Abstract
The bone morphogenetic protein (BMP) antagonist gremlin 1 plays a central role in the pathogenesis of hypoxic pulmonary hypertension (HPH). Recently, non-canonical functions of gremlin 1 have been identified, including specific binding to the vascular endothelial growth factor receptor-2 (VEGFR2). We tested the hypothesis that gremlin 1 modulates VEGFR2 signaling in the pulmonary microvascular endothelium.
We examined the effect of gremlin 1 haploinsufficiency on the expression of VEGF responsive genes and proteins in the hypoxic (10% O2) murine lung in vivo. Using human microvascular endothelial cells in vitro we examined the effect of gremlin 1 on VEGF signaling.
Gremlin 1 haploinsufficiency (Grem1+/–) attenuated the hypoxia-induced increase in gremlin 1 observed in the wild-type mouse lung. Reduced gremlin 1 expression in hypoxic Grem1+/– mice restored VEGFR2 expression and endothelial nitric oxide synthase (eNOS) expression and activity to normoxic values. Recombinant monomeric gremlin 1 inhibited VEGFA-induced VEGFR2 activation, downstream signaling, and VEGF-induced increases in Bcl-2, cell number, and the anti-apoptotic effect of VEGFA in vitro.
These results show that the monomeric form of gremlin 1 acts as an antagonist of VEGFR2 activation in the pulmonary microvascular endothelium. Given the previous demonstration that inhibition of VEGFR2 causes marked worsening of HPH, our results suggest that increased gremlin 1 in the hypoxic lung, in addition to blocking BMP receptor type-2 (BMPR2) signaling, contributes importantly to the development of PH by a non-canonical VEGFR2 blocking activity.
Keywords: pulmonary endothelium, VEGFA, VEGFR2, BMP, BMPR2, pulmonary hypertension, gremlin 1, GREM1, Grem1
Introduction
Chronic hypoxic lung diseases are frequently complicated by the development of hypoxic pulmonary hypertension (HPH), which leads to right ventricular overload and hypertrophy, right ventricular failure, and reduced life expectancy.1,2 While the molecular pathogenesis of HPH is incompletely understood, attenuated bone morphogenetic protein (BMP) signaling is centrally implicated.3–5 Loss of BMP signaling leads to dysfunction in both pulmonary artery endothelial cells and pulmonary artery smooth muscle cells resulting in the development of pulmonary hypertension (PH).3,6,7
Gremlin 1 is a glycoprotein whose canonical function is to bind to BMP2, BMP4, and BMP7 and thus prevent interaction of these ligands with BMP receptors (BMPR).8–13 It is expressed in monomeric and dimeric forms, both of which act to block BMP signaling.14–16 Increased gremlin 1 in the lung during the development of HPH and other forms of PH plays a key role in the pathogenesis of PH; to date, this action has been attributed to its blocking of BMP signaling.9,10
More recently, other non-canonical functions of gremlin 1 have been identified that are not mediated by modifying BMP signaling.17–20 Gremlin 1 binds with high affinity to vascular endothelial growth factor receptor 2 (VEGFR2) in human umbilical vein endothelial cells and acts as an agonist at this receptor leading to receptor phosphorylation and activation of downstream signaling;17,18,20 importantly, gremlin 1 does not bind to VEGFR1 or to VEGFR3.18 More recently, it has been reported that gremlin 1 in its monomeric form can act as an antagonist of VEGF activity at VEGFR2, in contrast to its previously reported agonist activity.14 In the context of PH, these actions at VEGFR2 are of particular interest since the VEGF–VEGFR2 pathway has important homeostatic roles in the pulmonary circulation.21,22 Inhibition of VEGFR2, with the VEGF receptor blocker SU5416, in combination with chronic hypoxia leads to the development of more severe hypertension than hypoxia alone, including the development of severe angio-obliterative pulmonary arterial hypertension (PAH) and right heart failure in animal models.21–23 Conversely, adenovirus mediated overexpression of VEGFA protects against the development of HPH.24
Taken together, these reports suggested that gremlin 1 might modulate the development of PH through an interaction with the VEGF–VEGFR2 axis, in addition to its canonical function of inhibition of BMP signaling. We report here a series of experiments undertaken to examine the role of gremlin 1 in modulating VEGFR2 signaling in the pulmonary microvascular endothelium and to examine its actions on endothelial cell function.
Materials and methods
Animal model
Gremlin 1 haploinsufficient knockout mice (Grem1+/–) and wild-type littermates (Grem1+/+) were bred and genotyped as described previously.9 All animal procedures conformed to National Institutes of Health guidelines and were approved by the University College Dublin Animal Research Ethics Committee. Male mice were exposed to hypoxia in a normobaric environmental chamber (FIO2 = 0.10) for periods of two days, a timepoint at which the majority of the structural remodeling of the pulmonary circulation is taking place and hypoxia-induced upregulation in gremlin 1 gene mRNA is greatest, or three weeks when the structural changes are established and hypoxia-induced gremlin 1 messenger RNA (mRNA) expression has returned to baseline levels;9,25 controls were maintained for the same periods of time in normoxia (FIO2 = 0.21). After experimental exposure, mice were anticoagulated with heparin, deeply anesthetized using intraperitoneal sodium pentobarbitone and then euthanized by exsanguination. Tissues were either immediately flash frozen in liquid nitrogen for later extraction of protein for immunoblotting or the lungs fixed for immunohistochemical analysis. Details of the primary and secondary antibodies used are given in the online supplement (Supplemental Tables 1 and 2).
In vitro cell culture
Primary human microvascular endothelial cells from the lung (HMVEC-L) were cultured according to the supplier's instructions (see Supplemental Table 3 for donor details) and used at passage six. For stimulation experiments, cells were seeded on six-well plates and used for experimentation when 80% confluent. Cells were serum starved before stimulation and then incubated with VEGF, gremlin 1, BMP2, BMP4, or noggin. When used, the BMP antagonists gremlin 1 and noggin were added 1 h before ligands (VEGFA and BMP2) to provide the optimum conditions for binding to and altering signaling through VEGFR2. The cells were then washed, pelleted, and lysed for extraction of protein or mRNA. Details of primary and secondary antibodies used are given in the online supplement (Supplemental Table 2).
Cell counting assay
Cells, plated on six-well plates, were serum starved overnight and then stimulated with VEGF or gremlin 1, separately or together. The medium was removed, cells fixed in situ, stained, rinsed, and microscopic images taken at predetermined locations. The images were digitized and rectangular stereological counting frames superimposed on each image. The number of cells within the counting frame was determined and the mean value used to calculate the cell density (number of cells/cm2) in each condition.
Endothelial apoptosis assay
Cells, seeded on 96-well plates for 24 h, were stimulated with VEGF or gremlin 1, separately or together, in serum starvation medium for 24 h. Caspase-Glo 3/7 reagent (Abgent, Cambridge, UK) was added in a 1:1 ratio to media. The plates were shaken briefly then incubated at room temperature for 1 h. The supernatant was transferred to a white 96-well plate and the luminescence recorded.
Statistical analyses
Normally distributed data are reported as mean (SD) and non-normally distributed data are presented as median (interquartile range [IQR]). For normally distributed data, the statistical significance of differences between means in a priori planned comparisons was determined with the use of paired or unpaired t-tests as appropriate. For non-normally distributed data, statistical significance was determined with the Mann–Whitney rank-sum (unpaired) or Wilcoxon signed-rank (paired) tests as appropriate; P values were computed with the exact (permutation) method. Multiple post hoc comparisons were corrected with the Holms–Sidak step-down test.26 Values of P < 0.05 were accepted as significant. For P > 0.001, exact P values are shown.
Results
We first examined gremlin 1 expression in mouse lungs and isolated human pulmonary microvascular endothelial cells in vitro and found that it is expressed in monomeric form in both the tissue homogenate and in endothelial cells (Supplemental Fig. 1). We next examined the actions of gremlin 1 in the lung in vivo using wild-type mice (Grem1+/+) and gremlin 1 haploinsufficient (Grem1+/–) mice.9 Gremlin 1 protein expression was not detectably different in normoxic wild-type and normoxic Grem1+/– lungs (Fig. 1a). Phosphorylation of SMAD 1/5/9 and expression of Kv1.5 were also unchanged in normoxic Grem1+/– lungs compared to normoxic wild-type lungs (Supplemental Fig. 2). In hypoxia, gremlin 1 expression was significantly reduced in the lungs of Grem1+/– mice in comparison to hypoxic wild-type controls (Fig. 1a). The BMP-dependent phosphorylation of SMADs 1/5/9 and the expression of the BMP-regulated potassium channel Kv1.5 in vascular smooth muscle in wild-type lungs was significantly reduced after 48 h of exposure to hypoxia. In contrast, both phosphorylation of SMAD 1/5/9 and Kv1.5 expression were preserved in the lungs of hypoxic Grem1+/– mice (Supplemental Fig. 2), in keeping with the augmentation of BMP activity resulting from the reduced gremlin 1 expression in these lungs (Supplemental Fig. 2). Normal expression of Kv1.5 plays an important role in the maintenance of normal pulmonary vascular resistance.27–29 These findings demonstrated that gremlin 1 was effectively reduced in the hypoxic haploinsufficient mouse and are in keeping with the canonical role of gremlin 1 in modulating BMP signaling.9,30,31
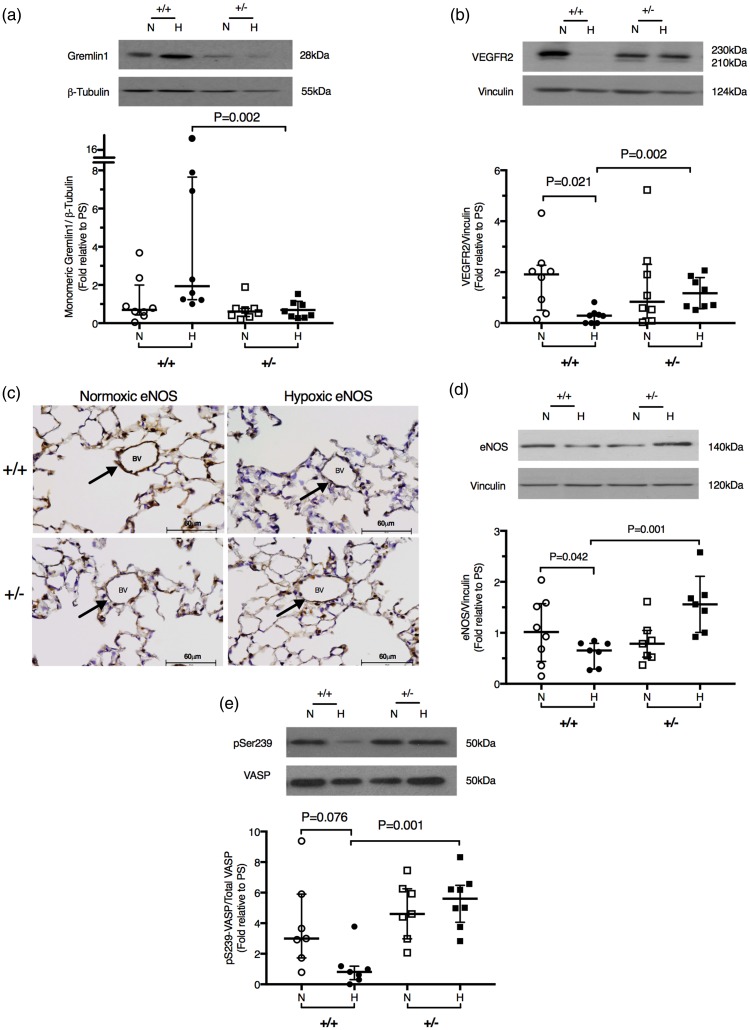
Fig. 1.
Gremlin 1 haploinsufficiency reduces gremlin 1 expression and restores VEGFR2 expression and eNOS expression and activity in the hypoxic lung in vivo. (a) Representative western blot and densitometric analysis of gremlin 1 expression in normoxic and hypoxic wild-type (+/+) and Grem1+/– lung lysate. (b) Representative western blot and densitometric analysis of VEGFR2 expression in normoxic and hypoxic wild-type (+/+) and Grem1+/– lung lysate. (c) Immunohistochemical localization of eNOS in the lungs of normoxic and hypoxic wild-type and Grem1+/– mice. (d) Representative western blot and densitometric analysis of eNOS expression in normoxic and hypoxic wild-type and Grem1+/– lung lysate. (e) Representative western blot and densitometric analysis of VASP phosphorylation in normoxic or hypoxic wild-type and Grem1+/– lung lysate. Densitometry values are normalized to a pooled standard. See Supplemental material for details. Bars represent median ± IQR.
In wild-type lungs, VEGFR2 expression was reduced by hypoxic exposure (Fig. 1b), whereas in the Grem1+/– mice, VEGFR2 expression was unaltered by hypoxia (Fig. 1b). Type III nitric oxide synthase or eNOS is a VEGF responsive protein expressed in endothelial cells.32,33 We found that expression of this protein in the pulmonary vascular endothelium was reduced in the hypoxic wild-type lung but was preserved in the hypoxic Grem1+/– lung (Fig. 1c and 1d). This preservation of eNOS protein expression in the hypoxic Grem1+/– mice was accompanied by increased eNOS activity compared to hypoxic wild-type mice, as shown by increased phosphorylation of vasodilator-stimulated phosphoprotein (VASP) at serine 239 (Fig. 1e). This phosphorylation site is a target of protein kinase G (PKG) and therefore an index of the activity of the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP)/PKG signaling pathway.34,35 These findings are compatible with an autocrine function of gremlin 1 to reduce VEGFR2-mediated signaling and thus inhibit eNOS expression and activity in the hypoxic lung in vivo, although gremlin 1 might also inhibit eNOS expression and activity by altering BMP signaling.
We have previously found that gremlin 1 reaches its highest expression during the first week of hypoxic exposure,9 at the time when vascular remodeling has been reported to be most active25 and subsequently returns to baseline values as stable HPH becomes established.9 At that later stage of hypoxic exposure, when there was no longer any difference in BMP-mediated signaling between the two genotypes, eNOS protein expression and phospho-VASP in the lungs were similar (Supplemental Fig. 3), suggesting that gremlin 1 was directly involved in mediating the changes in eNOS.
Given the central role of VEGF acting via VEGFR2 in regulating eNOS expression and activity,32,33,36 these findings in vivo suggested that in the hypoxic lung increased gremlin1 acts to block VEGFR2 signaling. To directly examine the effect of gremlin 1 on VEGF-mediated signaling in endothelial cells, we next assessed the effect of gremlin 1 on VEGFA-induced VEGFR2 phosphorylation in cultured human pulmonary microvascular endothelial cells. Gremlin 1 attenuated VEGF-induced phosphorylation of VEGFR2 at tyrosine 1059 (Fig. 2a), an essential step in VEGFR2 kinase activation and downstream signaling.37–39 There was no evidence of VEGFR2 phosphorylation induced by gremlin 1 in the absence of VEGFA (Fig. 2a).
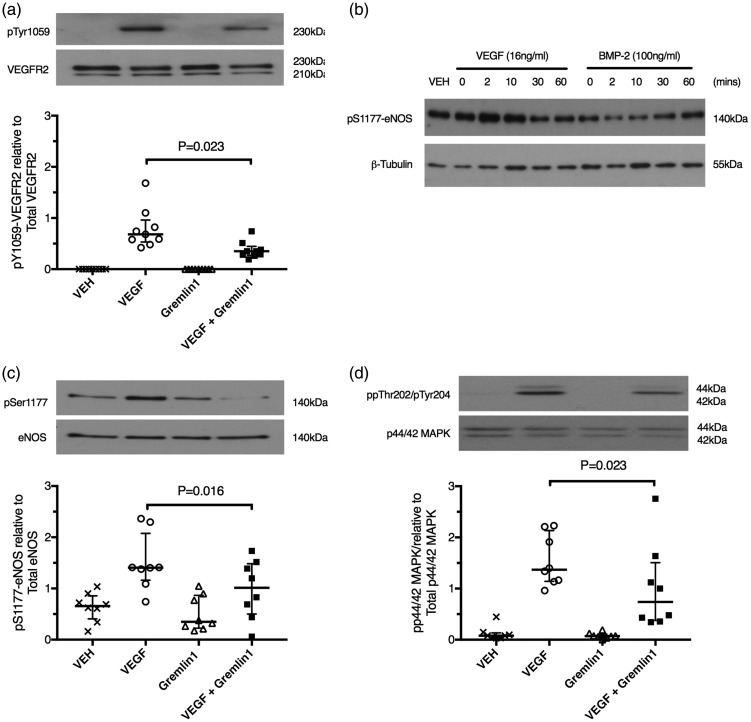
Fig. 2.
Monomeric gremlin 1 is not a VEGF agonist but blocks VEGFA-induced VEGFR2 phosphorylation and downstream signaling in vitro. (a) Representative western blot and densitometric analysis of VEGFR2 phosphorylation in human microvascular endothelial cells from the lung (HMVEC-L) exposed to VEGFA (16 ng/mL) for 2 min, gremlin 1 (2 µg/mL) for 60 min, or VEGFA (16 ng/mL) for 2 min after pre-incubation with gremlin 1 (2 µg/mL) for 60 min. (b) Representative western blot and densitometric analysis of eNOS serine1177 phosphorylation in HMVEC-L exposed to VEGFA (16 ng/mL) or BMP2 (100 ng/mL) stimulation for increasing periods from 2 to 60 min. VEGFA response was maximal after 2 min while the maximum BMP2 response was not observed until 60 min of stimulation. The image is representative of three independent experiments. (c) Representative western blot and densitometric analysis of eNOS phosphorylation in HMVEC-L exposed to VEGFA (16 ng/mL) for 2 min, gremlin 1 (2 µg/mL) for 60 min, or VEGFA (16 ng/mL) for 2 min after gremlin 1 (2 µg/mL) preincubation for 60 min. (d) Representative western blot and densitometric analysis of p44/42 MAPK (Erk 1/2) phosphorylation in HMVEC-L exposed to VEGFA (16 ng/mL) for 2 min, gremlin 1 (2 µg/mL) for 60 min, or VEGFA (16 ng/mL) for 2 min after gremlin 1 (2 µg/mL) preincubation for 60 min. Bars represent median ± IQR.
While one of the canonical actions of VEGF acting via VEGFR2 is to increase eNOS expression and activity,32,33,36 more recently it has been reported that activation of BMPR2 by BMPs also regulated eNOS activity.6,40–42 Furthermore, pulmonary vascular endothelial cells produce BMP2 (Supplemental Fig. 4a), raising the possibility that the effect of gremlin 1 that we had observed was mediated by inhibition of an autocrine BMP signaling pathway.9,43,44 However, regulation of eNOS by these two different pathways can be distinguished as the response produced by VEGFA is short latency (peak response at 2 min) and larger than the much longer latency (60 min) phosphorylation caused by BMP2 (Fig. 2b).40 The mean ( ± SD) maximal fold change in eNOS phosphorylation of 1.7 ± 0.8, expressed relative to vehicle-treated cells, was induced by 2 min of VEGF stimulation. By comparison, after 2 min of BMP2 stimulation, the mean ( ± SD) BMP2-induced fold change in eNOS phosphorylation relative to vehicle was reduced at 0.54 ± 0.39. Therefore, at this timepoint (2 min), VEGF induced an easily detectable increase in phosphorylation of eNOS while BMP2 did not alter eNOS phosphorylation. We found that gremlin 1 inhibited the short latency eNOS phosphorylation demonstrating that gremlin 1 acted to block VEGFA-induced phosphorylation (Fig. 2c). Furthermore, noggin, a potent BMP antagonist that binds and inhibits BMP2, BMP4, and BMP7, the same BMPs as gremlin 1 binds,45 did not block VEGFA-induced eNOS phosphorylation, demonstrating that the action of gremlin 1 was not mediated by blockade of autocrine BMP2 signaling (see Supplemental Fig. 5a). Gremlin 1 also inhibited VEGFA-induced p44/42 mitogen activated protein kinase (MAPK) phosphorylation (Fig. 2d), a second well-described downstream signaling response to VEGFR2 activation.37,46,47 Gremlin 1 alone did not have any detectable effect on these two phosphorylation reactions (Fig. 2c and 2d). Taken together with the previous demonstration that gremlin 1 binds with high affinity to VEGFR2,17,18 these data show that gremlin 1 directly blocked VEGFA signaling at VEGFR2.
We next examined the effect of gremlin 1 on VEGFA-induced gene expression and found that VEGFA increased the expression of Bcl-2 (Fig. 3a), as previously reported,48 and that this action was blocked by gremlin 1 (Fig. 3b). To exclude the possibility that this effect was in part the result of blocking the autocrine actions of BMPs produced by the endothelial cells (see Supplemental Fig. 4a), we undertook a further separate series of experiments in which we first confirmed that VEGF stimulation caused an increase in Bcl-2 expression and that gremlin 1 blocked this increase. Gremlin 1 alone did not alter Bcl-2 expression (Fig. 3c). Stimulation of the endothelial cells with BMP2 alone or BMP4 alone did not cause any increase in Bcl-2 expression compared to vehicle (Fig. 3c). Thus, the inhibition of VEGF-induced upregulation of Bcl-2 by gremlin 1 was independent of its antagonism of the BMP signaling pathway.
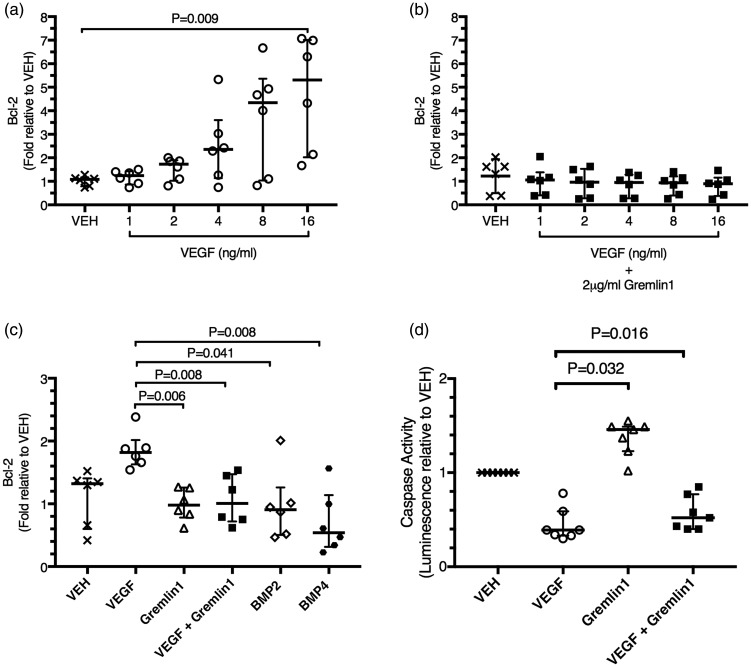
Fig. 3.
Gremlin 1 blocks the expression of the VEGFA responsive gene Bcl-2 in a BMP independent manner. (a) Relative Bcl-2 gene expression in human microvascular endothelial cells from the lung (HMVEC-L) after stimulation with increasing concentrations of VEGFA (1–16 ng/mL) for 24 h. (b) Relative Bcl-2 gene expression in HMVEC-L stimulated with increasing concentrations of VEGFA (1–16 ng/mL) and gremlin 1 (2 µg/mL) together for 24 h. (c) Relative Bcl-2 gene expression in HMVEC-L stimulated for 24 h with VEGFA (16 ng/mL) alone, gremlin 1 (2 µg/mL) alone, VEGFA (16 ng/mL) and gremlin 1 (2 µg/mL) together, BMP2 (100 ng/mL) alone, or BMP4 (80 ng/mL) alone. (d) Relative caspase activity in human microvascular endothelial cells from the lung (HMVEC-L) after stimulation with VEGF (16 ng/mL), gremlin 1 (2 µg/mL) alone, or VEGF and gremlin 1 together for 24 h. Caspase 3/7 activity is expressed as fold change in luminescence relative to vehicle-treated cells. Bars represent median ± IQR.
In keeping with these actions of VEGFA and gremlin 1 on Bcl-2 expression, VEGFA inhibited apoptosis in these endothelial cells and this action was significantly antagonized by gremlin 1 (Fig. 3d), which inhibited the anti-apoptotic effect of VEGF in all seven independent experiments. Interestingly, gremlin 1 alone increased endothelial apoptosis (Fig. 3d), an action that may have been due to its blocking of an autocrine effect of VEGFA produced by the endothelial cells (see Supplemental Fig 4b).
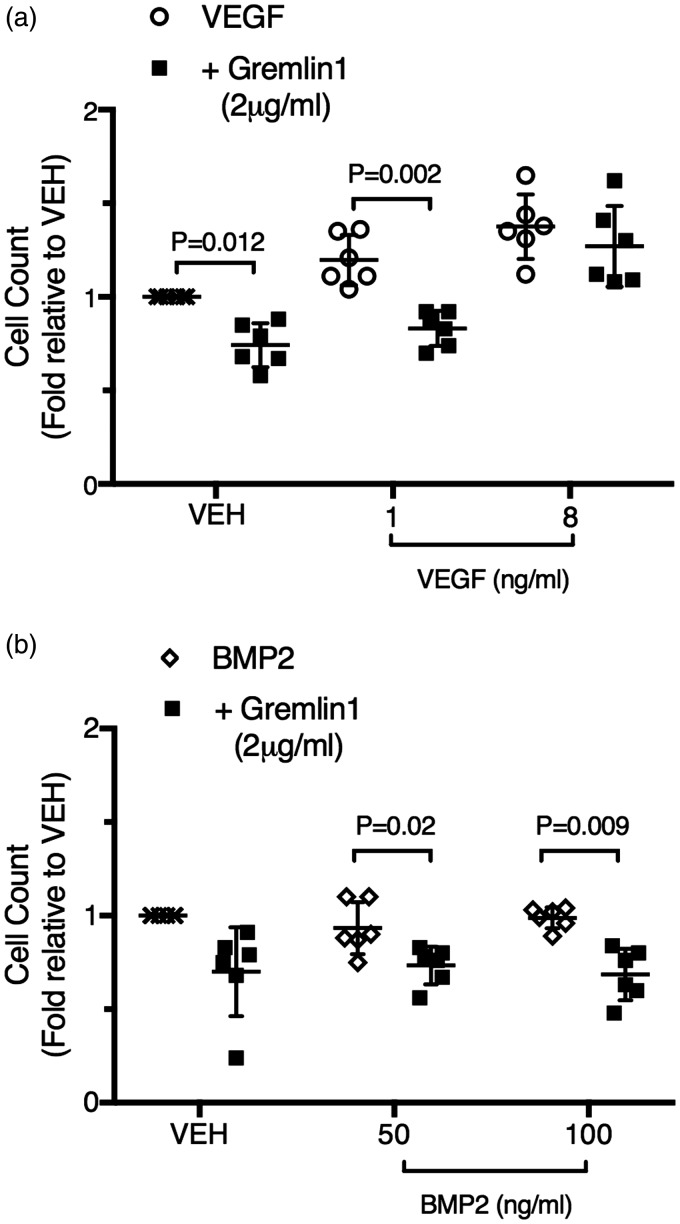
As expected, VEGFA induced an increase in endothelial cell number in six out of six independent experiments (Fig. 4a) when both a lower concentration (1 ng/mL) was used (P = 0.017) and when a higher concentration (16 ng/mL) was used (P = 0.003). This VEGFA-induced increase was blocked by gremlin 1 in the presence of the lower concentrations of VEGFA but not at the higher concentration (Fig. 4a). Interestingly, gremlin 1 also reduced endothelial cell numbers in the absence of added VEGFA. BMP2 alone had no effect on endothelial cell number (Fig. 4b) at either the lower concentration (50 ng/mL) used (P = 0.274) or the higher (100 ng/mL) concentration (P = 0.651). In the vehicle-treated cells, gremlin 1 did not cause a statistically significant reduction in endothelial cell number, although it reduced the number in each of the six independent experiments (Fig. 4B). In one experiment, the addition of gremlin 1 caused a much greater reduction than in any other, suggesting that this represented an outlier. When this result was excluded, the reduction in endothelial cell number caused by gremlin 1 alone was statistically significant (P = 0.018), in agreement with the results shown in Fig. 4a. Gremlin 1 reduced endothelial cell number in the presence of both concentrations of added BMP2 (Fig. 4b). Pulmonary microvascular endothelial cells produced both BMP2 and VEGFA (Supplemental Fig. 4). As BMP2 does not alter endothelial cell number and given the essential requirement for autocrine VEGF activity for endothelial cell survival,49,50 these findings suggest that gremlin 1 blocked the basal VEGFR2 activation caused by endogenous VEGFA production in these microvascular cells (Supplemental Fig. 4b).
Fig. 4.
Gremlin 1 attenuates VEGFA induced increases in human lung microvascular endothelial cell number. (a) Numbers of human microvascular endothelial cells from the lung (HMVEC-L) after exposure for 24 h to vehicle alone, vehicle and gremlin 1 (2 µg/mL), VEGFA (1 ng/mL) alone, VEGFA (1 ng/mL) together with gremlin 1 (2 µg/mL), VEGFA (8 ng/mL) alone, or VEGFA (8 ng/mL) together with gremlin 1 (2 µg/mL). Cell counts are expressed relative to control number (vehicle) in each experiment. (b) Numbers of HMVEC-L after exposure to vehicle alone, vehicle and gremlin 1 (2 µg/mL), BMP2 (50 ng/mL) alone, BMP2 (50 ng/mL) together with gremlin 1 (2 µg/mL), BMP2 (100 ng/mL) alone, or BMP2 (100 ng/mL) together with gremlin 1 (2 µg/mL) for 24 h. Cell counts are expressed relative to control number (vehicle) in each experiment. Bars represent mean ± SD.
Discussion
We report here that reduction of gremlin 1 in haploinsufficient mice (Grem1+/–) prevented the normal hypoxia-induced reduction of VEGFR2 expression and increased endothelial expression and activity of nitric oxide synthase within the hypoxic lung in vivo. In human pulmonary microvascular endothelial cells in vitro, gremlin 1 inhibited VEGFA-induced VEGFR2 phosphorylation, activation, and downstream signaling, Bcl-2 expression and increases in endothelial cell number and the anti-apoptotic effects of VEGFA. Both isolated pulmonary microvascular endothelial cells and whole lung expressed the monomeric form of gremlin 1. Taken together, these data suggest that increased gremlin 1 in the lung acts to cause PH, not alone by inhibiting BMP signaling, but also by blocking VEGFA actions at VEGFR2 and reducing VEGFR2 expression.
Gremlin 1 was first identified as a secreted glycoprotein that binds non-covalently to BMP2, BMP4, and BMP7, preventing the interaction of these ligands with their dimeric receptor (BMPR1-BMPR2) and reducing BMP signaling. Gremlin 1 is increased in the lung during the development of PH and plays an important pathogenetic role by antagonizing BMP signaling.8–11,13,51 Our findings in Grem1+/– mice confirmed the important BMP-blocking action caused by increased gremlin 1 during the development of HPH (Supplemental Fig. 2).
More recently, a second important action of gremlin 1 has been identified, that is binding to the VEGFR2 and altering signaling through that pathway.14,17,18,20,52 It has previously been reported that in its dimeric form gremlin 1 acts as a VEGFR2 agonist whereas in its monomeric form it acts as an antagonist, blocking VEGF mediated activation of the receptor in human umbilical vein endothelial cells by binding to VEGFR2 and preventing the interaction of VEGFA with the VEGFR2;14,18,53 importantly, gremlin 1 does not bind to VEGFR1 or to VEGFR3.18 In the context of PH, these actions at the VEGFR2 are of particular interest since blockade of the VEGF-VEGFR2 pathway with the VEGF receptor blocker SU5416, in combination with chronic hypoxia leads to the development of more severe hypertension than hypoxia alone, including the development of angio-obliterative PAH and right heart failure in animal models.21–23
Given this background, we sought evidence of augmented VEGF actions in hypoxic mice haploinsufficient for gremlin 1 during the development of PH. We confirmed that hypoxia reduced the expression of VEGFR2 in wild-type mice (Fig. 1b) as has previously been reported.54,55 We found that reduction of gremlin 1 in the Grem1+/– mice completely abolished the hypoxia-induced reduction in VEGFR2 expression observed in wild-type mice (Fig. 1b). Moreover, reduction of gremlin 1 in the Grem1+/– mice completely protected against the hypoxia-induced reduction of eNOS expression and signaling seen in wild-type mice (Fig. 1). As eNOS expression and activity in endothelial cells are increased by VEGF signaling,32,33 restoration of VEGFR2 expression may have contributed substantially to the augmented eNOS activity and expression observed in the Grem1+/– mice. It is important to remember that gremlin 1 can also regulate eNOS activity by its blocking actions on the BMP pathway, since BMPR2 activation can increase eNOS activity both by a direct action on eNOS, and by inducing increases in apelin, which subsequently increase eNOS expression and activity.6,40–42 Thus, the blocking action of increased gremlin 1 on BMP signaling could also have contributed to the reduced eNOS activity that we observed in the hypoxic wild-type lungs and to the restoration of eNOS expression and activity observed in the Grem1+/– mice in hypoxia (Fig. 1). Since the pulmonary vascular endothelium is a major source of gremlin 1 in the pulmonary vasculature in vivo, and since gremlin 1, when secreted, is largely bound to the extracellular glycocalyx on the cell surfaces close to the site of secretion,9,19,31,56 these findings are compatible with an autocrine action of gremlin 1 in the endothelial cells that mediates the early hypoxia-induced reduction in eNOS expression and activity. This reduction of eNOS activity is functionally important since hypoxia-induced impairment of endothelial nitric oxide synthase activity contributes to both vascular remodeling and to hypoxia-induced vasoconstriction in PH.23,57–65
In addition to reducing VEGFR2 expression, increased gremlin 1 in the hypoxic lung could also have altered VEGFR2 activity by binding directly to the receptor and altering downstream signaling.18,53 Given that gremlin 1 can act either as a VEGFR2 agonist or antagonist, increased expression of gremlin 1 in the hypoxic wild-type lung (Fig. 1c–e) might have reduced eNOS expression and activity if gremlin 1 acted predominantly to block VEGFR2 signaling or it might have increased eNOS expression and activity if it acted predominantly as an agonist at VEGFR2.32,36 The finding that reduction of gremlin 1 in Grem1+/– mice (Fig. 1a) completely abrogated the hypoxia-induced attenuation of VEGFR2 expression (Fig. 1b) and eNOS expression and activity (Fig. 1d and 1e), taken together with the presence of the monomeric, VEGFR2-blocking form within the pulmonary endothelium (Supplemental Fig. 1), suggests that the increase in gremlin 1 induced by hypoxia in wild-type mice blocked the VEGF-mediated activation of eNOS. In contrast, if gremlin 1 normally acted in the lung as a physiologically important VEGFR2 agonist, this complete restoration of eNOS expression and activity would not have occurred, since loss of the agonist activity of gremlin 1 in the haploinsufficient mice would have removed that component of eNOS activity that was due to VEGFR2 activation. Furthermore, it has been reported that reduction of BMPR2 signaling by inactivating BMPR2 mutations in mice did not reduce total eNOS expression.11 That implies that the increased eNOS expression that we observed in the hypoxic Grem1+/– lung compared to the hypoxic wild-type lung (Fig. 1d) could not be the result of the restoration of BMPR2 signaling (Supplementary Fig. 2).
Taken together, the results in the gremlin 1 haploinsufficient mouse were compatible with a reduction in VEGFR2 signaling caused by gremlin 1 in the hypoxic lung during the development of PH. To examine a possible direct blocking action of gremlin 1 at VEGFR2, we assessed the action of gremlin 1 in human pulmonary microvascular cells in vitro and found that it antagonized VEGFR2 activation and downstream signaling (Fig. 2), and antagonized VEGFA-induced Bcl-2 expression (Fig. 3), a gene known to be VEGFA-regulated.48 The inhibitory actions of gremlin 1 on endothelial cell number and the anti-apoptotic effects of VEGFA also demonstrate blockade of VEGFA actions (Figs. 3d and 4a). Furthermore, we found no evidence that gremlin 1 alone acted as a VEGFR2 agonist, in contrast to an agonist action reported in endothelial cells isolated from other vascular beds.14,17,18,52 Rather, we found that recombinant gremlin 1 antagonized VEGFR2 in a manner similar to that recently reported for monomeric gremlin 1.14 In support of this, we confirmed by immunoblotting that the recombinant gremlin 1 that we used was monomeric (Supplemental Fig. 1). Thus, our findings are in keeping with the recently reported blocking action of monomeric gremlin 1 on VEGFR2 activity in human umbilical vein endothelial cells and extends that observation to adult pulmonary microvascular endothelial cells.14
Since it has been previously reported that these endothelial cells produce BMP2,9,43,44 which we confirmed in the present experiments (Supplemental Fig. 4a), the possibility that the observed actions of gremlin 1 might have been mediated through its canonical function of inhibition of the BMP secreted by the cells themselves had to be excluded. We ensured that in vitro experiments were conducted at a timepoint (2 min) when VEGF induced marked eNOS phosphorylation and BMP2 was without effect (Fig. 2b). Furthermore, we demonstrated that the effects we examined were not caused by stimulation with BMP2 or BMP4 (i.e. short latency eNOS phosphorylation and increased Bcl-2 expression).66–69 Finally, we found that in contrast to gremlin 1, complete blockade of BMP2, BMP4, and BMP7 signaling by noggin did not change VEGFA-mediated short latency eNOS phosphorylation (Figs. 2b and 3c and Supplemental Fig. 5). Taken together, these data suggest that the effects of gremlin 1 on VEGFA-induced activation of VEGFR2 that we examined were not due to blockade of BMP signaling. An additional approach to demonstrate that the actions of gremlin 1 on VEGFR2 were independent of BMP signaling, which we did not use, would be to examine the effect of gremlin 1 on VEGFR2-mediated signaling after complete blockade of BMPR2 signaling, e.g. by knockdown of BMPR2.
Noggin, despite being an effective BMP antagonist (Supplemental Fig. 5b), did not have an effect on VEGF signaling (Supplemental Fig. 5a). Noggin has been shown to be responsive to the hypoxia mimetic, cobalt chloride, but has previously been shown to be unresponsive to levels of hypoxia typically found in the murine lung in vivo during the development of PH, in contrast to the robust increases in gremlin 1 under these conditions.9,70 Uniquely, among BMP antagonists, surface plasmon resonance analysis has shown that gremlin 1 can interact with VEGFR2 and exists as a monomer and dimer in vitro and in vivo.14,53 Interestingly, it has previously been reported that the inhibition of BMP2 by gremlin 1 occurs by a mechanism that is distinct from other known inhibitors of BMP signaling such as noggin and chordin.15 These reports suggest that gremlin 1 has a structure that is different from other BMP antagonists, which may account for its ability to bind VEGFR2, a property that has not been reported in other BMP antagonists.
It is important to note that most secreted glycosylated gremlin 1 remains close to the secreting cell as it binds to the extracellular glycocalyx on the cell surface.19,31 Thus, high local concentrations of gremlin 1 are present on the cell surface and in the interstitial spaces within intact tissues. Moreover, the glycosylated form, which is the predominant form in the lung (Supplemental Fig. 1), retains its BMP and VEGFR2 blocking actions.18,31 These high local concentrations of monomeric glycosylated gremlin 1 in the intact lung could result in blocking of VEGFR2 in addition to blocking BMPR2 ligands in the endothelium of the intact pulmonary vessels in vivo.
It is now well recognized that the development of HPH and other forms of PH differs markedly in males and females.71,72 Our study was conducted in male mice and does not provide information on the effects of gremlin 1 on VEGF signaling during the development of HPH in female mice. HPH is less severe in female rats than in male rats and the actions of endogenous estrogens have a central role in ameliorating PH in females.72,73 Of particular importance for this study, 17β-estradiol reduces gremlin 1 expression, in the hypoxic lung.11 Thus, the effect of gremlin 1 on VEGF signaling in females may be very different and needs to be separately studied. It is important that our findings should not be directly extrapolated to the development of HPH in females.
In summary, we have shown that the monomeric form of gremlin 1 acts as an antagonist of both BMP signaling through BMPR2 and of VEGF-mediated VEGFR2 activation in the pulmonary microvascular endothelium (Fig. 5). We found that the monomeric form is produced by the isolated pulmonary endothelium and in the whole lung suggesting that, in vivo, it can act in an autocrine manner to block these pathways. Further support for an autocrine blocking of VEGFR2 activity by native gremlin 1 in the lung is provided by the data obtained in gremlin 1 haploinsufficient mice. Taken together with the previous evidence that VEGFR2 signaling attenuates the development of HPH, the data presented here suggest that increases in gremlin 1 contribute importantly to the development of PH, not alone by blocking BMP signaling, but also by acting to block VEGFR2 signaling.
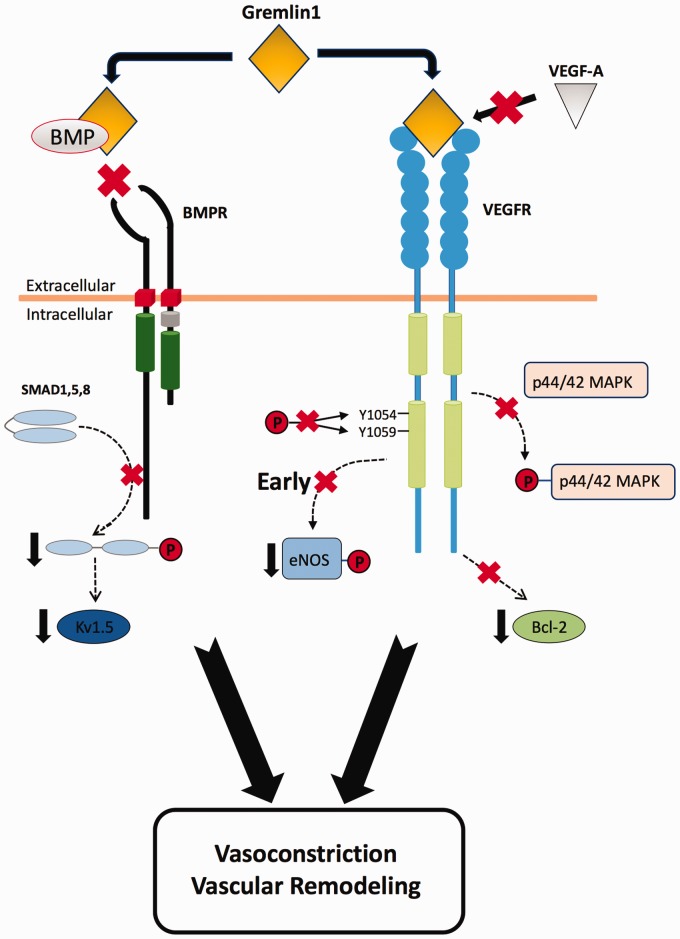
Fig. 5.
Proposed blocking action of gremlin 1 on both BMP and VEGF signaling during the development of hypoxic pulmonary hypertension (HPH). As confirmed in the present study, the canonical action of increased gremlin 1 during the development of HPH is to prevent BMP2 and BMP4 binding to BMPRs thus blocking BMP dependent signaling. Increased gremlin 1 simultaneously acts to block VEGF signaling by binding to VEGFR2.53 Reduced BMPR signaling74,75 and reduced VEGFR2 signaling23,65 both contribute importantly to the development of HPH. Thus, gremlin 1 mediated blockade of both pathways causes vasoconstriction and vascular remodeling. The signaling pathways illustrated in the schematic are only those used in the present study to demonstrate the separate blocking actions of gremlin 1 on BMPR signaling and on VEGFR2 signaling. Both agonists also exert multiple other actions that are not shown here.
Supplemental Material
Supplemental material, PUL807205 Supplemental Material1 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material2 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material3 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material4 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material5 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research was funded by Science Foundation Ireland.
ORCID iD
Simon C. Rowan https://orcid.org/0000-0002-4103-4421
Supplemental material
Supplemental material for this article is available online.
References
- 1.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–116. [DOI] [PubMed] [Google Scholar]
- 2.Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2: 20–22. [DOI] [PubMed] [Google Scholar]
- 3.Hong KH, Lee YJ, Lee E, et al. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 2008; 118: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado RD, Pauciulo MW, Thomson JR, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 2001; 68: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang K, Wang J, Lu W. Bone morphogenetic protein signalling in pulmonary hypertension: advances and therapeutic implications. Exp Physiol 2017; 102: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 6.Anderson L, Lowery JW, Frank DB, et al. Bmp2 and Bmp4 exert opposing effects in hypoxic pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol 2010; 298: R833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 2006; 13: 657–670. [DOI] [PubMed] [Google Scholar]
- 8.Barnes JW, Kucera ET, Tian L, et al. Bone morphogenic protein type 2 receptor mutation-independent mechanisms of disrupted bone morphogenetic protein signaling in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2016; 55: 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill E, Costello CM, Rowan SC, et al. Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 2012; 125: 920–930. [DOI] [PubMed] [Google Scholar]
- 10.Ciuclan L, Sheppard K, Dong L, et al. Treatment with anti-gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. Am J Pathol 2013; 183: 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frump AL, Albrecht ME, McClintick JN, et al. Estrogen receptor-dependent attenuation of hypoxia-induced changes in the lung genome of pulmonary hypertension rats. Pulm Circ 2017; 7: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu M, Shao NY, Sa S, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell 2017; 20: 490–504.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama H, Dewachter C, Belhaj A, et al. Endothelin-Bone morphogenetic protein type 2 receptor interaction induces pulmonary artery smooth muscle cell hyperplasia in pulmonary arterial hypertension. J Heart Lung Transplant 2015; 34: 468–478. [DOI] [PubMed] [Google Scholar]
- 14.Grillo E, Ravelli C, Corsini M, et al. Monomeric gremlin is a novel vascular endothelial growth factor receptor-2 antagonist. Oncotarget 2016; 7: 35353–35368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisonaite M, Wang X, Hyvonen M. Structure of Gremlin-1 and analysis of its interaction with BMP-2. Biochem J 2016; 473: 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsinkam AJ, Mulloy B, Rider CC. Mapping the heparin-binding site of the BMP antagonist gremlin by site-directed mutagenesis based on predictive modelling. Biochem J 2015; 470: 53–64. [DOI] [PubMed] [Google Scholar]
- 17.Lavoz C, Alique M, Rodrigues-Diez R, et al. Gremlin regulates renal inflammation via the vascular endothelial growth factor receptor 2 pathway. J Pathol 2015; 236: 407–420. [DOI] [PubMed] [Google Scholar]
- 18.Mitola S, Ravelli C, Moroni E, et al. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood 2010; 116: 3677–3680. [DOI] [PubMed] [Google Scholar]
- 19.Muller I, Schonberger T, Schneider M, et al. Gremlin-1 is an inhibitor of macrophage migration inhibitory factor and attenuates atherosclerotic plaque growth in ApoE-/- Mice. J Biol Chem 2013; 288: 31635–31645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stabile H, Mitola S, Moroni E, et al. Bone morphogenic protein antagonist Drm/gremlin is a novel proangiogenic factor. Blood 2007; 109: 1834–1840. [DOI] [PubMed] [Google Scholar]
- 21.Voelkel NF, Gomez-Arroyo J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol 2014; 51: 474–484. [DOI] [PubMed] [Google Scholar]
- 22.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006; 290: L209–221. [DOI] [PubMed] [Google Scholar]
- 23.Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001; 15: 427–438. [DOI] [PubMed] [Google Scholar]
- 24.Partovian C, Adnot S, Raffestin B, et al. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 2000; 23: 762–771. [DOI] [PubMed] [Google Scholar]
- 25.Meyrick B, Reid L. Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am J Pathol 1979; 96: 51–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol 1998; 25: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 27.Pozeg ZI, Michelakis ED, McMurtry MS, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 2003; 107: 2037–2044. [DOI] [PubMed] [Google Scholar]
- 28.Remillard CV, Tigno DD, Platoshyn O, et al. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 2007; 292: C1837–1853. [DOI] [PubMed] [Google Scholar]
- 29.Whitman EM, Pisarcik S, Luke T, et al. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K + channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 2008; 294: L309–318. [DOI] [PubMed] [Google Scholar]
- 30.Church RH, Krishnakumar A, Urbanek A, et al. Gremlin1 preferentially binds to bone morphogenetic protein-2 (BMP-2) and BMP-4 over BMP-7. Biochem J 2015; 466: 55–68. [DOI] [PubMed] [Google Scholar]
- 31.Topol LZ, Bardot B, Zhang Q, et al. Biosynthesis, post-translation modification, and functional characterization of Drm/Gremlin. J Biol Chem 2000; 275: 8785–8793. [DOI] [PubMed] [Google Scholar]
- 32.Papapetropoulos A, Garcia-Cardena G, Madri JA, et al. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997; 100: 3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziche M, Morbidelli L, Choudhuri R, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997; 99: 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibarra-Alvarado C, Galle J, Melichar VO, et al. Phosphorylation of blood vessel vasodilator-stimulated phosphoprotein at serine 239 as a functional biochemical marker of endothelial nitric oxide/cyclic GMP signaling. Mol Pharmacol 2002; 61: 312–319. [DOI] [PubMed] [Google Scholar]
- 35.Oelze M, Mollnau H, Hoffmann N, et al. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res 2000; 87: 999–1005. [DOI] [PubMed] [Google Scholar]
- 36.Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. J Biol Chem 1999; 274: 33057–33063. [DOI] [PubMed] [Google Scholar]
- 37.Clegg LW, Mac Gabhann F. Site-Specific Phosphorylation of VEGFR2 Is Mediated by Receptor Trafficking: Insights from a Computational Model. PLoS Comput Biol 2015; 11: e1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dougher M, Terman BI. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene 1999; 18: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 39.Koch S, Tugues S, Li X, et al. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011; 437: 169–183. [DOI] [PubMed] [Google Scholar]
- 40.Gangopahyay A, Oran M, Bauer EM, et al. Bone morphogenetic protein receptor II is a novel mediator of endothelial nitric-oxide synthase activation. J Biol Chem 2011; 286: 33134–33140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alastalo TP, Li M, Perez Vde J, et al. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest 2011; 121: 3735–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azizi Y, Faghihi M, Imani A, et al. Post-infarct treatment with [Pyr(1)]apelin-13 improves myocardial function by increasing neovascularization and overexpression of angiogenic growth factors in rats. Eur J Pharmacol 2015; 761: 101–108. [DOI] [PubMed] [Google Scholar]
- 43.Cola C, Almeida M, Li D, et al. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun 2004; 320: 424–427. [DOI] [PubMed] [Google Scholar]
- 44.Kaigler D, Krebsbach PH, West ER, et al. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J 2005; 19: 665–667. [DOI] [PubMed] [Google Scholar]
- 45.Song K, Krause C, Shi S, et al. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J Biol Chem 2010; 285: 12169–12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999; 399: 601–605. [DOI] [PubMed] [Google Scholar]
- 47.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 2016; 17: 611–625. [DOI] [PubMed] [Google Scholar]
- 48.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998; 273: 13313–13316. [DOI] [PubMed] [Google Scholar]
- 49.Domigan CK, Warren CM, Antanesian V, et al. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J Cell Sci 2015; 128: 2236–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007; 130: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costello CM, Howell K, Cahill E, et al. Lung-selective gene responses to alveolar hypoxia: potential role for the bone morphogenetic antagonist gremlin in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2008; 295: L272–284. [DOI] [PubMed] [Google Scholar]
- 52.Chiodelli P, Mitola S, Ravelli C, et al. Heparan sulfate proteoglycans mediate the angiogenic activity of the vascular endothelial growth factor receptor-2 agonist gremlin. Arterioscler Thromb Vasc Biol 2011; 31: e116–127. [DOI] [PubMed] [Google Scholar]
- 53.Maiolo D, Mitola S, Leali D, et al. Role of nanomechanics in canonical and noncanonical pro-angiogenic ligand/VEGF receptor-2 activation. J Am Chem Soc 2012; 134: 14573–14579. [DOI] [PubMed] [Google Scholar]
- 54.Nadeau S, Baribeau J, Janvier A, et al. Changes in expression of vascular endothelial growth factor and its receptors in neonatal hypoxia-induced pulmonary hypertension. Pediatr Res 2005; 58: 199–205. [DOI] [PubMed] [Google Scholar]
- 55.Yamaji-Kegan K, Su Q, Angelini DJ, et al. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2. Am J Physiol Lung Cell Mol Physiol 2006; 291: L1159–1168. [DOI] [PubMed] [Google Scholar]
- 56.Maciel TT, Melo RS, Schor N, et al. Gremlin promotes vascular smooth muscle cell proliferation and migration. J Mol Cell Cardiol 2008; 44: 370–379. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh S, Gupta M, Xu W, et al. Phosphorylation inactivation of endothelial nitric oxide synthesis in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2016; 310: L1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason NA, Springall DR, Burke M, et al. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol 1998; 185: 313–318. [DOI] [PubMed] [Google Scholar]
- 59.Hopkins N, McLoughlin P. The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis?. J Anat 2002; 201: 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Champion HC, Bivalacqua TJ, Greenberg SS, et al. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc Natl Acad Sci U S A 2002; 99: 13248–13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fagan KA, Fouty BW, Tyler RC, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 1999; 103: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fagan KA, McMurtry I, Rodman DM. Nitric oxide synthase in pulmonary hypertension: lessons from knockout mice. Physiol Res 2000; 49: 539–548. [PubMed] [Google Scholar]
- 63.Steudel W, Ichinose F, Huang PL, et al. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res 1997; 81: 34–41. [DOI] [PubMed] [Google Scholar]
- 64.Steudel W, Scherrer-Crosbie M, Bloch KD, et al. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 1998; 101: 2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vitali SH, Hansmann G, Rose C, et al. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulm Circ 2014; 4: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buckley S, Shi W, Driscoll B, et al. BMP4 signaling induces senescence and modulates the oncogenic phenotype of A549 lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol 2004; 286: L81–86. [DOI] [PubMed] [Google Scholar]
- 67.Hay E, Lemonnier J, Fromigue O, et al. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem 2001; 276: 29028–29036. [DOI] [PubMed] [Google Scholar]
- 68.Lagna G, Nguyen PH, Ni W, et al. BMP-dependent activation of caspase-9 and caspase-8 mediates apoptosis in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2006; 291: L1059–1067. [DOI] [PubMed] [Google Scholar]
- 69.Zhang S, Fantozzi I, Tigno DD, et al. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2003; 285: L740–754. [DOI] [PubMed] [Google Scholar]
- 70.Maruyama H, Dewachter C, Sakai S, et al. Bosentan reverses the hypoxia-induced downregulation of the bone morphogenetic protein signaling in pulmonary artery smooth muscle cells. Life Sci 2016; 159: 111–115. [DOI] [PubMed] [Google Scholar]
- 71.Batton KA, Austin CO, Bruno KA, et al. Sex differences in pulmonary arterial hypertension: role of infection and autoimmunity in the pathogenesis of disease. Biol Sex Differ 2018; 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mair KM, Johansen AK, Wright AF, et al. Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br J Pharmacol 2014; 171: 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lahm T, Albrecht M, Fisher AJ, et al. 17beta-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 2012; 185: 965–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spiekerkoetter E, Tian X, Cai J, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 2013; 123: 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi K, Kogaki S, Matsushita T, et al. Hypoxia induces alteration of bone morphogenetic protein receptor signaling in pulmonary artery endothelial cell. Pediatr Res 2007; 61: 392–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL807205 Supplemental Material1 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material2 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material3 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material4 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation
Supplemental material, PUL807205 Supplemental Material5 for Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium by Simon C. Rowan, Lucie Piouceau, Joanna Cornwell, Lili Li and Paul McLoughlin in Pulmonary Circulation