Abstract
Respiratory disturbances present in Parkinson’s disease (PD) are not well understood. Thus, studies in animal models aimed to link brain dopamine (DA) deficits with respiratory impairment are needed. Adult Wistar rats were lesioned with injection of 6-hydroxydopamine (6-OHDA) into the third cerebral ventricle. Two weeks after hypoxic test was performed in whole-body plethysmography chamber, phrenic (PHR) and hypoglossal (HG) nerve activities were recorded in normoxic and hypoxic conditions in anesthetized, vagotomized, paralyzed and mechanically ventilated rats. The effects of activation and blockade of dopaminergic carotid body receptors were investigated during normoxia in anesthetized spontaneously breathing rats. 6-OHDA injection affected resting respiratory pattern in awake animals: an increase in tidal volume and a decrease in respiratory rate had no effect on minute ventilation. Hypoxia magnified the amplitude and minute activity of the PHR and HG nerve of 6-OHDA rats. The ratio of pre-inspiratory to inspiratory HG burst amplitude was reduced in normoxic breathing. Yet, the ratio of pre-inspiratory time to total time of the respiratory cycle was increased during normoxia. 6-OHDA lesion had no impact on DA and domperidone effects on the respiratory pattern, which indicate that peripheral DA receptors are not affected in this model. Analysis of monoamines confirmed substantial striatal depletion of dopamine, serotonin and noradrenaline (NA) and reduction of NA content in the brainstem. In bilateral 6-OHDA model changes in activity of both nerves: HG (linked with increased apnea episodes) and PHR are present. Demonstrated respiratory effects could be related to specific depletion of DA and NA.
Keywords: Parkinson’s disease, 6-OHDA rat model, Hypoxic ventilatory response, Dopaminergic receptor, Phrenic and hypoglossal nerves
Introduction
Parkinson’s disease (PD) is a long-term neurodegenerative disorder of the central nervous system (CNS). Patients with PD apart from deficits in motor activity exhibit respiratory system impairments such as: dyspnea, sleep breathing disorders, restrictive pulmonary function, and upper airway dysfunction [1, 2]. Scarce respiratory studies in PD patients have not explained the origin of ventilatory abnormalities appearing in the disease [3–6]. Therefore, studies in animal models of PD, aimed to link brain DA deficits with respiratory disturbances are necessary to investigate mechanisms involved. They allow the researcher to obtain more homogenous stage of PD pathology and to eliminate the impact of antiparkinsonian drug treatment in modeled animals. To date 6-hydroxydopamine (6-OHDA) [7, 8] and reserpine models have been used [9] for studying respiratory impairments present in PD.
Recent study performed in 6-OHDA-induced PD model demonstrated decrease in expression of neurokinin 1 receptor in the pre-Bötzinger Complex and phox2b-expressing neurons in retrotrapezoid nucleus corresponding with frequency of breathing decline in normoxic breathing and decreased tachypneic response to hypercapnia [8, 10]. Our previous study in unilateral 6-OHDA model displayed increased tidal volume and attenuated respiratory rate responses during hypoxia and hypercapnia in comparison to sham-operated rats [11, 12].
DA and dopaminergic receptors have been indicated to play an important role in the regulation of breathing [13, 14]. Dopaminergic axons are distributed in the carotid bodies and within the brainstem where respiratory neurons are located [14]. It has been indicated that dopamine suppresses responsiveness to hypoxia, both in the central chemoreflex pathway and in the carotid body [15–18]. Therefore, it seems that dopamine neurons degeneration, DA deficiency and altered expression of dopaminergic receptors may lead to disturbed breathing control.
In the present study, we investigated normoxic pattern of breathing and hypoxic respiratory response in awake rats in the bilateral model evoked with intracerebroventricular (ICV) 6-OHDA injection, imitating advanced stage of PD [19]. Further we examined the activity of hypoglossal (HG) and phrenic (PHR) nerves after exposure to acute hypoxia, for the first time in bilateral PD model, having in mind that in unilateral one contralateral intact hemisphere may have compensatory influence on respiratory reflexes.
HG nerve innervates tongue muscles and its pre-inspiratory activity secures patency of the pharyngeal airway preparing upper airway muscles for inspiration [7]. Dysfunction of upper airway is one of the respiratory disturbances observed in PD patients [1], therefore analysis of HG nerve activity (pre-inspiratory time and amplitude) in PD model seems to be valuable tool for studying foregoing.
We have investigated previously the respiratory response to acute hypoxia after blockade of D2 receptors in 6-OHDA unilateral model [11]. All available studies in animal models of PD focus on the central effects of DA depletion. Potential changes in the peripheral dopaminergic receptors are neglected. Therefore, last purpose of the current study was examination of an impact of central DA depletion on stimulation and blockade of peripheral carotid body dopaminergic receptors in 6-OHDA-lesioned rats. Both tested agents dopamine and domperidone act only peripherally after systemic application [20, 21]. We decided to test this only during air breathing because in the current study ICV 6-OHDA lesion affected only normoxic pattern of breathing.
Behavioral test and contents of DA, noradrenaline (NA) and serotonin (5-HT) were analyzed in the striatum and brainstem to confirm lesion effectiveness and to allow interpretation of the respiratory test results. To avoid any compensation the study was performed 2 weeks after lesion. The rats were also pretreated with desipramine to protect noradrenergic neurons and obtain more selective degeneration of the dopaminergic system [19].
Material and methods
Animals and experimental protocol
All experimental procedures were approved by the local Ethics Committee for Animal Experimentation and conformed with the international/EU guidelines and regulations on the use and care of laboratory animals (EU Directive 2010/63/EU for animal experiments). Young adult male Wistar rats (n = 29) weighing 230–260 g (10–12 weeks old) at the beginning of the experiment were housed under standard laboratory conditions with a 12 h light/12 h dark cycle and unrestricted access to food and water.
The experiments were performed in the following scheme:
-
I.
Hypoxic tests in awake rats in plethysmographic chamber and open field test before and 14 days after 6-OHDA/vehicle ICV administration: Rats from plethysmographic chamber experiments were re-used the following day:
-
II.Hypoxic test in anesthetized animals with registration of phrenic and hypoglossal nerve activity 15 days after 6-OHDA/vehicle ICV administration.
- Sham rats injected ICV with vehicle (n = 7).
- ICV 6-OHDA-injected rats (n = 9).
-
III.Effects of D2 receptor agonist (dopamine) and antagonist (domperidone) (iv) injection, on normoxic ventilatory parameters in anesthetized rats 14 days after 6-OHDA/vehicle ICV administration.
- Sham rats injected ICV with vehicle (n = 6).
- ICV 6-OHDA-injected rats (n = 7).
6-OHDA bilateral model
Rats were anesthetized with an intraperitoneal injection of thiopentalum natricum (Sandoz GmbH, Austria) at a dose of 90 mg kg−1 and fixed to a stereotaxic frame (Digital Lab Standard Stereotaxic Stoelting, USA). Desipramine hydrochloride (25 mg kg−1, Sigma Aldrich, Poland) was given ip 30 min before the surgery to preclude the uptake of 6-OHDA by noradrenergic nerve terminals. Operation was performed using standard aseptic technique. After skin incision, the skull was trephined with a dental drill in specific stereotaxic coordinates according of the Paxinos and Watson Atlas [22]. Stereotaxic coordinates for the administration site were antero-posterior, bregma: − 0.85 mm, lateral: 0.0 mm, ventral dura: − 7.1 mm. Vehicle or 6-hydroxydopamine hydrochloride (250 μg dissolved in 0.9% NaCl containing 0.1% ascorbic acid—Sigma Aldrich, Poland) at a volume of 10 μl was injected into the third brain ventricle with a sterile Hamilton micro-syringe with rate of 1 μl min−1. The dose of 6-OHDA applied in the present study was selected based on the literature [23, 24]. After injection, the needle was left in the brain for 5 min to prevent the solution from flowing backward and then was slowly withdrawn. After the surgery, rats were left to recover with standard laboratory conditions, and unlimited access to food and water. One day before lesion and 2 weeks later behavioral and hypoxic tests were carried out.
Measurement of lung ventilation in awake rats
Ventilation and its response to acute hypoxia were investigated in a whole-body rodent plethysmography (WBP, model PLY 3223, Buxco Electronics, USA), as described previously [10]. Briefly, the calibration of volume was performed during each experiment by injecting of 1 ml of air into the chamber. The pressure signal was amplified, filtered, recorded, and analyzed with data analysis software (Biosystem XA for Windows, SFT3410 230 ver 2.9; Buxco Electronics, Wilmington, NC) generating tidal volume (VT, ml) and breathing frequency (f, breaths min−1). Tidal volume was calculated using approach of Epstein et al. [25]. Minute ventilation (VE, ml min−1, BTPS) was determined as a product of tidal volume and breathing frequency. VT and VE were normalized to body weight (ml kg−1 and ml kg−1 min−1, respectively). Rectal temperature was measured before and at the end of the experiments, and the values were averaged. All experiments were performed at room temperature (24–26 °C). Each rat was placed in the chamber (4.7 L) and left for 30 min of adaptation, while flushing with fraction of atmospheric air at 2.5 L min−1 to prevent CO2 accumulation. Acute hypoxia was achieved by a rapid flushing of gas mixture containing 8% of O2 in N2. Ventilation and its response to inspired hypoxia before and after implementation of PD model were registered. After 30 min of adaptation to breathing with the chamber air, pulmonary ventilation was taken as the baseline level of ventilation and recorded during 1 min before the introduction of hypoxia. Ventilation during 3 min of hypoxia and 5 min after switching to the air breathing was recorded. Period of 30 s breathing preceding hypoxia was calculated as a control normoxic breathing. Three-minute period of hypoxia breathing was averaged.
Electrophysiological experiments in anesthetized rats
Two weeks after 6-OHDA or vehicle ICV administration rats were anesthetized intraperitoneally with 750 mg/kg of urethane (Sigma Aldrich, Poland) and 150 mg/kg of α-chloralose (Fluka, Germany). Animals were cannulated into the femoral artery to monitor blood pressure and femoral vein to administer supplemental anesthesia and fluids as required. Rectal temperature was maintained throughout the experiment at 37–38 °C via external heating pad. Arterial blood pressure was measured with a BP-2 Columbus Instruments (Columbus, USA). After the tracheostomy and pipecuronium bromide (Arduan, Gedeon-Richter, Hungary) administration at initial dose of 0.08 mg/kg (supplemented every hour) paralyzed rats were artificially ventilated (7025 Rodent Ventilator, Ugo Basile, Comerio, Italy). End-tidal CO2 was maintained between 4.5 and 5.0% (Capstar-100, CWE, USA) by adjusting parameters of mechanical ventilation (tidal volume was 2.3 ml, respiratory rate ranged between 35–40 strokes/min). The exhalation port of the ventilator was attached to a positive pressure of 3 cm H2O to prevent alveolar collapse. Vagal nerves were isolated in the neck and cut to eliminate an adjustment of the respiratory activity with lung inflation caused by respiratory pump. The phrenic nerve root and the hypoglossal nerve were transected in the neck. Central ends of the whole phrenic nerve and the main hypoglossal trunk were placed on bipolar silver electrodes for recording. The activities of both nerves were amplified and filtered (5–2500 Hz) using a NeuroLog system (Digitimer Ltd., Welwyn, UK) and integrated with the time constant of 70 ms. Raw and integrated nervous activities and arterial blood pressure were digitized using a CED Power 1401 data acquisition interface, recorded on a computer and analyzed using the Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Acute hypoxia protocol consisted of ventilation with 8% oxygen in nitrogen. Each hypoxic exposure lasted 1.5 min or was stopped when episode of apnea occurred.
All analyzed data were calculated from the integrated phrenic and hypoglossal respiratory neurograms, as displayed previously [26]. Frequency (f), inspiratory time (Ti), expiratory time (Te), and the respiratory cycle time (Tc) were computed based on the activity of the phrenic nerve. The beginning of Ti was set as the start of the increase in the integrated activity of the PHR. The end of Ti was determined at a point where the maximal amplitude of the PHR decreased by 50%. Te was calculated as a period between the end and the beginning of Ti. Tc was a sum of Ti and Te. The amplitude of the pre-inspiratory HG activity (A pre-I HG) was determined based on the integrated hypoglossal activity at the time point corresponding to the start of the phrenic nerve activity. The time between the onset of the integrated HG activity and that of the PHR activity indicated the duration of the pre-inspiratory hypoglossal activity (T pre-I HG). All parameters were recorded and determined by averaging the variables measured for 15 s at the air-breathing, during each hypoxic episode (averaged periods of 15–30 s, 30–45 s, 45–90 s) and at recovery (1 min after the end of hypoxia). Changes in amplitude, frequency and minute activity were expressed as a percentage of the baseline of nerve activity and reported as means ± SEM. The duration of the T pre-I HG was calculated relative to the duration of the respiratory cycle measured between the onsets of integrated hypoglossal bursts (T pre-I/Tc), whereas the amplitude of the pre-I HG was presented as a fraction of the peak inspiratory hypoglossal amplitude (A pre-I/A HG).
Respiratory response after dopamine and domperidone injection in spontaneously breathing anesthetized rats
14 days after 6-OHDA lesion rats were anesthetized and prepared in similar way to procedure described in previous section excluding vagi nerve dissection, nerves preparation and arduan application. The tracheal cannula was connected to a pneumotachograph head, linked to Research Pneumotach System (RSS 100 h, Hans Rudolph Inc.) and a computerized recording system (Windows software version 3.07.02, KORR Medical Technologies Inc.) for measuring and recording tracheal airflow, respiratory frequency (f), tidal volume (VT), respiratory minute volume (VE).
Dopamine (Sigma Aldrich Poland) at a dose of 20 µg/kg and subsequently domperidone (Sigma Aldrich Poland) at a dose of 1 mg/kg, both dissolved in 0.9% saline (Sigma Aldrich Poland) were administered into the femoral vein in a volume of 0.2 ml. After injection catheter was immediately flushed with 0.2 ml of physiological saline. The gap between drug injections was 10 min.
The ventilatory parameters like VT and VE were calculated to body mass and by averaging the variables measured for 15, 30, 60 s, 2 min, 3 min and 5 min after the challenge just prior to drug injection and at selected time points of maximal respiratory change. Maximum of the response chosen from measured time points and recovery phase were presented.
Behavioral open field test
To test whether 6-OHDA/vehicle ICV injected rats present any behavioral changes the animals were examined in the open field before and 14 days after administration. The test gives possibility of estimation of animal locomotor activity, exploration and motivation. The open field test was performed in a plastic chamber (size 75 × 75 × 35 cm) in infrared light without daylight. Each animal was put on the central place of the open field and was registered during 15 min by video camera. Results were analyzed by associated software (EthoVision XT and BehaActive) to count the parameters describing the vertical movement and exploration.
High-performance liquid chromatography (HPLC) analysis: assay of dopamine, serotonin, and noradrenaline and parallel metabolites
The animals were killed by decapitation 14 days following 6-OHDA or sham operation to III ventricle, then the brains were rapidly removed. The left/right striata and brainstem were dissected. Each tissue sample was weighed, placed on dry ice and frozen (− 80 °C) until further biochemical analysis. Thereafter, the brain tissue samples were homogenized with the ice-cold 0.1 M HClO4 containing 0.05 mM ascorbic acid and centrifuged at 13,000×g for 15 min at 4 °C. The supernatant was filtered through 0.2-μm pore size filter (Whatman, UK). The high-performance liquid chromatography system with electrochemical detection (HPLC-ED) comprising an electrochemical detector (L-3500 detector; Merck, Germany) with a glassy carbon electrode, autosampler injector (Merck) and delivery pump (Knauer, Germany) was used to measure the content of either DA, 5-HT and NA, together with parallel metabolites: 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxyindolacetic acid (5-HIAA), 3-methoxy-4-hydroxyphenylglycol acid (MHPG). The voltage was setting at + 0.8 V with respect to an Ag/Ag Cl reference electrode. The aliquots (20 μl) were separated on the C–18 a reverse phase column (250 × 4.6 mm; Nucleosil, 5 μm, Macherey–Nagel, Germany). A mobile phase containing: 32 mM sodium phosphate (Sigma-Aldrich, USA), 34 mM citric acid (Sigma-Aldrich, USA), 1 mM octane sulfonic acid (Sigma-Aldrich, USA), and 54 μM ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich, USA) in deionized (18.3 mΩ) water with 16% methanol (Merck, Germany). The mobile phase was infused at a flow rate of 0.8 ml min−1. Quantitation was achieved by comparing the peak area ratios of analyte to the external standard calibration curve with ClarityChrom software (Knauer, Germany). The contents of monoamines and parallel metabolites were expressed as pg mg−1 fresh tissue.
Statistical analysis
Data were analyzed using non-parametric statistics. Differences between sham and 6-OHDA groups were evaluated with Whitney–Mann U test. For comparison within the group, Wilcoxon’s signed-ranks test was used. The confidence limit of p < 0.05 was considered to be statistically significant. The results are expressed as the means ± SEM.
Results
Hypoxic ventilatory response in vehicle and 6-OHDA-injected awake rats
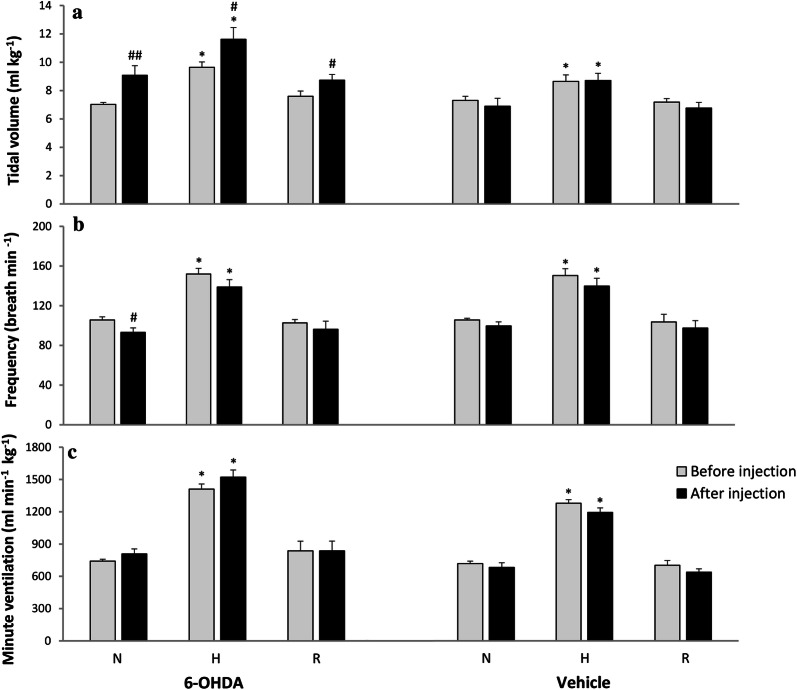
Vehicle injection into the third brain ventricle had no influence on normoxic and hypoxic values of all respiratory parameters in sham rats (Fig. 1). 6-OHDA administration produced a statistically significant 23% increase in tidal volume and 12% decrease in frequency of breathing during normoxia (Fig. 1a, b). In comparison to pre-6-OHDA state respiratory response of VT showed significant increase during hypoxia (18%), and at recovery (13%), while respiratory rate during hypoxia showed nonsignificant decline (9%). The alteration between pre-lesion and after 6-OHDA injection in hypoxic respiratory response seems to be the consequence of changes in resting respiratory variables, however in PD model rats VT response during hypoxia seems to be attenuated: it increased above 28% of its normoxic value, while before 6-OHDA injection VT was able to rise above 37% of its control. Changes in VT and f were inverse and produced nonsignificant increase of VE in normoxic (8%) and low oxygen condition (7%) in comparison to pre-lesion state (Fig. 1c).
Fig. 1.
Respiratory response to hypoxic stimulus before (grey bar) and 14 days (black bar) after ICV injection of vehicle (sham) or 6-OHDA. a Tidal volume; b frequency of breathing; c minute ventilation. N normoxia, H hypoxia, R recovery after the hypoxic stimulus. All values are mean ± SEM. *#p < 0.05; ##p < 0.01. *—statistical significance versus the respective normoxic baseline value (N), #—statistical significance versus the corresponding pre-injection with 6-OHDA value (n = 7–9 per group)
Phrenic nerve activity under normoxic and hypoxic condition in vehicle and 6-OHDA-treated rats
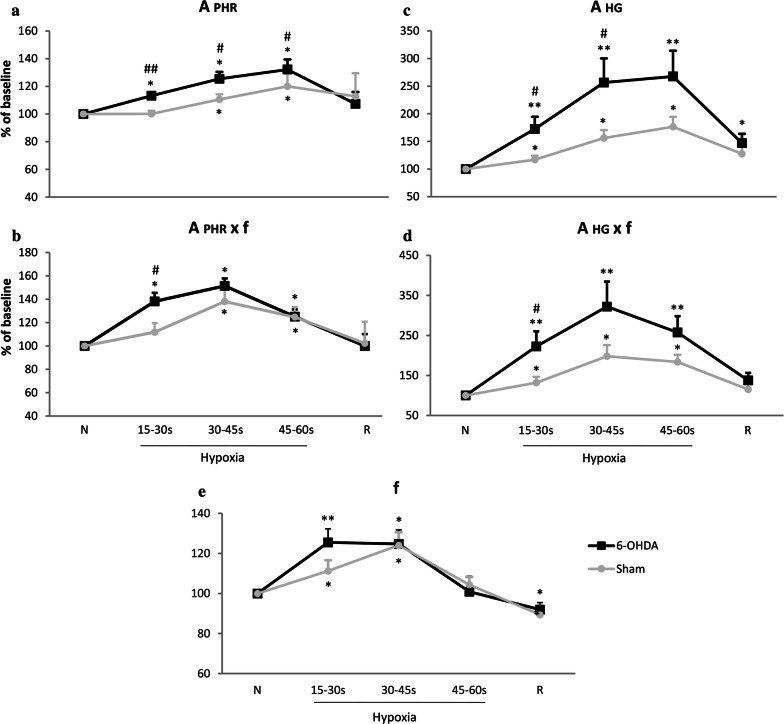
The frequency of nerve discharge was measured based on the activity of the phrenic nerve and it was the same as the frequency of the hypoglossal bursts. Raw values of nerve discharge frequency during normoxia did not differ between both analyzed groups; in 6-OHDA group it was 40 ± 4 and in sham rats 45 ± 4 bursts/min (p = 0.2). Baseline amplitude values of PHR activity were not significantly different between groups and in sham rats it was 1.16 ± 0.4 a.u., in 6-OHDA animals 1.43 ± 0.2 a.u. (p = 0.6). Figure 2 depicts changes in the phrenic nerve activity as percentage of control normoxic breathing. Frequency of phrenic nerve discharges increased during hypoxic stimulus and the increase was similar in both neurological states (Fig. 2e). Amplitude of the PHR in lesioned rats exhibited significantly higher values at 15 s (13%), 30 s (15%) and 45 s (12%) of response to hypoxia (Fig. 2a). PHR minute activity was augmented (26%) to a higher degree in 6-OHDA than in sham rats at first 15-s response to low oxygen (Fig. 2b).
Fig. 2.
Changes of amplitude (A), minute activity (A × f) and frequency of discharge (f) of the phrenic nerve (PHR) (a, b, e) and hypoglossal nerve (HG) (c, d) in normoxia and hypoxia in animals following vehicle (Sham), and 6-OHDA (6-OHDA) injection. Results are expressed as a percentage of the baseline nerve activity before hypoxia. All values are mean ± SEM. N normoxia, R recovery after the hypoxic stimulus. *#p < 0.05; **##p < 0.01, *—statistical significance in comparison to normoxic value expressed as 100% (N), #—statistical significance between corresponding points of hypoxic response present in 6-OHDA and Sham groups (n = 7–9 per group)
Experiments in anesthetized rats on respiratory nerve activity showed no differences between both neurological states in time of hypoxia application to apnea appearance. 6-OHDA rats achieved apnea after 35 ± 3 s, sham rats after 29 ± 6 s of hypoxia exposure (p = 0.1). Time duration of apnea did not vary between groups, as well (p = 0.6). For 6-OHDA rats it was 42 ± 4 s versus sham 53 ± 15.
Activity of hypoglossal nerve in normoxia and in response to hypoxia in vehicle and 6-OHDA-treated rats
Baseline values of amplitude of HG nerve did not differ significantly between tested groups; in sham rats it was 0.66 ± 0.3 and in 6-ODA-lesioned 1.07 ± 0.3 a.u. (p = 0.3). Hypoglossal nerve activity expressed as a percentage of baseline normoxic activity displayed augmented amplitude and minute activity in both groups of animals during hypoxia (Fig. 2c, d). However, changes in amplitude of 6-OHDA-treated rats in response to hypoxia were substantially magnified (Fig. 2c). Significant growth of amplitude of PD model rats exceeded amplitude of sham ones over 55% at 15 s and 100% at 30 s of the reaction.
Minute activity: product of amplitude and frequency of nerve discharge was much more increased in 6-OHDA rats, as well (Fig. 2d). Significantly higher response to hypoxia over 90% than in the sham rats was observed at 15 s.
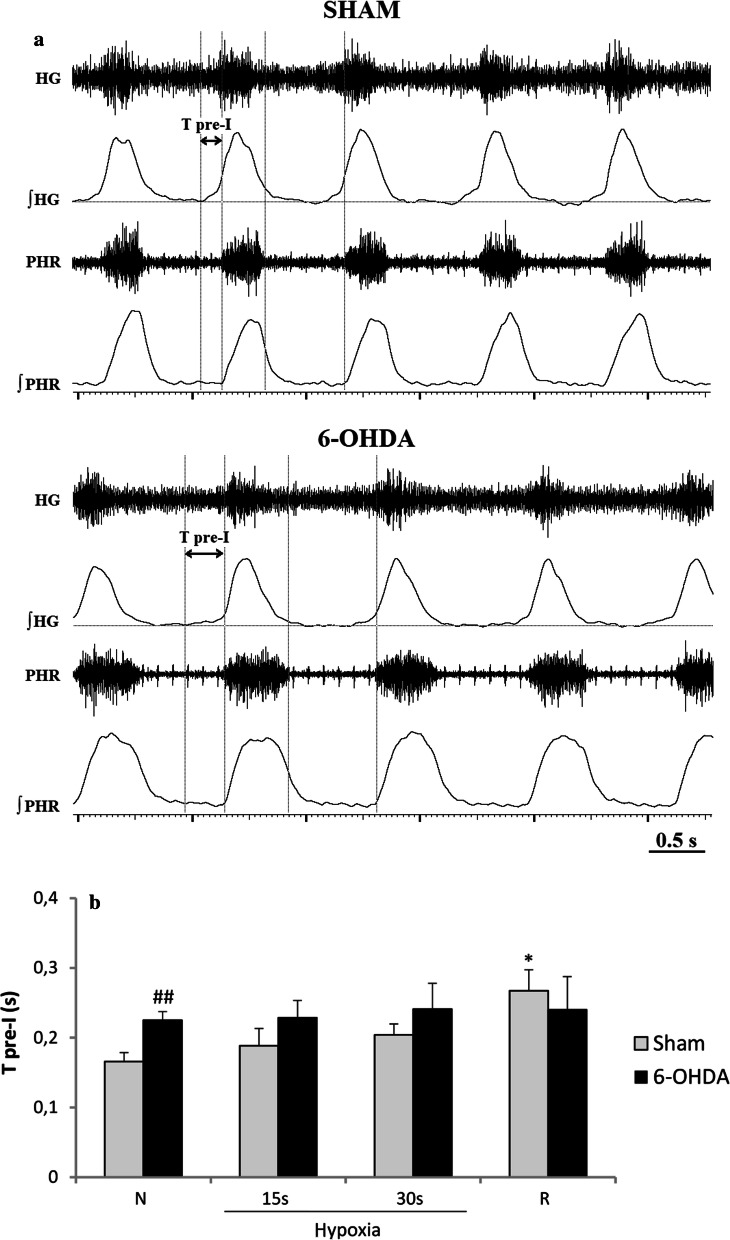
Analysis of pre-inspiratory time of HG nerve activity (T pre-I) showed that it was much prolonged in 6-OHDA rats in normoxia (Fig. 3a, b). There were no differences in length of T pre-I between both groups during hypoxia and recovery time. Remaining analyzed parameters such as: Ti, Te, Tc, A pre-I were not statistically different between 6-OHDA and sham rats (data not shown).
Fig. 3.
Sample recordings of PHR and HG nerve activity (raw and integrated signals—∫) during normoxia (a) and changes of pre-inspiratory time of HG nerve activity (T pre-I) in respiratory response to hypoxia (b) after injection of vehicle (Sham) or 6-OHDA into the third brain ventricle. Note visible more prolonged T pre-I in 6-OHDA-treated rats in normoxia. All values are given as mean ± SEM. N normoxia, R recovery after the hypoxic stimulus. *p < 0.05; ##p < 0.01. *—statistical significance versus the respective normoxia (N) value, #—significance between corresponding values in 6-OHDA and Sham group (n = 6–7)
Analysis of ratio of pre-inspiratory time of HG nerve to the total length of the respiratory cycle (T pre-I/Tc) displayed significant changes between examined neural states during normoxia and recovery time (Fig. 4a). PD model rats had increased ratio during air breathing and decreased while recovery from hypoxia. Ratio of pre-inspiratory amplitude to the inspiratory peak amplitude of HG (A pre-I/A HG) in 6-OHDA rats was significantly decreased in normoxia (20%) and during post-hypoxic recovery time (28%) (Fig. 4b).
Fig. 4.
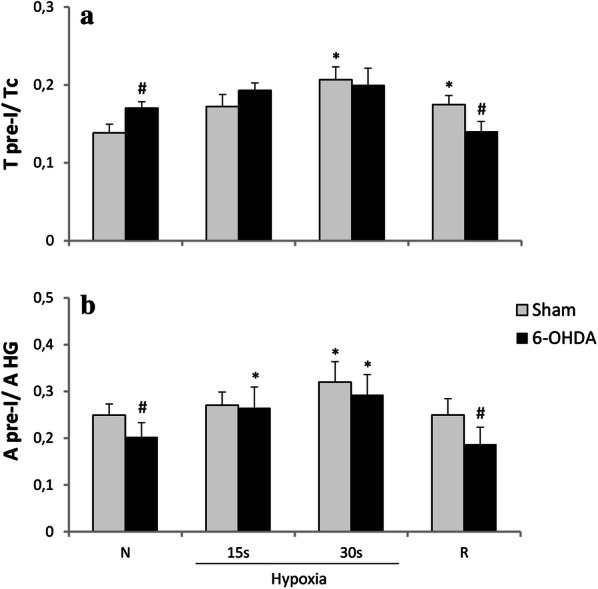
Changes in ratio of pre-inspiratory time of HG to a total length of the respiratory cycle (T pre-I/Tc) (a), and ratio of the pre-inspiratory hypoglossal nerve amplitude to the inspiratory HG peak amplitude (A pre-I/A HG) (b) in respiratory response to hypoxia after injection of vehicle (Sham) or 6-OHDA into the third brain ventricle. All values are given as mean ± SEM. N normoxia, R recovery after the hypoxic stimulus. *#p < 0.05. *—statistical significance versus normoxia (N) value, #—significance between corresponding values in 6-OHDA and Sham group (n = 6–7)
Ventilatory response after peripheral D2 receptors stimulation and blockade in vehicle and 6-OHDA-treated rats
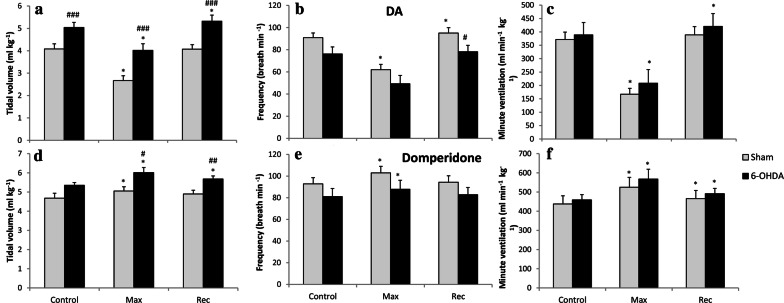
In conscious 6-OHDA-treated rats normoxic respiratory parameters presented in Fig. 1 were changed in comparison to sham-operated animals. In similar way in this experiment performed under anesthesia tidal volume was significantly augmented in PD animals in normoxic breathing (Fig. 5a). Frequency of breathing decline observed after anesthesia in PD model rats did not exhibit significance like in plethysmography experiment, however the trend is similar (Fig. 5b). Opposing changes in VT and f had no influence on changes in control minute ventilation (Fig. 5c).
Fig. 5.
Effect of dopamine (a–c) and domperidone (d–f) i.v. injections on tidal volume, frequency of breathing and minute ventilation during normoxia in Sham rats and 14 days after ICV 6-OHDA treatment. Control—before treatment, Max—maximal respiratory changes after drug administration, R recovery after max. All values are mean ± SEM. *#p < 0.05; ##p < 0.01; ###p < 0.001. *—statistical significance versus baseline value before drug injection, #—statistical significance versus the corresponding Sham value (n = 6–7 per group)
Intravenous injection of dopamine induced prompt, short-living decrease of all respiratory parameters in neurotoxin and vehicle-treated rats (Fig. 5a–c). DA treatment significantly declined VT, f and VE (to 46% of control values). The effect lasted 30 s.
Domperidone intravenous treatment caused increase in tidal volume and in frequency of breathing resulting in minute ventilation augmentation in sham (20%) and 6-OHDA (23%) rats (Fig. 5d–f). The effect lasted to 3 min after injection. PD model rats achieved more augmented VT in point of maximal response and during recovery (Fig. 5e), but the change was the consequence of augmented tidal volume in 6-OHDA-treated rats at baseline values. Overall these data show that there is no difference between sham and PD animals in response to activation and blockade of peripheral D2 receptors.
Body weight
Rats on the beginning of experiment before 6-OHDA lesion weighted 245 ± 2 g. Two weeks after neurotoxin injection body mass of animals increased to 259 ± 7 g. Sham group after vehicle injection presented increase in body weight from 256 ± 3 to 318 ± 10 g. Gain of body mass in both groups was significantly different (p < 0.01); in 6-OHDA rats it was 5% ± 3%, in sham rats 24% ± 4%.
Behavioral open field test
6-OHDA-injected rats showed decreased motor activity during 15 min of behavioral open filed test in comparison to vehicle-treated animals (Fig. 6). PD model rats presented reduced total distance length (33%) and time of moving (30%) and prolonged the time of immobility (43%) in comparison to sham animals.
Fig. 6.
Changes of the motor activity during 15 min of behavioral open field test between vehicle (Sham) and 6-OHDA-treated rats. All values are given as mean ± SEM. #p < 0.05; ##p < 0.01 significance versus Sham group (n = 7–9)
Content of monoamines and metabolites in the striatum and brainstem
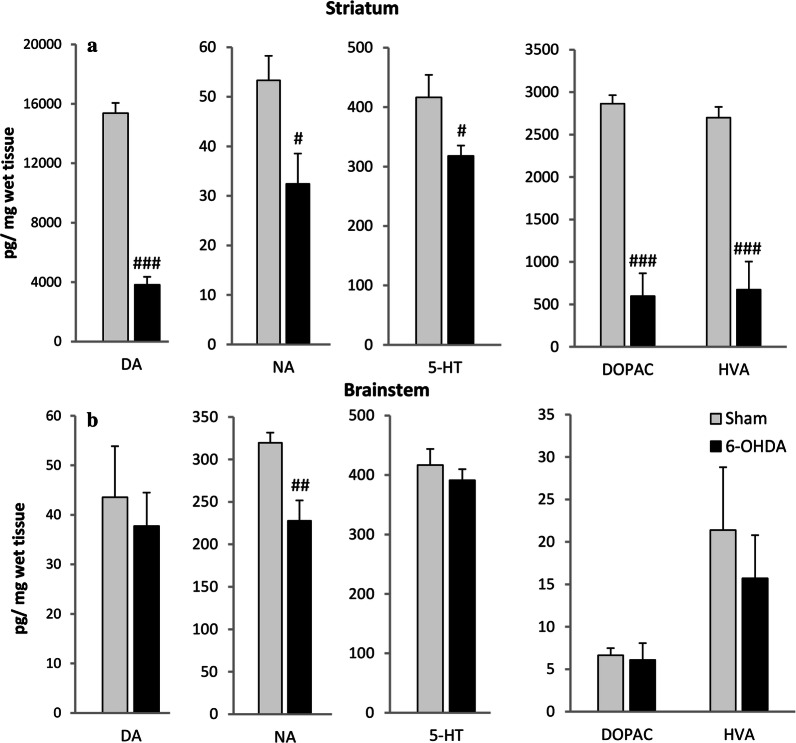
ICV injections of 6-OHDA resulted in 75% reduction of striatal DA concentration compared to the sham-operated rats accompanied with diminished metabolites: DOPAC level by 80% and HVA by 75%. Striatal NA and 5-HT were also reduced in the 6-OHDA-treated group by 40% and 24%, respectively (Fig. 7). In the brainstem only NA was significantly decreased by 28% in comparison to sham rats (Fig. 7). The content of 5-HIAA, metabolite of 5-HT was not significantly different between examined groups (data not shown). The level of MHPG, NA metabolite was undetectable.
Fig. 7.
Mean concentration of dopamine (DA), noradrenaline (NA), serotonin (5-HT), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the striatum (a) and brainstem (b) of Sham and 6-OHDA rats, evaluated by HPLC detection ex vivo. The data are expressed as mean ± SEM. ###p < 0.001, ##p < 0.01, #p < 0.05 significance versus Sham group (n = 7–8)
Discussion
Our study demonstrated that substantial depletion of DA in the striatum resulted in normoxic respiratory pattern changes characterized by slowing breathing and augmented tidal volume and altered activity of phrenic and hypoglossal nerve. The latter was investigated for the first time in bilateral PD model produced with ICV 6-OHDA injection. We also managed to confirm the lack of lesion effect on peripheral D2 receptor functioning.
Basic difference observed in the bilateral model in comparison to our previous study, when we applied 6-OHDA to one hemisphere [11, 26, 27], is altered to higher extent hypoglossal nerve activity and for the first time also augmented phrenic nerve amplitude during hypoxia. The pre-inspiratory time and the ratio of T pre-I/Tc were increased during baseline breathing, which was parallel to diminished ratio of pre-inspiratory HG nerve amplitude to the inspiratory HG peak amplitude. Hypoglossal pre-inspiratory activity is regarded as responsible for the patency of the pharyngeal airway before inspiration [28–30]. Hypoglossal nerve facilitates opening of the glottis and prevents collapse of the upper airways, which is the main reason of apnea [31]. Given these facts, altered ratios may indicate disturbances in maintenance of patency of the upper airway respiratory tract during the pre-inspiratory phase.
The analysis of the phrenic and the hypoglossal nerve activities in the bilateral 6-OHDA model demonstrated augmented response to hypoxia in amplitude and minute activity of both nerves. The increased amplitude of PHR activity during hypoxia was present only in the current model in contrast to previously studied unilateral ones [7, 26]. In these models the intact hemisphere plausibly compensated the depletion of DA and responded for the unaltered phrenic nerve activity.
Dopamine playing a crucial role in the oxygen detection in the peripheral chemoreceptors of the carotid body [32], in the CNS is regarded to be involved in the depressive phase of the respiratory response to hypoxia [16, 17]. Exogenously applied DA was reported to inhibit the activity of the hypoglossal and phrenic nerves [33], thus we suppose augmented amplitudes of both nerves can result from 6-OHDA-evoked DA depletion. In correspondence with our results, striatal DA loss evoked with this neurotoxin produced impairment of motoric tongue functions [34, 35], which indicated changes in control of tongue, innervated with the HG nerve. What is interesting, changed expression of D1 and D2 receptors have been demonstrated within the nucleus of the hypoglossal nerve (HG) of 6-OHDA-lesioned rats [36].
We found that stimulation or blockade of peripheral DA receptors produced similar respiratory responses in both sham and PD model rats, which means that carotid body DA receptors remained unaffected by central 6-OHDA injection. This is in contrast to Białkowska et al. [9] study, where lack of domperidone stimulatory effect on respiration was reported in reserpine rat model of parkinsonism. In this case, however reserpine depletes dopamine and other monoamines in the brain and in the periphery [37], thus most probably also in the carotid body. Dopamine respiratory effect has not been tested in PD model rats so far, and when applied i.v. it produced short-lived depression of ventilation as it was confirmed in sham group and earlier study in healthy cats [38]. The latter study showed also that carotid body denervation eliminated DA-induced respiratory depression, which confirmed that receptors located in these structures were involved.
Other neurotransmitter systems like serotonergic or noradrenergic have been postulated to contribute to respiratory PD abnormalities [8, 12, 39–41].
Previously in a unilateral model (medial forebrain injection), we demonstrated that 6-OHDA treatment depleted not only DA, but also decreased the content of 5-HT in the injured striatum and in both sides of the brainstem, with no effect on NA concentration [27]. ICV lesion reduced DA with lower impact on both NA and 5-HT levels in the striatum. The only change in the brainstem was significantly decreased NA regardless of desipramine pretreatment. Thus, it seems that depending on the site of 6-OHDA injection disturbance in various monoaminergic systems can be obtained. What is more important there is growing evidence that noradrenergic neurons of the brainstem locus coeruleus (LC) also degenerate in PD and can be responsible for motor and non-motor deficits in the course of disease [39]. The catecholaminergic terminals in the brainstem project to regions involved in respiratory control [42, 43] and NA provides a powerful stimulus for breathing in normoxia, hypoxia and hypercapnia [44–47]. What is more, α1-adrenergic receptors have been shown to be the main postsynaptic mediator of adrenergic excitation in HG motoneurons [48]. There is also evidence that activation of α1-adrenergic located near the phrenic motor nucleus elicits phrenic long-term facilitation [49].
We can speculate that NA reduced by 28% in the brainstem could be responsible at least is some part for respiratory changes present after 6-OHDA. Substantial lesion of noradrenergic neurons of LC [46] and most of the bulbospinal catecholaminergic neurons (C1 and A5 group) resulted in blunted ventilatory response to hypoxia [50]. We observed rather tendency to increased hypoxic minute ventilation, thus this moderate depletion of NA present in our study seems to be compensated. Intriguing fact is that 6-OHDA lesion of dopaminergic neurons evokes overactivation of LC noradrenergic neurons suggesting their impact could be augmented in this model [51]. It is plausible that in the present study 6-OHDA injection produced brainstem noradrenergic neurons loss, evidenced by declined concentration of NA, which in turn led to the overactivity of remaining noradrenergic neurons. This compensatory mechanism for the dopaminergic dysfunction in PD may be responsible for slight minute ventilation change with tendency to nonsignificant increase despite the increase of tidal volume and reduction of respiratory rate during air breathing and hypoxia. The same could be observed with augmented amplitudes of hypoglossal and phrenic nerves.
After striatal bilateral lesion, LC was not affected and the main observed respiratory effect was the decrease of frequency of breathing, without any changes in tidal volume [8]. Yet in our study ICV neurotoxin application was apparently able to affect brainstem noradrenergic system what was reflected in diminished NA concentration. Therefore, the normoxic tidal volume increase reported by us could be the effect of aforementioned 6-OHDA-induced overactivity of noradrenergic neurons [51]. The role of NA system in breathing modulation was confirmed by Oliveira et al. [10] in conclusion that spared NA neurons of LC could be responsible for maintaining chemoreflex sensitivity, which was reduced in response to CO2 in 6-OHDA-lesioned rats with additionally ablated noradrenergic LC neurons.
Respiratory alteration observed in the present study coexisted with behavioral changes, confirmed by the open field test estimating locomotor activity and motivation of animals to explore the surrounding environment. Significantly longer immobility time and shorter distance travelled by ICV 6-OHDA rats corresponded to results obtained after bilateral partial intranigral lesion [52, 53]. It is well known that 6-OHDA-treated rats revealed an increase in immobility time which is typical for anhedonia (i.e., decreased motivation/responsiveness to reward) [54]. We cannot exclude that immobility/anhedonia was partially mediated through the alterations in the mesolimbic dopamine system [55]. We did not examine the latter, but the study by Rodriguez-Rodríguez-Diaz et al. [56] reported that ICV 6-OHDA injection was effective in producing loss of dopaminergic neurons in the ventral tegmental area.
Conclusion
In conclusion, we suggest that respiratory changes appearing in ICV 6-OHDA model of PD results from the changed control of breathing at the brainstem level related to impairment in two neurochemical systems: substantial striatal depletion of DA and significant depletion of the brainstem NA. Magnified amplitudes of HG and PHR nerve activity during hypoxia and the changes in pre-inspiratory activity of HG nerve are more pronounced in the bilateral 6-OHDA model. Yet, we suggest cautious selection of 6-OHDA model to studying non-motor symptoms since depending on the site of the neurotoxin injection specific deficits beyond the dopaminergic systems can be achieved.
Acknowledgements
Not applicable.
Abbreviations
- AHG
Amplitude of hypoglossal nerve activity
- A pre-I HG
Amplitude of the pre-inspiratory hypoglossal nerve activity
- 5-HIAA
5-Hydroxyindolacetic acid
- 5-HT
Serotonin
- 6-OHDA
6-Hydroxydopamine
- CNS
Central nervous system
- DA
Dopamine
- DOPAC
3,4-Dihydroxyphenylacetic acid
- f
Frequency of breathing
- HG
Hypoglossal nerve
- HPLC
High-performance liquid chromatography
- HVA
Homovanillic acid
- ICV
Intracerebroventricular injection into III ventricle
- LC
Locus coeruleus
- MFB
Medial forebrain bundle
- MHPG
3-Methoxy-4-hydroxyphenylglycol acid
- NA
Noradrenaline
- NTS
Solitary tract nucleus
- PD
Parkinson’s disease
- PHR
Phrenic nerve
- Te
Expiratory time
- Ti
Inspiratory time
- Tc
Total respiratory cycle time
- T pre-I HG
Pre-inspiratory hypoglossal activity
- VE
Minute ventilation
- VT
Tidal volume
Authors’ contributions
KK and KA designed the experiments; KA, KK and MJ performed the experiments; KA, KK and MJ and drafted the manuscript; KA, KK, MJ, MZ, IJM and PMB were responsible for sample collection and data analysis. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Research involving animals. The study was approved by the IV Warsaw Ethics Committee for Animal Experimentation, and all experiments followed the guidelines of the committee.
Consent for publication
All authors approved this manuscript and consented for publication.
Competing interests
The authors declare that there are no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hovestadt A, Bogaard JM, Meerwaldt TJD, van der Meche FGA, Stigtt J. Pulmonary function in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1989;52:329–333. doi: 10.1136/jnnp.52.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baille G, De Jesus AM, Perez T, Devos D, Dujardin K, Charley CM, Defebvre L, Moreau C. Ventilatory dysfunction in Parkinson's disease. J Parkinsons Dis. 2016;16:463–471. doi: 10.3233/JPD-160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seccombe LM, Giddings HL, Rogers PG, Corbett AJ, Hayes MW, Peters MJ, Veitch EM. Abnormal ventilatory control in Parkinson’s disease-further evidence for non-motor dysfunction. Respir Physiol Neurobiol. 2011;179:300–304. doi: 10.1016/j.resp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Feinsilver SH, Friedman JH, Rosen JM. Respiration and sleep in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1986;49:964. doi: 10.1136/jnnp.49.8.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serebrovskaya T, Karaban I, Mankovskaya I, Bernardi L, Passino C, Appenzeller O. Hypoxic ventilatory responses and gas exchange in patients with Parkinson’s disease. Respiration. 1998;65:28–33. doi: 10.1159/000029224. [DOI] [PubMed] [Google Scholar]
- 6.Onodera H, Okabe S, Kikuchi Y, Tsuda T, Itoyama Y. Impaired chemosensitivity and perception of dyspnoea in Parkinson’s disease. Lancet. 2000;356:739–740. doi: 10.1016/S0140-6736(00)02638-6. [DOI] [PubMed] [Google Scholar]
- 7.Budzinska K, Andrzejewski K. Respiratory activity in the 6-hydroxydopamine model of Parkinson's disease in the rat. Acta Neurobiol Exp (Wars) 2014;74:67–81. doi: 10.55782/ane-2014-1973. [DOI] [PubMed] [Google Scholar]
- 8.Tuppy M, Barna BF, Alves-Dos-Santos L, Britto LR, Chiavegatto S, Moreira TS, Takakura AC. Respiratory deficits in a rat model of Parkinson's disease. Neuroscience. 2015;297:194–204. doi: 10.1016/j.neuroscience.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Białkowska M, Boguszewski P, Pokorski M. Breathing in Parkinsonism in the rat. Adv Exp Med Biol. 2016;884:1–11. doi: 10.1007/5584_2015_177. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira LM, Tuppy M, Moreira TS, Takakura AC. Role of the locus coeruleus catecholaminergic neurons in the chemosensory control of breathing in a Parkinson's disease model. Exp Neurol. 2017;293:172–180. doi: 10.1016/j.expneurol.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Andrzejewski K, Budzinska K, Zaremba M, Kaczyńska K. Hypoxic ventilatory response after dopamine D2 receptor blockade in unilateral rat model of Parkinson's disease. Neuroscience. 2016;316:192–200. doi: 10.1016/j.neuroscience.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Andrzejewski K, Budzińska K, Kaczyńska K. Effect of 6-OHDA on hypercapnic ventilatory response in the rat model of Parkinson's disease. Physiol Res. 2019;68:285–293. doi: 10.33549/physiolres.933949. [DOI] [PubMed] [Google Scholar]
- 13.Prieto-Lloret J, Donnelly DF, Rico AJ, Moratalla R, González C, Rigual RJ. Hypoxia transduction by carotid body chemoreceptors in mice lacking dopamine D(2) receptors. J Appl Physiol. 2007;103:1269–1275. doi: 10.1152/japplphysiol.00391.2007. [DOI] [PubMed] [Google Scholar]
- 14.Lalley PM. D1/D2-dopamine receptor agonist dihydrexidine stimulates inspiratory motor output and depresses medullary expiratory neurons. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1829–R1836. doi: 10.1152/ajpregu.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao C, Lahiri S, Mokashi A. Peripheral and central dopamine receptors in respiratory control. Respir Physiol. 1989;76:327–336. doi: 10.1016/0034-5687(89)90073-X. [DOI] [PubMed] [Google Scholar]
- 16.Goiny M, Lagercrantz H, Srinivasan M, Ungerstedt U, Yamamoto Y. Hypoxia-mediated in vivo release of dopamine in nucleus tractus solitarii of rabbits. J Appl Physiol. 1991;70:2395–2400. doi: 10.1152/jappl.1991.70.6.2395. [DOI] [PubMed] [Google Scholar]
- 17.Guner I, Yelmen N, Sahin G, Oruc T. The effect of intracerebroventricular dopamine administration on the respiratory response to hypoxia. Tohoku J Exp Med. 2002;196:219–230. doi: 10.1620/tjem.196.219. [DOI] [PubMed] [Google Scholar]
- 18.Niewinski P, Tubek S, Banasiak W, Paton JFR, Ponikowski P. Consequences of peripheral chemoreflex inhibition with low-dose dopamine in humans. J Physiol. 2014;592:1295–1308. doi: 10.1113/jphysiol.2013.266858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the Nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 20.Pinder RM. Possible dopamine derivatives capable of crossing the blood-brain barrier in relation to Parkinsonism. Nature. 1970;228:358. doi: 10.1038/228358a0. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen ME, Dorrington KL, Robbins PA. Effects of haloperidol on ventilation during isocapnic hypoxia in humans. J Appl Physiol. 1997;83:1110–1115. doi: 10.1152/jappl.1997.83.4.1110. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- 23.Rakovska A, Javitt D, Raichev P, Ang R, Balla A, Aspromonte J, Vizi S. Physiological release of striatal acetylcholine (in vivo): effect of somatostatin on dopaminergic–cholinergic interaction. Brain Res Bull. 2003;61:529–536. doi: 10.1016/S0361-9230(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 24.Shachar DB, Kahana N, Kampel V, Warshawsky A, Youdim MBH. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lesion in rats. Neuropharmacology. 2004;46:254–263. doi: 10.1016/j.neuropharm.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Epstein RA, Epstein MAF, Haddad GG, Mellins RB. Practical implementation of the barometric method for measurement of tidal volume. J Appl Physiol. 1980;49:1107–1115. doi: 10.1152/jappl.1980.49.6.1107. [DOI] [PubMed] [Google Scholar]
- 26.Andrzejewski K, Budzińska K, Kaczyńska K. Phrenic and hypoglossal nerve activity during respiratory response to hypoxia in 6-OHDA unilateral model of Parkinson’s disease. Life Sci. 2017;180:143–150. doi: 10.1016/j.lfs.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Andrzejewski K, Zaremba M, Kaczyńska K. Serotonergic system in hypoxic ventilatory response in unilateral rat model of Parkinson’s disease. J Biomed Sci. 2017;24:24. doi: 10.1186/s12929-017-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiter JC, St-John WM. Phrenic, vagal and hypoglossal activities in rat, pre-inspiratory, inspiratory, expiratory components. Respir Physiol Neurobiol. 2004;142:115–126. doi: 10.1016/j.resp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol. 2011;179:71–79. doi: 10.1016/j.resp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bautista TG, Dutschmann M. Inhibition of the pontine Kölliker-Fuse nucleus abolishes eupneic inspiratory hypoglossal motor discharge in rat. Neuroscience. 2014;267:22–29. doi: 10.1016/j.neuroscience.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 31.Strohl KP, Butler JP, Malhotra A. Mechanical properties of the upper airway. Compr Physiol. 2012;2:1853–1872. doi: 10.1002/cphy.c110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Comp Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Lunteren E, Haxhiu MA, Mitra J, Cherniack NS. Effects of dopamine, isoproterenol, and lobeline on cranial and phrenic motoneurons. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:737–745. doi: 10.1152/jappl.1984.56.3.737. [DOI] [PubMed] [Google Scholar]
- 34.Ciucci MR, Russell JA, Schaser AJ, Doll EJ, Vinney LM, Connor NP. Tongue force and timing deficits in a rat model of Parkinson disease. Behav Brain Res. 2011;222:315–320. doi: 10.1016/j.bbr.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nuckolls AL, Worley C, Leto C, Zhang H, Morris JK, Stanford JA. Tongue force and tongue motility are differently affected by unilateral vs bilateral nigrostriatal dopamine depletion in rats. Behav Brain Res. 2012;234:343–348. doi: 10.1016/j.bbr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L, Wang ZY, Lian H, Song HY, Zhang YM, Zhang XL, Fan RF, Zheng LF, Zhu JX. Altered expression of dopamine receptors in cholinergic motoneurons of the hypoglossal nucleus in a 6-OHDA-induced Parkinson's disease rat model. Biochem Biophys Res Commun. 2014;452:560–566. doi: 10.1016/j.bbrc.2014.08.104. [DOI] [PubMed] [Google Scholar]
- 37.Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wypych B, Szereda-Przestaszewska M. Depression of ventilation by dopamine in cats: effects of bilateral cervical sympathetic and vagal trunk section. Exp Physiol. 1995;80:255–263. doi: 10.1113/expphysiol.1995.sp003845. [DOI] [PubMed] [Google Scholar]
- 39.Delaville C, Navailles S, Benazzouz A. Effects of noradrenaline and serotonin depletions on the neuronal activity of globus pallidus and substantia nigra pars reticulata in experimental parkinsonism. Neuroscience. 2012;202:424–433. doi: 10.1016/j.neuroscience.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Lim SY, Fox SH, Lang AE. Overview of the extranigral aspects of Parkinson disease. Arch Neurol. 2009;66:167–172. doi: 10.1001/archneurol.2008.561. [DOI] [PubMed] [Google Scholar]
- 41.Buddhala C, Loftin SK, Kuley BM, Cairns NJ, Campbell MC, Perlmutter JS, Kotzbauer PT. Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol. 2015;2:949–959. doi: 10.1002/acn3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun QJ, Pilowsky P, Minson J, Arnolda L, Chalmers J, Llewellyn-Smith IJ. Close appositions between tyrosine hydroxylase immunoreactive boutons and respiratory neurons in the rat ventrolateral medulla. J Comp Neurol. 1994;340:1–10. doi: 10.1002/cne.903400102. [DOI] [PubMed] [Google Scholar]
- 43.Li A, Emond L, Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol. 2008;605:371–376. doi: 10.1007/978-0-387-73693-8_65. [DOI] [PubMed] [Google Scholar]
- 44.Milsom WK, Sadig T. Interaction between norepinephrine and hypoxia on carotid body chemoreception in rabbits. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1893–1898. doi: 10.1152/jappl.1983.55.6.1893. [DOI] [PubMed] [Google Scholar]
- 45.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- 46.Soulage C, Perrin D, Cottet-Emard JM, Pequignot JM. A6 noradrenergic cell group modulates the hypoxic ventilatory response. Adv Exp Med Biol. 2003;536:481–487. doi: 10.1007/978-1-4419-9280-2_61. [DOI] [PubMed] [Google Scholar]
- 47.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volgin DV, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat. 2001;22:157–166. doi: 10.1016/S0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- 49.Huxtable AG, MacFarlane PM, Vinit S, Nichols NL, Dale EA, Mitchell GS. Adrenergic α1 receptor activation is sufficient, but not necessary for phrenic long-term facilitation. J Appl Physiol. 2014;116:1345–1352. doi: 10.1152/japplphysiol.00904.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malheiros-Lima MR, Totola LT, Takakura AC, Moreira TS. Impaired chemosensory control of breathing after depletion of bulbospinal catecholaminergic neurons in rats. Pflugers Arch - Eur J Physiol. 2018;470:277–293. doi: 10.1007/s00424-017-2078-8. [DOI] [PubMed] [Google Scholar]
- 51.Wang T, Zhang OJ, Liu J, Zhong-Heng WU, Wang S. Firing activity of locus coeruleus noradrenergic neurons increases in a rodent model of Parkinsonism. Neurosci Bull. 2009;25:15–20. doi: 10.1007/s12264-009-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santiago RM, Barbieiro J, Lima MM, Dombrowski PA, Andreatini R, Vital MA. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson's disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1104–1114. doi: 10.1016/j.pnpbp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Sakai K, Gash DM. Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat. Brain Res. 1994;633:144–150. doi: 10.1016/0006-8993(94)91533-4. [DOI] [PubMed] [Google Scholar]
- 54.McDowell K, Chesselet MF. Animal models of the non-motor features of Parkinson’s disease. Neurobiol Dis. 2012;46:597–606. doi: 10.1016/j.nbd.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanton CH, Holmes AJ, Chang SWC, Joormann J. From stress to anhedonia: molecular processes through functional circuits. Trends Neurosci. 2019;42:23–42. doi: 10.1016/j.tins.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez-Díaz M, Abdala P, Barroso-Chinea P, Obeso J, González-Hernández T. Motor behavioural changes after intracerebroventricular injection of 6-hydroxydopamine in the rat: an animal model of Parkinson's disease. Behav Brain Res. 2001;122:79–92. doi: 10.1016/S0166-4328(01)00168-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.