Abstract
Background
Given presumed differences in disease severity between young (≤ 45 years) and elderly (≥ 75 years) women with breast cancer, we sought to compare tumor histopathology, stage at presentation, patterns of care, and survival at the extremes of age.
Methods
Adults with stages 0–IV breast cancer in the National Cancer Database (2004–2015) were categorized by age (18–45 years, 46–74 years, ≥ 75 years) and compared. Kaplan–Meier curves were used to visualize unadjusted overall survival (OS). A Cox proportional-hazards model was used to estimate the effect of age group, including adjustment for tumor subtype [hormone receptor [HR]+/HER2−, HER2+, triple-negative (TN)].
Results
Of the 1,201,252 patients identified, 13% were ≤ 45 years and 17.5% were ≥ 75 years. Women ≤ 45 years were more likely to have higher pT/N stages and grade 3 disease compared to older patients; however, rates of de novo cM1 disease were comparable (3.7% vs 3.5%). HER2+ and TN tumors were more common in those ≤ 45 years (HER2+ : 18.6% vs 9.2%; TN: 14.9% vs 8.2%), while HR+/HER2− tumors were more likely in women ≥ 75 years (69.3% vs 51.3%) (all p < 0.001). Younger patients were more likely to undergo mastectomy vs lumpectomy (56% vs 34%), and receive chemotherapy (65.8% vs 10.2%) and radiation (56.2% vs 39.5%). After adjustment, OS was worse in older patients (older HR 2.94, CI 2.86–3.03).
Conclusions
High-risk tumor subtypes and comprehensive multimodal treatment remain significantly more common among younger women (≤ 45 years) with breast cancer, yet, elderly women are similarly diagnosed with incurable de novo metastatic disease. Tailored screening and treatment strategies are critical to prevent age-related disparities in breast cancer care.
Keywords: Breast cancer, Elderly, Young women, Age extremes
Introduction
Among younger breast cancer patients, high-risk tumor subtypes and more advanced tumor and nodal stages at presentation have warranted comprehensive evaluation and treatment, while de-escalation of therapy has overwhelmingly been endorsed in older women with presumably favorable tumors. Breast cancer is the most common cancer in elderly women in the United States (US) [1], but it also affects more than 12,000 women < 40 years old each year [2]. Furthermore, breast cancer is the most common cause of cancer-related deaths in women under 40 years old in the US with survival rates often lower than those observed in older women [2].
Numerous studies have investigated the biology and treatment of breast cancer at the extremes of age. Young women in particular have been extensively studied with reports of more aggressive disease, more extensive treatment, and concerns for worse survival [3–5]. Breast cancer in “young” women has been ill-defined, and those diagnosed at younger ages are often compared to women of any age greater than the defined limit (usually 40 years or 45 years) [3, 6]. Similarly, elderly women have also been a population of strong interest with numerous large studies evaluating the optimal treatment for this aging population with competing comorbidities [7]. While some studies have focused on histology [8], most have evaluated treatments and outcomes with a particular focus on de-escalating therapy [9–12].
Given the aging population in the US and advancements in modern breast cancer treatments, we sought to compare tumor histopathology, disease stage, contemporary patterns of care, as well as survival trends in breast cancer patients at the extremes of age.
Methods
The National Cancer Database (NCDB) was used to select adult patients diagnosed with ductal carcinoma in situ (DCIS), invasive non-metastatic, and metastatic breast cancer from 2004 to 2015. Patients with missing or unknown clinical TNM stage, grade, estrogen receptor (ER) status, progesterone receptor (PR) status, treatment information (surgery, chemotherapy, radiation, endocrine therapy), or survival data (including all patients diagnosed in 2015) were excluded. Patients were categorized by age as ≤ 45 years, 46–74 years, and ≥ 75 years. Patients were categorized as DCIS if their ICD-O-3 behavior code was two (meaning ‘in situ’) and they were pT0/IS, pN0, and pM0/X if pathologic data were available, or cT0/IS, cN0, and cM0 if pathologic data were not available. Patients were categorized as invasive if their ICD-O-3 behavior code was three (meaning ‘invasive’). Invasive patients with pM1 or cM1 were categorized as metastatic, and all other invasive patients were categorized as invasive non-metastatic. Patients with in situ behavior who could not be categorized as DCIS were excluded.
Patient characteristics were summarized by N (%) for categorical variables, and median (interquartile range, IQR) for continuous variables, for all patients, and by age group. Analysis of variance (ANOVA) and Chi-square tests were used to test for differences between groups for continuous and categorical variables, respectively. Of note, although all groups were compared (all data included), we primarily report and discuss the comparisons between the extremes of age (≤ 45 years and ≥ 75 years).
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Kaplan–Meier curves were used to visualize the unadjusted OS, and 5-year and 10-year survival rates and 95% confidence intervals (CI) were reported. A Cox proportional-hazards model was used to estimate the effect of age group and diagnosis (DCIS, invasive non-metastatic, and invasive metastatic) on OS after adjustment for known covariates, and hazard ratios (HR) and 95% CI were reported. All models were adjusted for year of diagnosis, gender, race/ethnicity, insurance status, income level, education level, facility type, facility location, distance traveled, Charlson/Deyo Comorbidity Score, histology, tumor grade, cT stage, cN stage, ER status, PR status, surgery type, radiation therapy, chemotherapy, and endocrine therapy. Additional adjusted survival models were conducted separately for each diagnosis. In order to account for the correlation of patients treated at the same facility, a robust sandwich covariance estimator was used for all adjusted survival models. Only patients with available data for all covariates were included in each model, and effective sample sizes were reported for each table/figure.
Of note, HER2 (human epidermal growth factor receptor 2) status is only reliably reported for patients diagnosed in 2010 and after. Therefore, subset analyses of these patients were conducted, in order to allow for adjustment for tumor subtype [(1) hormone receptor-positive/HER2-negative, (2) HER2-positive, (3) triple-negative breast cancer].
A p value < 0.05 was considered statistically significant, and no adjustments were made for multiple comparisons. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary NC). This study was approved by the Institutional Review Board.
Results
Of the 1,201,252 patients identified (Supplemental Table 1), 13% were ≤ 45 years (‘younger’; N = 156,240) and 17.5% were ≥ 75 years (‘older’; N = 210,095) (Supplemental Fig. 1). Median follow-up was 58.7 months (95% CI 58.6–58.8) for all patients. Compared to women ≤ 45 years, those ≥ 75 years were more likely to be non-Hispanic white (82% vs 66.2%). By contrast, a higher proportion of patients ≤ 45 years were non-Hispanic Black (14% vs 7.5%) or Hispanic (8.5% vs 2.7%), compared to those ≥ 75 years. Although education levels were similar for both age groups, a higher proportion of younger patients had an income ≥ $48,000 (66.5% vs 61.6%). (Table 1).
Table 1.
Select patient and facility characteristics of women with breast cancer in the NCDB (2004–2015), stratified by age group
| All patients (N = 1,201,252) | Ages ≤ 45 years (N = 156,240) | Ages ≥ 75 years (N = 210,095) | p value | |
|---|---|---|---|---|
| Gender | < 0.001 | |||
| Female | 1,191,150 (99.2%) | 155,463 (99.5%) | 207,536 (98.8%) | |
| Male | 10,102 (0.8%) | 777 (0.5%) | 2559 (1.2%) | |
| Race/ethnicity | < 0.001 | |||
| Non-Hispanic White | 908,990 (75.7%) | 103,355 (66.2%) | 172,366 (82%) | |
| Non-Hispanic Black | 126,573 (10.5%) | 21,873 (14%) | 15,774 (7.5%) | |
| Hispanic | 57,113 (4.8%) | 13,279 (8.5%) | 5721 (2.7%) | |
| Other | 44,001 (3.7%) | 9485 (6.1%) | 3992 (1.9%) | |
| Charlson/Deyo Comorbidity Score | < 0.001 | |||
| 0 | 1,008,792 (84%) | 145,622 (93.2%) | 159,889 (76.1%) | |
| 1 | 156,501 (13%) | 9535 (6.1%) | 38,742 (18.4%) | |
| ≥ 2 | 35,959 (3%) | 1083 (0.7%) | 11,464 (5.5%) | |
| Income level | < 0.001 | |||
| < $48,000 | 424,193 (35.3%) | 51,145 (32.7%) | 78,913 (37.6%) | |
| ≥ $48,000 | 768,697 (64%) | 103,838 (66.5%) | 129,413 (61.6%) | |
| Education level | < 0.001 | |||
| ≤ 87% high school grad rate | 445,597 (37.1%) | 58,044 (37.2%) | 76,745 (36.5%) | |
| > 87% high school grad rate | 747,773 (62.2%) | 96,989 (62.1%) | 131,687 (62.7%) | |
| Insurance status | < 0.001 | |||
| Private | 634,577 (52.8%) | 123,753 (79.2%) | 19,358 (9.2%) | |
| Government | 526,525 (43.8%) | 23,941 (15.3%) | 187,884 (89.4%) | |
| Not insured | 23,942 (2%) | 5967 (3.8%) | 657 (0.3%) | |
| Facility type | < 0.001 | |||
| Academic | 362,684 (30.2%) | 56,323 (36%) | 51,418 (24.5%) | |
| Integrated network | 136,214 (11.3%) | 18,142 (11.6%) | 23,489 (11.2%) | |
| Comprehensive | 584,136 (48.6%) | 69,111 (44.2%) | 109,882 (52.3%) | |
| Community | 118,218 (9.8%) | 12,664 (8.1%) | 25,306 (12%) |
Data presented as N (%) unless otherwise specified. Percentages may not add up to 100 due to rounding or missing values. p values represent the comparison between patients ≤ 45 years and those ≥ 75 years
Anatomic stage at presentation
Clinical and pathological T/N stages were significantly different between age groups (all p < 0.001) and were generally more advanced in younger patients compared to those ≥ 75 years. The median tumor size in younger patients was 2 cm, compared to 1.5 cm in older patients (p < 0.001). Older patients were more likely to be cN0 (87.7% vs 74.2%, p < 0.001). Women ≤ 45 years were more likely to have higher pathological N stage, with 18.4% and 6% of younger women presenting with pN1 and pN2 disease, respectively, vs 10.5% and 3.3% of older women (p < 0.001). Notably, rates of de novo cM1 disease were comparable at the extremes of age (younger 3.7% vs older 3.5%, p < 0.001), although women ≤ 45 years had slightly higher rates of biopsy-proven pM1 disease (younger 1.4% vs older 0.8%, p < 0.001). (Table 2).
Table 2.
Select disease characteristics of women with breast cancer in the NCDB (2004–2015), stratified by age group
| All patients (N = 1,201,252) | Ages ≤ 45 years (N = 156,240) | Ages ≥ 75 years (N = 210,095) | p value | |
|---|---|---|---|---|
| Histology | < 0.001 | |||
| Ductal | 880,010 (73.3%) | 122,889 (78.7%) | 147,029 (70%) | |
| Lobular | 233,400 (19.4%) | 22,978 (14.7%) | 45,108 (21.5%) | |
| Other | 87,842 (7.3%) | 10,373 (6.6%) | 17,958 (8.5%) | |
| Behavior | < 0.001 | |||
| In situ | 169,773 (14.1%) | 20,961 (13.4%) | 22,263 (10.6%) | |
| Invasive | 1,031,479 (85.9%) | 135,279 (86.6%) | 187,832 (89.4%) | |
| Tumor type | < 0.001 | |||
| DCIS | 169,773 (14.1%) | 20,961 (13.4%) | 22,263 (10.6%) | |
| Invasive non-metastatic | 989,079 (82.3%) | 129,095 (82.6%) | 180,196 (85.8%) | |
| Invasive metastatic | 42,400 (3.5%) | 6184 (4%) | 7636 (3.6%) | |
| Tumor laterality | 0.48 | |||
| Bilateral | 417 (0%) | 59 (0%) | 70 (0%) | |
| Unilateral | 1,200,454 (99.9%) | 156,139 (99.9%) | 209,942 (99.9%) | |
| Tumor size (cm)-median (IQR) | 1.6 (1–2.6) | 2 (1.2–3.3) | 1.5 (1–2.5) | < 0.001 |
| # LNs Examined-median (IQR) | 3 (2–8) | 4 (2–12) | 3 (2–7) | < 0.001 |
| # Positive LNs-median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–0) | < 0.001 |
| Clinical T stage | < 0.001 | |||
| T0/IS | 192,356 (16%) | 24,558 (15.7%) | 25,151 (12%) | |
| T1 | 631,855 (52.6%) | 63,885 (40.9%) | 120,054 (57.1%) | |
| T2 | 276,529 (23%) | 48,677 (31.2%) | 47,759 (22.7%) | |
| T3 | 54,940 (4.6%) | 12,914 (8.3%) | 7320 (3.5%) | |
| T4 | 45,572 (3.8%) | 6206 (4%) | 9811 (4.7%) | |
| Clinical N stage | < 0.001 | |||
| N0 | 1,004,429 (83.6%) | 115,957 (74.2%) | 184,318 (87.7%) | |
| N1 | 146,788 (12.2%) | 30,770 (19.7%) | 18,989 (9%) | |
| N2 | 31,886 (2.7%) | 5973 (3.8%) | 4701 (2.2%) | |
| N3 | 18,149 (1.5%) | 3540 (2.3%) | 2087 (1%) | |
| Clinical M stage | < 0.001 | |||
| M0 | 1,161,206 (96.7%) | 150,439 (96.3%) | 202,831 (96.5%) | |
| M1 | 40,046 (3.3%) | 5801 (3.7%) | 7264 (3.5%) | |
| Pathologic T stage | < 0.001 | |||
| T0/IS | 154,529 (12.9%) | 23,077 (14.8%) | 18,163 (8.6%) | |
| T1 | 535,254 (44.6%) | 57,388 (36.7%) | 97,130 (46.2%) | |
| T2 | 224,479 (18.7%) | 34,172 (21.9%) | 41,640 (19.8%) | |
| T3 | 35,426 (2.9%) | 6518 (4.2%) | 5823 (2.8%) | |
| T4 | 16,365 (1.4%) | 1823 (1.2%) | 4756 (2.3%) | |
| TX | 203,595 (16.9%) | 29,493 (18.9%) | 34,525 (16.4%) | |
| Pathologic N stage | < 0.001 | |||
| N0 | 650,012 (54.1%) | 76,447 (48.9%) | 108,733 (51.8%) | |
| N1 | 165,391 (13.8%) | 28,705 (18.4%) | 22,142 (10.5%) | |
| N2 | 49,743 (4.1%) | 9309 (6%) | 6879 (3.3%) | |
| N3 | 25,057 (2.1%) | 4132 (2.6%) | 3662 (1.7%) | |
| NX | 267,390 (22.3%) | 33,055 (21.2%) | 57,302 (27.3%) | |
| Pathologic M stage | < 0.001 | |||
| M0 | 747,034 (62.2%) | 90,913 (58.2%) | 127,894 (60.9%) | |
| M1 | 12,485 (1%) | 2172 (1.4%) | 1779 (0.8%) | |
| MX | 441,733 (36.8%) | 63,155 (40.4%) | 80,422 (38.3%) | |
| Recurrence risk (oncotype DX) | < 0.001 | |||
| High | 13,660 (1.1%) | 1802 (1.2%) | 1073 (0.5%) | |
| Intermediate | 42,760 (3.6%) | 4973 (3.2%) | 2693 (1.3%) | |
| Low | 81,070 (6.7%) | 8384 (5.4%) | 5197 (2.5%) | |
| Oncotype DX Score-median (IQR) | 16 (11–22) | 17 (12–23) | 16 (10–23) | < 0.001 |
| Grade | < 0.001 | |||
| 1 | 258,029 (21.5%) | 19,825 (12.7%) | 54,390 (25.9%) | |
| 2 | 522,699 (43.5%) | 60,558 (38.8%) | 98,974 (47.1%) | |
| 3 | 420,524 (35%) | 75,857 (48.6%) | 56,731 (27%) | |
| ER status | < 0.001 | |||
| ER+ | 976,250 (81.3%) | 116,531 (74.6%) | 179,920 (85.6%) | |
| ER− | 225,002 (18.7%) | 39,709 (25.4%) | 30,175 (14.4%) | |
| PR status | < 0.001 | |||
| PR+ | 854,346 (71.1%) | 106,235 (68%) | 154,745 (73.7%) | |
| PR− | 346,906 (28.9%) | 50,005 (32%) | 55,350 (26.3%) | |
| HER2 status* | < 0.001 | |||
| HER2+ | 95,049 (12.6%) | 17,104 (18.6%) | 11,891 (9.2%) | |
| HER2− | 540,847 (71.7%) | 60,976 (66.2%) | 99,793 (77.5%) | |
| Tumor subtype* | < 0.001 | |||
| HR+/HER2− | 463,374 (61.4%) | 47,241 (51.3%) | 89,285 (69.3%) | |
| HER2+ | 95,049 (12.6%) | 17,104 (18.6%) | 11,891 (9.2%) | |
| TNBC | 77,473 (10.3%) | 13,735 (14.9%) | 10,508 (8.2%) |
Data presented as N (%) unless otherwise specified. Percentages may not add up to 100 due to rounding or missing values. p values represent the comparison between patients ≤ 45 years and those ≥ 75 years
LN lymph nodes, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, HR hormone receptor, TNBC triple-negative breast cancer
HER2 status was reported for patients diagnosed in 2010 and after
Tumor histopathology
Tumor grade was significantly different between younger and older patients, with women ≤ 45 years more likely to have grade 3 disease when compared to patients ≥ 75 years (48.6% vs 27%, p < 0.001). A lower proportion of younger patients were diagnosed with invasive lobular carcinoma compared to older patients (14.7% vs 21.5%, p < 0.001). Tumor subtype differed between younger and older patients, with hormone receptor-positive/HER2-negative tumors less likely in those ≤ 45 years (51.3% vs 69.3%), while HER2-positive and triple-negative tumors were more common in those ≤ 45 years (HER2-positive: younger 18.6% vs older 9.2%; triple-negative: younger 14.9% vs older 8.2%). When reported, Oncotype DX scores were similar between younger and older patients (median score: ≤ 45 years = 17 vs ≥ 75 years = 16) (Table 2).
Patterns of care
In general, younger patients were more likely to receive comprehensive multimodal treatment (surgery, systemic therapy, and radiation) compared to older patients (Fig. 1). A higher proportion of patients ≤ 45 years underwent mastectomy than those ≥ 75 years (56% vs 34%), and they were less likely to forego breast surgery when compare to older women (4.6% vs 8.8%). Younger patients were more likely to receive chemotherapy (65.8% vs 10.2%, p < 0.001) and radiation (56.2% vs 39.5%, p < 0.001). Among patients with ER-positive and/or PR-positive disease only, endocrine therapy was also more common among younger patients (75.7% vs 64.7%, p < 0.001).
Fig. 1.
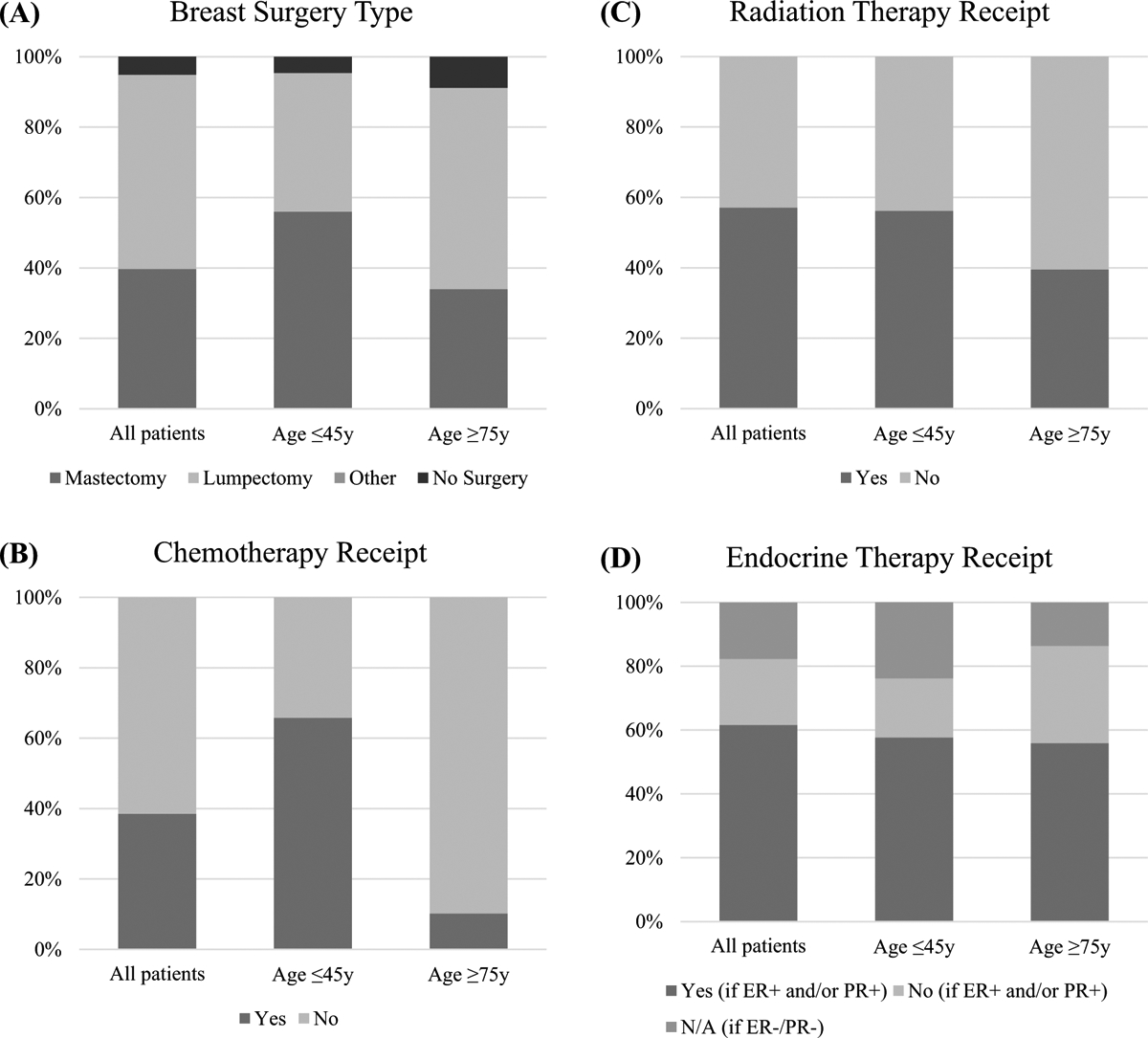
Select treatment characteristics of women with breast cancer in the NCDB (2004–2015), stratified by age group. Data presented as percentages and may not add up to 100 due to rounding or missing values. All p values < 0.001 and represent the comparison between patients ≤ 45 years and those ≥ 75 years. a Breast surgery type. b Chemotherapy receipt. c Radiation therapy receipt. d Endocrine therapy receipt, stratified by hormone receptor (ER/PR) status. ER estrogen receptor. PR progesterone receptor
Survival outcomes
Women ≥ 75 years had worse unadjusted OS compared to younger patients (Fig. 2), a finding that remained true after adjustment for all diagnoses (younger REF, older HR 2.944, CI 2.862–3.028, p < 0.001) (Supplemental Table 2). When stratified by stage (Stage 0: DCIS, Stages I–III: invasive non-metastatic, and Stage IV: invasive metastatic), the strength of the association weakened with increased disease severity (Table 3). For example, older patients with DCIS had a much higher risk of death compared to younger patients (younger REF, older HR 7.191, CI 6.280–8.235, p < 0.001), in contrast to those with invasive metastatic disease where the risk of death was more similar (younger REF, older HR 1.566, CI 1.479–1.659, p < 0.001) (Table 3).
Fig. 2.
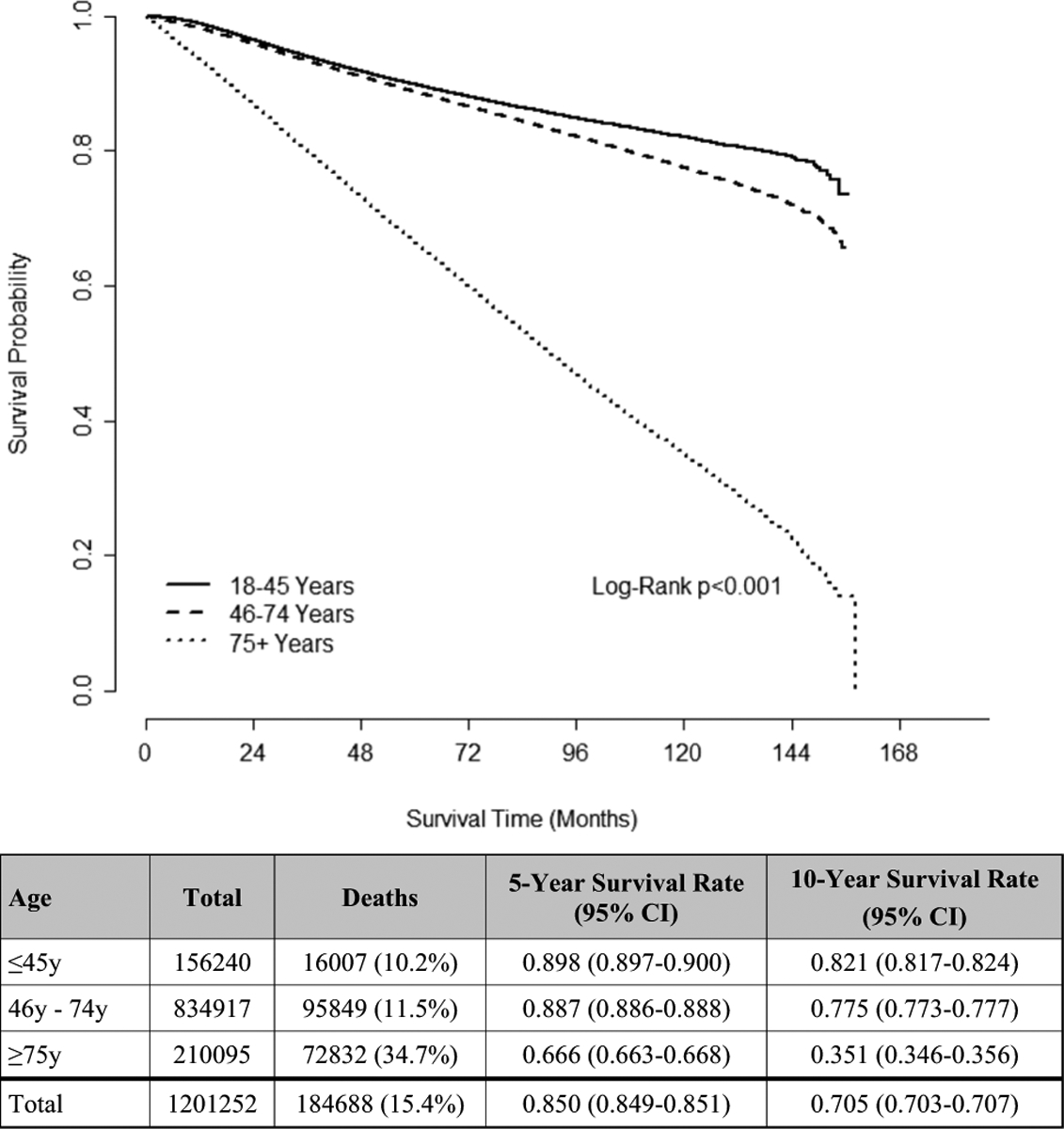
Unadjusted OS of women with breast cancer in the NCDB (2004–2015), stratified by age group (N = 1,201,252)
Table 3.
Adjusted OS by diagnosis: DCIS (N = 158,241), invasive non-metastatic (N = 915,994), and invasive metastatic (N = 38,813)
| Hazard ratio (95% CI) | p value | Overall p value | |
|---|---|---|---|
| DCIS | |||
| Age group (years) | < 0.001 | ||
| ≤ 45 | Ref | ||
| 46–74 | 2.187 (1.928–2.48) | < 0.001 | |
| ≥ 75 | 7.191 (6.28–8.235) | < 0.001 | |
| Invasive, non-metastatic | |||
| Age group (years) | < 0.001 | ||
| ≤ 45 | Ref | ||
| 46–74 | 1.207 (1.179–1.237) | < 0.001 | |
| ≥ 75 | 3.057 (2.965–3.151) | < 0.001 | |
| Invasive, metastatic | |||
| Age group (years) | < 0.001 | ||
| ≤ 45 | Ref | ||
| 46–74 | 1.166 (1.117–1.218) | < 0.001 | |
| ≥ 75 | 1.566 (1.479–1.659) | < 0.001 | |
All models adjusted for year of diagnosis, gender, race/ethnicity, insurance status, income level, education level, facility type, facility location, distance traveled, Charlson/Deyo Comorbidity Score, histology, tumor grade, clinical T stage, clinical N stage, estrogen receptor status, progesterone receptor status, surgery type, radiation therapy, chemotherapy, and endocrine therapy
DCIS ductal carcinoma in situ
Similar trends in OS were observed in subgroup analyses of all patients with invasive disease where HER2 status was routinely reported in 2010 and after (younger REF, older HR 2.732, CI 2.623–2.844, p < 0.001) (Supplemental Table 3). When stratified into invasive non-metastatic and metastatic subgroups, the strength of the association again weakened for older patients with invasive metastatic disease in this same period (younger REF, older HR 1.671, CI 1.548–1.804, p < 0.001) (Supplemental Table 4).
Discussion
Breast cancer is the most common cancer diagnosis in women in the US [1], and of the over one million patients initially identified, women ≤ 45 years and those ≥ 75 years represented comparable proportions (13% vs 17.5%, respectively). Although numerous studies have investigated breast cancer features at the extremes of age, few have compared them directly. As such, our study represents one of the largest to directly compare tumor histopathology, stage of presentation, and patterns of care in the young and elderly. Compared to women ≥ 75 years, we found that women ≤ 45 years are more likely to have high-risk tumor phenotypes and advanced stage at presentation. Accordingly, they were also more likely to receive multimodal treatment, including mastectomy, chemotherapy, and radiation. However, despite having breast cancers that were typically lower grade, node-negative, and hormone receptor-positive, a similar proportion of women ≥ 75 years presented with de novo metastatic disease compared to their younger counterparts.
In our cohort, young women were approximately two times more likely to have HER2-positive and triple-negative (TN) phenotypes and grade 3 tumors. Similar to our findings and other studies [13, 14], Collins et al. previously demonstrated that a greater proportion of young women (≤ 40 years) had luminal B tumors (ER-positive and/or PR-positive and HER2-positive, or ER and/or PR-positive, HER2-negative and grade 3) and a smaller proportion had luminal A tumors (ER-positive and/or PR-positive and HER2-negative, histologic grade 1 or 2) [15]. Colleoni et al. also found higher proportions of ER-negative, PR-negative, and grade 3 tumors in a cohort of 185 very young women (age ≤ 35 years) with breast cancer [16]. Beyond differences in molecular subtypes, others have demonstrated unique proliferation-related prognostic gene signatures [6] and oncogenic signaling pathways [17] in young breast cancer patients. Recently, Azim et al. compared genomic aberrations among 780 young and elderly breast cancer patients in The Cancer Genome Atlas dataset [18]; older patients had more somatic mutations and copy number variations, while younger patients had higher expression of gene signatures related to proliferation, stem cell features, and endocrine resistance [18]. Notably, among women with hormone receptor-positive invasive breast cancer, our study demonstrated similar Oncotype Dx scores between younger and older women. Although this may represent a biased subset of breast cancer patients being considered for chemotherapy, further investigation is warranted to characterize the genetic and genomic assay differences among women at the extremes of age.
Consistent with observed higher risk tumor phenotypes, younger women were more likely to have higher pathologic T/N stages at presentation. Although this may be related to age-specific disease biology, it may also reflect current breast cancer screening guidelines, which usually recommend initiation of routine mammograms at the age of 40 or 45, and are unclear about the benefits of screening in women ≥ 75 years [19, 20]. As a result, young women may be more likely to present with symptomatic disease, while older women may be more likely to have screen-detected breast cancers. While younger women were more likely to have TN and HER2-positive breast cancers, elderly women were as likely to present with incurable de novo metastatic disease. There was a slightly greater difference in the rates of biopsy-proven pM1 disease (higher in younger women, compared to cM1 disease); however, this may reflect clinicians’ tendency to obtain pathologic confirmation of metastatic sites in younger patients as opposed to relying solely on clinical or radio-graphic findings. Notably, our data suggests that even the “less aggressive” tumor phenotypes observed in the elderly population can still spread to distant sites, resulting in incurable disease. It may be that the HER2-positive and triple-negative tumors result in metastatic disease at a younger age, while the hormone receptor-positive tumors may take longer to cause a detectable metastatic tumor burden, although the exact oncogenic drivers of disease progression are understudied [21]. Interestingly, a recent study of 564 breast cancer patients noted a difference in the site of distant metastases by age: brain metastases were more common in the young, and lung metastases were more common in older patients, likely reflecting differing tropism for distant sites between tumor subtypes [22]. Regardless, once a patient is diagnosed with metastatic disease, we demonstrate that survival differences narrow regardless of age at diagnosis.
Aside from their cancer diagnosis, young women with breast cancer are typically healthy, and thus, their disease-specific mortality overwhelmingly aligns with their OS. In contrast, elderly women often suffer from pre-existing comorbidities and lower life expectancy, which may undoubtedly be of greater risk to their OS than an early-stage breast cancer diagnosis. Narod et al. found that the breast cancer-specific mortality was greater in young women (age ≤ 35 years) with DCIS when compared to older women, suggesting that death in the elderly is likely related to causes other than breast cancer [23]. Decreased baseline life expectancy and reduced rates of local recurrence have prompted the breast cancer community to de-escalate therapy that lacks meaningful medical benefit in older women with breast cancer [9–11]. Importantly, Van Leeuwen et al. evaluated 212 elderly (age ≥ 80) breast cancer patients and demonstrated that outcomes may have been compromised when less than complete combined modality treatment was undertaken [24]. While recent landmark clinical trials for metastatic breast cancer have largely not excluded women based on age alone, the median age of women was 54 years (range 22–89) in the CLEOPATRA study, and 56–57 years (range 29–88) in the PALOMA-3 trial [25, 26]. Furthermore, both trials excluded women with an ECOG (Eastern Cooperative Oncology Group) performance status ≥ 2, which likely affects older women more than younger and is a common exclusion criteria in clinical trials. Not surprisingly, Freyer et al. surveyed oncologists caring for elderly women with metastatic breast cancer, and found that treatment plans differed from those of younger women, were driven by age, and characterized by the oncologists’ subjective assessment of the patient and in isolation from the geriatric care team [27]. Given that Americans ages 65 and older are projected to increase from 16 to 23% of the total population by 2060 [28], the vast heterogeneity in this group is also expected to continue to expand and will undoubtedly require careful attention to provide adequate and appropriate care at the individual patient level.
Limitations
While our study is one of the largest studies to directly compare breast cancer at the extremes of age, it has several limitations. Although the NCDB captures ~ 70–80% of breast cancer patients in the United States [29], the NCDB lacks granularity related to patient comorbidities, treatments administered, treatment adherence, and breast cancer-specific outcomes, including disease-related recurrence and mortality [30].
Conclusion
Our findings demonstrated that young women (≤ 45 years) remain at greater risk of diagnosis with more biologically aggressive and locally advanced breast cancer, yet, elderly women (≥ 75 years) remain vulnerable to metastatic breast cancer that could threaten their health and survival. Clinical trials tailored to the treatment of metastatic disease in elderly patients remain scarce, but necessary. Additionally, identifying predictors of low-versus high-risk cancer in older women may further inform the care of this growing population, where functional status more accurately predicts health outcomes. In a changing demographic of older women with breast cancer, thoughtful screening and treatment are important to avoid age-related disparities in breast cancer care.
Supplementary Material
Acknowledgements
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding Dr. R. Greenup is supported by the National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Career Development Award, K12HD043446-11 (PI: Andrews). Dr. O. Fayanju is supported by the National Institutes of Health (NIH) under Award Number 1K08CA241390 (PI: Fayanju). This work is also supported by the Duke Cancer Institute developmental funds through NIH Grant P30-CA014236 (PI: Kastan; Office of Cancer Centers, NCI). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest Dr. Plichta received a research grant from the Color Foundation in 2017–2018. All other authors declare that they have no conflict of interest.
Ethical approval This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was not obtained from individual participants included in the study, as the data was de-identified from the National Cancer Database.
This work was presented at the May 2019 American Society of Breast Surgeons Annual Meeting.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-020-05542-4) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. [16 May 2018];How common is breast cancer? https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html. Accessed.
- 2. [23 Apr 2018];American Cancer Society: Breast Cancer Facts & Figures 2017–2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Accessed.
- 3.Azim HA Jr, Partridge AH (2014) Biology of breast cancer in young women. Breast Cancer Res 16(4):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SM, Partridge AH (2015) Management of breast cancer in very young women. Breast 24(Suppl 2):S154–158 [DOI] [PubMed] [Google Scholar]
- 5.Plichta JK, Rai U, Tang R, Coopey SB, Buckley JM, Gadd MA, Specht MC, Hughes KS, Taghian AG, Smith BL (2016) Factors associated with recurrence rates and long-term survival in women diagnosed with breast cancer ages 40 and younger. Ann Surg Oncol 23(10):3212–3220 [DOI] [PubMed] [Google Scholar]
- 6.Azim HA Jr, Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, Haibe-Kains B, Piccart MJ, Sotiriou C, Loi S (2012) Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 18(5):1341–1351 [DOI] [PubMed] [Google Scholar]
- 7.Karuturi M, VanderWalde N, Muss H (2016) Approach and management of breast cancer in the elderly. Clin Geriatr Med 32(1):133–153 [DOI] [PubMed] [Google Scholar]
- 8.Chatzidaki P, Mellos C, Briese V, Mylonas I (2011) Does primary breast cancer in older women (≥ 80 years) have unfavorable histological characteristics? Arch Gynecol Obstet 284(3):705–712 [DOI] [PubMed] [Google Scholar]
- 9.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, Muss HB, Smith BL, Hudis CA, Winer EP et al. (2013) Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 31(19):2382–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamirisa N, Thomas SM, Fayanju OM, Greenup RA, Rosenberger LH, Hyslop T, Hwang ES, Plichta JK (2018) Axillary nodal evaluation in elderly breast cancer patients: potential effects on treatment decisions and survival. Ann Surg Oncol 25:2890–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spazzapan S, Crivellari D, Bedard P, Lombardi D, Miolo G, Scalone S, Veronesi A (2011) Therapeutic management of breast cancer in the elderly. Expert Opin Pharmacother 12(6):945–960 [DOI] [PubMed] [Google Scholar]
- 12.DeChant CA, Thomas SM, Rosenberger LH, Fayanju OM, Greenup RA, Hwang ES, Plichta JK (2019) Ductal carcinoma in situ in the elderly: what is the ideal treatment plan? J Unexplored Med Data 4:2 [Google Scholar]
- 13.Rosenberg SM, Partridge AH (2015) Management of breast cancer in very young women. Breast (Edinburgh, Scotland) 24(Suppl 2):S154–158 [DOI] [PubMed] [Google Scholar]
- 14.Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA (2012) Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res 14(2):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, Brachtel EF, Schapira L, Come SE, Winer EP et al. (2012) Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat 131(3):1061–1066 [DOI] [PubMed] [Google Scholar]
- 16.Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, Luini A, Veronesi P, Intra M, Orecchia R et al. (2002) Very young women (%3c 35 years) with operable breast cancer: features of disease at presentation. Ann Oncol 13(2):273–279 [DOI] [PubMed] [Google Scholar]
- 17.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG et al. (2008) Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26(20):3324–3330 [DOI] [PubMed] [Google Scholar]
- 18.Azim HA Jr, Nguyen B, Brohee S, Zoppoli G, Sotiriou C (2015) Genomic aberrations in young and elderly breast cancer patients. BMC Med 13:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, Walter LC, Church TR, Flowers CR, LaMonte SJ et al. (2015) Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 314(15):1599–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monticciolo DL, Newell MS, Hendrick RE, Helvie MA, Moy L, Monsees B, Kopans DB, Eby PR, Sickles EA (2017) Breast cancer screening for average-risk women: recommendations from the ACR commission on breast imaging. J Am Coll Radiol 14(9):1137–1143 [DOI] [PubMed] [Google Scholar]
- 21.Arnedos M, Vicier C, Loi S, Lefebvre C, Michiels S, Bonnefoi H, Andre F (2015) Precision medicine for metastatic breast cancer–limitations and solutions. Nat Rev Clin Oncol 12(12):693–704 [DOI] [PubMed] [Google Scholar]
- 22.Akrami M, Sepahdar A, Arasteh P, Tahmasebi S, Zangouri V, Askari A, Pezeshki B, Talei A (2018) Do site and type of metastasis in breast cancer show a changing pattern with increased age? A cross comparison of clinicopathological characteristics between age groups. World J Surg Oncol 16(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P (2015) Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 1(7):888–896 [DOI] [PubMed] [Google Scholar]
- 24.Van Leeuwen BL, Rosenkranz KM, Feng LL, Bedrosian I, Hartmann K, Hunt KK, Kuerer HM, Ross M, Singletary SE, Babiera GV (2011) The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol 79(3):315–320 [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K et al. (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(3):209–219 [DOI] [PubMed] [Google Scholar]
- 27.Freyer G, Braud A-C, Chaibi P, Spielmann M, Martin J-P, Vilela G, Guerin D, Zelek L (2005) Dealing with metastatic breast cancer in elderly women: results from a French study on a large cohort carried out by the ‘Observatory on elderly patients’. Ann Oncol 17(2):211–216 [DOI] [PubMed] [Google Scholar]
- 28.Vespa J, Armstrong DM, Medina L (2018) Demographic turning points for the United States: population projections for 2020 to 2060. Population Estimates and Projections Current Population Reports. US Department of Commerce Economics and Statistics Administration, Washington, D.C. [Google Scholar]
- 29.Mallin K, Browner A, Palis B, Gay G, McCabe R, Nogueira L, Yabroff R, Shulman L, Facktor M, Winchester DP et al. (2019) Incident cases captured in the national cancer database compared with those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann Surg Oncol 26:1604–1612 [DOI] [PubMed] [Google Scholar]
- 30.Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN et al. (2017) Using the National cancer database for outcomes research: a review. JAMA Oncol 3(12):1722–1728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


