Summary
CD28H and B7‐H5 have been identified as receptor–ligand pairs in the B7/CD28 family, and have co‐stimulatory activity in immune cells. Here, we have systematically reviewed the research reports concerning the CD28H/B7‐H5 pathway. It was found that CD28H is mainly expressed in T cells and natural killer (NK) cells with naive and poorly differentiated properties, and repeated antigen stimulation leads to permanent loss of CD28H. In tumors, CD28H is mainly expressed in tissue‐resident memory (TRM) lymphocyte T cells, which is associated with improved tumor prognosis. B7‐H5 is a ligand for CD28H and is widely expressed in tumor cells. B7‐H5 expression is closely related to the prognosis of the tumor. Studies have shown that high expression of B7‐H5 in tumor is related to a worse prognosis for lung cancer, osteosarcoma, oral squamous cell carcinoma (OSCC), breast carcinoma, human clear cell renal cell carcinoma (ccRCC), intrahepatic cholangiocarcinoma (ICC), bladder urothelial carcinoma (BUC) and colorectal cancer (CRC), but is associated with a better prognosis for pancreatic ductal adenocarcinoma (PDAC) and glioma. Controversial views exist in studies on gastric cancer prognosis.
Keywords: cancer immunotherapy, cancer prognosis, CD28H/B7‐H5, immune checkpoints, phenotype
The B7/CD28 family regulates T lymphocyte response through co‐stimulation and co‐inhibition. CD28H/B7‐H5, as a new member of the family, has proven to be a costimulatory pathway. The expression of CD28H and B7‐H5 in tumors has certain relationship with tumor prognosis.

Introduction
CD28H was previously named ‘transmembrane and immunoglobulin domain containing 2’ (TMIGD2, gene ID 126259), and has been identified as a novel adhesion molecule involved in angiogenesis in 2012 1. In an extensive whole‐genome search, CD28H was identified based on preferential expression in T cells and homology to CD28 2. The human CD28H gene, constituted by five exons, is located on chromosome 19q13.3. However, no coding gene for CD28H has been found in mice or rats 2. In human peripheral blood cell subsets, CD28H expression in granulocytes, monocytes, bone marrow dendritic cells (MDCs) and B cells is negligible 2, 3. By contrast, high levels of CD28H were detected in T cells, natural killer (NK) cells, plasmacytoid dendritic cells (PDCs) and innate lymphoid cells (ILCs) 2, 3, 4. In human peripheral tissues, CD28H transcripts are preferentially enriched in lymphoid organs. The spleen and thymus have the highest levels of CD28H transcript 2, and the liver is also rich in CD28H transcripts. In human lungs, approximately 20% of CD8+ T cells express CD28H, while almost all CD8+ T cells express CD28H in the human small intestine 5. There is no difference in the moderately expression of CD28H in the tonsils and spleen 3. CD28H is expressed in tumor‐infiltrating lymphocytes (TILs) in different tissues, including pancreatic carcinoma, ovarian carcinoma, breast carcinoma, colorectal carcinoma, lung cancer, melanoma and glioma 3, 5.
The B7/CD28 family regulates lymphocyte responses through co‐stimulation and co‐inhibition 6, 7, 8 ( Fig. 1) and can be phylogenetically divided into three groups 9, 10. The first group consists of B7‐1/B7‐2 / CD28/cytotoxic T lymphocyte antigen 4 (CTLA‐4) and inducible co‐stimulator ligand L/inducible co‐stimulator ligand (ICOSL/ICOS); group II contains programmed cell death ligand 1/programmed cell death 1 (PD‐L1/PD‐L2/PD‐1); the third group includes B7‐H3, B7x (B7‐H4) and HERV–H long terminal repeat (LTR)‐associating protein 2 (HHLA2) (B7H7/B7‐H5)/CD28H (TMIGD2). Identifying the CTLA‐4 and PD‐1/PD‐L1 pathways has had a major impact on the immunotherapy of cancer, resulting in improved treatment and survival of cancer patients 11, 12. B7‐H5, which has also been called B7H7 13, has no specific function, is a ligand for CD28H and was formerly known as human endogenous retrovirus‐H long terminal repeat‐associating 2 (HHLA2) 14. It is the only member of the B7 family that can be found in humans but not in mice. By binding to unknown receptors, B7‐H5 has been reported to be effective in inhibiting the proliferation of human CD4 and CD8 T cells and inhibiting the production of T cell cytokines, including interferon (IFN)‐γ, tumor necrosis factor (TNF)‐α, interleukin (IL)‐5, IL‐10, IL‐13, IL‐17A and IL‐22 9. At the same time, it can also co‐stimulate T cells by the CD28H/B7‐H5 pathway 2. Similarly to the B7‐H3, both play a T cell co‐inhibitory role as well as a co‐stimulatory one. Based on homology searches, B7‐H5 was revealed as a new candidate for the B7 family in 2001 15. B7‐H5 was known as a binding protein for CD28H by screening for receptor sequences of more than 2300 transmembrane proteins 2. As well as B7‐H3 16, which has two repeats of immunoglobulin (Ig)V‐IgC, B7‐H5 differs from other known B7 members with two Ig domains and its extracellular region has three characteristic Ig domains (IgV‐Igc‐IgV), with the first IgV domain having the highest homology to other B7 family members 2. B7‐H5 is preferentially transcribed in peripheral tissues 2, with particularly high levels of B7‐H5 mRNA in the testes, colon, lungs, kidneys and pancreas. B7‐H5 is transcriptionally low in liver and skeletal muscle. In immune cells, B7‐H5 is not transcribed in T cells, whereas B cells, dendritic cells and macrophages are abundant in B7‐H5 mRNA. Studies have shown that the expression of B7‐H5 on B cells is induced by inflammatory stimuli 9. At the same time, studies have suggested that there is no expression of B7‐H5 on freshly isolated monocytes or T, B, NK cells on human peripheral blood mononuclear cells (PBMCs), but B7‐H5 expression is present on monocyte‐derived macrophages and dendritic cells 2. These results suggest that B7‐H5 is largely an inducer of antigen‐presenting cells (APCs) response to inflammation. B7‐H5 is also expressed on activated myeloid cells, and widely expressed in human cancer tissues 2, including pancreatic ductal adenocarcinoma (PDAC) 17, 18, lung cancer 19, osteosarcoma 20, breast cancer 21, oral squamous cell carcinoma (OSCC) 22, human clear cell renal cell carcinoma (ccRCC), intrahepatic cholangiocarcinoma (ICC), bladder urothelial (ccRCC) 23, 24, ICC 25, bladder urothelial carcinoma (BUC) 26, colorectal cancer (CRC) 27, glioma 28 and gastric cancer 29, 30.
Figure 1.
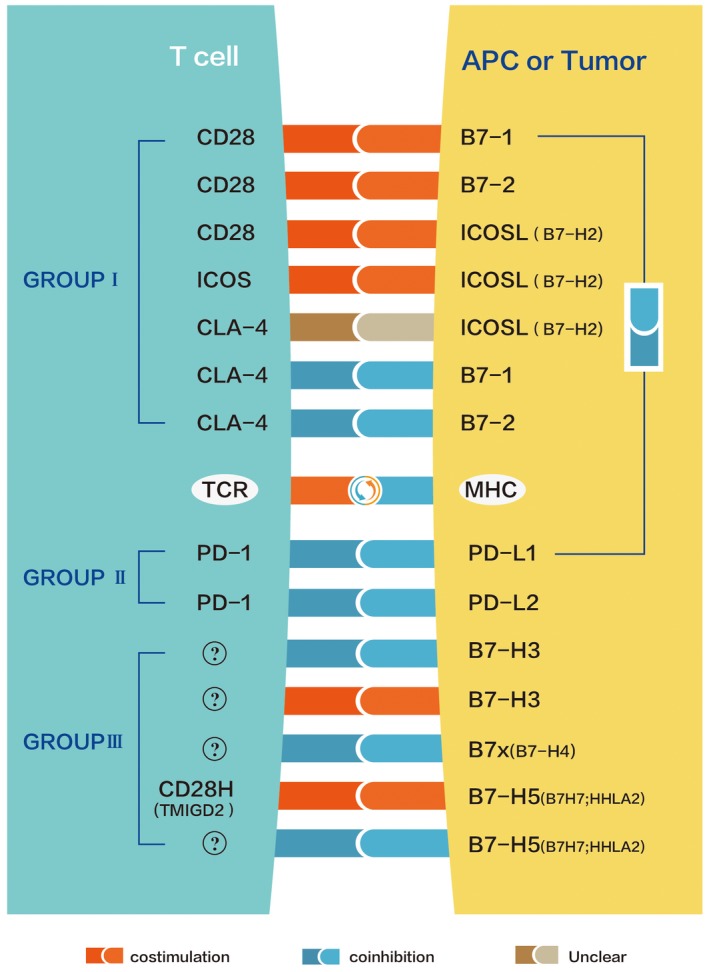
The B7/CD28 family regulates T lymphocyte responses through co‐stimulation and co‐inhibition. CD28 and cytotoxic T lymphocyte antigen 4 (CTLA‐4) compete for the same ligands B7‐1 and B7‐2. Inducible co‐stimulator ligand L (ICOSL) is a ligand for inducible co‐stimulator ligand (ICOS) and can also bind to CD28 and CTLA‐4 65. Programmed cell death 1 (PD‐1) is a common co‐inhibitory receptor for PD ligand 1 (PD‐L1) and PD‐L2. B7‐H3 has unknown co‐inhibitory and co‐stimulatory receptors. B7x has an unknown co‐suppressor receptor. B7‐H5 possesses the co‐stimulatory receptor CD28H and an unknown co‐inhibitory receptor 9. In addition, B7‐1 can interact with PD‐L1 to inhibit the T cell response 66, 67, 68.
Phenotypical characteristics of different immune cells expressing CD28H
Phenotypical characteristics of naive T cells expressing CD28H
It has been shown that CD28H is widely expressed in naive T cells, and studies have shown that only 6–15% of naive T cells are CD28H− 3. By comparing expression of CD31, which is the most recent thymic transplant marker 31, on CD28H+ and CD28H− naive T cells, it was found that CD31 was expressed on most CD28H+ but not CD28H− naive T cells. Furthermore, CD28H− naive T cells express higher levels of the T helper type 1 (Th1) regulatory transcription factor T‐bet, as well as the effectors IFN‐γ and TNF‐α. Similar expression levels of IL‐8 and IL‐2 were found in naive and memory T cells of CD4+CD28H+ and CD4+CD28H− 3. It can be concluded that CD28H+ naive T cells exhibit more abundant naive characteristics than CD28H− naive T cells.
Phenotypical characteristics of activated T cells expressing CD28H
Activation of T cells results in irreversible loss of CD28H. Twenty per cent of memory T cells express CD28H 3, while almost all T cells with a senescent phenotype (CD7lowCD27−CD28−CD57+) do not express CD28H 2, 32, 33, 34, 35. Studies have shown that IFN‐γ and CD57 levels in CD28H− T cells are higher than in CD28H+ T cells 3. CD57 can be used as a marker for T cell terminal differentiation 36. Based on the expression of CCR7 and CD45RA, CD8+ T cells can be divided into different subpopulations 37, including naive T cells (CD45RA+CCR7+), central memory T cells (CD45RA−CCR7+) and effector memory T cells (CD45RA−CCR7−; CD45RA+CCR7−). Nearly all (97·5%) naive T cells (CD45RA+CCR7+) were found to constitutively express CD28H 2. Approximately half (52·9 and 41·5%) of the T cells with central memory (CD45RA−CCR7+) and effector memory (CD45RA−CCR7−) phenotypes were CD28H+ 2. Most (73·6%) CD45RA+ effector memory cells (CD45RA+CCR7−), possibly terminally differentiated effector T cells, lose expression of CD28H 2, 37, 38. A similar finding was detected in the CD4+ T cell subset 2. By staining CD28H on T cells and various other T cell surface markers, it was found that CD28H− T cells have higher lateral scatter and CD45RO is expressed in most of them, which do not express CD45RA 2. Furthermore, expression of activation‐induced molecules, such as PD‐1, was shown to be increased in CD28− T cells, while expression levels of CD62L and CD27 were decreased 39, 40. Similarly, almost all CD4+ T helper cells producing effector cytokines or forkhead box protein 3 (FoxP3)+CD4+ T regulatory cells did not express CD28H. These results led to the conclusion that less differentiation and effector function are shown in CD28H+ memory T cells, and the senescence of T cell may lead to the loss of CD28H expression. In human peripheral tissues, It is worth noting that all CD8+ T cells in the human small intestine were CD28H+ and they all express CD103 and CD69 5, which is the main feature of tissue‐resident memory (TRM) T cells, which have recently been identified as a new subset of memory T cells 41, 42, 43. In human lungs, approximately 20% of CD8+ T cells express CD28H, but in TRM cells there are more CD28H+ T cells (27·4 versus 11·1%) 5. In the spleen, although few CD8+ T cells showed a TRM phenotype, CD28H expression was highest in TRM cells (33%) 5. These findings indicate that in human tissues T cells expressing CD28H are mainly T cells with a TRM phenotype. In this study, it was demonstrated that IL‐2‐stimulated T cells gradually lost CD28H expression during division, while stimulation of IL‐7 plus IL‐15 preserved the expression of CD28H 5. Regardless of the stimulation by OKT3 or IL‐15, stimulation with TGF‐β can attract higher expression of CD28H in activated CD8+ T cells 5. It was also found that the expression of CD103+CD69+ TRM cells in activated CD8+ T cells was significantly increased upon TGF‐β stimulation 5. Furthermore, TRM cells in activated CD8+ T cells have higher CD28H expression and a higher percentage of CD28H‐positive cells. Stimulation with TGF‐β also increased the percentage of cells expressing CD28H (72·5 versus 89·1%) and increased the CD28H expression in TRM cells 5. These results indicate that it is likely that IL‐15 and TGF‐β are able to maintain CD28H expression in T cells exposed to epithelial stimulation.
Phenotypical characteristics of NK cells expressing CD28H
CD28H expression was observed on the cell surface of most freshly isolated human NK cells 4. Based on CD56 expression, human NK cells can be divided into two subpopulations, CD56bright and CD56dim NK cells, which have different phenotypes and properties 44. Studies have shown that CD56bright subpopulations express a larger proportion of CD28H 4, and most CD56bright NK cells have a phenotype of CD56brightCD16−KIR−NKG2A+CD57−, a phenotype representing a less mature NK cell population 4. The expression of KIR and NKG2A is not related to the expression of CD28H in CD56dim NK cells 4. Based on CD57 expression, CD56dim NK cells can also be categorized 45. CD57+ NK cells exhibit maturation, terminal differentiation and reduced proliferative capacity 45. The CD56dimCD57−NK cell subset expressed a higher proportion of CD28H than the CD56dimCD57+NK cell subset 4. In‐vitro‐expanded and ‐activated NK cells are highly cytotoxic, and have been used in clinical and basic research for decades 46, 47. A combination of CD28H and 2B4 results in synergistic activation of freshly isolated NK cells for degranulation, target cell lysis and expression of cytokines and chemokines 4. CD28H is an addition to the NK cell co‐activator family that functions synergistically with 2B4 and NKp46, but does not interact with natural killer group 2D (NKG2D), CD2 or DNAX accessory molecule‐1 (DNAM‐1) 4. During prolonged activation of IL‐2, expression of CD28H is turned off. CD28H can also enhance NK‐cell activation through CD16 for antibody‐dependent cellular cytotoxicity (ADCC). Co‐engagement of CD16 with CD28H enhances its NK‐mediated cell degranulation and cytotoxicity. Unlike co‐acting receptors that require synergy, CD16 signaling in NK cells is sufficient to activate cytotoxicity 48, 49. Other activated receptors that enhance ADCC have also been reported, such as 2B4, CD2, NKG2D and DNAM‐1 48, 49, 50, 51. Mutation of tyrosine 192 on the cytoplasmic tail of CD28H abolished NK cell activation by CD28H. As B7‐H5 is widely expressed in tumor tissues, the CD28H chimeric antigen receptor (CD28H−CAR) has been designed 4, which consists of full‐length CD28H fused to the cytoplasmic domain of the T cell receptor ζ chain. Notably, CD28H− CAR expression in NK cells triggers the cleavage of B7‐H5+ human leukocyte antigen (HLA)‐E+ tumor cells by overcoming the inhibition of the HLA‐E receptor NKG2A. Both the CD28H and the cytoplasmic domains of the ζ chain are required for this activity. Thus, CD28H is a potent activating receptor for NK cells, which broadens their anti‐tumor activity, and is a potential component of NK‐based CAR for cancer immunotherapy.
Phenotypical characteristics of tumor‐infiltrating lymphocytes expressing CD28H
CD28H is expressed in TILs of different tumor tissues, including pancreatic carcinoma, ovarian cancer, breast carcinoma, colorectal carcinoma, lung carcinoma, melanoma and glioma 3, 5. Although TILs contain a lower percentage of CD28H than PBMCs, CD28H is still highly expressed in TILs. Unlike human peripheral blood, all CD28H+ cells in TILs are memory‐phenotype or antigen‐experienced T cells. They express CD45RO but do not express CD45RA, and the majority of them express ICOS. CD28H+ TILs do not express the aging‐related molecule killer cell lectin like receptor G1 (KLRG1) or CD57, which is similar to the compartment in peripheral blood 2. It is worth noting that after short‐term cell stimulation in vitro, CD28H+ T cells express less CCR7 and produce more IL‐5 and less IFN‐γ 5. Consistently, in TILs, CD28H+ cells produced less CD107a, perforin and granzyme B 5. All these results indicate that most CD28H+ T cells are effector/memory cells with younger and less differentiated phenotypes 3, 5. According to the expression of CD28H, CD69 and CD103, TILs from pancreatic cancer can be further divided into three CD8+ T cell subpopulations, namely CD28H− TRM, CD28H+ TRM and non‐TRM. According to gene enrichment analysis, cytokine IL‐2 is mainly transcribed on CD28H+ TRM cells. In CD28H+ TRM cells the expression of CD161 and CCR6, which are important for differentiation of memory T cells, was higher. CD49a has been recently associated with highly cytotoxic TRM cells, and exhibits significantly lower transcription levels in the CD28H+ TRM subpopulation 52. CD28H+ T cells express higher levels of IL‐7R (CD127), which is related to long‐term survival of T cells. Flow cytometric analysis of TILs from diverse tumor tissues indicated that CD28H+ TRM cells expressed less CD49a but had higher levels of IL‐7R. These results indicate that the CD28+ T cell subset consists of young TRM cells with lower cytotoxicity 5.
Relationship between expression of CD28H and cancer prognosis
CD28H is expressed in TILs in different tumor tissues, including pancreatic carcinoma, ovarian cancer, breast carcinoma, colorectal carcinoma, lung carcinoma, melanoma and glioma 3, 5. Recent research shows that many human TILs have the characteristics of TRM cells and may also be positively associated with cancer patient survival 53, 54, 55 and prognosis 56, 57. Physiologically, it is crucial for maintaining the integrity of epithelia that TRM cells interact with epithelial cells 58, 59. TRM cells respond quickly to attack pathogens at the barrier site before recruiting T cells from the blood 60. Recent clinical studies have suggested that TRM cells play a critical role in human cancer immunity 56, 57, 61. It has been proposed that CD28H+ T cells are characteristic of TRM cells in human TILs, and experiments have shown that there is a significant positive correlation between the percentage of TRM cells and CD28H+ T cells in CD8+ TILs from pancreatic cancer 5. This means that amplification of CD28H+ TILs can directly affect the number of TRM cells in TILs, which may help to improve the prognosis of cancer.
B7‐H5 expression in cancer and relationship with cancer prognosis
Pancreatic ductal adenocarcinoma
Reports on the prognosis of PDAC suggests that CD28H/B7‐H5 is a co‐stimulatory pathway that can improve the prognosis of PDAC 17, 18. Investigations found that when tumor specimens were co‐cultured with T cells, PDAC cells with higher expression of B7‐H5 induced a stronger immune reaction, indicated by increased proliferation of T lymphocytes and high cytokine release. Accordingly, patients with stronger expression of B7‐H5 had significantly longer overall survival (OS) 17. In Han’s study of 92 patients with PDAC 18, B7‐H5 was rarely detected in normal acinar, islet and ductal cells, but was widely expressed from early pancreatic pre‐cancerous lesions to invasive PDAC. In the low‐grade pancreatic intra‐epithelial neoplasia (PanIN), the overall B7‐H5 positive rate was 95% (19 of 20) and in intraductal papillary mucinous neoplasm (IPMN) was 70·73% (29 of 41). In this population, B7‐H5 expression was detected in 77·17% (71 of 92) of PDAC patients and was significantly associated with a better survival rate 18. It has been shown that B7‐H5 can act as a co‐stimulatory factor through its receptor CD28H. Therefore, overcoming the weak expression of CD28H in T lymphocytes which have infiltrated tumor tissue, or up‐regulating expression of B7‐H5 on PDCA cells, may constitute a new mechanism to improve the function of PDCA immunotherapy 17.
Lung cancer
In a retrospective study of lung cancer 19, it was found that B7‐H5 is not observed in most normal lung tissue, but expressed in more than 60% of cases of non‐small‐cell lung carcinoma among different subtypes. High TILs intensity and epidermal growth factor receptor (EGFR) mutation status were independently associated with B7‐H5 expression in lung adenocarcinoma by multivariate analysis. B7‐H5 may thus be a new target for lung cancer immunotherapy.
Osteosarcoma
In a study of the prognosis of osteosarcoma 20, it was found that B7‐H5 expresses in most of osteosarcoma samples. Studies have shown that B7‐H5 is more common in patients with advanced disease and metastatic tissue. Higher expression of B7‐H5 may lead to an increased capability to survive after leaving the primary tumor and entering the blood circulation. Patients whose tumors were ≥ 25% or ≥ 50% B7‐H5‐positive had a significantly worse 5‐year event‐free‐survival. In conclusion, higher levels of B7‐H5 expression in the tumor resulted in a worse prognosis.
Breast cancer
In studies of breast cancer 21, clinical features of patients with locally advanced breast cancer were collected. All these patients were first treated surgically, and followed by chemotherapy, radiation therapy or both. Triple‐negative breast cancer with high B7‐H5 expression is associated with lymph node positive and higher cancer stages at diagnosis, indicating that tumors with high B7‐H5 expression are more aggressive and lead to worse prognosis.
Oral squamous cell carcinoma
In a study on OSCC 22, it was found that B7‐H5 expression is increased in dysplasia and OSCC. Higher expression levels of B7‐H5 indicate poor OS. The study also found that B7H5 was positively correlated with the expression levels of T cell immunoglobulin and mucin domain‐containing protein 3 (TIM3), lymphocyte‐activation gene 3 (LAG3), B7H3 (CD276), B7H4 and V‐domain immunoglobulin‐containing suppressor of T cell activation (VISTA). These molecules are negative immunological checkpoint molecules with increased expression in OCSS. Based on these relationships, we can speculate that B7‐H5 acts as an immunosuppressive molecule in OCSS.
Human ccRCC
In studies into human ccRCC 23, 24 , it was found that the expression of B7‐H5 mRNA is higher in human ccRCC tissues compared with adjacent normal tissues according to TCGA data, and the staining intensity of B7‐H5 in human ccRCC tissues was significantly higher than that in adjacent normal tissues by immunohistochemistry study. It has been suggested that the OS rate of patients with high expression of B7‐H5 in human ccRCC is significantly lower than that of patients with low expression of B7‐H5 in human ccRCC, and the high B7‐H5 expression in human ccRCC tissues was significantly positively correlated with larger tumor size and late tumor–node–metastasis (TNM) stage.
ICC
In a study on ICC 25, B7‐H5 was identified as an independent prognostic indicator for OS. B7‐H5 was used as an inhibitory checkpoint and it was found that B7‐H5 expression was more frequent in ICC than PD‐L1 (49·0 versus 28·1%). Patients with over‐expression of B7‐H5 were more likely to relapse and metastasize because B7‐H5 expression was positively correlated with serum carcinoembryonic antigen and CA19‐9 levels. Two tumor biomarkers usually reflect the primary tumor and ICC cycle 62. It was also suggested that B7‐H5 may promote tumor progression by binding to CD28H, and was identified as an independent prognostic indicator for OS.
Bladder urothelial carcinoma
In a study on BUC 26, it was found that the expression of B7‐H5 in BUC tissues was significantly up‐regulated compared to normal bladder tissue by immunohistochemical staining. In BUC tissues, the expression of B7‐H5 was significantly related to tumor stage, tumor size, tumor grade and lymph node metastasis. B7‐H5 expression is an independent prognostic factor for tumor metastasis in BUC, and could be a useful diagnostic indicator.
CRC
In a study on human CRC 27, it was found that B7‐H5 expression is up‐regulated in CRC patients, and B7‐H5 is an independent prognostic factor for OS in CRC patients. B7‐H5 expression level was significantly related to the depth of invasion and CD8+ T cell infiltration status, and predicted a high mortality rate. High B7‐H5 expression corresponds to deeper depth of invasion and fewer numbers of CD8+ cells, and predicts poor prognosis in patients with CRC. Studies also point out that high expression of B7‐H5 is closely related to poor 5‐year recurrence‐free survival and OS in patients with BUC.
Glioma
In a recent study on gliomas 28, B7‐H5 expression was not present in glioblastoma multiforme. As the degree of malignancy of the tumor increases, the expression of B7‐H5 in glioma gradually decreases until it disappears. Down‐regulated B7‐H5 predicts a poor prognosis for gliomas. Patients with elevated B7‐H5 have better OS. Using B7‐H5 as an immunostimulant may become a valuable method for clinical treatment of glioma.
Gastric cancer
In a study on gastric cancer prognosis 29, it was found that B7‐H5 expression is increased in gastric cancer tissue specimens compared to normal gastric tissue specimens by analyzing of B7‐H5 expression data of gastric cancer tissue samples in the database. In addition, studies have found that high B7‐H5 expression in tumor tissue is associated with advanced clinical stage, deep tumor invasion, lymph node metastasis, distant metastasis and short OS. High expression of B7‐H5 has been shown to be a poor independent prognostic factor for OS in patients with gastric cancer. Different conclusions emerged in another study on the prognosis of gastric cancer 30. It was found that normal epithelial cells showed higher B7‐H5 expression than tumor cells by using immunohistochemistry, which is similar to the expression of B7‐H5 in PDAC. This study also compared the expression of B7‐H5 mRNA in the blood of patients with gastric cancer and normal subjects. The results suggest that the level of B7‐H5 mRNA in the blood of patients is significantly lower than that of normal people. This study indicated that B7‐H5 expression in blood was inversely correlated with tumor invasion depth, distant metastasis and disease stage. At the same time, it is suggested that high expression of B7‐H5 in the blood leads to a higher 5‐year survival rate.
Conclusions
As a new member of the B7/CD28 family, CD28H interacts with its ligand B7‐H5 and regulates T cell responses. Current studies have indicated that CD28H and its ligand B7‐H5 are the second signaling molecules of T lymphocytes 63. Here, we mainly summarize the phenotypical characteristics of different immune cells expressing CD28H (Table 1). The results suggest that CD28H was not expressed on monocytes, granulocytes, B cells or MDCs. High levels of CD28H are expressed on naive T cells, ILCs, NK cells and PDCs in human peripheral blood. Moderate levels of CD28H are expressed on memory T cells. There was irreversible loss of CD28H expression with activation of immune cells after repeated stimulation, which is related to the replicative aging of CD28H+ T cells 2, 64. In NK cells, CD28H is a strong activator which can be used for the lysis of B7‐H5+ tumor cells 4. In human tissues, CD28H expression identifies resident memory CD8+ T cells with less cytotoxicity. The role of TRM cells has recently become an active subject of investigation, and several recent clinical studies have suggested that TRM cells play a vital role in human cancer immunity. The close relationship between CD28H and TRM cells suggests a role for CD28H in tumor immunotherapy. As the ligand for CD28H, B7‐H5 interacts with CD28H to promote T cell responses, and it has also been demonstrated to deliver a suppressive signal to T cells via an unknown receptor 9. This shows that CD28H/B7‐H5 pathway both play a T cell co‐inhibitory as well as a co‐stimulatory role. In contrast to B7‐1 and B7‐2, which are limited on professional APCs, B7‐H5 is preferentially transcribed in peripheral tissues. This is likely to be essential for CD28H/B7‐H5 to co‐stimulate newly produced effectors or effector/memory T cells in peripheral tissues. B7‐H5 is not detected in T cells. However, B7‐H5 is likely to be expressed on B cells, dendritic cells and macrophages as an inducing molecule of the inflammatory response. This is different from other B7s. B7‐H5 is widely expressed in tumor cells (Table 2). High expression of B7‐H5 in tumor leads to a better prognosis of PDAC and glioma, but leads to a worse prognosis of lung cancer, osteosarcoma, breast cancer, OSCC, human ccRCC, ICC, BUC and CRC. There are contradictions between the two studies 29, 30 on the expression characteristics of B7‐H5 in gastric cancer tumor tissues and their relationship with tumor prognosis, and further research is needed. Based on current research, B7‐H5 and CD28H do not bind to other known B7 receptors or ligands, respectively, and the CD28H/B7‐H5 pathway is not present in rats and mice. This is different from other B7/CD28 pathways. Much remains to be discovered about this new CD28H/B7‐H5 pathway. The underlying mechanism regulating B7‐H5 or CD28H is still unknown.
Table 1.
Phenotypical characteristics of different immune cells expressing CD28H
| Immune cells | Naive T cells | Effector memory T cell | Central memory T cell | NK cell | ||
|---|---|---|---|---|---|---|
| CH28H expression | CD28H+ a | CD28H− b | CD28H+ | CD28H− | CD28H+ | CD28H+ |
| CD45RA | Mostc ,2 | Littlec ,2 | Mediumc ,2 | Medium2 | ||
| CA45RO | Most2 | Most2 | ||||
| CCR7 | Most2 | Medium2 | Medium2 | |||
| CD31 | Highd ,3 | Lowd ,3 | ||||
| T‐bet | Low3 | High3 | ||||
| IFN‐γ | Low3 | High3 | Low7 | Low7 | Low3 | |
| TNF‐α | Low3 | High3 | ||||
| CD28 | High3 | Low3 | ||||
| IL‐2 | Similard ,3 | Similar3 | ||||
| CXCL8 | Similar3 | Similar3 | ||||
| CD7 | Low2,3,32–35 | Low2,3,32–35 | ||||
| CD27 | Little2,3,32–35 | Little2 3,32–35 | ||||
| CD28 | Little2,3 32–35 | Little2,3,32–35 | ||||
| CD57 | Low3 | Most2,3,32–35 | Low3 | Most2,3 32–35 | Low3 | |
| KIR | Little4 | |||||
| NKG2A | Most4 | |||||
| CD56 | Little4 | |||||
CD28H+: immune cells express CD28H‐positive.
CD28H−: immune cells express CD28H‐negative.
Most, medium and little: percentage of CD28H+ or CD28H− immune cells expressing different cell surface molecules.
High, similar and low: comparison of expression levels of different cell surface molecules in CD28H+ and CD28H− T cells.
IFN = interferon; TNF = tumor necrosis factor; IL = interleukin; CXCL8 = C‐X‐C motif chemokine ligand 8; KIR = killer immunoglobulin‐like receptors.
Table 2.
Relationship between B7‐H5 expression and tumor pathology and prognosis
| Cancer type | Samples | B7‐H5 expression | Pathological correlates | Clinical outcomes | Refs |
|---|---|---|---|---|---|
| Pancreatic ductal adenocarcinoma | 136 | 68% (higha) | No correlation | Better OS | 17 |
| 32% (low) | |||||
| 92 | 77% (positiveb) | Highest expression in low‐grade pancreatic intra‐epithelial neoplasia (PanIN) | Better survival rate | 18 | |
| Lung cancer | 195 (cohort 1) | 61% (positive) | High TIL intensity and EGFR mutation status | Decreased OS (not statistically) | 19 |
| 197 (cohort 2) | 64% (positive) | ||||
| Osteosarcoma | 55 | 68% (positive) | High expression in metastatic tissue | Worse five‐year event‐free‐survival | 20 |
| Breast cancer | 50 | 56% (high) | Related to regional lymph node metastasis and advanced disease | Worse prognosis | 21 |
| Oral squamous cell carcinoma | 210 | 65% (high) | No correlation | Poor OS | 22 |
| Human clear cell renal cell carcinoma | 87 | Unclear | Related to larger tumor size and advanced TNM stage | Poor OS | 23 |
| 92 | 95% (positive) | Tumor size, clinical stage and histological grade | Poor OS | 24 | |
| Intrahepatic cholangiocarcinoma | 153 (cohort 1) | 49% high) | More likely to relapse and metastasize | Poor OS | 25 |
| 65 (cohort 2) | 68% (high) | ||||
| Bladder urothelial carcinoma | 212 | 84% (positive) | Significantly related to tumor stage, tumor size, tumor grade and lymph node metastasis | Poor OS and 5‐year recurrence‐free survival | 26 |
| 26% (high) | |||||
| Colorectal carcinoma | 63 | 48% (high) | Deeper infiltration depth | Poor OS | 27 |
| Glioma | 669 | Unclear | Low malignancy | Better OS | 28 |
| Gastric cancer | 124 | 53% (high) | Related to advanced clinical stages, deep tumor infiltration, lymph node metastasis and distant metastasis | Poor OS | 29 |
| 111 | 50% (high) | inversely related to the depth of tumor invasion, distant metastasis and disease stage | Higher 5‐year survival rate | 30 |
High: high B7‐H5 expression.
Positive: B7‐H5 express positive.
OS = overall survival; TNM = tumor–node–metastasis.
Disclosure
The authors declare no conflicts of interest.
Acknowledgements
This work supported in part by the Natural Science Foundation of China (81974377) and the Scientific Research Project of Education Department of Liaoning Province (JC2019017).
References
- 1. Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. Identification of IGPR‐1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell 2012; 23:1646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu Y, Yao S, Iliopoulou BP et al B7–H5 costimulates human T cells via CD28H. Nat Commun 2013; 4:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crespo J, Vatan L, Maj T, Liu R, Kryczek I, Zou W. Phenotype and tissue distribution of CD28H (+) immune cell subsets. Oncoimmunology 2017; 6:e1362529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhuang X, Long EO. CD28 homolog is a strong activator of natural killer cells for lysis of B7H7+ tumor cells. Cancer Immunol Res 2019; 7:939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian Y, Sun Y, Gao F et al CD28H expression identifies resident memory CD8 + T cells with less cytotoxicity in human peripheral tissues and cancers. Oncoimmunology 2018; 8:e1538440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Ann Rev Immunol 2005; 23:515–48. [DOI] [PubMed] [Google Scholar]
- 7. Linsley PS, Clark EA, Ledbetter JA. T‐cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB‐1. Proc Natl Acad Sci USA 1990; 87:5031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Ann Rev Immunol 1996; 14:233–58. [DOI] [PubMed] [Google Scholar]
- 9. Zhao R, Chinai JM, Buhl S et al HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T‐cell function. Proc Natl Acad Sci USA 2013; 110:9879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA 2003; 100:10388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chinai JM, Janakiram M, Chen F, Chen W, Kaplan M, Zang X. New immunotherapies targeting the PD‐1 pathway. Trends Pharmacol Sci 2015; 36:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janakiram M, Pareek V, Cheng H, Narasimhulu DM, Zang X. Immune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignancies. Immunotherapy 2016; 8:809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flajnik MF, Tlapakova T, Criscitiello MF, Krylov V, Ohta Y. Evolution of the B7 family: co‐evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7's historical relationship with the MHC. Immunogenetics 2012; 64:571–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mager DL, Hunter DG, Schertzer M, Freeman JD. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3). Genomics 1999; 59:255–63. [DOI] [PubMed] [Google Scholar]
- 15. Fahrer AM, Bazan JF, Papathanasiou P, Nelms KA, Goodnow CC. A genomic view of immunology. Nature 2001; 409:836–8. [DOI] [PubMed] [Google Scholar]
- 16. Ling V, Wu PW, Spaulding V et al Duplication of primate and rodent B7–H3 immunoglobulin V‐ and C‐like domains: divergent history of functional redundancy and exon loss. Genomics 2003; 82:365–77. [DOI] [PubMed] [Google Scholar]
- 17. Chen Q, Wang J, Chen W et al B7–H5/CD28H is a co‐stimulatory pathway and correlates with improved prognosis in pancreatic ductal adenocarcinoma. Cancer Sci 2019; 110:530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan H, Qiu W, Koehne de Gonzalez AK et al HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post‐surgical survival. Cancer Lett 2019; 442:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng H, Janakiram M, Borczuk A et al HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res 2017; 23:825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koirala P, Roth ME, Gill J et al HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep 2016; 6:31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janakiram M, Chinai JM, Fineberg S et al Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res 2015; 21:2359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao Y, Li H, Yang LL et al The expression patterns and associated clinical parameters of human endogenous retrovirus‐H long terminal repeat‐associating protein 2 and transmembrane and immunoglobulin domain containing 2 in oral squamous cell carcinoma. Dis Markers 2019; 2019:5421985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Zhu D, Feng J et al Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int 2019; 19:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen D, Chen W, Xu Y et al Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: a novel prognostic biomarker and potential therapeutic target. J Med Genet 2019; 56:43–9. [DOI] [PubMed] [Google Scholar]
- 25. Jing CY, Fu YP, Yi Y et al HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD‐L1. J Immunother Cancer 2019;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin G, Ye H, Wang J, Chen S, Chen X, Zhang C. Immune checkpoint human endogenous retrovirus‐H long terminal repeat‐associating protein 2 is upregulated and independently predicts unfavorable prognosis in bladder urothelial carcinoma. Nephron 2019; 141:256–64. [DOI] [PubMed] [Google Scholar]
- 27. Zhu Z, Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther 2018; 11:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qi Y, Deng G, Xu P et al HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol Rep 2019; 42:2309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wei L, Tang L, Chang H, Huo S, Li Y. HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Hum Cell 2019. 10.1007/s13577-019-00280-2. [DOI] [PubMed] [Google Scholar]
- 30. Shimonosono M, Arigami T, Yanagita S et al The association of human endogenous retrovirus‐H long terminal repeat‐associating protein 2 (HHLA2) expression with gastric cancer prognosis. Oncotarget 2018; 9:22069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kimmig S, Przybylski GK, Schmidt CA et al Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med 2002; 195:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aandahl EM, Sandberg JK, Beckerman KP, Taskén K, Moretto WJ, Nixon DF. CD7 is a differentiation marker that identifies multiple CD8 T cell effector subsets. J Immunol 2003; 170:2349–55. [DOI] [PubMed] [Google Scholar]
- 33. Hamann D, Kostense S, Wolthers KC et al Evidence that human CD8+CD45RA+CD27– cells are induced by antigen and evolve through extensive rounds of division. Int Immunol 1999; 11:1027–33. [DOI] [PubMed] [Google Scholar]
- 34. Brenchley JM, Karandikar NJ, Betts MR et al Expression of CD57 defines replicative senescence and antigen‐induced apoptotic death of CD8+ T cells. Blood 2003; 101:2711–20. [DOI] [PubMed] [Google Scholar]
- 35. Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T‐cell replicative senescence in human aging. Immunol Rev 2005; 205:147–57. [DOI] [PubMed] [Google Scholar]
- 36. Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell energy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 2013; 25:214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 38. Hamann D, Baars PA, Rep MH et al Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 1997; 186:1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dong H, Chen L. B7–H1 pathway and its role in the evasion of tumor immunity. J Mol Med 2003; 81:281–7. [DOI] [PubMed] [Google Scholar]
- 40. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Ann Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 41. Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001; 291:2413–7. [DOI] [PubMed] [Google Scholar]
- 42. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10:524–30. [DOI] [PubMed] [Google Scholar]
- 43. Gebhardt T, Whitney PG, Zaid A et al Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 2011; 477:216–9. [DOI] [PubMed] [Google Scholar]
- 44. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer‐cell subsets. Trends Immunol 2001; 22:633–40. [DOI] [PubMed] [Google Scholar]
- 45. Lopez‐Verges S, Milush JM, Pandey S et al CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK‐cell subset. Blood 2010; 116:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bachanova V, Miller JS. NK cells in therapy of cancer. Crit Rev Oncogen 2014; 19:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Granzin M, Wagner J, Kohl U, Cerwenka A, Huppert V, Ullrich E. Shaping of natural killer cell antitumor activity by ex vivo cultivation. Front Immunol 2017;8:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006; 107:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 2009; 114:2657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 2006; 214:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu LL, Landskron J, Ask EH et al Critical role of CD2 co‐stimulation in adaptive natural killer cell responses revealed in NKG2C‐deficient humans. Cell Rep 2016; 15:1088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheuk S, Schlums H, Gallais Sérézal I et al CD49a expression defines tissue‐resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity 2017; 46:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koh J, Kim S, Kim MY, Go H, Jeon YK, Chung DH. Prognostic implications of intratumoral CD103+ tumor‐infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget 2017; 8:13762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boddupalli CS, Bar N, Kadaveru K et al Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue‐resident memory T cells. JCI Insight 2016; 1:e88955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murray T, Fuertes Marraco SA, Baumgaertner P et al Very late antigen‐1 marks functional tumor‐resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol 2016; 7:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Djenidi F, Adam J, Goubar A et al CD8+CD103+ tumor‐infiltrating lymphocytes are tumor‐specific tissue‐resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol 2015; 194:3475–86. [DOI] [PubMed] [Google Scholar]
- 57. Komdeur FL, Prins TM, van de Wall S et al CD103+ tumor‐infiltrating lymphocytes are tumor‐reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology 2017; 6:e1338230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol 2009; 9:153–61. [DOI] [PubMed] [Google Scholar]
- 59. Ariotti S, Haanen JB, Schumacher TN. Behavior and function of tissue‐resident memory T cells. AdvImmunol 2012; 114:203–16. [DOI] [PubMed] [Google Scholar]
- 60. Schenkel JM, Masopust D. Tissue‐resident memory T cells. Immunity 2014; 41:886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Savas P, Virassamy B, Ye C et al Single‐cell profiling of breast cancer T cells reveals a tissue‐resident memory subset associated with improved prognosis. Nat Med 2018; 24:986–93. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Li J, Xia Y et al Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013; 31:1188–95. [DOI] [PubMed] [Google Scholar]
- 63. Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The third group of the B7‐CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7–H3. Immunol Rev 2017; 276:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vallejo AN, Weyand CM, Goronzy JJ. T‐cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med 2004; 10:119–24. [DOI] [PubMed] [Google Scholar]
- 65. Yao S, Zhu Y, Zhu G et al B7–h2 is a costimulatory ligand for CD28 in human. Immunity 2011; 34:729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death‐1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Butte MJ, Pena‐Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD‐L1 and B7–1. Mol Immunol 2008; 45:3567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chaudhri A, Xiao Y, Klee AN, Wang X, Zhu B, Freeman GJ. PD‐L1 binds to B7–1 only in cis on the same cell surface. Cancer Immunol 2018; 6:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


