Abstract
Purpose
PRIMARYS (NCT00690898) was a 48-week, open-label, phase 3b study, evaluating treatment with the somatostatin receptor ligand lanreotide autogel (stable dose: 120 mg/28 days) in treatment-naïve patients with growth hormone (GH)-secreting pituitary macroadenoma. This post hoc analysis aimed to evaluate factors predictive of long-term responses.
Methods
Potential predictive factors evaluated were: sex, age, and body mass index at baseline; and GH, insulin-like growth factor-1 (IGF-1), and tumor volume (TV) at baseline and week 12, using univariate regression analyses. Treatment responses were defined as hormonal control (GH ≤ 2.5 µg/L and age- and sex-normalized IGF-1), tight hormonal control (GH < 1.0 µg/L and normalized IGF-1), or ≥ 20% TV reduction (TVR). Receiver-operating-characteristic (ROC) curves were constructed using predictive factors significant in univariate analyses. Cut-off values for predicting treatment responses at 12 months were derived by maximizing the Youden index (J).
Results
At baseline, older age, female sex, and lower IGF-1 levels were associated with an increased probability of achieving long-term hormonal control. ROC area-under-the curve (AUC) values for hormonal control were high for week-12 GH and IGF-1 levels (0.87 and 0.93, respectively); associated cut-off values were 1.19 μg/L and 110% of the upper limit of normal (ULN), respectively. Results were similar for tight hormonal control (AUC values: 0.92 [GH] and 0.87 [IGF-1]; cut-off values: 1.11 μg/L and 125% ULN, respectively). AUC and J values associated with TVR were low.
Conclusions
The use of predictive factors at baseline and week 12 of treatment could inform clinical expectations of the long-term efficacy of lanreotide autogel.
Electronic supplementary material
The online version of this article (10.1007/s11102-019-01020-3) contains supplementary material, which is available to authorized users.
Keywords: Acromegaly, Predictive factors, Growth hormone, Baseline IGF-1, Hormonal response, Somatostatin receptor ligands
Introduction
Acromegaly is a disease characterized by the hypersecretion of growth hormone (GH)/insulin-like growth hormone-1 (IGF-1), typically as a result of a benign pituitary adenoma [1]. Long-acting forms of first-generation somatostatin receptor ligands (SRLs) are a well-established medical treatment for acromegaly. They are recommended in patients who do not achieve an adequate response following surgery, as well as in the first-line treatment of patients who are not suitable for or who refuse surgery [2, 3]. Long-acting SRLs have proven benefits in patients with acromegaly, reducing tumor volume (TV), decreasing GH and IGF-1 levels, and improving comorbidities [4–8]. However, not all patients respond to SRL treatment. In a meta–analysis conducted in 2005, it was found that approximately 50% of patients treated with SRLs achieved hormonal control [9]. One, more recent, systematic review reported considerably lower response rates than this (with an average response rate of 31%) [10], and individual analyses have reported values ranging between 17% and approximately 85% [5, 11]. Potential reasons for these disparities are numerous and include differences in patient populations, lack of standardization of GH and IGF-1 assays, and exclusion of treatment non-responders [5]. These data highlight the importance of being able to predict which patients are likely to respond to treatment with SRLs so as to avoid unnecessary treatment in those unlikely to respond. Previous studies have shown that several factors may influence the response to SRLs. These include: age and sex; pre-treatment and early post-treatment TV, GH, and IGF-1 levels; tumor histopathology (Ki-67, somatostatin receptor subtype 2 expression, AIP expression, granularity, β-arrestin expression); imaging characteristics (T2-weighted magnetic resonance imaging signal intensity); genetic factors; and treatment history [12–30].
PRIMARYS was an international, 48-week, open-label, phase 3b study evaluating primary treatment with the SRL lanreotide autogel (fixed dose of 120 mg/28 days) in patients with GH-secreting macroadenomas [4]. Lanreotide autogel provided early and sustained reductions in TV and GH/IGF-1 hypersecretion. Given its size (90 patients), homogeneous nature (all patients were treatment–naïve), and consistency of dosing (120 mg without dose titration throughout the 48-week study), the study data provide scope for further investigations. Here, we report post-hoc analyses to investigate factors predictive for hormonal control and clinically significant TV reduction (TVR) in patients primarily treated with lanreotide autogel 120 mg/28 days.
Patients and methods
Study design, patients, and interventions
The analyses described here were undertaken with the dataset from the PRIMARYS study (ClinicalTrials.gov NCT00690898; EudraCT 2007-000155–34), the methodology of which has been described previously [4].
In brief, PRIMARYS was a 48-week, open-label, single-arm, phase-3b study that was conducted in endocrine centers in nine countries. Men and women (aged 18–75 years) with acromegaly were included in the study if they had a mean GH level (mean of five samples for patients with diabetes mellitus) or nadir GH level (assessed by oral glucose tolerance test, for all other patients) > 1 µg/L, IGF-1 level above age- and sex-matched normal ranges, a macroadenoma (≥ 10 mm diameter), and no visual field defects. Patients were excluded if they had undergone or were likely to require pituitary surgery or radiotherapy, or they had received treatment with SRL, dopamine agonist, or GH receptor antagonist previously, or were likely to require any of these treatments (except lanreotide autogel). Patients were also excluded if prolactin co-secretion was > 100 µg/L.
Patients received a total of 12 doses of lanreotide autogel 120 mg (deep subcutaneous injection every 28 days). Patients could be withdrawn at any time if there was evidence of new visual field abnormalities or other safety concerns, insufficient reduction in IGF-1 levels at week 24 (reduction < 10% compared with the level at baseline, or if in the investigator’s judgement the response was inadequate), or prolactin levels after baseline were > 100 µg/L (for participants with levels of 20–100 µg/L at baseline).
Hormone levels and TVs were assessed centrally at screening, at day 1 (baseline; hormone levels only) and weeks 12, 24, and 48, and at early withdrawal, if applicable; TV at the screening visit was used as the baseline value. The assessments have been described previously [4]. Briefly, TV was measured using pre-specified methods, including magnetic resonance imaging and computer modelling, by three neuroradiologists blinded to the chronology of patients’ scans. IGF-1 levels were assessed at each visit using a radioimmunoassay (Esoterix/LabCorp Endocrine Sciences, CA, USA), and parameters were as follows: lower limit of detection, 7.7 μg/L; lower limit of quantitation, 15 μg/L; intra-assay precision, 5.3–14.1%; and interassay precision, 7.2–17.0%. Five consecutive samples were taken at 10- to 15-minute intervals to assess mean GH levels using a simultaneous one-step immunoenzymatic assay (Access Ultrasensitive GH assay; Beckman Coulter Inc, CA, USA), and parameters were as follows: lower limit of detection, 0.002 μg/L; intra-assay precision, 1.9–3.8%; and interassay precision, 2.7–3.9% [4]. The primary endpoint was the proportion of patients achieving ≥ 20% TVR at last post-baseline value available (LVA). Secondary efficacy endpoints included the proportions of patients achieving GH ≤ 2.5/< 1.0 µg/L, and age and sex-normalized IGF-1 levels at LVA.
Statistical analyses
Potential predictive factors evaluated were: sex; age and body mass index (BMI) at baseline; and GH, IGF-1, and TV at baseline and week 12. In the present analyses, treatment response was defined as: hormonal control (GH ≤ 2.5 µg/L and normalized IGF-1 levels), tight hormonal control (GH < 1.0 µg and normalized IGF-1 levels), or TV response (≥ 20% TVR) at LVA. Factors predictive for each of these treatment responses were investigated using a series of post hoc analyses. Firstly, potential predictive factors were examined using summary statistics for the proportions of patients achieving hormonal control (both definitions) at LVA according to baseline GH and IGF-1 levels, and the proportions achieving a TV response according to baseline TVs. Secondly, univariate logistic regression analyses were used to examine associations between potential predictive factors (at baseline, week 12, and for changes from baseline to week 12) and each of the three treatment responses. These were followed by correlation analyses with potential predictive factors from the univariate analyses to assess for the presence of multicollinearity among variables. Multicollinearity was detected between baseline and week-12 data, therefore, multivariate logistic regression analyses including baseline and week-12 variables were not performed.
Receiver-operating-characteristic (ROC) curves were then performed. ROC analysis is a well-accepted method that allows predictive accuracy to be tested across the full range of scores and does not require a single, pre-determined cut-off value to determine a true positive result [31, 32]. ROC curves were drawn for each of the three treatment responses, using predictive factors that were significant in the univariate analyses. For each factor in the ROC curves, a cut-off value for predicting a treatment response at LVA was derived by maximizing the Youden index (J = sensitivity + specificity–1). The J measure of sensitivity is a frequently used summary measure of the ROC curve that enables selection of an optimal threshold value (cut-off point) for predictive markers [33]. In the context of the present analyses, this approach minimizes the proportions of false positives (patients with levels below the cut-off value who did not achieve a treatment response) and false negatives (patients with levels above the cut-off who achieved a treatment response).
All analyses were based on data from patients in the intention-to-treat (ITT) population (patients receiving at least one injection of study medication and with at least one baseline efficacy assessment for the primary endpoint [TV] of the PRIMARYS study [4]). All post hoc analyses were hypothesis-generating, and no power calculations were performed. A p value of < 0.05 was considered significant.
Results
A total of 90 patients were enrolled in the PRIMARYS study and received treatment, and 18 of these withdrew due to an insufficient IGF-1 response [4]. At baseline, 47.8% were men, the mean (SD) age was 49.5 (12.4) years, BMI was 27.7 (4.6) kg/m2, and time since diagnosis of acromegaly was 121 (150) days [4]. Of the 90 patients, 89 fulfilled the criteria for inclusion in the ITT population, and 88 had LVA data for hormonal response and TV responder status.
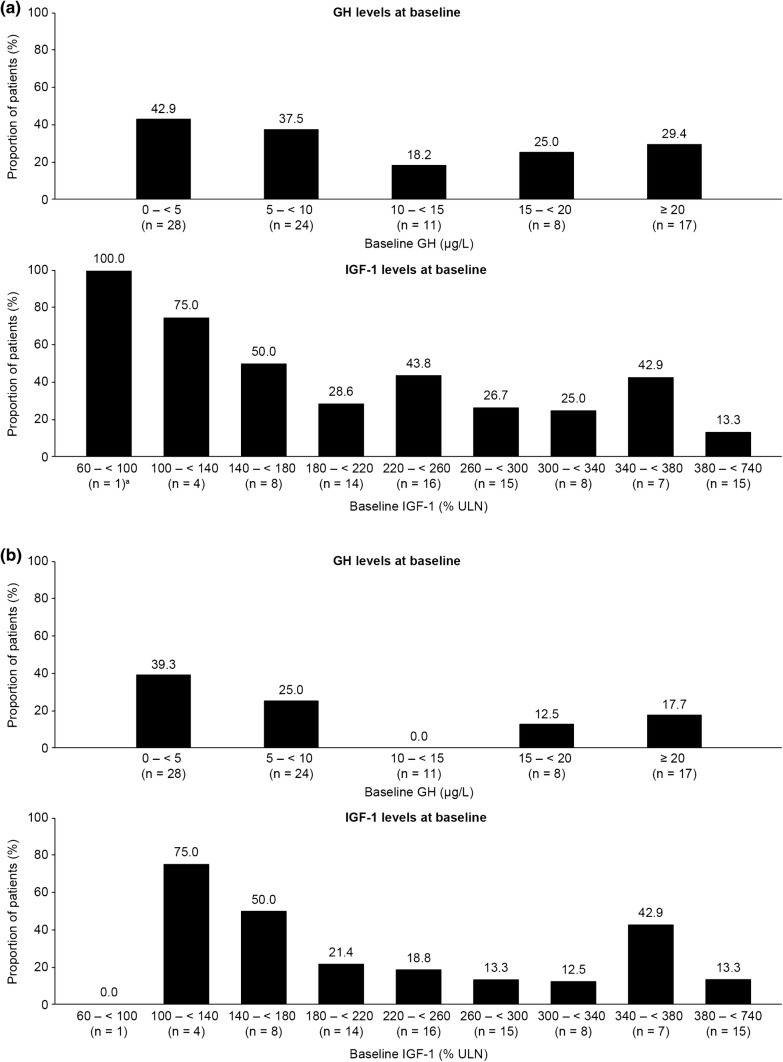
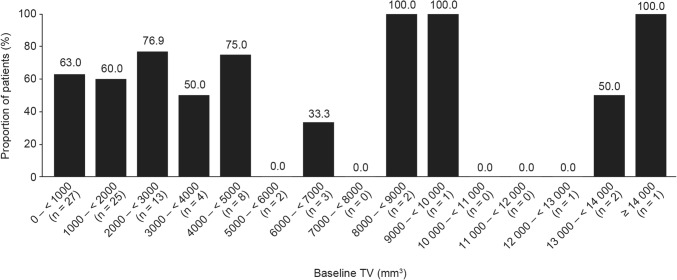
As reported previously, the proportion of patients achieving GH ≤ 2.5 µg/L and normalized IGF-1 levels at LVA was 34.1%, while 62.9% achieved a TV response at LVA (a priori analyses) [4]. The proportion achieving tight hormonal control as defined for the present analyses (GH < 1.0 µg/L and normalized IGF-1 levels) was 23.9% (post hoc analysis). Although some patients with higher baseline hormone levels did achieve hormonal control at LVA, the proportions of patients who achieved hormonal control at LVA were generally greater for those with lower baseline hormone levels (Fig. 1a, b). There was no clear relationship between TV responder status at LVA and TV at baseline; however, the majority of patients who achieved a TV response had a baseline TV of < 5000 mm3 (Fig. 2).
Fig. 1.
Proportions of patients achieving a hormonal control (defined as GH ≤ 2.5 µg/L and IGF-1 levels within normal ranges at LVA) and b tight hormonal control (defined as GH < 1.0 µg/L and IGF-1 levels within normal ranges at LVA), at LVA according to baseline GH and IGF-1 levels. GH growth hormone, IGF-1 insulin-like growth factor-1, LVA last post-baseline value available. Patients with baseline IGF-1 levels between 380 and < 740% ULN were grouped together. Of the two patients who achieved tight hormonal control at LVA, one patient had IGF-1 levels between 460 and 500% ULN, and the other between 540 and 580% ULN. Data are from the intention-to-treat population for patients with LVA data (n = 88)
Fig. 2.
Proportions of patients achieving TV responder status at LVA according to baseline TV. TV tumor volume, LVA last post-baseline value available. Data are from the intention-to-treat population for patients with LVA data (n = 89). TV responder status was defined as ≤ 20% reduction in TV
Univariate logistic regression analyses
The results of univariate analyses examining the associations between potential predictive factors (baseline, week-12, and change-from-baseline factors) and each treatment response are shown in Tables 1, 2, 3.
Table 1.
Univariate logistic regression analyses for hormonal control defined as GH ≤ 2.5 µg/L and IGF-1 levels within normal ranges
| Number of patients | Odds ratio [95% CI] | p-Value | |
|---|---|---|---|
| Baseline | |||
| Age (per 10-year higher age) | 88 | 2.20 [1.39; 3.48] | < 0.001 |
| Sex (women vs men) | 88 | 2.87 [1.13; 7.33] | 0.027 |
| BMI (≥ 25 vs 20–25 kg/m2) | 88 | 0.50 [0.20; 1.26] | 0.139 |
| GH (per 1-μg/L lower level) | 88 | 1.01 [0.98; 1.04] | 0.433 |
| IGF-1 (per 50% lower level ULN) | 88 | 1.28 [1.01; 1.63] | 0.040 |
| TV (per 100-mm3 smaller size) | 88 | 1.02 [1.00; 1.05] | 0.055 |
| Week 12 | |||
| GH (per 1-μg/L lower level) | 84 | 3.86 [1.87; 7.98] | < 0.001 |
| IGF-1 (per 50% lower level ULN) | 85 | 10.70 [3.59; 31.91] | < 0.001 |
| TV (per 100-mm3smaller size) | 85 | 1.04 [1.00; 1.08] | 0.046 |
| Change-from-baseline to week 12 | |||
| GH (per 10% increase) | 84 | 0.73 [0.59; 0.90] | 0.004 |
| IGF-1 (per 10% increase) | 85 | 0.48 [0.33; 0.68] | < 0.001 |
| TV (per 10% reduction) | 85 | 1.37 [0.99; 1.90] | 0.059 |
Factors in bold are statistically significant. Data are based on the number of patients with available data for each factor at each timepoint, and with p-values from Chi squared tests
Table 2.
Univariate logistic regression analyses for tight hormonal control defined as GH < 1.0 µg/L and IGF-1 levels within normal ranges
| Number of patients | Odds ratio [95% CI] | p-Value | |
|---|---|---|---|
| Baseline | |||
| Age (per 10-year higher age) | 88 | 2.50 [1.46; 4.26] | < 0.001 |
| Sex (women vs men) | 88 | 2.73 [0.95; 7.90] | 0.063 |
| BMI (≥ 25 vs 20–25 kg/m2) | 88 | 0.74 [0.27; 2.06] | 0.566 |
| GH (per 1-μg/L lower level) | 88 | 1.02 [0.98; 1.05] | 0.361 |
| IGF-1 (per 50% lower level ULN) | 88 | 1.22 [0.94; 1.58] | 0.132 |
| TV (per 100-mm3 smaller size) | 88 | 1.02 [0.99; 1.05] | 0.125 |
| Week 12 | |||
| GH (per 1-μg/L lower level) | 84 | 11.61 [3.12; 43.27] | < 0.001 |
| IGF-1 (per 50% lower level ULN) | 85 | 4.70 [2.05; 10.81] | < 0.001 |
| TV (per 100-mm3 smaller size) | 85 | 1.04 [0.99; 1.08] | 0.091 |
| Change-from-baseline to week 12 | |||
| GH (per 10% increase) | 84 | 0.78 [0.62; 0.98] | 0.030 |
| IGF-1 (per 10% increase) | 85 | 0.58 [0.42; 0.81] | 0.001 |
| TV (per 10% reduction) | 85 | 1.29 [0.91; 1.83] | 0.160 |
Factors in bold are statistically significant. Data are based on the number of patients with available data for each factor at each timepoint, and with p-values from Chi squared tests
Table 3.
Univariate logistic regression analyses for TV responder status
| Number of patients | Odds ratio [95% CI] | p-Value | |
|---|---|---|---|
| Baseline | |||
| Age (per 10-year higher age) | 89 | 1.17 [0.82; 1.66] | 0.380 |
| Sex (women vs men) | 89 | 1.32 [0.56; 3.12] | 0.531 |
| BMI (≥ 25 vs 20–25 kg/m2) | 89 | 0.68 [0.26; 1.73] | 0.413 |
| GH (per 1-μg/L lower level) | 89 | 0.98 [0.95; 1.01] | 0.116 |
| IGF-1 (per 50% ULN lower level) | 89 | 1.02 [0.86; 1.22] | 0.794 |
| TV (per 100-mm3 smaller size) | 89 | 1.00 [0.99; 1.01] | 0.976 |
| Week 12 | |||
| GH (per 1-μg/L lower level) | 85 | 1.12 [1.01; 1.25] | 0.039 |
| IGF-1 (per 50% lower level ULN) | 85 | 1.48 [1.13; 1.94] | 0.004 |
| TV (per 100-mm3 smaller size) | 85 | 1.00 [0.99; 1.02] | 0.491 |
| Change-from-baseline to week 12 | |||
| GH (per 10% increase) | 85 | 0.78 [0.67; 0.90] | < 0.001 |
| IGF-1 (per 10% increase) | 85 | 0.73 [0.62; 0.87] | < 0.001 |
| TV (per 10% reduction) | 85 | 7.15 [3.15; 16.20] | < 0.001 |
BMI body mass index, GH growth hormone, IGF-1 insulin-like growth factor-1, TV tumor volume, ULN upper limit of normal
Factors in bold are statistically significant. Data are based on the number of patients with available data for each factor at each timepoint, and with p-values from Chi squared tests. TV responder status was defined as ≥ 20% reduction in TV from baseline to last post-baseline value available
Associations were significant between three baseline factors and hormonal control, defined as GH ≤ 2.5 µg/L and IGF-1 levels within normal ranges at LVA. Specifically, the odds of achieving hormonal control were 2.20 times higher for each 10-year higher age, 2.87 times higher in women than men, and 1.28 times higher for each 50% lower IGF-1 level ULN (Table 1). No significant associations were identified for BMI, GH levels, or TV. Associations were also significant for 3 week-12 factors: the odds were 3.86 times higher for a 1-μg/L lower GH level; 10.70 times higher for a 50% lower IGF-1 level upper limit of normal (ULN); and 1.04 times higher for a 100-mm3 lower TV. Changes from baseline to week 12 in GH and IGF-1 levels, but not TVs, were significantly associated with a treatment response (Table 1).
Only one baseline factor was significantly associated with tight hormonal control, defined as GH < 1.0 µg/L and IGF-1 levels within normal ranges at LVA: the odds of achieving tight hormonal control were 2.50 times higher for each 10-year higher age at baseline. Two week-12 factors were significantly associated with a treatment response for tight hormonal control: the odds of achieving a treatment response were 11.61 times higher for a 1-μg/L lower week-12 GH level; and 4.70 times higher for a 50% lower week-12 IGF-1 level ULN. Changes from baseline to week 12 in GH and IGF-1 levels, but not TVs, were also significantly associated with a treatment response (Table 2).
There were no significant associations between baseline factors and TV responder status at LVA. Associations were significant, however, for 2 week-12 factors: the odds of achieving TV responder status were 1.12 times higher for a 1-μg/L lower GH level and 1.48 times higher for a 50% lower IGF-1 level ULN. No significant association was identified with a 100-mm3 lower TV. Changes from baseline to week 12 in GH and IGF-1 levels, and in TVs, were significantly associated with a TV treatment response (Table 3).
Receiver-operating-characteristic curves
Multiple models were examined for each analysis; however, the simplest model for a given area under the curve (AUC) was chosen according to the ‘parsimony principle’.
Hormonal control defined as GH ≤ 2.5 µg/L and IGF-1 levels within normal ranges at LVA
The final model incorporating baseline factors had an AUC of 0.79 (Supplementary Fig. 1). The baseline factors of IGF-1 level, age, and sex were significant in univariate analyses. However, using ROC curves, these factors were associated with relatively poor AUCs: 0.64, 0.74, and 0.63 for IGF-1, age, and sex, respectively. The IGF-1 level cut-off for predicting a treatment response using the Youden index was 225% ULN, but the value of J was low (0.24) and sensitivity and specificity were limited (sensitivity, 0.50; specificity, 0.74). Following multicollinearity between baseline and week-12 factors, and the poor AUCs from baseline ROC curves, the final model selected was based on ROC curves with only week-12 variables.
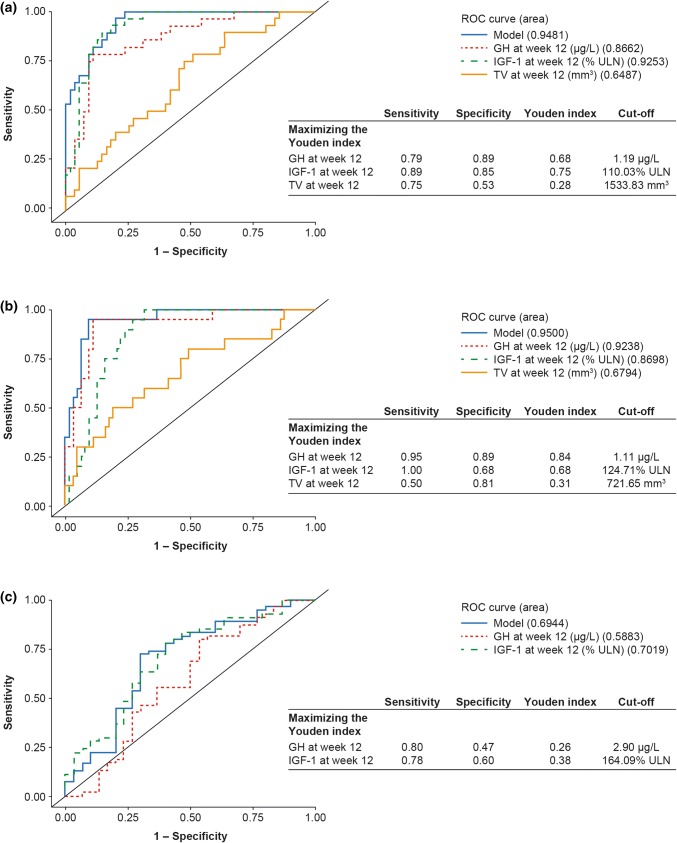
The final model incorporating week-12 factors had an AUC of 0.95 (Fig. 3a). Week-12 GH and IGF-1 levels were associated with high AUCs and, with J maximized, were associated with optimal cut-off values of 1.19 μg/L and 110% ULN, respectively (Fig. 3a). In contrast, the AUC for TV in the final model was poor (0.65) (Fig. 3a), and the maximized value of J was low (0.28). This model was not greatly improved by the addition of change-from-baseline factors (AUC of 0.95; Supplementary Fig. 2a) and J values were low. In this model, hormonal control was associated with an optimal cut-off of –55.3% in IGF-1 levels from baseline to week 12 and − 80.3% in GH levels.
Fig. 3.
ROC curves drawn for the calculation of week-12 cut-off values for predicting hormonal control and TV responder status at LVA when a hormonal control is defined as GH ≤ 2.5 µg/L and IGF-1 levels within normal ranges at LVA, b tight hormonal control is defined as GH < 1.0 µg/L and IGF-1 levels within normal ranges at LVA, and c TV responder status is defined as ≤ 20% reduction in TV at LVA. GH growth hormone, IGF-1 insulin-like growth factor-1, LVA last post-baseline value available, ROC receiver operating characteristic, TV tumor volume, ULN upper limit of normal. Data are from the intention-to-treat population for patients with LVA data (n = 88)
Tight hormonal control defined as GH < 1.0 µg/L and IGF-1 levels within normal ranges at LVA
ROC curves were not developed for baseline factors as age was the only factor that was significant in univariate analyses.
The final model with week-12 factors had an AUC of 0.95 (Fig. 3b). Twelve-week GH and IGF-1 levels in this model were associated with high AUCs and with optimal cut-off values of 1.11 μg/L and 125% ULN, respectively, with J maximized (Fig. 3b). In contrast, the AUC for TV in the final model was poor (0.68), the value of J when maximized was low (0.31). This model was not greatly improved by the addition of change-from-baseline factors (AUC of 0.95; Supplementary Fig. 2b), and J values were low. In this model, tight hormonal control was associated with an optimal cut-off of − 55.3% in IGF-1 levels from baseline to week 12 and − 72.6% in GH levels.
TV responder status at LVA
ROC curves were not developed for baseline factors as none were significant in the univariate analyses.
The final model with week-12 factors had an AUC of 0.69 (Fig. 3c). This model was improved by the addition of change-from-baseline factors (AUC, 0.94); however, the maximized J values were low for percent change-from-baseline to week 12 for both GH and IGF-1 (0.40 and 0.38, respectively, Supplementary Fig. 2C). A TV response was associated with an optimal cut-off of − 68.6% in GH levels from baseline to week 12, − 61.0% in IGF-1 levels, and 21% for TV. Twelve-week GH and IGF-1 levels in the final model (Fig. 3c) were associated with AUCs of 0.59 and 0.70, respectively. Optimal cut-off values to predict TV responder status at LVA were 2.90 µg/L and 164% ULN for 12-week GH and IGF-1, respectively, with J values maximized, but the J values were low in each case.
Discussion
Post-hoc analyses were undertaken with data from the PRIMARYS study to determine whether treatment responses to lanreotide autogel at 12 months could be predicted from baseline characteristics and/or from week-12 hormone concentrations and TVs. This week-12 information could be useful to clinicians as it could potentially identify “early-response” patients most likely to benefit, thus facilitating individualized management [18, 24], as well as managing the expectations of both patient and physician regarding likely treatment outcomes. The ability to distinguish patients likely and unlikely to respond early in the treatment could also mean that the unnecessary continuation of an ineffective treatment could be avoided in non-responsive patients. Treatment response was based on accepted measures (i.e. hormonal control and TV). Two levels of hormonal control were used (GH ≤ 2.5 μg/L or < 1.0 μg/L, with age- and sex-normalized IGF-1), reflecting the increasing sensitivity and specificity of assays in everyday clinical use [2]. To the best of our knowledge, this is one of the first studies to investigate potential factors at baseline and week 12 after initiation of treatment with lanreotide autogel 120 mg that may predict hormonal control at 12 months.
Older age, female sex, and lower IGF-1 levels were associated with an increased likelihood of achieving hormonal control defined as GH levels ≤ 2.5 µg/L and normalized IGF-1 levels at LVA. This may be related to the pattern of GH secretion in female patients with acromegaly, as well as the age-dependent decline in GH secretion in acromegaly [34]. Older patients were also more likely to achieve tight hormonal control (GH levels < 1.0 µg/L and normalized IGF-1 levels). Pre-treatment IGF-1 levels have also been shown to be an important predictive factor for acromegaly in a study by Bhayana et al., who reported that responders were more likely to have lower baseline levels of IGF-1 [35]. An Italian multicenter retrospective study also demonstrated that pre-treatment IGF-1 levels are predictors for both morbidity and mortality in patients with acromegaly [36].
Twelve-week GH and IGF-1 levels were associated with an increased likelihood of hormonal control, tight hormonal control, and a clinically significant TV response at LVA in univariate analyses. ROC analyses indicated that 12-week GH levels < 1.19 µg/L were predictive for hormonal control at LVA and levels < 1.11 µg/L were predictive for tight hormonal control. Corresponding data for IGF-1 levels were < 110% ULN and < 125% ULN, respectively. At week 12, TV response was not an accurate predictive factor for either hormonal control or tight hormonal control. The results obtained for GH and IGF-1 levels at week 12 resonate with established thresholds for disease control in acromegaly [3]. This model could therefore provide significant value to both clinician and patient by predicting the response to lanreotide autogel 120 mg at 12 months, equivalent to only three injections and with an acceptable safety profile, using data collected in the early stages of treatment (at week 12) [8]. However, patients may still be considered to benefit from lanreotide autogel even if they do not meet these targets for hormonal control if there are nevertheless marked improvements in biomarkers, clinical symptoms, or TV. Maximum effects may occur only after longer treatment periods. Together, the results from this study suggest that early efficacy may be predictive of long-term response; these data reflect the findings of Colao et al. who reported that tumor shrinkage and GH levels after 3 months of treatment with the SRL octreotide long-acting release (LAR), could predict the magnitude of tumor shrinkage at 12 months [37]. A study by Mercado et al. that looked at the efficacy of octreotide LAR treatment at 48 weeks, reported that the majority of patients showed a favorable biochemical response with substantial decreases in GH and IGF-1 levels at week 12. However, the achievement of GH level ≤ 2.5 µg/L and/or the normalization of IGF-1 at 12 weeks of treatment, was not predictive of a significant reduction in tumor volume by the end of study [38]. In 2015, Cuevas-Ramos et al. presented a new structural and functional acromegaly classification [39]. Using cluster analysis, they identified three acromegaly types, based primarily on immunohistochemical and radiological characteristics, with important prognostic implications. Unfortunately, as we do not have follow-up data on surgery and immunohistochemistry for the patients primarily treated with lanreotide autogel in the PRIMARYS study, we are unable to compare results with the proposed classification directly.
The interpretation of data from this study was limited by the post hoc nature of the analyses; the extent to which the results can be generalized to those beyond the study population (previously untreated patients with macroadenomas) is unclear. Furthermore, cut-offs provided here are assay-dependent, which is of special importance for IGF-1, and may be variable. It must also be considered that patients with high baseline GH or IGF-1 levels may have large reductions in hormone levels in response to treatment, but not be considered a responder if they do not reach the cut-off threshold; in this situation, treatment may still be regarded as beneficial. Also, the analysis evaluated only some of the possible predictive factors for response to acromegaly treatment; there are others, such as tumor histopathology (as discussed in the “Introduction”) or expression of somatostatin receptors that may also contribute to how patients respond. A recent post hoc analysis of the PRIMARYS data found that IGF-1 levels after treatment with lanreotide autogel were lower in patients with T2-signal hypointense GH-secreting macroadenomas compared with T2-signal isointense GH-secreting macroadenomas, hence assessment of T2-signal intensity was suggested as a predictive factor for long-term response [30]. It should also be noted that the decision to treat with SRLs may not only depend on predictive factors, but also on the cost of medication and availability of experienced surgeons; both of these factors may vary considerably between countries. Despite these limitations, these analyses are nonetheless valuable as they were conducted with data from a homogeneous population of 90 patients. In addition, both GH and IGF-1 levels and TVs were assessed in a centralized laboratory to reduce measurement variability. The information yielded may assist in the clinical management of treatment and the potential future personalization of therapeutic decisions, likely in combination with other predictive factors [40].
In summary, these post hoc analyses from the PRIMARYS study indicate that treatment-naïve patients with GH/IGF-1 hypersecretion and GH-secreting macroadenomas were more likely to achieve hormonal control at 12 months if they were female, older, or had lower IGF-1 levels at baseline, or if GH levels were less than 1.2 µg/L and IGF-1 levels less than 110% ULN at week 12. Thus, it may in the future be possible to predict as early as week 12 whether treatment-naïve patients with GH-secreting macroadenomas may show a longer-term hormonal response or control to lanreotide autogel 120 mg/28 days.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all patients involved in the original study, as well as their care team, investigators and research staff in participating institutions. The authors acknowledge John Bevan for contributing to the concept and design of the study; patient enrolment into the study; analysis and interpretation of the data.
Medical writing support
The authors thank Amy Watkins, PhD, of Watermeadow Medical for providing medical writing support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines.
Author contributions
PJC and SP were involved in: concept and design of the study; patient enrolment into the study; analysis and interpretation of the data; and drafting of the manuscript. AH and CS were involved in: analysis and interpretation of the data; and drafting of the manuscript. All authors additionally revised the work critically for important intellectual content, approved the final version to be published, and agree to be accountable for all aspects of the work.
Funding
This analysis was funded by Ipsen.
Data availability
Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.
Compliance with ethical standards
Conflicts of interest
SP has served as an advisory board member for Ipsen and Novartis, and has received honoraria for speaking at symposia for Ipsen, Novartis and Pfizer. AH and CS are employed by Ipsen. PC has served as a consultant and speaker for Ipsen, Novartis and Pfizer, and has served as an advisory board member for Ipsen.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119(11):3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, et al. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10(4):243–248. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 3.Katznelson L, Laws ER, Jr, Melmed S, Molitch ME, Murad MH, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 4.Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, et al. Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J Clin Endocrinol Metab. 2014;99(4):1282–1290. doi: 10.1210/jc.2013-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer. 2016;23(12):R551–R566. doi: 10.1530/ERC-16-0151. [DOI] [PubMed] [Google Scholar]
- 6.Feelders RA, Hofland LJ, van Aken MO, Neggers SJ, Lamberts SW, et al. Medical therapy of acromegaly: efficacy and safety of somatostatin analogues. Drugs. 2009;69(16):2207–2226. doi: 10.2165/11318510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Cozzi R, Attanasio R. Octreotide long-acting repeatable for acromegaly. Expert Rev Clin Pharmacol. 2012;5(2):125–143. doi: 10.1586/ecp.12.4. [DOI] [PubMed] [Google Scholar]
- 8.Burness CB, Dhillon S, Keam SJ. Lanreotide autogel((R)): a review of its use in the treatment of patients with acromegaly. Drugs. 2014;74(14):1673–1691. doi: 10.1007/s40265-014-0283-8. [DOI] [PubMed] [Google Scholar]
- 9.Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, et al. Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2005;90(8):4465–4473. doi: 10.1210/jc.2005-0260. [DOI] [PubMed] [Google Scholar]
- 10.Shanik MH, Cao PD, Ludlam WH. Historical response rates of somatostatin analogues in the treatment of acromegaly: a systematic review. Endocr Pract. 2016;22(3):350–356. doi: 10.4158/EP15913.RA. [DOI] [PubMed] [Google Scholar]
- 11.Colao A, Auriemma RS, Pivonello R, Kasuki L, Gadelha MR. Interpreting biochemical control response rates with first-generation somatostatin analogues in acromegaly. Pituitary. 2016;19(3):235–247. doi: 10.1007/s11102-015-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paragliola RM, Corsello SM, Salvatori R. Somatostatin receptor ligands in acromegaly: clinical response and factors predicting resistance. Pituitary. 2017;20(1):109–115. doi: 10.1007/s11102-016-0768-4. [DOI] [PubMed] [Google Scholar]
- 13.Colao A, Marek J, Goth MI, Caron P, Kuhn JM, et al. No greater incidence or worsening of cardiac valve regurgitation with somatostatin analog treatment of acromegaly. J Clin Endocrinol Metab. 2008;93(6):2243–2248. doi: 10.1210/jc.2007-2199. [DOI] [PubMed] [Google Scholar]
- 14.Mazziotti G, Giustina A. Effects of lanreotide SR and Autogel on tumor mass in patients with acromegaly: a systematic review. Pituitary. 2010;13(1):60–67. doi: 10.1007/s11102-009-0169-z. [DOI] [PubMed] [Google Scholar]
- 15.Colao A, Pivonello R, Auriemma RS, Briganti F, Galdiero M, et al. Predictors of tumor shrinkage after primary therapy with somatostatin analogs in acromegaly: a prospective study in 99 patients. J Clin Endocrinol Metab. 2006;91(6):2112–2118. doi: 10.1210/jc.2005-2110. [DOI] [PubMed] [Google Scholar]
- 16.Potorac I, Petrossians P, Daly AF, Alexopoulou O, Borot S, et al. T2-weighted MRI signal predicts hormone and tumor responses to somatostatin analogs in acromegaly. Endocr Relat Cancer. 2016;23(11):871–881. doi: 10.1530/ERC-16-0356. [DOI] [PubMed] [Google Scholar]
- 17.Puig-Domingo M, Resmini E, Gomez-Anson B, Nicolau J, Mora M, et al. Magnetic resonance imaging as a predictor of response to somatostatin analogs in acromegaly after surgical failure. J Clin Endocrinol Metab. 2010;95(11):4973–4978. doi: 10.1210/jc.2010-0573. [DOI] [PubMed] [Google Scholar]
- 18.Heck A, Ringstad G, Fougner SL, Casar-Borota O, Nome T, et al. Intensity of pituitary adenoma on T2-weighted magnetic resonance imaging predicts the response to octreotide treatment in newly diagnosed acromegaly. Clin Endocrinol (Oxf) 2012;77(1):72–78. doi: 10.1111/j.1365-2265.2011.04286.x. [DOI] [PubMed] [Google Scholar]
- 19.Heck A, Emblem KE, Casar-Borota O, Bollerslev J, Ringstad G. Quantitative analyses of T2-weighted MRI as a potential marker for response to somatostatin analogs in newly diagnosed acromegaly. Endocrine. 2016;52(2):333–343. doi: 10.1007/s12020-015-0766-8. [DOI] [PubMed] [Google Scholar]
- 20.Shen M, Zhang Q, Liu W, Wang M, Zhu J, et al. Predictive value of T2 relative signal intensity for response to somatostatin analogs in newly diagnosed acromegaly. Neuroradiology. 2016;58(11):1057–1065. doi: 10.1007/s00234-016-1728-4. [DOI] [PubMed] [Google Scholar]
- 21.Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M. Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary. 2013;16(4):490–498. doi: 10.1007/s11102-012-0445-1. [DOI] [PubMed] [Google Scholar]
- 22.Gatto F, Biermasz NR, Feelders RA, Kros JM, Dogan F, et al. Low beta-arrestin expression correlates with the responsiveness to long-term somatostatin analog treatment in acromegaly. Eur J Endocrinol. 2016;174(5):651–662. doi: 10.1530/EJE-15-0391. [DOI] [PubMed] [Google Scholar]
- 23.Gatto F, Feelders RA, van der Pas R, Kros JM, Waaijers M, et al. Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2013;98(1):E66–E71. doi: 10.1210/jc.2012-2609. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Levi AM, Bernabeu I, Sampedro-Nunez M, Marazuela M. Genetic predictors of response to different medical therapies in acromegaly. Prog Mol Biol Transl Sci. 2016;138:85–114. doi: 10.1016/bs.pmbts.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Kasuki L, Wildemberg LE, Neto LV, Marcondes J, Takiya CM, et al. Ki-67 is a predictor of acromegaly control with octreotide LAR independent of SSTR2 status and relates to cytokeratin pattern. Eur J Endocrinol. 2013;169(2):217–223. doi: 10.1530/EJE-13-0349. [DOI] [PubMed] [Google Scholar]
- 26.Kasuki L, Vieira Neto L, Wildemberg LE, Colli LM, de Castro M, et al. AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocr Relat Cancer. 2012;19(3):L25–L29. doi: 10.1530/ERC-12-0020. [DOI] [PubMed] [Google Scholar]
- 27.Baldys-Waligorska A, Krzentowska-Korek A, Golkowski F, Sokolowski G, Hubalewska-Dydejczyk A. The predictive value of the IGF-1 level in acromegaly patients treated by surgery and a somatostatin analogue. Endokrynol Polska. 2011;62(5):401–408. [PubMed] [Google Scholar]
- 28.Sherlock M, Fernandez-Rodriguez E, Alonso AA, Reulen RC, Ayuk J, et al. Medical therapy in patients with acromegaly: predictors of response and comparison of efficacy of dopamine agonists and somatostatin analogues. J Clin Endocrinol Metab. 2009;94(4):1255–1263. doi: 10.1210/jc.2008-1420. [DOI] [PubMed] [Google Scholar]
- 29.Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr Rev. 2011;32(2):247–271. doi: 10.1210/er.2010-0002. [DOI] [PubMed] [Google Scholar]
- 30.Bonneville F, Riviere LD, Petersenn S, Bevan J, Houchard A et al (2019) MRI T2 signal intensity and tumor response in patients with GH-secreting pituitary macroadenoma: PRIMARYS post hoc analysis. Eur J Endocrinol 180(3):155–164 [DOI] [PubMed]
- 31.Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12(2):132–139. doi: 10.1111/j.1365-2753.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 32.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 33.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro-Oliveira A, Jr, Abrantes MM, Barkan AL. Complex rhythmicity and age dependence of growth hormone secretion are preserved in patients with acromegaly: further evidence for a present hypothalamic control of pituitary somatotropinomas. J Clin Endocrinol Metab. 2013;98(7):2959–2966. doi: 10.1210/jc.2013-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S. The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab. 2005;90(11):6290–6295. doi: 10.1210/jc.2005-0998. [DOI] [PubMed] [Google Scholar]
- 36.Arosio M, Reimondo G, Malchiodi E, Berchialla P, Borraccino A, et al. Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol. 2012;167(2):189–198. doi: 10.1530/EJE-12-0084. [DOI] [PubMed] [Google Scholar]
- 37.Colao A, Pivonello R, Auriemma RS, Galdiero M, Savastano S, et al. Growth hormone-secreting tumor shrinkage after 3 months of octreotide-long-acting release therapy predicts the response at 12 months. J Clin Endocrinol Metab. 2008;93(9):3436–3442. doi: 10.1210/jc.2008-0424. [DOI] [PubMed] [Google Scholar]
- 38.Mercado M, Borges F, Bouterfa H, Chang TC, Chervin A, et al. A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol (Oxf) 2007;66(6):859–868. doi: 10.1111/j.1365-2265.2007.02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuevas-Ramos D, Carmichael JD, Cooper O, Bonert VS, Gertych A, et al. A structural and functional acromegaly classification. J Clin Endocrinol Metab. 2015;100(1):122–131. doi: 10.1210/jc.2014-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puig Domingo M. Treatment of acromegaly in the era of personalized and predictive medicine. Clin Endocrinol (Oxf) 2015;83(1):3–14. doi: 10.1111/cen.12731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.