Abstract
The natural estrogen 17β-estradiol (17β-E2) is a major endocrine disruptor. Accordingly, due to their frequent presence in global surface waters, prolonged exposure to estrogen-contaminated water may disrupt sexual development in animals. It has adverse effects on wildlife and humans. To date, the most effective strategy for estrogen removal from the environment is biodegradation using microorganisms. To this end, we isolated a strain of Lysinibacillus sphaericus, namely DH-B01, from a contraceptive factory in Beijing. The experimental results revealed that the bacterium has a high capacity to degrade estrogen, with a 17β-E2 degradation rate of about 97%, and produces the secondary metabolite estrone. In addition, a series of genes involved in steroid metabolism and stress response in L. sphaericus sp. DH-B01 were predicted, and several key genes with high similarity to those of other strains were subjected to sequence alignment to find their conserved regions. This is the first study of the ability of L. sphaericus strains to degrade estrogens and the degradation mechanism involved. This work advances the genomic study of estrogen-degrading strains and the study of bacterial estrogen degradation mechanisms. In this paper, a novel bacterial strain capable of degrading 17β-E2 was studied. L. sphaericus sp. DH-B01 can effectively degrade 17β-E2. During the degradation process, 17β-E2 can be gradually metabolized to a substance without estrogen activity. By analyzing the enzymatic reactions in the metabolic process, we found genes with high similarity to reported 17β-HSD. L. sphaericus sp. DH-B01 was found to degrade 17β-E2. There are many types of bacteria that are currently being studied for the degradation of estrogen, but L. sphaericus sp. DH-B01 is the only strain of L. sphaericus that has been shown to degrade estrogen. This work advances the genomic study of estrogen-degrading bacterial strains and the study of bacterial estrogen degradation mechanisms. Additionally, it explores the correlation between different L. sphaericus strains. The differences play an important role and further enrich the functionality and diversity of L. sphaericus strains. In subsequent studies, the specificity of L. sphaericus sp. DH-B01 can be applied to different environments for future environmental restoration.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2155-0) contains supplementary material, which is available to authorized users.
Keywords: 17β-E2, Lysinibacillus sphaericus, Degradation pathway, Sequence alignment
Introduction
At present, the negative effects of endocrine disrupting compounds (EDC), such as estrogens and alkyl phenols, on animal and human endocrine systems are a worldwide concern (Falconer et al. 2006; Liu et al. 2009). Estrogens are classified as natural estrogens, including estrone (E1), 17β-estradiol (17β-E2) and estriol; and synthetic estrogens, such as 17α-ethinyl estradiol (EE2), which are the main sources of estrogenic activity in wastewater and surface water (Solé et al. 2000). Among them, the natural estrogen 17β-E2 has the highest estrogenic activity (Xiong et al. 2018). Additionally, compared with other natural estrogens, 17β-E2 has stronger endocrine disruptive effect. Estrogens are excreted by humans and animals through the urine and feces and accumulate in sewage. Even after being subjected to currently available treatments, only a part of the estrogen content in domestic wastewater is removed. As a result estrogens are continuously being discharged into the environment where they remain for a long time, which ultimately has a significant negative impact on human health and the ecological environment (Kolodziej et al. 2003; Ternes et al. 1999). Several studies have shown that biodegradation is the most effective way to remove estrogen from the natural environment (Johnson and Sumpter 2013; Writer et al. 2012). For example, the use of microbial biodegradation has significantly improved the removal of estrogen contamination and bioremediation of polluted environments (Xiong et al. 2018). As a result of the concern for environmental estrogen, many bacterial strains that have the ability to efficiently degrade estrogen, such as Sphingomonas, Pseudomonas, Acinetobacter, Rhodococcus, and Bacillus, have been isolated from the environment (Roh and Chu 2010; Yu et al. 2013). The estrogen degradation capacity of each strain varies greatly, but little research has been reported on the degradation mechanism and products. Therefore, further research on the mechanism and products is needed.
In this study, we isolated a bacterial strain degrading 17β-E2, designated as DH-B01, from activated sludge obtained from a wastewater treatment plant that was associated with a steroid contraceptive plant in Beijing. The bacterium was identified as Lysinibacillus sphaericus, formerly known as Bacillus sphaericus, and is defined as having a spherical terminal spore. The L. sphaericus is a class of aerobic, spore-forming Gram-positive, mesophilic, rod-shaped bacteria suitable for growth at temperatures of 30 °C, and mild alkaline environments. Isolates of this species from nickel-contaminated soils (Abou-Shanab et al. 2007), industrial landfills (Desai et al. 2008), natural metal-bearing soils (Pal and Paul 2004), and uranium mines (Selenska-Pobell et al. 1999), they have been reported as potential metal bioremediation bacteria. Furthermore resistance to arsenic as high as 200 mM has been reported for certain L. sphaericus strains (Peña-Montenegro and Dussán 2013).
The process of estrogen degradation can be achieved through a variety of enzymes. To this end, after discovering the ability of L. sphaericus DH-B01 to degrade 17β-E2, we performed genome-wide sequencing of L. sphaericus DH-B01 to find degradation genes that play a key role in the estrogen degradation process. Through the alignment of amino acid sequences and prediction of protein structures, the similarity between highly conserved sequences and protein structures was evaluated to determine whether the selected key genes were homologous. This is the first report of estrogen-degrading strain of L. sphaericus, which besides contributing to the genomic database of estrogen-degrading microorganisms, reveals the biodegradation mechanism of estrogen, and suggests future environmental remediation applications.
Methods
Isolation and screening of estradiol degrading bacteria
Sludge was collected from a contraceptive factory in Beijing. 1 mL of the selected sample was diluted with sterile water from 10–2 to 10–8 step by step and was coated on LB solid medium; three parallel groups were made and cultured for 2 days and the colony was observed. The better growing colonies were separated on LB solid medium for many times until a single colony was obtained. Transfer the single colony to the test tube slope for preservation. After the OD600 value of bacterial suspension reached 1.0 or higher (about 48 h later), the bacterial suspension was transferred to an inorganic salt medium containing only 17β-E2 as the sole carbon source to test its growth and bacterial degradation ability. A single strain, designated DH-B01, with the ability to degrade estradiol was selected and purified.
Identification, physiological characterization and phylogenetic analysis of the novel bacterial strain
DH-B01 DNA was amplified using 16s rDNA primers and the nucleotide sequence of the PCR product was determined to identify the bacterial strain. Briefly, cells were dissolved in 50 μM of TaKaRa Lysis Buffer for Microorganism to Direct PCR (Code No. D304; TaKaRa, Dalian, China), and the lysate was centrifuged to obtain the DNA (template DNA). Then, 2 × TransTaq® High Fidelity (HiFi) PCR SuperMix I (Code No: AS131; TransGen Biotech Co., Ltd., Beijing, China) was used to amplify the 16S ribosomal DNA (rDNA) gene by polymerase chain reaction (PCR) amplification. The following oligos, 27F: 5′-AGAGTTTGATCMTGGCTCAG-3′ and 1492R: 5′-TACGGYTACCTTGTTACGACTT-3′ were used as primers in the PCR amplification, and the amplified products were sequenced using an ABI DNA Sequencer (Model: 3730XL; Applied Biosystems, Foster City, CA, USA). By comparing with the local database, the closest strains at the species level, based on the 16S rDNA sequence, were selected, and Neighbor-Joining (NJ) method was selected in the MEGA software. Using GGDC 2.1 (https://ggdc.dsmz.de) to predict DNA-DNA hybridization by intergenomic distances (Meierkolthoff et al. 2013). The strain was identified as belonging to the bacterial species L. sphaericus and given the name “DH-B01”. To further characterize the DH-B01strain, standard physiological, and biochemical tests were performed, including: (1) glucose; (2) mannitol; (3) 3% catalase activity detection; (4) glucose phosphate water; (5) semi-solid agar; (6) maltose; (7) nitrate (reduction); (8) sucrose; (9) lysozyme nutrient broth; (10) quality-controlled nutrient broth; (11) urea.
Determination of the optimal growth conditions of DH-B01
To determine the optimum growth conditions of L. sphaericus DH-B01, we evaluated four parameters: temperature, pH value, concentration of estradiol substrate, and inoculation volume of bacterial suspension to determine the optimal degradation capacity of the bacterial strain under the prescribed optimum conditions. The strain was cultured for 48 h in an Erlenmeyer flask containing 100 mL of Lysogeny broth (LB) medium, and subsequently inoculated into 100 mL of an inorganic salt medium at an initial OD600 of 1.0. To determine the optimal temperatures, degradation of 30 mg/L of 17β-E2 by each culture was assessed in inorganic salts medium at a temperature of 15, 20, 25, 30, 35, or 40 °C. To determine the optimal pH, 30 mg/L of 17β-E2 was degraded by each culture in inorganic salt medium at pH 4, 5, 6, 7, 8, or 9. In addition, the DH-B01 bacterial strain cells were cultured in inorganic salt medium containing 1, 5, 10, 30, 50, or 70 mg/L of the different types of estradiol tested. Also, different volumes of bacterial suspension inoculum (1, 3, 5, 7, or 9 mL), were inoculated into separate flasks containing inorganic salt medium and 30 mg/L of 17β-E2. The strain was cultured in the initial pH 7, 30 °C, 120 r/min constant temperature shaking incubator for 48 h. The growth of bacteria in the four sets of experiments was evaluated by measuring the optical density (OD600) to determine the optimal growth conditions of the DH-B01 strain. Then, the 17β-E2 degradation efficiency of strain DH-B01 was determined by detecting the residues of 17β-E2 in the culture by high performance liquid chromatography (HPLC). The HPLC detector was UV (DualλAbsorbance Detector, Water 2487), and the chromatographic column was a Zorbax Eclipse Plus C18 column (150 mm × 4.6 mm, 3.5 mm). The mobile phase volume ratio was V (acetonitrile): V (water) = 1: 1. The detector wavelength was 275 nm, the flow rate was 0.8 mL/min, and the injection volume was 10 μL. Each sample was repeated three times and the average value was taken. HPLC determination of the standard equation of E2, with different concentrations of E2 solution 1, 5, 10, 20, 50 mg/L, the standard equation is Y = 15001X + 2719.7, R2 = 0.998, where Y is the HPLC spectrum Peak area, X is the E2 concentration, and R2 is the correlation coefficient.
L. sphaericus DH-B01 genome sequencing
The strain L. sphaericus DH-B01 was inoculated in LB liquid medium, cultured at 30 °C, with shaking at 120 rpm for 24 h. Subsequently, the bacterial suspension was centrifuged at 4000 rpm for 10 min, the supernatant was discarded, the cells were resuspended in 0.2 M phosphate buffer (pH 7.0), and then centrifuged again. The centrifugation was repeated three times and the precipitate was collected each time. Then, the genomic DNA was extracted using a bacterial genomic DNA extraction kit, DNA extraction kit were obtained from Tiangen Biochemical Technology Co., Ltd. (Beijing, China). Next, a TBS380 or Nanodrop2500 spectrometer was used to determine the genomic DNA concentration and to assess the quality of the DNA (no degradation, OD 260/280 = 1.8–2.0, total not less than 10 μg). Genomic DNA was fragmented into 8–10 k fragments using the G-tubes method. The ends were filled in, and the two ends were respectively connected to a circular single strand: the two ends of the single strand were respectively ligated to the double-stranded positive and negative strands to construct a Single Molecule, Real-Time (SMRT) Bell library. The library single-stranded loop was annealed and bound to a polymerase at the bottom of a fixed ZMW (zero-mode waveguides). After binding, sequencing was performed using the PacBio third-generation sequencing platform. The raw data obtained by sequencing was filtered to obtain the clean data. Starting from Clean Data after quality control of each sample, the SMRT portal software was used to assemble the sequencing reads into single contig that represented the entire genome.
Liquid chromatography mass spectrometry
The E2 metabolites were analyzed by liquid chromatography/mass spectrometry (LC/MS) on a PE Sciex API 2000 MDS LC/MS System (Applied Biosystems), using the electrospray ionization (ESI) method in negative ion mode to scan in the 50–600 Da range. According to first-order mass spectrometry, the product is the mother ion, which enters the collision chamber and reacts with the high-purity nitrogen gas, and the ion mass spectra were obtained by the MS2 analyzer and receiver.
Sequence comparison
The homology of the nucleotide and the predicted amino acid sequences of the estrogen-degrading enzyme was analyzed using BLAST (NCBI, Bethesda, MD, USA). The L. sphaericus DH-B01 was aligned with the target strain to translate the nucleotide sequence into an amino acid sequence. The similarity between CLUSTALX and several genes with the same function with higher homology in the alignment results was compared, and several genes with higher similarity were combined with the predicted protein model using the ESPript software (https://espript.ibcp.fr/ESPript/ESPript/) to obtain the conserved region of the sequence and determine the protein. The secondary structure of the protein was also determined.
Results and discussion
Bacterial isolation identification and description
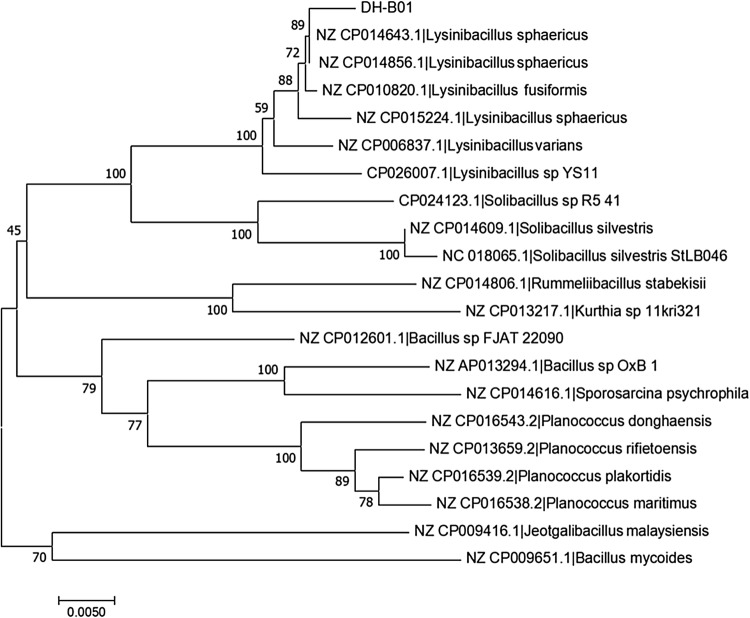
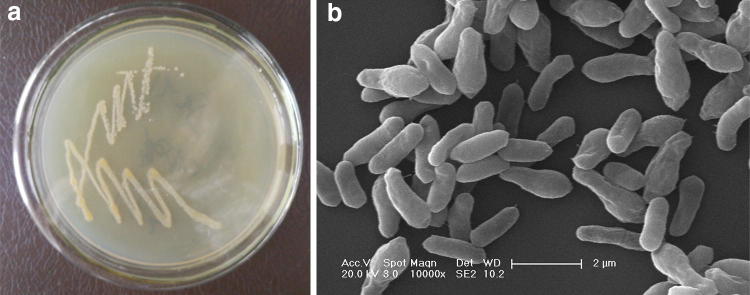
A strain capable of degrading 17β-E2 was isolated from the sludge of a pharmaceutical company in Beijing. The Neighbor-Joining method was used to construct a phylogenetic tree of 16S rDNA sequences (Fig. 1) showing that the bacterium belongs to the bacterial species Lysinibacillus sphaericus. The species L. sphaericus is a genetically heterogeneous species comprising strains that are divided into five DNA homologous groups. Strains that are pathogenic to mosquitoes have been found in the IIA subgroup, but these homologues also contain non-pathogenic isolates, and subgroup IIB has been assigned to the species Lysinibacillus fusiformis (Nakamura 2000). Among the five closely related strains of bacteria DH-B01, DNA-DNA relatedness value with L. sphaericus sp. OT4b.25, L. sphaericus sp. III (3)7 and L. sphaericus sp. 2362 is the highest, DNA-DNA relatedness value with L. fusiformis sp. RB-21 is 51.20 ± 6.80%, DNA-DNA relatedness value with L. varians sp. GY32 is 24.00 ± 18.19%, but with L. sphaericus sp. 2362 is 91.60 ± 7.21%, indicating the same species (Table 1). DH-B01 bacterial cells produce light yellow, smooth streaks on agar plates (Fig. 2a). The individual cells were rod-shaped, with a length of about 2 μm and their surface was slightly wrinkled (Fig. 2b). The biochemical analyses (Table 2) revealed that L. sphaericus DH-B01 reacted with urea was positive, indicating that the strain contained urease. In addition, L. sphaericus DH-B01 did not react with glucose, maltose or sucrose, indicating that DH-B01 cannot ferment sugars.
Fig. 1.
Lysinibacillus sphaericus DH-B01 16 s rDNA phylogenetic tree. The phylogenetic tree was constructed with MEGA 7.0 software using the neighbor-joining method with Kimura two-parameter model. The bootstrap consensus tree was performed with 1000 replicates
Table 1.
DNA-DNA hybridization values of DH-B01 with closely related strains
| Strains | %DNA-DNA hybridization |
|---|---|
| L. sphaericus sp. OT4b.25 | 91.50 ± 7.25 |
| L. sphaericus sp. III (3)7 | 91.50 ± 7.23 |
| L. fusiformis sp. RB-21 | 51.20 ± 6.80 |
| L. sphaericus sp. 2362 | 91.60 ± 7.21 |
| L. varians sp. GY32 | 24.00 ± 18.19 |
Fig. 2.
aLysinibacillus sphaericus DH-B01 plate culture. b DH-B01 scanning electron micrograph (SEM)
Table 2.
Biochemical characteristics of L. sphaericus DH-B01
| Test items | Positive ( +) Negative ( −) |
Test items | Positive ( +) Negative ( −) |
|---|---|---|---|
| Glucose | − | Mannitol | − |
| 3% Hydrogen peroxide | + | Glucose phosphate | − |
| Semi-solid agar | + | Maltose | − |
| Nitrate (reduction) | − | Sucrose | − |
| Lysozyme nutrient broth | + | Urea | + |
| Quality control nutrient broth | + |
HPLC detection of DH-B01 ability to degrade 17β-E2
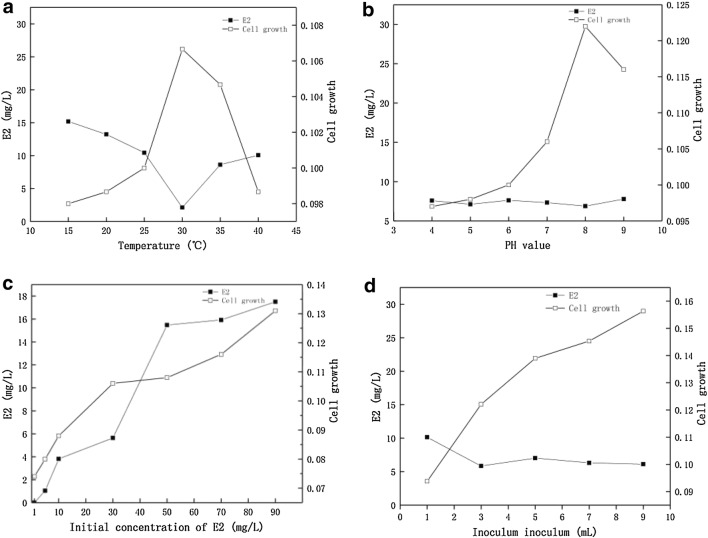
The degradation of 17β-E2 by L. sphaericus DH-B01, was assayed under different conditions growth and 17β-E2 degradation were maximum at 30 °C Fig. 3a. The growth rate was highest at pH 8 (Fig. 3b). Different concentrations, in the range from 1 to 70 mg/L, of 17β-E2 were degraded by DH-B01, as shown in Fig. 3c. From the residual amount of 17β-E2, the 1 mg/L residue of 17β-E2 was completely degraded, and the degradation efficiency of 17β-E2 at 30 mg/L was the highest (Fig. 3c). There was not much difference in the degradation effect of different amounts of bacteria. This may be due to insufficient carbon source in the growing medium, which causes the bacteria to have a protective mechanism to form spores and no longer participate in the degradation process (Fig. 3d).
Fig. 3.
Effect of degradation of 17β-E2 by DH-B01 under different conditions. a Different temperatures in the range from 15 to 40 °C. b Different pH values in the range 4–9. c Different concentrations of 17β-E2 in the range from 1 to 70 mg/L. d The dosage of bacteria is 1–9 mL. The solid line represents the residual content of 17β-E2 of 30 mg/L, and the hollow line represents the growth of DH-B01 (OD600)
The degradation rate of estrogen by L. sphaericus DH-B01 was verified by HLPC analysis. As shown in Fig. 4, L. sphaericus DH-B01 can utilize estradiol as the sole carbon source and exhibited a remarkable degradation effect, degrading 90% or more of 17β-E2 (30 mg/L) at 72 h After 17β-E2 is degraded to E1, E1 can continue to act as a carbon source for bacterial growth until the E1 is also completely degraded.
Fig. 4.

L. sphaericus sp. DH-B01 growth turbidity (OD600) and degradation of 17β-E2 in inorganic salt medium for 120 h. Each sample was repeated 3 times and the average value was taken
L. sphaericus DH-B01 whole genome sequencing analysis
L. sphaericus strains can transform into dormant spore cells under extreme conditions and switch to sporophyte reproduction under suitable conditions. Therefore, L. sphaericus strains have strong resistance to environmental hazards. It has been reported that L. sphaericus strains can still survive in heavy metal-containing environments. Numerous reports on the ability of L. sphaericus strains to inactivate Culex pipiens (common house mosquito) have been reported, but there has been no report on the ability of L. sphaericus strains to degrade estrogen chemicals. To gain a better insight into the mechanism underlying the degradation of estrogen by L. sphaericus DH-B01, we sequenced the whole genome and obtained a complete circular chromosome with a total length of 4,602,887 bp and an average GC content of 37.27%. GenBank accession number is no. CP045583 (chromosome, https://www.ncbi.nlm.nih.gov/nuccore/CP045583). The genome is predicted to contain 4395 coding genes, accounting for 83.38% of the total length. The genome also encodes 107 tRNAs and 37 rRNAs (13 5s rRNAs, 12 16s rRNAs, 12 23s rRNAs). A total of 4244 Cluster of Orthologous Groups (COG)-annotated genes were grouped into four categories and 21 types (Table S1). The seven most abundant types are: amino acid transport and metabolism (n = 382), transcription (n = 280), inorganic ion transport and metabolism (n = 258), signal transduction mechanisms (n = 218), replication, recombination and repair (n = 206), translation, ribosomal structure and biogenesis (n = 198), energy production and conversion (n = 180). The genomic characteristics of L. sphaericus DH-B01 were compared with those of six other strains with genome sizes ranging from 4.5 to 4.8 M bp. The number of coding genes in all the genomes is greater than 4000, and the number of RNA genes is between 130 and 150 (Table 3).
Table 3.
Genomic comparison of 7 Lysinibacillus sphaericus strains
| Genome features | L. sphaericus sp. DH-B01 | L. sphaericus sp. OT4b.25 | L. sphaericus sp. III (3)7 | L. fusiformis sp. RB-21 | L. sphaericus sp. 2362 | L. varians sp. GY32 | L. sphaericus sp. YS11 |
|---|---|---|---|---|---|---|---|
| Genome size(bp) | 4,602,887 | 4,665,575 | 4,663,526 | 4,843,789 | 4,692,801 | 4,662,822 | 4,584,915 |
| CDS genes | 4395 | 4565 | 4572 | 4476 | 4389 | 4506 | 4456 |
| Nucleotide (Genomic DNA) | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| Protein-coding | 4278 | 4452 | 4485 | 4461 | 4295 | 4506 | 4263 |
| RNA genes | 149 | 149 | 149 | 150 | 149 | 130 | 146 |
| rRNA genes | 37 | 37 | 37 | 42 | 37 | 31 | 34 |
| tRNA genes | 107 | 107 | 107 | 107 | 107 | 98 | 107 |
| ncRNA genes | 5 | 5 | 5 | 1 | 5 | 1 | 5 |
Lysinibacillus sphaericus strains are among the most popular and effective entomopathogenic bacteria and are a highly heterogeneous group. L. sphaericus strains can grow in arsenate, hexavalent chromium and lead, have a certain tolerance to heavy metals, and are also useful for biological control and bioremediation of mosquitoes (Lozano and Dussán 2013). In fact, they are available for biological control of malaria, filariasis, dengue fever, and West Nile fever (Porter et al. 1993; Vaidyanathan and Scott 2007). L. sphaericus DH-B01 also has multiple stress response genes; multidrug resistance, such as MATE (multidrug and toxin extrusion) family efflux pump gene norM; antibiotic resistance, such as gene fusA (extension factor G) (Peña-Montenegro and Dussán 2013). Based on the KEGG analysis, some predicted proteins may be involved in the degradation of sodium benzoate, aminobenzoate, quinolate, toluene, naphthalene, eugenol, limonene, decene, chloroalkane, chloroolefin, styrene B, benzene, caprolactam and atrazine compounds, and biosynthesis of streptomycin, neomycin, zeatin, amyloid, penicillin, and cephalosporin (Peña-Montenegro and Dussán 2013).
Analysis of the E2 degradation products
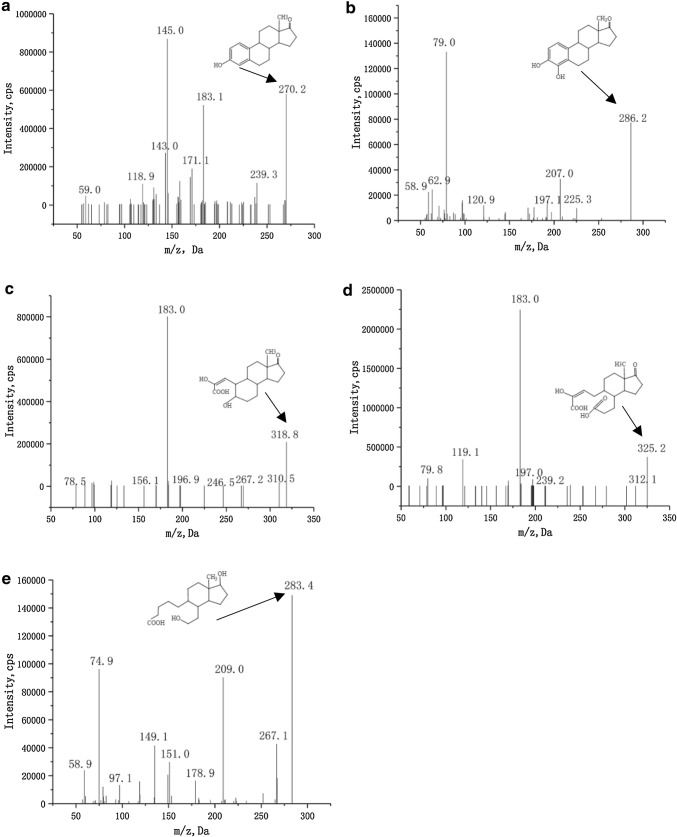
The 17β-E2 degradation products were separated and identified by High-performance liquid chromatography (HPLC) (Fig. 5). In the mass spectrometry (MS) analysis of 17β-E2 metabolites, the negative ion scanning mode was used to obtain the mass spectra of the cultured samples. The mass–charge ratio (m/z) corresponds to the molecular weight of the product minus subtracted from the proton, i.e.[M-H]. The spectra of HPLC peaks are shown in the Fig. 6. No additional metabolites were detected by LC–MS/MS analysis, possibly because their concentrations were below the detection range.
Fig. 5.
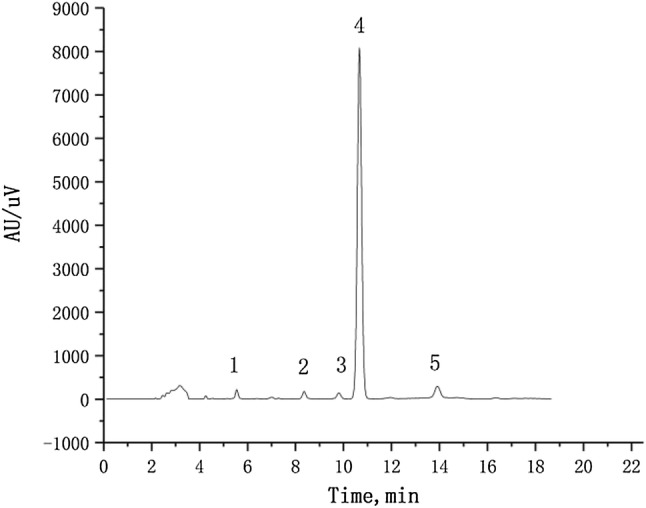
The 17β-E2 degradation products were separated by High-performance liquid chromatography (HPLC) and analyzed by LC–MS/MS. The degradation rate reached a stable state in 48 h. Therefore, the metabolites of 17β-E2 were identified at 48 h. Peak 4 corresponds to the retention time of the 17β-E2 substrate chromatographic peaks for 10.68 min. Peaks 1–3 and 5 correspond to 17β-E2 metabolites, and the other unmarked peaks are background peaks
Fig. 6.
LC–MS/MS spectra of metabolites of 17β-E2
The results of the mass spectrometry analysis of the 17β-E2 degradation products shown in Fig. 6 reveal that after 48 h of incubation in inorganic salt medium, the m/z values indicate that they may be the metabolites of 17β-E2. Through analysis of the secondary MS data and retrieval of reference data from the NIST mass spectrometry online database, five metabolites were found to be produced by 17β-E2 degradation and have been reported to appear in the same metabolic pathway of 17β-E2 (Wang et al. 2019). The values of the metabolites measured by m/z were 270.2, 283.4, 286.2, 318.8, and 325.2 (Fig. 6). By liquid chromatography-tandem mass spectrometry (LC–MS/MS) spectral and ion fragmentation analysis of the six metabolites, it was determined that these five metabolites are all complete molecules, and they were all new metabolites produced by 17β-E2.
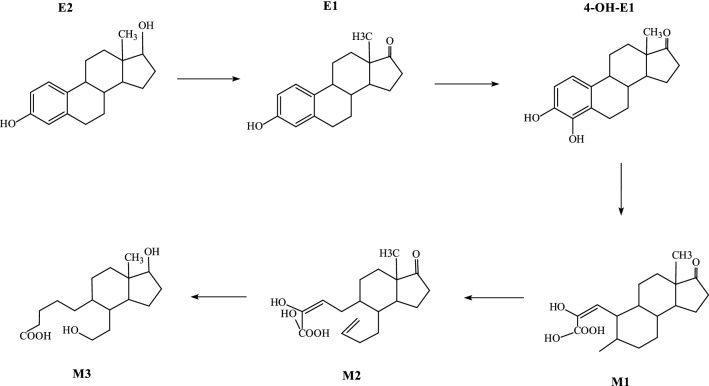
Estrogen degradation pathway and gene coding for catabolic enzymes
Microbial degradation of pollutants is an enzymatic process. The type and activity of the enzymes involved play a key role in E2 degradation. Microorganisms metabolize estrogen mainly through enzymatic degradation. At present, there are more than 60 known kinds of enzymes involved in estrogen catabolism, mainly dehydrogenases, monooxygenases (hydroxylases), dioxygenases, hydrolases, hydratases and some coenzymes (Fig. 7). Among them, 17β-hydroxysteroid dehydrogenase plays a key role in the degradation pathway of 17β-E2 to E1 (Chen et al. 2017). In L. sphaericus DH-B01, a total of 159 dehydrogenase-encoding genes, 9 monooxygenase-encoding genes, 6 dioxygenase-encoding genes, 16 hydratase-encoding genes, and 27 hydrolase encodings were predicted (Table S2).
Fig. 7.
Pathway of 17β-E2 degradation (Wang et al. 2019; Yu et al. 2013)
Based on the analysis of the estrogen metabolites, we can infer the estrogen metabolic pathway of L. sphaericus DH-B01. The degradation of 17β-E2 usually begins with the conversion of 17β-E2 to E1 (Fig. 7). This process involves the oxidation, in L. sphaericus DH-B01, of the C-17 hydroxyl group of 17β-E2 to a ketone group, possibly by a dehydrogenase, and then degradation to other products. It has been reported that E1 can be further degraded to 4-OH-E1. Coombre et al. (1966) first proposed in 1966 (Coombre et al. 1966), that the degradation of E1 is catalyzed through dioxygenase A-ring cleavage, which converts E1 into 4-OH-E1. Cyclic A cleaves 4-OH-E1 to form metabolite M1, which may be mediated by oxygenase. The degradation of M1 to metabolite M2 is a process in which L. sphaericus DH-B01 splits the ring B of M1 to M2 that may involve an oxygenase. It has been reported that metabolite M3 may be 5-(1-Hydroxy-4-(2-hydroxyethyl) -7a-methyloctahydro-1H-inden-5-yl) pentanoic acid (Wang et al. 2019).
Multiple sequence alignments to find conserved regions
At present, the conversion of 17β-E2 to E1 is considered as the first and key step in the degradation of this compound (Yu et al. 2007, 2013). 17β-hydroxysteroid dehydrogenase (17β-HSD) has been shown to catalyze the oxidation/reduction of 17β-E2 and estrone (Xiong et al. 2018). In Sphingomonas strain KC8, the putative 17β-HSD gene (oecA; KC8_09390) is responsible for C17 dehydrogenation of 17β-E2 (Yu et al. 2007). In L. sphaericus DH-B01, there are three coding genes [gene1936 (31.29%), gene3461 (32.11%), gene3569 (33.33%)], whose sequences are highly homologous to that of the Sphingomonas strain KC8 17β-HSD gene (Fig. 8). Indeed, sequence alignment and secondary structure analysis showed that the three genes had multiple highly conserved amino acid regions and motifs with Sphingomonas strain KC8 17β-HSD. Compared with the crystal structure of 17β-HSD of the Sphingomonas strain KC8, there are twelve α-helices and eleven β-sheet structures. Thus, these three genes may belong to the SDR superfamily together with 17β-HSD, which suggests that they may play the same role in the degradation of 17β-E2.
Fig. 8.
Sequence alignment of the predicted protein sequence of Sphingomonas strain KC8 gene (oecA: KC8 _09390) with the predicted protein sequences of three L. sphaericus DH-B01 (gene 1936, gene 3461, gene 3569)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the Natural Science Foundation of Jilin, China (20180101083JC) and the Natural Science Foundation of China (Grant No. 51978132)
Data availability
The data used to support the findings of this study are included within the article. The complete genome sequence of L. sphaericus sp. DH-B01 has been deposited at GeneBank under the accession no. CP045583 (chromosome). This strain has been deposited in China Type Culture Collection on January 14, 2019 under the accession number CCTCC M 2019039.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest, financial or otherwise.
References
- Abou-Shanab RAI, Berkum PV, Angle JS. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere. 2007;68(2):360–367. doi: 10.1016/j.chemosphere.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Chen YL, et al. Biochemical mechanisms and catabolic enzymes involved in bacterial estrogen degradation pathways. Cell Chem Biol. 2017;24(6):712. doi: 10.1016/j.chembiol.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Coombre RG, Tsong YY, Hamilton PB, Sih CJ. Mechanisms of steroid oxidation by microorganisms. X. Oxidative cleavage of estrone. J Biol Chem. 1966;241(7):1587–1595. [PubMed] [Google Scholar]
- Desai C, Jain K, Madamwar D. Evaluation of in vitro Cr(VI) reduction potential in cytosolic extracts of three indigenous Bacillus sp. isolated from Cr(VI) polluted industrial landfill. Bioresour Technol. 2008;99(14):6059–6069. doi: 10.1016/j.biortech.2007.12.046. [DOI] [PubMed] [Google Scholar]
- Falconer IR, Chapman HF, Moore MR, Ranmuthugala G. Endocrine-disrupting compounds: a review of their challenge to sustainable and safe water supply and water reuse. Environ Toxicol. 2006;21(2):181–191. doi: 10.1002/tox.20172. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Sumpter JP. Removal of endocrine-disrupting chemicals in activated sludge treatment works. Environ Sci Technol. 2013;35(24):4697–4703. doi: 10.1021/es010171j. [DOI] [PubMed] [Google Scholar]
- Kolodziej EP, Gray JL, Sedlak DL. Quantification of steroid hormones with pheromonal properties in municipal wastewater effluent. Environ Toxicol Chem. 2003;22(11):2622. doi: 10.1897/03-42. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Kanjo Y, Mizutani S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ. 2009;407(2):731–748. doi: 10.1016/j.scitotenv.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Lozano LC, Dussán J. Metal tolerance and larvicidal activity of Lysinibacillus sphaericus. World J Microbiol Biotechnol. 2013;29(8):1383–1389. doi: 10.1007/s11274-013-1301-9. [DOI] [PubMed] [Google Scholar]
- Meierkolthoff JP, Auch AF, Klenk HP, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013;14(1):60–60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura LK. Phylogeny of Bacillus sphaericus-like organisms. Int J Syst Evol Microbiol. 2000;50(Pt5):1715–1722. doi: 10.1099/00207713-50-5-1715. [DOI] [PubMed] [Google Scholar]
- Pal A, Paul AK. Aerobic chromate reduction by chromium-resistant bacteria isolated from serpentine soil. Microbiol Res. 2004;159(4):347–354. doi: 10.1016/j.micres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Peña-Montenegro TD, Dussán J. Genome sequence and description of the heavy metal tolerant bacterium Lysinibacillus sphaericus strain OT4b.31. Stand Genom Sci. 2013;9(1):42–56. doi: 10.4056/sigs.4227894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AG, Davidson EW, Liu JW. Mosquitocidal toxins of bacilli and their genetic manipulation for effective biological control of mosquitoes. Microbiol Rev. 1993;57(4):838–861. doi: 10.1128/MMBR.57.4.838-861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh H, Chu KH. A 17β-estradiol-utilizing bacterium, Sphingomonas strain KC8: part I—characterization and abundance in wastewater treatment plants. Environ Sci Technol. 2010;44(13):4943–4950. doi: 10.1021/es1001902. [DOI] [PubMed] [Google Scholar]
- Selenska-Pobell S, Panak P, Miteva V, Boudakov I, Bernhard G, Nitsche H. Selective accumulation of heavy metals by three indigenous Bacillus strains, B. cereus, B. megaterium and B. sphaericus, from drain waters of a uranium waste pile. FEMS Microbiol Ecol. 1999;29(1):59–67. doi: 10.1111/j.1574-6941.1999.tb00598.x. [DOI] [Google Scholar]
- Solé M, López de Alda MJ, Castillo M, Porte C, Ladegaard-Pedersen K, Barceló D. Estrogenicity determination in sewage treatment plants and surface waters from the Catalonian area (NE Spain) Environ Sci Technol. 2000;34(24):5076–5083. doi: 10.1021/es991335n. [DOI] [Google Scholar]
- Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken R-D, Servos M. Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany Canada and Brazil. Sci Total Environ. 1999;225(1–2):81–90. doi: 10.1016/S0048-9697(98)00334-9. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Scott TW. Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector Borne Zoonotic Dis. 2007;7(2):193–198. doi: 10.1089/vbz.2006.0589. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shao H, Zhu S, Tian K, Qiu Q, Huo H. Degradation of 17β-estradiol and products by a mixed culture of Rhodococcus equi DSSKP-R-001 and Comamonas testosteroni QYY20150409. Biotechnol Biotechnol Equip. 2019 doi: 10.1080/13102818.2019.1568913. [DOI] [Google Scholar]
- Writer JH, Ryan JN, Keefe SH, Barber LB. Fate of 4-nonylphenol and 17β-estradiol in the redwood River of Minnesota. Environ Sci Technol. 2012;46(2):860–868. doi: 10.1021/es2031664. [DOI] [PubMed] [Google Scholar]
- Xiong W, Peng W, Liang R. Identification and genome analysis of Deinococcus actinosclerus SJTR1, a novel 17β-estradiol degradation bacterium. 3 Biotech. 2018;8(10):433. doi: 10.1007/s13205-018-1466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CP, Deeb RA, Chu K-H. Microbial degradation of steroidal estrogens. Chemosphere. 2013;91(9):1225–1235. doi: 10.1016/j.chemosphere.2013.01.112. [DOI] [PubMed] [Google Scholar]
- Yu CP, Roh H, Chu K-H. 17β-estradiol-degrading bacteria isolated from activated sludge. Environ Sci Technol. 2007;41(2):486–492. doi: 10.1021/es060923f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article. The complete genome sequence of L. sphaericus sp. DH-B01 has been deposited at GeneBank under the accession no. CP045583 (chromosome). This strain has been deposited in China Type Culture Collection on January 14, 2019 under the accession number CCTCC M 2019039.