Abstract
Study Objectives
Neurocognitive impairment is one of the daytime symptoms of obstructive sleep apnea (OSA). We proposed to use tract-specific statistical analysis (TSSA) to investigate whether there are fiber tract abnormalities in OSA, which may be undiscovered using voxel-based approaches, and whether such tract-specific disruptions in brain connectivity are associated with neuropsychological deficits in patients with untreated OSA.
Methods
We enrolled 38 patients with OSA diagnosed by overnight polysomnography, and 41 healthy sleepers. Fractional anisotropy (FA) and mean diffusivity (MD) maps were obtained from whole-brain diffusion tensor imaging, and TSSA were used to assess regional deficits of white matter tracts. All participants underwent a battery of neuropsychological tests. To evaluate the association between FA values and clinical, polysomnographic, and neuropsychological parameters in the OSA group, permutation-based tests for correlation were performed preceding cluster-based statistics.
Results
Compared to healthy controls, patients with OSA showed decreased values of FA in the left and right anterior thalamic radiations, and right uncinate fasciculus (UNC) (p < 0.001, p = 0.005, and p = 0.008, respectively). A lower score of digit span backward was associated with lower FA values of right UNC in the OSA group (p = 0.023). The Rey Complex Figure Test copy score revealed a positive correlation with FA values in the right UNC (p = 0.010).
Conclusions
The TSSA method indeed identified previously unrevealed tract-specific disruptions in OSA. Furthermore, reduced FA values in the frontal lobe portion of the right UNC which has been known to be involved in working memory function were significantly associated with lower cognitive performance in patients with untreated OSA.
Keywords: obstructive sleep apnea; white matter, tractography, cognitive function
Statement of Significance.
Voxel-based analyses have discovered only subtle and non-tract specific changes in white matter fiber integrity due to obstructive sleep apnea (OSA). Our novel tract-specific statistical analysis (TSSA) method enabled us to find the specific portions and direction of disconnected fibers. Untreated OSA revealed impaired fiber integrity in bilateral anterior thalamic radiations and right uncinate fasciculus (UNC) compared to healthy controls. Interestingly, fractional anisotropy values of the right UNC revealed a significant association with performance of attention and working memory and visuospatial memory in patients with OSA. Our results of the TSSA method could provide evidence of disrupted integrity of white matter tracts on the neurocognitive pathomechanism of OSA.
Introduction
Obstructive sleep apnea (OSA) is one of the most prevalent sleep disorders, characterized by repetitive interruption of breathing during sleep due to complete or partial airway obstruction. Repetitive apneas and hypopneas cause intermittent hypoxemia, hypercapnia, microarousals, and fragmented sleep, resulting in sleep disruption and changes in neural activities [1]. OSA is associated with medical, neurocognitive, cardiovascular morbidity, and mortality. Patients with OSA have shown neurocognitive deficits in attention, episodic memory, and executive function, which were partially remediated by treatment [2, 3]. The pathophysiological mechanism of the neurocognitive deficits remains unclear, although the main contributors might be sleep fragmentation and intermittent nocturnal hypoxemia during recurrent episodes of OSA [4], which may consequently yield aberrations in integrity of brain structures and connectivity related to cognition.
High-resolution magnetic resonance imaging (MRI) has been a useful tool which provides quantification of brain structural changes in relation to OSA. In particular, analyses of diffusion tensor imaging (DTI) parameters including fractional anisotropy (FA), and mean diffusivity (MD) allow for the assessment of white matter fiber integrity and myelination. Previous DTI studies have described subtle structural white matter alterations in OSA patients [5–7]. A voxel-wise study of 41 OSA patients found decreased FA in peripheral white matter regions underlying the cortex as well as in focal cortical areas when compared to controls [6]. Other studies showed reduced brain MD values in newly diagnosed OSA patients [7], and increased radial diffusivity with no MD changes in severe OSA [5], suggesting acute and chronic inflammatory processes possibly due to apnea-related intermittent hypoxia [8]. However, whether these white matter structural impairments are associated with disease severity of OSA (i.e., manifestation on polysomnography or on neuropsychological test) has remained unclear. Furthermore, the voxel-wise approaches used in the aforementioned studies may lack the specificity of which white matter fiber tract is impaired, which is crucial to understanding the possible disruption in the connectivity between two specific brain cortical/subcortical gray matter regions.
Tract-based spatial statistics (TBSS) performed on DTI allow for the investigation of tract-specific microstructural alterations. In this method, white matter alterations are localized on a specific white matter fiber tract by projecting diffusion parameters onto a white matter skeleton [9]. However, this projection may generate convoluted diffusion parameters which are computed from multiple adjacent fibers oriented differently [10]. The tract-specific statistical analysis (TSSA), a method which our group developed and showed its clinical utility as it identified disrupted white matter integrity in patients with memory impairment [11], has improved the mapping of tract diffusion coefficients along the corresponding major tracts. In the current study, we aimed to investigate the tract-specific abnormalities in patients with untreated OSA using the TSSA method. We then assessed whether such abnormalities correlated with neuropsychological deficits in OSA.
Methods
Subjects
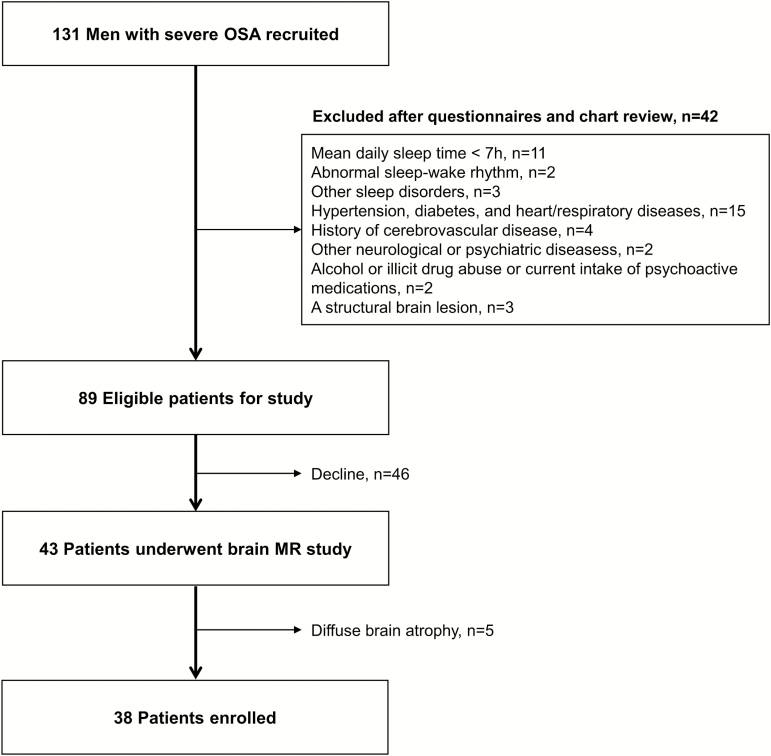
A total of 131 men with severe OSA (apnea–hypopnea index (AHI) greater than 30/h) were recruited from the sleep clinic of Samsung Medical Center, Seoul, South Korea. Each subject had a detailed clinical interview, sleep questionnaire, and overnight polysomnography. Forty-two patients were excluded according to the following criteria (Figure 1): (1) mean daily sleep time more than 7 h, (2) abnormal sleep–wake rhythm, (3) other sleep disorders, (4) hypertension, diabetes, and heart and respiratory diseases, (5) history of cerebrovascular disease, (6) other neurological (neurodegenerative diseases, epilepsy, head injury) or psychiatric diseases (psychosis, current depression), (7) alcohol or illicit drug abuse or current intake of psychoactive medications, and (8) a structural lesion on previous brain MRI. Forty-three patients underwent MR scans with a study protocol including T1-weighted, T2-weighted, and FLAIR images. Five patients who showed diffuse brain atrophy on brain MRI were excluded from the OSA group. Forty-one male good sleepers were recruited using an advertisement in a local community. Participants, if they exhibited evidence of OSA (AHI greater than 5/h) or evidence for other sleep disorders such as periodic limb movement disorders, were excluded from the control group. Even though patients and healthy controls were mostly middle-aged men, the control group was significantly younger than the OSA patients (p < 0.001). Age was thus considered as a covariate in our statistical analyses (details found below). Finally, 38 patients with OSA and 41 controls were included in our study. Written informed consent was obtained from all participants, and the Institutional Review Board of Samsung Seoul Hospital authorized the study protocol and design.
Figure 1.
Study algorithm of the patient selection.
Overnight polysomnography
Polysomnography was recorded with Somnologica (Embla, Denver, CO) using a six-channel electroencephalogram, a four-channel electrooculogram, electromyogram, and electrocardiogram. A thermistor, a nasal air pressure monitoring sensor, an oximeter, piezoelectric bands, and a body position sensor were also applied to the participants. Apnea was defined as a reduction in airflow by 90% or more lasting at least 10 s. Hypopnea was defined as a reduction in airflow by 30% or more lasting at least 10 s and accompanied by at least 4% oxygen desaturation compared to baseline [12].
Neuropsychological assessments
OSA and control groups underwent a battery (2.5 h) of neuropsychological tests and an individual standardized intelligence test. Neuropsychological tests consisted of the Korean California Verbal Test and the Rey Complex Figure Test for memory function; digit span tests from the Wechsler Memory Scale-Revised, the Corsi block tapping tests (forward and backward), the Trail Making Tests A and B, the digit symbol test for attention and working memory; the Stroop test and the Controlled Oral Word Association Test for executive function; and the Korean Boston naming test for verbal function. Composite scores and detailed information regarding these tests were described in a previous article [13].
Acquisition of MR images
T1 and diffusion-weighted images were acquired from all 38 patients and 41 healthy controls using the same 3.0 Tesla MRI scanner (Philips 3.0 T Achieva) within 1 week after the baseline visit. T1-weighted images were obtained using the following scanning variables: 1 mm sagittal slice thickness, overcontiguous slices with 50% overlap, no gap, repetition time (TR) of 9.9 ms, echo time (TE) of 4.6 ms, flip angle of 8° and matrix size of 240 × 240 pixels. Images were reconstructed to 480 × 480 over a 240 mm field of view. In the whole-brain diffusion-weighted MRI examination, sets of axial diffusion-weighted single-shot echo-planar images were collected with the following parameters: 128 × 128 acquisition matrix, 1.72 × 1.72 × 2 mm3 voxels reconstructed to 1.72 × 1.72 × 2 mm3, 70 axial slices, 220 × 220 mm2 field of view, TE 60 ms, TR 7383 ms, flip angle 90°, slice gap 0 mm and b-factor of 600 s mm−2. For the baseline image without diffusion weighting (the reference volume), diffusion-weighted images were acquired from 45 different directions. All axial sections were acquired parallel to the anterior commissure–posterior commissure line.
Tract-specific statistical analysis
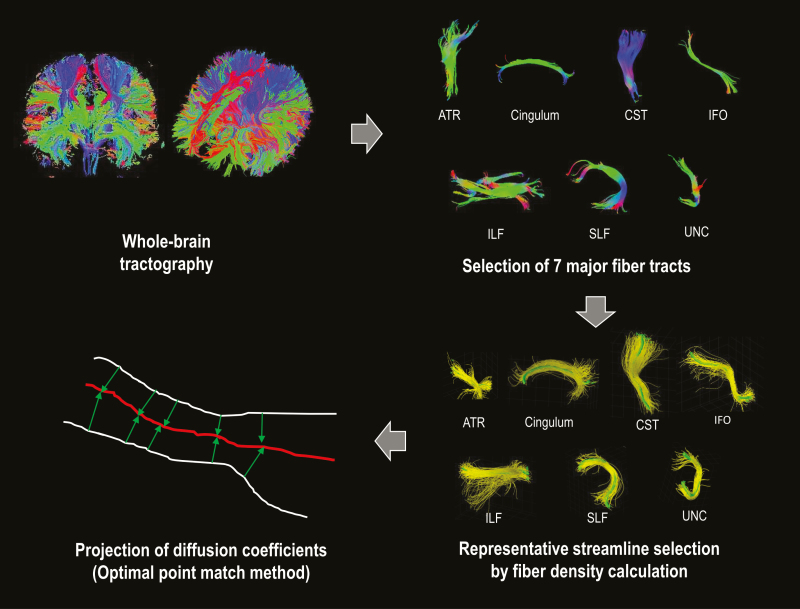
The TSSA includes three steps as follows: (1) identifying the major anatomic bundles from diffusion coefficients along the tractography-derived fiber bundles, (2) calculating Tract Profile, and (3) performing statistical analysis. The schematic process of the TSSA method was introduced in the previous report of Jung et al [11]. In the current study, we applied deterministic tractography with DTI reconstruction using the Diffusion toolkit [14].
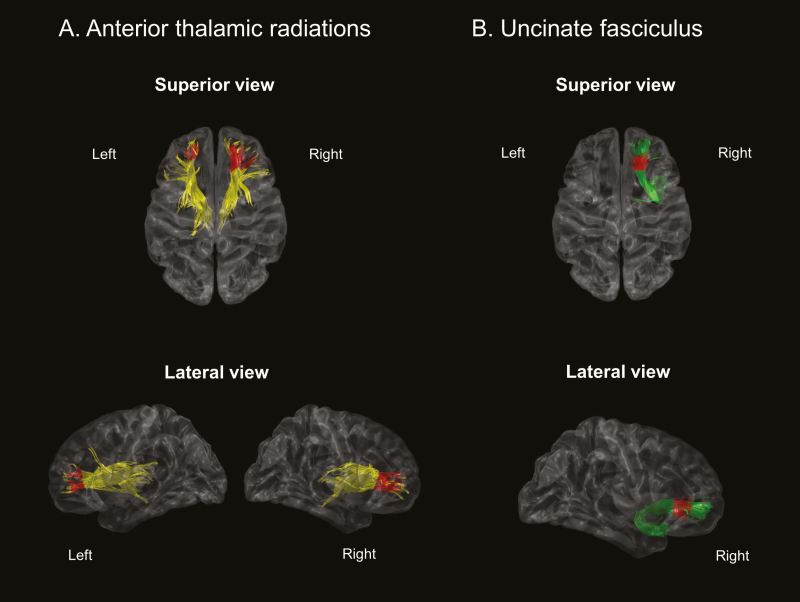
In the first step, the major anatomical tracts were identified in each subject’s brain from the DWI dataset. Fiber tracts were estimated with Diffusion toolkit using deterministic tractography based on Fiber Assignment by Continuous Tracking (FACT) [15]. FA and MD maps were also obtained using Diffusion toolkit. Next, the immense number of fiber tracts were automatically classified and labeled with seven major anatomic bundles according to their shape and position [16]. These seven tracts included anterior thalamic radiation (ATR) connecting the anterior and midline nuclear group of the thalamus with the frontal lobe, cingulum (CG) projecting from the cingulate gyrus to the entorhinal cortex in the brain, corticospinal tract (CST), a white matter motor pathway, inferior fronto-occipital fasciculus (IFO) connecting the occipital cortex to the frontal lobe, inferior-longitudinal fasciculus (ILF) connecting primarily the occipital cortex with the temporal lobe, superior-longitudinal fasciculus (SLF) passing from the frontal lobe through the operculum to the occipital lobe and radiating the projection to the temporal lobe, and uncinate fasciculus (UNC) connecting parts of the limbic system such as the hippocampus and amygdala in the temporal lobe with frontal cortices such as the orbitofrontal cortex (Figure 2).
Figure 2.
White matter tract-based approach of the tract-specific statistical analysis (TSSA). ATR, anterior thalamic radiation; CST, corticospinal tract; IFO, inferior fronto-occipital fasciculus; ILF, inferior-longitudinal fasciculus; SLF, superior-longitudinal fasciculus; UNC, uncinate fasciculus.
The second step was Tract Diffusion Profiling, which maps the diffusion measurements such as FA and MD onto each major tract [17]. After the major tract bundles were refined by removing the streamlines that are not part of the major fiber tracts, a representative tract was selected for use for the fiber density map [11]. Then a group-wise representative tract is further selected by accommodating more than 60% of subjects. For each representative tract, corresponding points across subjects were determined using the optimal point matching algorithm [10] that shows the robustness to spatial distortion and variability of diffusion values. Diffusion data for all fiber tracts were projected to the representative tract based on a weighted-averaging in which the Mahalanobis distance from the corresponding tract was used to weigh the given fiber tract.
In the last step, the statistical analysis of diffusion properties and clinical data was performed. To evaluate group difference of diffusion parameters along the tract between the OSA and control groups, permutation-based ANCOVA, along with controlling for age and body mass index (BMI), was applied with 10,000 permutations. Since FA or MD values in the Tract Profile were sampled at equidistant locations along the group-wise representative tract, a cluster-based statistics method was used for multiple comparisons correction [18, 19].
To estimate the association between FA values and clinical, polysomnographic, and neuropsychological parameters in the OSA group, permutation-based tests for correlation were performed with cluster-based statistics. First, partial correlation coefficients were calculated for FA values at each location along the representative tract with various parameters controlling for age and BMI as covariates. Then, the significance level of the correlation coefficient was adjusted by permutation testing, assuming a null distribution of the maximum cluster extent [20]. The permutation-based approach we employed in the current paper is proper for the analysis of a data distribution that is unknown or does not follow the normal distribution, which is the case in numerous neuroimaging studies like ours [18, 21, 22]. In addition, permutation testing, a nonparametric approach, is appropriate for relatively small sample studies in contrast to the classic tests [22, 23]. The validity of permutation-based cluster analysis for correlation with various scores was described in detail in the paper of Han et al [20]. The TSSA method was performed using a combination of the Diffusion toolkit, FMRIB Software Library (FSL), MATLAB, and our own code.
Results
Clinical and polysomnographic characteristics
Detailed clinical characteristics and polysomnographic findings between the two groups were summarized in Table 1.
Table 1.
Clinical and polysomnographic characteristics between OSA and control groups.
| OSA group | Control group | P | |
|---|---|---|---|
| Clinical factors | |||
| Subjects, No. | 38 | 41 | |
| Age, years, mean (SD) | 45.0 (6.6) | 37.2 (10.7) | 0.001 |
| Men, No. (%) | 38 (100) | 41 (100) | – |
| BMI, kg/m2, mean (SD) | 28.3 (4.0) | 24.0 (2.1) | <0.001 |
| ESS score, mean (SD) | 11.0 (4.5) | 4.5 (2.7) | <0.001 |
| BDI score, mean (SD) | 5.9 (6.3) | 2.9 (3.3) | 0.019 |
| BAI score, mean (SD) | 5.5 (5.7) | 4.3 (3.1) | 0.864 |
| Polysomnographic parameters | |||
| Time in bed, min, mean (SD) | 393.2 (67.8) | 425.5 (34.8) | 0.035 |
| Total sleep time, min, mean (SD) | 327.9 (65.9) | 383.5 (33.3) | <0.001 |
| Sleep latency, min, mean (SD) | 8.3 (10.2) | 8.5 (7.4) | 0.462 |
| REM sleep latency, min, mean (SD) | 89.4 (52.0) | 79.4 (31.5) | 0.471 |
| Sleep efficiency, %, mean (SD) | 81.5 (15.2) | 90.2 (3.7) | <0.001 |
| Arousal index, events/h, mean (SD) | 50.0 (20.0) | 12.8 (3.2) | <0.001 |
| WASO, min | 57.6 (38.0) | 40.6 (26.3) | 0.008 |
| N1 sleep, %, mean (SD) | 37.6 (16.2) | 12.4 (4.7) | <0.001 |
| N2 sleep, %, mean (SD) | 41.5 (12.8) | 53.2 (6.0) | <0.001 |
| N3 sleep, %, mean (SD) | 3.1 (4.8) | 10.7 (3.6) | <0.001 |
| REM sleep, %, mean (SD) | 17.7 (6.6) | 23.8 (5.4) | <0.001 |
| AHI, events/h, mean (SD) | 56.8 (26.2) | 2.3 (2.2) | <0.001 |
| PLM index, events/h, mean (SD) | 3.7 (5.7) | 2.7 (4.8) | 0.818 |
| Movement arousal index, events/h, mean (SD) | 0.3 (0.8) | 0.8 (1.2) | 0.007 |
| Lowest saturation of oxygen, % | 75.3 (9.4) | 92.7 (2.7) | <0.001 |
| Oxygen desaturation index, events/h, mean (SD) | 50.2 (26.8) | 0.4 (0.9) | <0.001 |
OSA, obstructive sleep apnea; SD, standard deviation; BMI, body mass index; ESS, Epworth sleepiness scale; BDI, Beck depression inventory; BAI, Beck anxiety inventory; WASO, wakefulness after sleep onset; AHI, apnea–hypopnea index; PLM, periodic limb movement. Bold values denote statistical significance at the p < 0.05 level.
Comparison of TSSA results between the OSA and control groups
Compared to controls, the OSA group showed significantly reduced FA values in bilateral ATR and right UNC between the two groups (Figure 3). Such decreased FA values in the left ATR and right UNC of OSA were exhibited in the anterior portions of each tract (occupying 20% of tract length; p < 0.001 and p = 0.008, respectively). Distribution of FA values in the right UNC were presented in Supplementary Figure S1. The overall standard deviation of controls and patients did not differ (F-test, p > 0.13). Supplementary Figure S1 also illustrates that the two groups have a similar pattern of decreases and increases in FA at equivalent locations along the tract. Therefore, the lack of association between FA and the cognitive test is not caused by a limited range of FA values in the control group.
Figure 3.
TSSA results presented different FA values between OSA and control groups for (A) anterior thalamic radiation and (B) uncinate fasciculus tracts. Red colors indicate the portions of fiber tracts where FA values significantly reduced in OSA group compared to controls (p < 0.05). FA, fractional anisotropy; OSA, obstructive sleep apnea.
The lower FA values in right ATR were present at its anterior portion and occupied 40% of the tract length (p = 0.005). In addition, the MD values were significantly increased in the inferior portion of left UNC (20% of tract length; p = 0.009). The results of group comparison for diffusion coefficients in major white matter tracts were presented in Table 2.
Table 2.
Group comparison of diffusion coefficients in major white matter tracts
| Major tract | OSA mMean FA (SD) | Control mean FA (SD) | Tract-wise corrected P* | OSA mean MD (×10−3 mm2/s) | Control mean MD (×10−3 mm2/s) | Tract-wise corrected P* |
|---|---|---|---|---|---|---|
| LH ATR | 0.248 (0.1506) | 0.271 (0.1476) | 0.0096 | 0.812 | 0.807 | 0.5197 |
| LH CST | 0.4478 (0.1567) | 0.423 (0.1478) | 0.5190 | 0.629 | 0.594 | – |
| LH CG | 0.2788 (0.1722) | 0.271 (0.1624) | 0.0859 | 0.658 | 0.631 | 0.6016 |
| LH IFO | 0.2420 (0.1159) | 0.251 (0.1220) | 0.4252 | 0.6510 | 0.621 | 0.4051 |
| LH ILF | 0.2392 (0.1027) | 0.246 (0.1022) | 0.1136 | 0.574 | 0.583 | 0.3701 |
| LH SLF | 0.3326 (0.1582) | 0.354 (0.1469) | 0.2698 | 0.606 | 0.568 | 0.2522 |
| LH UNC | 0.2098 (0.1120) | 0.2083 (0.1014) | 0.6680 | 0.750 | 0.710 | 0.0085 |
| RH ATR | 0.3133 (0.1768) | 0.3195 (0.1411) | 0.0047 | 0.579 | 0.553 | 0.0924 |
| RH CST | 0.3739 (0.1872) | 0.3772 (0.1818) | 0.0837 | 0.684 | 0.667 | 0.4474 |
| RH CG | 0.2506 (0.1228) | 0.2468 (0.1220) | 0.4067 | 0.554 | 0.536 | 0.1514 |
| RH IFO | 0.2455 (0.1514) | 0.2590 (0.1552) | 0.1042 | 0.656 | 0.626 | 0.6467 |
| RH ILF | 0.3012 (0.1379) | 0.3099 (0.1559) | 0.1940 | 0.594 | 0.560 | 0.2790 |
| RH SLF | 0.2917 (0.1526) | 0.2916 (0.1527) | 0.3353 | 0.570 | 0.539 | 0.5092 |
| RH UNC | 0.1813 (0.1052) | 0.1897 (0.1149) | 0.0083 | 0.758 | 0.719 | 0.6300 |
OSA, obstructive sleep apnea; FA, fractional anisotropy; SD, standard deviation; MD, mean diffusivity; LH, left hemisphere; RH, right hemisphere; ATR, anterior thalamic radiation; CST, corticospinal tract; CG, cingulum; IFO, inferior fronto-occipital fasciculus; ILF, inferior-longitudinal fasciculus; SLF, superior-longitudinal fasciculus; UNC, uncinate fasciculus. Bold values denote statistical significance at the p < 0.05 level.
*The lowest p-value from the cluster-based statistics for each fiber tract.
Further analyses showed that the FA and MD values in OSA in the hemisphere opposite to the tracts where we found significantly lower FA and higher MD in OSA presented the same trend. However, this pattern was not significant after the multiple comparison correction (p ≈ 0.1). Comparing the hemispheric asymmetry of FA and MD between OSA patients and controls, we found that there was no significant deviation of either FA or MD asymmetry for OSA patients from healthy controls (p > 0.1).
Comparison of neuropsychological tests
Patients with OSA showed a significantly slower speed of information processing composite score than controls (p = 0.008). Verbal and visual memory composite scores were also lower in the OSA group (p = 0.003 and p = 0.04, respectively). There were no differences in other composite scores found between the two groups. The details of the neuropsychological tests are summarized in Table 3.
Table 3.
Comparison of neuropsychological tests scores between OSA patients and healthy controls
| OSA group | Control group | P | |
|---|---|---|---|
| Attention and executive functioning composite score, mean (SD) | −0.08 (0.47) | 0.08 (0.41) | 0.071 |
| Digit span, forward, mean (SD) | 8.4 (2.2) | 9.6 (1.9) | 0.017 |
| Digit span, backward, mean (SD) | 7.4 (1.9) | 8.1 (2.0) | 0.128 |
| Corsi block, forward, mean (SD) | 8.4 (2.0) | 10.7 (9.9) | 0.071 |
| Corsi block, backward, mean (SD) | 8.3 (1.6) | 8.6 (1.4) | 0.336 |
| Trail-making test A, mean (SD) | 40.7 (12.7) | 32.7 (10.9) | 0.007 |
| Trail-making test B, mean (SD) | 102.2 (45.3) | 83.1 (31.9) | 0.045 |
| Stroop color test, mean (SD) | 104.4 (13.3) | 104.2 (11.6) | 0.793 |
| Information processing composite score, mean (SD) | −0.29 (0.73) | 0.27 (1.15) | 0.008 |
| Digit symbol test, mean (SD) | 56.7 (10.4) | 64.7 (16.4) | 0.008 |
| Verbal memory composite score, mean (SD) | −0.25 (0.75) | 0.23 (0.86) | 0.003 |
| Korean California Verbal Test | |||
| Total, mean (SD) | 49.6 (11.2) | 58.1 (9.2) | <0.001 |
| Short delay free recall, mean (SD) | 11.1 (2.7) | 12.7 (2.8) | 0.006 |
| Long delay free recall, mean (SD) | 11.7 (2.6) | 13.2 (2.4) | 0.001 |
| Recognition, mean (SD) | 14.6 (1.6) | 15.0 (1.3) | 0.374 |
| Visual memory composite score, mean (SD) | −0.17 (0.73) | 0.15 (0.84) | 0.039 |
| Rey Complex Figure Test | |||
| Copy, mean (SD) | 34.6 (2.2) | 35.2 (1.1) | 0.415 |
| Immediate recall, mean (SD) | 23.1 (6.5) | 24.4 (7.2) | 0.400 |
| Delayed recall, mean (SD) | 22.9 (6.1) | 25.0 (6.3) | 0.161 |
| Recognition, mean (SD) | 20.1 (1.9) | 20.9 (2.1) | 0.071 |
| Verbal fluency composite score, mean (SD) | −0.09 (0.78) | 0.08 (0.60) | 0.216 |
| Controlled Oral Word Association Test | |||
| Phonetic word fluency, mean (SD) | 31.6 (13.9) | 36.6 (13.2) | 0.076 |
| Semantic word fluency, mean (SD) | 35.3 (8.8) | 34.2 (6.9) | 0.888 |
OSA, obstructive sleep apnea; SD, standard deviation. Bold values denote statistical significance at the p < 0.05 level.
TSSA results associated with clinical, polysomnographic, and cognitive parameters
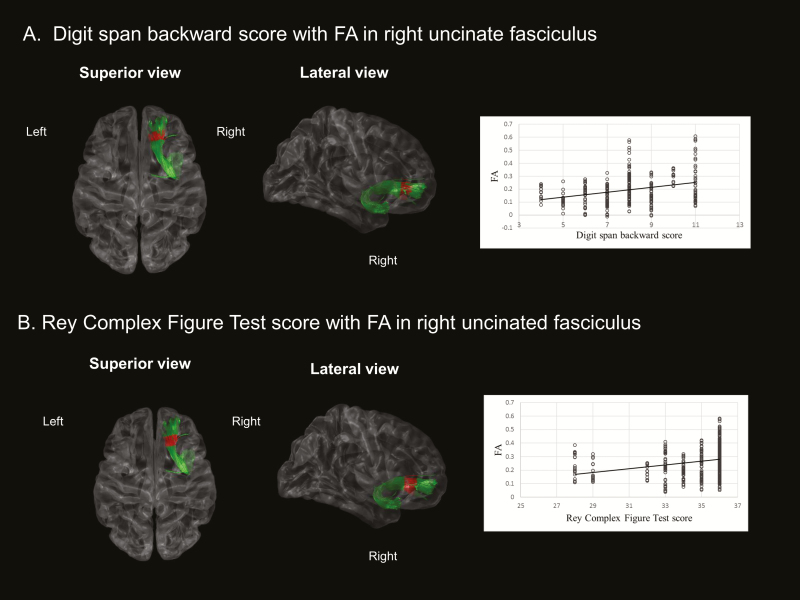
Correlations of FA/MD values with clinical/polysomnographic indices were not significant (p > 0.05). For the relationship between tract specific variables and neuropsychological parameters, the OSA group showed that FA values in the anterior portion of the right UNC were significantly associated with digit span backward (p = 0.02). The scores of the Rey Complex Figure Test copy were positively correlated with FA values in the anterior portion of the right UNC (p = 0.01). Using general linear models where we included one of the OSA severity variables (i.e., AHI, AI, the lowest O2 saturation (%)), and one of the cognitive scores (i.e., digit span backward, Rey Complex Figure Test copy) as independent variables and FA measurement as the dependent variable, we then analyzed an interaction term of OSA severity × cognitive score to assess the influence of OSA severity on the association between FA and a cognitive outcome. In the anterior portion of the right UNC where we found a correlation between FA and cognitive scores, the interaction analysis showed that the lowest level of O2 saturation significantly enhanced the association between the FA value and performance in Digit Span Backward and Rey Complex Figure tests (p < 0.02). In a partial correlation adjusting for age, BMI, and AHI, the FA values were still significantly correlated with digit span backward, but not with the scores of the Rey Complex Figure Test copy. The control group showed no significant correlation between FA values and neuropsychological measures. MD values in the left UNC showed no significant association with clinical, polysomnographic, and neuropsychological variables. The neuropsychological composite scores were not significantly correlated with FA/MD values in patients with OSA. The significant results of the correlation analyses were represented after controlling for age and BMI in Figure 4.
Figure 4.
Correlation analyses with adjusting age and BMI between FA values and neuropsychological parameters in OSA group. (A) Reduced FA values in right uncinate fasciculus were significantly correlated with impaired performance of digit span backward, and (B) Low FA values in right uncinate fasciculus were associated with impaired performance of Rey Complex Figure Test (p < 0.05). BMI, body mass index; FA, fractional anisotropy; OSA, obstructive sleep apnea.
Discussion
In this cross-sectional, case-control study, the TSSA method demonstrates tract-specific abnormalities in OSA and their correlations with polysomnographic parameters and neuropsychological deficits. More specifically, OSA patients showed significantly decreased FA values in bilateral ATR and the right UNC. Furthermore, the lower FA values in the right UNC in OSA was associated with the decreased scores of digit span backward and a low score of the Rey Complex Figure Test copy, suggesting that altered tract-specific changes in the connections from the anterior temporal lobe to the orbital cortex were related to worse cognitive function. Due to significant gender-related differences in the symptoms, diagnosis, consequences, and treatment of OSA [24], the female OSA patients may be a different phenotype and may not involve the same pathophysiology as the typical male OSA patient. Thus, mixing them with male OSA patients would create confounding effects in the finding and make interpretation difficult. To the best of our knowledge, our data has provided new findings of brain connectivity, in terms of fiber directions and local alterations of white matter tracts, with worse cognitive function in men with OSA.
FA, a measure indicating the overall directionality of water diffusion, presents a higher value for white matter tracts of high integrity [25]. FA is sensitive to microstructural tract changes, however, less specific to the type of changes. MD, which describes the magnitude of water diffusion within the brain tissue regardless of the tract orientation, presents a low value in healthy white matter tracts and becomes increased in abnormal white matter tracts that are affected, for example, by inflammation or edema. Our TSSA results demonstrated that the FA values of untreated OSA were significantly reduced in bilateral ATR and the right UNC compared to controls. The FA values of the left UNC also represented a decreased trend, but were not significant. The MD values of the left UNC were additionally increased in the untreated OSA group, although the MD values of the left UNC were not correlated with any clinical parameters. To elucidate the impact of OSA in lateralized difference, further studies with a larger population should be conducted. Although chronic intermittent hypoxia has been considered an important pathomechanism of OSA-consequencing brain injury [1, 26], our analysis dependent on cross-sectional data did not clarify the possible association between polysomnographic measures-related hypoxia and FA or MD values. However, our interaction analysis discovered that oxygen desaturation is an important mechanism in brain connectivity changes, because hypoxic brain injury rather than the frequency of sleep apnea abnormally enhances the association between decreased FA and worse cognitive function. Our result is also supported by a previous review study which provided collective evidence that chronic intermittent hypoxia and neuroinflammation are major contributing factors causing neuronal degeneration and subsequently provoking cognitive dysfunction [27].
Patel et al. reported that nocturnal hypoxemia predominantly due to OSA was associated with white matter hyperintesities independent of other risk factors such as hypertension, diabetes, and age [28]. Our data could be additional evidence of the influence of chronic intermittent hypoxia on the pathomechanism of OSA. Previous studies with DTI revealed multifocal alterations of white matter tracts in untreated OSA [6, 7, 29]. However, directional information, including the portion and length of altered white matter tracts, was not estimated in these studies. Our TSSA results of altered FA/MD values provide the focal portion and length of deteriorated white matter tracts in patients with untreated OSA.
Among the neuropsychological variables, a lower score of digit span backward reflecting function of attention and working memory was significantly associated with lower FA values of the right UNC in the OSA group. Digit span forward is an item that mainly evaluates focused attention, whereas digit span backward is focused on sustained attention. Attention is associated with medial/superior frontal cortical connectivity [30]. The frontal and temporal lobes are the main structures for the function of working memory [31], and the UNC is a major white matter tract connecting the temporal lobe with the frontal cortex [32, 33]. Also, the UNC is larger in the right hemisphere, indicating greater right-sided frontotemporal connectivity [34]. A study in normal aging confirms that working memory performance is associated with variation in connectivity of the UNC [35]. Studies analyzing DTI in various neurological disorders have reported that abnormalities in the UNC are associated with reduced working memory [36–38]. Our proposed DTI method also demonstrated that reduced FA in the right UNC was associated with impaired attention and working memory in patients with OSA.
The OSA group showed that a lower score of the Rey Complex Figure Test copy was significantly associated with lower FA values in the right UNC. A decreased score of the Rey Complex Figure Test copy is indicative of visuo-spatial dysfunction, although the Rey Complex Figure Test immediate, delayed, and recognition items are representative of visual memory function. Visuo-spatial dysfunction including visuo-spatial neglect has been associated with injury to the fronto-parietal attention network [39]. Furthermore, patients with more severe oxygen desaturation presented a stronger association between low FA value and worse cognitive performance. These results suggest that visuo-spatial dysfunction, which is associated with disrupted integrity of UNC connectivity, might be led by intermittent oxygen desaturation in OSA.
Our TSSA method was capable of identifying tract-specific white matter local deficits, unlike other methods such as connectivity analysis and TBSS. Whole brain connectivity analysis cannot investigate which part of fiber tracts undergoes changes in diffusion parameters, but rather investigates alterations for the whole white matter. On the other hand, TBSS captures regional microstructural alterations. For each individual, TBSS computes the maximum diffusion parameter value (e.g., FA) among the voxels located along the direction perpendicular to the mean skeleton, regardless of the tract specification, and projects the maximum value onto the corresponding voxel of the mean skeleton. However, this projection may include the diffusion values from non-target tracts if their values are maximum. Moreover, the skeleton in TBSS is extracted based on the distribution of the FA map for each tract without using the tensor orientation information, which may yield anatomically suboptimal point-wise correspondence across subjects [40]. To utilize the tensor orientation information and subsequently ensure the accurate classification of voxels into the corresponding tract, several approaches employ fiber tractography [41, 42]. Similar to these approaches, TSSA also considers the fiber orientation resulting from the tractography. TSSA could thus capture local alteration of the target tract more precisely than TBSS [10]. Moreover, the TSSA approach is a registration-free method as it classifies each streamline into one of the major tracts by clustering individual streamlines based on their shapes and trajectories without using predefined ROIs/atlases for masking streamlines. On the other hand, other methods are atlas-based [41, 42], requiring a spatial normalization process [10, 17, 43–45], which may make these methods involve additional registration errors.
Furthermore, cluster-based statistics were used to correct for multiple comparisons in our tract-specific analyses, which may provide results more efficacious [20] than other methods such as Bonferroni correction, or the false discovery rate procedure when variables in a probability space are mapped on a manifold (e.g., a collection of points forming the tract skeleton).
Our study has several limitations. First, the controls and OSA patients in the current study showed significant difference in age, although we recruited middle-aged patients and controls. Our statistical analyses took this into account by adjusting individual ages. Our study analyzed cross-sectional data which is not a representative sample for the investigation of the harmful effect of the severity of OSA-driven hypoxia on the brain. Next, the corpus callosum was not included as a major anatomic bundle in this study. The selection of commissural fiber tracts could be considered in following studies. Third, the effect of smoking was not adjusted for in our statistical analyses, though smoking has been considered a factor that can significantly impact white matter integrity [46]. Last, using the current dataset, it is difficult to conclude whether the FA and MD changes are lateralized exclusively to one hemisphere or not, and this may require a larger sample study to clarify.
In conclusion, the adopted TSSA method enabled us to find the specific portion and direction of disconnected fibers. Patients with untreated OSA revealed impaired fiber integrity in bilateral ATRs and the right UNC compared to controls. Furthermore, FA values of the right UNC were significantly associated with performance of attention and working memory and visuospatial memory in the patients. Our results might provide new insight into the pathomechanism of OSA with disrupted integrity of white matter tracts. A previous study from our group demonstrated that cortical atrophy led by OSA can be recovered by long-term treatment using continuous positive airway pressure [47]. Whether the impaired white matter fiber integrity found in the current study is reversible remains unclear; a further study is demanded to investigate the effect of treatments such as continuous positive airway pressure on the white matter integrity in OSA patients.
Supplementary Material
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (2017R1A2B4003120), by Samsung Biomedical Research Institute grant (OTC1190671), by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (No. 2016R1A2B4014398), and the National Research Council of Science & Technology (NST) grant by the Korea Government (MSIT) (No. CAP-18-01-KIST). HK was supported by the National Institutes of Health grant (P41EB015922) and BrightFocus Foundation grant (A2019052S).
Disclosure Statement
None declared.
Conflict of interest statement. None declared.
References
- 1. Koo DL, et al. . Sleep disturbances as a risk factor for stroke. J Stroke. 2018;20(1):12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Décary A, et al. . Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000;23(3):369–381. [PubMed] [Google Scholar]
- 3. Bucks RS, et al. . Reviewing the relationship between OSA and cognition: where do we go from here? Respirology. 2017;22(7):1253–1261. [DOI] [PubMed] [Google Scholar]
- 4. Torelli F, et al. . Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54(2):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen HL, et al. . White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38(3):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macey PM, et al. . Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977. [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar R, et al. . Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90(10):2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lavie L. Intermittent hypoxia: the culprit of oxidative stress, vascular inflammation and dyslipidemia in obstructive sleep apnea. Expert Rev Respir Med. 2008;2(1):75–84. [DOI] [PubMed] [Google Scholar]
- 9. Smith SM, et al. . Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 10. O’Donnell LJ, et al. . Tract-based morphometry for white matter group analysis. Neuroimage. 2009;45(3):832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jung NY, et al. . Tract-specific correlates of neuropsychological deficits in patients with subcortical vascular cognitive impairment. J Alzheimers Dis. 2016;50(4):1125–1135. [DOI] [PubMed] [Google Scholar]
- 12. Iber C, et al. . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed., Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 13. Noh HJ, et al. . The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol. 2012;8(2):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang R, et al. . Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med. 2007; 15. [Google Scholar]
- 15. Mori S, et al. . Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. [DOI] [PubMed] [Google Scholar]
- 16. Yoo SW, et al. . An example-based multi-atlas approach to automatic labeling of white matter tracts. PLoS One. 2015;10(7):e0133337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeatman JD, et al. . Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11):e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bullmore ET, et al. . Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18(1):32–42. [DOI] [PubMed] [Google Scholar]
- 19. Groppe DM, et al. . Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology. 2011;48(12):1711–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han CE, et al. . Cluster-based statistics for brain connectivity in correlation with behavioral measures. PLoS One. 2013;8(8):e72332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludbrook J, et al. . Why permutation tests are superior totandftests in biomedical research. Am Statist. 1998;52(2):127–132. [Google Scholar]
- 22. Nichols TE, et al. . Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Legendre P, et al. . Numerical ecology. 2nd ed. Amsterdam: Elsevier Science; 1998. [Google Scholar]
- 24. Wimms A, et al. . Obstructive sleep apnea in women: specific issues and interventions. Biomed Res Int. 2016;2016:1764837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pirko I. Multiple Sclerosis 3: Advances in Multiple Sclerosis Imaging. Vol. 35 1st ed: Saunders; 2009. [Google Scholar]
- 26. Sforza E, et al. . Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (Auckl). 2016;4:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daulatzai MA. Pathogenesis of cognitive dysfunction in patients with obstructive sleep apnea: a hypothesis with emphasis on the nucleus tractus solitarius. Sleep Disord. 2012;2012:251096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel SK, et al. . Nocturnal hypoxemia is associated with white matter hyperintensities in patients with a minor stroke or transient ischemic attack. J Clin Sleep Med. 2015;11(12):1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castronovo V, et al. . White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37(9):1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Filley CM. The neuroanatomy of attention. Semin Speech Lang. 2002;23(2):89–98. [DOI] [PubMed] [Google Scholar]
- 31. Stern CE, et al. . Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11(4):337–346. [DOI] [PubMed] [Google Scholar]
- 32. Catani M, et al. . Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. [DOI] [PubMed] [Google Scholar]
- 33. Schmahmann JD, et al. . Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(Pt 3):630–653. [DOI] [PubMed] [Google Scholar]
- 34. Highley JR, et al. . Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex. 2002;12(11):1218–1224. [DOI] [PubMed] [Google Scholar]
- 35. Charlton RA, et al. . White matter pathways associated with working memory in normal aging. Cortex. 2010;46(4):474–489. [DOI] [PubMed] [Google Scholar]
- 36. Diehl B, et al. . Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49(8):1409–1418. [DOI] [PubMed] [Google Scholar]
- 37. Hanlon FM, et al. . Frontotemporal anatomical connectivity and working-relational memory performance predict everyday functioning in schizophrenia. Psychophysiology. 2012;49(10):1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKenna BS, et al. . Fusing functional MRI and diffusion tensor imaging measures of brain function and structure to predict working memory and processing speed performance among inter-episode bipolar patients. J Int Neuropsychol Soc. 2015;21(5):330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Szczepanski SM, et al. . Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci. 2010;30(1):148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bach M, et al. . Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage. 2014;100:358–369. [DOI] [PubMed] [Google Scholar]
- 41. Yushkevich PA, et al. . Structure-specific statistical mapping of white matter tracts. Neuroimage. 2008;41(2):448–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang H, et al. . A tract-specific framework for white matter morphometry combining macroscopic and microscopic tract features. Med Image Anal. 2010;14(5):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson RT, et al. . Diffusion properties of major white matter tracts in young, typically developing children. Neuroimage. 2014;88:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maddah M, et al. . Findings in schizophrenia by tract-oriented DT-MRI analysis. Med Image Comput Comput Assist Interv. 2008;11(Pt 1):917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mezer A, et al. . Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med. 2013;19(12):1667–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin F, et al. . Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depend. 2013;129(1-2):82–87. [DOI] [PubMed] [Google Scholar]
- 47. Kim H, et al. . Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum Brain Mapp. 2016;37(1):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.