Abstract
Background
After a first ischaemic stroke, further vascular events due to thromboembolism are common and often fatal. Anticoagulants could potentially reduce the risk of such events, but any benefits could be offset by an increased risk of fatal or disabling haemorrhages.
Objectives
To assess the effect of prolonged anticoagulant therapy compared with placebo or open control following presumed non‐cardioembolic ischaemic stroke or transient ischaemic attack.
Search methods
We searched the Cochrane Stroke Group Trials Register in May 2008. In June 2008 we searched three online trial registers, used Web of Science Cited Reference Search to identify new citations of previously included studies, contacted a pharmaceutical company, and also contacted authors for additional information on included trials.
Selection criteria
Randomised and quasi‐randomised trials comparing at least one month of anticoagulant therapy with control in people with previous, presumed non‐cardioembolic, ischaemic stroke or transient ischaemic attack.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trial quality and extracted the data.
Main results
Eleven trials involving 2487 participants were included. The quality of the nine trials which predated routine computerised tomography (CT) scanning and the use of the International Normalised Ratio to monitor anticoagulation was poor. There was no evidence of an effect of anticoagulant therapy on either the odds of death or dependency (two trials, odds ratio (OR) 0.83, 95% confidence interval (CI) 0.52 to 1.34) or of 'non‐fatal stroke, myocardial infarction, or vascular death' (four trials, OR 0.96, 95% CI 0.68 to 1.37). Death from any cause (OR 0.95, 95% CI 0.73 to 1.24) and death from vascular causes (OR 0.86, 95% CI 0.66 to 1.13) were not significantly different between treatment and control. The inclusion of two recently completed trials did not alter these conclusions. There was no evidence of an effect of anticoagulant therapy on the risk of recurrent ischaemic stroke (OR 0.85, 95% CI 0.66 to 1.09). However, anticoagulants increased fatal intracranial haemorrhage (OR 2.54, 95% CI 1.19 to 5.45), and major extracranial haemorrhage (OR 3.43, 95% CI 1.94 to 6.08). This is equivalent to anticoagulant therapy causing about 11 additional fatal intracranial haemorrhages and 25 additional major extracranial haemorrhages per year for every 1000 patients given anticoagulant therapy.
Authors' conclusions
Compared with control, there was no evidence of benefit from long‐term anticoagulant therapy in people with presumed non‐cardioembolic ischaemic stroke or transient ischaemic attack, but there was a significant bleeding risk.
Keywords: Humans; Anticoagulants; Anticoagulants/adverse effects; Anticoagulants/therapeutic use; Cerebral Hemorrhage; Cerebral Hemorrhage/chemically induced; Cerebral Hemorrhage/mortality; Ischemic Attack, Transient; Ischemic Attack, Transient/drug therapy; Ischemic Attack, Transient/prevention & control; Randomized Controlled Trials as Topic; Secondary Prevention; Stroke; Stroke/drug therapy; Stroke/prevention & control
Plain language summary
Anticoagulants for preventing recurrence following presumed non‐cardioembolic ischaemic stroke or transient ischaemic attack
Most strokes are due to a sudden blockage of an artery in the brain (this type of stroke is called an ischaemic stroke). In most ischaemic strokes, the blockage is caused by a blood clot. In patients with an irregular heart rhythm (atrial fibrillation), anticoagulant drugs, such as warfarin, prevent such clots forming and prevent stroke. However, anticoagulant drugs may also cause bleeding in the brain and this harmful effect could outweigh any benefits in patients with a normal heart rhythm. This review identified 11 trials, involving 2487 participants who had had a stroke (and also had a normal heart rhythm), of anticoagulants to prevent further strokes. There was good evidence that anticoagulants could cause serious bleeding, and there was no evidence that, in such patients, anticoagulants were of benefit in the prevention of further strokes. Other trials have shown that, in a person with a normal heart rhythm who has had an ischaemic stroke, antiplatelet drugs such as aspirin are a safe and effective way to reduce the risk of further strokes and heart attacks.
Background
Community‐based studies have shown that approximately 60% to 90% of patients who have suffered a first‐ever ischaemic stroke survive the initial event (Sacco 1982; Bamford 1990; Ricci 1991; Dennis 1993; Weissbein 1994; Slowik 2007). However, among survivors, recurrent vascular events (especially myocardial infarction (MI) and recurrent stroke) are common and often fatal. One study showed that the cumulative risk of myocardial infarction or vascular death was 5.5% (95% confidence interval (CI) 3.7 to 7.3) in the first year after stroke, and 15.5% (95% CI 11.9 to 18.0) at five years after stroke (Dhamoon 2007). Three community‐based studies, in which only the minority of patients received antiplatelet or anticoagulant therapy, showed that the five‐year cumulative stroke recurrence rate after a first‐ever ischaemic stroke varied between 20% and 40% (Sacco 1982; Meissner 1988; Burn 1994; Hardie 2005), and that 15% to 20% of patients died within 30 days of their first recurrence (Burn 1994). The highest risk of recurrence was in the first year following the initial stroke but, even after four years of follow up, the risk of stroke in survivors was higher than that in the general population (Burn 1994).
Among patients from three different cohorts who had suffered a transient ischaemic attack (TIA), the actuarial risks ranged between 3% to 8% per year for death from any causes, 3% to 7% for stroke recurrence, 1% to 2% for myocardial infarction, and 6% to 9% for any stroke, myocardial infarction or vascular death (Hankey 1993). These risks do not represent the natural history of transient ischaemic attacks, however, because many patients were treated with antiplatelet agents. Once again, the risk of stroke was highest within the first year after transient ischaemic attack (Dennis 1990), rising from 11.5% at seven days to 18.5% at three months (Coull 2004). Recurrent ischaemic vascular disease therefore has an important impact on public health. Antithrombotic therapy may decrease the risk of recurrent ischaemia but it may also carry certain hazards, particularly an increase in the risk of haemorrhage.
Prolonged antiplatelet therapy has been shown to reduce the relative risk of non‐fatal recurrent stroke, myocardial infarction and vascular death in patients with a history of stroke or transient ischaemic attack by about 15% to 20% (APT 1994; Algra 1996; Algra 1999; ATT 2002). Among patients with non‐valvular atrial fibrillation (AF), anticoagulation has also been shown to reduce the relative risk of stroke by about 60% to 70% in both those with and without a previous history of stroke (AFI 1994; Benavente 2002; Saxena 2004b; Aguilar 2005). However, the balance of risk and benefit from long‐term anticoagulation following the acute stage of non‐cardioembolic stroke remains unclear. A previous meta‐analysis showed patients randomised to anticoagulation suffered more strokes and death than control patients (Jonas 1988). However, this review did not clearly define its inclusion criteria and did not use an extensive search strategy to identify all the trials.
A Cochrane review found that there was no evidence of net benefit from anticoagulation in the acute phase of ischaemic stroke (Gubitz 2004). Another Cochrane review of trials comparing oral anticoagulants with antiplatelet therapy for preventing further vascular events after transient ischaemic attack or minor stroke of presumed arterial origin has been completed (Algra 2006). The review concluded that, for the secondary prevention of further vascular events after transient ischaemic attack or minor stroke of presumed arterial origin, there was no clear advantage of oral anticoagulants over antiplatelet agents (Algra 2006). They also concluded that there was insufficient evidence to justify the routine use of low intensity oral anticoagulants (INR 2.0 to 3.6) and that more intense anticoagulation (INR 3.0 to 4.5) was unsafe and should not be used in this setting (Algra 2006).
To complement these reviews, we sought to perform a systematic review of all randomised controlled trials comparing prolonged anticoagulation with control in patients with a history of presumed non‐cardioembolic ischaemic stroke or transient ischaemic attack. Our aim was to determine whether there was any clear evidence of net benefit from anticoagulation in such patients.
Objectives
The objective of this review was to determine the effectiveness and safety of at least one month of oral anticoagulants in patients with a history of presumed non‐cardioembolic ischaemic stroke or transient ischaemic attack (that is, mainly among patients not in atrial fibrillation). In particular, we wished to test whether a policy of prolonged anticoagulation compared with no anticoagulation was associated with:
improved functional outcome at the end of follow up (that is, a reduced risk of being dead or dependent on others for activities of daily living);
a lower risk of recurrent stroke, myocardial infarction, vascular death and death from all causes during follow up;
a higher risk of haemorrhage (both intracranial and extracranial) during follow up.
We also wished to estimate the size of any such treatment effects.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all unconfounded randomised controlled trials in which prolonged anticoagulation was compared with control. We anticipated that many of the earlier trials may not have used strictly random allocation and, therefore, we also planned to include any trials that used a quasi‐randomised (or systematic) method of treatment allocation (such as alternation, by day of the week, by hospital number, by the patient's date of birth). However, these trials and randomised trials that used an open random number list do not adequately conceal the allocation from the person entering the patient in the trial and can lead to bias in treatment allocation which may invalidate the trial's results (Schulz 1995). We therefore planned to perform a sensitivity analysis including only those trials in which the method of allocation was both strictly random and adequately concealed (e.g. methods such as randomisation by telephone or on‐site computer; controlled by pharmacy; sequentially‐numbered bottles containing identical drug or placebo; and sequentially‐numbered, sealed, opaque envelopes).
Types of participants
Trials which included patients with a history of ischaemic stroke or transient ischaemic attack were eligible. Trials could include either patients in the acute phase of stroke (that is, within 14 days of stroke onset) or patients entered more than 14 days after onset. We excluded any trials which were designed to assess the efficacy of anticoagulation in the secondary prevention of stroke in patients with a cardiac embolic source such as atrial fibrillation; this is the topic of a separate review (Saxena 2004b), but we did include trials that did not specifically separate patients with a cardiac embolic source from those without such a source.
We included trials in which the pathological type of initial stroke had not been confirmed by CT (computerised tomography) scanning before entry into the trial, because approximately 80% of all first‐ever strokes are ischaemic (Warlow 1993). It is likely though, that some patients who initially had a haemorrhagic stroke were included in this review, and we therefore planned a sensitivity analysis excluding trials in which patients did not have CT confirmation of the initial ischaemic stroke.
Types of interventions
We included trials that compared any form of prolonged anticoagulation with no anticoagulation, regardless of the level of anticoagulation attempted. We defined 'prolonged' as a planned duration of anticoagulation of at least four weeks. We excluded trials that assessed the efficacy of a short period of anticoagulation (up to 14 days) in the treatment of patients during the acute phase of stroke; this is the topic of a separate review (Gubitz 2004). We excluded confounded trials (for example, those that compared an anticoagulant with an antiplatelet agent) and trials that compared one type of anticoagulant with another. However, we included trials where antiplatelet therapy was given to both treatment and control groups as these are unconfounded with respect to the anticoagulation comparison. In order to assess whether there was an interaction between anticoagulation and antiplatelet therapy we planned a sensitivity analysis comparing trials that did or did not use concomitant antiplatelet therapy. We also excluded trials in which the control group received a sub‐therapeutic dose of an anticoagulant as placebo as it is possible that even a sub‐therapeutic dose may have some anticoagulant effect. We defined anticoagulants as any agents which inhibit the coagulation cascade either directly or indirectly (that is, by inhibiting of synthesis of clotting factors). The following agents were eligible, classified by route of administration:
parenteral: unfractionated standard heparin, low‐molecular‐weight heparins (LMWH), heparinoids (e.g. danaparoid, mesoglycan, dermatan sulphate), and newer antithrombin agents (e.g. hirudin analogues);
oral: (e.g. warfarin and other coumarins, phenindione).
We expected most trials would include oral indirect agents due to the ease of administration for outpatient therapy.
Types of outcome measures
We aimed to extract from each trial the number of patients originally allocated to each treatment group to allow an intention‐to‐treat analysis. We chose two primary outcomes. The first was death or dependency since this is probably most important to the patient. The second was the composite outcome of any non‐fatal stroke, non‐fatal myocardial infarction or vascular death, since this assesses the effect of anticoagulation on the total number of serious vascular events during follow up (APT 1994).
Primary outcomes
1. Dead or dependent. Patients who were either dead or dependent on others for activities of daily living at the end of scheduled follow up. Patients dependent on others for any reason were included; that is, it was not restricted to those dependent because of a stroke.
2. Stroke, myocardial infarction or vascular death. The number of patients who developed any non‐fatal stroke, non‐fatal myocardial infarction or vascular death. Each patient was included only once in this composite outcome; that is, if a patient had a non‐fatal event and a fatal event, he or she was counted only once.
Secondary outcomes
1. Death from any cause during the treatment and scheduled follow‐up period.
2. Death from vascular causes during the treatment and scheduled follow‐up period. We defined vascular causes as:
stroke or any complication of stroke (e.g. pneumonia, pulmonary embolism, etc);
coronary artery disease (e.g. myocardial infarction, congestive cardiac failure, sudden death);
peripheral vascular disease;
haemorrhage (intracranial or extracranial);
other vascular cause.
3. Recurrent stroke (ischaemic or unknown pathologic type) during the treatment or scheduled follow‐up period; that is, a stroke in which haemorrhage had been excluded by imaging or autopsy or in which the pathology was unknown. Transient ischaemic attacks (lasting less than 24 hours) were not included. We also assessed the severity of the recurrent stroke by classifying them as fatal or non‐fatal. The definition of non‐fatal stroke was taken from each particular trial (rather than impose a set duration of survival following the stroke).
4. Definite intracranial haemorrhage confirmed by CT scanning, magnetic resonance imaging (MRI) or autopsy: (i.e. extradural extracerebral haemorrhage, subarachnoid haemorrhage, subdural haematoma, epidural haematoma or parenchymatous intracerebral haemorrhage). In trials performed before the CT era, non‐fatal intracranial haemorrhages diagnosed on the basis of a blood stained cerebrospinal fluid were reclassified as unknown strokes, whilst non‐fatal subarachnoid haemorrhages were not reclassified. We also planned to assess the clinical severity of the haemorrhage by separating fatal from non‐fatal haemorrhages.
5. Any recurrent stroke or symptomatic intracranial haemorrhage (that is, either 3 or 4 above).
6. Major extracranial haemorrhage. The definition of major was taken from the original trial or, if no definition was given, we defined it as a fatal bleed or one which required a transfusion, an operation, or a hospital admission.
7. Myocardial infarction. These were classified as fatal or non‐fatal.
8. Other embolic events (e.g. ischaemic bowel disease, peripheral arterial embolism). These were classified as fatal or non‐fatal.
9. Non‐fatal stroke/intracranial haemorrhage or vascular death during follow up.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Review Group Co‐ordinator in May 2008. In June 2008, we searched three online trials registers: the Internet Stroke Center’s Stroke Trial Registry (www.strokecenter.org), Clinicaltrials.gov (www.clinicaltrials.gov) and Current Controlled Trials (www.controlled‐trials.com). We searched for new citations of previously included trials using Web of Science’s Cited Reference Search facility (June 2008). We contacted Portola Pharmaceuticals Inc. for details of planned trials of PRT054021, a factor Xa inhibitor, and we wrote to authors for additional information on two included trials (SWAT 1998; LHSPS 1999) that had only previously been published in abstract form (June 2008).
For the first cycle of this review in 1997 we contacted the following manufacturers of anticoagulants in order to identify any unpublished trials: Alfa Wasserman (parnaparin and dermatan sulphate), Kabi (dalteparin), Knoll (reviparin), Leo (tinzaparin), Mediolanum (dermatan sulphate), Mitsubishi Chemical (agratroban/MD‐805), Novo (tinzaparin), Organon (danaparoid), Rhone‐Poulenc Rorer (enoxaparin), Sandoz (Sandoz LMWH), Sanofi Winthrop (nadroparin and CY 222) and DuPont pharmaceuticals (warfarin).
Data collection and analysis
Two authors (Ming Liu and Carl Counsell for the first version, Orell Mielke and PS for the previous update, PS and LG for this update) independently selected the trials to be included in the review and resolved disagreements by discussion. The same two review authors assessed the methodological quality of each trial by recording details of the randomisation method, blinding, whether an intention‐to‐treat analysis was possible from the published data and the number of randomised patients who were excluded from analysis for any reason. The same two review authors also extracted the outcome data, and other important details of the trial such as the inclusion criteria, the duration of follow up, the use of CT scanning, and the target level of anticoagulation and degree of compliance. The review authors double checked these data and resolved differences by discussion. We tried to extract the data on the number of patients with each outcome event, by allocated treatment group, irrespective of compliance, and whether or not the patient was subsequently deemed ineligible or otherwise excluded from treatment or follow up, in order to allow an intention‐to‐treat analysis. We assumed that patients who were randomised and then either excluded or lost to follow up had no event in the main analyses. We used only published data for the trials performed before 1980 because it was not possible to contact the authors to obtain any items of missing data. For two trials (SWAT 1998; LHSPS 1999), full publications are still awaited.
For all non‐zero trials (that is, trials where at least one outcome occurred), we calculated a weighted estimate of the odds ratio for each outcome using the Peto method. We calculated absolute risk reductions using the risk difference analysis and expressed them as events prevented per 1000 patients treated. We tested for heterogeneity between trials with the standard I2 test. When we compared two odds ratios (for example, in sensitivity analyses) we assessed whether the difference in the natural logarithms of the two odds ratios (lnOR) was significantly different from zero using a normal approximation. The variance of each lnOR was estimated as the reciprocal of the variance of the O‐E statistic given in the Cochrane 'Review Manager' software, RevMan 5.0 (RevMan 2008). There was variation in the duration of follow up in each trial and so, for each outcome, we calculated a mean duration of follow up weighted by study size by summing the total number of patient years of follow up for each trial contributing to that outcome and dividing the sum by the total number of patients in those trials.
The main analyses were based on all trials. However, we also planned a priori sensitivity analyses based on:
trials with good methodology (well‐concealed randomisation and blinded outcome assessment) versus those of poor methodology;
trials where all patients had their initial stroke confirmed as ischaemic by CT scan versus those without initial CT;
trials published before 1980 were compared with those which were published after that date;
trials using intensive anticoagulation (target INR > 3) versus those using a less intensive dose;
trials with follow up of more than one year versus those with shorter follow up;
trials which excluded patients with atrial fibrillation versus those that did not;
trials in which anticoagulation was given in the presence of antiplatelet therapy (that is, antiplatelet therapy was given to both treated and control groups) versus trials where no antiplatelet therapy was given.
We suspected that results may differ significantly between the groups in these analyses. We also performed post‐hoc sensitivity analyses based on:
only events that occurred whilst the allocated treatment was being taken, that is, excluding events in the anticoagulant group that occurred whilst the patient was not taking anticoagulant and excluding events in the control group that occurred whilst the patient was anticoagulated;
adjusting the event rates for differences in the duration of follow up between the treatment groups, that is, if the anticoagulant group was followed up for 12 months and the control group for 15 months, the event rate in the anticoagulant group was multiplied by a factor of 15/12;
comparing trials where the patients were anticoagulated within 14 days of the initial stroke with those in which treatment was started later.
Results
Description of studies
We have identified a total of 16 completed randomised controlled trials (RCTs) so far. We have included 11 trials, involving 2487 participants, in this review. We excluded five trials because they used an active control (Hill 1960; Hill 1962; Pearce 1965), or excluded large numbers of patients after randomisation (Eriksson 1983), or did not provide the numbers of patients and outcome events by treatment group, despite our efforts to contact the author (Vilenskii 1976). Two trials (SWAT 1998; LHSPS 1999) have been completed but, as yet, have only been published in abstract form. We have only been able to obtain limited details of these two trials from the authors despite repeated correspondence. Summary details of the 11 included trials are given in the 'Characteristics of included studies' table.
Nine included trials were performed before 1980 and two after that date. The patients were generally younger than the average stroke patients (mean age ranged from 52 to 75.5 years), and the majority were male. One trial included only transient ischaemic attack patients (Baker 1964) but the remainder included mainly patients with previous strokes. Five trials excluded patients with a possible cardiac embolic source (McDevitt 1959; VA Study 1961; Howard 1963; Wallace 1964; Enger 1965) and a further trial gave the results of patients with embolic stroke separately so these were excluded from this review (Nat‐Coop 1962). Nine trials were performed before routine computerised tomography (CT) was available and so some patients with initial haemorrhagic stroke will probably have been included. Five trials (824 patients) used lumbar puncture to try to rule out intracranial haemorrhage before randomisation (McDevitt 1959; Nat‐Coop 1962; Thygesen 1964; Wallace 1964; Bradshaw 1975) and three trials (Thygesen 1964; Enger 1965; Bradshaw 1975) performed an arteriogram in all participants. Six trials excluded patients with severe hypertension (McDevitt 1959; VA Study 1961; Howard 1963; Baker 1964; Enger 1965; Bradshaw 1975), three definitely excluded the elderly (Baker 1964; Enger 1965; Bradshaw 1975), and all trials excluded patients at high risk of bleeding, e.g. recent peptic ulcer. Seven of the trials entered most of the patients within 14 days of stroke onset (McDevitt 1959; VA Study 1961; Nat‐Coop 1962; Howard 1963; Baker 1964; SWAT 1998; LHSPS 1999).
Oral anticoagulants (coumarins or phenindione) were used in all but one trial (LHSPS 1999) and were usually started in hospital. Low‐dose heparin was compared with usual therapy in LHSPS 1999 (it appeared that a proportion of patients in the control group received aspirin, but it remained unclear how many). This trial was therefore potentially partly confounded. In SWAT 1998 a combination of oral anticoagulants and aspirin against aspirin alone was tested. Analysis of the SWAT 1998 trial's result is partly confounded by the use of different aspirin doses in the warfarin and aspirin arm (80 mg of aspirin) versus the aspirin arm (1300 mg). We therefore performed analyses with and without these trials. Only three trials (Enger 1965; SWAT 1998; LHSPS 1999) defined a scheduled treatment period (two years) whilst in the others the treatment period varied from three to 42 months.
The target prothrombin activity was 10% to 25% of normal (equivalent to 2 to 2.5 times the control in seconds) in all trials except one (Wallace 1964), which used less intensive anticoagulation (20% to 35% of normal, reducing to 50% after 10 months). However, it is difficult to compare the level of anticoagulation between trials since different techniques were used to monitor anticoagulation and no world‐wide standard control was available (Loeliger 1985). Compliance was generally poor. In two trials all patients randomised to anticoagulants remained on them (Howard 1963; Wallace 1964) but in the other trials anticoagulants were withdrawn in between 20% to 70% of patients for a variety of reasons.
The duration of follow up varied from nine to 45 months (mean follow up weighted for study size was 1.9 years). In five trials the follow‐up period was shorter by one to four months in the treated group than in the control group (VA Study 1961; Nat‐Coop 1962; Baker 1964; Enger 1965; Bradshaw 1975) whilst in two trials it was longer by four and five months in the treated group (McDevitt 1959; Thygesen 1964).
All trials used death as one of the main outcome measures and nine recorded the cause of death. Only two trials (McDevitt 1959; Enger 1965) reported the level of dependency in survivors. Ten trials reported recurrent stroke, nine trials reported fatal intracranial haemorrhage and eight recorded myocardial infarctions.
Risk of bias in included studies
The method of randomisation was poorly reported. Four trials (McDevitt 1959; VA Study 1961; Nat‐Coop 1962; Baker 1964) used sealed envelopes but only one reported that they were opaque (McDevitt 1959) and none stated that they were sequentially numbered. Two trials used definitely inadequate concealment of allocation (Enger 1965; Bradshaw 1975) and three trials did not mention the concealment of randomisation (Howard 1963; Thygesen 1964; Wallace 1964). In three trials the person assessing outcome was blind to treatment allocation (Howard 1963; Thygesen 1964; Enger 1965), and in two others the patients were blinded (McDevitt 1959; Nat‐Coop 1962). No serious imbalances in baseline prognostic factors were reported between groups, although in two trials the proportion of patients with cardiac disease varied between the groups: in one there were more patients with cardiac disease in the treated group (Thygesen 1964) whilst in the other there were more in the control group (VA Study 1961). No details on the methods of randomisation were available for SWAT 1998 and LHSPS 1999 but from the abstract our judgement was that they were probably truly randomised.
In two trials, the number of patients randomised to each group was unclear since exclusions after randomisation were not described according to treatment group (VA Study 1961; Nat‐Coop 1962). For these trials, we assumed the number excluded were roughly equally distributed between each group. Results were unavailable for about 53 (9%) of those randomised to anticoagulation and 42 (7%) of those in the control group. These patients were included in the main analyses and assumed not to have had any outcome events.
Effects of interventions
Since only one trial specified a pre‐planned treatment period, only analyses of events that occurred during the whole follow‐up period have been performed. The main analyses included all events regardless of whether they occurred whilst the patient was taking the treatment they were allocated to or not (that is, an intention‐to‐treat analysis).
Primary outcome measures
Comparison 1.1: Death or dependency at end of follow up
Only two trials recorded dependency in survivors and neither used a validated measure. In one, 'functional incapacity' was divided into five groups (0%, 25%, 50%, 75%,100% incapacitated) and we defined those as 50% or more incapacitated as dependent (Enger 1965). In the other, survivors were graded according to whether they were 'self caring' or 'not self caring' (McDevitt 1959). There was a non‐significant reduction in the numbers dead or dependent at the end of follow up (weighted mean duration 2.9 years) between those in the anticoagulation group (114/169) and those in the control group (111/157) but the confidence interval could not rule out significant benefit or hazard (odds ratio (OR) 0.83, 95% confidence interval (CI) 0.52 to 1.34).
Comparison 1.2: Non‐fatal stroke, myocardial infarction or vascular death during follow up
Only four trials provided data on this outcome (weighted mean follow up 2.3 years). There was significant heterogeneity in the results of these trials (I2 = 63%) which was largely due to two trials (VA Study 1961; Enger 1965). In the VA study there was an excess of non‐fatal recurrent ischaemic or unknown strokes and vascular deaths in the anticoagulated group. The reason for this was unclear since we found no obvious differences between this trial and the others. It may be due to chance. Overall, there was no evidence of an effect of anticoagulants on the odds of non‐fatal stroke, myocardial infarction or vascular death (OR 0.96, 95% CI 0.68 to 1.37) and, excluding the VA Cooperative study or the Enger study, or both, did not significantly change this result.
Secondary outcome measures
Comparison 1.3: Deaths from any cause during follow up
Ten trials provided data on death from any cause during follow up (weighted mean duration 1.8 years). The odds of any death in the treatment group were not significantly different from those in the control group: 163/679 patients died in the treatment group compared with 161/654 in control group (OR 0.95, 95% CI 0.73 to 1.24).
Comparison 1.4: Vascular deaths during follow up
There were slightly fewer vascular deaths (137/618) in the treated group than in the control group (146/596) but the reduction was not statistically significant (OR 0.86, 95% CI 0.66 to 1.13).
Comparison 1.5: Recurrent ischaemic/unknown stroke during follow up
Seven trials reported fatal recurrent ischaemic strokes and 10 trials reported fatal and non‐fatal recurrent ischaemic strokes (or recurrent stroke of unknown pathologic type) over a weighted mean follow up of 1.9 years. Given that CT scanning was not available for eight trials and that for the two trials where it was available the numbers of recurrent strokes were not reported by pathological type, all non‐fatal recurrences other than subarachnoid haemorrhages in these trials were classified as of unknown type. In trials which included patients with acute stroke, deaths due to the initial stroke were not included in this analysis. There was a non‐significant trend for fewer recurrences in the anticoagulant group. There were 130/1214, and 146/1184 fatal and non‐fatal recurrent strokes in the treated and control groups respectively (OR 0.85, 95% CI 0.66 to 1.09). There was a non‐significant trend in favour of treatment for fatal recurrences alone (OR 0.51, 95% CI 0.26 to 1.02).
Comparison 1.6: Symptomatic intracranial haemorrhage during follow up
Data on fatal symptomatic intracranial haemorrhage (most of which were confirmed at autopsy) were provided by nine trials and the results were remarkably consistent: anticoagulation more than doubled the odds of fatal intracranial bleeding (20/618, 3.2% versus 7/596, 1.2%, OR 2.54, 95% CI 1.19 to 5.45), which was statistically significant (2p = 0.02). This was equivalent to anticoagulation causing about an extra 20 (95% CI 3 to 38) fatal intracranial haemorrhages per 1000 patients treated during the whole of follow up (weighted mean follow up 1.8 years) and so about 11 extra per year per 1000 patients treated. However, data were missing for 53 treated patients and 42 controls. One option to assess how robust the result is in view of these missing patients would be to do a best‐case and a worst‐case sensitivity analysis. For an extreme best‐case analysis, one could assume all missing patients in the anticoagulated group did not have a fatal intracranial haemorrhage whilst all those in the control group did. This is not particularly sensible since the highest rate of fatal haemorrhage in the control patients was only 4% (Wallace 1964) rather than 100%. We therefore decided to perform a more realistic best‐case analysis. We assumed that the rate of fatal intracranial haemorrhage in the missing anticoagulated patients was the same as in the treatment arm of the trial with the lowest rate (0%) whilst the rate in the missing control patients was the same as in the control arm of the trial with highest rate (1/25 in the Enger trial) (Enger 1965). The additional two events in the control patients were then added to the trial with the biggest weight in the analysis to maximise their impact, and the overall odds ratio recalculated. The result of this analysis showed that the odds of fatal intracranial haemorrhage in the treatment group were still double that in the control group (OR 2.08, 2p = 0.05). A realistic worst‐case analysis (that is where the rate of intracranial haemorrhage in the 53 lost treated patients was 7% (the highest rate found in treated patients in the Wallace trial) and that the rate in control patients was 0%) gave a rate of fatal haemorrhage of 3.8% in the treated group compared to 1.2% in the control group (OR 2.93). As nine trials were performed before routine CT scanning was available and the two more recent trials did not report stroke recurrence by pathological type, there were no reliable data on the frequency of non‐fatal intracranial haemorrhages. One trial did report three non‐fatal subarachnoid haemorrhages (diagnosed with lumbar puncture) in the anticoagulated group. The SWAT 1998 trial reported eight non‐fatal haemorrhages in the anticoagulated group and six in the control group but from the way it was reported in the abstract these haemorrhages were probably extracranial and have been included in that analysis.
Comparison 1.7: Any recurrent stroke or symptomatic intracranial haemorrhage during follow up
Ten trials (weighted mean follow up 1.9 years) reported this composite outcome which assesses the balance between the potential for anticoagulants to prevent recurrent ischaemic strokes and to increase the risk of intracranial haemorrhage. For fatal and non‐fatal events together, there was no evidence of an effect of anticoagulation 155/1214 (12.8%) versus 162/1184 (13.7%) (OR 0.92, 95% CI 0.72 to 1.16). The confidence interval for the effect on fatal events was equally wide (OR 1.02, 95% CI 0.60 to 1.74).
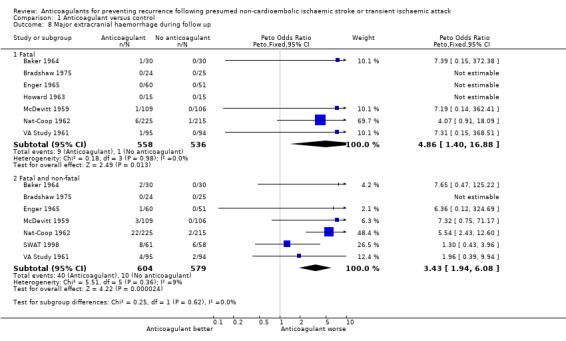
Comparison 1.8: Major extracranial haemorrhage during follow up
Seven trials reported fatal extracranial haemorrhage and seven recorded fatal and non‐fatal major extracranial haemorrhage. There was a large increase in the odds of fatal and non‐fatal major extracranial haemorrhage in the anticoagulated group (40/604) compared with the control group (10/579), which was highly significant although the confidence interval was wide because of the small number of events (OR 3.43, 95% CI 1.94 to 6.08, 2p < 0.0001). Restricting the analysis to fatal haemorrhages gave a similar result. Thus anticoagulation was associated with an additional 49 (95% CI 26 to 71) major extracranial haemorrhages per 1000 patients treated during a mean weighted follow up of 1.9 years (about 25 extra per year per 1000 patients) and an extra 14 (95% CI 0 to 28) fatal extracranial haemorrhages per 1000 patients. Once more this analysis was not affected by performing a modified best‐case (OR for major extracranial haemorrhage = 2.68, 2p = 0.0005) or worst‐case (OR 3.85, 2p < 0.00001) sensitivity analysis.
Comparison 1.9: Myocardial infarction during follow up
Seven trials reported fatal myocardial infarction (weighted mean follow up 1.8 years) and fatal and non‐fatal myocardial infarction (weighted mean follow up 2.2 years). No significant differences were observed between the treated and control groups for either outcome (OR 0.97, 95% CI 0.59 to 1.58 for fatal myocardial infarction alone and OR 1.02, 95% CI 0.62 to 1.70 for fatal and non‐fatal myocardial infarction).
Comparison 1.10: Other embolic events during follow up
Pulmonary embolism or peripheral arterial occlusion were reported by four trials but too few events occurred (16 fatal events, all of which were pulmonary emboli, four non‐fatal pulmonary emboli, and nine non‐fatal arterial occlusions) to give reliable estimates of the possible effects of long‐term anticoagulation (fatal and non‐fatal events: OR 0.83, 95% CI 0.38 to 1.78).
Comparison 1.11: Non‐fatal stroke/intracranial haemorrhage or vascular death during follow up
Eight trials (weighted mean follow up 2.0 years) provided data on the composite outcome of non‐fatal stroke or vascular death; there was no evidence of an effect of anticoagulation (OR 0.88, 95% CI 0.69 to 1.13).
Sensitivity analyses
We were not able to perform sensitivity analyses based on trial quality because none of the trials reported using both a well‐concealed method of treatment allocation and blinded outcome assessment. Similarly, we were not able to perform an analysis based on the level of anticoagulation (an INR range was defined in only one small trial). We did however perform other sensitivity analyses for the three outcomes with most data: death from any cause, any recurrent stroke, and non‐fatal stroke or vascular death. There were no significant differences between the results of:
those trials (LHSPS 1999; SWAT 1998) performed after 1980 that also had access to CT and those published before 1980 and that had no CT;
trials in which follow up was less than one year versus those in which it was more than one year;
trials that excluded patients with atrial fibrillation versus those that did not;
the single small trial where anticoagulation was given with antiplatelet therapy and the trials where no antiplatelet therapy was given;
analyses including only events that occurred whilst the allocated treatment was being taken versus intention‐to‐treat analyses;
analyses that adjusted for differences in the duration of follow up versus those that were unadjusted;
trials in which anticoagulants were started within 14 days of the initial stroke in most patients versus those that started treatment later.
Analyses including data from two trials which were excluded because the control group received very low‐dose anticoagulation as a placebo (Hill 1962; Pearce 1965) did not alter the results, and neither did analyses with and without the LHSPS 1999 and SWAT 1998 trials (to assess the confounding effects of different aspirin doses).
Systematic reviews are prone to publication bias. For the outcomes with statistically significant results (the increases in fatal intracranial haemorrhage and major extracranial haemorrhage with anticoagulation), we assessed how many patients would need to be included in unpublished null trials to overturn the significant results that we had found. We did this by adding a notional trial to the analysis in which the event rate in both groups was equal to the overall rate in the control group of the trials that were included in the review. We then altered the size of the trial until the overall result was no longer significant. The results showed that our results were robust. For fatal intracranial haemorrhage, 1200 patients would need to be included in unpublished null trials to overcome our result, whilst for major extracranial haemorrhage the number was 16,000.
Discussion
This review has demonstrated several important limitations in the trials comparing long‐term anticoagulation with control following non‐cardioembolic stroke or transient ischaemic attack.
Firstly, the trials were small and generally of poor quality and so the results may not be reliable. Two trials definitely used inadequate methods of randomisation (Enger 1965; Bradshaw 1975), and in the remaining trials we could not be sure that allocation was well‐concealed and truly random. This could have led to selection bias (Schulz 1995). It is difficult, although not impossible as shown in the WARSS trial (WARSS 2001), to blind the doctors treating patients in trials of anticoagulation, since the degree of anticoagulation needs to be monitored. However, outcome assessment can usually be blinded but most of the trials (six out of nine) did not attempt to do so. Therefore there may have been detection bias for some of the more subjective outcomes such as minor recurrent strokes. Knowledge of treatment may also have influenced who was sent for autopsy, for example patients in the anticoagulation group who died may have been more likely to have had an autopsy and so more likely to be diagnosed as having a fatal intracranial haemorrhage. We were also not able to perform true intention‐to‐treat analyses since 53 patients in the anticoagulation group and 42 in the control group (8% of those initially randomised) were excluded or lost to follow up. In two trials it was unclear how many patients had actually been randomised in each group. For our main analyses we assumed that none of those who were excluded had an adverse outcome although this is unlikely to be the case. A final methodological problem was that few trials assessed the level of dependency in survivors and those that did used poorly validated measures.
Secondly, the trials were all much too small to detect reliably moderate but important benefits with anticoagulation. When the data were combined to provide an overall estimate of effect, most of the results were not significant but the confidence intervals remained wide and so we could not exclude the possibility of significant benefit (or harm) from anticoagulation. The inclusion of the two larger more recent but still not fully published trials (SWAT 1998; LHSPS 1999) did not significantly change this. For example, the 95% confidence intervals demonstrated that anticoagulation could be associated with either about 50 fewer or 20 more patients dead or dependent per 1000 patients treated per year, or about 40 fewer or 15 more patients having a non‐fatal recurrent stroke (including intracranial haemorrhage) or vascular death per 1000 patients treated per year.
Thirdly, most of the trials were performed before 1980 and so their results may be difficult to generalise to present day practice. The management of patients who had suffered a stroke or transient ischaemic attack is likely to have been different to modern management (i.e. blood pressure reduction, antiplatelet therapy, carotid endarterectomy, cholesterol reduction, etc) and this may alter the effect of anticoagulation. CT scanning was not available for these patients and so some patients who had an initial haemorrhagic stroke could well have been included in these early trials. In addition, it is difficult to know the intensity of anticoagulation that was used in the trials because there was no world‐wide standard control until the INR was recommended by WHO in 1983 (Loeliger 1985). Although most of the trials aimed for prothrombin times of two to three times control, it cannot be assumed that these equate to INRs of between two and three: some may have been higher, others lower. It is clear that there were major problems with anticoagulant control in these trials as reflected in the poor compliance.
The lack of CT scanning and INRs in nine trials could therefore have increased the risk of major haemorrhage in these trials compared to present day. Further analysis only partially supports this. In this review, the average rate of only fatal intracranial haemorrhage in those allocated to anticoagulation (about 2% per year) was higher than the rate of both fatal and non‐fatal intracranial haemorrhage found in more recent studies in which the target INR ranged from two to four (EAFT 1993 0%, Hart 1995 1.7%). The average yearly rate of any fatal intracranial or major extracranial haemorrhage with anticoagulation in this review (5%) was higher than the rate of all major haemorrhages (including non‐fatal intracranial haemorrhages) in studies of anticoagulation following stroke (EAFT 1993 3.0%, ESPRIT 2007 1.8%). The risk of intracranial haemorrhage depends on the intensity of the anticoagulant, and rises with INR level (EAFT 1995; Algra 1997). The highest rate of major extracranial haemorrhage in this review came from the most recent trial (SWAT 1998) where an INR was used but this may have been due to concomitant use of aspirin. Moreover, the definition of major extracranial haemorrhage may have differed between the older and newer studies, and so such indirect comparisons need to be interpreted cautiously.
We aimed to include trials in which patients with a major cardiac source of embolism (chiefly atrial fibrillation) were excluded. However, the extent to which other cardiac sources of embolism had been sought prior to randomisation was unclear, and it is plausible that a proportion of the included cases had a cardio‐embolic source (for example, paroxysmal atrial fibrillation).
The above limitations make it difficult to draw any firm conclusions from these data about the use of long‐term anticoagulation following non‐embolic ischaemic cerebrovascular events. However, the evidence in this review does not support their routine or widespread use. None of the trends for benefit in terms of death or recurrent ischaemic events were significant and the largest trial that we identified (LHSPS 1999) has only ever been published as an abstract and the full publication is still awaited. However, in this review anticoagulation was associated with a 3% to 4% increase in the absolute risk of major haemorrhage per year which is unlikely to be solely due to bias (lack of blinding may have increased the detection of major haemorrhages in the treated group but selection bias could also have resulted in patients at lower risk of haemorrhage being allocated anticoagulation).
A Cochrane review of the trials comparing warfarin with antiplatelet therapy for stroke prevention did not provide evidence of net advantage of oral anticoagulants over antiplatelet agents (Algra 2006); Algra et al are awaiting data on vascular outcomes from the WARSS trial to include in their Cochrane review. Three trials comparing oral anticoagulants with antiplatelet therapy for stroke prevention have been completed but have yet to be included in the Cochrane review (WASID 2005; AVASIS 2006; ESPRIT 2007). The Aspirin Versus Anticoagulants in Symptomatic lntracranial Stenosis trial (AVASIS 2006) was stopped after slow recruitment (http://64.37.123.165/trials/TrialDetail.aspx?tid=3). Twenty‐eight patients with symptomatic middle cerebral artery stenosis were randomised to receive either warfarin (target INR 2.0 to 3.0) or aspirin (300 mg daily). Two patients in the warfarin group experienced vascular events (one patient had a myocardial infarction and the second patient had an intracerebral haemorrhage) compared to none in the aspirin group. This difference in incidence of vascular events between the two treatment groups was not significant (p = 0.48). The Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID 2005) study compared the effects of warfarin (targeted INR 2.0 to 3.0) to aspirin (1300 mg daily) in patients with transient ischaemic attack or stroke attributable to stenosis of a major intracranial artery. The trial was double‐blind and recruited 569 patients, after which enrolment ceased due to concerns about the safety of patients assigned to receive warfarin. No significant differences were found between the groups regarding incidence of ischaemic stroke, death from vascular causes other than stroke or brain haemorrhage (hazard ratio (HR) 1.04 (95% CI 0.73 to 1.48)). However, the rate of death was significantly higher in the warfarin treatment group (9.7% of patients assigned to warfarin died compared to 4.3% of patients assigned to aspirin, HR 0.46 (95% CI 0.23 to 0.90) (WASID 2005). The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT 2007) assigned patients with transient ischaemic attack or minor ischaemic stroke to receive either warfarin (target INR 2.0 to 3.0) or aspirin (30 mg to 325 mg daily). The anticoagulants versus aspirin comparison of ESPRIT was prematurely ended because ESPRIT reported previously that the combination of aspirin and dipyridamole was more effective than aspirin alone. Mean follow up was 46 years (SD 22). The mean achieved INR was 257 (SD 086). A primary outcome event occurred in 99 (19%) patients on anticoagulants and in 98 (18%) patients on aspirin (HR 102, 95% CI 0.77 to 1.35). The HR for ischaemic events was 0.73 (0.52 to 1.01) and for major bleeding complications 2.56 (148 to 443). The HR for the primary outcome event comparing anticoagulants with the combination treatment of aspirin and dipyridamole was 1.31 (0.98 to 1.75). The authors concluded that 'oral anticoagulants (target INR range 2.0 to 3.0) were not more effective than aspirin for secondary prevention after transient ischaemic attack or minor stroke of arterial origin. A possible protective effect against ischaemic events was offset by increased bleeding complications'.
The lack of evidence to support the use of anticoagulation following non‐cardioembolic ischaemic stroke should not detract from the evidence that does exist for the use of anticoagulation following presumed embolic cerebrovascular events in patients with non‐rheumatic atrial fibrillation (Saxena 2004a; Saxena 2004b). In addition, anticoagulants are beneficial for the primary prevention of ischaemic stroke in patients with non‐rheumatic atrial fibrillation (Aguilar 2005), and reduce the risk of stroke by about one‐third compared to antiplatelet drugs (Aguilar 2007). These benefits, however, are dependent on identifying patients with low risks of major intracranial or extracranial bleeding. We were unable to determine whether the cerebral bleeding risks were related to the presence of white matter changes (leukoaraiosis) as has been suggested in some studies (Algra 1997; Gorter 1999).
In summary, this review does not provide evidence that, in patients with recent non‐cardioembolic ischaemic stroke or transient ischaemic attack, anticoagulants offer any clear advantage over control, and the Cochrane review of the trials comparing anticoagulants with antiplatelet therapy does not show clear evidence of an advantage over antiplatelet therapy (Algra 2006). On the other hand, long‐term antiplatelet therapy is safe and effective and reduces the risk of serious vascular events by about a quarter (APT 1994; ATT 2002). The current trials of new orally active anticoagulant agents (chiefly in patients with atrial fibrillation) may help to identify agents with a more favourable risk‐benefit ratio which might be effective for secondary stroke prevention in people with non‐cardioembolic stroke. Similarly, further research may identify a specific category of patient where anticoagulants are more effective than antiplatelet therapy but, in the meantime, oral anticoagulants must be regarded as an unproven therapy for secondary stroke prevention in patients with stroke of presumed arterial origin (that is, no cardiac source of embolism).
Authors' conclusions
Implications for practice.
This review did not provide any evidence to support the routine use of anticoagulant therapy for secondary prevention in patients with non‐cardioembolic ischaemic stroke or transient ischaemic attack (that is, who are not in atrial fibrillation). Anticoagulation in this type of patient carried a definite risk of major intracranial and extracranial haemorrhage without reducing the risk of ischaemic vascular events and hence there was no evidence of net clinical benefit. Other reviews have shown the net benefit from long‐term antiplatelet therapy, which remains the antithrombotic treatment of first choice in these patients. Anticoagulation is of benefit for secondary stroke prevention in patients with atrial fibrillation and may have a role to play in patients who continue to have ischaemic vascular events whilst taking antiplatelet therapy, but this has not been evaluated in randomised trials.
Implications for research.
The data from this review do not support the concept of further randomised trials, aiming to reduce the long‐term risk of further vascular events after non‐cardioembolic ischaemic stroke, which compare currently available anticoagulant agents either with no anticoagulant or with antiplatelet therapy.
Feedback
Are further trials of anticoagulants versus control ethical?, 25 June 2007
Summary
This review, published in 2003, found no evidence for a net benefit of anticoagulants for secondary prevention of symptomatic cranial artery stenosis. Yet the implications for research included the point 'Since antiplatelet therapy has now been shown to be effective in secondary prevention, it is no longer ethical to include an untreated control group. The questions that now need to be answered are: (1) whether anticoagulant therapy alone is better than antiplatelet therapy alone, which is being addressed by three trials (ESPRIT, WASID and AVASIS).' In 2005, the Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis (WASID) trial was terminated early because of the high incidence of death in the warfarin group (4.3% in the aspirin group versus 9.7% in the warfarin group; hazard ratio for aspirin relative to warfarin, 0.46; 95% confidence interval, 0.23 to 0.90; P = 0.02)1. The authors concluded 'Aspirin should be used in preference to warfarin for patients with intracranial arterial stenosis.' This Cochrane review should be updated to indicate that vitamin K inhibitors should be contraindicated in acute ischemic stroke. No further anticoagulant drug research is warranted in this patient group.
The next point in the Implications for research was '(b) whether anticoagulation adds worthwhile benefit to antiplatelet therapy (without an unacceptable increase in the risk of haemorrhage)'. Further research involving adding anticoagulants to aspirin for this indication would be unethical because the lack of overall benefit of anticoagulants in multiple trials and the demonstrated increase in bleeding risk.
1. Chimowitz MI, Lynn MJ, Howlett‐Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. New England Journal of Medicine 2005;352(13):1305‐16.
Reply
This comment was submitted before the present update was performed (Note: the response to this feedback was delayed by a number of unavoidable administrative factors). The Cochrane systematic review of oral anticoagulants versus antiplatelet therapy for preventing further vascular events after transient ischaemic attack or minor stroke of presumed arterial origin1 is in the process of being updated to include the trials mentioned. Those trials, being comparisons with antiplatelet agents, were not eligible for inclusion in this review. However, the wording of the conclusions of this updated review had already been modified in the light of the trials mentioned by Dr Cundiff. Since the wording of the present version of the review is in keeping with the comment above, no further changes have been made to the review apart from the addition of this reply.
1. Algra A, De Schryver ELLM, van Gijn J, Kappelle LJ, Koudstaal PJ. Oral anticoagulants versus antiplatelet therapy for preventing further vascular events after transient ischaemic attack or minor stroke of presumed arterial origin. Cochrane Database of Systematic Reviews 2006 , Issue 3 . Art. No.: CD001342. DOI: 10.1002/14651858.CD001342
Contributors
Commenter: David A Cundiff MD
Reply: Peter Sandercock
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2009 | Feedback has been incorporated | Feedback and the authors' response have been included. |
| 24 July 2008 | New search has been performed | The searches have been updated to June 2008, no new trials have been added, and there is no change to the conclusions. |
| 24 July 2008 | New citation required but conclusions have not changed | The authorship of the review has changed. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 21 April 2008 | Amended | Converted to new review format. |
| 4 October 2002 | New search has been performed | The searches were updated to August 2002 and, as a result, two new trials were included (LHSPS 1999 and SWAT 1998), bringing the total of trials included to 11, including 2487 participants. The title of the review was modified slightly and the body of the text has been substantially revised. The methods for the review had to be adapted. Some planned sensitivity analyses could not be performed since the relevant data were not available. To take account of the deficiencies in the data available, we performed three new post‐hoc analyses. |
| 4 October 2002 | New citation required but conclusions have not changed | Change of authors. |
Acknowledgements
We thank Hazel Fraser for providing us with lists of the relevant trials from the Cochrane Stroke Group Trials Register, and Brenda Thomas for help with searches. For the first cycle of this review: Carl Counsell wrote the protocol, carried out searches, extracted the data, and performed the analyses. He was an active participant in each of the subsequent updates. Dr Prapan Rimdusid helped develop the protocol of the first version of this review, and Dr Ashfaq Shuaib and Elaine Barnhart provided additional information on the SWAT trial. Orell Mielke helped with a previous update of the review. If anyone knows of trials that we have omitted, we would be grateful if they could contact Professor Peter Sandercock.
Data and analyses
Comparison 1. Anticoagulant versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency at end of follow up | 2 | 326 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.52, 1.34] |
| 2 Non‐fatal stroke, myocardial infarction or vascular death during follow up | 4 | 575 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.68, 1.37] |
| 3 Deaths from any cause during follow up | 10 | 1333 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.73, 1.24] |
| 4 Vascular deaths during follow up | 9 | 1214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.66, 1.13] |
| 5 Recurrent ischaemic/unknown stroke during follow up | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fatal | 7 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.26, 1.02] |
| 5.2 Fatal and non‐fatal | 10 | 2398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.66, 1.09] |
| 6 Symptomatic intracranial haemorrhage during follow up | 9 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Fatal | 9 | 1214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.54 [1.19, 5.45] |
| 6.2 Fatal and non‐fatal | 1 | 215 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.81 [0.75, 19.23] |
| 7 Any recurrent stroke or symptomatic intracranial haemorrhage during follow up | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Fatal | 7 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.60, 1.74] |
| 7.2 Fatal and non‐fatal | 10 | 2398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.72, 1.16] |
| 8 Major extracranial haemorrhage during follow up | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 Fatal | 7 | 1094 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.86 [1.40, 16.88] |
| 8.2 Fatal and non‐fatal | 7 | 1183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.43 [1.94, 6.08] |
| 9 Myocardial infarction during follow up | 9 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 Fatal | 7 | 1132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.59, 1.58] |
| 9.2 Fatal and non‐fatal | 7 | 795 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.62, 1.70] |
| 10 Other embolic events during follow up | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 Fatal | 4 | 575 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.16, 1.20] |
| 10.2 Fatal and non‐fatal | 3 | 515 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.38, 1.78] |
| 11 Non‐fatal stroke/intracranial haemorrhage or vascular death during follow up | 8 | 1251 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
1.1. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 1 Death or dependency at end of follow up.
1.2. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 2 Non‐fatal stroke, myocardial infarction or vascular death during follow up.
1.3. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 3 Deaths from any cause during follow up.
1.4. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 4 Vascular deaths during follow up.
1.5. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 5 Recurrent ischaemic/unknown stroke during follow up.
1.6. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 6 Symptomatic intracranial haemorrhage during follow up.
1.7. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 7 Any recurrent stroke or symptomatic intracranial haemorrhage during follow up.
1.8. Analysis.
Comparison 1 Anticoagulant versus control, Outcome 8 Major extracranial haemorrhage during follow up.
1.9. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 9 Myocardial infarction during follow up.
1.10. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 10 Other embolic events during follow up.
1.11. Analysis.

Comparison 1 Anticoagulant versus control, Outcome 11 Non‐fatal stroke/intracranial haemorrhage or vascular death during follow up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baker 1964.
| Methods | C: sealed envelopes (? opaque and sequentially numbered) Blinding: none Results unavailable for: 2 in control group (lost to follow up) | |
| Participants | USA 60 patients (30% of screened patients) Mean age 62 years 100% male Any TIA No CT Time since TIA: unknown (probably within days) Comparability of groups: age, sex, cardiac disease and hypertension similar | |
| Interventions | Rx: unnamed anticoagulant, started in hospital Duration: not specified Target prothrombin activity: (Quick method) 15% to 25% of normal (2 to 2.5 times control in seconds) Monitoring: 2‐weekly Compliance: anticoagulation adequate 80% of time; anticoagulation stopped in 7 (1 bleed, 6 other) Control: no treatment | |
| Outcomes | Death + cause of death Fatal intracranial haemorrhage Any recurrent stroke or symptomatic intracranial haemorrhage Major extracranial haemorrhage MI Pulmonary embolism | |
| Notes | Ex crit: 'severe' hypertension, peptic ulcer, bleeding risk, > 80 years, poor life expectancy Follow up: mean 38 months treatment group versus 41 months control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bradshaw 1975.
| Methods | C: odd or even year of birth in 35 patients, random basis of selection (probable haphazard) for other 14 patients Blinding: none Results available for all patients randomised | |
| Participants | UK 49 patients (? number screened) Mean age 52 years 71% male Carotid TIA/minor stroke No CT; LP in all but 4; carotid arteriogram in all Time since stroke/TIA: < 28 days in 41, 3 to 12 months in 8 Comparability of groups: age, sex, time since onset, % with TIA similar | |
| Interventions | Rx: warfarin in 22; phenindione in 2, started in hospital Duration: mean 18 months (3 to 42 months) Target prothrombin time: 2 times fresh controls Monitoring: weekly or monthly Compliance: adequacy of anticoagulation unknown; anticoagulation stopped in 17 Control: no treatment | |
| Outcomes | Death + cause of death Fatal intracranial haemorrhage Any recurrent stroke or symptomatic haemorrhage Major extracranial haemorrhage MI | |
| Notes | Ex crit: > 65 years, diabetes, myxoedema, diastolic BP > 104 mm Hg, heart disease, peripheral vascular disease Follow up: mean: 41 months in Rx group, 45 months in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Enger 1965.
| Methods | C: open random number list Blinding: patient, assessor Results unavailable for: 3 in treated group, 2 in control group (excluded because of < 3 months follow up) | |
| Participants | Norway 111 patients (? number screened) Mean age: 62.6 years 64% male Non‐embolic stroke (108), TIA (3) No CT; carotid angiogram in all Time since stroke: mean 20 days (range 2 to 180) Comparability of groups: age, sex, hypertension, functional incapacity, stroke severity similar | |
| Interventions | Rx: phenindione; started in hospital Duration: scheduled for 2 years, mean 22.8 months in treated group, 22.6 months in control group Target prothrombin activity: thrombotest 10% to 25% of normal Monitoring: 1 to 2‐weekly in hospital, 3 to 5‐weekly after discharge Compliance: anticoagulation adequate 77% of time; anticoagulation stopped in 11 (1 drug sensitivity, 1 gastrointestinal bleeding, 9 other) Control: placebo (lactate and gelatine) Compliance: placebo withdrawn in 7 (3 coma, 4 other); anticoagulant started in 2 | |
| Outcomes | Death + cause of death Fatal intracranial haemorrhage Any recurrent stroke or symptomatic intracranial haemorrhage Major extracranial haemorrhage MI Death or dependency | |
| Notes | Ex crit: > 75 years, diastolic BP ≥ 120 mm Hg, peptic ulcer, poor life expectancy Follow up: mean 38 months in treated group versus 39 months in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Howard 1963.
| Methods | C: unknown Blinding: doctor, patient, assessor all blind Results available for all patients randomised | |
| Participants | USA 30 patients (24% of screened patients) Mean age 71 years 57% male Non‐embolic stroke No CT Time since stroke: unknown (probably days) Comparability of groups: age similar | |
| Interventions | Rx: dicumarol Duration: 1 year Target prothrombin activity: 15% to 25% of normal Monitoring: depended on physician Compliance: adequacy of anticoagulation not known; anticoagulation stopped none Control: placebo | |
| Outcomes | Death Fatal intracranial haemorrhage | |
| Notes | Ex crit: systolic BP > 200 mmHg, recent MI, bleeding risk Follow up: 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
LHSPS 1999.
| Methods | Unknown | |
| Participants | Italy 1095 patients Non‐embolic ischaemic stroke and without carotid stenosis, 21 to 210 days after CT‐confirmed ischaemic stroke | |
| Interventions | Rx: (550 patients) unfractionated heparin 12.500 IU/d plus 'usual therapy' for the mean duration of 2 years Control: (545 patients) usual therapy | |
| Outcomes | Cumulated stroke recurrence, general mortality | |
| Notes | Follow up: 2 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McDevitt 1959.
| Methods | C: sealed, opaque envelopes Blinding: patients Results not available for: 17 in treated group because never received anticoagulant (14 excluded, 3 lost to follow up), 7 in control group (1 excluded because of intracranial tumour, 6 lost to follow up) | |
| Participants | USA 215 patients (25% of screened patients) Mean age 68.7 years 65% male Non‐embolic stroke No CT; 100% LP Time since stroke: < 7 days to 2 months Comparability of groups: age, sex, incidence of associated disease (such as diabetes, hypertension, and peripheral vascular disease), site of occlusion and severity of stroke similar | |
| Interventions | Rx: dicumarol or warfarin Duration: 4 days to 62 months Target prothrombin activity: (Link‐Shapiro modification of the Quick test) 2 to 2.5 times control in seconds Monitoring: daily in hospital, weekly after discharge Compliance: on anticoagulation for 44% of total follow up Control: placebo | |
| Outcomes | Death + cause of death Fatal intracranial haemorrhage + non‐fatal subarachnoid haemorrhage Any recurrent stroke or symptomatic intracranial haemorrhage Major extracranial haemorrhage MI Pulmonary embolism Other embolic events Death or dependency | |
| Notes | Ex crit: severe hepatic or renal disease, bleeding risk, active peptic ulcer, BP > 180/110 mmHg, prolonged depression of consciousness, unlikely to survive Follow up: mean 36 months in treated group versus 31 months in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Nat‐Coop 1962.
| Methods | C: sealed envelope (? opaque or sequentially numbered) Blinding: patients only Results unavailable for at least 22 (? which group) who were excluded because they died within 24 hours, or did not receive Rx assigned to, or did not have ischaemic stroke | |
| Participants | USA 440 patients (? number screened) Mean age unknown (84% > 55 years) 65% male TIA (10%) or presumed non‐embolic stroke (90%) No CT; 100% LP Time since TIA/stroke: < 8 weeks Comparability of groups: age, sex, vascular risks similar | |
| Interventions | Rx: heparin 50 mg 4‐hourly iv then dicumarol Duration not specified Target prothrombin activity: (Quick method) 15% to 25% of control Monitoring: 1 to 2‐weekly Compliance: adequacy of control not specified; anticoagulation stopped in 96 (bleeding, poor compliance, difficulty monitoring) Control: placebo Compliance: 4 started anticoagulation | |
| Outcomes | Death + cause of death Fatal intracranial haemorrhage Any recurrent stroke or symptomatic intracranial haemorrhage Fatal extracranial haemorrhage Fatal MI | |
| Notes | Ex crit: active gastrointestinal/urinary bleeding, bleeding disorder, serious disease Follow up: mean 11 months treatment group versus 15 months control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
SWAT 1998.
| Methods | Unknown | |
| Participants | USA 178 patients Patients with non‐embolic TIA or mild stroke within 180 days of last event and without carotid stenosis > 70% Size: 178 (aspirin 58, warfarin 59, warfarin + aspirin 61) | |
| Interventions | Enteric‐coated aspirin 650 mg 12‐hourly versus warfarin (INR 2.0 to 3.0) versus warfarin plus aspirin 80 mg 24‐hourly | |
| Outcomes | Death, recurrent stroke, major haemorrhage | |
| Notes | Follow up: 2 years Only aspirin and aspirin + warfarin groups included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Thygesen 1964.
| Methods | C: unknown Blinding: patient, assessor Results available for all patients randomised | |
| Participants | Denmark 68 patients (? number screened) Mean age 60.5 years Approximately 58% male Predominantly non‐embolic stroke No CT; LP and arteriography in most Time since stroke: 6 weeks Comparability of groups: age, premorbid health, previous strokes similar; more cardiac disease in treated group. | |
| Interventions | Rx: phenindione Duration: not specified Target prothrombin activity: prothrombin‐proconvertin test 10% to 20% Monitoring: unknown Compliance: anticoagulation inadequate in at least 3 Control: placebo | |
| Outcomes | Death + cause of death Fatal intracranial haemorrhage Any recurrent stroke or symptomatic intracranial haemorrhage Fatal MI | |
| Notes | Ex crit: no major exclusions Follow up: mean 22 months in treated group versus 17 months in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
VA Study 1961.
| Methods | C: numbered sealed envelopes (? opaque) Blinding: none Results unavailable for 34 patients excluded (? which groups) because of wrong diagnoses or follow up < 1 month; and for 8 lost to follow up (Rx 5, control 3) | |
| Participants | USA 189 patients (13% of screened patients) Mean age unknown 100% male Presumed non‐embolic TIA (24%) or stroke (76%) No CT Time since TIA/stroke: < 1 month Comparability of groups: age similar, more cardiac problems in controls (48% versus 33%) | |
| Interventions | Rx: coumadin or dicumarol Duration: unspecified Target prothrombin activity: (one stage method) 20% of normal Monitoring: 1 to 3 weekly Compliance: adequate control 80% of time; anticoagulation stopped in 17 (6 bleeds, 11 other) Control: no treatment Compliance: 5 started anticoagulation | |
| Outcomes | Death + cause of death Fatal intracranial haemorrhage Any recurrent stroke or symptomatic intracranial haemorrhage Major extracranial haemorrhage MI Other thrombotic events (pulmonary embolism, peripheral vascular) | |
| Notes | Ex crit: 'severe' hypertension, bleeding risk, coma Follow up: mean 9 months treatment group versus 13 months control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wallace 1964.
| Methods | C: unknown Blinding: none Results available for all patients randomised | |
| Participants | Australia 52 patients (inpatients only) Mean age 75.7 years 25% male Non‐embolic stroke No CT; 100% LP Time since stroke: > 14 days Comparability of groups: age, sex, severity of stroke, BP similar | |
| Interventions | Rx: phenindione or warfarin Duration: until hospital discharge Target prothrombin activity: (Quick method) 20% to 35% of normal (50% after 10 months) Monitoring: unknown Compliance: adequacy of anticoagulation unclear; anticoagulation stopped in none Control: no treatment | |
| Outcomes | Death Recurrent stroke Fatal intracranial haemorrhage | |
| Notes | Ex crit: acute peptic ulcer, recent bleed, renal/liver disease Follow up: until hospital discharge: mean 9.5 months treatment group versus 10 months control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BP: blood pressure C: concealment of allocation CT: computerised tomography Ex crit: exclusion criteria iv: intravenous LP: lumbar puncture MI: myocardial infarction Rx: treatment TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Eriksson 1983 | Large number of patients excluded after treatment allocation (at least 56 (29%) in anticoagulant group, and 39 (28%) in control group). In addition, some of the patients in the anticoagulation group also received dextran (confounded). |
| Hill 1960 | Active control (phenindione 50 mg versus 1 mg identical tablet) |
| Hill 1962 | Active control (phenindione 50 mg versus 1 mg identical tablet); this is a continuation of the Hill 1960 trial |
| Pearce 1965 | Active control (phenindione 50 mg versus 1 mg identical tablet) |
| Vilenskii 1976 | Patients probably randomised by toss of a coin but marked imbalance between groups (anticoagulation 166 patients, control 138 patients). Numbers of outcome events not reported by treatment group, and not available despite contacting author. |
Contributions of authors
For this update Lorna Gibson carried out searches, extracted the data, performed the initial analyses, and redrafted the review. Ming Liu commented on drafts of the manuscript. For all stages of each version of the review, Peter Sandercock commented on analyses and draft versions and for this update checked the analyses and wrote the final text of the review. Peter Sandercock is the Guarantor of the review.
Sources of support
Internal sources
University of Edinburgh, UK.
External sources
Wellcome Trust, UK.
Medical Research Council, UK.
Faculty of Medine Mannheim ‐ University of Heidelberg, Germany.
Declarations of interest
Peter Sandercock was the principal investigator of the International Stroke Trial and Dr Counsell was also on the Steering Committee of this trial. Peter Sandercock has received, from a variety of manufacturers of antiplatelet and anticoagulant drugs (Sanofi, Bristol Meyer Squibb, Sanofi‐Synthelabo, Organon, Boehringer Ingelheim, Janssen): lecture fees and travel expenses for lectures delivered at conferences: consultancy fees; he has in the past received research grants from Glaxo‐Wellcome and Boehringer Ingelheim; the drug supply for the start‐up phase of the IST‐3 trial was donated to his department by Boehringer Ingelheim; he does not have any continuing contractual consultancy arrangements with any company, or any current research grants from any company, nor does he hold stock (or hold any other financial interests) in any pharmaceutical company.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Baker 1964 {published data only}
- Baker RN, Schwartz WS, Rose MD. Transient ischemic strokes. A report of a study of anticoagulant therapy. Neurology 1966;16:841‐7. [Google Scholar]
Bradshaw 1975 {published data only}
- Bradshaw P, Brennan S. Trial of long‐term anticoagulant therapy in the treatment of small stroke associated with a normal carotid angiogram. Journal of Neurology, Neurosurgery & Psychiatry 1975;38:642‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enger 1965 {published data only}
- Enger E, Boyesen S. Long‐term anticoagulant therapy in patients with cerebral infarction: a controlled clinical study. Acta Medica Scandinavica 1965;178 Suppl 438:7‐61. [PubMed] [Google Scholar]
Howard 1963 {published data only}
- Howard FA, Cohen P, Hickler RB, Locke S, Newcomb T, Tyler HR. Survival following stroke. JAMA 1963;183(11):921‐5. [DOI] [PubMed] [Google Scholar]
LHSPS 1999 {published data only}
- Fortini A, Bonechi G, Carnovali M, Olivo G, Rinaldi G, Gensini GF. Multi‐centric study on ischemic cerebral re‐infarct prevention with low‐dose heparin: the informatic system [Sistema informatico dello studio clinico controllato multicentrico sulla prevenzione del reinfarto cerebrale ischemico con eparina a basso dosaggio]. Rivista di Neurobiologica 1990;36(2):219‐21. [Google Scholar]
- Neri Serneri GG, Gensini GF, Carnovali M, Olivo G, Modesti PA, Inzitari D, et al. Low‐dose heparin stroke prevention study: preliminary efficacy analysis. Cerebrovascular Diseases 1999;9 Suppl 1:67. [Google Scholar]
McDevitt 1959 {published data only}
- Groch SN, McDevitt E, Wright IS. A long‐term study of cerebral vascular disease. Annals of Internal Medicine 1961;55:358‐67. [DOI] [PubMed] [Google Scholar]
- McDevitt E, Groch SN, Wright IS. A cooperative study of cerebrovascular disease. Circulation 1959;20:215‐23. [DOI] [PubMed] [Google Scholar]
- McDowell F, McDevitt E. Treatment of the completed stroke with long‐term anticoagulant: six and one‐half years experience. In: Siekert RG, Whisnant JP editor(s). Cerebral Vascular Diseases. Fourth Conference. New York: Grune & Stratton Inc, 1965:185‐99. [Google Scholar]
- McDowell F, McDevitt E, Wright IS. Anticoagulant therapy: five years experience with the patient with an established cerebrovascular accident. Archives of Neurology 1963;8:209‐14. [Google Scholar]
Nat‐Coop 1962 {published data only}
- Baker RN, Broward JA, Fang HC, Fisher CM, Groch SN, Heyman A, et al. Anticoagulant therapy in cerebral infarction. Report on co‐operative study. Neurology 1962;12:823‐9. [Google Scholar]
- Fisher CM. Anticoagulant therapy in cerebral thrombosis and cerebral embolism. A national cooperative study interim report. Neurology 1961;11:119‐31. [DOI] [PubMed] [Google Scholar]
SWAT 1998 {published data only}
- Stewart B, Shuaib F, Veloso F. Stroke Prevention with Warfarin or Aspirin Trial (SWAT). Stroke 1998;29:304. [Google Scholar]
Thygesen 1964 {published data only}
- Thygesen P, Christensen E, Dyrbye M, Eiken M, Franzen E, Gormsen J, et al. Cerebral apoplexy: a clinical, radiological, electroencephalographic and pathological study with special reference to the prognosis of cerebral infarction and the result of long‐term anticoagulant therapy. Danish Medical Bulletin 1964;11:233‐57. [PubMed] [Google Scholar]
VA Study 1961 {published data only}
- Baker RN. An evaluation of anticoagulant therapy in the treatment of cerebrovascular disease. Report of the Veterans Administration Cooperative Study of Atherosclerosis, Neurology Section. Neurology 1961;11:132‐8. [DOI] [PubMed] [Google Scholar]
Wallace 1964 {published data only}
- Wallace DC. Cerebral vascular disease in relation to long‐term anticoagulant therapy. Journal of Chronic Diseases 1964;17:527‐37. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Eriksson 1983 {published data only}
- Eriksson S‐E, Link H. Anticoagulants in patients with partial stroke. Proceedings of the 1st Scandinavian Meeting on Cerebrovascular Disease. 1982:11.
- Eriksson S‐E, Link H. Evaluation of anticoagulants in patients with cerebral infarction with slight to moderate neurological deficit. Acta Neurologica Scandinavica 1983;68:96‐106. [DOI] [PubMed] [Google Scholar]
Hill 1960 {published data only}
- Hill AB, Marshall J, Shaw DA. A controlled clinical trial of long‐term anticoagulant therapy in cerebrovascular disease. Quarterly Journal of Medicine 1960;29:597‐609. [PubMed] [Google Scholar]
Hill 1962 {published data only}
- Hill AB, Marshall J, Shaw DA. Cerebrovascular disease: trial of long‐term anticoagulant therapy. BMJ 1962;ii:103‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearce 1965 {published data only}
- Pearce JMS, Gubbay SS, Walton JN. Long‐term anticoagulant therapy in transient cerebral ischaemic attacks. Lancet 1965;i:6‐9. [DOI] [PubMed] [Google Scholar]
Vilenskii 1976 {published data only}
- Vilenskii BS. Ambulatory treatment with anticoagulants in the prevention of cerebral ischaemia. Zhurnal Nevropatologii i Psikhiatrii imeni S.S. Korsakova 1976;76:1143‐9. [PubMed] [Google Scholar]
Additional references
AFI 1994
- Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Archives of Internal Medicine 1994;154:1449‐57. [PubMed] [Google Scholar]
Aguilar 2005
- Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non‐valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001927.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aguilar 2007
- Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non‐valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006186.pub2] [DOI] [PubMed] [Google Scholar]
Algra 1996
- Algra A, Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. Journal of Neurology, Neurosurgery & Psychiatry 1996;60:197‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Algra 1997
- Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group. A randomised trial of anticoagulants versus aspirin after cerebral ischemia of arterial origin. Annals of Neurology 1997;42:857‐65. [DOI] [PubMed] [Google Scholar]
Algra 1999
- Algra A, Gijn J. Cumulative meta‐analysis of aspirin efficacy after cerebral ischaemia of arterial origin. Journal of Neurology, Neurosurgery & Psychiatry 1999;66:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Algra 2006
- Algra A, Schryver ELLM, Gijn J, Kappelle LJ, Koudstaal PJ. Oral anticoagulants versus antiplatelet therapy for preventing further vascular events after transient ischaemic attack or minor stroke of presumed arterial origin. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001342.pub2] [DOI] [PubMed] [Google Scholar]
APT 1994
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy‐I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81‐106. [PMC free article] [PubMed] [Google Scholar]
ATT 2002
- Antithrombotic Trialists' Collaboration. Collaborative meta‐analysis of antiplatelet therapy for prevention of death, myocardial infarction and stroke in high risk patients. BMJ 2002;324:71‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
AVASIS 2006
- Marti‐Fabregas J, Cocho D, Marti‐Vilalta J‐L, Gich I, Belvis R, Bravo Y, et al. Aspirin or anticoagulants in stenosis of the middle cerebral artery: a randomised trial. Cerebrovascular Diseases 2006;22:162‐9. [DOI] [PubMed] [Google Scholar]
Bamford 1990
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: The Oxfordshire Community Stroke Project ‐ 1981‐1986. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. Journal of Neurology, Neurosurgery & Psychiatry 1990;53:16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benavente 2002
- Benavente O, Hart R, Koudstaal P, Laupacis A, McBride R. Antiplatelet therapy for preventing stroke in patients with non‐valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001925.pub2] [DOI] [PubMed] [Google Scholar]
Burn 1994
- Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long‐term risk of recurrent stroke after a first‐ever stroke. The Oxfordshire Community Stroke Project. Stroke 1994;25:333‐7. [DOI] [PubMed] [Google Scholar]
Dennis 1990
- Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischaemic attacks in the Oxfordshire Community Stroke Project. Stroke 1990;21:848‐53. [DOI] [PubMed] [Google Scholar]
Dennis 1993
- Dennis MS, Burn JPS, Sandercock PAG, Bamford JM, Wade DT, Warlow CP. Long‐term survival after first‐ever stroke: The Oxfordshire Community Stroke Project. Stroke 1993;24:796‐800. [DOI] [PubMed] [Google Scholar]
Dhamoon 2007
- Dhamoon MS, Tai W, Boden‐Albala B, Rundek T, Paik MC, Sacco RL, et al. Risk of myocardial infarction or vascular death after first ischaemic stroke: the Northern Manhattan Study. Stroke 2007;38:1752‐8. [DOI] [PubMed] [Google Scholar]
EAFT 1993
- European Atrial Fibrillation Trial Study Group. Secondary prevention in non‐rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342:1255‐62. [PubMed] [Google Scholar]
EAFT 1995
- The European Atrial Fibrillation Trial Study Group. Optimal anticoagulant therapy in patients with nonrheumatic atrial fibrillation and recent cerebral ischemia. New England Journal of Medicine 1995;333:5‐10. [DOI] [PubMed] [Google Scholar]
ESPRIT 2007
- Schryver ELLM, on behalf of the European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) Group. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurology 2007;6:115‐24. [DOI] [PubMed] [Google Scholar]
Gorter 1999
- Gorter JW for the Stroke Prevention in Reversible Ischaemia Trial (SPIRIT) and European Atrial Fibrillation Study Groups. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Neurology 1999;53:1319‐27. [DOI] [PubMed] [Google Scholar]
Gubitz 2004
- Gubitz G, Counsell C, Sandercock P. Anticoagulants for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000024.pub2] [DOI] [PubMed] [Google Scholar]
Hankey 1993
- Hankey GJ, Dennis MS, Slattery JM, Warlow CP. Why is the outcome of transient ischaemic attacks different in different groups of patients?. BMJ 1993;306:1107‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardie 2005
- Hardie K, Jamrozik K, Hankey GJ, Broadhurst RJ, Anderson C. Trends in five‐year survival and risk of recurrent stroke after first‐ever stroke in the Perth community stroke study. Cerebrovascular Diseases 2005;19:179‐85. [DOI] [PubMed] [Google Scholar]
Hart 1995
- Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke 1995;26:1471‐7. [DOI] [PubMed] [Google Scholar]
Jonas 1988
- Jonas S. Anticoagulant therapy in cerebrovascular disease: review and meta‐analysis. Stroke 1988;19:1043‐8. [DOI] [PubMed] [Google Scholar]
Loeliger 1985
- Loeliger EA, Poller L, Samana M, Thomson JM, Besselaar AMHP, Vermylen J, et al. Questions and answers on prothrombin time standardisation in oral anticoagulant control. Thrombosis and Haemostasis 1985;54:515‐7. [PubMed] [Google Scholar]
Meissner 1988
- Meissner I, Whisnant JP, Garraway MW. Hypertension management and stroke recurrence in a community (Rochester, Minnesota, 1950‐1979). Stroke 1988;19:459‐63. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Ricci 1991
- Ricci S, Celani MG, Rosa F, Vitali R, Duca E, Ferraguzzi R, et al. SEPIVAC: a community‐based study of stroke incidence in Umbria, Italy. Journal of Neurology, Neurosurgery & Psychiatry 1991;54:695‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacco 1982
- Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The Framingham Study. Stroke 1982;13:290‐5. [DOI] [PubMed] [Google Scholar]
Saxena 2004a
- Saxena R, Koudstaal PJ. Anticoagulants versus antiplatelet therapy for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischaemic attack. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000187.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saxena 2004b
- Saxena R, Koudstaal P. Anticoagulants for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischemic attack. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000185.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Slowik 2007
- Slowik A, Turoj W, Zwolinska G, Rog T, Dziedzic T, Pera J, et al. Stroke attack rates and case fatality in the Krakow Stroke Registry. Eurologia I Neurochirurgia Polska 2007;41:291‐5. [PubMed] [Google Scholar]
Warlow 1993
- Warlow C. Disorder of the cerebral circulation. In: Walton J editor(s). Brain Diseases of the Nervous System. 10th Edition. Oxford: Oxford University Press, 1993. [Google Scholar]
WARSS 2001
- Mohr JP, Thompson JLP, Lazar RM, Pullicino P. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. New England Journal of Medicine 2001;345(20):1444‐51. [DOI] [PubMed] [Google Scholar]
WASID 2005
- Chimowitz MI, Lynn MJ, Howlett‐Smith MH, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. New England Journal of Medicine 2005;352:1305‐16. [DOI] [PubMed] [Google Scholar]
Weissbein 1994
- Weissbein T, Czlonkowska A, Popow J, Ryglewicz D, Heir DB. Analysis of 30‐day stroke mortality in a community‐based registry in Warsaw, Poland. Journal of Stroke and Cerebrovascular Diseases 1994;4:63‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Coull 2004
- Coull AJ, Lovett JK, Rothwell PM. Population‐based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 1997
- Liu M, Counsell C, Sandercock P. Anticoagulant therapy for secondary prevention following non embolic stroke or TIA: a systematic review of the randomised evidence. Cerebrovascular Diseases 1997;7 Suppl 4:3. [Google Scholar]
Mielke 2002
- Mielke O, Liu M, Counsell C, Sandercock P. Anticoagulants for preventing recurrence following ischaemic stroke or transient ischaemic attack. Cerebrovascular Diseases 2002;13 Suppl 3:7. [Google Scholar]


