are shown in S1 and S2 Movies.
Binding behavior of knobless/knobby, trophozoite-stage IEs to CHO-745CD36 cells
Knobby trophozoite-stage IEs of the two isolates (IT4-IEstroph+K and 3D7-IEstroph+K, respectively) were captured from the flow and tethered to CHO-745CD36 cells at high shear stress (4 dyn/cm2) in contrast to IEstroph−K. Trajectories of IEstroph+K could be constructed at 4 dyn/cm2 (an example for 3D7-IEstroph+K is shown in Fig. 2B), which was not the case for IEstroph−K. The number of trajectories was 6 ± 1 tracks/0.2 mm2 for 3D7-IEstroph+K and 10 ± 1/0.2 mm2 for IT4-IEstroph+K (Fig. 2C). At 3 dyn/cm2, longer trajectories (>30 µm) compared with those generated by IEstroph−K were observed (Fig. 2A,B; S3 Movie). The number of trajectories was 18 ± 2 tracks/0.2 mm2 for 3D7-IEstroph+K and 34 ± 6 tracks/0.2 mm2 for IT4-IEstroph+K (Fig. 2C). The number of trajectories was increased at 2 dyn/cm2 (Fig. 2C), with 36 ± 7 tracks/0.2 mm2 for 3D7-IEstroph+K and 49 ± 7 tracks/0.2 mm2 for IT4-IEstroph+K. The number of trajectories was even higher at 1.5 and 0.9 dyn/cm2 (76 ± 13 and 87 ± 25 tracks/0.2 mm2 for 3D7-IEstroph+K and 105 ± 6 and 167 ± 18 tracks/0.2 mm2 for IT4-IEstroph+K, respectively), although these trajectories were shorter (10–30 µm) (Fig. 2B).
Binding behavior of schizont-stage IEs to CHO-745CD36 cells
To compare binding behavior between the trophozoite and schizont stages, experiments were performed using knobby schizont-stage IEs (36–44 hpi) of the IT4 and 3D7 isolates (IT4-IEsschi+K and 3D7-IEsschi+K, respectively). As an example, 3D7-IEs are shown in Fig. 2B. Similar to 3D7-IEstroph+K, 3D7-IEsschi+K were captured from the flow by CHO-745CD36 cells at 4 dyn/cm2. Lowering the shear stress increased the number of trajectories. Specifically, the number of trajectories at 4, 3, 2, 1.5, and 0.9 dyn/cm2 was 9 ± 1, 26 ± 4, 22 ± 2, 65 ± 18, and 82 ± 6 tracks/0.2 mm2 for 3D7-IEstroph+K, and 20 ± 1, 38 ± 5, 43 ± 5, 71 ± 4, and 132 ± 10 tracks/0.2 mm2 for IT4-IEstroph+K, respectively (Fig. 2C and S4 Movie).
Effects of the presence of knobs and the parasite stage on the distance traveled by and velocity of 3D7-IEs rolling over CHO-745CD36 cells
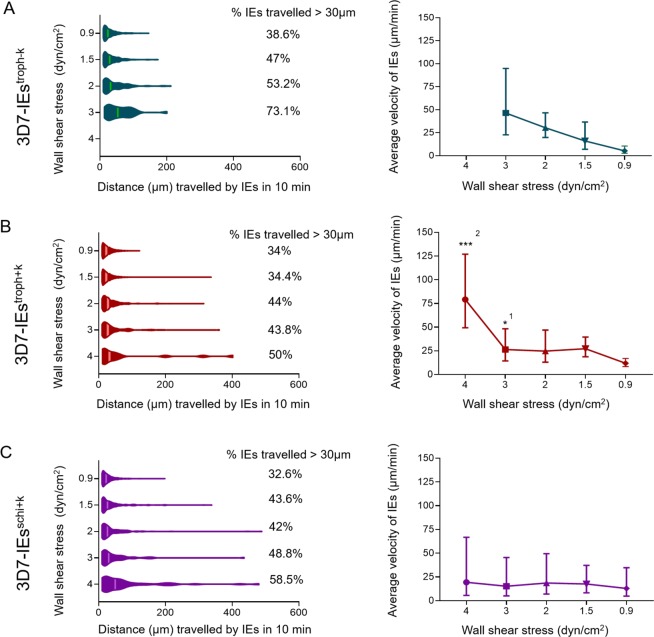
At 3 dyn/cm2, 3D7-IEstroph−K traveled a minimum distance of 13.43 µm and a maximum distance of 201 µm, with a geometric mean of 48 µm (Fig. 3A). At 2 dyn/cm2, 3D7-IEstroph−K traveled a minimum distance of 10 µm and a maximum distance of 212 µm, with a geometric mean of 34 µm. At 1.5 and 0.9 dyn/cm2, the minimum distance traveled remained the same (10 µm), while the maximum distance traveled decreased to 174 and 146 µm, respectively. The presence of knobs on the surface of 3D7-IEs affected the distance traveled. Specifically, the maximum distance traveled by 3D7-IEstroph+K was 330–400 µm at 4, 3, 2, and 1.5 dyn/cm2, while the minimum distance traveled was 11.5 µm at 4 dyn/cm2 and 10 µm at all other shear stresses (Fig. 3B). On the other hand, at the lowest shear stress (0.9 dyn/cm2), the maximum distance traveled was only 122.9 µm and the minimum distance traveled remained 10 µm (Fig. 3B). The maximum distance traveled by 3D7-IEsschi+K was longer (430–490 µm) at 4, 3, and 2 dyn/cm2 but decreased to 338 and 198 µm at 1.5 and 0.9 dyn/cm2, respectively. The minimum distance traveled by 3D7-IEsschi+K remained the same (10 µm) at all shear stresses (Fig. 3C).
Figure 3.
Tracking of 3D7-IEs over CHO-745CD36 cells. Knobless and knobby trophozoite-stage 3D7-IEs (3D7-IEstroph−K (A), 3D7-IEstroph+K (B) and knobby schizont-stage 3D7-IEschi+K (C)) were analyzed. The distance traveled and the average velocity of IEs were calculated using the trackdem R package38,58. The distance traveled by each IE was divided by the individual contact time with cells to calculate velocity (n = 20). Significance was assessed using the Kolmogorov-Smirnov test. *p < 0.01, ***p < 0.0001 (1statistical comparison of 3D7-IEstroph−K and 3D7-IEstroph+K, 2statistical comparison of 3D7-IEstroph+K and 3D7-IEsschi+K).
The rolling phenotype was analyzed in detail by calculating the velocity of individual IEs. The average velocity of 3D7-IEstroph−K was 46.41, 30.40, 16.07, and 5.3 µm/min at 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 3A). The average velocity of 3D7-IEstroph+K was 79.2, 26.38, 24.78, 27.30, and 11.94 µm/min at 4, 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 3B). The average velocity of 3D7-IEsschi+K was 19.44, 15.27, 18.66, 17.63, and 13.01 µm/min at 4, 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 4C).
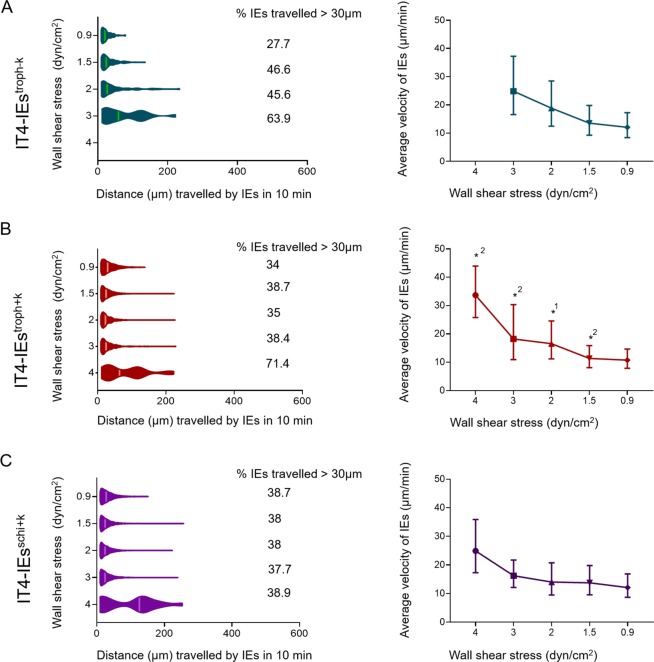
Figure 4.
Tracking of IT4-IEs over CHO-745CD36 cells. Knobless and knobby trophozoite-stage IT4-IEs (IT4-IEstroph−K (A), IT4-IEstroph+K (B) and knobby schizont stage IT4-IEschi+K (C)) were analyzed. The distance traveled and the average velocity of IEs were calculated using the trackdem R package38,58. The distance traveled by each IE was divided by the individual contact time with cells to calculate velocity (n = 20). Significance was assessed using the Kolmogorov-Smirnov test. *P < 0.01 (1statistical comparison of IT4-IEstroph−K and IT4-IEstroph+K, 2statistical comparison of IT4-IEstroph+K and IT4-IEsschi+K).
Effects of the presence of knobs and the parasite stage on the distance traveled by and velocity of IT4-IEs rolling over CHO-745CD36 cells
At 3 dyn/cm2, IT4-IEstroph−K traveled a minimum distance of 11.9 µm and a maximum distance of 222.7 µm, with a geometric mean of 48.4 µm (Fig. 4A). Although the minimum distance traveled (10 µm) was almost the same at all shear stresses, the maximum distance traveled decreased as shear stress decreased (234, 135, and 79 µm at 2, 1.5, and 0.9 dyn/cm2, respectively) (Fig. 4B). IT4-IEstroph+K traveled a maximum distance of 222–228 µm at 1.5–4 dyn/cm2 and 138.9 µm at 0.9 dyn/cm2 (Fig. 4C). The minimum distance traveled by IT4-IEstroph+K was 10 µm at all shear stresses, except for 4 dyn/cm2 where it was 12 µm (Fig. 4B). IT4-IEsschi+K traveled the same minimum distance (10 µm) at all shear stresses (Fig. 4C). The maximum distance traveled by IT4-IEsschi+K was 328.7 µm at 4 dyn/cm2, but remained almost constant at 3, 2, and 1.5 dyn/cm2 (229, 213, and 247 µm, respectively) (Fig. 4C).
The average velocity of IT4-IEstroph−K was 24.8, 18.82, 13.52, and 12.04 µm/min at 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 4A). The average velocity of IT4-IEstroph+K was 33.66, 18.24, 16.58, 11.38, and 10.76 µm/min at 4, 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 4B). The average velocity of IT4-IEsschi+K was 24.92, 16.24, 14.04, 13.78, and 12.10 µm/min at 4, 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 4C).
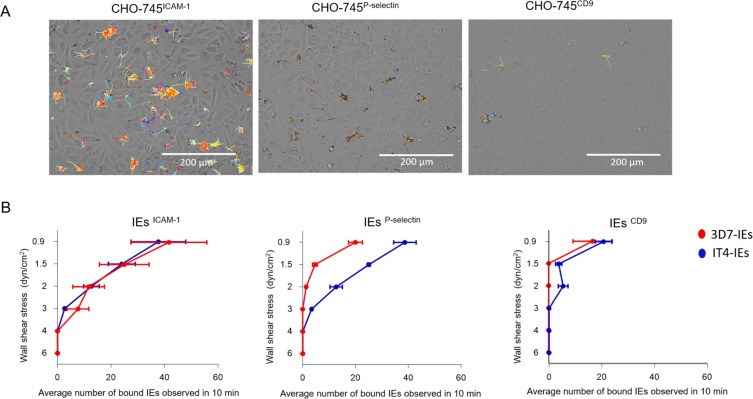
Binding behaviors of receptor-enriched trophozoite-stage IEs to CHO-745ICAM-1, CHO-745P-selectin, and CHO-745CD9 cells
Analysis of the binding behavior of the IEs to ICAM-1, P-selectin and CD9 revealed very short binding traces inverse to the rolling binding to CD36 (Fig. 5A). IEs tended to cluster in small conglomerates. We concluded that binding to these receptors was stationary (defined as capture of IEs from the flow without rolling or tethering) (S5 Movie). 3D7-IEsICAM-1 showed minimal binding to CHO-745ICAM-1 cells at 3 dyn/cm2, with 7 ± 7 IEs/0.2 mm2. This increased to 11 ± 10, 25 ± 16, and 41 ± 24 IEs/0.2 mm2 at 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 5B). 3D7-IEsP-selectin bound to CHO-745P-selectin cells at 2 and 1.5 dyn/cm2, with no more than 5 IEs/0.2 mm2. This increased to 20 ± 4 IEs/0.2 mm2 at 0.9 dyn/cm2. Binding of 3D7-IEsCD9 to CHO-745CD9 cells was less than 5 IEs/0.2 mm2 at 2 and 1.5 dyn/cm2, but increased to 20 ± 5 IEs/0.2 mm2 at 0.9 dyn/cm2 (Fig. 5B).
Figure 5.
Static adhesion of IEs over ICAM-1, P-selectin, and CD9. The trajectories by IEs over the 3 receptors. (A) Average number of bound IEs to the three receptors. (B) Cells were cultivated in a µ-slide I 0.8 Luer ibiTreat and the fluidic unit was connected with 1 × 107 IEs. Different shear stresses were applied using the ibidi pump system. Parasites were enriched to bind to the respective receptor via at least seven rounds of selection. The number of adherent IEs was determined after 10 min of flow under various shear stresses (6, 4, 3, 2, 1.5, and 0.9 dyn/cm2). Bound IEs were counted in the total image field (0.2 mm2) and are represented as mean ± SEM. Examples of selected trajectories are shown S5 Movie.
The binding capacity of IT4-IEs to the three ECRs is shown in Fig. 5B. At 6 dyn/cm2, IT4-IEs did not bind to CHO-745ICAM-1, CHO-745P-selectin, or CHO-745CD9 cells. IT4-IEsICAM-1 did not adhere until shear stress reached 3 dyn/cm2 (2 ± 0.5 IEs/0.2 mm2). The number of bound IT4-IEsICAM-1 increased as shear force decreased (12 ± 5, 24 ± 9, and 37 ± 18 IEs/0.2 mm2 at 2, 1.5, and 0.9 dyn/cm2, respectively) (Fig. 5B). IT4-IEsP-selectin began to bind at 4 dyn/cm2 (1 ± 0.5 IEs/0.2 mm2). The number of bound IT4-IEsP-selectin was 3 ± 0.5, 12 ± 4, 25 ± 1, and 38 ± 7 IEs/0.2 mm2 at 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 5B). IT4-IEsCD9 bound to CHO-745CD9 cells only at 0.9 dyn/cm2 (20 ± 6 IEs/0.2 mm2) (Fig. 5B).
As a control, static binding assays were performed for IT4 and 3D7 IEstroph+K over CHO-745GFP, where non-specific binding was not observed (Fig. S6B). Additionally, flow experiments were performed with IEs over CHO-745GFP cells. After 30 min at 0.9 dyn/cm2, very few bound IEs were detected (3–6 IEs/0.2 mm2). Another control was performed by allowing IT4-IEsICAM-1 and 3D7-IEsICAM-1 to bind to CHO-745CD36 cells under flow conditions using the same experimental set-up. Interestingly, IT4-IEsICAM-1 showed rolling binding behavior on CD36, as observed with the long-term cultured parasites.
Binding behaviors to HBEC-5i cells under controlled flow and temperature conditions
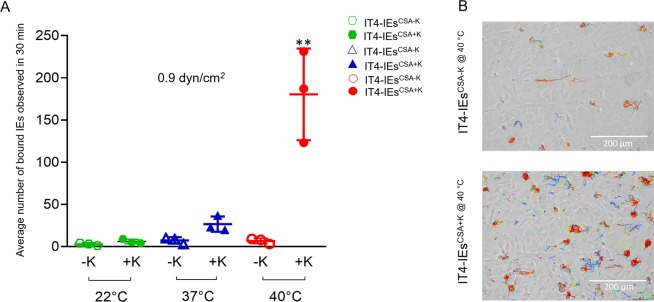
To analyze the binding behavior of IT4-IEs to human ECs, we used the HBEC-5i cell line. These cells express CSA on their surface due to immortalization processes, which lead to excessive surface production of CSA39. IT4-IEs were enriched for binding to HBEC-5i cells via at least seven rounds of selection to generate a CSA-binding parasite population (IT4-IEsCSA). Exclusive expression of var2csa was confirmed by qPCR and transcriptome analysis (Fig. S6A)36. To select IT4-IEsCSA with knobs (IT4-IEsCSA+K), seven rounds of enrichment were performed at 40 °C (Fig. 6A,B), which exclusively selects IT4-IEsCSA+K 36. Flow experiments with IT4-IEsCSA−K and IT4-IEsCSA+K were performed at room temperature (22 °C), body temperature (37 °C), and febrile temperature (40 °C). Binding was only observed at 0.9 dyn/cm2; therefore, we tracked IEs under this shear stress for 30 min. IT4-IEsCSA−K and IT4-IEsCSA+K only statically adhered to CSA on HBEC-5i cells (Fig. 6A,B; S7 and S8 Movies). Figure 6A shows the average numbers of bound IT4-IEsCSA−K and IT4-IEsCSA+K at the three temperatures. At 22 °C, IT4-IEsCSA−K had a very low binding capacity, with an average of 3 ± 1 IEs/0.2 mm2, while the average number of bound IT4-IEsCSA+K was 6 ± 1 IEs/0.2 mm2. Increasing the temperature to 37 °C only increased the binding of IT4-IEsCSA+K, with an average of 26 ± 5 IEs/0.2 mm2. At febrile temperature (40 °C), binding of IT4-IEsCSA+K significantly increased to an average of 180 ± 30 IEs/0.2 mm2. Statistical significance was done using ANOVA between the binding capacities at the three different temperature values (p = 0.0036).
Figure 6.
Adhesion of IEs to HBEC-5i cells under flow and controlled temperatures. Average number of bound IEs. (A) Trajectories generated by bound IT4-IEsCSA+K@40 °C and IT4-IEsCSA+K@37 °C at 0.9 dyn/cm2. (B) Experiments were performed using IT4-IEs enriched over HBEC-5i cells for at least seven rounds of enrichment. HBEC-5i cells were cultivated in a µ-slide I 0.8 Luer ibiTreat and the fluidic unit was connected with 1 × 107 IEs. Different shear stresses were applied using the ibidi pump system. Temperature was controlled using a hood over the microscope. An example is shown in S7 and S8 Movies. The number of bound IEs was counted using the trackdem R package38,58 in the total image field (0.2 mm2) and is represented as the mean ± SEM (**p < 0.001).
Rolling adhesion over CHO-745CD36 cells under controlled flow and temperature conditions
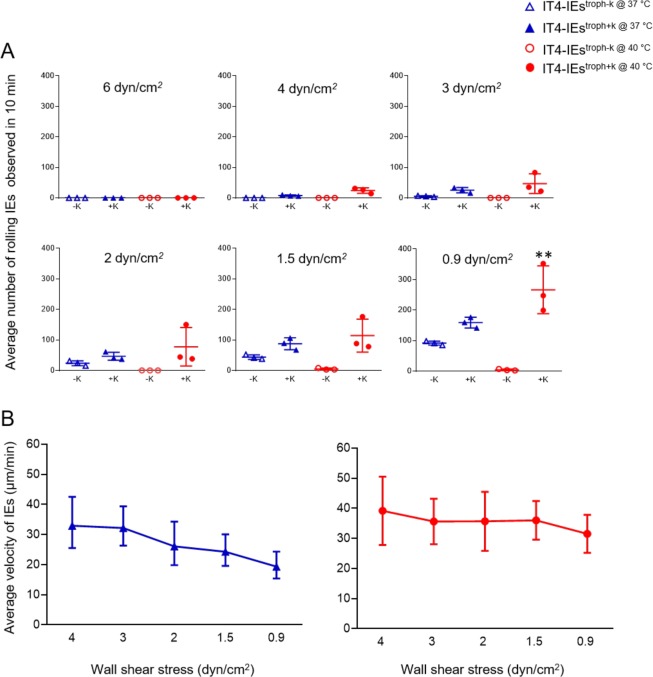
Next, we analyzed the rolling behavior of IT4-IEstroph−K and IT4-IEstroph+K at different temperatures (37 °C and 40 °C). Figure 7A shows the average number of rolling IEs at 4 dyn/cm2. Rolling of IT4-IEstroph−K@37° and IT4-IEstroph−K@40 °C was not observed. On the other hand, IT4-IEstroph+K@37 °C and IT4-IEstroph+K@40 °C rolled, with an average of 7 ± 1 and 34 ± 11 IEs/0.2 mm2, respectively. Decreasing shear stress to 3 dyn/cm2 increased the average number of rolling IT4-IEstroph+K@37 °C and IT4-IEstroph+K@40 °C to 25 ± 4 and 46 ± 18 IEs/0.2 mm2, respectively (Fig. 7A). The average number of rolling IEs was further increased at 2 dyn/cm2, with an average of 23 ± 4 IEs/0.2 mm2 for IT4-IEstroph−K@37° and 46 ± 7 IEs/0.2 mm2 for IT4-IEstroph+K@37 °C. Rolling of IT4-IEstroph−K@40 °C was not observed. On the other hand, IT4-IEstroph+K@40 °C rolled with an average of 77 ± 36 IEs/0.2 mm2. At 1.5 dyn/cm2, the average number of rolling IT4-IEstroph−K@37 °C, IT4-IEstroph+K@37 °C, IT4-IEstroph−K@40 °C, and IT4-IEstroph+K@40 °C was 43 ± 4, 87 ± 11, 4 ± 1, and 114 ± 31 IEs/0.2 mm2, respectively (Fig. 7D). Finally, at 0.9 dyn/cm2, the number of rolling IT4-IEstroph−K@37 °C, IT4-IEstroph+K@37 °C, IT4-IEstroph−K@40 °C, and IT4-IEstroph+K@40 °C was 91 ± 3, 158 ± 10, 4 ± 1, and 266 ± 45 IEs/0.2 mm2, respectively (Fig. 7A).
Figure 7.
Rolling adhesion behavior of knobless and knobby trophozoite-stage IEs over CHO-745CD36 under controlled temperatures. Average number of IEs adhering to CHO-745CD36 cells at 37 °C (blue) and 40 °C (red). (A) Cells were cultivated in a µ-slide I 0.8 Luer ibiTreat and the fluidic unit was connected with 1 × 107 IEs. Different shear stresses were applied using the ibidi pump system. Binding to cells was analyzed with long-term cultures. The number of adherent IEs was determined after flow for 30 min at 0.9 dyn/cm2. The number of rolling IEs was counted using the trackdem R package38,58 in the total image field (0.2 mm2) and is represented as mean ± SEM (**p < 0.001). (B) Average velocity of IT4troph+K@37 °C and IT4troph+K@40 °C, respectively. The distance travelled by each IE was divided by the individual contact time with cells to calculate velocity (n = 20).
Tracking of rolling IEs showed that the average velocity of IT4-IEstroph+K@37 °C was 33.98, 32.8, 26.94, 24.83, and 19.82 µm/min at 4, 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 7B). On the other hand, the average velocity of IT4-IEstroph+K@40 °C was 39.19, 35, 35, 36.02, and 31.53 µm/min at 4, 3, 2, 1.5, and 0.9 dyn/cm2, respectively (Fig. 7B).
We also observed that, on performing the flow assays at 22 and 37 °C at lower shear stresses (1.5–0.9 dyn/cm2) some of the rolling IEs stopped rolling and adhere to CD36 (about 60%). Whereas, this was not observed as we performed the assays at 40 °C, where a lower number of IT4-IEs (3–9 IEs/0.2 mm2 < 1%) adhered at all shear stresses.
Discussion
We developed a new approach to study the binding behavior of IEs. This method integrates a flow system, which mimics vascular mechanobiology under physiological and febrile temperatures, and bioinformatics image tracking procedures to accurately characterize the cytoadhesive behavior of IEs. Wall shear stress in vivo varies along the venular tree as vessel diameter and flow rate change. Therefore, we performed experiments under six magnitudes of shear stress. We demonstrated that IEs interacting with CD36 performed rolling adhesion under different shear stresses and at different temperatures. Additionally, the rolling behavior of disc-shaped trophozoite-stage IEs (flipping) differed from that of round-shaped schizont-stage IEs (smooth continuous rolling). Studies by Fedosov and colleagues using multiscale IE modeling are consistent with this finding20,23. However, binding behavior to CD36 is controversial. In agreement with the current study, Herricks and colleagues observed rolling adhesion of isogeneic IEs over a microfluidic slide coated with CD3640. Rolling adhesion was also observed over human dermal microvascular ECs, which express numerous CD36 molecules on their surface (86 ± 14 × 106 molecules/mm2)41. Lansche and colleagues reported that even deformed trophozoite-stage IEs with sickle cell traits perform rolling adhesion over human dermal microvascular ECs42. A chimeric SCID mouse model was used to mimic the human microvasculature in vivo. The authors also observed rolling adhesion performed of patients’ IEs on human skin grafts with CD36 on their surface19. On the other hand, other studies suggested that IEs only bind statically to CD3616,18. CD36 is the main interacting partner of IEs because more than 40 of the 60 PfEMP1 family members contain a CD36-binding domain (CIDRα2–6)35,43,44. This supports the theory that PfEMP1-CD36 interactions are essential for survival of the parasite in its host.
CD36 is a class B scavenger transmembrane glycoprotein found on the microvascular endothelium, macrophages, microglia, adipocytes, and platelets45. This receptor binds to a diverse range of ligands including pathogen-associated molecular patterns and modified self-molecules46. CD36 forms a ‘hairpin-like’ structure with a large ectodomain and two transmembrane domains45. The ectodomain of CD36 is capped by a three-α-helix bundle and an apex region that contains an accumulation of cationic residues, which are proposed to form a binding site for polyanionic ligands. Co-crystallization studies of CIDRα2-6 domains and the CD36 ectodomain identified a small conserved hydrophobic pocket in CIDRα peptides that forms a non-covalent hydrogen bond with a complementary hydrophilic phenylalanine residue (F153) in CD36. This combination generates a high affinity and stable binding site that allows the domains to interact with their ligands with a slow off rate, which stabilizes cytoadhesion of IEs against the strong forces generated by blood flow47. In the current study, cytoadhesive dynamics to CD36 differed according to the conditions. The absence of knobs on the surface of IEs shortened the rolling distance, whereas the presence of knobs led to maintenance of the rolling pattern for longer distances in the case of both trophozoite- and schizont-stage IEs. We also observed that the distance travelled by the IEs at higher shear stresses in both isolates have two peaks. This might be due to the control of the shear stresses to the rolling movement of the IEs. Some PfEMP1 molecules on the surface of IEs might perform a stronger bond with CD36, so that the IEs resist flow while others with weaker bond might not be able to roll longer distance and will be washed with the flow.
The mean rolling velocity of IEs over CD36 was different between different stages. We observed that schizonts roll more stable at different shear stresses than trophozoites. The flipping of trophozoites was quicker at higher shear stresses than the lower ones. In this study, all the recorded velocities at different stages and conditions were less than 1 µm/sec which was not recorded by Antia and colleagues. In their study, they observed higher velocities few to tens µm/sec. This might be due to the fact that they performed the assays over recombinant proteins (CD36 and ICAM-1) which might change the binding behavior16.
Comparing the mean rolling velocities of the knobby IT4-IEs at 37 °C versus 40 °C showed no statistical significance, in both cases they roll with a mean velocity of 30–50 µm/min which is higher than the mean velocity at room temperature (30–20 µm/min). This difference highlights the effect of temperature on binding dynamics between CD36 and PfEMP-1.
Shear stress significantly affected the velocity of IEs without knobs on their surface. It seems that flow influenced IEs lacking knobs. This might be because the density of PfEMP1 is supposed to be lower on the surface of IEs without knobs than on the surface of IEs with knobs48. On the other hand, tethering and receptor attachment of IEs with knobs seems to be controlled independently of shear stress. This was observed from the stable mean of velocity observed during the experiments performed with IEs+k (Figs. 3 and 4). Thus, the presence of knobs benefits parasites by stabilizing the ligand-receptor interaction due to the concentrated amount of PfEMP1 on the knob’s surface. Slip binding occurs between CD36 and PfEMP1, and its life time is decreased by external forces (e.g., shear stress)34, which might explains the rolling behavior on higher shear stresses and static binding on the lower ones. Interestingly, rolling of schizont-stage IEs was more stable and continued for longer distances than that of trophozoite-stage IEs. The surface of schizont-stage IEs is homogenously covered by knobs, which is assumed to slow down rolling movement41. In addition, the adhesion force is smaller at the schizont stage than at the trophozoite stage, despite the high concentration of adhesion proteins in the former stage49.
We made the following findings regarding binding of IEs to ICAM-1, P-selectin, and CD9: (i) binding occurs mostly at lower shear stresses (starting from 2 dyn/cm2), (ii) IEs mostly bind in a conglomerate pattern and resist flow, and (iii) no rolling pattern is observed. This suggests that catch bonds mediated binding. These are defined as non-covalent bonds that have increased lifetimes only when external force is applied27,28. On the other hand, IT4-IEsICAM-1 showed rolling binding behavior on CD36, this IEs population is known to express mainly IT4-var01, this encodes for a protein which have both head structure for CD36 binding and also an ICAM-1 binding domain33. In accordance with the current study, such static binding to ICAM-1 was previously reported19. The low binding capacity at higher shear stress might be due to the lack of CD36 on the surface of CHO-745 cells. We believe that CD36 is the main receptor responsible for tethering of IEs and allows them to roll slowly before being immobilized by other ECRs. In addition, the presence of knobs did not affect the binding capacity or pattern to ICAM-1, P-selectin, and CD9, suggesting that these receptors play a secondary role in sequestration of IEs and only when ECs are stimulated with inflammatory cytokines. Previous studies provided more details about the binding dynamics of ECs to different receptors (including selectins and ICAM-1). When an activation signal is detected, ECRs switch from a low to a high affinity state due to changes in bond formation rates and bond dissociation properties50.
We investigated the thermodynamics of IE-ECR interactions. Most of these interactions occur at body temperature (37 °C) and during fever (up to 40 °C). We used HBEC-5i cells, which express CSA on their surface. Our flow experiments demonstrated the importance of knobs for parasite survival. Only IEs with knobs could withstand the high temperature and bind under shear stress of 0.9 dyn/cm2. In addition, rolling adhesion to CD36 was preserved and the number of rolling IEs was increased at 40 °C and under higher shear stress (starting from 4 dyn/cm2). Normally, fever is a reaction to a pathogen in order to protect the host27,50. Fever induces expression of adhesive receptors (i.e., ICAM-1) on the surface of ECs and leukocyte trafficking27, but parasites are well prepared for these changes. Carvalho and colleagues measured the cytoadhesive force of IEs to CSA via force spectroscopy. They found that the binding force is notably decreased at febrile temperature, but the number of bound IEs increases51. They speculated that this increase in binding despite the decrease in force is due to non-specific binding. Another study demonstrated that, the binding affinities to both CD36 and ICAM-1 were decreased at febrile temperature (41 C°)34. The difference between our experiments and those performed by Lim and colleagues that34, they used recombinant proteins (ICAM-1 and CD36) in their binding studies and we used transfected CHO-cells that express the receptor of interest. We do believe that these two methods lead to different binding phenotypes between the receptor and ligand. On the other hand, it might be also that other proteins on the surface of the cells might help in increasing the binding capacity (for example heat shock proteins), but it will be difficult in the current study to confirm this explanation.
In conclusion, our data completely describe the cytoadhesive dynamics between IEs and ECRs in the presence and absence of knobs. IEs are first captured from the circulation via CD36, and this is followed by tethering and slow rolling. Other ECRs are expressed as the parasite load increases, which enhances immobilization of IEs to the surface of ECs. We also highlighted the importance of knobs for the thermodynamics of IE-ECR interactions. Higher temperatures also affect the binding dynamics between IEs and ECRs: (i) increased binding of knobby IEs to CSA, (ii) increased number of knobby IEs rolling over CD36, (iii) decreased number of immobilized knobby IEs over CD36.
Methods
Parasite culture
IT4 (FCR3S1.2) and 3D7 P. falciparum isolates were cultivated with human O+ erythrocytes (5% hematocrit; UKE, Hamburg, Germany) in the presence of 10% human serum A+ (Interstate Blood Bank, Inc. Memphis, TN, USA) according to standard procedures52. Parasite cultures were synchronized once per week using 5% sorbitol53.
Transfection and culture of CHO-745 cells
CHO-745 cells defective in glycosaminoglycan biosynthesis (American Type Culture Collection (ATCC); no. CRL-2242) were transfected and cultured as previously described8,31,33. CHO-745 cells transfected with GFP, CD36, ICAM-1, P-selectin, and CD9 were used. These five cell lines were routinely sorted for surface expression of the receptors via fluorescence-activated cell sorting.
Selection and enrichment of IEs that bind to ECRs of interest
As previously described33, IEs (10% parasitaemia) were pre-absorbed over CHO-745GFP cells for 60 min at 37 °C in binding medium (RPMI containing 2% glucose, pH 7.2). Thereafter, non-binding IEs were incubated with transfected CHO-745 cells for 60 min at 37 °C. Washing was then performed using binding medium for 7 times and then controlled under inverted microscope for any unbound IEs. Bound IEs were cultivated up to a parasitaemia of 10%. The entire enrichment procedure was repeated weekly for 7 weeks.
Selection and enrichment of IEs that bind to HBEC-5i cells
For enrichment of IEs for binding to HBEC-5i, the same procedure as described above was performed. At least seven rounds of selection were performed at 37 °C and 40 °C36. The HBEC-5i cell line was cultivated according to ATCC guidelines.
Enrichment of knob-containing IEs using gelatin flotation
IEs were resuspended in two volumes of 1% pre-warmed gelatin (175 g Bloom, Sigma-Aldrich, Germany) and incubated for 45 min at 37 °C54. The supernatant, which harbored knob-containing IEs, was washed with RPMI and cultivated as usual. This procedure was repeated each week. The presence of knobs was confirmed by electron microscopy.
qPCR
As a control, expression levels of var genes and kahrp in each parasite population were determined by qPCR. qPCR was performed using sense and antisense primers designed to amplify 100–120 bp fragments of the respective genes (S8 Text). After RNA isolation, cDNA was synthesized using SuperScript II Reverse Transcriptase (Invitrogen, Germany) and random hexamers (Invitrogen) at 50 °C for 1 h. The cDNA template was mixed with SYBR Green PCR Master Mix (QuantiTect SYBR Green PCR Systems, Qiagen, Germany) and 0.5 µM forward and reverse primers in a final volume of 10 µl. Samples were incubated at 95 °C for 15 min, and then subjected to 40 cycles of 95 °C for 15 sec and 60 °C for 1 min, followed by a melting step (60–95 °C). The specificity of each primer pair was confirmed after each qPCR run by performing dissociation curve analysis. var gene expression was normalized against expression of the conserved ring-stage gene Skeleton-binding protein 1 (sbp1) and the housekeeping genes Fructose-bisphosphate aldolase and arginyl-tRNA. Expression of each var gene was calculated relative to the geometric mean expression of these three normalizers55,56.
Electron microscopy
Trophozoite-stage P. falciparum cultures were separated by Percoll density gradient centrifugation and fixed with 2.5% glutaraldehyde and 2.5% paraformaldehyde (Electron Microscopy Science, Hatfield, PA, USA). Samples were washed with 50 mM sodium cacodylate trihydrate buffer, pH 7.4 (Electron Microscopy Science), and post-fixed with 2% OsO4 prepared in H2O for 45–60 min on ice in the dark. After heavy metal staining using 0.5–1% uranyl acetate (Electron Microscopy Science) for 45–60 min at room temperature, samples were dehydrated using an increasing ethanol series prepared in H2O. Embedding and polymerization were performed using increasing concentrations of epoxy resin (EPoN 812; Carl Roth, Karlsruhe, Germany) at 60 °C. Polymerized samples were cut into 55–60 nm sections (Ultracut UC7, Leica, Germany), placed on 300 mesh copper grids, and analyzed with a transmission electron microscope (Tecnai Sprit TEM, FEI, Netherland) at an acceleration voltage of 80 kV. By counting 150 IEs from each sample, the percentage of knobby to knobless IEs was determined.
Binding experiments under flow conditions
Seeding of CHO cells. A total of 1.5 × 105 CHO cells were seeded into a µ-slide I 0.8 Luer ibiTreat in 200 µl Ham’s F12 medium containing G418 as a selection marker 2 days before the assay. Cells were 90–100% confluent on the day of the assay.
Enrichment of IEs containing parasites at the trophozoite or schizont stage by magnetic-activated cell sorting. On the day of the assay, IEs containing trophozoites (28–32 hpi) or schizonts (36–44 hpi) were enriched using various MACS magnetic separation columns as described previously57. Briefly, cultures were centrifuged, resuspended in binding medium such that hematocrit was 10%, and loaded onto the top of the column. The outflow, which contained non-IEs and ring-stage and young trophozoites, was discarded. Finally, trophozoite- or schizont-stage IEs were eluted in binding medium and incubated over CHO-745GFP cells for 60 min at 37 °C33. Finally, unbound cells were washed with binding medium and the cell count was adjusted to 1 × 107 using a Neubauer chamber.
Flow assay set-up: a unidirectional laminar flow system (ibidi pump system) was used. According to the manufacturer’s instructions, IEs obtained in step 2 were resuspended in binding medium and added to the fluidic unit of the flow system. The fluidic unit was then connected to the CHO-745 cell slide and pump system. A range of wall shear stresses similar to those detected in venular microcapillaries26 were applied using software to control the pump. Specifically, 6, 4, 3, 2, and 1.5 dyn/cm2 was applied for 10 min, and 0.9 dyn/cm² was applied for 30 min. Imaging was performed using an EVOS FL Auto inverted microscope (Thermo Fisher Scientific, Waltham, USA). An image was acquired every 5 sec using a control program. The experiment was started by simultaneously switching on the pump system control program and the microscope control program.
Bioinformatics to track IEs and analyze trajectories
The trackdem (0.4.2) R package was used to characterize the behavior of IEs38. In each experiment, the image sequence recorded under each shear stress was loaded in the R‐environment (3.5.1)58. A background image containing all motionless objects was created. Moving particles were detected by subtracting this background from all images. Identification was optimized using machine learning. The Shiny R package was used with thresholding of 0.1. In the PixelRange argument, a range of 30–500 was used to filter any unwanted noise. Particle tracking was then performed to generate tracked segments connecting the moving IEs in each frame. Individual trajectories were subsequently reconstructed based on a minimum present (incThres) equal to six frames (i.e., 30 sec). The reconstructed trajectories were plotted as images and presented in AVI animations. The accumulated distance covered by each IE was calculated by multiplying the total displacement in pixels by 0.4572. The detailed R-script used is provided in S10 Text. To calculate the average velocity of each individual IE, the accumulated distance was divided by the calculated contact time with cells. IEs that traveled less than 50 µm were excluded from the analysis.
Robustness checks
Manual tracking was performed and compared with automated tracking. ImageJ (Version 1.48 V, National Institutes of Health, Maryland, USA) was used to evaluate each experiment. The accumulated distance traveled by individual IEs was tracked using the Manual Tracking and Chemotaxis plugins.
Statistical analysis
Geometric mean was calculated using GraphPad Prism 8. Statistical significance was assessed using the Kolmogorov-Smirnov test in flow experiments and the Kruskal Wallis test in qPCR experiments (GraphPad Prism 8).
Supplementary information
Acknowledgements
This work was supported by the Leibniz Center Infection, Jürgen Manchot Stiftung, Joachim Herz Stiftung and the Deutsche Forschungsgemeinschaft (Br1744/12-1, Bu956/16-1). We would like to thank Marjolein Bruijning for the support with trackdem R script. We also thank Heidrun von Thien and Birgit Förster for technical assistance.The publication of this article was funded by the open Access Fund of the Leibniz Association.
Author contributions
P.L., N.G.M., and I.B. conceived and designed the experiments. P.L., P.B., L.R., T.Re., K.H., J.S.W., and N.G.M. performed the experiments. P.L., P.B., T.T., I.B., and N.G.M. analyzed the data. I.B. and N.G.M. wrote the paper. P.L., M.D., L.R., T.Re., J.B., A.B., T.Ro., T.G., and T.B. reviewed and edited the manuscript. I.B. and N.G.M. supervised the study. All authors approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pedro Lubiana and Philip Bouws.
Contributor Information
Iris Bruchhaus, Email: bruchhaus@bnitm.de.
Nahla Galal Metwally, Email: metwally@bnitm.de.
Supplementary information
is available for this paper at 10.1038/s41598-020-61388-2.
References
- 1.WHO. World malaria report 2019 (2019).
- 2.Phillips MA, et al. Malaria. Nat. Rev. Dis. Prim. 2017;3:17050. doi: 10.1038/nrdp.2017.50. [DOI] [PubMed] [Google Scholar]
- 3.Craig AG, Khairul MF, Patil PR. Cytoadherence and severe malaria. Malays. J. Med. Sci. 2012;19:5–18. [PMC free article] [PubMed] [Google Scholar]
- 4.Kyes S, Horrocks P, Newbold C. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- 5.Newbold C, et al. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int. J. Parasitol. 1999;29:927–937. doi: 10.1016/s0020-7519(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 6.Turner L, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nat. 2013;498:502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert. Rev. Mol. Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esser C, et al. Evidence of promiscuous endothelial binding by Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2014;16:701–708. doi: 10.1111/cmi.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherf A, et al. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rug M, Prescott SW, Fernandez KM, Cooke BM, Cowman AF. The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes. Blood. 2006;108:370–378. doi: 10.1182/blood-2005-11-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruenberg J, Allred DR, Sherman IW. Scanning electron microscope-analysis of the protrusions (knobs) present on the surface of Plasmodium falciparum-infected erythrocytes. J. Cell Biol. 1983;97:795–802. doi: 10.1083/jcb.97.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutts EE, et al. Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions. PLoS Pathog. 2017;13:e1006552. doi: 10.1371/journal.ppat.1006552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann. Biomed. Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 14.Helms G, Dasanna AK, Schwarz US, Lanzer M. Modeling cytoadhesion of Plasmodium falciparum-infected erythrocytes and leukocytes-common principles and distinctive features. FEBS Lett. 2016;590:1955–1971. doi: 10.1002/1873-3468.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunkel EJ, Dunne JL, Ley K. Leukocyte arrest during cytokine-dependent inflammation in vivo. J. Immunol. 2000;164:3301–3308. doi: 10.4049/jimmunol.164.6.3301. [DOI] [PubMed] [Google Scholar]
- 16.Antia M, Herricks T, Rathod PK. Microfluidic modeling of cell-cell interactions in malaria pathogenesis. PLoS Pathog. 2007;3:e99. doi: 10.1371/journal.ppat.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flatt C, Mitchell S, Yipp B, Looareesuwan S, Ho M. Attenuation of cytoadherence of Plasmodium falciparum to microvascular endothelium under flow by hemodilution. Am. J. Trop. Med. Hyg. 2005;72:660–665. doi: 10.4269/ajtmh.2005.72.660. [DOI] [PubMed] [Google Scholar]
- 18.Li A, et al. Molecular mechanistic insights into the endothelial receptor mediated cytoadherence of Plasmodium falciparum-infected erythrocytes. PLoS One. 2011;6:e16929. doi: 10.1371/journal.pone.0016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yipp BG, et al. Differential roles of CD36, ICAM-1, and P-selectin in Plasmodium falciparum cytoadherence in vivo. Microcirculation. 2007;14:593–602. doi: 10.1080/10739680701404705. [DOI] [PubMed] [Google Scholar]
- 20.Fedosov DA, Caswell B, Karniadakis GE. Wall shear stress-based model for adhesive dynamics of red blood cells in malaria. Biophys. J. 2011;100:2084–2093. doi: 10.1016/j.bpj.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito A, et al. Quantitative imaging of human red blood cells infected with Plasmodium falciparum. Biophys. J. 2010;99:953–960. doi: 10.1016/j.bpj.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldecker Mailin, Dasanna Anil K., Lansche Christine, Linke Marco, Srismith Sirikamol, Cyrklaff Marek, Sanchez Cecilia P., Schwarz Ulrich S., Lanzer Michael. Differential time-dependent volumetric and surface area changes and delayed induction of new permeation pathways inP. falciparum-infected hemoglobinopathic erythrocytes. Cellular Microbiology. 2016;19(2):e12650. doi: 10.1111/cmi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedosov DA, Lei H, Caswell B, Suresh S, Karniadakis GE. Multiscale modeling of red blood cell mechanics and blood flow in malaria. PLoS Comput. Biol. 2011;7:e1002270. doi: 10.1371/journal.pcbi.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell GI. Models for the specific adhesion of cells to cells. Sci. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 25.Evans E, Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys. J. 1997;72:1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 27.Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat. Rev. Immunol. 2015;15:335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu C, Lou J, McEver RP. Catch bonds: physical models, structural bases, biological function and rheological relevance. Biorheology. 2005;42:443–462. [PubMed] [Google Scholar]
- 29.Udomsangpetch R, et al. Febrile temperatures induce cytoadherence of ring-stage Plasmodium falciparum-infected erythrocytes. Proc. Natl Acad. Sci. USA. 2002;99:11825–11829. doi: 10.1073/pnas.172398999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinkovic M, et al. Febrile temperature leads to significant stiffening of Plasmodium falciparum parasitized erythrocytes. Am. J. Physiol. Cell Physiol. 2009;296:C59–64. doi: 10.1152/ajpcell.00105.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilly AK, et al. Type of in vitro cultivation influences cytoadhesion, knob structure, protein localization and transcriptome profile of Plasmodium falciparum. Sci. Rep. 2015;5:16766. doi: 10.1038/srep16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Sci. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 33.Metwally NG, et al. Characterisation of Plasmodium falciparum populations selected on the human endothelial receptors P-selectin, E-selectin, CD9 and CD151. Sci. Rep. 2017;7:4069. doi: 10.1038/s41598-017-04241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim YB, Thingna J, Cao J, Lim CT. Single molecule and multiple bond characterization of catch bond associated cytoadhesion in malaria. Sci. Rep. 2017;7:4208. doi: 10.1038/s41598-017-04352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rask Thomas S., Hansen Daniel A., Theander Thor G., Gorm Pedersen Anders, Lavstsen Thomas. Plasmodium falciparum Erythrocyte Membrane Protein 1 Diversity in Seven Genomes – Divide and Conquer. PLoS Computational Biology. 2010;6(9):e1000933. doi: 10.1371/journal.pcbi.1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dörpinghaus Michael, Fürstenwerth Finn, Roth Lisa K., Bouws Philip, Rakotonirinalalao Maximilian, Jordan Vincent, Sauer Michaela, Rehn Torben, Pansegrau Eva, Höhn Katharina, Mesén-Ramírez Paolo, Bachmann Anna, Lorenzen Stephan, Roeder Thomas, Metwally Nahla Galal, Bruchhaus Iris. Stringent Selection of Knobby Plasmodium falciparum-Infected Erythrocytes during Cytoadhesion at Febrile Temperature. Microorganisms. 2020;8(2):174. doi: 10.3390/microorganisms8020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paszkowiak JJ, Dardik A. Arterial wall shear stress: observations from the bench to the bedside. Vasc. Endovasc. Surg. 2003;37:47–57. doi: 10.1177/153857440303700107. [DOI] [PubMed] [Google Scholar]
- 38.Bruijning M, Visser M, Hallmann M, Jongejans E. trackdem: Automated particle tracking to obtain populationcounts and size distributions from videos in R. Methods. Ecol. Evol. 2018;9:965–973. [Google Scholar]
- 39.Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect. Immun. 2006;74:645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herricks T, Avril M, Janes J, Smith JD, Rathod PK. Clonal variants of Plasmodium falciparum exhibit a narrow range of rolling velocities to host receptor CD36 under dynamic flow conditions. Eukaryot. Cell. 2013;12:1490–1498. doi: 10.1128/EC.00148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dasanna AK, Lansche C, Lanzer M, Schwarz US. Rolling Adhesion of Schizont Stage Malaria-Infected Red Blood Cells in Shear Flow. Biophys. J. 2017;112:1908–1919. doi: 10.1016/j.bpj.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lansche C, et al. The sickle cell trait affects contact dynamics and endothelial cell activation in Plasmodium falciparum-infected erythrocytes. Commun. Biol. 2018;1:211. doi: 10.1038/s42003-018-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith JD, Subramanian G, Gamain B, Baruch DI, Miller LH. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 2000;110:293–310. doi: 10.1016/S0166-6851(00)00279-6. [DOI] [PubMed] [Google Scholar]
- 44.Robinson BA, Welch TL, Smith JD. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 2003;47:1265–1278. doi: 10.1046/j.1365-2958.2003.03378.x. [DOI] [PubMed] [Google Scholar]
- 45.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 46.Cabrera A, et al. Plasmodium falciparum-CD36 Structure-Function Relationships Defined by Ortholog Scanning Mutagenesis. J. Infect. Dis. 2019;219:945–954. doi: 10.1093/infdis/jiy607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh FL, et al. The structural basis for CD36 binding by the malaria parasite. Nat. Commun. 2016;7:12837. doi: 10.1038/ncomms12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horrocks P, et al. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J. Cell Sci. 2005;118:2507–2518. doi: 10.1242/jcs.02381. [DOI] [PubMed] [Google Scholar]
- 49.Xu X, et al. Probing the cytoadherence of malaria infected red blood cells under flow. PLoS One. 2013;8:e64763. doi: 10.1371/journal.pone.0064763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang J, Hunt CA. Identifying the rules of engagement enabling leukocyte rolling, activation, and adhesion. PLoS Comput. Biol. 2010;6:e1000681. doi: 10.1371/journal.pcbi.1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carvalho PA, Diez-Silva M, Chen H, Dao M, Suresh S. Cytoadherence of erythrocytes invaded by Plasmodium falciparum: quantitative contact-probing of a human malaria receptor. Acta Biomater. 2013;9:6349–6359. doi: 10.1016/j.actbio.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trager W, Jensen JB. Human malaria parasites in continuous culture. 1976. J. Parasitol. 2005;91:484–486. doi: 10.1645/0022-3395(2005)091[0484:HMPICC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 53.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 54.Waterkeyn JG, Cowman AF, Cooke BM. Plasmodium falciparum: gelatin enrichment selects for parasites with full-length chromosome 2. implications for cytoadhesion assays. Exp. Parasitol. 2001;97:115–118. doi: 10.1006/expr.2000.4593. [DOI] [PubMed] [Google Scholar]
- 55.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 57.Ribaut C, et al. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 2008;7:45. doi: 10.1186/1475-2875-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.