Abstract
MAPK phosphatases (MKP) downregulate the activity of mitogen-activated protein kinases (MAPK), such as ERK1/2, and modulate the processes regulated by these kinases. ERK1/2 participate in a wide range of processes including tissue-specific hormone-stimulated steroidogenesis. H295R cells are a suitable model for the study of human adrenal cortex functions, particularly steroid synthesis, and respond to angiotensin II (Ang II) triggering ERK1/2 phosphorylation in a transient fashion. MKP-3 dephosphorylates ERK1/2 and, as recently reported, forkhead box protein 1 (FOXO1). Here, we analyzed MKP-3 expression in H295R cells and its putative regulation by Ang II. Results showed the expression of MKP-3 full length (L) and a short splice variant (S), and the upregulation of both isoforms by Ang II. L and S messenger and protein levels increased 30 min after Ang II stimulation and declined over the next 3 h, a temporal frame compatible with ERK1/2 dephosphorylation. In addition, FOXO1 activation is known to include its dephosphorylation and nuclear translocation. Therefore, we analyzed the effect of Ang II on FOXO1 modulation. Ang II induced FOXO1 transient phosphorylation and translocation and also the induction of p21, a FOXO1-dependent gene, whereas MKP-3 knock-down reduced both FOXO1 translocation and p21 induction. These data suggest that, through MKP-3, Ang II counteracts its own effects on ERK1/2 activity and also triggers the activation of FOXO-1 and the induction of cell cycle inhibitor p21. Taken together, the current findings reveal the participation of MKP-3 not only in turn–off but also in turn-on signals which control important cellular processes.
Keywords: Cell biology, Proteins, Biochemistry, Molecular biology, Biomolecules, MKP-3, DUSP6, FOXO1, p21, H295R, ERK1/2
Cell biology; Proteins; Biochemistry; Molecular biology; Biomolecules; MKP-3, DUSP6, FOXO1, p21, H295R, ERK1/2
1. Introduction
Multiple biological processes are regulated by the action of mitogen-activated protein kinases (MAPK), such as ERK1/2, JNK1/2 and p38 proteins. Indeed, MAPK signaling regulates functions such as cellular proliferation, differentiation and hypertrophia, among others, and even specific functions of specialized tissues, such as steroid production. MAPK are activated by phosphorylation on two residues, Thr and Tyr; therefore, their actions are counteracted by specific phosphatases [1, 2].
The MAP kinase phosphatase (MKP) family comprises enzymes which can be classified on the basis of sequence homology, subcellular localization and substrate specificity [3, 4]. Three well-characterized members of the MKP family are MKP-1, MKP-2 and MKP-3. While MKP-1 and MKP-2 (DUSP1 and DUSP4, respectively) are nuclear enzymes induced by different stimuli, MKP-3 or DUSP6 is an ERK-selective cytoplasmic phosphatase induced mainly by proliferative stimuli [3, 4, 5, 6]. MKP are highly regulated by transcriptional mechanisms and by posttranslational modifications, mainly protein phosphorylation [7, 8, 9]. In addition, splice variants of MKP-2 and MKP-3 have been described in human tissues [10, 11]. Regarding MKP-3, these splice variants included the full-length or long transcript (L) and the alternative or short transcript (S), the latter generated by the loss of exon 2 [11].
MKP are highly specific to MAPK family members. However, the action of MKP-3 on forkhead box protein 1 (FOXO1) has also and more recently been described. FOXO1 transcription factor regulates diverse cellular processes such as autophagy, proliferation, apoptosis and metabolism through the induction of specific genes [12, 13, 14]. Particularly, FOXO1 regulates cell proliferation in part through the expression of cell cycle inhibitor p21 [15]. In turn, FOXO1 is regulated by several posttranslational modifications which activate or inhibit its transcriptional activity. Among other modifications, protein phosphorylation is a key regulatory mechanism of FOXO1 subcellular localization and DNA binding affinity [13, 14, 15, 16]. While FOXO1 phosphorylation at specific sites promotes its translocation to the cytoplasm, its dephosphorylation leads to accumulation into the nucleus, where it triggers gene expression [16]. It has been reported that MKP-3 interacts with and dephosphorylates FOXO1 [17–19], leading to the induction of genes encoding gluconeogenic enzymes [18]. Previous work by our group focused on the action of MKP-3 on p21 expression, since both proteins are linked to cell proliferation. We demonstrated that, in steroid-producing MA-10 Leydig cells, the stimulation of the corresponding trophic hormone receptor upregulates the expression of MKP-3 and the expression of p21 by an MKP-3-dependent mechanism [9]. Also, we have demonstrated that proteasome inhibitor Bortezomib induces p21, probably through a mechanism involving MKP-3-mediated FOXO1 dephosphorylation in endothelial cells transformed by the viral G protein-coupled receptor associated to Kaposi's sarcoma [20].
In steroidogenic tissues, including adrenal cortex and testis, the corresponding trophic hormones trigger MAPK activation [21, 22, 23, 24] and upregulate the expression of enzymes involved in steroid synthesis pathways [25, 26, 27]. In agreement, the regulation of MKP by steroidogenic hormones has also been reported, with evidence including the rapid induction of MKP-1 by ACTH in human [28] and mouse adrenocortical cell lines [29], as well as MKP-1 induction in Leydig cells and its modulatory effect on key enzymes of the steroidogenic pathway [30, 31]. Also, as already mentioned, we have demonstrated the upregulation of MKP-3 by cAMP in Leydig cells [9]. However, the hormonal regulation of MKP-3 in adrenocortical cells has not been described yet.
Angiotensin II (Ang II) stimulates aldosterone production and hyperplasia of zona glomerulosa cells via the AT1 receptor [32]. The human adrenocortical carcinoma-derived cell line H295R is a suitable model widely used for the study of human adrenal cortex functions and responds to Ang II increasing aldosterone production [33]. Ang II signal transduction in this human experimental model has been widely described, with results showing transient ERK1/2 phosphorylation [34]. Nevertheless, MKP-3 and FOXO1 have not been studied as components of this pathway. Therefore, we aimed to analyze the expression of MKP-3 L and S variants and the putative regulatory effect of Ang II in H295R cells and to expand the study of FOXO1 regulation by MKP-3. Our findings could contribute to the characterization of Ang II signaling in a human cell line widely used to study adrenocortical cellular functions.
2. Materials and methods
2.1. Cell culture
The NCI–H295R cell line is a clonal strain of human adrenal carcinoma [33]. The cells used in this study were from American Type Culture Collection (ATCC, Manassas, VA, USA) and were handled as originally described [33]. The growth medium consisted of DMEM/Ham's F-12 1:1 containing 1.1 g/L NaHCO3, 20 mM HEPES, 200 IU/ml penicillin, 200 μg/ml streptomycin sulfate, and 5% COSMIC serum. Flasks and multiwell plates were maintained at 37°C in a humidified atmosphere containing 5% CO2. After 24 h of serum starvation, cells were incubated with 10−7 M Ang II for different times.
2.2. RNA extraction and real-time PCR
Total RNA was extracted using Tri Reagent following the manufacturer's instructions (Molecular Research Center Inc., Cincinnati, OH, USA).
For semiquantitative PCR assay the reaction conditions were one 5-min cycle at 95 °C followed by 27 cycles consisting of a 30 s step at 95 °C, 20 s at 60 °C and 60 s at 72 °C, using the following specific primers Forward: 5′-GCTATACGAGTCGTCGCACA-3´; Reverse: 5′-TAGGCATCGTTCATCGACAG-3´. PCR products were resolved on a 1.5% (wt/vol) agarose gel containing 0.5 μg/ml of ethidium bromide to determine the molecular sizes of the amplicons: 830 bp for MKP-3L and 403 bp for MKP-3S. The identity of the amplicons was confirmed by DNA sequencing (Macrogen, Seul, South Korea). For quantitative real time PCR assay, isolated RNA was deoxyribonuclease-treated using RNAse-free DNase RQ1 (Promega, Madison, USA). Reverse transcription was carried out on 2 μg total RNA using M-MLV Reverse Transcriptase following the manufacturer's instructions (Promega, Madison, USA). Reactions were carried out using the SYBR Green Master Mix reagent kit (Applied Biosystems, Carlsbad, CA, USA) and the following specific primers: for MKP-3L, forward 5′-CAAGCAAATCCCCATCTCG-3′ and reverse 5′-CCAGCCAAGCAATGTAACA-3′, for MKP-3S, forward 5′-TCTACGACGAGAGCAGCAG3′ and reverse 5′-GGCTTCATCTTCCAGGTA-3′, for p21 forward, 5′-GACTCTCAGGGTCGAAAACG-3′ and reverse 5′-ATGCC CAGCACTCTTAGGAA-3′, for 18S forward, 5′-TCAAGAACGAAAGTCGGAGG-3′ and reverse 5′-GGACATCTAAGGGCATCACA-3´. The reaction conditions were: one 5-min cycle at 95 °C, followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. Gene mRNA expression levels were normalized to human 18S RNA expression, performed in parallel as endogenous control. Real-time PCR data were analyzed by calculating the 2−ΔΔCt value (comparative Ct method) for each experimental sample.
2.3. Western blot analysis
Proteins were subjected to SDS–PAGE (10%) and electrotransferred onto polyvinylidine fluoride membranes as previously described [29]. The following specific antibody dilutions were used: mouse monoclonal anti-GAPDH, (1:1500) (Santa Cruz Biotechnology Cat#sc-47724, RRID:AB_627678), mouse monoclonal anti-MKP-3 (1:1500) (Santa Cruz Biotechnology, Cat# sc-377070, RRID:AB_2802089), rabbit polyclonal anti-P-ERK1/2 (1:5000) (Cell Signaling Technology Cat# 9101,RRID:AB_331646), rabbit polyclonal anti total ERK1/2 (1:5000) (Cell Signaling Technology Cat# 9102, RRID:AB_330744), rabbit polyclonal anti-FOXO1 (1:3000) (Cell Signaling Technology Cat# 9461, RRID:AB_329831) or mouse monoclonal anti-FLAG (1:10000) (Sigma-Aldrich Cat# F3165, RRID:AB_259529). In order to detect bound antibodies we used goat anti-rabbit or goat anti-mouse horseradish peroxidase-conjugated as secondary antibodies (Bio-Rad Cat# 170-6515, RRID: AB_11125142 and Cat# 170-6516,RRID:AB_11125547) and the enhanced chemiluminescence detection reagent Bio-Lumina (Kalium Technologies SRL, Buenos Aires, Argentina).
2.4. Plasmid constructs
A vector expressing FOXO1 fused with mVenus yellow fluorescent protein was constructed (pVENUS-FOXO1) to evaluate FOXO1 subcellular localization. FOXO1 full length sequence was obtained by digestion of pSG5L-HA-FOXO1 (Addgene, MA, USA) and cloned in the plasmid mVenus C1 (Addgene, MA, USA) utilizing the same restriction sites. A short hairpin small interfering RNA (shRNA) was constructed in order to knockdown human MKP-3 expression. The shRNA plasmid vector was constructed as follows: a pair of 66-nucleotide (nt)-annealed DNA oligonucleotides was inserted between the BglII and HindIII restriction sites of the pSUPER.retro vector (OligoEngine, Seattle, USA) to express shRNA under the control of the polymerase-III H1-RNA promoter following the manufacturer's instructions. Specific sequence for human MKP-3 is detailed: 5′-CTTATGCAGAAGCTCAATT-3`. An empty pSUPER.retro vector or a pSUPER.retro vector containing a non specific shRNA were used as control for knockdown experiments. The following sequence was used to produce pSUPER.retro non specific shRNA: 5′-TCATTCATTCGCTATCCGC-3`.
FLAG-tagged human MKP-3 construct (pFLAG-MKP-3) was generated using the p3xFLAG-CMV™-10 expression vector (Sigma-Aldrich, St. Louis, MO, USA). A full commercially available plasmid containing the human MKP-3 sequence in the pCMV-SPORT6 vector was acquired from GE Healthcare (Chicago, IL, USA). Using this plasmid as template, a fragment of 1303 kb corresponding to the full-length coding region of MKP-3 (NM_001943.3) was amplified using the following primers: forward, 5′-CTCAAGCTTATGATAGATACGCTCAGAC-3′ and reverse, 5′-TACGGATCCTTGGTAACCTTGTCTAGTA-3′, containing cleavage sites for HindIII and BamHI, respectively. This fragment was purified and fused into p3xFLAG-CMV™-10 using HindII/BamHI restriction sites.
2.5. Transfection assay
Cells were seeded the day before transfection, grown up to 80% confluence and transfected with the corresponding plasmids for 6 h using Lipofectamine 2000 reagent in Opti-MEM according to the manufacturer's instructions (Invitrogen, Life Technologies, Grand Island, NY, USA).
2.6. Fluorescence microscopy
In order to study FOXO1 subcellular localization, cells were transiently transfected with pVENUS-FOXO1 plasmid. To study MKP-3 effect on FOXO1 localization, cells were co-transfected with pVENUS-FOXO1 plasmid with MKP-3 shRNA, pSUPER.retro empty vector (Mock) or pSUPER.retro non specific shRNA (Control). Then cells were grown on poly-L-lysine-coated glass coverslips. The following day, cells were serum-starved for 24 h and then incubated with 10−7 M Ang II for the indicated times. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The glass coverslips were mounted in Dako fluorescence mounting medium (Santa Clara, CA, USA). FOXO1 localization was visualized by confocal laser scanning fluorescence microscopy. Images were captured on an Olympus FV1000 microscope and random microscopy fields were used for quantitative analysis Three independent experiments were carried out and triplicates were performed for each treatment. Subcellular localization of fluorescent proteins was measured as the following nucleus to cytoplasm fluorescence ratio: (Nuclear Mean Fluorescence Intensity -Background)/(Cytoplasmic Mean Fluorescence Intensity - Background).
2.7. Statistical analysis
Results are shown as the mean ± SEM. Statistical significance was evaluated using ANOVA followed by the corresponding post-test, as indicated in figure legends. Differences were deemed significant when P < 0.05.
3. Results
3.1. MKP-3L and S transcripts are expressed and induced by Ang II in H295R cells
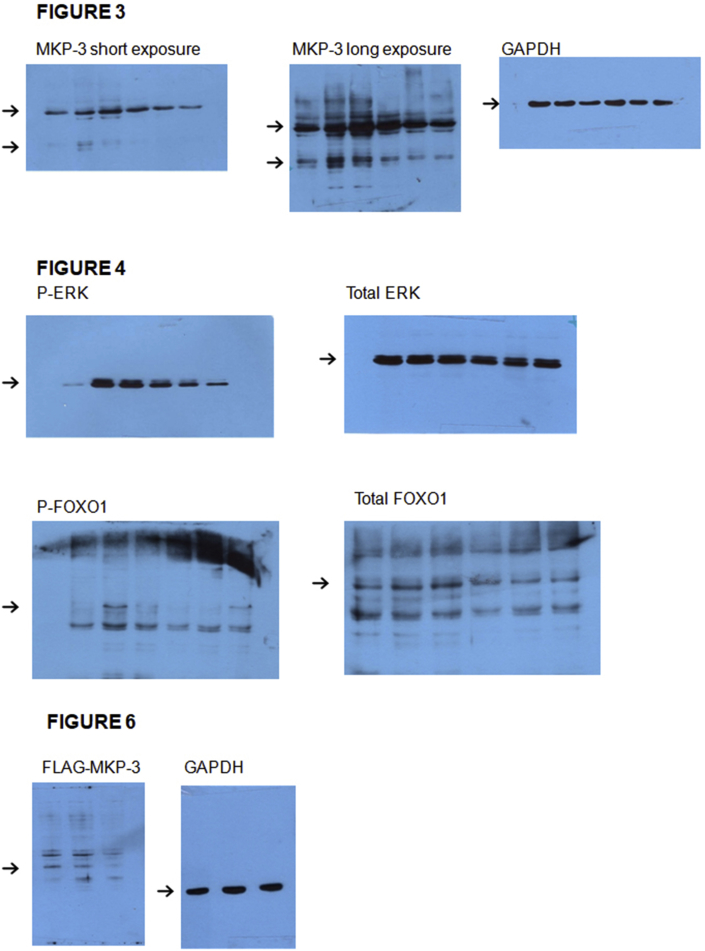
As we aimed to explore the expression of the S and L transcripts of MKP-3 in a human adrenocortical cell model, we used the H295R cell line. By means of a set of primers designed for the simultaneous detection of both transcripts (Figure 1, upper panel), two signals were detected by RT-PCR (Figure 1, lower panel) which had the expected size for MKP-3S and L and whose identity was subsequently confirmed by sequence analysis. Next, experiments were conducted in order to analyze the effect of Ang II on MKP-3L and S mRNA levels. Cells were serum-starved for 24 h, stimulated with Ang II for different times (0–3 h) and total RNA was processed for the evaluation of both transcripts. MKP-3L and S mRNA levels were upregulated by Ang II, showing a significant increase after 0.5 h stimulation and a decline after 1 h (Figure 2). We also analyzed the effect of Ang II on MKP-3 protein levels by Western blot. Ang II increased MKP-3L protein levels starting at 15 min and reaching maximal levels (~4-fold) after 30 min of stimulation (Figure 3). Ang II also had an effect on MKP-3S protein levels, although this variant was barely detected as compared to MKP-3L (Figure 3).
Figure 1.
Expression of MKP-3 splicing variants in adrenocarcinoma H295R cells. MKP-3 mRNA organization. Black lines represent introns. Boxes represent exons (white box = UTR, gray box = translated region). MKP-3 gene renders two splicing variants: MKP-3 large (L) and short (S). Arrows represent the localization of specific primers (upper panel). RNA from H295R cells was purified, cDNA obtained, and PCR performed using a set of primers for MKP-3 large and short isoforms (L and S). PCR products were resolved in ethidium bromide-stained agarose gels. Figure shows a representative gel with bands corresponding to both MKP-3 variants (lower panel).
Figure 2.
Effect of Ang II on MKP-3 mRNA levels in H295R cells. Cells were serum-starved for 24 h and then incubated with 10−7 M Ang II for the indicated times. Total RNA was isolated, subjected to reverse transcription and real-time PCR using specific primers for MKP-3 L (A) or S (B) and 18S as endogenous control. Data are expressed in arbitrary units and represent the mean ± SEM of three independent experiments. ∗p < 0.05 and ∗∗p < 0.01 vs. 0 h, by ANOVA followed by Dunnet's test.
Figure 3.
Effect of Ang II on MKP-3 protein levels in H295R cells. Cells were serum-starved for 24 h and then incubated with 10−7 M Ang II for the indicated times. Cells were lysed and protein levels analyzed by Western blot using antibodies against MKP-3 (1:2000) or GAPDH (1:500) as loading control. Specific bands were detected by chemiluminescence. The intensity of specific bands was quantified and normalized against GAPDH signal. The figure shows a representative Western blot (upper panel) and quantification in arbitrary units of three independent experiments, expressed as mean ± SEM (lower panel). ∗p < 0.05 and ∗∗p < 0.01 vs. 0 h, by ANOVA followed by Dunnet's test. The full, non-adjusted blot image is included in supplementary material (Figure Supplementary Material).
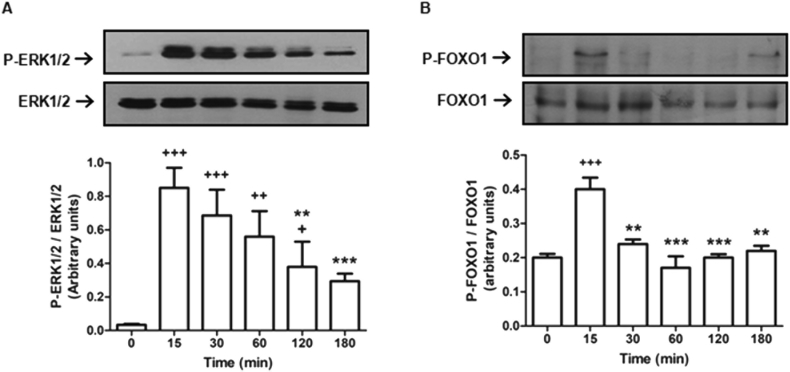
3.2. Ang II promotes transient ERK1/2 and FOXO1 phosphorylation
This set of experiments aimed to analyze the temporal profile of P-ERK1/2 and P-FOXO1 levels in Ang II-stimulated cells and the potential role of MKP-3 in the dephosphorylation of both proteins. As previously reported, Ang II increased P-ERK1/2 levels. In our experimental conditions, P-ERK1/2 peaked already after 15 min stimulation (~25-fold), decreasing thereafter (Figure 4A). Also, P-FOXO1 levels were sharply increased at 15 min of Ang II stimulation and decreased at 30 min (Figure 4B).
Figure 4.
Effect of Ang II on ERK1/2 and FOXO1 phosphorylation levels in H295R cells. Cells were serum starved for 24 h and then incubated with 10−7 M Ang II for the indicated times. Cells were lysed and protein levels analyzed by Western blot using antibodies against P-ERK (1:5000) and ERK (1:5000) (A) or P-FOXO1 (1:3000) and FOXO1 (1:3000) (B). Specific bands were detected by chemiluminescence. The intensity of specific bands was quantified and normalized against loading control. The figure shows a representative Western blot (upper panel) and quantification in arbitrary units of three independent experiments, expressed as mean ± SEM (lower panel).∗∗p < 0.01 and ∗∗∗p < 0.001 vs. 15 min; + p < 0.05, ++ p < 0.01 and +++ p < 0.001 vs 0 min by ANOVA followed by Tukey's test. The full, non-adjusted blot image is included in supplementary material (Figure Supplementary Material).
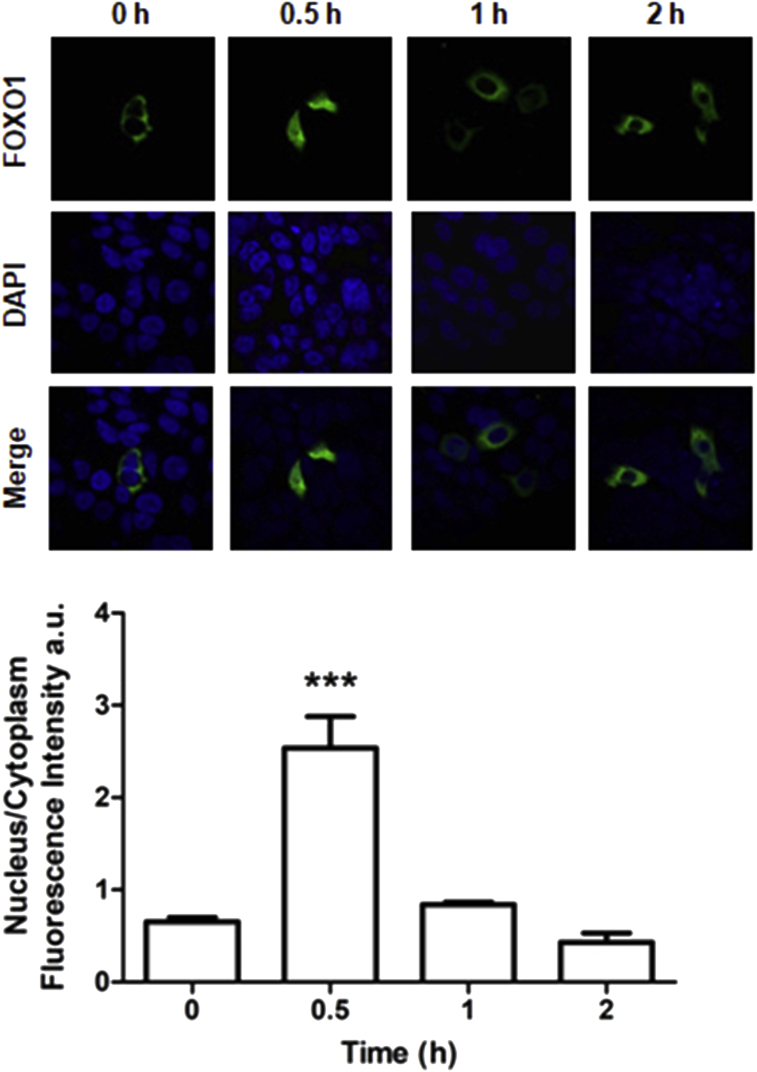
Given that dephosphorylation is required for FOXO1 translocation to the nucleus, where it promotes the transcription of specific genes [16], we next evaluated whether the expression of MKP-3 regulates the subcellular localization of this transcription factor. First, the effect of Ang II on the localization of FOXO1 was evaluated by fluorescence microscopy in pVENUS-FOXO1-transfected H295R cells at different times. After 0.5 h of Ang II stimulation, a significant increase was observed in nuclear signal as compared to the cytoplasm (Figure 5). These results demonstrate that Ang II promoted FOXO1 nuclear localization, probably mediated by MKP-3.
Figure 5.
Effect of Ang II on FOXO1 cellular localization in H295R cells. Cells were transfected with pVENUS-FOXO1 and, 24 h later, plated on coverslips. The following day, cells were serum-starved for 24 h and then stimulated with 10−7 M Ang II for the indicated times. Cells were fixed, stained with DAPI and subjected to fluorescence microscopy. FOXO1 localization was visualized by confocal laser scanning fluorescence microscopy. Images were captured on an Olympus FV1000 microscope (upper panel). Fluorescence intensity rate between cytoplasm and nuclear expression of mVenus-FOXO1. Intensities were measured in microscopy images, and expressed as calculated as (mean cytoplasm intensity - mean background intensity)/(mean nuclear intensity - mean background intensity). Data are expressed in arbitrary units and represent the mean ± SEM of three independent experiments (lower panel). ∗∗∗p < 0.001 vs. 0 h, by ANOVA followed Dunnet's test.
Next, a shRNA designed to knock down the expression of human MKP-3 L and S variants (MKP-3 shRNA) was used to determine whether MKP-3 indeed participates in Ang II-induced FOXO1 localization. Cells were co-transfected with pVENUS-FOXO1 and MKP-3 shRNA or pSUPER.retro empty vector (mock) or a non-specific shRNA (control) and, after 48 h, serum-starved and incubated with or without 10−7 M Ang II for 0.5 h. Results show that MKP-3 knockdown significantly reduced the nuclear signal of FOXO1 after 0.5 h of Ang II stimulation (Figure 6A and B). Worth pointing out, shRNA efficiency was previously assessed by co-transfecting cells with pFLAG-MKP-3 (for MKP-3 protein expression) and MKP-3 shRNA or pSUPER.retro empty vector (mock) or a non-specific shRNA (control). FLAG-MKP-3 expression was efficiently downregulated only by the shRNA (Figure 6C), as shown by Western blot analysis with anti-FLAG antibody. Finally, the specific shRNA also succeeded in downregulating MKP-3 mRNA levels (Figure 6D).
Figure 6.
Effect of MKP-3 on FOXO1 cellular localization in H295R cells. Cells were co-transfected with pVENUS-FOXO1 and pSUPER.retro-MKP-3 to express the shRNA against MKP-3 (MKP-3 shRNA) or the empty vector (mock) or the non-specific shRNA (control). Cells were plated on coverslips 24 h later. The following day, cells were serum-starved for 24 h and then stimulated with 10−7 M Ang II for 0.5 h. Cells were fixed, stained with DAPI and subjected to fluorescence microscopy. FOXO1 localization was visualized by confocal laser scanning fluorescence microscopy. Images were captured in an Olympus FV1000 microscope (A). Fluorescence intensity ratio of cytoplasm and nuclear expression of mVenus-FOXO1 was measured in microscopy images and expressed as (mean cytoplasm intensity - mean background intensity)/(mean nuclear intensity - mean background intensity). ∗p < 0.05 vs 0 h; + p < 0.05 vs mock 0.5 h and control 0.5 h by ANOVA followed by Tukey's test (B). Cells were co-transfected with pFLAG-MKP-3 and pSUPER.retro-MKP-3 or the empty vector (mock) or the non-specific shRNA (control). Cells were lysed and protein levels analyzed by Western blot using antibodies against FLAG tag (1:10000) or GAPDH (1:500) as loading control. Specific bands were detected by chemiluminescence. The figure shows a representative Western blot of two independent experiments (The full, non-adjusted blot image is included in supplementary material) (C). Cells were transfected with pSUPER.retro-MKP-3 or the empty vector (mock) or the non-specific shRNA (Control). Total RNA was isolated, subjected to reverse transcription and real-time PCR using specific primers for MKP-3 and 18S as endogenous control. Data are expressed in arbitrary units and represent the mean ± SEM of two independent experiments (D).∗p < 0.05 vs Mock and Control, by ANOVA followed by Tukey's test.
3.3. Ang II upregulates p21 mRNA levels
Since p21 transcription is dependent on FOXO1 activation [15], studies were conducted on the effect of Ang II on p21 mRNA. Ang II increased p21 mRNA levels, which peaked at 0.5 h and declined thereafter (Figure 7A). Then, cells were transiently transfected with MKP-3 shRNA or the corresponding empty vector or a non-specific shRNA (control) and then stimulated with Ang II (0–30 min). Knocking down the expression of MKP-3 significantly reduced the Ang II-induced increment in p21 mRNA levels (Figure 7B).
Figure 7.
Effect of Ang II and MKP-3 on p21 mRNA levels in H295R cells. Cells were serum-starved for 24 h, then incubated with 10−7 M Ang II for the indicated times (A). Cells were transfected with the vector pSUPER.retro-MKP-3 to express the shRNA against MKP-3 (MKP-3 shRNA) or the empty vector (mock) or the non-specific shRNA (control), serum starved and incubated with or without 10−7 M Ang II for the indicated times (B). Total RNA was isolated and subjected to reverse transcription and real-time PCR using specific primers for p21 and 18S as endogenous control. Data are expressed in arbitrary units and represent the mean ± SEM of three independent experiments. ∗∗p < 0.01 vs. 0 h (A), ∗∗p < 0.01 vs. 0 h Mock, ∗∗∗p < 0.001 vs. 0 h Control, ++ p < 0.01 vs. 0.5 h Control and #p < 0.05 vs. 0.5 h Mock (B) by ANOVA followed by Tukey's test.
4. Discussion
This work demonstrates the expression of ERK-specific dual phosphatase MKP-3, its regulation by Ang II and its connection with FOXO1 activation in the human adrenocortical carcinoma cell line H295R. To our understanding, these novel data have true potential in the knowledge of the biology of H295R, a cell line widely used to study adrenocortical cell physiology and metabolism. Even when the regulation of FOXO1 by MKP-3 has been previously reported [17, 18, 19], our data expand this regulation to a human cell model.
It has been demonstrated that ERK1/2 participates in MKP-3 gene induction [35] and mRNA stability [36]. Ang II is known to promote ERK activation in H295R cells [34] and, in our experimental conditions, it also induced a ~20-fold increase in P-ERK levels after 15 min of stimulation, the shortest time tested. Therefore, Ang II-mediated ERK activation could be involved in the upregulation of MKP-3 mRNA levels detected in the current study after 30 min of Ang II stimulation. In MA-10 Leydig cells, luteinizing hormone (LH) receptor activation and 8Br-cAMP promote an increase in MKP-3 mRNA levels by a transcriptional action after 2 h stimulation [9]. In contrast, the kinetic profile of MKP-3 mRNA levels in H295R cells over 3 h of Ang II stimulation only showed a raise in messenger levels at 30 min, an early effect which could respond to ERK-mediated messenger stabilization rather than de novo gene transcription.
Two scarcely analyzed aspects of MKP are the expression of alternative transcripts of MKP-2 and MKP-3 in human tissues and their biological implication. A variant of MKP-2 with a divergent N-terminal, product of the alternative splicing of the gene, has been described in some cellular types of human origin such as prostate and breast cancer cells [10]. Both transcripts of MKP-3 have been detected in brain, liver, kidney and pancreas and in tumoral cells [11]. In addition, our work shows both MKP-3L and S transcripts in H295R cells and their increase upon Ang II stimulation.
Ang II also increased MKP-3L and S protein levels after 15 min of stimulation. This rapid increase in protein levels could be explained by posttranslational protein stabilization, as previously reported in MA-10 Leydig cells exposed to hCG or 8Br-cAMP [9]. Furukawa et al. have studied the ratio between MKP-3L and S transcripts in twenty pancreatic cancer cell lines, showing reduced expression of the full-length transcript, or even both transcripts, in several of the cell lines investigated. In turn, normal kidney and liver expressed comparable L and S transcript levels, while normal pancreatic tissue revealed a much higher expression of the full-length transcript as compared to the alternative one [11]. MKP-3 protein levels observed here for H295R cells are consistent with those obtained in pancreatic messenger levels. Indeed, Western blot analysis showed a prominent signal corresponding to MKP-3L and a weak signal corresponding to MKP-3S, only detected after long exposure.
Since the S variant lacks sites related to the regulation and localization of the enzyme, it is likely that L and S proteins are differently regulated, supporting a putative regulatory and functional difference between L and S isoforms. This highlights a role for the alternative splicing of MKP-3 gene in MAPK signaling and associated events. Nevertheless, whether normal and tumoral adrenocortical tissues exhibit comparable MKP-3 L/S ratios has not been determined yet.
It has been reported that MKP-3 dephosphorylates the non-MAPK substrate transcription factor FOXO1, an effect which leads to the activation of FOXO1-dependent genes encoding for gluconeogenic enzymes [18]. In this sense, we have previously analyzed FOXO1-dependent genes as potential targets of MKP-3 in Leydig cells and demonstrated that LH receptor stimulation triggers the expression of cell cycle regulator p21 in an MKP-3-dependent manner [9]. The present work further reports an early increase in P-FOXO1 levels and a decline at 30 min accompanied by nuclear translocation upon Ang II stimulation. In addition, we show an increase in p21 messenger levels already after 30 min of Ang II stimulation, an effect partially counteracted by the expression of a shRNA against MKP-3 even upon low transfection efficiency. This increase in p21 mRNA levels suggests an antiproliferative effect of Ang II on H295R cells, which is in accordance with results showing a hypertrophic but non-proliferative action of Ang II in cultured rat glomerulosa cells [37]. However, while the in vivo proliferative effect of Ang II is well recognized, its effect on cultured cells remains controversial [37].
FOXO1 transcriptional activity, subcellular localization and DNA binding are regulated by posttranslational modifications [38]. In this context, our current results show that Ang II increased FOXO1 nuclear signal with respect to the cytoplasm, an effect partly mediated by MKP-3. Nevertheless, given that the shRNA targets both MKP-3 L and S, further studies will be necessary to elucidate isoform-specific participation in FOXO1 translocation. As a matter of fact, MKP-3 residues 200-260 have been identified as critical for mediating MKP-3/FOXO1 interaction in FAO rat hepatoma cells [17]. Of note, hMKP-3 S protein conserves the catalytic domain intact but lacks these sites required for MKP-3/FOXO1 interaction. Therefore, MKP-3L and S variants could exhibit different capability to promote FOXO1 dephosphorylation and activation.
In summary, our work demonstrates the role of MKP-3 in FOXO1 regulation and p21 induction mediated by Ang II in human adrenocortical carcinoma H295R cells. We also report the expression of MKP-3L and its alternative variant S and their regulation by Ang II. Our current efforts focus on the characterization of MKP-3 proteins encoded by L and S transcripts at biochemical and functional levels.
Declarations
Author contribution statement
TBC M. Garcia: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
P. Maloberti and C. Paz: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
J. Sabban: Performed the experiments; Analyzed and interpreted the data.
M. Dattilo: Performed the experiments.
P. Mele and S. Nudler: Performed the experiments; Contributed reagents, materials, analysis tools or data.
C. Mendez: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the University of Buenos Aires (UBACYT20020170100438BA and 20020170200350BA), CONICET (PIP 11220150100335CO) and ANPCyT (PICT 2014-1006).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank María M. Rancez for providing language help and writing assistance with the manuscript.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Material.
References
- 1.Raman M., Chen W., Cobb M.H. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 2.Osborne J.K., Zaganjor E., Cobb M.H. Signal Control through Raf: in Sickness and in Health. Cell Res. 2012;22:14–22. doi: 10.1038/cr.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidger A.M., Keyse S.M. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs) Semin. Cell Dev. Biol. 2016;50:125–132. doi: 10.1016/j.semcdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutros T., Chevet E., Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase Regulation : roles in cell growth , death , and cancer. Pharmacol. Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 5.Huang C.Y., Tan T.H. DUSPs, to MAP kinases and beyond. Cell Biosci. 2012;2:24. doi: 10.1186/2045-3701-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y., Zhang Z.Y. The mechanism of dephosphorylation of extracellular signal-regulated kinase 2 by mitogen-activated protein kinase phosphatase 3. J. Biol. Chem. 2001;276:32382–32391. doi: 10.1074/jbc.M103369200. [DOI] [PubMed] [Google Scholar]
- 7.Brondello J.M., Pouysségur J., McKenzie F.R. Reduced MAP kinase phosphatase-1 degradation after p42/p44(MAPK)- dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti S., Gimond C., Chambard J.-C., Touboul T., Roux D., Pouyssegur J., Pages G. Extracellular signal-regulated kinases phosphorylate mitogen-activated protein kinase phosphatase 3/DUSP6 at serines 159 and 197, two sites critical for its proteasomal degradation. Mol. Cell Biol. 2005;25:854–864. doi: 10.1128/MCB.25.2.854-864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori Sequeiros García M., Gómez N.V., Gorostizaga A., Acquier A., González-Calvar S.I., Mendez C.F., Paz C. MAP kinase phosphatase-3 (MKP-3) is transcriptionally and post-translationally up-regulated by hCG and modulates cAMP-induced p21 expression in MA-10 Leydig cells. Mol. Cell. Endocrinol. 2013;371:174–181. doi: 10.1016/j.mce.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Cadalbert L.C., Sloss C.M., Cunningham M.R., Al-Mutairi M., McIntire A., Shipley J., Plevin R. Differential regulation of MAP kinase activation by a novel splice variant of human MAP kinase phosphatase-2. Cell. Signal. 2010;22:357–365. doi: 10.1016/j.cellsig.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa T., Yatsuoka T., Youssef E.M., Abe T., Yokoyama T., Fukushige S., Soeda E., Hoshi M., Hayashi Y., Sunamura M., Kobari M., Horii A. Genomic analysis of DUSP6, a dual specificity MAP kinase phosphatase, in pancreatic cancer. Cytogenet. Cell Genet. 1998;82:156–159. doi: 10.1159/000015091. [DOI] [PubMed] [Google Scholar]
- 12.Wang S., Xia P., Huang G., Zhu P., Liu J., Ye B., Du Y., Fan Z. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat. Commun. 2016;7:11023. doi: 10.1038/ncomms11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y.Q., Li A., Yang Y., Li X.X., Zhang L.N., Guo H.C. Vol. 193. Life Sciences; 2018. The Regulation of FOXO1 and its Role in Disease Progression; pp. 124–131. [DOI] [PubMed] [Google Scholar]
- 14.Huang H., Tindall D.J. Dynamic FoxO transcription factors. J. Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 15.Roy S.K., Srivastava R.K., Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J. Mol. Signal. 2010;5:10. doi: 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K., Guo X., Yan H., Wu Y., Pan Q., Shen J.Z., Li X., Chen Y., Li L., Qi Y., Xu Z., Xie W., Zhang W., Threadgill D., He L., Villarreal D., Sun Y., White M.F., Zheng H., Guo S. Phosphorylation of forkhead protein FoxO1 at S253 regulates glucose homeostasis in mice. Endocrinology. 2019;160(5):1333–1347. doi: 10.1210/en.2018-00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao P., Feng B., Xu H. Mapping MKP-3/FOXO1 interaction and evaluating the effect on gluconeogenesis. PloS One. 2012;7(7):e41168. doi: 10.1371/journal.pone.0041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z., Jiao P., Huang X., Feng B., Feng Y., Yang S., Hwang P., Du J., Nie Y., Xiao G., Xu H. MAPK phosphatase-3 promotes hepatic gluconeogenesis through dephosphorylation of forkhead box O1 in mice. J. Clin. Invest. 2010;120:3901–3911. doi: 10.1172/JCI43250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rodrigues B.A., Kuga G.K., Muñoz V.R., Gaspar R.C., Tavares M.R., Botezelli J.D., da Silva A.S.R., Cintra D.E., de Moura L.P., Simabuco F.M., Ropelle E.R., Pauli J.R. Overexpression of Mitogen-activated protein kinase phosphatase-3 (MKP-3) reduces FoxO1 phosphorylation in mice hypothalamus. Neurosci. Lett. 2017;659:14–17. doi: 10.1016/j.neulet.2017.08.067. [DOI] [PubMed] [Google Scholar]
- 20.Suares A., Mori Sequeiros Garcia M., Paz C., González-Pardo V. Antiproliferative effects of Bortezomib in endothelial cells transformed by viral G protein-coupled receptor associated to Kaposi’s sarcoma. Cell. Signal. 2017;32:124–132. doi: 10.1016/j.cellsig.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe G., Pena P., Albanese C., Wilsbacher L.D., Young J.B., Pestell R.G. Adrenocorticotropin induction of stress-activated protein kinase in the adrenal cortex in vivo. J. Biol. Chem. 1997;272:20063–20069. doi: 10.1074/jbc.272.32.20063. [DOI] [PubMed] [Google Scholar]
- 22.Le T., Schimmer B.P. The regulation of MAPKs in Y1 mouse adrenocortical tumor cells. Endocrinology. 2001;142:4282–4287. doi: 10.1210/endo.142.10.8441. [DOI] [PubMed] [Google Scholar]
- 23.Hirakawa T., Ascoli M. The lutropin/choriogonadotropin receptor-induced phosphorylation of the extracellular signal-regulated kinases in leydig cells is mediated by a protein kinase a-dependent activation of ras. Mol. Endocrinol. 2003;17:2189–2200. doi: 10.1210/me.2003-0205. [DOI] [PubMed] [Google Scholar]
- 24.McNeill H., Whitworth E., Vinson G.P., Hinson J.P. Distribution of extracellular signal-regulated kinases 1 and 2 in the rat adrenal and their activation by angiotensin II. J. Endocrinol. 2005;187(1):149–157. doi: 10.1677/joe.1.06347. [DOI] [PubMed] [Google Scholar]
- 25.Gyles S.L., Burns C.J., Whitehouse B.J., Sugden D., Marshll P.J., Persaud S.J., Jones P.M. ERKs regulate cyclic AMP-induced steroid synthesis through transcription of the steroidogenic acute regulatory (StAR) gene. J. Biol. Chem. 2001;276:34888–34895. doi: 10.1074/jbc.M102063200. [DOI] [PubMed] [Google Scholar]
- 26.Szekeres M., Turu G., Orient A., Szalai B., Süpeki K., Cserzo M., Várnai P., Hunyady L. Mechanisms of angiotensin II-mediated regulation of aldosterone synthase expression in H295R human adrenocortical and rat adrenal glomerulosa cells. Mol. Cell. Endocrinol. 2009;302:244–253. doi: 10.1016/j.mce.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Matzkin M.E., Yamashita S., Ascoli M. The ERK1/2 pathway regulates testosterone synthesis by coordinately regulating the expression of steroidogenic genes in Leydig cells. Mol. Cell. Endocrinol. 2013;370(1–2):130–137. doi: 10.1016/j.mce.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sewer M.B., Waterman M.R. Adrenocorticotropin/cyclic adenosine 3′,5′-monophosphate-mediated transcription of the human CYP17 gene in the adrenal cortex is dependent on phosphatase activity. Endocrinology. 2002;143:1769–1777. doi: 10.1210/endo.143.5.8820. [DOI] [PubMed] [Google Scholar]
- 29.Bey P., Gorostizaga A.B., Maloberti P.M., Lozano R.C., Poderoso C., Maciel F.C., Podestá E.J., Paz C. Adrenocorticotropin induces mitogen-activated protein kinase phosphatase 1 in Y1 mouse adrenocortical tumor cells. Endocrinology. 2003;144:1399–1406. doi: 10.1210/en.2002-220987. [DOI] [PubMed] [Google Scholar]
- 30.Brion L., Maloberti P.M., Gomez N.V., Poderoso C., Gorostizaga A.B., Mori Sequeiros Garcia M.M., Acquier A.B., Cooke M., Mendez C.F., Podesta E.J., Paz C. MAPK phosphatase-1 (MKP-1) expression is up-regulated by hCG/cAMP and modulates steroidogenesis in MA-10 Leydig cells. Endocrinology. 2011;152:2665–2677. doi: 10.1210/en.2011-0021. [DOI] [PubMed] [Google Scholar]
- 31.Gómez N.V., Gorostizaga A.B., García M.M.M.S., Brion L., Acquier A., González-Calvar S.I., Méndez C.F., Podestá E.J., Paz C. MAPK phosphatase-2 (MKP-2) is induced by hCG and plays a role in the regulation of CYP11A1 expression in MA-10 leydig cells. Endocrinology. 2013;154:1488–1500. doi: 10.1210/en.2012-2032. [DOI] [PubMed] [Google Scholar]
- 32.Er L. Angiotensin and aldosterone. Regul. Pept. 1999;80:91–100. doi: 10.1016/s0167-0115(99)00026-9. [DOI] [PubMed] [Google Scholar]
- 33.Rainey W.E., Bird I.M., Mason J.I. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol. Cell. Endocrinol. 1994;100:45–50. doi: 10.1016/0303-7207(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan R., Yang D.C., Nadler J.L. Key role of P38 mitogen-activated protein kinase and the lipoxygenase pathway in angiotensin II actions in H295R adrenocortical cells. Endocrine. 2002;18:295–301. doi: 10.1385/ENDO:18:3:295. [DOI] [PubMed] [Google Scholar]
- 35.Ekerot M., Stavridis M.P., Delavaine L., Mitchell M.P., Staples C., Owens D.M., Keenan I.D., Dickinson R.J., Storey K.G., Ekerot M., Stavridis M.P., Delavaine L., Mitchell M.P., Staples C., Owens D.M., Keenan I.D., Dickinson R.J., Storey K.G., Keyse S.M. Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem. J. 2008;412:287–298. doi: 10.1042/BJ20071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bermudez O., Jouandin P., Rottier J., Bourcier C., Pagès G., Gimond C. Post-transcriptional regulation of the DUSP6/MKP-3 phosphatase by MEK/ERK signaling and hypoxia. J. Cell. Physiol. 2011;226:276–284. doi: 10.1002/jcp.22339. [DOI] [PubMed] [Google Scholar]
- 37.Otis M., Campbell S., Payet M.D., Gallo-Payet N. Angiotensin II stimulates protein synthesis and inhibits proliferation in primary cultures of rat adrenal glomerulosa cells. Endocrinology. 2005;146:633–642. doi: 10.1210/en.2004-0935. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Zhou Y., Graves D.T. FOXO transcription factors: their clinical significance and regulation. BioMed Res. Int. 2014:1–13. doi: 10.1155/2014/925350. [DOI] [PMC free article] [PubMed] [Google Scholar]