Summary
Immune tolerance is one of the leading causes of chemotherapy resistance in carcinoma cases. Studies have shown that programmed cell death ligand‐1 (PD‐L1), an inhibitory molecule expressed by cancer cells, plays a significant role in immune tolerance through the induction of T cell dysfunction. The results of our RNA sequencing in previous studies revealed that microRNA‐145 (miR‐145), which is known to be down‐regulated by cisplatin in cisplatin‐resistant ovarian cancer cells, also represses gene PD‐L1 expression. However, the mechanism by which miR‐145 contributes to regulate PD‐L1 expression in cisplatin resistance of ovarian cancer is yet to be fully understood. Here, we show that cisplatin‐mediated miR‐145 down‐regulation increased PD‐L1 expression via targeting the c‐Myc transcription factor, thereby inducing T cell apoptosis in vitro. We also report that expression of miR‐145 is negatively correlated with PD‐L1 expression in human ovarian cancer tissues, malignant grades and the recurrent risks of ovarian cancer after chemotherapy. In summary, our findings suggest that the miR‐145/c‐Myc/PD‐L1 axis contributes to cisplatin resistance in ovarian cancer and support that miR‐145 might act as an adjuvant therapeutic target in chemotherapy of ovarian cancer.
Keywords: c‐Myc, cisplatin resistance, microRNA‐145, PD‐L1, ovarian cancer
Cisplatin‐induced PD‐L1 expression in cisplatin‐resistant ovarian cancer cells contributes to T lymphocytes dysfunction
![]()
Introduction
Ovarian cancer is the leading cause of mortality in gynecological malignancies 1. Besides the lack of screening techniques for early diagnosis of ovarian cancer, chemoresistance is yet another major challenge associated with high mortality, although the initial response to platinum‐based chemotherapy is encouraging 2, 3. Previous studies have shown that chemoresistance involves different mechanisms, such as reduction in drug accumulation inside the tumor cells, elevated levels of cellular detoxification, mutations in drug targets and abnormalities in DNA damage and repair systems, etc. 4. In recent years, immune tolerance induced by tumor antigen‐specific cytotoxic T lymphocytes (CTLs) dysfunction has also been considered a critical cause of chemoresistance 5. Generally, chemotherapy drugs kill most cancer cells while the remainder of these cells are often cleared by the immune system. However, studies have shown that remaining tumor cells after chemotherapy can also acquire immune‐tolerance ability, thereby evading the immune surveillance successfully via T lymphocyte dysfunction 6, 7, 8. Programmed cell death protein ligand‐1 (PD‐L1), which is expressed in a number of tumor cells, can induce CTL apoptosis to help tumor cells evade immunosurveillance by activating its receptor, PD‐1, on CTLs 9. Furthermore, several studies have revealed that PD‐L1 up‐regulation, following treatment with chemotherapeutic agents, increases the immune tolerance ability of tumor cells 10, 11, 12. However, the mechanism of PD‐L1 activity after cisplatin therapy remains unclear.
MicroRNAs are small non‐coding RNAs with conserved sequence, which are 19–25 base pairs (bp) in length, and regulate expression of their target genes either through accelerating mRNA degradation or blocking mRNA translation 13. The expression of PD‐L1 has been found to be directly regulated by a variety of microRNAs, thus influencing the pathological processes of tumor development. More specifically, the miR‐200 family suppresses PD‐L1 in epithelial–mesenchymal transition (EMT) in lung cancer cells 14; miR‐143 down‐regulation promotes radiation‐induced thymic lymphoma by targeting PD‐L1 15; up‐regulation of miR‐138 facilitates colorectal cancer cell viability and invasion via targeting PD‐L1 16; and miR‐142‐5p represses PD‐L1 expression in tumor cells, thereby enhancing anti‐tumor immunity 17. Protein p53 targeting PD‐1/PD‐L1 signaling by miR‐34 plays a critical role in tumor immune evasion of non‐small cell lung cancer (NSCLC) 18.
In our previous study, miR‐145 has been found to be significantly down‐regulated in cisplatin‐treated A2780cis cells compared with untreated cells using a next‐generation sequencing analysis. Although down‐regulating miR‐145 has been reported to suppress the proliferation, metastasis, differentiation and angiogenesis of many malignant tumors by targeting estimated glomerular filtration rate (EGFR) 19, integrin‐linked kinase (ILK) 20, mucin 1 (MUC1) 21, SRY‐box transcription factor 1 (SOX9) 22 and infected‐cell polypeptide 4 (ICP4) 23, no prior studies have shown the effect of miR‐145 on PD‐L1 expression in ovarian carcinoma.
In this paper, we sought to unveil a new mechanism of cisplatin resistance in ovarian cancer by studying how miR‐145 negatively regulates PD‐L1, thereby influencing the chemoresistance of ovarian cancer cells. Our data may provide references for weakening immune tolerance and improving the effect of chemotherapy for ovarian cancer.
Patients and methods
Patients and tissues
Fresh tumor tissues from 73 patients with recurrent ovarian epithelial carcinoma (ROC) treated at Dongfang Hospital of Xiamen University between January 2014 and December 2017 were stored at −80°C, including high‐grade serous carcinoma (SC) (n = 48) and mucinous carcinoma (MC) (n = 25) 24. Fifteen platinum‐resistant SC cases recurred in 6 months upon chemotherapy completion, according to 2012 NCCN guidelines 25.
Cell culture and cisplatin treatment
A2780 and A2780cis were purchased from the European Collection of Authenticated Cell Cultures (ECACC) and were cultured in RPMI‐1640 with 10% fetal bovine serum (FBS) (gibco, Carlsbad, ca, usa). HEK293T were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS. A2780cis cells were treated with cisplatin (Sigma, St Louis, MO, USA) with a concentration gradient (0, 5, 10, 20, 100, 150, 200 μg/ml). Based on the expression level of PD‐L1 in A2780cis cells, the optimal concentration of cisplatin was validated.
Plasmids, RNAs and lentivirus
Synthetic DNA sequences of pre‐miR‐145 and scramble microRNA (Sangon Biotech, Shanghai, China) were annealed and inserted into a vector pcDNA3.1 to construct recombinant pcDNA3.1‐scramble and pcDNA3.1‐pre‐miR‐145. Synthesized wild‐type and mutant‐type promoter and coding sequence (CDs) of gene c‐Myc, as templates, were amplified using the polymerase chain reaction (PCR) system and inserted into vectors pMIR or pcDNA3.1 to construct recombinant pMIR‐wt‐c‐Myc, pMIR‐mu‐c‐Myc and pcDNA3.1‐c‐Myc, respectively. Sequences of all plasmids were confirmed using a sequencing system (Sangon Biotech). c‐Myc shRNA lentiviral particles and its control were purchased from Santa Cruz Biotech (Dallas, TX, USA); miR‐145 mimics and its inhibitor were obtained from Ambion (Austin, TX, USA).
Antibodies and reagents
Commercial antibodies used in this study were purchased as follows: rabbit anti‐PD‐L1 monoclonal antibody (mAb) (Abcam, Cambridge, UK), phycoerythrin (PE) mouse anti‐human PD‐1 mAb (Thermo Fisher Scientific, Waltham, MA, USA), mouse anti‐human CD8 mAb (Thermo Fisher Scientific), rabbit anti‐c‐Myc antibody (Abcam), mouse anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) mAb, mouse anti‐tubulin mAb and mouse anti‐lamin B (Santa Cruz Biotech), horseradish peroxidase (HRP) anti‐mouse immunoglobulin (Ig)G and HRP anti‐rabbit IgG (ZSGB‐BIO, Beijing, China). Reagents were bought as follows: Dynabeads M‐280 sheep anti‐mouse IgG (Life Tech, Carlsbad, CA, USA), Pierce fast Western blot kit (Thermo Fisher Scientific), annexin V apoptosis detection kit fluorescein isothiocyanate (FITC) (Thermo Fisher Scientific), lipofectamine 2000 reagent (Thermo Fisher Scientific), dual‐luciferase reporter assay (Promega, Madison, WI, USA), TriZol (Invitrogen, Carlsbad, CA, USA), TransScript One‐Step gDNA removal and cDNA Synthesis SuperMix (Transgen Biotech, Beijing, China), SYBR Premix Ex Taq II (TaKaRa, Dalien, China), nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific) and MaxVision HRP‐polymer anti‐mouse IHC kit (MXB Biotech; Santa Cruz Biotech).
Quantification of mRNAs and miRNAs with real‐time PCR
Total RNAs from tissues and cells were extracted using TRIzol Reagent (Invitrogen). cDNAs were synthesized using a TransScript One‐Step gDNA removal and cDNA Synthesis SuperMix (Transgen Biotech). Real‐time quantitative polymerase chain reaction (qPCR) was performed using an ABI 7500 fast qPCR system with SYBR® Premix Ex TaqTMⅡ(TaKaRa) under the following conditions: 95°C for 30 s, 40 cycles of 95°C for 10 s, 60°C for 1 min and 72°C for 45 s. The relative expressions of PD‐L1 and miR‐145 were normalized to housekeeping genes, GAPDH and U6, respectively. Real‐time qPCR reactions were performed in triplicate. The sequences of primers used are as follows: PD‐L1 5′‐TCCTACACGGTCTCCATC AAG‐3′/5′‐CTGTTCTCCTTCCTTACCCG‐3′;GAPDH 5′′‐CACGTGGGCTCCAGC ATT‐3′/5′‐TCACCAGTCATTTCTGCCTTTG‐3′; miR145 5′‐ATCGTCCAGTTTTC CCAGG‐3′/5′‐CGCCTCCACACACTCACC‐3′; and U6 snRNA 5′‐CTCGCTTCGGCA GCACA‐3′/5′‐AACGCTTCACGAATTTGCGT‐3′.
Immunoblotting
Total cellular proteins or nucleic proteins were extracted from cells or tissues using a nuclear and cytoplasmic extraction reagents kit (Thermo Fisher Scientific). The protein samples were separated by 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, MA, USA). Blots were probed with PD‐L1 mAb (rabbit; Abcam), c‐Myc antibody (rabbit; Abcam), GAPDH Ab (mouse), tubulin antibody (mouse), lamin B antibody (mouse; Santa Cruz Biotech) and HRP‐conjugated secondary antibodies (ZSGB‐BIO). Bands were developed with a SuperSignal West Pico chemiluminescent substrate kit (Thermo Fisher Scientific).
Immunohistochemistry (IHC)
Ovarian cancer tissue chips (Outdo Biotech, Shanghai, China) were dewaxed, rehydrated and underwent antigen retrieval successively, and were then detected with PD‐L1 antibody (Thermo Fisher Scientific) and the MaxVision HRP‐Polymer IHC kit (MXB Biotech; Santa Cruz Biotech) to evaluate its expression and distribution under a microscope (Olympus, Tokyo, Japan).
CD8+ T cell sorting and treatment
Lymphocytes were separated from healthy human peripheral blood using Lymphoprep (Solarbio, Beijing, China). CD8+ T lymphocyte were sorted by Dynabeads M‐280 sheep anti‐mouse IgG (Life Tech) with human CD8 mAb (Thermo Fisher Scientific). A2780cis cells were pre‐treated with cisplatin (100 μg/ml) for 24 h and were then washed three times with phosphate‐buffered saline (PBS). After the cells were cultured with fresh media for 48 h, the conditioned media were collected and filtered to remove cell debris, and then added into CD8+ T lymphocytes to induce apoptosis. PD‐1 antibody (Thermo Fisher Scientific) was used to block the PD‐1‐mediated pathway of T lymphocytes.
CD8+ T cell proliferation assay
Cell counting kit‐8 (CCK‐8) (Beyotime Biotech, Shanghai, China) was used to evaluate cell proliferation activity. Sorted CD8+ T cell suspension (20 000 cells/ml) with the conditioned media from cisplatin‐treated A2780cis cells were seeded into 96‐well plates (100 μl/well) to be cultured overnight. According to the instructions, 10 µl of the CCK‐8 solution were added to each well of the plate and incubated for 2 h in the incubator. The absorbance at 450 nm was measured using a microplate reader and a proliferation curve was drawn.
CD8+ T cell apoptosis assay
After CD8+ T cells were treated with conditioned media from A2780cis for 48 h, flow cytometry analysis was performed to detect apoptosis of PD‐1+/CD8+/T lymphocytes using the annexin V apoptosis detection kit, FITC (Thermo Fisher Scientific) and PE‐conjugated PD‐1 mAb (Thermo Fisher Scientific).
Dual‐luciferase reporter assay
HEK‐293T cells were seeded in 48‐well plates (1~2 × 105/well) and were transfected with the pLG3–PD‐L1 promoter (wild‐type) or pGL3–PD‐L1 mutant promoter (mutant), either alone or in combination with miR‐145 mimics or inhibitors. Firefly luciferase (FLuc) and Renilla luciferase (RLuc) activities in cell lysates were measured using a dual‐luciferase reporter assay kit (Promega).
Statistical analysis
All measurement data are presented as the mean ± standard deviation (s.d.) from at least three independent experiments and were analyzed by one‐way analysis of variance. Correlation between two variables were evaluated using Pearson’s r‐value. Differences were considered statistically significant at P < 0·05.
Results
Cisplatin‐induced PD‐L1 expression in cisplatin‐resistant ovarian cancer cells contributes to T lymphocyte dysfunction
It is well known that PD‐L1, which can induce T lymphocytes anergy, is one of the triggers of tumor immune tolerance 26. As shown in Fig. 1a, mRNA (up) and protein (down) of PD‐L1 expression in cisplatin‐resistant ovarian cancer cells (A2780cis) was significantly higher than in cisplatin‐sensitive ovarian cancer cells (A2780) (P < 0·001). Moreover, cisplatin induced a dose‐dependent up‐regulation of PD‐L1 gene to 100 μg/ml [⅖ half maximal inhibitory concentration (IC50) dose of cisplatin on A2780cis cells], while more than 100 μg/ml concentration of cisplatin repressed PD‐L1 expression (Fig. 1b). Interestingly, the conditioned media from cisplatin‐treated A2780cis cells showed a significant inhibition of cell proliferation (P < 0·01) (Fig. 1c) and induced CD8+ T lymphocyte apoptosis (Fig. 1d), but PD‐1 antibody treatment mitigated the effects on these T lymphocytes.
Figure 1.
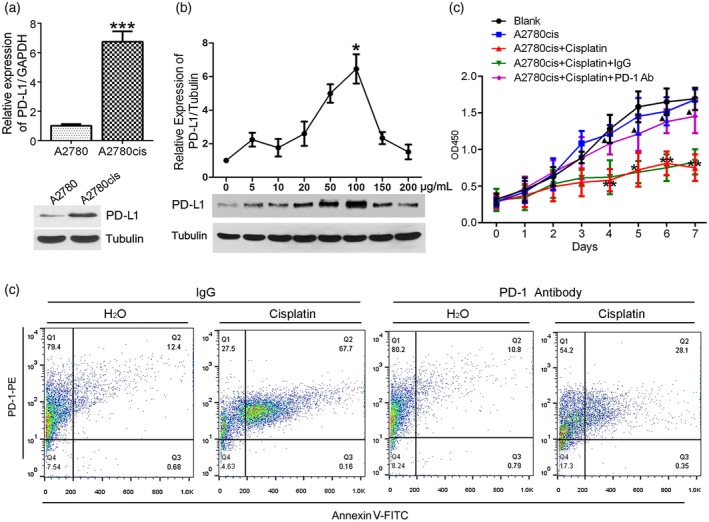
Cisplatin‐induced programmed cell death ligand‐1 (PD‐L1) expression in A2780cis cells and contributed to T lymphocyte disfunction. (a) Difference of relative expression of PD‐L1 between A2780 and A2780cis by real‐time polymerase chain reaction (PCR) (up) and Western blot (down). (b) An optimal concentration of cisplatin‐induced PD‐L1 maximal expression in A2780cis cells by Western blot assay. (c,d) Conditioned media from the cisplatin‐treated A2780cis was used to culture CD8+ T lymphocytes. Media from H2O‐treated A2780cis was used as negative control. PD‐1 antibody or mouse immunoglobulin (Ig)G was used to block the PD‐1‐dependent pathway on T cells. Effects of the conditioned media on CD8+ T lymphocyte proliferation and apoptosis were evaluated by cell count kit‐8 (c) and flow cytometry (d), respectively. * P < 0·05 and ** P < 0·01 versus A2780cis; ▲ P < 0·05 versus A2780cis + cisplatin + IgG.
miR‐145 down‐regulation negatively correlated with PD‐L1 expression in ovarian carcinoma tissues
To evaluate the clinical significance of miR‐145 and PD‐L1, we collected the tumor tissues from SC (n = 48) and MC (n = 25) patients. As shown in Fig. 2a, real‐time PCR assay revealed significant miR‐145 down‐regulation in the SC group (P < 0·001). IHC straining confirmed this result (Fig. 2c). To further explore the relationship between miR‐145 and PD‐L1, we detected their expression in each pair of ovarian carcinoma tissues, including unilateral ovarian cancers, and the recurrent tumor tissues in 6 months after unilateral adnexal resection combined with cisplatin chemotherapy. As shown in Fig. 2b, compared to the group before cisplatin chemotherapy, miR‐145 (left) was down‐regulated (P < 0·05) and PD‐L1 was up‐regulated in recurrent cancer tissues (middle) (P < 0·05). miR‐145 negatively correlated with PD‐L1 expression in ovarian carcinoma tissues (right) (r = −0·767, P < 0·01). These data support that miR‐145 plays a critical role in cisplatin resistance and tumor recurrence.
Figure 2.
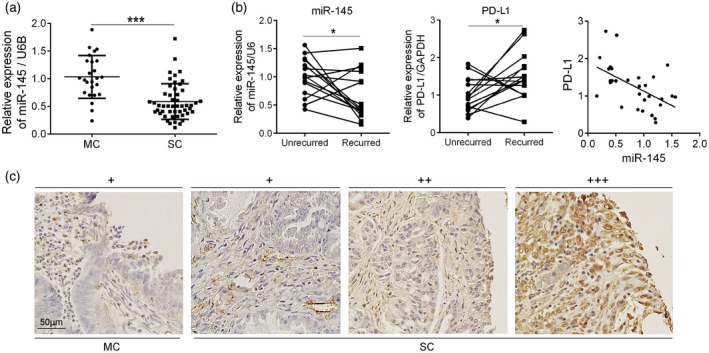
miR‐145 down‐regulation negatively correlated with programmed cell death ligand‐1 (PD‐L1) expression in human ovarian carcinoma tissues. (a) mRNA levels of miR‐145 in mucinous carcinoma (MC) (n = 25) and SC (n = 48) tissues were analyzed by real‐time polymerase chain reaction (PCR). (b) mRNA levels of miR‐145 (left) and PD‐L1(middle) in serous carcinoma (SC) tumor tissue before and after recurrence (n = 15) by real‐time PCR. The relationship between miR‐145 and PD‐L1 expression in SC tissues by Pearson’s correlation coefficient (right). (c) Immunohistochemistry (IHC) staining of PD‐L1 in human ovarian carcinoma tissues. Cases of ovarian cancer are graded from low to high with +, ++ and +++, respectively. Scale bars 50 μm. *P < 0·05; ***P < 0·001.
Cisplatin‐mediated down‐regulation of miR‐145‐induced PD‐L1 via targeting c‐Myc in A2780cis cells
To further explore the role of miR‐145 in cisplatin up‐regulating PD‐L1, we confirmed the effect of cisplatin on miR‐145 expression in vitro. As shown in Fig. 3a, cisplatin repressed miR‐145 expression. Further, we found that miR‐145 also repressed PD‐L1 expression (Fig. 3b, left). However, there are no binding sites of miR‐145 in the PD‐L1 promoter region, so we focused on a transcription factor named c‐Myc, which has been reported to regulate the transcription of PD‐L1 27. Indeed, miR‐145 could repress c‐Myc expression (Fig. 3b, right). To define a directly regulating target of miR‐145, we examined the effects of miR‐145 mimics and its inhibitor on the activity of c‐Myc promoter and its mutant. As shown in Fig. 3c, the results of dual luciferase reporter gene assay indicated that miR‐145 only inhibited wild‐type promoter of c‐Myc, not its mutant promoter. Finally, we confirmed the role of c‐Myc in miR‐145 repressing PD‐L1 by real‐time PCR assay. As demonstrated in Fig. 3d, up‐regulation of c‐Myc in A2780cis cells enhanced the transcription of PD‐L1 (left). Cisplatin induced PD‐L1 expression in A2780cis cells but not in A2780cis cells without c‐Myc (middle). Similarly, miR‐145 inhibitor increased PD‐L1 expression via c‐Myc in A2780cis cells (right).
Figure 3.
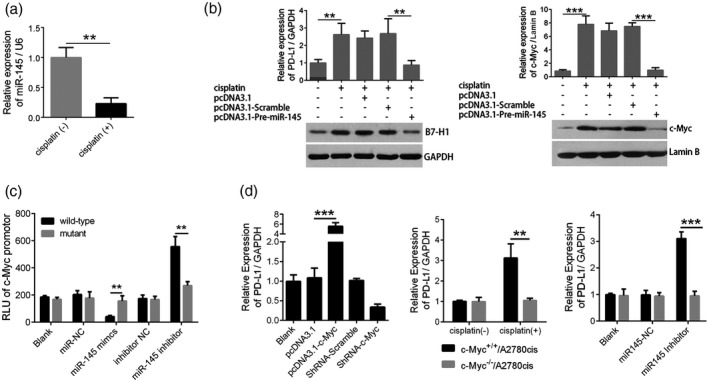
Cisplatin‐mediated down‐regulation of miR‐145 contributes to up‐regulation of programmed cell death ligand‐1 (PD‐L1) via targeting c‐Myc in A2780cis cells. (a) Effects of cisplatin on miR‐145 expression in A2780cis cells by real‐time polymerase chain reaction (PCR); (b) effects of miR‐145 on PD‐L1 (left) and c‐Myc (right) expression in A2780cis cells by Western blot; (c) effects of miR‐145 on promoter activity of c‐Myc in HEK293 cells by dual‐luciferase reporter assays; (d) effects of c‐Myc (left), cisplatin (middle) and miR‐145 (right) on PD‐L1 expression in c‐Myc+/+/A2780cis or c‐Myc−/−/A2780cis cells by real‐time PCR assays. The data were representative of three independent experiments. **P < 0·01; ***P < 0·001.
Discussion
Chemoresistance is one of the challenging issues in the clinical treatment of ovarian cancer 1. Although recent studies have shown that immune tolerance induced by PD‐1/PD‐L1 pathway activation was involved in chemoresistance 28, the detailed mechanisms remain unclear.
PD‐L1, a negative co‐stimulatory molecule on several cancer cells, induces immune cell anergy and evades immune surveillance 29. Hence, the expression level of PD‐L1 was consistent with the immune tolerance of tumor cells 30. Our observations revealed a significant difference in the expression level of PD‐L1 between the cisplatin‐sensitive (A2780) and cisplatin‐resistant (A2780 cis) ovarian cancer cell lines. Here, the relative expression level of PD‐L1 was seven times higher in A2780cis cells than in A2780 cells. This suggests that cisplatin may have cell‐selective effects on PD‐L1 expression. Although some studies have shown a significant up‐regulation of PD‐L1 after chemotherapy 11, 12, our current data demonstrate that a sequential increase in the final concentration of cisplatin from 0 to 100 μg/ml elicited a corresponding increase in the expression level of PD‐L1, but significant repression was observed beyond this concentration range. This is a novel finding, as no previous study, to our knowledge, has demonstrated that the induction effect of cisplatin on the PD‐L1 expression in A2780cis is influenced by cisplatin concentration.
We also observed that the conditioned media from cisplatin‐treated A2780cis cells induced PD‐1+/CD8+ T lymphocyte apoptosis. We speculated that this observed effect on the T lymphocytes was mediated by the exosomal PD‐L1 present in the conditioned media. Several studies have demonstrated that PD‐L1 was up‐regulated in cancer cell‐derived extracellular vesicles, which assist cellular communication between cancer cells and immune cells 31. Interestingly, the effect of conditioned media from cisplatin‐treated A2780cis cells on these lymphocytes was significantly reduced by PD‐1 antibody. We speculated that this was due to the inhibition of PD‐1 signal transduction on T cells, thereby stopping the PD‐1 response to PD‐L1. This further supports the claim that PD‐L1 could play a pivotal role in A2780cis‐induced T lymphocyte apoptosis. However, more studies need to be conducted to elucidate the molecular mechanisms underlying cisplatin upregulation of PD‐L1 expression in ovarian cancer cells.
MicroRNAs are considered to be one of the major factors involved in the post‐transcriptional regulation of genes. Studies have shown that PD‐L1 expression is directly regulated by several microRNAs, such as miR‐200 14, miR‐143 15, miR‐138 16, miR‐142‐5p 17 and miR‐34 18. In our comparative study on the expression level of miR‐145, we confirmed that it was four times lower in cisplatin‐treated A2780cis cells than in untreated A2780cis cells using real‐time PCR analysis. Although miR‐145 down‐regulation has been reported in several studies, including colonic adenocarcinoma 32, lung adenocarcinoma 33, breast cancer 34, prostate carcinomas 35, colorectal cancer 36 and ovarian carcinoma 37, etc. and its tumor‐suppressive role via targeting many genes, including EGFR 19, ILK 20, MUC1 21, SOX9 22and ICP4 23, no prior studies have shown the effect of miR‐145 on PD‐L1 expression in ovarian carcinoma. Hence, in this research, we first report that cisplatin‐mediated down‐regulation of miR‐145 contributes to PD‐L1 up‐regulation in cisplatin‐resistant ovarian cancer cells. Over‐expression of miR‐145 significantly reduces the cisplatin‐induced up‐regulation of PD‐L1 in vitro. This result is supported by the negative correlation between miR‐145 and PD‐L1 expression in human ovarian carcinoma tissues.
To further explore the molecular mechanism of miRNA‐145 suppressing PD‐L1 expression, we carried out a bioinformatics analysis on potential target genes that could be directly regulated by miR‐145 and a transcription factor, c‐Myc, was found to be one of its potential target genes. As a member of the Myc family, c‐Myc controls stability and transcriptional activity of target gene via binding DNA on heterodimerization with Myc‐associated factor X 38. We confirmed the direct regulatory effect of miR‐145 on c‐Myc using luciferase reporter gene detection. Sachdeva et al. 39 also reported that c‐Myc was a direct target for miR‐145 in both HCT‐116 and MCF‐7 cells, which strongly corroborates our study results. As expected, when we knocked down the c‐Myc gene expression in A2780cis cells, we observed an interruption in the negative regulation of miR‐145 on PD‐L1.
In this paper, we describe new findings on cisplatin resistance of ovarian cancer. We report that cisplatin‐mediated down‐regulation of miR‐145 is responsible for PD‐L1 expression via c‐Myc in ovarian cancer cells. Our data suggest that miR‐145 might serve as an adjuvant therapeutic target for chemotherapy of ovarian cancer.
Disclosure
The authors have no competing interests to disclose.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81402156); Natural Science Foundation of Ningxia Province (NZ17206); Excellent Young Teachers Program of Education department of Ningxia (NGY2015095) and ‘Parental Affection Plan’ Project of Ministry of Education (Z2016042).
Contributor Information
Y. Song, Email: yanfengsong123@hotmail.com.
W. Zhao, Email: zhaowei@nxmu.edu.cn.
References
- 1. Ayen A, Jimenez Martinez Y, Marchal JA, Boulaiz H. Recent progress in gene therapy for ovarian cancer. Int J Mol Sci 2018; 19:1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet 2014; 384:1376–88. [DOI] [PubMed] [Google Scholar]
- 3. Holmes D. Ovarian cancer: beyond resistance. Nature 2015; 527:S217. [DOI] [PubMed] [Google Scholar]
- 4. Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol 2016; 43:723–37. [DOI] [PubMed] [Google Scholar]
- 5. Smith HJ, Straughn JM, Buchsbaum DJ, Arend RC. Epigenetic therapy for the treatment of epithelial ovarian cancer: a clinical review. Gynecol Oncol Rep 2017; 20:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szczepanski MJ, Szajnik M, Czystowska M et al Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res 2009; 15:3325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dobrzanski MJ, Reome JB, Hylind JC, Rewers‐Felkins KA, Abulsamad K, Adams SL. Ag‐specific type 1 CD8 effector cells enhance methotrexate‐mediated antitumor responses by modulating differentiated T cell localization, activation and chemokine production in established breast cancer. Clin Immunol 2008; 128:205–18. [DOI] [PubMed] [Google Scholar]
- 8. Shalapour S, Font‐Burgada J, Di Caro G et al Immunosuppressive plasma cells impede T‐cell‐dependent immunogenic chemotherapy. Nature 2015; 521:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Anti‐PD‐L1/PD‐1 immune therapies in ovarian cancer: basic mechanism and future clinical application. Int J Clin Oncol 2016; 21:456–61. [DOI] [PubMed] [Google Scholar]
- 10. Inaguma S, Wang Z, Lasota J et al Comprehensive immunohistochemical study of programmed cell death ligand 1 (PD‐L1): analysis in 5536 cases revealed consistent expression in trophoblastic tumors. Am J Surg Pathol 2016; 40:1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong W, Song Q, Lu X, Zhao J, Min P, Yi X. Paclitaxel induced B7–H1 expression in cancer cells via the MAPK pathway. J Chemother 2011; 23:295–9. [DOI] [PubMed] [Google Scholar]
- 12. Peng J, Hamanishi J, Matsumura N et al Chemotherapy induces programmed cell death‐ligand 1 overexpression via the nuclear factor‐kappab to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res 2015; 75:5034–45. [DOI] [PubMed] [Google Scholar]
- 13. Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis 2015; 35:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grenda A, Krawczyk P. New dancing couple: PD‐L1 and MicroRNA. Scand J Immunol 2017; 86:130–4. [DOI] [PubMed] [Google Scholar]
- 15. Zhao H, Cheng Y, Dong S et al Down regulation of miR‐143 promotes radiation – induced thymic lymphoma by targeting B7H1. Toxicol Lett 2017; 280:116–24. [DOI] [PubMed] [Google Scholar]
- 16. Zhang XL, Xu LL, Wang F. Hsa_circ_0020397 regulates colorectal cancer cell viability, apoptosis and invasion by promoting the expression of the miR‐138 targets TERT and PD‐L1. Cell Biol Int 2017; 41:1056–64. [DOI] [PubMed] [Google Scholar]
- 17. Jia L, Xi Q, Wang H et al miR‐142‐5p regulates tumor cell PD‐L1 expression and enhances anti‐tumor immunity. Biochem Biophys Res Commun 2017; 488:425–31. [DOI] [PubMed] [Google Scholar]
- 18. Cortez MA, Ivan C, Valdecanas D et al PDL1 Regulation by p53 via miR‐34. J Natl Cancer Inst 2016; 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho WC, Chow AS, Au JS. MiR‐145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol 2011; 8:125–31. [DOI] [PubMed] [Google Scholar]
- 20. Noguchi S, Yasui Y, Iwasaki J et al Replacement treatment with microRNA‐143 and ‐145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett 2013; 328:353–61. [DOI] [PubMed] [Google Scholar]
- 21. Wu H, Xiao Z, Wang K, Liu W, Hao Q. MiR‐145 is downregulated in human ovarian cancer and modulates cell growth and invasion by targeting p70S6K1 and MUC1. Biochem Biophys Res Commun 2013; 441:693–700. [DOI] [PubMed] [Google Scholar]
- 22. Yu CC, Tsai LL, Wang ML et al miR145 targets the SOX9/ADAM17 axis to inhibit tumor‐initiating cells and IL‐6‐mediated paracrine effects in head and neck cancer. Cancer Res 2013; 73:3425–40. [DOI] [PubMed] [Google Scholar]
- 23. Lee CY, Rennie PS, Jia WW. MicroRNA regulation of oncolytic herpes simplex virus‐1 for selective killing of prostate cancer cells. Clin Cancer Res 2009; 15:5126–35. [DOI] [PubMed] [Google Scholar]
- 24. Lu Z. Introduction of WHO classification of tumours of female reproductive organs, fourth edition. Zhonghua Bing Li Xue Za Zhi 2014; 43:649–50. [PubMed] [Google Scholar]
- 25. Morgan RJ Jr, Armstrong DK, Alvarez RD et al NCCN clinical practice guidelines in oncology. J Natl Compr Canc Network 2016; 14:1134–63. [DOI] [PubMed] [Google Scholar]
- 26. Yu MC, Chen CH, Liang X et al Inhibition of T‐cell responses by hepatic stellate cells via B7‐H1‐mediated T‐cell apoptosis in mice. Hepatology 2004; 40:1312–21. [DOI] [PubMed] [Google Scholar]
- 27. Casey SC, Tong L, Li Y et al MYC regulates the antitumor immune response through CD47 and PD‐L1. Science 2016; 352:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA‐4 and PD‐1 blockade and new combinations. Semin Oncol 2015; 42:363–77. [DOI] [PubMed] [Google Scholar]
- 29. Gibbons Johnson RM, Dong H. Functional expression of programmed death‐ligand 1 (B7–H1) by immune cells and tumor cells. Front Immunol 2017; 8:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gordon SR, Maute RL, Dulken BW et al PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017; 545:495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen G, Huang AC, Zhang W et al Exosomal PD‐L1 contributes to immunosuppression and is associated with anti‐PD‐1 response. Nature 2018; 560:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 2003; 1:882–91. [PubMed] [Google Scholar]
- 33. Cho WC, Chow AS, Au JS. Restoration of tumour suppressor hsa‐miR‐145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer 2009; 45:2197–206. [DOI] [PubMed] [Google Scholar]
- 34. Radojicic J, Zaravinos A, Vrekoussis T, Kafousi M, Spandidos DA, Stathopoulos EN. MicroRNA expression analysis in triple‐negative (ER, PR and Her2/neu) breast cancer. Cell Cycle 2011; 10:507–17. [DOI] [PubMed] [Google Scholar]
- 35. Hart M, Nolte E, Wach S et al Comparative microRNA profiling of prostate carcinomas with increasing tumor stage by deep sequencing. Mol Cancer Res 2014; 12:250–63. [DOI] [PubMed] [Google Scholar]
- 36. Tan YG, Zhang YF, Guo CJ, Yang M, Chen MY. Screening of differentially expressed microRNA in ulcerative colitis related colorectal cancer. Asian Pac J Trop Med 2013; 6:972–6. [DOI] [PubMed] [Google Scholar]
- 37. Vaksman O, Stavnes HT, Kaern J, Trope CG, Davidson B, Reich R. miRNA profiling along tumour progression in ovarian carcinoma. J Cell Mol Med 2011; 15:1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caforio M, Sorino C, Iacovelli S, Fanciulli M, Locatelli F, Folgiero V. Recent advances in searching c‐Myc transcriptional cofactors during tumorigenesis. J Exp Clin Cancer Res 2018; 37:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sachdeva M, Zhu S, Wu F et al p53 represses c‐Myc through induction of the tumor suppressor miR‐145. Proc Natl Acad Sci USA 2009; 106:3207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


