Abstract
Study Objectives
Stress is associated with poor and short sleep, but the temporal order of these variables remains unclear. This study examined the temporal and bi-directional associations between stress and sleep and explored the moderating role of baseline sleep complaints, using daily, intensive longitudinal designs.
Methods
Participants were 326 young adults (Mage = 23.24 ± 5.46), providing >2,500 nights of sleep altogether. Prospective total sleep time (TST), sleep onset latency (SOL), wake after sleep onset (WASO), and sleep efficiency (SE) were measured using actigraphy and sleep diaries. Perceived stress was reported three times daily between: 11:00–15:00, 15:30–19:30, and 20:00–02:00. Sleep complaints were measured at baseline using the PROMIS sleep disturbance scale. Within- and between-person sleep and stress variables were tested using cross-lagged multilevel models.
Results
Controlling for covariates and lagged outcomes, within-person effects showed that higher evening stress predicted shorter actigraphic and self-reported TST (both p < .01). Conversely, shorter actigraphic and self-reported TST predicted higher next-day stress (both p < .001). Longer self-reported SOL and WASO (both p < .001), as well as lower actigraphic (p < .01) and self-reported SE (p < .001), predicted higher next-day stress. Between-person effects emerged only for self-reported TST predicting stress (p < .01). No significant results were found for the moderating role of baseline sleep complaints.
Conclusions
Results demonstrated bi-directional relations between stress and sleep quantity, and a consistent direction of worse sleep quantity and continuity predicting higher next-day stress. Results highlighted within-individual daily variation as being more important than between-individual differences when examining sleep and daytime functioning associations.
Keywords: stress, sleep continuity, sleep quantity, daily design
Statement of Significance.
This study examined the temporal and bi-directional associations between stress and sleep (self-report and objective estimates) in a large sample of young adults using one of the strongest tests of directionality possible in observational designs (daily, intensive longitudinal design). Even after accounting for covariates and previous-night outcomes, higher evening stress predicted subsequent shorter sleep quantity, and shorter sleep quantity and continuity predicted higher next-day stress. These findings highlight the vicious daily cycle between high stress and short or discontinuous sleep, which may increase the risk or accelerate the progression of mental and physical disorders. In addition to the behavioral indices of sleep, future research should explore the associations between daily stress and sleep architecture.
Introduction
Stress and sleep are two important determinants of health and well-being, both linked with health outcomes including cardiovascular diseases, diabetes, depression, and anxiety [1–6]. Current understanding of the associations between perceived stress and sleep is primarily based on studies that examined between-person differences, such as cross-sectional associations and between-group comparisons [7–13]. Despite daily variations in both stress and sleep within individuals, whether stress and sleep bi-directionally influence each other on a day-to-day basis is unclear. This study aimed to test the temporal and bi-directional associations between stress and both self-reported and actigraphic sleep across 12 days. Daily repeated ecological momentary assessments (EMA) used in this study provide a stronger test of directionality between daily stress and sleep in a naturalistic environment than cross-sectional studies [14].
Stress and sleep
Cross-sectional studies show a consistent association between high perceived stress and poor, disturbed, or short sleep, particularly self-reported sleep [7–13]. For example, employees who experienced higher work-related stressors that affected their family relationships reported lower sleep sufficiency, poorer sleep quality, more insomnia symptoms, and had shorter actigraphic sleep duration [9]. Similarly, some prospective longitudinal studies have demonstrated that greater psychosocial stress, such as more stressful life events, stress-related cognitive intrusion, and work-related stressors, is associated with the development and maintenance of insomnia [15, 16]. However, due to the lack of daily stress and sleep, these studies do not inform whether daily, dynamic changes in stress influence sleep or vice versa.
To date, only a few studies examined the stress–sleep association on a daily basis. On stress predicting subsequent sleep, a 42-day study showed that higher severity of stress or worries at bedtime, but not the average stress across the day, predicted poorer self-reported sleep quality that night [17]. Similarly, a seven-day study using actigraphic sleep measures in young adults found that on days of higher-than-usual stress, those with high childhood family risk had shorter actigraphic total sleep time (TST) [18].
Few studies tested the bi-directional associations between stress and sleep [19], with even fewer studies using objective estimates of sleep [20]. In an 8-day study in working parents [19], within-person effects showed that greater work-related stress predicted longer self-reported sleep onset latency (SOL) that night, but the reverse direction was nonsignificant (i.e. longer SOL the previous night did not predict higher next-day stress). Further, self-reported poorer sleep quality and shorter duration predicted higher next-day work-related stress, whereas the opposite direction was nonsignificant. When actigraphic sleep measures were utilized, Doane and Thurston [20] found a significant bi-directional association between perceived stress and TST in adolescents. Specifically, higher perceived stress predicted shorter TST that night, and shorter TST predicted higher next-day perceived stress. Finally, lower sleep efficiency (SE) predicted higher next-day stress, but not the reverse direction.
These inconsistent findings show the complexity of the bi-directional associations and temporal order between stress and sleep, which may differ across sleep parameters (i.e. SOL, TST, and SE) and measurement (i.e. self-report vs. actigraphy). Despite differences in self-reported and objectively measured sleep parameters [21], current literature increasingly recognizes both as representing unique features of sleep and its associated experiences. Thus, there is a need for daily studies on stress and sleep that incorporate both objective and self-reported sleep measures.
Current study
The primary aim of this study is to examine the bi-directional, temporal associations between daily stress and sleep across 12 days, using both objective actigraphic and self-report measures of sleep. It was hypothesized that: (1) Higher evening stress would predict shorter sleep duration (TST) and worse sleep continuity (i.e. longer SOL, higher wake after sleep onset [WASO], and lower SE [22]) on the same night. (2) Shorter sleep duration and worse sleep continuity would predict higher next-day stress. These a priori hypotheses were preregistered on the Open Science Framework on July 7, 2017, prior to the completion of data collection (https://osf.io/h47yb/).
As a secondary exploratory aim, this study examined whether baseline levels of sleep complaints would moderate the prospective associations between nightly sleep and next-day stress. We hypothesized that individuals with greater sleep complaints at baseline would report higher stress following a night of shorter or lower than usual sleep duration and continuity. This hypothesis was made based on the consistent associations between sleep complaints and unhelpful thoughts and beliefs about sleep (e.g. catastrophizing about the consequences of poor sleep on daytime functioning) [23, 24]. Thus, individuals with high sleep complaints may be more vulnerable to the effects of discontinuous or short sleep the previous night and experience higher stress the following day.
Methods
Participants
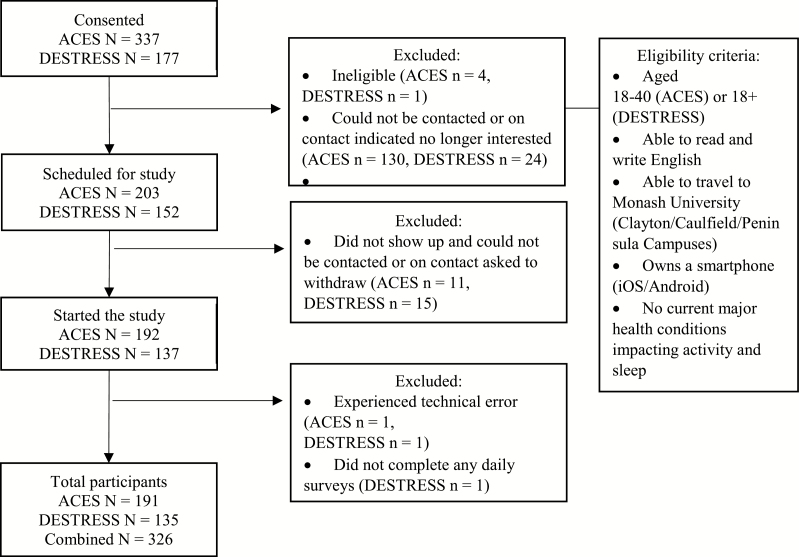
Participants in this study were drawn from two studies: (1) The Activity, Coping, Emotions, Stress, and Sleep (ACES) study and (2) Diet, Exercise, Stress, Emotion, Speech, and Sleep (DESTRESS) study. Both studies utilized similar eligibility criteria, daily EMA designs, and recruitment strategies (see Figure 1). The main differences were the number of days observed (12 vs. 7 days for ACES and DESTRESS, respectively) and the age range (see Figure 1). The final sample consisted of 326 participants (191 from ACES and 135 from DESTRESS). A priori power analyses conducted in G*Power [25] showed a sample of 60 participants (assuming 75% completion rate) provide 80% power to detect a small-medium effect size at the within-person level. A larger sample was collected to support other aims and subgroup analyses not related to the current paper. ACES was conducted from April 2017 to December 2017, whereas DESTRESS was conducted from May 2018 to August 2018. Figure 1 shows a participant flow chart.
Figure 1.
Summary of recruitment process. The ACES study recruited from April 13, 2017, to December 5, 2017. The DESTRESS study recruited from May 22, 2018, to August 13, 2018.
Design and procedure
All procedures were approved by the Monash University Human Research Ethics Committee. Both studies employed daily, intensive, longitudinal design with repeated EMA. Through EMA, participants report their real-time experiences in their natural environment, which maximizes ecological validity and reduces recall biases [26]. By centering within-person data on an individual’s average across time, repeated assessments across days allowed participants to serve as their own control, providing rigorous testing of the temporal relations between stress and sleep.
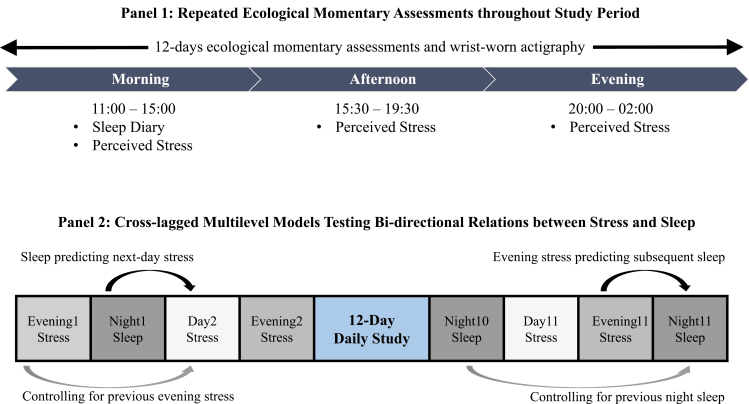
Most participants participated in both studies during school or semester periods. Participants first completed a baseline questionnaire consisting of demographic information, covariates, and other measures related to the overall study. For ACES, participants started on a Thursday or Friday and end on a Monday or Tuesday, whereas DESTRESS started on any weekday. Participants then completed the daily EMA component where they wore an actigraphy device throughout the study period and completed three surveys per day in the mornings (11:00–15:00), afternoons (15:30–19:30), and evenings (20:00–02:00) via a mobile application (MetricWire). Specifically, participants reported their stress levels in the morning, afternoon, and evening surveys and completed sleep diary in the morning surveys within the stated time windows (see Figure 2). Automated reminders were sent to participants when surveys were available, and all surveys were closed outside their respective time windows to ensure participants reported their real-time experiences.
Figure 2.
Panel 1 shows the repeated ecological momentary assessments throughout the study period, with participants completing three surveys a day and wearing an actigraphy watch. Panel 2 illustrates the cross-lagged multilevel models testing bi-directional relations of stress and sleep. All relationships were tested prospectively and controlled for lagged outcomes (i.e. previous evening stress or previous night sleep).
Measures
Sleep
Objective estimates of sleep were determined at 60-s epoch using ActiGraph wGT3X-BT, an actigraphy-device with good validity and reliability against polysomnography estimates [27]. The sleep data were scored using the ActiLife software (v.6.13.3) following an established protocol based on activity, light, and sleep diary as well as integrated approximations from the Cole–Kripke scoring algorithm [28]. The parameters included TST, SOL, WASO, and SE. For self-reported sleep, participants reported bed and rise times, SOL, the number of and total time of WASO as part of the daily morning survey. These items were adapted from the Consensus Sleep Diary [29].
Perceived Daily Stress
Perceived daily stress was measured three times a day (mornings, afternoons, and evenings as described above) using a single item ranging from 0 (Not at all stressful) to 10 (Very stressful) adapted from the Daily Inventory of Stressful Events scale [30, 31]. For example, “Since the afternoon survey (or since 3:30 pm if you did not do the afternoon survey), how stressful has your day been?”. Morning and afternoon stress were averaged to create the composite of next-day stress. Evening stress was reported an average of 3.30 (SD = 1.60) h prior to actigraphic bedtime.
Sleep Complaints
Sleep complaints, or self-reported sleep disturbance symptoms within the past 7 days were measured at baseline using the eight-item, Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance Short-Form 8a scale [32]. Example item includes “My sleep was restless,” and responses were rated on a 5-point Likert scale. The raw sum scores were converted to a standardized T-score following PROMIS guidelines which have a population mean of 50 and standard deviation of 10, with higher scores indicating greater sleep complaints or disturbance. This scale showed strong internal consistency reliability (Cronbach’s α = .89 and α = .90 for ACES and DESTRESS, respectively).
Covariates
Given that sociodemographic characteristics, smoking, and alcohol consumption may relate to stress and sleep, age (years) [33], sex (male/female) [33–35], education level (university graduate and below/postgraduate) [36, 37], race/ethnicity (White/Asian/other) [37], body mass index (BMI) [38, 39], employment status (working/not working) [36, 37], school status (in school/not in school) [36, 37, 40], smoking (current/former vs. never) [39, 41], and alcohol consumption (abstainers/moderate/at-risk) [39, 42] assessed at baseline were included as covariates. Alcohol consumption was measured using the World Health Organization alcohol use identification test [43]. Questions 9 and 10 were removed to exclude probing potentially sensitive questions regarding harms caused by participants’ alcohol use. The first three items were used to classify participants as abstainers, moderate, or at-risk based on the National Institute on Alcohol Abuse and Alcoholism recommendations [44]. Daily covariates included study day and day of the week (weekend/weekday), given that individuals reported longer sleep duration, higher positive and lower negative affect during weekends as compared to weekdays [45, 46].
Analytic approach
Cross-lagged multilevel linear models were used to examine the bi-directional relationship between stress and sleep, which tested between- and within-person effects as well as fixed and random effects. All models were estimated using restricted maximum likelihood [47]. A homogenous, independent, residual covariance matrix was used given autoregressive effects were explicitly modeled by including lagged variables as described below. Previous study has shown the association between sleep and waking health behaviors (e.g. caffeine and alcohol consumption) was the strongest in weekly patterns compared to immediate influence [48]. Thus, this study validated the appropriate number of lags to be included in the models through step-wise addition of lags (e.g. first to fourth order lags stress and sleep variables) and compared the models through Bayesian information criterion (BIC). All analyses were run in R software (v.3.4.4) [49], using lme4 v1.1–13 (to estimate the models) [50] and lmerTest v2.0–33 (to estimate degrees of freedom and p-values) [51].
To provide a strong test of directionality, all relationships were tested prospectively and controlled for lagged outcomes (see Figure 2). Specifically, the first set of models tested daily evening stress as a predictor of both actigraphic and self-reported TST, SOL, WASO, and SE, while controlling for the previous night sleep outcomes. The second set of models tested actigraphic and self-reported TST, SOL, WASO, and SE as predictors of next-day stress (average from morning and afternoon surveys), while controlling for previous evening stress. All within-person variables were centered on the individual’s own average. Effect sizes were calculated as follows. For every model, marginal and conditional R2 values were calculated [52, 53]. The marginal R2 is the proportion of total variance explained by the fixed effects, while the conditional R2 is the proportion of total variance explained by both the fixed and random effects combined. For each predictor, nested models were run dropping predictors one at a time, and these were used to calculate the unique change in marginal and conditional R2 values attributable to each predictor, which were then used to calculate a Cohen’s f2 type effect size for mixed models as:
where R2AB is the marginal R2 from the full model, and R2AB is the marginal R2 from the restricted model dropping the relevant predictor.
For the exploratory analysis, the third set of models tested the interaction effects of baseline sleep complaint and daily sleep on next-day stress. Follow-up analyses for significant interactions were then examined using simple slopes tests for high (+1 SD from the mean) and low (−1 SD from the mean) sleep complaint [54].
Separate models were tested for each sleep parameter and type of sleep measure (i.e, actigraphic and self-reported). All covariates and between- and within-person predictors were included as fixed effects. Intercepts, lagged-dependent variables, and within-persons predictors were included as random effects. All repeatedly measured predictors were separated into between- and within-person levels of analyses. Between-person levels examined the differences between individuals (i.e. the participants’ own mean), whereas within-person levels tested deviations from the individual’s own average levels calculated across the 7 or 12 days. Alpha was set at .01 to reduce false discovery and control Type 1 errors. All dependent variables were examined for normality violations. Both actigraphic and self-reported SOL, WASO, and SE showed skew, and they were winsorized and all but actigraphic SE were square-root transformed for all subsequent analyses.
The number of daily observations varied from 2040 to 2610. Sample sizes and number of observations varied across the models due to missing data, type of sleep measures (i.e. actigraphic and self-reported), and the cross-lagged design. For instance, when testing stress predicting same night sleep, evening stress on the last study day (i.e. day 12) was excluded due to missing same-night sleep variable. Likewise, when testing sleep predicting next-day stress, reports of next-day stress (morning and afternoon stress on the first study day) were excluded due to missing previous day stress and prior night sleep.
Results
Descriptive results
Table 1 shows the descriptive sociodemographic profile of the participants from ACES and DESTRESS. The participants were mostly young adult females, with a majority of university students and of Asian descent. Overall, the sample was healthy, with most participants never having smoked, being moderate drinkers, and having an average BMI within the healthy range for adults.
Table 1.
Descriptive results for demographic variables by study
| Participant characteristic | ACES (N = 191) | DESTRESS (N = 135) | P-value |
|---|---|---|---|
| Age, M (SD) | 22.55 (4.13) | 24.76 (7.51) | <.001 |
| Body mass index (BMI), M (SD) | 22.30 (3.59) | 22.63 (3.51) | .40 |
| Female, N (%) | 127 (66.50) | 102 (75.60) | .86 |
| Race/ethnicity, N (%) | .16 | ||
| White/European | 44 (23.20) | 42 (31.10) | |
| Asian | 111 (58.40) | 65 (48.10) | |
| Others | 35 (18.40) | 28 (20.70) | |
| Level of education, N (%) | .02 | ||
| Undergraduate and below | 139 (72.80) | 82 (60.70) | |
| Postgraduate | 52 (27.20) | 53 (39.3) | |
| School status, N (%) | <.001 | ||
| In school | 175 (91.60) | 100 (76.30) | |
| Not in school | 16 (8.40) | 31 (23.70) | |
| Work status, N (%) | .08 | ||
| Working | 61 (31.90) | 55 (42.00) | |
| Not Working | 130 (68.10) | 76 (58.00) | |
| Smoking status, N (%) | .04 | ||
| Current | 2 (1.10) | 2 (1.50) | |
| Former | 5 (2.60) | 12 (8.90) | |
| Never | 183 (96.30) | 121 (89.60) | |
| Alcohol risk, N (%) | .05 | ||
| Abstainer | 35 (18.40) | 39 (29.10) | |
| Moderate | 128 (67.40) | 74 (55.20) | |
| At-risk | 27 (14.20) | 21 (15.70) |
Examining the daily study variables (Table 2), there were low missing daily surveys on average. Comparing across days and surveys, there were more missing evening surveys compared to mornings and afternoons (Figure S1). Rates of missing surveys were consistent across days except for Wednesday and Thursday evenings for ACES due to the lagged design.
Table 2.
Means and standard deviations for main variables by study
| Variables | ACES (N = 191) | DESTRESS (N = 135) | p-value |
|---|---|---|---|
| Stress | |||
| Morning | 2.08 (1.50) | 2.06 (1.65) | .91 |
| Afternoon | 2.47 (1.65) | 2.22 (1.54) | .17 |
| Evening | 2.32 (1.60) | 2.10 (1.61) | .22 |
| Actigraphic sleep | |||
| Total sleep time (hours) | 7.32 (0.97) | 7.33 (0.92) | .91 |
| Sleep onset latency (mins) | 7.14 (4.24) | 5.61 (4.66) | .01 |
| Wake after sleep onset (mins) | 57.55 (28.19) | 52.95 (24.18) | .22 |
| Sleep efficiency (%) | 87.27 (4.98) | 88.52 (4.45) | .90 |
| Time in bed (hours) | 8.40 (1.11) | 8.31 (1.03) | .56 |
| Self-reported sleep | |||
| Total sleep time (hours) | 7.86 (0.94) | 7.75 (1.14) | .34 |
| Sleep onset latency (mins) | 28.72 (35.36) | 27.68 (34.26) | .79 |
| Wake after sleep onset (mins) | 9.64 (12.14) | 9.84 (14.30) | .22 |
| Sleep efficiency (%) | 93.00 (0.05) | 93.00 (0.06) | .06 |
| Time in bed (hours) | 8.46 (1.15) | 8.32 (1.08) | .27 |
| Sleep Disturbance Index (T-score) | 46.89 (8.15) | 48.50 (6.10) | .05 |
| Proportion of Missing Daily Surveys | 0.08 (0.09) | 0.13 (0.13) | <.001 |
Note. The actigraphic and self-reported sleep efficiency, sleep onset latency, and wake after sleep onset presented are raw and untransformed values. p-Values are based on independent samples t-tests after first averaging values for each participant.
On average, participants reported relatively low stress levels throughout the mornings, afternoons, and evenings. On average, participants slept 7.32 and 7.80 h based on actigraphic and self-reported, respectively, both of which are within the recommended hours of sleep duration for adults [55]. Large discrepancies were observed between actigraphic and self-reported SOL and WASO. Nonetheless, both actigraphic and self-reported SE were above the 85% threshold, indicating that participants, on average, were sleeping well [56].
Evening stress predicting same-night sleep
All models with step-wise addition of evening stress lags were compared through BIC, and results showed that the first-order lag model was the most appropriate model with lowest BIC values. Table 3 shows the adjusted cross-lagged multilevel models of evening stress predicting same-night actigraphic and self-reported TST, SOL, WASO, and SE. Even after controlling for 11 covariates and lagged outcomes, within-person effects showed that one-unit higher evening stress (out of a 0 to 10-point scale) than average significantly predicted a −0.05 h (3-min) shorter actigraphic and self-reported TST. However, evening stress was not a significant predictor of actigraphic and self-reported SOL, WASO, and SE. No significant relations were found for between-person stress and actigraphic and self-reported sleep.
Table 3.
Multilevel modeling examining evening stress as a predictor of actigraphic and self-reported sleep (N = 326)
| TST | SOL | WASO | SE | |
|---|---|---|---|---|
| Between-person effects | ||||
| Actigraphic | −0.03, < .01 [−0.10, 0.05] | −0.01, < .01 [−0.09, 0.07] | −0.14, 0.01 [−0.29, 0.01] | 0.39, 0.01 [−0.02, 0.08] |
| Self-reported | −0.06, <.01 [−0.14, 0.02] | 0.07, <.01 [−0.09, 0.24] | −0.04, <.01 [−0.16, 0.07] | −0.27, <.01 [−1.00, 0.46] |
| Within-person effects | ||||
| Actigraphic | −0.05, 0.01* [−0.08, −0.01] | −0.01, 0.02 [−0.05, 0.02] | −0.02, 0.01 [−0.07, 0.03] | −0.02, 0.01 [−0.15, 0.11] |
| Self-reported | −0.05, 0.02 * [−0.08, −0.01] | 0.02, < .01 [-0.02, 0.06] | 0.01, 0.01 [−0.02, 0.06] | −0.22, 0.01 [−0.44, −0.01] |
Note. Results are unstandardized regression coefficients, Cohen’s f2, [95% confidence intervals]. * p < .01, ** p < .001. TST, Total Sleep Time; SOL, Sleep Onset Latency (square-root transformed); WASO, Wake After Sleep Onset (square-root transformed); SE, Sleep Efficiency (square-root transformed). Covariates were adjusted in all models including age, sex, body mass index, race, alcohol use, smoking status, education level, school status, employment status, day of week, and study days 1 to 12. The predictor for all models is within-person centered evening stress.
Sleep predicting next-day stress
All models with step-wise addition of sleep variable lags were compared through BIC, and results showed that the first-order lag was the most appropriate model with lowest BIC values. The adjusted models of actigraphic and self-reported TST, SOL, WASO, and SE predicting next-day stress are summarized in Table 4. At the between-person level, shorter self-reported TST significantly predicted higher next-day stress (b = −0.23). No significant relations were found between other actigraphic and self-reported sleep parameters and next-day stress. Similarly, at the within-person level, shorter actigraphic and self-reported TST predicted higher next-day stress (b = −0.13 and −0.15, respectively). Longer self-reported SOL and WASO (both b = 0.07) predicted higher stress the next day. Lower actigraphic and self-reported SE also predicted higher next-day stress (both b = −0.02). In additional exploratory analyses, a quadratic relationship between TST (actigraphic and self-reported) and next-day stress was tested to examine whether sleep duration demonstrated a J-shaped association with stress. All quadratic effects were not significant and are not reported. Further, sensitivity analyses revealed that within-person effects of the relations between self-reported WASO and next-day stress, as well as actigraphic SE and next-day stress, were significantly stronger during weekends compared to weekdays (both p < .01). All other stress–sleep relations were not significantly different during weekdays vs. weekends.
Table 4.
Multilevel modeling examining actigraphic and self-reported sleep as predictors of next-day stress (N = 326)
| TST | SOL | WASO | SE | |
|---|---|---|---|---|
| Between-person effects | ||||
| Actigraphic | −0.15, <.01 [−0.37, 0.07] | −0.08, < .01 [−0.30, 0.14] | −0.12, 0.01 [−0.23, 0.00] | 0.04, 0.01 [−0.01, 0.08] |
| Self-Reported | −0.23, 0.01* [−0.40, −0.06] | 0.04, <.01 [−0.04, 0.12] | −0.05, <.01 [−0.17, 0.08] | −0.01, <.01 [−0.03, 0.01] |
| Within-person effects | ||||
| Actigraphic | −0.13, 0.04** [−0.08, −0.01] | 0.04, 0.01 [0.00, 0.08] | 0.02, 0.01 [−0.01, 0.06] | −0.02, 0.02* [−0.04, −0.01] |
| Self-Reported | −0.15, 0.07** [−0.20, −0.10] | 0.07, 0.05** [0.03, 0.11] | 0.07, 0.01** [0.03, 0.10] | −0.02, 0.06** [−0.03, −0.02] |
Note. Results are unstandardized regression coefficients, Cohen’s f2, [95% confidence intervals]. Covariates were adjusted in all models including age, sex, body mass index, race, alcohol use, smoking status, education level, school status, employment status, day of week, and study days 1 to 12. The outcome in all models is next-day stress.
TST, total sleep time (square-root transformed); SOL, Sleep Onset Latency (square-root transformed); WASO, Wake After Sleep Onset (square-root transformed); SE, sleep efficiency (square-root transformed).
* p < .01, **p < .001.
Moderating role of baseline sleep complaint
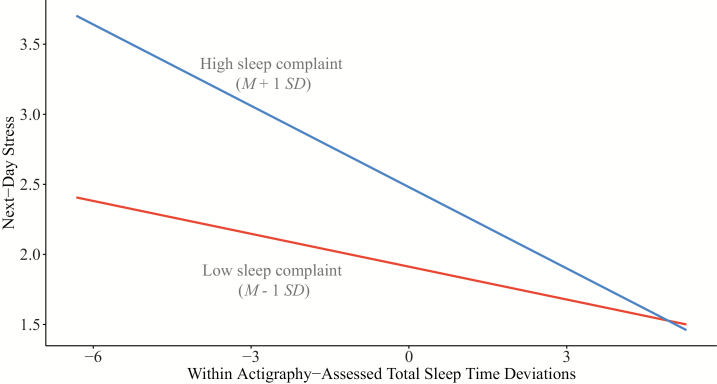
Overall, baseline sleep complaint did not significantly moderate the effects of either actigraphic or self-reported sleep on next-day stress. However, we did observe an interaction between baseline sleep complaint and actigraphic and self-reported TST on next-day stress in the hypothesized direction (both b = −0.01, p = .03, nonsignificant on a priori alpha of .01). Figure 3 shows the simple slopes of the relations between actigraphic TST and next-day stress at high and low baseline sleep complaint. For those with greater baseline sleep complaint, days with shorter-than-average actigraphic TST were associated with higher perceived stress (compared to those with lower levels of baseline sleep complaint).
Figure 3.
Simple slopes plot for next-day stress by within-person actigraphic total sleep time for high and low baseline sleep complaint.
Discussion
This study investigated the bi-directional, temporal associations between daily stress and sleep, with sleep measured using both actigraphy and self-report measures. We also explored the moderating role of baseline sleep complaint on the daily sleep–stress relationships. Findings showed a bi-directional relationship between sleep duration and stress: higher evening stress predicted shorter actigraphic and self-reported TST, whereas shorter actigraphic and self-reported TST predicted higher next-day stress. Data in this study supported poorer sleep continuity (i.e. longer self-reported SOL and WASO, as well as lower actigraphic and self-reported SE) as predictors of higher next-day stress, but evening stress did not predict same-night sleep continuity. No significant relations were found for the moderating role of baseline sleep complaint on the relations between sleep and next-day stress, although some trend in the hypothesized direction was noted.
Between- versus within-person effects
First and foremost, in this study, only shorter self-reported TST significantly predicted higher next-day stress on the average levels (i.e. between-person effects). The weak between-person stress–sleep relationship highlighted the importance of considering each individual’s daily within-person experience. These within- and between-person effects are entirely independent of each other, but each provides unique information. The lack of between-person effects suggests that those who typically experience higher stress compared to others do not necessarily have more disturbed sleep on average; and vice versa. The within-person effects discussed below should be interpreted, such that regardless of a person’s average sleep and stress levels, days with greater changes in sleep or stress were associated with greater changes in the other.
Daily evening stress predicting sleep
Comparing among the number of evening stress lags (i.e. first- to fourth order lags), results indicated that first-order evening stress was the best predictor of subsequent sleep. Individuals who experience higher than usual stress on a given evening had shorter sleep that night (both actigraphic and self-reported), even after controlling for covariates and previous night TST. These results support previous daily studies showing decreased objectively measured sleep duration following higher than usual stress days [20] and strengthened the temporal directionality between evening stress and sleep. In contrast to the study by Lee and colleagues in working adults [19], the current findings showed evening stress as a significant predictor of shorter self-reported TST. Differences between our results and Lee and colleagues’ could be due to the sampled population, such that working adults have fixed wake and sleep schedule and different types of stressors as compared to mostly university students in this study [19]. Further, in Lee and colleagues, participants reported their prior-night sleep on the following evening (vs. the following morning in this study), which may have influenced the accuracy of report [19].
The effects of stress on sleep could be explained through the framework of hyperactivation of the hypothalamic–pituitary–adrenal axis and presleep cognitive arousals. For example, previous research has demonstrated that elevated evening cortisol and flatter diurnal cortisol slopes are associated with shorter sleep duration [57–59]. Experiencing high psychological stress in the evening or near bedtime can cause spikes in evening cortisol, which are associated with physiological arousal that can impair sleep. Further, the experience of evening stress may cause emotional and cognitive arousal to affect sleep. Previous studies have demonstrated an association between preoccupation with stress at bedtime and subsequent poorer sleep, such as rumination and “not letting go of problems” [10, 11], and presleep arousal significantly mediated the stress–sleep relationship [15]. It is also possible that experiencing higher evening stress (e.g. before an upcoming examination) may require greater effort and time to manage or accommodate the increased demands (e.g. spending time resolving the issue), thus delaying bedtime and reducing sleep duration. Together, preoccupation with the stressor may amplify physiological activation, emotional reactivity, and presleep arousals, thus delaying sleep onset and reducing sleep duration.
Surprisingly, higher evening stress did not predict subsequent actigraphic and self-reported sleep continuity. These findings differed from previous cross-sectional [9] and daily-diary [19, 20] studies demonstrating the association between higher stress and subsequent poorer sleep quality or continuity. As the previous studies examined the stress–sleep relationship in working adults [9, 19] or adolescents [20], the types of stressor experienced may be different and may affect sleep quality or continuity differently compared to university students. As previously discussed, experiencing higher evening stress may require greater efforts to accommodate the increased demands and subsequently delay bedtime. For instance, a student who is stressed about completing assignments may stay up late resulting in a delayed bedtime and sleep deprivation. A delayed bedtime could result in a stronger sleep drive and reduced circadian alerting signal, thus leading to a shorter time to fall asleep and possibly a shorter sleep duration. Consistent with this interpretation, the findings showed that evening stress did indeed predict shorter TST. Another explanation may be that evening stress was reported on an average of 3 hours before bedtime, which may not capture the rumination of stressful experiences during or pre-bedtime that can prolong SOL.
Poor Sleep Predicting Next-Day Stress
Similar to the stress as predictor models, results showed that the first-order sleep lag was the best predictor of next-day stress. Findings showed a consistent relationship of shorter sleep duration and worse sleep continuity (actigraphic and self-reported) predicting next-day stress. These results are in accordance with previous daily studies using objective [20] and subjective measures of sleep [19] linking shorter TST with higher stress. In this study, self-reported longer SOL and WASO, as well as lower actigraphic and self-reported SE, predicted significantly higher stress the next day, extending the null findings from previous research [19].
One potential underlying mechanism is that discontinuous and short sleep may impair the emotional regulatory system, which is critical for regulating the negative emotional experiences caused by stressors. For instance, neuroimaging evidence shows decreased capacity in regulating negative emotional responses following sleep deprivation [60]. Specifically, there is significantly greater amygdala reactivity toward negative stimuli and lower functional connectivity between the amygdala and the medial prefrontal cortex (an area with projections to the amygdala which inhibits amygdala reactivity) in sleep-deprived individuals compared to controls [60]. Supporting this explanation, research has also shown that sleep-deprived individuals respond with greater psychological distress than well-rested individuals following exposure to minor (but not high-intensity) stressors [61]. This suggests that poor or discontinuous sleep may impair the emotional regulatory system and increase the likelihood of perceiving events or demands as stressful [61]. Hence, individuals with discontinuous and short sleep may react more strongly to daily stressors and perceive them as more severe.
Moderating role of sleep complaint
Results did not support the exploratory hypothesis that baseline levels of sleep complaint would moderate the sleep and next-day stress within-person relationships. However, we did observe a tendency for individuals with higher baseline sleep complaints to report higher stress the following day when they had shorter than usual TST (on both actigraphic and self-reported, both p = .03), compared to individuals with lower baseline sleep complaints. Although nonsignificant at the conservative alpha of .01, the results suggest that individuals with greater sleep complaints may be somewhat more vulnerable to the effects of short sleep on their stress levels following day. As the current sample consisted of relatively healthy individuals with low sleep complaints, these effects may be stronger in populations with higher sleep complaints and concerns (e.g. those with insomnia) and should be explored in future studies.
Limitations and strengths
Findings from this study should be interpreted considering several limitations. First, the nonsignificant findings could be partially due to a relatively low stress level in this sample, thus causing a floor effect. Second, like all other actigraphy-based studies, quiet wakefulness (i.e. lying on bed with eyes closed without activity) may be counted toward sleep, thus underestimating SOL and affect its associated results. Although partially addressed by including self-reported measures of sleep, self-reported SOL and TST are often overestimated [21]. Third, this study included mainly young, healthy university students with relatively low stress levels. Thus, these findings may not generalize to other individuals experiencing high stress (e.g. people with cancer), working adults with relatively fixed sleep and wake schedule, or individuals with more severe sleep problems (e.g. people with insomnia). Fourth, it is not possible to examine seasonal influence due to data collection was mostly carried out in one season: for ACES, most participants (85%) completed the study from April to October (mostly winter in Australia), while all participants completed the study in winter for DESTRESS. Finally, the lack of in-home electroencephalography sleep monitoring means that it is not possible to explore how stress and sleep architecture may be related on a daily basis. Future daily studies are needed that explicitly measure and compare sleep during restricted (e.g. semester term) and unrestricted (e.g. vacation; weekend), which may influence sleep/wake schedule and stress levels [62].
Despite these limitations, this study had notable strengths. The core strength of this study is the use of intensive, longitudinal daily design with EMA as well as both objective and subjective estimates of sleep, which extended findings from previous cross-sectional and daily studies. The use of repeated, real-time assessments maximize ecological validity and reduce recall biases [26]. Further, this study employed vigorous methodologies to test the temporal and bi-directional relations between stress and sleep by including lagged outcomes and separating between- and within-person effects in the analyses along with effect sizes, thus strengthening the confidence in the findings [14]. Together, this study provides one of the strongest tests of directionality and causality possible in observational designs, extending the current literature by demonstrating the bi-directionality between daily stress and nightly sleep in a large sample of young adults.
Conclusion
Using a daily repeated measures design with rigorous analytical methods, this study demonstrated a bi-directional relationship between stress and TST for both objective and subjective sleep measures. Although stress did not predict subsequent sleep continuity, the opposite direction emerged, such that longer SOL, WASO, and poorer SE predicted higher next-day stress. The weak between-person effects highlighted the importance of considering the daily variations of stress and sleep of each individual.
The magnitude of bi-directional effects between stress and sleep may be relatively small from one day to the next. However, the cumulative impact of potentially vicious cycles of high stress and short/poor sleep is not to be underestimated given the importance of both stress and sleep to physical and mental health [1–6]. On a positive note, the bi-directional links between lower daily stress with longer sleep duration are consistent with the notion of good sleep as a source of resilience and replenishment of energy and emotional regulation [19, 63, 64].
Considering these findings, behavioral interventions that can be embedded and applied in daily routines to either reduce modifiable stressors or improve sleep could help break the vicious cycles. Encouraging awareness of stress levels and sleep quantity/continuity in everyday life may help adopt timely countermeasures and coping, which may be especially helpful for individuals who often experience significant stressors or changes in sleep/wake routines.
Supplementary Material
Acknowledgments
We would like to acknowledge all the participants who volunteered their time and energy to make this possible.
Funding
D.C.S. and D.JT are supported by R01AI128359-01 from the National Institute of Health. BB is supported by National Health and Medical Research Council Health Professional Research Fellowship.
References
- 1. Richardson S, et al.. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110(12):1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cappuccio FP, et al.. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. [DOI] [PubMed] [Google Scholar]
- 3. Kendler KS, et al.. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60(8):789–796. [DOI] [PubMed] [Google Scholar]
- 4. Cappuccio FP, et al.. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly SJ, et al.. Stress and type 2 diabetes: a review of how stress contributes to the development of type 2 diabetes. Annu Rev Public Health. 2015;36:441–462. [DOI] [PubMed] [Google Scholar]
- 6. Benca RM, et al.. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–68; discussion 669. [DOI] [PubMed] [Google Scholar]
- 7. Lund HG, et al.. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46(2):124–132. [DOI] [PubMed] [Google Scholar]
- 8. Kashani M, et al.. Perceived stress correlates with disturbed sleep: a link connecting stress and cardiovascular disease. Stress. 2012;15(1):45–51. [DOI] [PubMed] [Google Scholar]
- 9. Buxton OM, et al.. Work-family conflict and employee sleep: evidence from IT workers in the work, family and health study. Sleep. 2016;39(10):1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akerstedt T, et al.. Impaired sleep after bedtime stress and worries. Biol Psychol. 2007;76(3):170–173. [DOI] [PubMed] [Google Scholar]
- 11. Hall M, et al.. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62(2):227–230. [DOI] [PubMed] [Google Scholar]
- 12. Akerstedt T, et al.. Sleep disturbances, work stress and work hours: a cross-sectional study. J Psychosom Res. 2002;53(3):741–748. [DOI] [PubMed] [Google Scholar]
- 13. Wiklund M, et al.. Subjective health complaints in older adolescents are related to perceived stress, anxiety and gender—a cross-sectional school study in Northern Sweden. BMC Public Health. 2012;12:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolger N, et al. Intensive longitudinal methods: An introduction to diary and experience sampling research. New York, NY: The Guilford Press; 2013. [Google Scholar]
- 15. Drake CL, et al.. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37(8):1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jansson M, et al.. Psychosocial work stressors in the development and maintenance of insomnia: a prospective study. J Occup Health Psychol. 2006;11(3):241–248. [DOI] [PubMed] [Google Scholar]
- 17. Åkerstedt T, et al.. Predicting sleep quality from stress and prior sleep—a study of day-to-day covariation across six weeks. Sleep Med. 2012;13(6):674–679. [DOI] [PubMed] [Google Scholar]
- 18. Hanson MD, et al.. Daily stress, cortisol, and sleep: the moderating role of childhood psychosocial environments. Health Psychol. 2010;29(4):394–402. [DOI] [PubMed] [Google Scholar]
- 19. Lee S, et al.. Daily antecedents and consequences of nightly sleep. J Sleep Res. 2017;26(4):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doane LD, et al.. Associations among sleep, daily experiences, and loneliness in adolescence: evidence of moderating and bidirectional pathways. J Adolesc. 2014;37(2):145–154. [DOI] [PubMed] [Google Scholar]
- 21. Silva GE, et al.. Relationship between reported and measured sleep times: the sleep heart health study (SHHS). J Clin Sleep Med. 2007;3(6):622–630. [PMC free article] [PubMed] [Google Scholar]
- 22. Mezick E. Sleep Continuity. In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. New York, NY: Springer New York; 2013:1805–1806. [Google Scholar]
- 23. Yang CM, et al.. The association of dysfunctional beliefs about sleep with vulnerability to stress-related sleep disturbance in young adults. Behav Sleep Med. 2011;9(2):86–91. [DOI] [PubMed] [Google Scholar]
- 24. Morin CM, et al.. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8(3):463–467. [DOI] [PubMed] [Google Scholar]
- 25. Faul F, et al.. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 26. Shiffman S, et al.. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 27. Quante M, et al.. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018;10:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole RJ, et al.. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. [DOI] [PubMed] [Google Scholar]
- 29. Carney CE, et al.. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Almeida DM, et al.. The daily inventory of stressful events: an interview-based approach for measuring daily stressors. Assessment. 2002;9(1):41–55. [DOI] [PubMed] [Google Scholar]
- 31. Koffer RE, et al.. Stressor diversity: Introduction and empirical integration into the daily stress model. Psychol Aging. 2016;31(4):301–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cella D, et al.; PROMIS Cooperative Group The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reyner LA, et al.. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep. 1995;18(2):127–134. [PubMed] [Google Scholar]
- 34. Chaplin TM, et al.. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32(7):1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fatima Y, et al.. Exploring Gender Difference in Sleep Quality of Young Adults: Findings from a Large Population Study. Clin Med Res. 2016;14(3-4):138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arber S, et al.. Gender and socio-economic patterning of self-reported sleep problems in Britain. Soc Sci Med. 2009;68(2):281–289. [DOI] [PubMed] [Google Scholar]
- 37. Grandner MA, et al.. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harding JL, et al.. Psychosocial stress is positively associated with body mass index gain over 5 years: evidence from the longitudinal AusDiab study. Obesity (Silver Spring). 2014;22(1):277–286. [DOI] [PubMed] [Google Scholar]
- 39. Strine TW, et al.. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Med. 2005;6(1):23–27. [DOI] [PubMed] [Google Scholar]
- 40. Lemma S, et al.. Sleep quality and its psychological correlates among university students in Ethiopia: a cross-sectional study. BMC Psychiatry. 2012;12:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naquin MR, et al.. College students’ smoking behavior, perceived stress, and coping styles. J Drug Educ. 1996;26(4):367–376. [DOI] [PubMed] [Google Scholar]
- 42. Ebrahim IO, et al.. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37(4):539–549. [DOI] [PubMed] [Google Scholar]
- 43. Saunders JB, et al.. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 44. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. Bethesda, MD: National Institutes of Health Publication; 2005. [Google Scholar]
- 45. Ryan RM, et al. Weekends, work, and well-being: Psychological need satisfactions and day of the week effects on mood, vitality, and physical symptoms. J Soc Clin Psychol. 2010;29(1):95–122. [Google Scholar]
- 46. Lee SY, et al.. Stress and sleep disturbances in female college students. Am J Health Behav. 2013;37(6):851–858. [DOI] [PubMed] [Google Scholar]
- 47. Singer JD, et al. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford university press; 2003. [Google Scholar]
- 48. Irish LA, et al.. A 24-hour approach to the study of health behaviors: temporal relationships between waking health behaviors and sleep. Ann Behav Med. 2014;47(2):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2013. [Google Scholar]
- 50. Bates D, et al. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 51. Kuznetsova A, et al. lmerTest Package: tests in linear mixed effects models. Journal of Statistical Software. 2017. 2017;82(13):26. [Google Scholar]
- 52. Johnson PC. Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol Evol. 2014;5(9):944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakagawa S, et al. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–142. [Google Scholar]
- 54. Aiken LS, et al. Multiple regression: Testing and interpreting interactions. Newbury Park: Sage; 1991. [Google Scholar]
- 55. Watson NF, et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep. 2015;38(6):843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perlis ML, et al. Cognitive behavioral treatment of insomnia: A session-by-session guide. New York, NY: Springer; 2005. [Google Scholar]
- 57. Zeiders KH, et al.. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescence. J Adolesc Health. 2011;48(6):566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kumari M, et al.. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94(12):4801–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Castro-Diehl C, et al.. Association of Sleep Duration and Quality With Alterations in the Hypothalamic-Pituitary Adrenocortical Axis: The Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Endocrinol Metab. 2015;100(8):3149–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoo SS, et al.. The human emotional brain without sleep–a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–R878. [DOI] [PubMed] [Google Scholar]
- 61. Minkel JD, et al.. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12(5):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bei B, et al.. Actigraphy-assessed sleep during school and vacation periods: a naturalistic study of restricted and extended sleep opportunities in adolescents. J Sleep Res. 2014;23(1):107–117. [DOI] [PubMed] [Google Scholar]
- 63. Scharf MT, et al.. The energy hypothesis of sleep revisited. Prog Neurobiol. 2008;86(3):264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldstein AN, et al.. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10:679–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.